Abstract
The Panel on Food Additives and Nutrient Sources added to Food (ANS) was requested from the European Commission to provide a statement on the validity of the conclusions of a mouse study on the carcinogenic potential of sucralose (E 955) performed by the Ramazzini Institute (Soffritti et al., 2016). Sucralose (E 955) is authorised as a food additive in the EU in accordance with Annex II to Regulation (EC) No 1333/2008 on food additives. According to Commission Regulation (EU) No 257/2010, the full re‐evaluation of sucralose shall be completed by December 2020. Taking into consideration the publication from Soffritti et al. (2016), the technical report and additional information provided by the Ramazzini Institute and other information available for sucralose (E 955), the Panel noted: (i) the design of the bioassay that considers exposure from gestation up to natural death of animals implies an increase in background pathology that results in the possibility of misclassifications and a difficult interpretation of data, especially in the absence of both an appropriate concurrent control group and a recent historical database; (ii) the lack of a dose–response relationship between the exposure to sucralose and incidence of lymphomas and leukaemias (combined); (iii) the lack of a mode of action and failure to meet all the Bradford‐Hill considerations for a cause–effect relationship between intake of sucralose and the development of tumours in male mice only; (iv) a comprehensive database was available for sucralose and no carcinogenic effect was reported in adequate studies in rats and mice. Moreover, there was no reliable evidence of in vivo genotoxicity. Therefore, the Panel concluded that the available data did not support the conclusions of the authors (Soffritti et al., 2016) that sucralose induced haematopoietic neoplasias in male Swiss mice.
Keywords: sucralose, E 955, food additive, sweetener, carcinogenicity
1. Introduction
The present document is an assessment of the study by Soffritti et al. (2016) in order to provide a statement on the validity of its conclusions.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
The use of food additives is regulated under the European Parliament and Council Regulation (EC) No 1333/20081 on food additives. Only food additives that are included in the Union list, in particular in Annex II to that regulation, may be placed on the market and used in foods under the conditions of use specified therein. Moreover, food additives shall comply with the specifications as referred to in Article 14 of that Regulation and laid down in Commission Regulation (EU) No 231/20122.
Sucralose is an artificial sweetener, which is authorised for use as a food additive in the Union. Since sucralose was permitted in the Union before 20 January 2009, it belongs to the group of food additives which will be subject to a new risk assessment by the European Food Safety Authority (EFSA), in line with the provisions of Regulation (EC) No 1333/2008. According to Commission Regulation (EU) No 257/20103, which sets up the programme for the re‐evaluation of food additives, the re‐evaluation of sucralose shall be completed by 31 December 2020. Nevertheless, authorised food additives should be kept under continuous observation and must be re‐evaluated whenever necessary in the light of changing conditions of use and new scientific information.
A new study describing a potential adverse health effect of sucralose has recently been published (sucralose administered in feed, beginning prenatally through lifespan, induces haematopoietic neoplasias in male Swiss mice, Soffritti et al., published on line on 29 January 2016, International Journal of Occupational and Environmental Health4). This study reports that sucralose is a carcinogenic agent in mice. Consequently, the European Commission has decided to consult EFSA on this matter.
1.1.2. Terms of Reference
In accordance with Article 29(1)(A) of Regulation (EC) No 178/20025, the European Commission requests EFSA to provide a scientific opinion in relation to a new study on the carcinogenic potential of the food additive sucralose (E 955) by Soffritti et al.4 In particular, EFSA is requested to carry out an assessment of the above mentioned study and to provide a statement on the validity of its conclusions.
1.2. Information on previous evaluations on sucralose
Sucralose (E 955) is authorised as a food additive in the European Union (EU) in accordance with Annex II to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/20122.
In 1990, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) first allocated an acceptable daily intake (ADI) of 0–15 mg/kg body weight (bw) for sucralose based on the no observe effect level (NOEL) of 1,500 mg/kg bw per day from a long‐term study in rats and applying a safety factor of 100 (JECFA, 1991). The Scientific Committee on Food (SCF) confirmed this ADI of 0–15 mg/kg bw based on an overall NOEL of 1,500 mg/kg bw per day and the application of a 100‐fold safety factor (SCF, 2000). The EFSA ANS Panel recently considered an extension of use for sucralose, which is not yet re‐evaluated, using the existing ADI based on the evaluation by the SCF (EFSA ANS Panel, 2016).
2. Data and methodologies
2.1. Data
The EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) considered for its assessment the publication from Soffritti et al. (2016) and the technical report and its annexes provided by the Ramazzini Institute – Cesare Maltoni Cancer Research Center (CMCRC, Documentation provided to EFSA No. 1).
The Working Group on sucralose of the ANS Panel had also the opportunity to further clarify experimental details during a technical hearing with representatives of the Ramazzini Institute held on 10 November 20166 and requested additional documentation to supplement the hearing (Documentation provided to EFSA No. 2). Following the technical hearing, additional data were requested which were provided on 16 January 2017 (Documentation provided to EFSA No. 3). A final request for further data was made in January, and these were provided on 15 February 2017 (Documentation provided to EFSA No. 4).
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant guidance documents from the EFSA Scientific Committee, both existing and under development.
3. Assessment
3.1. Biological and toxicological data
3.1.1. The study from Ramazzini Institute
The study design and the results were described both in the Soffritti et al. (2016) and in the technical report requested from the Ramazzini Institute – CMCRC (Documentation provided to EFSA No. 1). The present statement is based on the information included in both documents and on additional information provided in support to and after the technical hearing (Documentation provided to EFSA Nos. 2, 3 and 4).
