Abstract
In April and May of 2016, Norway confirmed two cases of chronic wasting disease (CWD) in a wild reindeer and a wild moose, respectively. In the light of this emerging issue, the European Commission requested EFSA to recommend surveillance activities and, if necessary, additional animal health risk‐based measures to prevent the introduction of the disease and the spread into/within the EU, specifically Estonia, Finland, Iceland, Latvia, Lithuania, Norway, Poland and Sweden, and considering seven wild, semidomesticated and farmed cervid species (Eurasian tundra reindeer, Finnish (Eurasian) forest reindeer, moose, roe deer, white‐tailed deer, red deer and fallow deer). It was also asked to assess any new evidence on possible public health risks related to CWD. A 3‐year surveillance system is proposed, differing for farmed and wild or semidomesticated cervids, with a two‐stage sampling programme at the farm/geographically based population unit level (random sampling) and individual level (convenience sampling targeting high‐risk animals). The current derogations of Commission Implementing Decision (EU) 2016/1918 present a risk of introduction of CWD into the EU. Measures to prevent the spread of CWD within the EU are dependent upon the assumption that the disease is already present; this is currently unknown. The measures listed are intended to contain (limit the geographic extent of a focus) and/or to control (actively stabilise/reduce infection rates in an affected herd or population) the disease where it occurs. With regard to the zoonotic potential, the human species barrier for CWD prions does not appear to be absolute. These prions are present in the skeletal muscle and other edible tissues, so humans may consume infected material in enzootic areas. Epidemiological investigations carried out to date make no association between the occurrence of sporadic Creutzfeldt–Jakob disease in humans and exposure to CWD prions.
Keywords: chronic, wasting, cervids, surveillance, risk, introduction, spread
Summary
In April and May of 2016, Norway confirmed two cases of chronic wasting disease (CWD) in a wild reindeer and a wild moose, respectively. This was the first time CWD had been detected in Europe and the first natural case in reindeer in the world. In the light of the sensitivity of this emerging issue, the European Food Safety Authority (EFSA) was asked by the European Commission to deliver its scientific opinion by 31 December 2016 on the following Terms of Reference (ToRs): (1) to provide recommendations on surveillance of cervid populations at the country level aimed at detecting CWD and/or estimating the prevalence of CWD in Norway, Sweden, Finland, Iceland, Estonia, Latvia and Poland, which are the European Union (EU) and European Economic Area (EEA) countries with reindeer and/or moose populations, depending on the level of prevalence which is wished to be detected; (2) has new evidence become available with regard to possible public health risks due to the occurrence of CWD in cervids since the publication of the 2011 joint EFSA/ECDC opinion? Does the natural exposure of consumers to cervid products originating from regions where CWD cases are detected represent a risk for public health? (3) EFSA is asked to recommend, if necessary, additional animal health risk‐based measures to prevent the introduction of CWD into the EU cervid populations and to prevent its spread within the EU.
It was agreed with the requestor to also include Lithuania in the mandate, and to consider the following seven wild, semidomesticated and farmed cervids, namely Eurasian tundra reindeer (Rangifer tarandus tarandus), Finnish (Eurasian) forest reindeer (Rangifer tarandus fennicus), moose (Eurasian/European elk) (Alces alces alces), roe deer (Capreolus capreolus), white‐tailed deer (Odocoileus virginianus), red deer (Cervus elaphus) and fallow deer (Dama dama).
The data used in this assessment have been sourced via different literature searches, looking at the new evidence from experimental studies examining the transmissibility of transmissible spongiform encephalopathies (TSE) agents to humans, as well as the epidemiology, surveillance and control of CWD. The figures on wild and hunted population abundance were obtained from different sources and represent various methodologies used for census and estimates. Surveillance data in Europe in 2015 have been extracted from annual reports submitted by the Member States (MS) and from the background information provided by the European Commission and included in the mandate.
It was agreed, due to limited time and resources, to carry out a qualitative evaluation by means of literature reviews based on the knowledge and expertise of the Working Group (WG) members. The experts in the WG selected relevant references starting from review papers, books chapters, non‐peer‐review papers known by the experts themselves or retrieved through non‐systematic searches until the information of the subject was considered sufficient to undertake the assessment by the WG. The literature search was used to support the expert review of these areas, and additional scientific information known by the experts was also considered in the assessment.
The surveillance system proposed for the countries concerned is based on the experience of CWD surveillance in North America and the knowledge of the different structures and management systems of the cervid populations of some of the countries concerned, in particular Norway and Sweden. The aims of the proposed surveillance system are to detect disease in countries where CWD has not yet been detected using a predefined design prevalence and to estimate prevalence in areas where disease has been detected. It is intended to overcome the shortcomings highlighted in the evaluation of the previous surveillance programme implemented in Europe 2006–2010. A two‐stage sampling programme is proposed based on the application of random sampling at the first stage (for wild/semidomesticated cervids the ‘primary sampling units’ (PSU) will correspond to geographical areas containing cervid populations, whereas for farmed cervids they will correspond to farms) and the application of convenience sampling of high‐risk animals within PSU (found dead, hunted or slaughtered animals considered not fit for human consumption, road/predator kills and animals killed because they are sick or in poor body condition and not fit for human consumption) of any of the selected species at the second stage.
With regard to the public health risks, there is currently no experimental model that encompasses all the potential host and agent variability required to assess zoonotic potential directly for any animal prion disease, including CWD. Although CWD has been experimentally transmitted to squirrel monkeys; in vivo transmission of CWD to other animal models including macaques and humanised mice has not yet been reported. CWD strains, their prevalence, host range and zoonotic potential remain incompletely understood. All currently available data pertaining to host range and human risk are derived from isolates obtained from North American cervid species, but preliminary evidence from the Norwegian CWD cases raises the possibility that European and North American isolates are different from each other.
There is no evidence of an absolute species barrier between CWD‐affected cervids and humans. CWD prions are present in the skeletal muscle and other edible tissues, which means that humans may consume infected material in enzootic areas. However, from the epidemiological investigations carried out to date, no association has been made between the occurrence of sporadic Creutzfeldt–Jakob disease (sCJD) in humans and exposure to CWD.
Since it was concluded that the most likely pathway of introduction of CWD into the EU is the movement of live cervids, the current derogations of Article 2.2 of the Commission Implementing Decision (EU) 2016/1918 present a risk of introduction of CWD into the EU. The probability of introduction of CWD into and spread within the EU associated with the movement of live cervids for direct slaughter is considered to be lower than situations in which live animals are translocated for other purposes. Minimising movements of live cervids would reduce the probability of introduction of CWD into the EU. The use of natural cervid urine lures is considered to increase the probability of introduction of CWD into the EU. Compliance with recommendations included in awareness campaigns targeting both local Norwegian hunters, and hunters visiting Norway from (and returning to) other countries, would reduce the probability of introduction of CWD into the EU.
Measures to prevent the spread of CWD within the EU are dependent upon the assumption that the disease is already present in some part of the EU territory. At the time of writing this opinion, this is unknown. The uncertainties associated with the limited knowledge of the situation of CWD in the countries concerned could render any of the recommended measures inadequate or insufficient. Moreover, strategies to prevent the introduction and/or the spread of CWD must be based on a combination of measures.
A list of measures has been included. Some aim to reduce animal‐to‐animal contact and lower population densities; others relate to increasing the disease awareness among stakeholders, reducing environmental contamination and developing contingency plans, including quarantine and other measures in infected and management premises/areas. Specific prohibitions within Norway, affecting silage, feed and lichen to prevent the spread of CWD, have been mentioned.
Additional activities have been recommended, such as reviewing the design of the surveillance system after 1 year of implementation, conducting a risk assessment to estimate the likelihood of introduction of CWD into the EU, collecting data on the cervid populations in Europe, implementing an individual identification system and record‐keeping of farmed and semidomesticated cervids together with an ad hoc data collection system for surveillance. Any positive case detected through surveillance should be genotyped and characterised by strain typing, with representative material archived for future reference. It is also recommended that all or a representative subset of cervids tested negative by surveillance are genotyped. Consideration is given to implementing surveillance programmes in other MS.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Previous opinions on zoonotic aspects of CWD and surveillance
The former Scientific Steering Committee of the European Commission (SSC)1 adopted on 6–7 March 2003 an opinion ‘on chronic wasting disease (CWD) and tissues that might carry a risk for human and animal feed chains’. In summary, it highlighted that a risk of prion transmissions to humans consuming products of CWD‐affected cervids could not be excluded.
In its scientific opinion of 3 June 2004 on a surveillance programme for CWD in the European Union (EU) (EFSA, 2004), the European Food Safety Authority (EFSA) stressed ‘a potential risk to consumers if a transmissible spongiform encephalopathy (TSE) would be present in European cervids’. EFSA further highlighted that ‘it might be prudent considering appropriate measures to reduce such a risk, e.g. excluding tissues such as central nervous system (CNS) and lymphoid tissues from the human food chain, which would greatly reduce any potential risk for consumers. However, it is stressed that currently, no data regarding a risk of TSE infections from cervid products for humans are available’.
In its 2011 scientific opinion on possible associations between TSEs in animals and humans (EFSA BIOHAZ Panel (2011), EFSA concluded regarding CWD that, although CWD agents have failed to induce disease in transgenic mice expressing human prion protein (PrP), experimental transmission to certain non‐human primate species has been reported. EFSA also mentioned ongoing experiments to assess the zoonotic potential of CWD strains in primate models.
The SSC Opinion of 6–7 March 2003 also recommended the instigation of a surveillance programme for TSE in cervids in the EU. As a result, the Commission asked EFSA for recommendations concerning such surveillance, and EFSA recommended in its opinion of June 2004 to initiate an EU‐wide experimental screening, targeting at‐risk groups of animals.
On that basis, a survey on CWD in the EU was launched by Commission Decision 2007/182/EC2 and implemented between 2007 and 2010. In this framework, more than 13,000 samples were collected from 21 Member States (MS) and Norway, mainly from red deer and white‐tailed deer (the survey also included 74 samples from reindeer), without any sample found positive to TSE. Therefore, EFSA concluded in 2010 that, while occurrences of cases of TSEs in cervids in the EU could not be excluded, especially in remote and presently unsampled geographical areas, there was no cervid TSE epidemic in the EU.
In mid‐March 2016, a sick animal was observed during an exercise of identification and registration of wild reindeers (Rangifer tarandus) by the Norwegian Institute for Nature Research, in the locality of Laerdal. The animal subsequently died and its carcass was sent to the Norwegian Veterinary Institute for necropsy. The necropsy included testing for TSE. On 4 April 2016, the Norwegian NRL for TSEs confirmed the presence of TSE by enzyme‐linked immunosorbent assay (ELISA), western blot (WB) and immunohistochemistry (IHC). On 7 April 2016, the European Reference laboratory (EURL) for TSE confirmed that the samples received were strongly positive for TSE and were presumptive for CWD. On 27 April 2016, the International Organization of Animal Health (OIE) Reference Laboratory for CWD in Canada (Canadian Food Inspection Agency) confirmed the CWD‐positive diagnosis, noting that the sample was consistent with CWD in farmed and wild cervids in Canada, and reindeer experimentally infected with CWD by the oral route.
On 25 May 2016, a second case of CWD was confirmed in Norway, this time in a wild moose, in the locality of Selbu. The moose (Alces alces) was a young adult and pregnant female, which was killed due to abnormal behaviour. The animal was dehydrated, cachectic and had increased urination. It was found in Selbu in south Norway. The Norwegian NRL for TSE performed ELISA and WB, which were both positive.
Following these cases, Norway has expanded its surveillance of cervids for TSEs. Norway's objective is to test those cervids found sick or that died but were not slaughtered for human consumption. In addition, the Norwegian authorities encourage hunters in the two concerned regions to bring heads of animals killed during the hunting season to control points in view of TSE sampling and testing. Furthermore, Norway plans to start a surveillance programme for farmed reindeer, during the slaughter season which starts in September 2016.
Additional information provided by the MS at request of the Commission included the UK informing the Commission on an updated qualitative risk assessment (RA) on the risk that CWD is being introduced into Great Britain (GB). The assessment is available at: https://www.gov.uk/govemment/up1oads/svstem/uploads/attachmentdata/file/514401/qra-chronic-wasting-disease.pdf
1.1.2. Terms of Reference
EFSA is requested by the European Commission to provide a scientific opinion on the following questions:
EFSA is asked to provide recommendations on surveillance of the cervid populations at the country level aimed at detecting CWD and/or estimating the prevalence of CWD in Norway, Sweden, Finland, Iceland, Estonia, Latvia and Poland, which are the EU and EEA countries with reindeer and/or moose populations, depending on the level of prevalence which is wished to be detected.
Has new evidence become available with regard to possible public health risks due to the occurrence of CWD in cervids since the publication of the 2011 joint EFSA/ECDC opinion? Does the natural exposure of consumers to cervid products originating from regions where CWD cases are detected represent a risk for public health?
EFSA is asked to recommend, if necessary, additional animal health risk‐based measures to prevent the introduction of CWD into the EU cervid populations and to prevent its spread within the EU?
Are the conclusions and recommendations in the EFSA opinion of June 2004 on diagnostic methods for CWD still valid? If not, an update should be provided.
EFSA is asked to update the conclusions of the 2010 EFSA opinion on the results of the EU survey on CWD in cervids, as regards the occurrence of CWD in the cervid population in the EU.
1.2. Interpretation of the Terms of Reference (if appropriate)
In the light of the sensitivity of this emerging issue, EFSA was asked by the European Commission to deliver its scientific opinion as soon as possible and according to the following schedule:
EFSA is asked to provide its scientific opinion on the Terms of Reference No 1 (surveillance), 2 (public health) and 3 (risk mitigation measures) by 31 December 2016;
EFSA is asked to provide its scientific opinion on the Terms of Reference No 4 (diagnostic of CWD) and 5 (review of 2010 EFSA opinion) by 31 December 2017.
It was agreed with the requestor to include Lithuania in the scope of the mandate due to the significant moose population in this country and its geographical location. Thus, the countries considered in this assessment are: Estonia, Finland, Iceland, Latvia, Lithuania, Norway, Poland and Sweden.
Taxonomically, cervids belong to the family Cervidae. The family Cervidae has two subfamilies: Cervinae and Capreolinae (or Odocoileinae).
Since there may be confusion and misunderstanding regarding the common names on cervid species (e.g. ‘Eurasian elk’ is the moose in Europe (Alces alces alces) but ‘North American elk’ (wapiti; Cervus elaphus nelsoni) are more equivalent to European ‘red deer’, etc.), it is necessary to refer to their Latin names. The cervid species below are referenced by common names hereafter. Unless preceded by a descriptor (e.g. ‘red’, ‘mule’) or otherwise denoted, the term ‘deer’ refers generically to animals of North American species in the genus Odocoileus.
The following species or subspecies in Europe are referred to in this report:
-
Subfamily Capreolinae:
-
1
— Eurasian tundra reindeer (Rangifer tarandus tarandus)
-
2
— Finnish (Eurasian) forest reindeer (Rangifer tarandus fennicus)
-
3
— Moose (or Eurasian/European elk) (Alces alces alces).
-
4
— Roe deer (Capreolus capreolus)
-
5
— White‐tailed deer (Odocoileus virginianus)
-
1
-
Subfamily Cervinae:
-
1
— Red deer (Cervus elaphus)
-
2
— Fallow deer (Dama dama).
-
1
For the countries of interest for this report, the management of cervid populations are considered for three different systems (where applicable): (a) wild cervid populations (i.e. all species, free ranging, no private owners); (b) semidomesticated reindeer, which constitutes those reindeer that are herded (i.e. Eurasian tundra reindeer); and (c) farmed cervids (i.e. red deer and fallow deer, held in fixed enclosures all year around and throughout their lifetime). In contrast to cervids in all these three management systems, cervids in parks and zoos are not raised for human consumption. However, such cervids have, in practical terms, a management system that is similar to that of farmed cervids and will thus not be dealt with separately in this report. These three management systems are considered to be of significant potential relevance to the epidemiology of CWD, and thus to surveillance and control.
1.3. Additional information (if appropriate)
Additional background information contained in the mandate, namely, current measures and import data on cervid meat, has been included in Appendix A.
2. Data and methodologies
2.1. Data
The data used in parts of Section 3.1 and in Section 3.2 have been sourced via different literature searches, looking at new evidence on the experimental studies showing the transmissibility of the TSE agents to humans, as well the epidemiology, surveillance and control of CWD, as described in Section 2.2. Additional data have been extracted from scientific papers that were out of the scope of the search due to the specific subject.
The figures on population abundance included in Section 3.3.4 were obtained from different sources and represent various methodologies used for census and estimates of wildlife population. Additionally, some of the data presented is several years old since more recent data could not be obtained, and the actual population sizes may have changed since the data were recorded. Therefore, the population data presented may be incomplete or outdated, reflecting the stated difficulties. Hunting statistics presented in the table below are mostly sourced from national official statistics websites.
According to Part I.A, Chapter B.I Annex III of Regulation (EC) 999/20013, the information to be presented by the MS in their annual report should include animals other than bovine, ovine and caprine, and the number of samples and confirmed TSE cases per species. Surveillance data in Europe in 2015 have been extracted from the above‐mentioned annual reports submitted by the MS and from the background information provided by the European Commission and included in the mandate.
2.2. Methodologies
A literature search was performed in the framework of this mandate to inform the review of the evidence in the scientific literature on the topics covered by the three ToRs. The literature search was used to support the expert review of these areas, and additional scientific information known by the experts was also considered in the assessment.
The search string used for the literature search was: (‘chronic wasting disease’ OR CWD OR wasting OR TSE* OR BSE OR scrapie OR PrP* OR PRNP OR prion*) AND (surveillance OR prevalence OR incidence OR epidem* OR introduc* OR spread OR risk OR ‘public health’ OR zoono*) AND (deer* OR cervid* OR moose* OR elk* OR reindeer*). These terms were searched in the titles and abstracts of the scientific publications. The search was conducted in the PubMed/MEDLINE database. The search was restricted to the following languages: English, Estonian, Finnish, French, Icelandic, Latvian, Norwegian, Polish and Swedish. The publication dates were unrestricted. A total of 238 references was retrieved and screened for studies of interest, mainly with regard to the tissue distribution of CWD. A subset of 26 references were considered potentially relevant and reviewed in full.
A literature search was performed in the framework of this mandate to inform the review of the evidence in the scientific literature on the in vivo and in vitro experimental studies showing the transmissibility of the TSE agents to humans. In particular, the search was aimed at identifying new scientific evidence that has become available subsequent to the publication of the 2015 EFSA scientific opinion ‘on a request for a review of a scientific publication concerning the zoonotic potential of ovine scrapie prions’ (EFSA BIOHAZ Panel, 2015). The literature search was used to support the expert review of these areas, and additional scientific information known by the experts was also considered in the review.
The search string used for the literature search of in vivo transmission studies of TSE in animal models exploring the zoonotic potential of CWD was: (BSE OR TSE OR scrapie OR CWD OR *CJD OR Nor98 OR Nor‐98 OR spongiform encephalopa* OR ‘chronic wasting disease’ OR ‘creutzfeldt‐jakob’ OR ‘creutzfeldt jakob’ OR prion OR prp*) AND (transmissible OR transmission OR transmitted OR transgenic OR barrier OR passage* OR tg OR humanised OR humanized). These terms were searched in the titles of the scientific publications. The search was conducted in the following databases: ISI Web of Knowledge; CAB Abstracts; Current Contents; FSTA; Journal Citation Report and Web of Science. The search was restricted to English language and from 1 January 2015 until 9 September 2016, since the opinion covered extensively the topic until 2015. A total of 101 references were retrieved and screened for studies of interest. A subset of 10 references were considered potentially relevant and reviewed.
The search string used for the literature search of in vitro transmission studies of TSE in animal models exploring the zoonotic potential of CWD was: (BSE OR TSE OR scrapie OR CWD OR *CJD OR Nor98 OR Nor‐98 OR spongiform encephalopa* OR ‘chronic wasting disease’ OR ‘creutzfeldt‐jakob’ OR ‘creutzfeldt jakob’ OR prion OR prp*) AND (misfold* OR conversion OR ‘in vitro’ OR ‘in‐vitro’ OR amplification OR passage* OR cycl* OR substrate OR *quic OR ‘asa’ OR pmca OR quaking). These terms were searched in the titles of the scientific publications. The search was conducted in the following databases: ISI Web of Knowledge; CAB Abstracts; Current Contents; FSTA; Journal Citation Report and Web of Science. The search was restricted to English language and from 1 January 2015 until 9 September 2016, since the opinion covered extensively the topic until 2015. A total of 119 references was retrieved and screened for studies of interest. A subset of 4 references was considered potentially relevant and reviewed in detail.
It was agreed, due to limited time and resources, to carry out a qualitative evaluation, by means of literature reviews based on the knowledge and expertise of the Working Group (WG) members. In these cases, the experts in the WG selected relevant references starting from scientific papers including review papers, books chapters, non‐peer‐review papers known by the experts themselves or retrieved through non‐systematic searches, until the information of the subject was considered sufficient to undertake the assessment by the WG.
3. Assessment
3.1. Chronic wasting disease (CWD): background
3.1.1. Origin of the disease
The origins of CWD are not clear. How different TSE have spread and become established in populations is reasonably well understood, but where the ‘index case’ came from has never been identified. It is also unclear whether all of the known foci can be tied to a single event or whether there have been multiple ‘origin’ events (Williams and Young, 1992; Williams and Miller, 2002, 2003). It has been speculated for some other animal TSE, in particular the atypical forms of scrapie and bovine spongiform encephalopathy (BSE), that disease may have a spontaneous origin, but this has not been conclusively proved (Casalone et al., 2004; Fediaevsky et al., 2008, 2010; Baron et al., 2011; Ortiz‐Pelaez et al., 2016). The origins of classical forms of TSE have never been established, and although it has been speculated that naturally occurring TSE may at some point have crossed between animal species, there is again no unequivocal evidence to support or refute these hypotheses.
This lack of understanding of the origins of disease means that we do not know whether the recent identification of disease in European cervids is necessarily linked to the pre‐existing disease in North America, or if it could be unrelated. The absence of any previous systematic monitoring or surveillance of European deer might have allowed disease to exist undetected for a long time (EFSA BIOHAZ Panel, 2010).
While the source of disease in many settings in North America almost certainly relates to the highly contagious transmission of CWD prions from diseased animals and to inadvertent or natural movements of infected animals, the ultimate origins of CWD remain a mystery in both Norway and North America.
Possible explanations of the origin of CWD include the spontaneous conversion of normal cervid prion protein (PrP) into abnormal form of PrP (PrPSc) that is transmissible to other wapiti and deer (Odocoileus spp.), or the transmission of prions causing diseases in other species to cervids. CWD could also have originated by infection with an as‐yet‐unrecognised prion. Consistent with the first hypothesis, high resolution structural studies showed the loop region linking the second beta‐sheet (β2) with the alpha2‐helix (α2) of cervid PrP to be extremely well defined compared with most other species, raising the possibility that this structural characteristic correlates with the ease with which contagious transmission of CWD occurs (Gossert et al., 2005). Consistent with this, transgenic (Tg) mice expressing mouse PrP, in which the β2–α2 loop was replaced by the corresponding region from cervid species, spontaneously developed prion disease (Sigurdson et al., 2009). Additional studies consistently point to the importance of the β2–α2 loop in regulating transmission barriers, including that of CWD to humans (Kurt et al., 2009; Sigurdson et al., 2010; Kurt et al., 2014, 2015). However, subsequent work suggested a more complex mechanism in which the β2–α2 loop participates with the distal region of α‐helix 3 to form a solvent‐accessible contiguous epitope (Perez et al., 2010). These and other studies (Christen et al., 2009) ascribed greater importance to the plasticity of this discontinuous epitope.
There has been long‐standing speculation that CWD derived from the interspecies transmission of scrapie, a disease that has been recognised in domestic sheep in the United States since 1947. Several studies have been performed to address this possibility (Race et al., 2002), including experimental intracerebral inoculation of deer and wapiti with scrapie prions (Hamir et al., 2003, 2004; Greenlee et al., 2011). Of six wapiti inoculated with scrapie, three presented with neurological signs and neuropathology, but only after long and variable times to disease onset, ranging from 25 to 46 months. The brains of these scrapie‐infected wapiti were indistinguishable from CWD by histopathological examination or IHC (Hamir et al., 2004). Two subsequent studies showed relatively easy transmission of scrapie to white‐tailed deer; in both cases all inoculated animals developed disease within 19–20 months (Greenlee et al., 2011). Additional findings indicate other similarities between CWD and scrapie in white‐tailed deer. While there was no lymphoid spread of scrapie in wapiti (Hamir et al., 2004), the early and widespread presence of PrPSc in lymphoid tissues of scrapie‐challenged deer (Greenlee et al., 2011) is similar to CWD (Sigurdson et al., 1999). Finally, western blot showed that PrPSc in the obex region of scrapie‐infected deer have a molecular profile consistent with CWD, but distinct from tissues of the cerebrum or the scrapie inoculum (Greenlee et al., 2011).
While these data indicate that scrapie can transmit intracerebrally to deer, the number of scrapie isolates and recipient animals is very small, and the outcome is a disease that presents some differences from CWD, so no conclusions can be drawn with regard to any causal relationship.
3.1.2. History of CWD
CWD was first identified in the late 1960s as a fatal wasting syndrome of mule deer (Odocoileus hemionus hemionus) in a northern Colorado research facility (Williams and Young, 1980, 1992). Although the disease was initially considered to be a nutritional deficiency and/or related to stresses caused by captivity, CWD was ultimately recognised as a TSE in 1978 by histopathological assessment (Williams and Young, 1980, 1982). Subsequent to its initial recognition in the northern Colorado research facility, CWD was identified in mule deer in a research facility in Wyoming. Soon thereafter CWD was diagnosed in captive wapiti in both the Colorado and Wyoming facilities.
