Abstract
Following a request from the European Commission, the EFSA Panel on Food Additives and Nutrient sources added to Food (ANS) was asked to deliver a scientific opinion on the re‐evaluation of pectin (E 440i) and amidated pectin (E 440ii) as food additives. An acceptable daily intake (ADI) ‘not specified’ was allocated by the Scientific Committee for Food (SCF) for E 440i and E 440ii. Pectin and amidated pectin would not be absorbed intact, but extensively fermented by intestinal microbiota in animals and humans; products formed from pectins in the gastrointestinal tract are similar to manufactured pectin‐derived acidic oligosaccharides (pAOS). There is no indication of genotoxicity for pectin and amidated pectin, although the available data were limited. No adverse effects were reported in a chronic toxicity study in rats at levels up to 5,000 mg pectin/kg bw per day, the highest dose tested. No treatment‐related effects were observed in a dietary one‐generation reproductive toxicity study with pAOS in rats at up to 6,200 mg/kg body weight (bw) per day, the highest dose tested. The Panel did not consider E 440i and E 440ii as having allergenic potential. A dose of 36 g/day (equivalent to 515 mg/kg bw per day) for 6 weeks in humans was without adverse effects. Exposure to pectins from their use as food additives ranged up to 442 mg/kg bw per day for toddlers at the 95th percentile (brand‐loyal scenario). The Panel concluded that there is no safety concern for the use of pectin (E 440i) and amidated pectin (E 440ii) as food additives for the general population and that there is no need for a numerical ADI.
Keywords: pectin, E 440i, amidated pectin, E 440ii, food additive
Summary
Following a request from the European Commission, the Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to deliver a scientific opinion re‐evaluating the safety of pectin (E 440i) and amidated pectin (E 440ii) when used as food additives.
Pectin (E 440i) and amidated pectin (E 440ii) are authorised as food additives in the European Union (EU) in accordance with Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives, and specific purity criteria have been defined in Commission Regulation (EU) No 231/2012. E 440 includes both pectin (E 440i) and amidated pectin (E 440ii).
Pectin (E 440i) consists of the partial methyl esters of polygalacturonic acid, while amidated pectin (E 440ii) consists of both partial methyl esters and amides of polygalacturonic acid. The Panel noted that the average molecular weight and the degree of methylation of pectins are highly variable, being their physicochemical properties, such as viscosity and gel strength, largely dependent on these parameters.
The Panel noted that pectins are natural compounds present in fruit and vegetables, the citrus peels and apple pomace being the main sources for the production of the food additive, which can be further modified enzymatically to produce pectins with different degrees of esterification or polymerisation. In addition, amidated pectins are modified forms of pectins produced by an amidation process and cannot be considered as a natural compound.
The Panel noted that according to the EU specifications for pectin (E 440i) and amidated pectin (E 440ii), impurities of the toxic elements arsenic, lead, mercury and cadmium are accepted up to concentrations of 3, 5, 1 and 1 mg/kg, respectively. Contamination at such levels could have a significant impact on the exposure to these metals, for which the exposure is already close to the health‐based guidance values or benchmark doses (lower confidence limits) established by EFSA. The Panel noted that, in June 2016, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) lowered the limit for lead to 2 mg/kg for general use and 0.5 mg/kg for use in infant formula.
Data on in vitro degradation of pectins and amidated pectins indicated that their digestibility was low in the upper parts of the digestive tract, but they would be fermented during their passage through the large intestine. These in vitro data are in agreement with in vivo studies demonstrating the absence of degradation of pectins in germ‐free rats by comparison to conventional animals. As demonstrated in ileostomy patients, the main end products of this colonic anaerobic digestive process are short‐chain fatty acids (SCFA), such as acetic, propionic and butyric acids, which are absorbed from the colon and considered of no safety concern by the Panel. These data indicated that pectins and amidated pectins would not be absorbed intact but extensively fermented by intestinal microbiota in animals and humans.
As products formed from pectins in the gastrointestinal tract are similar to manufactured pectin‐derived acidic oligosaccharides (pAOS), in 2015, JECFA concluded that studies using pAOS were relevant for the evaluation of pectins in infant formulae. The Panel agreed with this conclusion.
The acute oral toxicity of pectin is low. Data on amidated pectin were not available, but acute oral toxicity was expected to be low, based on the structural similarity to pectin.
The oral exposure to non‐amidated and amidated pectin at a dose level up to 13,500 mg/kg body weight (bw) per day did not result in effects of toxicological relevance in a subchronic feeding study in rats. No adverse effects were detected in a subchronic drinking water study in rats at a concentration of 5% in the water, equal to 3,366 mg/kg bw per day in males and 3,916 mg/kg bw per day in females, the highest dose tested. The reduction of protein digestibility and calcium absorption was shown in a subacute feeding study in rats at a dose of 12,000 mg/kg bw per day. In a 13‐week dietary study with pAOS in F1 rats, according to the OECD Guideline 408 and good laboratory practice (GLP), an increased level of urinary calcium and a minimal degree of urothelial hyperplasia was observed in the 5% group (3,400 mg/kg bw per day). In an additional 13‐week study in rats with pAOS, a no‐observed‐adverse‐effect level (NOAEL) of 1,700 mg/kg bw per day was identified.
The Panel noted that, although the available in vitro and in vivo data were limited, no genotoxic activity has been observed for pectin. This conclusion was also supported by the negative results obtained with manufactured pAOS. Data on amidated pectin were not available, but considering its chemical structure and its negligible absorption, the Panel considered that there is no concern with respect to genotoxicity for amidated pectin.
A chronic dietary toxicity study in rats with pectin or amidated pectin showed no adverse effects at 10% in the diet, equivalent to 5,000 mg/kg bw per day, the highest dose tested.
Two reproductive toxicity studies and one developmental toxicity study with pectin, which were considered inadequate for risk assessment, were available. In a dietary one‐generation reproductive toxicity study with pAOS in rats, a NOAEL of 6,200 mg/kg bw per day, the highest dose tested, was identified.
The Panel considered that there is no indication that the reported immune‐modulatory properties of pectin may lead to an adverse response, the data being rather indicative of an effect which would limit the hypersensitivity response. Therefore, the Panel did not consider pectin (E 440i) and amidated pectin (E 440ii) as having an allergenic potential.
A daily dose of 36,000 mg pectin (equivalent to 515 mg/kg bw per day) for 6 weeks in humans was associated with abdominal distension and increasing flatus in some individuals, effects which were considered by the Panel as undesirable, but not adverse.
No adverse effects were noted in four studies in infants with infant formulae containing pectin and pAOS. Pectin (4 mg/kg bw per day) improved the permeability of the small intestine in young infant boys (5–12 months) with persistent diarrhoea.
The safety of pectin (high‐ester pectin extracted from citrus peel and standardised by the addition of sucrose) in a milk replacer was tested in neonatal pigs (Yorkshire‐bred, age 2 days). The NOAEL for this study was 1,049 mg/kg bw per day. Based on the data available, the Panel considered that this conclusion appeared appropriate. JECFA (2016a) concluded from the data of a new piglet study that the reduced milk replacer consumption observed in both studies at a dose level of 1% pectin in milk replacer was likely due to delayed gastric emptying and/or prolonged gut transit resulting from consumption of the highly viscous 1% pectin diet. The data available from this study were not sufficient for the Panel to confirm the conclusion of JECFA.
To assess the dietary exposure to pectins (E 440) from their use as food additives, the exposure was calculated based on (1) maximum use levels provided to EFSA (defined as the maximum level exposure assessment scenario), and (2) reported use levels or analytical data (defined as the refined exposure assessment scenario, brand‐loyal and non‐brand‐loyal).
Pectins (E 440) are authorised in a wide range of foods. The Panel did identify brand loyalty to specific food categories in toddlers (e.g. flavoured fermented milk products and flavoured drinks). Further, the Panel considered that the non‐brand‐loyal scenario covering other population groups was the most appropriate and realistic scenario for risk characterisation, because it is assumed that the population would probably be exposed long‐term to the food additive present at the mean reported use level in processed food.
A maximum estimated exposure assessment scenario taking into account the food for special medical purposes for infants and young children (Food category (FC) 13.5.1. Dietary foods for infants for special medical purposes and special formulae for infants and FC 13.1.5.2 Dietary foods for babies and young children for special medical purposes as defined by Commission Directive 1999/21/EC) was also performed using food additive producers data to estimate exposure for infants and toddlers who may be on a specific diet. This exposure scenario considered products belonging to food categories 13.1.2, 13.1.3 and 13.1.4, excluding FC 13.1.1 (infant formulae), where pectins (E 440), according to the EU regulation, are not authorised. Considering that this diet is required due to specific needs, it is assumed that consumers are loyal to the food brand, therefore only the maximum brand‐loyal estimated exposure scenario was performed.
A refined estimated exposure assessment scenario taking into account the consumption of food supplements was also performed to estimate exposure for children, adolescents, adults and the elderly, for consumers only, as exposure via food supplements may deviate largely from that via food, and the number of food supplement consumers may be low depending on the population and survey.
The refined estimates were based on 23 out of 81 food categories in which pectins (E 440) are authorised. Overall, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the real exposure to pectins (E 440) as food additives in European countries for the maximum level exposure scenario and for the refined exposure assessment scenarios when considering only food additive uses for which data have been provided.
However, the Panel noted that, given the information from the Mintel Global New Products Database (GNPD), it may be assumed that pectins (E 440) are used in several food categories (n = 9) for which no data have been provided by food industry. The Panel noted that out of these nine food categories, fruit juices and fruit nectars are products highly consumed. If this would be confirmed, it would therefore result in an underestimation of the exposure.
A possible additional exposure to pectins (E 440) from their use directly by the consumer (adding pectins to thicken foods, e.g. jams, marmalades) and from their use as food additives authorised in accordance with Annex III to Regulation (EC) No 1333/2008 were not considered in any of the above exposure assessment scenarios.
The Panel also noted that the refined exposure estimates were based on information reported on the use levels of pectins (E 440) by food industry. If actual practices change, these refined estimates may no longer be representative and should be updated.
Following the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA ANS Panel, 2014), and given that:
the data received for the 23 food categories were adequate for a combined exposure assessment for these categories;
based on these reported use levels, a refined exposure of up to 442 mg/kg bw per day for toddlers in these categories (brand‐loyal scenario) was estimated;
pectin and amidated pectin are not absorbed intact, but extensively fermented by intestinal microbiota in animals and humans;
adequate toxicity data were available;
in oral subchronic studies with pectins in rats, no adverse effects were observed at doses ranging from 3,366 mg/kg bw per day to 13,500 mg/kg bw per day, the highest doses tested. In subchronic studies with pAOS in the diet in rats, a NOAEL of 1,700 mg/kg bw per day was identified;
no effects on body weight and food intake were observed in male neonatal pigs exposed to 0.3% pectin in formula (equal to 1,049 mg pectin/kg bw per day) for 28 days, while in the same study such effects were observed at 1% pectin in formula (equal to 4,015 mg pectin/kg bw per day);
no adverse effects were reported in a chronic study in rats at up to 5,000 mg pectin/kg bw per day, the highest dose tested;
there is no concern with respect to genotoxicity for pectin and amidated pectin;
a daily dose of 36,000 mg pectin (equivalent to 515 mg/kg bw per day) for 6 weeks in humans was associated with abdominal distension and increasing flatus in some individuals, effects which were considered by the Panel as undesirable, but not adverse,
the Panel concluded that there is no need for a numerical ADI for pectin (E 440i) and amidated pectin (E 440ii) and that there is no safety concern for the general population at the refined exposure assessment for the reported uses and use levels of pectins (E 440) as food additives.
Concerning the use of pectins (E 440) in ‘dietary foods for special medical purposes and special formulae for infants’ (FC 13.1.5.1) and in ‘dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC’ (FC 13.1.5.2), and given that:
for populations consuming foods for special medical purposes and special formulae, the 95th percentile of maximum exposure assessments calculated based on the maximum reported data from food additive producers were up to 1,349 mg/kg bw per day for infants;
infants and young children consuming these foods may be exposed to a greater extent to pectins (E 440) than their healthy counterparts because the permitted levels of pectins (E 440) in formulae for special medical purposes are 2‐fold higher (1%) than in formulae for healthy individuals;
infants and young children consuming foods belonging to these food categories may show a higher susceptibility to the gastrointestinal effects of pectins than their healthy counterparts due to their underlying medical condition;
no effects on body weight and food intake were observed in male neonatal pigs exposed to 0.3% pectin in formula (equal to 1,049 mg pectin/kg bw per day) for 28 days, while in the same study, such effects were observed at 1% pectin in formula (equal to 4,015 mg pectin/kg bw per day);
in infants and young children (< 18 months of age), a formula containing 0.5% pectin (E 440) given for 3 months was well tolerated without adverse effects;
no human studies investigating the adverse effects of formulae containing pectins (E 440) at the maximum permitted level (MPL) of 1% for the relevant age group were available,
the Panel concluded, that the available data do not allow for an adequate assessment of the safety of use of pectins (E 440) in infants and young children consuming these foods for special medical purposes at the presently authorised maximum use levels of 1%.
The Panel recommended that:
the European Commission considers lowering the maximum limits for the impurities of toxic elements arsenic, lead, mercury and cadmium in the EU specifications for pectin (E 440i) and amidated pectin (E 440ii) in order to ensure that pectin (E 440i) and amidated pectin (E 440ii) as food additives will not be a significant source of exposure to those toxic elements in food; special requirements might be defined in the specifications for pectin (E 440i) and amidated pectin (E 440ii) to be used in formulae or food for infants, toddlers and other young children;
limits for aluminium should be considered for inclusion in the EU specifications, as aluminium can be used in the manufacturing process;
the European Commission considers harmonising the microbiological specifications for polysaccharidic thickening agents, such as pectins, and including criteria for the absence of Salmonella spp. and Escherichia coli, for total aerobic microbial count (TAMC) and for total combined yeasts and moulds count (TYMC) in the EU specifications for pectin (E 440i) and amidated pectin (E 440ii);
additional clinical data should be generated to assess the safety of pectins (E 440) when used in ‘dietary foods for special medical purposes and special formulae for infants’ (FC 13.1.5.1) and in ‘dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC’ (FC 13.1.5.2);
due to the discrepancies observed between the data reported from industry and the Mintel database, where pectins are labelled in more products than in food categories for which data were reported from industry, the Panel recommended collection of data on usage and use levels of pectins (E 440) in order to perform a more realistic exposure assessment.
1. Introduction
The present opinion deals with the re‐evaluation of the safety of pectin (E 440i) and amidated pectin (E 440ii) when used as food additives. Pectin (E 440i) and amidated pectin (E 440ii) are authorised food additives in the European Union (EU) according to Annex II and Annex III of Regulation (EC) No 1333/20081. E 440 includes both pectin (E 440i) and amidated pectin (E 440ii).
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union (EU). In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the EU before 20 January 2009 has been set up under Regulation (EU) No 257/20102. This Regulation also foresees that food additives are re‐evaluated whenever necessary in the light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU3 of 2001. The report ‘Food additives in Europe 2000’ submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010, the 2003 Terms of Reference are replaced by those below.
1.1.2. Terms of Reference
The Commission asks EFSA to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.1.3. Interpretation of Terms of Reference
The Panel on Food Additives and Nutrient Sources added to Food (ANS Panel) described its risk assessment paradigm in its Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012). This Guidance states, that in carrying out its risk assessments, the Panel sought to define a health‐based guidance value e.g. an acceptable daily intake (ADI) (IPCS, 2004) applicable to the general population. According to the definition above, the ADI as established for the general population does not apply to infants below 12 weeks of age (JECFA, 1978; SCF, 1998). In this context, the re‐evaluation of the use of food additives, such as pectin and amidated pectin, in food for infants below 12 weeks represents a special case for which specific recommendations were given by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 1972, 1978) and by the SCF (1996, 1998). The Panel endorsed these recommendations.
In the current EU legislation (Regulation (EC) No 1333/2008), use levels of additives in food for infants under the age of 12 weeks in categories 13.1.1 and 13.1.5.14 (Annex II) and uses of food additives in nutrient preparations for use in food for infants under the age of 12 weeks and maximum levels for the carry‐over from these uses (Annex III, Part 5, section B) are included. The Panel considers that these uses would require a specific risk assessment in line with the recommendations given by JECFA and the SCF and endorsed by the Panel in its current Guidance for submission for food additives evaluations (EFSA ANS Panel, 2012). Therefore risk assessments for the general population are not considered applicable for infants under the age of 12 weeks and will be performed separately.
This re‐evaluation refers exclusively to the uses of pectin (E 440i) and amidated pectin (E 440ii) as food additives in food, including food supplements and does not include a safety assessment of other uses of pectins.
1.2. Information on existing authorisations and evaluations
In 1978, the SCF (1978) endorsed the ADI ‘not specified’ established by JECFA (1974) for non‐amidated pectins. The Committee was prepared to accept a temporary ADI of 0–25 mg/kg body weight (bw) for amidated pectin, provided that the results of further toxicological studies be received by 1982. The Committee recommended that these studies should include adequate reproduction, embryotoxicity and teratology studies in rats and an adequate long‐term study in a rodent species, preferably in the rat.
The SCF (1985) endorsed the evaluation of JECFA (1981) in which additional long‐term feeding studies in rats, as well as multi‐generation studies, showed no toxicological differences between pectins and amidated pectins. The Committee concluded that the database was sufficient, and established a group ADI ‘not specified’ for non‐amidated and amidated pectin. However, no toxicological data were specified in the document.
The SCF (2003) recommended that pectins should not be used in infant formulae and follow‐on formulae in view of limited information on their potential effects in infants. The Committee had no objections against the continued use of pectins up to a maximum level of 10 g/L in dietary foods for special medical purposes for infants to be used under medical supervision.
In 2014, JECFA evaluated the safety of using non‐amidated pectin in infant formula and formula for special medical purposes intended for infants (JECFA, 2015). In this document, it was stated that ‘the Committee was made aware that a further pectin product is available on the market. This product, known as pectin‐derived acidic oligosaccharides (pAOS), is produced by enzymatic hydrolysis of pectin. pAOS has not been evaluated by the Committee and is not covered by the existing specifications for pectins.’ JECFA concluded that the use of non‐amidated pectin in infant formulas at the maximum proposed use levels (0.5%) is of concern. This conclusion was based on the decreased food intake and body weight gain in neonatal pigs.
In the updated safety evaluation (JECFA, 2016a), the Committee concluded that at the new maximum proposed use level of pectin in infant formula (0.2%), the estimated exposure of infants aged 0–12 weeks would be up to 360 and 440 mg pectin/kg bw per day at mean and high consumption, respectively. The margins of exposure for average and high consumers were 2.9 and 2.4, respectively, when compared with the no‐observed‐effect level (NOAEL) of 1,049 mg/kg bw per day, identified in the piglet study. On the basis of a number of considerations (low toxicity of pectin, NOAEL derived from a relevant study in neonatal piglets, relation of adverse effects in piglet study to viscosity, support of clinical studies for tolerance of infants to pectin up to the concentration of 0.2% and conservative exposure estimates), the Committee concluded that the margins of exposure calculated for the use of pectin at a concentration of 0.2% in infant formula indicate a low risk for the health of infants aged 0–12 weeks and is not of concern. JECFA further stated that ‘there is variability in medical conditions among infants requiring formula for special medical purposes and that these infants would be normally under medical supervision’.
An evaluation of pectin and amidated pectin is also available from the Nordic Council of Ministers (TemaNord, 2002). It was stated that pectin has been a natural component of human diet throughout evolution and there is no indication of toxic effects induced by pectin or amidated pectin.
The EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) established a cause and effect relationship between the consumption of pectins and a reduction of post‐prandial glycaemic responses and maintenance of normal blood cholesterol concentrations (EFSA NDA Panel, 2010). In order to obtain these physiological effects, 10 g of pectins per meal or 6 g of pectins per day, in one or more servings, respectively, are required.
2. Data and methodologies
2.1. Data
The ANS Panel was not provided with a newly submitted dossier. EFSA launched public calls for data,5 , 6 , 7 to collect relevant information from interested parties.
The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up to the last Working Group meeting before the adoption of the opinion.8 Attempts were made to retrieve relevant original study reports on which previous evaluations or reviews were based, however, these were not always available to the Panel.
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database9) was used to estimate the dietary exposure.
The Mintel Global New Products Database (GNPD) is an online resource listing food products and compulsory ingredient information that should be included in labelling. This database was used to verify the use of pectin (E 440i) and amidated pectin (E 440ii) in food products.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
The ANS Panel assessed the safety of pectin (E 440i) and amidated pectin (E 440ii) as food additives in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the SCF (2001) and taking into consideration the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
When the test substance was administered in the feed or drinking water, but doses were not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012) for studies in rodents or, in the case of other animal species, by JECFA (2000). In these cases, the daily intake is expressed as equivalent. When, in human studies in adults (aged above 18 years), the dose of the test substance administered was reported in mg/person per day, the dose in mg/kg bw per day was calculated by the Panel using a body weight of 70 kg as default for the adult population, as described in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012).
Dietary exposure to pectin (E 440i) and amidated pectin (E 440ii) from their use as food additives was estimated combining the food consumption data available within the EFSA Comprehensive European Food Consumption Database with the maximum levels according to Annex II to Regulation (EC) No 1333/2008 and/or reported use levels and analytical data submitted to EFSA following a call for data. Different exposure scenarios were used to calculate exposure (see Section 3.4.1). Uncertainties in the exposure assessment were identified and discussed.
3. Assessment
3.1. Technical data
3.1.1. Identity of the substances
According to Commission Regulation (EU) No 231/201210, pectin (E 440i) consists mainly of the partial methyl esters of polygalacturonic acid and their ammonium, sodium, potassium and calcium salts. It is obtained by extraction, in an aqueous medium, of strains of appropriate edible plant material, usually citrus fruits peels or apple pomace. Pectin has the Chemical Abstract Service (CAS) Registry No 9000‐69‐5 and the EC (EINECS) No 232‐553‐0.
Amidated pectin (E 440ii) consists of both partial methyl esters and amides of polygalacturonic acid. Amidated pectin has the CAS Registry No 56645‐02‐4; an EC (EINECS) No has not been assigned.
The structure of pectins is based on a backbone of 1,4‐linked α‐d‐galactopyranosyluronic acid units intercalated by 2‐linked l‐rhamnosyl residues. Parts of the carboxyl groups are esterified with methanol. The degree of esterification (DE) can range theoretically from 0% to 100%. Commercial pectins are divided into low‐methoxyl pectins (LM‐pectins), where less than 50% (typically 20–40%) of the carboxyl groups are methylated, whereas in high‐methoxy pectins (HM‐pectins), more than 50% (typically 55–75%) are methylated. Pectins with a degree of methylation of less than 10% are called pectic acid or pectate. In addition, the galacturonic acid monomers may be esterified with acetyl groups to varying degrees, depending on the plant source (Pilnik and Voragen, 1992; Voragen, 2007). Neutral sugars are covalently linked as side‐chains to the rhamnogalacturonan via the C3 of galacturonosyl and/or the C4 of the rhamnosyl residues. Side‐chain sugars are mainly galactose and arabinose, forming galactan, arabinan and arabinogalactan, and appear to be concentrated along certain regions of the polymer (‘hairy regions’), in contrast to side‐chain free parts (‘smooth regions’) (Thibault, 1983). Arabinogalactan I is a backbone of mainly β‐(1,4)‐galactopyranosyl linkages with α‐(1,5)‐linked arabinosyl residues (as single arabinose side‐chain). This arabinogalactan is the one present in pectins. Arabinogalactan II is mainly linked to proteins or free and the β‐galactopyranosyl residues are predominantly α‐1,3‐linked. The 1,4‐linked α‐d‐galactosyluronic residues can also be O‐acetylated at the C2 and C3 positions (Fincher et al., 1983; Huisman et al., 2001; Voragen et al., 2009).
The structural formula of the galacturonic acid chain (40% methylated), the major structure unit of pectins, is exemplarily presented in Figure 1.
Figure 1.
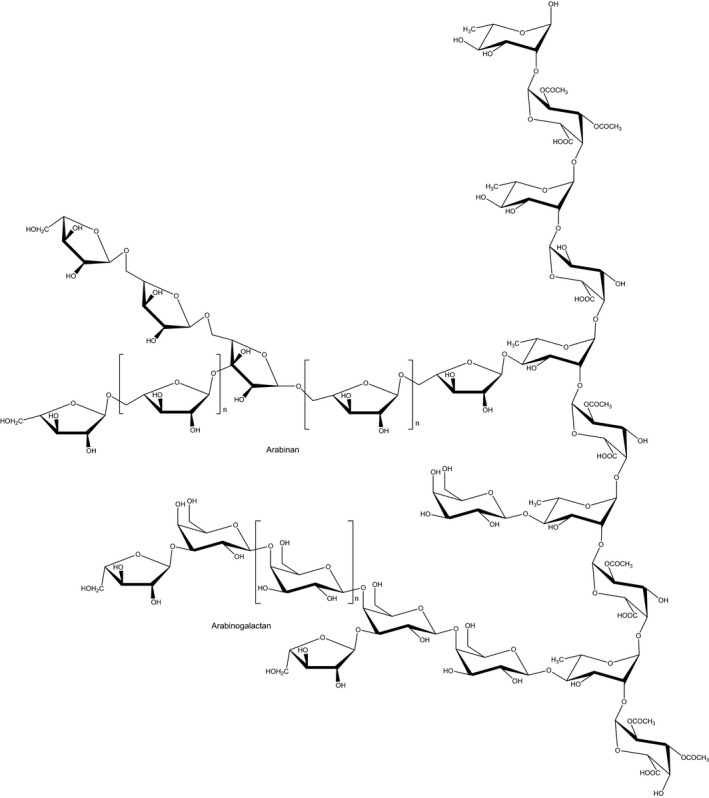
Structural formula of pectins. The backbone is composed of the disaccharide repeating unit of α‐(1,2)‐d‐galacturonic acid‐α‐(1,4)‐l‐rhamnose. Side chains are polymers containing linear and branched α‐l‐arabinose and/or β‐d‐galactose residues to form arabinan and arabinogalactan I of varying chain lengths (adapted from Ochoa‐Villarreal et al., 2012; CC BY 3.0)
In three commercial samples, the content of neutral sugars varied between 8.5% and 27.0% (Rolin et al., 1998).
In amidated pectins (E 440ii), up to 25% of the carboxyl groups are amidated [Documentation provided to EFSA, n. 14]. Amidated pectins are derived from HM‐pectins. Two types of amidated pectins are commercially available. The distinction between the types is made on the basis of the rate of setting (i.e. rate of gelification) in the presence of calcium ions: (i) ‘slow‐set amidated low‐methoxyl pectins’ (degree of methylation 35%; degree of amidation 15%) and (ii) ‘rapid‐set amidated high‐methoxyl pectins’ (degree of methylation 30%; degree of amidation 20%) (Voragen et al., 1995).
The average molecular weight of pectins, based on viscosity measurements, is reported to be in the range between 50,000 and 200,000 (Rolin et al., 1998).
According to Commission Regulation (EU) No 231/2012, pectins are white, light yellow, light grey or light brown or brownish powders, which are soluble in water, forming a colloidal, opalescent solution, and insoluble in ethanol. They are practically odourless, with a mucilaginous taste (Merck Index, 2012).
