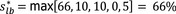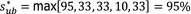Abstract
The European Commission has requested EFSA to assess animal diseases according to the criteria as laid down in Articles 5, 7, 8 and Annex IV for the purpose of categorisation of diseases in accordance with Article 9 of the Regulation (EU) No 2016/429 (Animal Health Law). This scientific opinion addresses the ad hoc method developed for assessing any animal disease for the listing and categorisation of diseases within the Animal Health Law (AHL) framework. The assessment of individual diseases is addressed in distinct scientific opinions that are published separately. The assessment of Articles 5, 8 and 9 criteria is performed on the basis of the information collected according to Article 7 criteria. For that purpose, Article 7 criteria were structured into parameters and the information was collected at parameter level. The resulting fact sheets on the profile and impact of each disease were compiled by disease scientists. A mapping was developed to identify which parameters from Article 7 were needed to inform each Article 5, 8 and 9 criterion. Specifically, for Articles 5 and 9 criteria, a categorical assessment was performed, by applying an expert judgement procedure, based on the mapped information. The judgement was performed by EFSA Panel experts on Animal Health and Welfare in two rounds, individual and collective judgement. The output of the expert judgement on the criteria of Articles 5 and 9 for each disease is composed by the categorical answer, and for the questions where no consensus was reached, the different supporting views are reported. To take better account of the uncertainties involved, a quantitative instead of a categorical approach has been followed from 2021. The output henceforth consists in the individual probability ranges provided by the experts and their computed median range.
Keywords: Animal Health Law, listing, categorisation, disease profile, disease impact
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Article 5 of the Regulation (EU) No 429/20161 of the European Parliament and of the Council on transmissible animal diseases (Animal Health Law), hereinafter referred as AHL, provides the list of animal diseases to which the rules set out in the AHL apply. These rules include the categorisation of those diseases into different groups depending on the appropriate measures, as provided for in Article 9 of that Regulation.
In addition to the list of five significant diseases laid down in Article 5 (1) of the AHL, there is a list of other diseases in Annex II that is amendable by the Commission by means of a delegated act.
Furthermore, there are transmissible animal diseases for which certain control or trade measures apply today but are not included in the mentioned Annex II and diseases that might be relevant for forthcoming Union intervention.
Criteria for listing of animal diseases are laid down in Article 5(3), for the assessment in Article 7 and for the categorisation of animal diseases in Annex IV of the AHL.
Those criteria constitute the benchmarks for the exercises of listing and categorisation and for determining the disease prevention and control rules to be applied to the different categories of listed diseases.
Furthermore, Article 8 of the AHL envisages that disease specific rules for listed diseases apply to listed animal species. Those species, or groups of animal species, are those that are either susceptible species or they have the capability to carry specific diseases.
Specific criteria for listing of species are provided for in Article 8(3) of the AHL.
The Commission needs a scientific advice to enable the assessment of the following diseases within the framework of the listing and categorisation according to the AHL, although the same methodology could be applied in the future for further request:
Aujeszky's disease
Enzootic bovine leukosis (EBL)
Bovine viral diarrhoea (BVD)
Infectious bovine rhinotracheitis (IBR)
Porcine reproductive and respiratory syndrome (PRRS)
Paratuberculosis
Koi herpes virus disease (KHV)
Anthrax
Infection with Brucella abortus, Brucella melitensis and Brucella suis
Japanese encephalitis
West Nile fever
Trypanosoma evansi infections (including Surra)
Equine encephalomyelitis (eastern and western)
Venezuelan equine encephalomyelitis
Borna disease
Bovine tuberculosis
Infection with low pathogenic avian influenza virus
Avian mycoplasmosis (Mycoplasma gallisepticum, Mycoplasma meleagridis)
Salmonella infection in poultry with serotypes of animal health relevance (Salmonella Pullorum, Salmonella Gallinarum and Salmonella arizonae)
Ebola
Bluetongue (all serotypes or group of serotypes)
Bovine genital campylobacteriosis
Trichomonosis
Border disease
Ovine epididymitis (Brucella ovis)
Contagious bovine pleuropneumonia
Contagious caprine pleuropneumonia
Infestation with Varroa spp. (Varroosis)
Batrachochytrium salamandrivorans (Bsal).
The criteria, provided in the Appendix A of this opinion, shall be used as a basis for the analytical assessment. The risk manager needs an updated scientific advice in order to:
assess if the above mentioned animal diseases are diseases for which control measures at the European Union (EU) level are justified;
proceed profiling each disease with a view to its categorisation;
assign listed species to the diseases identified as relevant for the EU intervention.
In view of the above, and in accordance with Article 29 of Regulation (EC) No 178/2002, the Commission asks EFSA for a scientific opinion:
ToR 1: for each of the diseases an assessment, following the criteria laid down in Article 7 of the AHL, on its eligibility to be listed for Union intervention as laid down in Article 5(3) of the AHL.
-
ToR 2: for each of those diseases which was found eligible to be listed for Union intervention:
an assessment of its compliance with each of the criteria in Annex IV to the AHL for the purpose of categorisation of diseases in accordance with Article 9 of the AHL;
a list of animal species that should be considered candidates for listing in accordance with Article 8 of the AHL.
1.2. Interpretation of the Terms of Reference (ToR)
An assessment of the animal diseases listed in Section 1.1 according to the criteria of Articles 5, Annex IV for the purpose of categorisation of diseases in accordance with Article 92 and 8 of the AHL is requested by the European Commission in five similar mandates, to be done on the basis of the information available according to the assessment criteria of Article 7 (profile and impact). The criteria of Articles 5, 7, 8 and 9, are provided in the Appendix A of the present opinion.
The first ToR requires an assessment of each disease against the eligibility criteria as listed in Article 5. The Article 5 of the Regulation (EU) No 429/2016 provides the list of animal diseases to which the rules set out in the AHL apply. A disease is eligible to be listed if it has been assessed according to the criteria listed in Article 7 and if it meets the criteria of Article 5 of the AHL. The criteria of Article 7 concern the disease profile, impact of the disease, potential to generate a crisis situation and its potential for use in bioterrorism, the feasibility, availability, effectiveness and the impact of disease prevention and control measures.
The second ToR requires an assessment of each disease against the criteria as in Annex IV of the AHL for the purpose of categorisation of diseases in accordance with Article 9. These criteria provide the rules for the categorisation of the diseases into different groups depending on the appropriate measures (eradication, control, etc.), as provided for in Article 9 of the AHL. There are five categories in Article 9 of the AHL, each with a defined purpose as follows:
category A: listed diseases that do not normally occur in the Union and for which immediate eradication measures must be taken as soon as they are detected.
category B: listed diseases which must be controlled in all Member States with the goal of eradicating them throughout the Union.
category C: listed diseases which are of relevance to some Member States and for which measures are needed to prevent them from spreading to parts of the Union that are officially disease‐free or that have eradication programmes for the listed disease concerned.
category D: listed diseases for which measures are needed to prevent them from spreading on account of their entry into the Union or movements between Member States.
category E: listed diseases for which there is a need for surveillance within the Union.
Each category foresees the application of certain disease prevention and control rules to the respective listed diseases when the disease in question fulfils the criteria laid down in the relevant Section of Annex IV of AHL (Sections 1–5 which correspond to categories A–E, respectively).
The same ToR requires the provision of the list of animal species that should be considered candidates for listing in accordance with Article 8(3). This Article envisages that specific rules for listed diseases apply to listed animal species. Those species, or groups of animal species, are those that are either susceptible species or those that have the capability to carry specific pathogenic agents.
For the first ToR, the disease and impact profile following Article 7 criteria requires compilation for each disease. This represents the baseline information for the assessment as requested by ToR 1 and ToR 2, i.e. to enable determination of the eligibility for listing the disease in the AHL, the category into which each of the diseases fits according to Article 9 of AHL, and the animal species that should be considered as relevant for that disease.
The use and the purpose of the assessment for the European Commission should be to provide clear and robust information to qualify the importance of each disease in the Union, and if justified, which measures should be taken in the EU.
The answer to the mandates will be structured in one document providing the description of the methodology for the assessment of diseases and an individual document providing the information and assessment for each disease.
2. Data and methodologies
In order to address the ToRs as provided by the Commission, an ad hoc method of assessment has been developed and used for each disease for collecting information on the profile and impacts of the targeted diseases and conducting the assessment of the disease within the framework of AHL.
The framework used to elaborate the method for information collection and assessment of diseases are the criteria laid down in Articles 5, 7, 8(3) and 9 of the AHL as provided to EFSA and as reported in the Appendix A of the present opinion.
The AHL foresees that the assessment of each disease, based on the criteria listed in Articles 5 (eligibility for listing), 9 (categorisation into control classes) and 8 (listing species) should be performed on the basis of the information collected according to Article 7 (disease profile and impacts). Accordingly, the proposed and developed method consists of three main steps as summarised in the following Table 1:
Table 1.
Assessment steps
| ToR | Section | Main steps | Description | Result | |
|---|---|---|---|---|---|
| 1 | 2.1 | Data collection for disease profile and impacts based on Article 7 | Structuring Article 7 criteria into parameters | Transcription of Article 7 criteria to allow data collection | Fact sheet of the disease profile and impacts |
| Disease Fact sheet compilation | Disease scientist recruited to collect data and compile Fact sheet | ||||
| 1 | 2.2 | Use of Article 7 data for disease assessment based on Articles 5, 8 and 9 | Article 5 based on Article 7 | Mapping each Article 5 criterion as a function of Article 7 criteria | Article 5 by Article 7 matrix |
| 2 | Article 9 based on Article 7 | Mapping each Article 9 criterion as a function of Article 7 criteria | Article 9 by Article 7 matrix | ||
| Article 8 based on Article 7 | Mapping each Article 8 criterion as a function of Article 7 criteria | Assessment of candidate animal species for listing (Article 8 by Article 7 matrix) | |||
| 1 & 2 | 2.3 | Disease assessment | Expert selection | AHAW Panel members for expert judgement | Disease assessment for listing and categorisation |
| Assessment method | Implementation of a two rounds approach (individual judgement + collective behavioural aggregation) on the basis of Sections 2.1 and 2.2 | ||||
These steps are described in detail in the following sections.
2.1. Data collection for disease profile and impacts based on Article 7 criteria
In order to compile the information on (a) the diseases profile, (b, c) the impact of the diseases, (d) the availability, feasibility and effectiveness of control measures, and (e) the impact of disease prevention and control measures according to Article 7 of AHL,
the Article 7 criteria (in Appendix A) are broken down and structured into parameters;
data/information is collected at parameter level by selected disease scientists and drafted in a fact sheet format.
2.1.1. Restructuring of Article 7 criteria into parameters
For each criterion in Article 7 of the AHL, subcriteria are identified on which information is needed to fully characterise the disease. For each subcriterion, a list of parameters characterising the sub‐criterion and related parameters are defined by the working group (WG) experts. Existing frameworks on disease and impact assessment using a multi criteria approach are consulted in order to support the definition and identification of appropriate parameters to assess diseases according to Article 7 criteria of the AHL (DEFRA, 2006; RIVM, 2006; WHO, 2006; European Commission, 2007; ETPGAH, 2007; Council of the European Union, 2008; Krause et al., 2008; Cardoen et al., 2009; Havelaar et al., 2010; OIE, 2010; Del Rio Vilas et al., 2011; ECDC, 2011; DISCONTOOLS, 2012; EFSA BIOHAZ Panel (2012); FAO, 2012; Humblet et al., 2012; Ng and Sargeant, 2012; Cox et al., 2013; Del Rio Vilas et al., 2013; Ng and Sargeant, 2013; ECDC, 2015; Gao et al., 2015; McFadden et al., 2016; Ng and Sargeant, 2016). The result of the restructuring of Article 7 is shown in Table 2.
Table 2.
