Abstract
The Panel on Food Additives and Nutrient Sources added to Food (ANS) provides a scientific opinion re‐evaluating the safety of guar gum (E 412) as a food additive. In the EU, guar gum was evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1970, 1974 and 1975, who allocated an acceptable daily intake (ADI) ‘not specified’. Guar gum has been also evaluated by the Scientific Committee for Food (SCF) in 1977 who endorsed the ADI ‘not specified’ allocated by JECFA. Following the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010, the Panel considered that adequate exposure and toxicity data were available. Guar gum is practically undigested, not absorbed intact, but significantly fermented by enteric bacteria in humans. No adverse effects were reported in subchronic and carcinogenicity studies at the highest dose tested; no concern with respect to the genotoxicity. Oral intake of guar gum was well tolerated in adults. The Panel concluded that there is no need for a numerical ADI for guar gum (E 412), and there is no safety concern for the general population at the refined exposure assessment of guar gum (E 412) as a food additive. The Panel considered that for uses of guar gum in foods intended for infants and young children the occurrence of abdominal discomfort should be monitored and if this effect is observed doses should be identified as a basis for further risk assessment. The Panel considered that no adequate specific studies addressing the safety of use of guar gum (E 412) in food categories 13.1.5.1 and 13.1.5.2 were available. Therefore, the Panel concluded that the available data do not allow an adequate assessment of the safety of guar gum (E 412) in infants and young children consuming these foods for special medical purposes.
Keywords: guar gum, E 412, food additive, CAS Registry Number 9000‐30‐0
Summary
Following a request from the European Commission, the Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to re‐evaluate the safety of guar gum (E 412) when used as a food additive.
The Panel was not provided with a newly submitted dossier and based its evaluation on previous evaluations and reviews, additional literature that has come available since then and the data available following a public call for data. The Panel noted that not all original studies on which previous evaluations were based were available for re‐evaluation by the Panel.
Guar Gum (E 412) is authorised as a food additive in the European Union (EU) in accordance with Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives. Specific purity criteria on guar gum (E 412) have been defined in Commission Regulation (EU) No 231/2012.
In the EU, guar gum was evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1970, 1974 and 1975 (JECFA, 1970, 1974, 1975a,b). Based on the lack of adverse effects in the toxicity studies available at the time, an acceptable daily intake (ADI) ‘not specified’ was allocated. Guar gum has been also evaluated by the Scientific Committee for Food in 1977 (SCF, 1978) who endorsed the ADI ‘not specified’ allocated by JECFA. In 1998, the SCF accepted the use of guar gum in foods for special medical purposes (FSMP) for infants and young children at levels up to 10 g/L in ready‐to‐use liquid formulae containing extensively hydrolysed protein and in ready‐to‐use liquid formulae containing partially hydrolysed proteins for infants in good health at levels up to 1 g/L. In 2001, the SCF accepted the use of guar gum in all weaning foods at levels up to 10 and up to 20 g/kg in gluten‐free cereal‐based foods, singly or in combination (SCF, 2001). In 2003, the SCF re‐evaluated guar gum in the revision of the essential requirements of infant formulae and follow‐on formulae intended for the feeding of infants and young children (SCF, 2003).
Guar gum is the ground endosperm of the seeds of the guar plant (Cyamopsis tetragonoloba L. Taub). Commercial food‐grade guar gum is reported to contain usually about 80% guaran, 5–6% crude protein, 8–15% moisture, 2.5% crude fibre, 0.5–0.8% ash, and small amounts of lipids composed mainly of free and esterified plant fatty acids.
The in vitro degradation and the in vivo digestibility of guar gum have been investigated in animals and humans which demonstrated that guar gum would not be absorbed unchanged and would not be metabolised by enzymes present in the gastrointestinal tract. However, it would be partially fermented to short‐chain fatty acids (SCFAs) during its passage through the large intestine by the action of the intestinal tract microflora. The rate of hydrolysis in the gastrointestinal tract in humans is unknown; however, it is expected that fermentation of guar gum would lead to the production of products such as SCFAs which were considered of no concern by the Panel.
Guar gum is regarded as not acutely toxic, based on the results of acute oral toxicity studies.
In short‐term and subchronic studies in mice, rats, dogs and monkeys, no adverse effects were observed at the highest dose tested.
The Panel considered the available genotoxicity data on guar gum (E 412) to be sufficient to conclude that there is no concern with respect to genotoxicity.
Overall, the Panel considered guar gum as not carcinogenic.
Guar gum did not show reproductive effects (fertility) or developmental toxicity effects in the available studies. From a combined fertility/developmental study in rats (Collins et al., 1987), the Panel could identify a no‐observed‐adverse‐effect‐level (NOAEL) of 5,200 mg/kg body weight (bw) per day for reproductive effects based on decreased number of corpora lutea and a NOAEL for developmental toxicity of 11,800 mg/kg bw per day the highest dose tested.
The present re‐evaluation includes the use of guar gum (E 412) in foods for infants from 12 weeks of age and for young children. The Panel acknowledged that consumption to the concerned food categories would be short and noted that it is prudent to keep the number of additives used in foods for infants and young children to the minimum necessary and that there should be strong evidence of need as well as safety before additives can be regarded as acceptable for use in infant formulae and foods for infants and young children. If guar gum is added in combination with locust bean gum and carrageenan to a follow‐on formula (food category 13.1.2), the maximum level recommended by the SCF for guar gum should not be exceeded by the total concentration of these three substances. The Panel noted that it may be considered to establish specific purity criteria for the use of guar gum in food for infants and young children (food category 13.1).
From the refined brand‐loyal estimated exposure scenario taking into account the FSMP, mean exposure to guar gum (E 412) from its use as a food additive ranged for infants between 325 and 609 mg/kg bw per day and between 120 and 457 mg/kg bw per day for toddlers. The 95th percentile of exposure ranged for infants between 912 and 1,555 mg/kg bw per day and for toddlers between 310 and 743 mg/kg bw per day.
The refined estimates are based on 51 out of 86 food categories in which guar gum (E 412) is authorised. The main food categories, in term of amount consumed, not taken into account were breakfast cereals, gluten‐free dietary foods for infants and young children, snacks and most of alcoholic beverages. However, based on the information in the Mintel Global New Products Database (GNPD) (Appendix C), in the EU market, no breakfast cereals are labelled with guar gum (E 412), and few alcoholic drinks are labelled with the additive. Therefore, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure to guar gum (E 412) as a food additive according to Annex II in European countries for all scenarios.
The Panel noted that in Annex II of Regulation (EC) No 1333/2008 use levels of guar gum (E 412) in food for infants under the age of 12 weeks are included in categories 13.1.1, 13.1.5.1 and 13.1.5.2. The Panel considered that these uses would require a specific risk assessment in line with the recommendations given by JECFA (1978) and the SCF (1998) and endorsed by the Panel (EFSA ANS Panel, 2012). Therefore, the current re‐evaluation of guar gum (E 412) as a food additive is not considered to be applicable for infants under the age of 12 weeks and will be performed separately.
General population
Following the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA ANS Panel, 2014), and given that:
adequate exposure data were available; in the general population, the highest refined exposure assessments calculated based on the reported data from food industry were for infants (12 weeks–11 months) up to 812 mg/kg bw per day (brand‐loyal scenario),
guar gum is practically undigested, not absorbed intact, but significantly fermented by enteric bacteria in humans,
adequate toxicity data were available,
no adverse effects were reported in subchronic studies in rodents at the highest dose tested of 15,000 mg guar gum/kg bw per day in mice and 18,000 mg guar gum/kg bw per day in rats,
there is no concern with respect to the genotoxicity of guar gum,
no carcinogenic effects were reported at the highest dose tested of 7,500 mg guar gum/kg bw per day in mice and 2,500 mg guar gum/kg bw per day in rats,
oral intake of large amount of guar gum in (9,000–30,000 mg/person corresponding to 128–429 mg/kg bw per day) was well tolerated in adults. In most studies after consumption of around 15,000 mg per day in adults corresponding to 214 mg/kg bw per day, some individuals experienced abdominal discomfort which was considered by the Panel as undesirable but not adverse,
in one interventional study with diabetic children abdominal discomfort was reported in 5 out of 22 children given 13,500 mg guar gum per day corresponding to 314 mg/kg bw per day,
using the refined exposure assessment (non brand‐loyal scenario), the Panel noted that exposures for high level consumers (children and adults) would be below the level at which some abdominal discomfort was reported,
no data on abdominal discomfort were available for infants and young children,
the Panel concluded that there is no need for a numerical ADI for guar gum (E 412), and that there is no safety concern for the general population at the refined exposure assessment for the reported uses of guar gum (E 412) as a food additive.
The Panel considered that for uses of guar gum in foods intended for infants and young children the occurrence of abdominal discomfort should be monitored and if this effect is observed doses should be identified as a basis for further risk assessment.
Infants and young children consuming foods for special medical purposes and special formulae
Concerning the use of guar gum (E 412) in ‘dietary foods for special medical purposes and special formulae for infants’ (Food category 13.1.5.1) and ‘in dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC’ (Food category 13.1.5.2), and given that:
for populations consuming dietary foods for special medical purposes and special formulae, the highest refined exposure estimate (p95) calculated based on the reported data from food industry are for infants (12 weeks‐11 months) consuming dietary FSMP and special formulae up to 1,555 mg/kg bw per day (brand‐loyal scenario),
infants and young children consuming these foods may be exposed to a greater extent to guar gum (E 412) than their healthy counterparts because the permitted levels of guar gum (E 412) in products for special medical purposes are 10‐fold higher than in infant formulae and follow‐on formulae for healthy individuals,
infants and young children consuming foods belonging to these food categories may show a higher susceptibility to the gastrointestinal effects of guar gum than their healthy counterparts due to their underlying medical condition,
no adequate specific studies addressing the safety of use of guar gum (E 412) in this population under certain medical conditions were available,
it was not possible to assess at which exposure level of guar gum the gastrointestinal effects developed in this specific population,
the Panel concluded that the available data do not allow an adequate assessment of the safety of guar gum (E 412) in infants and young children consuming these foods for special medical purposes.
The Panel recommended that the maximum limits for the impurities of toxic elements (lead, mercury and arsenic) in the EC specification for guar gum (E 412) should be revised in order to ensure that guar gum (E 412) as a food additive will not be a significant source of exposure to those toxic elements in food in particular for infants and children. The Panel noted that currently detected levels of these toxic elements were orders of magnitude below those defined in the EU specifications, and therefore, the current limits could be lowered.
The Panel recommended to harmonise the microbiological specifications in the EU Regulation for polysaccharidic thickening agents, such as gums, and to include criteria for the absence of Salmonella spp. and Escherichia coli for total aerobic microbial count (TAMC) and for total combined yeasts and moulds count (TYMC) into the EU specifications of guar gum (E 412).
The Panel recommended to give separate specifications in the EU regulation for guar gum and clarified guar gum differing significantly in the protein content.
The Panel considered that no threshold dose can be established for allergic reactions. Therefore, it is advisable that exposure to eliciting allergens, such as proteinaceous compounds, is avoided as much as possible and therefore the Panel recommended that their content should be reduced as much as possible, which can be achieved, for example, by clarification of guar gum.
The Panel recommended that additional data should be generated to assess the potential health effects of guar gum (E 412) when used in ‘dietary foods for infants for special medical purposes and special formulae for infants’ (Food category 13.1.5.1) and in ‘dietary foods for babies and young children for special medical purposes’ as defined in Directive 1999/21/EC (Food category 13.1.5.2).
1. Introduction
The present opinion deals with the re‐evaluation of guar gum (E 412) when used as a food additive.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background as provided by the European Commission
Regulation (EC) No 1333/20081 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the EU. In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the EU before 20 January 2009 has been set up under the Regulation (EU) No 257/20102. This Regulation also foresees that food additives are re‐evaluated whenever necessary in the light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU3 of 2001. The report ‘Food additives in Europe 2000’ submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010 the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference as provided by the European Commission
The Commission asks EFSA to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.1.3. Interpretation of Terms of Reference
The Panel on Food Additives and Nutrient Sources added to Food (ANS) described its risk assessment paradigm in its Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012). This Guidance states, that in carrying out its risk assessments, the Panel sought to define a health‐based guidance value, e.g. an acceptable daily intake (ADI) (IPCS, 2004) applicable to the general population. According to the definition above, the ADI as established for the general population does not apply to infants below 12 weeks of age (JECFA, 1978; SCF, 1998). In this context, the re‐evaluation of the use of food additives, such as thickening agents and certain emulsifiers, in food for infants below 12 weeks represents a special case for which specific recommendations were given by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (JECFA, 1972, 1978) and by the SCF (SCF, 1996, 1998). The Panel endorsed these recommendations.
In the current EU legislation (Annex II of Regulation (EC) No 1333/2008), use levels of additives in food for infants under the age of 12 weeks are included in categories 13.1.1 and 13.1.5.1. The Panel considers that these uses would require a specific risk assessment in line with the recommendations given by JECFA and the SCF and endorsed by the Panel in its current Guidance for submission for food additives evaluations (EFSA ANS Panel, 2012). Therefore, a risk assessment as for the general population is not considered to be applicable for infants under the age of 12 weeks and will be performed separately.
This re‐evaluation refers exclusively to the uses of guar gum (E 412) as a food additive in food, including food supplements, and does not include a safety assessment of other uses of guar gum.
1.2. Information on existing evaluations and authorisations
Guar Gum (E 412) is authorised as a food additive in the EU in accordance with Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives. Specific purity criteria on guar gum (E 412) have been defined in Commission Regulation (EU) No 231/20124.
In the EU, guar gum was evaluated by JECFA in 1970, 1974 and 1975 (JECFA, 1970, 1974, 1975a,b). Based on the lack of adverse effects in the toxicity studies available at the time, an ADI ‘not specified’ was allocated.
Guar gum has been also evaluated by the SCF in 1977 (SCF, 1978) who endorsed the ADI ‘not specified’ allocated by JECFA. No detailed information was given on the basis for the evaluation. In 1998, the SCF accepted the use of guar gum in foods for special medical purposes (FSMP) for infants and young children at levels up to 10 g/L in ready‐to‐use liquid formulae containing extensively hydrolysed protein and in ready‐to‐use liquid formulae containing partially hydrolysed proteins for infants in good health at levels up to 1 g/L. According to the SCF, FSMP for infants and young children encompass a wide variety of different products in powdered, liquid or semisolid forms, each with a specific formulation and hence each with its own technological requirements. Thus, the technological requirements for additives in FSMP may differ considerably from those for foods for infants and young children in good health (SCF, 1999). In 2001, the SCF accepted the use of guar gum in all weaning foods at levels up to 10 and up to 20 g/kg in gluten‐free cereal‐based foods, singly or in combination (SCF, 2001). In these two reports (SCF, 1999, 2001), the SCF endorsed its principles that it is prudent to keep the number of additives used in foods for infants and young children to the minimum necessary and that there should be strong evidence of need as well as safety before additives can be regarded as acceptable for use in infant formulae and foods for infants and young children.
In 2003, the SCF re‐evaluated guar gum in the revision of the essential requirements of infant formulae and follow‐on formulae intended for the feeding of infants and young children (SCF, 2003).
The Committee recommends guar gum should not be used in infant formulae.
Considering that guar gums have been used for quite some time in follow‐on formulae without the appearance of reports on adverse events, the Committee finds it acceptable to maintain the current maximum level of the use of guar gums in follow‐on formulae of 1 g/L.
The Committee further recommends maintaining the concept that if more than one of the three substances, locust bean gum, guar gum or carrageenan, are added to a follow‐on formula, the maximum level established for each of those substances is lowered with that relative part as is present of the other substances together.
According to the Codex Alimentarius, guar gum is used as thickener, stabiliser, and emulsifier in many food categories (GSFA, 2011). The same functional uses are stated in JECFA (2008).
Guar gum has also been reviewed by the Nordic Council of Ministers (TemaNord, 2002), who concluded that no immediate re‐evaluation of guar gum is necessary. However, it was suggested that the aspects of allergy/intolerance and purity should be included in the next evaluation and the conduction of a multigeneration study including reproduction should be considered.
Guar gum is one of the food additives that composed jelly mini‐cups which were suspended in 2004 by the European Commission to be placed on the market and import (Commission Decision 2004/37/EC, EC 2004), following the measures taken and information provided by different Member States. Jelly mini‐cups are defined as ‘jelly confectionery of a firm consistence, contained in semi rigid mini‐cups or mini‐capsules, intended to be ingested in a single bite by exerting pressure on the mini‐cups or mini‐capsule to project the confectionery into the mouth’.
In 2004, the EFSA Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) prepared a scientific opinion on a request from the European Commission related to the use of certain food additives derived from seaweed or non‐seaweed origin, including guar gum (E 412) in jelly mini‐cups (EFSA AFC Panel, 2004). The AFC Panel concluded that any of these gel‐forming additives or of any other type that gave rise to a confectionery product of a similar size, with similar physical and/or physicochemical properties and that could be ingested in the same way as the jelly mini‐cups, would give rise to a risk for choking (EFSA AFC Panel, 2004). The use of these additives in jelly mini‐cups is not authorised in the EU.5
In 2007, the EFSA AFC Panel issued an opinion on the use of partially depolymerised guar gum as a food additive (EFSA, 2007). A manufacturer requested the use of partially hydrolysed guar gum produced from one of three manufacturing process consisting of heat treatment, acid hydrolysis or alkaline oxidation, which all exert a partial depolymerisation of the native guar gum. The AFC Panel noted that partially depolymerised guar gums have been shown to be very similar to native guar gum with respect to the structure of galactomannan polysaccharide and the composition of the final product, except the level of salts arising from the neutralisation steps involved in the manufacturing process. Furthermore, the average molecular weight of all depolymerised guar gums preparations examined matched the criteria set for the molecular weight of food grade specified to be between 50,000 and 8,000,000 g/mol. Based on detailed analysis of polydispersity, the percentages of components with molecular mass below 50,000 g/mol was reported to be 0% for depolymerised guar gum prepared by heat treatment and acid hydrolysis and 7.6% for alkaline oxidation process. Based on specifications for guar gum defined by JECFA, it appeared to the AFC Panel that the depolymerised guar gum prepared by alkaline oxidation matches those specifications. The safety of depolymerised guar gum was assessed from a 90‐day study in rats fed with a depolymerised guar gum prepared by alkaline oxidation which showed no adverse effect up to dose levels of 50 g/kg diet, estimated to be equal to 2,500 mg/kg body weight (bw) per day. Furthermore, based on the documented safety of native guar gum and considering that depolymerised guar gum appears to fall within the specifications of native guar gum, the Panel concluded that there is no safety concern for the partially depolymerised guar gum prepared by either heat treatment, acid hydrolysis or alkaline oxidation at the estimated levels of intake (between 41 and 57 mg/kg bw per day based on a worst case scenario). Finally, the Panel considered that the specifications for guar gum may need to be modified to take account of the increased level of salts and the possible undesirable by‐products, e.g. furfural and peroxides, that may result from the described processes for the production of partially depolymerised guar gum.
In 2010, the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) prepared a scientific opinion on the substantiation of health claims related to guar gum (EFSA NDA Panel, 2010). No cause and effect relationship could be established between the consumption of guar gum and maintenance of normal blood glucose concentrations, increase in satiety and maintenance of normal blood cholesterol concentrations. In 2011, the EFSA NDA Panel prepared a scientific opinion on the substantiation of health claims related to partially hydrolysed guar gum (EFSA NDA Panel, 2011). No cause and effect relationship could be established with decreasing potentially pathogenic gastrointestinal microorganisms, changes in short‐chain fatty acid (SCFA) production and/or pH in the gastrointestinal tract, changes in bowel function and reduction in gastrointestinal discomfort.
2. Data and methodologies
2.1. Data
The Panel on Food Additives and Nutrient Sources added to Food (ANS) was not provided with a newly submitted dossier. EFSA launched public calls for data6 , 7 , 8 and, if relevant, contacted other scientific risk assessment bodies to collect relevant information from interested parties.
The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up to the last Working Group meeting before the adoption of the opinion.9 Attempts were made at retrieving relevant original study reports on which previous evaluations or reviews were based; however, these were not always available to the Panel.
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database10) was used to estimate the dietary exposure.
The Mintel's Global New Products Database (GNPD) is an online resource listing food products and compulsory ingredient information that should be included in labelling. This database was used to verify the use of guar gum (E 412) in food products.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
The ANS Panel assessed the safety of guar gum (E 412) as a food additive in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the Scientific Committee on Food (SCF, 2001), and taking into consideration, the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
When the test substance was administered in the feed or in the drinking water, but doses were not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012) for studies in rodents or, in the case of other animal species, by JECFA (2000). In these cases, the daily intake is expressed as equivalent. When in human studies in adults (aged above 18 years), the dose of the test substance administered was reported in mg/person per day, the dose in mg/kg bw per day was calculated by the Panel using a body weight of 70 kg as default for the adult population as described in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012).
Dietary exposure to guar gum (E 412) from its use as a food additive was estimated combining food consumption data available within the EFSA Comprehensive European Food Consumption Database with the maximum levels according to Annex II to Regulation (EC) No 1333/200811 and/or reported use levels and analytical data submitted to EFSA following a call for data. Different scenarios were used to calculate exposure (see Section 3.3.1). Uncertainties on the exposure assessment were identified and discussed.
In the context of this re‐evaluation, the Panel followed the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EC) No 257/2010 (EFSA ANS Panel, 2014).
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
Guar gum is the ground endosperm of the seeds of natural strains of the guar plant, Cyamopsis tetragonoloba (L.) Taub. (family: Leguminosae).
Guar gum (E 412) has the CAS Registry No 9000‐30‐0, EINECS No 232‐536‐0.
