Abstract
Benzophenone [FL‐no: 07.032] has been evaluated as a flavouring substance, in FGE.69, by the EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food in 2008. Benzophenone was evaluated also by JECFA (2011) and by IARC (2013) based on studies that were not considered in the EFSA opinion on FGE.69. Therefore, the Commission requested the CEF Panel to carry out a review of existing literature on the safety of this flavouring substance. In the framework of the evaluation of benzophenone as a food contact material, the CEF Panel established a tolerable daily intake (TDI) of 0.03 mg/kg body weight (bw) per day (2009). In the present Opinion, the Panel considered the already existing evaluations by EFSA, JECFA, IARC and available literature data on benzophenone toxicity. Moreover, new data on the use levels of benzophenone as a flavouring substance have been provided. The Panel considers that there is no concern with respect to genotoxicity. The Panel considers the endocrine activities of benzophenone and its metabolite 4‐hydroxybenzophenone as weak and not directly related to the observed toxic effects including the neoplastic effects in rodents. The Panel confirms that the conservative approach taken by EFSA (2009) to derive a TDI of 0.03 mg/kg bw for benzophenone is appropriate to cover the non‐neoplastic effects in the chronic toxicity studies and the neoplastic effects induced in the rodent carcinogenicity studies. The TDI is in the same order of magnitude as the chronic dietary exposure of adults and children to benzophenone (10–20 μg/kg bw per day) for the amount of added flavouring substance. The Panel considers that the calculated TDI and exposure estimate are based on conservative assumptions. The Panel concludes that there is no safety concern for benzophenone under the current condition of use as a flavouring substance.
Keywords: benzophenone, [FL‐no: 07.032], FGE.69, 119‐61‐9, genotoxicity, carcinogenicity, flavourings
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The use of flavourings is regulated under Regulation (EC) No 1334/20081 of the European Parliament and Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation, an evaluation and approval are required for flavouring substances.
The Union list of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/20122.
The substance benzophenone, [FL no. 07.032] (CAS No. 119‐61‐9) is currently included in this Union List. It is a substance which is not currently under evaluation. This substance is also known as diphenyl ketone. This substance was included in the Union list on the basis of the EFSA evaluation in FGE 69 of 2008. FGE 69 includes this substance. Benzophenone was evaluated by JECFA as a flavouring substance, with JECFA No. 831. The studies evaluated by IARC were not considered in the EFSA opinion on FGE 69. There may be also additional studies on the safety of this substance.
The substance ethyl acrylate, [FL no. 09.037], (CAS No. 140‐88‐5) was included in the Union list on the basis of the EFSA evaluation in FGE 71 of 2010. FGE 71 includes this substance. Ethyl acrylate was evaluated by JECFA as a flavouring substance, with JECFA No. 1351. The studies evaluated by IARC were not considered in the EFSA opinion of FGE 71. There may be also additional studies on the safety of this substance.
1.1.2. Terms of Reference
In accordance with Art. 29 (1) (a) of Regulation (EC) No 178/20023, the European Commission requests the European Food Safety Authority (EFSA) to carry out a review of existing published literature on the safety of the flavouring substances benzophenone, [FL. No 07.032], (CAS No. 119‐61‐9) and ethyl acrylate, [FL 09.037], (CAS No. 140‐88‐5), and advise on their safety when used as flavouring substances.
1.2. Interpretation of the Terms of Reference
Since benzophenone [FL‐ no: 07.032] and ethyl acrylate [FL‐no: 09.037] are not structurally related and were evaluated in different FGEs (FGE.69 and FGE.71), they will be evaluated in separate opinions. The present document will address the question of the Commission on benzophenone. Meanwhile, EFSA has also received information on use levels which will be taken into consideration. Considering the Terms of Reference, the Panel will not address in this assessment, the exposure that may result from the use as food contact material.
In respect to the approach to be followed for the assessment of benzophenone used as a flavouring substance, the Panel was of the view that previous assessments should be used as starting points for this scientific opinion. The previous assessment will be updated with new information that is connected to the concerns identified in the background (Section 1.1).
In the background (Section 1.1), it is especially mentioned that the studies evaluated by IARC in the report on carcinogenicity of benzophenone from 2013 were not considered in the EFSA evaluation from 2008, and that there may be additional studies on the safety of benzophenone when used as a flavouring substance. Thus, the present evaluation is mainly focusing on the genotoxicity and carcinogenicity of benzophenone, in addition to considering any new data on toxicity, especially effects on the endocrine system, which may have an impact on the safety assessment of benzophenone as flavouring. In addition, previous assessments of benzophenone by JECFA (2011) and EFSA (2009) are briefly presented. A number of benzophenone derivatives are used as UV‐filters in sunscreens; however, the safety of these compounds will not be assessed in the present evaluation, which will focus on benzophenone, the compound used as a flavouring substance.
1.3. Information on evaluations from other organisations
In the JECFA report on ‘Aromatic substituted secondary alcohols, ketones and related esters’ (JECFA, 2011), additional eight substances were evaluated and new carcinogenicity data (NTP, 2006) which have become available since JECFA evaluated benzophenone at their 57th meeting (2001) were included.
The European Chemical Agency (ECHA) issued a Decision on Substance Evaluation for benzophenone (ECHA, 2015) in which oestrogenic, anti‐androgenic and thyroidal activities of the substance were addressed. While ECHA acknowledged that the submitted long‐term studies on benzophenone did not provide evidence for ‘endocrine disruption due to its estrogenic properties’, it was also outlined that these studies did not include some endpoints (e.g. on mammary gland histology or follicular maturation) which are ‘especially sensitive to estrogenic substances’. The EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) noted that the ECHA document was mainly focussed on aquatic toxicity and the studies on endocrine activities were already discussed in the previous EFSA opinion (EFSA, 2009).
The endocrine effects of benzophenone and its metabolites were also discussed by IARC (2013) as potential mechanisms of tumour induction. Based on the results from NTP carcinogenicity studies (NTP, 2006), IARC (2013) concluded that there was sufficient evidence in experimental animals for carcinogenicity and classified benzophenone as possibly carcinogenic to humans (Group 2B).
2. Data and methodologies
The search for literature was done by EFSA on benzophenone [FL‐no: 07.032] to review existing literature on the safety of this substance and to advise on its safety when used as flavouring substance. A literature search was carried out through Web of Science database, until August 2017, using keywords ‘benzophenone’ or ‘119‐61‐9’ and ‘genotox*’, ‘canc*’, ‘carc*’, ‘tumor*’, ‘toxicokin*’, ‘metabol*’, ‘absorb*’, ‘distrib*’, ‘excret*’, ‘estrogen*’, ‘endocr*’, ‘diet*’ and ‘reproduct*’ while searching in ‘all databases’. Additional searches were carried out through Scopus database, using the keyword combination of ‘benzophenone’ and ‘tox’, and the database of Decernis, using the keyword ‘119‐61‐9’.
3. Procedure of the safety assessment
3.1. Assessment
The Panel considered studies already evaluated (EFSA, 2008, 2009; JECFA, 2011) and new data from literature search. Except for genotoxicity, no new relevant studies were identified for short‐term toxicity, chronic toxicity and carcinogenicity.
3.2. Technical data
Information on identity of the substance and specifications are based on data already described in FGE.69 (EFSA, 2008) and are presented in Table 1.
Table 1.
Summary of specifications for the substance benzophenone (EFSA, 2008)
| FL‐no JECFA‐no | EU Register name | Structural formula | FEMA no CoE no CAS no | Phys. form Mol. formula Mol. weight | Solubilitya Solubility in ethanolb | Boiling point, °Cc Melting point, °C ID test Assay minimum | Refrac. Indexd Spec. gravitye |
|---|---|---|---|---|---|---|---|
|
07.032 831 |
Benzophenone |
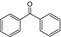
|
2134 166 119‐61‐9 |
Solid C13H10O 182.22 |
Insoluble Very soluble |
305 48 IR 98% |
n.a. n.a. |
FL‐no: FLAVIS number; JECFA: The Joint FAO/WHO Expert Committee on Food Additives; FEMA: Flavor and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service; IR: infrared spectroscopy; n.a.: not applicable.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1,013.25 hPa, if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
3.3. Information on existing evaluations from EFSA
Benzophenone [FL‐no: 07.032] has been evaluated by JECFA (2002). EFSA considered this evaluation and concluded, in FGE.69, that benzophenone is of no safety concern at estimated level of intake as flavouring substance based on the Maximised Survey‐derived Daily Intake (MSDI) approach (EFSA, 2008).
Benzophenone is also used as an additive in food contact materials (FCM) and was evaluated by the CEF Panel in 2009 (EFSA, 2009). The Panel considered that the non‐neoplastic kidney effects observed in male rats (NTP, 2006) were adverse and applied a benchmark dose (BMD) analyses on this endpoint, deriving a tolerable daily intake (TDI) of 0.03 mg/kg body weight (bw).
3.4. Exposure
3.4.1. Concentration in processed and non‐processed foods from natural sources
Benzophenone [FL‐no: 07.032] has been reported to occur in bullocks heart, cherimoya, grape, mountain papaya, passion fruit, soursop, tea and vanilla (Triskelion, 2017). Quantitative data are reported for benzophenone in mountain papaya (< 0.01 mg/kg), passion fruit (0.045 mg/kg), grape (up to 0.13 mg/kg) and vanilla (up to 0.48 mg/kg) (Triskelion, 2017).
3.4.2. Chronic dietary exposure
The exposure assessment to be used in the Procedure for the safety evaluation of the candidate substance is the chronic added portions exposure technique (APET) estimate (EFSA CEF Panel, 2010). The chronic APET for benzophenone has been calculated for adults and children (see Table 2 and Appendix C). The chronic APET calculation is based on the combined normal occurrence level.
Table 2.
APET – Chronic dietary exposure
| Chronic APET | Addeda | Other dietary sourcesb | Combinedc | |||
|---|---|---|---|---|---|---|
| μg/kg bw per day | μg/person per day | μg/kg bw per day | μg/person per day | μg/kg bw per day | μg/person per day | |
| Adultsd | 8.5 | 512 | 0.30 | 18.2 | 8.5 | 512 |
| Childrene | 22 | 323 | 0.76 | 11.5 | 22 | 323 |
APET: added portions exposure technique; bw: body weight.
APET Added is calculated on the basis of the normal amount of flavouring added to a specific food category.
APET Other Dietary Sources is calculated based on the natural occurrence of the flavouring in a specified food category.