Article 7 criteria structured into sub‐criteria and related parameters
| Article 7 criteria as provided by AHL | The structure for data collection | ||
|---|---|---|---|
| Subcriteria | Parameters | ||
| (a) The disease profile | (i) Animal species concerned by the disease | Susceptible animal species | 1. Naturally susceptible wildlife species (or families/orders) |
| 2. Naturally susceptible domestic species (or families/orders) | |||
| 3. Experimentally susceptible wildlife species (or families/orders) | |||
| 4. Experimentally susceptible domestic species (or families/orders) | |||
| Reservoir animal species | 5. Wild reservoir species (or families/orders) | ||
| 6. Domestic reservoir species (or families/orders) | |||
| (ii) Morbidity and mortality rates of the disease in animal populations | Morbidity | 1. Prevalence/incidence | |
| 2. Case‐morbidity rate (% clinically diseased animals out of infected ones) | |||
| Mortality | 3. Case‐fatality rate | ||
| (iii) Zoonotic character of the disease | Presence | 1. Report of zoonotic human cases (anywhere) | |
| (iv) Resistance to treatments, including antimicrobial resistance | Resistance to treatment | 1. Resistant strain to any treatment even at laboratory level | |
| (v) Persistence of the diseasea in an animal population or in the environment | Animal population | 1. Duration of infectious period in animals | |
| 2. Presence and duration of latent infection period | |||
| 3. Presence and duration of the pathogen in healthy carriers | |||
| Environment | 4. Length of survival (dpi) of the agent and/or detection of DNA in selected matrices (soil, water, air) from the environment (scenarios: high and low temperature) | ||
| (vi) The routes and speed of transmission of the disease between animals and, when relevant, between animals and humans | Routes of transmission | 1. Type of routes of transmission from animal to animal (types of horizontal and vertical routes) | |
| 2. Type of routes of transmission between animals and humans (types of direct, indirect routes, including food‐borne) | |||
| Speed of transmission | 3. Incidence between animals and, when relevant, between animals and humans | ||
| 4. Transmission rate (beta) (from R0 and infectious period) between animals and, when relevant, between animals and humans | |||
| (vii) The absence or presence and distribution of the disease in the Union, and, where the disease is not present in the Union, the risk of its introduction into the Union | Presence and distribution | 1. Map where the disease is present in EU | |
| 2. Type of epidemiological occurrence (sporadic, epidemic, endemic) at MS level | |||
| Risk of introductionb | 3. Routes of possible introduction | ||
| 4. Number of animal moving and/or shipment size | |||
| 5. Duration of infectious period in animal and/or commodity (see (a)(v)1; (a)(v)4) | |||
| 6. List of control measures at border (testing, quarantine, etc.) | |||
| 7. Presence and duration of latent infection and/or carrier status see (a)(v)2; (a)(v)3 | |||
| 8. Risk of introduction by possible entry routes (considering parameters from 3 to 7) | |||
| (viii) Existence of diagnostic and disease control tools | Diagnostic tools | 1. Existence of diagnostic tools | |
| Control tools | 2. Existence of disease control tools | ||
| (b) the impact of the disease | (i) On agricultural and aquaculture production and other parts of the economy, as regards: | Level of presence of the disease in the Union | 1. Number of MSs where the disease is present |
| Loss of production due to the disease | 2. Proportion of production losses (%) by epidemic/endemic situation (milk, growth, semen, meat, etc.) | ||
| (ii) On human healthc as regards: | Transmissibility between animals and humans | 1. Type routes of transmission between animals and humans (see (a)(vi)2) | |
| 2. Incidence of zoonotic cases | |||
| Transmissibility between humans | 3. Occasional or sustainable? | ||
| 4. Epidemic or pandemic? | |||
| Severity of human form of the disease | 5. Disability‐adjusted life year (DALY) | ||
| Availability of effective prevention or medical treatment | 6. Availability of medical treatment and their effectiveness (therapeutic effect and any resistance) | ||
| 7. Availability of vaccines and their effectiveness (reduced morbidity) | |||
| (iii) On animal welfare | Welfare | 1. Severity of clinical signs at case level and related level and duration of impairment | |
| (iv) On biodiversity and the environment | Biodiversity | 1. Endangered wild species affected: listed species as in CITES and/or IUCN list | |
| 2. Mortality in wild species | |||
| Environment | 3. Capacity of the pathogen to persist in the environment and cause mortality in wildlife | ||
| (c) Potential to generate a crisis situation and its potential use in bioterrorism | Potential use in bioterrorism | 1. Listed in OIE/CFSPH classification of pathogens | |
| 2. Listed in the Encyclopaedia of Bioterrorism Defense of Australia Group | |||
| 3. Included in any other list of potential bio‐ agro‐terrorism agents | |||
| (d) Feasibility, availability and effectiveness of disease prevention and control measures | (i) Diagnostic tools and capacities | Availability | 1. Officially/internationally recognised diagnostic tool, OIE certified |
| Efficacy | 2. Se and Sp of diagnostic test | ||
| Feasibility | 3. Type of sample matrix to be tested (blood, tissue, etc.) | ||
| (ii) Vaccination | Availability | 1. Types of vaccines available on the market (live, inactivated, DIVA, etc.) | |
| 2. Availability/production capacity (per year) | |||
| Effectiveness | 3. Protection as reduced morbidity (as reduced susceptibility to infection and/or to disease). (alternatively efficacy of vaccines combined with coverage could be considered) | ||
| 4. Duration of protection | |||
| Feasibility | 5. Way of administration | ||
| (iii) Medical treatments | Availability | 1. Types of drugs available on the market | |
| 2. Availability/production capacity (per year) | |||
| 3. Therapeutic effect in the field (effectiveness) | |||
| Feasibility | 4. Way of administration | ||
| (iv) Biosecurity measures | Availability | 1. Available biosecurity measures | |
| Effectiveness | 2. Effectiveness of biosecurity measure (description) in preventing the pathogen introduction | ||
| Feasibility | 3. Feasibility of biosecurity measure (description) | ||
| (v) Restrictions on the movement of animals and products, as control measure | Availability | 1. Available restriction movement measures | |
| Effectiveness | 2. Effectiveness of restriction of animal movement in preventing the between farm spread (description) | ||
| Feasibility | 3. Feasibility of restriction of animal movement (description) | ||
| (vi) Killing of animals | Availability | 1. Available methods of killing of animal | |
| Effectiveness | 2. Effectiveness of killing animals (at farm level or within the farm) for reducing/stopping spread of the disease (description) | ||
| Feasibility | 3. Feasibility of killing animals (description) | ||
| (vii) Disposal of carcasses and other relevant animal by‐products | Availability | 1. Disposal options available | |
| Effectiveness | 2. Effectiveness of disposal option (description) | ||
| Feasibility | 3. Feasibility of disposal option (description) | ||
| (e) The impact of disease prevention and control measures, as regards: | (i) The direct and indirect costs for affected sectors and economy as a whole | Cost | Description of:
|
| (ii) Their societal acceptanced | Society | ||
| (iii) The welfare of affected subpopulations of kept and wild animals | Welfare | 1. Welfare impact of control measures on domestic (description) | |
| 2. Wildlife depopulation as control measure | |||
| (iv) The environment and biodiversity | Environment | 1. Use and potential residuals of biocides or medical drugs in environmental compartments (soil, water, feed, manure) | |
| Biodiversity | 2. mortality due to control measures in wild species | ||
i.e. infective agent.
Only for diseases not already present in the EU.
Only for zoonotic diseases.
Elements characterising the costs for the affected sectors and the types of prevention and control measures that may entail issues about societal acceptance.
Concerning the criteria e(i) and e(ii), i.e. the impact of disease prevention and control measures as regards the costs for affected sectors and the economy as a whole, and the societal acceptance, respectively, an assessment of all direct and indirect costs cannot be provided in this opinion in a general framework, since the analysis of costs of control measures and of their societal acceptance is not science‐driven but it is linked to the different socioeconomical contexts and to the different management decisions to be applied. In order to assess this criterion, a description is provided about the elements characterising the costs for the affected sectors and the types of prevention and control measures that may entail issues about societal acceptance, by bringing some cases studies, where available.
Regarding the criterion about impact of disease prevention and control measures as regards environment and biodiversity, the outcomes of interest concern the use and potential residues of biocides and medical treatments and reference to already available environmental risk assessment is provided where available.
The evidence collected on the different criteria is summarised in narrative and in summary tables.
2.1.2. Disease fact sheet compilation
Disease fact sheet
A disease fact sheet is defined as a document containing all relevant information on a specific disease following the structure of the Article 7 criteria of the AHL. The aim is to draft a comprehensive, succinct and up to date fact sheet on the profile, impact, and control options of each disease requested by the mandate. The fact sheet covers all criteria of Article 7 of the AHL according to the parameters as mentioned above.
The fact sheet is used as scientific basis to allow the EFSA panel of experts on animal health and welfare to assess the criteria for listing and categorisation of a certain disease in the framework of AHL (Articles 5 and 9 of AHL), and for the identification of the animal species concerned (Article 8 of the AHL).
Disease Scientist
The disease fact sheet compilation has been outsourced to disease scientists (DSs). A DS is a scientist who has the expertise to retrieve, extract, collate and interpret relevant scientific information related to a specific disease, covering all AHL (Article 7) criteria and related parameters.
The required expertise of the DS should cover the criteria on the disease profile as laid down in Article 7 of the AHL, which are listed below:
Epidemiology
Economics of animal health and production
Ecology/ecosystems/dependencies/impact on
The above listed fields of expertise, deemed to be necessary, implies also certain knowledge on the following domains:
Clinical expression
Diagnostic methods
Disease control strategies
Monitoring/surveillance strategies
Pharmacology
Risk assessment
Statistics
Zoology
The expertise of a candidate DS is expected to cover most of the required fields for at least one of the diseases that EFSA is requested to assess.
DSs with the required scientific profile have been identified and selected by a search in databases of scientific literature, by giving priority to authors of the most recent publications and reviews and by considering scientists working in the OIE, EU or national reference laboratories.
In this specific case of the AHL disease profiling, the search string for retrieving the relevant literature had to be general enough to cover all aspects of each disease. The search string used is the following:
[name of the disease] OR [name of the pathogen] OR [acronym of the disease] OR [acronym of the pathogen] OR [common name of the disease]
Once the list of authors is returned by the search, the selection of the DS among the potential candidates, as described above, is based on number of publications, date of publication, affiliation and availability.
Fact sheet compilation
Sources of information:
The DS could use scientific literature as sources of information, giving priority to peer‐reviewed literature and based on the evidence pyramid, where most reliance can be given to systematic reviews and meta‐analyses, followed by critically‐appraised topics with syntheses, critically‐appraised articles (synopses), randomised controlled trials, cohort studies, case–control studies, case‐series and reports, and expert opinions. Least reliance can be given to the latter. Furthermore, the following sources of information could be used:
EFSA databases (Data Collection Framework and/or ad hoc databases)
EFSA scientific outputs published on the same topic (Scientific Opinions, Scientific Reports, Technical Reports, other outputs)
EMA outputs relevant for treatments and vaccination
ECDC outputs relevant for the zoonotic aspects
EUROSTAT for information related to trade
Information by MSs
OIE manuals and any other output relevant for the diagnostic tests and the vaccines
The DS should also identify for which Article 7 criteria and related parameters the data and the information available, at the time of the fact sheet compilation, are lacking or not up to date. The data and the information should be combined and narratively described, interpreted and commented, following the structure of the fact sheet. The DS should complete and update each of them by reporting in detail the sources of information used.
Completed fact sheets are then reviewed by two independent experts selected from the EFSA Panel on Animal Health and Welfare (AHAW) experts or from the EFSA ad hoc WG, whose role is to assess the integrity of the documents. The reviewers assess that the content of the fact sheets sufficiently covers what is requested by the parameters of Article 7, and highlighted any knowledge gap, any missing or wrong information, any missing critical or relevant references and possible expert bias introduced by the DS. The comments provided by reviewers are addressed and endorsed by DSs.
2.2. Use of Article 7 criteria and parameters to inform Articles 5, 8 and 9 criteria: mapping
As mentioned, the assessment of each disease, based on the criteria listed in Article 5 (eligibility for listing), should be performed on the basis of the information collected according to Article 7. For this purpose, a mapping is developed to identify which elements from Article 7 are needed to inform each Article 5 criterion. The table based on Article 7 criteria at parameter level (disease fact sheet) is used to build a matrix linking sets of relevant Article 7 parameters to each relevant criterion of Article 5.
Likewise, the assessment of the diseases in view of their categorisation based on Article 9, and of animal species that should be considered candidates for listing in accordance with Article 8 of the AHL are performed on the basis of the information collected under Article 7. In the same way, a similar mapping is developed to build a matrix linking sets of relevant Article 7 parameters to each relevant criterion of Article 9 and Article 8. For Article 8, the mapped Article 7 information constitutes directly the outcome of the assessment.
2.3. Disease assessment according to Article 5 and Article 9
The assessment of each disease according to Articles 5 and 9 criteria is carried out using the procedure of expert judgement that involves expert selection and judgement as described below.
2.3.1. Expert selection
The AHAW Panel is composed of 21 members who have been selected according to their expertise on animal health and welfare, and their absence of conflict of interest has been screened according to the EFSA rules on declarations of interest for the experts.3 Furthermore, the AHAW Panel adopts and is the author of the EFSA opinions according to Article 29 of the Regulation (EC) No 178/20024. For this reason, the AHAW Panel is the group of experts considered as the most entitled to perform such a judgement. Accordingly, a group of at least 10 experts from the AHAW Panel are requested to participate to the expert judgement.
2.3.2. Assessment method
Expert judgement is performed using the Individual and Collective Behavioural Aggregation (ICBA) approach described below. Experts are individually provided with the disease fact sheet prior to the judgement together with interpretations and definitions of some wording used in Articles 5 and 9. Wording that has not been defined is up to the individual experts' interpretation.