In 1980, following this description of CWD as a TSE, a case was confirmed by neuropathological assessment of a mule deer that had died at the Toronto Zoo in 1978, at which time the diagnosis was ‘spongiform encephalopathy’. This animal had clinical signs now known to be compatible with CWD. A retrospective study of cervids resident at the Toronto Zoo between 1973 and 2003 revealed additional CWD cases, the last case being in 1981 (Dubé et al., 2006). Analyses included examination of management, animal health and post‐mortem records, and immunohistochemical studies. CWD was ultimately diagnosed in eight out of 105 animals tested: seven mule deer and one black‐tailed deer (O. hemonius columbianus). The most likely method of introduction was the importation of CWD‐infected animals from a zoo in the United States. Animal‐to‐animal contact and environmental contamination were the most likely methods of spread of CWD at the Toronto zoo. Although CWD was not diagnosed in cervid species other than mule or black‐tailed deer, the Toronto zoo either donated or sold a total of 108 wapiti, 8 moose, 83 reindeer/caribou and 31 white‐tailed deer to other zoos, government agencies, or universities from 1976 to 2002 (Dubé et al., 2006).
CWD is unique among prion diseases as being the only known prion disease of free‐ranging, as well as captive animals. Disease was found in free‐ranging mule deer and wapiti in south‐eastern Wyoming and north‐eastern Colorado soon after its recognition as a TSE (Williams and Young, 1992; Spraker et al., 1997). Subsequent surveillance and modelling studies indicated that CWD occurred endemically among free‐ranging deer and wapiti in a contiguous area in north‐eastern Colorado, south‐eastern Wyoming and western Nebraska, and that CWD most likely had been present in free‐ranging cervids in this ‘endemic region’ for several decades prior to its eventual recognition (Miller et al., 2000). In recent years, CWD has been detected in wild (Baeten et al., 2007) and captive moose (Alces alces shirasi) (Kreeger et al., 2006) in the endemic region.
Although first thought to be limited in to the endemic region in the wild, additional foci of disease in free‐ranging animals, distant from the endemic region, have been identified. In 2000, CWD was detected in Saskatchewan, Canada, and in 2002, a third free‐ranging population with CWD was identified in southern Wisconsin (Joly et al., 2003). Ultimately additional, apparently separate foci were reported in New Mexico, New York, West Virginia, Missouri, Minnesota and Arkansas. While identification of CWD‐affected animals in areas previously thought to be free of infection may be partly related to increased surveillance, spread of the disease by natural migration of infected animals, and by the translocation of infected cervids by humans, have almost certainly played a role in the emergence of disease in new locations. The latter mechanism is exemplified by CWD outbreaks occurring in South Korea, which resulted from the importation of subclinically infected Canadian animals (Sohn et al., 2002; Kim et al., 2005).
The occurrence of CWD in captive herds in new locations has, in some cases, preceded detection of the disease in free‐ranging animals. This raised the possibility that the origins of CWD in wild animals in Saskatchewan, New York state and Missouri were spillovers of disease from captive facilities in or before 1996–2001, 2005 and 2010, respectively. Disease continues to be detected in new geographic locations and with increasing prevalence in some areas where the disease has been monitored the longest (Miller and Fischer, 2016). At the time of writing, CWD has been documented in captive and free‐ranging deer in 24 states of the USA and three Canadian provinces. Since the beginning of 2016, CWD has been documented in free‐ranging deer and wapiti populations in new geographic locations within several endemic jurisdictions (Alberta, Nebraska, Texas, Missouri, Colorado and Wyoming), and was detected for the first time in Arkansas. The disease was also detected in new facilities for captive white‐tailed deer in Texas and Wisconsin. A map of the current known distribution of CWD in North America, available from the United States Geological Survey (USGS) National Wildlife Health Center (NWHC), is shown in Figure 1. In addition, the detection of CWD in wild reindeer (Benestad et al., 2016) in April 2016 and subsequently moose in May 2016, announced by the Norwegian Veterinary Institute and the Norwegian Environment Agency, represent the first documented cases of CWD in Europe.
Figure 1.
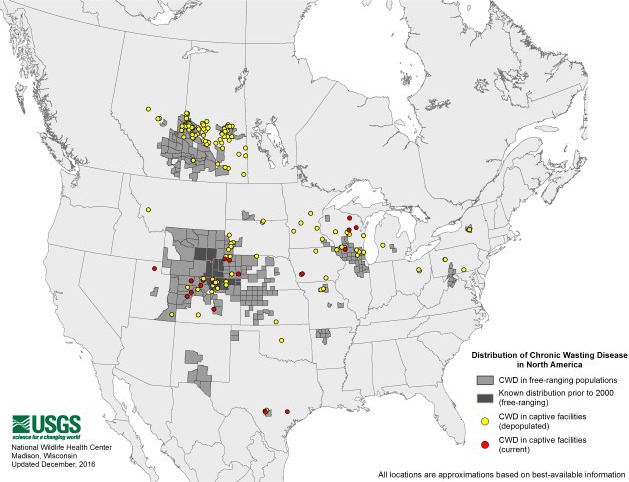
Distribution of CWD in North America in as of October 2016. Source: USGS
- The historical occurrence at the Toronto Zoo (Ontario, Canada) is not shown on the map (https://www.nwhc.usgs.gov/disease_information/chronic_wasting_disease)
CWD can have a variety of effects on jurisdictions where cervids represent an important economic, ecologic or sociologic resource. CWD has both a significant economic impact and influences wildlife conservation. The US Fish and Wildlife Service estimated that a total of $33.7 billion was spent on hunting items in 2011, and an estimated 11.6 million hunters pursued big game such as deer and wapiti (U.S. Department of the Interior, U.S. Fish and Wildlife Service, and U.S. Department of Commerce, U.S. Census Bureau, 2011). The Wisconsin Department of National Resources spent approximately $5 million annually on CWD management from 2002 until 2006 (Wisconsin Department of Natural Resources, 2012). Saskatchewan spent ~$30 million to eradicate the disease within infected commercially operated game farms (Oklahoma Department of Wildlife Conservation, 2002).
Beyond the direct effect on productivity of infected cervid herds or populations when prevalence becomes high, infection at any level has variable impact on animal movements, management options, and public perceptions about food safety. To date these impacts have not been catastrophic, but the potential for greater impacts remains a possibility given experiences with other animal prion diseases.
3.1.3. Pathogenesis
Data indicate that the general pathogenesis of CWD is similar to that recorded for classical scrapie, with detectable involvement of the lymphoreticular system (LRS) preceding that of the central nervous system (CNS) (Sigurdson et al., 1999; Fox et al., 2006). As in sheep, host genotype has an effect on how disease develops but, unlike the situation for scrapie, this seems to relate more to the length of incubation period than to the pathogenesis or ultimate dissemination of the agent within tissues (Fox et al., 2006; Johnson et al., 2011; Miller et al., 2012).
Like other prion diseases, lesions are confined to the CNS and consist of intraneuronal vacuolation, neuropil spongiosis, astrocytic hypertrophy and hyperplasia (Williams and Young, 1993). Florid amyloid plaques also feature in the neuropathology of diseased deer (Liberski et al., 2001). CWD is characterised by extensive CNS and lymphoid tissue deposition of PrPSc, the latter being detectable at early stages of the disease (Sigurdson et al., 1999; Fox et al., 2006); however, again, CWD pathogenesis seems to vary between deer and wapiti with less PrPSc deposition in the lymphoid tissues of wapiti compared with deer (Race et al., 2007). This early peripheral PrPSc accumulation can hypothetically be used to improve surveillance sensitivity, but involvement of tissues readily accessible in the live animal, such as the rectoanal mucosa‐associated lymphoid tissue (RAMALT) are not consistently affected in earliest stages of infection. Infectivity has been detected in saliva and in urine, supporting a role for these body fluids in transmission and dissemination. Faecal material from subclinical deer also harbours infectivity (Haley et al., 2009a; Tamgüney et al., 2009). These excreta may also offer some potential for surveillance/screening. The list of tissues affected by the time clinical disease develops includes edible tissues such as the heart, liver, kidney, tongue, pancreas (Fox et al., 2006; Sigurdson et al., 2001), blood (Mathiason et al., 2006) adipose tissue and LRS, and those used as dietary supplements (e.g. antler velvet).
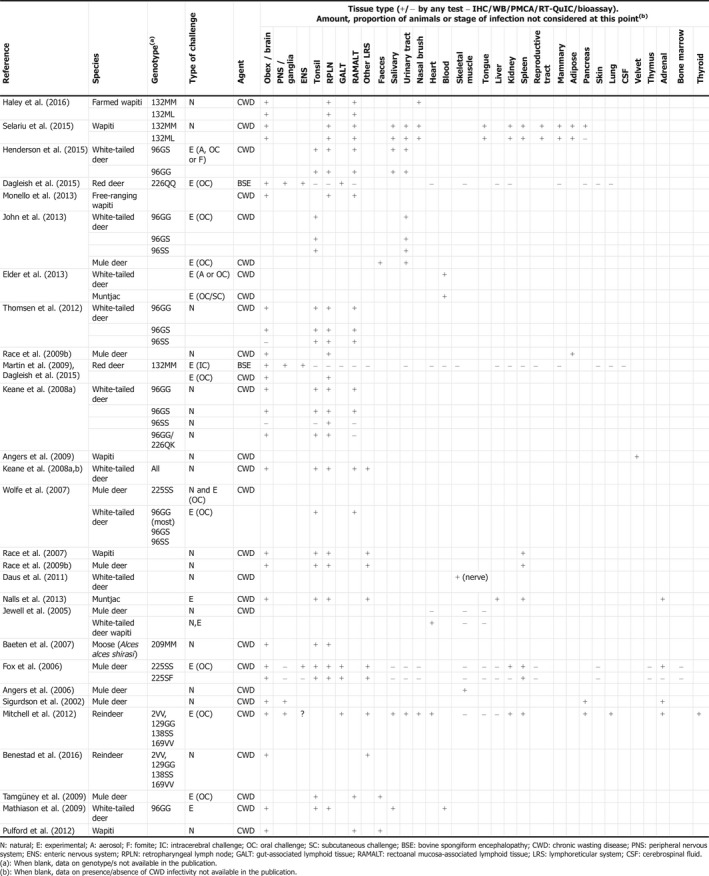
Table 1 summarises the documented evidence for total detectable tissue distribution of PrPSc and/or infectivity, as determined by a range of methods (IHC, WB, protein misfolding cyclic amplification (PMCA), real‐time quaking‐induced conversion (RT‐QuIC) or bioassay), regardless of the stage of disease. A detailed analysis of the timescale and specific pathogenesis in individual species is beyond the scope of this opinion.
Table 1.
Distribution of CWD infectivity in cervids by species, genotype and route of exposure
Signs in clinically affected animals include weight loss, behavioural alterations, apparent ruminal atony and salivary drooling in late stages of disease. Clinical features also include gradual loss of body condition, resulting in emaciation, hence the term ‘wasting disease’, and behavioural changes that include generalised depression, and loss of fear of humans (Williams, 2003). At later stages, affected animals may display polydipsia and polyuria, sialorrhoea and generalised incoordination. The clinical course in captive animals is slowly progressive, and after diagnosis most animals survive for a few weeks up to 3–4 months.
3.1.4. Genetics
‘The PRNP gene is remarkably conserved within the family Cervidae; only 16 amino acid polymorphisms have been reported within the 256 amino acid open reading frame in the third exon of the PRNP gene. Some of these polymorphisms have been associated with lower rates of CWD infection and slower progression of clinical CWD’. (Robinson et al., 2012)
As demonstrated in other species in which prion diseases occur naturally, susceptibility to CWD is highly dependent on polymorphic variation in the deer and wapiti PRNP gene. In mule deer, the polymorphism at codon 225 encoding serine (S) or phenylalanine (F) influences CWD susceptibility, the 225F allele being partially protective. The occurrence of CWD was found to be 30‐fold higher in deer homozygous for serine at position 225 (225SS) than in heterozygous (225SF) animals; the frequency of 225SF and 225FF genotypes in CWD‐negative deer was 9.3%, but only 0.3% in CWD‐positive deer (Jewell et al., 2005). Recent studies comparing CWD susceptibility in mule deer of the two residue 225 genotypes (225SS, 225FF) showed that 225FF mule deer had differences in clinical disease presentation, as well as more subtle, atypical traits (Wolfe et al., 2014). Immediately adjacent to the protective mule deer PrP polymorphism at 225, residue 226 encodes the singular primary structural difference between wapiti/red deer and North American deer (Odocoileus spp.) PrP. Wapiti PrP contains glutamate (E), and deer PrP glutamine (Q) at this position.
Polymorphisms at codons 95 glutamine (Q) or histidine (H) (Johnson et al., 2003), 96 glycine (G) or serine (S) (Raymond et al., 2000; Johnson et al., 2003) and 116 alanine (A) or glycine (G) (Heaton et al., 2003) in white‐tailed deer have been reported. While all major genotypes were found in deer with CWD, the Q96, G96, A116 allele (QGA) was more frequently found in CWD‐affected deer than the QSA allele (Johnson et al., 2003; O'Rourke et al., 2004). The wapiti PRNP coding sequence is also polymorphic at codon 132 encoding either methionine (M) or leucine (L) (Schatzl et al., 1997; O'Rourke et al., 1998). This position is equivalent to human PRNP codon 129. Studies of free‐ranging and captive wapiti with CWD (O'Rourke et al., 1999), as well as oral transmission experiments (Hamir et al., 2006a; O'Rourke et al., 2007), indicate that the 132 L allele partially protects against CWD.
Transgenic (Tg) mouse modelling has provided a means of assessing the effect of cervid PrP gene polymorphisms on CWD. Investigations combining studies in Tg mice, the natural host, cell‐free prion amplification and molecular modelling approaches analysed the effects of deer polymorphic amino acid variations on CWD propagation and susceptibility to prions from different species (Angers et al., 2014). The properties of CWD prions were consistently maintained in deer following their passage through Tg mice expressing cognate PrP, reflecting the general authenticity of the Tg modelling approach. Moreover, the protective influences of naturally occurring PrP polymorphisms on CWD susceptibility were accurately reproduced in Tg mice, or during cell‐free amplification. The resistance to CWD of Tg mice expressing deer PrP S96, referred to as Tg(DeerPrP‐S96)7511 mice, is consistent with previously generated Tg60 mice expressing serine at residue 96 (Meade‐White et al., 2007). In the studies of Angers et al. (2014), whereas substitutions at residues 95 and 96 affected CWD propagation, their protective effects were negated during replication of sheep prions in Tg mice and, in the case of residue 96, deer.
To more fully address the influence of the wapiti 132 polymorphism, transmissibility of CWD prions was assessed in Tg mice expressing cervid cellular PrP (PrPC) with L or M at residue 132 (Green et al., 2008). While Tg mice expressing CerPrP‐L132 afforded partial resistance to CWD, SSBP/1 sheep scrapie prions transmitted efficiently to Tg mice expressing CerPrP‐L132, suggesting that the wapiti 132 polymorphism controls prion susceptibility at the level of prion strain selection. The susceptibility of Tg mice expressing deer PrP with S at residue 96, referred to as Tg(DeerPrP‐S96)7511, albeit with incomplete attack rates and long incubation times, is at odds with previous work showing complete resistance of Tg60 mice (Meade‐White et al., 2007; Race et al., 2011). This apparent discrepancy is most likely related to the low transgene expression in Tg60 mice, reported to be 70% of the levels found in deer. CWD occurs naturally in deer homozygous for the PrP‐S96 allele (Keane et al., 2008a), which is clearly inconsistent with a completely protective effect of this substitution, suggesting that Tg(DeerPrP‐S96)7511 mice represent an accurate Tg model in which to assess the effects of the S96 substitution.
Tg mice expressing wild‐type deer PrP (Tg33) or Tg60 were challenged with CWD prions from experimentally infected deer with varying polymorphisms at residues 95 and 96 (Duque Velasquez et al., 2015). Passage of deer CWD prions into Tg33 mice expressing wild‐type deer PrP resulted in 100% attack rates, with CWD prions from deer expressing H95 or S96 having significantly longer incubation periods. Remarkably, otherwise resistant Tg60 mice (Meade‐White et al., 2007; Race et al., 2011) developed disease only when inoculated with prions from deer expressing H95/Q95 and H95/S96 PrP genotypes. Serial passage in Tg60 mice resulted in propagation of a novel CWD strain, referred to as H95(+), while transmission to Tg33 mice produced two disease phenotypes consistent with propagation of two strains.
Recent findings show that residue 225, which is polymorphic in mule deer, and 226, which differs between wapiti and deer, play a critical role in PrPC‐to‐PrPSc conversion and strain propagation, but that their effects are distinct from those produced by the H95Q, G95S and M132L polymorphisms (Angers et al., 2014). Structural analyses confirm that residues 225 and 226 are located in the distal region of α‐helix 3 that participates with the β2–α2 loop to form a solvent‐accessible contiguous epitope (Perez et al., 2010). Consistent with a role for this epitope in PrP conversion, these polymorphisms severely impact replication of both the scrapie isolate SSBP/1 and, to variable degrees, CWD. In the case of Tg mice expressing deerPrP‐F225, referred to as Tg(DeerPrP‐F225), SSBP/1 incubation times were prolonged threefold, whereas inoculation with CWD produced incomplete attack rates or prolonged and variable incubation times in small numbers of mice. In those Tg(DeerPrP‐F225) mice that did succumb to CWD, PrPSc distribution patterns were altered compared with Tg(DeerPrP) mice.
To address the effects of substitution of glutamic acid (E) for glutamine (Q) at residue 226, recent studies assessed whether Tg mice expressing wild‐type wapiti or deer PrP differed in their responses to CWD. These studies showed that differences at residue 226 affected CWD replication, but to a lesser degree than the residue 225 polymorphism, with disease onset prolonged by 20–46% in CWD‐inoculated Tg(Deer PrP) compared with Tg(ElkPrP) mice, and PrPSc distribution and neuropathology varying in each case (Angers et al., 2009). In contrast to Tg(DeerPrP) mice which are susceptible to SSBP/1 (Green et al., 2008), Tg(ElkPrP) were completely resistant (Angers et al., 2009), although the resistance of wapiti PrPC to propagation of SSBP/1 was overcome following adaptation in deer or Tg(DeerPrP) mice. Passage in Tg mice expressing E226 or Q226 profoundly affected the ability of SSBP/1 to reinfect Tg mice expressing sheep PrPC. These studies paralleled aspects of previously published studies indicating that amino acid differences at residue 226 controlled the manifestation of CWD quasispecies or closely related strains (Angers et al., 2010). These findings therefore collectively point to an important role for residues 225 and 226 in PrPC‐to‐PrPSc conversion and the manifestation of prion strain properties, and substantiate the view that long‐range interactions between the β2–α2 loop and α‐helix 3 provide protection against prion infection and suggest a likely mechanism to account for the protective effects of the F225 polymorphism. Molecular dynamics analyses (Angers et al., 2014) showed that the S225F and E226Q substitutions in deer alter the orientations of D170 in the β2–α2 loop and Y228 in α‐helix 3. This structural change allows hydrogen bonding between the side chains of these residues, which results in reduced plasticity of the β2–α2 loop/α‐helix 3 epitope compared with deer or wapiti PrP structures. This suggests that the increased stability of this tertiary structural epitope precludes PrPC‐to‐PrPSc conversion of deerPrP‐F225. However, some clinical cases in animals with this genotype have been reported (Wolfe et al., 2014).
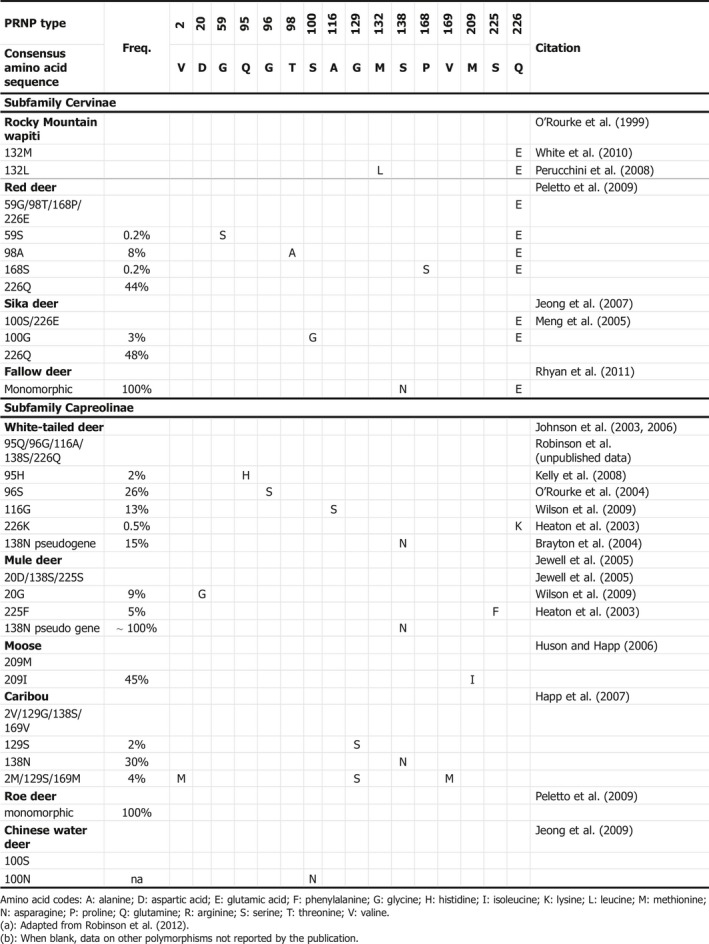
The three Norwegian reindeer confirmed positive to CWD (up to 21 November 2016) had the same PrP genotype as the two successfully orally inoculated reindeer in the Canadian study (Mitchell et al., 2012), GG129 SS138 VV169. Nevertheless other PrP genotypes also seem to be susceptible following either IC inoculation or contact, if not even more susceptible to CWD (Moore et al., 2016). Table 2 shows a summary of the polymorphisms identified in the PRNP gene of cervid species in North America (adapted from Robinson et al., 2012).
Table 2.
Variability in the PRNP genotypes of cervids(a) , (b)
3.1.5. Mechanisms of natural transmission
Chronic wasting disease is infectious (Williams and Young, 1980, 1992; Miller and Williams, 2003), and infected individuals shed prions naturally via multiple routes. CWD has been detected in saliva and blood by bioassay (Mathiason et al., 2006), and urine by PMCA (Haley et al., 2009a) and bioassay (Haley et al., 2009b), suggesting a role for these body fluids in transmission and dissemination. Faecal material from subclinical deer also harbours infectivity (Haley et al., 2009b; Tamgüney et al., 2009), which is consistent with the mechanism of contagious lateral transmission. The detection of CWD prions in wapiti antler velvet by transgenic bioassay, and the annual shedding of this material, raises the possibility that it may also play a role in CWD transmission (Angers et al., 2009). Nasal secretions, milk and semen seem likely to be additional sources of agent shedding (Gough and Maddison, 2010). Prion shedding occurs intermittently throughout much of the disease course (Tamgüney et al., 2009).
Oral exposure appears to be the predominant route of natural infection (reviewed by Williams and Miller, 2002; Williams, 2005; Saunders et al., 2012), although maternal transmission has also been shown to occur experimentally in Reeves’ muntjac deer (Muntiacus reevesi) (Nalls et al., 2013), and possibly contributes to the efficient transmission of CWD in naturally exposed cervid populations (Chapuis et al., 2016). The highly efficient transmission of CWD appears unparalleled among prion diseases (Williams and Young, 1980; Miller et al., 2000; Miller and Williams, 2003).
Susceptible hosts may be exposed either directly through interaction with an infectious host or indirectly through prion‐contaminated food, water, or environments (Miller and Williams, 2003; Miller et al., 2004; Mathiason et al., 2009). Its’ remarkably contagious nature has been documented in a captive mule deer population wherein 90% of the mule deer present for more than 2 years ultimately developed disease (Williams and Young, 1980). Ingestion of forage or water contaminated by secretions, excretions, or other sources, for example, CWD‐infected carcasses (Miller et al., 2004), has long been thought the most plausible natural route. In addition to prions shed by an infectious host, central nervous and lymphoid tissues (Sigurdson et al., 1999; Wolfe et al., 2012; also see Table 1) as well as many others (reviewed by Williams, 2005; Haley and Hoover, 2015) carry infectivity that may also represent sources of transmission if consumed by susceptible animals.
However, unlike BSE and transmissible mink encephalopathy (TME) in mink, none of the CWD outbreaks investigated thus far has incriminated prion‐contaminated feed as the driving force behind transmission. Pertinent to this issue is the well‐known persistence of prions in the environment, a feature that is linked to their unusual resistance to degradation. The CWD prions in excreta and carcass remains can persist for years in the environment (Williams and Young, 1992; Miller and Williams, 2003; Miller et al., 2004). Coupled with this, prions bound to soil particles remain infectious after oral consumption (Saunders et al., 2012; Johnson et al., 2007). Moreover, prion binding to soil elements (e.g. clay) enhances persistence and infectivity (Johnson et al., 2007). Indirect transmission greatly complicates CWD control strategies (Williams and Young, 1992; Williams et al., 2002; Miller et al., 2004).
3.1.6. Epidemiology
The occurrence of CWD in deer populations or in particular species may be very different but caution is needed when considering field data. As reported by Williams (2005) in captive herds where introduction of disease is thought to be recent, prevalence may be low (1%) but may approach 100% in CWD endemic research facilities; in free‐ranging populations, it may range between 1% and 30% but is more frequently under 5% (Saunders et al., 2012). Mule deer seem to experience the highest prevalence rates than other species (Spraker et al., 1997; Saunders et al., 2012). However, although North American deer (Odocoileus spp.) generally show higher prevalence than wapiti or moose, extraordinarily high rates have been described in captive wapiti so this may be more a function of social and foraging behaviour differences.
There is no clear information on temporal trends. In general, until the mid‐1990s only two or three US states had reported cases; then over the subsequent 15 years an apparently rapid spread of the disease has been observed in the country (Saunders et al., 2012), although it has been argued that this reflects the efforts to detect disease (increased surveillance), and is not a ‘real time’ representation of disease spread (Miller and Fischer, 2016).
Based on experimental oral infections, the incubation period for the onset of clinical signs in mule deer has been estimated about 15 months and in wapiti between 12 and 34 months with most of the clinical cases observed in animals between 2 and 7 years of age (Williams et al., 2002). In general, male deer experience higher risk than females: e.g. in a cohort of mule deer (Miller et al., 2008) the prevalence among the sampled adult male deer was about twice the prevalence among adult females. That is likely to be explained by different behaviour: males interact with more groups or roam more widely while seeking to establish social dominance, increasing the contact rate with other animals in general and with infected animals in particular, by their associating with numerous females, or by fighting with other males (Koutnik, 1981).