APET Combined is calculated based on the combined amount of added flavouring and naturally occurring flavouring in a specified food category.
For the adult APET calculation, a 60‐kg person is considered representative.
For the child APET calculation, a 3‐year‐old child with a 15 kg bw is considered representative.
The Panel noted that the contribution from natural occurrence of benzophenone to the overall exposure is approximately 3–5% of the APET.
The Panel noted that there are other sources of exposure, e.g. from FCM as described in the EFSA 2009 opinion. According to Regulation (EU) 10/20114 on plastics, benzophenone has a specific migration limit (SML) of 0.6 mg/kg food corresponding to a maximum exposure of 600 μg/person per day. Based on this SML, the maximum exposure to benzophenone from FCM would be one‐third of the present TDI (0.03 mg/kg bw per day). The Panel considers that the calculated TDI is conservative and that the exposure data is based on worst‐case scenarios.
3.4.3. Acute dietary exposure
Acute exposure is not evaluated, because this opinion addresses only chronic exposure.
3.5. Biological and toxicological data
3.5.1. Absorption, distribution, metabolism and excretion
A rapid absorption of benzophenone [FL‐no: 07.032] from the gastrointestinal tract in rats was observed after a single oral dose of 100 mg/kg bw (Jeon et al., 2008).
The metabolism of benzophenone has been investigated in vitro in freshly isolated rat hepatocytes (Nakagawa et al., 2000) and in rats (Nakagawa and Tayama, 2002; Jeon et al., 2008). In rat hepatocytes, benzophenone was converted to benzhydrol, 4‐hydroxybenzophenone and the sulphate conjugate of 4‐hydroxybenzophenone (Nakagawa et al., 2000).
Also in vivo, benzhydrol and 4‐hydroxybenzophenone were reported to be the main metabolites in male rats (Figure 1) after a single oral dose of 100 mg/kg bw (Jeon et al., 2008). The same two metabolites were identified in plasma of female rats after oral doses of 100 and 400 mg/kg bw with benzhydrol being the major metabolite (Nakagawa and Tayama, 2002).
Figure 1.
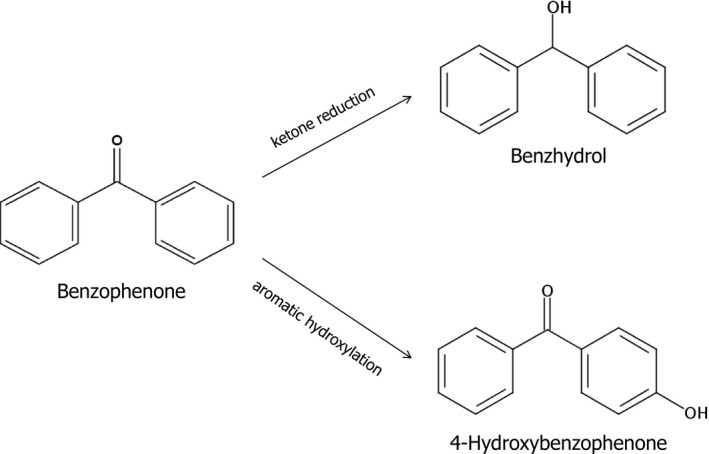
Proposed metabolism of benzophenone in rats (Nakagawa and Tayama, 2002; Jeon et al., 2008)
Overall, from studies in rats benzophenone has been demonstrated to be rapidly absorbed in the gastrointestinal tract and metabolised mainly to benzhydrol and 4‐hydroxybenzophenone.
3.5.2. Short‐term and subchronic toxicity
Short‐term animal studies, previously described in the EFSA scientific opinion (2009), suggested that the liver and kidneys were target organs for benzophenone toxicity, as well as the haematopoietic system (USEPA, 1984; Burdock et al., 1991). In 14‐week toxicity studies by NTP (2000), evaluated by EFSA (2009) and JECFA (2011), B6C3F1 mice and F344/N rats were exposed to benzophenone in feed at concentrations of 0, 1,250, 2,500, 5,000, 10,000 and 20,000 mg/kg. The highest dose was unpalatable and most of the animals either died or were terminated for humane reasons.
In mice, these concentrations were estimated by the authors to correspond to a daily intake of 0, 200, 800, 400, 1,600 or 3,300 mg/kg bw for males and 0, 270, 540, 1,000, 1,900 or 4,200 mg/kg bw for females. The kidney weight was increased in both sexes exposed to 2,500 mg/kg feed. Increased liver weight in all dose groups was associated with centrilobular hypertrophy of hepatocytes and induction of CYP2B enzymes by benzophenone, which may be considered an adaptive response of the liver to treatment. Based on the increases in kidney weights, JECFA (2011) considered the no observed adverse effect level (NOAELs) in mice to be 200 mg/kg bw per day (males) and 270 mg/kg bw per day (females).
In the 14‐week NTP oral rat study, the doses were 0, 75, 150, 300, 700 or 850 mg/kg bw per day in males and 0, 80, 160, 300, 700 or 1,000 mg/kg bw per day in females, as estimated by the authors. Treatment‐related increases in liver and kidney weights were reported in all exposed groups, except the kidney weight in females at the lowest dose. Histopathological changes were observed in the liver (hypertrophy and/or cytoplasmic vacuolisation of hepatocytes) and the kidney (tubule dilatation and foci of tubule regeneration). Benzophenone‐induced increases in CYP2B activity were reported. Anaemia, as indicated by decreased haematocrit values, haemoglobin concentrations and erythrocyte levels, was observed in males and females exposed to 150 and 160 mg/kg bw per day and higher, respectively. The authors considered the haematological effects to be ‘of minimal severity’ and not ‘clinically relevant’. However, they also mentioned that dehydration may have masked the severity of anaemia. Based on the haematological findings, JECFA considered the NOAELs in rats to be 75 mg/kg bw per day (males) and 80 mg/kg bw per day (females).
Overall, the above‐mentioned studies in mice and rats together with the already discussed (EFSA, 2009) short‐term/subchronic studies in rats (USEPA, 1984; Burdock et al., 1991) have demonstrated that the liver, kidney and haematopoietic system are targets for benzophenone toxicity. The Panel noted that increased liver and kidney weights along with microscopic changes were observed in all dose groups, except in the 20 mg/kg bw per day group in the Burdock study. While the liver effects were considered to be adaptive changes (Maronpot et al., 2010; Hall et al., 2012), the Panel took a conservative approach concerning the kidney effects in the male rat because there is still an ongoing debate on the relevance of rat kidney effects for humans (Melnick et al., 2012; Hard et al., 2013). Taking these considerations into account, the lowest dose in the 14‐week NTP study (75 and 80 mg/kg bw per day for male and female rats, respectively) was considered a lowest‐observed‐adverse‐effect level (LOAEL).
3.5.3. Genotoxicity
Summary of in vitro data assessed in FGE.69
Benzophenone evaluation in FGE.69 was based on JECFA (2002).
No reverse mutation was reported in the standard Ames assay with various strains of Salmonella Typhimurium (TA98, TA100, TA1535, TA1537 and TA1538,) incubated with 3–1,000 μg/plate of benzophenone [FL‐no: 07.032] (Mortelmans et al., 1986; Appendix A, Table A.1).
Table A.1.
Genotoxicity data on benzophenone [FL‐no: 07.032] evaluated by JECFA (2002) and considered by EFSA in FGE.69 (2008)
| Chemical name FL‐no JECFA‐no | End‐point | Test system | Concentration | Results | Reference | Comments |
|---|---|---|---|---|---|---|
|
Benzophenone 07.032 831 |
Bacterial reverse mutation | S. Typhimurium TA97, TA98, TA100, TA1535 and TA1537 | 3–1,000 μg/plate | Negativea | Mortelmans et al. (1986) | Reliable with the following restriction: the study complied with current recommendations with the exception that tester strains TA102 or E. coli WP2uvrA were not used |
With and without metabolic activation.
In vitro data not previously considered
Benzophenone and its metabolites benzhydrol and 4‐hydroxybenzophenone induced umu gene expression, which may be indicative of DNA damage in S. Typhimurium TA1535/pSK1002 when tested in the presence of recombinant human P450s expressed on Escherichia coli membranes (Takemoto et al., 2002); in the same study, negative results were obtained with human, rat or mouse microsomes, as well as with human liver S9 and cytosolic fractions, suggesting the detoxication of P450‐mediated metabolites by other enzymes present in liver preparations. Overall, the relevance for genotoxicity assessment of the positive findings reported by Takemoto et al. (2002) is limited in view of the artificial metabolic activation system used.
Benzophenone was tested in the SOS/umuC assay in the range of 7.81–1,000 mg/mL (Kotnik et al., 2016). It showed positive results at the highest concentration only in the presence of metabolic activation with rat S9. The Panel noted that the umu assay (Takemoto et al., 2002; Kotnik et al., 2016) is an indicator assay, it is not a validated method for the evaluation of bacterial gene mutation and it is not one of the assays recommended by the EFSA Scientific Committee for genotoxicity assessment (EFSA Scientific Committee, 2011).
Negative results were reported with benzophenone in a mammalian mutation assay with mouse lymphoma L5178Y/tk+/− cells (CCRIS, 2009).
Benzophenone at a concentration range of 8.9–142.8 μg/mL (up to 80% inhibitory concentration (IC80)) in the presence and absence of metabolic activation, did not induce a significant increase in mutation frequencies in L5178Y (tk+/−) mouse lymphoma cell line (Jeon et al., 2007).
These studies are summarised in Appendix B, Table B.1.
Table B.1.
In vitro genotoxicity studies on benzophenone [FL‐no: 07.032]
| Chemical name FL‐no JECFA‐no | End‐point | Test system | Concentration | Results | Reference | Comments |
|---|---|---|---|---|---|---|
|
Benzophenone 07.032 831 |
SOS/umuC assay | S. Typhimurium TA1535 | 0–1,000 μMa | Positive | Takemoto et al. (2002) | Study is reliable. Positive at the higher concentrations (100–1,000 μM) in the presence of metabolic activation. However, the relevance of this endpoint is low |
| 7.8–1,000 μg/mLa | Positive | Kotnik et al. (2016) | Study is reliable. Positive at the highest concentration in the presence of metabolic activation. However, the relevance of this endpoint is low | |||
| Bacterial reverse mutation assay | S. Typhimurium TA98, TA100, TA1535, TA1537 | 10–2,000 μg/platea,d | Negative | CCRIS (2009) | Reliability cannot be evaluated (full study report not available) | |
| 3–333 μg/plateb,e | Negative | |||||
| 10–1,000 μg/plateb,e | Negative | |||||
| 1–166 μg/platec,e | Negative | |||||
| Gene mutation in mammalian cells | L5178Y (tk+/−) mouse lymphoma cells |
33–90 μg/mLc 35–145 μg/mLb |
Negative | |||
|
8.9–142.8 μg/mLc 8.9–141.7 μg/mLb |
Negative | Jeon et al. (2007) |
Reliable with limitations (experimental details are not provided) 80% inhibitory concentration (IC80) was used as maximum concentration |
With and without metabolic activation.