The criteria of Articles 5 and 9 are phrased in a way that they could be translated into questions to which Yes/No answers could be given (fulfilment check). In order to facilitate the risk managers' decision for listing and categorisation, a Yes/No/na (na: ‘not available’, meaning insufficient evidence or irrelevant to judge) assessment for the criteria of Articles 5 and 9 was developed. Accordingly, an assessment table is constructed, where a mapping of Article 5 and 9 in relation to Article 7, with the possible Yes/No/na outcomes, is displayed.
Using that assessment table, the experts indicate their Y/N or ‘na’ judgement on each criterion of Articles 5 and 9, and they may provide the different reasoning arguments supporting their judgement.
The ICBA method to perform the expert judgement for each disease is implemented in two rounds:
Round 1: Individual judgement
Each expert performs his/her individual judgement. On the basis of the evidence collected from Article 7 criteria/parameters and mapped into Article 5 and Article 9 criteria, the experts are asked individually to provide categorical answers (Y/N/na) (using Table 2 and Appendix B). For questions where no consensus is reached at the individual level, the experts are requested to provide the view/reasoning leading them to the final Y/N/na answer.
Round 2: Collective judgement
The collective judgement consists of a behavioural aggregation where the individual judgements produced in Round 1 are discussed in a physical meeting, where additional material/information may be supplied by the experts present, in order to seek for consensus. Following the discussion, for each Article 5 and 9 criterion, the final answer is provided (consensus for Yes, No or ‘na’ or no consensus) together with the supporting views where the consensus is not reached. The following are taken into account:
only the responses from experts having taken part in both individual and collective judgement are counted per question in the final output;
the questions where full consensus is reached during the individual judgement are not further discussed in the physical meeting.
The overall output of the judgement achieved for each Article 5 and 9 criterion for each disease is composed by a categorical answer (Y/N/na), and for the no consensus questions, the outcome includes all the supporting views and the %Y, %N and %na. To take better account of the uncertainties involved, a quantitative instead of the above‐described categorical approach has been followed from 2021. The output henceforth consists in the individual probability ranges provided by the experts and their computed median range. Details are given in Appendix C.
Fact sheet amendment: following the individual judgment, and during the collective judgment until the final adoption, additional information or clarification of certain points can be added into the fact sheet for consideration. The fact sheet can also be revised when necessary.
Reassessment: In the case of substantial changes in the fact sheet that can impact the outcome of the assessment already done, the target question has to be reassessed under collective judgment (not necessarily involving the same judges).
3. Results
The main objective of this work is to develop an ad hoc methodological approach fit for the purpose of assessing each disease under rules prescribed in the AHL. As a result, the method that has been developed consists in three chronological main steps as summarised in Figure 1 and described below.
Figure 1.
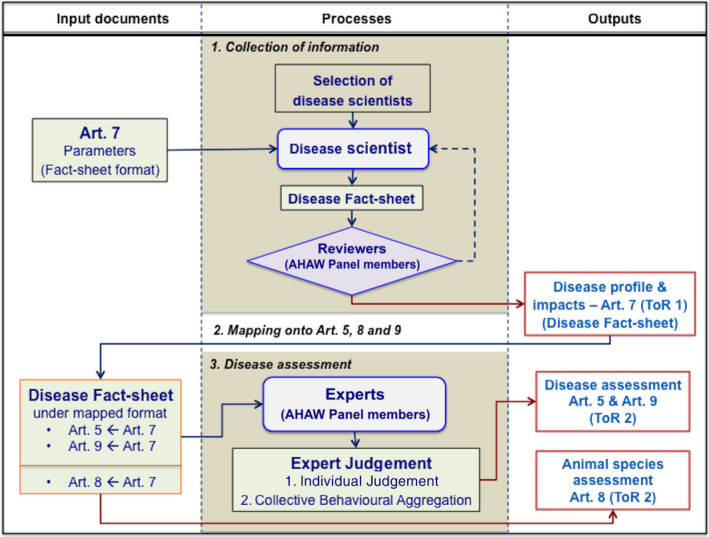
Flow chart of the algorithm for the assessment of diseases within the AHL framework according to the ad‐hoc method developed in this opinion
The three processes in Figure 1 are described in detail in the following Sections 3.1, 3.2 and 3.3.
3.1. Collection of information
At the commencement of the assessment of any disease, a disease scientist (DS) is selected as described in Section 2. The DS is provided with a template of a fact sheet to be completed following the framework determined from the structured Article 7 (input) (see Table 2). The result of breaking down the Article 7 criteria of the AHL into sub‐criteria and parameters is given below in Table 2.
The DS collects information and drafts the disease fact sheet according to the criteria they have been provided (structured Article 7 criteria of the AHL).
The disease fact sheet is then reviewed by two independent reviewers (AHAW Panel experts or WG experts) and DS endorses the comments provided.
Input: Structured Article 7 (Table 2).
Output: Disease profile and impacts in a fact sheet format (Result for ToR 1).
3.2. Mapping of Article 7 criteria in relation to Article 5, Article 9 and Article 8 criteria
The disease fact sheet as in step 1 (structured table of Article 7 criteria) is reorganised using mapping matrices that allow assigning each set of information related to each Article 7 criteria (and related parameters) to the relevant Articles 5, 9 and 8 criteria. The mapping matrices providing each criterion of Articles 5, 9 and 8 as a function of Article 7 criteria (and related parameters) are given below in Tables 3, 4 and 5, respectively.
Table 3.
Mapping matrix of criteria for listing the disease as in Article 5 of the AHL vs Article 7 parameters
| Article 5 criteria for listing | Mapped Article 7 criteria | ||
|---|---|---|---|
| A(i) | Scientific evidence indicates that the disease is transmissible | (a)(vi) | The routes and speed of transmission of the disease between animals and, when relevant, between animals and humans |
| (a)(v) | Persistence of the disease in an animal population or in the environment* | ||
| A(ii) | Animal species are either susceptible to the disease or vectors and reservoirs thereof exist in the Union | (a)(i) | Animal species concerned by the disease |
| A(iii) | The disease causes negative effects on animal health or poses a risk to public health due to its zoonotic character | (a)(ii) | Morbidity and mortality rates of the disease in animal populations |
| (a)(iii) | Zoonotic character of the disease | ||
| (a)(iv) | Resistance to treatments, including antimicrobial resistance | ||
| (b)(ii) | Impact of the disease on human health as regards transmissibility between animal s and humans, and between humans, and the severity of human form of the diseasea | ||
| (b)(iii) | Impact of the disease on animal welfare | ||
| (c) | Potential to generate a crisis situation and its potential use in bioterrorism | ||
| A(iv) | Diagnostic tools are available for the disease | (a)(viii) | Existence of diagnostic and disease control tools |
| A(v) | Risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union | (a)(viii) | Existence of diagnostic and disease control tools |
| (b)(ii) | IMPACT of the disease on human health as regards the availability of effective prevention or medical treatmenta | ||
| (d)(i) | Feasibility, availability and effectiveness of diagnostic tools and capacities | ||
| (d)(ii) | Feasibility, availability and effectiveness of vaccination | ||
| (d)(iii) | Feasibility, availability and effectiveness of medical treatments | ||
| (d)(iv) | Feasibility, availability and effectiveness of biosecurity measures | ||
| (d)(v) | Feasibility, availability and effectiveness of restrictions on the movement of animals and products, as control measure | ||
| (d)(vi) | Feasibility, availability and effectiveness of killing of animals | ||
| (d)(vii) | Feasibility, availability and effectiveness of disposal of carcasses and other relevant animal by‐products | ||
| B(i) | The disease causes or could cause significant negative effects in the Union on animal health, or poses or could pose a significant risk to public health due to its zoonotic character | (a)(ii) | Case‐morbidity and case‐fatality rates of the disease in animal populations |
| (a)(iii) | Zoonotic character of the disease | ||
| (a)(iv) | Resistance to treatments, including antimicrobial resistance | ||
| (b)(ii) | IMPACT of the disease on Human health as regards transmissibility between animal s and humans, and between humans, and the severity of human form of the disease | ||
| B(ii) | The disease agent has developed resistance to treatments and poses a significant danger to public and/or animal health in the Union | (a)(iv) | Resistance to treatments, including antimicrobial resistance |
| B(iii) | The disease causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union | (a)(ii) | Morbidity and mortality rates of the disease in animal populationsb |
| (b)(i) | The impact of the disease on agricultural and aquaculture production and other parts of the economy | ||
| B(iv) | The disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism | (c) | Potential to generate a crisis situation and its potential use in bioterrorism |
| B(v) | The disease has or could have a significant negative impact on the environment, including biodiversity, of the Union | (b)(iv) | Impact of the disease on biodiversity and the environment |
| (e)(iv) | The impact of disease prevention and control measures, as regards the environment and biodiversity | ||
| (a)(v) | Persistence of the disease in an animal population or in the environment* | ||
Mapped at subcriterion level.
Only the parameter a(ii) 3 ‘case‐fatality rate’ is considered.
Table 4.
Mapping matrix of criteria for disease categorisation as in Annex IV according to Article 9 of the AHL vs Article 7 criteria
| Article 9 categories | Mapped Article 7 criteria | ||||
|---|---|---|---|---|---|
| Category A | Category B | Category C | |||
| 1 | The disease is not present in the territory of the Union OR present only in exceptional cases (irregular introductions) OR present in only in a very limited part of the territory of the Union | The disease is present in the whole or part of the Union territory with an endemic character. However, several Member States or zones of the Union are free of the disease |
In terrestrial animals: present in whole or part of the Union territory with an endemic character; in aquatic animals: several Member States or zones of the Union are free of the disease |
(a)(vii) | The absence or presence and distribution of the disease in the Union, and, where the disease is not present in the Union, the risk of its introduction into the Union |
| (b)(i) | The impact of the disease on agricultural and aquaculture production and other parts of the economy, as regards level of presence of the disease in the Uniona | ||||
| 2.1 | The disease is highly transmissible | The disease is moderately to highly transmissible | The disease is moderately to highly transmissible | (a)(vi) | The routes and speed of transmission of the disease between animals and, when relevant, between animals and humansb |
| (a)(v) | Persistence of the disease in an animal population or in the environment | ||||
| 2.2 | Direct and indirect transmission and airborne or waterborne or vector‐borne spread | Direct and indirect transmission and airborne or waterborne or vector‐borne spread. |
In terrestrial animals: mainly by direct and indirect transmission in aquatic animals: mainly direct and indirect transmission. |
(a)(vi) | The routes and speed of transmission of the disease between animals and, when relevant, between animals and humansc |
| 2.3 | The disease affects multiple species of kept and wild animals or single species of kept animals of economic importance | The disease affects single or multiple animal species |
In terrestrial animals: it mainly affects multiple or single animal species in aquatic animals: it affects multiple or single animal species; |
(a)(i) | Animal species concerned by the disease |
| 2.4 | The disease may result in high morbidity and significant mortality rates | The disease may result in high morbidity and in general low mortality |
In terrestrial animals: it does not result in high morbidity and negligible or no mortality, often the most observed effect is production loss; In aquatic animals: it has high morbidity and usually low mortality, often the most observed effect is production loss |
(a)(ii) | Morbidity and mortality rates of the disease in animal populations |
| (b)(i) | Impact of the disease on agricultural and aquaculture production and other parts of the economy | ||||
| 3 | The disease in question has a zoonotic potential with significant consequences for public health, including epidemic or pandemic potential or possible significant threats to food safety | The disease in question has a zoonotic potential with significant consequences for public health, or possible significant threats to food safety | (a)(iii) | Zoonotic character of the disease | |
| (a)(vi) | The routes and speed of transmission of the disease between animals and, when relevant, between animals and humansd | ||||
| (b)(ii) | Impact of the disease on Human health resistance as regards the severity of human form of the disease and availability of effective prevention or medical treatment | ||||
| (c) | Potential to generate a crisis situation and its potential use in bioterrorism | ||||
| 4 | The disease in question has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | The disease in question has a significant impact on the economy of the Union, mainly related to its direct impact on the health and productivity of animals | (a)(ii) | Morbidity and mortality rates of the disease in animal populations | |
| (b)(i) | Impact on agricultural and aquaculture production and other parts of the economy | ||||
| 5(a) | The disease in question has a significant impact on society, with in particular an impact on labour markets | (b)(i) | The impact of the disease on agricultural and aquaculture production and other parts of the economy | ||
| 5(b) | The disease in question has a significant impact on animal welfare, by causing suffering to large numbers of animals | (a)(ii) | Morbidity and mortality rates of the disease in animal populationse | ||
| (b)(iii) | Impact of the disease on animal welfare | ||||
| 5(c) | The disease in question has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | (b)(iv) | Impact of the disease on biodiversity and the environmentf | ||
| (e)(iv) | The impact of disease prevention and control measures, as regards the environment and biodiversity | ||||
| (a)(v) | Persistence of the disease in an animal population or in the environment(1) | ||||
| 5(d) | The disease in question has a significant impact on in the long term, biodiversity or the protection of endangered species or breeds, including the possible disappearance of, or long‐term damage to, those species or breeds | (b)(iv) | Impact of the disease on biodiversity and the environment | ||
| Category D | |||||
| The disease fits category A or B or C or E and where the risk posed by the disease in question can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread | – | As from the criteria of category A in Article 9 | |||
| – | As from the criteria of category B in Article 9 | ||||
| – | As from the criteria of category C in Article 9 | ||||
| – | As from the criteria of category E in Article 9 | ||||
| (d)(v) | Feasibility, availability and effectiveness of restrictions on the movement of animals and products, as control measure | ||||
| Category E | |||||
| If the disease fits category A or B or C and/or the surveillance of the disease is necessary for reasons relating to animal health, animal welfare, human health, the economy, society or the environment (compliance with all criteria of Article 5(A) and at least one of Article 5(B)) | – | As from the criteria of category A in article 9 | |||
| – | As from the criteria of category B in article 9 | ||||
| – | As from the criteria of category C in article 9 | ||||
| – | As all criteria in article 5(A) and at least one criterion of article 5(B) | ||||
For this criterion in this question, only the parameter b(i) 1 ‘number of Member States where the disease is present’ is considered.