3.1.1.1. Study design and conduct as reported by Ramazzini Institute – CMCRC
The experimental in vivo phase of the study started on 8 October 2005 (from 12th day of gestation) and ended on 23 April 2008. It is reported that the study was conducted following the principles of Good Laboratory Practices (GLP) in all its phases, including planning, conducting and reporting and that Standard Operating Procedures (SOPs) of the laboratories involved were considered. A person from Quality Assurance (QA) Unit is also listed within the Study Personnel. According to the technical report, an internal quality control procedure was implemented during the study in order to guarantee the reliability of the data obtained covering for scientific, structural and operative aspects. The Ramazzini Institute – CMCRC adopted all reasonable measures to record data in accordance to the protocol.
The experimental design used by the Ramazzini Institute – CMCRC consisted of treatment with sucralose prenatally (specifically in utero exposure from the 12th day of pregnancy) until either spontaneous death of the animals or 130 weeks of age. At this age, the remaining mice were sacrificed since the number of animals alive was < 10%.
As reported in the technical report, sucralose was produced by Nutraceutica srl (Monterenzio, Bologna, Italy, an international company specialised in the production of raw materials),7 its purity was 99.4% with a specification for impurities of 0.05% for methanol and < 0.1 mg/kg diet for heavy metals. Sucralose was pulverised and incorporated in a standard pelleted diet. The diet was analysed to determine the amount of nutritional components, microorganisms and potential contaminants (e.g. pesticides, heavy metals) every 6 months, and the pelleted diet was used within 2 months from the date of production. It is reported that at the beginning of the experiment, the various concentrations and the stability of sucralose in the feed were analysed.
The pelleted diet contained sucralose at 0, 500, 2,000, 8,000 and 16,000 mg/kg, leading to – according to the technical report and on the basis of a mouse average weight of 40 g and a standard daily feed consumption of 5 g – estimated daily doses of 0, 62.5, 250, 1,000 and 2,000 mg/kg bw, respectively.
Drinking water from the public supply was available ad libitum during the study and it was periodically analysed for the possible presence of bacteria and pollutants. It is reported that values were ‘always within the acceptable limits by law’.
Pathogen‐free Swiss mice were provided by Charles River Laboratories (Milan, Italy). After a quarantine period, the breeders were randomly distributed by weight in a total of five groups: three of 40 and two of 60 animals (the same number of males and females) for a total of 240 mice. At 13 weeks of age, one male and one female breeder were placed in a cage for 5 days; after that the male mouse was removed. Diet with sucralose was made available to female breeder starting from the 12th day of gestation, thus exposure to sucralose started prenatally. All male and female pups from all litter were included in the study in order to reach the planned number per sex per group and to allow the evaluation of a potential family effect in the carcinogenic process. This approach resulted in an unequal distribution of pups among groups as follows (Table 1):
Table 1.
Experimental design
| Group | Sucralose mg/kg diet | Sucralose mg/kg bw | Mice per sex | Total no. of mice | No of breeders |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 117 M + 102 F | 219 | 30 M + 30 F |
| 2 | 500 | 62.5 | 114 M + 105 F | 219 | 30 M + 30 F |
| 3 | 2,000 | 250 | 80 M + 60 F | 140 | 20 M + 20 F |
| 4 | 8,000 | 1,000 | 66 M + 65 F | 131 | 20 M + 20 F |
| 5 | 16,000 | 2,000 | 70 M + 64 F | 134 | 20 M + 20 F |
bw: body weight; M: male; F: female.
Pups were weaned at 4–5 weeks of age when they were identified and assigned to the respective dose group. Treatment with sucralose in the diet was uninterrupted from the 12th day of gestation until spontaneous death or 130 week of age. Control animals received the same diet without sucralose.
It was reported that the animals (10 per cage) were kept in rooms designated for this experiment with environmental conditions monitored and deviations duly recorded (temperature 23 ± 2°C, relative humidity ranging from 50% to 60% and 12 h light/dark lighting cycle was maintained).
Clinical appearance and behaviour of animals included in the experiment were observed three times daily with the exception of Sundays and holidays (twice daily). According to the technical report, in case of infectious pathologies mice were isolated and kept under continuous observation.
It was reported that animals were checked for mortality four times a day and any animal considered ill or moribund was duly isolated or sacrificed for humane reasons. Mice found dead were kept refrigerated until necropsy; necropsies were performed no more than 16 h after death, all the days of the week, including Sunday mornings. A qualified pathologist performed or supervised the necropsies. All animals were subjected to complete necropsy. Necropsy included a physical examination of external surface and orifices followed by examination of internal organs in situ. Selected organs and tissues were collected and preserved in 70% ethyl alcohol, except for bones, which were fixed in 10% formalin and then decalcified with 10% formaldehyde and 20% formic acid in water solution. Samples were prepared according to relevant SOPs. Histopathology was routinely performed on a comprehensive range of organs and tissues from each animal from each group. The technical report specifies that all the organs and tissues that were collected were histopathologically examined to detect any pathological change. All the slides were examined and histopathologically evaluated first by two junior pathologists and then by a senior pathologist. In addition, all the malignant tumours and the borderline lesions were peer reviewed by two senior pathologists. It is reported that the results were recorded in a systematic and standardised way.