For both sexes, the risk of infection appears to increase in early adulthood, resulting in relatively high prevalence in adult (> 2‐year‐old) mule deer as compared with yearlings, and in a decline in older age classes (Miller and Conner, 2005).
3.1.7. Risk factors
Many risk factors have been suggested to facilitate the introduction and spread of the disease (Williams and Miller, 2003). They play a role in enhancing the ability of CWD prions to transmit both directly (animal‐to‐animal) and indirectly via the contaminated environment.
The incursion of the disease into unaffected populations may be due to the natural migration of cervids and/or the translocation of infected animals or soil by humans (Sohn et al., 2002; Department for Environment Food & Rural Affairs (Defra, 2016)). Once introduced, the spread of the agent is associated with the rate of contacts between animals. Behavioural and social factors have already been mentioned to explain the higher risk for males than for females.
It has been shown that rates of between‐group contacts increase with deer density (Habib et al., 2011). For instance, the risk of spread in young (< 2‐year‐old) white‐tailed deer in south‐central Wisconsin was non‐linearly but positively associated with both frequency of disease (prevalence) and density of infected deer (Storm et al., 2013). Increasing densities of the cervid populations by human intervention, such as concentrating deer in captivity or by baiting or feeding them artificially, e.g. over the winter season, (Miller and Williams, 2003; Sorensen et al., 2014) or by providing lick blocks, may facilitate transmission. The local environmental characteristics (e.g. deer habitat) may increase the probability of aggregation of animals and therefore of disease transmission (Miller and Conner, 2005). In a study by Storm et al. (2013), landscape factors (e.g. deciduous forest cover and forest edge density) were positively associated with infection rates. Areas where deer congregate seasonally may be particularly important, as well as their tendency to concentrate and to become sedentary on their winter range.
It has been observed that the prevalence of CWD at any particular point is correlated with distance from the introduction point, as a surrogate for the time required for disease spread or ‘disease history’ (Joly et al., 2006).
As highlighted by the Norwegian Scientific Committee for Food Safety (Vitenskapskomiteen for Mattrygghet (VKM), 2016 and Defra, 2016), CWD prions might enter the environment via carcass decomposition, antler velvet and skin, saliva, urine, faeces, blood, and most probably via placenta and milk. Exposure to environmental fomites (e.g. bedding and water) is sufficient to transmit CWD (Miller et al., 2004; Mathiason et al., 2009). It has been suggested that people (in particular deer hunters, given their increased contact with deer and their environment) travelling from a CWD‐affected area with potentially contaminated clothing/boots and/or equipment may act as long distance vectors of disease (Defra, 2016).
A potential role in the transmission of disease has been suggested for cervid urine, collected and distributed widely for use by hunters as an attractant/lure (Defra, 2016): the captive populations from which the urine is collected may include preclinical cases of infection. This hypothesis drove a number of US states to ban the use of natural deer urine for lures. Additional feed for reindeer, such as lichens, can be harvested and traded and could be a potential source of infection. Other types of feed, including pet food containing cervid protein, have also been hypothesised as a potential source of incursion in CWD‐free areas (Defra, 2012).
Finally, as mentioned earlier, genetics must be considered among the risk factors. Polymorphisms in the PRNP gene appear to influence susceptibility (see section 3.1.4), even though this remains less understood for CWD than the well‐documented and strong genetic influence of the sheep PRNP on scrapie.
3.2. Considerations on the host range of CWD and risk to human health
3.2.1. The species barrier
In addition to its increased geographic distribution, the known host range of CWD is also expanding. Naturally infected species include while‐tailed deer, mule deer, black‐tailed deer, reindeer, moose and wapiti. Other cervid species are susceptible to CWD following experimental challenge. These include European red deer (Martin et al., 2009) and muntjac deer (Nalls et al., 2013). Brain material from CWD‐infected white‐tailed deer and wapiti produced disease in only four of 13 intracerebrally inoculated fallow deer (Hamir et al., 2008), and the same species remained free of disease when cohoused in paddocks with CWD‐affected mule deer (Rhyan et al., 2011), suggesting relative resistance of this cervid species to CWD.
Experimental transmissions to species outside the cervid family either intracerebrally or orally, have given mixed results. Studies have demonstrated that the CWD agent transmitted poorly to Syrian golden hamsters, ferrets and mink (Bartz et al., 1998; Marsh et al., 2005; Sigurdson, 2008). Experimentally susceptible species include several species of voles, white‐footed mice, deer mice, cats, raccoons and squirrel monkeys (Hamir et al., 2003, 2007; Race et al., 2009a Heisey et al., 2010; Di Bari et al., 2013; Mathiason et al., 2013; Race et al., 2014; Seelig et al., 2015). While non‐transgenic mice have been reported to be resistant to CWD infection (Browning et al., 2004), limited infection of the VM/Dk inbred strain of mice by wapiti CWD has been reported (Lee et al., 2013). Bank voles (Myodes glareoulos) are particularly susceptible to CWD (Di Bari et al., 2013).
Inoculation of prions into individuals of the same species will typically cause disease with remarkably reproducible clinical signs. Whether the natural host range of CWD extends beyond the family Cervidae is currently unclear. Barriers to transmission between species are characterised by protracted incubation times compared with the permissive host, and/or low rates of infection with variable intervals to disease. Further passage of the resulting pathogenic prions to animals expressing PrP of the new species results in shorter, synchronous times to disease in all inoculated recipients, reflecting the adaptation of prions to ensure continued propagation in the new host (reviewed in Baskakov, 2014).
The recognised importance of PrP primary structure in controlling prion adaptation across species barriers paved the way for development of Tg mouse models that recapitulate natural prion diseases (Telling, 2011), and model the susceptibility of at risk, or seemingly resistant species (Vidal et al., 2015; Espinosa et al., 2009). While species‐related primary structural incompatibilities between PrPSc and PrPC expressed in the new host have a major impact on prion transmission barriers (Prusiner et al., 1990), strain properties of the infectious agent have significant additional influence on the outcome of interspecies prion transmissions, as exemplified by the extensive host range properties of BSE. In the absence of an informational nucleic acid component in prions, heritable properties, including incubation times and neuropathological profiles, are enciphered within the conformation of PrPSc, which varies among different strains (Bessen and Marsh, 1994; Telling et al., 1996). The emergence of newly adapted strains, which generally occurs as prions transit species barriers, is accompanied by changes in the conformation of PrPSc constituting prions from the original host, compared to the PrPSc of prions produced in the species of adaptation (Peretz et al., 2002). To reconcile the influences of conformationally enciphered strain properties and species‐dependent variations in PrP primary structure on adaptive transmission, the conformational selection model postulated that PrP expressed in a newly infected host selects a species‐optimised prion from an ensemble of quasispecies conformations produced during replication in the host of origin (Collinge and Clarke, 2007).
Tg mice are also used to model interspecies transmissions (Vickery et al., 2014), which are generally inefficient, and usually characterised by low attack rates and/or long and variable incubation times on primary transmission. Such Tg mouse models cannot always be considered as an accurate proxy for the natural host, so a failure to transmit needs to be interpreted with caution (for review, see EFSA BIOHAZ Panel, 2015). The initial barrier to propagation is thought to result from primary structural incompatibilities between donor PrPSc and recipient PrPC resulting in inefficient PrPC conversion. Ultimately, nascent PrPSc readily converts PrPC, and efficient prion propagation ensues in the recipient species. The relative ease of transmission on serial passage reflects adaptation in the new species (Telling, 2011).
Recent studies cast light on the importance of specific secondary structural elements during adaptive trans‐species transmissions, in particular the loop connecting the β2 and α2 regions (Sigurdson et al., 2010; Kurt et al., 2015).
The remarkably high rate of CWD prion transmission brings into question the risk posed to livestock from developing a novel CWD‐related prion disease via shared grazing of CWD‐contaminated rangeland. This issue has been investigated by transmitting CWD via intracerebral inoculation to cattle (Hamir et al., 2001, 2005, 2006b, 2007) and to sheep (Hamir et al., 2006b). In the case of cattle, PrPSc was detected in approximately 40% of intracerebrally inoculated animals. Low rates of disease also occurred in intracerebrally inoculated sheep and host genotype appeared to affect transmission efficiency. Tg mice expressing bovine PrP have also been challenged with CWD, thus far with negative outcomes (Tamguney et al., 2006). However, Tg mice expressing ovine PrP challenged with CWD have resulted in highly efficient, life‐long asymptomatic replication of these prions in the spleen tissue, with brain involvement at late stage (Béringue et al., 2012). Davenport and co‐workers employed the RT‐QuIC assay to compare the conversion properties of CWD and BSE, as well as feline‐adapted versions of these prions (Davenport et al., 2015). CWD, BSE and feline‐adapted CWD most effectively seeded cervid, bovine and feline bacterially expressed recombinant PrP (rPrP), respectively, while feline spongiform encephalopathy (FSE) prions converted more efficiently bovine than feline rPrP. To model the potential of these prions to convert human PrPC, human rPrP was used as a substrate in RT‐QuIC. Remarkably, CWD, feline‐adapted CWD, BSE and FSE all converted human rPrP, although not as efficiently as sporadic CJD prions. These findings are similar to those in low‐level, cell‐free conversions of human prion protein by BSE, CWD and scrapie prions (Raymond et al., 2000). Overall, these data indicate that CWD can (or at least has the molecular potential to) infect other species following experimental challenge, but with varying degrees of ease. These data cannot be directly extrapolated to the likelihood of such infection occurring naturally.
3.2.2. Agent strains
Field observations of natural disease and epidemiology indicate, anecdotally, that there are at least two, and possibly three distinct strains of CWD in North America. Early pathology data from the Norwegian cases suggests that (in moose at least) a strain distinct from any seen in North America may be involved (Section 3.3.2).
Although seminal studies in Tg mice (Browning et al., 2004), and subsequent work (LaFauci et al., 2006) raised the possibility of CWD strain variation, the limited number of isolates and the lack of detailed strain analyses in those studies meant that this hypothesis remained speculative. Subsequent studies supported the feasibility of using Tg(CerPrP)1536+/− mice for characterising naturally occurring CWD strains, and novel cervid prions generated by PMCA (Green et al., 2008). To address whether different CWD strains occur in various geographic locations or in different cervid species, bioassays in Tg mice were used to analyse CWD in a large collection of captive and wild mule deer, white‐tailed deer and wapiti from various geographic locations in North America (Angers et al., 2010). These findings provided substantial evidence for two prevalent CWD prion strains, referred to as CWD1 and CWD2, with different clinical and neuropathological properties. Remarkably, primary transmissions of CWD prions from wapiti produced either CWD1 or CWD2 profiles, while transmission of deer inocula favoured the production of mixed intrastudy incubation times of CWD1 and CWD2 neuropathologies. These findings indicate that wapiti may be infected with either CWD1 or CWD2, whereas deer brains tend to harbour CWD1/CWD2 strain mixtures.
The different primary structures of deer and wapiti PrP at residue 226 provide a framework for understanding these differences in strain profiles of deer and wapiti. Because of the role played by residue 226, the description of a lysine polymorphism at this position in deer, and its possible effect on strain stability may be significant (Angers et al., 2010). It is unknown whether CWD1 and CWD2 interfere or act synergistically, or whether their coexistence contributes to the unparalleled efficiency of CWD transmission. Interestingly, transmission results reported in previous studies suggested that cervid brain inocula might be composed of strain mixtures (Tamgüney et al., 2006).
Additional studies support the existence of multiple CWD strains. CWD has also been transmitted, albeit with varying efficiency, to Tg mice expressing mouse PrP (Sigurdson et al., 2006; Tamgüney et al., 2006). In the former study, a single mule deer isolate produced disease in all inoculated Tga20 mice, which express mouse PrP at high levels. On successive passages, incubation times dropped. In the second study, one wapiti isolate from a total of eight deer and wapiti CWD isolates induced disease in 75% of inoculated Tg4053 mice, which also overexpress mouse PrP. The distribution of lesions in both studies appeared to resemble the CWD1 pattern. Low efficiency CWD prion transmission was also recorded in hamsters and Tg mice expressing Syrian hamster PrP (Raymond et al., 2007). In that study, during serial passage of mule deer CWD, fast and slow incubation time strains with different patterns of brain pathology and PrPSc deposition were also isolated. In yet other studies, serial passages of CWD from white‐tailed deer into Tg mice expressing hamster PrP, and then Syrian golden hamsters, produced a strain, referred to as ‘wasting’ (WST), characterised by a prominent preclinical wasting disease, similar to cachexia, which the authors proposed is due to a prion‐induced endocrinopathy (Bessen et al., 2011). These same investigators identified a second strain, defined as ‘cheeky’ (CKY), derived from infection of Tg mice that express hamster PrP (Crowell et al., 2015). The CKY strain had a shorter incubation period than WST, but after transmission to hamsters, the incubation period of CKY became around ∼ 150 days longer than WST. In this case, proteinase K (PK) digestion revealed strain‐specific PrPSc signatures that were maintained in both hosts, but the solubility and conformational stability of PrPSc differed for the CWD strains in a host‐dependent manner. In addition to supporting the view that there are multiple CWD strains, these findings suggest the importance of host‐specific pathways, independent of PrP, that participate in the selection and propagation of distinct strains.
In one study, infection of hamsters with CWD from white‐tailed deer, mule deer or wild white‐tailed deer resulted in phenotypic differences between isolates that were interpreted as indicative of different strains (Triscott et al., 2015).
3.2.3. Approaches for the study of the zoonotic potential of CWD
There are many factors which influence the ability of any TSE agent to infect an animal (including man), but the precise roles and potential for interdependence of these factors are not clear. It is impossible to define an experimental model that encompasses all this potential variability and that directly measures the likely transmission across a species barrier. Trying to assess the human species barrier is even more difficult. There are therefore two main approaches to assessing zoonotic potential: looking at the permeability of the species barrier and examining the link between animal and human TSE cases.
The effectiveness of the species barrier is determined not only by the host PRNP gene but also by the infecting strain of the agent. This is potentially confounded by the observation that isolates may change some or all of their phenotypic characteristics following either intra‐ or interspecies transmission.
For a comprehensive overview on the background, developments and principles of the factors influencing the species barrier and the experimental approaches available as a proxy for assessing zoonotic potential, see EFSA's Scientific Opinion ‘on a request for a review of a scientific publication concerning the zoonotic potential of ovine scrapie prions’ (EFSA BIOHAZ Panel, 2015).
3.2.3.1. Modelling the permeability of the transmission barrier
As noted above, experimental transmission of CWD to other species has yielded mixed results. The resistance of mice (Browning et al., 2004) and the inefficient transmission to ferrets (Bartz et al., 1998) are examples of species barriers to CWD prions, albeit of varying extent.
Seminal studies in Tg mice with sheep scrapie prions experimentally adapted to mice or Syrian hamsters showed that optimal disease progression requires related PrPSc and PrPC primary structures (Prusiner et al., 1990; Scott et al., 1993), which paved the way for the development of Tg models in which to study human prions (Telling et al., 1995), and subsequently other naturally occurring mammalian prions (reviewed in Telling, 2011). Tg approaches also suggested that PrPSc tertiary structure enciphers strain information (Bessen and Marsh, 1994; Telling et al., 1996). Tg mice have also been used to model interspecies transmissions (Vickery et al., 2014; EFSA BIOHAZ Panel, 2015).
Several Tg mouse models expressing either wapiti or deer PrP have been produced in which the species barrier to CWD has been eliminated. Prototype Tg mice expressing deer PrP, designated Tg(CerPrP)1536+/− (Browning et al., 2004), recapitulated the cardinal neuropathological, clinical and biochemical features of CWD, an observation subsequently confirmed in comparable transgenic mouse models expressing deer or wapiti PrP (Kong et al., 2005; LaFauci et al., 2006; Tamgüney et al., 2006; Meade‐White et al., 2007; Angers et al., 2009). The generation of CWD‐susceptible Tg mice, in concert with the development of PMCA‐based approaches for amplifying CWD infectivity using PrPC expressed in the CNS of those mice (Green et al., 2008; Meyerett et al., 2008), has also provided crucial information about the biology of CWD and cervid prions. Amplification in vitro was shown to maintain CWD prion strain properties, and provided a means of generating novel cervid prion strains (Kurt et al., 2007; Green et al., 2008; Meyerett et al., 2008; Kurt et al., 2009).
Tg mice and in vitro amplification approaches (reviewed in EFSA BIOHAZ Panel, 2015) have also facilitated our understanding of the mechanism of CWD transmission among deer and wapiti (Mathiason et al., 2006; Haley et al., 2009a,b; Tamgüney et al., 2009). Transmission studies in Tg(CerPrP)1536+/− and similar Tg mice demonstrated that CWD prions were present in urine and faeces and saliva (Tamgüney et al., 2006; Haley et al., 2009a), and these findings are substantiated by in vitro amplification techniques (Haley et al., 2009b; Pulford et al., 2012; Henderson et al., 2013).
Tg approaches have been essential for assessing the potential risk of human exposure to CWD prions (Angers et al., 2006, 2009; Race et al., 2009a). The identification and characterisation of distinct CWD strains, and the influence of PrP primary structure on their stabilities, is of importance when considering the potential for interspecies transmission. The appearance of variant Creutzfeldt–Jakob (vCJD) disease following human exposure to BSE (Bruce et al., 1997; Hill et al., 1997), placed the human species barrier to other animal prion diseases, particularly CWD, at the forefront of public health concerns during a period of time.
3.2.3.2. Establishing the link between human and animal TSE cases
North American hunters harvest thousands of deer and wapiti each year, and it is not mandatory to have these animals tested, although the Centers for Disease Control and Prevention (CDC) publishes guidance4 on the handling and dressing of carcases, and disposal of waste, designed to minimise exposure to the highest risk tissues. However, the demonstration of CWD prions in the skeletal muscle and fat of deer (Angers et al., 2006; Race et al., 2009b), means that humans may consume CWD prions in enzootic areas. The substantial market for wapiti antler velvet in traditional Asian medicine also warrants concern (Angers et al., 2009).
Estimates of the zoonotic potential of CWD are currently mixed. While initial cell‐free conversion studies suggested that the ability of CWD prions to transform human PrPC into PK‐resistant PrP was low (Raymond et al., 2000), subsequent results showed that cervid PrPSc induced the conversion of human PrPC by PMCA, following CWD prion strain stabilisation by successive passages in vitro or in vivo (Barria et al., 2011). These results have implications for the human species barrier to CWD, and underscore the role of strain adaptation on interspecies transmission barriers. Wapiti CWD converted human PrPC from the human brain, humanised mouse brain and human‐derived PrPC overexpressing cell lines in a PRNP‐dependent manner, with higher efficiency for 129M. Most interestingly, the resulting PrPSc resembled that of sporadic CJD (sCJD) of the MM1 subtype providing evidence that a switch of the phenotype had occurred (Barria et al., 2014).
Additional studies using Tg mice expressing human PrPC showed that CWD failed to induce disease following intracerebral inoculation (Kong et al., 2005; Tamgüney et al., 2006; Sandberg et al., 2010). CWD transmission was reported to non‐human primates through intracerebral (80% attack rate) and oral inoculation (15% attack rate) of squirrel monkeys (Saimiri sciureus) (Marsh et al., 2005; Race et al., 2009a). Subpassage experiments demonstrated a reduction in the incubation period indicating an initial species barrier. However, squirrel monkeys are evolutionarily quite far from humans. Macaques are closer to humans, and are susceptible to BSE and L‐type atypical BSE (L‐BSE), and classical scrapie. Current data suggests that macaques are more resistant to CWD, but challenges are ongoing. Challenge with scrapie in the macaque model resulted in disease after 10 years (four times longer than BSE) (Comoy et al., 2015), so it is still too early to draw conclusions based on the current data regarding CWD (Mussil et al., 2015), as these animals have a lifespan of up to 30 years.
Moreover, using RT‐QuIC to model the transmission barrier of CWD to human rPrP suggest that, at the level of protein–protein interactions, CWD adapts to new species more readily than does BSE, and that the barrier preventing transmission of CWD to humans may be less robust than estimated (Davenport et al., 2015).
Epidemiosurveillance of human populations currently shows no evidence of transmission (Belay et al., 2004; Mawhinney et al., 2006), but there is still no confirmed aetiology for sporadic CJD cases that occur worldwide. Comparison of the CJD prevalence rates in North America relative to other countries does not suggest a CWD‐related problem in man. For example, the mean annual mortality rates from CJD are relatively consistent at 1–1.5 cases per million in both Europe and North America.5
Longitudinal studies following known dietary exposure to CWD have not identified any causal links with human disease (Mawhinney et al., 2006; Anderson et al., 2007), but given the very long incubation periods for TSE in man, and the lack of robust global surveillance data for either the human or animal populations over the appropriate time periods, it is not possible to rule out conclusively the hypothetical possibility of transmission.
One means of assessing the potential causal links between animal and human TSEs that has been used previously (EFSA BIOHAZ Panel (2011, 2015)) is to apply the Bradford Hill guidelines (Hill, 1965), which continue to be used in public health to assess the strength of causal relationships in epidemiology. The guidelines include consideration of the following criteria: strength, consistency, specificity, temporality, biological gradient, biological plausibility, coherence, experiment and analogy. Not all criteria in the guidelines need to be fulfilled to suggest a causal link and some of the criteria are now judged to be less important than others, notably analogy and consistency. For disease with a long latency, temporality is of greater importance. Table 3, first presented in 2011 and updated for classical scrapie (EFSA BIOHAZ Panel, 2015), has been reviewed based on evidence currently available and the assessment of the zoonotic potential of CWD by these criteria remains unchanged.
Table 3.
Assessment of putative links between animal and human TSEs according to the criteria of the Bradford Hill guidelines
| Criteria | Cattle BSE | Small ruminant BSEa | Atypical BSE (L‐BSE) | Atypical BSE (H‐BSE) | CWD | Classical scrapieb , c | Atypical scrapie |
|---|---|---|---|---|---|---|---|
| 1. Strength d | + | ||||||
| 2. Consistency | + | ||||||
| 3. Specificity | + | ||||||
| 4. Temporality | + | ||||||
| 5. Biological gradient | + | ||||||
| 6. Plausibility | + | + | + | + | + | + | + |
| 7. Coherence | + | +/− | |||||
| 8. Experiment | + | + | + | +/− | + | ||
| 9. Analogy | + | + | + | + | + | + | + |
BSE: bovine spongiform encephalopathy; L‐BSE: L‐type atypical BSE; H‐BSE: H‐type atypical BSE; CWD: chronic wasting disease;
+: Some scientific evidence is available for a positive interpretation.
+/−: Debatable or conflicting evidence is available.
Classical BSE has not been identified in sheep, but two cases of BSE in goat have been reported in France and the UK.
There are multiple strains of the Classical scrapie agent.
A single study has reported transmission of a natural sheep classical scrapie isolate to primates (Comoy et al., 2015).
When blank, no scientific evidence is available for a positive interpretation.
3.3. Surveillance and control of CWD: current situation
3.3.1. Surveillance
North American experiences with CWD surveillance and control have been reviewed recently (Miller and Fischer, 2016; Uehlinger et al., 2016). Surveillance activities have been applied extensively to CWD. Detection of cases, prevalence estimation, geographical spread and monitoring over time are the main aims pursued and require the investigation of representative (i.e. valid) samples from the populations of interest so that data can be interpreted.
However, farmed cervids may be considered as a particular type of livestock for which classical surveillance designs are applicable, wildlife populations deserve a dedicated study design. Probability sampling (i.e. a procedure like simple, systematic, or stratified random sampling that assures that each unit in the population has equal probability of being selected), applied to obtain a representative set of animals is not straightforward for these target populations, as sampling frames are not available. To overcome this caveat, proxy (i.e. the random sampling of mutually exclusive areas containing animals) is commonly used (Salman, 2008; Morrison et al., 2008). This strategy also ensures that the geographical coverage is as comprehensive as possible, acknowledging the difficulties of achieving it. This was a shortcoming identified in the evaluation of the previous surveillance exercise (EFSA BIOHAZ Panel, 2010). As highlighted by Miller and Fischer (2016), one of the most common flaws in CWD control efforts to date has been an initial underestimation of the affected area (often based on inadequate surveillance and erroneous assumptions about how long disease has been present).
Alternative sampling strategies include, for instance, the application of two‐stage cluster probability sampling where, after the random selection of the land areas, individual deer are examined within each sampled land area. As an alternative, convenience sampling based on road‐killed or apparently healthy hunter‐shot animals can be applied. However, these samples may be biased since in the former they may over‐represent diseased animals (Krumm et al., 2005), and in the latter they may over‐represent either non‐affected animals (avoidance) or diseased (vulnerable) animals (Conner et al., 2000). An apparently sick animal could be easier to hunt, but hunters may refuse to shoot them because they would count as part of their hunting quota and may not be suitable for human consumption. Over‐representation of diseased animals would be a helpful outcome that could be used to enhance disease detection, but it would be less appropriate if prevalence estimates in the general population (i.e. at individual level) are required. However, estimating herd‐level prevalence (i.e. the prevalence of affected farms or of land areas containing population units) would be possible.
Nusser et al. (2008) suggested that a partial implementation of probability sampling mitigating biases from convenience samples could be obtained by combining in two‐stage cluster sample designs the probability sampling of land areas and the convenience sampling of deer opportunistically shot in each selected land area. Biases based on the apparent health of the animals, if so desired, may be mitigated by training the hunters to avoid such biases by encouraging hunters to kill ‘sick’ animals for control purposes.
Moreover, this design may also be more efficient by including an unequal probability sampling, i.e. a probability‐based oversampling of more informative first‐stage sample areas (i.e. areas where deer are deemed to show higher density). In this case, weighted estimates have to be used to account for oversampling.
As mentioned above, for areas or countries where the disease has not been reported, the objective of surveillance should be to detect cases, whereas where the disease has been confirmed, to set up a continuous monitoring and the temporal and spatial characterisation of the disease should be the priority. Thus, surveillance may be implemented for CWD detection in a country or (as is the case in Norway) in areas other than those of original detection; then for initial and periodical estimation of prevalence in the cervids of the affected areas.
Surveillance for CWD should be continuous in jurisdictions where foci have not been detected, whereas monitoring may be episodic (e.g. at multiyear intervals) in other scenarios. Strategies for detecting ‘new’ foci vs those for following temporal trends have been reviewed in detail by Samuel et al. (2003).