Commercial food‐grade guar gum is reported to contain usually about 80% galactomannan guaran, 5–6% crude protein, 8–15% moisture, 2.5% crude fibre, 0.5–0.8% ash, and small amounts of lipids composed mainly of free and esterified plant fatty acids (Leung and Foster, 1996). Other literature references indicate that guaran content in guar gum may reach 84–95%, with a protein content from 4% to 6% with no α‐amylase enzymatic activity reported (Merck Index, 2006; Europ. Pharm. Comment., 2005; JECFA 2008a; Document provided to EFSA n. 7). In 2008, JECFA described a guar gum (clarified) in which the gum is purified, reducing the protein content by dissolution in water, precipitation and recovery with ethanol or isopropanol. These preparations should comply with a maximum value of 1% protein (JECFA, 2008b).
The galactomannan guaran is commonly defined as a high‐viscosity water‐soluble polysaccharide fraction consisting of linear chains of (1→4)‐β‐d‐mannopyranosyl units with α‐d‐galactopyranosyl units attached by (1→6) linkages. Mannose and galactose units are contained with a mass ratio of approximately 2:1 (Merck Index, 2006). The molecular weight of the polysaccharide is reported to be approximately 50,000–8,000,000 g/mol (JECFA, 2008; Regulation (EU) 231/2012), while other sources refer to a narrower range: around 220,000 (Leung and Foster, 1996; Merck Index, 2006), 220,000–250,000 (CRC, 1972), and 200,000–300,000 (Glicksman, 1969 as reported by Yoon et al., 2008).
The structural formula of the polysaccharide of guar gum is presented in Figure 1.
Figure 1.
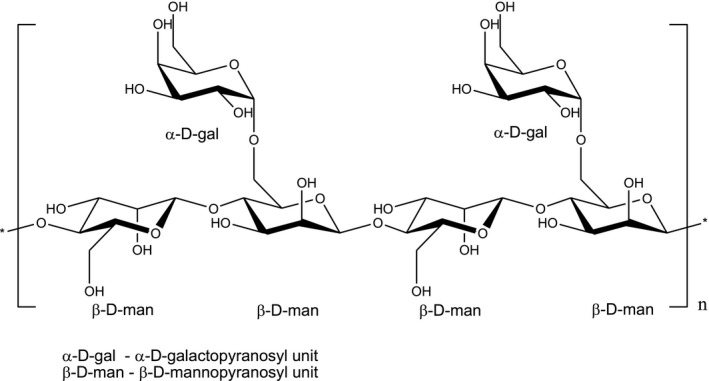
Structural formula of guar gum E 412
Guar gum is described by JECFA (2008) as a white to yellowish‐white, nearly odourless, free‐flowing powder insoluble in ethanol. According to EC specifications, guar gum is soluble in cold water (Commission Regulation (EU) No 231/2012). It is practically insoluble in organic solvents like oils, greases, hydrocarbons, ketones and esters (Lewis, 2007). Water dispersions are tasteless and odourless (Merck Index, 2006; Lewis, 2007).
A 1% dispersion of a typical commercial guar gum may reach a viscosity between 2,700 and 3,500 mPa.s (CRC, 1972; Ullmann, 2007). The 1% dispersion shows a pH of 5.5–6.1. It is relatively stable over the range of pH 4–10.5, and exhibits a slight buffering action. Solutions are thixotropic (become thin, less viscous over time), and the viscosity is relatively unaffected by the presence of electrolytes (CRC, 1972). Guar gum has a high water binding capacity with a swelling index of 36 mL/g (Huth, 1985).
Guar gum solutions show high viscosity, which changes with shear rate under all operational conditions, so that a pseudoplastic behaviour is observed. The apparent viscosity increases with gum concentration and decreases as the temperature increases (Casas et al., 2000).
Mixtures of xanthan and guar gum showed a higher combined viscosity than that occurring in each separate gum. This synergistic interaction was affected by the gum ratio in the mixture and dissolution temperature of both gums. The highest viscosities were observed when 2.0 kg/m3 gum concentration was used together with a ratio of xanthan/guar gum of 3/3 (w/w) and dissolution temperature of 40 and 80°C for xanthan and guar gum, respectively (Casas et al., 2000).
According to Tako (1992), guar gum as a galactomannan with a high galactose content (33%) showed a slight degree of interaction with xanthan gum. This might be due to the high number of side chains presented on the guar gum molecule. These side chains might prevent the insertion of the charged trisaccharide side chains of the xanthan molecule into the backbone of the guar gum molecule (Tako, 1992).
Guar gum can be distinguished from locust bean gum by microscopic examination. Locust bean gum contains long stretched tubiform cells, separated or slightly interspaced while guar gum shows close groups of round to pear shaped cells with yellow to brown contents (Specifications for locust bean gum from Commission Regulation (EU) 231/2012).
Guar gum may be partially hydrolysed by heat treatment, mild acid or alkaline oxidative treatment for viscosity adjustment and as such it is part of EU definition of E 412 food additive (Commission regulation, 2012).
Guar gum is also known by the synonyms Guarkernmehl, gum cyamopsis, guar flour, jaguar gum among others (Sci Finder online ed.; ChemIdPlus online ed.).
Upon request of the Panel for information on the particle size distribution, data were provided by two interested parties (Document provided to EFSA n. 7; Document provided to EFSA n. 8) regarding guar gum. According to the submitted results of batch analysis by laser diffraction in dry dispersion and electronic microscopy, the great majority of guar gum particle size distribution is in micrometre (μm) range.
3.1.2. Specifications
The Panel noted that JECFA has established particular specifications for a guar gum called clarified which has not yet been identified in the EU Regulation (EU) 231/2012. The definition of this clarified guar gum is described by JECFA as arising from the native guar gum clarified by dissolution in water, filtration and precipitation with ethanol and isopropanol. Clarified guar gum does not contains cell wall materials and clarified guar gum in the market is normally standardised with sugars (2008a,b).
Specifications for partially hydrolysed guar gum have been addressed by the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) in its opinion related to an application on the use of partially depolymerised guar gum as a food additive (EFSA, 2007). Data provided for all depolymerised guar gum preparations examined by AFC show average molecular weights matching the criteria set for food grade guar gum, specified to be between 50,000 and 8,000,000 g/mol. Furthermore, the AFC Panel noticed that ‘partially hydrolysed guar gums have been shown to be very similar to native guar gum with respect to the structure of the galactomannan polysaccharide and the composition of the final products, except for the level of salts present in the samples made by acid hydrolysis and alkaline oxidation because of the neutralisation step involved in the manufacturing process. The resulting salts, either sodium citrate (E311) or sodium phosphate (E339) have well established safety records when used as a food additive in food products (EC, 1995).12 During the manufacturing process potential undesirable compounds such as furfural, organic peroxides and small molecular weight organic acid can be formed from oxidation and are controlled by the manufacturer below specific levels. The AFC Panel considered that specifications for guar gum may need to be modified to take account of the possible production of undesirable by‐products. The AFC Panel noticed that the intake of furfural, resulting from its presence at levels below 1 mg/kg in partially depolymerised guar gum will be below the group ADI of 0–0.5 mg/kg bw per day established for furfural, furfuryl alcohol and other furfuryl derivatives’ (EFSA, 2007).
The European Pharmacopeia 8th ed. (2014) also indicates that in guar gum Escherichia coli and Salmonella should not be detectable and that loss on drying is limited to 15%, because mould infestation increases with higher moisture content (Europ. Pharm. Comment., 2005). Loss of drying measured in batches of guar gum ranged from 8.9% to 9.8% (Document provided to EFSA n. 7), meeting the EU specifications in Table 1. The European Pharmacopeia 8th ed. (2014) defines 8% as limits for proteins in guar gum. It is reported that insufficient elimination of the germ tissue from endosperm during the manufacturing process leads to higher percentages in protein content (Europ. Pharm. Comment., 2005). The European Pharmacopoeia and JECFA specify lower content in ash for guar gum (1.8%) than the EU regulation No 231/2012 (5.5%). Information provided by the industry explains that this difference is due to the fact that EU regulation considers also partially hydrolysed guar gum in the specifications of guar gum in contrast to the other two specifications (Document provided to EFSA n. 7). According to this information, the higher content of salts in the partially hydrolysed guar gum coming from its preparation process causes a higher amount of total ash.
Table 1.
Specifications of guar gum have been defined in Commission Regulation (EU) 231/2012 and by JECFA (2008). The available specifications are listed in Table 1. Commission Regulation No 231/2012/EC and JECFA specifications of guar gum (E 412) (JECFA, 2008a) and guar gum (clarified) (JECFA, 2008b)
| Regulation (EU) 231/2012 on guar gum | JECFA on guar gum (2008a) | JECFA on clarified guar gum (2008b) | |
|---|---|---|---|
| Definition | Guar gum is the ground endosperm of the seeds of natural strains of the guar plant, Cyamopsis tetragonolobus (L.) Taub. (family Leguminosae). Consists mainly of a high molecular weight hydrocolloidal polysaccharide composed of galactopyranose and mannopyranose units combined through glycosidic linkages, which may be described chemically as a galactomannan. The gum may be partially hydrolysed by either heat treatment, mild acid or alkaline oxidative treatment for viscosity adjustment | Primarily, the ground endosperm of the seeds from Cyamopsis tetragonolobus a (L.) Taub. (family. Leguminosae) mainly consisting of high molecular weight (50,000–8,000,000) polysaccharides composed of galactomannans; the mannose:galactose ratio is about 2:1. The seeds are crushed to eliminate the germ; the endosperm is dehusked, milled and screened to obtain the ground endosperm (native guar gum). The gum may be washed with ethanol or isopropanol to control the microbiological load (washed guar gum) |
Primarily, the ground endosperm of the seeds from Cyamopsis tetragonolobus (L.) Taub. (family Leguminosae) mainly consisting of high molecular weight (50,000–8,000,000) polysaccharides composed of galactomannans; the mannose:galactose ratio is about 2:1. The seeds are crushed to eliminate the germ; the endosperm is dehusked, milled and screened to obtain the ground endosperm (native guar gum). The gum is clarified by dissolution in water, filtration and precipitation with ethanol or isopropanol Clarified guar gum does not contain cell wall materials. Clarified guar gum in the market is normally standardised with sugars |
| MW | 50,000–8,000,000 | ||
| Assay | Galactomannan content not less than 75% | – | |
| Description | A white to yellowish‐white, nearly odourless powder | White to yellowish‐white, nearly odourless, free‐flowing powder | White to yellowish white, nearly odourless, free‐flowing powder |
| Functional uses | – | Thickener, stabiliser, emulsifier | Thickener, stabiliser, emulsifier |
| Identification | – | ||
| Tests for galactose and for mannose | Passes tests | – | |
| Solubility | Soluble in cold water | Insoluble in ethanol | Insoluble in ethanol |
| Gel formation | – | Add small amounts of sodium borate TS to an aqueous dispersion of the sample; a gel is formed | Add small amounts of sodium borate TS to an aqueous solution of the sample; a gel is formed |
| Viscosity | – | [test] | [test] |
| Gum constituents | – | Proceed as directed under Gum Constituents Identification using 100 mg of the sample instead of 200 mg and 1–10 μL of the hydrolysate instead of 1–5 μL. Use galactose and mannose as reference standards. These constituents should be present | Proceed as directed under Gum Constituents Identification using 100 mg of the sample instead of 200 mg and 1–10 μL of the hydrolysate instead of 1–5 μL. Use galactose and mannose as reference standards. These constituents should be present |
| Microscopic examination | – | [test] | – |
| Purity | |||
| Loss on drying | Not more than 15% (105°C, 5 h) | Not more than 15.0% (105°, 5 h) | Not more than 15.0% (105°, 5 h) |
| Ash | Not more than 5.5% determined at 800°C | Not more than 1.5% (800°, 3–4 h) | Not more than 1.0% (800°, 3–4 h) |
| Acid‐insoluble matter | Not more than 7% | Not more than 7.0% | Not more than 1.2% |
| Protein (N × 6.25) | Not more than 10% |
Not more than 10.0% Proceed as directed under Nitrogen Determination (Kjeldahl Method) in Volume 4 (under ‘General Methods, Inorganic components’). The percentage of nitrogen determined multiplied by 6.25 gives the percentage of protein in the sample |
Not more than 1.0% Proceed as directed under Nitrogen Determination (Kjeldahl Method) in Volume 4 (under ‘General Methods, Inorganic components’). The percentage of nitrogen determined multiplied by 6.25 gives the percentage of protein in the sample |
| Starch | Not detectable by the following method: to a 1 in 10 solution of the sample add a few drops of iodine solution (no blue colour is produced) | – | |
| Organic peroxides | Not more than 0.7 meq active oxygen/kg sample | – | |
| Furfural | Not more than 1 mg/kg | – | – |
| Pentachlorophenol | Not more than 0.01 mg/kg | ||
| Lead | Not more than 2 mg/kg |
Not more than 2 mg/kg Determine using an AAS/ICP‐AES technique appropriate to the specified level. The selection of sample size and method of sample preparation may be based on the principles of the methods described in Volume 4 (under ‘General Methods, Metallic Impurities’) |
Not more than 2 mg/kg Determine using an AAS/ICP‐AES technique appropriate to the specified level. The selection of sample size and method of sample preparation may be based on the principles of the methods described in Volume 4 (under ‘General Methods, Metallic Impurities’) |
| Arsenic | Not more than 3 mg/kg | – | – |
| Mercury | Not more than 1 mg/kg | – | – |
| Cadmium | Not more than 1 mg/kg | – | – |
| Borate | – |
Absent by the following test Disperse 1 g of the sample in 100 mL of water. The dispersion should remain fluid and not form a gel on standing. Mix 10 mL of dilute hydrochloric acid with the dispersion, and apply one drop of the resulting mixture to turmeric paper. No brownish red colour is formed |
Absent by the following test Disperse 1 g of the sample in 100 mL of water. The dispersion should remain fluid and not form a gel on standing. Mix 10 mL of dilute hydrochloric acid with the dispersion, and apply one drop of the resulting mixture to turmeric paper. No brownish red colour is formed |
| Residual solvents | – | Not more than 1% of ethanol or isopropanol, singly or in combination | Not more than 1% of ethanol or isopropanol, singly or in combination See description under TESTS |
| Microbiological criteria | – |
Test: Initially prepare a 10‐1 dilution by adding a 50 g sample to 450 mL of Butterfield's phosphate‐buffered dilution water and homogenising the mixture in a high‐speed blender Total (aerobic) plate count: Not more than 5,000 CFU/g E. coli: Negative in 1 g Salmonella: Negative in 25 g Yeasts and moulds: Not more than 500 CFU/g |
Initially prepare a 10‐1 dilution by adding a 50 g sample to 450 mL of Butterfield's phosphate‐buffered dilution water and homogenising the mixture in a high‐speed blender Total (aerobic) plate count: Not more than 5,000 CFU/g E. coli: Negative in 1 g Salmonella: Negative in 25 g Yeasts and moulds: Not more than 500 CFU/g |
AAS: atomic absorption spectroscopy; ICP‐AES: inductively coupled plasma atomic emission spectroscopy; CFU: colony‐forming units.
Current accepted name Cyamopsis tetragonoloba (ILDIS, 2013).
The Panel noted some case reports of hypersensitivity reactions associated with guar gum (Section 3.5.7). The Panel considered that these hypersensitivity reactions might be due to the guar gum proteins. The Panel noted that guar gum (E 412) is also produced in a purified form as clarified guar gum containing not more than 1% proteins.
In contrast to the JECFA specifications, the EU specifications do not define limits for microbiological contaminations of guar gum (E 412). Because of both the botanical origin and the polysaccharidic nature of gums, they can be a substrate of microbiological contamination and of field and storage fungal development. The latter has been recently demonstrated by the mycotoxin contaminations of gums (Zhang et al., 2014).
The Panel noted that, different from other gums, no microbiological criteria were defined for guar gum by the EU Regulation. The Panel also noted that the microbiological specifications for polysaccharidic thickening agents, such as gums, should be harmonised and that for guar gum criteria for the absence of Salmonella spp. and E. coli, for total aerobic microbial count (TAMC) and for total combined yeasts moulds count (TYMC) should be included into the EU specifications, as it is the case for other polysaccharidic thickening agents (e.g. alginic acids and its salts (E 400–E 404), agar (E 406), carrageenan (E 407), processed eucheuma sea weed (E 407a), xanthan gum (E 415), gellan gum (E 418)).
Information provided by the industry on analysis in batches of guar gum showed total plate count between 10 and 4,000 CFU/g, yeast and moulds between < 10 and 110 CFU/g, of E. coli between < 10 CFU/g and negative in 10 g and negative Salmonella in 25 g of guar gum (Document provided to EFSA n. 7; Document provided to EFSA n. 5).
Information provided by the industry on analysis in batches of guar gum of aflatoxins B1, B2, G1, G2 showed all less than 0.1 μg/kg, all results were reported to be below quantification levels of the analytical method (Document provided to EFSA n. 7). The other interested party provided analytical data for aflatoxins B1 and G1 of ≤ 1 μg/kg and for aflatoxins B2 and G2 of ≤ 0.5 μg/kg. All results were below the limit of quantification (LOQ) of the analytical method used (Document provided to EFSA n. 5).
According to the Commission Regulation (EU) No 258/2010 of 25 March 2010 imposing special conditions on the imports of guar gum originating in or consigned from India due to contamination risks by pentachlorophenol (PCP) and dioxins, maximum limits were set for the contaminant pentachlorophenol in guar gum (E 412) in the EC specifications (explanation in the point 23 of the Preamble of the Commission Regulation (EU) 231/2012). In July 2007, high levels of PCP and dioxins have been found in the EU in certain batches of guar gum originating in or consigned from India. The initially found levels of up to 480 pg WHO‐PCDD/F‐TEQ/g product and 4 mg PCP/kg gave reason for serious concern. The Community Reference Laboratory for Dioxins and PCBs in Feed and Food has carried out a study on the correlation between PCP and dioxins in contaminated guar gum from India. From this study, it can be concluded that guar gum containing a level of PCP below the level of 0.01 mg/kg does not contain unacceptable levels of dioxins (Wahl et al., 2007). Information provided by the industry on analysis of PCP in batches of guar gum confirmed that levels were all less than 0.01 mg/kg (Document provided to EFSA n. 7).
In view of the botanical origin of guar gum (E 412), limitations of possible contamination with pesticides should also be considered.
According to information provided by the industry on analysis of pesticides in batches of guar gum all results for organochlorine, pyrethroides and organophosphorus pesticides were below LOQ of the analytical methods used (Document provided to EFSA n. 7; Document provided to EFSA n. 5). However, in view of the use of guar gum in food for infant and young children, the Panel considered it particularly necessary to pay attention on the compliance of guar gum raw material to the existing EU regulation on pesticides.
The Panel noted that, according to the EC specifications for guar gum (E 412), impurities of the toxic elements lead, mercury, cadmium and arsenic are accepted up to concentrations of 2, 1, 1 and 3 mg/kg, respectively. Contamination at those levels could have a significant impact on the exposure to these metals, for which the intake is already close to the health‐based guidance values established by EFSA (EFSA CONTAM Panel, 2009a,b, 2010, 2012). However, information provided by the industry on analysis of lead, cadmium, arsenic and mercury in batches of guar gum ranged from < 0.02 to 0.21 mg/kg, < 0.01 to < 0.1 mg/kg, < 0.005 to < 0.1 mg/kg and < 0.01 to < 0.1 mg/kg, respectively (Document provided to EFSA n. 7; Document provided to EFSA n. 5).
Other components of guar gum such as acid‐insoluble material measured in batches of guar gum ranged from 1.9% to 2.8% (Document provided to EFSA n. 7), meeting the EU specifications in Table 1.
3.1.3. Manufacturing process
Guar gum is isolated from the seeds of the guar plant (Cyamopsis tetragonoloba L. Taub.), which mainly grows in India and Pakistan. For the separation of the germ and the endosperm halves, guar seeds are first screened to remove foreign matter and then fed to an attrition mill to split the seeds in two endosperm halves; finally, the germ material is sifted off by sieving. Despite their relatively low galactomannan content, the remaining endosperm halves, covered with hull (splits), are called ‘guar splits’ and are suitable for many technical applications. The quality of the splits is determined by the amount of husk still on the endosperm (degree of purity), the amount of physical protein particles in the splits, the amount of broken endosperm particles, the colour of the splits and the amount of small traces of field material like bits of the pod, stalks, etc. (Document provided to EFSA n. 7). For higher quality demands, and particularly for food applications, the endosperm must be further purified and liberated from the adhering hull. Various processes are used for this; in general, the splits are treated with moist or dry, hot air, which results in the loosening of the hull as a result of different swelling properties. After removal of the hull by sifting or by sieving, the pure endosperm is obtained with a galactomannan content of 85–95% based on dry matter. By suitable milling and screening techniques, the endosperm can be worked up to the desired commercial products (Ullmann, 2007). Bleaching of guar splits with peroxide, pH adjustment with acid, flaking, grounding, drying, sieving and blending have also been described (Document provided to EFSA n. 7). Sodium hypochlorite can also be added as processing aid in the process to maintain the quality of used water (Document provided to EFSA n. 7).
Other purification techniques described in the literature include washing with ethanol or isopropanol or dispersing in boiling water, followed by filtering, evaporation and drying (TemaNord, 2002). The purified gum, which complies with the JECFA specifications for guar gum (clarified), is obtained by dissolution of guar gum in hot water and then recovery by precipitation in ethanol or isopropanol solutions. Clarified guar gum in the market is normally standardised with sugars (JECFA, 2008b). Guar gum manufacturing flowchart according to JECFA (2008cc) is presented in Figure 2.