According to the technical report, ‘the following parameters were evaluated:
water and feed consumption,
body weight variations,
cumulative mortality,
clinical observations (status, behaviour, toxic effects),
general pathological lesions, both macroscopic and microscopic,
types of tumours and tumour precursors,
number of benign and malignant tumours and tumours precursors for each tumour bearing animal,
number of malignant tumours per 100 animals,
number and percentage of animals bearing different types of tumours and tumour precursor,
latency time of benign and malignant tumours and tumours precursors’.
The statistical approach was briefly described in the technical report: ‘statistical analysis of survival and of the malignant neoplastic lesions were based on the Cox proportional hazard regression model (Cox DR 1972) which adjust for possible differential survival among experimental groups’. The test and the p‐values (as being either ≤ 0.05 or ≤ 0.01) were reported in the tables. Estimation of the survival function and cumulative hazard curves by the dose group according to the Kaplan–Meier approach were mentioned and shown in Figures 6 and 7, respectively, in the Soffritti et al. (2016); a test for trend was mentioned in a footnote to Table 3 in the paper and in the results section from the Technical report.
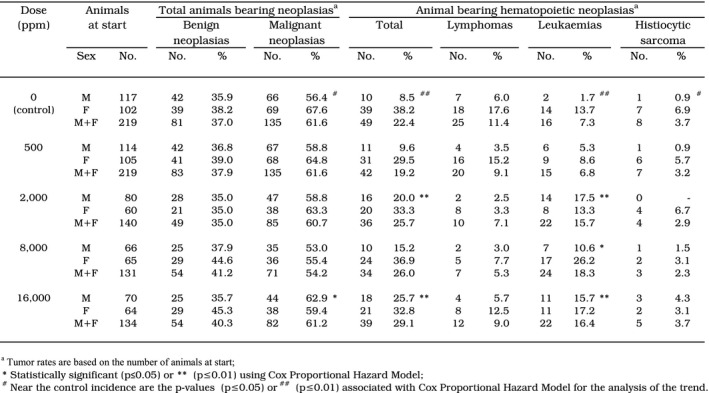
Table 3.
Benign and malignant neoplasias observed in the sucralose mouse study (from Soffritti et al., 2016, reprinted by permission of the publisher (Taylor & Francis Ltd, http://www.tandfonline.com))
It was also reported that an internal quality control was implemented in order to guarantee the reliability of data obtained, according to GLP. These procedures apply to scientific, structural and operative aspects. The SOPs of the laboratories involved were followed. The Ramazzini Institute – CMCRC ‘adopted all the reasonable measures to record data in accordance to the protocol. All the specimens and primary data are retained in compliance with GLP’.
3.1.1.2. ANS Panel's comments on study design and conduct
The Panel noted that the study started more than 10 years ago and that the technical report made available was from June 2016. This appears a very long delay even considering the intrinsic complexity of the study. The main results were published in January 2016 (Soffritti et al., 2016). The Panel also noted that the outcome of the study was presented during a scientific conference ‘Childhood Cancer 2012’ in London (April 2012), approximately 4 years before the publication of the paper and the technical report.
The experimental design applied by Ramazzini Institute deviated from that in OECD guidelines (OECD TG 451 and TG 453) with respect to duration of the in vivo phase and termination criteria (standard duration of 18 or 24 months, or termination when < 25% of survivors in the control or low‐dose group). This design was claimed to be more sensitive than the standard ones (Soffritti et al., 1999, 2007, 2008; Haseman et al., 2001; Huff et al., 2008; Gift et al., 2013; Manservisi et al., 2016). The Panel noted, however, that, although a life‐span experimental design can benefit from a possible increased sensitivity, it may suffer from increased background pathologies in late‐life period that may act as a confounding factor in the interpretation of results (EFSA ANS Panel, 2009; Gift et al., 2013). In addition, it is of paramount relevance to have a solid database with historical control data proximate to the study under evaluation and generated in the same facility, experimental approach and conditions with animals from the same supplier (OECD Carcinogenicity Guideline TG 451). The Panel noted that the data made available by the Ramazzini Institute as regards historical control data for neoplastic lesions were for the period 1973 up to 1983. The Ramazzini Institute stated: ‘historical data should be contemporary to the study being evaluated (e.g. within a period of up to around five years of the study). Historical data older than this should be used with caution and acknowledgment of its lower relevance and reliability’.
According to the Panel, the proposed study design (exposure prenatally till spontaneous death) appeared to be unique and is supported by a very limited database. Furthermore, the potential advantage of the increased sensitivity of the design based on the exposure to the chemical during fetal life or nursing seemed to be of limited value in cases where the substance showed very low absorption and exposure of fetuses and pups to the test compound was not demonstrated.
From the information provided, the facility was not GLP accredited at the time the study was carried out. Although stated to be conducted ‘following the principles of GLP’, the study (protocol BT 6012) has to be considered non‐GLP compliant, since no quality inspections have been conducted as indicated by the lack of a GLP statement. GLP is a quality system which is not directly correlated with the scientific quality and outcome of an experimental activity. GLP is internationally recognised in order to ensure adequate planning, performance, monitoring, recording, archiving and reporting of experimental studies to decrease the chances of mistakes, and, at the same time, ensuring completeness and transparency. The GLP environment is specifically intended to non‐clinical safety testing of test items including food additives.8
The Panel did not have comments on the selection of the doses and the way of administering sucralose.