The physicochemical properties of pectins are largely dependent on molecular mass, degree of esterification, degree of acetylation, degree of amidation and the neutral sugar content (Voragen, 2007). Commercial pectins are soluble in water, forming a viscous solution which contains hydrated particles. They dissolve more readily in water if first moistened with alcohol, glycerol or sugar syrup, or if first mixed with three or more parts of sucrose (Merck Index, 2012). The pH of the aqueous solution is 2.9–3.2, depending on the degree of esterification (Pilnik and Voragen, 1992). The pKa values vary between 3 and 4, and depend on the degree of methylation (Ralet et al., 2002). Pectins are insoluble in alcohol, diluted alcohol and other organic solvents (Pilnik and Voragen, 1992; Voragen, 2007; Merck Index, 2012). Gelling is related to viscosity of pectin solutions. Viscosity and gel strength of the aqueous solutions increase with increasing molecular weight (Voragen, 2007). It is also affected by the degree of methylation, pH and presence of counter‐ions (Phatak et al., 1988). Viscosity also changes with concentration and temperature. HM‐pectins form gels if the pH is below the value of, approximately, 3.6 and if a cosolute is present, typically sucrose, at a concentration greater than 55% by weight (Oakenful and Scott, 1984). LM‐pectins form electrostatically stabilised gel networks with whey proteins that can be further stabilised by heat treatment (Krzeminski et al., 2014).
According to Gilsenan et al. (2003a,b) and Sharma et al. (2006), pectins can be added to food together with other hydrocolloids to improve or modify the gelation conditions and the physicochemical properties and texture for certain food applications, improving some quality parameters. HM‐pectins can be used in combination with guluronic‐rich alginates to form firm gels in acid conditions without Ca2+ ions. According to Helgerud et al. (2010), fruits naturally rich in pectin, such as apples, form gels when a sodium alginate solution is added after cooking. In contrast to thermally stable calcium alginate gels, alginate‐pectin gels are thermo‐reversible.
Pectins can also interact with other polymers such as starch, gelatine, agar‐agar, guar gum, locust bean gum, oxidised starch, potato maltodextrin and gum arabic (acacia gum). According to Sharma et al. (2006), the combination of gelatine with pectin in confectionery products, compensates the disadvantage of a low‐melting temperature when gelatine is used alone, improving the product stability to higher temperatures. The texture of the products can be regulated by the pectin/gelatine ratio, and the texture becomes more elastic and brittle with the increasing pectin share, while the products become also more viscous with increasing gelatine share. The addition of pectin also reduces the setting time in respect to a pure gelatine system. It has been also reported by Gilsenan et al. (2003a,b) that gelatine does not develop sufficient net positive charge to allow the electrostatic association with pectin until the pH is below the isoelectric point of gelatine of 4.9.
Pectins can also interact with starch, and this combination is used to produce jelly beans. The pectin/starch ratio regulates the consistency in these products. The combination of pectins with agar‐agar is used in aerated confectionery products such as marshmallows to produce a more viscous texture, enhancing the mouth‐feel attribute, and can influence positively the water binding capacity leading to better preservation and longer stability at storage. Pectin can also interact with other polymers as guar, locust bean gum, oxidised starch, potato maltodextrin and gum arabic. Interactions with gum arabic are hydrophobic and lead to differing gel properties. Branched hydrocolloids cause a faster destabilisation of calcium‐induced low methoxyl pectin than linear polymers. Interactions of branched regions in pectins with other branched regions are highly hydrophobic and non‐ionic. The interaction of pectin and guar gum can improve some quality parameters in partially baked frozen bread such as moisture content and crumb cohesiveness, decreasing volume and increasing crumb hardness and chewiness (Skara et al., 2013).
Synonyms of pectins are Genu Pectin, pectic acid, polypectate.
3.1.2. Specifications
The specifications for pectin (E 440i) and amidated pectin (E 440ii), as defined in Commission Regulation (EU) No 231/2012 and by JECFA (2016b), are listed in Table 1.
Table 1.
Specifications for pectin (E 440i) and amidated pectin (E 440ii) according to Commission Regulation (EU) No 231/2012 and JECFA (2016b)
| Commission Regulation (EU) No 231/2012 | JECFA (2016b) | ||
|---|---|---|---|
| Pectin (E 440i) | Amidated pectin (E 440ii) | Pectins (INS 440) | |
| Definition | Pectin consists mainly of the partial methyl esters of polygalacturonic acid and their ammonium, sodium, potassium and calcium salts. It is obtained by extraction in an aqueous medium of strains of appropriate edible plant material, usually citrus fruits or apples. No organic precipitant shall be used other than methanol, ethanol and propan‐2‐ol | Amidated pectin consists mainly of the partial methyl esters and amides of polygalacturonic acid and their ammonium, sodium, potassium and calcium salts. It is obtained by extraction in an aqueous medium of appropriate strains of edible plant material, usually citrus fruits or apples and treatment with ammonia under alkaline conditions. No organic precipitant shall be used other than methanol, ethanol and propan‐2‐ol | Consists mainly of the partial methyl esters of polygalacturonic acid and their sodium, potassium, calcium and ammonium salts; obtained by extraction in an aqueous medium of appropriate edible plant material, usually citrus fruits or apples; no organic precipitants shall be used other than methanol, ethanol and isopropanol; in some types, a portion of the methyl esters may have been converted to primary amides by treatment with ammonia under alkaline conditions. Sulfur dioxide may be added as a preservative |
| Assay | Content not less than 65% of galacturonic acid on the ash‐free and anhydrous basis after washing with acid and alcohol | Content not less than 65% of galacturonic acid on the ash‐free and anhydrous basis after washing with acid and alcohol | Not less than 65% of galacturonic acid calculated on the ash‐free and dried basis |
| Description | White, light yellow, light grey or light brown powder | White, light yellow, light greyish or light brownish powder | White, yellowish, light greyish or light brownish powder |
| Identification | |||
| Solubility | Soluble in water forming a colloidal, opalescent solution. Insoluble in ethanol | Soluble in water forming a colloidal, opalescent solution. Insoluble in ethanol | – |
| Test for pectins | – | – | Passes test |
| Test for amide group | – | – | Passes test (amidated pectins only) |
| Purity | |||
| Loss on drying | Not more than 12% (105°C, 2 h) | Not more than 12% (105°C, 2 h) | Not more than 12% (105°C, 2 h) |
| Acid insoluble ash | Not more than 1% (insoluble in approximately 3N hydrochloric acid) | Not more than 1% (insoluble in approximately 3N hydrochloric acid) | Not more than 1% |
| Degree of amidation | – | Not more than 25% of total carboxyl groups | Not more than 25% of total carboxyl groups of pectin |
| Sulfur dioxide | Not more than 50 mg/kg on the anhydrous basis | Not more than 50 mg/kg on the anhydrous basis | Not more than 50 mg/kg |
| Nitrogen content | Not more than 1.0% after washing with acid and ethanol | Not more than 2.5% after washing with acid and ethanol | Not more than 2.5% after washing with acid and ethanol |
| Total insolubles | Not more than 3% | Not more than 3% | Not more than 3% |
| Solvent residues | Not more than 1% of free methanol, ethanol and propan‐2‐ol, singly or in combination, on the volatile matter‐free basis | Not more than 1% of free methanol, ethanol and propan‐2‐ol, singly or in combination, on the volatile matter‐free basis | Not more than 1% methanol, ethanol and isopropanol, singly or in combination |
| Arsenic | Not more than 3 mg/kg | Not more than 3 mg/kg | – |
| Lead | Not more than 5 mg/kg | Not more than 5 mg/kg | Not more than 2 mg/kg for general use and 0.5 mg/kg for use in infant formula |
| Mercury | Not more than 1 mg/kg | Not more than 1 mg/kg | – |
| Cadmium | Not more than 1 mg/kg | Not more than 1 mg/kg | – |
The Panel noted that, in addition, JECFA made the following remarks: the commercial product is normally diluted with sugars for standardisation purposes. In addition to sugars, pectins may be mixed with suitable food‐grade buffer salts required for pH control and desirable setting characteristics. The article of commerce may be further specified as to pH value, gel strength, viscosity, degree of esterification and setting characteristics (JECFA, 2016b).
The Panel noted that limits for aluminium should be considered for inclusion in the EU specifications, as aluminium can be used in the manufacturing process (Section 3.1.3).
The Panel noted that, according to the EU specifications for pectin (E 440i) and amidated pectin (E 440ii), impurities of the toxic elements arsenic, lead, mercury and cadmium are accepted up to concentrations of 3, 5, 1 and 1 mg/kg, respectively. Contamination at such levels could have a significant impact on the exposure to these metals, for which exposure is already close to the health‐based guidance values or benchmark doses (lower confidence limits) established by EFSA (EFSA, 2009; EFSA CONTAM Panel, 2009, 2010, 2012).
In its report, JECFA (2014) noted that the Eighth Session of the Codex Committee on Contaminants in Foods (CCCF) agreed to a maximum level (ML) of 0.01 mg/kg for lead in infant formula (as consumed) (FAO/WHO, 2014). The Committee also noted that the use of pectin as a food additive at the proposed use levels could result in an exceedance of the ML for lead in infant formula. This situation was estimated to occur if lead were present in pectin at the specified limit of 5 mg/kg. This estimate was calculated without considering the contribution of other ingredients to the overall lead level in infant formulas. The Committee also noted that the responsibility for ensuring that the final infant formulas comply with the ML for lead remains with infant formula producers. Furthermore, the Committee noted that data provided by the sponsors indicate that the food additive can be produced with lead levels below the limit of 5 mg/kg in pectin. Considering this, the Committee noted that a lower lead limit in the specifications, for instance 1 mg/kg pectin, would not result in the additive exceeding the ML for lead in the final infant formula (i.e. 0.01 mg/kg). According to the recent JECFA (2016a) evaluation, a limit of 0.5 mg/kg for lead was introduced for pectins for use in infant formula.
In view of the botanical origin of pectins, possible contamination with pesticides should be considered. The Panel considered it necessary to pay attention to the compliance of pectins raw material to the existing EU regulation on pesticides.
Because of both the botanical origin and the polysaccharidic nature of pectins, they can be a substrate of microbiological contamination. The Panel noted that no microbiological criteria were defined for pectins in the EU Regulation. The Panel also noted that the microbiological specifications for polysaccharidic thickening agents should be harmonised, and that for pectins, criteria for the absence of Salmonella spp., Escherichia coli, total aerobic microbial count (TAMC) and total combined yeast and mould count (TYMC), should be included in the EU specifications.
3.1.3. Manufacturing process
The most important raw materials for pectin production are sugar beets or citrus peels and apple pomace, which are available as by‐products of juice manufacturing. The raw material undergoes extraction in water which has been acidified with pure mineral acid (e.g. hydrochloric acid and nitric acid) or organic acid (e.g. citric acid or oxalic acid) to pH 1–3 at 50–90°C for 3–12 h. During extraction, limited depolymerisation and hydrolysis of methyl ester and acetate groups which are present in natural pectin takes place, and the pectin dissolves. The remaining insoluble plant material and undesirable components (e.g. colours) are removed by centrifugation and/or filtration in one or more stages. The pectin‐containing extract is passed through a cation‐exchange column, concentrated by evaporation and then precipitated with alcohol (methanol, ethanol or isopropanol). Alternatively to alcohol precipitation, aluminium salts are added to the extract, forming insoluble salts with pectin. The aluminium ions are removed by washing with acidified aqueous alcohol (Pilnik and Voragen, 1992; Rolin et al., 1998; Voragen, 2007). Commercial pectin products are often standardised (e.g. to a specific gelling strength) by the addition of food grade sugars. Further enzymatic processing of pectins by addition of small amounts of, e.g. esterases, polygalacturonases, pectin lyases and amylases is used in industry to clarify the extract or to facilitate the hydrolysis of glycosidic bonds or ester groups. These enzymes may come also from the raw material where they naturally also exist. For the production of low ester pectin types, the extract may be held for hours, allowing for the spontaneous hydrolysis of the ester groups with the enzymes from the raw material present in the extract. This process could be facilitated by temperature and by other added esterases. Different degrees of esterification or polymerisation can be produced through different enzymatic treatments. The enzymes are irreversibly inactivated later in the manufacturing process by low pH, high temperatures or alcohol precipitation. The precipitated pectin is washed with diluted alcohol to remove acid, salts and other impurities, before drying and milling. The Panel noted that, as a result of pectin manufacture, inactivated enzymes, either from the aqueous extract or added, could be present in the product in low amounts, together with other proteins from the raw material, in amounts ranging from 0% to 5%.
The Panel was informed by one interested party (IPPA, 2015) that aluminium‐based purification is no longer used. However, according to the information provided by some interested parties (IPPA, 2017), some commercial pectin types are produced using a traditional ‘salt flocculation process’ consisting in the addition of flocculation agents (calcium di‐chloride and/or aluminium trichloride), before the precipitation phase with ethanol or propan‐2‐ol. The Panel was informed by this interested party that the added cations (Al3+ and/or Ca2+) are removed after several purification steps using acidified alcohol/water solutions. Moreover, for the production of some low ester pectin types, some variations in the process are introduced, consisting in the treatment of the precipitated pectin with diluted alcohol followed by the washing, drying and milling steps. The alcohol used may be acidic by the addition of mineral acids or organic acids or the alcohol may be alkaline by addition of sodium‐ or potassium hydroxide or carbonate (in combination with ammonia, in the case of amidated pectin). The Panel noted that, as a consequence, residual aluminium could be present as a result of the processing of pectins using the ‘salt flocculation process’ method.
Amidated pectins (E 440ii) are produced by treatment of an alcoholic suspension with ammonia, which leads to conversion of carboxylate methyl esters to primary amides. By choosing proper conditions with respect to ammonia concentration, water activity and temperature, pectins with various proportions of amidated, methyl esterified and free carboxylate groups can be produced (Pilnik and Voragen, 1992; Rolin et al., 1998; Voragen, 2007). In addition, the Panel noted that amidated pectins are modified forms of pectins produced by an amidation process and therefore cannot be considered as natural compounds.
3.1.4. Methods of analysis in food
There are a number of analytical methods available for the determination of pectin in foodstuff, including gas chromatography (GC), high‐performance liquid chromatography (HPLC), electrophoresis and colorimetry.
Most of the methods are based on the determination of the degree of polymerisation and methylation, through the quantification of the number of galacturonic acid‐based units.
The extraction of pectin from different foodstuffs before analysis is influenced by extraction parameters such as pH, time, temperature, material/solvent ratio, type of solvent.
3.1.4.1. Gas‐chromatographic methods
An analytical method for pectin and other polysaccharides (agar, carrageenan, sodium alginate, locust bean gum, guar gum, gum arabic, gum tragacanth, xanthan, carboxymethyl cellulose, propylene glycol alginate, gum ghatti, tamarind, gum karaya and larch arabinogalactan) in a variety of foods like blancmange powder, glaze, ice cream, and cream cheese was described by Preuss and Thier (1982, 1983). According to this method, polysaccharides are extracted from the foodstuff, and then fat, starch, proteins and carbohydrates are removed by extraction or degradation. The resulting polysaccharide fraction is analysed by gas chromatography after hydrolysis with methanolic HCl, and derivatisation of the resulting monosaccharides with trimethylchlorosilane. The polysaccharides can be qualitatively identified by their characteristic monosaccharide pattern, and quantified via the single monosaccharide peaks. In the case of pectin, the major hydrolysis product is galacturonic acid. Recoveries for most of the thickeners and gums were about 60–85%, with a coefficient of variation of 2–8% (Preuss and Thier, 1982, 1983).
A similar analytical method without extraction from foodstuff was previously described by Mergenthaler and Scherz (1976). The monosaccharides resulting from hydrolysis were derivatised to their aldonitrilacetates and analysed by gas chromatography.
Mergenthaler and Scherz (1980) improved the separation of different polysaccharides. After extraction of fat and proteins, the polysaccharides are eluted through a chromatographic column filled with diethylaminoethyl (DEAE)‐cellulose. The resulting fractions can be further analysed either by thin‐layer chromatography or, after derivatisation, by GC.
According to the method described by Melton and Smith (2001) for the determination of the neutral sugars in the extracted pectin samples, pectins are hydrolysed with trifluoroacetic acid under nitrogen and then phenyl‐β‐d‐glucopyranoside is added as an internal standard. After filtration, the filtrate is evaporated with nitrogen and the sugars are silylated by adding N,N‐dimethylformamide and N,O‐bis(trimethylsilyl) trifluoroacetamide (BSTFA) before analysis with GC/mass spectrometry (MS) is carried out.
3.1.4.2. Liquid chromatographic methods
Hunziker and Miserez (1980) published a method for the quantitative determination of different polysaccharides using gel permeation chromatography (GPC). After extraction of fat, pectin is purified by precipitation with methanol, and the precipitate is further washed and dried. The polysaccharide is chromatographed as a 0.04% solution.
Koswig et al. (1997) have analysed pectin and other thickening agents in fruit preparations using high‐performance anion‐exchange chromatography with pulsed amperometric detection (HPAE‐PAD). Pectin is isolated from the food by ethanol precipitation, hydrolysed with sulfuric acid, and the resulting monosaccharides are analysed by HPAE‐PAD. The presence of galacturonic acid in the chromatogram proves the existence of pectin.
White et al. (1999) have used a high‐performance size‐exclusion chromatography (HPSEC) method as a rapid technique to determine the average molecular weight range of polygalacturonic acid commercially available as apple and citrus pectin, using a refractive index detector. The molecular weight was calculated using a calibration curve of log of the molecular weight versus the HPSEC retention time.
Quemener et al. (2000) determined pectin and other polysaccharides in several foods (yoghurt, gelling milk or pâté) by reversed phase HPLC. The polysaccharides are extracted from the food, hydrolysed with methanolic HCl, and the resulting methylglycosides are analysed by HPLC using refractometric detection. Recoveries were generally higher than 80%, with a detection limit of about 0.03%.
3.1.4.3. Electrophoretic methods
An electrophoretic method for qualitative and quantitative analysis of gelling agents in food (pudding, milk‐based baby food, sugar fruits, ice cream, ketchup, cream stabiliser) was published by Pechanek et al. (1982). After removal of fat, starch and proteins, the polysaccharides are precipitated and separated via electrophoresis using polyacrylamide gel, agarose gel or a cellulose acetate membrane. After electrophoresis, the gels are stained with toluidine blue. The polysaccharides are quantified using a scanner.
Similar electrophoretic methods, using silylated glass fibre as supporting material, were published by Bettler et al. (1985) and Schaefer and Scherz (1983). Both methods have been tested successfully on a number of foods (tomato sauce, tomato soup, grapefruit juice, ice cream powder mixtures, cacao powder, gelated fruits, meat sauce).
3.1.4.4. Spectrophotometric methods
The isolation method for pectins according to Melton and Smith (2001) has been applied by Müller‐Maatsch et al. (2016) in 26 food waste streams such as apple pomace, pumpkin kernel, whole apples, etc. It consists, briefly, in a cell wall isolation, with 25 g of sample being ground to a powder and mixed with 100 mL of 80% phenol/HEPES (4‐(2‐hydroxyethyl)piperazine‐1‐ethanesulfonic acid). A sequential extraction of soluble pectic polysaccharides was also performed, according to Melton and Smith, 2001. The cell wall samples (25 g) were treated with 50 mM trans‐1,2‐diaminocyclohexane‐N,N,N′,N′‐tetraacetic acid (CDTA) and potassium acetate buffer (pH 6,5). The supernatant was separated after centrifugation for 20 min at 3,220 g. The pellet was treated with 50 mM Na2CO3/20 mM NaBH4, centrifuged, neutralised and dialysed with water. The chelating agent‐soluble solids and dilute alkaline‐soluble solids solutions were freeze‐dried and the total pectin yield was taken as the combined weights.
For the determination of uronic acid content, the freeze‐dried pectin samples were hydrolysed by adding concentrated sulfuric acid according to the method of Melton and Smith (2001). After dilution with water and centrifugation, the supernatant was used for the colorimetric assay. The supernatant and the standard (galacturonic acid in water at different concentrations) were mixed with sulfamic acid/potassium sulfamate solution and sodium tetraborate/sulfuric acid solution. After heating the mixture 20 min at 100°C, NaOH was added, and the absorbance was measured at 525 nm.
List et al. (1985) analysed pectin in fruit juices and in commercial pectin preparations as a complex with m‐phenylphenol, which can be determined spectrophotometrically. The applied method is sensitive for uronic acids and is not affected by the presence of neutral mono‐ and disaccharides.
3.1.4.5. Tritimetric methods
Nazaruddin et al. (2013) have also described a tritimetric determination of methoxyl and anhydro‐uronic acid contents and degree of esterification in extracted pectins. The sample of pectin is mixed with ethanol, sodium chloride and deionised water. After pectin is dissolved, the mixture is titrated with 0.1 M NaOH, and phenol red as indicator. Subsequently, 0.25 M NaOH is added to de‐esterify pectin, and then 0.25 M HCL is added to neutralise NaOH. The solution is titrated again until the colour changes.
3.1.4.6. NMR and FTIR spectroscopic methods
Grassino et al. (2016) made the extraction and characterisation of pectin from tomato waste. Pectin was isolated from raw, dried and milled tomato waste using ammonium oxalate/oxalic acid as an extracting solvent. The extraction was performed at 60°C and 80°C during 24 h and 12 h in a second step with a new volume of extracting solvent. At the end, the mixture was filtered and pectins were precipitated with ethanol and dried at 40°C in a vacuum oven. For the determination of the pectin structure, the 1H nuclear magnetic resonance (NMR) spectra and Fourier transform infrared (FTIR) spectra are registered and compared with the spectra obtained from commercial apple pectin samples. The FTIR spectra show the presence of esterified forms of pectin, depending on the absorption bands.
The degree of methylation and acetylation can be measured according to the method described by Müller‐Maatsch et al. (2014). The freeze‐dried pectin samples extracted according to the method of Melton and Smith (2001) described previously, are combined with 0.4 M NaOH and centrifuged, followed by the addition of the internal standard solution (3‐(trimethylsilyl)propionate (TSP)‐d4) in D2O, used to quantify the methanol and the acetic acid. The supernatant is filtered and transferred in NMR‐tubes for measurement.
A simple and validated method has been developed by Müller‐Maatsch et al. (2014), for the determination of methylation, acetylation and feruloylation degree of pectin (isolated according to Melton and Smith, 2001) from various food sources. The pectin esters are hydrolysed in NaOH/D2O, and the obtained methanol, acetic acid and ferulic acid are directly measured by 1H NMR. This method presented high accuracy, repeatability and reproducibility and the time for the analysis was reduced compared to the conventional chromatography of titration‐based methods. This quantitative methodology can be applied to pectic polysaccharides from a wide range of sources with different physical properties and can detect small amounts of methanol, acetic acid and ferulic acid. It is more accurate than titration for the determination of the methylation degree, as there is not any confusion with other esterified forms in the pectin. Another advantage of this method is the structural information that can be obtained on other compounds possibly linked with ester bonds to pectin.
Overall, acceptable and reliable methods are available for the determination of pectin in foodstuff, which allow discrimination from other polysaccharides.
3.1.5. Stability of the substance, and reaction and fate in food
Pectins can interact with other food components in different ways, such as the hydrophobic interactions of methyl groups on adjacent pectins when jams are made from fruit and sugar with the addition of commercial pectins (BeMiller, 2007). The gelling mechanism of pectins includes several types of bonds: OH hydrogen bonds, NH2 hydrogen bonds, CH3 hydrophobic bonds, Na/K/Ca binding and the specific Ca‐binding mechanism of gelation for LMP based on the ‘egg‐box’ mechanism, in which a section of two pectin chains, which must be free of ester groups (homogalacturonic ‘smooth regions’ of different chains), is held together by a number of calcium ions (Einhorn‐Stoll et al., 2014).
Long‐term storage of citrus pectins under different humidity conditions at room temperature or a two‐week period at high temperature (60°C) and humidity (80%) (Joye and Luzio, 2000), altered the molecular parameters and some of the properties of the pectins in a large extent. Among these, browning, demethoxylation and depolymerisation can occur, resulting in an increased number of hydrophilic carboxyl and hydroxyl groups. This increase in the number of hydrophilic groups might increase their water‐binding capacity. Furthermore, the behaviour in thermal analysis and dissolution and the gel formation properties can be altered (May, 1990; Joye and Luzio, 2000).
The pectin–water interactions and their water‐binding capacity are influenced by the number and type of hydrophilic groups. In a carbohydrate polymer, each hydrophilic group can bind about one molecule of non‐freezing water (Hatakeyama and Hatakeyama, 1998; Einhorn‐Stoll et al., 2012). Pectins from different suppliers, produced from varying raw materials and under different processing conditions have also varying properties and water‐binding behaviour. In a study performed by Einhorn‐Stoll et al. (2014) in eight commercial pectins from three suppliers (three high‐methoxylated pectin (HMP) and five low‐methoxylated pectin (LMP)) stored in a chamber at 60°C and 80% humidity, the water content was, as expected, higher for HMP than LMP, and varied depending on the supplier. It was also reported that water‐binding was determined not only by molecular structure but also by pectin material properties, such as particle size and morphology, surface area and porosity. These properties depend on the preparation conditions.
Pectin is a negatively charged polysaccharide and therefore it can be used in milk drinks to avoid protein aggregation (flocculation) through its adsorption onto the casein micelles (Maroziene and de Kruif, 2000) which is strongly depending on pH, starting at pH 5.0 (Tuinier et al., 2002). It has been demonstrated that, in pectins with a degree of esterification (DE) of 61 ± 1% and in reconstituted milk from low‐heat skim milk powder, the attachment of pectin onto casein micelles is driven by electrosorption in multilayers, with thickness increasing as the pH decreases in the range of 3,5–5 (Tuinier et al., 2002). These authors also demonstrated the thermodynamic incompatibility of casein micelles/pectin mixture above pH 5 and the adsorption of pectins on casein micelles, leading to stabilisation below pH 5.
Kratchanova et al. (2004) have investigated the interaction of pectin with amino acids and other amino compounds in aqueous solution, demonstrating that pectin can interact with some amino acids as lysine, methyl glycine, guanidine and some amines at different temperatures. The authors stated that amidation or hydrolysis of the pectin ester groups, decarboxylation of free carboxyl groups, β‐elimination or hydrolysis of glycosidic bonds can occur also during thermal treatment of pectins and protein‐rich plant raw materials. The authors also showed the effect of microwave heating on the alcohol‐insoluble residue of peas that resulted in a reduction of the free amino groups of proteins.
Kar and Arslan (1999a) have studied the effect of sugars such as dextrose and maltose on the viscosity of pectin solutions. Pectin was obtained from orange peel with a DE of 74.46%, and therefore was a high‐methoxyl and rapid‐set pectin. Pectin was dissolved in a 0.1 M sodium phosphate buffer (pH = 7) and the pectin solutions were mixed with sugars to give solutions with a sugar content of 0–50 g/100 g. Maltose and dextrose increased the viscosity of the pectin solution up to a sugar concentration of 30%, through the formation of aggregates of pectin molecules. This is either due to polyhydroxyl combinations by the formation of cross‐linking hydrogen bonds between the hydroxyl groups of pectin and those of sugars, or to the dehydrating action of sugars, that prevents water from taking part in the hydrogen bonding with pectin, allowing pectin molecules to form cross‐linked bonds. The effect of dextrose in increasing pectin viscosity is higher than that of maltose, as this last one contains less primary alcohols that are more reactive.