For this criterion in this question, only the parameter (a)(vi) 3 ‘Incidence between animals and, when relevant, between animals and humans’ and (a)(vi) 4 ‘Transmission rate (beta) (from R0 and infectious period) between animals and, when relevant, between animals and humans’ are considered.
For this criterion in this question, only the parameter (a)(vi) 1 ‘type of routes of transmission from animal to animal (horizontal, vertical)’ is considered.
For this criterion in this question, only the parameter (a)(vi) 2 ‘type of routes of transmission between animals and humans (direct and indirect including food‐borne); (a)(vi) 3 ‘Incidence between animals and when relevant between animals and humans’ and (a)(vi) 4 ‘Transmission rate (beta) (from R0 and infectious period) between animals and when relevant between animals and humans’ are considered.
For this criterion in this question, only the parameter (a)(ii) 2 ‘Case‐morbidity rate (% clinically diseased animals out of infected ones)’ is considered.
For this criterion in this question only the parameter (b)(iv) 1 ‘Endangered wild species affected: listed species as in CITES and/or IUCN list’ and (b)(iv) 2 ‘Mortality in wild species’ are considered.
(1): For this criterion in this question, only the parameter (a)(v) 4 ‘Length of survival (dpi) of the agent and/or detection of DNA in selected matrices (soil, water, air) from the environment (scenarios: high and low temperature)’ is considered.
Table 5.
Mapping matrix of criteria for listing the animal species or group of animal species affected or that pose a risk for the spread of a specific disease as in Article 8 of the AHL vs Article 7 parameters
| Article 8 criteria for listing | Mapped Article 7 criteria | ||
|---|---|---|---|
| 3(a) | Animal species or group of animal species are susceptible to a specific disease or scientific evidence indicates that such susceptibility is likely | (a)(i) | Animal species concerned by the disease |
| (a)(v) | Persistence of the disease (i.e. infective agent) in an animal population or in the environmenta | ||
| 3(b) | Animal species or group of animal species are vector species or reservoirs for that disease, or scientific evidence indicates that such role is likely | (a)(i) | Animal species concerned by the disease |
| (a)(vi) | The routes of transmission of the disease between animals and, when relevant, between animals and humansb | ||
Parameters used in the mapping are from 1 to 3.
Mapped at subparameter level.
As a result, the disease fact sheet is reshaped into disease assessment table, an example is shown in Table 6 where the first question of Article 5 is reported, while, in Appendix B, the tables for all other questions are reported.
Table 6.
Example of disease assessment table
|
Question A(i) scientific evidence indicates that the disease is transmissible Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Article 7 criteria | Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(vi) The routes and speed of transmission of the disease between animals and, when relevant, between animals and humans | (a)(vi) 1 Type of routes of transmission from animal to animal (horizontal, vertical) | <Information from the fact sheet> |
| (a)(vi) 2 Type of routes of transmission from animal to humans (direct, indirect) | <Information from the fact sheet> | |
Output: Mapping matrices (Tables 3, 4 and 5) and a disease assessment table (Table 6). For Article 8, mapping of the disease fact sheet into Table 5 provides directly the outcome of the assessment.
In Appendix B, the instructions for the judgement including definitions and interpretations of terms and questions are provided. Appendix C highlights the changes adopted from 2021.
3.3. Disease assessment
The disease assessment is performed using the ICBA approach as described in Section 2 on methodology. In the first round of judgement, the disease assessment table (Table 6) in step 2 is provided to experts (AHAW panel experts) for individual assessment of the disease. In the second round of judgement, the results of the expert judgement obtained in first round are used for the collective behavioural aggregation.
The final result of the expert judgement on each criterion of article 5 and article 9 is the output of the collective judgement, which combines the outcome of the individual judgements, their discussion and any additional relevant material known to the experts participating in the judgement. The final outcome can be revised by the AHAW Panel on the basis of any new available evidence before the adoption of the scientific opinion, and the judgment can be amended accordingly. The result of assessment is reported in Tables 7 and 8.
Table 7.
Final result of the expert judgement on each criterion of Article 5 or Article 9
| Question | Final outcome | |
|---|---|---|
| A(i) | Is the disease is transmissible? | Y/N |
| NC | ||
| na | ||
Colour code: green = consensus (Yes/No), yellow = no consensus (NC), red = not available (na), i.e. insufficient evidence or irrelevant to judge (na).
Table 8.
Final result of the expert judgement in case of no consensus (NC) outcome for each criterion of Article 5 or Article 9
| Question | Final outcome | Response | |||
|---|---|---|---|---|---|
| Y (%) | N (%) | na (%) | |||
| B(v) | Disease has or could have a significant negative impact on the environment, including biodiversity, of the Union (Example question) | NC | % of Yes | % of No | % of na |
NC: no consensus.
As from the legal text of the AHL, a disease is considered eligible to be listed as laid down in Article 5 if it fulfils all criteria indicated in point A and at least one criterion as in point B of Article 5(3) (see Appendix A.2). Likewise, a disease is considered to belong or classified in a category according to Article 9 if it fulfils all criteria indicated at points a and b and at least one of the criteria indicated at points c, d, e as in Annex IV (see Appendix A.3). According to the assessment methodology, a criterion is considered fulfilled when the outcome is ‘Yes’.
For each outcome without consensus, an additional table (Table 8) is provided followed by the detailed list of different supporting views.
To take better account of the uncertainties involved, a quantitative instead of the above‐described categorical approach has been followed from 2021. The output henceforth consists in the individual probability ranges provided by the experts and their computed median range. Details are given in Appendix C. For each disease, the final results of the assessment according to the assessment steps as shown in Figure 1 are the following:
Disease fact sheet: provides information on the disease profile and impacts. The format is shown in Table 2.
Disease assessment according to Article 5: it provides information on the eligibility of the disease to be listed. The format is shown in Tables 6 and 7.
Disease assessment according to Article 9: it provides information on the compliance of the disease with each of the criteria in Annex IV to the AHL for the purpose of categorisation in accordance with Article 9. The format is shown in Tables 6 and 7.
Disease assessment according to article 8: a list of animal species that should be considered candidates for listing in accordance with Article 8 of the AHL, based on the criteria as displayed in Table 5 of the present document.
The assessment results are drafted in a separated opinion, one for each disease.
Conclusions
An ad hoc method for disease listing and categorisation according to the framework of Articles 7, 5 and Annex IV in accordance with Articles 9 and 8 of the AHL has been developed in order to allow the AHAW Panel to perform the requested assessment on each disease.
The assessment methodology includes: (i) the procedure for collecting the data and compiling the information in a disease fact sheet, (ii) the mapping matrices of Articles 5, 9 and 8 based on Article 7 providing the directions for use of the relevant information from the disease fact sheets, and (iii) the expert judgement for the disease assessment for listing and categorisation.
This methodology is used for the following assessment steps: the assessment according to the Article 7 criteria (disease fact sheet); the expert judgement according to Articles 5 and 9 criteria; and the assessment according to Article 8 criteria on the species concerned by the disease, which is derived from the related criteria of the fact sheet.
The expert judgement approach that was followed during the disease assessment combines the individual and collective knowledge and opinion of experts and seeks consensus, when possible.
This method has been developed and adapted to a set of predefined and prestructured criteria from the legislative text of the AHL. Therefore, the structure of the method follows the legislative texts of the AHL. Initially, data are collected on the disease profile and impacts according to Article 7, next, the eligibility of the disease for listing is assessed and categorised according to Articles 5 and 9, respectively, and finally the animal species candidates for listing are assessed according to Article 8. The categorical approach has been replaced by a quantitative approach from 2021.
Finally, the assessment framework as proposed above (see Figure 1) does not target nor exclude a formal and automated procedure.
Glossary and Abbreviations
- AHL
Animal health law
- AHAW
EFSA Panel on Animal Health and Welfare
- CITES
Convention on International Trade in Endangered Species of Wild Fauna and Flora
- Disease fact sheet
A document containing all relevant information on a specific disease. The framework of the document follows the structure of the Article 7 criteria of the new AHL
- Disease scientist (DS)
A scientist who is able to retrieve, extract, collate and interpret relevant scientific information related to a specific disease, covering all domains required to address the AHL (Article 7) criteria and related parameters
- DIVA
Differentiating Infected from Vaccinated Animals (vaccine)
- ICBA
Individual and collective behavioural aggregation
- IUCN
International Union for Conservation of Nature
- MSs
Member States
- OIE
World Organisation for Animal Health
- OIE/CFSPH
The Center for Food Security and Public Health (OIE Collaborating Centre for Day‐One Veterinary Competencies and Continuing Education)
Appendix A – Criteria of Articles 7, 5, 8 and Annex IV for the purpose of categorisation of diseases in accordance with Article 9 of the AHL
A.1. Article 7 – Assessment parameters
The Commission shall use the following assessment parameters in order to determine whether a disease meets the conditions requiring it to be listed in accordance with Article 5(2):
-
the disease profile, which shall comprise the following:
the animal species concerned by the disease;
the morbidity and mortality rates of the disease in animal populations;
the zoonotic character of the disease;
the resistance to treatments, including antimicrobial resistance;
the persistence of the disease in an animal population or in the environment;
the routes and speed of transmission of the disease between animals and, when relevant, between animals and humans;
the absence or presence and distribution of the disease in the Union, and, where the disease is not present in the Union, the risk of its introduction into the Union;
the existence of diagnostic and disease control tools;
-
the impact of the disease on:
-
agricultural and aquaculture production and other parts of the economy, as regards:
-
1
– the level of presence of the disease in the Union;
-
2
– the loss of production due to the disease;
-
3
– other losses;
-
1
-
human health, as regards:
transmissibility between animals and humans;
transmissibility between humans;
the severity of human forms of the disease;
the availability of effective prevention or medical treatment in humans;
animal welfare;
biodiversity and the environment;
-
its potential to generate a crisis situation and its potential use in bioterrorism;
-
the feasibility, availability and effectiveness of the following disease prevention and control measures:
diagnostic tools and capacities;
vaccination;
medical treatments;
biosecurity measures;
restrictions on the movement of animals and products;
killing of animals;
disposal of carcasses and other relevant animal by‐products;
-
the impact of disease prevention and control measures, as regards:
the direct and indirect costs for the affected sectors and the economy as a whole;
their societal acceptance;
the welfare of affected subpopulations of kept and wild animals;
the environment and biodiversity.
A.2. Article 5 – Criteria for listing of diseases
1. The disease‐specific rules for the prevention and control of diseases provided for in this Regulation shall apply to:
-
the following listed diseases:
foot and mouth disease;
classical swine fever;
African swine fever;
highly pathogenic avian influenza;
African horse sickness; and
the listed diseases set out in the list in Annex II.
2. The Commission shall adopt delegated acts in accordance with Article 264 concerning amendments to the list referred to in point (b) of paragraph 1 of this Article.
3. A disease shall be included on the list referred to in point (b) of paragraph 1 of this Article if it has been assessed in accordance with Article 7 and it meets:
-
all of the following criteria:
scientific evidence indicates that the disease is transmissible;
animal species are either susceptible to the disease or vectors and reservoirs thereof exist in the Union;
the disease causes negative effects on animal health or poses a risk to public health due to its zoonotic character;
diagnostic tools are available for the disease; and
risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union; and
-
at least one of the following criteria:
the disease causes or could cause significant negative effects in the Union on animal health, or poses or could pose a significant risk to public health due to its zoonotic character;
the disease agent has developed resistance to treatments which poses a significant danger to public and/or animal health in the Union;
the disease causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union;
the disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism; or
the disease has or could have a significant negative impact on the environment, including biodiversity, of the Union.
4. The Commission shall adopt delegated acts in accordance with Article 264 concerning the removal of a disease from the list referred to in point (b) of paragraph 1 of this Article when that disease no longer fulfils the criteria provided for in paragraph 3 of this Article.