According to the technical report the diet was provided by ‘Laboratorio Dottori Piccioni’, a firm specialised in diets mainly for cats and dogs.9 The batches of pelleted test diets with various content (%/w) of sucralose (provided by Nutraceutica), were changed every 2 months. The Panel noted, that six certificates of sucralose were provided; however, no information on impurities or degradation products during the manufacturing of the pelleted diet was available.
Analyses of the various concentrations of sucralose in the diet were performed at different time points (approximately every 6 months) during the experiment. The Panel estimated that at least 15 batches of pelleted diet with different concentrations of sucralose were prepared to supply the in life phase (lasting for 130 weeks) of the study. It appeared from the report that only a single analytical check of the concentration of the test material in the test diets was performed at the start of the study. Furthermore, the stability of the test material in the diet was not determined. In addition, data on animals health checks and environmental condition were not included in the report.
In the technical report under ‘Biophase results’, it was noted that the breeding phase proceeded without any setbacks, and that the average number of pups per litter was between 12 and 13 with a percentage of pregnant females of 83% (highest dose) or from 90% to 96% in the remaining groups. However, the number of pups entering in the experimental phase appeared to be lower (i.e. 90% of pregnant control females (27) multiplied by 12 pups (lower range) results in 324 pups and not 219 as reported in the experimental design; the same applies for the top dose with 17 pregnant females (83%) that multiplied by 12 pups would suggest 204 instead of 134 as reported) (Table 2).
Table 2.
Experimental design
| Group | Sucralose mg/kg diet | Sucralose mg/kg bw | ADI 15 mg/kg | Mice per sex | Total no. of mice | No of breeders | No of pups (expected)a |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | – | 117 M + 102 F | 219 | 30 M + 30 F | 324 |
| 2 | 500 | 62.5 | 4x | 114 M + 105 F | 219 | 30 M + 30 F | 324 |
| 3 | 2,000 | 250 | 17x | 80 M + 60 F | 140 | 20 M + 20 F | 216 |
| 4 | 8,000 | 1,000 | 67x | 66 M + 65 F | 131 | 20 M + 20 F | 216 |
| 5 | 16,000 | 2,000 | 133x | 70 M + 64 F | 134 | 20 M + 20 F | 204 |
bw: body weight; ADI: acceptable daily intake; M: male; F: female.
Assuming 90% pregnant females (except Group 5 with 83%) and an average of 12 pups.
The Panel noted that there was no explanation of the apparent difference between the number of animals bred and the number used nor was the selection of test animals and their allocation to groups sufficiently clear for the Panel to exclude the possibility that results could be influenced by an unrecognised bias in group selection. No further explanation was provided even during the technical hearing.
It was also reported that the control group served as reference not only for the study under assessment (named BT 6012) but also for two other studies conducted with either aspartame or formaldehyde. Although this experimental approach seemed to be of value from a point of view of animal welfare (3Rs), at the same time, it was not suggestive of an enrichment of the database and posed questions on the appropriateness of the experimental design especially in absence of a solid historical database (adequacy of both, the group size of the controls in comparison with the total number of treated animals in the three studies and the power calculations due to multiple analyses) and quality assurance. The control group was housed with the animals from study BT 6010 (aspartame) and only these groups of animals had identical environmental conditions (Soffritti et al., 2010). The possibility that reported differences between controls and treated groups in the other two studies were artefacts arising from differences in environmental conditions cannot be excluded.
3.1.1.3. Results as reported by Ramazzini Institute – CMCRC
No substantial differences were observed between control and treated animals at clinical observations, water and feed consumption. Concerning the body weight, a slight increase was observed in males at the dose of 500 mg/kg diet limited to the period from 40 to 104 weeks. In the females, a slight increase was also observed in animals treated with 500 or 2,000 mg/kg diet from week 71 to week 104.
For the survival, a statistically significant dose‐related decrease was observed among treated male mice at 2,000 and 16,000 mg/kg diet. A non‐significant decrease was also observed in females at 2,000 mg/kg diet. This difference in survival was based on the Kaplan–Meier survival function in Figure 6 from Soffritti et al. (2016).
No relevant differences were observed among treated and non‐treated animals for non‐neoplastic lesions.
The descriptive occurrence of benign and malignant tumours is reported in Table 3.
The Panel noted a mistake in the published table above and in the technical report regarding the total percentage of female bearing lymphomas at the dose of 2,000 mg/kg diet, which should be 13.3% rather than 3.3%.
According to the technical report, the data showed in males an increased incidence in tumour‐bearing animals exposed at the top dose of 16,000 mg/kg diet (p ≤ 0.05), with a significant dose‐related trend (p ≤ 0.05). The most common tumour types observed were haematopoietic neoplasias. An increased incidence of cortical adenoma in the various treated groups was also observed. Specifically males exposed to 2,000 and 16,000 mg/kg diet (both with a p ≤ 0.01) showed an increased incidence of haematopoietic neoplasias already visible at necropsies (multiple organs involved such as thymus, spleen, liver and lymph nodes). At histopathological examination, most of neoplasias observed in these two dose groups were leukaemias. In addition to the above‐mentioned organs involved, diffuse permeation of vessels and extensive infiltration of adjacent tissues was reported.