To study the effect of L‐ascorbic acid on the viscosity of pectic solutions, the pectic solutions (0.1 M in phosphate buffer at pH = 7) were mixed with l‐ascorbic acid to give solutions with final concentrations of 0.33–3.3 mM l‐ascorbic acid (Kar and Arslan, 1999b). Orange peel pectin degrades due to the depolymerisation effect of l‐ascorbic acid, resulting in an increase in the specific fluidity of the pectin solution. The higher the concentration, the greater the increase. The specific viscosity of the pectin solution in the presence of 3.3 mM l‐ascorbic acid decreased by 66.7% after 2 h compared with the control solution. The oxidation of pectins by reducing agents such as l‐ascorbic acid in aqueous media is considered to be a free radical reaction, being the free radicals produced responsible for the depolymerisation.
Gelation of LMP with a degree of methylation of 50% is induced by lowering the temperature in the presence of Ca2+. It is caused by complexation of sections of two different pectin chains with the ions (Garnier et al., 1993). LMP can also gel in the absence of Ca2+ by lowering the pH below ~ 3.3 (Gilsenan et al., 2000). According to a study by Capel et al. (2006) on the gelation of LMP and partially amidated LMP as a function of pH and Ca2+ concentration, the acid‐induced gelation occurs below pH 3.5, while the gelation induced by calcium is most effective at pH > 4.5. In the intermediate range, both types of gelation occur. The acid‐induced gelation is enhanced by amidation.
Stability of pectin is depending on temperature and pH. Pectin has optimal stability at pH 3.5–4.0. Under acidic conditions and low temperatures, deacetylation, demethoxylation and hydrolysis of the polymer chain occur, while at temperatures above 50°C, cleavage of the glycosidic linkages becomes predominant (Pilnik and Voragen, 1992; Voragen, 2007).
In a shelf‐life stability test, three samples of high ester pectin were stored at 5, 25 and 40°C, and characteristic parameters were monitored during a period of 26–50 weeks. After 40 weeks, the DE had decreased by 10% at 40°C, while the samples stored at 5°C and 25°C were stable. After 49 weeks, viscosity had decreased by 40% at 40°C and by 5% at 25°C, while the sample stored at 5°C was stable. After 26 weeks, the gelling power had decreased by 10% at 40°C, while the pectin stored at 5°C and 25°C was stable (IPPA, 2010). In conclusion, the shelf‐life of pectins’ functionality is at least 25 weeks when stored at the recommended conditions (room temperature).
In a study on non‐enzymatic degradation, citrus pectin and pectate with degrees of esterification of 93% and < 5%, buffered from pH 4.0 to 8.5, were heated at 75, 85, 95 and 110°C for 300 min (Diaz et al., 2007). The major degradation mechanism was acid hydrolysis for pectate and β‐elimination for pectin. A summary of the observed changes is given in Table 2.
Table 2.
Effect of pH and heat (95°C) on pectate and pectin (Diaz et al., 2007)
| Loss of viscosity (%) | Loss of molecular weight (%) | |||
|---|---|---|---|---|
| pH 4.5 | pH 8.5 | pH 4.5 | pH 8.5 | |
| Pectate | 40 | 20 | 80 | 70 |
| Pectin | 20 | 60 | 40 | 84 |
3.2. Authorised uses and use levels
Maximum levels of pectins (E 440) have been defined in Annex II to Regulation (EC) No 1333/2008 on food additives, as amended. In this document, these levels are named maximum permitted levels (MPLs).
Currently, pectin (E 440i) and amidated pectin (E 440ii) are authorised food additives in the EU at quantum satis (QS) in almost all food categories listed in Table 3, apart from foods for infants and young children, fruit juices and fruit nectars (at levels of 3,000 up to 10,000 mg/kg). Pectin (E 440i) and amidated pectin (E 440ii) are included in the Group I of food additives authorised at QS.
Table 3.
MPLs of pectins (E 440) in foods according to Annex II to Regulation (EC) No 1333/2008
| Food category number | Food category name | E‐number/group | Restrictions/exceptions | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|---|
| 01.3 | Unflavoured fermented milk products, heat‐treated after fermentation | Group I | QS | |
| 01.4 | Flavoured fermented milk products including heat‐treated products | Group I | QS | |
| 01.6.2 | Unflavoured live fermented cream products and substitute products with a fat content of less than 20% | E 440 | QS | |
| 01.6.3 | Other creams | Group I | QS | |
| 01.7.1 | Unripened cheese excluding products falling in category 16 | Group I | Except mozzarella | QS |
| 01.7.5 | Processed cheese | Group I | QS | |
| 01.7.6 | Cheese products (excluding products falling in category 16) | Group I | QS | |
| 01.8 | Dairy analogues, including beverage whiteners | Group I | QS | |
| 02.2.2 | Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions | Group I | QS | |
| 02.3 | Vegetable oil pan spray | Group I | QS | |
| 03 | Edible ices | Group I | QS | |
| 04.2.1 | Dried fruit and vegetables | Group I | QS | |
| 04.2.2 | Fruit and vegetables in vinegar, oil, or brine | Group I | QS | |
| 04.2.4.1 | Fruit and vegetable preparations excluding compote | Group I | QS | |
| 04.2.4.2 | Compote, excluding products covered by category 16 | E 440 | Only fruit compote other than apple | QS |
| 04.2.5.1 | Extra jam and extra jelly as defined by Directive 2001/113/EC | E 440 | QS | |
| 04.2.5.2 | Jam, jellies and marmalades and sweetened chestnut purée as defined by Directive 2001/113/EC | E 440 | QS | |
| 04.2.5.3 | Other similar fruit or vegetable spreads | E 440 | QS | |
| 04.2.5.4 | Nut butters and nut spreads | Group I | QS | |
| 04.2.6 | Processed potato products | Group I | QS | |
| 05.1 | Cocoa and Chocolate products as covered by Directive 2000/36/EC | E 440 | As glazing agent only | QS |
| 05.1 | Cocoa and Chocolate products as covered by Directive 2000/36/EC | Group I | Only energy‐reduced or with no added sugar | QS |
| 05.2 | Other confectionery including breath freshening microsweetsa | Group I | QS | |
| 05.3 | Chewing gum | Group I | QS | |
| 05.4 | Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | Group I | QS | |
| 06.2.2 | Starches | Group I | QS | |
| 06.3 | Breakfast cereals | Group I | QS | |
| 06.4.2 | Dry pasta | Group I | Only gluten free and/or pasta intended for hypoproteic diets in accordance with Directive 2009/39/EC | QS |
| 06.4.4 | Potato gnocchi | Group I | Except fresh refrigerated potato gnocchi | QS |
| 06.4.5 | Fillings of stuffed pasta (ravioli and similar) | Group I | QS | |
| 06.5 | Noodles | Group I | QS | |
| 06.6 | Batters | Group I | QS | |
| 06.7 | Pre‐cooked or processed cereals | Group I | QS | |
| 07.1 | Bread and rolls | Group I | Except products in 7.1.1 and 7.1.2 | QS |
| 07.2 | Fine bakery wares | Group I | QS | |
| 08.3.1 | Non‐heat‐treated meat products | Group I | QS | |
| 08.3.2 | Heat‐treated meat products | Group I | Except foie gras, foie gras entier, blocs de foie gras, Libamáj, libamáj egészben, libamáj tömbben | QS |
| 08.3.3 | Casings and coatings and decorations for meat | Group I | QS | |
| 09.2 | Processed fish and fishery products including molluscs and crustaceans | Group I | QS | |
| 09.3 | Fish roe | Group I | Only processed fish roe | QS |
| 10.2 | Processed eggs and egg products | Group I | QS | |
| 11.2 | Other sugars and syrups | Group I | QS | |
| 11.4.1 | Table‐top sweeteners in liquid form | E 440 | QS | |
| 11.4.2 | Table‐top sweeteners in powder form | E 440 | QS | |
| 11.4.3 | Table‐top sweeteners in tablets | E 440 | QS | |
| 12.1.2 | Salt substitutes | Group I | QS | |
| 12.2.2 | Seasonings and condiments | Group I | QS | |
| 12.3 | Vinegars | Group I | QS | |
| 12.4 | Mustard | Group I | QS | |
| 12.5 | Soups and broths | Group I | QS | |
| 12.6 | Sauces | Group I | QS | |
| 12.7 | Salads and savoury‐based sandwich spreads | Group I | QS | |
| 12.8 | Yeast and yeast products | Group I | QS | |
| 12.9 | Protein products, excluding products covered in category 1.8 | Group I | QS | |
| 13.1.2 | Follow‐on formulae as defined by Directive 2006/141/EC | E 440 | Only acidified follow‐on formulae | 5,000 |
| 13.1.3 | Processed cereal‐based foods and baby foods for infants and young children as defined by Directive 2006/125/EC | E 440 | Only processed cereal‐based foods and baby foods | 10,000b |
| 13.1.3 | Processed cereal‐based foods and baby foods for infants and young children as defined by Directive 2006/125/EC | E 440 | Only gluten‐free cereal‐based foods | 20,000 |
| 13.1.4 | Other foods for young children | E 440 | 5,000b | |
| 13.1.5.1 | Dietary foods for infants for special medical purposes and special formulae for infants | E 440 | From birth onwards in products used in case of gastrointestinal disorders | 10,000 |
| 13.1.5.2 | Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC | E 440 | From birth onwards in products used in case of gastrointestinal disorders | 10,000 |
| 13.2 | Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5) | Group I | QS | |
| 13.3 | Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet) | Group I | QS | |
| 13.4 | Foods suitable for people intolerant to gluten as defined by Regulation (EC) No 41/2009 | Group I | Including dry pasta | QS |
| 14.1.2 | Fruit juices as defined by Directive 2001/112/EC and vegetable juices | E 440 | Only pineapple and passion fruit juice | 3,000 |
| 14.1.2 | Fruit juices as defined by Directive 2001/112/EC and vegetable juices | Group I | Only vegetable juices | QS |
| 14.1.3 | Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products | E 440 | Only pineapple and passion fruit | 3,000 |
| 14.1.3 | Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products | Group I | Only vegetable nectars | QS |
| 14.1.4 | Flavoured drinks | Group I | QS | |
| 14.1.5.2 | Other | Group I | Excluding unflavoured leaf tea; including flavoured instant coffee | QS |
| 14.2.3 | Cider and perry | Group I | QS | |
| 14.2.4 | Fruit wine and made wine | Group I | QS | |
| 14.2.5 | Mead | Group I | QS | |
| 14.2.6 | Spirit drinks as defined in Regulation (EC) No 110/2008 | Group I | Except whisky or whiskey | QS |
| 14.2.7.1 | Aromatised wines | Group I | QS | |
| 14.2.7.2 | Aromatised wine‐based drinks | Group I | QS | |
| 14.2.7.3 | Aromatised wine‐product cocktails | Group I | QS | |
| 14.2.8 | Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol | Group I | QS | |
| 15.1 | Potato‐, cereal‐, flour‐ or starch‐based snacks | Group I | QS | |
| 15.2 | Processed nuts | Group I | QS | |
| 16 | Desserts excluding products covered in category 1, 3 and 4 | Group I | QS | |
| 17.1c | Food supplements supplied in a solid form including capsules and tablets and similar forms, excluding chewable forms | Group I | QS | |
| 17.2c | Food supplements supplied in a liquid form | Group I | QS | |
| 17.3c | Food supplements supplied in a syrup‐type or chewable form | Group I | QS | |
| 18 | Processed foods not covered by categories 1–17, excluding foods for infants and young children | Group I | QS |
MPL: maximum permitted level.
May not be used in jelly mini‐cups.
E 410, E 412, E 414, E 415 and E 440 are authorised individually or in combination.
FCS 17 refers to food supplements as defined in Directive 2002/46/EC of the European Parliament and of the Council, excluding food supplements for infants and young children.
Table 3 summarises foods that are permitted to contain pectin (E 440i) and amidated pectin (E 440ii) and the corresponding MPLs as set by Annex II to Regulation (EC) No 1333/2008.
According to Annex III, Part 1 of Regulation (EC) No 1333/2008, pectin (E 440i) and amidated pectin (E 440ii) are also authorised as carriers in all food additives at QS. According to Annex III, Part 3 of Regulation (EC) No 1333/2008, they are also authorised as food additives (including carriers) in food enzymes at QS.
In addition, according to Annex III, Part 5, Section A of Regulation (EC) No 1333/2008, pectin (E 440i) and amidated pectin (E 440ii) are also authorised to be used as food additives (including carriers) in all nutrients at QS, except for nutrients intended to be used in foodstuffs for infants and young children listed in point 13.1 of Part E of Annex II. However, according to Annex III, Part 5, Section B, they are authorised as food additives in all nutrients intended to be used in foodstuffs for infants and young children listed in point 13.1 of Part E of Annex II under the condition that the maximum level in foods mentioned in point 13.1 is not exceeded. The food categories in which this use is permitted are follow‐on formulae and processed cereal‐based foods and baby foods for infants and young children as defined by Directive 2006/125/EC.
3.3. Exposure data
3.3.1. Reported use levels or data on analytical levels
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment, especially for those food additives for which no MPL is set and which are authorised according to QS.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued public calls,7 , 11 for occurrence data (usage level and/or concentration data) on pectins (E 440). In response to these calls, use levels and analytical of data on pectin (E 440i) and amidated pectin (E 440ii) were submitted to EFSA by industry and Member States, respectively.
3.3.2. Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with updated data on use levels of pectin (E 440i) (n = 281) and amidated pectin (E 440ii) (n = 288) in foods for 53 and 38 food categories, respectively, out of the 81 food categories in which they are authorised.
Updated information on the actual use levels of pectin (E 440i) in foods was made available to EFSA by FoodDrinkEurope (FDE) [Documentation provided to EFSA, n. 5], the European Dairy Association (EDA) [Documentation provided to EFSA, n. 2], the European Federation of Health Products Manufacturers Associations (EHPM) [Documentation provided to EFSA, n. 3], the International Pectin Producers’ Association (IPPA) [Documentation provided to EFSA, n. 16], Specialised Nutrition Europe (SNE) [Documentation provided to EFSA, n. 20], the International Chewing Gum Association (ICGA) [Documentation provided to EFSA, n. 6] and the Association of the European Self‐Medication Industry (AESGP) [Documentation provided to EFSA, n. 1]. Updated information on the actual use levels of amidated pectin (E 440ii) in foods was made available to EFSA by FDE [Documentation provided to EFSA, n. 5], IPPA [Documentation provided to EFSA, n. 16] and ICGA [Documentation provided to EFSA, n. 6].
The Panel noted that a data provider (IPPA) is not a food industry (i.e. using pectins in their food products), but an association of food additive producers. Usage levels reported by food additive producers are not be considered at the same level as those provided by food industry. Food additive producers might recommend usage levels to the food industry, but the final levels might, ultimately, be different. Therefore, unless food additive producers confirm that the recommended levels are used by food industry, they are not considered in the refined exposure scenarios. Data from food additive producers will only be used in the maximum level exposure assessment scenario in case of QS authorisation and when no data are available from food industry. In this way, the most complete exposure estimates are calculated.
Usage levels related to the combination of different food additives, including pectins (E 406, agar; E 407, carrageenan; E 410, locust bean gum; E 412, guar gum; E 415, xanthan gum; E 440, pectins; E 1442, hydroxypropyl distarch phosphate), have been provided to EFSA in previous calls. These data are not presented or used in this opinion, since their use would have required assumptions with respect to the level of pectins compared to the other food additives.
Appendices A and B provide data on the use levels of pectin (E 440i) and amidated pectin (E 440ii) in foods as reported by industry.
3.3.3. Summarised data on concentration levels in food provided by Member States
In total, 20 analytical results were reported to EFSA by one country: Germany (n = 20). These data were referring to the food category FCS 14.1.2 (Fruit juices as defined by Directive 2001/112/EC and vegetable juices). Foods were sampled between 2010 and 2011 and all of them were analysed the year that they were collected.
Complete information on the methods of analysis (e.g. validation) was not made available to EFSA, but all samples were analysed in accredited laboratories. The Panel noted that one analytical result was reported in orange juice, in which pectins (E 440) are not authorised for direct addition, according to Annex II of Regulation (EC) No 1333/2008. It should, however, be noted that pectin is a natural constituent of citrus fruit (peel).
Overall, 19 analytical results reported for pectin (E 440i) in foods were considered by the Panel in the exposure assessment.
Appendix C shows the analytical results of pectins (E 440) in foods as reported by Member States.
3.3.4. Summarised data extracted from the Mintel Global New Products Database
The Mintel GNPD is an online database which monitors product introductions in consumer packaged goods markets worldwide. It contains information of over 2 million food and beverage products of which more than 900,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 20 out of its 28 member countries and Norway presented in the Mintel GNPD.12
For the purpose of this Scientific Opinion, the Mintel GNPD13 was used for checking the labelling of products containing pectins (E 440) within the EU's food products, as the Mintel GNPD shows the compulsory ingredient information presented in the labelling of products. The Panel noted that within the Mintel GNPD, the labelling of products with pectin (E 440i) or amidated pectin (E 440ii) is not differentiated.
According to Mintel, 463,856 foods, drinks and food supplement products were labelled with pectins (E 440) between 2011 and 2016.
Appendix D presents the percentage of the food products labelled with E 440 between 2011 and 2016, out of the total number of food products per food subcategory according to the Mintel food classification.
3.3.5. Food consumption data used for exposure assessment
3.3.5.1. EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). New consumption surveys recently14 added in the Comprehensive database were also taken into account in this assessment.9
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database represents the best available source of food consumption data across Europe at present.
Food consumption data from the following population groups: infants, toddlers, children, adolescents, adults and the elderly were used for the exposure assessment. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 4).
Table 4.
Population groups considered for the exposure estimates of pectins (E 440)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlers | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrena | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlya | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Sweden, UK |
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure estimates. In practice, the FoodEx food codes were matched to the FCS food categories.
3.3.5.2. Food categories selected for the exposure assessment of pectins (E 440)
The food categories in which the use of pectins (E 440) is authorised were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
Some food categories are not referenced in the EFSA Comprehensive Database and could therefore not be taken into account in the present estimate. This was the case for 12 food categories (Appendix E) and may have resulted in an underestimation of the exposure. The food categories which were not taken into account are described below (in ascending order of the FCS codes):
02.3 Vegetable oil pan spray
04.2.5.1 Extra jam and extra jelly as defined by Directive 2001/113/EC
06.4.4 Potato gnocchi
06.4.5 Fillings of stuffed pasta (ravioli and similar)
06.6 Batters
06.7 Pre‐cooked or processed cereals
08.3.3 Casings and coatings and decorations for meat
12.1.2 Salt substitutes
14.1.3 Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products, only vegetable nectars
14.2.5 Mead
14.2.7.2 Aromatised wine‐based drinks
14.2.7.3 Aromatised wine‐product cocktails
Foods for special medical purposes (FSMP) (FC 13.2 and FC 13.3) could not be considered because of very limited information on consumption levels being available in the EFSA Comprehensive database.
For the following food categories, the restrictions/exceptions which apply to the use of pectins (E 440) could not be taken into account, and therefore, the whole food category was considered in the exposure assessment. This applies to six food categories (Appendix E) and may have resulted in an overestimation of the exposure:
05.1. Cocoa and cocoa products, only energy‐reduced or with no added sugar and as glazing agent only
06.4.2 Dry Pasta, only gluten free and/or pasta intended for hypoproteic diets in accordance with Directive 2009/39/EC
08.3.2 Heat‐treated meat products, except foie gras, foie gras entier, blocs de foie gras, Libamáj, libamáj egészben, libamáj tömbben
09.3 Fish roe, only processed fish roe
11.4.1/11.4.2/11.4.3 Table‐top sweeteners in liquid form, powder form and in tablets
17.1/17.2/17.3 Food supplements, in solid, liquid, syrup‐type or chewable form.
No information on the usage levels of pectins (E 440) has been reported to EFSA for the following 18 food categories and therefore, these could not be taken into account (Appendix E). This may have resulted in an underestimation of the exposure, if E 440 is used in these food categories. Comparing these food categories with the data extracted from the Mintel database (information on the use of E 440 was found for categories: 04.2.5.4, 06.5, 11.2, 12.3, 12.7, 14.1.2, 14.1.3, 15.1, 15.2), the Panel noted that the use of pectins in these food categories was limited (Appendix E):
04.2.1 Dried fruit and vegetables
04.2.5.4 Nut butters and nut spreads
06.2.2 Starches
06.5 Noodles
10.2 Processed eggs and egg products
11.2 Other sugars and syrups
12.3 Vinegars
12.4 Mustard
12.7 Salads and savoury‐based sandwich spreads
12.8 Yeast and yeast products
13.1.5.1 Dietary foods for infants for special medical purposes and special formulae for infants
13.1.5.2 Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC
14.1.2 Fruit juices as defined by Directive 2001/112/EC and vegetable juices, only vegetable juices
14.1.3 Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products, only vegetable nectars
14.1.5.2 Other, excluding unflavoured leaf tea; including flavoured instant coffee
14.2.7.1 Aromatised wines
15.1 Potato‐, cereal‐, flour‐ or starch‐based snacks
15.2 Processed nuts
Considering that the FC 18 (Processed foods not covered by categories 1–17, excluding foods for infants and young children) is extremely unspecific (e.g. composite foods), processed foods prepared or composite dishes belonging to the FC 18 were reclassified under other food categories in accordance to their main component. Therefore, FC 18 is not taken into account as a contributor to the total exposure estimates.
Overall, 12 food categories were not taken into account in the exposure assessment because these are not referenced in the EFSA Comprehensive Database. Six food categories were included in the exposure assessment without considering the restrictions/exceptions as set in Annex II to Regulation (EC) No 1333/2008. Eighteen food categories were not taken into account because no information on the usage levels of pectins (E 440) has been reported to EFSA. For the refined exposure assessment scenario, 19 additional food categories were not taken into account because only concentration data provided by food additives manufacturers were made available to EFSA. For the remaining food categories, the refinements considering the restrictions/exceptions as set in Annex II to Regulation No 1333/2008 were applied.
Overall, for the maximum level exposure assessment scenario, 44 food categories were included, while for the refined exposure assessment scenarios, 23 food categories were included in the present exposure assessment to pectins (E 440) (Appendix E).
3.4. Exposure estimates
3.4.1. Exposure to pectins (E 440) from their use as food additives
The Panel estimated chronic exposure to pectins (E 440) for the following population groups: infants, toddlers, children, adolescents, adults and the elderly. A combined dietary exposure estimate was calculated for pectins (E 440), by selecting within each food category the highest level among the levels of each of the two types of pectins (E 440i and E 440ii).
Dietary exposure to pectins (E 440) was calculated by multiplying pectins (E 440) concentrations for each food category (Appendix E) with their respective consumption amount per kilogram of body weight for each individual in the Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only one day per subject were excluded, as they are considered as not adequate to assess repeated exposure.
This was carried out for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 4). On the basis of these distributions, the mean and 95th percentiles of exposure were calculated per survey and per population group. The 95th percentile of exposure was only calculated for those population groups where the sample size was sufficiently large to allow this calculation (EFSA, 2011a). Therefore, in the present assessment, the 95th percentile of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain were not included.
Exposure assessment to pectins (E 440) was carried out by the ANS Panel based on two different sets of concentration data: (1) maximum levels of use reported to EFSA (defined as the maximum level exposure assessment scenario) and (2) reported use levels and/or analytical data (defined as the refined exposure assessment scenarios). These two scenarios are discussed in detail below.
These scenarios do not consider the consumption of food supplements (FC 17.1, FC 17.2 and FC 17.3), which is covered in an additional refined exposure scenario detailed below (food supplements consumers only scenario).
As pectins (E 440) are also authorised in the food categories 13.1.5.1 and 13.1.5.2, an exposure assessment scenario taking into account these two food categories was performed to estimate the exposure of infants and toddlers who may eat and drink FSMP.
The consumption of FSMP is not reported in the EFSA Comprehensive database. To consider the exposure to food additives via consumption of these foods, the Panel assumed that the amount of FSMP consumed by infants and toddlers resembles that of comparable foods consumed by infants and toddlers from the general population. Thus, the consumption of FSMP categorised as FC 13.1.5 is assumed to be equal to that of formulae and food products categorised under food categories 13.1.2, 13.1.3 and 13.1.4.
FSMP consumed by other population groups (children, adolescents, adults and the elderly) may be very diverse; they cannot be considered because of very limited information on consumption and use levels.
A possible additional exposure to pectins (E 440) from their use directly by the consumer (adding pectins to thicken foods, e.g. jams, marmalades) and from their use as food additives authorised in accordance with Annex III to Regulation (EC) No 1333/2008 were not considered in any of the above exposure assessment scenarios.
3.4.1.1. Maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario is based on the MPLs as set in Annex II to Regulation (EC) No 1333/2008. As pectins (E 440) are authorised at QS in almost all food categories, a ‘maximum level exposure assessment’ scenario was estimated based on the maximum reported use levels provided by food industry or food additive producers, excluding exposure via food supplements and FSMP, as described in the EFSA Conceptual framework (EFSA ANS Panel, 2014). An additional scenario for FSMP consumers only was estimated as follows:
Consumers only of FSMP were assumed to be exposed to pectins (E 440) present at the maximum reported use level (data provided by food additive producers, reported values equal to the MPL) on a daily basis via consumption of the food categories 13.1.5.1 and 13.1.5.2. For the remaining food categories, the maximum reported use levels were used.
The Panel considers the exposure estimates derived following this scenario as the most conservative, as it is assumed that the population group will be exposed to pectins (E 440) present in food at the maximum reported use levels over a longer period of time, assuming that pectins are only used in the food categories for which data were submitted.
3.4.1.2. Refined exposure assessment scenarios
The refined exposure assessment scenarios are based on use levels reported by food industry and analytical results reported by Member States (for one FC: 14.1.2). These exposure scenarios can consider only food categories for which the above data were available to the Panel.
Appendix E summarises the concentration levels of pectins (E 440) used in the refined exposure assessment scenarios. Based on the available dataset, the Panel calculated two refined exposure estimates based on different model populations:
-
The brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to pectins (E 440) present at the maximum reported use/analytical level for one food category. This exposure estimate is calculated as follows:
-
1
— Combining food consumption with the maximum of the reported use levels for the main contributing food category at the individual level.
-
2
— Using the mean of the typical reported use levels for the remaining food categories. For FC 14.1.2, and specifically for pineapple fruit juice, the P75 of the analytical results was used for the exposure calculation (EFSA, 2011a).
-
1
The non‐brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to pectins (E 440) present at the mean reported use/analytical levels in food. This exposure estimate is calculated using the mean of the typical reported use levels or the mean of analytical results for all food categories.