However, random sampling (e.g. from harvested animals) seems better suited for providing relatively unbiased prevalence or incidence estimates (Samuel et al., 2003), a risk‐based strategy focusing on subpopulations at expected higher risk may be particularly efficient when detection is the primary aim. The detection of Norwegian CWD cases through screening of ‘suspect’ cervids illustrates the potential effectiveness of this approach for detecting foci in other parts of Norway or in other European countries. Experience in Colorado, Wyoming and Wisconsin has shown that the probability of finding a CWD‐positive animal may be greater among sick‐looking animals than the general population, and those involved in road accidents or killed by predators (Krumm et al., 2005, 2009; Miller et al., 2008). As an example of the relative vulnerability of CWD‐infected individuals to vehicle collisions, CWD prevalence among vehicle‐killed mule deer in northern Colorado was between 1.6 and 15.9 times higher than the estimated CWD prevalence among mule deer of the same sex sampled in the vicinity of collision sites.
In wildlife, passive surveillance based on the reporting of ‘indicator animals’ (sick or suspicious or fallen stock) may be particularly helpful in detecting new cases of a disease (Thulke et al., 2009; Guberti et al., 2014). However, when sampling collection depends on the voluntary reporting of sick or dead wild animals it may be difficult to obtain a large number of samples for several reasons: (a) most animals with clinical signs will not be observed/detected in the wild; (b) it may be difficult to gain access to the carcass (deep in the forest, in rivers); (c) the need of transport from remote areas; (d) low quality of the material (advanced autolysis so there is no brain left) (Bollinger et al., 2004). Moreover in Europe, CWD is a new disease and, without awareness campaigns, little knowledge is expected among stakeholders about the clinical signs and the species/subpopulations likely to be affected.
The addition of other sources of animals in surveillance may improve the ability to detect cases, for example, with road‐killed cervids or hunted sick animals. The results of the CWD surveillance conducted in the EU between 2006 and 2010 showed that 81 wild red deer and white‐tailed deer tested were in the clinical/sick group (1.1%), 277 were fallen animals (3.8%) and 215 were road injured/killed animals (2.9%) (EFSA BIOHAZ Panel, 2010). Therefore, the sampling of vehicle‐killed animals may be exploited to increase the efficiency of surveillance programmes designed to detect new foci of CWD infection (Miller and Conner, 2005). Other subpopulations at expected higher risk are those including any animal discarded at hunting (hunted but not used for consumption because of anything abnormal, or poor body condition) or discarded at slaughter inspection (game slaughtering, reindeer slaughtering).
Finally, effective surveillance should also account for the characteristics of the disease of interest, i.e. it is necessary to apply an appropriate disease distribution model within the population. For instance, a random disease distribution model would assume that cases are distributed without depending on any specific factor such as neighbouring infected animals or environmental contamination. This model is unrealistic if applied to CWD: Miller and Conner (2005) showed that a more realistic disease distribution model is given by the clustering of diseased animals within the cervid population. Nusser et al. (2008) used the expression ‘hot spot and spark model’ suggesting a CWD distribution with most of the diseased animals clustered and with a few additional outlying cases. Surveillance should occur at biologically relevant spatial scales in view of the highly clustered distribution of CWD in wild cervids. Prevalence in foci of disease is usually lower than 5% (Saunders et al., 2012) requiring continuous monitoring over several years to detect disease. However, prevalence may be as high as 30% (Saunders et al., 2012) and therefore the sample size needed to detect the presence of disease may be relatively low.
In the US, it has been shown that targeting sample sources known to have a relatively high infection probability in endemic areas can be a cost‐effective surveillance approach (Miller et al., 2000; Samuel et al., 2003; Walsh and Miller, 2010) and spatial targeting via risk‐based assessments to focus sampling on landscapes of highest relative risk may enhance surveillance (Bollinger, 2004; Rees et al., 2012; Nobert et al., 2016).
3.3.2. Surveillance in Europe: past and present
3.3.2.1. In Europe
During the period 2006–2010, a survey was carried out in the EU with the aim of detecting the possible presence of CWD and other TSE in the EU farmed and wild cervid populations, with no TSE positive results recorded.
The framework of the survey was established by the Commission Decision 2007/182/EC (see footnote in Section 1.1.1) on the basis of the recommendations provided by a SSC Opinion (Scientific Steering Committee (SSC), 2003) and an EFSA Opinion (EFSA, 2004). The MS were asked to complete the testing activities by the end of 2008.
It was recommended that:
’all MS should take samples for CWD from clinical/sick cervids and fallen/culled cervids, as a priority, as well as from road‐injured or killed cervids of all cervid species. The competent authority of the Member States should endeavour to maximise awareness of these cervids and to ensure that as many such cervids were tested for CWD as possible.
based on sufficiently large deer populations, a subset of 13 target MS should randomly test wild and/or farmed red deer and in Finland wild white‐tailed deer were considered as susceptible i.e. target species; all cervids should be over 18 months of age with a preference for male animals; these MS were assigned a minimum sample size of deer to be collected (close to 600 animals per MS in either wild or, where present, farmed cervids) based on a design prevalence at national level equal to 0.5%.’
The criteria used to design and implement the survey along with some critical comments included in a subsequent EFSA Opinion (EFSA BIOHAZ Panel, 2010) are summarised in Table 4.
Table 4.
Comparison of the features of the 2004 EFSA Opinion, 2007 EC Decision and 2010 EFSA Opinion
| Criteria | EFSA (2004) | 2007/182/EC Decision | EFSA BIOHAZ Panel (2010) |
|---|---|---|---|
| Scope | n/a | Detection of the CWD presence in animals of the deer family | n/a |
| Target disease | All forms of TSE | CWD | n/a |
| Target MS | n/a | Wild: AT, CZ, DE, FR, UK, FI, HU, IT, LV, PL, SK | n/a |
| n/a | Farmed: AT, CZ, DE, FR, IE, UK | n/a | |
| Target species | Red deer + white‐tailed deer | In target MS: red deer + white‐tailed deer | n/a |
| Sampling design | n/a | Random | Not fully geographically representative |
| Tests | Rapid tests for BSE + IHC & WB | Rapid tests for TSE in cattle and small ruminants + IHC | n/a |
| Target populations | Farmed + wild FS | Farmed + wild | n/a |
| Target groups | Sick, FS | Sick, road‐killed, FS/culled, healthy slaughtered and healthy shot | n/a |
| Tissue | Obex (dorsal vagus nuclei) + retropharyngeal lymph nodes (cortex area) + entire head | Obex (dorsal vagus nuclei) | Low sensitivity when targeting only obex |
| Age | > 18 months of age | > 18 months of age | n/a |
| Sex | n/a | Males (when shot) | n/a |
| Slaughter | n/a | Older males and females | n/a |
| Risk factors to determine sample selection | High BSE and scrapie incidence, exposure to feeding stuffs, import from areas affected by CWD | Densely populated deer areas, high BSE and scrapie incidence, exposure to feeding stuffs, import from areas affected by CWD | n/a |
| Genotyping | n/a | Only if positive result obtained | Lack of collection of PrP gene polymorphism frequencies in the EU cervid populations |
| Design prevalence | 0.5% for risk populations, otherwise 1% | 598 samples | n/a |
| All MS | n/a |
Target species: all cervids Target groups: sick animals, road‐killed, culled or FS Sample size: as many samples as possible |
Limited testing in: moose, roe deer, reindeer |
TSE: Transmissible spongiform encephalopathy; BSE: bovine spongiform encephalopathy; CWD: chronic wasting disease; FS: fallen stock; IHC: immunohistochemistry; MS: Member State; WB: western blot; AT: Austria; CZ: the Czech Republic; DE: Germany; FR: France; UK: the United Kingdom; FI: Finland; HU: Hungary; IE: Ireland; IT: Italy; LV: Latvia; PL: Poland; SK: Slovakia.
The survey was carried out mainly between 2007 and 2009 (few samples were collected in 2006 and 2010). A total of 21 MS reported some testing activities on wild animals and 14 MS tested farmed animals with a total of 10,109 tests in the two target species – red deer and white‐tailed deer – (including 612 animals from Norway). However, not all target MS were able to meet the minimum sample size. An additional 2,602 other farmed/wild cervids were also submitted to rapid test (mostly roe deer but also fallow deer, moose, reindeer or other).
As mentioned, no TSE positive results were found. A subsequent EFSA opinion (EFSA BIOHAZ Panel, 2010) reviewed the results and concluded that there is not a cervid TSE epidemic in the EU. However, the same opinion also concluded that, based on available data, the ‘occurrence of cases of TSEs, especially in remote and presently unsampled geographic areas, may not be excluded in cervids in the EU’.
Prior to this survey, another study was carried out in wild and farmed cervid species in Germany, which did not record any TSE positive results (Schettler et al., 2006).
Between 2011 and 2014, the testing activity in the MS (see Appendix A) was minimal. In 2015, based on available official data from the TSE annual reports submitted by the MS to the European Commission, only Finland and Hungary and one non‐MS (Norway) reported test results for TSE in cervids (Table 5). None of the samples tested positive. However, the number of tested animals over the 2011–2015 period was insufficient to draw any epidemiological conclusions.
Table 5.
Number of samples tested in cervids in 2015 in the EU and EFTA countries
| Reporting country | Species | Other | ||||||
|---|---|---|---|---|---|---|---|---|
| Red deer | White‐tailed deer | Reindeer | Fallow deer | Roe deer | Moose | Wild ruminants | ||
| DK | – | – | – | – | – | – | – | 25 |
| FI | – | 4 | 3 | 1 | – | 6 | – | – |
| HU | – | – | – | – | – | – | 9 | – |
| LV | – | – | – | – | – | – | – | 2 |
| NO | 4(1)a | – | 1(1)a | – | 8(7)a | 4(2)b | – | – |
| SE | – | – | 1 | – | – | – | – | – |
DK: Denmark; FI: Finland; HU: Hungary; LV: Latvia; NO: Norway; SE: Sweden.
Total (wild).
One of the moose tested was classified as ‘unspecified’. All test results were negative. The data were extracted from annual reports submitted by reporting countries, and from background information provided by the European Commission and included in the mandate.
3.3.2.2. The Norwegian TSE situation
In 1994, a cat was diagnosed with FSE (Bratberg et al., 1995). Atypical/Nor98 scrapie was first diagnosed in Norwegian sheep in 1998 (Benestad et al., 2003) and since then 3–15 cases are identified each year in sheep. A single case of atypical/Nor98 scrapie was diagnosed in a goat in 2005. Classical scrapie is rare in Norway now and only six single‐flock outbreaks (in 2001, 2002, 2003, 2004, 2006 and 2009) have been diagnosed during the last 15 years. In 2015, atypical BSE (H‐type) was diagnosed in a 15‐year‐old cow of the Scottish Highland breed, which was killed due to injury (Table 6).
Table 6.
Number and year of detection of animal prion disease cases diagnosed in Norway (between 1981 and 6/10/2016)
| Species | Classical scrapiea | FSE | Atypical/Nor98 scrapie | Atypical BSE (H type) | CWD |
|---|---|---|---|---|---|
| Sheep | 64 (last in 2009) | – | 143 | – | – |
| Goat | – | – | 1 (2005) | – | – |
| Cattle | – | – | – | 1 (2015) | – |
| Domestic cat | – | 1 (1994) | – | – | – |
| Reindeer (free‐ranging) | – | – | – | – | 3 (2016) |
| Moose | – | – | – | – | 2 (2016) |
FSE: feline spongiform encephalopathy; BSE: bovine spongiform encephalopathy; CWD: chronic wasting disease.
Prior to 1998, classical and atypical/Nor98 were not distinguished, but based on the diagnostic criteria, most of the diagnosed cases were classical scrapie.
During the slaughter season for semidomesticated reindeer in the municipalities of Kautokeino and Karasjok (Finnmark County), from December 2003 until February 2004, samples from 792 animals were collected and screened for prion diseases, with negative results by ELISA analysis (Tryland et al., 2014). Altogether a total of 2,163 cervids were found negative for CWD between 2004 and 2015 (Table 7).
Table 7.
Results of the CWD surveillance programme in Norway in the period 2004–2015
| Species | Roe deer | Red deer | Fallow deer | Reindeer (free‐ranging) | Reindeer (semidomestic) | Moose | Musk ox | Total |
|---|---|---|---|---|---|---|---|---|
| 2004 | 21 | 11 | – | 1 | 792 | 10 | 13 | 848 |
| 2005 | 17 | 10 | – | 1 | 93 | 14 | 10 | 145 |
| 2006 | 9 | 129 | – | – | 48 | 12 | 13 | 211 |
| 2007 | 34 | 612 | 8 | – | 30 | 35 | 1 | 720 |
| 2008 | 26 | 9 | – | 2 | – | 9 | 1 | 47 |
| 2009 | 31 | 9 | – | 2 | – | 11 | – | 53 |
| 2010 | 17 | 5 | – | 2 | – | 13 | 4 | 41 |
| 2011 | 12 | 13 | – | 1 | 1 | 11 | – | 38 |
| 2012 | 3 | 10 | 3 | – | – | 5 | – | 21 |
| 2013 | 4 | 4 | 1 | – | – | 1 | – | 10 |
| 2014 | 1 | 4 | – | – | – | 5 | – | 10 |
| 2015 | 8 | 4 | – | 3 | – | 4 | – | 19 |
| Total | 183 | 820 | 12 | 10 | 966 | 130 | 42 | 2,163 |
This led to the conclusion that there was no CWD epidemic in Norwegian cervid populations. However, looking retrospectively the sample size was very limited as only 10 free‐ranging reindeer and 130 moose were analysed (Table 7), none of them originating from the CWD areas, and thus low‐level occurrence during this period of time cannot be excluded.
The surveillance programme put in place after the detection of CWD in reindeer and moose in Norway has as its main goals:
’To get a better understanding of the distribution of the disease in the country and whether it is really limited to the two areas of Nordfjella (reindeer) and Selbu (moose). This is being addressed by testing found sick/dead and traffic‐killed animals from the whole country.
To determine whether reindeer and moose are the only affected species, by testing all cervid species.
To ascertain if CWD is only present in free‐ranging species by also testing some captive reindeer.
To measure the frequency of the disease in the affected species in the vicinity of known cases. This would give some information about how long the disease may have gone undetected. This is currently addressed by targeting hunter‐killed (i.e., randomly sampled) animals from the two affected areas’.
The targeted number of cervids to be sampled was originally set as 15,000 for 2016. However, it is expected that it will not be higher than 10,000. This is due to the substantial logistical challenges associated with the collection of samples from free‐ranging animals, and the difficult Norwegian geographical conditions. To date (21‐Nov‐2016), 8,912 cervids have been analysed (Table 8): 4,264 moose, 1,785 reindeer, 2,442 red deer and 336 fallow deer.6
Table 8.
Results of the CWD surveillance programme in Norway in 2016 (up to 21/11/2016)
| Year/species | Roe deer | Red deer | Fallow deer | Reindeer (free‐ranging) | Reindeer (semidomestic) | Moose | Musk ox | Total |
|---|---|---|---|---|---|---|---|---|
| Positives | – | – | – | 3 | – | 2 | – | |
| Total | 336 | 2,442 | – | 1,785 | – | 4,264 | – | 8,912 |
| From CWD‐affected areas | – | – | – | 338 (Nordfjella) | – | 492 (around Selbu) | – | 830 |
CWD: Chronic wasting disease.
If the apparent prevalence in the affected areas is as low as 1%, then the prevalence in other regions may be as low, and the chance of detecting one case would be very limited, considering the number of animals tested. Another limitation of the current surveillance system is the poor quality of some brain samples collected in the wild, which makes the identification of the brain stem and the specific target area, the obex, more difficult. The sensitivity of surveillance, reliant on the collection of brain samples only, may also be reduced in some cases if the earliest detectable accumulation of PrPSc is in lymphoreticular tissue and not the brainstem. However, a caveat to this is that not every CWD case presents with early lymphoid involvement (the Norwegian moose being an example), so this is equivalent to the situation in sheep scrapie, where PrPSc can be identified early in the incubation period, but only in certain genotypes or scrapie strains.
The first case of CWD in Norway was the first case of naturally occurring CWD in reindeer worldwide (Benestad et al., 2016), but it has been known that the species is susceptible to the disease since researchers have reported successful oral transmission of CWD to reindeer (Mitchell et al., 2012; Moore et al., 2016).
It is also notable that CWD cases in moose seem to be a rare occurrence in North America, whereas two of the five CWD cases detected thus far in Norway were moose. This difference might be due to the fact that the Scandinavian moose population is much larger than that in the parts of North America where CWD occurs, or due to differences in CWD strains or exposure probabilities in moose between the two continents.
Baeten et al. (2007) described a natural case of CWD in a hunted moose in Colorado. The IHC in this case demonstrated the presence of accumulations of CWD‐associated prion protein (PrPCWD) in tissue sections of the medulla oblongata at the level of the obex (dorsal motor nucleus of the vagus) and in retropharyngeal lymph node (RPLN). This immunolabelling is therefore indistinguishable from that described in other CWD cases. In contrast, preliminary unpublished results from the PrPCWD immunostaining obtained in the two Norwegian moose show clear differences. An unusual PrPCWD distribution is found throughout the brain where the vast majority of PrPCWD is detected only intraneuronally, and with no special labelling in the dorsal motor nucleus of the vagus. The lymph nodes, available from only one of the two moose, were negative. The western blot glycoprofile of the PrPCWD of the two moose show a lower molecular weight than those of other CWD isolates from different North American species but quite similar to each other's. A similar low molecular weight of PrPCWD has been reported in wapiti with an M‐to‐L polymorphism at codon 132 (O'Rourke et al., 2007). Bioassay studies are ongoing to verify whether these differences could reflect a new or unidentified strain/phenotype in the Norwegian moose.
3.3.3. Control of CWD
The science available to inform effective CWD management and control strategies remains relatively incomplete (Miller and Fischer, 2016; Uehlinger et al., 2016). Approaches for CWD management generally fall into three categories: (a) prevention, for regions where CWD is not believed to occur or is assumed absent; (b) containment, focused on limiting the geographic extent of a focus; and (c) control/suppression, to actively stabilise or reduce infection rates in an affected herd or population. Preventive and containment strategies tend to focus on regulations (e.g. bans on movements of live animals, carcasses, or specified risk materials), whereas control/suppression tends to be more active (e.g. selective or random culling, reduction in affected populations via harvest, depopulation). As an overarching observation, attempts to manage and control CWD in North America thus far have been more successful in captive situations than in free‐ranging ones. Efforts to prevent the introduction of CWD should consider whether prior surveillance data are sufficient to assure that the disease has not already spread into the area of interest.
Eradicating CWD from North America appears infeasible, given its extensive distribution and other epidemiological attributes. Free‐ranging foci have persisted despite varied control attempts with only two exceptions: Minnesota and New York (Minnesota Department of Natural Resources, 2016).
CWD was detected during routine surveillance in captive white‐tailed deer in Oneida County, New York State, in 2005. A further case was detected in a second captive herd that had received deer from the first one, and both herds were depopulated. Intensive efforts were made to assess whether CWD occurred in wild deer in the area. These included operations by a multiagency team under the Incident Command, which ran 14 teams of sharpshooters in a 10‐mile radius around the index case. Two further cases of CWD were found in wild deer. A containment area of 850 square miles was established, in which intensive testing and a number of activities to hinder the spread of CWD were conducted following an emergency regulation. These included the prohibition of: (a) processing traffic‐killed deer by private citizens, to make the animals available for testing; (b) rehabilitating deer at facilities housing cervids and (c) moving animal parts or deer urine out of the area. The testing of hunter‐killed deer in the containment area in the 2015 hunting season was made compulsory, and waste from deer carcasses was disposed of in a landfill. The sale of white‐tailed deer feed was prohibited in the whole state. Capture and possession of free‐ranging white‐tailed deer by deer or wapiti farm owners, or taxidermists working with deer or wapiti, was also forbidden. New requirements for reporting and record‐keeping were implemented for taxidermists (Brown et al., 2006). In the 5 years following the detection of the first case of CWD in New York State, 7,335 deer from the containment area and 21,867 from the rest of the state were killed and tested (New York State Department of Environmental Conservation, 2013). Surveillance has continued since then and no further cases have been detected to date.
Intensive surveillance and culling leading to apparent elimination or to an undetectable level of the infection were also applied in Minnesota, where one case of CWD was found in a wild white‐tailed deer. More than 4,000 deer were tested in the area where the case was confirmed from 2011 to 2013. No further cases were detected until autumn 2016 (Minnesota Department of Natural Resources, 2016).
These experiences underscore the importance of early detection and intervention. Once CWD is well‐established and has become endemic in the cervid population and the environment, elimination is considered unattainable. In these circumstances, controlling the spread – in particular by human activities – and suppressing infection rates seem far more realistic goals than eradication.
Even where eradication is infeasible, combinations of intensive focal deer removal and more sustained herd suppression may offer some measure of control (Manjerovic et al., 2014; Geremia et al., 2015; Nobert et al., 2016). Acquiring reliable distribution and prevalence data early on may improve the efficacy of subsequent CWD control efforts (Miller and Fischer, 2016). Jurisdictions should also consider how cervid population management practices (e.g. harvest regimes, artificial feeding and congregating, translocations or movements in commerce), may be contributing to disease emergence or persistence (Miller and Fischer, 2016).
In Europe, in addition to the permanent measures as described in Appendix A, the Commission Implementing Decision (EU) 2016/19187 enforces temporary safeguard measures in relation to CWD (until 31 December 2017), subject to review of the epidemiological situation and of the necessity of the prohibition.
According to Article 2.1 of the Commission Implementing Decision, the movement of live cervids from Norway into the Union is prohibited from 28 October 2016.
-
According to Article 2.2, by way of derogation from paragraph 1, the following movements of live cervids are permitted:
-
1
— movements of live reindeer for seasonal grazing from Norway to the areas in Sweden listed in the Annex of the Commission Implementing Decision, or back to the areas in Sweden listed in the Annex the Commission Implementing Decision after seasonal grazing in Norway, provided that the competent authority of Sweden gives its prior written consent to such movement;
-
2
— movements of live reindeer for seasonal grazing from Norway to the areas in Finland listed in the Annex of the Commission Implementing Decision;
-
3
— movements of live reindeer from Finland which have grazed in Norway in the area located between the Norwegian‐ Finnish border and the Norwegian‐Finnish Reindeer Fence and return to Finland;
-
4
— movements of live cervids from Norway to Sweden or Finland for direct slaughter, provided that the competent authority of the Member State of destination gave its prior written consent to such movement;
-
5
— movements of live reindeer from Norway to the areas in Sweden listed in the Annex the Commission Implementing Decision for sportive or cultural events, or after having taken part in sportive or cultural events, provided that the competent authority of Sweden gives its prior written consent to the movement of each consignments;
-
6
— transit of live cervids from Norway through Sweden or Finland and destined to Norway, provided that the competent authority of the Member State of transit gave its prior written consent.
-
1
By way of derogation, the dispatch of live cervids for direct slaughter from certain areas in Sweden to the rest of Sweden or to Finland and from certain areas in Finland to Sweden or to the rest of Finland are permitted, provided that the competent authority gives prior written consent. Equally, the dispatch of live cervids from certain areas to Norway is permitted provided that the competent authority of Norway gave its prior written consent.
3.3.4. Cervid populations in the concerned countries
3.3.4.1. Cervid populations – wild/free ranging
The numbers of individual animals in free‐ranging wildlife populations could not be accurately ascertained, so, estimates of population size are presented in Table 9. There is not a single best method to estimate population density and/or abundance. Both direct (for example, direct counts) and indirect methods (for example, counting dung) can be used and the choice of method depends on local conditions, habitat, resource availability, accuracy requirements and other factors. Hunting statistics can provide information on population abundance, in particular at large spatial scales, such as at country level, and also on their long‐term temporal trends, provided that the hunting effort is maintained.
Table 9.
Total number of hunted and estimated wild population of the cervid species of interest in the countries covered by the mandate
| Species | Reindeer (wild) | Moose | White‐tailed deer | Roe deer | Red deer | Fallow deer |
|---|---|---|---|---|---|---|
| Sweden‐population | 0 | 300,000c | Occasional | 350,000r | 35,000r | 130,000r |
| Sweden‐hunted | 0 | 87,094d | 0 | 106,024d | 7,751d | 30,382d |
| Norway‐population | 25,000a , e | 120,000f | 0 | 150,000f | 125,000q | Very few |
| Norway‐hunted | 6,507a , e | 31,137e | 0 | 19,996e | 33,799e | 0 |
| Finland‐population | 2,555b , g | 54,187g | 46,162g | 30,000–50,000p | 0 | 612g |
| Finland‐hunted | 18b , h | 44,122h | 26,578h | 4,771h | 0 | 61h |
| Estonia‐population | 0 | 13,500i | 0 | 46,400i | 3,260i | 0 |
| Estonia‐hunted | 0 | 6,873j | 0 | 6,264j | 1,252j | 0 |
| Latvia‐population | 0 | 21,700k | 0 | 133,200k | 53,200k | 0 |
| Latvia‐hunted | 0 | 5,810k | 0 | 13,170k | 11,805k | 0 |
| Lithuania‐population | 0 | 10,903l | 0 | 111,427l | 30,056l | 2,325l |
| Lithuania‐hunted | 0 | 8,660m | 0 | 109,707m | 25,672m | 1,631m |
| Poland‐population | 0 | 11,714n | 0 | 829,000n | 203,000n | 27,225n |
| Poland‐hunted | 0 | 0n | 0 | 166,889n | 60,307n | 7,042n |
| Iceland‐population | – | 0 | 0 | 0 | 0 | 0 |
| Iceland‐hunted | 1,291o | 0 | 0 | 0 | 0 | 0 |
Wild Eurasian tundra reindeer (Rangifer tarandus tarandus).
Finnish forest reindeer (Rangifer tarandus fennicus).
Eurasian tundra reindeer (R. t. tarandus) (2015) http://www.hjorteviltregisteret.no
VKM (2016). CWD in Norway. Opinion of the Panel on biological hazards, ISBN: 978‐82‐8259‐216‐1, Oslo, Norway.
https://riistaweb.riista.fi/riistatiedot/riistatietohaku.mhtml Report of population left (after hunting), for year 2015.
http://www.amvmt.lt/images/veikla/stat/miskustatistika/2012/07%20Misku%20ukio%20statistika%202012_m.pdf, (2011/2012).
http://stat.gov.pl/cps/rde/xbcr/gus/rl_lesnictwo_2012.pdf, (2011/2012)
Mr Jyrki Pusenius, Natural Resources Institute Finland, confirmed this by email on 21 November 2016.