With metabolic activation.
Without metabolic activation.
Plate‐incorporation.
Pre‐incubation.
Summary of in vivo data assessed in FGE.69
No data available.
In vivo data not previously considered
In male B6C3F1 mice, intraperitoneal treatment with 0, 200, 300, 400 and 500 mg benzophenone/kg body weight (three injections at 24‐h intervals) did not induce statistically significant increase in the frequencies of micronucleated polychromatic erythrocytes (PCEs) in bone marrow (NTP, 2006). No increases in micronucleated normochromatic erythrocytes were observed in peripheral blood of male or female mice administered benzophenone for 14 weeks in feed over a concentration range of 1,250–20,000 ppm. No significant alterations in the percentage of PCEs among total erythrocytes were noted in either micronucleus test, indicating no toxicity to the bone marrow from benzophenone treatment (NTP, 2006).
Benzophenone administered intraperitoneal as a single dose at 0, 500, 1,000 and 2,000 in CBA mice and at 0,100, 250, 400 and 600 mg/kg bw in NMRI male mice did not induce increase in the frequency of micronucleated PCEs in peripheral blood, evaluated using the flow cytometer‐based assay. No significant decrease in %PCE was associated with benzophenone treatment (Abramsson‐Zetterberg and Svensson, 2011).
These studies are summarised in Appendix B, Table B.2.
Table B.2.
In vivo genotoxicity studies on benzophenone [FL‐no: 07.032]
| Chemical name FL‐no JECFA‐no | Test system in vivo | Test object | Route | Dose | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|
|
Benzophenone 07.032 831 |
Micronucleus assay in bone marrow | B6C3F1 male mice | Intraperitoneal | 200, 300, 400, 500 mg/kg bw (solvent: corn oil) | Negative | NTP (2006) | Reliable without restriction. Three injections at 24 h intervals; sacrifice 24 h after 3rd injection. No toxicity to the bone marrow |
| Micronucleus assay in peripheral blood polychromatic erythrocytes | B6C3F1 male and female mice | Oral (feed) | 1,250, 2,500, 5,000, 10,000, 20,000 ppm | Negative | Reliable without restriction. Harvest at end of 14‐week dosing regimen. No toxicity to the bone marrow | ||
| Male CBA mice | Intraperitoneal | 500, 1,000, 2,000 mg/kg bw | Negative | Abramsson‐Zetterberg and Svensson (2011) | Reliable without restriction. Single intraperitoneal injection, peripheral blood sampled after 42 h | ||
| Male NMRI mice | 100, 250, 400, 600 mg/kg bw | Negative | Reliable without restriction. Single intraperitoneal injection, peripheral blood sampled after 42 h |
Conclusion on genotoxicity
Overall, the Panel considered that based on the available data, which covers all relevant genetic endpoints (i.e. gene mutations, structural and numerical chromosomal aberrations) there is no concern with respect to genotoxicity of benzophenone.
3.5.4. Chronic toxicity and carcinogenicity
In toxicity and carcinogenesis studies by NTP (2006; Rhodes et al., 2007), evaluated by EFSA (2009) and JECFA (2011), mice and rats were exposed during 105 weeks to benzophenone in feed at concentrations of 0, 312, 625 and 1,250 mg/kg.
In mice the corresponding doses, estimated by the authors, were 40, 80 and 160 mg/kg bw per day in males and 35, 70 and 150 mg/kg bw per day in females. Statistically significant increases in centrilobular hepatocyte hypertrophy were observed in all exposed groups of mice. Increased incidences of hepatocellular adenoma (including multiple adenomas) were reported in the high‐ and mid‐dose groups in males. Incidence of kidney nephropathy was increased in all dose groups of female mice and there was a dose‐related increase in the severity of nephropathy in males.
The corresponding doses in rats were 15, 30 and 60 mg/kg bw per day in males and 15, 30 and 65 mg/kg bw per day in females. Statistically significant increases in centrilobular hepatocyte hypertrophy were observed in all exposed groups of rats and in females there was also an increased incidence of bile duct hyperplasia in all treated groups. In male rats, there was a positive trend in the incidence of renal tubule adenoma, associated with a significantly increased incidence of renal tubule hyperplasia in the mid‐ and high‐dose groups. In males, the severity of chronic nephropathy increased with dose and the increases were statistically significant in all exposed groups compared to controls. In females, the severity of nephropathy was significantly increased in the mid‐ and high‐dose groups. Increased incidence of mononuclear cell leukaemia was observed in male rats in the low‐ and mid‐dose groups and in female rats in the mid‐dose group. Rare histiocytic sarcomas were seen in female rats and mice in the two higher dose groups.
The conclusions by NTP on carcinogenicity of benzophenone were: some evidence in male rats based on the incidence of renal tubule adenoma; equivocal evidence in female rats based on the marginal increased incidence of mononuclear cell leukaemia (MNCL) and histiocytic sarcoma; some evidence in male mice based on the increased incidence of hepatocellular adenoma; some evidence in female mice based on increased incidence of histiocytic sarcoma.
EFSA (2009) noted that benzophenone caused kidney adenoma, including hyperplasia and nephropathy in rats and applied BMD analysis for the non‐neoplastic kidney effects in male rats from the NTP study (2006) to derive a TDI for benzophenone.
JECFA (2011) noted that histiocytic sarcomas occurred only in female mice and rats and only at dose levels inducing toxicity and possibly affecting hormonal balance. A NOAEL was not defined. The sex specificity of renal pathology in rats was suggested by JECFA to be due to differences in renal clearance of metabolites and more severe ageing chronic nephropathy in males compared to females, possibly due to higher concentration of proteins, primarily α‐2μ‐globulin, in male rats. A conclusion from JECFA was that the increasing severity of ageing chronic nephropathy is largely responsible for the renal tubular proliferation in male rats and that this mode of action is not relevant to human renal carcinogenesis.
Overall, in 2‐year studies in rats and mice administered benzophenone in the feed, neoplastic responses were reported in kidney, liver and haematopoietic system. Species‐ and sex‐specific differences in effects were observed. Effects were seen in all dose groups and no NOAEL was defined.
3.5.5. Reproductive and developmental toxicity
In a NTP developmental toxicity study, benzophenone was administered by gavage to timed pregnant rats at doses of 100, 200 or 300 mg/kg bw per day on days 6–19 of gestation (NTP, 2002). Maternal toxicity was observed at all doses, including clinical signs (lethargy, piloerection, weight loss) and significantly increased maternal liver and kidney weights. Average fetal body weight per litter was significantly lower in the high‐dose group compared to controls. No effects on prenatal viability or overall incidences of fetal malformations were observed. The incidences of unossified sternebrae were increased in all dose groups and the incidence of extra rib was increased in the two highest dose groups. The conclusion was that no NOAEL could be defined, but that the developmental toxicity was limited to mild developmental delays with a high probability of recovery.
Developmental toxicity of benzophenone was also investigated in rabbits, administered benzophenone by gavage in doses of 5, 25 and 45 mg/kg bw per day on gestational days 6–29 (NTP, 2004). Maternal body weights and feed consumption decreased in a dose‐related manner, but no effects on liver and kidney weights were observed. There were no effects on prenatal viability. However, the number of successful deliveries was decreased with increasing dose of benzophenone. Fetal body weight was significantly decreased in the highest dose group. The conclusion by the authors was that developmental toxicity was only noted in the presence of maternal toxicity.
A two‐generation reproductive toxicity study on benzophenone was performed in Sprague–Dawley rats in accordance with OECD test guideline 416 (OECD, 2001), including extra parameters to detect endocrine‐disrupting activity (Hoshino et al., 2005). Rats were administered benzophenone via feed at concentrations of 0, 100, 450 or 2,000 mg/kg. According to the authors the corresponding doses were 0, 6.5, 29 and 130 mg/kg bw per day in males and 0, 8.4, 38 and 167 mg/kg bw per day in females of the F0 generation (Hoshino et al., 2005). In the F1 generation, the parental exposure was somewhat higher.
All exposed groups in the F0 and F1 parental animals had increased liver weights (relative liver weight increases in the high‐dose groups: up to 39% and 32% in F0 and F1 males, respectively, and up to 49% in the high‐dose groups of F0 and F1 females compared with controls) along with a dose‐dependent increase of incidences/severity of centrilobular hepatocytic hypertrophy. However, no toxicologically relevant liver weight increases were observed in the low‐dose group 6.5 mg/kg bw per day (relative liver weight increase: 4% in the F0 males and females, and 5% and 3% in the groups F1 males and females, respectively, compared with controls). Considering both the induction of CYP enzymes by benzophenone and the absence of liver tumours in benzophenone‐treated rats, the Panel considers the liver effects as an adaptive response. At the two highest doses, body weight gain and food consumption were reduced, renal weights were increased, dilatation of renal proximal tubules and regeneration of proximal tubular epithelium were observed. In the offspring of both F1 and F2, a statistically significant decreased body weight gain was reported in males and females of the highest dose group.
In both generations, no effects were observed on male and female reproduction (sperm analysis, oestrous cycle, serum levels of testosterone, oestradiol, follicle‐stimulating hormone (FSH) and luteinising hormone (LH), mating and fertility index, gestational length, number of implantation sites, number of offspring at birth and sex ratio). No effect of treatment was found on viability, physical development, including vaginal opening and preputial separation of the penis, results of reflex and response tests or on external abnormalities. Anogenital distance (AGD) was statistically significantly decreased in the low‐ and mid‐dose group in females of the F1 generation, but not in the high dose or in the F2 generation or in males. The decrease in F1 female AGD was up to 11%, statistically significant and based on reasonable numbers (n = 22–24) that accounted for the litter effect. A decreased female AGD may be adverse and could be an indication of developmental and/or endocrine consequences. However, the effect on AGD was not dose‐dependent and no effects on fertility were observed.