-
‘Food supplement consumers only’ scenario: This scenario is estimated as follows:
-
1
— Consumers only of food supplements were assumed to be exposed to pectins (E 440) present at the maximum reported use level on a daily basis via consumption of food supplements. For the remaining food categories, the mean of the typical reported use levels was used.
-
1
As FC 17 does not consider food supplements for infants and toddlers, as defined in the legislation, exposure to pectins (E 440) from food supplements was not estimated for these two population groups.
3.4.1.3. Dietary exposure to pectins (E 440)
Table 5 summarises the estimated exposure to pectins (E 440) from their use as food additives in the different population groups (Table 4) according to the different exposure scenarios (Section 3.4.1). Detailed results per population group and survey are presented in Appendix F.
Table 5.
Summary of anticipated exposure to pectins (E 440) from their use as food additives in the maximum level exposure assessment scenario and in the refined exposure scenarios, in the different population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Maximum level exposure assessment scenario | ||||||
| Mean | 27.2–170.6 | 91.7–320.0 | 54.7–268.8 | 33.7–117.1 | 20.9–70.7 | 16.0–61.6 |
| 95th percentile | 64.8–628.9 | 272.4–549.4 | 112.0–518.4 | 66.1–233.7 | 45.9–142.9 | 36.1–116.3 |
| Refined estimated exposure assessment scenario | ||||||
| Brand‐loyal scenario | ||||||
| Mean | 5.4–43.6 | 29.8–167.9 | 23.0–146.1 | 10.8–66.3 | 6.1–32.3 | 4.3–20.8 |
| 95th percentile | 41.7–215.5 | 112.1–442.0 | 65.8–380.1 | 31.9–150.7 | 23.3–83.6 | 17.0–55.4 |
| Non‐brand‐loyal scenario | ||||||
| Mean | 2.5–37.6 | 19.7–99.5 | 16.2–87.7 | 8.9–46.5 | 4.3–22.6 | 2.8–15.6 |
| 95th percentile | 25.4–215.5 | 61.9–232.4 | 41.5–204.8 | 25.0–100.7 | 14.9–58.5 | 10.8–41.7 |
From the maximum level exposure assessment scenario, mean exposure to pectins (E 440) from their use as food additives ranged from 16.0 mg/kg bw per day in the elderly to 320.0 mg/kg bw per day in toddlers. The 95th percentile of exposure to pectins (E 440) ranged from 36.1 mg/kg bw per day in the elderly to 628.9 mg/kg bw per day in infants.
From the refined estimated exposure assessment scenario, in the brand‐loyal scenario, mean exposure to pectins (E 440) from their use as food additives ranged from 4.3 mg/kg bw per day in the elderly to 167.9 mg/kg bw per day in toddlers. The 95th percentile of exposure to pectins (E 440) ranged from 17.0 mg/kg bw per day in the elderly to 442.0 mg/kg bw per day in toddlers.
In the non‐brand‐loyal scenario, mean exposure to pectins (E 440) from their use as food additives ranged from 2.5 mg/kg bw per day in infants to 99.5 mg/kg bw per day in toddlers. The 95th percentile of exposure to pectins (E 440) ranged from 10.8 mg/kg bw per day in the elderly to 232.4 mg/kg bw per day in toddlers.
From the exposure scenario taking into account the foods for special medical purposes, for consumers only, mean exposure to pectins (E 440) from their use as food additives ranged from 111 mg/kg bw per day to 897 mg/kg bw per day for infants and from 93 mg/kg bw per day to 516 mg/kg bw per day for toddlers. The 95th percentile of exposure to pectins (E 440) for infants ranged from 797 mg/kg bw per day to 1,349 mg/kg bw per day, and for toddlers, from 364 mg/kg bw per day to 831 mg/kg bw per day. The food categories contributing the most at the exposure to pectins (E 440) at the mean, for infants, were foods for infants and young children, and unflavoured and flavoured fermented milk products for toddlers.
From the refined estimated exposure scenario taking into account the consumption of food supplements, for consumers only, among children, adolescents, adults and the elderly, mean exposure to pectins (E 440) from their use as food additives ranged from 7.2 mg/kg bw per day in the elderly to 188.8 mg/kg bw per day in children. The 95th percentile of exposure ranged from 28.4 mg/kg bw per day in the elderly to 169.1 mg/kg bw per day in children.
3.4.1.4. Main food categories contributing to exposure to pectins (E 440) using the maximum level exposure assessment scenario
In the maximum level exposure assessment scenario, the main contributing food categories to the mean exposure estimates for infants were foods for infants and young children and unflavoured fermented milk products. For toddlers, the main contributing food categories were unflavoured and flavoured fermented milk products. For children and adolescents, the main contributing food categories were flavoured fermented milk products, meat products and flavoured drinks; for adults, meat products and flavoured drinks; and for the elderly, meat products.
The main food categories contributing to the combined exposure to pectins (E 440) using the maximum level exposure assessment scenario are presented in Appendix G.
3.4.1.5. Main food categories contributing to exposure to pectins (E 440) using the refined exposure assessment scenarios
In the brand‐loyal scenario, the main contributing food categories were flavoured fermented milk products and processed fruit and vegetables for infants and the elderly; for toddlers, children, adolescents and adults, the main contributing food categories were flavoured fermented milk products and flavoured drinks.
In the non‐brand‐loyal scenario, the main contributing food categories were: flavoured fermented milk products and processed fruit and vegetables for infants; flavoured fermented milk products and desserts for toddlers and children; flavoured fermented milk products and flavoured drinks for adolescents and adults; processed fruit and vegetables and desserts for the elderly.
The main food categories contributing to the combined exposure to pectins (E 440) using the refined exposure assessment scenarios are presented in Appendix G.
3.4.1.6. Uncertainty analysis
Uncertainties in the exposure assessment of pectins (E 440) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 6.
Table 6.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/– |
| Use of data from food consumption survey of a few days to estimate long‐term (chronic) exposure for high percentiles (95th percentile) | + |
| Correspondence of reported use levels and analytical data to the food items in the EFSA Comprehensive Food Consumption Database: uncertainties to which types of food the levels refer to | +/– |
| Uncertainty in possible national differences in use levels of food categories | +/– |
| 3–99% of the amount of food consumed taken into account in the refined exposure assessment scenarios out of all authorised food (n = 23/81 total number of food categories) | – |
| Food categories selected for the exposure assessment: exclusion of food categories due to missing FoodEx linkage (n = 12/81 food categories) | – |
| Food categories selected for the exposure assessment: inclusion of food categories without considering the restriction/exception (n = 6 for the maximum level scenario/n = 6 for the refined scenarios, out of 81 food categories) | + |
| Food categories included in the exposure assessment: data not available for certain food categories which were excluded from the exposure estimates (n = 18 for the maximum level scenario/n = 37 for the refined scenarios, out of 81 food categories). Information on the use of pectins was found in the Mintel database for 9 of these categories. | – |
Concentration data:
|
+ +/− |
Maximum level exposure assessment scenario:
|
+ − |
|
Refined exposure assessment scenarios:
|
+/− − − |
|
Food supplements consumers only scenario:
|
+ − − |
|
FSMP, consumers only scenario:
|
+ + − |
FSMP: foods for special medical purposes; MPL: maximum permitted level.
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
Pectins (E 440) are authorised as a Group I food additive in 67 food categories and have specific authorised uses in 14 other categories (Table 3). Since the majority of food categories correspond to the general Group I food additives authorisation, pectins (E 440) may not necessarily be used in some of these food categories. This may explain why use levels for pectins (E 440) were not reported by the food industry for 18 food categories. However, the Panel noted that information from the Mintel GNPD (Appendix D) indicated that some of these 18 food categories were labelled with pectins (noodles, vinegar, spreads, etc.).
Overall, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the real exposure to pectins (E 440) as food additives in European countries considered in the EFSA European database for the maximum level exposure assessment scenario and for the refined scenarios, when considering only food additive uses for which data have been provided.
However, the Panel noted that, given the information from the Mintel GNPD, it may be assumed that pectins (E 440) are used in several food categories (n = 9) for which no data have been provided by food industry. The Panel noted that out of these nine food categories, fruit juices and fruit nectars are products highly consumed. Therefore, if this would be confirmed, it would result in an underestimation of the exposure.
The Panel noted that food categories which may contain pectins (E 440) due to carry‐over (Annex III, Part 1, 3, 5 (Sections A and B)) were not considered in the current exposure assessment.
Concerning the exposure to pectins (E 440) of infants and young children eating FSMP, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure in European countries.
3.4.2. Exposure to pectins via the regular diet
To estimate dietary intake to pectin from the regular diet, the Panel considered data on pectin content used in the EFSA scientific Opinion on the re‐evaluation of aspartame (E 951) (Appendix B‐III) (EFSA ANS Panel, 2013). In average, across population groups, estimates of dietary intake to pectin from the regular diet ranged from 2 to 14 mg/kg bw per day and from 6 to 38 mg/kg bw per day at the high level.
3.4.3. Exposure via other sources
This re‐evaluation refers exclusively to the use of pectins (E 440) as food additives in food, including food supplements, and does not include a safety assessment of other uses of pectins.
3.4.3.1. Pectins as ingredient in slimming products
Pectins are described as ingredients in slimming products assuming a capacity to reduce the rate of digestion by immobilising food components in the intestine, resulting in lower nutrient absorption (Srivastava and Rishabha, 2011).
Cause and effect relationships have been established between the consumption of pectins and a reduction of post‐prandial glycaemic responses and maintenance of normal blood cholesterol concentrations. In order to obtain these physiological effects, 10 g of pectins per meal or 6 g/day of pectins, in one or more servings, respectively, are required (EFSA NDA Panel, 2010; Brouns et al., 2012).
3.4.3.2. Pharmaceutical uses
From data provided by the European Medicines Agency (EMA), information about the current medicinal usage of pectins and their usage as an excipient, was retrieved [Documentation provided to EFSA, n. 4].
Pectin is used as an active ingredient in antidiarrheal medicinal products and for the treatment of regurgitation in infants due to its water‐binding properties (Brejnholt, 2010; Srivastava and Rishabha, 2011; Martindale, 2016). According to EMA, several combination products exist, in which pectin is one of the active ingredients. The indications are unspecific diarrhoea in children and adults or symptomatic treatment of regurgitation in babies. Special warnings are not mentioned.
Pectin is used in medicinal products mainly as an excipient, due to its gelling and thickening properties. This function of pectin is described as carrier for drug delivery to the gastrointestinal tract, as matrix in tablets, gel beads and film‐coated pills. Pectin is used in controlled‐release formulations, also together with other polymers. Pectin is considered as a carrier material in colon‐specific drug delivery systems (for systemic action or local treatment in diseases such as ulcerative colitis, Crohn's disease, colon carcinomas) (Brejnholt, 2010; Srivastava and Rishabha, 2011).
3.4.4. Exposure from all sources
To estimate dietary intake of pectin from all sources, the exposure via the use of pectins as food additive at the mean level (non‐brand loyal scenario) for the age group of toddlers (Table 5) and exposure to pectins via the regular diet (Section 3.4.2) were considered. Based on these values, the use of pectins as food additives is contributing seven times more (87.7% from the use of pectins as food additive versus 12.3% from the intake via the regular diet) at the mean level, and at the high level, six times more (86% from the use of pectins as food additive versus 14% from the intake via the regular diet), to the exposure from all sources, when compared to the exposure from the regular diet.
3.4.5. Exposure to methanol
In human volunteers, consumption of 10–15 g isolated pectin or of 1 kg apples (containing approximately 10 g natural pectin) induced a significant increase in methanol in the breath and, by inference, in the blood. Consumption of 1 kg apples was estimated to release 500 mg methanol. It has been estimated that humans may be exposed to approximately 1,000 mg methanol per day from fruits and vegetables; riper fruit was found to release more methanol than unripe fruit (COT, 2011).
An exposure assessment to methanol from the intake of pectins present in the regular diet was prepared by the ANS Panel in the frame of the re‐evaluation of aspartame (E 951) as a food additive (EFSA ANS Panel, 2013). The Panel estimated the anticipated combined exposure to methanol from endogenous sources (basal endogenous pathway, endogenously metabolised pectin) and exogenous sources (aspartame as a food additive and natural food occurrence) in five population groups (toddlers, children, adolescents, adults and the elderly).
Exposure to methanol from natural occurrence of pectins in food ranged from 0.2–1.4 mg/kg bw per day at the mean to 0.6–3.8 mg/kg bw per day for high level consumers (EFSA ANS Panel, 2013).
In addition to the direct contribution of methanol from fruits and vegetables, there is clear evidence for the internal production of methanol in the gastrointestinal tract from ingested pectins and endogenous production in other parts of the body, such as liver and brain (WHO, 1997). The Panel estimated the internal exposure to methanol due to endogenous sources to range from 7.6 to 18 mg/kg bw per day at the mean and from 14.1 to 33.1 mg/kg bw per day at the high level (EFSA ANS Panel, 2013).
The exposure to methanol from all endogenous pathways (basal, endogenously metabolised pectin) and natural food occurrence of pectins ranged from 7.8 to 19.4 mg/kg bw per day at the mean and from 14.8 to 36.9 mg/kg bw per day at the high level.
Exposure to methanol from pectins used as food additives was calculated based on the degradation factor from pectin to methanol (Lindinger et al., 1997; EFSA ANS Panel, 2013) to be up to 9 mg/kg bw per day for the age group of toddlers at the mean (non‐brand loyal scenario).
Overall, the exposure to methanol from all sources (endogenous pathways, natural occurrence and pectins used as food additives), ranged from 9.6 to 28.4 mg/kg bw per day at the mean.
3.4.5.1. Formaldehyde
The Panel assumed that all methanol was converted into formaldehyde, which then formed a formaldehyde acetal with water. The increase in formaldehyde acetal associated with 9.6–28.4 mg/kg per day methanol given as a single dose (the Panel noted that this was an unlikely scenario for dietary pectin) would result in the same increase of less than 7% and less than 35% (for the steady state level) of the normal intracellular endogenous formaldehyde level (EFSA ANS Panel, 2013).
This can be used to make an estimate of the additional burden of formaldehyde acetal associated with the intake of pectin. Such an additional burden should be evaluated in the light of the naturally occurring interspecies and intraspecies variation in the internal level of methanol, formaldehyde and formaldehyde acetal, which by far exceed the difference between internal concentration of these endogenous substances and the additional exposure by oral intake of pectin. Kleinnijenhuis et al. (2013) using a sensitive and specific method have measured a formaldehyde concentration in blood of 2.25 ± 0.67 mg/L in rats. This corresponds to a coefficient of variation of 30% in endogenous formaldehyde blood levels in rats (EFSA ANS Panel, 2013).
3.5. Biological and toxicological data
3.5.1. Absorption, distribution, metabolism and excretion
There is evidence that certain high molecular weight dietary polysaccharides, such as pectins, starches and gums, could be partially broken down in the large intestine of man. In addition to intermediate metabolites, such as lactic, acrylic or fumaric acid, the main end products of this colonic anaerobic digestive process are short‐chain fatty acids (SCFA), such as acetic, propionic and butyric acids, which are absorbed from the colon (Cummings and Englyst, 1987).
3.5.1.1. In vitro studies
Kertesz (1940) conducted in vitro tests to determine the possible digestion of citrus pectin by various digestive enzymes of dog and human origin. Both saliva and gastric juice do not contain enzymes acting upon pectin. Pectin passed through the stomach and part of the small intestine of a dog and could be recovered without loss. Trypsin, pepsin and rennet had no effect on pectin in vitro, but pectin incubated with faeces was rapidly degraded. According to the author, it appeared probable that pectin taken orally is not attacked until it reaches the large intestine, where it is completely hydrolysed by bacterial enzymes.
Bacteria capable of metabolising pectin in vitro were isolated from dog faeces (Werch et al., 1942). The more active bacteria belonged to the following groups: Aerobacillus, Lactobacillus, Micrococcus and Enterococcus. These bacteria contained heat‐labile, pectinase‐like enzymes and/or pectase‐like (coagulating) enzyme. The main products of degradation by the bacterial enzymes were formic and acetic acids. Galacturonic acid seemed to be an intermediary product.
A total of 188 strains of 10 species of Bacteroides found in the human colon were surveyed for their ability to ferment mucins and plant polysaccharides (Salyers et al., 1977a). Many of the Bacteroides strains tested were able to ferment a variety of plant polysaccharides, including pectin, amylose, dextran and gums. The ability to utilise mucins and plant polysaccharides varied considerably among the Bacteroides species tested. Pectin (Sunkist Inc., USA) was shown to be fermented by five species of Bacteroides.
A total of 154 strains of 22 species of Bifidobacterium, Peptostreptococcus, Lactobacillus, Ruminococcus, Coprococcus, Eubacterium and Fusobacterium, which are present in high concentrations in the human colon, were surveyed for their ability to ferment 21 different complex carbohydrates (Salyers et al., 1977b). Among them, pectin (Sunkist Inc., USA) was only fermented by strains of Eubacterium eligens.
Fermentation of 10 polysaccharides, including pectin, identified as polypectate, by species of the family Enterobacteriaceae (Klebsielleae and other gram‐negative facultative bacteria) was examined by Ochuba and Von Riesen (1980). Polypectate (Sigma, USA) was fermented by three species (Klebsiella, Pectobacterium and Yersinia strains).
Adiotomre et al. (1990) investigated the effects of dietary fibres, including pectin, on caecal fermentations, by using fresh human microflora. Evolution of SCFA and water‐holding capacity after fermentation were also measured. Among other fibres, pectin (E 440 grade) yielded the largest amount of total SCFA (82.2 vs 15.5 mmol/L for controls). The major SCFA produced were acetic, propionic and butyric acid, with smaller amounts of isobutyric, valeric, and isovaleric acids. By contrast, the amount of water held by 1 g of the fermented residue was lower in the case of pectin in comparison to other fibres (2.15 vs 0.91 g/g for controls).
A total of 290 strains of 29 species of bifidobacteria of human and animal origin (mainly of faecal origin) were tested for their ability to ferment complex carbohydrates (Crociani et al., 1994). Pectin (Sigma, USA) was fermented by 11 species out of the 29 tested.
In another in vitro study, citrus pectin (TIC Gums, USA) was fermented using dog faeces as the source of inoculum (Sunvold et al., 1995a). Organic matter disappearance and SCFA production were measured after 6, 12 or 24 h of incubation. Irrespectively of the duration of incubation, organic matter disappearance and acetic, propionic and butyric acid production were higher for pectin than for other polysaccharides like gums, rice bran, oat fibre or beet pulp. Identical conclusions were drawn from a similar study using the same substrates fermented by cat faecal microflora (Sunvold et al., 1995b).
In vitro experiments gave information on the metabolic pathway of citrus pectin (Dongowski and Anger, 1996). In these studies, a 150 mL pectin solution (DE: 34.4%; containing 5 mg/mL galacturonan; Copenhagen Pectin A:S, Lille Skensved) was incubated with human faecal microflora in 4 g faeces at 37°C for 24 h. Samples were collected after 4, 6, 8, 12 and 24 h, and analysed. During degradation by the microflora, the pectin concentration diminished continuously. The fraction of oligogalacturonic acids increased in the first 8 h and decreased afterwards. No pectin or oligogalacturonic acid was measured after 24 h. Accordingly, the concentration of SCFA (typical products of fermentation of dietary fibres) increased and reached a maximum after 24 h. The Panel noted the relevance of these data, since oligogalacturonic acids formed in this study from pectin via fermentation with human faecal microflora are similar to galacto‐oligosaccharides manufactured as pAOS.
Knaup et al. (2008) investigated the human gastrointestinal metabolism of amidated pectin (DE 30%, degree of amidation 20%; Herbstreith and Fox, Germany) and d‐galacturonic acid (the major intestinal metabolite of pectin; Fluka, Germany). A number of in vitro experiments were carried out. Both substrates were incubated under aerobic conditions at 37°C using saliva (5 mg test item in 2.5 mL diluted saliva, exposure duration 2 min) collected from three healthy subjects (23–35 years old) or artificial gastric juice (5 mg test item in 2.5 mL diluted juice, exposure duration 4 h). Under anaerobic conditions, 5 mg amidated pectin or 5 mg d‐galacturonic acid were incubated for up to 10 h at 37°C with 2.5 mL filtered human ileostomy fluid provided by three healthy subjects (34–39 years old; donors 1–3) with a terminal ileostomy, undergone colectomy 5–6 years prior to the study. Both substrates were also incubated under the same conditions for up to 24 h with filtered colostomy fluids obtained from three donors (30–60 years old; donors 4–6) with left‐sided colostomies, undergone colectomy 12–30 years prior to the study. Amidated pectin, d‐galacturonic acid, SCFA (acetic, propionic and butyric acid), as well as methanol concentrations, were analysed. Amidated pectin and d‐galacturonic acid were found to be stable during incubation with saliva and simulated gastric juice. In contrast, both test items underwent degradation in the course of incubation with ileostomy and colostomy fluid. Amidated pectin was only degraded in part, revealing stable amounts of 22–35% in ileostomy fluid after 10 h and 3–17% in colostomy fluid after 24 h. SCFA were generated up to 59% of the applied amidated pectin. In parallel, 19–60% (ileostomy fluid) and 52–67% (colostomy fluids) of the available methyl ester groups were cleaved in the course of incubations. d‐Galacturonic acid was practically completely decomposed within 10 h and SCFA (mainly acetic acid) were formed up to 98% of the incubated substrate in colostomy inoculum. Complete degradation of d‐galacturonic acid was observed (after 6 h of incubation) using the ileostomy fluids from donors 1 and 2, whereas a partial degradation (after 10 h) occurred using the ileostomy fluids from donor 3. Methanol liberation was observed, reaching concentrations of up to 16, 36 or 53 mg/L in donor 3, 2 and 1, respectively. In conclusion, amidated pectin and d‐galacturonic acid are stable in human saliva and simulated gastric juice. The expected partial fermentation of amidated pectin and the complete degradation of d‐galacturonic acid by the microflora of the colon were shown. The Panel noted that, in this study, the low degree of fermentation of amidated pectin in ileostomy fluid might be related to altered microflora of the ileum 6 years after terminal ileostomy.
3.5.1.2. In vivo studies
Animal studies
Rats
The digestibility of pectin was estimated in a subchronic feeding study (Til et al., 1972) by measuring the faeces dry matter (pectin dry matter = total dry matter minus dry matter from basal diet and cellulose) in weanling rats (groups of 10 males and 10 females). After 11 weeks of exposure to 5%, 10% or 15% pectin in the diet, the amount of indigestible pectin (presumably not degraded by the microflora) ranged between 25% and 40%.
The metabolism and fate of pectin‐labelled plant cell walls (PCW) was followed in male Wistar rats (Buchanan et al., 1994). The PCW were 14C‐labelled in the galacturonic acid residues (using d‐[6‐14C]glucuronic acid, more than 85% of the 14C was confined to the C6 position of pectic galacturonic acid residues) or in the methyl ester groups (using l‐[methyl‐14C]methionine, 90% of the 14C was confined to methyl ester groups and 10% of the 14C in methyl ether groups). Four rats were dosed by gavage with 1 mL suspension containing 5 mg 14C‐PCW. Each rat was maintained in a metabolic cage to collect CO2, faeces and urine. The study was terminated after 18 h, rats sacrificed and radioactivity in excreta and rat body measured. When [uronate‐6‐14C]pectin was used, only small amounts of radioactivity were detected in stomach (0.4% of applied radioactivity), small intestine (0.4%), caecum (1.6%) and colon (0.7%). In faeces, 5.8% of the applied radioactivity was recovered. Most of the applied radioactivity was detected in exhaled CO2 (37.4%) and in body tissues (20.8% in removed organs and in the remaining carcass), while 2.2% was excreted via urine. The total recovery was 69.3%. Thus, 87.7% of the recovered pectin‐labelled PCW was metabolised in the rat and not recovered in the gut contents or faeces. In rats receiving [methyl‐14C]pectin, similar data were obtained in the gastrointestinal tract (0.3, 0.6, 0.6 and 0.5% of applied radioactivity detected in the stomach, small intestine, caecum and colon, respectively), in faeces (3.0%) and urine (1.9%). However, less radioactivity was recovered as exhaled in CO2 (18.1%) and more distributed in body tissues (59.8%). In these animals, the total recovery was 84.6%. According to the authors, these results demonstrated that PCW polysaccharides in pectin are extensively degraded in the rat gut. The authors stated that the material derived from the polysaccharides may enter specific metabolic pathways in rats and provide contributions to both energy and precursors of structural compounds.
The absorption of oligogalacturonic acids in rats was demonstrated in a preliminary study (Anger et al., 1994, data from abstract, no further details available). About 9–45% of oligogalacturonides injected directly into the caecum of rats was recovered in urine within 16 h after application, suggesting absorption of the oligogalacturonides.
In the same laboratory, Dongowski and Anger (1996) compared the degradation of pectins in the gastrointestinal tract in germ‐free rats (without intestinal microflora) and in conventional rats. Groups of 10 conventional rats or six germ‐free rats (no further details about test species) were fed 0% or 6.5% pectin in the diet (with different degrees of esterification: 34%, 71% or 93%) for 21 days. Germ‐free rats received sterilised diet. Faeces were collected during a 3‐day period at the end of week 2 and week 3. At termination, the content of ileum, caecum and colon were also analysed. In germ‐free rats, no relevant decrease in the degree of esterification was found in ileum, caecum and colon, independently of the degree of esterification; similar results were reported in the ileum of conventional rats (no data on caecum and colon). When compared to conventional rats, high amounts of pectin (measured as galacturonan) were detected in caecum, colon and faeces of germ‐free rats. In conventional rats, no oligogalacturonic acids were identified in the faeces, but unsaturated di‐ and trigalacturonic acids were identified in the colon of a few rats. The authors concluded that pectin passes through the small intestine as a macromolecule and is intensively degraded by the action of the microflora in the large intestine. The intermediate products of degradation are oligogalacturonic acids which could be absorbed by the host or further metabolised by the microflora. Pectins with low degree of esterification were degraded faster than high‐esterified pectins.
Dogs
Werch and Ivy (1941) compared the metabolism of citrus pectin (California Fruit Growers Exchange Research Laboratories, free of pentoses, pentosans and colours) in four normal dogs and in two dogs with ileostomy. The normal dogs received 20 g pectin mixed with the diet for 7 days. Faeces were collected and analysed by three different methods. The two dogs with ileostomy were fed 20 g pectin mixed with beef. Faeces were collected for the next 24 h. In normal dogs, 3–9% of ingested pectin was recovered, depending on the analytical method. In dogs with ileostomy, more than 85% of ingested pectin was recovered in excreta, independently of the analytical method used. These results indicated that a nearly complete breakdown of pectin would occur in the large intestine of normal dogs. According to the authors, bacterial enzymes are involved, rather than the enzymes of the animal organism.
Human studies
Werch and Ivy (1941) compared pectin metabolism in six healthy volunteers with data from two patients with ileostomy (no further details about subjects). The study was performed under controlled dietary conditions. Each healthy subject ingested, once, 50 g pure citrus pectin (no further details) in gelatine capsules, whereas ileostomy patients received 20 g of pectin with a low‐crude fibre diet. Samples of faeces and excreta were analysed by three different methods. In healthy subjects, 4% of ingested pectin was recovered by the pectic acid method, 14% by the uronic acid method and 12% by the furfural method. In ileostomy patients, more than 85% of ingested pectin was recovered in excreta, independently of the method used. According to the authors, these results indicated that in healthy volunteers, nearly complete breakdown of pectin occurs in the large intestine rather than in the small intestine, and that bacterial enzymes are involved, rather than endogenous enzymes.
