Abstract
Following a request from the European Commission, the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) was asked to deliver a scientific opinion on synthetic N‐acetyl‐d‐neuraminic acid (NANA) as a novel food (NF) submitted pursuant to Regulation (EC) No 258/97. The information on the composition, the specifications, the batch‐to‐batch variability, stability and production process of the NF is sufficient and does not raise concerns about the safety of the NF. The NF is intended to be marketed as an ingredient in formulae and foods for infants and young children as well as an ingredient in a variety of foods and in food supplements for the general population. NANA is naturally present in human milk, in a bound and free form. The Margin of Exposure, which was based on the no‐observed‐adverse effect level (NOAEL) of 493 mg/kg body weight (bw) per day from a subchronic study and the anticipated daily intake of the NF, was considered to be sufficient for fortified foods for the general population and for food supplements for individuals above 10 years of age, as the anticipated daily intake was in the range of the exposure to free NANA from the consumption of early human milk, which is considered to be safe. The Panel concludes that the NF is safe when added to foods other than food supplements at the proposed uses and use levels for the general population; is safe in food supplements alone at the proposed uses and use levels for individuals above 10 years of age; is safe at the combined intake from fortified foods plus food supplements in individuals above 10 years of age; the safety of the NF is not established in food supplements alone at the proposed uses and use levels for individuals below 10 years of age.
Keywords: N‐acetyl‐d‐neuraminic acid, NANA, novel food, ingredient, safety
Summary
Following a request from the European Commission, the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) was asked to deliver a scientific opinion on synthetic N‐acetyl‐d‐neuraminic acid as a novel food (NF) submitted pursuant to Regulation (EC) No 258/97 of the European Parliament and of the Council. The assessment follows the methodology set in Commission Recommendation 97/618/EC of 29 July 1997 concerning the scientific aspects and the presentation of information necessary to support applications for the placing on the market of novel foods and novel food ingredients and the preparation of initial assessment reports under Regulation (EC) No 258/97 of the European Parliament and of the Council. The assessment is based on the data supplied in the original application, the initial assessment by the competent authority of Ireland, the concerns and objections of a scientific nature raised by the other Member States and the responses of the applicant.
The NF which is the subject of the application is synthetic N‐acetyl‐d‐neuraminic acid dihydrate (NANA·2H2O).
The information provided on the composition, the specifications, the batch‐to‐batch variability, stability and production process of the NF is sufficient and does not raise concerns about the safety of the NF.
The applicant intends to market synthetic NANA (which is in a free form) as an ingredient in infant formula, follow‐on formula, and foods for infants and young children as well as an ingredient in a variety of foods for the general population. The applicant also intends to market synthetic NANA in food supplements (as solid, liquid, syrup‐type or chewable forms) for the general population with the intended maximum daily use levels of 300 mg.
NANA is an endogenously produced monosaccharide, which is present in human milk and in foods of animal origin. In human milk, NANA is predominantly bound to human milk oligosaccharides, to proteins and lipids, but it is also present in its free (unbound) form. In foods of animal origin, NANA is also present in free and bound forms. Taking into account that the extent of the amount of free NANA released from bound NANA in human milk and in foods is unknown, the Panel considers only the exposure of free NANA from human milk and from the background diet.
For teenagers and adults, the combined anticipated daily intake of NF from fortified foods, food supplements and free NANA from the background diet is in the range of the daily intake of free NANA from early human milk, whereas for individuals below 10 years of age the combined intake exceeds the range of the daily intake of free NANA from early human milk.
The information provided does not raise concerns with respect to genotoxicity of the NF.
An oral toxicity study in rats with the NF, which consisted of an initial in‐utero and lactational phase which was followed by a subchronic 90‐day oral toxicity study in the first generation offspring, was provided. Based on the observations from this study, the Panel considers that the no‐observed‐adverse effect level (NOAEL) of the NF is 493 mg/kg body weight (bw) per day.
The margin of exposure (MoE) for the NF (i.e. the ratio between the NOAEL and the anticipated daily intake of the NF), for each population group, was calculated for fortified foods and food supplements.
Taking into account that the anticipated daily intake of the NF from fortified foods would be in the range of the exposure to free NANA from the consumption of early human milk, which is considered to be safe, the Panel considers that the MoE for the NF at the intended uses and use levels in fortified foods is sufficient for the general population.
Taking into account that the anticipated daily intake of the NF from food supplements in individuals above 10 years of age would be in the range of the exposure to free NANA from the consumption of early human milk, which is considered to be safe, the Panel considers that the MoE for the NF at the intended uses and use levels in food supplements is sufficient for individuals above 10 years of age.
The Panel concludes that:
the NF, synthetic N‐acetyl‐d‐neuraminic acid, is safe when added to foods other than food supplements at the proposed uses and use levels for the general population;
the NF is safe in food supplements alone at the proposed uses and use levels for individuals above 10 years of age;
the NF is safe at the combined intake from fortified foods plus food supplements, at the proposed uses and use levels, in individuals above 10 years of age.
the safety of the NF is not established in food supplements alone at the proposed uses and use levels for individuals below 10 years of age because the intake would exceed the level which the Panel considers as safe (11 mg/kg bw) by 5.4 times in infants, 2.3 times in toddlers and 1.2 times in children up to 10 years of age.
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
On 22 September 2015, the company Glycom A/S submitted a request under Article 4 of the Novel Food Regulation (EC) No 258/971 to place on the market synthetic N‐acetyl‐d‐neuraminic acid (NANA) as a novel food (NF).
On 8 March 2016, the competent authority of Ireland forwarded to the Commission its initial assessment report, which came to the conclusion that synthetic N‐acetyl‐d‐neuraminic acid meets the criteria for acceptance of a novel food defined in Article 3(1) of Regulation (EC) No 258/97.
On 15 March 2016, the Commission forwarded the initial assessment report to the other Member States (MS). Several of the MS submitted comments or raised objections.
The concerns of a scientific nature raised by the MS can be summarised as follows:
Residual solvents and heavy metals should be included in the specifications and the limit of residual proteins should be reduced.
Information on the production process to obtain N‐acetylmannosamine, which is the precursor for the production of NANA, should be provided.
A MS noted the impurities eluted at 2.2 min in the 3‐year stability study report. The 5‐year stability report, which was ongoing at the time of the submission of the dossier, was requested to be provided.
Information was needed on the accreditation to internationally recognised systems of the testing facilities, in which chemical and microbiological analyses were performed.
The proposed use level for synthetic NANA in infant and follow‐on formula is based on its presence in breast milk. However, the proposed use levels seem to be at the high end of the range of the reported average values for free NANA levels in breast milk.
Although the consumption of food supplements is proposed as an alternative to foods fortified with the NF, the possibility for combined intake from both types of products should be considered. No information has been provided on the overall intake of total NANA (free and bound) from the background diet.
The amount of synthetic NANA to be added to foods is based on the amount contained in colostrum. Infants who consume transitional and, in particular, mature breast milk are naturally exposed to lower levels of free NANA than infants who consume colostral milk, as the NANA level falls considerably the longer the mother nurses the infant. A MS questioned the natural overall exposure of other age groups, whether the NANA content in colostrum is an appropriate reference point for assessing tolerance in these age groups and whether the doses of NANA which are normal for infants are automatically safe for other age groups.
Some MS commented that owing to the consistently lower body weight (bw) in the high‐dose male group in the 90‐day toxicity study in rats, the no‐observed‐adverse effect level (NOAEL) should be set at the mid‐dose (493 mg/kg bw synthetic NANA).
Tolerance to synthetic NANA in the target population has not been investigated in human studies.
In accordance with Article 29(1)(a) of Regulation (EC) No 178/20022, the European Food Safety Authority (EFSA) is asked to carry out the additional assessment for synthetic N‐acetyl‐d‐neuraminic acid as a NF in the context of Regulation (EC) No 258/97.
EFSA is asked to carry out the additional assessment and to consider the elements of a scientific nature in the comments raised by the other MS.
2. Data and methodologies
2.1. Data
The assessment of the safety of this NF is based on data supplied in the original application, the initial assessment by the competent authority of Ireland, the concerns and objections of a scientific nature of the other MS and the responses of the applicant.
In accordance with Commission Recommendation 97/618/EC,3 synthetic N‐acetyl‐d‐neuraminic acid is allocated to Class 1.2, i.e. ‘foods and food components that are single chemically defined substances or mixtures of these which are not obtained from plants, animals or microorganisms that have been genetically modified. The source of the NF has no history of food use in the Community'. The data are required to comply with the information required for novel foods of Class 1.2, i.e. structured schemes I, II, III, IX, XI, XII and XIII of Commission Recommendation 97/618/EC. In the current scientific opinion, these structured schemes are listed in Sections 3.1–3.8. The intention is to use the NF as an ingredient in formulae and foods for infants and young children, and in foods and food supplements for the general population. This assessment concerns only risk that might be associated with consumption of the NF under the proposed conditions of use, and is not an assessment of the efficacy of the NF with regard to any claimed benefit.
2.2. Methodologies
The assessment follows the methodology set out in Commission Recommendation 97/618/EC of 29 July 1997 concerning the scientific aspects and the presentation of information necessary to support applications for the placing on the market of novel foods and novel food ingredients and the preparation of initial assessment reports under Regulation (EC) No 258/97 of the European Parliament and of the Council.
3. Assessment
3.1. Specification of the NF
The NF which is the subject of the application is synthetic N‐acetyl‐d‐neuraminic acid dihydrate (NANA·2H2O) (chemical formula: C11H23NO10; molecular weight: 345.31 Da; CAS number: 50795‐27‐2).
N‐Acetyl‐D‐neuraminic acid, (IUPAC name: 5‐acetamido‐3,5‐dideoxy‐d‐glycero‐d‐galacto‐non‐2‐ulopyranosonic acid; trivial name sialic acid) (NANA or Neu5Ac or SA), is the most dominant member of the family of sialic acids which comprises over 60 nine‐carbon acidic monosaccharides consisting of N‐ or O‐substituted derivatives of d‐neuraminic acid (Angata and Varki, 2002; Schauer, 2004). NANA is naturally present in human milk and in foods of animal origin.
The molecular structure of NANA was firstly reported by Kuhn and co‐workers in 1962 (Kuhn and Baschang, 1962; Kuhn and Brossmer, 1962) and was confirmed by X‐ray crystallography (Flippen, 1973). In addition, a number of publications have further reported on the structure characterisation of NANA by 1H‐ and 13C‐nuclear magnetic resonance (NMR) techniques (Brown et al., 1975; Dabrowski et al., 1979; Friebolin et al., 1980, 1981; Ogura et al., 1984; Klepach et al., 2008a,b).
The X‐ray single crystal diffraction data indicate that NANA·2H2O is crystallised with two molecules of water, and is the β‐anomer. The dihydrate form of NANA was also confirmed by Karl Fischer titration data. The NMR investigations indicate that when these crystals are dissolved in D2O an equilibrium of the α‐ and the β‐anomers occurs with a 1:9 ratio. Mass spectrometry data indicate that the molecular mass and the fragmentation pattern of the NF correspond to those of NANA as isolated from human origin. The chemical structure of the NF is indicated in Figure 1.
Figure 1.
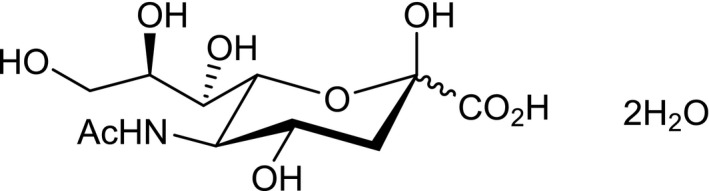
Chemical structure of the NF (NANA·2H2O)
The specifications of the NF are presented in Table 1.