5. The Commission shall review the listing of each disease in the light of newly available significant scientific data.
A.3. Annex IV – Criteria for the application of the disease prevention and control rules referred to in Article 9(1) to diseases listed in accordance with Article 5
The scope of this Annex is to detail the criteria to be considered by the Commission when determining the disease prevention and control rules to be applied to the different categories of diseases listed in accordance with Article 5.
The process of categorisation shall take into account the profile of the disease in question, the level of the impact of that disease on animal and public health, animal welfare and the economy, and the availability, feasibility and effectiveness of the diagnostic tools and different sets of disease prevention and control measures provided for in this Regulation with respect to the disease.
A.3.1. Section 1 Criteria for the application of the disease prevention and control rules referred to in point (a) of Article 9(1)
The diseases for which the disease prevention and control rules referred to in point (a) of Article 9(1) apply shall be considered to have the most severe animal health, public health, economic, social or environmental impacts on the Union. Those diseases need to fulfil the following criteria:
-
a
the disease in question is: and
not present in the territory of the Union;
present only in exceptional cases (irregular introductions); or
present in only in a very limited part of the territory of the Union;
-
b
the disease in question is highly transmissible; in addition to direct and indirect transmission, there may also be possibilities of airborne, waterborne or vector‐borne spread. The disease may affect multiple species of kept and wild animals, or a single species of kept animals of economic importance, and may result in high morbidity and significant mortality rates.
In addition to the criteria set out in points (a) and (b), those diseases need to fulfil one or more of the following criteria:
-
c
the disease in question has a zoonotic potential with significant consequences for public health, including epidemic or pandemic potential or possible significant threats to food safety;
-
d
the disease in question has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals;
-
e
the disease in question has a significant impact on one or more of the following:
society, with in particular an impact on labour markets;
animal welfare, by causing suffering to large numbers of animals;
the environment, due to the direct impact of the disease or due to the measures taken to control it;
in the long term, biodiversity or the protection of endangered species or breeds, including the possible disappearance of, or long‐term damage to, those species or breeds.
A.3.2. Section 2 Criteria for the application of the disease prevention and control rules referred to in point (b) of Article 9(1)
The diseases for which the disease prevention and control rules referred to in point (b) of Article 9(1) apply shall be controlled in all Member States with the goal of eradicating them throughout the Union.
Those diseases need to fulfil the following criteria:
-
a
the disease in question is endemic in nature and is present in the whole or part of the Union territory. However, several Member States or zones of the Union are free of the disease; and
-
b
the disease is moderately to highly transmissible; in addition to direct and indirect transmission, there may also be possibilities of airborne, waterborne or vector‐borne spread. It may affect single or multiple animal species and may result in high morbidity, with in general low mortality.
In addition to the criteria set out in points (a) and (b), those diseases need to fulfil one or more of the following criteria:
-
c
the disease in question has a zoonotic potential with significant consequences for public health, including epidemic potential or possible significant threats to food safety;
-
d
the disease in question has a significant impact on the economy of the Union causing substantial costs, mainly related to its direct impact on the health and productivity of animals;
-
e
the disease has a significant impact on one or more of the following:
society, with in particular an impact on labour markets;
animal welfare, by causing suffering to large numbers of animals;
the environment, due to the direct impact of the disease or due to the measures taken to control it;
in the long term, biodiversity or the protection of endangered species or breeds, including the possible disappearance of, or long‐term damage to, those species or breeds.
A disease to which the measures referred to in point (a) of Article 9(1) apply, which has not been successfully and promptly eradicated in a part of the Union, and has, in that part of the Union, obtained an endemic character, may be subject to disease prevention and control measures under point (b) of Article 9(1), in that part of the Union.
A.3.3. Section 3 Criteria for the application of the disease prevention and control rules referred to in point (c) of Article 9(1)
The diseases for which the disease prevention and control rules referred to in point (c) of Article 9(1) apply are of relevance to some Member States and measures are needed to prevent them from spreading to parts of the Union that are officially disease‐free or that have eradication programmes for the listed disease in question.
Those diseases need to fulfil the following criteria:
-
a
in terrestrial animals, the disease in question is endemic in nature and is present in the whole or part of the Union territory; or in aquatic animals, several Member States or zones of the Union are free of the disease; and
-
b
i) in terrestrial animals, the disease in question is moderately to highly transmissible, mainly through direct and indirect transmission. The disease mainly affects multiple or single animal species, usually does not result in high morbidity, and has a negligible or no mortality rate. Often the most observed effect is production loss;
ii) in aquatic animals, the disease is moderately to highly transmissible, mainly through direct and indirect transmission. The disease affects multiple or single animal species and may result in high morbidity and usually low mortality. Often the most observed effect is production loss.
In addition to the criteria set out in points (a) and (b), those diseases need to fulfil one or more of the following criteria:
-
c
the disease in question has a zoonotic potential with significant consequences for public health, or possible threats to food safety;
-
d
the disease in question has a significant impact on the economy of parts of the Union, mainly related to its direct impact on certain types of animal production systems.
-
e
the disease in question has a significant impact on one or more of the following:
society, with, in particular, an impact on labour markets;
animal welfare, by causing suffering to large numbers of animals;
the environment, due to the direct impact of the disease or of the measures taken to control it;
in the long term, biodiversity or the protection of endangered species or breeds, including the possible disappearance of, or long‐term damage to, those species or breeds.
A.3.4. Section 4 Criteria for the application of the disease prevention and control rules referred to in point (d) of Article 9(1)
The disease prevention and control rules referred to in point (d) of Article 9(1) shall apply to diseases that fulfil the criteria set out in Sections 1, 2 or 3 and to other diseases fulfilling the criteria set out in Section 5 where the risk posed by the disease in question can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread.
A.3.5. Section 5 Criteria for the application of the disease prevention and control rules referred to in point (e) of Article 9(1)
The disease prevention and control rules referred to in point (e) of Article 9(1) shall apply to diseases that fulfil the criteria set out in Sections 1, 2 or 3 and to other diseases where surveillance of the disease is necessary for reasons relating to animal health, animal welfare, human health, the economy, society or the environment.
A.4. Article 8 – Listed species
Animal species or groups of animal species shall be added to the list if they are affected or if they pose a risk for the spread of a specific listed disease because:
they are susceptible to a specific listed disease or scientific evidence indicates that such susceptibility is likely; or
they are vector species or reservoirs for that disease, or scientific evidence indicates that such role is likely.
Appendix B – Expert Judgement document
1.
Here below the instructions for the judgement and, where needed, the interpretations of the questions is reported.
B.1. Instructions
All evidence provided by question should be read and considered when answering the question.
Sequence of the task for judging:
read the question, including any internal interpretation of this question
read the information/data
answer the question.
B.2. Interpretation of terms and questions
The following interpretations were considered for the assessment of criteria of Article 5 for listing and of Annex IV referring to categories of Article 9 of the AHL:
Interpretation of the term ‘significant’ when it is used in relation to the impact or the effect of the disease or of the disease control or preventive measure on animal health, public health, economy, society, environment:
A disease will be considered as having a significant impact on a system (animal health, public health, society, environment, economic) when it causes overall additional effects (damages, losses, costs, disturbance, …) compared to the situation in the absence of the disease either,
at local scale (a single country for EU) but with long‐term (> 1 year) effects
at larger scale (> 2 countries) regardless the effect duration (short or long‐terms)
or that require mitigation and control (authority) actions to restoring the system in its state in the absence of the disease.
Interpretation of questions in Article 5 B(i), B(iii) – ‘The disease causes or could cause …effect/impact…’ and B(v) – ‘The disease has or could have…impact…’:
the disease impact or negative effects have to be assessed without considering any effect of interventions or control measures that are (if the disease is already present in the EU) or can be put in place (if the disease is exotic and assuming it is introduced into the EU).
All Article 9 criteria/questions refer to the EU regardless ‘in the Union’ is mentioned or not in the question.
Interpretation of questions in Article 9 2.4A, 2.4B – ‘The disease may result in high morbidity…’:
For the judgment of this question, the potential for the disease to have high morbidity and/or high mortality even in absence of control measures are considered.
Interpretation of questions in Article 9 4AB, 4C, 5A, 5B, 5C, 5D starting with ‘the disease has a significant impact on…’:
-
Case 1: if the disease is not present (exotic) or present only in exceptional cases, or only in a very limited part of EU:
-
☐
— Potential impact: Use the fact sheet and collective opinion to assess the impact of the disease assuming it is introduced into the EU and without considering the effects of any interventions or control measures.
-
☐
-
Case 2: if the disease is endemic/present in the whole or part of the Union
-
☐
— Current impact: Use the fact sheet (which provides information of the current status) and collective opinion to assess the impact of the disease by taking into account the effects of the interventions or control measures that are in place (if any);
-
☐
— Potential impact: when relevant, assess the impact of the disease considering the potential situation in EU as the interventions or control measures in place (if any) would be removed.
-
☐
For the disease related to case 2 the different type of impact (current and potential, as explained above), is assessed separately in the table collecting the answers of the judges or explained in the reasoning points.
Interpretation of question D in Article 9 ‘ the risk posed by the disease in question can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread’: for exotic diseases, the assessment of the effectiveness of movement of animals and products to mitigate the risk should take into account also the control of movements into the EU (import checks).
For questions related to Article 9
The following interpretations were used for the assessment of the criteria across the categories A, B and C. If criterion 1 about disease distribution is fulfilled in category B, then the same available data would most likely also support fulfillment of category. For criterion 2.1 about transmissibility, the options in category B and C are the same. The criterion 2.2 is the same in category A and B, thus with same outcome. The criterion 2.3 in category B and C is considered always fulfilled, because a disease is dealt here if it affects at least one animal species. Criteria 5(a‐d) are the same in category A, B and C and have the same outcome. The evidence extrapolated from the relevant criteria of Article 7 (fact sheet) should always support such interpretation.
B.3. Information provided to the experts for performing the assessment on each disease: information mapping
Here below all the example tables are reported, which show the criteria (questions) of Articles 5 and 9 next to the related criteria and parameters of Art. 7, based on which the judgement on each question should be performed.