The digestion of pectin in the human gut was examined in a group of five healthy male volunteers aged 21–24 years (Cummings et al., 1979). The subjects received a controlled diet over 3 weeks, which was supplemented for the next 6 weeks with 36 g pectin per day (National Formulary; pure high‐methoxy pectin; source: Bulmers Ltd.), followed by an ad libitum diet in week 10. The volunteers ingested the pectin in divided doses as a powder, mixed into either the food or drink of each meal. Throughout the 10 weeks, the subjects collected their stools, which were cooled to −20°C until analysis. During the control period, only 15% of the dietary fibre ingested was excreted in faeces. When pectin was added to the diet, there was no increase in fibre excretion. Stool frequency and mean transit time were unchanged, but stool wet weight increased by 33%, and faecal excretion of fatty acids increased by 80%. The authors concluded that pectin could not be recovered from the faeces, suggesting that it is totally metabolised in the gut, probably as the result of bacterial fermentation.
The digestibility of pectin in human subjects was also investigated by comparison of the results in a group of healthy volunteers (five men and five women; 21–35 years old) with data in patients (three men and three women; 25–49 years old) who had undergone total colectomy, followed by ileostomy, in the management of ulcerative colitis (Holloway et al., 1983). Both groups were given a fixed diet for 10 days. The pectin content of the individual foods of plant origin in the diet and the pectin content of the ileal excreta or faeces (sampling started after 4 days being on diet) were determined by measuring the uronic acid content. In ileostomy female subjects, 85% of ingested pectin was excreted, while in male subjects, 53% (n = 3, high variability; possibly bacterial growth within the ileostomy bag). In contrast, in healthy volunteers, only 3.5% of ingested pectin was found in the faeces in women and 4.8% in men. The authors concluded that some loss of pectin occurred in the small intestine but most of the pectin was degraded in the large intestine.
In a clinical study (Saito et al., 2005), the authors compared the amount of pectin in the human terminal ileum with that of orally administered apple pectin (molecular weight 20,000–400,000; 81% galacturonan; Sigma Chemical Co. Ltd.). Seven healthy male volunteers (20–27 years old; no history of gastrointestinal disease) received a test meal containing 5 g of pectin (corresponding to 4.05 g galacturonan) after 15 h fast and thorough intestinal lavage. A tube was placed in the terminal ileum using the endoscopic retrograde bowel insertion method. The ileal contents were aspirated through the tube for 9 h starting immediately after tube insertion; samples were frozen to inhibit degradation by microflora. Amounts of pectin orally administered and collected from the terminal ileum were determined by measuring galacturonic acid concentrations. The amount of pectin collected in the terminal ileum was 3.58 ± 0.43 g (mean ± standard deviation) or 88.4% ± 10.5% of the pectin administered. Due to large interindividual differences, the recovery ranged from 76.8% to 105.1%. In conclusion, about 90% of ingested pectin was recovered in the terminal ileum in this study. The authors stated that only minor amounts may have been degraded by bacteria in the terminal ileum.
Overall, data on in vitro degradation of pectins and amidated pectin by digestive enzymes contained in saliva, gastric juice or ileal fluids indicated that their digestibility was low in the upper parts of the digestive tract. By contrast, additional in vitro studies using fresh human microflora or isolated colonic bacteria demonstrated that pectins would be fermented during their passage through the large intestine by strains of bacteria found in the human colon. All these in vitro data are in agreement with rat studies demonstrating the absence of degradation of pectin in germ‐free animals by comparison to conventional animals.
Moreover, the absence of unchanged pectin in the faeces of animals and humans would give evidence of the complete in vivo degradation of pectin. As demonstrated in ileostomy patients, the main end products of this colonic anaerobic digestive process are SCFA, such as acetic, propionic and butyric acids, which are absorbed from the colon. Based on the available knowledge on the role of SCFA as end products of the fermentation of dietary fibres by the anaerobic intestinal microflora (Topping and Clifton, 2001; Den Besten et al., 2013), the Panel considered that their potential formation as fermentation products from pectins does not raise any safety concern. The Panel considered that pectin and amidated pectin would not be absorbed intact but mainly fermented by the intestinal microbiota in animals and humans.
3.5.2. Acute toxicity
Acute oral toxicity of ‘natural’ food additives, including pectin (no details available about the test item), was tested in mice and rats at a dose level of 5,000 mg/kg bw and a post‐exposure observation period of 14 days (Shimizu et al., 1993). No deaths were reported (data from abstract in English, original publication in Japanese). The LD50 values exceeded 5,000 mg/kg bw.
The acute oral toxicity of Jeju citrus rind pectin (purity 99%) was studied in groups of 10 male and 10 female Sprague–Dawley rats (Shim and Choung, 2003). Rats were dosed by gavage with 0, 100, 250 and 500 mg/kg bw at a volume of 10 mL/kg bw. The highest dose level was limited by the solubility of pectin in the solvent water. Clinical signs were recorded daily and body weight at day 1, 3, 7 and 14. No deaths occurred during the post‐exposure observation period of 14 days. The treatment resulted in soft stool and reduced body weight (no details) at 500 mg/kg bw during the first day after gavage.
Based on these data, the Panel considered that the acute oral toxicity of pectin is low. Although data on amidated pectin were not available, the acute oral toxicity was expected to be low, based on the structural similarity to pectin.
3.5.3. Short‐term and subchronic toxicity
Rats
Groups of male weanling Sprague–Dawley rats (totally 50 rats per group, no further details) were fed a diet containing 0% or 5% pectin (National Formulary; degree of methoxylation 10–12%; equivalent to 0 or 6,000 mg/kg bw per day) (Gilmore, 1965). The rats were sacrificed after 2, 4, 6 or 8 weeks of feeding. The treatment caused a decrease in food consumption and body weight gain at ≥ week 5. Serum albumin was increased at week 2 but not at week 6. Serum globulin was elevated at week 6 (no further details available; data from abstract).
The utilisation of protein and the absorption of calcium were investigated, besides toxic effects, in a subacute study with pectin‐S (pure citrus pectin, 55% esterified) and pectin‐M (pure citrus pectin, 65% esterified) (Viola et al., 1970). Groups of three male and three female CD rats (data combined) received 0%, 5% (only pectin‐S) or 10% of pectin‐S or pectin‐M in the diet for 10 days (equivalent to 0, 6,000 or 12,000 mg/kg bw per day). Body weight gain decreased significantly at the 10% level (pectin‐S: 71% of control; pectin‐M: 52% of control), accompanied by a significant reduction of food intake (pectin‐S: 82% of control; pectin‐M: 70% of control). The amount of excreted faeces increased significantly in all treatment groups (dose‐dependent in pectin‐S groups). The high‐dose level of pectin‐M resulted in a significant decrease of the weight gain per g protein intake. The digestibility of protein was reduced to 79% of the control value at the 10% level of pectin‐S and pectin‐M and to 85% at the 5% level of pectin‐S. Both pectin‐S and pectin‐M resulted in a reduced absorption of calcium admitted with the food.
In a subacute study (Andoh et al., 1999), the authors investigated the effects of pectin (citrus pectin, source: Sigma Chemical Co.; no further details) on the morphological parameters of the small intestine. Male Wistar rats (four per group) were fed a diet supplemented with 0% or 2.5% pectin (equivalent to 3,000 mg/kg bw per day). After a 2‐week treatment period, the rats were sacrificed, blood samples collected and the small intestines removed and processed for histological analysis. The treatment did not result in significant differences in body weight gain or calorie intake. In pectin‐fed rats, the length of the small intestine and the depth of crypts and villus height were significantly increased. The Panel considered this as an adaptive, rather than an adverse effect. There was no significant difference in plasma gastrin levels, but a significant increase in plasma enteroglucagon levels in the pectin‐fed group.
In a 90‐day subchronic feeding study (Til et al., 1972), two types of pectin were tested: pectin A (Melange A2) and pectin C (Melange C2; the Panel noted that JECFA, 1974 stated that Melange C2 is 21% amidated, thus the Panel considered this to be amidated pectin; no further details about the test item). Groups of 10 male and 10 female weanling Wistar rats received ad libitum a diet containing 0%, 5%, 10% or 15% pectin A or pectin C (totally 8 groups, including two control groups). The tested concentrations in the diet were equivalent to 0, 4,500, 9,000 or 13,500 mg/kg bw per day. The test diets were prepared once a fortnight. The authors assumed that the usable calorie content decreased with increasing dose levels. In a preliminary dose‐range study, a concentration of 20% in the diet resulted in growth depression, reduced food consumption and decreased food efficiency (no details about experimental design). No clinical signs occurred in any treatment group. During the exposure period, pectin A and pectin C resulted in a significant decrease of > 10% in body weight of males at a dose level of 15%. Female rats receiving 15% pectin A in the diet showed also a significant reduction in body weights, which was > 10% compared with controls. A slight (< 10% reduction), but significant, decrease in body weight was measured in high‐dose females exposed to pectin C. The food consumption was decreased (> 10% reduction) the first 2–3 weeks of exposure in males and females fed 15% pectin C and in males receiving 15% pectin A in the first week of exposure. Food efficiency data showed a reduction of > 10% in high‐dose rats of either sex exposed to pectin A during weeks 1–4. The relative organ weight of the empty caecum increased in male and female rats dose‐dependently and significantly at ≥ 5% pectin C. A similar, significant, but less pronounced effect on the empty caecum weight was detected in males at ≥ 5% pectin A and in females at 15% pectin A. Gross examination did not reveal any test compound‐related effects. No findings were reported in histopathology, with the exception of a slight degree of hyperkeratosis of the forestomach in males and females receiving ≥ 10% pectin C (incidences in males 0/10, 0/10, 3/10 and 6/10, and in females 0/10, 0/10, 2/10 and 5/10, respectively). No such effects occurred after exposure to pectin A. The enlargement of the caeca was not paralleled by histopathological alterations and was considered by the authors of the study to be an adaptive response rather than a toxic effect. A NOAEL was not allocated by the authors of the study. The Panel considered the reduced body weights at 15% pectin A or C to be related to the decreased food consumption and/or the reduced calorie intake. The slight hyperkeratosis of the forestomach was not considered to be of toxicological relevance. Overall, pectin and amidated pectin did not induce adverse effects in rats at dose levels of up to 15% in the diet, corresponding to 13,500 mg/kg bw per day, the highest dose tested.
Repeated oral exposure of rats to pectin in drinking water was tested (data from abstract and detailed Tables in English; original publication in Japanese) (Takagi et al., 1997). Ten male and 10 female F‐344 rats received for 13 weeks drinking water containing 0%, 0.15%, 0.5%, 1.5% or 5% pectin (‘pectin digest’, no further details) (equal to 0, 148, 545, 1,231 or 3,366 mg/kg bw per day in males and 0, 204, 657, 1,290 or 3,916 mg/kg bw per day in females, respectively). The treatment resulted in a slight reduction of food consumption (< 10%) and body weight (3% reduction of terminal weight) in high‐dose males, but 32% reduction in water intake (presumably due to altered taste and/or odour; no statistical analysis). Similar results were obtained in high‐dose females, but in contrast to males, a slight increase in the final body weight was observed. Haematology and clinical chemistry were performed according to current standards at termination. Blood parameters did not show any findings of toxicological relevance. In clinical chemistry, serum creatinine was elevated in males at ≥ 1.5% and blood urea nitrogen at 5%; no such effects were detected in females. Data on historical controls of this laboratory are lacking. Absolute and relative liver weights were significantly increased in females at ≥ 1.5%. However, no treatment‐related effects were detected in histopathology, and clinical chemistry did not reveal signs of hepatotoxicity. A NOAEL was not allocated by the authors. The Panel considered 5% pectin in drinking water (corresponding to 3,366 mg/kg bw per day in males and 3,916 mg/kg bw per day in females) as a NOAEL, the highest dose tested.
In addition, the safety of sodium pectate as food ingredient was described by Borzelleca et al. (1996). In this publication, two unpublished studies (a 14‐day study; Zoetis, 1991; and a 13‐week study; Moore et al., 1994) were described, in which no remarkable effects were observed; these studies were not available to the Panel.
3.5.3.1. Pectin‐derived acidic oligosaccharides
As products formed from manufactured pAOS are similar to products formed from pectin in the gastrointestinal tract, JECFA concluded that studies with pAOS were relevant for the evaluation of pectin in infant formulae (JECFA, 2016c). The Panel agreed with this conclusion.
A 13‐week repeated‐dose study with 0%, 5% and 10% pAOS in the diet (equal to 0, 3,400 and 7,100 mg/kg bw per day) was performed according to OECD Guideline 408 and good laboratory practice (GLP) in F1 rats (10/sex/group, Wistar) which were exposed in utero (Garthoff et al., 2010). An extra control group receiving 10% short‐chain fructo‐oligosaccharides (scFOS), which mimic the neutral oligosaccharides in human milk, was included in this study (this study is further described in the reproductive and developmental toxicity section). The F1 animals were selected after weaning, and were exposed for an additional 13 weeks. No treatment‐related clinical signs were observed during the study. Body weight and food intake were comparable in all groups. Increased water intake in the males of all groups and females of the reference control group and the high‐dose group, were considered to be a response to the feeding of high‐dose oligosaccharides. Effects were seen on the urinary volume in females of the high‐dose group. Urinary calcium was increased in the 10% scFOS (both sexes), 5% pAOS (females) and 10% pAOS groups. For males of the 5% and 10% pAOS group, the urinary density was increased, resulting in a high sodium content in these groups. The urinary pH was increased in the females of the 10% pAOS group. No treatment‐related macroscopic changes were seen at necropsy. Absolute and relative caecum weights (empty and full) were increased in males and females of the scFOS group and in the 10% pAOS group, when compared to controls. The kidney weights (absolute and relative) were increased in males of the pAOS groups compared to both controls. In the high‐dose groups, a minimal degree of urothelial hyperplasia at the 5% level and a diffuse hyperplasia at the 10% level was observed. No other treatment‐related effects were observed.
An additional 13‐week study was performed in Wistar rats (age 4 weeks) with 0%, 1%, 2.5% and 10% pAOS in the diet (equal to 0, 700, 1,700 and 7,200 mg/kg bw per day) and an additional high‐dose (10%) group supplemented with NH4Cl as acidifying substance (equal to 7,100 mg/kg bw per day) in the diet (Garthoff et al., 2010). The study was also performed according to OECD Guideline 408 and GLP. No treatment‐related clinical signs were observed during the study. Body weight and food intake were comparable in all groups except in the males of the 10% pAOS + NH4Cl group. Similarly to the first study, water intake was increased in both 10% pAOS groups. Urinary pH was constantly high in pAOS‐treated groups. The addition of NH4Cl was increased after day 11 from 1% to 2%, because the acidifying effect was less marked than anticipated. Urinary sodium excretion was increased in both high‐dose groups due to the high sodium content of the diet (3.1%). The urinary calcium excretion was increased in the 10% pAOS + NH4Cl group in females at all stages and in males at day 90. The caecum weights (absolute and relative) and the relative kidney weights of the high‐dose groups were increased. No treatment‐related macroscopic changes were seen at necropsy. Histopathological examination showed diffuse urothelial hyperplasia in four males of the 10% pAOS group and in two females of this group. The authors considered 2.5% pAOS in the diet (equal to 1,700 mg pAOS/kg bw per day) as the NOAEL. The Panel agreed with this NOAEL.
Overall, the oral exposure to non‐amidated and amidated pectin at a dose level up to 13,500 mg/kg bw per day did not result in effects of toxicological relevance in a subchronic feeding study in rats (Til et al., 1972). Accordingly, no adverse effects were detected in a subchronic drinking water study in rats at a concentration of 5% pectin in the water, corresponding to a NOAEL of 3,366 mg/kg bw per day in males and 3,916 mg/kg bw per day in females (Takagi et al., 1997). The reduction of protein digestibility and calcium absorption was shown in a subacute feeding study in rats at a dose of 12,000 mg/kg bw per day (Viola et al., 1970). A common phenomenon in feeding studies was the reduced food consumption at a dose level of 10% (Viola et al., 1970) or 15% (Til et al., 1972) in the diet or the reduced water consumption at a concentration of 5% in the drinking water (Takagi et al., 1997). In a 13‐week dietary study with pAOS in F1 rats according to the OECD Guideline 408 and GLP, an increased level of urinary calcium and a minimal degree of urothelial hyperplasia was observed in the 5% group (3,400 mg/kg bw per day) and kidney weights were increased (Garthoff et al., 2010). In an additional 13‐week study in rats with pAOS, a NOAEL of 1,700 mg/kg bw per day was identified (Garthoff et al., 2010).
3.5.4. Genotoxicity
In vitro
In a screening of food ingredients, citrus pectin (CAS 9000‐69‐5, no further details) was tested in a bacterial reverse mutation test in Salmonella typhimurium strains TA98, TA100, TA1535, TA1537, TA1538 and E. coli WP2uvrA (Mortelmans and Griffin, 1982; also reported in Prival et al., 1991). The substance was tested with the plate incorporation method at six doses (0.033, 0.10, 0.33, 1.0, 3.3 and 10 mg/plate) with and without metabolic activation by Aroclor‐induced rat liver S9. Concurrent positive controls were run with each test. The substance was tested in two independent trials using two plates per concentration. The solvent used was sodium phosphate buffer. Pectin was reported not to induce any increase in revertant counts in any bacterial strain, with and without S9, and was evaluated as not mutagenic in this assay. The Panel noted that no raw data are presented in these publications.
Further results from unpublished in vitro genotoxicity tests with sodium and/or calcium pectates are quoted without details in a review by Borzelleca et al. (1996) and are summarised below.
In a bacterial reversion test, the S. typhimurium strains TA98, TA100, TA1535, TA1537 and TA1538 were exposed to sodium pectate or a mix (1:1) of sodium/calcium pectate, with and without a metabolic activation system (no data about dose). The treatment resulted in no increase of revertants. No mutagenic activity was also detected in similar tests with sodium pectate heated to 170°C for 3 min or bleached sodium pectate. Negative results were also reported in a mammalian cell gene mutation test in mouse lymphoma cells with bleached sodium pectate (Borzelleca et al., 1996).
In vivo
No standard in vivo genotoxicity assays on pectins were available for evaluation. Negative results were reported, without further details (dose, treatment conditions, etc.), in an in vivo micronucleus test in mice with bleached sodium pectate (Borzelleca et al., 1996).
Other studies were performed to evaluate the influence of dietary intake of pectins on the occurrence of DNA‐adduct‐like indigenous compounds (I‐compounds). In these studies, groups of germ‐free (six per group) and conventional Wistar rats (10 per group; no data about sex) received diets containing 0% or 7.5% pectin of different degrees of esterification (pectin A: 92.6%; pectin B: 70.8%; pectin C: 34.5%). Rats were sacrificed after an exposure period of 21 days and DNA was isolated from colonic mucosa, liver, kidney and lung. DNA adducts were measured by the 32P‐post‐labelling assay. In germ‐free animals, DNA‐adduct‐like compounds were detected in all tissue samples in animals fed the low‐esterified pectin C, but also in controls. Under the higher‐esterified pectin A and B, a weak adduct formation could be demonstrated only in the liver. In conventional animals, DNA adducts occurred in all samples of colonic mucosa but with the highest intensity in the control group, followed by the low‐esterified pectin C group, and a weak intensity under the higher‐esterified pectin A and B (Zunft et al., 1997; Goldin‐Lang et al., 1998). Beyond the decrease of I‐compounds, which are considered products of reaction of endogenous metabolites with DNA, no evidence for DNA‐adduct formation was observed in the tissues examined.
3.5.4.1. Pectin‐derived acidic oligosaccharides
Further in vitro and in vivo genotoxicity studies were performed with pAOS, consisting of small polymers similar to products formed from pectin in the gastrointestinal tract. In these studies, summarised in Garthoff et al. (2010), pAOS were negative in a bacterial reverse mutation assay and in a mouse lymphoma assay, in which non‐reproducible increases of mutants were only observed at cytotoxic doses exceeding the maximum recommended dose according to the OECD Guideline 490. In a chromosomal aberration test in Chinese hamster ovary cells, pAOS were negative after short (4 h) treatment, with and without metabolic activation, whereas a significant increase of aberrant cells was observed after continuous treatment (18 h). The Panel noted that the increase in aberrant cells with pAOS dissolved in dimethyl sulfoxide (DMSO) was only observed above the maximum recommended dose according to OECD Guideline 473, and evaluated the overall result of this study as negative. In vivo, negative results were obtained in a micronucleus assay in rats treated in utero and for 13 weeks within a one‐generation dietary study with pAOS, at a dose corresponding to 7 g/kg bw. Based on these results, the Panel concluded that pAOS are not genotoxic.
In summary, only limited genotoxicity data were available on pectins. Negative results were obtained in a bacterial reversion assay with pectin performed with acceptable standards (Mortelmans and Griffin, 1982; also reported in Prival et al., 1991). Negative results, without further details, were also reported in a review by Borzelleca et al. (1996), in bacterial reversion assays with sodium and calcium pectates and with bleached sodium pectate; the last was also reported to be negative in a mammalian cell gene mutation test in vitro.
No results from validated and adequately reported in vivo genotoxicity assays were available. However, bleached sodium pectate was reported to be negative in a micronucleus test in mice and no evidence for DNA‐adduct formation was obtained in colonic mucosa, liver, kidney and lung of rats fed a diet with 7.5% pectin (Zunft et al., 1997; Goldin‐Lang et al., 1998).
Negative results were obtained with manufactured pAOS, representative of fermentation products of pectins in the gastrointestinal tract, in a battery of in vitro and in vivo assays (Garthoff et al., 2010).
Data on amidated pectin were not available, but considering its chemical structure and its negligible absorption, the Panel considered that there is no concern with respect to genotoxicity for amidated pectin. Overall, the Panel concluded that pectins do not raise concern for genotoxicity.
3.5.5. Chronic toxicity and carcinogenicity
Rats
In a chronic study (Cohen et al., 1967) with citrus pectin, a group of nine male Sprague–Dawley rats was fed for 18 months a basal diet containing 20% pectin (equivalent to 10,000 mg/kg bw per day) and five rats received the basal diet (control). Data on survival were not reported. A 20% reduction of the terminal body weight in the treatment group was observed, compared to control. Water consumption (about 30% increase) and dry weight of faeces (about 40% increase) were altered in treated rats. The increase in relative testes weight was probably related to the reduced body weight. In clinical chemistry, reduced cholesterol levels were measured. The authors reported a pneumonitis in both groups. Therefore, this study is of limited value for risk assessment.
In a chronic study (Palmer et al., 1974; referred to in Borzelleca et al., 1996), groups of 20 male weanling Wistar rats received for 2 years a diet (Purina laboratory meal) containing 10% α‐cellulose (Alphacel®; control group), 10% non‐amidated pectin (no further details about the test item) or 10% amidated pectin (18% amidated, low molecular weight pectin; no further details). A dose of 10% in the diet was equivalent to 5,000 mg/kg bw per day. The diets were made isocaloric by supplementation of the control diet (Alphacel®) with dextrose, assuming a caloric equivalent for pectin (0.6187 cal/g). Survival did not vary significantly and body weights for both pectin‐fed groups ‘were similar but significantly less than those of the control animals’ (no further details). Rats in both treatment groups showed lower food consumption, but a slight increase in food efficiency compared with controls. At necropsy, the relative organ weight of adrenal, heart, kidney, liver and spleen was not changed, but in both treatment groups the relative testis weight was increased, presumably due to reduced body weights. Serum alanine transaminase and aspartate transaminase analysed at sacrifice showed no abnormalities in the pectin‐fed groups. No unusual findings were detected at necropsy. Histopathology of all gross lesions and adrenal, heart, kidney, liver, lung, spleen and testes revealed no compound‐related effects (no complete histopathology). No adverse effects were noted at a dose of 10% non‐amidated or amidated pectin in the diet (equivalent to 5,000 mg/kg bw per day).
In a feeding study (Mosinger, 1974; cited in JECFA, 1981) with amidated pectin (degree of amidation: 24.5%; DE: 21%), groups of 20 male and 20 female Wistar rats received a diet containing 0% or 16.6% amidated pectin for 2 years (equivalent to 8,300 mg/kg bw per day). In the control group, nine rats died before termination of the study and 25% of all animals showed Mycoplasma pneumoniae infection. In the treated rats, 7/20 animals were infected. In historical controls (n = 4,000) of this laboratory, 58% of all rats had pulmonary mycoplasmosis. The author of the study compared body weight gain and final body weight in treated rats with the historical control data, which gave no differences of toxicological relevance. In histopathology, the incidences of non‐neoplastic and neoplastic lesions in treated rats were comparable to the historical controls. No data are available on other parameters. Due to the M. pneumoniae infection, the Panel considered the study not reliable for risk assessment.
The objective of a further chronic feeding study (Industrial‐Biotest, 1979a–d) was also the comparison of biological effects of amidated pectin with non‐amidated pectin. No additional control group was applied. Groups of 50 male and 50 female weanling Charles River strain albino rats were exposed to amidated pectin (88.7% galacturonic acid, degree of amidation: 20.5%, DE: 28.5%) or non‐amidated pectin (high methoxy pectin, 87% galacturonic acid, DE: 62%) at dietary levels of 2% or 5% for 2 years (corresponding to 1 or 2.5 g/kg bw per day). The Panel considered the study not adequate for risk assessment because it lacked a control group.
Overall, a feeding study on chronic toxicity of pectin or amidated pectin in rats with sufficient information was available for evaluation of this endpoint, including data on a concurrent control (Palmer et al., 1974; cited in Borzelleca et al., 1996). The NOAEL was 10% in the diet, equivalent to 5,000 mg/kg bw per day, the highest dose tested.
Initiation–promotion studies
The Panel noted a series of studies which had investigated potential modulating effects of pectin administration on the development of cancer in animal models treated with a number of initiating agents (Bauer et al., 1978, 1981; Watanabe et al., 1979; Freeman et al., 1980; Goldsworthy et al., 1986; Jacobs and Lupton, 1986; Jiang et al., 1996). The Panel considered the outcome of these studies, which showed either no or inhibitory effects on the development of cancer, not relevant for the risk assessment of pectins as food additives.
3.5.6. Reproductive and developmental toxicity
3.5.6.1. Reproductive toxicity studies
In a study on reproductive toxicity of amidated pectin (degree of amidation: 24.5%, DE: 21%), groups of five male and five female Wistar rats were fed at a level of 16.6% in the diet (equivalent to 8,300 mg/kg bw per day) for 4 months, followed by mating of one male with five females (Mosinger, 1976). One set of litters was produced (totally 46 rats, 22 males and 24 females). The growth of the F1 generation was normal. All F1 pups were examined for external malformations and 10 pups were analysed for further teratogenic effects via macroscopic examination. No abnormalities were found. Further parameters, examined in standard studies on developmental toxicity, were not presented. Data on a concurrent, treated or untreated, control group were not reported. The presence of mycoplasmosis, the low number of animals and the limited data presented in the report prevent the use of the data from this study for risk assessment.