Table 1.
Specifications of the NF
| Parameter | Specification | Method |
|---|---|---|
| Appearance | Crystalline powder | Visual |
| Colour | White to off white | Visual |
| Identification | RT corresponds to RT of standard ± 3% | Glycom method HPLC‐003‐002 |
| NANA·2H2O (additional water and solvent free) | Min. 97.0% | Glycom method HPLC‐003‐002 |
| pH (20°C, 5% solution) | 1.7–2.5 | WBSE‐77:2012 |
| Water (dihydrate calculates to 10.4%) | Max. 12.5% | Karl Fischer (EP 2.5.32) |
| Ash, sulfated | Max. 0.2% (w/w) | EP 6.7 04/2010:20414 |
| Acetic acid (as free acid and/or sodium acetate) | Max. 0.5% (w/w) | MSZ EN ISO 10304‐1:2009 |
| 2‐Propanola | Max. 0.1% (w/w) | EP GC 2.4.24 |
| Acetonea | Max. 0.1% (w/w) | EP GC 2.4.24 |
| Ethyl acetatea | Max. 0.1% (w/w) | EP GC 2.4.24 |
| Residual proteinsb | Max. 0.01% (w/w) | Bradford Assay; Glycom method UV‐001 |
| Ironc | Max. 20 mg/Kg | EPA 6010C:2007 |
| Leadd | Max. 0.1 mg/Kg | EPA 6020A:2007 |
| Microbiological specifications | ||
| Salmonella | Absent in 25 g | MSZ‐EN‐ISO 6579:2006 |
| Aerobic mesophilic total count | Max. 500 CFU/g | MSZ‐EN‐ISO 4833:2003 |
| Enterobacteriaceae | Absent in 10 g | MSZ‐ISO 21528‐2:2007 |
| Cronobacter (Enterobacter) sakazakii | Absent in 10 g | ISO‐TS 22964:2006 |
| Listeria monocytogenes | Absent in 25 g | MSZ‐EN‐ISO 11290‐1:1996, 1998/A1:2005 |
| Bacillus cereus | Max. 50 CFU/g | MSZ‐EN‐ISO 7932:2005 |
| Yeasts | Max. 10 CFU/g | MSZ‐ISO 7954:1999 |
| Moulds | Max. 10 CFU/g | MSZ‐ISO 7954:1999 |
| Residual endotoxins | Max. 10 EU/mg | LAL Kinetic chromogenic assay (EP 2.6.14) |
CFU: colony forming units; EP: European Pharmacopeia; EU: endotoxin units; GC: gas chromatography; HPLC: high‐performance liquid chromatography; ISO: International Organization for Standardization; LAL: Limulus Amebocyte Lysate; LOQ: limit of quantification; RT: retention time.
a LOQ = 0.001%; b LOQ = 0.0017%; c LOQ = 1%; d LOQ = 0.1%.
Upon request by MS and EFSA, residual solvents (2‐propanol, acetone and ethyl acetate) and heavy metals (iron and lead) were included in the specifications and the limit of residual proteins was reduced to 0.01%.
Impurities which may potentially remain in the NF include residual starting materials (N‐acetyl‐d‐mannosamine and sodium pyruvate), and other carbohydrate‐type compounds which may stem from anhydrous NANA. These impurities are controlled by additional internal product specifications (not more than 1.0% for N‐acetyl‐d‐mannosamine and sodium pyruvate; not more than 0.4% for each single other impurity; not more than 1% for total other impurities) and by the Hazard Analysis and Critical Control Points (HACCP) procedure which is in place.
Because of the sensitivity of infants to microbial toxins, the endotoxin specification for the NF is set to not exceed the levels reported for infant formula powder (Townsend et al., 2007).
In reply to a comment from a MS, the applicant assured that all analytical measurements were performed using internationally recognised methods in accredited external laboratories, or, where no internationally recognised methods existed (for NANA itself, N‐acetyl‐d‐mannosamine and sodium pyruvate), these were developed by Glycom and validated according to Good Laboratory Practice (GLP) standards.
In order to confirm that the manufacturing process is reproducible and adequate to produce a product that is within the specifications as set in Table 1, the applicant provided batch‐to‐batch analyses of five commercial batches of the NF (Table 2).
Table 2.
Batch‐to‐batch analyses of the NF
| Parameter | Specification | Batch results | ||||
|---|---|---|---|---|---|---|
| L12058K | L3A075K | L3A076K | L3B014K | L3B015K | ||
| Appearance | Crystalline powder | Crystalline powder | Crystalline powder | Crystalline powder | Crystalline powder | Crystalline powder |
| Colour | White to off‐white | White | White | White | White | White |
| NANA identification | RT corresponds to RT (standard) ± 3% | Complies | Complies | Complies | Complies | Complies |
| NANA·2H2O by HPLC (additional water free) (%; w/w) | Min. 97.0 | 98.6 | 98.9 | 99.0 | 98.5 | 99.2 |
| pH (20°C, 5% solution) | 1.7 to 2.5 | 2.1 | 1.84 | 1.83 | 1.83 | 1.84 |
| Water (%; w/w) | Max. 12.5 | 10.6 | 10.9 | 10.6 | 10.6 | 10.5 |
| Ash, sulfated (%; w/w) | Max. 0.2 | 0.03 | 0.11 | 0.01 | 0.04 | 0.01 |
| Acetic acid (as free acid and/or sodium acetate) (%; w/w) | Max. 0.5 | < 0.01 | < 0.2 | < 0.2 | < 0.2 | < 0.2 |
| 2‐Propanola (%; w/w) | Max. 0.1 | 0.07 | 0.046 | 0.055 | 0.055 | 0.044 |
| Acetone (%; w/w) | Max. 0.1 | < LOQa | < LOQa | < LOQa | < LOQa | < LOQa |
| Ethyl acetate (%; w/w) | Max. 0.1 | < LOQa | < LOQa | < LOQa | < LOQa | < LOQa |
| Residual proteinsb (%) | Max. 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
| Ironc | Max. 20 mg/Kg | 1 | 10 | 7 | 6 | 7 |
| Leadd | Max. 0.1 mg/Kg | < LOQd | < LOQd | < LOQd | < LOQd | < LOQd |
| Microbiological specifications | ||||||
| Salmonella | Absent in 25 g | Complies | Complies | Complies | Complies | Complies |
| Aerobic mesophilic total count (CFU/g) | Max. 500 | < 10 | < 10 | < 10 | < 10 | < 10 |
| Enterobacteriaceae | Absent in 10 g | Complies | Complies | Complies | Complies | Complies |
| Cronobacter (Enterobacter) sakazakii | Absent in 10 g | Complies | Complies | Complies | Complies | Complies |
| Listeria monocytogenes | Absent in 25 g | n/a | Complies | Complies | Complies | Complies |
| Bacillus cereus (CFU/g) | Max. 50 | n/a | < 10 | < 10 | < 10 | < 10 |
| Yeasts (CFU/g) | Max. 10 | n/a | < 10 | < 10 | < 10 | < 10 |
| Moulds (CFU/g) | Max. 10 | n/a | < 10 | < 10 | < 10 | < 10 |
| Residual endotoxins (EU/mg) | Max. 10 | < LORe | < LORe | < LORe | < LORe | < LORe |
CFU: colony forming units; EU: endotoxin units; HPLC: high‐performance liquid chromatography; LOQ: limit of quantification; LOR: level of reporting; n/a: not applicable; RT: retention time.
a LOQ for residual solvents = 0.001%; b LOQ for proteins = 0.0017%; c LOQ for iron = 1%; d LOQ for lead = 0.1%; e LOR = 0.01 EU/mg.
The Panel considers that the information provided on the composition, the specifications and the batch‐to‐batch variability of the NF is sufficient and does not raise safety concerns.
3.1.1. Stability of the NF
As requested by a MS, a 5‐year long‐term stability study on crystalline NANA·2H2O from a single batch has been provided. In this study, samples were packed into polyethylene bags as the primary packaging material, which was then packed into polyethylene/aluminium/polyester triple layer foil bags as the secondary packaging material and stored under the following storage conditions: 25°C/60% relative humidity (RH%), 5°C or −20°C. NANA·2H2O was analysed by high‐performance liquid chromatography (HPLC) and water content by Karl Fischer titration. No significant change was observed in the value of NANA·2H2O during the 60 month storage at −25°C and at 5 C°, whereas at 25°C a slight decrease of the value was observed after 48 and 60 months storage at this temperature. An unidentified impurity eluting at 2.2 min in the HPLC chromatogram is present at the three storage conditions just over the 0.1% (max 0.13%) and the amount is not increasing with the time of storage; therefore, this impurity is not considered as a degradation product by the Panel. No other degradation products above 0.1% were indicated by HPLC during the stability study.
The presence of potential degradation products during storage of NANA·2H2O was also investigated by high‐performance anion exchange chromatography with pulsed amperometric detection (HPAEC‐PAD). The analysis revealed three carbohydrate‐type impurities that were detected at 36 months of storage. Only one of the impurities, eluting at 8.2 min, showed increasing intensity with the increasing temperature of storage indicating that it is degradation product which was isolated and its structure was characterised as a dehydrated species of NANA (4‐epi‐NANA‐2en) (Sugiyama et al., 1991). In any case, values remained within the established internal product specifications (see Section 3.1).
No significant change in the water content was observed under any of the storage conditions throughout the 36 months of storage, indicating that the packaging is appropriate in protecting NANA·2H2O from water absorption. Microbiological purity was also maintained for up to 36 months under all storage conditions. No change was also observed by sensory testing during the 60 month storage at three different conditions.
The results of this study indicate that NANA·2H2O is stable with no significant degradation when stored at a temperature of 25°C at 60% RH for periods up to 36 months and at temperature of 5°C or −20°C for periods up to 60 months. The current shelf‐life of crystalline NANA·2H2O has been established as 36 months when stored at 25°C at 60% RH, and as 60 months when stored at 5°C or −20°C.
A 24‐month accelerated stability study (40°C and 75% RH in a climatic chamber) was performed on crystalline NANA·2H2O from a single batch which was packed as in the long‐term real‐time stability study described above. NANA·2H2O was analysed by HPLC and water content by Karl Fischer titration. No significant change in the water content was observed throughout the 24 months of storage, as well as in the microbiological purity. The only change observed was the discoloration of the samples from white to brownish‐white throughout the course of the study. Degradation products were measured by HPAEC at the 24‐month time point only and were found to be the same as those detected in the long‐term real‐time stability study at the 36‐month time‐point and remained within the established internal product specifications. These results indicate that NANA·2H2O is stable with no significant degradation when stored under accelerated conditions (40°C and 75% RH) for periods of up to 24 months. However, the shelf‐life of crystalline NANA·2H2O is established based on the long‐term real‐time stability study.
The Panel considers that the data provided sufficient information with respect to the stability of the NF.
3.1.2. Stability under the intended conditions of use
The stability of NANA·2H2O in a representative powdered infant formula (IF) is being evaluated in an on‐going 3‐year study. The IF powder used in the study is a whey‐based formula also containing skimmed milk powder, vegetable oil, lactose, a vitamin/mineral premix, and lecithin. The composition of the IF powder is representative of commercial IF on the market and is in accordance with EFSA's guidelines on the essential composition of infant and follow‐on formulae (EFSA NDA Panel, 2014).
NANA·2H2O from a single batch was added to IF powder. One IF batch with no added NANA·2H2O was used as a control. IF samples were collected, blended to ensure homogeneity, and packaged in aluminium milk powder cans, which were then sealed and filled with nitrogen gas. Cans were then stored at 5°C, 25°C/60% RH, 30°C/65% RH, or 40°C/75% RH under standard storage conditions according to the International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use.