Figure 2.
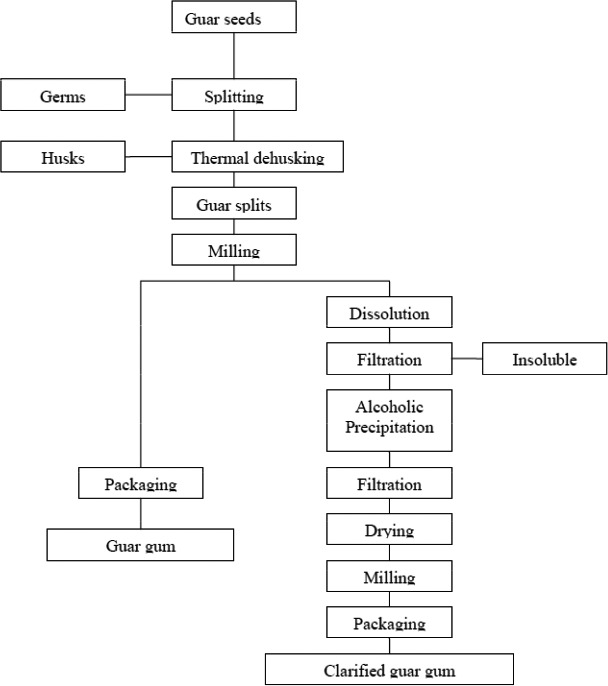
Guar gum manufacturing flow chart (JECFA, 2008c; prepared by Kawamura for FAO/WHO)
3.1.4. Methods of analysis in food
No analytical methods for the quantification of guar gum in foods were identified in the literature. Instead, methods for the estimation of the total gum content in foodstuff and gum mixtures as well as for the distinction of different gums are reported.
For the qualitative test of gums in mayonnaise and French dressing, the AOAC Official Method 937.12 is reported by the Association of Official Agricultural Chemists (AOAC, 2002). The gums are precipitated from the food sample, hydrolysed to monosaccharides which are qualitatively identified. This method is not applicable in the presence of starch. A similar method (AOAC Official Method 935.61) for qualitative determination of gums in salad dressing based on a precipitation reaction is applicable in the presence of starch (AOAC, 2002). Both methods are usable for determination of the sum of different gums used in foodstuff.
Methods for quantitative measurements of monosaccharides after hydrolysis of the polymers are described by Scherz and Mergentaler (1980). The monosaccharides are analysed using thin‐layer chromatography (TLC) or, after derivatisation, by gas chromatography. An anion‐exchange liquid chromatography method coupled to pulse amperometric detection is described to determine quantitatively complex carbohydrates mixtures (neutral saccharides, aminosacharides, glucoronic acids and disaccharides) in nutritional products (Eberendu et al., 2005).
A method establishing protein profiles of several gums including guar gum, by capillary electrophoresis was applied to estimate its presence in caroba‐guar gum mixture in samples (Ramis‐Ramos et al., 2003).
A polymerase chain reaction (PCR)‐based method was developed to differentiate the thickening agents locust bean gum and guar gum in finished food products. By applying this method, guar and/or locust bean gum remaining plant DNA can be detected in ice cream stabilisers and in foodstuffs, such as dairy products, ice cream, dry seasoning mixes, a finished roasting sauce and a fruit jelly product, but not in products with highly degraded DNA, such as tomato ketchup and sterilised chocolate cream. With both methods, guar and locust bean gum can be detected in ice cream and fresh cheese at levels < 0.1% (Meyer et al., 2001). A similar PCR‐based method is reported for the identification of guar and locust bean gum in 22 finished food products (ice cream, dehydrated desserts, milk derivatives, dehydrated soups, salad dressing, marmalade and meat) (Urdiain et al., 2004, 2005). The described PCR‐based methods are useful for the qualitative but not for quantitative determination of guar gum in foodstuff.
A Fourier transform infrared spectroscopy (FTIR) method was developed for quality control of selected gums and gum mixtures used in the food industry (Prado et al., 2005). This method allows differentiation of guar, locust bean, tara and fenugreek gums. Quantification of individual gums in gum mixtures (0.5–15%, w/w) was possible.
3.1.5. Stability of the substance, and reaction and fate in food
No specific information on reaction and fate in foods was identified. However, since the technological interest of using guar gum is related to its stability under most conditions, guar gum it is not expected to react with food under normal conditions. It has been reported that guar gum when heated to decomposition emits acrid smoke and irritating fumes (Sax, 1984).
Galactomannans are rapidly degraded in acidic aqueous solutions at elevated temperatures, resulting in a rapid loss of viscosity. They can also be degraded by oxidants and by microbial enzymes (mannanases) (Ullmann, 2007). Galactomannans are rather stable under alkaline conditions (Ullmann, 2007).
The reaction and fate in food of partially depolymerised guar gum was not specifically addressed by the AFC Panel in its 2007 opinion but it was mentioned that mild hydrolysis processing allowing to selectively decrease molecular weight, ending in different flow characteristics in solution, without affecting the chemical nature of the gum has been developed (EFSA, 2007).
3.2. Authorised uses and use levels
Maximum permitted levels (MPLs) of guar gum (E 412) have been defined in Annex II to Regulation (EC) No 1333/2008 on food additives, as amended. In this document, these levels are named maximum permitted levels (MPLs).
Currently, guar gum (E 412) is an authorised food additive in the EU at quantum satis (QS) in most foods apart from jam, jellies and similar fruit or vegetables and foods for infants and young children. Guar gum (E 412) is included in the Group I of food additives authorised at QS.
Table 2 summarises foods that are permitted to contain guar gum (E 412) and the corresponding MPLs as set by Annex II to Regulation (EC) No 1333/2008.
Table 2.
MPLs of guar gum (E 412) in foods according to the Annex II to Regulation (EC) No 1333/2008
| Food category number | Food category name | E number/group | Restrictions/exceptions | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|---|
| 01.3 | Unflavoured fermented milk products, heat‐treated after fermentation | Group I | QS | |
| 01.4 | Flavoured fermented milk products including heat‐treated products | Group I | QS | |
| 01.6.2 | Unflavoured live fermented cream products and substitute products with a fat content of less than 20% | E 412 | QS | |
| 01.6.3 | Other creams | Group I | QS | |
| 01.7.1 | Unripened cheese excluding products falling in category 16 | Group I | Except mozzarella | QS |
| 01.7.5 | Processed cheese | Group I | QS | |
| 01.7.6 | Cheese products (excluding products falling in category 16) | Group I | QS | |
| 01.8 | Dairy analogues including beverage whiteners | Group I | QS | |
| 02.2.2 | Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions | Group I | QS | |
| 02.3 | Vegetable oil pan spray | Group I | QS | |
| 03 | Edible ices | Group I | QS | |
| 04.2.1 | Dried fruit and vegetables | Group I | E 412 may not be used to produce dehydrated foods intended to rehydrate on ingestion | QS |
| 04.2.2 | Fruit and vegetables in vinegar, oil or brine | Group I | QS | |
| 04.2.3 | Canned or bottled fruit and vegetables | E 412 | Only chestnuts in liquid | QS |
| 04.2.4.1 | Fruit and vegetable preparations excluding compote | Group I | QS | |
| 04.2.5.2 | Jam, jellies and marmalades and sweetened chestnut puree as defined by Directive 2001/113/EC | E 412 | a | 10,000 |
| 04.2.5.3 | Other similar fruit or vegetable spreads | E 412 | a | 10,000 |
| 04.2.5.4 | Nut butters and nut spreads | Group I | QS | |
| 04.2.6 | Processed potato products | Group I | QS | |
| 05.1 | Cocoa and Chocolate products as covered by Directive 2000/36/EC | Group I | Only energy‐reduced or with no added sugar | QS |
| 05.2 | Other confectionery including breath refreshening microsweets | Group I | E 412 may not be used in jelly mini‐cups, defined, for the purpose of this Regulation, as jelly confectionery of a firm consistence, contained in semi‐rigid mini‐cups or mini‐capsules, intended to be ingested in a single bite by exerting pressure on the mini‐cups or mini‐capsule to project the confectionery into the mouth; E 412 may not be used to produce dehydrated foodstuffs intended to rehydrate on ingestion | QS |
| 05.3 | Chewing gum | Group I | QS | |
| 05.4 | Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | Group I | QS | |
| 06.2.2 | Starches | Group I | QS | |
| 06.3 | Breakfast cereals | Group I | QS | |
| 06.4.2 | Dry pasta | Group I | Only gluten‐free and/or pasta intended for hypoproteic diets in accordance with Directive 2009/39/EC | QS |
| 06.4.4 | Potato Gnocchi | Group I | Except fresh refrigerated potato gnocchi | QS |
| 06.4.5 | Fillings of stuffed pasta (ravioli and similar) | Group I | QS | |
| 06.5 | Noodles | Group I | QS | |
| 06.6 | Batters | Group I | QS | |
| 06.7 | Precooked or processed cereals | Group I | QS | |
| 07.1 | Bread and rolls | Group I | Except products in 7.1.1 and 7.1.2 | QS |
| 07.2 | Fine bakery wares | Group I | QS | |
| 08.2 | Meat preparations as defined by Regulation (EC) NO 853/2004 | E 412 | Only preparations in which ingredients have been injected; meat preparations composed of meat parts that have been handled differently: minced, sliced or processed and that are combined together. Except bifteki, soutzoukaki, kebap, gyros and souvlaki | QS |
| 08.3.1 | Non‐heat‐treated meat products | Group I | QS | |
| 08.3.2 | Heat‐treated meat products | Group I | Except foie gras, foie gras entier, blocs de foie gras, Libamáj, libamáj egészben, libamáj tömbben | QS |
| 08.3.3 | Casings and coatings and decorations for meat | Group I | QS | |
| 09.2 | Processed fish and fishery products including molluscs and crustaceans | Group I | QS | |
| 09.3 | Fish roe | Group I | Only processed fish roe | QS |
| 10.2 | Processed eggs and egg products | Group I | QS | |
| 11.2 | Other sugars and syrups | Group I | QS | |
| 11.4.1 | Table Top Sweeteners in liquid form | E 412 | QS | |
| 11.4.2 | Table Top Sweeteners in powder form | E 412 | QS | |
| 12.1.2 | Salt substitutes | Group I | QS | |
| 12.2.2 | Seasonings and condiments | Group I | QS | |
| 12.3 | Vinegars | Group I | QS | |
| 12.4 | Mustard | Group I | QS | |
| 12.5 | Soups and broths | Group I | QS | |
| 12.6 | Sauces | Group I | QS | |
| 12.7 | Salads and savoury‐based sandwich spreads | Group I | QS | |
| 12.8 | Yeast and yeast products | Group I | QS | |
| 12.9 | Protein products excluding products covered in category 1.8 | Group I | QS | |
| 13.1.1 | Infant formulae as defined by Directive 2006/141/EC | E 412 | Only where the liquid product contains partially hydrolysed proteins | 1,000 |
| 13.1.2 | Follow‐on formulae as defined by Directive 2006/141/EC | E 412 | b | 1,000 |
| 13.1.3 | Processed cereal‐based foods and baby foods for infants and young children as defined by Directive 2006/125/EC | E 412 | Only gluten‐free cereal‐based foodsc | 20,000 |
| 13.1.3 | Processed cereal‐based foods and baby foods for infants and young children as defined by Directive 2006/125/EC | E 412 | Only processed cereal‐based foods and baby foodsc | 10,000 |
| 13.1.4 | Other foods for young children | E 412 | c | 10,000 |
| 13.1.5.1 | Dietary foods for infants for special medical purposes and special formulae for infants | E 412 | From birth onwards in products in liquid formulae containing hydrolysed proteins, peptides or amino acids | 10,000 |
| 13.1.5.1 | Dietary foods for infants for special medical purposes and special formulae for infants | E 412 | Only where the liquid product contains partially hydrolysed proteins | 1,000 |
| 13.1.5.1 | Dietary foods for infants for special medical purposes and special formulae for infants | E 412 | b | 1,000 |
| 13.1.5.2 | Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC | E 412 | From birth onwards in products in liquid formulae containing hydrolysed proteins, peptides or amino acids | 10,000 |
| 13.1.5.2 | Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC | E 412 | b | 1,000 |
| 13.1.5.2 | Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC | E 412 | Only gluten‐free cereal‐based foodsc | 20,000 |
| 13.1.5.2 | Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC | E 412 | Only processed cereal‐based foods and baby foodsc | 10,000 |
| 13.2 | Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5) | Group I | QS | |
| 13.3 | Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet) | Group I | QS | |
| 13.4 | Foods suitable for people intolerant to gluten as defined by Regulation (EC) No 41/2009 | Group I | Including dry pasta | QS |
| 14.1.2 | Fruit juices as defined by Directive 2001/112/EC and vegetable juices | Group I | Only vegetable juices | QS |
| 14.1.3 | Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products | Group I | Only vegetable nectars | QS |
| 14.1.4 | Flavoured drinks | Group I | QS | |
| 14.1.5.2 | Other | Group I | Excluding unflavoured leaf tea; including flavoured instant coffee | QS |
| 14.2.3 | Cider and perry | Group I | QS | |
| 14.2.4 | Fruit wine and made wine | Group I | QS | |
| 14.2.5 | Mead | Group I | QS | |
| 14.2.6 | Spirit drinks as defined in Regulation (EC) No 110/2008 | Group I | Except whisky or whiskey | QS |
| 14.2.7.1 | Aromatised wines | Group I | QS | |
| 14.2.7.2 | Aromatised wine‐based | Group I | QS | |
| 14.2.7.3 | Aromatised wine‐product cocktails | Group I | QS | |
| 14.2.8 | Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol | Group I | QS | |
| 15.1 | Potato‐, cereal‐, flour‐ or starch‐based snacks | Group I | QS | |
| 15.2 | Processed nuts | Group I | QS | |
| 16 | Desserts excluding products covered in category 1, 3 and 4 | Group I | QS | |
| 17.1d | Food supplements supplied in a solid form including capsules and tablets and similar forms, excluding chewable forms | Group I | E 412 may not be used to produce dehydrated foods intended to rehydrate on ingestion | QS |
| 17.2d | Food supplements supplied in a liquid form | Group I | QS | |
| 17.3d | Food supplements supplied in a syrup‐type or chewable form | Group I | QS | |
| 18 | Processed foods not covered by categories 1–17, excluding foods for infants and young children | Group I | QS |
MPL: maximum permitted level.
Maximum individually or in combination with E 400–404, E 406, E 407, E 410, E 412, E 415 and E 418.
If more than one of the substances E 407, E 410 and E 412 is added to a foodstuff, the maximum level established for that foodstuff for each of those substances is lowered with that relative part as is present of the other substances together in that foodstuff.
E 410, E 412, E 414, E 415 and E 440 are authorised individually or in combination.
FCS 17 refers to food supplements as defined in Directive 2002/46/EC of the European Parliament and of the Council excluding food supplements for infants and young children.
According to Annex III, Part 1, to Regulation (EC) No 1333/2008, guar gum (E 412) is also authorised in all food additives as a carrier at QS.
According to Annex III, Part 2, to Regulation (EC) No 1333/2008, guar gum (E 412) is also authorised as a food additive other than carriers in all foods additives at QS.
In addition, according to Annex III, Part 3, to Regulation (EC) No 1333/2008, guar gum (E 412) is authorised as a food additive including as a carrier, in food enzymes with a maximum level in enzyme preparation and in final food (beverages or not) at QS.
According to Annex III, Part 4, to Regulation (EC) No 1333/2008, guar gum (E 412) is also authorised as a food additive including carriers in all foods flavourings at QS.
Finally, according to Annex III, Part 5, Section A, to Regulation (EC) No 1333/2008, guar gum (E 412) is authorised as a food additive in all nutrients (except nutrients intended to be used in foodstuffs for infants and young children listed in point 13.1 of Part E to Annex II) at QS.
The Regulation (EC) No 1333/2008 stipulates that guar gum (E 412), as a food additive, belonging to group I, is not authorised for the use in jelly mini‐cups and may not be used to produce dehydrated foods intended to rehydrate on ingestion.
The Panel noted that these restrictions have to be seen against the background of human cases on severe adverse effects, such as oesophageal obstruction or asphyxiation, after oral intake of guar gum or other gums/hydrocolloids with similar physicochemical properties as guar gum in the form of granules or pills without enough liquid (e.g. guar gum: Ranft and Imhof, 1983; Morse and Malloy, 1990; Opper et al., 1990; Seidner et al., 1990; Lewis, 1992; Halama and Mauldin, 1992; Taylor et al., 1998; FDA, 2016) or in the form of jelly mini‐cups (konjac gum/glucomannan: EFSA AFC Panel, 2004).
3.3. Exposure data
3.3.1. Reported use levels or data on analytical levels of guar gum (E 412)
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment, especially for those food additives for which no MPL is set and which are authorised according to QS.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued public calls13 , 14 for occurrence data (usage level and/or concentration data) on guar gum (E 412). In response to this public call, updated information on the actual use levels of guar gum (E 412) in foods was made available to EFSA by the food industry and also by gums producers. No analytical data on the concentration of guar gum (E 412) in foods were made available by the Member States.
Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with data on use levels (n = 363) of guar gum (E 412) in foods for 79 out of the 86 food categories in which guar gum (E 412) is authorised.
Updated information on the actual use levels of guar gum (E 412) in foods was made available to EFSA by Association for International Promotion of Gums (AIPG, Document provided to EFSA n. 11), BABBI Confectionary Industry (Document provided to EFSA n. 14), Biovegan GmbH (Document provided to EFSA n. 12), EUROGUM A/S (Document provided to EFSA n. 13), Food and Drink Europe (FDE, Document provided to EFSA n. 9), the International Chewing Gum Association (ICGA, Document provided to EFSA n. 16), Rudolf Wild GmbH & Co. KG (Document provided to EFSA n. 15) and Specialised Nutrition Europe (SNE, Document provided to EFSA n. 10).
The Panel noted that some data providers (e.g. AIPG: ‘international association representing producers, processors and traders in natural tree exudate gums’) are not food industry using gums in their food products but association/food additive producers. Usage levels reported by food additive producers should not be considered at the same level as those provided by the food industry. Food additive producers might recommend usage levels to the food industry but the final levels might, ultimately, be different, unless food additive producers confirm that these levels are used by the food industry. In all other cases, data from food additive producers will only be used in the MPL scenario in case of QS authorisation when no data are available from the food industry in order to have the most complete exposure estimates.
For instance, data from AIPG are all identical: for food categories authorised at QS, maximum levels are 10,000 and typical levels 8,000 for food categories authorised with a numerical MPL, maximum equals the MPL. For Eurogum A/S, all the submitted data are theoretical amounts suggested or recommended; they are ‘based on [their] own technical know‐how regarding adequate/recommended levels of use in different food applications’.
Therefore, the Panel decided to use the data from AIPG and Eurogum A/S only in the maximum level exposure assessment scenario for the food categories in which the use of guar gum (E 412) is permitted at QS and when no other data are available.
Appendix A provides data on the use levels of guar gum (E 412) in foods as reported by industry (food industry and gum producers).
3.3.2. Summarised data extracted from the Mintel GNPD
The Mintel's GNPD is an online database which monitors products introductions in consumer packaged goods markets worldwide. It contains information of over 2 million food and beverage products of which more than 900,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 20 out of its 28 member countries and Norway presented in the GNPD.15
For the purpose of this Scientific Opinion, the GNPD16 was used for checking the labelling of products containing guar gum (E 412) within the EU's food products as the GNPD shows the compulsory ingredient information presented in the labelling of products.
According to the Mintel GNPD, guar gum (E 412) is labelled on more than 36,000 food, drink and supplement products with over 20,000 of them published in between 2011 and 2016.
Appendix B presents the percentage of the food products labelled with guar gum (E 412) between 2011 and 2016, out of the total number of food products per food subcategories according to Mintel food classification.
3.3.3. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). New consumption surveys recently17 added in the Comprehensive database were also taken into account in this assessment.18
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database represents the best available source of food consumption data across Europe at present.
Food consumption data from the following population groups: infants, toddlers, children, adolescents, adults and the elderly were used for the exposure assessment. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 3).
Table 3.
Population groups considered for the exposure estimates of guar gum (E 412)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlers | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrena | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlya | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Sweden, UK |
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure estimates. In practice, FoodEx food codes were matched to the FCS food categories.
Food categories considered for the exposure assessment of guar gum (E 412)
The food categories in which the use of guar gum (E 412) is authorised were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
Some food categories or their restrictions/exceptions are not referenced in the EFSA Comprehensive Database and could therefore not be taken into account in the present estimate. This was the case for 14 food categories and may result in an underestimation of the exposure. The food categories which were not taken into account are described below (in ascending order of the FCS codes):
01.7.6 Cheese products (excluding products falling in category 16);
02.3 Vegetable oil pan spray;
04.2.3 Canned or bottled fruit and vegetables, only chestnuts in liquid;
06.4.4 Potato gnocchi;
06.6 Batters;
06.7 Precooked or processed cereals;
08.3.3 Casings and coatings and decorations for meat;
12.1.2 Salt substitutes;
12.8 Yeast and yeast products
13.1.3 Processed cereal‐based foods and baby foods for infants and young children as defined by Directive 2006/125/EC, only gluten‐free cereal‐based foods;
13.1.5.2 Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC, only gluten‐free cereal‐based foods;
14.1.3 Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products, only vegetable nectars;
14.2.4 Fruit wine and made wine;
14.2.5 Mead.
For the following food categories, the restrictions/exceptions which apply to the use of guar gum (E 412) could not be taken into account, and therefore the whole food category was considered in the exposure assessment. This was the case for nine food categories and may result in an overestimation of the exposure:
05.1 Cocoa and Chocolate products as covered by Directive 2000/36/EC;
05.2 Other confectionery including breath refreshening microsweets;
06.4.2 Dry pasta;
07.1 Bread and rolls;
08.2 Meat preparations as defined by Regulation (EC) No 853/2004
08.3.2 Heat‐treated meat products;
09.3 Fish roe;
13.1.1 Infant formulae as defined by Commission Directive 2006/141/EC;
13.1.3 Processed cereal‐based foods and baby foods for infants and young children as defined by Commission Directive 2006/125/EC.