Two‐generation reproductive toxicity studies were performed in rats with nine chemicals, including extra parameters to detect endocrine‐disrupting activity (Yamasaki et al., 2005). Rats were given benzophenone via the diet at concentrations of 0, 100, 450 and 2,000 mg/kg feed, equal to 9, 40.5, and 180 mg/kg bw per day, using default conversion factors by EFSA (EFSA Scientific Committee, 2012). According to the authors, no obvious effects on endocrine system and reproductive toxicological effects were detected in the F0 and F1 parents of F1 and F2 offspring.
Overall, no effects of benzophenone on reproductive parameters, including reproductive endocrine system, were demonstrated and developmental effects were only seen in the presence of maternal toxicity. Concerning the liver effects the CEF Panel reconfirms the view of the EFSA opinion 2009 that liver hypertrophy in rats is an adaptive liver response.
Oestrogenic activity
Data on oestrogenic activity have been addressed in the CEF Panel opinion of 2009 (EFSA, 2009), IARC (2013) and ECHA (2015). These data are briefly summarised below.
In vitro studies
Oestrogenic activity of benzophenone and its metabolites benzhydrol and 4‐hydroxybenzophenone has been tested in several studies. Nakagawa et al. (2000) treated human breast cancer MCF‐7 cells with 10 nM–500 μM benzophenone or its metabolites for 6 days. Increased cell proliferation, indicating estrogenic activity, was found at 10–100 μM 4‐hydroxybenzophenone, but not after treatment with benzophenone or benzhydrol. By using oestrogen competitive ligand binding assay Nakagawa and Tayama (2001) demonstrated binding of 4‐hydroxybenzophenone (IC50 50 μM), but no binding of benzophenone or benzhydrol at concentrations up to 500 μM. Reporter gene assays, measuring oestrogen receptor‐alpha (ERα) activity, was negative for benzophenone and positive for 4‐hydroxybenzophenone (Yamasaki et al., 2002; Suzuki et al., 2005; Kerdivel et al., 2013). In two more recent studies, benzophenone showed a weak oestrogenic potency (more than five orders of magnitude less potent that 17β‐estradiol) in the yeast oestrogen (YES) assay (Zhang et al., 2017) and in a chemokine (CXCL12 as an indicator of oestrogen‐dependent proliferation) releasing assay in breast cancer cells (Habauzit et al., 2017). The latter report showed that 4‐hydroxybenzophenone is more potent than benzophenone which is qualitatively in line with the above reported findings.
In vivo studies
The rat uterotrophic assay (OECD test guideline 440, 2007) has been used to test oestrogenic activity of benzophenone and its metabolites in vivo. Yamasaki et al. (2002) did not observe any effects in the immature rat uterotrophic assay, when benzophenone was injected subcutaneously at doses of 2, 20 and 200 mg/kg bw for 3 days. In contrast, Nakagawa and Tayama (2002) demonstrated oestrogenic activity of benzophenone in a study with ovariectomised rats. Sprague–Dawley rats were administered benzophenone orally at doses of 100 or 400 mg/kg bw per day for 3 days. The high dose resulted in increased uterine weight as well as an increase in luminal epithelial height and stromal cells in the uterus and an increase in thickness of vaginal epithelial cell layers with cornification.
The same authors reported a dose‐dependent increase in uterine weight in juvenile female rats administered 4‐hydroxybenzophenone subcutaneously in doses of 100, 200 and 400 mg/kg bw for 3 days (Nakagawa and Tayama, 2001). The uterine response was accompanied by an increase in luminal epithelial height and stromal cells in the uterus and an increase in thickness of vaginal epithelial cell layers with cornification. No effects were observed after treatment with benzophenone or benzhydrol at 400 mg/kg bw. Yamasaki et al. (2003) demonstrated a dose‐related increase in uterine weight at doses ≥ 200 mg/kg bw of 4‐hydroxybenzophenone, injected subcutaneously for 3 days in juvenile rats.
In summary, oestrogenic activity of 4‐hydroxybenzophenone, but not of benzophenone or the main metabolite benzhydrol, was demonstrated in in vitro and in vivo model systems, i.e. uterotrophic assay, except in one assay with ovariectomised rats at a high dose of benzophenone or at high concentrations in vitro which are of questionable relevance for risk assessment. The Panel noted that reproductive and developmental toxicity studies on benzophenone, including extra parameters to detect endocrine‐disrupting activity of benzophenone, did not detect any obvious effects on endocrine system and reproductive toxicity despite the demonstrated oestrogenic activity of its metabolite 4‐hydroxybenzophenone.
3.5.6. Other endocrine effects
Anti‐androgenic activity of 4‐hydroxybenzophenone and benzophenone was reported by Suzuki et al. (2005) in an in vitro study using a reporter gene assay, where the inhibitory effect of the test compounds on the androgenic activity of dihydrotestosterone was examined.
No androgenic activity of 4‐hydroxybenzophenone was demonstrated, in vivo, studied in rats by the Hershberger assay (Yamasaki et al., 2003).
An inhibitory effect of benzophenone on thyroid peroxidase activity, in vitro, although without a clear dose‐response, was reported by Song et al. (2012), using a reporter gene assay.
Using the yeast two‐hybrid assay, Mikamo et al. (2003) showed that benzophenone activated pregnane X receptor (PXR) in vitro. The same authors also reported that benzophenone induced CYP2C11, CYP2B1/2 and CYP3A1 gene expression in liver of rats administered with benzophenone by intraperitoneal injection for 3 days. The Panel considers that this might affect levels of endogenous hormones via the production of active metabolites.
3.5.7. Derivation of a TDI
According to the CEF Panel opinion of 2009 (EFSA, 2009), liver and kidney were identified as the primary target organs of benzophenone toxicity in rats and in mice, based on results from chronic carcinogenicity (NTP, 2006) and reproductive toxicity studies (Hoshino et al., 2005).
Liver hypertrophy in the rat at the lowest dose level (~ 6 mg/kg bw per day) in a two‐generation study (Hoshino et al., 2005) was considered the most sensitive effect of benzophenone. However, in the absence of liver tumours in rats in the chronic NTP carcinogenicity study (even at doses yielding severe liver damage), the Panel concluded that the liver hypertrophy in rat is an adaptive but not an adverse response as also discussed by Maronpot et al. (2010) and by Hall et al. (2012).
While benzophenone caused liver adenomas in the B6C3F1 mouse, at a dose of 40 mg/kg bw per day, the Panel noted that this effect is a less sensitive endpoint than the kidney effects in rats at the lowest dose of 15 mg/kg bw per day in a chronic carcinogenicity study (NTP, 2006). Along with dose‐related increases in the incidences of renal tubule hyperplasia and in the severity of chronic nephropathy, a positive trend in the incidences of renal tubule adenoma was observed in the male rats. In female rats, the severity of nephropathy was significantly increased in the mid and high dose groups. Given that proliferative lesions and adenomas may be considered as a biological and morphological continuum in the progression to kidney neoplasia and the contribution of nephropathy to kidney tumour development cannot be ruled out (Rhodes et al., 2007; Melnick et al., 2012), the Panel considered that the non‐neoplastic effects are adverse and used them as a point of departure for the risk assessment of benzophenone. As reported in the CEF Panel opinion (EFSA, 2009) ‘Benchmark dose (BMD) analyses were applied for the non‐neoplastic kidney effects in male rats (NTP, 2006), and the lower 95% confidence limits of the benchmark dose for a 10% effect (BMDL10) were calculated to be 3.1–7.4 mg/kg b.w. per day. The models used in the analysis were consistent, and passed statistical validation. The Panel decided that the BMDL10 value of 3.1 mg/kg b.w. per day was the most appropriate departure point for derivation of the TDI. By applying an uncertainty factor of 100, a TDI of 0.03 mg/kg body weight is derived’. Based on the incidence data for non‐neoplastic kidney effects, the BMDL was recalculated in accordance to the update on the use of the BMD approach (EFSA Scientific Committee, 2017) using the EFSA web‐tool5 and the same BMDL was obtained as in the previous assessment (Appendix D). In the light of an ongoing debate on the relevance of the rat chronic nephropathy to humans (Melnick et al., 2012; Hard et al., 2013), the Panel considers the EFSA approach used in the 2009 opinion to derive a TDI for benzophenone conservative and appropriate to cover the potential induction of toxic effects including tumours in humans.
3.5.8. Discussion
In the NTP carcinogenicity studies with rats and mice, tumour induction by benzophenone was observed in liver, kidney and the haematopoietic system. The Panel concluded that based on the negative results from studies on mutagenicity or chromosomal damage a genotoxic mechanism of tumour induction by benzophenone can be excluded. A potential involvement of endocrine effects of benzophenone or its metabolite 4‐hydroxybenzophenone in tumour induction, e.g. via oestrogenic, anti‐androgenic modulation or the potential activation of nuclear constitutive androstane receptor and PXR (as discussed e.g. by IARC and ECHA) cannot be ruled out. However, the Panel noted that the effective concentrations of these substances in vitro and the effective dose of benzophenone in the uterotrophic assay with ovariectomised rats were relatively high indicating only weak endocrine effects. In addition, the Panel noted that no oestrogenic effects of benzophenone were reported in (sub)chronic, reproductive and developmental toxicity studies. The Panel noted that there is no concern with respect to genotoxicity. Accordingly, a threshold mechanism of toxicity can be assumed. Therefore, the Panel concludes that the carcinogenicity is also covered by the TDI (0.03 mg/kg bw) derived from the most sensitive endpoint, non‐neoplastic effects of benzophenone in rat kidneys observed in the chronic NTP study (NTP, 2006).
The incidences of histiocytic sarcoma observed in female rats and mice in the NTP carcinogenicity study showed positive trends. Histiocytic sarcomas are considered as extremely rare neoplasms in mice (historical control range for feed studies: 0–2%) and rats (not observed in historical controls). This neoplastic lesion was observed at 625 mg/kg feed (mice: 80 and 70 mg/kg bw per day for males and females, respectively, and rats: 30 mg/kg bw per day) and 1,250 mg/kg feed (mice: 160 and 150 mg/kg bw per day for males and females, respectively, and rats: 60 and 65 mg/kg bw per day for males and females, respectively). The low incidences of this lesion (3/50 and 2/50 at the highest doses in mice and rats, respectively) are associated with a high uncertainty in risk assessment.