3.1.1.4. ANS Panel's comments on the reported results
According to the technical report (Documentation provided to EFSA no. 1), no relevant differences were observed among treated and non‐treated animals for non‐neoplastic lesions. However, the Panel noted that the incidence of severe bronchiolar/alveolar and peribronchiolar inflammation in the lungs of both males and females was rather high, especially in the controls. The incidence of severe bronchiolar/alveolar inflammation in the low‐dose group males (500 mg/kg diet) was similar to that in controls (20% vs 19%), whereas the incidences in the male higher dose groups were much lower. The incidence of the severe bronchiolar/alveolar inflammation in the other three groups was statistically significantly (p < 0.001) lower compared to the controls. The peribronchiolar inflammation was similar among all groups, controls included. In females, the incidences of severe bronchiolar/alveolar inflammation and peribronchiolar inflammation were similar among all groups (not statistically significantly different from controls).
Furthermore, the incidence of chronic reactive lymphadenitis in the thymus (Documentation provided to EFSA Nos. 2 and 3) was also high in male controls (39%), and statistically significantly lower in all treated groups, at 500 mg/kg diet (18%), 2,000 mg/kg diet (12%), 8,000 mg/kg diet (7.6%) and 16,000 mg/kg diet (7%) groups, compared to controls. In females, the incidence of chronic reactive lymphadenitis in the thymus showed a similar pattern (46%; 38%; 20%; 26% and 16% in the controls; 500; 2,000; 8,000 and 16,000 mg/kg diet groups, respectively), which resulted to be statistically significant in all treated groups compared to controls.
In males, the incidences of chronic reactive lymphadenitis in lymph nodes (Documentation provided to EFSA Nos. 2 and 3) was 91% in controls, whereas, the incidences in the treated groups were statistically significantly lower (58%; 48%; 47% and 36% in the 500; 2,000; 8,000; and 16,000 mg/kg diet groups, respectively). In females, the incidences of chronic reactive lymphadenitis in lymph nodes was high in the controls (76%) and 500 mg/kg diet group (66%), but statistically significantly lower in the 2,000 mg/kg diet (33%), the 8,000 mg/kg diet (43%) and 16,000 mg/kg diet (34%) groups, compared to controls.
The major effect observed in the study was an increased incidence in haematopoietic neoplasias (i.e. lymphomas and leukaemias; histiocytic sarcomas not included) in male animals only (Table 3).
The increased incidence did not show a dose correlation and started from Group 3 (2,000 mg/kg diet). A statistically significant increase (p < 0.05) in the percentage of haematopoietic neoplasias (leukaemias + lymphomas) in male animals was reported at two dose levels (i.e. 2,000 and 16,000 mg/kg diet) but not at 8,000 mg/kg diet.
The incidence of lymphomas and leukaemias in control males (7.7%) was within the normal range for these tumours observed in (very old) historical control mice (0–12.5%) in the Ramazzini Institute but rather low in comparison with the incidence of haematopoietic neoplasias in CD‐1 mice at 104 weeks of age (15.7%) (Charles River Laboratories, 2010; Bradley et al., 2012).
At the end of the study (spontaneous death of more than 90% of mice at 130 weeks), 37% and 62% in control mice (males and females together) were bearing benign or malignant tumours, respectively. The combined incidence of benign and malignant tumours observed among all groups including controls varied between 95% and 100%.
3.1.1.5. Mammary gland neoplasms
With respect to the adenomas, fibromas and fibroadenomas observed in the mammary gland of female mice, the Panel noted that there was a dose‐related trend towards a decreased incidence of these benign tumours in treated groups. In particular, a higher incidence was recorded in control animals that was even outside the database values (1.3–2% incidence at 104 weeks for ‘mammary gland: fibroadenoma’ (Charles River Laboratories, 2010)) and specifically the following data were recorded: 9.8% (control), 4.8%, 5.0%, 6.2% and 1.6% (at 500, 2,000, 8,000 and 16,000 mg/kg diet, respectively). This demonstrates the variability of the results obtained and that data outside the (historical control) range considered of normality might be observed incidentally.
4. Discussion
Soffritti et al. (2016) published an oral carcinogenicity study with sucralose in mice. The animals were exposed to sucralose from the 12th day of pregnancy (in utero) until spontaneous death or 130 weeks of age. The authors reported that ‘sucralose administered in feed, beginning prenatally through lifespan, induces hematopoietic neoplasias in male Swiss mice’.
The Panel did not share this view based on the following arguments:
The only effect observed in the study was an increased incidence in haematopoietic neoplasias (i.e. leukaemias) in male animals. The increased incidence did not show a dose–response relationship and started from Group 3 (2,000 mg/kg diet). The incidence of leukaemias in control males was lower than, whereas in control females the incidence was close to, the incidence observed in the same strain and sex after 104 week of age (Charles River Laboratories, 2010). The background data on incidence of leukaemia in this strain suggested that females were more prone to develop leukaemia than males. This was contrary to the findings in the study by Soffritti et al.(2016). Furthermore, there was a lack of plausibility for a possible neoplastic effect, and lack of a mode of action (Smith et al., 2016). In addition, the majority of the basic Bradford‐Hill considerations (Lucas and McMichael, 2005; Fedak et al., 2015) were not met such as strength, consistency, biological gradient and plausibility. If sucralose indeed had the suggested carcinogenic potential, it should have been observed in females rather than in males since female Swiss mice are more prone to develop lymphomas and leukaemias than males (31.3% in female controls vs 7.7% in male controls).