In a three‐generation reproductive toxicity study (Industrial‐Biotest, 1979e), the effects of amidated pectin were compared with those of non‐amidated pectin. Groups of eight male and 16 female weanling albino rats (no data about strain) were fed a diet containing 2% or 5% amidated pectin (88.7% galacturonic acid, degree of amidation: 20.5%, DE: 28.5%) or 2% or 5% non‐amidated pectin (high‐methoxy pectin, 87% galacturonic acid, DE: 62%), equivalent to 1,000 or 2,500 mg/kg bw per day. No additional control group was added. The Panel considered the study not adequate for risk assessment because it lacked a control group and because no historical data were presented in the report.
Pectin‐derived acidic oligosaccharides
In a dietary one‐generation reproductive toxicity study with pAOS in Wistar rats (GLP compliant, according to OECD Guideline 415) (Garthoff et al., 2010), eight males and 16 females were fed diets containing 0%, 5% and 10% pAOS/kg diet (equal to 3,100 and 6,200 mg/kg bw per day for the F0 females during premating and gestation; during the lactation period the intake was doubled). An extra control group receiving 10% scFOS (which mimic the neutral oligosaccharides in human milk) was included in this study. The diets were administered 4 weeks before mating, during mating, gestation, lactation and until weaning. No treatment‐related effects on clinical signs, body weight, body weight gain, feed intake and neurobehavioural observations were shown. Water consumption was increased in the males of all groups and females of the scFOS reference control group. No effects on reproductive or developmental parameters were observed. The Panel considered 6,200 mg/kg bw per day, the highest dose tested, to be the NOAEL of this study.
3.5.6.2. Developmental toxicity studies
In a prenatal developmental toxicity study (Industrial‐Biotest, 1976), the effects of amidated pectin were compared to those of non‐amidated pectin. Groups of 17–20 pregnant Charles River albino rats were fed on gestation days (GD) 6–15 a diet containing 2% or 5% amidated pectin (88.7% galacturonic acid, degree of amidation: 20.5%, DE: 28.5%) or 2% or 5% non‐amidated pectin (high‐methoxy pectin, 87% galacturonic acid, DE: 62%), equivalent to 1,000 or 2,500 mg/kg bw per day (see Industrial‐Biotest, 1979a). No additional control group was added. The Panel considered the study not adequate for risk assessment because it lacked a control group and because no historical data were presented in the report.
Overall, two reproductive toxicity studies and one developmental toxicity study with pectins, considered inadequate for risk assessment, were available. In a dietary one‐generation reproductive toxicity study with pAOS in rats (GLP‐compliant, according to OECD Guideline 415), a NOAEL of 6,200 mg/kg bw per day, the highest dose tested, was identified (Garthoff et al., 2010).
3.5.7. Hypersensitivity, allergenicity and food intolerance
In three case reports (Kraut et al., 1992; Cohen et al., 1993; Jaakkola et al., 1997), data on occupational asthma caused by pectin inhalation were presented. In a further case report, an atopic woman, occupationally sensitised to pectin, got rhinitis, conjunctivitis and contact urticaria from pectin. The woman developed anaphylaxis after eating cashew nuts. The authors discussed cross‐reacting allergens in cashew nut and pectin (Rasanen et al., 1998).
Ferdman et al. (2006) reported a case of a child with hypersensitivity reaction after ingestion of pectin. The authors considered also this as possibly due to a cross‐reactivity with cashew and pistachio allergens.
Several publications have reported immune‐modulatory effects of acidic oligosaccharides extracted from various foods, including pectin, in animals and humans (Popov and Ovodov, 2013). This immunomodulation tended to enhance Th1‐related and suppress Th2‐related parameters (Vos et al., 2007; Eiwegger et al., 2010). In this context, dietary pectin was expected to alleviate type 1 allergy reaction possibly leading to prevention of atopic dermatitis, food allergy, and allergic asthma (Lim et al., 2002; Kerperien et al., 2014; Hogenkamp et al., 2015). It is hypothesised that the immune‐modulating effects of these oligosaccharides may be assisted via alteration of the intestinal microbiota or in a microbiota‐independent manner by direct interaction on immune cells, or both (Fanaro et al., 2005; Jeurink et al., 2013). However, the Panel noted that there is no indication that these effects were associated with pectin extracts that complied with the specifications of the food additives E 440i and E 440ii.
Overall, in view of the available data, the Panel considered that there is no indication that the reported immune‐modulatory properties of pectin may lead to an adverse response, the data being rather indicative of an effect which would limit the hypersensitivity response. Therefore, the Panel did not consider the food additives pectin (E 440i) and amidated pectin (E 440ii) as having an allergenic potential.
3.5.8. Other studies
3.5.8.1. Animal studies
Five groups of pigs (n = 10, body weight 8.6 ± 1.4 kg, 4 weeks of age) were fed a low‐fibre (LF) (raw wheat and barley flour), mid‐fibre (MFH) and high‐fibre (HFH) diets by adding barley hulls, or mid‐ (MFP) and high‐ (HFP) fibre diets containing pectin (Genu pectin type B, 75% methylation) for 9 days (Hedemann et al., 2006). The pectin diets contained 71 g/kg pectin; the HFP diet contained, in addition, 96 g/kg barley hulls. After 9 days, the animals were sacrificed and the entire gastrointestinal tract was removed. The feed intake in the pectin‐fed groups was decreased. The mucosal‐enzyme activity was affected by the fibre content of the diets in the MFH, MFP, HFH and HFP diet groups. The villi and the crypts were shorter in the pectin groups, but the villous height/crypt ratio was unaltered. The area of mucins in the crypts was decreased, suggesting that pigs fed pectin‐containing diets are more susceptible to pathogenic bacteria.
Special studies in young animals
The safety of pectin (high‐ester pectin extracted from citrus peel and standardised by the addition of sucrose) in a milk replacer was tested in pre‐weaning farm piglets (Yorkshire‐bred, age 2 days) (MPI, 2013; as referred to in JECFA, 2015). The milk replacer containing 0, 0.5, 3.0 or 10 g/L was given 6 times per day to groups of six males and six female piglets, at a dose volume of 500 mL/kg bw (equal to 0, 142, 847 or 3,013 mg/kg bw per day for males and 0, 114, 870 or 3,094 mg/kg bw per day for females, respectively) for 3 weeks. These doses were recalculated based on measured concentrations of pectin instead of target concentrations (doses equal to 0, 131, 1,049 or 4,015 mg/kg bw per day for males and 0, 130, 1,088 or 4,123 mg/kg bw per day for females) (JECFA, 2016c). No treatment‐related clinical signs were observed. At day 21 of the study, the body weight of the males of the high‐dose group was decreased (19.3%) when compared to the control. This decrease was accompanied by a decrease in feed intake of 30%. Feed efficiency was decreased in the high‐dose group. In females of this group, a slightly decreased body weight gain was observed from day 15 onwards. No effects were observed on body weight and food intake in the low‐ and mid‐dose groups. At necropsy, caecum and colon weights were increased in the mid‐ and high‐dose groups. Furthermore, there was a decrease in pH level of the caecum and colon contents, which may be related to the production of SCFA by endogenous microbiota. There were no histopathological effects observed in the intestinal tract, apart from a slight increase of subacute inflammation in the high‐dose group. No differences in blood cytokines were observed. According to the authors, the NOAEL for this study was the mid‐dose, 1,049 mg/kg bw per day. Based on the data available, the Panel considered that this conclusion appeared appropriate.
A new study in neonatal pigs (6/sex per group) administered pectin in milk replacer as their sole source of nutrition for 3 weeks at a target concentration of 0.2% or 1% (equal to 704 and 4,461 mg/kg bw per day, respectively, for males and females combined) was referred to in JECFA (Dilger, 2015; cited in JECFA, 2016c). Consumption of milk replacer and growth were significantly reduced in the 1% pectin group. The NOAEL for this new study was 0.2% pectin, however the NOAEL of the older study (0.3%) was still considered to be relevant. JECFA (2016) concluded that the reduced milk replacer consumption observed in both studies at a dose level of 1% pectin in milk replacer was likely due to delayed gastric emptying and/or prolonged gut transit resulting from consumption of the highly viscous 1% pectin diet. The data available from this study were not sufficient for the Panel to confirm the conclusion of JECFA.
3.5.8.2. Observations in humans
Contraindications, warnings and undesirable effects for pectin as excipient are not known in dosages used.
A decline in serum cholesterol (approx. 5%) after repeated ingestion of pectin was shown after daily intake of 15 g pectin (pure citrus pectin, National Formulary; source: Sunkist Growers) incorporated in special biscuits, in studies with healthy, middle‐aged, male volunteers (no further details) under controlled conditions in a metabolic unit (Keys et al., 1961). Data on clinical symptoms after repeated ingestion of pectin were not reported.
In a clinical study (Durrington et al., 1976), 12 healthy men (22–45 years old, mean 25 years; no medication) ingested daily 12 g of powdered pectin (source: Bulmer Ltd., no further data) for 3 weeks. No subjective symptoms were reported. A small, but statistically significant, decrease in total serum cholesterol (approx. 8%) was shown after oral exposure to pectin.
Mild clinical signs after ingestion of large doses of pectin after daily intake of 36 g pectin given in separate doses for 6 weeks (National Formulary; pure high methoxy pectin; source: Bulmers Ltd.) were reported in a study on the digestion of pectin in the human gut (Cummings et al., 1979). The dose corresponded to about 515 mg/kg bw per day. The five male healthy volunteers reported no subjective symptoms, except a feeling of abdominal distension associated with a considerable increase in flatus production. The calcium balance remained unchanged. Stool frequency and mean transit time (measured by markers) were unchanged by pectin administration.
The effects of intake of 6 g pectin (high‐methoxyl undiluted citrus pectin) per day for 24 days was studied in 12 healthy male and female adults (age 23–60 years) (Spiller et al., 1980). The subjects were chosen on the basis of slow intestinal transit time and low faecal output when consuming their normal diets. Faecal weight, stool pH and transit time were comparable to the placebo group. In the pectin group, an increase in the volatile fatty acids (SCFA) was observed.
After intake of 12 g pectin per day for 4 weeks by two males (age 21 years) and eight females (age 26–42 years), the effects on stool pH, transit time and stool weight were measured (Hillman et al., 1983). Because of the gelling properties of pectin, the subjects had great difficulty in taking the full amount of pectin. Side effects described in females were laxative effect (n = 1), constipation (n = 2), nausea (n = 1) and heartburn (n = 1). No effects on stool pH, stool transit time or stool weight were reported.
To evaluate the effect of oral administration of pectin on the gastric emptying rates and the blood glucose levels, a double‐blind, randomised cross‐over study was conducted in volunteers (Iftikhar et al., 1994). No effects on blood glucose levels were reported. The authors concluded that a dose of 2 g pectin can significantly delay the rate at which the terminal phase of a meal is emptied from the stomach.
The effects of dietary pectin (18 g/day; high‐methoxyl pectin, source: Bulmers Ltd.; no further details) on human faecal microflora were investigated in a clinical study (Mallett et al., 1987). The total number of bacteria per gram faeces was significantly reduced, but the ammonia per g faeces, as well as the endogenous ammonia production (increased enzyme activity) were significantly increased by the pectin treatment. The activity of bacterial β‐glucosidase and β‐glucuronidase was significantly decreased. In conclusion, the enzymatic activity of the large intestine microflora was altered after daily ingestion of 18 g pectin for 3 weeks (260 mg/kg bw per day, assuming a body weight of 70 kg). Data on clinical signs of toxicity were not reported.
Overall, a slight but significant decline (5–8% reduction) in serum cholesterol after repeated ingestion of pectin was shown in clinical studies after daily ingestion of 12 g (Durrington et al., 1976) or 15 g pectin (Keys et al., 1961). A single oral dose of 2 g pectin can significantly delay the rate at which the terminal phase of a meal is emptied from the stomach (Iftikhar et al., 1994). In a clinical study, the enzymatic activity of the large intestine microflora was altered after daily ingestion of 18 g pectin (Mallett et al., 1987). In a study with pectin (Cummings et al., 1979), the volunteers reported no subjective symptoms, except a feeling of abdominal distension associated with a considerable increase in flatus production, at a dose level of 515 mg pectin/kg bw per day. In other studies, 6 g or 12 g pectin for 24 days did not affect faecal weight, stool pH and transit time and the adverse effects were minimal (Spiller et al., 1980; Hillman et al., 1983).
Studies in infants
In a multicenter, third‐party‐blinded, randomised‐controlled, prospective study, preterm infants (n = 74) were fed human milk with a pectin‐containing liquid milk fortifier (0.085% pectin) or human milk fortified with a control fortifier (n = 72) (Moya et al., 2012). The infants fed pectin‐containing milk achieved a higher linear growth rate, and greater increase in weight, length and head circumference.
Furthermore, in a prospective, multicentre, double‐blind randomised control trial (Dupont et al., 2014, 2015), the addition of pectin (0.5 g/100 mL) to amino‐acid based formulae (thickened amino‐acid‐based formula (TAAF)) for children (n = 75) allergic to cow's milk and intolerant to extensively hydrolysed milk proteins, was studied. A commercially available ‘reference’ amino‐acid based formula (RAAF) served as a reference. Infants and young children were < 18 month of age. The children were fed for 3 months TAAF or RAAF. Thereafter, all infants were fed TAAF for 3 months. The study was including a safety evaluation. Anthropometric data were carefully scored. Parameters included paediatric assessments and monitoring of cutaneous, respiratory and digestive symptoms (regurgitations, vomiting, stool consistency). In the TAAF group, two adverse events occurred: one case of oesophagitis and one case of gastroenteritis. No differences in growth parameters between the two groups were observed. TAAF reduced the Scoring Atopic Dermatitis Index and the number of infants with skin dryness. In addition, TAAF improved stool consistency, gave less irritability signs and better night‐sleep quality in more infants. The authors concluded that the pectin‐containing formula was well tolerated and ensured appropriate growth.
The effects of 4 g pectin/kg bw per day on intestinal permeability were studied in 57 boys (5–12 months) with persistent diarrhoea (≥ 14 days) (Rabbani et al., 2004). Children were given a rice‐based diet containing pectin (n = 17) or rice diet alone (n = 21) for a week. Intestinal permeability was assessed before and after treatment. Treatment with pectins significantly (p < 0.05) reduced lactulose recovery, increased mannitol recovery and decreased the lactulose/mannitol ratio, indicating improvement of permeability. Permeability changes were associated with a 50% reduction in stool weights.
Studies with pectin‐derived acidic oligosaccharides
In a double‐blind, placebo‐controlled, randomised intervention trial, 82 healthy, full‐term infants (age 1 week to 3 months) were given whey‐based formula (control group, n = 23), whey‐based formula with galacto‐ and long‐chain fructo‐oligosaccharides (scGOS/IcFOs group, n = 25) or the last formula with pectin‐derived acidic oligosaccharides (scGOS/IcFOs/pAOS group, n = 24) (Magne et al., 2008). The groups were partially breastfed and the pAOS group consumed at least 300 mL per day containing 0.2% pAOS (equal to 240 mg/kg bw per day). No adverse effects were noted.
Piemontese et al. (2011), studied the effects of addition of pAOS (1,200 mg/L) in the diet of healthy infants (age 20–42 days) in a multicentre, randomised, double‐blind, placebo‐controlled trial. A group of 300 infants was fully breastfed. The formula‐fed infants were randomised in a group receiving regular formula with a mixture of neutral oligosaccharides (n = 416), and a group receiving regular formula with pAOS (n = 414). No differences were reported in growth, gastrointestinal tolerance and stool frequency between infants fed the pAOS formula and infants fed the control formula; stool consistency was softer in infants fed the pAOS formula, but more similar to that of the breastfed infants.
Overall, no adverse effects were noted in the four studies in infants with infant formulae containing pectin (Moya et al., 2012; Dupont et al., 2014, 2015) and pAOS (Magne et al., 2008; Piemontese et al., 2011). Pectin (4 g/kg bw per day) improved the permeability of the small intestine in young infant boys (5–12 months) with persistent diarrhoea (Rabbani et al., 2004).
3.6. Discussion
Pectin (E 440i) consists of the partial methyl esters of polygalacturonic acid, while amidated pectin (E 440ii) consists of both partial methyl esters and amides of polygalacturonic acid. Neutral sugars are covalently linked as side‐chains to the rhamnogalacturonan via the C3 of galacturonosyl and/or the C4 of the rhamnosyl residues. Side‐chain sugars are mainly galactose and arabinose, forming galactan, arabinan and arabinogalactan. The Panel noted that there is a large structural variation within the arabinogalactans.
The Panel considered that, as a result of the pectin manufacturing process, inactivated enzymes could be present in trace amounts in the product together with other proteins (0–5%) from the raw materials.
Pectins can be applied together with other hydrocolloids to modify the physicochemical properties and texture for certain food applications. The combination with alginates results in the formation of firm gels. The combination with gelatine in confectionery products reduces the viscosity and makes the texture more elastic and brittle. The interaction with agar‐agar produces a viscous texture in aerated confectionery products. Pectins can also interact with starch, guar, locust bean gum, oxidised starch, potato maltodextrin and gum arabic, leading to different gel properties and to improved product characteristics, as the interaction with guar gum in partially baked frozen bread that improves some quality parameters as crumb cohesiveness.
The Panel noted that according to EU specifications for pectin (E 440i) and amidated pectin (E 440ii), impurities of the toxic elements arsenic, lead, mercury and cadmium are accepted up to concentrations of 3, 5, 1 and 1 mg/kg, respectively. Contamination at such levels could have a significant impact on exposure to these metals, for which the exposure is already close to the health‐based guidance values or benchmark doses (lower confidence limits) established by EFSA.
The Panel noted that the limit for lead in pectin (E 440i) and amidated pectin (E 440ii) was the same (5 mg/kg) according to EU Commission Regulation No 231/2012 and JECFA (2009). However, it was reviewed by JECFA in 2016, and the limit for general use was lowered from 5 to 2 mg/kg and to 0.5 mg/kg for use in infant foods. However, the Panel noted that the EU regulatory limits have not been updated.
Data on in vitro degradation of pectins and amidated pectins indicated that their digestibility was low in the upper parts of the digestive tract, but they would be fermented during their passage through the large intestine. These in vitro data are in agreement with in vivo studies demonstrating the absence of degradation of pectins in germ‐free rats by comparison to conventional animals. As demonstrated in ileostomy patients, the main end products of this colonic anaerobic digestive process are SCFA, such as acetic, propionic and butyric acids, which are absorbed from the colon and considered of no safety concern by the Panel. These data indicated that pectins and amidated pectins would not be absorbed intact but extensively fermented by intestinal microbiota in animals and humans.
As products formed from pectins in the gastrointestinal tract are similar to manufactured pAOS, JECFA (2015) concluded that studies using pAOS were relevant for the evaluation of pectins in infant formulae. The Panel agreed with this conclusion.
The acute oral toxicity of pectin is low. Data on amidated pectin were not available, but a low acute oral toxicity is expected based on the structural similarity to pectin.
In subchronic studies, after oral exposure (in the diet or drinking water) to non‐amidated and/or amidated pectins, the NOAEL ranged from 3,366 mg/kg bw per day (Takagi et al., 1997) to 13,500 mg/kg bw per day (Til et al., 1972). In subchronic studies with pAOS in the diet, the NOAEL ranged from 1,700 to 3,400 mg/kg bw per day (Garthoff et al., 2010).
Although the available data were limited, there was no indication of genotoxicity for pectins. This conclusion was also supported by the negative results obtained with manufactured pAOS (Garthoff et al., 2010). Data on amidated pectin were not available, but considering its chemical structure and its negligible absorption, the Panel considered that there is no concern with respect to genotoxicity for amidated pectin.
A feeding study on chronic toxicity of pectin or amidated pectin in rats was available with sufficient information for the evaluation of this endpoint, including data on a concurrent control (Palmer et al., 1974; cited in Borzelleca et al., 1996). The NOAEL was 10% in the diet, equivalent to 5,000 mg/kg bw per day.
Two reproductive toxicity studies and one developmental toxicity study with pectins, considered inadequate for risk assessment, were available. In a dietary one‐generation reproductive toxicity study with pAOS in rats (GLP‐compliant, according to OECD Guideline 415), a NOAEL of 6,200 mg/kg bw per day, the highest dose tested, was identified (Garthoff et al., 2010).
The Panel considered that there was no indication that the reported immune‐modulatory properties of pectin may lead to an adverse response, the data being rather indicative of an effect which would limit the hypersensitivity response. Therefore, the Panel did not consider the food additives pectin (E 440i) and amidated pectin (E 440ii) as having an allergenic potential.
In human volunteers, a slight but significant decline in serum cholesterol after repeated ingestion of pectins was shown in clinical studies, after daily ingestion of 12,000 mg (Durrington et al., 1976) or 15,000 mg pectins (Keys et al., 1961). A single oral dose of 2,000 mg pectins can significantly delay the rate at which the terminal phase of a meal is emptied from the stomach (Iftikhar et al., 1994). In a clinical study, the enzymatic activity of the large intestine microbiota was altered after daily ingestion of 18,000 mg pectins (Mallett et al., 1987). In a study with pectins (Cummings et al., 1979), the volunteers reported no subjective symptoms, except a feeling of abdominal distension associated with a considerable increase in flatus production, at a dose level of 36,000 mg per day (equivalent to 515 mg/kg bw per day). In other studies, 6,000 mg or 12,000 mg (equivalent to 86 mg/kg bw per day and 172 mg/kg bw per day, respectively) pectin for 24 days did not affect faecal weight, stool pH and transit time and the adverse effects were minimal (Spiller et al., 1980; Hillman et al., 1983).
No adverse effects were noted in the four studies in infants with infant formulae containing pectins (Moya et al., 2012; Dupont et al., 2014, 2015) and pAOS (Magne et al., 2008; Piemontese et al., 2011). Pectin (4 g/kg bw per day) improved the permeability of the small intestine in young infant boys (5–12 months) with persistent diarrhoea (Rabbani et al., 2004). In the study of Dupont et al. (2014, 2015) in infants and young children (< 18 months of age), receiving a formula containing 0.5% pectin for 3 months, safety and anthropometric parameters were carefully evaluated and no relevant adverse effects were observed.
The Panel noted that infants and young children (up to 3 years) consuming FSMP may be exposed to a greater extend to pectins (E 440) than their healthy counterparts, because the permitted levels of pectins (E 440) in formulae for special medical purposes are twofold higher than in formulae for healthy individuals. The Panel further noted that, given their medical condition, infants and young children consuming foods belonging to these food categories may show a different susceptibility, compared to their healthy counterparts, to the gastrointestinal effects of pectins and of other compounds having similar physicochemical properties. No sufficient data with formulae containing pectin at the MPL of 1% for this relevant population group are available. Thus, monitoring of any side effects, including those in the gastrointestinal system, in infants and young children consuming these foods under medical supervision, could be helpful to reduce this uncertainty.
The safety of pectin (high‐ester pectin extracted from citrus peel and standardised by the addition of sucrose) in a milk replacer was tested in neonatal pigs (Yorkshire‐bred, age 2 days) (MPI, 2013; as referred to in JECFA, 2016c). The NOAEL for this study was 1,049 mg/kg bw per day (JECFA, 2016c). Based on the data available, the Panel considered that this conclusion appeared appropriate. JECFA (2016) concluded from the data of a new piglet study that the reduced milk replacer consumption observed in both studies at a dose level of 1% pectin in milk replacer was likely due to delayed gastric emptying and/or prolonged gut transit resulting from consumption of the highly viscous 1% pectin diet. The data available from this study were not sufficient for the Panel to confirm the conclusion of JECFA.
In the safety evaluation by JECFA (2016c), the Committee concluded that at the new maximum proposed use level of 0.2% pectin in infant formula, the estimated exposure of infants aged 0–12 weeks would be up to 360 and 440 mg pectin/kg bw per day at mean and high consumption. The Panel noted that, according to the EU regulation, pectins are not authorised in infant formulae (FC 13.1.1).
To assess the dietary exposure to pectins (E 440) from their use as food additives, the exposure was calculated based on (1) maximum use levels provided to EFSA (defined as the maximum level exposure assessment scenario), and (2) reported use levels or analytical data (defined as the refined exposure assessment scenario, brand‐loyal and non‐brand‐loyal).
Pectins (E 440) are authorised in a wide range of foods. The Panel did identify brand loyalty to specific food categories in toddlers (e.g. flavoured fermented milk products and flavoured drinks). Further, the Panel considered that the non‐brand‐loyal scenario covering other population groups was the most appropriate and realistic scenario for risk characterisation, because it is assumed that the population would probably be exposed long‐term to the food additive present at the mean reported use level in processed food.
A maximum estimated exposure assessment scenario taking into account the FSMP for infants and young children (FC 13.5.1. Dietary foods for infants for special medical purposes and special formulae for infants and FC 13.1.5.2 Dietary foods for babies and young children for special medical purposes as defined by Commission Directive 1999/21/EC) was also performed using food additive producers data to estimate exposure for infants and toddlers who may be on a specific diet. This exposure scenario considered products belonging to food categories 13.1.2, 13.1.3 and 13.1.4, excluding FC 13.1.1 (infant formulae), where pectins (E 440), according to EU regulation, are not authorised. Considering that this diet is required due to specific needs, it is assumed that consumers are loyal to the food brand, therefore only the maximum brand‐loyal estimated exposure scenario was performed.
A refined estimated exposure assessment scenario taking into account the consumption of food supplements was also performed to estimate exposure for children, adolescents, adults and the elderly, for consumers only, as exposure via food supplements may deviate largely from that via food, and the number of food supplement consumers may be low depending on the population and survey.
The refined estimates were based on 23 out of 81 food categories in which pectins (E 440) are authorised. Overall, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the real exposure to pectins (E 440) as food additives in European countries for the maximum level exposure scenario and for the refined exposure assessment scenarios, when considering only food additive uses for which data have been provided.
However, the Panel noted that, given the information from the Mintel GNPD, it may be assumed that pectins (E 440) are used in several food categories (n = 9) for which no data have been provided by food industry. The Panel noted that out of these nine food categories, fruit juices and fruit nectars are products highly consumed. If this would be confirmed, it would therefore result in an underestimation of the exposure.
A possible additional exposure to pectins (E 440) from their use directly by the consumer (adding pectins to thicken foods, e.g. jams, marmalades) and from their use as food additives authorised in accordance with Annex III to Regulation (EC) No 1333/2008, were not considered in any of the above exposure assessment scenarios.
The Panel also noted that the refined exposure estimates were based on information reported on the use levels of pectins (E 440) by food industry. If actual practices change, these refined estimates may no longer be representative and should be updated.