Solberg et al. (2008).
Mr Jonas Malmsten, Viltkonsult Jonas Malmsten AB, confirmed this by email on 21 November 2016.
Since diverse population estimation methods are used according to the circumstances and in different countries, and also for different cervids species, direct comparison of such data is not reliable.
The figures on population abundance provided in Table 9 have been obtained from different sources and represent various methodologies used for census and estimates. Additionally, some of the data are several years old since more recent data could not be obtained, and the actual population size may have changed since the data were recorded. Therefore, the population data presented may be incomplete or outdated, reflecting the difficulties stated. Hunting statistics presented in Table 9 are mostly sourced from national official statistics websites.
These data are presented to illustrate the diversity in population sizes, by species, for the various countries under consideration, and to demonstrate how difficult it is to obtain/estimate such data.
3.3.4.2. Cervid populations – captive and farmed
Cervids are also kept in captivity in deer farms, animal parks, zoological gardens and other holdings. Farmed cervids in the countries concerned are mostly red deer and fallow deer. Smaller numbers of cervids are kept in parks for educational purposes, recreation and tourism, for example, moose parks. Data on the captive cervid populations in the countries were not readily available and therefore are not presented in this opinion.
3.4. Ecology and husbandry of wild, semidomesticated and farmed cervids in Fennoscandia8
Cervids are deer species of the family Cervidae. Cervids have played important roles throughout human history, representing important sources of meat and materials. In addition to reindeer herding, wild cervid populations are very important to the region, especially as a source of recreational hunt, and hunting is also the most effective management and control measure in the cervid populations of these regions.
Cervids are ruminant plant eaters. Some species feed mostly on the ground (grazers) and others on trees and bushes (browsers). The choice of feed will to a large extent depends on the local ecosystems and availability, and usually also varies throughout the year. Reindeer are gregarious animals, forming large herds, whereas the other cervid species in question mostly live in smaller groups or are solitary animals. Cervids, such as reindeer, may also conduct seasonal migrations, sometimes over large distances, and sometimes between summer and winter habitats on a more local scale.
In the following sections, the ecology and husbandry of wild and semidomesticated cervids in Fennoscandia are described. Due to shortage of time, similar information for other regions and countries included in the mandate have not been collated and described in this opinion.
3.4.1. Wild cervid populations
3.4.1.1. Eurasian tundra reindeer and Finnish forest reindeer
Reindeer are mostly grey to brown in colour, and, in contrast to all other cervid species, both sexes have antlers, which are shed after the rut in October (males) or later during winter and spring (females). Reindeer give birth to one calf (twin calves are extremely rare) in late April to early June. They become sexually mature at approximately 1.5 years of age, females usually giving birth as a 2‐year‐old. Reindeer vary considerably in size, both between and within the different subspecies and their geographical range. The body length of reindeer may reach 220 cm from nose to tail, a wither height (height of the ridge between the shoulder blades) of 125 cm and a body weight of 270 kg for large males, whereas females are considerably smaller. In winter, as much as 40–80% of the food may be lichens (Cladina spp. and others), whereas in summer they eat grass, sedges, herbs and some shoots and leaves, depending on habitats and availability. In general, food is plentiful in summer, but often limited on winter pastures.
Reindeer, commonly called caribou in North America, all belong to the same species, Rangifer tarandus. This species is divided in seven subspecies, of which only two are relevant to this report: the Eurasian tundra reindeer (R. t. tarandus) and the Finnish (Eurasian) forest reindeer (R. t. fennicus).
The Eurasian tundra reindeer is almost continuously distributed in Eurasia, across the tundra region and mountain areas, and may in some places overlap with the Finnish forest reindeer. This subspecies comprises the cornerstone of the reindeer herding industry and cultures, as semidomesticated reindeer, in mainland Norway, Sweden, Finland and parts of Russia. This subspecies is also found as wild reindeer in Norway, comprising 23 separated populations of approximately 25,000 animals in total (Figure 2). Of these, the Hardangervidda population is the largest, with about 10–11,000 animals.
Figure 2.
Distribution of the 23 populations of wild Eurasian tundra reindeer of Norway. Source: Norwegian Institute for Nature Research
- The three clinical cases (up 21 November 2016) of CWD have all been detected in Nordfjella (region 11). (http://www.nina.no/english/Home).
Wild reindeer usually conduct seasonal migrations between summer and winter pastures, although, for some of the populations, infrastructure (e.g. roads and railways) and natural boundaries (e.g. rivers, lakes and valleys) limit such migrations to a large extent.
The wild reindeer population in the Nordfjella region (Figure 2, region 11), in which the three CWD cases up to now have been detected, is estimated to consist of approximately 2,500 animals, and is restricted to an area of 2,995 km2 region north of the Hardangervidda mountain plateau. During the 18th and 19th centuries and into the first half of the 20th century, there was also reindeer herding (i.e. semidomesticated reindeer) in several regions of what today is defined as the Nordfjella wild reindeer region, with frequent mingling between wild and semidomesticated reindeer (Figure 3), and there is still some exchange of animals between wild and semidomesticated reindeer herds today (Strand et al., 2011). The status and development of the 23 different wild reindeer populations in Norway varies. The harvest from the Nordfjella population during the last 6 years has varied from 266 animals in 2012 to 522 animals in 2014 (mean 2012–2014: 448) (http://www.hjorteviltregisteret.no).
Figure 3.
Distribution of the Nordfjella wild reindeer population (red), where the three CWD cases had been found, and the borders to the Hardangervidda wild reindeer population (dark green) and the neighbouring herd of semidomesticated reindeer (pink)
- Map: Bernt Johansen, Norwegian Research Institute (NORUT).
Some of the wild reindeer populations, but not the Nordfjella population, are included in the national surveillance programme for deer species of the Norwegian Institute for Nature Research (NINA) (http://www.nina.no/english/Home). Some investigations on health and diseases have been conducted through the surveillance programme for cervid health of the Norwegian Veterinary Institute (NVI) (http://wwweng.vetinst.no/eng/index.html). There are no previous reports of diseased reindeer with CNS symptoms from the Nordfjella population.
The Finnish forest reindeer is found in two defined populations in Finland, as well as in Russia, but it remains unresolved whether reindeer of other forest regions should also be included within the same subspecies. This subspecies is larger than the tundra reindeer, has longer legs and narrow v‐shaped antlers, and prefers boreal forest habitats.
3.4.1.2. Moose
Moose (A. alces) is further divided into several subspecies. In Europe, they are therefore sometimes called Eurasian moose or Eurasian elk/European elk (A. a. alces), which is mostly distributed in Finland, Sweden, Norway, Latvia, Lithuania, Estonia and Russia. In Norway, moose were previously restricted to the southern and eastern parts of the country, but during the past decades have spread to most parts of the country (Figure 4).
Figure 4.
Number of moose shot in Norway per 10 km2 hunting area (2016), and geographical distribution. Source: Statistics Norway (https://www.ssb.no/en/jord-skog-jakt-og-fiskeri/statistikker/elgjakt)
The moose is the largest cervid species (males 400–550 kg and females 320–400 kg) and may reach an age of 15–25 years. It is brown to grey in colour and only males have antlers. They are commonly found in boreal coniferous and deciduous forests in temperate and subarctic regions in the Northern hemisphere. However, they may also exist in many other habitats, including regions with a mountainous and alpine character. Moose are solitary animals, with strong bonds between mother and calves, but dozens of animals may be observed together, especially during winter, when they congregate for feeding or under heavy snow conditions to save energy. The total winter population of moose in Norway is about 120,000 animals (Table 9).
Males are polygamous, serving several females during the mating season. Moose usually give birth to one or two calves in May–June. The most common predators of moose in Fennoscandia are the wolf (Canis lupus lupus) and the brown bear (Ursus arctos). Moose hunting is a very popular activity, with long traditions in many regions and is the main regulator of the populations, as predator numbers are generally low. The harvest of moose in Norway has been stable over the last decade, and approximately 32,000 animals were shot annually during 2005–2015 (http://www.hjorteviltregisteret.no).
Moose are selective feeders. In spring/summer, they typically not only feed on fresh shoots of deciduous trees and shrubs and herbs but also on fresh grass, and in winter, they mostly feed on older branches, including pine trees. During summer, a full‐grown moose may eat as much as 50 kg of biomass each day in summer, but only 8–16 kg of biomass per day in winter, when the metabolism is lower. During winter, an adult moose may lose 20–25% of its body weight (http://www.hjorteviltregisteret.no). A moose may spend large amounts of energy seeking food, and the hair coat on the legs and the sides of the animal may be worn out. This sign is often found along with emaciation (lack of fat resources, i.e. subcutaneous, intraabdominal, coronary grooves and bone marrow).
Since 1985, a wasting syndrome (moose wasting syndrome (MWS), also called Älvsborgsjukan) has been observed throughout Sweden, but with an elevated occurrence in the county of Älvsborg, with clinical symptoms resembling CWD. More than 1,000 moose were found dead or euthanised in this county by the end of 1992 (Merza et al., 1994).
This condition is characterised by starved and emaciated animals with a lack of normal fight behaviour, ataxia, lack of coordination and circling. The aetiology of MWS is unclear (Frank, 2004). Investigations of brain material from affected animals for histopathological alterations revealed no spongiform changes, characteristic of prion disease, and the immunohistochemical analysis for prion protein aggregates was negative. Brain areas investigated included the forebrain, cerebellum and, most importantly, the brain stem at the level of the obex. Based on this, it was concluded that MWS was not caused by a prion agent like CWD (Rehbinder et al., 1991). A retrovirus was previously isolated from diseased moose thought to cause the wasting syndrome (Merza et al., 1994), but this has gained little support as the only cause of the syndrome. Other hypotheses regarding the aetiology have been numerous, among others, several types of nutritional deficiencies (Frank, 1998).
3.4.1.3. Roe deer
Among the cervid species included in this scientific opinion, the roe deer is the smallest (18–36 kg) with a wither height of 70–85 cm. The roe deer was almost extinct in Scandinavia around 1830, but is again abundant in the region covered by this report, and has expanded into new regions. Roe deer not only inhabit agricultural land and grass lands, but also different types of forest ecosystems, feeding on grass, sedges and berries, as well as grain, heather, leaves and young shoots. Only the males grow antlers. They use small trees to clean the velvet from new antlers (April–May) and to mark territory during the rut (August). The antlers are shed in November–December, and new velvet‐covered antlers are soon established. Roe deer is an important game species in Europe (see Table 9).
3.4.1.4. White‐tailed deer
White‐tailed deer is a medium‐sized cervid species, native to the United States, Canada, Mexico, Central America, and South America, and is the most widely distributed wild ungulate in America. This species has also been introduced to a wide range of other countries, including some European countries, such as Finland, the Czech Republic and Serbia. In 1934, four white‐tailed deer, three females and one male were imported to Finland, as a gift from Minnesota, USA. Since then, this population has grown tremendously, to approximately 25,000 animals, and is today one of most economically important game species in Finland, second only to moose (Table 9). The Finnish population is isolated from other white‐tail deer populations (Kekkonen et al., 2016).
In Finland, white‐tailed deer feed mainly on grass, shoots and leaves in deciduous forest habitats. They may also eat feed associated with farms, and, in Finland, white‐tailed deer are often and to a large extent fed through the winter, with hay or ensilage. Feeding spots for white‐tailed deer are also often visited by other cervids, and may thus be a very important contact point between animals and species with regards to the potential transmission of CWD.
3.4.1.5. Red deer
The red deer is distributed in most parts of Europe, the Asian part of Turkey, the Middle‐East, Asia and North Africa. A full‐grown male can have a body length of 260 cm, a wither height of 150 cm and weigh up to 250 kg. The female typically weighs around 120 kg. Red deer can live longer than 20 years in captivity, but rarely grow older than 10 years in the wild. The hair coat of red deer is short and brown in summer, but in winter the guard hair becomes longer and greyer. In summer, red deer mainly feed on grass and sedges. In winter, they still prefer grass, if available, but also feed on heather and other plants on the ground, as well as shoots and branches from deciduous trees. Red deer females give birth to one calf (7–10 kg) in May, and feed the calf for about 7 months. Only males grow antlers, which are shed in April–May, followed by growth of new antlers.
Wild red deer can live solitary lives or they can form smaller herds of a few animals or some dozens of animals. Some animals tend to stay in the same area all year around, whereas others migrate between summer and winter pastures, usually from lowlands and forest areas on the coast and through valleys, reaching more mountainous ecosystems in the spring, summer and autumn.
The harvest of red deer in Norway has been stable over the past years and on average, approx. 33,700 animals were shot annually in Norway during 2011–2015 (http://www.hjorteviltregisteret.no). Red deer is also an important game species for the other countries relevant to this report (Table 9).
Wild red deer can be infected with gammaherpesvirus, known to cause malignant catarrhal fever (MCF) in cattle, and having sheep as a reservoir. This may be relevant, since this infection may generate CNS signs, such as abnormal behaviour, apathy, incoordination, circling, staggering gait, convulsions and impaired vision (Vikøren et al., 2006). In a screening in Norway, 5% (13 animals) had antibodies against gammaherpesvirus.
3.4.1.6. Fallow deer
The fallow deer is native to western Eurasia, but has been introduced to numerous other countries. In Norway, wild fallow deer originate from individuals that have escaped from deer farms, and a small population has settled on Hankø Island (Østfold County) and individuals are sometimes observed east of the river Glomma (Hedmark County), but this species is numerous in Sweden and represents an important game species (Table 9). A fallow deer is smaller than the red deer but larger than the roe deer. An adult female fallow deer (> 3 years) weighs about 40–65 kg and an adult male (> 5 years) weighs approximately 80–120 kg. Fallow deer have characteristic white spots on the sides and over the back. Only males have antlers, which are shed in April–May. After approximately 8 months pregnancy, one calf is born. Females and calves typically form their own groups in other periods of the year than the rut, which starts in October–November, when males may fight and gather a harem of females. Fallow deer are found in open agricultural land with grass pastures in spring and summer, but usually prefer forest during autumn and winter to seek more shelter, and to feed on nuts, berries and bark. They are regarded as more stationary compared with other deer species.
3.4.2. Semidomesticated reindeer
Herded reindeer are not wild, since they are owned, and most of them are gathered a few times during the year for slaughter, or tagging and transport between summer and winter pastures. But they are not regarded as domesticated, as compared with cattle, sheep and goats, since they are always outdoors, most of the time as free‐ranging animals. Herded reindeer are therefore commonly referred to as semidomesticated.
Except for a few smaller regions in Finland inhabited by wild Eurasian forest reindeer, all other reindeer in Fennoscandia (Norway, Sweden, Finland and Kola Peninsula, Russia) belong to the Eurasian tundra reindeer subspecies. Except for the 23 wild reindeer populations in southern Norway, and the forest reindeer in Finland, all other reindeer in Fennoscandia are herded, i.e. semidomesticated.
In Fennoscandia, reindeer herding is a traditional cornerstone of the Sami culture. In Norway and Sweden, only people with a Sami heritage can own reindeer, whereas in Finland, there are both Sami and non‐Sami reindeer herders. In Russia, the Sami people are one of many indigenous people that live from reindeer herding, often combined with fishing, hunting and trapping. The area traditionally inhabited by the Sami people, which grossly also reflects the core region of the reindeer herding, that in Sami is called Sápmi (Figure 5).
Figure 5.
The Sápmi region, the part of Fennoscandia traditionally inhabited by the Sami people, and where the majority of the reindeer herding of this region is conducted. Source: (http://www.sim1.se/background/samer/samer_01.html)
Reindeer feed on many different plants during summer, depending on the local habitats and resources, and they have shown a great ability to adapt to changing environments. The summer pastures support most of the production (i.e. the calves) whereas the winter pastures, often high mountain plateaus, such as Finnmarksvidda in Norway, often consists of lichens and other plants with poorer nutritious content, which represents maintenance feed, with carbohydrates for winter survival but little protein for growth. The winter pastures are often the restricting resource during the herding year, determining how many animals the herder/pasture can maintain. Semidomesticated reindeer were traditionally kept in a seminomadic way, shifting between winter and summer pastures. This practice was, however, challenged when the country borders were drawn in the northern regions during the 18th century, shutting down some of the traditional coast – inland migration. However, such migrations are still used in many regions of Norway and some places in Sweden, where animals are either herded by foot, or transported by car or boat between summer and winter pastures, whereas in other parts of Sweden and in Finland, animals are kept at the same pasture year around, and feeding, either as supplementary feeding or on a daily basis, becomes more and more common (Figure 6).
Figure 6.
Reindeer herding in Norway, Sweden and Finland, indicating the development of reindeer numbers the past century, the stocking density (animals/km2) and the seasonal migration in the spring (arrows) or year‐round pastures (circles)
- Between Norway and Sweden, cross‐border herding takes place, with many herders having pasture rights on both sides (Source: Pape and Löffler, 2012; © Royal Swedish Academy of Sciences).
In Norway, the total number of semidomesticated reindeer is about 211,000 animals (Table 10). Reindeer herding is organised in six reindeer pasture regions, namely Øst‐Finnmark, Vest‐Finnmark, Troms, Nordland, Nord‐Trøndelag and Sør‐Trøndelag, with approximately 80 herding districts, each consisting of several reindeer herding units (i.e. siida) for summer and winter pastures, respectively (Figure 7).
Table 10.
Some key features of reindeer herding in Norway, Sweden and Finland (2014–2015)
| Norwayb | Swedenc | Finlandd | Total | |
|---|---|---|---|---|
| Number of animals a | 211,606 | 250,332 | 194,652 | 656,590 |
| Animals slaughtered/year | 75,382 | 56,333 | 84,997 | 216,712 |
| Reindeer meat/year (×1,000 Kg) | 1,748 | 1,459 | 2,000 | 5,207 |
| Land area used as reindeer pastures (%) | 40 | 50 | 36 | – |
Reindeer heads in spring 2015 (after slaughter, before calving).
Ressursregnskap for reindrift 2014–2015; Landbruksdirektoratet, Norway (Anonymous, 2016).
Sametinget, Sweden (https://www.sametinget.se/statistik_rennaring).
Reindeer Herders’ Association (Finland) http://paliskunnat.fi/reindeer
Figure 7.
Reindeer herding areas of Norway (semidomesticated reindeer; red areas)
- Map: Bernt Johansen, NORUT. Reindeer herding is conducted in 140 municipalities in Norway, using around 140,000 km2 or about 40% of the land area.
For the reindeer herding year 2014–2015, 3,150 persons were registered with a siida unit. A total of 69% of the semidomesticated reindeer in Norway are found in Finnmark County, whereas 11,065 animals are found in Troms, 14,465 in Nordland, 14,031 in Nord‐Trøndelag, 13,024 in Sør‐Trøndelag and Hedmark Counties, and 12,640 in the non‐Sami herding units (‘tamreinlagene’). Of these animals, 79% were females, 5% males and 16% calves. The mean carcass weight was 18.7 kg (calf), 29.7 kg (female > 2 year) and 43.8 kg (male > 2 year). Due to differences in natural pasture resources, animal density, and loss of animals (predators, road kill, etc.), the annual production of meat per female animal in the herd varied between districts, from almost nothing to up to 16.4 kg (Anonymous, 2016).
In Sweden, reindeer herders are organised in a ‘sameby’, which is an economic and administrative unit in a given region (the parallel to ‘siida’ in Norway). There are 51 such units in Sweden. Reindeer pastures all together constitute approximately 50% of the land area of Sweden.
The reindeer herding units are designated to specific summer and winter pastures. A reindeer herder may share winter pastures with 5–10 other herds, and summer pastures with these and/or other herds. In a recent questionnaire about the disease keratoconjunctivitis in reindeer among reindeer herders in Norway and Sweden (63 respondents), almost 30% reported that their animals had contact with as many as 25 other herds or more during a year by sharing pastures, corrals transport vehicles, etc. (Tryland et al., 2016).
Although reindeer herding is organised in a siida or sameby, animals in most districts are free ranging and without daily inspection. They can also be left without observation for longer periods to avoid disturbances, such as during calving. Although fences are sometimes used to limit mingling between herds, these are generally not common in Norway and parts of Sweden. Thus, reindeer of different herds will be in contact and to some extent intermingle. It is also common to exchange animals across herding unit borders, by foot and also over longer distances by road or boat transport. Further, reindeer owners often have to transport animals for slaughter for longer distances. Thus, during natural herding conditions, there will be contact between reindeer of different herds, both neighbouring and geographically more distant.
The nature of reindeer herding in Fennoscandia, using vast land areas, migrating between summer and winter pastures, crossing municipality‐, county‐ and country borders, the large degree of contact between herds, and the fact that free‐ranging animals may not be observed closely for long periods of time are regarded as highly relevant factors for the epidemiology and control of CWD, should this disease enter the reindeer herding industry.
3.4.2.1. Shared habitats and contact between free‐ranging cervid species in Norway
The different cervid species have somewhat differing dietary preferences and these may change during the seasons. Searching for food, together with seeking shelter and safety, are important drivers of animal movement and seasonal migrations. Free‐ranging cervids generally have a restricted energy uptake during winter, and in the spring, they seek pastures and regions with an early onset of greenery, such as in coastal and low land regions. It is thus common to see cervids of different types feeding on farmland pastures that are free of snow early in the spring. In summer, domestic animals, such as sheep and goats, graze more and more at higher altitude and mountainous pastures, thus increasing the potential for contact with wild cervids. Some free‐ranging cervids, such as moose, may also visit farmland during winter and feed upon silage bales.
Feeding wild cervids, such as roe deer and red deer, is rather common in the countries considered in this scientific opinion. The purpose of this may be to keep animals away from traffic points and busy roads but also to increase the number of animals that survive the winter. If winter feed is made available in a sheltered spot, moose, red deer and roe deer may have greater contact than would normally occur through natural feeding. Such feeding areas should be considered as potential environmental hot spots that could facilitate direct and indirect transmission of infectious agents, including prions and CWD.
3.4.3. Farmed cervids
In Sweden, both fallow deer (imported) and red deer (indigenous) are farmed, but most of the farmed cervids (80%, 2005) are fallow deer. Today, there are approximately 270 deer farms in Sweden, keeping about 30,000 animals (http://www.fedfa.org/en/fedfa-org.-data/member-countries/sweden-2767771). Tuberculosis, caused by Mycobacterium bovis, was identified in farmed fallow deer in 1991 and the source of infection was traced back to an import of fallow deer in 1987. At least 13 farms with fallow deer were affected, and a TB programme was implemented. Meat inspection became compulsory for farmed fallow deer in 1990 and for hunted red deer and fallow deer in 1994 (Bölske et al., 2006).
In Norway, cervid farming started during the 1980s, and today, approximately 80 cervid farms are in production, distributed mostly in the southern part of Norway, north to Fauske in Nordland County, including about 8,400 animals (http://www.hjortesenteret.no). In 2013, deer farmers were responsible for providing the Norwegian market with nearly 170 tonnes of venison (http://www.fedfa.org/en/fedfa-org.-data/member-countries/norway-2767748). Most farms have red deer, but some keep fallow deer, or in a few cases, both species. It is common to shoot farmed cervids inside the fence, bleed and transport them to special facilities that do not normally slaughter farmed animals for carcase dressing.
Farmed cervids are kept in enclosures and normally do not have contact with free‐ranging cervids, but during the mating period, wild males may be able to enter enclosures to get access to females. Captive deer may escape enclosures, which is how the wild population of fallow deer was established in Norway.
4. Answers to the ToRs
4.1. Answer to Term of Reference 1
The proposed surveillance system for the concerned countries is based on the experience of CWD surveillance in North America and the knowledge of the structure and management system of the cervid population of some of the concerned countries, in particular Norway and Sweden.
The aims of the proposed surveillance system is twofold: (a) to detect disease in countries where CWD has not yet been detected using a predefined design prevalence based on previous known occurrence in newly infected areas in NA; (b) to estimate prevalence in areas where disease has been detected.
The proposed surveillance system is intended to overcome the shortcomings highlighted in the evaluation of the previous surveillance programme implemented in Europe 2006–2010, where it was concluded that there was not full geographical coverage, and some species were under‐represented (EFSA BIOHAZ Panel, 2010). The sampling strategy applied in it did not take into account the known clustered and uneven distribution of the disease, resulting in foci in which the disease prevalence is higher. A multiyear surveillance programme may be the best strategy to effectively address the potential for an emerging disease with expected initial low prevalence and clustered distribution.
Based on the above, the surveillance system is proposed to be implemented during a 3‐year cycle, using a combination of random and convenience sampling. This would help to address the problems encountered by the previous scheme where an extension of a second year was required in order to achieve the targeted sample size. This will also contribute to ensuring geographical representativeness, which was another identified shortcoming of the previous scheme.
A further refinement of the proposed surveillance system is that it is tailored specifically for two different management systems: farmed and wild/semidomesticated cervids.
A two‐stage sampling programme is proposed based on the application of random sampling at the first stage (for wild/semidomesticated cervids the ‘primary sampling units’ (PSU) will correspond to geographical areas containing cervid populations, whereas for farmed cervids they will correspond to farms) and the application of convenience sampling within PSU corresponding to high‐risk animals (found dead, hunted or slaughtered animals considered not fit for human consumption, road/predator kills and animals killed because they are sick or in poor body condition and not fit for human consumption) of any selected species at the second stage. PSU may be defined as subsets of the population based on the geographical aggregation of animals or the outputs of national RA. Since there is no prior knowledge of differences in species susceptibility (with the exception of fallow deer which is likely to have reduced susceptibility to CWD) and multiple species coexist within primary sampling units, all species could contribute. However, a subset of these species may be selected based on the outputs of national RA.
The characteristics of the proposed surveillance programme for wild cervids, semidomesticated reindeer and farmed cervids in the countries covered by the mandate are displayed in Table 11 below.
Table 11.