B.3.1. Article 5
Question A(i)
|
Question A(i) scientific evidence indicate that the disease is transmissible Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Article 7 criteria | Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(vi) The routes and speed of transmission of the disease between animals and, when relevant, between animals and humans | (a)(vi) 1 type of routes of transmission from animal to animal (horizontal, vertical) | <Information from the fact sheet> |
| (a)(vi) 2 type of routes of transmission from animal to humans (direct, indirect) | <Information from the fact sheet> | |
| (a)(v) Persistence of the disease in an animal population or in the environment | (a)(v) 1 duration of infectious period in animals | <Information from the fact sheet> |
| (a)(v) 2 presence and duration of latent infection period | ||
| (a)(v) 3 presence and duration of the pathogen in healthy carriers | ||
Question A(ii)
|
Question A(ii) animal species are either susceptible to the disease or vectors and reservoirs thereof exist in the Union Interpretation‐ indicate if animal species susceptible to the disease or vector or reservoir are present in the Union Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Article 7 criteria | Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(i) Animal species concerned by the disease | (a)(i) 1 naturally susceptible wildlife species (or families/orders) | <Information from the fact sheet> |
| (a)(i) 2 naturally susceptible domestic species (or families/orders) | <Information from the fact sheet> | |
| (a)(i) 3 experimentally susceptible wildlife species (or families/orders) | <Information from the fact sheet> | |
| (a)(i) 4 experimentally susceptible domestic species (or families/orders) | ||
| (a)(i) 5 wild reservoir species (or families/orders) | <Information from the fact sheet> | |
| (a)(i) 6 domestic reservoir species (or families/orders) | <Information from the fact sheet> | |
Question A(iii)
|
Question A(iii) disease causes negative effects on animal health OR poses a risk to public health due to its zoonotic character Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Article 7 criteria | Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(ii) Morbidity and mortality rates of the disease in animal populations | (a)(ii) 1 Prevalence/incidence | <Information from the fact sheet> |
| (a)(ii) 2 Case‐morbidity rate (% clinically diseased animals out of infected ones) | <Information from the fact sheet> | |
| (a)(ii) 3 Case‐fatality rate | <Information from the fact sheet> | |
| (a)(iii) Zoonotic character of the disease | (a)(iii) 1 report of zoonotic human cases | <Information from the fact sheet> |
| (a)(iv) Resistance to treatments, including antimicrobial resistance | (a)(iv) 1 resistant strain to any treatment even at laboratory level | <Information from the fact sheet> |
| (b)(ii) Impact of the disease on human health | (b)(ii) 1 type of routes of transmission between animals and humans ‐ see a(vi)2 | <Information from the fact sheet> |
| (b)(ii) 2 Incidence of zoonotic cases | ||
| (b)(ii) 3 Occasional or substantial? | ||
| (b)(ii) 4 Epidemic or pandemic? | ||
| (b)(ii) 5 DALY | ||
| (b)(iii) Impact of the disease on animal welfare | (b)(iii) 1 severity of clinical signs at case level and related level and duration of impairment | <Information from the fact sheet> |
| (c) Potential to generate a crisis situation and its potential use in bioterrorism | (c) 1 listed in OIE/CFSPH classification of pathogens | <Information from the fact sheet> |
| (c) 2 listed in the Encyclopedia of Bioterrorism Defense of Australia Group | <Information from the fact sheet> | |
| (c) 3 included in any other list of potential bio‐ agro‐terrorism agents | ||
Question A(iv)
|
Question A(iv) diagnostic tools are available for the disease Interpretation‐ diagnostic tools are available for the disease in the Union Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Article 7 criteria | Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(viii) Existence of diagnostic and disease control tools | (a)(viii) 1 Existence of diagnostic tools | <Information from the fact sheet> |
| (a)(viii) 2 Existence of disease control tools | <Information from the fact sheet> | |
Question A(v)
|
Question 5 A(v) the risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Article 7 criteria | Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(viii) Existence of diagnostic and disease control tools | (a)(viii) 1 Existence of diagnostic tools | <Information from the fact sheet> |
| (a)(viii) 2 Existence of disease control tools | <Information from the fact sheet> | |
| (b)(ii) Impact of the disease on human health | (b)(ii) 7 Availability of medical treatment and their effectiveness (therapeutic effect and any resistance) | <Information from the fact sheet> |
| (b)(ii) 8 Availability of vaccines and their effectiveness (reduced morbidity) | ||
| (d)(i) Feasibility, availability and effectiveness of diagnostic tools and capacities | (d)(i) 1 officially/internationally recognised diagnostic tool, OIE certified | <Information from the fact sheet> |
| (d)(i) 2 Se and Sp of diagnostic test | <Information from the fact sheet> | |
| (d)(i) 3 type of sample matrix to be tested (blood, tissue, etc.) | <Information from the fact sheet> | |
| (d)(ii) Feasibility, availability and effectiveness of vaccination | (d)(ii) 1 types of vaccines available on the market | <Information from the fact sheet> |
| (d)(ii) 2 availability/production capacity (per year) | <Information from the fact sheet> | |
| (d)(ii) 3 Field protection as reduced morbidity (reduced susceptibility to infection and/or to disease) | <Information from the fact sheet> | |
| (d)(ii) 4 Duration of protection | <Information from the fact sheet> | |
| (d)(ii) 5 Way of administration | <Information from the fact sheet> | |
| (d)(iii) Feasibility, availability and effectiveness of medical treatments | (d)(iii) 1 types of drugs available on the market and/or allowed by the EU regulatory system | <Information from the fact sheet> |
| (d)(iii) 2 availability/production capacity (per year) | <Information from the fact sheet> | |
| (d)(iii) 3 therapeutic effect in the field (effectiveness) | ||
| (d)(iii) 4 Way of administration | ||
| (d)(iv) Feasibility, availability and effectiveness of biosecurity measures | (d)(iv) 1 available biosecurity measures | <Information from the fact sheet> |
| (d)(iv) 2 effectiveness of biosecurity measure (description) | <Information from the fact sheet> | |
| (d)(iv) 3 feasibility of biosecurity measure (description) | <Information from the fact sheet> | |
| (d)(v) Feasibility, availability and effectiveness of restrictions on the movement of animals and products, as control measure | (d)(v) 1 available restriction movement measures | <Information from the fact sheet> |
| (d)(v) 2 effectiveness of restriction of animal movement in preventing the between farm spread (description) | <Information from the fact sheet> | |
| (d)(v) 3 feasibility of restriction of animal movement (description) | <Information from the fact sheet> | |
| (d)(vi) Feasibility, availability and effectiveness of killing of animals | (d)(vi) 1 available killing of animal measures | <Information from the fact sheet> |
| (d)(vi) 2 effectiveness of killing animals (at farm level or within the farm) for reducing/stopping spread of the disease (description) | <Information from the fact sheet> | |
| (d)(vi) 3 feasibility of killing animals (description) | <Information from the fact sheet> | |
| (d)(vii) feasibility, availability and Effectiveness of disposal of carcasses and other relevant animal by‐products | (d)(vii) 1 disposal options available | <Information from the fact sheet> |
| (d)(vii) 2 effectiveness of disposal option (description) | <Information from the fact sheet> | |
| (d)(vii) 3 feasibility of disposal option (description) | <Information from the fact sheet> | |
Question B(i)
|
Question 5 B(i) disease causes or could cause significant negative effects in the Union on animal health, OR poses or could pose a significant risk to public health due to its zoonotic character? Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Article 7 criteria | Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(ii) Morbidity and mortality rates of the disease in animal populations | (a)(ii) 1 Prevalence/Incidence | <Information from the fact sheet> |
| (a)(ii) 2 Case‐morbidity rate (% clinically diseased animals out of infected ones) | <Information from the fact sheet> | |
| (a)(ii) 3 Case‐fatality rate | <Information from the fact sheet> | |
| (a)(iii) Zoonotic character of the disease | (a)(iii) 1 report of zoonotic human cases | <Information from the fact sheet> |
| (a)(iv) Resistance to treatments, including antimicrobial resistance | (a)(iv) 1 resistant strain to any treatment even at laboratory level | <Information from the fact sheet> |
| (b)(ii) Impact of the disease on Human health | (b)(ii) 1 type of routes of transmission between animals and humans ‐ see a(vi)2 | <Information from the fact sheet> |
| (b)(ii) 2 Incidence of zoonotic cases | <Information from the fact sheet> | |
| (b)(ii) 3 Occasional or substantial? | <Information from the fact sheet> | |
| (b)(ii) 4 Epidemic or pandemic? | <Information from the fact sheet> | |
| (b)(ii) 5 DALY | <Information from the fact sheet> | |
Question B(ii)
|
Question B(ii) disease agent has developed resistance to treatments WHICH poses a significant danger to public and/or animal health in the Union? Interpretation‐ disease agent has developed resistance to treatments AND therefore poses a significant danger to public and/or animal health. If no treatment exists the answer should be na Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Article 7 criteria | Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(iv) Resistance to treatments, including antimicrobial resistance | (a)(iv)1 list of any resistant strain to any treatment even at laboratory level | <Information from the fact sheet> |
Question B(iii)
|
Question B(iii) disease causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union? Interpretation‐ disease and/or infection causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union if no intervention is in place Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Article 7 criteria | Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(ii) Morbidity and mortality rates of the disease in animal populations | (a)(ii) 3 Case‐fatality rate | <Information from the fact sheet> |
| (b)(i) The impact of the disease on agricultural and aquaculture production and other parts of the economy | (b)(i) 1 Number of MSs where the disease is presence | <Information from the fact sheet> |
| (b)(i) 2 Proportion of production losses (%) by epidemic/endemic situation (milk, growth, semen, meat, etc.…) | <Information from the fact sheet> | |
Question B(iv)
|
Question B(iv) disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Article 7 criteria | Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (c) potential to generate a crisis situation and its potential use in bioterrorism | (c) 1 listed in OIE/CFSPH classification of pathogens | <Information from the fact sheet> |
| (c) 2 listed in the Encyclopaedia of Bioterrorism Defense of Australia Group | <Information from the fact sheet> | |
| (c) 3 included in any other list of potential bio‐ agro‐terrorism agents | ||
Question B(v)
|
Question B(v) disease has or could have a significant negative impact on the environment, including biodiversity, of the Union Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Article 7 criteria | Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (b)(iv) Impact of the disease on biodiversity and the environment | (b)(iv) 1 endangered wild species affected: listed species as in CITES and/or IUCN list | <Information from the fact sheet> |
| (b)(iv) 2 mortality in wild species | <Information from the fact sheet> | |
| (b)(iv) 3 capacity of the pathogen to persist in the environment and cause mortality in wildlife | <Information from the fact sheet> | |
| (e)(iv) The impact of disease prevention and control measures, as regards the environment and biodiversity | (e)(iv) Mortality in wild species | <Information from the fact sheet> |
| (a)(v) Persistence of the disease in an animal population or in the environment | (a)(v) 4 Length of survival (dpi) of the agent and/or detection of DNA in selected matrices (soil, water, air) from the environment (scenarios: high and low temperature) | <Information from the fact sheet> |
B.3.2. Article 9
Question 1
The answer to the question 1CAq can be Y only for diseases affecting aquatic animal species, therefore do not assess this question for diseases affecting terrestrial animal species
|
Question 1A the disease is not present in the territory of the Union OR present only in exceptional cases (irregular introductions) OR present in only in a very limited part of the territory of the Union Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
|
Question 1B the disease is present in the whole OR part of the Union territory with an endemic character AND (at the same time) several Member States or zones of the Union are free of the disease Answer Y ☐ N ☐ na ☐ | ||
|
Question 1C the disease is present in the whole OR part of the Union territory with an endemic character Answer Y ☐ N ☐ na ☐ | ||
|
Question 1CAq several Member States or zones of the Union are free of the disease Answer Y ☐ N ☐ na ☐ | ||
| Mapped Article 7 criteria | Mapped Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (b)(i) The impact of the disease on agricultural and aquaculture production and other parts of the economy, as regards level of presence of the disease in the Union | (b)(i)1 Number of MSs where the disease is presence | <Information from the fact sheet> |
| (a)(vii) The absence or presence and distribution of the disease in the Union, and, where the disease is not present in the Union, the risk of its introduction into the Union | (a)(vii) 1 Map of MSs where the disease is present | <Information from the fact sheet> |
| (a)(vii) 2 Type of epidemiological occurrence | <Information from the fact sheet> | |
Questions 2.1
|
Question 2.1A the disease is highly transmissible Answer: Y ☐ N ☐ na ☐ | ||
|---|---|---|
|
Question 2.1BC the disease is moderately to highly transmissible
Answer Y ☐ N ☐ na ☐ | ||
| Mapped Article 7 criteria | Mapped Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(vi) The routes and speed of transmission of the disease between animals and, when relevant, between animals and humans | (a)(vi) 3 Incidence between animals and, when relevant, between animals and humans | <Information from the fact sheet> |
| (a)(vi) 4 Transmission rate (beta) (from R0 and infectious period) between animals and, when relevant, between animals and humans | ||
| (a)(v) Persistence of the disease in an animal population or in the environment | (a)(v) 1 duration of infectious period in animals | <Information from the fact sheet> |
| (a)(v) 2 presence and duration of latent infection period | ||
| (a)(v) 3 presence and duration of the pathogen in healthy carriers | ||
Questions 2.2
|
Question 2.2AB there be possibilities of airborne or waterborne or vector‐borne spread Interpretation – the disease or the infection can be transmitted via airborne or waterborne or vector‐borne (mechanical or biological vector) spread Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Mapped Article 7 criteria | Mapped Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(vi) The routes and speed of transmission of the disease between animals and, when relevant, between animals and humans | (a)(vi) 1 type of routes of transmission from animal to animal (horizontal, vertical) | <Information from the fact sheet> |
Questions 2.3
|
Question: 2.3A the disease affect multiple species of kept and wild animals OR single species of kept animals of economic importance Interpretation – …OR at least one single species of kept animals of economic importance Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Mapped Article 7 criteria | Mapped Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(i) Animal species concerned by the disease | (a)(i) 1 naturally susceptible wildlife species (or families/orders) | <Information from the fact sheet> |
| (a)(i) 2 naturally susceptible domestic species (or families/orders) | <Information from the fact sheet> | |
| (a)(i) 3 experimentally susceptible wildlife species (or families/orders) | <Information from the fact sheet> | |
| (a)(i) 4 experimentally susceptible domestic species (or families/orders) | ||
| (a)(i) 5 wild reservoir species (or families/orders) | <Information from the fact sheet> | |
| (a)(i) 6 domestic reservoir species (or families/orders) | <Information from the fact sheet> | |
Questions 2.4