Although the parental generation of the mice was originally from Charles River laboratories, the animals used were bred at Ramazzini Institute – CMCRC. Therefore, potential health issues in an internal colony could not be totally ruled out (EFSA ANS Panel, 2009, 2013; Gift et al., 2013).
For example, the background incidence of severe bronchiolar/alveolar and peribronchiolar inflammation was very high, in both males and females of all groups, controls included, which was also observed in previous studies performed in rats at the Ramazzini Institute – CMCRC (EFSA ANS Panel, 2013). The differential diagnosis between severe pulmonary inflammation and leukaemia is occasionally difficult to be done and it is well known that such tumours may arise as a result of severe lymphoid hyperplasia in the lungs of animals suffering from chronic respiratory disease. It was, therefore, not illogical to assume that pulmonary inflammation might have been misdiagnosed for leukaemia.
The available in vitro and in vivo genotoxicity data did not provide reliable evidence of mutagenicity for sucralose (Jeffrey and Williams, 2000; Brusick et al., 2010). Positive results were only reported with an indicator assay (comet assay) in experiments in vitro (Van Eyk, 2014) and in vivo in mouse stomach, colon and lung cells following oral administration of a single high dose of sucralose (2,000 mg/kg) (Sasaki et al., 2000). Concerning the results reported by Sasaki's group, the Panel noted that the results produced in this laboratory, which used an in‐house developed methodology, have been evaluated ‘to be interpreted with caution’ by an international expert group (Hartmann et al., 2003). Moreover, the Panel considered that mutagenicity data should receive greater weight than results in indicator assays (ECHA, 2016; OECD, 2016), and that in the framework of a definition of mutagenic mode of action results in the target tissue for carcinogenicity should receive greater consideration (Dearfield and Moore, 2005; Butterworth, 2006). In this respect, the Panel noted that uniformly negative results have been obtained in clastogenicity tests in haemopoietic cells of rats (chromosomal aberrations) and mice (micronuclei) (Brusick et al., 2010; Berry et al., 2016), and that negative results in bone marrow have also been reported in the Sasaki's study in mice. Although sucralose is resistant to hydrolysis and particularly to attack from glycosidic enzymes in the gut, it is sensitive to thermal degradation with possible production of chlorinated byproducts (SCF, 2000; Dong et al., 2013a,b; De Oliveira et al., 2015). One of the degradation products of sucralose, namely 1,6‐dichlorofructose (1,6‐DCF), was weakly mutagenic in vitro in bacteria and in mammalian cells, mainly in the absence of a source of mammalian metabolism, but ineffective in other studies in vitro and in vivo in rodent liver (DNA damage) and bone marrow (sister chromatid exchanges, micronuclei and DNA binding), and overall 1,6‐DCF was evaluated as non genotoxic (SCF, 2000).
Overall, the Panel concluded that the results of in vitro and in vivo assays of sucralose revealed no genotoxic activity, which is consistent with the chemical structure of sucralose that predicts a low order of reactivity and no identified structural alerts for genotoxic or carcinogenic activity (Berry et al., 2016).
Carcinogenicity studies in rats and mice with a duration of 104 weeks and performed according to GLP and designed according to the regulatory guidance and protocols for conducting studies to evaluate the safety of new food ingredients (Hayes et al., 2011) produced no evidence that even very high doses of sucralose (30,000 mg/kg diet) induced carcinogenicity (Mann et al., 2000a,b).
5. Conclusions
Taking into consideration the paper from Soffritti et al. (2016), the technical report and additional information provided by the Ramazzini Institute and other information available for sucralose (E 955), the Panel noted:
the lack of a dose–response relationship between the exposure to sucralose and incidence of lymphomas and leukaemias (combined);
the lack of a mode of action and failure to meet all the Bradford‐Hill considerations for a cause–effect relationship between intake of sucralose and the development of tumours in male mice only;
the design of the bioassay that considers exposure from gestation up to natural death of animals implies an increase in background pathology that results in the possibility of misclassifications and a difficult interpretation of data, especially in the absence of both an appropriate concurrent control group and a recent historical database;
A comprehensive database was available for sucralose and no carcinogenic effect was reported in adequate studies in rats and mice. Moreover, there was no reliable evidence of in vivo genotoxicity. Furthermore, negative results have been obtained in genotoxicity tests for various end‐points in haematopoietic cells of mice and rats, both with sucralose and its degradation product 1,6‐DCF;
therefore, the Panel concluded that the available data did not support the conclusions of the authors (Soffritti et al., 2016) that sucralose induced haematopoietic neoplasias in male Swiss mice.
Documentation provided to EFSA
Ramazzini Institute – Cesare Maltoni Cancer Research Center (CMCRC). Technical Report: Long‐term carcinogenicity bioassay to evaluate the potential biological effects, in particular carcinogenic, of sucralose administered in feed to Swiss mice study protocol: BT 6012. Submitted to EFSA in June 2017.
Ramazzini Institute – Cesare Maltoni Cancer Research Center (CMCRC). Additional information requested to supplement the technical hearing of the 10 November 2016. Letter sent on 21 October 2016, Ref. CRP/PC/MP 2016. Submitted to EFSA on 4 November 2016.
Ramazzini Institute – Cesare Maltoni Cancer Research Center (CMCRC). Additional information requested. Letter sent on 23 November 2016, Ref. CRP/PC/LF 2016. Submitted to EFSA on 16 January 2017.