A statistically significant increase in the incidences of MNCL was observed in male rats in the low‐ (15 mg/kg bw per day) and in the mid‐ (30 mg/kg bw per day) dose groups, whereas the incidences at the highest dose (60 mg/kg bw per day) in males were slightly decreased compared to controls. In female rats, the incidence of MNCL exceeded the historical control range with a significant increase in the mid‐dose group. An extended assessment of the lesions in males showed that the extent of multiple organ involvement of leukaemia decreased in the higher exposure groups (Rhodes et al., 2007). The Panel noted that while MNCL is a common neoplasm in F344 rats used in the NTP study, it is species‐specific and no histological comparable tumour is observed in humans. Thus, this tumour may not be appropriate for human health risk assessment (Maronpot et al., 2016).
In the NTP (2006) carcinogenicity study with mice, there was a positive trend in the incidence of hepatocellular adenoma in males. Moreover, IARC (2013) also noted that hepatoblastomas in the high‐dose group of males – even though not statistically different from controls – exceeded the historical control range. In addition, non‐neoplastic lesions in the livers were significantly increased in mice. In contrast to mice, no liver tumours were reported for rats while the hypertrophic effect of benzophenone in the liver was observed in the Hoshino study (Hoshino et al., 2005) at all doses. Considering that no neoplastic liver lesions were observed in rats, the CEF Panel reconfirms the view of the EFSA 2009 opinion that benzophenone‐induced liver hypertrophy is an adaptive response, i.e. not adverse (Maronpot et al., 2010; Hall et al., 2012).
In the NTP (2006) carcinogenicity study, treatment‐related increases in the severity of chronic nephropathy, in the incidences of renal tubule adenomas and renal tubule hyperplasia were reported. The potential impact of nephropathy to the formation of neoplastic lesions in the kidney was noted by several authors (Seely et al., 2002; Rhodes et al., 2007; Travlos et al., 2011) while the relevance of the rat chronic nephropathy to humans was discussed controversially (Melnick et al., 2012; Hard et al., 2013). For the risk assessment of benzophenone, the Panel considered the statistically significant increases in renal tubule hyperplasia in all treated males and females (single and step sections, combined) as an adverse effect and reconfirmed as a conservative approach the lowest BMDL10 of 3.1 mg/kg bw per day derived in the EFSA 2009 opinion. Having applied the usual uncertainty factor of 100 to the BMDL10, the Panel considers the resulting TDI of 0.03 mg/kg bw sufficient to cover neoplastic effects induced in the rodent carcinogenicity studies observed at 40 mg/kg bw per day and above.
4. Conclusions
Based on the negative results from studies on the relevant genetic endpoints, the Panel concluded that there is no concern with respect to genotoxicity of benzophenone.
Having reviewed the data related to endocrine activities of benzophenone and its metabolite 4‐hydroxybenzophenone, the Panel considered them as weak and not directly related to the observed toxic effects including the neoplastic effects of these substances.
Overall, the Panel concludes that the conservative approach taken by the CEF Panel in 2009 (EFSA, 2009) to derive a TDI of 0.03 mg/kg bw for benzophenone is appropriate to cover the non‐neoplastic effects in the chronic toxicity studies and the neoplastic effects induced in the rodent carcinogenicity studies.
The Panel notes that the TDI is in the same order of magnitude as the chronic dietary exposure of adults and children to benzophenone (10–20 μg/kg bw per day) calculated by the APET approach for the amount of added flavouring. The Panel considers that the calculated TDI and exposure estimate are based on conservative assumptions. The Panel concludes that there is no safety concern for benzophenone under the current condition of use as a flavouring substance.
5. Recommendations
The Panel takes note of other potential sources of dietary exposure such as from FCM. Exposure from FCM could be up to 10 μg/kg bw per day, bringing the potential exposure from both plastic and flavouring up to the TDI for children. The Commission may wish to take this into account.
Abbreviations
- AGT
anogenital distance
- APET
added portions exposure technique
- BMD
benchmark dose
- Bw
body weight
- CAS
Chemical Abstract Service
- CCRIS
Chemical Carcinogenesis Research Information System
- CEF
Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CoE
Council of Europe
- ER
oestrogen receptor
- FAO
Food and Agriculture Organization of the United Nations
- FCM
Food Contact Materials
- FEMA
Flavor and Extract Manufacturers Association
- FGE
Flavouring Group Evaluation
- FLAVIS (FL)
Flavour Information System (database)
- FSH
follicle‐stimulating hormone
- IARC
International Agency for Research on Cancer
- IC
inhibitory concentration
- ID
Identity
- IR
infrared spectroscopy
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- LOAEL
lowest‐observed‐adverse‐effect level
- LH
luteinising hormone
- MNCL
mononuclear cell leukaemia
- MSDI
Maximised Survey‐derived Daily Intake
- NOAEL
no observed adverse effect level
- NTP
National Toxicology Program
- OECD
Organisation for Economic Cooperation and Development
- PCE
polychromatic erythrocyte
- PXR
pregnane X receptor
- SCF
Scientific Committee on Food
- SML
specific migration limit
- SPET
single portion exposure technique
- TDI
tolerable daily intake
- USEPA
United States Environmental Protection Agency
- UV
ultraviolet
- WHO
World Health Organization
- YES
yeast oestrogen
Appendix A – Genotoxicity data evaluated in FGE.69
1.
Appendix B – Previously not considered genotoxicity data
1.
Appendix C – Exposure
Calculation of the Dietary Exposure ‐ APET
1.
Chronic Dietary Exposure – ‘Added Portions Exposure Technique’ (APET)6
The chronic APET calculations are based on the normal combined occurrence level by adding the highest contributing portion of food and highest contributing portion of beverages (either among soft drinks or alcoholic beverages). APET for children is calculated by adding the highest contributing portion of food and highest contributing portion of beverages (among soft drinks). Furthermore, in the APET calculation for children the portion sizes listed in Table C.1 is adjusted by a factor 0.63 to take into account the smaller portion sizes consumed by the child.
Table C.1.
Normal and maximum occurrence levels for refined categories of foods and beverages
| Food categoriesa | Standard portionsb (g) | Occurrence level as added flavouring substance (mg/kg) | Occurrence level from other sourcesc (mg/kg) | Combined occurrence level from all sourcese (mg/kg) | ||||
|---|---|---|---|---|---|---|---|---|
| Normal | Maximum | Averaged | Maximum | Normal | Maximum | |||
| 01.1 | Milk‐ and dairy‐based drinks | 200 | 1.06 | 5 | 1.06 | 5 | ||
| 01.2 | Fermented and renneted milk products (plain), excluding food category 01.1.2 (dairy‐based drinks) | 200 | 1.06 | 5 | 1.06 | 5 | ||
| 01.3 | Condensed milk and analogues (plain) | 70 | 1.06 | 5 | 1.06 | 5 | ||
| 01.4 | Cream (plain) and the like | 15 | 1.06 | 5 | 1.06 | 5 | ||
| 01.5 | Milk powder and cream powder and powder analogues (plain) | 30 | 1.06 | 5 | 1.06 | 5 | ||
| 01.6 | Cheese and analogues | 40 | 1.06 | 5 | 1.06 | 5 | ||
| 01.7 | Dairy‐based desserts (e.g., pudding, fruit or flavoured yoghurt) | 125 | 1.06 | 5 | 1.06 | 5 | ||
| 01.8 | Whey and whey products, excluding whey cheeses | 200 | 1.06 | 5 | 1.06 | 5 | ||
| 02.1 | Fats and oils essentially free from water | 15 | ||||||
| 02.2 | Fat emulsions mainly of type water‐in‐oil | 15 | ||||||
| 02.3 | Fat emulsions mainly of type water‐in‐oil, including mixed and/or flavoured products based on fat emulsions | 15 | ||||||
| 02.4 | Fat‐based desserts excluding dairy‐based dessert products of category 1.7 | 50 | ||||||
| 03.0 | Edible ices, including sherbet and sorbet | 50 | 0.01 | 0.089 | 0.01 | 0.089 | ||
| 04.1.1 | Fresh fruit | 140 | 0.13 | 0.13 | ||||
| 04.1.2 | Processed fruit | 125 | 0.13 | 0.13 | ||||
| 04.1.2.5 | Jams, jellies, marmalades | 30 | ||||||
| 04.2.1 | Fresh vegetables (including mushrooms and fungi, roots and tubers, pulses and legumes, and aloe vera), seaweed, and nut and seed | 200 | ||||||
| 04.2.2 | Processed vegetables (including mushrooms and fungi, roots and tubers, pulses and legumes, and aloe vera), seaweed, and nut and seed purees and spreads (e.g. peanut butter) and nuts and seeds | 200 | ||||||
| 04.2.2.5 | Vegetables (including mushrooms and fungi, roots and tubers, pulses and legumes, and aloe vera), seaweed, and nut and seed purees and spreads (e.g. peanut butter) | 30 | ||||||
| 05.1 | Cocoa products and chocolate products, including imitations and chocolate substitutes | 40 | 1.15 | 2.42 | 1.15 | 2.42 | ||
| 05.1.3 | Cocoa‐based spreads, including fillings | 30 | 1.15 | 2.42 | 1.15 | 2.42 | ||
| 05.2 | Confectionery, including hard and soft candy, nougats, etc., other than 05.1, 05.3 and 05.4 | 30 | 1.15 | 2.42 | 1.15 | 2.42 | ||
| 05.3 | Chewing gum | 3 | ||||||
| 05.4 | Decorations (e.g. for fine bakery wares), toppings (non‐fruit) and sweet sauces | 35 | 1.15 | 2.42 | 1.15 | 2.42 | ||
| 06.1 | Whole, broken or flaked grain, including rice | 200 | 1 | 5 | 1 | 5 | ||
| 06.2 | Flours and starches (including soya bean powder) | 30 | 1 | 5 | 1 | 5 | ||
| 06.3 | Breakfast cereals, including rolled oats | 30 | 1 | 5 | 1 | 5 | ||
| 06.4 | Pastas and noodles and like products (e.g. rice paper, rice vermicelli, soya bean pastas and noodles) | 200 | 1 | 5 | 1 | 5 | ||
| 06.5 | Cereal‐ and starch‐based desserts (e.g. rice pudding, tapioca pudding) | 200 | 1 | 5 | 1 | 5 | ||
| 06.6 | Batters (e.g. for breading or batters for fish or poultry) | 30 | 1 | 5 | 1 | 5 | ||