Characteristics of the proposed surveillance system for CWD in the countries covered by the mandate
| Management system | Wild cervids and semidomesticated reindeer | Farmed/captive cervids |
|---|---|---|
| Time frame |
|
|
| Species to be tested |
|
|
| Sampling units |
As sampling frames are not available, the design is based on the testing of animals (subunits) from geographically based ‘primary sampling units’ (PSU) PSU are geographical areas defined by each country using a geographical criterion that has to be based:
For example, in the case of semidomesticated reindeer, siida in Norway and sameby in Sweden may be considered the correct level of aggregation to define the PSU. For wild cervids, each of the 23 different wild reindeer populations in Norway could be considered a PSU |
Farms or other captive wildlife facilities |
| Aims |
The surveillance system should have a two‐fold aim: Detection:
PSU prevalence estimation:
|
The surveillance system should have a twofold aim: Detection:
Farm prevalence estimation:
|
| Target groups |
Sampling of semidomesticated reindeer may be concentrated seasonally during the period/s of maximal aggregation of animals |
|
| Sampling design |
Two‐stage sampling aiming at testing at a national level a total of 3,000 animals of all or the subset of selected species over the 3‐year period, which corresponds to an overall design prevalence at a population level of 0.1% and a 95% confidence level.
PSU‐level and animal‐level sensitivity/specificity are assumed to be equal to 100% |
Two‐stage sampling aiming at testing at a national level a total of 3,000 animals of all or the subset of selected species over the 3‐year period, which corresponds to an overall design prevalence at a population level of 0.1% and a 95% confidence level.
Farm‐level and animal‐level sensitivity/specificity are assumed to be equal to 100% |
| Tissue to be collected |
|
|
A proper (qualitative/quantitative) RA to estimate the likelihood of introduction of CWD into the EU taking into account primarily movements of live cervids across the border is necessary. Surveillance activities would be better designed after considering the outputs of such RA.
4.2. Answer to Term of Reference 2
There are many factors that influence the ability of any TSE agent to infect a host, regardless of whether the infection occurs across a species barrier. Currently, there is no experimental model that encompasses all the potential host and agent variability to directly assess zoonotic potential for any animal prion disease, including CWD.
Although CWD has been transmitted to squirrel monkeys, in vivo transmission of CWD to other animal models, including macaques and humanised mice, has not yet been reported.
New evidence shows that in vitro amplified CWD can convert human PrP by PMCA. Experiments using RT‐QuIC also suggest that at the level of protein–protein interactions the molecular barrier preventing transmission of CWD to humans may be less robust than previously thought.
Based on the previous two bullet points, there is no evidence of an absolute species barrier between CWD‐affected cervids and humans.
CWD strains, their prevalence, host range and zoonotic potential remain incompletely understood. All currently available data pertaining to host range and human risk are derived from isolates from cases of CWD identified in North American cervid species. Preliminary evidence from the Norwegian CWD cases raises the possibility that European and North American isolates are different.
CWD prions are present in the skeletal muscle and other edible tissues which means that humans may consume infected material in enzootic areas.
There are no data on the effective infectious ‘dose’ of CWD for humans, or how that might vary by agent strain/host species. The level of oral exposure is largely determined by the prevalence of animal TSE and by the amount of infectivity in animal tissues entering the food chain. The latter is reduced for BSE and scrapie by the specified risk materials (SRM) measures, but such measures are not mandatory for cervids consumed in North America.
From the epidemiological investigations carried out to date, no association has been made between the occurrence of sCJD in humans and exposure to CWD. Surveillance of sCJD indicates that the mean overall annual mortality rates are relatively consistent at 1–1.5 cases per million in both Europe and North America.
4.3. Answer to Term of Reference 3
4.3.1. Additional animal health risk‐based measures to prevent the introduction of CWD into EU cervid populations
In order to propose risk‐based measures, an accurate knowledge of the epidemiological situation of the disease and a RA to estimate the likelihood of introduction of CWD into the EU would be necessary. This exercise would consider the most likely routes of introduction and spread so that the transmission pathways are identified and ranked based on their likelihood.
Although not a requirement of this mandate, a preliminary RA has been performed in a qualitative fashion by reviewing what is known about the husbandry of wild, semidomesticated and farmed cervids in Norway, the mechanisms of natural transmission, the epidemiology of the disease and risk factors associated to the introduction, persistence and spread of CWD, and the review of past and present surveillance and control measures in North America.
It was concluded that the most likely pathway of introduction of CWD into the EU is the movement of live cervids, either by deliberate transportation or by the movement of wild animals across the border of Norway to Sweden or Finland.
Although CWD has not been detected in semidomesticated reindeer, it is known that semidomesticated reindeer and wild cervids have overlapping habitats. Thus, husbandry practices applied through semidomesticated reindeer herding that could increase the risk of introduction of CWD in to the EU via Sweden and Finland must be considered when proposing preventive measures for introduction of CWD into the EU.
Based on the above and the current uncertainties of the actual distribution of the disease in Norway, the following options are for consideration:
Since it was concluded that the most likely pathway of introduction of CWD into the EU is the movement of live cervids, the current derogations of Article 2.2 of the Commission Implementing Decision (EU) 2016/1918 (see details in Section 3.3.3), currently present a risk of introduction of CWD into the EU. The probability of the introduction of CWD into and spread within the EU associated with the movement of live cervids for direct slaughter is considered to be lower than situations in which live animals are translocated for other purposes. Minimising movements of live cervids would reduce the probability of introduction of CWD into the EU.
The use of natural cervid urine lures is considered to increase the probability of introduction of CWD into the EU, hence discontinuing its use would reduce the probability of introduction of CWD into the EU.
Compliance with recommendations included in awareness campaigns targeting both local Norwegian hunters, and hunters visiting Norway from (and returning to) other countries, with regard to personal protective equipment (PPE), disinfection, the safe dressing of carcasses and the appropriate disposal of carcase trimmings, would reduce the probability of introduction of CWD into the EU.
4.3.2. Additional animal health risk‐based measures to prevent the spread of CWD within the EU
Measures to prevent the spread of CWD within the EU would require the assumption that the disease is already present in some part of the EU territory and affecting one or more wild, semidomesticated or farmed cervid populations. At the time of writing this opinion, this is unknown. Measures to prevent disease spread would be very different depending on the distribution, prevalence and the population at the time and place in which it was detected in the EU for the first time. No specific programme of preventive measures can therefore be proposed.
As stated in Section 3.3.1, acquiring reliable distribution and prevalence data early on may improve the efficacy of future CWD control efforts. The uncertainties associated with the limited knowledge of the situation of CWD in the concerned countries could render any of the recommended measures inadequate or insufficient. Moreover, strategies to prevent the introduction and/or the spread of CWD must be based on a combination of measures. It is also important to emphasise the fact that the experience in North America shows that once CWD is well established and has become endemic in the cervid population and the environment, elimination is considered unattainable.
However, some measures listed below are intended to contain (limit the geographic extent of a focus) and/or to control/suppress (actively stabilise or reduce infection rates in an affected herd or population) CWD in a region or country where the disease is present. The list is not exhaustive and measures should be applied according to the circumstances, as described above, and in addition to the ones already mentioned aimed to prevent the introduction of CWD into the EU.
-
Measures to reduce transmission by minimising animal‐to‐animal contact and lowering population densities include:
-
1
— Forbid baiting and supplementary feeding of wild cervids.
-
2
— Forbid use of lick blocks.
-
3
— Non‐specific cull: partial or total depopulation of captive, semidomesticated and wild cervids in infected areas.
-
4
— General, non‐selective reduction in wild population by: increasing harvest permits, and/or extending hunting season or changing the harvest season (it should be species‐specific).
-
5
— Selective culling: selective, preferential or planned removal of cervids in infected areas, defined using geographical or any other criterion.
-
6
— Trace cervid movements from infected farms (at least 5 years prior to the identification of CWD) and/or epidemiologically linked farms in order to apply surveillance and control measures. This measure is only applicable to farmed cervids.
-
1
Implement awareness campaigns to educate herders, hunters and other stakeholders about the disease, impact, epidemiology and risk factors measures in place, consequences, safe and appropriate disposal of PPE and carcase trimmings, among others.
Ban the movement of dead cervids to feed wildlife and to use them as baits for hunting or viewing, unless they have been tested, and found negative, prior to movement.
Ensure appropriate disposal of carcasses and parts (offal) of hunted animals, animal by‐products from slaughtered animals, clinical suspects, found dead, etc., even if not tested or not official clinical suspects.
Develop a contingency plan, including specific measures to be enforced in premises under quarantine, and any other measures considered by the competent authority to be applied in infected farms and management zones around them (to be defined by the competent authority).
-
Additional specific measures to prevent the spread of CWD within Norway:
-
1
— Avoid the movement of silage and feed, harvested or produced using plants originating in counties where CWD has been detected, to other counties of Norway and abroad.
-
2
— Avoid lichen removal from counties where CWD has been detected.
-
1
5. Further recommendations
Review the design of the surveillance system after 1 year of implementation
Conduct a RA to estimate the likelihood of introduction of CWD into the EU. Surveillance would be better designed and risk mitigation measures would be better tailored should the outputs of such a RA be available.
Collect data on population size, geographical distribution, spatial subdivision and density for each cervid species in the concerned countries, concurrent with the implementation of the proposed surveillance system. Such data would facilitate the design of future surveillance activities and the analysis and interpretation of surveillance data.
Implement an individual identification system and record‐keeping requirements for farmed and semidomesticated cervids.
Set up a fit‐for‐purpose data collection system for the surveillance system, allowing for the following minimal set of epidemiological data to be collected, including: species, age, sex, geographical location (coordinates or postal code), body condition, target group, sample type, management type (farmed, wild, semi), primary sampling unit and farm code if farmed.
Characterise, by strain typing, all positive cases detected by surveillance.
Archive representative tissue samples from all positive cases detected by surveillance for future analysis and comparison.
Genotype all cervids tested positive by surveillance and all or a representative subset of cervids tested negative by surveillance in order to generate information on the PRNP gene in European cervid populations, and collate data regarding the probable susceptibility or resistance of these species to CWD.
Consider the implementation of similar surveillance programmes to the one proposed here in other MS not explicitly considered in this opinion.
Abbreviations
- AT
Austria
- BIOHAZ
EFSA Panel on Biological Hazards
- BSE
bovine spongiform encephalopathy
- CDC
Centers for Disease Control and Prevention
- CJD
Creutzfeldt–Jakob disease
- CKY
cheeky
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CWD
chronic wasting disease
- CZ
Czech Republic
- DE
Germany
- Defra
Department for Environment Food & Rural Affairs
- ECDC
European Centre for Disease Prevention and Control
- EEA
European Economic Area
- ELISA
enzyme‐linked immunosorbent assay
- ENS
enteric nervous system
- EURL
European Reference Laboratory
- FI
Finland
- FR
France
- FSE
feline spongiform encephalopathy
- FS
fallen stock
- GALT
gut‐associated lymphoid tissue
- GB
Great Britain
- H‐BSE
H‐type atypical BSE
- HU
Hungary
- IE
Ireland
- IHC
immunohistochemistry
- IT
Italy
- L‐BSE
L‐type atypical BSE
- LRS
lymphoreticular system
- LV
Latvia
- MCF
malignant catarrhal fever
- MS
Member state
- MWS
moose Wasting Syndrome
- NINA
Norwegian Institute for Nature Research
- NO
Norway
- NORUT
Norwegian Research Institute
- NVI
Norwegian Veterinary Institute
- NWHC
National Wildlife Health Center
- OIE
International Organization of Animal Health
- PK
proteinase K
- PL
Poland
- PMCA
protein misfolding cyclic amplification
- PNS
peripheral nervous system
- PPE
personal protective equipment
- PRNP
gene encoding for the major prion protein PrP
- PrP
prion protein
- PrPC
cellular PrP
- PrPCWD
CWD‐associated prion protein
- PrPRes
protease‐resistant form of PrP, also used as synonymous of PrPSc
- PrPSc
abnormal form of PrP, also used as synonymous of PrPRes
- PSU
primary sampling units
- RA
risk assessment
- RAMALT
rectoanal mucosa‐associated lymphoid tissue
- RPLN
retropharyngeal lymph node
- rPrP
recombinant PrP
- RT‐QuIC
real‐time quaking‐induced conversion
- sCJD
sporadic Creutzfeldt–Jakob Disease
- SK
Slovakia
- SSC
Scientific Steering Committee of the European Commission
- SRM
specified risk material
- Tg
transgenic
- TME
transmissible mink encephalopathy
- ToR
Terms of Reference
- TSE
transmissible spongiform encephalopathies
- UK
United Kingdom
- USA
United States of America
- USGS
United States Geological Survey
- vCJD
variant Creutzfeldt–Jakob disease
- VKM
Norwegian Scientific Committee for Food Safety (Vitenskapskomiteen for Mattrygghet)
- WB
western blot
- WG
Working Group
- WST
wasting
Appendix A – Additional information provided in the mandate
A.1. Current measures
The main provisions in the TSE Regulation currently applicable to CWD based on the preceding scientific opinions can be summarised as follows:
passive surveillance is mandatory also for cervids, as ‘any animal suspected of being infected by a TSE shall be either placed under an official movement restriction until the results of a clinical and epidemiological examination carried out by the competent authority are known, or killed for laboratory examination under official control’ (Article 12(1) of Regulation (EC) No 999/2001);
on a voluntary basis, the Member States may carry out additional TSE surveillance in cervids (Part III of Chapter A of Annex III to Regulation (EC) No 999/2001);
all parts of the body of a cervid positive for TSE must be sent to disposal as category 1 materials in accordance with the Animal By‐Products Regulation 10 (Article 13.1.(a) of Regulation (EC) No 999/2001);
TSE‐positive cases in cervids must be notified to the Commission and the Member States (Article 11 of Regulation (EC) No 999/2001);
in the EU, the feeding to cervids of proteins derived from animals is prohibited, with the exception of milk and milk products, eggs and egg products, hydrolysed proteins from non‐ruminants or from ruminant hides and skins, gelatine and collagen from non‐ruminants (Article 7 and Annex IV to Regulation (EC) No 999/2001);
-
at import into the EU, an attestation is required for meat and meat products from wild and farmed cervids coming from the USA or Canada (Chapter F of Annex IX to Regulation (EC) No 999/2001), confirming that the products:
-
1
— exclude the offal and spinal cord,
-
2
— are derived from animals tested for CWD with negative results, and
-
3
— are derived from animals which do not come from a herd (for farmed animals) or a region (for wild animals) where CWD has been confirmed or officially suspected.
-
1
In addition, in accordance with Regulation (EU) No 206/2010, the import into the EU of live cervids from the USA and Canada is prohibited.
The conditions for imports into the EU of certain animal by‐products derived from cervid materials can be summarised as follows:
The import of unprocessed urine for hunting lures is prohibited when derived from farmed cervids. The import of processed urine from farmed animals is subject to treatment requirements laid down in the ABP Regulations. The import of urine from wild cervids is out of the scope of the EU ABP Regulations.
The import of pet food containing cervid materials and of products derived from cervids (including PAP) and destined for the manufacturing of pet food is permitted provided that the requirements of the ABP Regulations are met. Raw materials must be derived from cervids slaughtered for human consumption.
-
For hides and skins, blood and blood products, animal by‐products intended for technical uses, rendered fats, gelatine and collagen, hydrolysed protein, di‐ and tricalcium phosphate, fat derivatives, the principle followed in the ABP Regulations can be summarised as follows:
-
1
— For raw products: imports are permitted only from third countries that are authorised for the import of fresh meat of cervids;
-
2
— For processed products derived from cervids: imports are permitted from all third countries listed in the Part I of Annex II to Regulation (EC) No 206/2010;
-
3
— For fully processed game trophies or hides and skins: imports are permitted from any third countries.
-
1
A.2. Import data
During the last 10 years, no meat of cervids was imported in the EU or EEA countries from the USA. Import data from Canada are in Table A.1 (extracted from TRACES).
Table A.1.
Cervid meat imported from Canada into the EU
| Year | Importing country | Amount (Kg) |
|---|---|---|
| 2006 | – | 0 |
| 2007 | FR | 368 |
| CH | 2,732 | |
| 2008 | FR | 17,634 |
| DE | 75 | |
| 2009 | FR | 2,815 |
| DE | 175 | |
| CH | 46,198 | |
| 2010 | FR | 693 |
| CH | 48,978 | |
| 2011 | FR | 1,351 |
| DE | 40 | |
| CH | 42,878 | |
| 2012 | FR | 1,357 |
| CH | 1,069 | |
| 2013 | BE | 2,613 |
| FR | 2,207 | |
| CH | 3,363 | |
| 2014 | BE | 334 |
| FR | 801 | |
| 2015 | FR | 1,261 |
| Total | BE | 2,947 |
| FR | 28,488 | |
| DE | 290 | |
| CH | 145,218 | |
| All | 176,942 |
BE: Belgium; DE: Germany; CH: Switzerland; FR: France.
The following information has been providing on surveillance after 2010:
Table A.2.
Number of species of cervids tested in Finland for the period 2011–2015
| Husbandry type | Species | Surveillance target group | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|---|---|
| Farmed reindeer | Rangifer tarandus tarandus | Slaughtered | – | – | – | – | – |
| Fallen stock | 2 | 1 | 4 | 13 | 3 | ||
| Forest reindeer | Rangifer tarandus fennicus | Found deada | – | – | – | – | – |
| Moose | Alces alces | Hunted | – | – | – | – | – |
| Found dead | 4 | 9 | 3 | 3 | 6 | ||
| White‐tailed deer | Odocoileus virginianus | Found dead | 1 | 2 | 5 | 3 | 4 |
| Hunted | – | – | – | – | – | ||
| Roe deer | Capreolus capreolus | Found dead | 1 | 2 | 2 | 2 | – |
| Slaughtered | – | – | – | – | – | ||
| Fallow deer | Dama dama | Found dead | – | – | – | 1 | 1 |
| Red deer | Cervus elaphus | Slaughtered | 1 | – | – | – | – |
| TOTAL | 9 | 14 | 14 | 22 | 14 |
Found dead = sick, road kill or found dead.
Table A.3.
Number of species of cervids tested in Denmark for the period 2011–2015
| Year | Number of cervids |
|---|---|
| 2011 | 3 |
| 2012 | 2 |
| 2013 | 0 |
| 2014 | 46 |
| 2015 | 25 |
Poland, the Netherland, Lithuania, the United Kingdom Portugal: no TSE test in cervids in the period 2011–2015.
Table A.4.
Number of species of cervids tested in Norway for the period 2010–2015
| Year | Farmed deer | Wild deer | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fallow deer | Red deer | Reindeer | Moose | Red deer | Musk | Reindeer | Roe deer | ||
| 2010 | – | 2 | – | 13 | 3 | 4 | 2 | 17 | 41 |
| 2011 | – | 11 | 1 | 11 | 2 | – | 1 | 12 | 38 |
| 2012 | 3 | 6 | – | 5 | 4 | – | – | 3 | 21 |
| 2013 | 1 | 4 | – | 1 | – | – | – | 4 | 10 |
| 2014 | – | 2 | – | 5 | 2 | – | – | 1 | 10 |
| 2015 | – | 3 | – | 4 | 1 | – | – | 8 | 19 |
Table A.5.
Number of species of cervids tested in Latvia for the period 2010–2015
| Year | Number of cervids |
|---|---|
| 2011 | – |
| 2012 | – |
| 2013 | 2 (Alces alces) |
| 2014 | – |
| 2015 | 2 |
Sweden: one clinical suspicion in a reindeer in the period 2011–2015.
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernández Escámez PS, Gironés R, Herman L, Koutsoumanis K, Lindqvist R, Nørrung B, Robertson L, Sanaa M, Skandamis P, Snary E, Speybroeck N, Kuile BT, Threlfall J, Wahlström H, Benestad S, Gavier‐Widen D, Miller MW, Ru G, Telling GC, Tryland M, Ortiz Pelaez A and Simmons M, 2017. Scientific opinion on chronic wasting disease (CWD) in cervids. EFSA Journal 2017;15(1):4667, 62 pp. doi: 10.2903/j.efsa.2017.4667
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00387
Panel members: Antonia Ricci, Ana Allende, Declan Bolton, Marianne Chemaly, Robert Davies, Pablo Salvador Fernández Escámez, Rosina Gironés, Lieve Herman, Kostas Koutsoumanis, Roland Lindqvist, Birgit Nørrung, Lucy Robertson, Moez Sanaa, Panagiotis Skandamis, Emma Snary, Niko Speybroeck, Benno Ter Kuile, Giuseppe Ru, Marion Simmons, John Threlfall and Helene Wahlström.
Acknowledgements: The Panel wishes to thank the members of the Working Group on a request for a scientific opinion on Chronic Wasting Disease (CWD) in cervids: Sylvie Benestad, Dolores Gavier‐Widen, Michael W Miller, Giuseppe Ru, Marion Simmons, Glenn C Telling and Morten Tryland for the preparatory work on this scientific opinion, and Angel Ortiz Pelaez for the support provided to this scientific opinion. The Panel wishes to acknowledge all organisations and individuals that provided data for this scientific opinion.
Amendment: The following changes were made: (i) the following sentence was added to p. 22: “However, Tg mice expressing ovine PrP challenged with CWD have resulted in highly efficient, life‐long asymptomatic replication of these prions in the spleen tissue, with brain involvement at late stage (Béringue et al., 2012)”; (ii) the reference to Béringue et al., 2012 was added to the Reference list and (iii) wapiti was added to the top of p.21. These changes do not materially affect the contents or outcome of this scientific output. To avoid confusion, the older version has been removed from the EFSA Journal, but is available on request, as is a version showing all the changes made.
Adopted: 2 December 2016
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © USGS; Figure 2: © Norwegian Institute for Nature Research; Figures 3 and 7: © Bernt Johansen (NORUT); Figure 4: © Statistics Norway; Figure 5: © Sim1; Figure 6: © Royal Swedish Academy of Sciences
Corrected: 9 February 2017
Notes
Commission Decision of 19 March 2007 on a survey for chronic wasting disease in cervids. OJ L 84/37 24.3.2007.
Regulation (EC) No 999/2001 of the European Parliament and of the Council of 22 May 2001 laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. OJ L 147 31.5.2001, p. 1.
Commission Implementing Decision (EU) 2016/1918 of 28 October 2016 concerning certain safeguard measures in relation to chronic wasting disease. OJ L 296. 1.11.2016, p. 21–24.
Nordic region comprising Finland, Norway and Sweden in their entireties as well as Murmansk Oblast, much of the Republic of Karelia and parts of northern Leningrad Oblast in Russia (http://www.wikipedia.org)
References
- Anderson CA, Bosque P, Filley CM, Arciniegas DB, Kleinschmidt‐DeMasters BK, Pape WJ and Tyler KL, 2007. Colorado surveillance program for chronic wasting disease transmission to humans. Lessons from 2 highly suspicious but negative cases. Archives of Neurology, 64, 439–441. [DOI] [PubMed] [Google Scholar]
- Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA and Telling GC, 2006. Prions in skeletal muscles of deer with chronic wasting disease. Science, 311, 1117. [DOI] [PubMed] [Google Scholar]
- Angers RC, Seward TS, Napier D, Green M, Hoover E, Spraker T, O'Rourke K, Balachandran A and Telling GC, 2009. Chronic wasting disease prions in elk antler velvet. Emerging Infectious Diseases, 15, 696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers RC, Kang HE, Napier D, Browning S, Seward T, Mathiason C, Balachandran A, McKenzie D, Castilla J, Soto C, Jewell J, Graham C, Hoover EA and Telling GC, 2010. Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science, 328, 1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers R, Christiansen J, Nalls AV, Kang HE, Hunter N, Hoover E, Mathiason CK, Sheetz M and Telling GC, 2014. Structural effects of PrP polymorphisms on intra‐ and interspecies prion transmission. Proceedings of the National Academy of Sciences of the United States, 111, 11169–11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous , 2016. Ressursregnskap for reindriftsnæringen (April 1, 2014 ‐ March 31, 2015). Landbruksdirekoratet, Report 14.
- Baeten LA, Powers BE, Jewell JE, Spraker TR and Miller MW, 2007. A natural case of chronic wasting disease in a free‐ranging moose (Alces alces shirasi). Journal of Wildlife Diseases, 43, 309–314. [DOI] [PubMed] [Google Scholar]
- Baron T, Vulin J, Biacabe A‐G, Lakhdar L, Verchere J, Torres J‐M and Bencsik A, 2011. Emergence of classical BSE strain properties during serial passages of H‐BSE in wild‐type mice. PLoS ONE, 6, e15839. doi: 10.1371/journal.pone.0015839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria MA, Telling GC, Gambetti P, Mastrianni JA and Soto C, 2011. Generation of a new form of human PrP(Sc) in vitro by interspecies transmission from cervid prions. Journal of Biological Chemistry, 286, 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria MA, Ironside JW and Head MW, 2014. Exploring the zoonotic potential of animal prion diseases: in vivo and in vitro approaches. Prion, 8, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JC, Marsh RF, McKenzie DI and Aiken JM, 1998. The host range of chronic wasting disease is altered on passage in ferrets. Virology, 251, 297–301. [DOI] [PubMed] [Google Scholar]
- Baskakov IV, 2014. The many shades of prion strain adaptation. Prion 8, 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay ED, Maddox RA, Williams ES, Miller MW, Gambetti P and Schonberger LB, 2004. Chronic wasting disease and potential transmission to humans. Emerging Infectious Diseases, 10, 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benestad SL, Sarradin P, Thu B, Schönheit J, Tranulis MA and Bratberg B, 2003. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet Rec. 153, 202–208. [DOI] [PubMed] [Google Scholar]
- Benestad SL, Mitchell G, Simmons M, Ytrehus B and Vikoren T, 2016. First case of chronic wasting disease in Europe in a Norwegian free‐ranging reindeer. Veterinary Research, 47, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béringue V, Herzog L, Jaumain E, Reine F, Sibille P, Le Dur A, Vilotte J‐L and Laude H, 2012. Facilitated Cross‐Species Transmission of Prions in Extraneural Tissue. Science 335.6067 (2012): 472–475. [DOI] [PubMed] [Google Scholar]
- Bessen RA and Marsh RF, 1994. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. Journal of Virology 68, 7859–7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen RA, Robinson CJ, Seelig DM, Watschke CP, Lowe D, Shearin H, Martinka S and Babcock AM, 2011. Transmission of chronic wasting disease identifies a prion strain causing cachexia and heart infection in hamsters. PLoS ONE, 6, e28026. doi: 10.1371/journal.pone.0028026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger T, Caley P, Merrill E, Messier F and Miller MW, 2004. Expert Scientific Panel on Chronic Wasting Disease. Chronic wasting disease in Canadian wildlife: an expert opinion on the epidemiology and risks to wild deer. Canadian Cooperative Wildlife Health Centre, 2004.