Measurement for NANA·2H2O content was scheduled at different time points up to 108‐day time point. However, since the study is currently on‐going, data are available up to the 720‐day time point. NANA was analysed by HPAEC‐PAD. The presence of carbohydrate‐type degradation products was not assessed, on account of the complex IF matrix.
The analytical data demonstrate no significant loss of NANA when added to an IF powder and stored at temperatures of up to 40°C over a period of 360 days when compared to the initial NF concentration. Microbiological purity also was maintained during the 360‐day storage period. Based on an acceptance criteria of 90% recovery, a shelf life of 2 years is proposed by the applicant for storage at 25 C° and below, and a shelf life of 1 year for storage in tropical countries (35 C°/65% RH).
The stability of synthetic NANA in other foods (such as yogurts, ready‐to‐drink flavoured milk, citrus fruit drinks, and cereal bars) was assessed under these foods' storage conditions. The synthetic NANA content was measured by HPLC with fluorescence detection. These stability studies showed that the NF is stable when added to yogurt, citrus fruit drinks and ready‐to‐drink chocolate‐flavoured milk following typical processing conditions and when stored at 4°C over the shelf‐life of these foods. The analytical data further demonstrate that the NF is stable when added to cereal bars following storage under ambient conditions over a 3‐month period.
The Panel considers that the data provided sufficient information with respect to the stability of the NF under the intended conditions of use.
3.2. Effect of the production process applied to the NF
The manufacturing process consists of two steps. In the first step, highly pure N‐acetyl‐d‐mannosamine, produced by Glycom from d‐fructose in a two‐step process, and sodium pyruvate are coupled using an isolated and purified enzyme. The product of the enzymatic reaction is then subjected to a series of processing steps, including removal of the enzyme, to produce crude NANA. The anhydrous NANA product spontaneously crystallises and is washed with solvent. Upon EFSA request, details on the production process to obtain N‐acetyl‐d‐mannosamine have been provided together with its certificate of analysis. The applicant also provided details on the enzyme, for which an application according to Regulation (EC) 1332/2008 has not yet been submitted.
In the second step, anhydrous NANA undergoes a series of processes which include purification with activated charcoal and subsequent filtration to remove colour and potential impurities. Microfiltration using a 0.2 μm filter is applied to ensure the absence of microbiological contamination. The raw NANA is then subjected to a crystallisation step (with the aid of 2‐propanol), and the crystals washed and dried to generate the final NANA dihydrate crystalline powdered product (NANA·2H2O).
NANA·2H2O is manufactured in compliance with current Good Manufacturing Practice and the principles of HACCP. Key intermediates are isolated in purified form and analytically characterised. Specifications for key intermediates are set according to HACCP principles, and conformity is monitored by certificates of analysis at each key‐stage of the production process.
The Panel considers that the production process is sufficiently described and does not raise safety concerns.
3.3. History of the organism used as a source of the NF
The NF which is the subject of this application is synthetically produced NANA and it is not extracted from a biological source. Thus, this section is not applicable for the NF under evaluation.
3.4. Anticipated intake/extent of use of the NF
The applicant intends to market synthetic NANA (which is in a free form) as an ingredient in formulae and foods for infants and young children (toddlers; aged 1–3 years old), as well as an ingredient in a variety of foods for the general population.
The applicant also intends to market synthetic NANA in food supplements in solid forms (e.g. capsules, tablets), liquid or syrup‐type or chewable forms for the general population.
3.4.1. Uses for infants and young children
The applicant intends to market synthetic NANA (which is in a free form) as an ingredient in IF (including formulae for special medical purposes), follow‐on formula (FOF) and foods for infants and young children (Table 3). The proposed maximum use level in IF is based on providing a similar level of free NANA as it occurs naturally in breast milk. The proposed maximum use level of synthetic NANA in all other foods is based on providing similar intake of free NANA from breast milk in infants on a mg/kg bw basis. Information on NANA levels in breast milk is provided in Section 3.4.7.
Table 3.
Proposed food uses and maximum use levels for synthetic NANA for infants and young children
| EU food category | Food category name | Proposed maximum use level |
|---|---|---|
| Foods covered by Regulation (EU) No 609/2013 | Infant formulae as defined by Regulation (EU) No 609/2013 | 0.05 g/L of reconstituted formula |
| Follow‐on formulae as defined by Regulation (EU) No 609/2013 | 0.05 g/L of reconstituted formula | |
| Processed cereal‐based foods and baby foods for infants and young children as defined by Regulation (EU) No 609/2013 | 0.05 g/kg for solid foods | |
| Foods for infants and young children for special medical purposes as defined by Regulation (EU) No 609/2013 | As specified on a case by case basis in accordance with Commission Directive 1999/21 |
3.4.2. Anticipated intake of the NF in infants up to 6 months of age from fortified IF
For infants aged 0–6 months, the estimated intake of the NF was calculated from the use of synthetic NANA in IF alone, under the assumption that it would be the only source of NANA in this population group. The daily intake of liquid IF was estimated to be 1,060 mL for infants aged 0–6 months based on the food consumption data (Kersting et al., 1998) previously used by EFSA (EFSA AFC Panel, 2007). This food intake scenario was based on a 3‐month‐old infant weighing 6.1 kg consuming 174 mL/kg bw per day of IF at the 95th percentile. Assuming an intended use level of 50 mg synthetic NANA/L formula, the 95th percentile daily intake of synthetic NANA from IF alone is estimated to be 53 mg/day (8.7 mg/kg bw) in infants aged 0–6 months.
3.4.3. Anticipated intake of the NF in infants and young children aged 4–17 months from fortified formulae and foods
For infants and young children aged 4–17 months, the applicant estimated the intake of the NF from the data on consumption of IF, FOF, other foods for infants and young children from the UK Diet and Nutrition Survey on Infants and Young Children DNSIYC conducted in 2011 (NatCen Social Research et al., 2013)4 and the proposed maximum use levels in foods for infants and young children (Table 3).
The anticipated daily intake of synthetic NANA in infants and young children in the UK is presented in Tables 4 and 5 (on a mg/day and mg/kg bw per day basis, respectively). Infants have the highest mean (30 mg/day) and 95th percentile (60 mg/day) intakes of synthetic NANA on an absolute basis (Table 4). On a body weight basis, infants aged 4 to 6 months have the highest mean and 95th percentile intakes (4.1 and 7.0 mg/kg bw per day, respectively) (Table 5).
Table 4.
Estimated daily intake (mg) of synthetic NANA for infants and young children in the UK, based on data from DNSIYC 2011
| Population group | Age group (months) | All‐users consumptiona (mg per day) | |||
|---|---|---|---|---|---|
| % consumers | Number of consumers | Mean | 95th percentile | ||
| Infants | 4–6 | 98.1 | 323 | 30 | 60 |
| Infants | 7–12 | 94.8 | 1,252 | 30 | 50 |
| Young children | 13–17 | 67.5 | 688 | 10 | 40 |
For the DNSIYC, only intakes based on the category ‘Foods for infants and young children’ were included in the assessment.
Table 5.
Estimated daily intake (mg/kg bw) of synthetic NANA for infants and young children in the UK, based on data from DNSIYC 2011
| Population group | Age group (months) | All‐users consumptiona (mg/kg bw per day) | |||
|---|---|---|---|---|---|
| % consumers | Number of consumers | Mean | 95th percentile | ||
| Infants | 4–6 | 98.1 | 323 | 4.1 | 7.0 |
| Infants | 7–12 | 94.8 | 1252 | 2.9 | 5.7 |
| Young children | 13–17 | 67.5 | 688 | 1.1 | 3.5 |
body weight.
For the DNSIYC, only intakes based on the category ‘Foods for infants and young children’ were included in the assessment.
3.4.4. Fortified foods
Intended food uses and maximum use levels of the NF are presented in Table 6. The proposed maximum use levels are based on providing similar intake of free NANA from breast milk in infants on a mg/kg bw basis. Information on NANA levels and intakes from breast milk in infants is provided in Section 3.4.7.
Table 6.
Proposed uses and maximum use levels for synthetic NANA (g/kg)a
| Food category | Food category name | Maximum level per serving | Suggested serving size | Proposed maximum use level (g/kg) |
|---|---|---|---|---|
| Dairy products and analogues | Unflavoured pasteurised and sterilised (including UHT) milk | 0.05 g/L | – | 0.05 g |
| Unflavoured fermented milk products |
0.05 g/L for beverages Other foods: 0.05 g /serving |
125 g for solid foods |
0.05 g beverages 0.4 g solids |
|
| Unflavoured fermented milk products, heat‐treated after fermentation | 125 g | |||
| Flavoured fermented milk products including heat‐treated products | 125 g | |||
| Dairy analogues, including beverage whiteners |
0.05 g/L for beverages Other foods: 0.05 g/serving |
200 g for solid foods |
0.05 g beverages 0.25 g solids |
|
| 0.05 g per sachet whitener | 3 g | 16.7 g | ||
| Bakery wares | Fine bakery wares. Cereal bars only | 0.5 g/kg | – | 0.5 g |
| Sugars, syrups, honey and table‐top sweeteners | Table top sweeteners | 0.05 g per sachet | 6 g sachet | 8.3 g |
| Foods covered by Regulation (EU) No 609/2013 | Infant formulaec as defined by Regulation (EU) No 609/2013 | 0.05 g/L of reconstituted formula | –b | 0.05 g |
| Follow‐on formulaec as defined by Regulation (EU) No 609/2013 | 0.05 g/L of reconstituted formula | –b | 0.05 g | |
| Processed cereal‐based foods and baby foodsc for infants and young children as defined by Regulation (EU) No 609/2013 | 0.05 g/kg for solid foods | – | 0.05 g | |
| Foods for special medical purposes as defined by Regulation (EU) No 609/2013 (excluding products from food category 13.1.5)d | As specified on a case by case basis in accordance with Commission Directive 1999/21 | Case‐by case basis | Case‐by case basis | |
| Total diet replacement for weigh control as defined by Regulation (EU) No 609/2013 | 0.05 g/meal replacement |
250 g drinks 30 g bars |
0.2 g (drinks) 1.7 g (bars) |
|
| Foods bearing statements on the absence or reduced presence of gluten in accordance with the requirements of Commission Implementing Regulation (EU) No 828/2014 | 0.05 g/serving | Bread products 40 g, pastas 55 (unprepared) to 110 g (prepared) | 0.5–1.25 g | |
| Beverages | Fruit juices as defined by Directive 2001/112/EC and vegetable juices (Commission of the European Communities, 2001) | 0.05 g/L | – | 0.05 g |
| Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products (Commission of the European Communities, 2001) | 0.05 g/L | – | 0.05 g | |
| Flavoured drinks | 0.05 g/L | – | 0.05 g | |
| Speciality coffee, tea, herbal and fruit infusions, chicory; tea, herbal and fruit infusions and chicory extracts; tea, plant, fruit and cereal preparations for infusions | 0.05 g/serving | 250 | 0.2 g |
While it is proposed that synthetic NANA will be added to these food categories, including ‘Dairy products and analogues’, once placed on the market, all food products with added synthetic NANA would be appropriately reclassified and labelled.
A serving size is not provided on the basis that the use level is specified on a per L/kg basis and not on a per serving basis. The suggested serving size for IF is according to the manufacturer's instructions and according to the baby's age and weight.
These categories were also assessed using food consumption data from the DNSIYC.
This category was not included in the UK NDNS intake assessment as these products should be assessed on a case‐by‐case basis, and are not widely consumed by the general population.