4. Conclusions
General population
Following the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA, 2014), and given that:
the data received for the 23 food categories were adequate for a combined exposure assessment for these categories;
based on these reported use levels, a refined exposure of up to 442 mg/kg bw per day for toddlers in these categories (brand‐loyal scenario) was estimated;
pectin and amidated pectin are not absorbed intact, but extensively fermented by intestinal microbiota in animals and humans;
adequate toxicity data were available;
in oral subchronic studies with pectins in rats, no adverse effects were observed at doses ranging from 3,366 mg/kg bw per day to 13,500 mg/kg bw per day, the highest doses tested. In subchronic studies with pAOS in the diet in rats, a NOAEL of 1,700 mg/kg bw per day was identified;
no effects on body weight and food intake were observed in male neonatal pigs exposed to 0.3% pectin in formula (equal to 1,049 mg pectin/kg bw per day) for 28 days, while in the same study, such effects were observed at 1% pectin in formula (equal to 4,015 mg pectin/kg bw per day);
no adverse effects were reported in a chronic study in rats at up to 5,000 mg pectin/kg bw per day, the highest dose tested;
there is no concern with respect to genotoxicity for pectin and amidated pectin;
a daily dose of 36,000 mg pectin (equivalent to 515 mg/kg bw per day) for 6 weeks in humans was associated with abdominal distension and increasing flatus in some individuals, effects which were considered by the Panel as undesirable, but not adverse,
the Panel concluded that there is no need for a numerical ADI for pectin (E 440i) and amidated pectin (E 440ii) and that there is no safety concern for the general population at the refined exposure assessment for the reported uses and use levels of pectins (E 440) as food additives.
Infants and young children consuming foods for special medical purposes and special formulae
Concerning the use of pectins (E 440) in ‘dietary foods for special medical purposes and special formulae for infants’ (FC 13.1.5.1) and in ‘dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC’ (FC 13.1.5.2), and given that:
for populations consuming foods for special medical purposes and special formulae, the 95th percentile of maximum exposure assessments calculated based on the maximum reported data from food additive producers was up to 1,349 mg/kg bw per day for infants;
infants and young children consuming these foods may be exposed to a greater extent to pectins (E 440) than their healthy counterparts because the permitted levels of pectins (E 440) in formulae for special medical purposes are twofold higher (1%) than in formulae for healthy individuals;
infants and young children consuming foods belonging to these food categories may show a higher susceptibility to the gastrointestinal effects of pectins than their healthy counterparts due to their underlying medical condition;
no effects on body weight and food intake were observed in male neonatal pigs exposed to 0.3% pectin in formula (equal to 1,049 mg pectin/kg bw per day) for 28 days, while in the same study, such effects were observed at 1% pectin in formula (equal to 4,015 mg pectin/kg bw per day);
in infants and young children (< 18 months of age), a formula containing 0.5% pectin (E 440) given for 3 months was well tolerated without adverse effects;
no human studies investigating the adverse effects of formulae containing pectins (E 440) at the MPL of 1% for the relevant age group were available,
the Panel concluded that the available data do not allow for an adequate assessment of the safety of use of pectins (E 440) in infants and young children consuming these FSMP at the presently authorised maximum use levels of 1%.
5. Recommendations
The Panel recommended that:
the European Commission considers lowering the maximum limits for the impurities of toxic elements arsenic, lead, mercury and cadmium in the EU specifications for pectin (E 440i) and amidated pectin (E 440ii) in order to ensure that pectin (E 440i) and amidated pectin (E 440ii) as food additives will not be a significant source of exposure to those toxic elements in food; special requirements might be defined in the specifications for pectin (E 440i) and amidated pectin (E 440ii) to be used in formulae or food for infants, toddlers and other young children;
limits for aluminium should be considered for inclusion in the EU specifications, as aluminium can be used in the manufacturing process;
the European Commission considers harmonising the microbiological specifications for polysaccharidic thickening agents, such as pectins, and including criteria for the absence of Salmonella spp. and Escherichia coli, for TAMC and for TYMC in the EU specifications for pectin (E 440i) and amidated pectin (E 440ii);
additional clinical data should be generated to assess the safety of pectins (E 440) when used in ‘dietary foods for special medical purposes and special formulae for infants’ (FC 13.1.5.1) and in ‘dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC’ (FC 13.1.5.2);
due to the discrepancies observed between the data reported from industry and the Mintel database, where pectins are labelled in more products than in food categories for which data were reported from industry, the Panel recommended collection of data on usage and use levels of pectins (E 440) in order to perform a more realistic exposure assessment.
Documentation provided to EFSA
AESGP (Association of the European Self‐Medication Industry), 2016. Data on usage levels of pectin (E 440i) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2015). Submitted to EFSA on 27 May 2016.
EDA (European Dairy Association), 2016. Data on usage levels of pectin (E 440i) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2015). Submitted to EFSA on 30 May 2016.
EHPM (European Federation of Health Products Manufacturers Associations), 2016. Data on usage levels of pectin (E 440i) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2015). Submitted to EFSA on 31 May 2016.
EMA (European Medicines Agency). Communication to EFSA request of 4 May 2015, for information on a certain group of substances used as food additives.
FDE (FoodDrinkEurope), 2016. Data on usage levels of pectin (E 440i) and amidated pectin (E 440ii) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2015). Submitted to EFSA on 31 May 2016.
ICGA (International Chewing Gum Association), 2016. Data on usage levels of pectin (E 440i) and amidated pectin (E 440ii) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2015). Submitted to EFSA on 31 May 2016.
IMR (Industrial Market Research), 2009. Special Profile: Pectin. IMR International Quarterly Review of Food Hydrocolloids. Submitted to EFSA on 11 November 2010.
Industrial‐Biotest (Industrial Biotest Laboratories), 1976. Report to International Pectin Producers Association: Teratogenic study with amidated pectin in albino rats. IBT No. 623‐07584. Submitted to EFSA on 11 November 2010.
Industrial‐Biotest (Hercules Incorporated), 1979a. Report to International Pectin Producers Association: Two‐year chronic oral toxicity study with amidated pectin in albino rats. Vol. I. Submitted to EFSA on 11 November 2010.
Industrial‐Biotest 1979b. Report to International Pectin Producers Association: Two‐year chronic oral toxicity study with amidated pectin in albino rats. Vol. IV. Submitted to EFSA on 11 November 2010.
Industrial‐Biotest (Hercules Incorporated), 1979c. Report to International Pectin Producers Association: Two‐year chronic oral toxicity study with amidated pectin in albino rats. Vol. II, IBT No. 8532‐07582. Submitted to EFSA on 11 November 2010.
Industrial‐Biotest (Hercules Incorporated), 1979d. Report to International Pectin Producers Association: Two‐year chronic oral toxicity study with amidated pectin in albino rats. Vol. III, Study 8532‐07582. Submitted to EFSA on 11 November 2010.
Industrial‐Biotest (Industrial Biotest), 1979e. Report to International Pectin Producers Association: Three generation reproduction study with amidated pectin in albino rats. Vol. I. Submitted to EFSA on 11 November 2010.
IPPA (International Pectin Producers Association), 2010. Draft: Pectin feed additives dossier that fulfils the requirements set out in Regulation 1831/2003 and the EFSA technical and administrative guidance documents (DHI, 2010). Submitted to EFSA on 11 November 2010.
IPPA (International Pectin Producers Association), 2015. Pectins. E 440i, ii. International Pectin Producers Association's (IPPA's) response to EFSA's ‘Call for technical data on certain thickening agents permitted as food additives in the EU’. Submitted to EFSA on 17 December, 2015.
IPPA (International Pectin Producers Association), 2016. Data on usage levels of pectin (E 440i) and amidated pectin (E 440ii) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2015). Submitted to EFSA on 30 May 2016.
IPPA (International Pectin Producers Association), 2017. Pectins (E 440i,ii). Additional data provided in response to the EFSA call for technical data on certain thickening agents permitted as food additives in the EU. Submitted to EFSA on 19 January 2017.
Mars, 2010. Food Additive (Emulsifier, Stabilisers and Gelling Agents) ‐ Present Usage − E 440. Pectins. Submitted to EFSA on 19 May 2010.
Mosinger M 1976. Two year chronic feeding study and multi‐generation reproductive ‐ Teratology study with amidated pectin (24.5% amidation). Unpublished report submitted to the International Pectin Producers Association, 97p. Submitted to EFSA on 11 November 2010.
SNE (Specialised Nutrition Europe), 2016. Data on usage levels of pectin (E 440i) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2015). Submitted to EFSA on 30 May 2016.
Til HP, Seinen W and de Groot AP, 1972. Sub‐chronic (90‐day) toxicity study with two samples of pectin (Melange A2 and C2) in rats. TNO Rapport Nr. R 3843. Submitted to EFSA on 11 November 2010.
Abbreviations
- ADI
acceptable daily intake
- AESGP
Association of the European Self‐Medication Industry
- ANS
Panel on Food Additives and Nutrient Sources
- BSTFA
N,O‐bis(trimethylsilyl) trifluoroacetamide
- bw
body weight
- CAS
Chemical Abstract Service
- CCCF
Codex Committee on Contaminants in Foods
- CDTA
trans‐1,2‐diaminocyclohexane‐N,N,N′,N′‐tetraacetic acid
- DEAE
diethylaminoethyl
- DE
degree of esterification
- DMSO
dimethyl sulfoxide
- EDA
European Dairy Association
- EHPM
European Federation of Health Products Manufacturers Associations
- EINECS
European Inventory of Existing Commercial Chemical Substances
- EMA
European Medicines Agency
- FAO/WHO
Food and Agriculture Organization/World Health Organisation
- FC
Food category
- FDE
FoodDrinkEurope
- FSMP
foods for special medical purposes
- FTIR
Fourier transform infrared spectrometry
- GC
gas chromatography
- GD
gestation day
- GLP
good laboratory practice
- GNPD
Mintel Global New Products Database
- GPC
gel permeation chromatography
- HEPES
4‐(2‐hydroxyethyl)piperazine‐1‐ethanesulfonic acid
- HF
high‐fibre
- HMP
high‐methoxylated pectins
- HPAE‐PAD
high‐performance anion‐exchange chromatography with pulsed amperometric detection
- HPLC
high‐performance liquid chromatography
- HPSEC
high‐performance size‐exclusion chromatography
- ICGA
International Chewing Gum Association
- IPPA
International Pectin Producers’ Association
- IR
infrared (spectrometry)
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LD50
lethal dose, median
- LF
low‐fibre
- LMP
low‐methoxylated pectins
- MF
mid‐fibre
- ML
maximum level
- MPL
maximum permitted level
- MS
mass spectrometry
- NDA
Panel on Dietetic Products, Nutrition and Allergies
- NMR
nuclear magnetic resonance
- NOAEL
no‐observed‐adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- pAOS
pectin‐derived acidic oligosaccharides
- PCW
plant cell walls
- QS
quantum satis
- RAAF
‘reference’ amino‐acid based formula
- SCF
Scientific Committee on Food
- SCFA
short‐chain fatty acids
- scFOS
short‐chain fructo‐oligosaccharides
- SNE
Specialised Nutrition Europe
- TAAF
thickened amino‐acid‐based formula
- TAMC
total aerobic microbial count
- TSP
3‐(trimethylsilyl)propionate
- TYMC
total combined yeast and mould count
- UV/VIS
ultraviolet/visual (spectrometry)
Appendix A – Summary of reported use levels (mg/kg or mg/L as appropriate) of pectin (E 440i) provided by industry
Appendix B – Summary of reported use levels (mg/kg or mg/L as appropriate) of amidated pectin (E 440ii) provided by industry
Appendix C – Summary of analytical results (mg/kg) of pectins (E 440) provided by Members States
Appendix D – Number and percentage of food products labelled with pectins (E 440) out of the total number of food products present in the Mintel GNPD per food subcategory between 2011 and 2016
Appendix E – Concentration levels of pectins (E 440) used in the maximum level exposure scenario, the refined exposure assessment scenarios and food supplements consumers only scenario (mg/kg or mL/kg as appropriate)
Appendix F – Summary of total estimated exposure of pectins (E 440) from their use as food additive for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Appendix G – Main food categories contributing to exposure to pectins (E 440) using the maximum level exposure scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
6.
Appendices A–G can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.4866
Supporting information
Summary of reported use levels (mg/kg or mg/L as appropriate) of pectin (E 440i) provided by industry
Summary of reported use levels (mg/kg or mg/L as appropriate) of amidated pectin (E 440ii) provided by industry
Summary of analytical results (mg/kg) of pectins (E 440) provided by Members States
Number and percentage of food products labelled with pectins (E 440) out of the total number of food products present in the Mintel GNPD per food subcategory between 2011 and 2016
Concentration levels of pectins (E 440) used in the maximum level exposure scenario, the refined exposure assessment scenarios and food supplements consumers only scenario (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of pectins (E 440) from their use as food additive for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to pectins (E 440) using the maximum level exposure scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Suggested citation: EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Dusemund B, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Lambré C, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Oskarsson A, Parent‐Massin D, Stankovic I, Waalkens‐Berendsen I, Wright M, Younes M, Tobback P, Ioannidou S, Tasiopoulou S and Woutersen RA, 2017. Scientific Opinion on the re‐evaluation of pectin (E 440i) and amidated pectin (E 440ii) as food additives. EFSA Journal 2017;15(7):4866, 62 pp. 10.2903/j.efsa.2017.4866
Requestor: European Commission
Question numbers: EFSA‐Q‐2011‐00528, EFSA‐Q‐2011‐00758
Panel members: Fernando Aguilar, Riccardo Crebelli, Alessandro Di Domenico, Birgit Dusemund, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Claude Lambré, Jean‐Charles Leblanc, Oliver Lindtner, Peter Moldeus, Alicja Mortensen, Pasquale Mosesso, Agneta Oskarsson, Dominique Parent‐Massin, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Jacqueline Wiesner. The ANS Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 17 May 2017
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
COM(2001) 542 final.
Food category 13.1.1: Infant formulae as defined by Directive 2006/141/EC; Food category 13.1.5.1: Dietary foods for infants for special medical purposes and special formulae for infants. This interpretation also applies to those food additives in food category 13.1.5.2 (Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC) for which exceptional uses in food for infants under the age of 12 weeks are indicated.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents. Published: 22 November 2009. Available online: http://www.efsa.europa.eu/en/dataclosed/call/ans091123
Call for technical data on certain thickening agents permitted as food additives in the EU. Published: 19 December 2014. Available online: http://www.efsa.europa.eu/it/data/call/141219
Call for food additives usage level and/or concentration data in food and beverages intended for human consumption. Published: 12 October 2015. Available online: http://www.efsa.europa.eu/en/data/call/151012
2 May 2017.
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1–295.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents. Published: 22 November 2009. Available online: https://www.efsa.europa.eu/en/dataclosed/call/ans091123
Missing Bulgaria, Cyprus, Estonia, Latvia, Lithuania, Luxembourg, Malta and Slovenia.
http://www.gnpd.com/sinatra/home/ accessed on 5/10/2016.
Available online: http://www.efsa.europa.eu/en/press/news/150428.htm
References
- Adiotomre J, Eastwood MA, Edwards CA and Brydon WG, 1990. Dietary fiber: in vitro methods that anticipate nutrition and metabolic activity in humans. American Journal of Clinical Nutrition, 52, 128–134. [DOI] [PubMed] [Google Scholar]
- Andoh A, Bamba T and Sasaki M, 1999. Physiological and anti‐inflammatory roles of dietary fiber and butyrate in intestinal functions. Journal of Parenteral and Enteral Nutrition, 23, S70–S73. [DOI] [PubMed] [Google Scholar]
- Anger H, Walzel E and Kahrmann B, 1994. About the absorption of oligo‐galacturonides from caecum of rats. The FASEB Journal, 8, A152. [Google Scholar]
- Bauer HG, Asp NG, Dahlqvist A, Fredlund P and Oste R, 1978. Effect of dietary fiber from various sources on the inducement of colonic tumors in the rat. European Surgical Research, 10, 112–113. [Google Scholar]
- Bauer HG, Asp NG, Dahlqvist A, Fredlund PE, Nyman M and Oste R, 1981. Effect of two kinds of pectin and guar gum on 1,2‐dimethylhydrazine initiation of colon tumors and on fecal beta‐glucuronidase activity in the rat. Cancer Research, 41, 2518–2523. [PubMed] [Google Scholar]
- BeMiller JN, 2007. Carbohydrate chemistry for food scientists, 2nd Edition. ACC International, Stl Paul, MM. pp. 303–311. [Google Scholar]
- Bettler B, Amado R and Neukom H, 1985. Electrophoretic determination of gelling and thickening agents on silylated glass fiber paper. Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und Hygiene, 76, 69–82. [Google Scholar]
- Borzelleca JF, Filer LJ Jr, Kinoshita FK, Gerrish TC, Kuo PK and La Du BN, 1996. Evaluation of the safety of sodium pectate as a food ingredient [published erratum appears in Food and Chemical Toxicology, 1996;34(4):432]. Food and Chemical Toxicology, 34, 21–25. [DOI] [PubMed] [Google Scholar]
- Brejnholt SM, 2010. Pectin. In: Imeson A (ed.). Food Stabilizers, Thickeners and Gelling Agents. Wiley‐Blackwell, Chichester. pp. 237–265. [Google Scholar]
- Brouns F, Theuwissen E, Adam A, Bell M, Berger A and Mensink RP, 2012. Cholesterol‐lowering properties of different pectin types in mildly hyper‐cholesterolemic men and women. European Journal of Clinical Nutrition, 66, 591–599. [DOI] [PubMed] [Google Scholar]
- Buchanan CJ, Fry SC and Eastwood MA, 1994. The metabolism and fate of [methyl‐14C] and [uronate‐6‐14C]pectin‐labelled dietary plant cell walls in the rat. Journal of the Science of Food and Agriculture, 66, 163–173. [Google Scholar]
- Capel F, Nicolai T, Durand D, Boulenguer P and Langendroff V, 2006. Calcium and acid induced gelation of (amidated) low methoxyl pectin. Food Hydrocolloids, 20, 901–907. [DOI] [PubMed] [Google Scholar]
- Cohen L, Perkins EG and Dobrilovic L, 1967. The manifold effects of different dietary carbohydrates. Progress in Biochemical Pharmacology, 2, 182–202. [Google Scholar]
- Cohen AJ, Forse MS and Tarlo SM, 1993. Occupational asthma caused by pectin inhalation during the manufacture of jam. Chest, 103, 309–311. [DOI] [PubMed] [Google Scholar]
- COT (Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment), 2011. COT statement on the effects of chronic dietary exposure to methanol. Available online: https://cot.food.gov.uk/sites/default/files/cot/cotstatementmethanol201102revjuly.pdf
- Crociani F, Alessandrini A, Mucci MM and Biavati B, 1994. Degradation of complex carbohydrates by Bifidobacterium spp. International Journal of Food Microbiology, 24, 199–210. [DOI] [PubMed] [Google Scholar]
- Cummings JH and Englyst HN, 1987. Fermentation in the human large intestine and the available substrates. American Journal of Clinical Nutrition, 45, 1243–1255. [DOI] [PubMed] [Google Scholar]
- Cummings JH, Southgate DA, Branch WJ, Wiggins HS, Houston H, Jenkins DJ, Jivraj T and Hill MJ, 1979. The digestion of pectin in the human gut and its effect on calcium absorption and large bowel function. British Journal of Nutrition, 41, 477–485. [DOI] [PubMed] [Google Scholar]
- Den Besten G, van Eunen K, Groen A, Venema K, Reijngoud DJ and Bakker M, 2013. The role of short‐chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. The Journal of Lipid Research, 54, 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz JV, Anthon GE and Barrett DM, 2007. Nonenzymatic degradation of citrus pectin and pectate during prolonged heating: effects of pH, temperature, and degree of methyl esterification. Journal of Agricultural and Food Chemistry, 55, 5131–5136. [DOI] [PubMed] [Google Scholar]
- Dilger RN, 2015. Effects of pectin on growth and digestibility in neonatal pigs. Study No. 1506. Unpublished study carried out at the Department of Animal Sciences, University of Illinois, Urbana, IL, USA. Submitted to WHO by Mead Johnson Nutrition. Cited in JECFA, 2016c.
- Dongowski G and Anger H, 1996. Metabolism of pectin in the gastrointestinal tract. Progress in Biotechnology, 14, 659–666. [Google Scholar]
- Dupont C, Kalach N, Soulaines P, Bradatan E, Lachaux A, Payot F, de Blay F, Guénard‐Bilbault L, Hatahet R and Mulier S, 2014. A thickened amino‐acid formula in infants with cow's milk allergy failing to respond to protein hydrolysate formulas: a randomized double‐blind trial. Paediatric Drugs, 16, 513–522. [DOI] [PubMed] [Google Scholar]
- Dupont C, Kalach N, Soulaines P, Bradatan E, Lachaux A, Payot F, De Blay F, Guénard‐Bilbault L, Hatahet R, Mulier S, Kapel N, Waligora‐Dupriet AJ and Butel MJ, 2015. Safety of a new amino acid formula in infants allergic to cow's milk and intolerant to hydrolysates. Journal of Pediatric Gastroenterology and Nutrition, 61, 456–463. [DOI] [PubMed] [Google Scholar]
- Durrington PN, Bolton CH, Manning AP and Hartog M, 1976. Effect of pectin on serum lipids and lipoproteins, whole gut transit time, and stool weight. Lancet, 2, 394–396. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Scientific Opinion of the Panel on Contaminants in the Food Chain (CONTAM) on cadmium in food. EFSA Journal 2009;7(3):980, 139 pp. 10.2903/j.efsa.2009.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012; 10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2013. Scientific Opinion on the re‐evaluation of aspartame (E 951) as a food additive. EFSA Journal 2013;11(12):3496, 263 pp. 10.2903/j.efsa.2013.3496 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2014. Statement on a conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010. EFSA Journal 2014;12(6):3697, 11 pp. 10.2903/j.efsa.2014.3697 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351, 199 pp. 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 241 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2010. Scientific Opinion on the substantiation of health claims related to pectins and reduction of post‐prandial glycaemic responses (ID 786), maintenance of normal blood cholesterol concentrations (ID 818) and increase in satiety leading to a reduction in energy intake (ID 4692) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2010;8(10):1747, 17 pp. 10.2903/j.efsa.2010.1747 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general Principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- Einhorn‐Stoll U, Hatakeyama H and Hatakeyama T, 2012. Influence of pectin modification on water binding properties. Food Hydrocolloids, 27, 494–502. [Google Scholar]
- Einhorn‐Stoll U, Prinz C and Drusch S, 2014. Gums and stabilisers for the food industry 17. Williams PA and Phillips GO. pp. 147–154. [Google Scholar]
- Eiwegger T, Stahl B, Haidl P, Schmitt J, Boehm G, Dehlink E, Urbanek R and Szépfalusi Z, 2010. Prebiotic oligosaccharides: in vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatric Allergy and Immunology, 21, 179–188. [DOI] [PubMed] [Google Scholar]
- Fanaro S, Jelinek J, Stahl B, Boehm G, Kock R and Vigi V, 2005. Acidic oligosaccharides from pectin hydrolysate as new component for infant formulae: effect on intestinal flora, stool characteristics, and pH. Journal of Pediatric Gastroenterology and Nutrition, 41, 186–190. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agricultural Organization of the United Nations/World Health Organization), 2014. Report of the eighth session of the codex committee on contaminants in foods. The Hague, the Netherlands, 31 March – 4 April 2014.
- Ferdman RM, Ong PY and Church JA, 2006. Pectin anaphylaxis and possible association with cashew allergy. Annals of Allergy, Asthma and Immunology, 97, 759–760. [DOI] [PubMed] [Google Scholar]
- Fincher GB, Stone BA and Clarke AE, 1983. Arabinogalactan‐proteins: structure, biosynthesis and function. Annual Review of Plant Physiology, 34, 47–70. [Google Scholar]
- Freeman HJ, Spiller GA and Kim YS, 1980. A double‐blind study on the effects of differing purified cellulose and pectin fiber diets on 1,2‐dimethylhydrazine‐induced rat colonic neoplasia. Cancer Research, 40, 2661–2665. [PubMed] [Google Scholar]
- Garnier C, Axelos MAV and Thibault JF, 1993. Phase diagrams of pectin–calcium systems: influence of pH, ionic strength and temperature on the gelation of pectins with different degrees of methylation. Carbohydrate Research, 240, 219–232. [Google Scholar]
- Garthoff JA, Heemskerk S, Hempenius RA, Lina BA, Krul CA, Koeman JH and Speijers GJ, 2010. Safety evaluation of pectin‐derived acidic oligosaccharides (pAOS): genotoxicity and sub‐chronic studies. Regulatory Toxicology and Pharmacology, 57, 31–42. [DOI] [PubMed] [Google Scholar]
- Gilmore NM, 1965. Effect of 5 per cent pectin N.F. or 5 per cent pectin L.M. upon growth, excretion, serum proteins and mineral contents in liver and kidney tissues of weanling male rats of Sprague‐Dawley strain (PhD thesis ‐ Abstract). Dissertation Abstract, 26, 4209–4210.
- Gilsenan PM, Richardson RK and Morris ER, 2000. Thermally reversible acid‐induced gelation of low‐methoxy pectin. Carbohydrate Polymers, 41, 339–349. [Google Scholar]
- Gilsenan PM, Richardson RK and Morris ER, 2003a. Associative and segregative interactions between gelatin and low‐methoxy pectin: part 1. Associative interactions in the absence of Ca2+ . Food Hydrocolloids, 17, 723–737. [Google Scholar]
- Gilsenan PM, Richardson RK and Morris ER, 2003b. Associative and segregative interactions between gelatin and low‐methoxy pectin: part 2. Co‐gelation in the presence of Ca2+ . Food Hydrocolloids, 17, 739–749. [Google Scholar]
- Goldin‐Lang P, Dongowski G and Zunft HJ, 1998. Influence of dietary fiber on DNA adduct formation in rat tissues. Zeitschrift für Ernährungswissenschaft, 37, 88–91. [PubMed] [Google Scholar]
- Goldsworthy TL, Hamm TE Jr, Rickert DE and Popp JA, 1986. The effect of diet on 2,6‐dinitrotoluene hepatocarcinogenesis. Carcinogenesis, 7, 1909–1915. [DOI] [PubMed] [Google Scholar]
- Grassino AN, Brnčić M, Vikić‐Topić D, Roca S, Dent M and Brnčić SR, 2016. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chemistry, 198, 93–100. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H and Hatakeyama T, 1998. Interaction between water and hydrophilic polymers. Themochimica Acta, 308, 3–22. [Google Scholar]
- Hedemann MS, Eskildsen M, Laerke HN, Pedersen C, Lindberg JE, Laurinen P and Knudsen KE, 2006. Intestinal morphology and enzymatic activity in newly weaned pigs fed contrasting concentrations and fiber properties. Journal of Animal Science, 6, 1375–1386. [DOI] [PubMed] [Google Scholar]
- Helgerud T, Gaserød O, Fjæreide T, Andersen PO and Larsen CK, 2010. Alginates. In: Imeson A (ed.). Food Stabilisers, Thickeners and Gelling Agents. Wiley‐Blackwell, UK. pp. 50–72. [Google Scholar]
- Hillman L, Peters S, Fisher A and Pomare EW, 1983. Differing effects of pectin, cellulose and lignin on stool pH, transit time and weight. British Journal of Nutrition, 50, 189–195. [DOI] [PubMed] [Google Scholar]
- Hogenkamp A, Knippels LM, Garssen J and van Esch BC, 2015. Supplementation of mice with specific nondigestible oligosaccharides during pregnancy or lactation leads to diminished sensitization and allergy in the female offspring. Journal of Nutrition, 145, 996–1002. [DOI] [PubMed] [Google Scholar]
- Holloway WD, Tasman‐Jones C and Maher K, 1983. Pectin digestion in humans. American Journal of Clinical Nutrition, 37, 253–255. [DOI] [PubMed] [Google Scholar]
- Huisman MMH, Brüll LP, Thomas‐Oates JE, Haverkamp J, Schols HA and Voragen AGJ, 2001. The occurrence of internal (1→5)‐linked arabinofuranose and arabinopyranose residues in arabinogalactan side chains from soybean pectic substances. Carbohydrates Research, 330, 103–114. [DOI] [PubMed] [Google Scholar]
- Hunziker HR and Miserez A, 1980. Gel permeation chromatography for the detection of gelling and thickening agents. Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und Hygiene, 71, 87–94. [Google Scholar]
- Iftikhar SY, Washington N, Wilson CG, MacDonald IA and Homer‐Ward MD, 1994. Effect of pectin on the gastric emptying rates and blood glucose levels after a test meal. Journal of Pharmacy and Pharmacology, 46, 851–853. [DOI] [PubMed] [Google Scholar]
- Industrial‐Biotest (Industrial Biotest Laboratories), 1976. Report to International Pectin Producers Association: teratogenic study with amidated pectin in albino rats. IBT No. 623‐07584.