| 06.7 | Pre‐cooked or processed rice products, including rice cakes (Oriental type only) | 200 | 1 | 5 | 1 | 5 | ||
| 06.8 | Soya bean products (excluding soya bean products of food category 12.9 and fermented soya bean products of food category 12.10) | 100 | 1 | 5 | 1 | 5 | ||
| 07.1 | Bread and ordinary bakery wares | 50 | 1 | 5 | 1 | 5 | ||
| 07.2 | Fine bakery wares (sweet, salty, savoury) and mixes | 80 | 1 | 5 | 1 | 5 | ||
| 08.1 | Fresh meat, poultry and game | 200 | ||||||
| 08.2 | Processed meat, poultry and game products in whole pieces or cuts | 100 | ||||||
| 08.3 | Processed comminute meat, poultry and game products | 100 | ||||||
| 08.4 | Edible casings (e.g. sausage casings) | 1 | ||||||
| 09.1.1 | Fresh fish | 200 | ||||||
| 09.1.2 | Fresh molluscs, crustaceans and echinoderms | 200 | ||||||
| 09.2 | Processed fish and fish products, including molluscs, crustaceans and echinoderms | 100 | ||||||
| 09.3 | Semi‐preserved fish and fish products, including molluscs, crustaceans and echinoderms | 100 | ||||||
| 09.4 | Fully preserved, including canned or fermented, fish and fish products, including molluscs, crustaceans and echinoderms | 100 | ||||||
| 10.1 | Fresh eggs | 100 | 1 | 5 | 1 | 5 | ||
| 10.2 | Egg products | 100 | 1 | 5 | 1 | 5 | ||
| 10.3 | Preserved eggs, including alkaline. salted and canned eggs | 100 | 1 | 5 | 1 | 5 | ||
| 10.4 | Egg‐based desserts (e.g. custard) | 125 | 1 | 5 | 1 | 5 | ||
| 11.1 | Refined and raw sugar | 10 | ||||||
| 11.2 | Brown sugar excluding products of food category 11.1 | 10 | ||||||
| 11.3 | Sugar solutions and syrups, and (partially) inverted sugars, including molasses and treacle, excluding products of food category 11.1.3 (soft white sugar, soft brown sugar, glucose syrup, dried glucose syrup, raw cane sugar) | 30 | ||||||
| 11.4 | Other sugars and syrups (e.g. xylose, maple syrup, sugar toppings) | 30 | ||||||
| 11.5 | Honey | 15 | ||||||
| 11.6 | Table‐top sweeteners, including those containing high‐intensity sweeteners | 1 | ||||||
| 12.1 | Salt and salt substitutes | 1 | ||||||
| 12.10 | Protein products other than from soybeans | 15 | ||||||
| 12.2 | Herbs, spices, seasonings and condiments (e.g. seasoning for instant noodles) | 1 | 0.48 | 0.48 | ||||
| 12.3 | Vinegars | 15 | ||||||
| 12.4 | Mustards | 15 | ||||||
| 12.5 | Soups and broths | 200 | ||||||
| 12.6 | Sauces and like products | 30 | ||||||
| 12.7.a | Salads 120 g (e.g. macaroni salad, potato salad) excluding cocoa‐ and nut‐based spreads of food categories | 120 | ||||||
| 12.7.b | Sandwich spreads (20 g), excluding cocoa‐ and nut‐based spreads of food categories | 20 | ||||||
| 12.8 | Yeast and like products | 1 | ||||||
| 12.9 | Soybean‐based seasonings and condiments | 15 | ||||||
| 12.9.1 | Fermented soya bean products (e.g. miso) | 40 | ||||||
| 12.9.2 | Soybean sauce | 15 | ||||||
| 12.9.3 | Fermented soybean sauce | 15 | ||||||
| 13.2.a | Complementary foods for infants and young children: Dry instant cereals (with or without milk), including pasta | 110 | ||||||
| 13.2.b | Complementary foods for infants and young children: Meat‐based or fish‐based dinner | 170 | ||||||
| 13.2.c | Complementary foods for infants and young children: Dairy‐based dessert | 110 | ||||||
| 13.2.d | Complementary foods for infants and young children: Vegetables, potatoes, broth, soups, pulses | 170 | ||||||
| 13.2.e | Complementary foods for infants and young children: Biscuits and cookies | 20 | ||||||
| 13.2.f | Complementary foods for infants and young children: Fruit purée | 110 | ||||||
| 13.2.g | Complementary foods for infants and young children: Fruit juice | 120 | ||||||
| 13.2.h | Milk for young children | 200 | ||||||
| 13.3 | Dietetic foods intended for special medical purposes (excluding food products of category 13.1 ‘Infant formulae, follow‐up formulae and other formulae for special medical purposes for infants’) | 200 | 1 | 5 | 1 | 5 | ||
| 13.4 | Dietetic formulae for slimming purposes and weight reduction | 200 | 1 | 5 | 1 | 5 | ||
| 13.5 | Dietetic foods (e.g. supplementary foods for dietary use), excluding products of food categories 13.1 (Infant formulae, follow‐up formulae and other formulae for special medical purposes for infants), 13.2–13.4 and 13.6 | 200 | 1 | 5 | 1 | 5 | ||
| 13.6 | Food supplements | 5 | ||||||
| 14.1 | Other non‐alcoholic (‘soft’) beverages (expressed as liquid) | 300 | 1 | 5 | 1 | 5 | ||
| 14.2.1 | Beer and malt beverages | 300 | ||||||
| 14.2.2 | Cider and perry | 300 | ||||||
| 14.2.3 | Grape wines | 150 | ||||||
| 14.2.4 | Wines (other than grape) | 150 | ||||||
| 14.2.5 | Mead | 150 | ||||||
| 14.2.6 | Distilled spirituous beverages containing more than 15% alcohol | 30 | ||||||
| 14.2.7 | Aromatised alcoholic beverages (e.g. beer, wine and spirituous cooler‐type beverages, low alcoholic refreshers) | 300 | ||||||
| 15.1 | Snacks, potato‐, cereal‐, flour‐ or starch‐based (from roots and tubers, pulses and legumes) | 30 | ||||||
| 15.2 | Processed nuts, including coated nuts and nut mixtures (with e.g. dried fruit) | 30 | ||||||
| 15.3 | Snacks – fish‐based | 30 | ||||||
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) – foods that could not be placed in categories 01–15 | 300 | 0.02 | 0.15 | 0.02 | 0.15 | ||
Most of the categories reported are the sub‐categories of Codex GSFA (General Standard for Food Additives, available at http://www.codexalimentarius.net/gsfaonline/CXS_192e.pdf) used by the JECFA in the SPET technique (FAO/WHO, 2008). In the case of category 13.2 (complementary foods for infants and young children), further refined categories have been created so that a specific assessment of dietary exposure can be performed in young children.
For Adults. In case of foods marketed as powder or as concentrates, occurrence levels must be reported for the reconstituted product, considering the instructions reported on the product label or one of the standard dilution factors established by the JECFA (FAO/WHO 2008):
– 1/25 for powder used to prepare water‐based drinks such as coffee, containing no additional ingredients,
– 1/10 for powder used to prepare water‐based drinks containing additional ingredients such as sugars (ice tea, squashes, etc.),
– 1/7 for powder used to prepare milk, soups and puddings,
– 1/3 for condensed milk.
As natural constituent and/or developed during the processing and/or as carry over resulting from their use in animal feed.
In order to estimate normal values in each category, only foods and beverages in which the substance is present in significant amount will be considered (e.g. for the category ‘Fresh fruit’ 04.1.1., the normal concentration will be the median concentration observed in all kinds of fruit where the flavouring substance is known to occur).
As added flavouring or from other sources. The normal and maximum combined occurrence levels of the substance will be assessed by the applicant either by adding up occurrence levels from added use to that from other sources or by expert judgement based on the likelihood of their concomitant presence. This will be done both for normal use levels and for maximum use levels.
Adults (‘Added Portions Exposure Technique’ (APET))
On the basis of normal occurrence level from the added flavouring only
Solid Food: The maximum intake will be from category 1.0 (Dairy products) with the normal combined occurrence level of 212 μg/adult per day.
Beverage: The category 14.1 (Non‐alcoholic (‘soft’) beverages, excl. dairy products) to which the candidate substance is added have the same normal combined occurrence level of 300 μg/adult per day.
The total APET will be 512 μg/adult per day corresponding to 8.5 μg/kg bw per day for a 60‐kg person.
Children (3‐year‐old child of 15 kg body weight)
Solid Food: The maximum intake will be from category 1.0 (Dairy products) with the normal combined occurrence level of 212 × 0.63 = 134 μg/child per day.
Beverage: The category 14.1 (Non‐alcoholic (‘soft’) beverages, excl. dairy products) to which the candidate substance is added have the same normal combined occurrence level of 300 × 0.63 = 189 μg/child per day.
The total APET will be 323 μg/child per day corresponding to 22 μg/kg bw per day for a 15‐kg child.
Conclusion
The higher of the two values among adults and children, expressed per kg/bw per day, should be used as the basis for the safety evaluation of the candidate substance, i.e. the value of 22 μg/kg bw per day for a 15‐kg child should be compared to the appropriate NOAEL for the candidate substance.
Combined Dietary Exposure
This is an estimate of total dietary exposure deriving from both the addition of the flavouring substance to foods and beverages and other dietary sources. To estimate the APET for combined dietary exposure, the occurrence of the substance in grapes and vanilla was also taken into account in the estimation.
Adults (‘Added Portions Exposure Technique’ (APET))
On the basis of normal occurrence level from the added flavouring only
Solid Food: The maximum intake will be from category 1.0 (Dairy products) with the normal combined occurrence level of 212 μg/adult per day.
Beverage: The category 14.1 (Non‐alcoholic (‘soft’) beverages, excl. dairy products) to which the candidate substance is added have the same normal combined occurrence level of 300 μg/adult per day.
The total APET will be 512 μg/adult per day corresponding to 8.5 μg/kg bw per day for a 60‐kg person.
Children (3‐year‐old child of 15 kg body weight)
Solid Food: The maximum intake will be from category 1.0 (Dairy products) with the normal combined occurrence level of 212 × 0.63 = 134 μg/child per day.
Beverage: The category 14.1 (Non‐alcoholic (‘soft’) beverages, excl. dairy products) to which the candidate substance is added have the same normal combined occurrence level of 300 × 0.63 = 189 μg/child per day.
The total APET will be 323 μg/child per day corresponding to 22 μg/kg bw per day for a 15‐kg child.