3.4.5. Anticipated intake of the NF from fortified foods
In a tiered approach, the applicant estimated the mean and high‐intake of the NF by using the EFSA Food Additive Intake Model (FAIM) tool. The Panel notes that the methodology of the FAIM tool, which is based on summary statistics of consumption data of the EFSA Comprehensive Food Consumption Database, generally leads to an overestimation of the estimated intakes as described in the EFSA instructions for the use of the FAIM tool (EFSA, 2012).
Thus, for a refined intake estimate of the NF, the applicant used individual consumption data recorded by the UK National Diet and Nutrition Survey (NDNS) for the years 2008–2012 (NatCen Social Research et al., 2015) and the proposed maximum use levels of the NF. For this refined intake estimate, individual food codes representative of each proposed food use were taken from the food code list associated with the UK NDNS food consumption survey and grouped according to the proposed uses for synthetic NANA as presented in Table 6. The daily intake of synthetic NANA was calculated at individual level, and represented projected 4‐day averages for each individual from days 1 to 4 of NDNS data, in the UK population. Anticipated daily intake estimates are presented in Table 7 (on a mg basis) and Table 8 (on a mg/kg bw basis).
Table 7.
Estimate daily intake of synthetic NANA (mg) from the proposed food categories by different population groups in the UK, based on the data from the NDNS 2008–2012
| Population group | Age group (years) | All‐users consumption (mg per day) | |||
|---|---|---|---|---|---|
| % of consumers | Number of consumers | Mean | 95th percentile | ||
| Toddlers | 1–3 | 100.0 | 386 | 50 | 100 |
| Children | 4–10 | 100.0 | 803 | 50 | 100 |
| Teenagers | 11–18 | 99.8 | 881 | 50 | 110 |
| Women of childbearing age | 19–40 | 99.9 | 419 | 50 | 130 |
| Female adults | 19–64 | 99.7 | 941 | 40 | 120 |
| Male adults | 19–64 | 99.6 | 706 | 50 | 130 |
| Elderly adults | above 65 | 99.6 | 425 | 40 | 120 |
Table 8.
Estimate daily intake of synthetic NANA (mg/kg bw) from the proposed food categories by different population groups in the UK, based on the data from the NDNS 2008–2012
| Population group | Age group (years) | All‐users consumption (mg/kg bw per day) | |||
|---|---|---|---|---|---|
| % of consumers | Number of consumers | Mean | 95th percentile | ||
| Toddlers | 1–3 | 100.0 | 337 | 3.6 | 7.1 |
| Children | 4–10 | 100.0 | 774 | 1.9 | 4.2 |
| Teenagers | 11–18 | 99.8 | 850 | 0.9 | 2.1 |
| Women of childbearing age | 19–40 | 99.9 | 396 | 0.7 | 1.9 |
| Female adults | 19–64 | 99.7 | 874 | 0.6 | 1.7 |
| Male adults | 19–64 | 99.5 | 663 | 0.6 | 1.5 |
| Elderly adults | 65+ | 99.5 | 390 | 0.6 | 1.7 |
bw: body weight.
With regard to intakes on an absolute basis (Table 7), the anticipated mean daily intake of the NF ranges between 40 mg and 50 mg, whereas the anticipated 95th percentile daily intake of the NF ranges between 100 mg and 130 mg among population groups.
On a body weight basis (Table 8), toddlers have the highest mean and 95th percentile daily intake of the NF among population groups (3.6 and 7.1 mg/kg bw, respectively), followed by children (1.9 and 4.2 mg/kg bw, respectively) and teenagers (0.9 and 2.1 mg/kg bw, respectively). For adults and elderly, the mean daily intake of the NF is 0.6‐0.7 mg/kg bw and the 95th percentile daily intake of the NF ranges from 1.5 to 1.9 mg/kg bw.
The Panel considers that the refined intake estimate based on UK individual consumption data is sufficiently conservative, as it is based on the assumption that all proposed food items consumed by an individual contain the NF at the maximum proposed use levels as presented in Table 6.
3.4.6. Food supplements
The applicant intends to market synthetic NANA in food supplements (as solid, liquid, syrup‐type or chewable forms) for the general population (infants, toddlers, children, teenagers and adults). The intended maximum daily use levels of the NF in food supplements is 300 mg.
Based on the mean body weight as recommended by the EFSA Scientific Committee (2012), the maximum daily intake of the NF from food supplements results in 60 mg/kg bw for infants, 25 mg/kg bw for toddlers, 13 mg/kg bw for children, 7 mg/kg bw for teenagers and 4.3 mg/kg bw for adults.
Following a comment from a MS, the applicant agreed that information should be given that food supplements with the NF intended for toddlers should not be used if breast milk or other foods with added NANA are consumed the same day.
3.4.7. Intake of NANA from breast milk
Sialic acids comprise over 60 nine‐carbon acidic monosaccharides consisting of N‐ or O‐substituted derivatives of d‐neuraminic acid (Angata and Varki, 2002; Schauer, 2004). Sialic acids occur naturally in human breast milk only in the N‐acetylated form of the D‐neuraminic acid (NANA), whereas in foods of animal origin and in mammalian milk sialic acids occur as a mixture of the N‐acetylated and N‐glycolylated forms.
In breast milk, NANA occurs predominantly bound to human milk oligosaccharides (HMOs), proteins (glycoproteins), and lipids (gangliosides), as well as in its free (unbound) form (Sabharwal et al., 1991; Hayakawa et al., 1993; Rueda et al., 1995; Puente et al., 1996; Thurl et al., 1996; Wang et al., 2001; Wiederschain and Newburg, 2001; Martín‐Sosa et al., 2004; Oriquat et al., 2011; Galeotti et al., 2012).
In human milk, free NANA constitutes only a fraction of the total NANA, as NANA is mainly bound to HMOs. The average levels of free NANA range approximately between 20 and 60 mg/L in early milk (colostrum and first month milk) and 10 and 40 mg/L in mature milk (> 1 month milk) (Hayakawa et al., 1993; Thurl et al., 1996; Wang et al., 2001; Wiederschain and Newburg, 2001; Martín‐Sosa et al., 2004; Oriquat et al., 2011; Qiao et al., 2013). Some publications were excluded by the applicant as they reported significantly high levels of free NANA in human breast milk (up to 960 mg/L) (Sabharwal et al., 1991; Galeotti et al., 2012).
The average levels of total NANA (bound and free) ranges between 900 and 1,800 mg/L in early milk and between 300 and 800 mg/L in mature milk (Hayakawa et al., 1993; Wang et al., 2001; Martín‐Sosa et al., 2004; Oriquat et al., 2011; Qiao et al., 2013). The Panel notes that the extent of the amount of free NANA released from bound NANA is unknown.
The intake of free and total NANA from early and mature human milk in infants was calculated. Based on the above free NANA levels in early milk, the daily intake of free NANA ranges between 3.5 and 11 mg/kg bw for a 3.4 kg bw newborn infant drinking 600 mL of breast milk per day (Hester et al., 2012; WHO, 2013). Based on the above free NANA levels in mature milk, the daily intake of free NANA ranges between 1.5 and 6 mg/kg bw for a 6.5‐kg bw infant drinking 1 L of breast milk per day (Davies et al., 1994; Hester et al., 2012). Likewise, based on the above total NANA levels in early and mature human milk, the daily intake of total NANA ranges between 160 and 320 mg/kg bw for early milk and between 45 and 125 mg/kg bw for mature milk.
3.4.8. Intake of NANA from the background diet
A background dietary exposure to free NANA for infants results from the IF consumption. Free NANA has been detected in food‐grade d‐lactose, a nutritional ingredient added to IF, at amounts between 20 and 50 mg/kg d‐lactose (Spichtig et al., 2010, 2011). The addition of d‐Lactose in IF results in free NANA levels of approximately 1.3–3.3 mg/L in IF. Therefore, a low background exposure to exogenous free NANA (0.6 mg/kg bw) from the consumption of IF exists.
Foods of animal origin contain NANA in free and bound forms (e.g. glycosidic conjugates, gangliosides, glycolipids or glycoproteins).
The free NANA content in foods of animal origin has been presented by Rohrig et al. (2017): fish eggs (caviar) from whitefish and salmon (459 and 149 mg/kg, respectively); fish (e.g. 117 mg/kg in mahi‐mahi, 106 mg/kg in wild salmon, 45 mg/kg in tuna, 30 mg/kg in swordfish); red meat, milk and cheese (10–40 mg/kg); poultry meat (7 mg/kg).
Based on the content of free NANA in foods and the consumption data from the UK NDNS rolling programme (2008‐2012), background dietary exposure to free NANA was calculated for the UK population.5 Tables 9 and 10 present the estimated dietary daily intakes of free NANA for the UK population on an absolute and body weight basis, respectively (mean and 95th percentile intakes).
Table 9.
Estimated background dietary daily intakes of free NANA (mg) in the UK by population group (NDNS Data, 2008–2012)
| Population group | Age group (years) | Daily dietary intake of free NANA (mg/day) | |||
|---|---|---|---|---|---|
| All‐users | |||||
| % consumers | N consumers | Mean | 95th percentile | ||
| Toddlers | 1–3 | 100.0 | 386 | 9.2 | 19.9 |
| Children | 4–10 | 99.5 | 799 | 7.4 | 16.2 |
| Teenagers | 11–18 | 100.0 | 884 | 6.0 | 16.5 |
| Adults | 19–64 | 99.9 | 1,653 | 6.6 | 14.7 |
| Elderly | ≥ 65 | 99.4 | 426 | 8.1 | 15.6 |
Table 10.
Estimated background dietary daily intakes of free NANA (mg/kg bw) in the UK by population group (NDNS Data, 2008–2012)
| Population group | Age group (years) | Daily dietary intake of free NANA (mg/kg bw per day) | |||
|---|---|---|---|---|---|
| All‐users | |||||
| % consumers | N consumers | Mean | 95th percentile | ||
| Toddlers | 1–3 | 100.0 | 337 | 0.65 | 1.57 |
| Children | 4–10 | 99.5 | 770 | 0.30 | 0.75 |
| Teenagers | 11–18 | 100.0 | 853 | 0.11 | 0.31 |
| Adults | 19–64 | 99.9 | 1,543 | 0.09 | 0.20 |
| Elderly | ≥ 65 | 99.4 | 391 | 0.11 | 0.25 |
bw: body weight.
Among population groups, toddlers have the highest mean and 95th percentile dietary daily intakes of free NANA (9.2 and 19.9 mg, respectively), while teenagers have the lowest mean dietary daily intakes of free NANA (6.0 mg) and adults have the lowest 95th percentile dietary daily intakes of free NANA (14.7 mg) (Table 9).
On a body weight basis, toddlers have the highest mean and 95th percentile dietary daily intakes of free NANA (0.65 and 1.57 mg/kg bw, respectively), whereas adults have the lowest mean and 95th percentile dietary daily intakes of free NANA (0.09 and 0.20 mg/kg bw, respectively) (Table 10).
NANA is also present in bound forms in foods of animal origin. NANA can be released from its bound forms owing to the limited acid stability of the conjugates or through enzymatic cleavage (Rohrig et al., 2017). Therefore, dietary NANA in its bound form would contribute to the systemic exposure to free NANA. However, the extent of the amount of free NANA released from bound NANA is unknown.
Upon a MS's comment on the dietary exposure to bound NANA, the applicant calculated the background dietary exposure to total NANA (bound and free) for the UK population based on the consumption data NDNS rolling programme (2008–2012) and the content of total NANA in foods (Rohrig et al., 2017) (e.g. crucian carp caviar 4,600 mg/kg; whitefish caviar 2,700 mg/kg; cheddar cheese 1,800 mg/kg; salmon caviar 1,200 mg/kg; mozzarella cheese 420 mg/kg; egg white 250 mg/kg; swordfish 250 mg/kg; cow milk 190 mg/kg; tuna 83 mg/kg).