For the following food categories, the differences between subgroups could not be taken into account, and therefore, the whole category was considered in the exposure assessment. This was the case for nine food categories and may result in an overestimation of the exposure:
-
01.6 Cream
-
1
— 01.6.2 Unflavoured live fermented cream products and substitute products with a fat content of less than 20%;
-
2
— 01.6.3 Other creams
-
1
-
08.3 Processed Meat
-
1
— 08.3.1 Non‐heat‐treated processed meat;
-
2
— 08.3.2 Heat‐treated processed meat
-
1
-
11.4 Table top sweeteners
-
1
— 11.4.1 Table Top Sweeteners in liquid form;
-
2
— 11.4.2 Table Top Sweeteners in powder form
-
1
17.1/17.2/17.3 Food supplements, in solid, liquid, syrup‐type or chewable form.
According to Regulation (EC) No 1333/2008, the food supplement category (FC 17) excludes ‘food supplements for infants and young children’. However, in the EFSA Comprehensive database, food supplements are consumed by infants and young children with no information provided on the food supplement type. In the exposure assessment, it was therefore assumed that the food supplements consumed in these population groups were the same as those consumed in the older population groups for which concentration data were supplied, resulting in an overestimation of the exposure to guar gum (E 412) in these two population groups.
Considering that the food category 18 (processed foods not covered by categories 1–17, excluding foods for infants and young children) being by definition unspecific (e.g. composite foods), processed foods, prepared or composite dishes belonging to the food category 18, were reclassified under their main component food categories. Therefore, no food products remain under the food category 18 and the food category as such will not appear as a food contributor of the total exposure estimates.
Overall, 71 food categories were included in the maximum level exposure scenario and 51 in the refined exposure assessment scenario to guar gum (E 412) (Appendix C).
3.4. Exposure estimate
3.4.1. Exposure to guar gum (E 412) from its use as a food additive
The Panel estimated chronic exposure to guar gum (E 412) for the following population groups: infants; toddlers, children, adolescents, adults and the elderly. Dietary exposure to guar gum (E 412) was calculated by multiplying guar gum (E 412) concentrations for each food category (Appendix C) with their respective consumption amount per kilogram of body weight for each individual in the Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only 1 day per subject were excluded as they are considered as not adequate to assess repeated exposure.
This was carried out for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 3). On the basis of these distributions, the mean and 95th percentile of exposure were calculated per survey and per population group. The 95th percentile of exposure was only calculated for those population groups where the sample size was sufficiently large to allow this calculation (EFSA, 2011a). Therefore, in the present assessment, the 95th percentile of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain were not included.
Exposure assessment to guar gum (E 412) was carried out by the ANS Panel based on (1) maximum levels of data provided to EFSA (defined as the maximum level exposure assessment scenario) and (2) reported use levels (defined as the refined exposure assessment scenario) as provided by industry. These two scenarios are discussed in detail below.
As guar gum (E 412) is also authorised in the food categories 13.1.5.1 and 13.1.5.2, a refined estimated exposure assessment scenario taking into account these two food categories was performed to estimate the exposure of infants and toddlers who may eat and drink these FSMP.
Considering that these specific foods are not reported in the EFSA Comprehensive database, but that foods for infants and young children in good health are, the Panel assumed that the amount consumed of FSMP in infants and toddlers is similar to the consumption of comparable foods in infants and toddlers from the general population. Thus, the consumption of FSMP under the food category 13.1.5 was assumed to be the same amount as the formulae and food products of food categories 13.1.1, 13.1.2, 13.1.3 and 13.1.4 (e.g. the consumption of ‘special’ infant formulae for medical purposes was assumed to be the same amount than the infant formulae of the FC 13.1.1).
Concerning the uses of guar gum (E 412) as carriers, there might be food categories where guar gum is used according to annex III and not to annex II. These food categories can only be addressed by analytical data or limits set in the Regulation No 1333/2008 that were not available to the Panel. Therefore, a possible additional exposure from the use of guar gum (E 412) as a food additive in Annex III to Regulation (EC) No 1333/2008 was not considered in any of the exposure assessment scenario.
Maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario is based on the MPLs as set in Annex II to Regulation (EC) No 1333/2008. As guar gum (E 412) is authorised according to QS in almost all food categories, a ‘maximum level exposure assessment’ scenario was estimated based on the maximum reported use levels provided by industry, as described in the EFSA Conceptual framework (EFSA ANS Panel, 2014) (Appendix C).
The Panel considers the exposure estimates derived following this scenario as the most conservative as it is assumed that the population group will be exposed to guar gum (E 412) present in food at the maximum reported use levels over a longer period of time.
Refined exposure assessment scenario
The refined exposure assessment scenario is based on use levels reported by industry. This exposure scenario can consider only food categories for which the above data were available to the Panel.
Appendix C summarises the concentration levels of guar gum (E 412) used in the refined exposure assessment scenario. Based on the available data set, the Panel calculated two refined exposure estimates based on different model populations:
-
The brand‐loyal consumer scenario: It was assumed that a consumer is exposed long term to guar gum (E 412) present at the maximum reported use for one food category. This exposure estimate is calculated as follows:
-
1
— Combining food consumption with the maximum of the reported use levels for the main contributing food category at the individual level.
-
2
— Using the mean of the typical reported use levels for the remaining food categories.
-
1
The non‐brand‐loyal consumer scenario: It was assumed that a consumer is exposed long term to guar gum (E 412) present at the mean reported use. This exposure estimate is calculated using the mean of the typical reported use levels for all food categories.
For the scenario taking into account the FSMP, considering that it is very specific diet, it is assumed that consumers are brand‐loyal and only the results of the brand‐loyal scenario were presented.
Dietary exposure to guar gum (E 412)
Table 4 summarises the estimated exposure to guar gum (E 412) from its use as a food additive in six population groups (Table 3) according to the different exposure scenario's. Detailed results per population group and survey are presented in Appendix D.
Table 4.
Summary of dietary exposure to guar gum (E 412) from its use as a food additive in the maximum level exposure assessment scenario and in the refined exposure scenarios, in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants | Toddlers | Children | Adolescents | Adults | The elderly | |
|---|---|---|---|---|---|---|
| (12 weeks–11 months) | (12–35 months) | (3–9 years) | (10–17 years) | (18–64 years) | (≥ 65 years) | |
| Maximum level exposure assessment scenario | ||||||
| Mean | 122.8–445.0 | 224.0–711.7 | 114.0–517.1 | 61.1–355.7 | 48.5–273.1 | 56.3–290.8 |
| 95th percentile | 247.1–894.2 | 424.2–1021.9 | 228.3–1,182.8 | 106.1–741.6 | 96.6–542.5 | 110.3–586.3 |
| Refined estimated exposure assessment scenario | ||||||
| Brand‐loyal scenario | ||||||
| Mean | 13.7–335.8 | 109.9–449.3 | 48.5–329.7 | 24.1–215.0 | 18.6–154.8 | 19.7–159.9 |
| 95th percentile | 45.1–812.3 | 258.7–663.2 | 110.4–865.0 | 46.7–562.8 | 38.8–380.2 | 38.2–392.9 |
| Non‐brand‐loyal scenario | ||||||
| Mean | 6.0–311.6 | 24.6–93.1 | 23.7–54.4 | 12.2–31.4 | 7.3–20.6 | 6.9–19.8 |
| 95th percentile | 23.4–609.8 | 63.5–280.5 | 47.1–122.5 | 26.8–67.6 | 15.3–41.5 | 14.7–44.1 |
Considering the general population, from the maximum level exposure assessment scenario, mean exposure to guar gum (E 412) from its use as a food additive ranged from 48 mg/kg bw per day in adults to 712 mg/kg bw per day in toddlers. The 95th percentile of exposure to guar gum (E 412) ranged from 97 mg/kg bw per day in adults to 1,183 mg/kg bw per day in children. From the refined estimated exposure assessment brand‐loyal scenario, mean exposure to guar gum (E 412) from its use as a food additive ranged from 14 mg/kg bw per day in infants to 449 mg/kg bw per day in toddlers. The 95th percentile of exposure to guar gum (E 412) ranged from 38 mg/kg bw per day in the elderly to 865 mg/kg bw per day in children. From the refined estimated exposure assessment non‐brand‐loyal scenario, mean exposure to guar gum (E 412) from its use as a food additive ranged from 6 mg/kg bw per day to 312 mg/kg bw per day in infants. The 95th percentile of exposure to guar gum (E 412) ranged from 15 mg/kg bw per day in adults and the elderly to 610 mg/kg bw per day in infants.
From the refined estimated exposure scenario taking into account the foods for special medical purposes, in the brand‐loyal scenario, mean exposure to guar gum (E 412) from its use as a food additive ranged for infants between 325 and 609 mg/kg bw per day, and between 120 and 457 mg/kg bw per day for toddlers. The 95th percentile of exposure to guar gum (E 412) ranged for infants between 912 and 1,555 mg/kg bw per day, and for toddlers between 310 and 743 mg/kg bw per day.
Main food categories contributing to exposure for the general population (i.e. not taking into account the food categories 13.1.5)
Main food categories contributing to exposure to guar gum (E 412) using the maximum level exposure assessment scenario
Table 5: Main food categories contributing to exposure to guar gum (E 412) using maximum usage levels (> 5% to the total mean exposure) and number of surveys in which each food category is contributing
Main food categories contributing to exposure to guar gum (E 412) using the refined exposure assessment scenario
Table 6: Main food categories contributing to exposure to guar gum (E 412) using the brand‐loyal refined exposure scenario (> 5% to the total mean exposure) and number of surveys in which each food category is contributing
Table 7: Main food categories contributing to exposure to guar gum (E 412) using the non‐brand‐loyal refined exposure scenario (> 5% to the total mean exposure) and number of surveys in which each food category is contributing
Main food categories contributing to refined estimated exposure assessment scenario taking into account FCS 13.1.5. Dietary foods for infants and young children for special medical purposes as defined by Commission Directive 1999/22/EC and special formulae for infants to guar gum (E 412) using the refined exposure assessment scenario
For infants, the main food category contributing is foods for infants and young children. For toddlers, the main contributing food categories are foods for infants and young children, soups and broths, unflavoured fermented milk products and flavoured fermented milk products.
Uncertainty analysis
Uncertainties in the exposure assessment of guar gum (E 412) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 9.
Table 9.
Statistically significant changes in the incidence of primary tumours in rats; number of tumour bearing animals/number of animals examined (incidence in %)
| Tumour type | 0 mg/kg diet | 25,000 mg/kg diet | 50,000 mg/kg diet |
|---|---|---|---|
| Male rats | |||
| Adenomas of the pituitary gland | 8/45 (18%) | 17/46 (37%)* | 17/43 (40%)* |
| Carcinomas of the pituitary gland | 5/45 (11%) | 0/46 (0)** | 2/43 (5%) |
| Adenomas and carcinomas of the pituitary gland (all) | 13/45 (29%) | 17/46 (37%) | 19/43 (44%) |
| Female rats | |||
| Phaeochromocytoma of the adrenal glands (benign) | 0/50 (0) | 5/50 (10%)* | 6/50 (12%)* |
| Phaeochromocytoma of the adrenal glands (all) | 1/50 (2%) | 5/50 (10%) | 6/50 (12%) |
* Significant increase (p < 0.05); ** significant decrease (p < 0.05).
Table 8.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/− |
| Use of data from food consumption survey of a few days to estimate long‐term (chronic) exposure for high percentiles (95th percentile) | + |
Correspondence of reported use levels to the food items in the EFSA Comprehensive Food Consumption Database:
|
+/− |
| Food categories excluded from the exposure assessment due to missing FoodEx linkage (n = 14/86 food categories) | − |
| Food categories selected for the exposure assessment: inclusion of food categories without considering the restriction/exception (n = 9/86 food categories) | + |
| Food categories included in the exposure assessment: data not available for certain food categories which were excluded from the exposure estimates (n = 71/86 food categories taken into account for the maximum level exposure scenario, n = 51/86 food categories taken into account for the refined exposure scenario) | − |
Maximum level exposure assessment scenario:
|
− + |
Refined exposure assessment scenarios:
|
− +/− |
| Uncertainty in possible national differences in use levels of food categories | +/− |
+, uncertainty with potential to cause overestimation of exposure; −, uncertainty with potential to cause underestimation of exposure.
Overall, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the real exposure to guar gum (E 412) as a food additive in European countries for the maximum level exposure scenario and for the refined scenario if it is considered that the food additive may not be used in food categories for which no usage data have been provided. The Panel noted that food categories which may contain guar gum (E 412) due to carry‐over (Annex III, Part 1, 2, 3, 4, 5 (section A)) were not considered in the current exposure assessment.
Considering the exposure to guar gum (E 412) for infants and young children eating FSMP, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure in European countries for the brand‐loyal refined scenario.
3.4.2. Exposure via other sources
Exposure to guar gum due to the following uses were not considered in this opinion.
Guar gum as ingredient in food supplements, slimming products and other foods
Uses of guar gum in dietary fibre supplements, e.g. for body weight–energy regulation and for supportive treatment of obesity are described. In these products, guar gum is regarded as a satiating material (Papathanasopoulos and Camilleri, 2010; Slavin and Green, 2007; Saper et al., 2004; Wielinga, 2010).
Pharmaceutical uses
Information on pharmaceutical uses was obtained by searches of the literature, the websites of national competent authorities for medicinal products and publicly available SmPC (summary of product characteristics) on the nationally available authorised products indicated to EFSA by EMA communication (Documentation provided to EFSA, document 4).
Guar gum is used as an active ingredient or excipient in pharmaceutical products (Martindale, 2014).
As an active ingredient, in authorised medicinal products for oral use, the daily dosage of guar gum is reported to be up to 25 g (up to five times 5 g daily). The indications list the medical use in addition to special diets and/or exercise in hypercholesterolaemia and hyperglycaemia and/or the use for reduction in postprandial serum glucose values. In most cases, the usage in children and adolescent is not foreseen, while some products would include adolescents of 15 years of age and older. Usage in pregnancy and lactation is possible if medically needed.
As an excipient, the daily dosage of guar gum is much less than the dosages used as active ingredient.
Further uses of guar gum, include uses in cosmetic products (emulsions, lotions, creams, toothpastes), oil industry, textile industry, paper manufacturing, production of explosives and cables, and in the processing of minerals and effluent purification (Merck Index, 2006; Ullmann, 2007).
3.5. Biological and toxicological data
3.5.1. Absorption, distribution, metabolism and excretion
There is evidence that certain high molecular weight dietary polysaccharides, such as gums, could be partially broken down in the large intestine of man. In addition to intermediate metabolites, such as lactate, acrylate or fumarate, the main end products of this colonic anaerobic digestive process are SCFAs such as acetic, propionic and butyric acids, which are absorbed from the colon (Cummings and Englyst, 1987).
In vitro studies
In vitro gastrointestinal digestion models showed that guar gum was resistant to digestion when contact to juices of the stomach and small intestine of humans (Semenza, 1975; unpublished, as reported in JECFA, 1975b). Ex vivo, guar gum may be degraded by the intestinal microflora after feeding to rats (Tsai and Whistler, 1975; unpublished, as reported in JECFA, 1975b, Booth et al., 1963).
A total of 188 strains from 11 species of Bacteroides found in the human colon were surveyed for their ability to ferment mucins and plant polysaccharides including gums (Salyers et al., 1977a). Many of the Bacteroides strains tested were able to ferment a variety of plant polysaccharides, including amylose, dextran, pectin and gums. The ability to utilise mucins and plant polysaccharides varied considerably among the Bacteroides species tested. Under the experimental conditions, guar gum was found to be fermented by three different strains of Bacteroides.
A total of 154 strains from 22 species of Bifidobacterium, Peptostreptococcus, Lactobacillus, Ruminococcus, Coprococcus, Eubacterium and Fusobacterium, which are present in high concentrations in the human colon, were surveyed for their ability to ferment 21 different complex carbohydrates including gums (Salyers et al., 1977b). Among them, guar gum was only fermented by one strain of Bifidobacterium and by five strains of Ruminococcus bacterial species.
Fermentations of 10 polysaccharides including guar gum, by species of the family Enterobacteriaceae (Klebsielleae and other facultative Gram‐negative Bacilli) were examined by Ochuba and Von Riesen (1980). Guar gum was not fermented by any of the species tested.
TomLin et al. (1988) determined the range of human colonic bacteria that could ferment guar gum, and sought evidence for collaboration between different strains of colonic bacteria. Single strains of a variety of species of bacteria isolated from human faeces were incubated with guar gum. Only seven Bacteroides on 57 different strains of bacteria tested could alter the viscosity and pH of guar gum. However, only two strains (one Bacteroides distasonis and one Bacteroides thetaiotaomicron) fermented guar gum. The results of this study suggest that different species of Bacteroides can interact to degrade and ferment guar gum in the intestinal tract.
Adiotomre et al. (1990) investigated the effects of dietary fibres, including gums, on caecal fermentations using fresh human microflora. Evolution of SCFAs and water‐holding capacity after fermentation were also measured. Among other gums, guar gum yielded a large amount of total SCFAs (71.4 vs 15.5 mmol/L for controls). The major SCFAs produced were acetic, propionic and butyric acids, with smaller amounts of isobutyric, valeric and isovaleric acids. By contrast, the amount of water held by 1 g of the fermented residue was low in case of guar gum (1.87 vs 0.91 g/g for controls).
A total of 290 strains of 29 species of bifidobacteria of human and animal origin (mainly of faecal origin) were surveyed for their ability to ferment complex carbohydrates including gums (Crociani et al., 1994). The substrates fermented by the largest number of species were d‐galactosamine, d‐glucosamine, amylose and amylopectin. Guar gum was shown to be fermented by only one species tested.
In another in vitro study, guar gum was fermented using dog faeces as the source of inoculum (Sunvold et al., 1995a). Organic matter disappearance and SCFAs production were measured after 6, 12 or 24 h of incubation. Whatever the duration of incubation was, the organic matter disappearance, and acetate, propionate and butyrate productions were higher in case of guar gum than for other gums like acacia gum and particularly karaya or xanthan gum. Identical conclusions were drawn from a similar study using the same substrates fermented by cat faecal microflora (Sunvold et al., 1995b).
In vivo studies
The digestibility of guar gum was reported to be efficient in rats (Booth et al., 1963). Weight losses of weanling albino rats (n = 5; no more information available fed 400 mg/rat per day for 7 days (equivalent to approximately 23 mg/kg bw per day) that were returned to basal diet for 2 days showed very low net weight gain (+ 1,000 mg), whereas digestibility19 of guar gum was high (76%) in this assay.
Rats (strain and sex not specified) were fed guar gum (concentration not specified) for 21 days. Thereafter, rat large gut microflora of these conditioned animals partially hydrolysed guar gum in vitro (Towle and Schranz, 1975; unpublished, as reported in JECFA, 1975b).
Purdue rats (n = 5/sex) on a mannose‐free diet were given 1% guar gum in the diet for 18 h in order to test the digestibility of the gum (Tsai and Whistler, 1975; unpublished, as reported in JECFA, 1975b). The main part of the mannose (88–100%) fed as guar gum was excreted in the faeces over a total of 30 h. The observed decrease in chain length of galactomannan may have occurred due to the activity of the intestinal microflora of the rats, since mammals are not known to possess β‐mannosidase. The rate of galactose production was not determined in this study.
One study in rats suggests that guar gum is bioavailable (Harmuth‐Hoene et al., 1978). Sprague–Dawley rats (n = 10) were fed 5% guar gum in a diet containing 10% casein. The apparent digestibility of casein was decreased as suggested by statistically significant (p < 0.01) decreases in nitrogen excretion from urine and faeces in animals supplemented with guar gum. According to the authors, the observed statistically significant (p < 0.001) increase in total faecal dry matter in animals supplemented with guar gum over control values (accounting for 30% of ingested guar gum), implies that 70% of guar gum was absorbed (bioavailable), following degradation by intestinal microflora.
Edwards and Eastwood (1995) investigated the caecal and faecal SCFAs and stool output in rats fed on diets containing non‐starch polysaccharides, including guar gum. The basal diet of male Wistar rats (n = 7) was supplemented or not with 50 g/kg of guar gum for 28 days. Faeces were then collected over 2 days and caecal contents obtained post‐mortem. Caecal and faecal wet and dry weights, and SCFA were measured. Guar gum increased caecal but not faecal SCFA in the rat in vivo. According to the authors, guar gum is rapidly fermented and increased significantly the molar proportion of acetic and butyric acids in faeces.
Overall, data on in vitro degradation by human gastrointestinal fluids and on in vivo digestibility of guar gum in animals demonstrated that this compound would not be absorbed intact or hydrolysed by digestive enzymes. However, guar gum would be fermented with production of SCFAs such as acetic, propionic and butyric acids, during its passage through the large intestine by strains of bacteria found in the human colon. Based on the available knowledge on the role of SCFA as end products of the fermentation of dietary fibres by the anaerobic intestinal microflora (den Besten et al., 2014; Topping and Clifton, 2001), the Panel considered that their potential formation as fermentation products from guar gum does not raise any concern. Despite the absence of convincing in vivo study in humans, the Panel considered that these data indicate that guar gum would most probably not be absorbed but significantly fermented by enteric bacteria in humans.