Appendix D – Benchmark Dose Modelling: Report
Data description
The endpoint to be analysed is: pelvis, transitional epithelium hyperplasia.
Data used for analysis:
| Dose | Hyperepith | Total |
|---|---|---|
| 0 | 1 | 50 |
| 15 | 11 | 50 |
| 30 | 29 | 50 |
| 60 | 34 | 50 |
Selection of the BMR
The benchmark dose (BMD) is defined as the dose that corresponds with an extra risk of 10% compared with the background risk. The benchmark response (BMR) is the estimated risk corresponding with the BMD of interest.
A 90% confidence interval around the BMD will be estimated, the lower bound is reported by BMDL and the upper bound by BMDU.
Software used
Results are obtained using the EFSA web‐tool for BMD analysis
Fitting benchmark dose models is based on the R‐package http://www.rivm.nl/en/Documents_and_publications/Scientific/Models/PROAST, version 64.9.
Averaging results from multiple fitted benchmark dose models is based on the methodology in https://www.jstatsoft.org/article/view/v026i05.
Specification of deviations from default assumptions
None.
Dose–response models
Default set of fitted models:
| Model | Number of parameters | Formula | |
|---|---|---|---|
| Null | 1 | y = a | |
| Full | No. of groups | y = group mean | |
| Logistic | 2 |
|
|
| Probit | 2 |
|
|
| Log‐logistic | 3 |
|
|
| Log‐probit | 3 |
|
|
| Weibull | 3 |
|
|
| Gamma | 3 | y = pgamma(bx; c) | |
| Two‐stage | 3 |
|
Procedure for selection of BMDL
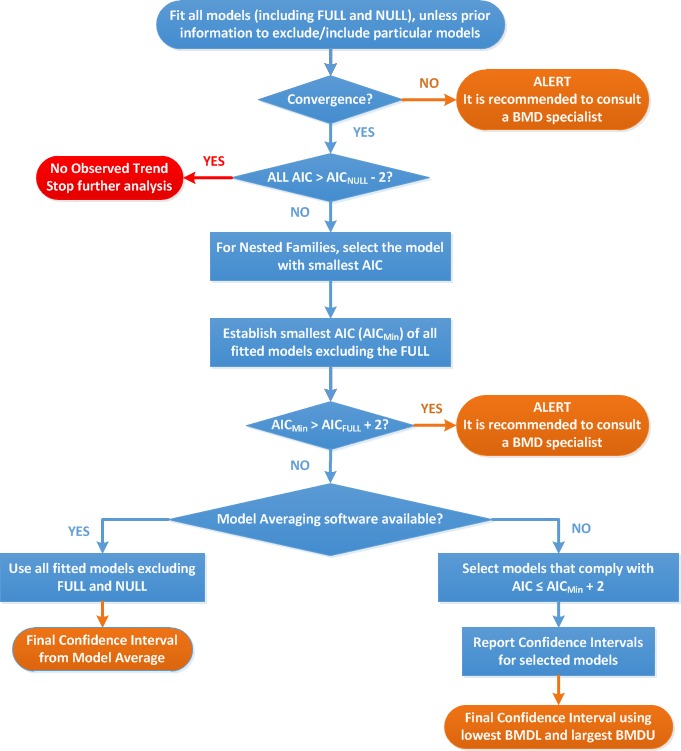
Flow chart for selection of BMDL
Results
Response variable: Hyperepith
Fitted Models
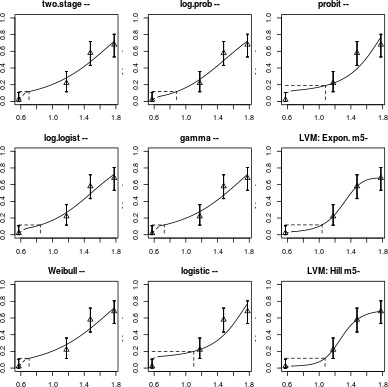
| Model | No.par | loglik | AIC | accepted | BMDL | BMDU | BMD | conv |
|---|---|---|---|---|---|---|---|---|
| null | 1 | −132.31 | 266.62 | NA | NA | NA | NA | |
| full | 4 | −96.61 | 201.22 | NA | NA | NA | NA | |
| two.stage | 3 | −98.40 | 202.80 | Yes | 4.13 | 6.29 | 5.1 | Yes |
| log.logist | 3 | −97.92 | 201.84 | Yes | 2.90 | 11.20 | 7.0 | Yes |
| Weibull | 3 | −98.40 | 202.80 | Yes | 1.59 | 9.33 | 5.1 | Yes |
| log.prob | 3 | −97.90 | 201.80 | Yes | 3.38 | 11.80 | 7.7 | Yes |
| gamma | 3 | −98.40 | 202.80 | Yes | 1.29 | 10.20 | 5.3 | Yes |
| logistic | 2 | −104.29 | 212.58 | No | NA | NA | 13.0 | Yes |
| probit | 2 | −103.78 | 211.56 | No | NA | NA | 12.0 | Yes |
| LVM: Expon. m5‐ | 4 | −96.61 | 201.22 | Yes | 5.30 | 14.60 | 11.0 | Yes |
| LVM: Hill m5‐ | 4 | −96.61 | 201.22 | Yes | 5.76 | 14.70 | 12.0 | Yes |
Estimated model parameters
two.stage
estimate for a‐: 0.01894
estimate for BMD‐: 5.05
estimate for c: 1e‐06
log.logist
estimate for a‐: 0.019
estimate for BMD‐: 7.026
estimate for c: 1.462
Weibull
estimate for a‐: 0.01895
estimate for BMD‐: 5.064
estimate for c: 1.001
log.prob
estimate for a‐: 0.0192
estimate for BMD‐: 7.662
estimate for c: 0.9019
gamma
estimate for a‐: 0.01916
estimate for BMD‐: 5.337
estimate for cc: 1.038
logistic
estimate for a‐: ‐2.078
estimate for BMD‐: 12.6
probit
estimate for a‐: ‐1.286
estimate for BMD‐: 12.04
EXP
estimate for a‐: 1.671
estimate for CED‐: 10.88
estimate for c‐: 0.5321
estimate for d‐: 1.527
estimate for th: 0
estimate for sigma: 0.25
HILL
estimate for a‐: 1.671
estimate for CED‐: 11.87
estimate for c‐: 0.526
estimate for d‐: 2.871
estimate for th: 0
estimate for sigma: 0.25
Weights for Model Averaging
| two.stage | log.logist | Weibull | log.prob | gamma | logistic | probit | EXP | HILL |
|---|---|---|---|---|---|---|---|---|
| 0.09 | 0.15 | 0.09 | 0.15 | 0.09 | 0 | 0 | 0.21 | 0.21 |
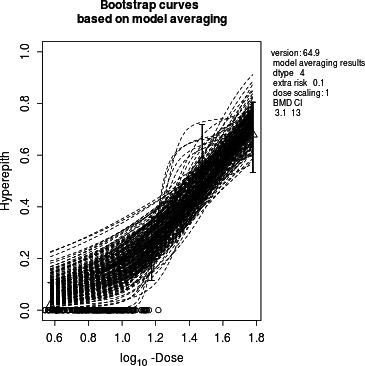
Final BMD Values
| BMD | BMDL | BMDU |
|---|---|---|
| 8.4 | 3.1 | 13 |
Confidence intervals for the BMD are based on 200 generated data sets.
Visualisation
Suggested citation: EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , Silano V, Bolognesi C, Castle L, Chipman K, Cravedi J‐P, Engel K‐H, Fowler P, Franz R, Grob K, Gürtler R, Husøy T, Kärenlampi S, Milana MR, Pfaff K, Riviere G, Srinivasan J, Tavares Poças MF, Tlustos C, Wölfle D, Zorn H, Benigni R, Binderup M‐L, Brimer L, Marcon F, Marzin D, Mosesso P, Mulder G, Oskarsson A, Svendsen C, Anastassiadou M, Carfì M, Saarma S and Mennes W, 2017. Scientific Opinion on safety of benzophenone to be used as flavouring. EFSA Journal 2017;15(11):5013, 33 pp. 10.2903/j.efsa.2017.5013
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00425
Panel members: Claudia Bolognesi, Laurence Castle, Kevin Chipman, Jean‐Pierre Cravedi, Karl‐Heinz Engel, Paul Fowler, Roland Franz, Konrad Grob, Rainer Gürtler, Trine Husøy, Sirpa Kärenlampi, Wim Mennes, Maria Rosaria Milana, Karla Pfaff, Gilles Riviere, Jannavi Srinivasan, Maria de Fátima Tavares Poças, Vittorio Silano, Christina Tlustos, Detlef Wölfle and Holger Zorn.
Adopted: 20 September 2017
Notes
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. OJ L 12, 15.1.2011, p. 1–89.
The APET has been calculated based on the occurrence levels in the food sub‐categories reported in the above table, with the exclusion of categories 13.2 (complementary foods for infants and young children).
References
- Abramsson‐Zetterberg L and Svensson K, 2011. 4‐Methylbenzophenone and benzophenone are inactive in the micronucleus assay. Toxicology Letters, 201, 235–239. [DOI] [PubMed] [Google Scholar]
- Burdock GA, Pence DH and Ford RA, 1991. Safety evaluation of benzophenone. Food and Chemical Toxicology, 29, 741–750. [DOI] [PubMed] [Google Scholar]
- CCRIS (Chemical Carcinogenesis Research Information System), 2009. Online database of the National Library of Medicine's Toxicology Data Network (TOXNET®) maintained by the National Cancer Institute (NCI). Available online: http://toxnet/nlm.nih.gov
- ECHA (European Chemicals Agency), 2015. Decision on substance evaluation pursuant to Article 46(1) of Regulation (EC) NO 1907/2006 for benzophenone, CAS No 119–61‐9 (EC No 204–337‐6). Substance evaluation CoRAP, Available online: https://echa.europa.eu/documents/10162/91bde36a-b42e-4c15-bc1e-19fb1dfc9bfd
- EFSA (European Food Safety Authority), 2008. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to Flavouring Group Evaluation 69. Consideration of aromatic substituted secondary alcohols, ketones and related esters evaluated by JECFA (57th meeting) structurally related to aromatic ketones from chemical group 21 evaluated by EFSA in FGE.16 (2006). EFSA Journal 2008;869, 1–35, 869 pp. 10.2903/j.efsa.2008.869 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Scientific Opinion of EFSA prepared by the Panel on food contact materials, enzymes, flavourings and processing aids (CEF) on Toxicological evaluation of benzophenone. EFSA Journal 2009;1104, 1–30. 10.2903/j.efsa.2009.1104 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2010. Guidance on the data required for the risk assessment of flavourings. EFSA Journal 2010;8(6):1623, 38 pp. 10.2093/j.efsa.2010.1623 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017. Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR. Update: Guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO , 2008. Evaluation of certain food additives. Sixty‐ninth report of the joint FAO/WHO expert committee on food additives. Rome, 17–26 June 2008. WHO technical report series, No 952.