The estimated background dietary daily intakes of total NANA is presented in Tables 11 and 12 (on a mg and mg/kg bw basis, respectively).
Table 11.
Estimated daily background dietary intakes of total NANA (mg/day) in the UK by population group (NDNS Data, 2008–2012)
| Population group | Age group (years) | Dietary intake of total NANA (mg/day) | |||
|---|---|---|---|---|---|
| % consumers | N consumers | Mean | 95th percentile | ||
| Toddlers | 1–3 | 100.0 | 386 | 76.0 | 147.2 |
| Children | 4–10 | 99.5 | 799 | 66.2 | 137.6 |
| Teenagers | 11–18 | 100.0 | 884 | 83.4 | 138.6 |
| Adults | 19–64 | 99.9 | 1,653 | 74.6 | 159.2 |
| Elderly | 65+ | 99.4 | 426 | 82.7 | 155.6 |
Table 12.
Estimated daily background dietary intakes of total NANA (mg/kg bw per day) in the UK by population group (NDNS Data, 2008–2012)
| Population group | Age group (years) | Dietary intake of total NANA (mg/kg bw per day) | |||
|---|---|---|---|---|---|
| % consumers | N consumers | Mean | 95th percentile | ||
| Toddlers | 1–3 | 100.0 | 337 | 5.35 | 11.84 |
| Children | 4–10 | 99.5 | 770 | 2.69 | 6.09 |
| Teenagers | 11–18 | 100.0 | 853 | 1.14 | 2.77 |
| Adults | 19–64 | 99.9 | 1,543 | 0.99 | 2.20 |
| Elderly | 65+ | 99.4 | 391 | 1.13 | 2.34 |
bw: body weight.
Among population groups, teenagers have the highest mean and adults have the highest 95th percentile dietary daily intake of total NANA (83.4 mg and 159.2 mg, respectively), whereas children have the lowest mean and 95th percentile dietary daily intake of total NANA (66.2 mg and 137.6 mg, respectively) (Table 11).
On a body weight basis, toddlers have the highest mean and 95th percentile dietary intakes of total NANA (5.35 and 11.84 mg/kg bw per day, respectively), whereas adults have the lowest mean and 95th percentile dietary intakes of total NANA (0.99 and 2.20 mg/kg bw per day, respectively) (Table 12).
3.4.9. Combined intake of NANA from all sources
The combined anticipated daily intake (95th percentile) of the NF from fortified foods, food supplements and the anticipated daily intake of free NANA from the diet for all population groups is presented in Table 13.
Table 13.
Combined intake of the NF from fortified foods, food supplements and free NANA from the background diet (based on the 95th percentile anticipated daily intake on a mg/ kg bw)
| Population group | 95th percentile of the anticipated daily intake of the NF from fortified foods (mg/kg bw) | 95th percentile of the daily intake of free NANA from the diet (mg/kg bw) | Anticipated daily intake of the NF from food supplements (mg/kg bw) | Combined intake of 95th percentile daily intake of the NF from fortified foods, food supplements and free NANA from the diet (mg/kg bw) |
|---|---|---|---|---|
|
Infants (Up to 1 year) |
8.7 (for infants 0–6 months of age who consume IF only) 7.0 (for infants 4–6 months of age) 5.7 (for infants 7–12 months of age) |
0.6 | 60 | 69.3 |
|
Toddlers (1–3 years) |
7.1 | 1.57 | 25 | 33.7 |
|
Children (4–10 years) |
4.2 | 0.75 | 13 | 18.0 |
|
Teenagers (11–18 years) |
2.1 | 0.31 | 7 | 9.4 |
|
Adults (19–64 years) |
1.9 | 0.2 | 4.3 | 6.4 |
|
Elderly (above 64 years) |
1.7 | 0.25 | 4.3 | 6.3 |
bw: body weight.
Although NANA in its bound form would contribute to the systemic exposure to free NANA, the extent of the amount of free NANA released from the bound NANA from the diet is unknown. Therefore, the Panel considers only free NANA from the diet for the calculation of the combined intake of NANA from all sources.
The Panel notes that combined anticipated daily intake of NF from fortified foods, food supplements and free NANA from the background diet is in the range of the daily intake of free NANA from early human milk (i.e. between 3.5 and 11 mg/kg bw) for teenagers and adults, whereas for individuals below 10 years of age the combined intake exceeds the range of the daily intake of free NANA from early human milk.
With respect to individuals below 10 years of age, the Panel notes that the anticipated daily intake from food supplements alone would exceed the range of the daily intake of free NANA from early human milk, whereas the anticipated daily intake from fortified foods (plus free NANA from the background diet) is in the range of the daily intake of free NANA from early human milk.
3.5. Nutritional information on the NF
As a monosaccharide, the caloric value of NANA is theoretically 4 kcal/g; however, unlike common dietary monosaccharides, NANA does not enter the glycolysis pathway, and therefore, should be considered as a non‐caloric monosaccharide.
The Panel considers that consumption of the NF is not nutritionally disadvantageous.
3.6. Microbiological information on the NF
Microbiological and residual endotoxin specifications of the NF are presented in Table 1. The endotoxin specification is set to not exceed the levels reported for IF powder (Townsend et al., 2007). Analyses of five batches of the NF showed compliance with the microbiological and residual endotoxin specifications (Table 2).
The Panel considers that the microbiological information provided does not raise safety concerns.
3.7. Toxicological information on the NF
3.7.1. Absorption, distribution, metabolism and excretion
The applicant provided two kinetic studies in which a radiolabelled and enzymatically‐produced NANA was orally administered by gavage to mice and rats (Witt et al., 1979; Nöhle and Schauer, 1981).
In the study by Witt et al. (1979), ten 3‐day‐old Sprague–Dawley rats were administered 0.1 mL of a 0.7% N‐acetyl‐[14C]neuraminic acid solution by gavage, and radioactivity was measured in blood, organs (gastrointestinal tract, heart, liver, brain, kidneys, spleen and lungs), carcass and urine at different time points (0.5, 1, 1,5, 2 and 6 h after administration). Radioactivity in the gastrointestinal tract declined over time (7.8% of the given radiolabelled dose after 6 h). Radioactivity was detected in plasma within 30 min after administration, with a peak at 1.5 h. Radioactivity was detected in the kidneys and carcass at 30 min and in other organs at 1 h. Organs and carcass attained their maximal levels of radioactivity at 1.5–2 h (10% and 23.8% of the applied dose, respectively). Radioactivity levels declined until 3 h and then remain almost constant in the carcass, kidneys and liver, and slightly increased in heart, brain and spleen. The radiolabelled product(s) were distributed mainly to the heart, liver, and brain (maximum levels of 0.86%, 3.9% and 2.8% of administered dose, respectively, at 1.5–2 h). Radioactivity in urine, which was detected after 1 h, increased linearly with time. Within 6 h, 75% of the administered radioactivity dose was excreted in the urine, while 25% was retained in the body (carcass, gastrointestinal tract and organs).
In the study by Nöhle and Schauer (1981), 20‐day‐old fasted C57 mice were administered by gavage 1.96 mg of NANA in the form of a mixture containing N‐acetyl‐[2‐14C]neuraminic acid and N‐acetyl‐[9‐3H]neuraminic acid (isotopic ratio 1:1). The 14C‐radioactivity was expected to follow the metabolic pathway of the pyruvic acid moiety, whereas 3H‐radioactivity was expected to follow the metabolic pathway of the N‐acetyl mannosamine. Radioactivity was measured in blood, organs (liver, brain, kidneys, and spleen), urine and exhaled air at different time points from 0.5 up to 72 h. A total of 95% of the administered dose applied in the stomach entered the intestine within 15 min, 90% of which was absorbed within 6 h. In the intestine, the 14C‐radioactivity disappeared faster than the 3H‐radioactivity. 3H‐ and 14C‐radioactivity were detected in blood within 1 h after administration. The overall 3H‐radioactivity in blood and organs (which corresponded to 10% of the administered dose) reached a peak at 3–4 h, whereas the overall 14C‐radioactivity in blood and organs (which corresponded to 3% of administered dose) reached a peak at 2 h. The 3H‐radioactivity in organs was greater than the 14C‐radioactivity. Radioactivity was detected in the liver, brain, the kidneys and spleen (maximum 3H‐radioactivity levels of 3%, 2.9%, 0.8% and 0.3% of administered dose, respectively).
Radioactivity in urine was detected 1 h after administration (isotopic ratio of 1:1 of 3H‐ and 14C‐ radioactivity). The amount excreted in urine varied among animals: some animals excreted 90% of the dose administered within 1‐h and others excreted 30% after 6 h. The authors indicated that the radioactive material in the urine was the NANA compound administered to the animals (determined through high‐voltage paper electrophoresis and radio thin‐layer chromatography), indicating that the compound is excreted in urine unchanged. By 24 h, only 0.5% of the administered 3H‐radioactivity and 0.2% of the administered 14C‐radioactivity remained in the body (blood and organs).
Overall, the applied 3H‐radioactivity was recovered quantitatively in the organs, blood and excreted urine. However, a marked relative loss (up to 70%) of the 14C‐radioactivity when compared with the remaining 3H‐radioactivity was observed. This apparent 14C‐radioactivity loss was correlated to the 14CO2 formed, which was measured in the exhaled air.
The authors investigated the N‐acetylneuraminate lyase activity following incubation of 14C‐ and 3H‐labelled NANA in liver, kidney, spleen, brain and intestinal tissues. N‐Acetylmannosamine and acetic acid were detected in all tissues. Radioactive pyruvic acid also was detected in kidney tissues. The authors indicated that NANA is likely metabolised by N‐acetylneuraminate lyase in organs.
The authors also reported that after intravenous injection of the mixture of 14C‐ and 3H‐ radiolabelled NANA in rats, 90% of the radioactivity was excreted in the urine within 10 min.
Overall, the provided studies on the kinetics suggest that synthetic NANA is rapidly absorbed following oral administration, with peak plasma levels reached within 2 h after administration, and is largely excreted unchanged in the urine.
3.7.2. Genotoxicity
The potential mutagenicity of synthetic NANA (98.6% purity) was assessed in a bacterial reverse mutation test using Salmonella Typhimurium test strains TA98, TA100, TA1535 and TA1537, and Escherichia coli WP2 uvrA in the absence and presence of metabolic activation (S9), using the plate‐incorporation and pre‐incubation methods (unpublished study report dated 2012a; Choi et al., 2014). The study was conducted in compliance with the OECD principles of GLP and in accordance with the ICH Guidelines for genotoxicity testing and OECD Guideline Test No. 471 (ICH, 1996, 1997; OECD, 1997, 1998a). Water was used as a negative control for all strains. Based on the results of a preliminary test, synthetic NANA concentrations up to 5,000 μg/plate were selected for the main test. Neither cytotoxicity nor precipitation of the test article was observed at any concentration in either the preliminary or the main test. Treatment with synthetic NANA did not result in significant increases in the number of revertants compared with the negative control at any concentration in both tests either in the presence or absence of S9. Thus, synthetic NANA was determined to be non‐mutagenic in the Ames test at concentrations up to 5,000 μg/plate.