The answer to the question 2.4CAq can be Y only for diseases affecting aquatic animal species, therefore do not assess this question for diseases affecting terrestrial animal species
|
Question 2.4A the disease may results in high morbidity and significant mortality rates Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
|
Question 2.4B the disease may result in high morbidity and in general low mortality Answer Y ☐ N ☐ na ☐ | ||
|
Question 2.4C the disease usually does not result in high morbidity and has negligible or no mortality AND often the most observed effect of the disease is production loss Answer Y ☐ N ☐ na ☐ | ||
|
Question 2.4CAq the disease may result in high morbidity and usually low mortality AND often the most observed effect of the disease is production loss Answer Y ☐ N ☐ na ☐ | ||
| Mapped Article 7 criteria | Mapped Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(ii) Morbidity and mortality rates of the disease in animal populations | (a)(ii) 1 Prevalence/Incidence | <Information from the fact sheet> |
| (a)(ii) 2 Case‐morbidity rate | <Information from the fact sheet> | |
| (a)(ii) 3 Case‐fatality rate | <Information from the fact sheet> | |
| (b)(i) Impact of the disease on agricultural and aquaculture production and other parts of the economy | (b)(i) 1 Number of MSs where the disease is present | <Information from the fact sheet> |
| (b)(i) 2 Proportion of production losses (%) by epidemic/endemic situation (milk, growth, semen, meat, etc.…) | <Information from the fact sheet> | |
Questions 3
|
Question 3A the disease has a zoonotic potential with significant consequences on public health, including epidemic or pandemic potential OR possible significant threats to food safety Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
|
Question 3B the disease has a zoonotic potential with significant consequences on public health, including epidemic potential OR possible significant threats to food safety Answer Y ☐ N ☐ na ☐ | ||
|
Question 3C the disease has a zoonotic potential with significant consequences for public health or possible significant threats to food safety Answer Y ☐ N ☐ na ☐ | ||
| Mapped Article 7 criteria | Mapped Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(iii) Zoonotic character of the disease | (a)(iii) 1 report of zoonotic human cases | <Information from the fact sheet> |
| (a)(vi) The routes and speed of transmission of the disease between animals and, when relevant, between animals and humans | (a)(vi) 2 type of routes of transmission between animals and humans (direct and indirect including foodborne) | <Information from the fact sheet> |
| (a)(vi) 3 Incidence between animals and when relevant between animals and humans | <Information from the fact sheet> | |
| (a)(vi) 4 Transmission rate (beta) (from R0 and infectious period) between animals and when relevant between animals and humans | ||
| (b)(ii) Impact of the disease on Human health | (b)(ii) 5 Disability‐adjusted life year (DALY) | <Information from the fact sheet> |
| (b)(ii) 6 Availability of medical treatment and their effectiveness (therapeutical effect and any resistance) | ||
| (b)(ii) 7 Availability of vaccines and their effectiveness (reduced morbidity) | ||
| (c) Potential to generate a crisis situation and its Potential use in bioterrorism | (c) 1 listed in OIE/CFSPH classification of pathogens | <Information from the fact sheet> |
| (c) 2 listed in the Encyclopaedia of Bioterrorism Defense of Australia Group | <Information from the fact sheet> | |
| (c) 3 included in any other list of potential bio‐ agro‐terrorism agents | ||
Questions 4
|
Question 4AB the disease in question has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals Interpretation – due to the substantial costs related to the disease direct impact on the health and productivity of animals, the disease has a significant impact on the economy Answer current impact Y ☐ N ☐ na ☐ Answer potential impact Y ☐ N ☐ na ☐ | ||
|---|---|---|
|
Question 4C the disease has a significant impact on the economy of the Union, mainly related to its direct impact on certain types of animal production systems Interpretation – due to its direct impact on how many types of animal production systems (not related to animal species but only to the type of products e.g. dairy, beef, reproduction, fattening, etc. and production system: e.g. indoor, outdoor, organic, etc.), the disease has a significant impact on the economy Answer current impact Y ☐ N ☐ na ☐ Answer potential impact Y ☐ N ☐ na ☐ | ||
| Mapped Article 7 criteria | Mapped Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (a)(ii) Morbidity and mortality rates of the disease in animal populations | (a)(ii) 1 Prevalence/Incidence | <Information from the fact sheet> |
| (a)(ii) 2 Case‐morbidity rate (% clinically diseased animals out of infected ones) | <Information from the fact sheet> | |
| (a)(ii) 3 Case‐fatality rate | <Information from the fact sheet> | |
| (b)(i) Impact on agricultural and aquaculture production and other parts of the economy | (b)(i) 1 Number of MSs where the disease is presence | <Information from the fact sheet> |
| (b)(i) 2 Proportion of production losses (%) by epidemic/endemic situation (milk, growth, semen, meat, etc.…) | <Information from the fact sheet> | |
Questions 5(a)
|
Question 5(a) the disease has a significant impact on society, with in particular an impact on labour markets Interpretation‐ the disease has a significant impact on society with (as the most important but not the only one) an impact on labour markets Answer current impact Y ☐ N ☐ na ☐ Answer potential impact Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Mapped Article 7 criteria | Mapped Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (b)(i) Impact on agricultural and aquaculture production and other parts of the economy | (b)(i) 1 Number of MSs where the disease is presence | <Information from the fact sheet> |
| (b)(i) 2 Proportion of production losses (%) by epidemic/endemic situation (milk, growth, semen, meat, etc.…) | <Information from the fact sheet> | |
Questions 5(b)
|
Question 5(b) the disease has a significant impact on animal welfare, by causing suffering to large numbers of animals Interpretation – due to the suffering of large number of animals caused by the disease, the disease has a significant impact on animal welfare Answer current impact Y ☐ N ☐ na ☐ Answer potential impact Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Mapped Article 7 criteria | Mapped Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (b)(iii) Impact of the disease on animal welfare | (b)(iii) 1 severity of clinical signs at case level and related level and duration of impairment | <Information from the fact sheet> |
| (a)(ii) 2 Case‐morbidity rate (% clinically diseased animals out of infected ones) | <Information from the fact sheet> | |
Questions 5(c)
|
Question 5(c) the disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it Interpretation – due to the direct impact of the disease OR to the impact of the measures taken to control it, the disease has a significant impact on the environment Answer current impact Y ☐ N ☐ na ☐ Answer potential impact Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Mapped Article 7 criteria | Mapped Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (b)(iv) Impact of the disease on biodiversity and the environment | (b)(iv) 1 endangered wild species affected: listed species as in CITES and/or IUCN list | <Information from the fact sheet> |
| (b)(iv) 2 Mortality in wild species | <Information from the fact sheet> | |
| (e)(iv) The impact of disease prevention and control measures | (e)(iv) 1 Mortality in wild species (e)(iv) 1 use and potential residuals of biocides or medical drugs in environmental compartments (soil, water, feed, manure | <Information from the fact sheet> |
| (a)(v) Persistence of the disease in an animal population or in the environment | (a)(v) 4 Length of survival (dpi) of the agent and/or detection of DNA in selected matrices (soil, water, air) from the environment (scenarios: high and low temperature) | <Information from the fact sheet> |
Questions 5(d)
|
Question 5(d) The disease has a significant impact on in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds Interpretation‐ the consequences of the impact of the disease can even lead to the possible disappearance or long‐term damage of endangered species or breeds Answer current impact Y ☐ N ☐ na ☐ Answer potential impact Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Mapped Article 7 criteria | Mapped Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (b)(iv) Impact of the disease on biodiversity and the environment | (b)(iv) 1 endangered wild species affected: listed species as in CITES and/or IUCN list | <Information from the fact sheet> |
| (b)(iv) 2 Mortality in wild species | <Information from the fact sheet> | |
| (b)(iv) 3 Capacity of the pathogen to persist in the environment and cause mortality in wildlife | <Information from the fact sheet> | |
Question D
|
Question D The risk posed by the disease in question can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread Answer Y ☐ N ☐ na ☐ | ||
|---|---|---|
| Article 7 criteria | Article 7 parameters | Assessment of the Article 7 parameters from fact sheet |
| (d)(v) Feasibility, availability and effectiveness of restrictions on the movement of animals and products, as control measure | (d)(v) 1 available restriction movement measures | <Information from the fact sheet> |
| (d)(v) 2 effectiveness of restriction of animal movement in preventing the between farm spread (description) | <Information from the fact sheet> | |
| (d)(v) 3 feasibility of restriction of animal movement (description) | <Information from the fact sheet> | |
Appendix C – Modified methodology from 2021
1.
This Appendix has been added in 2022 and the new authors are EFSA Panel on Animal Health and Welfare (AHAW), Søren Saxmose Nielsen, Julio Alvarez, Paolo Calistri, Elisabetta Canali, Julian Ashley Drewe, Bruno Garin‐Bastuji, José Luis Gonzales Rojas, Christian Gortázar, Mette Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Barbara Padalino, Paolo Pasquali, Helen Clare Roberts, Hans Spoolder, Karl Ståhl, Antonio Velarde, Arvo Viltrop, Christoph Winckler, Francesca Baldinelli, Alessandro Broglia, Lisa Kohnle and Dominique Bicout.
To take better account of the uncertainties identified during the judgement, the criteria of Articles 5 and 9 are translated into questions of the form: ‘How certain are you that the statement X is true?’, to which a probability range can be given as answer based on Table C.1 (EFSA, 2018).
Table C.1.
Approximate probability scale recommended for harmonised use in EFSA
| Probability term | Subjective probability range |
|---|---|
| Almost certain | 99–100% |
| Extremely likely | 95–99% |
| Very likely | 90–95% |
| Likely | 66–90% |
| About as likely as not | 33–66% |
| Unlikely | 10–33% |
| Very unlikely | 5–10% |
| Extremely unlikely | 1–5% |
| Almost impossible | 0‐1% |
Using Table C.1, the experts should indicate their answer for each of the criteria of Articles 5 and 9. Multiple ranges can be chosen to reflect a wider uncertainty (e.g. 90–95% and 95–99%, which would correspond to 90–99%).
In situations where there is complete uncertainty (due to e.g. total lack of evidence), the possible answer could be 0–100%. This would be different from 33% to 66%, which expresses a lower degree of uncertainty excluding certain probability ranges due to e.g. a larger amount of evidence available). The answer ‘not applicable’ (na) is an option and should be used when the question is irrelevant to be judged or not applicable, or when it is impossible to answer the question (e.g. antibiotic resistance for a viral disease, or public health impact for a non‐zoonotic disease).
The method to perform the expert judgement for each disease is implemented in two rounds:
Round 1: Individual judgement
First, each expert performs his/her individual judgement. On the basis of the evidence collected from Article 7 criteria, mapped into Article 5 and 9 criteria, the experts are asked to provide their answers individually, which are the probability ranges (or combinations thereof) described in Table C.1, as well as the reasoning for each of their individual answers.
In order to provide advice about a certain disease to be listed and categorised according to the AHL, it should be assessed whether or not the criteria of Articles 5 and 9 are fulfilled. In order to define whether the criterion related to each question is fulfilled, two thresholds were set on the probability scale, at 33% and 66%. Therefore, the probability zone 66–100% (green zone) corresponds to ‘criterion fulfilled’ and the probability zone 0–33% (red zone) corresponds to 'criterion not fulfilled'. The 33–66% zone (yellow zone) or any probability range that crosses into this zone corresponds to ‘uncertainty about criterion fulfilment’.
| 0–33% | 33–66% | 66–100% |
|---|---|---|
| Criterion not fulfilled | Uncertainty about criterion fulfilment | Criterion fulfilled |
| Any probability range that crosses into the 33–66% zone | ||
According to this definition, when all individual answers are within one probability zone, this would mean that the Panel has reached consensus on that criterion.
When the individual answers fall into more than one of these three probability zones, i.e. the (upper and/or lower) bounds of at least one answer lay in more than one probability zone, no consensus about criterion fulfilment is reached. In this case, a collective discussion based on the provided reasoning is needed (see Round 2: Collective judgement). When the Panel has a consensus on a criterion, the question is not further discussed collectively.
The individual answers are summarised and displayed visually (see Appendix D.1 for the method used to summarise individual answers). Experts are informed about the collective outcome of their individual judgement.
Round 2: Collective judgement
The collective judgement consists of a ‘behavioural aggregation’ of the experts, i.e. a discussion among all experts to reach an agreement, when the individual judgements produced in the first round have not reached a consensus. These are discussed in a meeting where additional material/information may be supplied by the experts that are present, and the collected reasoning points from each expert are discussed.
At the beginning of the discussion of each Article 5 and 9 criterion, the individual probability ranges are graphically shown. Based on the reasoning points provided, the experts discuss and may finally change their individual answers.
The aim of the collective discussion is to drive the experts consensually towards one of the probability zones and, in each case, reasoning points related to a possible change of probability zone are reported.
The outcome of the collective judgement, for each criterion, is the median of the final probability ranges provided by the experts, which indicates whether the criterion is fulfilled or not, or is uncertain. Criterion fulfilment is therefore based on the probability zone where the overall median range lays. The criteria about impact (Criteria 4 and 5(a)‐(d)) are assessed both for the current and potential impact, but in the final results these are then combined by taking the largest range of probability of the two.
The median of the series of probability ranges is computed as the mid‐value of the series ordered in descending order (for details see Appendix D.1). Only the responses from experts having participated in both individual and collective judgement are counted per question in the final output.
For reasons of transparency and completeness of displaying the distribution of the individual answers provided by each expert, these are reported as figures in the Annex of each opinion. For the criteria for which the median falls into more than one of the probability zones and for which there is uncertainty about fulfilment of the criterion, the figures are supplemented by the supporting reasoning points.
For the overall outcome on listing and categorisation of the disease according to Article 5 and 9 criteria, the median ranges for each set of questions were aggregated as described in Appendix D.2.
Appendix D – Operations for the modified methodology
1.
This Appendix has been added in 2022 and the new authors are EFSA Panel on Animal Health and Welfare (AHAW), Søren Saxmose Nielsen, Julio Alvarez, Paolo Calistri, Elisabetta Canali, Julian Ashley Drewe, Bruno Garin‐Bastuji, José Luis Gonzales Rojas, Christian Gortázar, Mette Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Barbara Padalino, Paolo Pasquali, Helen Clare Roberts, Hans Spoolder, Karl Ståhl, Antonio Velarde, Arvo Viltrop, Christoph Winckler, Francesca Baldinelli, Alessandro Broglia, Lisa Kohnle and Dominique Bicout.