Ramazzini Institute – Cesare Maltoni Cancer Research Center (CMCRC). Additional information requested. Letter sent out on 31 January 2017, Ref. CRP/CS/LF 2017. Submitted to EFSA on 15 February 2017.
Abbreviations
- ADI
acceptable daily intake
- ANS
Scientific Panel on Food Additives and Nutrient Sources added to Food
- bw
body weight
- DCF
1,6‐dichlorofructose
- CMCRC
Cesare Maltoni Cancer Research Center
- FAO
Food and Agriculture Organization of the United Nations
- GLP
Good Laboratory Practices
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- NOEL
no observed effect level
- OECD
Organisation for Economic Co‐operation and Development
- QA
Quality Assurance
- SCF
Scientific Committee on Food
- SOPs
Standard Operating Procedures
- WHO
World Health Organization
Suggested citation: EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Aguilar F, Crebelli R, Di Domenico A, Dusemund B, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Lambré C, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Parent‐Massin D, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Younes M, Ciccolallo L, Colombo P, Lodi F and Mortensen A, 2017. Statement on the validity of the conclusions of a mouse carcinogenicity study on sucralose (E 955) performed by the Ramazzini Institute. EFSA Journal 2017;15(5):4784, 14 pp. 10.2903/j.efsa.2017.4784
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00251
Panel members: Fernando Aguilar, Riccardo Crebelli, Alessandro Di Domenico, Birgit Dusemund, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Claude Lambré, Jean‐Charles Leblanc, Oliver Lindtner, Peter Moldeus, Alicja Mortensen, Pasquale Mosesso, Agneta Oskarsson, Dominique Parent‐Massin, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Adopted: 4 April 2017
Reproduction of the table listed below is prohibited and permission must be sought directly from the copyright holder:
Table 3: © Taylor & Francis
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1–295.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up the program for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Directive 2004/10/EC of the European Parliament and the Council of 11 February 2004; OJ L 50/44–59.
References
- Berry C, Brusik D, Cohen S, Hardisty JF, Grotz VL and Williams GM, 2016. Sucralose non‐carcinogenicity: a review of the scientific and regulatory rationale. Nutrition and Cancer, 68, 1247–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley A, Mukaratirwa S and Petersen‐Jones M, 2012. Incidence and range of spontaneous findings in the lymphoid and haemopoietic system of control Charles River CD‐1 Mice (Crl: CD‐1(ICR) BR) used in chronic toxicity studies. Toxicologic Pathology, 40, 375–381. [DOI] [PubMed] [Google Scholar]
- Brusick D, Borzelleca JF, Gallo M, Williams G, Kille J, Hayes AW, Pi‐Sunyer FX, Williams C and Burks W, 2010. Experts Panel report on a study of Splenda in male rats. Regulatory Toxicology and Pharmacology, 55, 6–12. [DOI] [PubMed] [Google Scholar]
- Butterworth BE, 2006. A classification framework and practical guidance for establishing a mode of action for chemical carcinogens. Regulatory Toxicology and Pharmacology, 45, 9–23. [DOI] [PubMed] [Google Scholar]
- Charles River Laboratories , 2010. Spontaneous neoplastic lesions in the Crl:CD1 (ICR) mouse in control groups from 18 month to 2 year studies. March 2010. Prepared by Giknis MLA and Clifford CB. 22 pp.
- De Oliveira DN, de Menezes M and Catharino RR, 2015. Thermal degradation of sucralose: a combination of analytical methods to determine stability and chlorinated byproducts. Scientific Reports, 5, 9598. 10.1038/srep09598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearfield K and Moore MM, 2005. Use of genetic toxicology information for risk assessment. Environmental and Molecular Mutagenesis, 46, 236–245. [DOI] [PubMed] [Google Scholar]
- Dong S, Liu G, Hu J and Zheng M, 2013a. Polychlorinated dibenzo‐p‐dioxin and dibenzofurans formed from sucralose at high temperatures. Scientific Reports, 3, 2946. 10.1038/srep02946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Liu G, Zhang B, Gao L and Zheng M, 2013b. Formation of polychlorinated naphthalenes during the heating of cooking oil in the presence of high amounts of sucralose. Food Control, 32, 1e5. [Google Scholar]
- ECHA (European Chemical Agency), 2016. Guidance on information requirements and chemical safety assessment. Chapter R.7a: Endpoint specific guidance. Version 5. December 2016. Available online from: https://echa.europa.eu/documents/10162/13632/information_requirements_r7a_en.pdf
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2009. Updated Scientific Opinion of the Panel on Food Additives and Nutrient Sources added to Food on a request from the European Commission related to the 2nd ERF carcinogenicity study on aspartame taking into consideration study data submitted by the Ramazzini Foundation in February 2009. EFSA Journal 2009;7(4):1015, 18 pp. 10.2903/j.efsa.2009.1015 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2013. Scientific Opinion on the re‐evaluation of aspartame (E 951) as a food additive. EFSA Journal 2013;11(12):3496, 263 pp. 10.2903/j.efsa.2013.3496 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to food), 2016. Safety of the proposed extension of use of sucralose (E 955) in foods for special medical purposes in young children. EFSA Journal 2016;14(1):4361, 11 pp. 10.2903/j.efsa.2016.4361 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- Fedak KM, Bernal A, Capshaw ZA and Gross S, 2015. Applying the Bradford Hill criteria in the 21st century: how data integration has changed casual inference in molecular epidemiology. Emerging Themes in Epidemiology, 12, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gift JS, Caldwell JC, Jinot J, Evans MV, Cote I and Vandenberg JJ, 2013. Scientific considerations for evaluating cancer bioassays conducted by the Ramazzini Institute. Environmental Health Perspective, 121, 1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Agurell E, Beevers C, Brendler‐Schwaab S, Burlinson B, Clay P, Collins A, Smith A, Speit G, Thybaud V and Tice RR, 2003. Recommendations for conducting the in vivo alkaline Comet assay. Mutagenesis, 18, 45–51. [DOI] [PubMed] [Google Scholar]
- Haseman J, Melnick R, Tomatis L and Huff J, 2001. Carcinogenesis bioassays: study and biological relevance. Food and Chemical toxicology, 39, 739–744. [DOI] [PubMed] [Google Scholar]
- Hayes AW, Dayan AD, Hall WC, Kodell RL and Williams GM, 2011. A review of mammalian carcinogenicity study design and potential effects of alternate test procedures on the safety evaluation of food ingredients. Regulatory Toxicology and Pharmacology 60 (1 Suppl), S1–S34. [DOI] [PubMed] [Google Scholar]
- Huff J, Jacobson MF and Lee David D, 2008. The limits of two‐year bioassay exposure regimens for identifying chemical carcinogens. Environmental Health Perspectives, 116, 1439–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1991. Toxicological evaluation of certain food additives and contaminants. WHO Food Additive Series, No 28. WHO, Geneva.