The genotoxic potential of synthetic NANA (98.6% purity) was further investigated in an in vitro mammalian cell micronucleus test conducted in human peripheral blood lymphocytes (unpublished study report, 2012b; Choi et al., 2014). The test was conducted in compliance with the OECD principles of GLP and in accordance with OECD Test No. 487 (OECD, 1998a, 2010) using the cytokinesis‐block method as described by Fenech and Morley (1986). Water was used as a negative control. Based on the results of a preliminary test, synthetic NANA concentrations up to 3,450 μg/mL were used in the main test. Synthetic NANA did not induce cytotoxicity in human peripheral blood lymphocytes compared to the negative control at any concentration or under any of the exposure conditions in the preliminary or main test. The three highest concentrations of synthetic NANA (1,690, 2,420 and 3,450 μg/mL) tested in the main test were selected for micronucleus examination. There were no statistically significant differences observed in the percentage of micronucleated cells at any of the synthetic NANA concentrations analysed compared to the negative control. Synthetic NANA was therefore determined to be non‐clastogenic and non‐aneugenic in human peripheral blood cells.
The Panel considers that the information provided does not raise concerns with respect to genotoxicity of the NF.
3.7.3. Subchronic/chronic toxicity studies
The NF was investigated in an oral toxicity study which consisted of an initial in‐utero and lactational phase, which was followed by a subchronic 90‐day oral toxicity study in the F1 (first generation) offspring (unpublished study report, 2013; Choi et al., 2014). The study was conducted in compliance with the OECD Principles of GLP, and the 13‐week dietary toxicity phase of the overall study was conducted according to OECD test guideline No. 408 (OECD, 1998a,b).
In‐utero and lactational phase
Male and female (nulliparous and non‐pregnant) CD® [Crl:CD®(SD)] rats were randomised into four groups (26 animals/sex per group). Parental (P) females were fed a diet containing 0% (control), 0.5%, 1.0% or 2.0% synthetic NANA (98.6% purity) for 28 days prior to mating and throughout the mating, gestation and lactation periods up until weaning of the F1 animals on post‐natal day (PND) 21. P males received the test diet only during the mating period and were euthanised after mating. During mating, P males and females were paired in a 1:1 ratio for 7 days. On PND 0, eight live pups (4 males and 4 females) from each litter were randomly selected for inclusion in the study, and on PND 4, pups were weighed and litters were culled to include only the selected pups. At weaning on PND 21, a minimum of one male and/or one female from each litter were randomly selected to continue on the 13‐week dietary toxicity phase. Off‐spring animals were fed a diet containing 0% (control), 0.5% (low‐dose), 1.0% (mid‐dose) or 2.0% (high‐dose) synthetic NANA for 13‐weeks. The control and high‐dose F1 groups each consisted of 30 animals/sex and the low‐ and mid‐dose groups each consisted of 20 animals/sex. A total of 10 animals/sex were selected from the control and high‐dose groups to continue in the 4‐week recovery period.
The following female reproductive parameters were recorded: number of females paired, mated, and pregnant; female mating, fertility, and fecundity indices; and number of females with confirmed mating day. For each litter, the total number of pups born, number of stillborn pups, number of live pups, number of male and female pups, individual body weights and any gross abnormalities were recorded. The gestation index, stillborn index and pup sex ratio also were calculated. Following parturition, the incidence of dead pups was recorded and pup survival indices (viability and lactation indices) were calculated. In addition, the following parameters were recorded at regular intervals: number of male and female pups, pup sex ratio, body weight, and observations of external examinations. The number of implantations was recorded upon necropsy of P females.
All animals were observed for mortality and morbidity twice daily. A detailed clinical examination was performed weekly, and body weight and food consumption were measured weekly for P females during the premating, gestation and lactation periods and for F1 animals during the 13‐week administration period and recovery period. Body weight changes, food efficiency, and test article consumption also were assessed for P females and F1 animals. Opthalmoscopic examinations were performed on F1 animals shortly after weaning and prior to necropsy. F1 animals (10/sex per group) also were subjected to a functional observational battery (FOB) test in the last week of the 13‐week administration period. Haematology, coagulation, clinical chemistry and urinalysis were performed on samples from 10 F1 males and 10 F1 females per group in the last week of the administration period and on samples from five F1 males and five F1 females per recovery group at the end of the recovery period. A detailed macroscopic examination was conducted on all animals at necropsy, except for P males. Terminal body weights and organ weights were recorded for all animals. Histopathological examination of all organs and tissues was conducted for 10 F1 males and 10 F1 females of the control and high‐dose groups at the end of the administration period.
Observations in P females
The daily intake of synthetic NANA for the combined pre‐mating, gestation, and lactation period for P females was 472, 946, and 1,895 mg/kg bw in the 0.5%, 1.0% and 2.0% synthetic NANA groups, respectively. No mortalities were observed in P females. No compound‐related macroscopic findings, statistically significant effects on reproductive performance, fertility, parturition and litter parameters were observed between the synthetic NANA and the control groups. Some transient statistically significant differences, which were not dose‐related, were reported (body weight gains, food consumption, cumulative food efficiency).
Observations in F1 pups up to PND 21
No compound‐related signs of toxicity or gross external abnormalities were noted in F1 offspring at birth. Shortly after birth, a dose‐related but slightly increased incidence of clinical observations in the F1 pups of synthetic NANA administered dams was noted when compared to F1 pups of control dams. These included a decrease in activity and appearance of cold skin upon touch in two males of the mid‐dose group (2 out of a total of 322 pubs) and four males of the high‐dose group (4 out of a total of 324 pubs); of these, one was found dead and two were missing (reported to be likely due to maternal cannibalism).
During the preweaning period, the mean body weight of male pups in the high‐dose group was slightly lower (by 4–8%) than the control group, with statistical significance noted at PND 7 and 21. Growth rates, however, were similar among groups throughout the pre‐weaning period. Notwithstanding the small magnitude of effect and the similarity in growth rates between groups, the effect on male F1 pup body weights is considered as adverse. No statistically significant differences in mean body weight were noted for female pups in the synthetic NANA groups as compared to the control group.
13‐week oral toxicity study in F1 rats
During the 13‐week period, the average daily intake of synthetic NANA in F1 males corresponded to 247, 493, and 974 mg/kg bw and in F1 females to 318, 646, 1,246 mg/kg bw at in the 0.5%, 1.0% and 2.0% synthetic NANA groups, respectively.
No mortality was observed. Several clinical signs were observed in 3–5 males of the high‐dose group during the last 3 weeks of the study, including aggressive behaviour, malocclusion and black material around the eyes. Two of these males had several other clinical signs. One of these two males was euthanised moribund (on day 74) due to a non‐compound related‐self‐induced injury to the palate. The other male continued on the study and exhibited a lower body weight when compared to the other males of the high‐dose group. No clinical findings were seen for the remaining male rats in the high‐dose group. No notable clinical signs were observed in females throughout the 13‐week administration period or in animals of the recovery group. Furthermore, no compound‐related ophthalmoscopic findings were noted.
The few statistically significant changes observed in the FOB tests and locomotor activity assessment were considered to be incidental and not dose‐related.
As a result of a reduced body weight in the pre‐weaning period, males in the high‐dose group entered the 13‐week study with a statistically significantly lower body weight (−6.3%) than control, which was maintained during the 13 weeks. Body weights in high‐dose males were statistically significantly lower at weeks 1, 2, 6–11, 13 (−6.5%) and throughout the recovery period (‐10% at weeks 15, 16, 17 and 18). The growth rates were similar among all male groups.
Food consumption in high‐dose males was statistically significantly lower than the control group (6.7% for weeks 1–13). It is likely that the decrease in food consumption and body weight in the high‐dose male group is related to the treatment. It could be expected that the high‐dose male group will compensate for their lower body weight and increase their food consumption. However, this is not the case as no difference in body weight growth rates between groups was observed.
There were no statistically significant differences in mean body weight or food consumption between the synthetic NANA and control females groups.
The food efficiency for the cumulative period (weeks 1–13) in any groups, both in males and in females, was not statistically significantly different from control.
Statistically significant changes were observed in some haematology and clinical chemistry parameters in the high‐dose group compared to the control group. These changes included a decrease (15%) in platelet counts in the high‐dose males. The decrease was partly attributed to one animal with a platelet decrease of 62% compared to the control mean. However, without this animal the mean platelet count was still reduced (9%). At the end of the recovery period, platelet counts were not statistically different between groups. Another finding was a decreased prothrombin time seen in the high‐dose male recovery group. This decrease was of small magnitude and not observed after 90‐days. In clinical chemistry, a significantly decreased creatinine level was observed in the high‐dose female group. This effect is not seen after the recovery period and is considered as incidental.
No statistically significant differences in urinalysis parameters were observed between the synthetic NANA and the control groups.
A statistically significant decrease in kidney weights relative to body weight and a statistically significant increase in pituitary gland weight relative to brain weight were observed in mid‐dose females as compared to control females. A statistically significant decrease (about 15%) in absolute and relative ovary weights to bw and to brain weight, was observed in high‐dose females when compared to control females. In mid‐dosed males, relative (brain weight) salivary gland mandibular weights were increased by about 10%. All organ weight changes except for the changes in ovary weight were small in magnitude (< 10%) and only seen in the mid‐dose group. No other statistically significant differences in organ weights relative to body weight were observed. No compound‐related macroscopic or histopathological findings were observed. One male in the 2.0% group in the recovery group had an undifferentiated sarcoma in the skeletal muscle. This finding was considered incidental.
In the first part of the study (in‐utero and lactational phase), a decrease in activity and cold skin upon touch, and decreased body weight were observed in the male pups of dams receiving the high dose. The 13‐week oral toxicity study in F1 rats, reported changes in the high‐dose male rats as compared to the control group in some parameters: a decrease in food consumption, body weight and platelet counts; an increase in aggressiveness and presence of black material around the eyes. Furthermore, decreased ovary weights were seen in high‐dose female rats. Based on the above observations in the high‐dose group, the Panel considers that the NOAEL for the subchronic toxicity of synthetic NANA is the mid dose which corresponds to 493 mg/kg bw per day in male rats. This NOAEL is not based on just one of the above‐mentioned effects but on the combination of these effects, which all appear in the high‐dose group.
3.7.4. Other animal studies
Three animal studies with synthetic NANA were provided by the applicant (Sakai et al., 2006; Wang et al., 2007; Hiratsuka et al., 2013). These studies were of short‐term exposure and were not specifically designed to assess toxicity and are therefore of limited value for the safety assessment of the NF.
3.7.5. Human studies
No human studies with synthetic NANA have been provided by the applicant.
3.8. Allergenicity
No information has been provided regarding allergenicity. However, considering that the amount of residual proteins in the NF included in the specifications is very low (0.01%), the Panel considers that the likelihood of allergic reactions to the NF is low.
4. Discussion
The NF which is the subject of the application is synthetic N‐acetyl‐d‐neuraminic acid dihydrate (NANA·2H2O).
NANA is an endogenously produced monosaccharide. In human milk, NANA is predominantly bound to HMOs, to proteins (glycoproteins) and lipids (gangliosides); but it is also present in its free (unbound) form. NANA is also present in foods of animal origin.
The information provided on the composition, the specifications, the batch‐to‐batch variability, stability and production process of the NF is sufficient and does not raise concerns about the safety of the NF.
The applicant intends to market synthetic NANA (which is in a free form) as an ingredient in IF, FOF, and foods for infants and young children as well as an ingredient in a variety of foods for the general population. The applicant also intends to market synthetic NANA in food supplements (as solid, liquid, syrup‐type or chewable forms) for the general population with the intended maximum daily use levels of 300 mg.
Assuming that an infant would only consume IF added with the synthetic NANA at the intended maximum use levels, the 95th percentile daily intake of synthetic NANA from IF is estimated to be 8.7 mg/kg.
Based on the UK NDNS (2008–2012) data and the intended maximum use levels in foods, the applicant estimated that toddlers have the highest mean and 95th percentile daily intake of the NF among population groups (3.6 and 7.1 mg/kg bw, respectively), followed by children (1.9 and 4.2 mg/kg bw, respectively) and teenagers (0.9 and 2.1 mg/kg bw, respectively). Adults and elderly have an estimated mean daily intake of the NF of 0.6–0.7 mg/kg bw per day, and an estimated 95th percentile daily intake of the NF of 1.5–1.9 mg/kg bw.