3.5.2. Acute toxicity
In acute toxicity studies B6C3F1 mice (5 animals/sex per group) and F344 rats (5 animals/sex per group) were given a single oral dose of 420 mg/kg bw per day guar gum in water by gavage. All animals were killed on day 15. In both species, no toxicity or compound‐related toxic effects were observed (NTP, 1982).
In a range finding study groups of B6C3F1 mice and F344 rats (5 animals/sex per group) were fed diets containing 0, 6,300, 12,500, 25,000, 50,000 or 100,000 mg guar gum/kg diet (equal to 945, 1,875, 3,750, 7,500, 15,000 mg/kg bw per day for mice and 315, 625, 1,250, 2,500, 5,000 mg/kg bw per day for rats and) guar gum for 2 weeks. All animals were killed on day 15. In both species, no compound‐related toxic effects were observed (NTP, 1982).
It is reported that guar gum given orally as a suspension in corn oil at a single dose of 5,000 and 10,000 mg/kg bw per day for 5 days to Sprague–Dawley rats (5 males) caused no toxic effects or deaths. Only a transient depression (not specified but presumably of the animal's behaviour) for a few hours after application was observed at the dose of 10,000 mg/kg bw per day (Stanford Research Inst., 1972).
A total of 18 rats (sex and strain not specified) were given guar gum in cocoa butter at a concentration of 30% of their diet for 48 h (equivalent to 36,000 mg/kg bw per day). No adverse effects were reported (Krantz et al., 1948; as reported by JECFA, 1975). Feeding 27% (equivalent to 32,400 mg/kg bw per day) guar gum to rats (number, sex and strain not specified) for 7 days was reported to have caused 7/10 deaths probably due to intestinal blockage (Anonymous, 1964 as reported by JECFA, 1975).
Ten adult Osborne–Mendel rats (both sexes) received by gavage a suspension in corn oil of 0.2 g guar gum/mL (Graham et al., 1981). Overnight fasted male and female rats weighting approximately 450 and 225 g, respectively, received an average of 7,060 mg/kg bw per day (5,790–8,570 mg/kg bw per day) in multiple equal applications (not exceeding three) separated by periods of at least 2 h. Observation of surviving animals after treatment lasted 14 days. The LD50 for males was reported to be 7,350 mg/kg bw per day and for females 6,770 mg/kg bw per day.
In summary, guar gum is regarded as presenting low acute oral toxicity. No acute toxicity was observed in studies in mouse, rat, rabbit and hamster at doses amounting up to 6,000–9,000 mg/kg bw per day (Stanford Research Inst., 1972; NTP, 1982; Sax, 1984).
3.5.3. Short‐term and subchronic toxicity
Mice
In a dose‐finding study groups of B6C3F1 mice (10 animals/sex per group) were fed diets containing 0, 6,300, 12,500, 25,000, 50,000 or 100,000 mg guar gum/kg diet (equal to 945, 1,875, 3,750, 7,500, 15,000 mg/kg bw per day) for 13 weeks (NTP, 1982). The investigations included clinical signs, body weights, feed consumption and histopathology of all major organs. Haematology, clinical chemistry and urine were not investigated. Mortality was checked twice daily and animals were weighed weekly. All animals were sacrificed at day 91 and gross and microscopic examinations were performed on major tissues, organs and all gross lesions from killed animals and from animals found dead unless precluded in whole or in part due by autolysis or cannibalism. Histopathological examinations of more than 20 tissues of all controls and animals were performed. One female mouse of the highest dose group died. In female mice receiving 50,000 and 100,000 mg guar gum/kg diet, the weight gain was depressed by 15% and 26%, respectively, compared to controls. Male mice showed a depressed weight gain not higher than 9%. In treated mice of either sex, the feed consumption was comparable with or higher than that of control mice. No compound‐related clinical signs or histopathological effects were observed. On the basis of this data, a no‐observed‐adverse‐effect‐level (NOAEL) for mice of 100,000 mg guar gum/kg diet (equal to 15,000 mg/kg bw per day, the highest dose tested) could be derived by the Panel.
Rats
Albino rats (n = 5; no more information available) fed 6% (w/w) guar gum in the diet for 91 days (equivalent to approximately 7,200 mg/kg bw per day) did not show significant changes in weight gains compared to controls (Stanford Research Inst., 1972).
Groups of rats (10 animals/sex per group, strain not identified) were fed diets containing 0, 1%, 2% or 5% guar flour in the diet for 90 days (equivalent to 0, 1,200, 2,400 or 6,000 mg/kg bw per day) (Til et al., 1974 as referenced by JECFA, 1975b). General behaviour, appearance and survival were not noticeably affected by feeding guar gum. In males fed 2% and 5% guar gum, growth was decreased and feed efficiency was slightly diminished in males of the highest dose group. Blood urea nitrogen values were slightly increased in males receiving 5% guar gum. In both sexes of animals from the 2% and 5% groupsm the relative weight of the caecum was increased and a relative weight increase in the thyroid in males from those groups was also reported. Haematology, urinalysis, serum enzyme activities, blood sugar levels, gross and histopathology was reported not showing effects attributable to the ingestion of guar gum. The Panel noted that the reference of this study as given by JECFA refers to locust bean gum, and therefore, it could not be possible to derive a toxicological reference value given the lack of precise information from this study.
The Panel noted that an increased caecum weight in animals fed high amounts of carbohydrates is considered a physiological response to an increased fermentation due to a carbohydrate‐induced modification on the composition of the intestinal microbiota. Increased caecum weight has been observed in rats fed carbohydrates other that guar gum (Leegwater et al., 1974; Licht et al., 2006). Animals fed diets containing potato starch, inulin or oligofructose had significantly higher caecum weights and lower pH values than the reference animal group (Licht et al., 2006). Different groups of animals fed modified diets containing increased concentration of potato starch, hydroxypropyl starch and hydroxypropyl distarch glycerol showed increases in the relative caecal weights, filled and emptied, with increasing concentrations of the various hydroxypropyl starches. These increases were accompanied by increased severities of diarrhoea that was related to an increased osmotic activity of the caecal fluid in the animals (Leegwater et al., 1974). The authors hypothesised that dietary components not completely digested and/or absorbed in the small intestine, and further fermented by the gut microflora, enhance the amounts of osmotically active material resulting in an increase in water retention and the animals drinking more water leading to the caecum distention to a size larger than normal.
Weanling albino rats (5 males/group, strain not specified) received basal diet (control) or basal diet plus 6% guar gum for 91 days (equivalent to 7,200 mg/kg bw per day). Between the two groups, no differences in feed efficiency (weight gain/feed intake) were observed. No significant alterations in haemoglobin, in erythrocyte and leucocyte counts or in organ weights were observed (Booth et al., 1963).
A total of 15 rats (no more information) were fed a diet containing 0.5% guar gum (equivalent to 600 mg/kg bw per day) and varying amounts of water for 21 days (Keane et al., 1962 cited by JECFA, 1975b). Increased weight gain and protein efficiency with higher water content were reported.
Newly weaned Sprague–Dawley rats (10 males) were fed a diet containing 2% guar gum for 36 days (equivalent to 2,400 mg/kg bw per day). Guar gum was reported as not influencing the growth of rats or the digestibility of the diet (Vohra et al., 1979).
Wistar rats (10 young and 15 old males) were fed 8% guar gum in diet for 6 weeks (equivalent to 9,600 mg/kg bw per day). Young rats responded to guar gum consumption with reduced body weight gain and improved carbohydrate tolerance; these responses were not seen in older rats (Track et al., 1985).
Growing Wistar rats (5 males) were fed on a diet containing 5% guar gum for 3 weeks. In comparison to the control group, feed consumption slightly decreased, resulting in a lower increase in body weight. Feeding guar gum resulted in increased weight of the gastrointestinal tract, the value for digestible and metabolisable energy and efficiency of energy utilisation declined but protein utilisation remained unchanged (Takahashi et al., 1994).
Six groups of Osborne–Mendel rats (approximately 25 animals/sex per group, 4 weeks old) were fed guar gum at 0%, 1%, 2%, 4%, 7.5%, and 15% dietary levels for 13 weeks (equivalent to approximately 1,200, 2,400, 4,800, 9,000 and 18,000 mg/kg bw per day) (Graham et al., 1981). Feed and water were allowed ad libitum, animals were observed daily and records of weights were kept weekly. An analysis of covariance was used to adjust body weights at weeks 4, 8 and 12 for initial weight. Twenty‐three males and 19 females died between weeks 2 and 5 and were evenly distributed among all the groups. The mortality was not only attributed to treatment but also to housing conditions that changed during the study. Male rats fed guar gum at the highest doses (7.5% and 15%) were reported to consume less diet (4.9% and 8.8% less, respectively) than controls. Female rats did not consume significantly less diet at those doses (1.2% and 0.3% less, respectively). At those highest doses (7.5% and 15%), male rats had significantly less body weights than controls at week 12. Female rats from all doses tested showed significantly less body weight compared to controls at week 12. Since weight gain was significantly reduced without ‘drastic’ reductions in feed consumption, it was suggested by the authors that ‘utilisation’ of guar gum by the rats was probably poor. Haematology analysis conducted on 10 males and 10 females of each group showed lower haematocrit values in males at all doses tested but with borderline statistically significance. In females, haemoglobin levels and erythrocyte counts were significantly decreased at one dose but not dose‐related. Clinical chemistry on 22 different parameters showed irregular changes and only glucose values in males showed a reduced linear trend with doses. From adjusted mean organ weights measured (livers, kidneys, spleens, hearts and testes), only the liver and kidney weights of males showed significant changes. However, only kidney weights showed a dose‐related decreasing trend. In females, a dose‐related decreasing trend was observed for liver weights, although the authors considered as borderline the significance of decreased organ weight at the dose 7.5% guar gum. Full histological examination of the control group (n = 44) and group fed 15% guar gum (n = 36), as well as short screen of some tissues from the remaining doses were normal for the liver and kidney. Only bone marrow showed atrophy characterised by proportional reductions of all cellular elements. No other tissue examined was reported having consistent histopathological alterations that could be attributed to exposure to guar gum. The authors concluded that the lack of more consistent linear trends in some parameters may suggest that the duration of the feeding was too short for patterns to be established in this study. Based on the lack of histopathological effects for most organs and a lack of a dose‐related effect on bone marrow, the Panel could identified a NOAEL of 18,000 mg/kg bw per day from this study.
In a dose‐finding study groups of F344 rats (10 animals/sex per group) were fed diets containing 0, 6,300, 12,500, 25,000, 50,000 or 100,000 mg guar gum/kg diet (equal to 315, 625, 1,250, 2,500, 5,000 mg/kg bw per day) for 13 weeks (NTP, 1982). The investigations included clinical signs, body weights, feed consumption and histopathology of all major organs. Haematology, clinical chemistry and urine were not investigated. Mortality was checked twice daily and animals were weighed weekly. It is reported that all animals were sacrificed at day 91, and gross and microscopic examinations were performed on major tissues, organs and all gross lesions from killed animals and from animals found dead excepted those in whole or in part due by autolysis or cannibalism. Histopathological examinations of more than 20 tissues of all controls and animals were performed. Two female rats died, one in the 50,000 mg guar gum/kg diet and one in the 100,000 mg guar gum/kg diet group. A dose‐related slight decrease in feed consumption was reported for rats of either sex; feed consumption by rats in the 100,000 mg guar gum/kg diet group was 80% that of the controls. The weight gain of male rats in this group was decreased by 16% as compared to controls. In female rats, weight gain was not affected by treatment. No compound‐related clinical signs or histopathological effects were reported. On the basis of this data, a NOAEL for rats of 100,000 mg guar gum/kg diet (equal to 5,000 mg/kg bw per day, the highest dose tested) could be derived by the Panel.
Dogs
JECFA evaluation of guar gum refers to a study on four groups of five male and five female Beagle dogs fed 0%, 1%, 5% or 10% (equivalent to approximately 0, 750, 3,750 and 7,500 mg/kg bw per day) of a precooked mixture of gum blend (no further details available) for 30 weeks (Cox et al., 1974, unpublished report as cited by JECFA, 1975b). Gut hypermotility, soft bulky faeces and reduced digestibility were observed at the highest dose and were considered of no toxicological significance. No adverse effects were noted on haematological and urinary parameters (no further details), and on gross and histopathological and ophthalmological examinations.
Monkeys
Two monkeys (no further information) were given 1 g guar flour daily in their diet. Growth, well‐being and haematology parameters as RBC, WBC, Hb, and urea were reported to remain normal. One animal died after 16 months and the other was sacrificed after 24 months. Gross and histopathology showed no abnormal effects (Krantz, 1948; unpublished, cited in JECFA, 1975b).
Overall, in short‐term and subchronic studies in mice, rats, dogs and monkeys, no adverse effects were observed at the highest dose tested.
3.5.4. Genotoxicity
In vitro studies
Guar gum was tested in vitro in bacterial test with Salmonella Typhimurium strains G46 and TA1530 (Stanford Research Inst., 1972). Results were reported as negative but the assay was limited only testing two strains with apparently no metabolic activation included.
Guar gum was tested in an in vitro yeast assay for mitotic recombination frequency in Saccharomyces cerevisiae D3 at doses of 1% and 5%, both concentrations being toxic for the indicator organisms (no information provided on metabolic activation) (Stanford Research Inst., 1972). Results showed an increased mitotic recombination of approximately three‐ and sixfold increases, but the doses tested induced cell toxicity. The Panel observes that the doses tested in this assay are higher than normally recommended and that nowadays yeast test are considered obsolete, the OECD technical guideline on yeast assays (OECD TG 481) being recently withdrawn.
Guar gum was tested in vitro in Salmonella Typhimurium strains TA97, TA98, TA100, TA1535 and TA1537 with and without metabolic activation (liver S9 fractions from Aroclor‐induced male Sprague–Dawley rats and Syrian hamsters) (Zeiger et al., 1992). The concentrations of guar gum tested ranged from 100 to 10,000 μg/plate. It is stated that chemicals that were poorly soluble were tested up to doses defined by their solubilities; the solvent used for guar gum was dimethylsulfoxide (DMSO). The assays were performed first using 10% of S9 and if the results were negative a second test was performed using 30% of S9. Guar gum was negative with all strains with and without metabolic activation. The Panel noted that guar gum was not tested in TA102 or E. coli WP2 and considered the negative results reliable with limitations.
Guar gum was further tested in an in vitro cytogenetic test in human fetal normal lung fibroblast cells (WI‐38) only performed without metabolic activation (Stanford Research Inst., 1972). Dose levels tested were 10, 100 and 1,000 μg/mL guar gum. Reported results showed a large number of aberrations (multipolar spindles, fragments, multiple aberrations) in cells treated with guar gum. The total number of cells with aberrations was even greater than that found with the positive control, a known genotoxic carcinogen (triethylenemelamine (TEM)). However, the Panel noted that the assay did not receive further validation and is presently not used in genotoxicity testing.
In vivo studies
Guar gum was assessed for its genotoxic properties in vivo in a host‐mediated assay in mice, in a rat bone marrow chromosomal aberration assay and in a rat dominant lethal assay. In all assays performed, the treatment regime used consisted of three dose levels 30, 2,500 and 5,000 mg/kg bw administered acutely by oral gavage, as single dose or subacutely using the same dosages as those in the acute study, each day for five consecutive days, 24 h apart (Stanford Research Inst., 1972).
In the host‐mediated assay, a number of 6–10 mice were allocated to each of the five groups for acute treatment and the four groups for subacute treatments. Three dose levels as described above and negative controls for both acute and subacute treatments were employed. Positive control groups were also included in the acute treatment. The indicator organisms used in this study were two histidine auxotroph S. Typhimurium strains (his G‐46, TA1530) for induction of reverse mutation and a diploid strain (D3) of S. cerevisiae for mitotic recombination. Results obtained indicated that tester strains S. Typhimurium TA1530, G‐46 and S. cerevisiae D3 did not show any increase in revertants and mitotic recombination, respectively, both in the acute and subacute treatments. The Panel noted that this assay did not receive further validation and it is not currently employed for genotoxicity testing.
In the rat bone marrow chromosomal aberration assay, a total of 59 animals in the acute treatment and 16 in the subacute treatment were employed. In the acute studies, animals were sacrificed 6, 24 and 48 h after dosing and in the subacute treatment 6 h after the last dose. Bone marrow erythrocytes were used to prepare cytogenetic slides for chromosome analyses. Fifty metaphase spread for animal were scored for chromosomal aberration analyses. Results obtained indicated that guar gum did not induce statistically significant increases in chromosomal aberrations at any dose level and sampling time employed both in the subacute and acute treatments. However, no indication of bone marrow toxicity was reported. The Panel noted that the study is close to current criteria for acceptability of this type of assays with the exception that a limited number of 50 metaphases per animal were scored.
In the rat dominant lethal assay, a total of 10 male random‐bread rats were allocated to each of the five groups, (e.g. three dose levels as described above and positive and negative control) for both acute and subacute treatments. Following treatments, the males were sequentially mated with two untreated virgin females per 7 days a week for 8 weeks. At the end of 7 days, females were removed from the males and housed separately until sacrifice. Females were sacrificed at 15 days after separation from males, and at necropsy, the uterus were analysed for early deaths, late fetal deaths and total implantations. Results obtained showed clear negative results.
The results obtained in these in vivo assays indicate that guar gum is not genotoxic. However, the Panel doubts about the relevance of these results due to the negligible absorption of guar gum.
Based on the available information, the Panel considers that guar gum is unable to elicit genotoxic effects at the gene and chromosomal level. This lack of genotoxic effects has been observed in assays in vitro and in vivo. However, from an in vitro cytogenetic assay, marked and statistically significant increases in cells showing multipolar spindles have been observed indicating a possible interference with the mitotic spindle functioning and consequent induction of aneuploidy in vitro, not covered by the available in vivo studies. On this basis, the Panel concludes that, although the findings observed in vitro have been obtained from a study following ‘non‐standard’ methodology no longer used and without indication of the purity of test substance, they cannot be dismissed but rather clarified in an additional robust in vitro study using a guar gum sample complying with the current specifications.
To this aim, an in vitro micronucleus test compliant with the OECD TG 487 and Good Laboratory Practice (GLP) regulations was then requested and subsequently performed following a public call for data.
In an unpublished report (Document provided to EFSA n. 8), guar gum (lot OG/0280/06), a powder with no indication of purity, was evaluated in an in vitro micronucleus assay in human peripheral blood lymphocytes for its ability to induce chromosomal damage or aneuploidy in the presence and absence of rat liver S9‐mix fraction as an in vitro metabolising system. Cells were stimulated for 44–48 h with phytohaemagglutinin to produce exponentially growing cells, and then treated for 4 h (followed by 24–28 h recovery) with 0, 62.3, 125.3, 249.8 and 500.3 of guar gum powder, the latter being the maximum practicable concentration in culture medium, in the absence and presence of S9‐mix. The levels of cytotoxicity (reduction in replication index) at the top concentrations were 15.8% and 16.2%, respectively. In a parallel assay, cells were treated for 24–28 h with 0, 62.3, 125.3, 249.8 and 500.3 of guar gum powder in the absence of S9‐mix with no recovery period. The top concentration induced 14.1% cytotoxicity. There were two replicate cultures per treatment and 1,000 binucleate cells per replicate (i.e. 2,000 cells per dose) were scored for micronuclei. Thus, the study design complies with current recommendations (OECD Guideline 487), and acceptable top concentrations in terms of maximum solubility of test compound in culture medium, were achieved in all parts of the study. No evidence of chromosomal damage or aneuploidy was observed as frequencies of MNBN cells were not significantly different from concurrent controls and fell within historical control ranges for all treatments with guar gum powder in the presence or absence of S9‐mix metabolic activation.
Overall, the Panel noted that based also on the new available data guar gum does not raise a concern for genotoxicity.
3.5.5. Chronic toxicity and carcinogenicity
Mice
Groups of B6C3F1 mice (50 animals/sex per day) were fed diets containing 0, 25,000 and 50,000 mg guar gum/kg diet (equal to 0, 3,750 and 7,500 mg/kg bw per day) for 103 weeks (NTP, 1982, Melnick et al., 1983). After week 20 of treatment, mean body weights of females from the highest dose group were statistically significant lower than those of untreated controls. In treated mice of either sex, feed consumption was statistically significant lower than controls. Upon histopathological examination, male mice from both treated groups showed statistically significant lower incidence of hepatocellular carcinomas than controls. No statistically significant differences in hepatocellular adenomas incidences were reported in those male mice. The combined incidence of hepatocellular adenomas or carcinomas in male mice remained statistically significantly lower (p < 0.05) in the highest dose group compared to controls. In this study, female mice did not show any statistically significant increases in neoplasms.
Guar gum was considered by the authors as not carcinogenic in mice under the conditions of this bioassay (NTP, 1982).
Rats
Groups of F344 rats (50 animals/sex per dose) were fed diets containing 0, 25,000 and 50,000 mg guar gum/kg diet (equal to 0, 1,250 and 2,500 mg/kg bw per day) for 103 weeks (NTP, 1982, Melnick et al., 1983). The investigations included clinical signs, body weights, feed consumption and histopathology of all major organs. Haematology, clinical chemistry and urinalysis have not been performed. No substance‐related effects on survival or clinical signs were reported. After week 40 of treatment, mean body weights of females from the highest dose group were statistically significant lower than those of untreated controls. In female rats receiving 50,000 mg guar gum/kg diet, the weight gain was depressed by 13% compared to controls. Male rats did not show a marked depressed weight gain at the end of the study. In treated rats of either sex, feed consumption was statistical significantly less than controls. Upon histopathological examination, the only major finding in male rats was statistically significant increased incidences of adenomas (benign) of the pituitary gland as compared to control animals from the study (see Table 9). The increased incidences showed an apparent dose‐dependency. However, the same male rats showed decreased incidences of carcinomas (malignant) of the pituitary gland in both treated groups as compared to control animals from the study. This decrease incidence of carcinomas of the pituitary gland became statistical significant for the intermediate dose animals (25,000 mg guar gum/kg diet). When both incidences of adenomas and carcinomas of the pituitary gland were considered together, no statistically significant differences were noticed compared to controls animals from the study. Other neoplasms, non‐neoplastic degenerative and inflammatory lesions were observed in male rats but they were reported as commonly of the type, incidences and distribution observed in ageing rats of this strain and in control animals.