- Bölske G, Wahlström H, Larsson B and Robertsson JÅ, 2006. A tuberculosis outbreak in farmed deer in Sweden and its economic consequences In: Thoen CO, Steele JH, Gilsdorf MJ. (eds.). Mycobacterium Bovis Infection in Animals and Humans. 2nd Edition, Blackwell Publishing, Oxford, UK: pp. 84–88. [Google Scholar]
- Bratberg B, Ueland K and Wells GA, 1995. Feline spongiform encephalopathy in a cat in Norway. Vet Rec. 136, 444. [DOI] [PubMed] [Google Scholar]
- Brayton KA, O'Rourke KI, Lyda AK, Miller MW and Knowles DP, 2004. A processed pseudogene contributes to apparent mule deer prion gene heterogeneity. Gene 326, 167–173. [DOI] [PubMed] [Google Scholar]
- Brown RD, Decker DJ, Major JT, Curtis PD, Shanahan JE and Siemer WF, 2006. Hunters’ and Other Citizens’ Reactions to Discovery of CWD in Central New York. Human Dimensions of Wildlife 11, 203–214. [Google Scholar]
- Browning SR, Mason GL, Seward T, Green M, Eliason GAJ, Mathiason C, Miller MW, Williams ES, Hoover E and Telling GC, 2004. Transmission of prions from mule deer and Elk with chronic wasting disease to transgenic mice expressing cervid PrP. Journal of Virology, 78, 13345–13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H and Bostock CJ, 1997. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature, 389, 498–501. [DOI] [PubMed] [Google Scholar]
- Casalone C, Zanusso G, Acutis P, Ferrari S, Capucci L, Tagliavini F, Monaco S and Caramelli M, 2004. Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeld‐Jakob disease. Proceedings of the National Academy of Sciences of the United States, 101, 3065–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis J, Moudjou M, Reine F, Herzog L, Jaumain E, Chapuis C, Quadrio I, Boulliat J, Perret‐Liaudet A, Dron M, Laude H, Rezaei H and Béringue V, 2016. Emergence of two prion subtypes in ovine PrP transgenic mice infected with human MM2‐cortical Creutzfeldt‐Jakob disease prions. Acta Neuropathologica Communications, 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen B, Hornemann S, Damberger FF and Wuthrich K, 2009. Prion protein NMR structure from tammar wallaby (Macropus eugenii) shows that the beta2‐alpha2 loop is modulated by long‐range sequence effects. Journal of Molecular Biology, 389, 833–845. [DOI] [PubMed] [Google Scholar]
- Collinge J and Clarke AR, 2007. A general model of prion strains and their pathogenicity. Science, 318, 930–936. [DOI] [PubMed] [Google Scholar]
- Comoy EE, Mikol J, Luccantoni‐Freire S, Correia E, Lescoutra‐Etchegaray N, Durand V, Dehen C, Andreoletti O, Casalone C, Richt JA, Greenlee JJ, Baron T, Benestad SL, Brown P and Deslys JP, 2015. Transmission of scrapie prions to primate after an extended silent incubation period. Scientific Reports, 5, 11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner MM, McCarty CW and Miller MW, 2000. Detection of bias in harvest‐based estimates of chronic wasting disease prevalence in mule deer. Journal of Wildlife Diseases, 36, 691–699. [DOI] [PubMed] [Google Scholar]
- Crowell J, Hughson A, Caughey B and Bessen RA, 2015. Host determinants of prion strain diversity independent of prion protein genotype. Journal of Virology 89, 10427–10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagleish MP, Martin S, Steele P, Finlayson J, Eaton SL, Siso S, Stewart P, Fernandez‐Borges N, Hamilton S, Pang Y, Chianini F, Reid HW, Goldmann W, Gonzalez L, Castilla J and Jeffrey M, 2015. Susceptibility of European red deer (Cervus elaphus elaphus) to alimentary challenge with bovine spongiform encephalopathy. PLoS ONE, 10, e0116094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daus ML, Breyer J, Wagenfuehr K, Wemheuer WM, Thomzig A, Schulz‐Schaeffer WJ and Beekes M, 2011. Presence and seeding activity of pathological prion protein (PrP TSE) in skeletal muscles of white‐tailed deer infected with chronic wasting disease. PLoS ONE, 6, e18345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport KA, Henderson DM, Bian J, Telling GC, Mathiason CK and Hoover EA, 2015. Insights into chronic wasting disease and bovine spongiform encephalopathy species barriers by use of real‐time conversion. Journal of Virology, 89, 9524–9531. doi: 10.1128/JVI.01439-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defra , 2012. What is the risk of chronic wasting disease being introduced into Great Britain? A Qualitative Risk Assessment. October 2012. Available online: http://webarchive.nationalarchives.gov.uk/20140507133914/http:/www.defra.gov.uk/animal-diseases/files/qra_chronic-wasting-disease-121029.pdf
- Defra , 2016. What is the risk of a cervid TSE being introduced from Norway into Great Britain? Qualitative Risk Assessment. September 2016. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/557205/qra-tse-reindeer-norway.pdf
- Di Bari MA, Nonno R, Castilla J, D'Agostino C, Pirisinu L, Riccardi G, Conte M, Richt J, Kunkle R, Langeveld J, Vaccari G and Agrimi U, 2013. Chronic wasting disease in bank voles: characterisation of the shortest incubation time model for prion diseases. PLoS Pathogens, 9, e1003219. doi: 10.1371/journal.ppat.1003219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé C, Mehren KG, Barker IK, Peart BL and Balachandran A, 2006. Retrospective investigation of chronic wasting disease of cervids at the Toronto Zoo, 1973–2003. The Canadian Veterinary Journal, 47, 1185–1193. [PMC free article] [PubMed] [Google Scholar]
- Duque Velasquez C, Kim C, Herbst A, Daude N, Garza MC, Wille H, Aiken J and McKenzie D, 2015. Deer prion proteins modulate the emergence and adaptation of chronic wasting disease strains. Journal of Virology, 89, 12362–12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Opinion of the European Food Safety Authority on a surveillance programme for Chronic Wasting Disease in the European Union. EFSA Journal 2004;2(7):70, 32 pp. doi: 10.2903/j.efsa.2004.70 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010. Scientific Opinion on the results of the EU survey for Chronic Wasting Disease (CWD) in cervids. EFSA Journal 2010;8(10):1861, 29 pp. doi: 10.2903/j.efsa.2010.1861 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011. Joint Scientific Opinion on any possible epidemiological or molecular association between TSEs in animals and humans. EFSA Journal 2011;9(1):1945, 111 pp. doi: 10.2903/j.efsa.2011.1945 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2015. Scientific Opinion on a request for a review of a scientific publication concerning the zoonotic potential of ovine scrapie prions. EFSA Journal 2015;13(8):4197, 58 pp. doi: 10.2903/j.efsa.2015.4197 [DOI] [Google Scholar]
- Elder AM, Henderson DM, Nalls AV, Wilham JM, Caughey BW, Hoover EA, Kincaid AE, Bartz JC and Mathiason CK, 2013. In vitro detection of prionemia in TSE‐infected cervids and hamsters. PLoS ONE, 8, e80203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JC, Herva ME, Andréoletti O, Padilla D, Lacroux C, Cassard H, Lantier I, Castilla J and Torres J‐M, 2009. Transgenic mice expressing porcine prion protein resistant to classical scrapie but susceptible to sheep bovine spongiform encephalopathy and atypical scrapie. Emerging Infectious Diseases, 15, 1214–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fediaevsky A, Tongue SC, Noremark M, Calavas D, Ru G and Hopp P, 2008. A descriptive study of the prevalence of atypical and classical scrapie in sheep in 20 European countries. BMC Veterinary Research, 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fediaevsky A, Maurella C, Noremark M, Ingravalle F, Thorgeirsdottir S, Orge L, Poizat R, Hautaniemi M, Liam B, Calavas D, Ru G and Hopp P, 2010. The prevalence of atypical scrapie in sheep from positive flocks is not higher than in the general sheep population in 11 European countries. BMC Veterinary Research, 6, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KA, Jewell JE, Williams ES and Miller MW, 2006. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). The Journal of general virology, 87(Pt. 11), 3451–3461. [DOI] [PubMed] [Google Scholar]
- Frank A, 1998. `Mysterious’ moose disease in Sweden. Similarities to copper deficiency and/or molybdenosis in cattle and sheep. Biochemical background of clinical signs and organ lesions. Science of The Total Environment 209, 17–26. [DOI] [PubMed] [Google Scholar]
- Frank A, 2004. A review of the “Mysterious” wasting disease in Swedish moose (Alces alces L.) related to molybdenosis and disturbances in copper metabolism. Biological Trace Element Research, 102, 143–159. [DOI] [PubMed] [Google Scholar]
- Geremia C, Hoeting JA, Wolfe LL, Galloway NL, Antolin MF, Spraker TR, Miller MW and Hobbs NT, 2015. Age and repeated biopsy influence antemortem prpcwd testing in mule deer (Odocoileus hemionus) in Colorado, USA. Journal of Wildlife Diseases 51, 801–810. doi: 10.7589/2014-12-284 [DOI] [PubMed] [Google Scholar]
- Gossert AD, Bonjour S, Lysek DA, Fiorito F and Wuthrich K, 2005. Prion protein NMR structures of elk and of mouse/elk hybrids. Proceedings of the National Academy of Sciences of the United States America, 102, 646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough KC and Maddison BC, 2010. Prion transmission. Prion, 4, 275–282. doi: 10.4161/pri.4.4.13678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KM, Browning SR, Seward TS, Jewell JE, Ross DL, Green MA, Williams ES, Hoover EA and Telling GC, 2008. The elk PRNP codon 132 polymorphism controls cervid and scrapie prion propagation. The Journal of general virology, 89(Pt 2), 598–608. [DOI] [PubMed] [Google Scholar]
- Greenlee JJ, Smith JD and Kunkle RA, 2011. White‐tailed deer are susceptible to the agent of sheep scrapie by intracerebral inoculation. Veterinary Research, 42, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guberti V, Stancampiano L and Ferrari N, 2014. Surveillance, monitoring and surveys of wildlife diseases: a public health and conservation approach. Hystrix, the Italian Journal of Mammalogy 25, 3–8. [Google Scholar]
- Habib TJ, Merrill EH, Pybus MJ and Coltman DW, 2011. Modelling landscape effects on density–contact rate relationships of deer in eastern Alberta: implications for chronic wasting disease. Ecological Modelling, 222, 2722–2732. [Google Scholar]
- Haley NJ, Siepker C, Walter WD, Thomsen BV, Greenlee JJ, Lehmkuhl AD and Richt JA, 2016. Antemortem detection of chronic wasting disease prions in nasal brush collections and rectal biopsy specimens from white‐tailed deer by real‐time quaking‐induced conversion. J Clin Microbiol, 54, 1108–1116, doi: 10.1128/JCM.02699-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley NJ and Hoover EA, 2015. Chronic wasting disease of cervids: current knowledge and future perspectives. Annual Review of Animal Biosciences, 3, 305–325. [DOI] [PubMed] [Google Scholar]
- Haley NJ, Seelig DM, Zabel MD, Telling GC and Hoover EA, 2009a. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS ONE, 4, e4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley NJ, Mathiason CK, Zabel MD, Telling GC and Hoover EA, 2009b. Detection of sub‐clinical CWD infection in conventional test‐negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS ONE, 4, e7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamir AN, Cutlip RC, Miller JM, Williams ES, Stack MJ, Miller MW, O'Rourke KI and Chaplin MJ, 2001. Preliminary findings on the experimental transmission of chronic wasting disease agent of mule deer to cattle. Journal of Veterinary Diagnostic Investigation 13, 91–96. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Miller JM, Cutlip RC, Stack MJ, Chaplin MJ and Jenny AL, 2003. Preliminary observations on the experimental transmission of scrapie to elk (Cervus elaphus nelsoni) by intracerebral inoculation. Veterinary Pathology, 40, 81–85. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Miller JM, Cutlip RC, Kunkle RA, Jenny AL, Stack MJ and Chaplin MJ, 2004. Transmission of sheep scrapie to elk (Cervus Elaphus Nelsoni) by intracerebral inoculation: final outcome of the experiment. Journal of Veterinary Diagnostic Investigation, 16, 316–321. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Kunkle RA, Cutlip RC, Miller JM, O'Rourke KI, Williams ES, Miller MW, Stack MJ, Chaplin MJ and Richt JA, 2005. Experimental transmission of chronic wasting disease agent from mule deer to cattle by the intracerebral route. Journal of Veterinary Diagnostic Investigation, 2005, 276–281. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Gidlewski T, Spraker TR, Miller JM, Creekmore L, Crocheck M, Cline T and O'Rourke KI, 2006a. Preliminary observations of genetic susceptibility of elk (Cervus elaphus nelsoni) to chronic wasting disease by experimental oral inoculation. Journal of Veterinary Diagnostic Investigation, 18, 110–114. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Kunkle RA, Cutlip RC, Miller JM, Williams ES and Richt JA, 2006b. Transmission of chronic wasting disease of mule deer to Suffolk sheep following intracerebral inoculation. Journal of Veterinary Diagnostic Investigation, 18, 558–565. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Miller JM, Kunkle RA, Hall SM and Richt JA, 2007. Susceptibility of cattle to first‐passage intracerebral inoculation with chronic wasting disease agent from white‐tailed deer. Veterinary Pathology, 44, 487–493. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Richt JA, Miller JM, Kunkle RA, Hall SM, Nicholson EM, O'Rourke KI, Greenlee JJ and Williams ES, 2008. Experimental transmission of chronic wasting disease (CWD) of Elk (Cervus elaphus nelsoni), white‐tailed deer (Odocoileus virginianus), and mule deer (Odocoileus hemionus hemionus) to white‐tailed deer by intracerebral route. Veterinary Pathology 45, 297–306. [DOI] [PubMed] [Google Scholar]
- Happ GM, Huson HJ, Beckmen KB and Kennedy LJ, 2007. Prion protein genes in caribou from Alaska. Journal of Wildlife Diseases, 43, 224–228. [DOI] [PubMed] [Google Scholar]
- Heaton MP, Leymaster KA, Freking BA, Hawk DA, Smith TPL, Keele JW, Snelling WM, Fox JM, Chitko‐McKown CG and Laegreid WW, 2003. Prion gene sequence variation within diverse groups of U.S. sheep, beef cattle, and deer. Mammalian Genome, 14, 765–777. [DOI] [PubMed] [Google Scholar]
- Heisey DM, Mickelsen NA, Schneider JR, Johnson CJ, Johnson CJ, Langenberg JA, Bochsler PN, Keane DP and Barr DJ, 2010. Chronic wasting disease (CWD) susceptibility of several North American rodents that are sympatric with cervid CWD epidemics. Journal of Virology, 84, 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DM, Manca M, Haley NJ, Denkers ND, Nalls AV, Mathiason CK, Caughey B and Hoover EA, 2013. Rapid antemortem detection of CWD prions in deer saliva. PLoS ONE, 8, e74377. doi: 10.1371/journal.pone.0074377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DM, Denkers ND, Hoover CE, Garbino N, Mathiason CK and Hoover EA, 2015. Longitudinal detection of prion shedding in saliva and urine by chronic wasting disease‐infected deer by real‐time quaking‐induced conversion. Journal of Virology, 89, 9338–9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AB, 1965. The environment and disease: association or causation? Proceedings of the Royal society of Medicine, 58, 295. [PMC free article] [PubMed] [Google Scholar]
- Hill AF, Desbruslais M, Joiner S, Sidle KCL, Gowland I, Collinge J, Doey LJ and Lantos P, 1997. The same prion strain causes vCJD and BSE. Nature, 389, 448–450. [DOI] [PubMed] [Google Scholar]
- Huson HJ and Happ GM, 2006. Polymorphisms of the prion protein gene (PRNP) in Alaskan moose (Alces alces gigas). Animal Genetics, 37, 425–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HJ, Lee JB, Park SY, Song CS, Kim BS, Rho JR, Yoo MH, Jeong BH, Kim YS and Choi IS, 2007. Identification of single‐nucleotide polymorphisms of the prion protein gene in sika deer (Cervus nippon laiouanus). Journal of veterinary science. 8, 299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HJ, Park SY, Kim BS, Yoo MH and Kim YS, 2009. Single‐nucleotide polymorphisms in prion protein gene of the Korean subspecies of Chinese water deer‐Hydropotes inermis argyropus. 대한수의학회지. 49, 59–62. [Google Scholar]
- Jewell JE, Conner MM, Wolfe LL, Miller MW and Williams ES, 2005. Low frequency of PrP genotype 225SF among free‐ranging mule deer (Odocoileus hemionus) with chronic wasting disease. The Journal of general virology, 86(Pt 8), 2127–2134. [DOI] [PubMed] [Google Scholar]
- John TR, Schatzl HM and Gilch S, 2013. Early detection of chronic wasting disease prions in urine of pre‐symptomatic deer by real‐time quaking‐induced conversion assay. Prion, 7, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Johnson J, Clayton M, McKenzie D and Aiken J, 2003. Prion protein gene heterogeneity in free‐ranging white‐tailed deer within the chronic wasting disease affected region of Wisconsin. Journal of Wildlife Diseases, 39, 576–581. [DOI] [PubMed] [Google Scholar]
- Johnson C, Johnson J, Vanderloo JP, Keane D, Aiken JM and McKenzie D, 2006. Prion protein polymorphisms in white‐tailed deer influence susceptibility to chronic wasting disease. Journal of General Virology 87, 2109–2114. [DOI] [PubMed] [Google Scholar]
- Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D and Aiken JM, 2007. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathogens, 3, e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CJ, Herbst A, Duque‐Velasquez C, Vanderloo JP, Bochsler P, Chappell R and McKenzie D, 2011. Prion protein polymorphisms affect chronic wasting disease progression. PLoS ONE, 6, e17450. doi: 10.1371/journal.pone.0017450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly DO, Ribic CA, Langenberg JA, Beheler K, Batha CA, Dhuey BJ, Rolley RE, Bartelt G, Van Deelen TR and Samuel MD, 2003. Chronic wasting disease in free‐ranging Wisconsin White‐tailed Deer. Emerging Infectious Diseases, 9, 599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly DO, Samuel MD, Langenberg JA, Blanchong JA, Batha CA, Rolley RE, Keane RD and Ribic CA, 2006. Spatial epidemiology of chronic wasting disease in Wisconsin white‐tailed deer. Journal of Wildlife Diseases, 42, 578–588. [DOI] [PubMed] [Google Scholar]
- Keane DP, Barr DJ, Bochsler PN, Hall SM, Gidlewski T, O'Rourke KI, Spraker TR and Samuel MD, 2008a. Chronic wasting disease in a Wisconsin white‐tailed deer farm. Journal of Veterinary Diagnostic Investigation, 20, 698–703. [DOI] [PubMed] [Google Scholar]
- Keane DP, Barr DJ, Keller JE, Hall SM, Langenberg JA and Bochsler PN, 2008b. Comparison of retropharyngeal lymph node and obex region of the brainstem in detection of chronic wasting disease in white‐tailed deer (Odocoileus virginianus). Journal of Veterinary Diagnostic Investigation, 20, 58–60. [DOI] [PubMed] [Google Scholar]
- Kekkonen J, Wikström M, Ala‐Ajos L, Lappalainen V and Brommer JE, 2016. Growth and age structure in an introduced and hunted cervid population: white‐tailed deer in Finland. Annales Zoologici Fennici, 53, 69–80. [Google Scholar]
- Kelly AC, Mateus‐Pinilla NE, Diffendorfer J, Jewell E, Ruiz MO, Killefer J, Shelton P, Beissel T and Novakofski J, 2008. Prion sequence polymorphisms and chronic wasting disease resistance in Illinois white‐tailed deer (Odocoileus virginianus). Prion, 2, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TY, Shon HJ, Joo YS, Mun UK, Kang KS and Lee YS, 2005. Additional cases of chronic wasting disease in imported deer in Korea. Journal of Veterinary Medical Science, 67, 753–759. [DOI] [PubMed] [Google Scholar]
- Kong QZ, Huang SH, Zou WQ, Vanegas D, Wang ML, Wu D, Yuan J, Zheng MJ, Bai H, Deng HY, Chen K, Jenny AL, O'Rourke K, Belay ED, Schonberger LB, Petersen RB, Sy MS, Chen SG and Gambetti P, 2005. Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. Journal of Neuroscience, 25, 7944–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutnik DL, 1981. Sex‐related differences in the seasonality of agonistic behavior in mule deer. Journal of Mammalogy, 62, 1–11. [Google Scholar]
- Kreeger TJ, Montgomery DL, Jewell JE, Schultz W and Williams ES, 2006. Oral transmission of chronic wasting disease in captive Shira's moose. Journal of Wildlife Diseases, 42, 640–645. [DOI] [PubMed] [Google Scholar]
- Krumm CE, Conner MM and Miller MW, 2005. Relative vulnerability of chronic wasting disease infected mule deer to vehicle collisions. Journal of Wildlife Diseases, 41, 503–511. [DOI] [PubMed] [Google Scholar]
- Krumm CE, Conner MM, Hobbs NT, Hunter DO and Miller MW, 2009. Mountain lions prey selectively on prion‐infected mule deer. Biology Letters, rsbl20090742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt TD, Perrott MR, Wilusz CJ, Wilusz J, Supattapone S, Telling GC, Zabel MD and Hoover EA, 2007. Efficient in vitro amplification of chronic wasting disease PrPRES . Journal of Virology 81, 9605–9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt TD, Telling GC, Zabel MD and Hoover EA, 2009. Trans‐species amplification of PrP(CWD) and correlation with rigid loop 170N. Virology, 387, 235–243. [DOI] [PubMed] [Google Scholar]
- Kurt TD, Bett C, Fernandez‐Borges N, Joshi‐Barr S, Hornemann S, Rulicke T, Castilla J, Wuthrich K, Aguzzi A and Sigurdson CJ, 2014. Prion transmission prevented by modifying the beta2‐alpha2 loop structure of host PrPC. Journal of Neuroscience, 34, 1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt TD, Jiang L, Fernandez‐Borges N, Bett C, Liu J, Yang T, Spraker TR, Castilla J, Eisenberg D, Kong Q and Sigurdson CJ, 2015. Human prion protein sequence elements impede cross‐species chronic wasting disease transmission. Journal of Clinical Investigation, 125, 1485–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFauci G, Carp RI, Meeker HC, Ye X, Kim JI, Natelli M, Cedeno M, Petersen RB, Kascsak R and Rubenstein R, 2006. Passage of chronic wasting disease prion into transgenic mice expressing Rocky Mountain elk (Cervus elaphus nelsoni) PrPC. Journal of General Virology, 87, 3773–3780. [DOI] [PubMed] [Google Scholar]
- Lee Y‐H, Sohn H‐J, Kim M‐J, Kim H‐J, Park K‐J, Lee W‐Y, Yun E‐I, Tark D‐S, Choi Y‐P, Cho I‐S and Balachandran A, 2013. Experimental chronic wasting disease in wild type VM mice. Journal of Veterinary Medical Science 75, 1107–1110. [DOI] [PubMed] [Google Scholar]
- Liberski PP, Guiroy DC, Williams ES, Walis A and Budka H, 2001. Deposition patterns of disease‐associated prion protein in captive mule deer brains with chronic wasting disease. Acta neuropathologica, 102, 496–500. [DOI] [PubMed] [Google Scholar]
- Malmstem J, 2016. Re: cervids population estimates to EFSA? Message to Dolores Gavier‐Widen. 21 November 2016.