NANA occurs naturally in human milk, predominantly bound to HMOs, proteins and lipids as well as in its free (unbound) form. The Panel notes that the extent of the amount of free NANA released from bound NANA is unknown. Based on the levels of free NANA in early and mature human milk, the daily intake of free NANA ranges between 3.5 and 11 mg/kg bw for early milk and between 1.5 to 6 mg/kg bw for mature milk.
NANA is present in foods of animal origin in free and bound forms. Although NANA in its bound form would contribute to the systemic exposure to free NANA from the diet, the extent of the amount of free NANA released from bound NANA is unknown. Therefore, the Panel considers only free NANA from the diet for the calculation of the combined intake of NANA from all sources (i.e. fortified foods, food supplements, background diet).
Table 13 in Section 3.4.9 presents the combined intake of the NF from fortified foods, food supplements and free NANA from the background diet.
The Panel notes that the combined anticipated daily intake of NF from fortified foods, food supplements and free NANA from the background diet is in the range of the daily intake of free NANA from early human milk (i.e. between 3.5 and 11 mg/kg bw) for teenagers and adults, whereas for individuals below 10 years of age the combined intake exceeds the range of the daily intake of free NANA from early human milk.
With respect to individuals below 10 years of age, the Panel notes that the anticipated daily intake from food supplements alone would exceed the range of the daily intake of free NANA from early human milk, whereas the anticipated daily intake from fortified foods (plus free NANA from the background diet) is in the range of the daily intake of free NANA from early human milk.
The provided studies on the kinetics suggest that synthetic NANA is rapidly absorbed following oral administration, with peak plasma levels reached within 2 h after administration, and is largely excreted unchanged in the urine.
The information provided does not raise concerns with respect to genotoxicity of the NF.
An oral toxicity study in rats with the NF, which consisted of an initial in‐utero and lactational phase which was followed by a subchronic 90‐day oral toxicity study in the F1 offspring, was provided. Based on the observations from this study, the Panel considers that the NOAEL of the NF is 493 mg/kg bw per day.
Fortified foods
The margin of exposure (MoE, i.e. the ratio between the NOAEL and the anticipated daily intake of the NF), for each population group, was calculated based on the NOAEL in the subchronic toxicity study and the mean and 95th percentile anticipated daily intake of NF from fortified foods. The MoE results in 57 for infants (only based on 95th percentile daily intake), 137 and 70 for toddlers (1–3 years old), whereas for children (age 4–10 years old) the MoE results in 260 and 117. For individuals above 11 years of age, the MoE for fortified foods would result in at least 200.
Taking also into account that the anticipated daily intake of the NF from fortified foods would be in the range of the exposure to free NANA from the consumption of early human milk, which is considered to be safe, the Panel considers that the MoE for the NF at the intended uses and use levels in fortified foods is sufficient for the target population, including infants, toddlers and children.
Food supplements
The MoE was calculated for food supplements based on the intended maximum daily use levels: 8 for infants, 20 for toddlers, 38 for children, 70 for teenagers and 115 for adults. The Panel notes that the maximum daily intake of the NF from food supplements would be in the range of the exposure to free NANA from the consumption of early human milk with the exception of infants and children up to 10 years of age, for which the maximum daily intake for food supplements would exceed the daily intake of free NANA from early human milk.
Taking also into account that the anticipated daily intake of the NF from food supplements in individuals above 10 years of age would be in the range of the exposure to free NANA from early human milk, which is considered to be safe, the Panel considers that the MoE for the NF at the intended uses and use levels in food supplements is sufficient for individuals above 10 years of age.
5. Conclusions
The Panel concludes that:
the NF, synthetic N‐acetyl‐d‐neuraminic acid, is safe when added to foods other than food supplements at the proposed uses and use levels for the general population;
the NF is safe in food supplements alone at the proposed uses and use levels for individuals above 10 years of age;
the NF is safe at the combined intake from fortified foods plus food supplements, at the proposed uses and use levels, in individuals above 10 years of age;
the safety of the NF is not established in food supplements alone at the proposed uses and use levels for individuals below 10 years of age because the intake would exceed the level which the Panel considers as safe (11 mg/kg bw) by 5.4 times in infants, 2.3 times in toddlers and 1.2 times in children up to 10 years of age.
Documentation provided to EFSA
Letter from the European Commission to the European Food Safety Authority with the request for a scientific opinion on the safety of N‐acetyl‐d‐neuraminic acid. Ref. Ares(2016)3440191, dated 14 July 22015.
On 28 July 2016, EFSA received the following documentation: technical dossier ‘application for the approval of the human‐identical milk monosaccharide N‐acetyl‐d‐neuraminic acid (NANA, sialic acid) as a Novel Ingredient for use in infant formulae and in foods’, which was submitted by Glycom A/S; initial assessment report carried out by the Food Safety Authority of Ireland ‘safety assessment of N‐acetyl‐d‐neuraminic acid (NANA)’; Member States’ comments and objections; response by the applicant to the initial assessment report and the Member States’ comments and objections.
On 9 September 2016, EFSA sent a request to the applicant to provide missing information to accompany the application.
On 15 September 2016, EFSA received the missing information as submitted by the applicant.
On 28 September 2016, after checking the content of the full dossier, including the missing information, EFSA considered the application valid.
Additional data were provided by the applicant on 7 February 2017.
During its meeting on 28 June 2017, the NDA Panel, having evaluated the data, adopted a scientific opinion on the safety of synthetic N‐acetyl‐d‐neuraminic acid as a novel food pursuant to Regulation (EC) No 258/97.
Abbreviations
- bw
body weight
- CAS
Chemical Abstracts Service
- CFU
colony forming units
- EP
European Pharmacopeia
- EU
endotoxin units
- F1
first generation
- FAIM
food additive intake model
- FOB
functional observational battery
- FOF
follow‐on formula
- GC
gas chromatography
- GLP
Good Laboratory Practice
- HACCP
Hazard Analysis and Critical Control Points
- HMO
human milk oligosaccharides
- HPAEC
high‐performance anion exchange chromatography
- HPAEC‐PAD
high‐performance anion exchange chromatography with pulsed amperometric detection
- HPLC
high‐performance liquid chromatography
- ICH
International Conference on Harmonisation
- IF
infant formula
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- LAL
Limulus Amebocyte Lysate
- LOR
level of reporting
- LOQ
limit of quantification
- MoE
margin of exposure
- MS
Member State
- NANA
N‐acetyl‐d‐neuraminic acid
- NDA
EFSA Panel on Dietetic Products, Nutrition and Allergies
- NDNS
National Diet and Nutrition Survey
- NF
novel food
- NMR
nuclear magnetic resonance
- NOAEL
no‐observed‐adverse effect level
- OECD
organisation for economic co‐operation and development
- P
parental
- PND
post‐natal day
- RH
relative humidity
- RT
retention time
Suggested citation: EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , Turck D , Bresson J‐L, Burlingame B, Dean T, Fairweather‐Tait S, Heinonen M, Hirsch‐Ernst KI, Mangelsdorf I, McArdle HJ, Naska A, Neuhäuser‐Berthold M, Nowicka G, Pentieva K, Sanz Y, Siani A, Sjödin A, Stern M, Tomé D, Vinceti M, Willatts P, Engel K‐H, Marchelli R, Pöting A, Poulsen M, Schlatter JR, Turla E and van Loveren H, 2017. Scientific Opinion on the safety of synthetic N‐acetyl‐d‐neuraminic acid as a novel food pursuant to Regulation (EC) No 258/97. EFSA Journal 2017;15(7):4918, 28 pp. 10.2903/j.efsa.2017.4918
Requestor: European Commission following an application by Glycom A/S
Question number: EFSA‐Q‐2016‐00488
Panel members: Jean‐Louis Bresson, Barbara Burlingame, Tara Dean, Susan Fairweather‐Tait, Marina Heinonen, Karen Ildico Hirsch‐Ernst, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Monika Neuhäuser‐Berthold, Grażyna Nowicka, Kristina Pentieva, Yolanda Sanz, Alfonso Siani, Anders Sjödin, Martin Stern, Daniel Tomé, Dominique Turck, Henk van Loveren, Marco Vinceti and Peter Willatts.
Adopted: 28 June 2017
Notes
Regulation (EC) No 258/97 of the European Parliament and of the Council of 27 January 1997 concerning novel foods and novel food ingredients. OJ L 43, 14.2.1997, p. 1–6.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Commission Recommendation 97/618/EC: Commission Recommendation of 29 July 1997 concerning the scientific aspects and the presentation of information necessary to support applications for the placing on the market of novel foods and novel food ingredients and the preparation of initial assessment reports under Regulation (EC) No 258/97 of the European Parliament and of the Council. OJ L 253, 16.9.1997, p. 1–36.
Statistical analysis and data management were conducted in DaDiet Software.
References
- Angata T and Varki A, 2002. Chemical diversity in the sialic acids and related alpha‐keto acids: an evolutionary perspective. Chemical Reviews, 102, 439–469. [DOI] [PubMed] [Google Scholar]
- Brown EB, Brey WS Jr and Weltner W Jr, 1975. Cell‐surface carbohydrates and their interactions. I. NMR or N‐acetyl neuraminic acid. Biochimica et Biophysica Acta, 399, 124–130. [DOI] [PubMed] [Google Scholar]
- Choi SS, Baldwin N, Wagner VO 3rd, Roy S, Rose J, Thorsrud BA, Phothirath P and Rohrig CH, 2014. Safety evaluation of the human‐identical milk monosaccharide sialic acid (N‐acetyl‐d‐neuraminic acid) in Sprague‐Dawley rats. Regulatory Toxicology and Pharmacology, 70, 482–491. [DOI] [PubMed] [Google Scholar]
- Dabrowski U, Friebolin H, Brossmer R and Supp M, 1979. 1H‐NMR studies of N‐acetyl‐D‐neuraminic acid ketosides for the determination of the anomeric configuration II. Tetrahedron Letters, 48, 4637–4640. [Google Scholar]
- Davies PS, Wells JC and Lucas A, 1994. Adjusting milk intake for body size in early infancy. Early Human Development, 36, 61–67. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Food Additives Intake Model (FAIM) Template ‐ Version 1.0 ‐ December 2012. EFSA ‐ FIP Unit ‐ FAIM ‐ Instructions for use. Available online: https://www.efsa.europa.eu/sites/default/files/assets/faimtemplateinstructions.pdf
- EFSA AFC Panel (EFSA Panel on Food additives, flavourings, processing aids and materials in contact with food), 2007. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to 2,2‐bis(4‐hydroxyphenyl)propane. EFSA Journal 2007;5(1):428, 75 pp. 10.2903/j.efsa.2007.428 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2014. Scientific Opinion on the essential composition of infant and follow‐on formulae. EFSA Journal 2014;12(7):3760, 106 pp. 10.2903/j.efsa.2014.3760 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- Fenech M and Morley AA, 1986. Cytokinesis‐block micronucleus method in human lymphocytes: effect of in vivo ageing and low dose X‐irradiation. Mutation Research, 161, 193–198. [DOI] [PubMed] [Google Scholar]
- Flippen JL, 1973. The crystal structure of β‐D‐N‐acetylneuraminic acid dihydrate (sialic acid), C11H19NO9.2H20. Acta Crystallographica, B29, 1881–1886. [Google Scholar]
- Friebolin H, Supp M, Brossmer R, Keilich G and Ziegler D, 1980. 1H‐NMR investigations on the mutarotation of N‐acetyl‐D‐neuraminic acid. Chemie International Edition in English, 19, 208–209. [Google Scholar]
- Friebolin H, Kunzelmann P, Supp M, Brossmer R, Keilich G and Ziegler D, 1981. 1H‐NMR‐Spektroskopische untersuchungen zur mutarotation der N‐acetyl‐D‐neuraminsäure‐ph‐abhängigkeit der mutarotationsgeschwindigkeit. Tetrahedron Letters, 22, 1383–1386. [Google Scholar]
- Galeotti F, Coppa GV, Zampini L, Maccari F, Galeazzi T, Padella L, Santoro L, Gabrielli O and Volpi N, 2012. On‐line high‐performance liquid chromatography‐fluorescence detection‐electrospray ionization‐mass spectrometry profiling of human milk oligosaccharides derivatized with 2‐aminoacridone. Analytical Biochemistry, 430, 97–104. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, De Felice C, Watanabe T, Tanaka T, Iinuma K, Nihei K, Higuchi S, Ezoe T, Hibi I and Kurosawa K, 1993. Determination of free N‐acetylneuraminic acid in human body fluids by high‐performance liquid chromatography with fluorimetric detection. Journal of Chromatography, 620, 25–31. [DOI] [PubMed] [Google Scholar]
- Hester SN, Hustead DS, Mackey AD, Singhal A and Marriage BJ, 2012. Is the macronutrient intake of formula‐fed infants greater than breast‐fed infants in early infancy? Journal of Nutrition and Metabolism, 2012, 891201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka S, Honma H, Saitoh Y, Yasuda Y and Yokogoshi H, 2013. Effects of dietary sialic acid in n‐3 fatty acid‐deficient dams during pregnancy and lactation on the learning abilities of their pups after weaning. Journal of Nutritional Science and Vitaminology, 59, 136–143. [DOI] [PubMed] [Google Scholar]
- ICH (International Conference on Harmonisation), 1996. Guidance on Specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals: S2A. (ICH Harmonised Tripartite Guideline ‐ Recommended for adoption at Step 4 of the ICH Process on 19 July 1995 by the ICH Steering Committee). Geneva, Switz., International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).