Female rats did not show statistically significant increases in adenoma or in carcinoma incidences of the pituitary gland. The only major finding in female rats from both treated groups (25,000 and 50,000) was an increased incidence of benign phaeochromocytomas as compared to control animals of the study. However, no changes in the incidences of malignant phaeochromocytomas were reported and the combined incidences of benign and malignant phaeochromocytomas in these animals were not statistically significant. Male rats did not show any statistically significant incidence differences on phaeochromocytomas of the adrenal gland (NTP, 1982). Guar gum was considered by the authors as not carcinogenic in rats under the conditions of this bioassay (NTP, 1982). The Panel agreed with these conclusions.
Phaeochromocytomas originate from chromaffin cells of the adrenal medulla. They are rare in all species except rats, occurring spontaneously with high frequencies to up 32% in males of some rat strains (Greim et al., 2009). Phaeochromocytomas can be induced by hormones and other non‐genotoxic agents in rats (Tischler et al., 2004). The underlying biochemical mechanisms are not only clearly defined but also has been suggested that they might originate from interference with several enzymes including those involved in the catecholamine synthesis pathway in a genetically predisposed environment, receptor kinase, hypoxia‐inducible factor, succinate dehydrogenase or fumarate hydratase (Greim et al., 2009). Hyperplasia changes in the adrenal medulla can give rise to benign or malignant phaeochromocytomas, but it is not clear if the hyperplasia changes are rather a physiological adaptation or a direct effect of a chemical since, in most cases, no correlation has been observed between exposure to a chemical and increased incidences of phaeochromocytomas (Greim et al., 2009). Intriguingly, spontaneous phaeochromocytomas are much less frequent in female rats, the historical controls incidences ranging from 0.5% to 6.3% (Greim et al. (2009). The authors observed that in the 1990s the spontaneous incidences of phaeochromocytomas in long‐term studies carried out by NTP ranged between 28% and 38% becoming lower in studies carried out from 2000 on, the spontaneous incidences ranging from 12% to 16%. According to the authors, the main reason for this was a change in the diet in the NTP studies which also influenced the survival rate and also reduced the incidences of pituitary gland tumours and the incidence and severity of nephropathy in males (Greim et al., 2009). The Panel noted that the NTP studies evaluated in this opinion where carried out by NTP in the early 1980s.
Overall, the Panel considered guar gum as not carcinogenic. The Panel could derive a NOAEL of 2,500 mg/kg bw per day, the highest dose tested, from the rats study and a NOAEL of 7,500 mg/kg bw per day, the highest dose tested, from the mice study.
3.5.6. Reproductive and developmental toxicity
In all studies performed by the Food and Drug Res. Lab. (FDRL 1972) described below, body weights were recorded at regular intervals during gestation and all animals were observed daily for appearance and behaviour. Animals were administered different doses of guar gum of unknown specifications suspended in anhydrous corn oil by gavage (dose volume 1 mL/kg bw per day in all groups except in the highest dose group with dose volume 3 mL/kg bw per day in the rabbit study); the control groups were vehicle‐treated. All dams were subjected to caesarean section, and the numbers of implantation sites, resorption sites, live and dead fetuses, and body weights of live pups were recorded. All fetuses were examined grossly for sex distribution and for external abnormalities (one‐third detailed visceral examination and two‐third stained and examined for skeletal defects).
Mice
Pregnant CD‐1 mice (18–21 animals/group) were treated by oral gavage once daily from gestation day (GD) 6 to 15 with doses of 0, 8, 37, 170 and 800 mg/kg bw/day of guar gum in corn oil (FDRL, 1972). Mortality was 6/19 mice dosed with 800 mg/kg bw per day and three litters lost. At necropsy on GD 17, the dams appeared to be completely normal and the number of implantations and live fetuses was comparable to the control group. The surviving dams of the 800 mg/kg bw per day group appeared to be completely normal. Doses up to 170 mg/kg bw per day had no noticeable effects on implantation or on maternal and fetal survival. The numbers of live or dead fetuses, the average implantation sites and fetal weights did not differ among the groups. The sex distribution of fetuses was not affected by the treatment. The two highest doses tested the percentages of litters with resorptions were increased but the highest dose tested showed litters with complete resorptions (14%). The number of abnormalities seen in skeletons at fetal pathological examination of the guar gum‐treated groups did not differ from the number in vehicle‐treated dams of the control group. Moreover, no abnormalities were observed in the soft tissues of the fetuses examined from the treated groups. The Panel noted that the effects related to developmental toxicity, mainly resorptions, fetal death and retardation, were due to the administration of guar gum at the maternal lethal level, being the highest dose tested (800 mg/kg bw per day).
Rats
Pregnant Wistar rats (20–24 animals/group) were treated by oral gavage once daily from GD 6 to 15 with doses of 9, 42, 200 and 900 mg/kg bw per day of guar gum in corn oil (FDRL, 1972). At necropsy on GD 20, doses up to 900 mg guar gum/kg bw per day appeared to be completely normal and had no noticeable effects on implantation or on maternal and fetal survival. The numbers of live or dead fetuses, resorptions, average implant sites and fetal weights did not differ among the groups. The sex distribution of fetuses was not affected by the treatment. The number and type of variants and abnormalities seen at fetal pathological examination of the guar gum groups in either soft tissues or skeletons, did not differ from the number in vehicle‐treated dams of the control group.
Guar gum was added in the diet at levels of 0%, 1%, 2%, 4%, 7.5% or 15% (equal to 0, 0.7, 1.4, 2.7, 5.2 and 11,800 mg/kg bw per day, respectively) to female and male Osborne–Mendel rats for 13 weeks before mating (Collins et al., 1987). This reproductive test is not only set up as a study of the developmental toxicity but studies also potential female fertility effects. The treatment was continued during mating and throughout gestation. After mating was confirmed (day 0), pregnant females were separated in groups of 38–42 animals per treatment group. The dams were killed on day 20 of gestation. This protocol provided an opportunity to assess any effects on female fertility and reproduction. Guar gum did not affect female mating. The Panel concluded that there was no effect upon female mating. No changes in behaviour and mortalities were reported. Clinical findings were unremarkable. During gestation, feed consumption was reduced in all treated groups compared to control animals, but this reduction was only statistically significant at the 2,700 and 5,200 mg/kg bw per day doses and was not dose‐related. Initial maternal body weights were significantly decreased in the 2,700, 5,200 and 11,800 mg/kg bw per day groups after 13 weeks of treatment by approximately 5%, 10% and 15%, respectively. The Panel considered the effects in the two highest doses as biologically relevant. Maternal body weight gain was decreased significantly only in the highest dose group animals (p < 0.05). This decrease appears to be solely a reflection of the smaller mean number of implantations per female in this dose group, because the numbers of early deaths and late deaths, singly or combined (as average percentage of resorptions), did not differ significantly from the control values. No effect on the number of pregnant females or sex ratio was observed in the treated groups when compared to the controls. The dams fed 11,800 mg/kg bw per day guar gum had a slightly lower number (statistically significant) corpora lutea and implantations than the controls, but no effect on implantation efficiency was observed. Hence, the number of viable fetuses/litter was also reduced (though not statistically significant) in the 11,800 mg/kg bw per day group and interpreted as an effect of the decreased number of corpora lutea. The number of abnormalities seen in either soft tissues or skeletons at fetal pathological examination of the guar gum‐treated groups, did not differ from the number in vehicle‐treated dams of the control group. The authors considered the highest dose as a no effect level in this study. The Panel identified a NOAEL of 2,700 mg/kg bw per day for general toxicity based on decreased body weight on GD 0, a NOAEL of 5,200 mg/kg bw per day for other reproductive (fertility) effects based on the decreased number of corpora lutea and a NOAEL for developmental toxicity of 11,800 mg/kg bw per day the highest dose tested.
Hamsters
Pregnant golden hamsters (20–21 animals/group) were treated by oral gavage once daily from GD 6 to 10 of gestation with doses of 6, 28, 130 and 600 mg/kg bw per day of guar gum in corn oil (FDRL, 1972). At necropsy on GD 14, doses up to 600 mg guar gum/kg bw per day appeared to be completely normal and showed no noticeable effects on implantation nor on maternal and fetal survival. The numbers of live or dead fetuses, resorptions, average implant sites or fetal weights did not differ among the groups. The sex distribution of fetuses was not affected by the treatment. The number of abnormalities seen in either soft tissues or skeletons at fetal pathological examination of the guar gum‐treated groups, did not differ from the number in vehicle‐treated dams of the control group.
Rabbits
Artificially inseminated Dutch‐belted rabbits (14 animals/group; and with 21 animals/in the 700 mg/kg group) were treated by oral gavage once daily from GD 6 to 18 with doses of 0, 7, 33, 150 or 700 mg/kg bw per day of guar gum in corn oil (dose volume 1 mL/kg bw per day in all groups except in the highest dose group with dose volume 3 mL/kg bw per day in the rabbit study) (FDRL, 1973). In the 7 mg/kg bw per day group, one female died (pregnancy was not established). In the 33 mg/kg bw per day group, two pregnant females died. In the 150 mg/kg bw per day group, two non‐pregnant females died. In the 700 mg/kg bw per day group, 5 out of 14 pregnant rabbits and 4 of the 7 non‐pregnant animals died. The majority of deaths occurred late in the dosing period or after dosing had been completed. According to the authors, the animals showed 48–72 h before death anorexia; no feed consumption data or individual body weights were reported. Those surviving appeared normal throughout the observation period and had normal young. At necropsy on GD 29 up to the dose of 150 mg/kg guar gum bw per day, the animals appeared to be completely normal and there were no noticeable effects on the numbers of live or dead fetuses, resorptions and fetal weights. In animals from the highest dose tested, small decreases in the average numbers of live fetuses and implantation sites per dam were observed, as well an increase in the sex ratio distribution of fetuses at this dose tested (M/F 1.6 vs 0.72 in the control group). The effects on the fetuses of the highest dose such as sex ratio cannot be used for the safety evaluation as only nine litters were available for examination at necropsy. Effects observed in the skeletal tissues in the offspring of all guar gum treated dams did not differ from the number in the fetuses of controls. No abnormalities were observed in the soft tissues of fetuses examined from the treated groups. The Panel noted that this study with maternal lethality in all treated groups cannot be used for the safety evaluation.
Overall, the Panel considered maternal toxicity at high dose levels as not relevant for hazard characterisation of guar gum.
From a dietary combined fertility and developmental 13‐weeks toxicity study on female and male Osborne–Mendel rats (Collins et al., 1987), the Panel could identify a NOAEL for general toxicity of 2,700 mg/kg bw per day (based on decreased body weight on GD 0), a NOAEL of 5,200 mg/kg bw per day for fertility effects based on decreased number of corpora lutea and a NOAEL for developmental toxicity of 11,800 mg/kg bw per day the highest dose tested.
3.5.7. Hypersensitivity, allergenicity and food intolerance
Several case reports in workers describe guar gum as inducing allergic rhinitis, asthma and sleep apnoea (Kanerva et al., 1988; Lagier et al., 1990; Leznoff et al., 1986). Exposure to guar gum presumable via inhalation, of workers from the pharmaceutical industry (use of guar gum as a hardener in tablets, Lagier et al., 1990), the carpet manufacturing (Lagier et al., 1990), in a power cable laboratory, where guar gum was used as an insulator (Kanerva et al., 1988), the paper industry (Kanerva et al., 1988), and the food processing industry (Leznoff et al., 1986) resulted in allergic reactions. Allergy was confirmed in most cases by nasal provocation tests or in an inhalation chamber (Kanerva et al., 1988; Lagier et al., 1990; Leznoff et al., 1986), by assessment of specific IgE (Kanerva et al., 1988; Lagier et al., 1990) and by skin prick tests (Lagier et al., 1990; Leznoff et al., 1986). The duration of occupation before the allergy occurred was usually 1–2 years (Leznoff et al., 1986; Kanerva et al., 1988; Cartier et al., 1992). In several cases, it was stressed that affected workers had no history of atopy.
Employees of a carpet manufacturing plant (162), in which guar gum used to adhere the dye to fibre was present as an aerosol, participated in a survey including a skin prick test and measuring of IgE and IgG antibodies to guar gum (Malo et al., 1990). Thirty‐seven employees (23%) had a history suggestive of occupational asthma and 59 (36%) had a history of occupational rhinitis. Eight (5%) showed a skin reaction to guar gum in skin testing and 11 (8.3%) had serum IgE antibodies to guar gum.
Seventy‐six subjects who first participated in the precedent survey were followed up 2 years after the initial study in order to document the rate of sensitisation to guar gum (Cartier et al., 1992). One subject had developed skin sensitisation and IgE levels were slightly increased.
An allergic reaction was reported in one patient after eating a local meat speciality (Seebach, 1987). The testing of guar gum by intracutaneous administration was negative, while testing of guar gum by nasal provocation test produced swelling of the mucosa and an oral stress test induced pruritus, oppression and headache after a latent period of 5 h. No testing was reported on the other components of the local meat speciality.
A severe anaphylactic reaction in one patient was attributed to the ingestion of a substitute meal containing guar gum (Papanikolaou et al., 2007). In this patient, positive skin prick test and specific IgE were reported towards soybean, guar gum and carob gum. Positive skin prick test but no specific IgE were reported towards the meal substitute.
The Panel noted that the use of guar gum (E 412) in the food categories 13.1.1., 13.1.5.1. and 13.1.5.2. containing hydrolysed or partially hydrolysed proteins may represent an additional exposure to potential allergenic proteins to infants at higher risks of allergy.
Overall, the Panel noted that most of the reported cases of allergic reaction to guar gum were after inhalation in occupational settings. In addition, there was no indication that the guar gum used under these conditions complied with the requirement of the specifications of the food additive. Very few cases were reported after consumption of foods containing guar gum. Thus, it is clear that guar gum may induce allergic reactions, likely due to the proteins that are present in the gum. Therefore, the Panel considered that the allergenic potential of guar gum used as a food additive should be reduced as much as possible, e.g. by decreasing the presence of proteins in the guar gum used as a food additive (E 412), which can be achieved by clarification of the gum.
3.5.8. Other studies
Animal studies
Young male rats (strain not reported) were fed a normal diet for 3 days, then fasted for 48 h and divided into the following experimental groups according to diet: cacao butter controls (15 rats), cacao butter plus 30% guar flour (18 rats), cacao butter plus 30% wheat flour (15 rats) and cacao butter plus 30% locust bean gum (12 rats). The animals received their diet for 2 days and were then sacrificed. The glycogen in the livers was determined. It was observed that guar flour and locust bean gum are available as glycogen precursors in the liver of rats in comparable amounts (average liver glycogen 0.8% and 0.62%). Their capacity is much less than that of wheat flour (average liver glycogen 2.6%, control: < 0.1%) (Krantz et al., 1948).
Guar gum has been reported to reduce serum total cholesterol and low‐density lipoprotein (LDL) in hypercholesterolaemic animals via acidification of the bulk medium in the caecum contributing to accelerated caecal bile acid reabsorption in rats fed guar gum (Moriceau et al., 2000). The degree of viscosity of guar gum has been postulated to modulate specific gut microbiota in Wistar rats affecting metabolism of SCFAs leading to a reduction in cholesterol, liver steatosis and blood glucose levels (Fǻk et al., 2015).
The effects on serum lipids of minipigs after addition of guar gum (15 and 30 g) to diets containing either with cornstarch or sucrose were studied (Ahrens et al., 1991). Serum total cholesterol, triglycerides and cholesterol concentrations in the lipoprotein fractions were not affected by guar gum in the cornstarch supplemented diet. Total cholesterol concentrations and cholesterol in the lipoprotein fractions were decreased by the 30 g guar gum treatment when added to the sucrose supplemented diet.
The effects of guar gum on digesta passage rate, energy and diet crude protein (CP) digestibility, digesta characteristics and ileal microbial populations, plasma glucose, ghrelin concentrations and feed intake and growth performance were studies in grower pigs (Owusu‐Asiedu et al., 2006). Animals were fed by gavage with an ileal T‐cannula. Guar gum dosed 7% in the diet (as well as cellulose) slowed the passage rate, increased total tract retention time and increase the viscosity of the ileal digesta. Plasma glucose, ghrelin hormone concentration (before and after feeding), CP digestibility and body weight after 14 days were also reduced by both treatments. Ileal bifidobacteria and enterobacteria microbiota were increased by both treatments, except for clostridia microbiota increased only by guar gum.
In C57B1/6J mice, the feeding of high diet guar gum (5%, 7.5% and 10% in the diet) decreased markers of the metabolic syndrome (body weight, adipose weight, triglycerides, glucose, insulin levels and HOMA‐IR20) (den Besten et al., 2014).
The effect of polysaccharides, including guar gum, fed at the 10% level in a semi‐synthetic diet on absorption of Ca, Fe, Zn, Cu, Cr and Co, on weight gain and on faecal dry matter excretion was studied over a period of 8 days in five groups of 12 weanling male rats each and compared to a control group (Harmuth‐Hoene and Schelenz, 1980). Guar gum reduced significantly the absorption of Zn, Cr, Cu and Co. A portion of guar gum was metabolised, presumably due to the action of intestinal bacteria.
Human data
Food uses
The EFSA NDA Panel (2010) reviewed the health claims on guar gum and found no cause and effect relationship between the consumption of guar gum and health claims such as glucose maintenance, satiety or maintenance of cholesterol blood concentrations. No cause–effect relationship was found between the consumption of partially hydrolysed guar gum and decreasing potentially pathogenic gastrointestinal microorganisms, changes in SCFA production and/or pH in the gastrointestinal tract, changes in bowel function and reduction in gastrointestinal discomfort (EFSA NDA Panel, 2011).
Pharmaceutical uses
Information on pharmaceutical uses was obtained by searches of the literature, the websites of national competent authorities for medicinal products and publicly available SmPC on the nationally available authorised products indicated to EFSA by EMA communication (Documentation provided to EFSA, documentation 4).
Guar gum has been reported to reduce serum total cholesterol and LDL in healthy subjects and diabetic patients, without any significant effect on serum high‐density lipoprotein (HDL)‐cholesterol or triglyceride levels. It is postulated by the authors that reduced cholesterol absorption and increased bile acid excretion may lead to an increased hepatic cholesterol turnover (Todd et al., 1990, Leung and Foster, 1996). The usage of guar gum in the treatment of hyperlipidaemia have been reported by some studies to lower serum total cholesterol and LDL cholesterol concentrations, while leaving unaffected HDL cholesterol and triglyceride concentrations, however, other studies have been unable to find those effects (Martindale, 2014). Publications have associated guar gum intake with an decrease in the postprandial serum glucose values due to, e.g. delayed gastric emptying, decreased small bowel motility, decreased glucose absorption resulting from increased viscosity of the contents of the gastrointestinal tract and/or inhibition of gastrointestinal hormones (Gruenwald et al., 2007; Martindale, 2014). Gruenwald et al. (2007) and Martindale (2014) consider that hypolipidaemic effects are still not clinically proven and that the reported lowering effects on blood glucose levels, either in healthy subjects or diabetic patients, are small.
Posology of guar gum is given in literature as consisting of three times 5 g/day together with liquids to avoid the formation of a bolus in the stomach (Gruenwald et al., 2007).
Taken from the SmPC from the products on the market reported by EMA, possible side‐effects related to the consumption of guar gum used as active ingredients in pharmaceutical products (up to doses of 25 g per person per day equivalent to 360 mg/kg bw per day) are hypersensitivity, and reduced oesophagus mobility as well as reduced passage in the digestive system and pancreatic insufficiency. Contraindications warn that the blood glucose level might change and in the case of diabetic patients there might be need to change the doses of other antidiabetic drugs (especially oral antidiabetics). Guar gum must be administered with ample volumes of liquid, avoiding alcoholic liquids and other medication should be consumed 2 h before or after intake of guar gum, since guar gum might influence absorption of other substances. Adverse effects reported after consumption of guar gum are gastrointestinal discomforts including gas in the intestine (flatulence) and diarrhoea, especially in the beginning of the use, with a frequency of very common (≥ 1/10) and nausea with a frequency of uncommon (≥ 1/1,000 to ≤ 1/100). To reduce these adverse effects, it is advised to take only half the dosage/intake (or to start only with 1 intake/day) at the beginning of consumption and to increase the dosage within a week to the recommended therapeutic dosage. Other reported unwanted side effects in the gastrointestinal tract after intake of guar gum include regurgitation, constipation, abdominal cramps and abdominal pain (Todd et al., 1990; Aronson, 2009; Martindale, 2014).
It is reported that ingestion of guar gum could reduce the absorption of vitamins, minerals and some medications (such as contraceptives) (Gruenwald et al., 2007).