D.1. Median aggregation
Let R = {r 1, r 2, … r n} be the collection of outcomes from individual assessments involving n experts, where the response or assessment r i of each expert is drawn from the EFSA approximate probability scale (Table C.1). The median of R is obtained as:
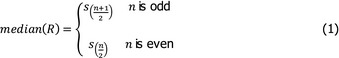
where s i is the i‐th largest of elements in S obtained by reordering or ranking R in descending order.
Example 1: Let {R = r 1, r 2, … r 7} = {50%, 10%, 66%, 95%, 66%, 10%, 95%}, we have the following steps to calculate the median:
Reordering or ranking R in descending order gives:S = {s 1, s 2, … s 7} = {95%, 95%, 66%, 66%, 50%, 10%, 10%}
Since n = 7 then, median(R) = s 4 = 66%.
In the case where the r i are uncertain probabilities or ranges, the median is calculated for both lower and upper bounds.
Example 2:
| Criterion | Certainty range | Expert | |
|---|---|---|---|
| Lower bound (%) | Upper bound (%) | ||
| 2.1A | 66 | 99 | A |
| 2.1A | 33 | 66 | B |
| 2.1A | 33 | 100 | C |
| 2.1A | 1 | 5 | D |
| 2.1A | 33 | 100 | E |
| 2.1A | 0 | 1 | F |
| 2.1A | 0 | 1 | G |
| 2.1A | 95 | 99 | H |
| 2.1A | 33 | 66 | I |
| 2.1A | 33 | 66 | J |
| 2.1A | 10 | 90 | K |
| 2.1A | 10 | 33 | L |
| 2.1A | 10 | 33 | M |
| 2.1A | 5 | 10 | N |
| 2.1A | 1 | 5 | O |
| Median | 10 | 66 | |
Figure D.1.
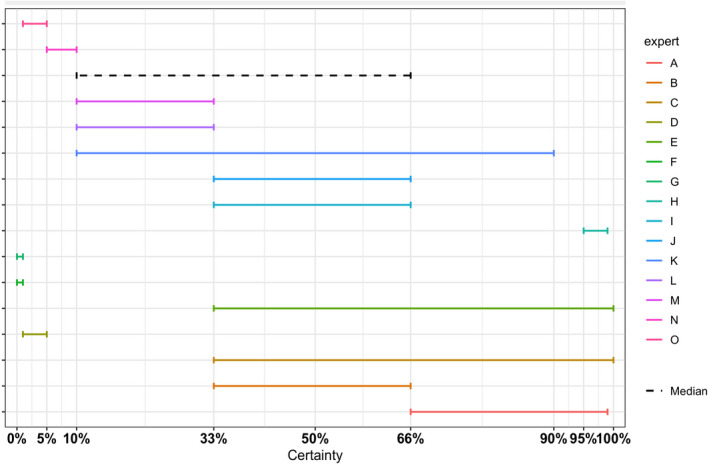
Illustration of the median aggregation
D.2. Aggregation according to AHL categories
The general structure of Articles 5 and 9 of the AHL can be summarised as in the table below.
| Criteria | AHL – aggregation | ||
|---|---|---|---|
| Set 1: all of them | r 1 | r* = r 1 ∧ r 2 ∧ … ∧ rn | Final result: R = r * ∧ s * |
| r 2 | |||
| ⫶ | |||
| r n | |||
| Set 2: at least one | s 1 | s* = s 1 ∨ s 2 ∨ … ∨ s m | |
| s 2 | |||
| ⫶ | |||
| s m | |||
|
∧ = AND operation; ∨ = OR operation When r i and s i are uncertain probabilities or ranges, the aggregation is calculated for both lower and upper bounds. | |||
In what follows, we will assume independence of probability ranges and use:
Example 1: Article 5
| Criteria | Certainty range | AHL – aggregation | |||
|---|---|---|---|---|---|
|
Lower bound (%) |
Upper bound (%) | ||||
| Set 1: all of them | A(i) | 99 | 100 |


|

|
| A(ii) | 99 | 100 | |||
| A(iii) | 95 | 100 | |||
| A(iv) | 99 | 100 | |||
| A(v) | 33 | 90 | |||
| Set 2: at least one | B(i) | 66 | 95 |
|
|
| B(ii) | 10 | 33 | |||
| B(iii) | 10 | 33 | |||
| B(iv) | 0 | 10 | |||
| B(v) | 5 | 33 | |||
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Candiani D, Gervelmeyer A, Zancanaro G, Kohnle L, Morgado J and Bicout D, 2017. Scientific opinion on an ad hoc method for the assessment on listing and categorisation of animal diseases within the framework of the Animal Health Law. EFSA Journal 2017;15(7):4783, 42 pp. 10.2903/j.efsa.2017.4783
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00603
Panel members: Dominique Bicout, Anette Bøtner, Andrew Butterworth, Paolo Calistri, Klaus Depner, Sandra Edwards, Bruno Garin‐Bastuji, Margaret Good, Christian Gortázar Schmidt, Virginie Michel, Miguel Angel Miranda, Simon More, Søren Saxmose Nielsen, Mohan Raj, Liisa Sihvonen, Hans Spoolder, Jan Arend Stegeman, Hans‐Hermann Thulke, Antonio Velarde, Preben Willeberg and Christoph Winckler.
Acknowledgements: The AHAW Panel wishes to thank the EFSA staff member: Frank Verdonck.
Adopted: 5 April 2017
Amendment note: We have added information on the quantitative approach followed from 2021 in Appendices C and D, which contain a different author list: EFSA Panel on Animal Health and Welfare (AHAW), Søren Saxmose Nielsen, Julio Alvarez, Paolo Calistri, Elisabetta Canali, Julian Ashley Drewe, Bruno Garin‐Bastuji, José Luis Gonzales Rojas, Christian Gortázar, Mette Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Barbara Padalino, Paolo Pasquali, Helen Clare Roberts, Hans Spoolder, Karl Ståhl, Antonio Velarde, Arvo Viltrop, Christoph Winckler, Francesca Baldinelli, Alessandro Broglia, Lisa Kohnle and Dominique Bicout. To avoid confusion, the original version of the output has been removed from the EFSA Journal, but is available on request.
Amended: 1 January 2022
Notes
Regulation (EC) No 429/2016 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). OJ L 84, 31.3.2016, p. 1–208.
In the opinion the ‘criteria of Annex IV for the purpose of categorisation of diseases in accordance with Article 9’ are indicated as ‘Article 9 criteria’.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
References
- Cardoen S, Van Huffel X, Berkvens D, Quoilin S, Ducoffre G, Saegerman C, Speybroeck N, Imberechts H, Herman L, Ducatelle R and Dierick K, 2009. Evidence‐based semiquantitative methodology for prioritization of foodborne zoonoses. Foodborne Pathogens and Disease, 6, 1083–1096. [DOI] [PubMed] [Google Scholar]
- Council of the European Union , 2008. Addendum to the outcome of proceedings of the working party of chief veterinary officers: non‐paper on “prioritisation of animal‐related threats and biosecurity”. Available online: http://register.consilium.europa.eu/doc/srv?l=EN&f=ST%209536%202008%20ADD%201 [Accessed: 16 September 2015]
- Cox R, Sanchez J and Revie CW, 2013. Multi‐criteria decision analysis tools for prioritising emerging or re‐emerging infectious diseases associated with climate change in Canada. PLoS ONE, 8, e68338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEFRA , 2006. Documentation for prototype AHW prioritisation decision support tool. Available online: http://webarchive.nationalarchives.gov.uk/20130822084033/http://www.defra.gov.uk/animalh/diseases/vetsurveillance/programme/prioritisation.htm [Accessed: 13 October 2015]
- Del Rio Vilas VJ, Montibeller G and Franco LA, 2011. Letter to the editor: prioritisation of infectious diseases in public health: feedback on the prioritisation methodology, 15 July 2008 to 15 January 2009. Available online: http://www.eurosurveillance.org/images/dynamic/EE/V16N27/art19911.pdf [Accessed: 6 October 2015] [PubMed]
- Del Rio Vilas VJ, Voller F, Montibeller G, Franco LA, Sribhashyam S, Watson E, Hartley M and Gibbens JC, 2013. An integrated process and management tools for ranking multiple emerging threats to animal health. Preventive Veterinary Medicine, 108, 94–102. [DOI] [PubMed] [Google Scholar]
- DISCONTOOLS , 2012. Work package 2 ‐ final paper on disease prioritisation‐ approaches to the prioritisation of diseases to focus and prioritise research in animal health: a worldwide review of existing methodologies. Available online: http://www.discontools.eu/Workgroups/Group/28 [Accessed: 13 October 2015]
- European Commission , 2007. A new animal health strategy for the European Union (2007–2013) where “prevention is better than cure.” Communication from the Commission to the Council, the European Parliament, the European Economic and Social Committee, and the Committee of the Regions. Available online: http://ec.europa.eu/food/animals/health/index_en.htm [Accessed: 13 October 2015]
- ECDC (European Centre for Disease Prevention and Control), 2011. Current and future burden of communicable diseases in the European Union and EEA/EFTA countries ‐ methodological protocol. Available online: http://ecdc.europa.eu/en/publications/Publications/1106_TER_Burden_of_disease.pdf [Accessed: 13 October 2015]
- ECDC (European Centre for Disease Prevention and Control), 2015. Best practices in ranking emerging infectious disease threats: a literature review. Available online: http://ecdc.europa.eu/en/publications/Publications/emerging-infectious-disease-threats-best-practices-ranking.pdf [Accessed: 9 October 2015]
- EFSA (European Food Safety Authority) Scientific Committee, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. https://doi.org/ 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2012. Scientific Opinion on the development of a risk ranking framework on biological hazards. EFSA Journal 2012;10(6):2724, 88 pp. 10.2903/j.efsa.2012.2724 [DOI] [Google Scholar]
- ETPGAH , 2007. Action plan. Available online: http://www.etpgah.eu/action-plan.html [Accessed: 13 October 2015]
- FAO (Food and Agriculture Organization of the United Nations), 2012. Multicriteria‐based ranking for risk management of food‐born parasites. Available online: http://www.fao.org/3/a-i3649e.pdf [Accessed: 13 October 2015]
- Gao T, Wang XC, Chen R, Ngo HH and Guo W, 2015. Disability adjusted life year (DALY): a useful tool for quantitative assessment of environmental pollution. Science of the Total Environment, 511, 268–287. [DOI] [PubMed] [Google Scholar]
- Havelaar AH, van Rosse F, Bucura C, Toetenel MA, Haagsma JA, Kurowicka D, Heesterbeek JH, Speybroeck N, Langelaar MF, van der Giessen JW, Cooke RM and Braks MA, 2010. Prioritizing emerging zoonoses in the Netherlands. PLoS ONE, 5, e13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humblet MF, Vandeputte S, Albert A, Gosset C, Kirschvink N, Haubruge E, Fecher‐Bourgeois F, Pastoret PP and Saegerman C, 2012. Multidisciplinary and evidence‐based method for prioritizing diseases of food‐producing animals and zoonoses. Emerging Infectious Diseases, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause G and the working group on prioritisation at the Robert Koch Institute (RKI), 2008. Prioritisation of infectious diseases in public health ‐ call for comments. Eurosurveillance, 13, 6 pp. [DOI] [PubMed] [Google Scholar]
- McFadden AM, Muellner P, Baljinnyam Z, Vink D and Wilson N, 2016. Use of multicriteria risk ranking of zoonotic diseases in a developing country: case study of Mongolia. Zoonoses and Public Health, 63, 138–151. [DOI] [PubMed] [Google Scholar]
- Ng V and Sargeant JM, 2012. A stakeholder‐informed approach to the identification of criteria for the prioritization of zoonoses in Canada. PLoS ONE, 7, e29752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng V and Sargeant JM, 2013. A quantitative approach to the prioritization of zoonotic diseases in North America: a health professionals' perspective. PLoS ONE, 8, e72172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng V and Sargeant JM, 2016. Prioritizing zoonotic diseases: differences in perspectives between human and animal health professionals in North America. Zoonoses and Public Health, 63, 196–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE (World Organisation for Animal Health), 2010. Listing and categorisation of priority animal diseases, including those transmissible to humans. Available online: http://www.oie.int/fileadmin/Home/eng/Support_to_OIE_Members/docs/ppt/OIE_study_priori-catego_mission_report.pdf [Accessed: 13 October 2015]
- RIVM (National Institute for Public Health and the Environment), 2006. RIVM rapport 330080001/2006 ‐ priority setting of foodborne pathogens. Available online: http://www.rivm.nl/bibliotheek/rapporten/330080001.pdf [Accessed: 17 September 2015]
- WHO (World Health Organization), 2006. Setting priorities in communicable disease surveillance. Available online: http://www.who.int/csr/resources/publications/surveillance/WHO_CDS_EPR_LYO_2006_3/en/ [Accessed: 6 October 2015]