- ICH (International Conference on Harmonisation), 1997. Genotoxicity: A Standard Battery for Genotoxicity Testing of Pharmaceuticals: S2B. (ICH Harmonised Tripartite Guideline ‐ Recommended for adoption at Step 4 of the ICH Process on 16 July 1997 by the ICH Steering Committee). Geneva, Switz., International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceutical for Human Use (ICH).
- Kersting M, Alexy U, Sichert_Hellert W, Manz F and Schoch G, 1998. Measured consumption of commercial infant food products in German infants: results from the DONALD study. Dortmund Nutritional and Anthropometrical Longitudinally Designed. Journal of Pediatric Gastroenterology and Nutrition, 27, 547–552. [DOI] [PubMed] [Google Scholar]
- Klepach T, Carmichael I and Serianni AS, 2008a. 13C‐labeled N‐acetyl‐neuraminic acid in aqueous solution: detection and quantification of acyclic keto, keto hydrate, and enol forms by 13C NMR spectroscopy. Journal of the American Chemical Society, 130, 11892–11900. [DOI] [PubMed] [Google Scholar]
- Klepach T, Zhang W, Carmichael I and Serianni AS, 2008b. (13)C‐(1)H and (13)C‐(13)C NMR J‐couplings in (13)C‐labeled N‐acetyl‐neuraminic acid: correlations with molecular structure. Journal of Organic Chemistry, 73, 4376–4387. [DOI] [PubMed] [Google Scholar]
- Kuhn R and Baschang G, 1962. Die Konfiguration der Sialinsäuren am C‐Atom 4. Chemische Berichte, 95, 2384–2385. [Google Scholar]
- Kuhn R and Brossmer R, 1962. Die Konfiguration der Sialinsäuren. Angewandte Chemie, 74, 252–253. [Google Scholar]
- Martín‐Sosa S, Martin MJ, Garcia‐Pardo LA and Hueso P, 2004. Distribution of sialic acids in the milk of spanish mothers of full term infants during lactation. Journal of Pediatric Gastroenterology and Nutrition, 39, 499–503. [DOI] [PubMed] [Google Scholar]
- NatCen Social Research, Medical Research Council (MRC) ‐ Resource Centre for Human Nutrition Research, MRC Epidemiology and Medical Care Unit, Newcastle University Institute for Ageing and Health , 2013. Diet and Nutrition Survey of Infants and Young Children, 2011. UKDA study number:7263. Available online: https://doi.org/esds.ac.uk/doi/?sn=7263
- NatCen Social Research, MRC Human Nutrition Research, University College London, Medical School , 2015. National Diet and Nutrition Survey years 1‐4, 2008/09‐2011/12. [data collection], 7th edition. UK Data Service. SN: 6533. Available online: 10.5255/ukda-sn-6533-6 (Accessed 05 October 2016). [DOI]
- Nöhle U and Schauer R, 1981. Uptake, metabolism and excretion of orally and intravenously administered, 14C‐ and 3H‐labeled N‐acetylneuraminic acid mixture in the mouse and rat. Hoppe‐Seylers Zeitschrift fur Physiologische Chemie, 362, 1495–1506. [DOI] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 1997. Test No. 471: Bacterial reverse mutation test. In: OECD guidelines for the testing of chemicals, Section 4: Health effects, 11 pp.
- OECD (Organisation for Economic Co‐operation and Development), 1998a. OECD Principles of good laboratory practice. OECD series on principles of good laboratory practice and compliance monitoring, number 1, ENV/MC/CHEM(98)17, 41 pp.
- OECD (Organisation for Economic Co‐operation and Development), 1998b. Test No. 408: Repeated dose 90‐day oral toxicity study in rodents. In: OECD guidelines for the testing of chemicals, Section 4: Health effects, 10 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2010. Test No. 487: In Vitro Mammalian Cell Micronucleus Test. In: OECD guidelines for the testing of chemicals, Section 4: Health effects, 23 pp.
- Ogura H, Furuhata K, Saitô H, Izumi G, Itoh M and Shitori Y, 1984. Stereochemical characterization of hydrated and dehydrated crystals of N‐acetylneuraminic acid as revealed by the IR, CD, and 13C cross polarization‐magic angle spinning NMR spectroscopy. Chemistry Letters, 6, 1003–1006. [Google Scholar]
- Oriquat GA, Saleem TH, Abdullah ST, Soliman GT, Yousef RS, Hameed AMA and Salim ML, 2011. Soluble CD14, sialic acid and l‐fucose in breast milk and their role in increasing the immunity of breast‐fed infants. American Journal of Biochemistry and Biotechnology, 7, 21–28. [Google Scholar]
- Puente R, Garcia‐Pardo LA, Rueda R, Gil A and Hueso P, 1996. Seasonal variations in the concentration of gangliosides from different mammalian species. International Dairy Journal, 6, 315–322. [Google Scholar]
- Qiao Y, Feng J, Yang J and Gu G, 2013. The relationship between dietary vitamin A intake and the levels of sialic acid in the breast milk of lactating women. Journal of Nutritional Science and Vitaminology, 59, 347–351. [DOI] [PubMed] [Google Scholar]
- Rohrig CH, Choi SS and Baldwin N, 2017. The nutritional role of free sialic acid, a human milk monosaccharide, and its application as a functional food ingredient. Critical Reviews in Food Science and Nutrition, 57, 1017–1038. [DOI] [PubMed] [Google Scholar]
- Rueda R, Puente R, Hueso P, Maldonado J and Gil A, 1995. New data on content and distribution of gangliosides in human milk. Biological Chemistry Hoppe‐Seyler, 376, 723–727. [DOI] [PubMed] [Google Scholar]
- Sabharwal H, Sjoblad S and Lundblad A, 1991. Sialylated oligosaccharides in human milk and feces of preterm, full‐term, and weaning infants. Journal of Pediatric Gastroenterology and Nutrition, 12, 480–484. [DOI] [PubMed] [Google Scholar]
- Sakai F, Ikeuchi Y, Urashima T, Fujihara M, Ohtsuki K and Yanahira S, 2006. Effects of feeding sialyllactose and galactosylated N‐acetylneuraminic acid on swimming learning ability and brain lipid composition in adult rats. Journal of Applied Glycoscience, 53, 249–254. [Google Scholar]
- Schauer R, 2004. Sialic acids: fascinating sugars in higher animals and man. Zoology (Jena), 107, 49–64. [DOI] [PubMed] [Google Scholar]
- Spichtig V, Michaud J and Austin S, 2010. Determination of sialic acids in milks and milk‐based products. Analytical Biochemistry, 405, 28–40. [DOI] [PubMed] [Google Scholar]
- Spichtig V, Rohfritsch P and Austin S, 2011. Lactose does not interfere with the analysis of sialic acids as their 1,2‐diamino‐4,5‐methylenedioxybenzene derivatives. Analytical and Bioanalytical Chemistry, 399, 1917–1922. [DOI] [PubMed] [Google Scholar]
- Sugiyama N, Saito KI, Fujikura K, Sugai K, Yamada N, Goto M, Ban C, Hayasaka E and Tomita K, 1991. Thermal and photochemical degradation of sodium N‐acetylneuraminate. Carbohydrate Research, 212, 25–36. [Google Scholar]
- Thurl S, Muller‐Werner B and Sawatzki G, 1996. Quantification of individual oligosaccharide compounds from human milk using high‐pH anion‐exchange chromatography. Analytical Biochemistry, 235, 202–206. [DOI] [PubMed] [Google Scholar]
- Townsend S, Caubilla Barron J, Loc‐Carrillo C and Forsythe S, 2007. The presence of endotoxin in powdered infant formula milk and the influence of endotoxin and Enterobacter sakazakii on bacterial translocation in the infant rat. Food Microbiology, 24, 67–74. [DOI] [PubMed] [Google Scholar]
- Unpublished study report , 2012a. Bacterial Reverse Mutation Assay. Study Number: AD41YL.503.BTL.
- Unpublished study report , 2012b. The In Vitro Mammalian Cell Micronucleus Test in Human Peripheral Blood Lymphocytes (HPBL). Study Number: AD41YL.348.BTL.
- Unpublished study report , 2013. Sialic acid: a 13‐week dietary toxicity study in rats with an in utero phase and a 4‐week recovery period. Study Number: 2018‐004.
- Wang B, Brand‐Miller J, McVeagh P and Petocz P, 2001. Concentration and distribution of sialic acid in human milk and infant formulas. American Journal of Clinical Nutrition, 74, 510–515. [DOI] [PubMed] [Google Scholar]
- Wang B, Yu B, Karim M, Hu H, Sun Y, McGreevy P, Petocz P, Held S and Brand‐Miller J, 2007. Dietary sialic acid supplementation improves learning and memory in piglets. American Journal of Clinical Nutrition, 85, 561–569. [DOI] [PubMed] [Google Scholar]
- WHO (World Health organisation), 2013. WHO Child Growth Standards. Geneva, Switzerland, WHO Multicentre Growth Reference Study Group, World Health Organization (WHO). Available online: http://www.who.int/childgrowth/standards/weight_for_age/en/index.html
- Wiederschain GY and Newburg DS, 2001. Glycoconjugate stability in human milk: glycosidase activities and sugar release. Journal of Nutritional Biochemistry, 12, 559–564. [DOI] [PubMed] [Google Scholar]
- Witt W, von Nicolai H and Zilliken F, 1979. Uptake and distribution of orally applied N‐acetyl‐(14C)neuraminosyl‐lactose and N‐acetyl‐(14C)neuraminic acid in the organs of newborn rats. Nutrition and Metabolism, 23, 51–61. [DOI] [PubMed] [Google Scholar]