- Jeffrey AM and Williams GM, 2000. Lack of DNA‐damaging activity of five non‐nutritive sweeteners in the rat hepatocyte/DNA repair assay. Food Chemical Toxicology, 38, 335–338. [DOI] [PubMed] [Google Scholar]
- Lucas RM and McMichael AJ, 2005. Association or causation: evaluating links between “environment and disease”. Public Health Classics, 83, 792–795. [PMC free article] [PubMed] [Google Scholar]
- Mann SW, Yuschak MM, Amyes SJG, Aughton P and Finn JP, 2000a. A carcinogenicity study of sucralose in the CD‐1 mouse. Food and Chemical Toxicology, 38, S91±S98. [DOI] [PubMed] [Google Scholar]
- Mann SW, Yuschak MM, Amyes SJG, Aughton P and Finn JP, 2000b. A combined chornic toxicity carcinogenicity study of sucralose in Sprague‐Dawley rats. Food and Chemical Toxicology, 38, S71–S89. [DOI] [PubMed] [Google Scholar]
- Manservisi F, Marquillas CB, Buscaroli A, huff J, Lauriola M, Mandrioli D, Manservigi A, Panzacchi S, Silbetgeld EK and Belpoggi F, 2016. An integrated experimental design for the assessment of multiple toxicological end points in rat bioassays. Environmental health perspectives, 125, 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2016. Overview of the set of OECD Genetic Toxicology Test Guidelines and updates performed in 2014–2015. Series on Testing and Assessment N. 238. ENV/JM/MONO(2016)33.
- Sasaki YF, Kawaguchi S, Kamaya A, Ohshita M, Kabasawa K, Iwama K, Taniguchi K and Tsuda S, 2000. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutation Research, 519, 103–119. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food), 2000. Opinion of the Scientific Committee on Food on sucralose. SCF/CS/ADDS/EDUL/190 Final. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out68_en.pdf
- Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, DeMarini DM, Caldwell JC, Kavloc RJ, Lambert PF, Hecht SS, Bucher JR, Stewart BW, Baan RA, Cogliano VJ and Straif K, 2016. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environmental Health Perspectives, 124, 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffritti M, Belpoggi F, Minardi F, Bua L and Maltoni C, 1999. Mega‐experiments to identify and assess diffuse carcinogenic risk. Annals New York Academy of Sciences, 895, 34–55. [DOI] [PubMed] [Google Scholar]
- Soffritti M, Belpoggi F, Degli Esposti D, Tibaldi E and Lauriola M, 2007. Lifespan exposure to low doses of aspartame beginning during prenatal life increases cancer effects in rats. Environmental Health Perspectives, 115, 1293–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffritti M, Belpoggi F, Degli Esposti D, Falcioni L and Bua L, 2008. Consequences of exposure to carcinogens beginning during developmental life. Basic and Clinical Pharmacology and Toxicology, 102, 118–124. [DOI] [PubMed] [Google Scholar]
- Soffritti M, Belpoggi F, Manservigi M, Tibaldi E, Lauriola M, Falcioni L and Bua L, 2010. Aspartame administered in feed, beginning prenatally through life span, induces cancers of the liver and lung in male swiss mice. American Journal of Industrial Medicine, 53, 1197–1206. [DOI] [PubMed] [Google Scholar]
- Soffritti M, Padovani M, Tibaldi E, Falcioni L, Manservisi F, Lauriola M, Bua L, Manservigi M and Belpoggi F, 2016. Sucralose administered in feed, beginning prenatally through lifespan, induces hematopoietic neoplasias in male swiss mice. International Journal of Occupational and Environmental Health, 22, 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyk AD, 2014. The effect of five artificial sweeteners on Caco‐2, HT‐29 and HEK‐293 cells. Drug and Chemical Toxicology, 38, 318–327. [DOI] [PubMed] [Google Scholar]