- Habauzit D, Martin C, Kerdivel G and Pakdel F, 2017. Rapid assessment of estrogenic compounds by CXCL‐test illustrated by the screening of the UV‐filter derivative benzophenones. Chemosphere, 173, 253–260. [DOI] [PubMed] [Google Scholar]
- Hall AP, Elcombe CR, Foster JR, Harada T, Kaufmann W, Knippel A, Küttler K, Malarkey DE, Maronpot RR, Nishikawa A, Nolte T, Schulte A, Strauss V and York MJ, 2012. Liver hypertrophy: a review of adaptive (adverse and non‐adverse) changes–conclusions from the 3rd International ESTP Expert Workshop. Toxicologic Pathology, 40, 971–994. [DOI] [PubMed] [Google Scholar]
- Hard GC, Banton MI, Bretzlaff RS, Dekant W, Fowles JR, Mallett AK, McGregor DB, Roberts KM, Sielken RL Jr, Valdez‐Flores C and Cohen SM, 2013. Consideration of rat chronic progressive nephropathy in regulatory evaluations for carcinogenicity. Toxicological Sciences, 132, 268–275. 10.1093/toxsci/kfs305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino N, Tani E, Wako Y and Takahashi K, 2005. A two‐generation reproductive toxicity study of benzophenone in rats. The journal of Toxicological Sciences, 30, Special Issue, 5–20. [DOI] [PubMed] [Google Scholar]
- IARC , 2013. Benzophenone. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 101, 285–304. [PMC free article] [PubMed] [Google Scholar]
- JECFA , 2002. Safety evaluation of certain food additives and contaminants. Fifty‐seventh meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 48. IPCS, WHO, Geneva.
- JECFA , 2011. Safety evaluation of certain food additives and contaminants. Seventy‐third meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 64. IPCS, WHO, Geneva.
- Jeon H‐K, Sarma SN, Kim Y‐J and Rhy J‐C, 2007. Forward gene mutation assay of seven benzophenone‐type UV filters using L5178Y mouse lymphoma cell. Molecular and Cellular Toxicology, 3, 23–30. [Google Scholar]
- Jeon H‐K, Sarma SN, Kim Y‐J and Ryu J‐C, 2008. Toxicokinetics and metabolisms of benzophenone‐type UV filters in rats. Toxicology, 248, 89–95. [DOI] [PubMed] [Google Scholar]
- Kerdivel G, Le Guevel R, Habauzit D, Brion F, Ait‐Aissa S and Pakdel F, 2013. Estrogenic potency of benzophenone UV Filters in breast cancer cells: proliferative and transcriptional activity substantiated by docking analysis. PLoS ONE, 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotnik K, Kosjek T, Žegura B, Filipič M and Heath E, 2016. Photolytic fate and genotoxicity of benzophenone‐derived compounds and their photodegradation mixtures in the aqueous environment. Chemosphere, 147, 114–123. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Yoshizawa K, Nyska A, Harada T, Flake G, Mueller G, Singh B and Ward JM, 2010. Hepatic enzyme induction: histopathology. Toxicologic Pathology, 38, 776–795. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Nyska A, Foreman JE and Ramot Y, 2016. The legacy of the F344 rat as a cancer bioassay model (a retrospective summary of three common F344 rat neoplasms). Critical Reviews in Toxicology, 46, 641–675. 10.1080/10408444.2016.1174669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick RL, Burns KM, Ward JM and Huff J, 2012. Chemically exacerbated chronic progressive nephropathy not associated with renal tubular tumor induction in rats: an evaluation based on 60 carcinogenicity studies by the national toxicology program. Toxicological Sciences, 128, 346–356. 10.1093/toxsci/kfs156 [DOI] [PubMed] [Google Scholar]
- Mikamo E, Harada S, J‐i Nishikawa and Nishihara T, 2003. Endocrine disruptors induce cytochrome P450 by affecting transcriptional regulation via pregnane X receptor. Toxicology and Applied Pharmacology, 193, 66–72. 10.1016/j.taap.2003.08.001 [DOI] [PubMed] [Google Scholar]
- Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B and Zeiger E, 1986. Salmonella mutagenicity tests II. Results from the testing of 270 chemicals. Environmental and Molecular Mutagenesis, 8(Suppl. 7), 1–119. [PubMed] [Google Scholar]
- Nakagawa Y and Tayama K, 2001. Estrogenic potency of benzophenone and its metabolites in juvenile female rats. Archives of Toxicology, 75, 74–79. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y and Tayama K, 2002. Benzophenone‐induced estrogenic potency metabolites in ovariectomized rats. Archives of Toxicology, 76, 727–731. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Suzuki T and Tayama S, 2000. Metabolism and toxicity of benzophenone in isolated rat hepatocytes and estrogenic activity of its metabolites in MCF‐7 cells. Toxicology, 156, 27–36. [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program), 2000. NTP technical report on the toxicity studies of benzophenone (CAS No. 119‐61‐9) administered in feed to F344/N rats and B6C3F1 mice. NTP Toxicity Report Series 61. [PubMed]
- NTP (National Toxicology Program), 2002. Developmental Toxicity Evaluation for Benzophenone (CAS No. 119‐61‐9) Administered by Gavage to Sprague‐Dawley (CD) Rats on Gestational Days 6 through 19. NTP Study No. TER‐98‐005. National Institute of Environmental Health Sciences, Research Triangle Park, NC.
- NTP (National Toxicology Program), 2004. Developmental Toxicity Evaluation for Benzophenone (CAS No. 119‐61‐9) Administered by Gavage to New Zealand White Rabbits on Gestational Days 6 through 29. Final Study Report. NTP Study No. TER‐99‐001. National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA.
- NTP (National Toxicology Program), 2006. NTP technical report on the toxicology and carcinogenesis studies of benzophenone (CAS no. 119‐61‐9) in F344/N rats and B6C3F1 mice (feed studies). February 2006. NTP TR 533. NIH Publication No. 06‐4469. [PubMed]
- OECD (Organisation for Economic Co‐operation and Development), 2001. Test No. 416: Two‐Generation Reproduction Toxicity. OECD Guideline for testing of chemicals, section 4. Available online: http://www.oecd-ilibrary.org/environment/test-no-416-two-generation-reproduction-toxicity_9789264070868-en
- OECD (Organisation for Economic Co‐operation and Development), 2007. Test No. 440: Uterotrophic Bioassay in Rodents. OECD Guideline for testing of chemicals, section 4. Available online: http://www.oecd-ilibrary.org/environment/test-no-440-uterotrophic-bioassay-in-rodents_9789264067417-en
- Rhodes MC, Bucher JR, Peckham JC, Kissling GE, Hejtmancik MR and Chhabra RS, 2007. Carcinogenesis studies of benzophenone in rats and mice. Food and Chemical Toxicology, 45, 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely JC, Haseman JK, Nyska A, Wolf DC, Everitt JI and Hailey JR, 2002. The effect of chronic progressive nephropathy on the incidence of renal tubule cell neoplasms in control male F344 rats. Toxicologic Pathology, 30, 681–686. [DOI] [PubMed] [Google Scholar]
- Song M, Kim YJ, Park YK and Ryu JC, 2012. Changes in thyroid peroxidase activity in response to various chemicals. Journal of Environmental Monitoring, 14, 2121–2126. 10.1039/c2em30106g [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kitamura S, Khota R, Sugihara K, Fujimoto N and Ohta S, 2005. Estrogenic and antiandrogenic activities of 17 benzophenone derivatives used as UV stabilizers and sunscreens. Toxicology and Applied Pharmacology, 203, 9–17. [DOI] [PubMed] [Google Scholar]
- Takemoto K, Yamazaki H, Nakajima M and Yokoi T, 2002. Genotoxic activation of benzophenone and its two metabolites by human cytochrome P450s in SOS/umu assay. Mutation Research, 519, 199–204. [DOI] [PubMed] [Google Scholar]
- Travlos GS, Hard GC, Betz LJ and Kissling GE, 2011. Chronic progressive nephropathy in male F344 rats in 90‐day toxicity studies: its occurrence and association with renal tubule tumors in subsequent 2‐year bioassays. Toxicologic Pathology, 39, 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triskelion , 2017. VCF online. Volatile Compounds in Food. Nijssen B, van Ingen‐Visscher K and Donders J (eds.). Database version 16.3. Triskelion, Zeist, The Netherlands. 1992–2017.
- USEPA (United States Environmental Protection Agency), 1984. Information Review; Benzophenone. Submitted by CRCS, Rockville, MD, in collaboration with Dynamac Corporation Environmental Control Division, Rockville, MD, to USEPA, TSCA Interagency Testing Committee.
- Wheeler MW and Bailer AJ, 2008. Model Averaging Software for Dichotomous Dose Response Risk Estimation. Journal of Statistical Software, 26, 1–15. 10.18637/jss.v026.i05 19777145 [DOI] [Google Scholar]
- Yamasaki K, Takeyoshi M, Yoshikuni Y, Sawaki M, Imatanaka N and Takatsuki M, 2002. Comparison of reporter gene assay and immature rat uterotrophic assay of twenty‐three chemicals. Toxicology, 170, 21–30. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Takeyoshi M, Sawaki M, Imatanaka N, Shinoda K and Takatsuki M, 2003. Immature rat uterotrophic assay of 18 chemicals and Hershberger assay of 30 chemicals. Toxicology, 183, 93–115. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Takahashi M and Yasuda M, 2005. Two‐generation reproductive toxicity studies in rats with extra parameters for detecting endocrine disrupting activity: introductory overview of results for nine chemicals. The Journal of Toxicological Sciences, 30, Special Issue, 1–4. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ma X, Dzakpasu M and Wang XC, 2017. Evaluation of ecotoxicological effects of benzophenone UV filters: luminescent bacteria toxicity, genotoxicity and hormonal activity. Ecotoxicology and Environmental Safety, 142, 338–347. [DOI] [PubMed] [Google Scholar]