The FDA concluded that for OTC drug products for human use, containing guar gum or other water‐soluble/hydrophilic gums, or hydrophilic mucilloids as an active ingredient, when marketed in a dry or incompletely hydrated form (e.g. capsules, granules, powders, tablets, wafers) the following labelling is needed to prevent asphyxiation: ‘Taking this product without adequate fluid may cause it to swell and block your throat or esophagus and may cause choking. Do not take this product if you have difficulty in swallowing. If you experience chest pain, vomiting, or difficulty in swallowing or breathing after taking this product, seek immediate medical attention’ (FDA, 2016).
Case reports
Oesophageal and small bowel obstructions have been reported after intake of guar gum containing diet pills (Morse and Malloy, 1990; Opper et al., 1990; Seidner et al., 1990; Lewis, 1992; Halama and Mauldin, 1992). The mechanism of obstruction was related to the water‐holding capacity, the gel‐forming tendency and the rapid expansion of the material (10‐ to 20‐fold) when in contact with water or gastric juice.
Lewis (1992) analysed 26 cases of oesophageal and small bowel obstruction from guar gum‐containing ‘diet pills’, a weight reduction product, reported to the FDA. There were 18 instances of oesophageal obstruction, seven instances of small bowel obstruction, and one individual who was reported to have died after ingestion of the guar gum containing preparations, but for whom insufficient details were provided to assess causation. Pre‐existing oesophageal or gastric disorders were only present in 50% of those with oesophageal obstruction, including peptic stricture, pyrosis, hiatal hernia, oesophagitis, gastric stapling procedure, Schatzki ring and muscular dystrophy. For the seven patients with small bowel obstruction, no specific predisposing factors were mentioned. These cases were considered to be very similar to those already reported in the literature (Morse and Malloy, 1990; Opper et al., 1990).
Ingestion of 5 g guar gum granules led to a complete obstruction of the distal oesophagus in a 63‐year‐old diabetic patient without predisposing oesophageal disease. The intake of three times 5 g guar gum with 250 mL of water per day had been tolerated without problems for half a year. When the obstruction was realised, a spirituous liquor had been consumed. The authors assumed that the swelling of the guar gum was enhanced by the ethanol contained in the beverage (Ranft and Imhof, 1983).
Clinical studies
Adults
Five volunteers ingested once daily for 10 days capsules containing 1 g guar flour without any apparent adverse effects (Krantz, 1947; unpublished, as reported in JECFA, 1975b).
The hypolipidaemic effect of guar gum (30 g/day) was studied in nine subjects (mean age 62 years, 6 females and 3 males) with primary hyperlipidaemia by a 12‐week double‐blind placebo‐controlled crossover study (Turner et al., 1990). There were no changes in the weight of the subjects at the end of the study. Plasma cholesterol, LDL‐cholesterol and plasma LDL‐apo B concentrations were statistically decreased (p < 0.05 or < 0.06) upon guar gum treatment, but overall LDL‐apo B increased in mg/kg bw per day was not statistically significant. Other parameters measured remained unchanged (plasma triglycerides, plasma VLDL, IDL‐cholesterol, HDL‐cholesterol). However, there were important variations between patients in responsiveness to guar gum, ranging from no decrease to a 24% reduction in plasma cholesterol for instance.
The hypolipidaemic effect of guar gum (30 g/day) was examined in a double‐blind placebo‐controlled crossover study in nine patients with primary hyperlipidaemia (Turner et al., 1990). Plasma cholesterol, cholesterol levels in LDL and in intermediate density lipoprotein (IDL) were reduced, the authors suggesting that observed effects of guar gum could be explained by the binding of bile acid.
A review on regulation of cholesterol metabolism by soluble fibres concludes that guar gum affects cholesterol metabolism, by reducing total and LDL‐plasma cholesterol in humans and pigs, increasing hepatic LDL receptor expression in pigs fed an antherogenic diet, reducing hepatic free and esterified cholesterol by affecting faecal loss of bile acids and reducing the enterohepatic bile acid pool size that may stimulate the liver to produce bile acids from cholesterol, (Rideout et al., 2008).
Some smaller studies with patients or healthy subjects have been published on the effect of guar gum preload on glycaemic control in people with type 2 diabetes (Clifton et al., 2014), on maintenance therapy with guar gum in the conservative treatment of chronic anal fissure (Brillantino et al., 2014), on effects of guar gum on blood glucose and brachial artery flow‐mediated dilatation (Thazhath et al., 2014), on usage of guar gum in the treatment of irritable bowel syndrome with constipation (Russo et al., 2015) or on effects of guar gum ingestion on postprandial blood pressure in older adults (Jang et al., 2015). Dosages mentioned in these publications were between 5 and 9 g/day, no information on dosage was available in two of those studies.
Eight patients with non‐insulin‐dependent diabetes mellitus (NIDDM) consumed at least 30 g guar gum for at least 16 weeks without any change in haematologic, hepatic or renal function. According to a serologic screening, no change in lipid, protein or mineral metabolism, and no change in electrolyte balance were found (McIvor et al., 1985).
A meta‐analysis of randomised trials on guar gum used in body weight reduction preparations found undesirable effects reported, such as flatulence, soft stools, diarrhoea and abdominal pain (cramps), in trials where the average daily dose of guar gum consumed ranged between 9 and 30 g (Pittler and Ernst, 2001). Thirty‐four double‐blind placebo‐controlled, randomised trials were identified in this publication. Eleven trials were suitable for statistical pooling; nine other studies were processed separately since data reported were not suitable for statistically pooling. Sensitivity analyses were performed to test the robustness of the overall analysis. A total of approximately 367 cases were concerned by these trials and in 3% (11 cases) of the patients gastrointestinal complains, such as flatulence, diarrhoea and nausea, were severe enough to withdraw them for the trial. Hypercholesterolaemic or hyperlipidaemic patients, diabetic patients, menopausal women and healthy volunteers were assessed. In six trials, the patients were described as overweight or obese. Effects experienced by patients were reported in 17 trials. Only two trials reported the absence of effects. In general, in most studies after consumption of around 15,000 mg/day corresponding to 214 mg/kg bw per day, some individual's experienced abdominal discomfort which was considered by the Panel as undesirable but not adverse (Pittler and Ernst, 2001). Overall, it was concluded that guar gum is not efficient for lowering body weight.
Infants and children
The effects of guar gum on plasma viscosity were studied in 10 diabetic children (median age 10 years, median duration of diabetes 5 years) supplemented with 0.45 g guar gum/kg bw per day (maximum 20 g guar gum/day in bread) for 4 weeks (Koepp and Hegewisch, 1981). Body weight remained unchanged during the study. As a whole plasma viscosity, insulin requirements and fibrinogen concentrations decreased during the guar gum treatment period. Albumin concentrations increased, whereas serum total protein, mean serum cholesterol, serum sodium, potassium and calcium did not change during the guar gum treatment period. It is reported that the diabetic children tolerated the guar diet without difficulty.
A randomised crossover study in 22 children (mean age 12.2 years) with IDDM (mean duration of diabetes 4.4 years) evaluated the effects of guar gum diet on their metabolic balance (Paganus et al., 1987). Diets given to children were supplemented with palatable guar gum 5% of daily carbohydrate intake (mean daily dose of guar gum 13.5 g, range 12–19 g/day) for 3 weeks followed by a diet containing guar gum + fructose for another 3 weeks. A control group of eight children (mean age 12.3 years, mean duration of diabetes 4.3 years) followed the same protocol but without guar gum supplementation. Glucosuria index was measured in urine and red cell glycohaemoglobin A1c (HbA 1c), serum total and HDL cholesterol, plasma C‐peptide, pancreatic and enteroglucagon were also measured. The height and weight of the children in the guar gum diet were normal. All children completed the study although five complained of abdominal swelling and flatulence. HbA 1c levels decreased during the guar gum diet intake but the changes were not significantly different compared to controls. The glycosuria indexes increased during the experimental period. Serum total cholesterol decreased in guar gum diet and HDL cholesterol was not affected. Postprandial plasma C‐peptide and the plasma pancreatic glucagon concentrations did not change during the treatment period. Mean plasma enteroglucagon concentrations increased during the guar gum diet treatment.
Possible effects of guar gum on the bioavailability of nutrients
Several authors have indicated the need to explore further the effect of thickening agents on the nutrition and health of infants as some studies in in vitro model have suggested that the bioavailability of calcium, iron and zinc may be affected by these compounds (Bosscher et al., 2001; Vandenplas et al., 2011).
An in vitro continuous flow dialysis model with a preliminary intraluminal digestive phase21 was used to measure calcium, iron and zinc availability from casein‐based and whey‐based infant formulas supplemented with soluble fibre fractions, among them guar gum (Bosscher et al., 2001). Availability was calculated from the amount of guar gum (and other soluble fibre fractions) that had passed the dialysis membrane proportional to the total element content of the sample. In whey‐based formula, the addition of guar gum at 1.44 g/100 mL influenced the availabilities of calcium and iron not of zinc. No results are shown from casein‐based formula but the authors mention that guar gum reduced calcium and iron availability as well.
Platt and Clydesdale (1987) studied the relative binding strengths and the number of binding sites for guar gum bound Fe under simulated duodenal pH conditions. The stability constants for the binding sites of lignin and guar show that iron absorption can be inhibited under these conditions. Although, it is mentioned that lignin has two high affinity binding sites for Fe, Zn and Cu no potential mode of action of guar gum in the binding of these minerals is postulated by the authors.
Wölbling et al. (1980) studied in situ the effect of guar gum in the absorption of 59Fe‐labelled iron in tied‐off jejunal segments of normal or iron‐deficient anesthetised female Wistar rats. Tied‐off jejunal segments were filled with 2 mL of a solution containing 360 nmol 59Fe‐labelled ferric chloride (pH 2) and increasing amounts of 1.2, 8 and 30 mg of guar gum. After 1 h of exposure, the animals were sacrificed and the tied‐off segments removed. The 59Fe activity in the carcasses was determined in a whole body counter. The amount of 59Fe detected (called ‘absorption’) in the carcasses was calculated in per cent of the dose administered. Increasing doses of guar gum inhibit the ‘absorption’ of iron in normal rats. The highest dose of guar gum inhibits iron ‘absorption’ by 60%. In iron‐deficient rats the ‘absorption’ of iron was reduced by not as much as in the normal iron rats. When 59Fe was given in the diet containing 10% of guar gum for 3 days and on the sixth day after administration of iron the retention of 59Fe was measured. Under these conditions, guar gum inhibits the ‘absorption’ of iron in normal rats (by about 40%) but did not in iron‐deficient rats.
Regarding the effect of guar gum on the bioavailability of minerals, the Panel noted that a human study with another glucomannan (locust bean gum) did not confirm this effect (Behall et al., 1987).
Riedl et al. (1999) studied the effect of different kinds of dietary fibre, including guar gum, on the bioavailability of carotenoids in six healthy female volunteers. The bioavailability was measured following the increases in the areas‐under‐curves (AUC) over 24 h. Guar gum was administered once in a meal enriched with 0.15 g guar gum/kg bw in combination with an antioxidant supplement. The test day was followed by a wash‐out period of at least 2 weeks. The antioxidant supplement contained all‐trans‐β‐carotene (0.4 mg/kg bw), lycopene (0.7 mg/kg bw), canthaxanthin (0.2 mg/kg bw) and α‐tocopherol (1.4 mg/kg bw). Results showed that addition of any kind of dietary fibre tested, including guar gum, to the standard meal resulted in decreased AUC of carotenoids, α‐tocopherol, lycopene and lutein. Canthaxanthin AUC was not affected by dietary fibres tested.
4. Discussion
Guar gum is the ground endosperm of the seeds of the guar plant (Cyamopsis tetragonoloba L. Taub). Commercial food‐grade guar gum is reported to contain usually about 80% guaran, 5–6% crude protein, 8–15% moisture, 2.5% crude fibre, 0.5–0.8% ash and small amounts of lipids composed mainly of free and esterified plant fatty acids. The glucomannan guaran, the major component of guar gum contains d‐mannose and d‐galactose as structural units with a ratio of approximately 2:1. The protein concentration in clarified guar gum preparations complies with a maximum value of 1%.
Specifications for guar gum have been defined in Commission Regulation (EU) 231/2012. It is reported that insufficient elimination of the germ tissue from endosperm during the manufacturing process leads to higher percentages in protein content (Europ. Pharm. Comment., 2005). The Panel noted some case reports of hypersensitivity (Section 3.5.6) associated with guar gum. The Panel considered that this hypersensitivity might be due to the guar gum proteins and therefore their content should be reduced as much as possible.
Because of both the botanical origin and the polysaccharidic nature of gums, they can be a substrate of microbiological contamination and of field and storage fungal development. The Panel noted that therefore for guar gum criteria for the absence of Salmonella spp. and E. coli, for TAMC and for TYMC should be included into the EU specifications.
The in vitro degradation and the in vivo digestibility of guar gum have been investigated in animals and humans. These studies demonstrated that guar gum would not be absorbed intact and would not be metabolised by enzymes present in the gastrointestinal tract. However, it would be partially fermented to SCFAs during its passage through the large intestine by the action of the intestinal tract microflora. The rate of hydrolysis in the gastrointestinal tract in humans is unknown; however, it is expected that fermentation of guar gum would lead to the production of products such as SCFAs which were considered of no concern by the Panel.
Guar gum is regarded as not acutely toxic, based on the results of acute oral toxicity studies.
Short‐term and subchronic studies on guar gum have not shown major adverse effects under the conditions of the tests. Repeated oral administration of guar gum caused some growth reduction in rats, mice and rabbits at high doses, but these effects can partially be attributed to the bulk properties of guar gum when in contact with water or intestinal juices and have not been considered as adverse effects. Increased caecum weight in animals fed high amounts (2–5% of the diet) of guar gum was also reported. NOAELs identified in short‐term and subchronic studies correspond to the highest dose tested of approximately 18,000 mg guar gum/kg bw per day for rats and of approximately 15,000 mg/kg bw per day for mice (Graham et al., 1981; NTP, 1982).
The Panel considered the available genotoxicity data on guar gum (E 412) to be sufficient to conclude that there is no concern with respect to genotoxicity.
Guar gum has been tested in several species in long‐term chronic and carcinogenicity studies up to doses of 7,500 mg/kg bw per day in mice and 2,500 mg/kg bw per day in rats (NTP, 1982). In female rats, statistically significant increased incidences of benign phaeochromocytomas of the adrenal gland were reported. However, no changes in the incidences of malignant phaeochromocytomas were observed, and the combined incidences of benign and malignant phaeochromocytomas in these animals were not statistically significant different. Male rats did not show any statistically significant difference on the incidence of phaeochromocytomas of the adrenal gland (NTP, 1982). The Panel considered that incidences of phaeochromocytomas and pituitary gland tumours occurring in the carcinogenicity study with F344 rats on guar gum, carried out in the early 1980s, are not relevant for human risk assessment. Only non‐malignant proliferations were increased in that rat study not leading to an increase incidence of carcinomas. The Panel considered guar gum as not carcinogenic. The Panel could derive a NOAEL of 2,500 mg/kg bw per day, the highest dose tested, from this study. The carcinogenicity study with guar gum in mice did not show carcinogenicity potential either. The Panel could derive a NOAEL of 7,500 mg/kg bw per day, the highest dose tested, from this study.
Guar gum did not show reproductive effects (fertility) or developmental toxicity effects in the available studies (FDRL, 1972, 1973). From a combined fertility/developmental study in rats (Collins et al., 1987), the Panel could identify a NOAEL of 5,200 mg/kg bw per day for reproductive effects based on decreased number of corpora lutea and a NOAEL for developmental toxicity of 11,800 mg/kg bw per day the highest dose tested.
The Panel noted that most of the reported cases of allergic reaction to guar gum were after inhalation in occupational settings. In addition, there was no indication that the guar gum used under these conditions complied with the requirement of the specifications of the food additive. Very few cases were reported after consumption of foods containing guar gum. Thus, it is clear that guar gum may induce allergic reactions, likely due to the proteins that are present in the gum. Therefore, the Panel considered that the allergenic potential of guar gum used as a food additive should be reduced as much as possible, e.g. by decreasing the presence of proteins in the guar gum used as a food additive (E 412), which can be achieved by clarification of the gum.
Human undesirable effects, such as flatulence, regurgitation, abdominal pain (cramps), bowel obstruction, constipation or on the contrary soft stools and diarrhoea, have also been reported upon consumption of guar gum as preparations (bolus dose) (Todd et al., 1990; Lewis, 1992; Aronson, 2009). Oral intake of large amount of guar gum (9,000–30,000 mg/person corresponding to 128–429 mg/kg bw per day) was well tolerated in humans. In general, in most studies after consumption of around 15,000 mg per day corresponding to 214 mg/kg bw per day, some individuals experienced abdominal discomfort (Pittler and Ernst, 2001). In one interventional study with diabetic children, abdominal discomforts were reported in 5 out of 22 children given 13,500 mg guar gum per day corresponding to 314 mg/kg bw per day (Paganus et al., 1987). The Panel considered the abdominal discomfort as undesirable but not adverse.
The present re‐evaluation includes the use of guar gum (E 412) in foods for infants from 12 weeks of age and for young children.
Concerning uses of guar gum in food for infants and young children, the Panel concurs with the SCF opinion (SCF, 1998, 2003) ‘….the SCF considered it prudent that the number and amounts of additives used in foods for infants and young children should be kept at the minimum necessary. The SCF confirmed its long standing view that additives should not be permitted in foods specially prepared for infants. Rarely, exceptional technological circumstances may justify the use of an additive’. And, ‘The Committee further recommends maintaining the concept that if more than one of the three substances locust bean gum, guar gum or carrageenan are added to a follow‐on formula, the maximum level established for each of those substances is lowered with that relative part as is present of the other substances together’. The Panel acknowledged that consumption to the concerned food categories would be short and noted that it is prudent to keep the number of additives used in foods for infants and young children to the minimum necessary and that there should be strong evidence of need as well as safety before additives can be regarded as acceptable for use in infant formulae and foods for infants and young children.
The Panel noted reports suggesting a putative effect of guar gum to decrease the bioavailability of certain nutrients; however, the data are sparse. Furthermore, a human study with another glucomannan (locust bean gum) did not confirm the reduced bioavailability of minerals.
Furthermore, the Panel noted that no specific clinical data addressing the safety of use of guar gum (E 412) in ‘dietary foods for infants for special medical purposes and special formulae for infants’ (food category 13.1.5.1) and in ‘dietary foods for baby and young children for special medical purposes as defined in Directive 1999/21/EC’ (food category 13.1.5.2) considering the defined maximum use levels were available to the Panel.
The Panel also noted that infants and young children consuming these foods may be exposed to a greater extent to guar gum (E 412) than their healthy counterparts because the permitted levels of guar gum (E 412) in products for special medical purposes are 10‐fold higher than in follow‐on formulae for healthy individuals. The Panel further noted that, given their medical condition, infants and young children consuming foods belonging to these food categories may show a higher susceptibility to the gastrointestinal effects of guar gum than their healthy counterparts. Thus, monitoring of any adverse effects including those in the gastrointestinal system in infants and young children consuming these foods under medical supervision could be helpful to reduce this uncertainty.
According to the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA, 2014), the Panel considered that sufficient toxicity data were available in animals showing no adverse effects at highest doses tested up to 7,500 mg/kg bw per day in mice and 2,500 mg/kg bw per day in rats. Therefore, the Panel considered that there is no need to allocate a numerical ADI for guar gum (E 412).
To assess the dietary exposure to guar gum (E 412) from its use as a food additive, the exposure was calculated based on (1) maximum levels of data provided to EFSA (defined as the maximum level exposure assessment scenario) and (2) reported use levels (defined as the refined exposure assessment scenario).
Based on the available data set, the Panel calculated two refined exposure estimates based on different assumptions: a brand‐loyal consumer scenario and a non‐brand‐loyal scenario. The Panel considered that the refined exposure assessment approach resulted in more realistic long‐term exposure estimates compared to the maximum level exposure assessment scenario as it is based on the range of data made available to EFSA by the food industry.
The exposure estimates in the maximum level exposure assessment scenario, at the mean level exposure to guar gum (E 412) from its use as a food additive ranged from 48 mg/kg bw per day for the adults to 712 mg/kg bw per day in toddlers. The 95th percentile of exposure to guar gum (E 412) ranged from 97 mg/kg bw per day for adults to 1,183 mg/kg bw per day in children (Table 4). The main contributing food categories to the mean exposure estimates for infants in this scenario were foods for infants and young children (FCS 13.1), and processed fruits and vegetables; for toddlers, milk products, soups and broth and bread and rolls For children and adolescents, the main contributing food categories were soups and broth, milk products and bread and rolls, and fine bakery wares, while for adults and the elderly, they were soups and broth and coffee, tea. The Panel noted that the estimated long‐term exposures based on this scenario are very likely conservative as this scenario assumes that all foods and beverages listed under the annex II to regulation No 1333/2008 contain guar gum (E 412) as a food additive at the maximum reported use level.
From the refined estimated exposure scenario considering only food categories for which direct addition of guar gum (E 412) to food is authorised, in the brand‐loyal scenario, mean exposure to guar gum (E 412) ranged from 14 mg/kg bw per day in infants to 449 mg/kg bw per day in toddlers. The 95th percentile of exposure ranged from 38 mg/kg bw per day for the elderly to 865 mg/kg bw per day in children. The main contributing food categories for infants were foods for infants and young children; for toddlers and children, fermented milk products, and for the other populations groups, soups and broth. In the non‐brand‐loyal scenario, mean exposure to guar gum (E 412) from its use as a food additive ranged from 6 mg/kg bw per day to 312 mg/kg bw per day for infants. The 95th percentile of exposure ranged from 15 mg/kg bw per day for adults and the elderly to 610 mg/kg bw per day in infants. The main contributing food for infants and toddlers were foods for infants and young children and fermented milk products; for children, fermented milk products, for adolescents, cocoa products and bread and rolls and for the other populations groups, bread and rolls.