- Manjerovic MB, Green ML, Mateus‐Pinilla N and Novakofski J, 2014. The importance of localized culling in stabilizing chronic wasting disease prevalence in white‐tailed deer populations. Preventive Veterinary Medicine 113, 139–145. [DOI] [PubMed] [Google Scholar]
- Marsh RF, Kincaid AE, Bessen RA and Bartz JC, 2005. Interspecies transmission of chronic wasting disease prions to squirrel monkeys (Saimiri sciureus). Journal of Virology, 79, 13794–13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Jeffrey M, González L, Sisó S, Reid HW, Steele P, Dagleish MP, Stack MJ, Chaplin MJ and Balachandran A, 2009. Immunohistochemical and biochemical characteristics of BSE and CWD in experimentally infected European red deer (Cervus elaphus elaphus). BMC Veterinary Research, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes‐Klug J, Seelig DM, Wild MA, Wolfe LL, Spraker TR, Miller MW, Sigurdson CJ, Telling GC and Hoover EA, 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science, 314, 133–136. [DOI] [PubMed] [Google Scholar]
- Mathiason CK, Hays SA, Powers J, Hayes‐Klug J, Langenberg J, Dahmes SJ, Osborne DA, Miller KV, Warren RJ, Mason GL and Hoover EA, 2009. Infectious prions in pre‐clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS ONE, 4, e5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiason CK, Nalls AV, Seelig DM, Kraft SL, Carnes K, Anderson KR, Hayes‐Klug J and Hoover EA, 2013. Susceptibility of domestic cats to chronic wasting disease. Journal of Virology, 87, 1947–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawhinney S, Pape WJ, Forster JE, Anderson CA, Bosque P and Miller MW, 2006. Human prion disease and relative risk associated with chronic wasting disease. Emerging Infectious Diseases, 12, 1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade‐White K, Race B, Trifilo M, Bossers A, Favara C, Lacasse R, Miller M, Williams E, Oldstone M, Race R and Chesebro B, 2007. Resistance to chronic wasting disease in transgenic mice expressing a naturally occurring allelic variant of deer prion protein. Journal of virology, 81, 4533–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng LP, Zhao DM, Liu HX, Yang JM, Ning ZY, Wu CD and Han CX, 2005. Polymorphisms of the prion protein gene (PRNP) in Chinese domestic sika deer (Cervus nippon hortulorum). Animal Genetics, 36, 266–267. [DOI] [PubMed] [Google Scholar]
- Merza M, Larsson E, Steen M and Morein B, 1994. Association of a retrovirus with a wasting condition in the Swedish moose. Virology, 202, 956–961. [DOI] [PubMed] [Google Scholar]
- Meyerett C, Michel B, Pulford B, Spraker TR, Nichols TA, Johnson T, Kurt T, Hoover EA, Telling GC and Zabel MD, 2008. In vitro strain adaptation of CWD prions by serial protein misfolding cyclic amplification. Virology 382, 267–276. [DOI] [PubMed] [Google Scholar]
- Miller MW and Conner MM, 2005. Epidemiology of chronic wasting disease in free‐ranging mule deer: spatial, temporal, and demographic influences on observed prevalence patterns. Journal of Wildlife Diseases, 41, 275–290. [DOI] [PubMed] [Google Scholar]
- Miller MW and Fischer JR, 2016. The first five (or more) decades of chronic wasting disease: lessons for the five decades to come. Transactions of the North American Wildlife and Natural Resources Conference 81, (in press). [Google Scholar]
- Miller MW and Williams ES, 2003. Prion disease: horizontal prion transmission in mule deer. Nature, 425, 35–36. [DOI] [PubMed] [Google Scholar]
- Miller MW, Williams ES, McCarty CW, Spraker TR, Kreeger TJ, Larsen CT and Thorne ET, 2000. Epizootiology of chronic wasting disease in free‐ranging cervids in Colorado and Wyoming. Journal of Wildlife Diseases, 36, 676–690. [DOI] [PubMed] [Google Scholar]
- Miller M, Williams E, Hobbs N and Wolfe L, 2004. Environmental sources of prion transmission in mule deer. Emerging Infectious Diseases, 10, 1003–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Swanson HM, Wolfe LL, Quartarone FG, Huwer SL, Southwick CH and Lukacset PM, 2008. Lions and prions and deer demise. PLoS ONE, 3, e4019. doi: 10.1371/journal.pone.0004019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Wolfe LL, Sirochman TM, Sirochman MA, Jewell JE and Williams ES, 2012. Survival patterns in white‐tailed and mule deer after oral inoculation with a standardized, conspecific prion dose. Journal of Wildlife Diseases, 48, 526–529. [DOI] [PubMed] [Google Scholar]
- Minnesota Department of Natural Resources , 2016. Chronic wasting disease response plan. Available online: http://files.dnr.state.mn.us/wildlife/research/health/disease/cwd/cwd_responseplan.pdf
- Mitchell GB, Sigurdson CJ, O'Rourke KI, Algire J, Harrington NP, Walther I, Spraker TR and Balachandran A, 2012. Experimental oral transmission of chronic wasting disease to reindeer (Rangifer tarandus tarandus). PLoS ONE, 7, e39055. doi: 10.1371/journal.pone.0039055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monello RJ, Powers JG, Hobbs NT, Spraker TR, O'Rourke KI and Wild MA, 2013. Efficacy of antemortem rectal biopsies to diagnose and estimate prevalence of chronic wasting disease in free‐ranging cow elk (Cervus elaphus nelsoni). Journal of Wildlife Diseases, 49, 270–278. [DOI] [PubMed] [Google Scholar]
- Moore SJ, Kunkle R, Greenlee MHW, Nicholson E, Richt J, Hamir A, Waters WR and Greenlee J, 2016. Pathological features of chronic wasting disease in reindeer and demonstration of horizontal transmission. Journal: Emerging Infectious Diseases EID‐16‐0635.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison ML, Block WM, Strickland MD, Collier BA and Peterson MJ, 2008. Wildlife study design. Springer Science & Business Media; 2008 May 25. [Google Scholar]
- Mussil B, Motzkus D, Hesse G, Borchert S, Schiller B, Stahl‐Hennig C, Schulz‐Schaeffer W, Beekes M, Daus M, Schatzl HM and Dudas S, 2015. Transmission of CWD to non‐human primates: interim results of a 6 year risk assessment study on the transmissibility to humans. In Prion, 9, S87–S87. [Google Scholar]
- Nalls AV, McNulty E, Powers J, Seelig DM, Hoover C, Haley NJ, Hayes‐Klug J, Anderson K, Stewart P, Goldmann W, Hoover EA and Mathiason CK, 2013. Mother to offspring transmission of chronic wasting disease in reeves’ muntjac deer. PLoS ONE, 8, e71844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New York State Department of Environmental Conservation , 2013. “Surveillance plan for chronic wasting disease in New York State 2013–2014”. Cornell University Animal Health Diagnostic Center. August 2013. Available online: (http://www.dec.ny.gov/docs/wildlife_pdf/cwdsurplan13web.pdf) [Accessed: 18 November 2016].
- Nobert BR, Merrill EH, Pybus MJ, Bollinger TK and Hwang YT, 2016. Landscape connectivity predicts chronic wasting disease risk in Canada. Journal of Applied Ecology, 53, 1450–1459. [Google Scholar]
- Nusser SM, Clark WR, Otis DL and Huang L, 2008. Sampling considerations for disease surveillance in wildlife populations. The Journal of Wildlife Management, 72, 52–60. [Google Scholar]
- Oklahoma Department of Wildlife Conservation , 2002. Wildlife Department to recommend suspending import of deer and elk. Available online: http://www.wildlifedepartment.com/facts_maps/archives/2002Whitetailarticles.htm [Accessed: 5 December 2016].
- O'Rourke KI, Baszler TV, Miller JM, Spraker TR, Sadler‐Riggleman I and Knowles DP, 1998. Monoclonal antibody F89/160.1.5 defines a conserved epitope on the ruminant prion protein. Journal of Clinical Microbiology, 36, 1750–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke KI, Besser TE, Miller MW, Cline TF, Spraker TR, Jenny AL, Wild MA, Zebarth GL and Williams ES, 1999. PrP genotypes of captive and free‐ranging Rocky Mountain elk (Cervus elaphus nelsoni) with chronic wasting disease. The Journal of general virology, 80(Pt. 10), 2765–2769. [DOI] [PubMed] [Google Scholar]
- O'Rourke KI, Spraker TR, Hamburg LK, Besser TE, Brayton KA and Knowles DP, 2004. Polymorphisms in the prion precursor functional gene but not the pseudogene are associated with susceptibility to chronic wasting disease in white‐tailed deer. Journal of General Virology, 85, 1339–1346. [DOI] [PubMed] [Google Scholar]
- O'Rourke KI, Spraker TR, Zhuang D, Greenlee J, Gidlewski TE and Hamir AN, 2007. Elk with a long incubation prion disease phenotype have a unique PrPd profile. NeuroReport, 18, 1935–1938. [DOI] [PubMed] [Google Scholar]
- Ortiz‐Pelaez A, Arnold ME and Vidal‐Diez A, 2016. Epidemiological investigations on the potential transmissibility of a rare disease: the case of atypical scrapie in Great Britain. Epidemiology and Infection, 144, 2107–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape R and Löffler J, 2012. Climate change, land use conflicts, predation and ecological degradation as challenges for reindeer husbandry in Northern Europe: what do we really know after half a century of research? Ambio, 41, 421–434. doi: 10.1007/s13280-012-0257-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peletto S, Perucchini M, Acín C, Dalgleish MP, Reid HW, Rasero R, Sacchi P, Stewart P, Caramelli M, Ferroglio E, Bozzetta E, Meloni D, Orusa R, Robetto S, Gennero S, Goldmann W and Pier Luigi Acutis PL, 2009. Genetic variability of the prion protein gene (PRNP) in wild ruminants from Italy and Scotland. Journal of Veterinary Science 10, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz D, Williamson RA, Legname G, Matsunaga Y, Vergara J, Burton DR, DeArmond SJ, Prusiner SB and Scott MR, 2002. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 34, 921–932. [DOI] [PubMed] [Google Scholar]
- Perez DR, Damberger FF and Wuthrich K, 2010. Horse prion protein NMR structure and comparisons with related variants of the mouse prion protein. Journal of Molecular Biology, 400, 121–128. [DOI] [PubMed] [Google Scholar]
- Perucchini M, Griffin K, Miller MW and Goldmann W, 2008. PrP genotypes of free‐ranging wapiti (Cervus elaphus nelsoni) with chronic wasting disease. Journal of General Virology, 89, 1324–1328. [DOI] [PubMed] [Google Scholar]
- Prusiner SB, Scott M, Foster D, Pan K‐M, Groth D, Mirenda C, Torchia M, Yang S‐L, Serban D, Carlson GA, Hoppe PC, Westaway D and DeArmond SJ, 1990. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell Volume 63, 673–686. [DOI] [PubMed] [Google Scholar]
- Pulford B, Spraker TR, Wyckoff C, Meyerett C, Bender H, Ferguson A, Wyatt B, Lockwood K, Powers J, Telling GC, Wild MA and Zabel MD, 2012. Detection of PrPcwd in feces from naturally exposed rocky mountain elk (Cervus elaphus nelsoni) using protein misfolding cyclic amplification. Journal of Wildlife Diseases 48, 425–434. doi: 10.7589/0090-3558-48.2.425 [DOI] [PubMed] [Google Scholar]
- Pusenius J, 2016. Re: Please help with cervids populations data Finland for EFSA report. Message to Dolores Gavier‐Widen. 21 November 2016. E‐mail
- Race RE, Raines A, Baron TGM, Miller MW, Jenny A and Williams ES, 2002. Comparison of abnormal prion protein glycoform patterns from transmissible spongiform encephalopathy agent‐infected deer, elk, sheep, and cattle. Journal of virology, 76, 12365–12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race BL, Meade‐White KD, Ward A, Jewell J, Miller MW, Williams ES, Chesebro B and Race RE, 2007. Levels of abnormal prion protein in deer and elk with chronic wasting disease. Emerging Infectious Diseases, 13, 824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race B, Meade‐White KD, Miller MW, Barbian KD, Rubenstein R, LaFauci G, Cervenakova L, Favara C, Gardner D, Long D, Parnell M, Striebel J, Priola SA, Ward A, Williams ES, Race R and Chesebro B, 2009a. Susceptibilities of nonhuman primates to chronic wasting disease. Emerging Infectious Diseases, 15, 1366–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race B, Meade‐White K, Race R and Chesebro B, 2009b. Prion infectivity in fat of deer with chronic wasting disease. Journal of Virology 83, no. 18 9608‐9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race B, Meade‐White K, Miller MW, Fox KA and Chesebro B, 2011. In vivo comparison of chronic wasting disease infectivity from deer with variation at prion protein residue 96. Journal of virology, 85, 9235–9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race B, Meade‐White K, Phillips K, Striebel J, Race R and Chesebro B, 2014. Disease agents in nonhuman primates. Emerging Infectious Diseases, 20, 833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond GJ, Bossers A, Raymond LD, O'Rourke KI, McHolland LE, Bryant PK, Miller MW, Williams ES, Smits M and Caughey B, 2000. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO Journal, 19, 4425–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond GJ, Raymond LD, Meade‐White KD, Hughson AG, Favara C, Gardner D, Williams ES, Miller MW, Race RE and Caughey B, 2007. Transmission and adaptation of chronic wasting disease to hamsters and transgenic mice: evidence for strains. Journal of Virology, 81, 4305–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees EE, Merrill EH, Bollinger TK, Hwang YT, Pybus MJ and Coltman DW, 2012. Targeting the detection of chronic wasting disease using the hunter harvest during early phases of an outbreak in Saskatchewan, Canada. Preventive Veterinary Medicine 104, 149–159. [DOI] [PubMed] [Google Scholar]
- Rehbinder C, Gimeno E, Belak KS, Stéen M, Rivera E and Nikkilii T, 1991. A bovine viral ′diarrhoearmucosal disease‐like syndrome in moose (Alces alces L.): investigations on the central nervous system. The Veterinary Record 129, 552–554. [PubMed] [Google Scholar]
- Rhyan JC, Miller MW, Spraker TR, McCollum M, Nol P, Wolfe LL, Davis TR, Creekmore L and O'Rourke KI, 2011. Failure of fallow deer (Dama dama) to develop chronic wasting disease when exposed to a contaminated environment and infected mule deer (Odocoileus hemionus). Journal of Wildlife Diseases 47, 739–744. doi: 10.7589/0090-3558-47.3.739 [DOI] [PubMed] [Google Scholar]
- Robinson SJ, Samuel MD, O'Rourke KI and Johnson CJ, 2012. The role of genetics in chronic wasting disease of North American cervids. Prion, 6, 153–162. doi: 10.4161/pri.19640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman M (ed.), 2008. Animal Disease Surveillance and Survey Systems: Methods and Applications. John Wiley & Sons, Ames, Iowa: pp. 222. [Google Scholar]
- Samuel MD, Joly DO, Wild MA, Wright SD, Otis DL, Werge RW and Miller MW, 2003. Surveillance strategies for detecting chronic wasting disease in free‐ranging deer and Elk. Results of a CWD Surveillance Workshop Madison, Wisconsin December 10–12, 2002. Available online: http://www.nwhc.usgs.gov/publications/fact_sheets/pdfs/cwd/CWD_Surveillance_Strategies.pdf
- Sandberg MK, Al‐Doujaily H, Sigurdson CJ, Glatzel M, O'Malley C, Powell C, Asante EA, Linehan JM, Brandner S, Wadsworth JDF and Collinge J, 2010. Chronic wasting disease prions are not transmissible to transgenic mice overexpressing human prion protein. Journal of General Virology, 91, 2651–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders SE, Bartelt‐Hunt SL and Bartz JC, 2012. Occurrence, transmission, and zoonotic potential of chronic wasting disease. Emerging Infectious Diseases, 18, 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzl HM, Wopfner F, Gilch S, von Brunn A and Jager G, 1997. Is codon 129 of prion protein polymorphic in human beings but not in animals? Lancet, 349, 1603–1604. [DOI] [PubMed] [Google Scholar]
- Schettler E, Steinbach F, Eschenbacher‐Kaps I, Gerst K, Meussdoerffer F, Risch K, Streich WJ and Frölich K, 2006. Surveillance for prion disease in cervids, Germany. Emerging Infectious Diseases, 12, 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M, Groth D, Foster D, Torchia M, Yang S‐L, DeArmond SJ and Prusiner SB, 1993. Propagation of prions with artificial properties in transgenic mice expressing chimeric PrP genes. Cell Volume 73, 979–988. [DOI] [PubMed] [Google Scholar]
- Seelig DM, Nalls AV, Flasik M, Frank V, Eaton S, Mathiason CK and Hoover EA, 2015. Lesion profiling and subcellular prion localization of cervid chronic wasting disease in domestic cats. Veterinary Pathology, 52, 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selariu A, Powers JG, Nalls A, Brandhuber M, Mayfield A, Fullaway S, Wyckoff CA, Goldmann W, Zabel MM, Wild MA, Hoover EA and Mathiason CK, 2015. In utero transmission and tissue distribution of chronic wasting disease‐associated prions in free‐ranging Rocky Mountain elk. Journal General Virology, 96, 3444–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdson CJ, 2008. A prion disease of cervids: chronic wasting disease. Veterinary Research, 39, 1–12. [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O'Rourke KI and Hoover EA, 1999. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). The Journal of General Virology, 80(Pt 10), 2757–2764. [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Spraker TR, Miller MW, Oesch B and Hoover EA, 2001. PrP(CWD) in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. The Journal of General Virology, 82(Pt 10), 2327–2334. [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Barillas‐Mury C, Miller MW, Oesch B, van Keulen LJM, Langeveld JPM and Hoover EA, 2002. PrPCWD lymphoid cell targets in early and advanced chronic wasting disease of mule deer. Journal of General Virology, 83, 2617–2628. [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Manco G, Schwarz P, Liberski P, Hoover EA, Hornemann S, Polymenidou M, Miller MW, Glatzel M and Aguzzi A, 2006. Strain fidelity of chronic wasting disease upon murine adaptation. Journal of Virology 80, 12303–12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdson CJ, Nilsson KPR, Hornemann S, Heikenwalder M, Manco G, Schwarz P, Ott D, Rülicke T, Liberski PP, Julius C, Falsig J, Stitz L, Wüthrich K and Aguzzi A, 2009. De novo generation of a transmissible spongiform encephalopathy by mouse transgenesis. Proceedings of the National Academy of Sciences of the United States of America, 106, 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdson CJ, Nilsson KPR, 3 Hornemann S, Manco G, Fernández‐Borges N, Schwarz P, Castilla J, Wüthrich K and Aguzzi A, 2010. A molecular switch controls interspecies prion disease transmission in mice. The Journal of Clinical Investigation 120, 2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn HJ, Kim J‐H, Choi K‐S1, Nah J‐J, Joo Y‐S, Jean Y‐H, Ahn S‐W, Kim O‐K, Kim D‐Y and Balachandran A, 2002. A case of chronic wasting disease in an elk imported to Korea from Canada. Journal of Veterinary Medical Science 64, 855–858. [DOI] [PubMed] [Google Scholar]
- Solberg EJ, Veiberg V, Strand O, Andersen R, Langvatn R, Heim M, Rolandsen CM, Holmstrøm F and Solem MI, 2008. Wild cervides 2007 – Annual report from the National monitoring program for wild cervides in Norway. NINA Report 380. 65 pp. (population 2007).
- Sorensen A, van Beest FM and Brook RK, 2014. Impacts of wildlife baiting and supplemental feeding on infectious disease transmission risk: a synthesis of knowledge. Preventive Veterinary Medicine, 113, 356–363. [DOI] [PubMed] [Google Scholar]
- Spraker TR, Miller MW, Williams ES, Getzy DM, Adrian WJ, Schoonveld GG and Merz PA, 1997. Spongiform encephalopathy in free‐ranging mule deer (Odocoileus hemionus), white‐tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. Journal of Wildlife Diseases 33, 1–6. [DOI] [PubMed] [Google Scholar]
- SSC (Scientific Steering Committee), 2003. Opinion on Chronic Wasting Disease and tissues that might carry a risk for human and animal feed chains. MEETING OF 6 – 7 MARCH 2003. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_ssc_out323_en.pdf
- Storm DJ, Samuel MD, Rolley RE, Shelton P, Keuler NS, Richards BJ and Van Deelen TR, 2013. Deerdensity and disease prevalence influence transmission of chronic wasting disease in white‐tailed deer. Ecosphere, 4, 10. doi: 10.1890/ES12-00141.1 [DOI] [Google Scholar]
- Strand O, Jordhøy P, Mossing A, Knudsen PA, Nesse L, Skjerdal H, Panzacchi M, Andersen R and Gundersen V, 2011. Villreinen i Nordfjella – Status og leveområde. NINA Rapport 634, 100 pp. Available online: http://www.nina.no/archive/nina/PppBasePdf/rapport%5C2011%5C634.pdf [Accessed: 8 December 2016].
- Tamgüney G, Giles K, Bouzamondo‐Bernstein E, Bosque PJ, Miller MW, Safar J, DeArmond SJ and Prusiner SB, 2006. Transmission of Elk and deer prions to transgenic mice. Journal of Virology, 80, 9104–9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamgüney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, Lemus A, DeArmond SJ and Prusiner SB, 2009. Asymptomatic deer excrete infectious prions in faeces. Nature, 461, 529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telling GC, 2011. Transgenic mouse models and prion strains. Topics in Current Chemistry 305, 79–100. [DOI] [PubMed] [Google Scholar]
- Telling GC, Scott M, Mastrianni J, Gabizon R, Torchia M, Cohen FE, DeArmond SJ and Prusiner SB, 1995. Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell, 83, 79–90. [DOI] [PubMed] [Google Scholar]
- Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P and Prusiner SB, 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274, 2079–2082 [DOI] [PubMed] [Google Scholar]
- Thomsen BV, Schneider DA, O'Rourke KI, Gidlewski T, McLane J, Allen RW, McIsaac AA, Mitchell GB, Keane DP, Spraker TR and Balachandran A, 2012. Diagnostic accuracy of rectal mucosa biopsy testing for chronic wasting disease within white‐tailed deer (Odocoileus virginianus) herds in North America: effects of age, sex, polymorphism at PRNP codon 96, and disease progression. Journal of Veterinary Diagnostic Investigation, 24, 878–887. [DOI] [PubMed] [Google Scholar]
- Thulke H‐H, Eisinger D, Freuling C, Fröhlich A, Globig A, Grimm V, Müller T, Selhorst T, Staubach C and Stephan Zips, 2009. Situation‐based surveillance: adapting investigations to actual epidemic situations. Journal of Wildlife Diseases, 45, 1089–1103. [DOI] [PubMed] [Google Scholar]
- Triscott E, Duque‐Velasquez C, Aiken JM and McKenzie D, 2015. Chronic wasting disease (CWD) transmission into hamsters. Prion, 9, S86–S86. [Google Scholar]
- Tryland M, Das Neves CG and Klein J, 2014. Virusinfeksjoner hos reinsdyr. Norsk veterinærtidsskrift, 126, 230–241. [Google Scholar]
- Tryland M, Stubsjøen SM, Ågren E, Johansen B and Kielland C. 2016. Herding conditions related to infectious keratoconjunctivitis in semi‐domesticated reindeer: a questionnaire‐based survey among reindeer herders. Acta Veterinaria Scandinavica 58, 22. doi: 10.1186/s13028-016-0203-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehlinger FD, Johnston AC, Bollinger TK and Waldner CL, 2016. Systematic review of management strategies to control chronic wasting disease in wild deer populations in North America. BMC Veterinary Research, 12, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of the Interior, U.S. Fish and Wildlife Service, and U.S. Department of Commerce, U.S. Census Bureau . 2011. National Survey of Fishing, Hunting, and Wildlife‐Associated Recreation. Available online: https://www.census.gov/prod/2012pubs/fhw11-nat.pdf
- Vickery CM, Lockey R, Holder TM, Thorne L, Beck KE, Wilson C, Denyer M, Sheehan J, Marsh S, Webb PR, Dexter I, Norman A, Popescu E, Schneider A, Holden P, Griffiths PC, Plater JM, Dagleish MP, Martin S, Telling GC, Simmons MM and Spiropoulos J, 2014. Assessing the susceptibility of transgenic mice overexpressing deer prion protein to bovine spongiform encephalopathy. Journal of Virology 88, 1830–1833. doi: 10.1128/JVI.02762-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal E, Fernández‐Borges N, Pintado B, Eraña H, Ordóñez M, Márquez M, Chianini F, Fondevila D, Sánchez‐Martín MA, Andreoletti O, Dagleish MP, Pumarola M and Castilla J, 2015. Transgenic mouse bioassay: evidence that rabbits are susceptible to a variety of prion isolates. PLoS Pathogens, 11, e1004977. doi: 10.1371/journal.ppat.1004977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikøren T, Li H, Lillehaug A, Jonassen CM, Bockerman I and Handeland K, 2006. Malignant catarrhal fever in free‐ranging cervids associated with OvHV‐2 and CpHV‐2 DNA. Journal of wildlife diseases 42, 797–807. [DOI] [PubMed] [Google Scholar]
- VKM (Vitenskapskomiteen for Mattrygghet), 2016. CWD in Norway. Opinion of the Panel on biological hazards, ISBN: 978‐82‐8259‐216‐1, Oslo, Norway. [Google Scholar]
- Walsh DP and Miller MW, 2010. A weighted surveillance approach for detecting chronic wasting disease foci. Journal of Wildlife Diseases, 46, 118–135. [DOI] [PubMed] [Google Scholar]
- White SN, O'Rourke KI, Gidlewski T, VerCauteren KC, Mousel MR, Phillips GE and Spraker TR, 2010. Increased risk of chronic wasting disease in Rocky Mountain elk associated with decreased magnesium and increased manganese in brain tissue. Canadian Journal of Veterinary Research, 74, 50–53. [PMC free article] [PubMed] [Google Scholar]
- Williams ES, 2003. Scrapie and chronic wasting disease. Clinics in laboratory medicine, 23, 139–159. [DOI] [PubMed] [Google Scholar]
- Williams ES, 2005. Chronic wasting disease. Veterinary Pathology, 42, 530–549. [DOI] [PubMed] [Google Scholar]
- Williams ES and Miller MW, 2002. Chronic wasting disease in deer and elk in North America. Revue Scientifique et Technique, 21, 305–316. [DOI] [PubMed] [Google Scholar]
- Williams ES and Miller MW, 2003. Transmissible spongiform encephalopathies in non‐domestic animals: origin, transmission and risk factors. Revue Scientifique et Technique (International Office of Epizootics) [2003, 22(1):145‐156]. [DOI] [PubMed] [Google Scholar]
- Williams ES and Young S, 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. Journal of Wildlife Diseases, 16, 89–98. [DOI] [PubMed] [Google Scholar]
- Williams ES and Young S, 1982. Spongiform encephalopathy of Rocky Mountain Elk. Journal of Wildlife Diseases, 18, 465–471. [DOI] [PubMed] [Google Scholar]
- Williams ES and Young S, 1992. Spongiform encephalopathies in Cervidae. Revenue Scientific Technical Review of The Office International Epizooties, 11, 551–567. [DOI] [PubMed] [Google Scholar]
- Williams ES and Young S, 1993. Neuropathology of chronic wasting disease of mule deer (Odocoileus hemionus) and Elk (Cervus elaphus nelsoni). Veterinary Pathology, 30, 36–45. [DOI] [PubMed] [Google Scholar]
- Williams ES, Miller MW, Kreeger TJ, Kahn RH and Thorne ET, 2002. Chronic wasting disease of deer and elk: a review with recommendations for management. The Journal of Wildlife Management, 66, 551–563. [Google Scholar]
- Wilson GA, Nakada SM, Bollinger TK, Pybus MJ, Merrill EH and Coltman DW, 2009. Polymorphisms at the PRNP gene influence susceptibility to chronic wasting disease in two species of deer (Odocoileus Spp.) in western Canada. Journal of Toxicology Environment Health A, 72, 1025–1029. [DOI] [PubMed] [Google Scholar]
- Wisconsin Department of Natural Resources , 2012. Wisconsin's chronic wasting disease response plan: 2010–2025. Available online: http://dnr.wi.gov/about/nrb/2013/February/02-13-8B1.pdf [Accessed: 5 December 2016].
- Wolfe LL, Spraker TR, Gonzalez L, Dagleish MP, Sirochman TM, Brown JC, Jeffrey M and Miller MW, 2007. PrPCWD in rectal lymphoid tissue of deer (Odocoileus spp.). Journal of General Virology, 88, 2078–2082. [DOI] [PubMed] [Google Scholar]
- Wolfe LL, Kocisko DA, Caughey B and Miller MW, 2012. Assessment of prospective preventive therapies for chronic wasting disease in mule deer. Journal of Wildlife Diseases 48, 530–533. doi: 10.7589/0090-3558-48.2.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe LL, Fox KA and Miller MW, 2014. “Atypical” chronic wasting disease in PRNP genotype 225FF mule deer. Journal of Wildlife Diseases, 50, 660–665. [DOI] [PubMed] [Google Scholar]