- Industrial‐Biotest (Hercules Incorporated), 1979a. Report to International Pectin Producers Association: two‐year chronic oral toxicity study with amidated pectin in albino rats. Vol. I.
- Industrial‐Biotest , 1979b. Report to International Pectin Producers Association: Two‐year chronic oral toxicity study with amidated pectin in albino rats. Vol. IV. Submitted to EFSA on 11 November 2010.
- Industrial‐Biotest (Hercules Incorporated), 1979c. Report to International Pectin Producers Association: Two‐year chronic oral toxicity study with amidated pectin in albino rats. Vol. II, IBT No. 8532‐07582. Submitted to EFSA on 11 November 2010.
- Industrial‐Biotest (Hercules Incorporated), 1979d. Report to International Pectin Producers Association: Two‐year chronic oral toxicity study with amidated pectin in albino rats. Vol. III, Study 8532‐07582. Submitted to EFSA on 11 November 2010.
- Industrial‐Biotest (Industrial Biotest), 1979e. Report to International Pectin Producers Association: three‐generation reproduction study with amidated pectin in albino rats. Vol. I.
- IPCS , 2004. IPCS Risk Assessment Terminology (Part 1: IPCS/OECD Key Generic terms used in Chemical Hazard/Risk Assessment. Available online: http://www.inchem.org/documents/harmproj/harmproj/harmproj1.pdf
- Jaakkola MS, Tammivaara R, Tuppurainen M, Lahdenne L, Tupasela O and Keskinen H, 1997. Asthma caused by occupational exposure to pectin. Journal of Allergy and Clinical Immunology, 100, 575–576. [DOI] [PubMed] [Google Scholar]
- Jacobs LR and Lupton JR, 1986. Relationship between colonic luminal pH, cell proliferation and colon carcinogenesis in 1,2‐dimethylhydrazine treated rats fed high fiber diets. Cancer Research, 46, 1727–1734. [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1972. WHO Technical Report Series, Evaluation of certain food additives and the contaminants mercury, lead, and cadmium. Sixteenth report of the Joint FAO/WHO Expert Committee on Food Additives. No. 505. World Health Organization, Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1974. Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. 340. Pectin. WHO Food Additives Series, No. 5. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1978. Evaluation of certain food additives. Twenty‐first report of the Joint FAO/WHO Expert Committee on Food Additives. No. 617. World Health Organization, Geneva, Switzerland.
- JECFA (Joint FAO, WHO Expert Committee on Food Additives), 1981. Evaluation of certain food additives. Twenty‐fifth report of the Joint FAO/WHO Expert Committee on Food Additives, WHO Technical Report Series, No 669. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2009. Compendium of food additive specifications. Joint FAO/WHO Expert Committee on Food Additives, 71st Meeting. FAO/WHO, Rome, 2009. Available online: http://www.fao.org/3/a-i0971e.pdf
- JECFA (Joint FAO/WHO Expert Committee on Food Additive), 2014. Evaluation of certain food additives. Seventy‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, No. 990, 140 pp. Available online: http://apps.who.int/iris/bitstream/10665/150883/1/9789241209908_eng.pdf#page=61
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2015. Safety evaluation of certain food additives. Pectins (addendum). Seventy‐ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additives Series, No. 70, 139 pp. Available online: http://apps.who.int/iris/bitstream/10665/171781/3/9789240693982_eng.pdf#page=147
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2016a. Summary report of the 82nd Joint FAO/WHO Expert Committee on Food Additives (JECFA) meeting – Food additives. JECFA/82/SC. Available online: http://www.who.int/foodsafety/areas_work/chemical-risks/JECFA82_SummaryReport.pdf?ua=1
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2016b. Compendium of food additive specifications. Joint FAO/WHO Expert Committee on Food Additives, 82nd Meeting. FAO/WHO, Rome, 2016. Available online: http://www.fao.org/3/a-i6413e.pdf
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2016c. Evaluation of certain food additives. Eighty‐second report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, No. 1000, 176 pp. Available online: http://apps.who.int/iris/bitstream/10665/250277/1/9789241210003-eng.pdf#page=46
- Jeurink PV, van Esch BC, Rijnierse A, Garssen J and Knippels LMJ, 2013. Mechanisms underlying immune effects of dietary oligosaccharides. American Journal of Clinical Nutrition, 98, 572S–577S. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Lupton JR, Chang W‐CL, Jolly CA, Aukema HM and Chapkin RS, 1996. Dietary fat and fiber differentially alter intracellular second messengers during tumor development in rat colon. Carcinogenesis (Oxford), 17, 1227–1233. [DOI] [PubMed] [Google Scholar]
- Joye DD and Luzio GA, 2000. Process for selective extraction of pectins from plant material by differential pH. Carbohydrate Polymers, 43, 337–342. [Google Scholar]
- Kar F and Arslan N, 1999a. Effect of temperature and concentration on viscosity of orange peel pectin solutions and intrinsic viscosity‐molecular weight relationship. Carbohydrate Polymers, 40, 277–284. [Google Scholar]
- Kar F and Arslan N, 1999b. Characterization of orange peel pectin and effect of sugars, L‐ascorbic acid, ammonium persulfate, salts on viscosity of orange peel pectin solutions. Carbohydrate Polymers, 40, 285–291. [Google Scholar]
- Kerperien J, Jeurink PV, Wehkamp T, van der Veer A, van de Kant HJ, Hofman GA, van Esch EC, Garssen J, Willemsen LE and Knippels LM, 2014. Non‐digestible oligosaccharides modulate intestinal immune activation and suppress cow's milk allergic symptoms. Pediatric Allergy and Immunology, 25, 747–754. [DOI] [PubMed] [Google Scholar]
- Kertesz ZI, 1940. Pectic enzymes V. The fate of pectins in the animal body. Journal of Nutrition, 20, 289–296. [Google Scholar]
- Keys A, Grande F and Anderson JT, 1961. Fiber and pectin in the diet and serum cholesterol concentration in man. Proceedings of the Society for Experimental Biology and Medicine, 106, 555–558. [DOI] [PubMed] [Google Scholar]
- Kleinnijenhuis AJ, Staal YCM, Duistermaat E, Engel R and Woutersen RA, 2013. The determination of exogenous formaldehyde in blood of rats during and after inhalation exposure. Food and Chemical Toxicology, 52, 105–112. [DOI] [PubMed] [Google Scholar]
- Knaup B, Kempf M, Fuchs J, Valotis A, Kahle K, Oehme A, Richling E and Schreier P, 2008. Model experiments mimicking the human intestinal transit and metabolism of D‐galacturonic acid and amidated pectin. Molecular Nutrition and Food Research, 52, 840–848. [DOI] [PubMed] [Google Scholar]
- Koswig S, Fuchs G, Hotsommer HJ and Graefe U, 1997. The use of HPAE‐PAD for the analysis of thickening agents in fruit juice and food analysis. Seminars in Food Analysis, 2, 71–83. [Google Scholar]
- Kratchanova M, Slavov A and Kratchanov C, 2004. Interaction of pectin with amino acids and other amino compounds in aqueous solution. Food Hydrocolloids, 18, 677–683. [Google Scholar]
- Kraut A, Peng Z, Becker AB and Warren CPW, 1992. Christmas Candy Maker's Asthma. IgG4‐Mediated pectin allergy. Chest, 102, 1605–1607. [DOI] [PubMed] [Google Scholar]
- Krzeminski A, Prell KA, Weiss J and Hinrichs J, 2014. Environmental response of pectin‐stabilized whey protein aggregates. Food Hydrocolloids, 35, 332–340. [Google Scholar]
- Lim OB, Choue RW, Ki Park D, Kim HC, Kim SY, Yamada K and Sugano M, 2002. Effect of dietary level of pectin on immunoglobulin and cytokine production by mesenteric lymph node lymphocytes and Interleukin‐2 receptor in rats. Food Science and Technology Research, 8, 14–16. [Google Scholar]
- Lindinger W, Taucher J, Jordan A, Hansel A and Vogel W, 1997. Endogenous production of methanol after the consumption of fruit. Alcoholism, Clinical and Experimental Research, 21, 939–943. [PubMed] [Google Scholar]
- List D, Buddruss S and Bodtke M, 1985. Determination of pectin with m‐phenylphenol. Zeitschrift für Lebensmittel‐Untersuchung und–Forschung, 180, 48–52. [Google Scholar]
- Magne F, Hachelaf W, Suau A, Boudraa G, Bouziane‐Nedjadi K, Rigottier‐Gois L, Touhami M, Desjeux JF and Pochart P, 2008. Effects on faecal microbiota of dietary and acidic oligosaccharides in children during partial formula feeding. Journal of Pediatric Gastroenterology and Nutrition, 46, 580–588. [DOI] [PubMed] [Google Scholar]
- Mallett AK, Rowland IR and Farthing MJ, 1987. Dietary modification of intestinal bacterial enzyme activities − potential formation of toxic agents in the gut. Scandinavian Journal of Gastroenterology, Supplement, 129, 251–257. [DOI] [PubMed] [Google Scholar]
- Maroziene A and de Kruif CG, 2000. Interaction of pectin and casein micelles. Food Hydrocolloids, 14, 391–394. [Google Scholar]
- Martindale , 2016. The complete drug reference. 38th Edition, edited by Alison Brayfield, Pharmaceutical Press, London, Pectin, 2167 pp. [Google Scholar]
- May CD, 1990. Industrial pectins: sources, production and applications. Carbohydrate Polymers, 12, 79–99. [Google Scholar]
- Melton LD and Smith BG, 2001. Determination of neutral sugars by gas chromatography of their alditol acetates. Current Protocols in Food Analytical Chemistry. E3.2.1–E3.2.13. [Google Scholar]
- Merck Index , 2012. Pectin. In: The Merck Index − An Encyclopedia of Chemicals, Drugs, and Biologicals. Merck & Co., Inc., Whitehouse Station, NJ. [Google Scholar]
- Mergenthaler E and Scherz H, 1976. Analysis of polysaccharides used as food additives. IV. Gas chromatographic identification and determination of neutral components in polysaccharide hydrolysates. Zeitschrift für Lebensmittel‐Untersuchung und–Forschung, 162, 25–29. [DOI] [PubMed] [Google Scholar]
- Mergenthaler E and Scherz H, 1980. [Detection and determination of gelling and thickening agents]. In: Neukom H, W Pilnik (ed.). Gelier‐ und Verdickungsmittel in Lebensmitteln, S 191, LWT‐Edition 5, 191‐211. Forster Verlag AG, Zürich. pp. 191–211. [Google Scholar]
- Moore MR, 1994. [Unpublished report (no further bibliographic data); cited in Borzelleca JF, Filer LJ Jr, Kinoshita FK, Gerrish TC, Kuo PK and La Du BN, 1996. [Google Scholar]
- Mortelmans KE and Griffin A, 1982. Microbial mutagenesis testing of substances. FDA (Food and Drug Administration), NTIS Report: PB89‐169106.
- Mosinger M, 1974. Expérimentation d’épreuve concernant les effets de l'administration orale prolongée du produit pectine L.H. NST de la Société Unipectine SA. Unpublished report from the ‘Central d'explorations et de recherches médicales’, Marseille; cited in JECFA, 1981.
- Mosinger M, 1976. Two year chronic feeding study and multi‐generation reproductive‐teratology study with amidated pectin (24.5% amidation). Unpublished report submitted to the International Pectin Producers Association, 97 pp.
- Moya F, Sisk PM, Walsh KR and Berseth CL, 2012. A new liquid human milk fortifier and linear growth in preterm infants. Pediatrics, 130, e928–e935. [DOI] [PubMed] [Google Scholar]
- MPI , 2013. Pectin: a dietary 3‐week safety study in farm piglets. MPI Research Study No. 1981‐002. Unpublished study carried out at MPI Research Inc., Mattawan, MI, USA. Submitted to WHO by International Special Dietary Foods Industries; cited in JECFA, 2015.
- Müller‐Maatsch J, Caligiani A, Tedeschi T, Elst K and Sforza S, 2014. Simple and validated quantitative ¹H NMR method for the determination of methylation, acetylation, and feruloylation degree of pectin. Journal of Agricultural and Food Chemistry, 62, 9081–9087. [DOI] [PubMed] [Google Scholar]
- Müller‐Maatsch J, Bencivenni M, Caligiania A, Tedeschi T, Bruggeman G, Bosch M, Petrusan J, Van Droogenbroeck B, Elstf K and Sforza S, 2016. Pectin content and composition from different food waste streams. Food Chemistry, 201, 37–45. [DOI] [PubMed] [Google Scholar]
- Nazaruddin R, Noor Baiti AA, Foo SC, Tan YN and Ayob MK, 2013. Comparative chemical characteristics of hydrochloric acid‐ and ammonium oxalate‐extracted pectin from roselle (Hibiscus sabdariffa L.) calyces. International Food Research Journal, 20, 281–284. [Google Scholar]
- Oakenful D and Scott A, 1984. Hydrophobic interaction in the gelation of high methoxyl pectins. Journal of Food Science, 49, 1039–1098. [Google Scholar]
- Ochoa‐Villarreal M, Aispuro‐Hernández E, Martínez‐Téllez MA and Vargas‐Arispuro I, 2012. Plant Cell Wall Polymers: function, Structure and Biological Activity of Their Derivatives. In: Polymerization, Dr. Ailton De Souza Gomes (Ed.). [Google Scholar]
- Ochuba and Von Riesen , 1980. Fermentation of polysaccharides by Klebsielleae and other facultative bacilli. Applied and Environmental Microbiology, 39, 988–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 1981. OECD Guideline for the testing of chemicals, 408. Subchronic Oral Toxicity – Rodent: 90‐day Study. Adopted 12 May 1981.
- OECD (Organisation for Economic Co‐operation and Development), 1983. OECD Guideline for the testing of chemicals, 415. One‐Generation Reproduction Toxicity Study. Adopted 26 May 1983.
- OECD (Organisation for Economic Co‐operation and Development), 2014. OECD Guideline for the testing of chemicals, 473. In Vitro Mammalian Chromosomal Aberration Test. Adopted 26 September 2014.
- OECD (Organisation for Economic Co‐operation and Development), 2016. OECD Guideline for the testing of chemicals, 490. In Vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene. Adopted 29 July 2016.
- Pechanek U, Blaicher G, Pfannhauser W and Woidich H, 1982. Electrophoretic method for qualitative and quantitative analysis of gelling and thickening agents. Journal of the Association of Official Analytical Chemists, 65, 745–752. [Google Scholar]
- Phatak L, Chang KC and Brown G, 1988. Isolation and characterization of pectin in sugar‐beet pulp. Journal of Food Science, 53, 830–833. [Google Scholar]
- Piemontese P, Giannì ML, Braegger CP, Chirico G, Grüber C, Riedler J, Arslanoglu S, van Stuijvenberg M, Boehm G, Jelinek J and Roggero P and MIPS 1 Working Group , 2011. Tolerance and safety evaluation in a large cohort of healthy infants fed an innovative prebiotic formula: a randomized controlled trial. PLoS ONE, 6, e28010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilnik W and Voragen AGJ, 1992. Gelling agents (pectins) from plants for the food industry. Advances in Plant Cell Biochemistry and Biotechnology, 1, 219–270. [Google Scholar]
- Popov SV and Ovodov YS, 2013. Polypotency of the immunomodulatory effect of pectins. Biochemistry (Mosc), 78, 823–835. [DOI] [PubMed] [Google Scholar]
- Preuss A and Thier HP, 1982. Quantitative analysis of natural thickeners and gums by methanolysis and capillary column gas chromatography. Zeitschrift für Lebensmittel‐Untersuchung und–Forschung, 175, 93–100. [Google Scholar]
- Preuss A and Thier HP, 1983. Isolation of natural thickeners from food by capillary gas chromatographic determination. Zeitschrift für Lebensmittel‐Untersuchung und–Forschung, 176, 5–11. [Google Scholar]
- Prival MJ, Simmon VF and Mortelmans KE, 1991. Bacterial mutagenicity testing of 49 food ingredients gives very few positive results. Mutation Research, 260, 321–329. [DOI] [PubMed] [Google Scholar]
- Quemener B, Marot C, Mouillet L, Da RV and Diris J, 2000. Quantitative analysis of hydrocolloids in food systems by methanolysis coupled to reverse HPLC. Part 2. Pectins, alginates and xanthan. Food Hydrocolloids, 14, 19–28. [Google Scholar]
- Rabbani GH, Teka T, Saha SK, Zaman B, Majid N, Khatun M, Wahed MA and Fuchs GJ, 2004. Green banana and pectin improve small intestinal permeability and reduce fluid loss in Bangladeshi children with persistent diarrhea. Digestive Diseases and Sciences, 49, 475–484. [DOI] [PubMed] [Google Scholar]
- Ralet M‐C, Bonnin E and Thibault J‐F, 2002. Pectins. In: De Baets s, Vandamme EJ, Alexander Steinbuchel A (eds.). Biopolymers, Volume 6, Polysaccharides II: polysaccharides from Eukaryotes, Wiley‐VCH, Weinheim, Germany. pp. 345–380. [Google Scholar]
- Rasanen L, Makinen‐Kiljunen S and Harvima RJ, 1998. Pectin and cashew nut allergy cross‐reacting allergens? Allergy (Copenhagen), 53, 626–628. [DOI] [PubMed] [Google Scholar]
- Rolin C, Nielsen BU and Glahn P‐E, 1998. Pectin. In: Dumitriu S (ed.). Polysaccharides: Structural Diversity and Functional Versatility. Dekker, New York. pp. 377–431. [Google Scholar]
- Saito D, Nakaji S, Fukuda S, Shimoyama T, Sakamoto J and Sugawara K, 2005. Comparison of the amount of pectin in the human terminal ileum with the amount of orally administered pectin. Nutrition, 21, 914–919. [DOI] [PubMed] [Google Scholar]
- Salyers AA, Vercellotti JR, West SE and Wilkins TD, 1977a. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Applied and Environmental Microbiology, 33, 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers AA, West SE, Vercellotti JR and Wilkins TD, 1977b. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Applied and Environmental Microbiology, 34, 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food), 1978. Report of the Scientific Committee for Food. 7th Series, 44 pp.
- SCF (Scientific Committee for Food), 1985. Opinion expressed 1983. Reports from the Scientific Committee for Food, 15th Series.
- SCF (Scientific Committee for Food), 1996. Opinion on additives in nutrient preparations for use in infant formulae, follow‐on formulae and weaning foods. 7 June 1996.
- SCF (Scientific Committee for Food), 1998. Opinion of the Scientific Committee of Food on the applicability of the ADI (Acceptable Daily Intake) for food additives to infants. 17 September 1998.
- SCF (Scientific Committee on Food), 2001. Guidance on submissions for food additive evaluations by the Scientific Committee on Food. Opinion expressed on 11 July 2001.
- SCF (Scientific Committee for Food), 2003. Report of the Scientific Committee on Food on the Revision of Essential Requirements of Infant Formulae and Follow‐on Formulae (adopted on 4 April 2003).
- Schaefer H and Scherz H, 1983. Analysis of polysaccharides used as food additives. VII. Zone electrophoresis on dimethyldichlorosilane‐pretreated glass‐fiber sheets. Zeitschrift für Lebensmittel‐Untersuchung und–Forschung, 177, 193–195. [Google Scholar]
- Sharma BR, Naresh L, Dhuldhoya NC, Merchant SU and Merchant UC, 2006. An overview on pectins. Times Food Processing Journal, June‐July. pp. 44–51. [Google Scholar]
- Shim KJ and Choung SY, 2003. Single oral toxicity of Jeju citrus rind pectin in Sprague‐Dawley rats. Journal of Applied Pharmacology, 11, 109–111. [Google Scholar]
- Shimizu M, Noda T, Yamano T, Yamada A and Morita S, 1993. Acute oral toxicity of natural food additives in mice and rats. Seikatsu Eisei, 37, 215–220. [Google Scholar]
- Skara N, Novotni D, Cukelj N, Smerdel B and Curic D, 2013. Combined effects of inulin, pectin and guar gum on the quality and stability of partially baked frozen bread. Food Hydrocolloids, 30, 428–436. [Google Scholar]
- Spiller GA, Chernoff MC, Hill RA, Gates JE, Nassar JJ and Shipley EA, 1980. Effect of purified cellulose, pectin, and a low‐residue diet on fecal volatile fatty acids, transit time, and fecal weight in humans. American Journal of Clinical Nutrition, 33, 754–759. [DOI] [PubMed] [Google Scholar]
- Srivastava P and Rishabha M, 2011. Sources of pectin, extraction and its applications in pharmaceutical industry − an overview. Indian Journal of Natural Products and Resources, 2, 10–18. [Google Scholar]
- Sunvold GD, Fahey GC Jr, Merchen NR, Titgemeyer EC, Bourquin LD, Bauer LL and Reinhart GA, 1995a. Dietary fiber for dogs: IV. In vitro fermentation of selected fiber sources by dog fecal inoculum and in vivo digestion and metabolism of fiber‐supplemented diets. Journal of Animal Science, 73, 1099–1109. [DOI] [PubMed] [Google Scholar]
- Sunvold GD, Fahey GC Jr, Merchen NR, Bourquin LD, Titgemeyer EC, Bauer LL and Reinhart GA, 1995b. Dietary fiber for cats: in vitro fermentation of selected fiber sources by cat fecal inoculum and in vivo utilization of diets containing selected fiber sources and their blends. Journal of Animal Science, 73, 2329–2339. [DOI] [PubMed] [Google Scholar]
- Takagi H, Yasuhara K, Mitsumori K, Onodera H, Takegawa K and Takahashi M, 1997. A 13‐week subacute oral toxicity study of pectin digests in rats. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku, 115, 119–124. [PubMed] [Google Scholar]
- TemaNord (Nordic Council of Ministers), 2002. Food Additives in Europe 2000 − Status of safety assessments of food additives presently permitted in the EU. Pectin and amidated pectin.
- Thibault J‐F, 1983. Enzymatic degradation and β‐elimination of the pectic substances in cherry fruits. Phytochemistry, 22, 1567–1571. [Google Scholar]
- Til HP, Seinen W and de Groot AP, 1972. Sub‐chronic (90‐day) toxicity study with two samples of pectin (Melange A2 and C2) in rats. TNO Rapport Nr. R 3843.
- Topping DL and Clifton PM, 2001. Short‐chain fatty acids and human colonic function: roles and resistant starch and nonstarch polysaccharides. Physiological Reviews, 81, 1031–1064. [DOI] [PubMed] [Google Scholar]
- Tuinier R, Rolin C and de Kruif CG, 2002. Electrosorption of pectin onto casein micelles. Biomacromolecules, 3, 632–638. [DOI] [PubMed] [Google Scholar]
- Viola S, Zimmermann G and Mokady S, 1970. Effect of pectin and algin upon protein utilization, digestibility of nutrients and energy in young rats. Nutrition Reports International, 1, 367–375. [Google Scholar]
- Voragen ACJ, 2007. Polysaccharides. In: Ullmann's Encyclopedia of Industrial Chemistry, Online version. Wiley‐VCH Verlag GmbH & Co. KGaA. [Google Scholar]
- Voragen AGJ, Pilnik W, Thibault J‐F, Axelos MAV and Renard CMGC, 1995. Pectins. In: Stephen AM (ed.). Food Polysaccharides and Their Applications. Marcel Dekker Inc., New York, USA. pp. 287–339. [Google Scholar]
- Voragen AGJ, Coenen G‐J, Verhoef RP and Schols HA, 2009. Pectin, a versatile polysaccharide present in plant cell walls. Structural Chemistry, 20, 263–275. [Google Scholar]
- Vos AP, van Esch BC, Stahl B, M'Rabet L, Folkerts G, Nijkamp FP and Garssen J, 2007. Dietary supplementation with specific oligosaccharide mixtures decreases parameters of allergic asthma in mice. International Immunopharmacology, 7, 1582–1587. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Reddy BS, Weisburger JH and Kritchevsky D, 1979. Effect of dietary alfalfa, pectin and wheat bran on azoxymethane‐induced or methylnitrosourea‐induced colon carcinogenesis in F‐344 rats. Journal of the National Cancer Institute, 63, 141–146. [PubMed] [Google Scholar]
- Werch SC and Ivy AC, 1941. On the fate of ingested pectin. Journal of Digestive Diseases, 101–105. [Google Scholar]
- Werch SC, Jung RW, Day AA, Friedemann TE and Ivy AC, 1942. The decomposition of pectin and galacturonic acid by intestinal bacteria. The Journal of Infectious Diseases, 70, 231–242. [Google Scholar]
- White GW, Katona T and Zodda JP, 1999. The use of high‐performance size exclusion chromatography (HPSEC) as a molecular weight screening technique for polygalacturonic acid for use in pharmaceutical applications. Journal of Pharmaceutical and Biomedical Analysis, 20, 905–912. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 1997. Methanol. Environmental Health Criteria 196. Available online: http://www.inchem.org/documents/ehc/ehc/ehc196.htm
- Zoetis T, 1991. Unpublished report (no further bibliographic data); cited in Borzelleca et al., 1996.
- Zunft HJ, Goldin‐Lang P and Dongowski G, 1997. DNA adducts in tissues of germ‐free and conventional rats fed high‐ and low‐esterified pectins. Cancer Letters, 114, 43–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of reported use levels (mg/kg or mg/L as appropriate) of pectin (E 440i) provided by industry
Summary of reported use levels (mg/kg or mg/L as appropriate) of amidated pectin (E 440ii) provided by industry
Summary of analytical results (mg/kg) of pectins (E 440) provided by Members States
Number and percentage of food products labelled with pectins (E 440) out of the total number of food products present in the Mintel GNPD per food subcategory between 2011 and 2016
Concentration levels of pectins (E 440) used in the maximum level exposure scenario, the refined exposure assessment scenarios and food supplements consumers only scenario (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of pectins (E 440) from their use as food additive for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to pectins (E 440) using the maximum level exposure scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)