A refined estimated exposure assessment scenario taking into account the FSMP for infants and young children (FCS 13.1.5. ‘Dietary foods for infants and young children for special medical purposes as defined by Commission Directive 1999/22/EC and special formulae for infants’) was also performed to estimate exposure for infants and toddlers who may be on a specific diet. Considering that this diet is required due to specific needs, it is assumed that consumers are loyal to the food brand, therefore only the refined brand‐loyal estimated exposure scenario was performed.
From this refined brand‐loyal estimated exposure scenario taking into account the FSMP, mean exposure to guar gum (E 412) from its use as a food additive ranged for infants between 325 and 609 mg/kg bw per day, and between 120 and 457 mg/kg bw per day for toddlers. The 95th percentile of exposure ranged for infants between 912 and 1,555 mg/kg bw per day, and for toddlers, between 310 and 743 mg/kg bw per day.
The refined estimates are based on 51 out of 86 food categories in which guar gum (E 412) is authorised. The main food categories, in term of amount consumed, not taken into account were breakfast cereals, gluten‐free dietary foods for infants and young children, snacks and most of alcoholic beverages. However, based on the information in the Mintel GNPD (Appendix C), in the EU market, no breakfast cereals are labelled with guar gum (E 412), and few alcoholic drinks are labelled with the additive. Therefore, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure to guar gum (E 412) as a food additive according to Annex II in European countries for all scenarios.
Guar gum (E 412) is used as a thickener and stabiliser agent in a wide range of foods. In specific populations consuming dietary FSMP and special formulae and in infants and young children consuming infant formulae and/or follow‐on formulae, brand‐loyalty may be relevant. Because these food groups are main contributors to mean exposure, the Panel therefore selected the brand‐loyal refined scenario as the most relevant exposure scenario for this additive in these specific situations.
The Panel noted that in Annex II of Regulation (EC) No 1333/2008 use levels of guar gum (E 412) in food for infants under the age of 12 weeks are included in categories 13.1.1, 13.1.5.1 and 13.1.5.2. The Panel considered that these uses would require a specific risk assessment in line with the recommendations given by JECFA (1978) and the SCF (1998) and endorsed by the Panel (EFSA ANS Panel, 2012). Therefore, the current re‐evaluation of guar gum (E 412) as a food additive is not considered to be applicable for infants under the age of 12 weeks and will be performed separately.
5. Conclusions
General population
Following the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA, 2014), and given that:
adequate exposure data were available; in the general population, the highest refined exposure assessments calculated based on the reported data from the food industry were for infants (12 weeks–11 months) up to 812 mg/kg bw per day (brand‐loyal scenario),
guar gum is practically undigested, not absorbed intact, but significantly fermented by enteric bacteria in humans,
adequate toxicity data were available,
no adverse effects were reported in subchronic studies in rodents at the highest dose tested of 15,000 mg guar gum/kg bw per day in mice and 18,000 mg guar gum/kg bw per day in rats,
there is no concern with respect to the genotoxicity of guar gum,
no carcinogenic effects were reported at the highest dose tested of 7,500 mg guar gum/kg bw per day in mice and 2,500 mg guar gum/kg bw per day in rats;
oral intake of large amount of guar gum in (9,000–30,000 mg/person corresponding to 128–429 mg/kg bw per day) was well tolerated in adults. In most studies after consumption of around 15,000 mg per day in adults corresponding to 214 mg/kg bw per day, some individuals experienced abdominal discomfort which was considered by the Panel as undesirable but not adverse,
in one interventional study with diabetic children abdominal discomfort was reported in 5 out of 22 children given 13,500 mg guar gum per day corresponding to 314 mg/kg bw per day,
using the refined exposure assessment (non‐brand‐loyal scenario), the Panel noted that exposures for high level consumers (children and adults) would be below the level at which some abdominal discomfort was reported,
no data on abdominal discomfort were available for infants and young children,
the Panel concluded that there is no need for a numerical ADI for guar gum (E 412), and that there is no safety concern for the general population at the refined exposure assessment for the reported uses of guar gum (E 412) as a food additive.
The Panel considered that for uses of guar gum in foods intended for infants and young children the occurrence of abdominal discomfort should be monitored and if this effect is observed doses should be identified as a basis for further risk assessment.
Infants and young children consuming foods for special medical purposes and special formulae
Concerning the use of guar gum (E 412) in ‘dietary foods for special medical purposes and special formulae for infants’ (Food category 13.1.5.1) and ‘in dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC’ (Food category 13.1.5.2), and given that:
for populations consuming dietary foods for special medical purposes and special formulae, the highest refined exposure estimate (p95) calculated based on the reported data from the food industry are for infants (12 weeks‐11 months) consuming dietary foods for special medical purposes and special formulae up to 1,555 mg/kg bw per day (brand‐loyal scenario),
infants and young children consuming these foods may be exposed to a greater extent to guar gum (E 412) than their healthy counterparts because the permitted levels of guar gum (E 412) in products for special medical purposes are 10‐fold higher than in infant formulae and follow‐on formulae for healthy individuals,
infants and young children consuming foods belonging to these food categories may show a higher susceptibility to the gastrointestinal effects of guar gum than their healthy counterparts due to their underlying medical condition,
no adequate specific studies addressing the safety of use of guar gum (E 412) in this population under certain medical conditions were available,
it was not possible to assess at which exposure level of guar gum the gastrointestinal effects developed in this specific population,
the Panel concluded that the available data do not allow an adequate assessment of the safety of guar gum (E 412) in infants and young children consuming these FSMP.
6. Recommendations
The Panel recommended that the maximum limits for the impurities of toxic elements (lead, mercury and arsenic) in the EC specification for guar gum (E 412) should be revised in order to ensure that guar gum (E 412) as a food additive will not be a significant source of exposure to those toxic elements in food in particular for infants and children. The Panel noted that currently detected levels of these toxic elements were orders of magnitude below those defined in the EU specifications and therefore the current limits could be lowered.
The Panel recommended to harmonise the microbiological specifications in the EU Regulation for polysaccharidic thickening agents, such as gums, and to include criteria for the absence of Salmonella spp. and E. coli, for TAMC and for TYMC into the EU specifications of guar gum (E 412).
The Panel recommended to give separate specifications in the EU regulation for guar gum and clarified guar gum differing significantly in the protein content.
The Panel considered that no threshold dose can be established for allergic reactions. Therefore, it is advisable that exposure to eliciting allergens, such as proteinaceous compounds, is avoided as much as possible, and therefore, the Panel recommended that their content should be reduced as much as possible, which can be achieved for example by clarification of guar gum.
The Panel recommended that additional data should be generated to assess the potential health effects of guar gum (E 412) when used in ‘dietary foods for infants for special medical purposes and special formulae for infants’ (Food category 13.1.5.1) and in ‘dietary foods for babies and young children for special medical purposes’ as defined in Directive 1999/21/EC (Food category 13.1.5.2).
Documentation provided to EFSA
Pre‐evaluation document on guar gum (E 412). Frauenhofer ITEM. March 2012.
MARS Chocolate UK. Data submitted to EFSA on 19 May 2010.
Cutisin. Data submitted to EFSA on 29 October 2010.
EMA (European Medicines Agency): communication to EFSA request in 4 May 2015, for information on a certain group of substances used as food additives, June 2014.
Shree Ram Gum, 2015. Reply to EFSA: Call for technical data on certain thickening agents permitted as food additive in the EU. Submitted on 23 March 2015.
INEC, 2015–2016. Reply to EFSA: Call for technical data on certain thickening agents permitted as food additive in the EU. Submitted on 16 December 2015 and 20 January 2016.
AIPG (Association for International Promotion of Gums), 2015–2016. Reply to EFSA: Call for technical data on certain thickening agents permitted as food additive in the EU. Submitted on 22 December 2015 and 15 February 2016.
Shefexil, 2016. Reply to EFSA: Call for technical data on certain thickening agents permitted as food additive in the EU. Submitted on 28 January 2016, 15 February 2016 and 23 March 2016.
FDE (Food Drink Europe), 2013. Data on usage levels of guar gum (E 412) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 29 November 2013.
SNE (Specialised Nutrition Europe), 2014. Data on usage levels of guar gum (E 412) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 30 September 2014.
AIPG (Association for international Promotion of Gums), 2014. Data on usage levels of guar gum (E 412) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 17 September 2014.
Biovegan GmbH, 2014. Data on usage levels of guar gum (E 412) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 4 June 2014.
EUROGUM A/S, 2014. Data on usage levels of guar gum (E 412) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 30 September 2014.
BABBI Confectionery Industry, 2014. Data on usage levels of guar gum (E 412) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 12 August 2014.
Rudolf Wild GmbH & Co. KG, 2014. Data on usage levels of guar gum (E 412) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 29 September 2014.
ICGA (International Chewing Gum Association), 2014. Data on usage levels of guar gum (E 412) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2014). Submitted to EFSA on 30 September 2014.
Abbreviations
- AAS
atomic absorption spectroscopy
- ADI
acceptable daily intake
- AFC
EFSA Former Panel on Additives, Flavourings, Processing Aids and Materials in Contact with Food
- AIPG
Association for International Promotion of Gums
- ANS Panel
EFSA Panel on Food Additives and Nutrient Sources added to Food
- AOAC
Association of Official Agricultural Chemists
- AUC
areas‐under‐curves
- CAS
Chemical Abstracts Service
- CFU
colony‐forming unit
- CP
crude protein
- DMSO
dimethylsulfoxide
- EINECS
European Inventory of Existing Commercial Chemical Substances
- EMA
European Medicines Agency
- FCS
Food Classification System
- FDA
Food and Drug Administration
- FDE
Food Drink Europe
- FDRL
Food and Drug Research Laboratories
- FSMP
foods for special medical purposes
- FTIR
Fourier transform infrared spectroscopy
- GD
gestation day
- GLP
Good Laboratory Practice
- GNPD
Global New Products Database
- Hb
haemoglobin
- HDL
high‐density lipoprotein
- HOMA‐IR
homoeostasis model assessment for insulin resistance
- ICGA
International Chewing Gum Association
- ICP‐AES
inductively coupled plasma atomic emission spectroscopy
- IDL
intermediate density lipoprotein
- Ig
Immunoglobulin
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LD50
lethal dose
- LDL
low‐density lipoprotein
- LOQ
limit of quantification
- MNBN
micronucleated binucleated
- MPL
maximum permitted level
- NDA
EFSA Panel on Dietetic Products, Nutrition and Allergies
- NIDDM
Non‐insulin‐dependent diabetes mellitus
- NOAEL
no‐observed‐adverse‐effect‐level
- OECD
Organisation for Economic Co‐operation and Development
- PCB
polychlorinated biphenyl
- PCP
pentachlorophenol
- PCR
polymerase chain reaction
- QS
quantum satis
- RBC
red blood cell
- SCF
Scientific Committee for Food
- SCFA
short‐chain fatty acids
- SmPC
summary of product characteristics
- SNE
specialised Nutrition Europe
- TAMC
total aerobic microbial count
- TDI
tolerable daily intake
- TEM
triethylenemelamine
- TLC
thin‐layer chromatography
- TMDI
theoretical maximum daily intake
- TYMC
total combined yeasts and moulds
- WBC
white blood cell
Appendix A – Summary of reported use levels of guar gum (E 412) provided by industry (mg/L or mg/kg as appropriate)
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section).
Appendix B – Number and percentage of food products labelled with guar gum (E 412) out of the total number of food products present in the Mintel GNPD per food subcategory between 2011 and 2016
1.
| Mintel subcategorya | Total number of products | Products labelled with guar gum (E 412) | |
|---|---|---|---|
| Dairy‐Based Frozen Products | 8,077 | 5,295 | 65.6 |
| Soy‐Based Frozen Products | 82 | 51 | 62.2 |
| Water‐Based Frozen Desserts | 1,235 | 589 | 47.7 |
| Sandwich Fillers/Spreads | 1,055 | 413 | 39.1 |
| Salads | 2,699 | 907 | 33.6 |
| Soft Cheese Desserts | 1,552 | 519 | 33.4 |
| Mayonnaise | 929 | 283 | 30.5 |
| Instant Noodles | 1,128 | 251 | 22.3 |
| Sandwiches/Wraps | 2,672 | 515 | 19.3 |
| RTD (Iced) Coffee | 895 | 161 | 18.0 |
| Meal Kits | 2,130 | 370 | 17.4 |
| Other Frozen Desserts | 1,596 | 264 | 16.5 |
| Liquid Dairy Other | 130 | 19 | 14.6 |
| Table Sauces | 6,076 | 791 | 13.0 |
| Chilled Desserts | 6,362 | 815 | 12.8 |
| Meal Replacements & Other Drinks | 1,266 | 162 | 12.8 |
| Dips | 1,525 | 194 | 12.7 |
| Flavoured Milk | 1,423 | 169 | 11.9 |
| Spoonable Yogurt | 9,995 | 1,126 | 11.3 |
| Hors d'oeuvres/Canapes | 4,124 | 426 | 10.3 |
| Meat Substitutes | 2,388 | 244 | 10.2 |
| Cream | 1,635 | 157 | 9.6 |
| Meat Pastes & Pates | 3,082 | 290 | 9.4 |
| Bread & Bread Products | 10,247 | 938 | 9.2 |
| Fresh Cheese & Cream Cheese | 2,753 | 251 | 9.1 |
| Soy‐Based Drinks | 665 | 57 | 8.6 |
| Cooking Sauces | 5,008 | 426 | 8.5 |
| Soy Yogurt | 437 | 37 | 8.5 |
| Potato Products | 3,204 | 257 | 8.0 |
| Dressings & Vinegar | 3,338 | 263 | 7.9 |
| Pizzas | 4,427 | 345 | 7.8 |
| Drinking Yogurt & Liquid Cultured Milk | 3,237 | 250 | 7.7 |
| Prepared Meals | 11,211 | 852 | 7.6 |
| Dry Soup | 1,662 | 119 | 7.2 |
| Wet Soup | 4,236 | 290 | 6.8 |
| Cakes, Pastries & Sweet Goods | 13,450 | 887 | 6.6 |
| Stuffing, Polenta & Other Side Dishes | 2,442 | 159 | 6.5 |
| Savoury Vegetable Pastes/Spreads | 1,753 | 112 | 6.4 |
| Malt & Other Hot Beverages | 1,039 | 66 | 6.4 |
| Processed Cheese | 2,138 | 135 | 6.3 |
| Instant Rice | 135 | 8 | 5.9 |
| Rice/Nut/Grain & Seed Based Drinks | 1,190 | 67 | 5.6 |
| Shelf‐Stable Desserts | 3,247 | 182 | 5.6 |
| Fish Products | 12,755 | 690 | 5.4 |
| Poultry Products | 6,525 | 326 | 5.0 |
| Curd & Quark | 1,026 | 51 | 5.0 |
| Dessert Toppings | 649 | 30 | 4.6 |
| Pastry Dishes | 1,959 | 77 | 3.9 |
| Baking Ingredients & Mixes | 9,207 | 300 | 3.3 |
| Meat Snacks | 1,012 | 32 | 3.2 |
| Fruit/Flavoured Still Drinks | 2,905 | 85 | 2.9 |
| Instant Pasta | 601 | 17 | 2.8 |
| Beverage Mixes | 853 | 22 | 2.6 |
| Pickled Condiments | 5,618 | 135 | 2.4 |
| Meat Products | 16,349 | 391 | 2.4 |
| Carbonated Soft Drinks | 5,713 | 132 | 2.3 |
| Noodles | 585 | 13 | 2.2 |
| Wheat & Other Grain‐Based Snacks | 1,967 | 43 | 2.2 |
| Other Snacks | 143 | 3 | 2.1 |
| Sports Drinks | 813 | 17 | 2.1 |
| Stocks | 1,398 | 29 | 2.1 |
| Confiture & Fruit Spreads | 4,828 | 92 | 1.9 |
| Sweet Biscuits/Cookies | 17,756 | 320 | 1.8 |
| Snack/Cereal/Energy Bars | 5,065 | 90 | 1.8 |
| Savoury Biscuits/Crackers | 4,816 | 76 | 1.6 |
| Pasta Sauces | 3,861 | 54 | 1.4 |
| Bean‐Based Snacks | 224 | 3 | 1.3 |
| Other Sauces & Seasonings | 945 | 12 | 1.3 |
| Seasonings | 9,554 | 121 | 1.3 |
| Rice | 3,352 | 42 | 1.3 |
| Baby Fruit Products, Desserts & Yogurts | 1,633 | 20 | 1.2 |
| Butter | 1,454 | 16 | 1.1 |
| Beverage Concentrates | 2,356 | 25 | 1.1 |
| Liquorice | 768 | 8 | 1.0 |
| Vegetables | 10,802 | 105 | 1.0 |
| Other Baby Food | 104 | 1 | 1.0 |
| Eggs & Egg Products | 1,547 | 14 | 0.9 |
| Soft Cheese & Semi‐Soft Cheese | 5,781 | 51 | 0.9 |
| Nectars | 4,075 | 34 | 0.8 |
| Boiled Sweets | 974 | 7 | 0.7 |
| Flavoured Alcoholic Beverages | 2,016 | 14 | 0.7 |
| Gum | 1,372 | 9 | 0.7 |
| Pasta | 10,255 | 60 | 0.6 |
| Corn‐Based Snacks | 2,278 | 13 | 0.6 |
| Energy Drinks | 1,699 | 9 | 0.5 |
| Popcorn | 1,147 | 5 | 0.4 |
| Nuts | 4,710 | 20 | 0.4 |
| Chocolate Countlines | 2,370 | 10 | 0.4 |
| Growing Up Milk (1–4 years) | 267 | 1 | 0.4 |
| Baby Snacks | 317 | 1 | 0.3 |
| Baby Biscuits & Rusks | 318 | 1 | 0.3 |
| Mixed Assortments | 318 | 1 | 0.3 |
| Cassava & Other Root‐Based Snacks | 319 | 1 | 0.3 |
| Margarine & Other Blends | 1,016 | 3 | 0.3 |
| Fruit | 2,841 | 8 | 0.3 |
| Toffees, Caramels & Nougat | 1,942 | 5 | 0.3 |
| Seasonal Chocolate | 5,686 | 14 | 0.2 |
| Rice Snacks | 408 | 1 | 0.2 |
| Lollipops | 410 | 1 | 0.2 |
| Non‐Individually Wrapped Chocolate Pieces | 5,355 | 13 | 0.2 |
| Snack Mixes | 1,485 | 3 | 0.2 |
| Oils | 4,513 | 9 | 0.2 |
| Chocolate Spreads | 1,156 | 2 | 0.2 |
| Vegetable Snacks | 650 | 1 | 0.2 |
| Baby Cereals | 700 | 1 | 0.1 |
| Nut Spreads | 782 | 1 | 0.1 |
| Hard Cheese & Semi‐Hard Cheese | 6,907 | 8 | 0.1 |
| Cold Cereals | 6,357 | 7 | 0.1 |
| Other Sugar Confectionery | 1,122 | 1 | 0.1 |
| Fruit Snacks | 3,382 | 3 | 0.1 |
| Hot Cereals | 1,237 | 1 | 0.1 |
| Pastilles, Gums, Jellies & Chews | 3,864 | 2 | 0.1 |
| Beer | 8,278 | 4 | 0.0 |
| Coffee | 7,770 | 3 | 0.0 |
| Juice | 8,103 | 2 | 0.0 |
| Chocolate Tablets | 8,529 | 2 | 0.0 |
| Potato Snacks | 5,065 | 1 | 0.0 |
| Tea | 8,943 | 1 | 0.0 |
| Total sample | 411,735 | 23,438 | 5.7b |
According to the Mintel food categorisation.
In total, between 2011 and 2016,15 in the food categories where food products can be labelled with guar gum (E 412), around 5.7% of the products available on the Mintel GNPD are labelled with guar gum (E 412).
Appendix C – Concentration levels of guar gum (E 412) used in the refined exposure scenarios (mg/L or mg/kg as appropriate)
1.
Appendix C can be found in the online version of this output (‘Supporting information’ section).
Appendix D – Summary of total estimated exposure of guar gum (E 412) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and high level (mg/kg bw per day)
1.
Appendix D can be found in the online version of this output (‘Supporting information’ section).
Supporting information
Summary of reported use levels of guar gum (E 412) provided by industry (mg/L or mg/kg as appropriate)
Concentration levels of guar gum (E 412) used in the refined exposure scenarios (mg/L or mg/kg as appropriate)
Summary of total estimated exposure of guar gum (E 412) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and high level (mg/kg bw per day)
Suggested citation: EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Lambré C, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Oskarsson A, Parent‐Massin D, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Younes M, Brimer L, Peters P, Wiesner J, Christodoulidou A, Lodi F, Tard A and Dusemund B, 2017. Scientific Opinion on the re‐evaluation of guar gum (E 412) as a food additive. EFSA Journal 2017;15(2):4669, 62 pp. doi: 10.2903/j.efsa.2017.4669
Requestor: European Commission
Question number: EFSA‐Q‐2011‐00511
Panel members: Fernando Aguilar, Riccardo Crebelli, Alessandro Di Domenico, Birgit Dusemund, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Claude Lambré, Jean‐Charles Leblanc, Oliver Lindtner, Peter Moldeus, Alicja Mortensen, Pasquale Mosesso, Agneta Oskarsson, Dominique Parent‐Massin, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific opinion: Dario Battacchi and Lemmetyinen Juho. The ANS Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific opinion.
Adopted: 6 December 2016
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 2: © FAO/WHO, prepared by Kawamura
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.27.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
COM(2001) 542 final.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1.
Annex II to Regulation (EC) No 1333/2008.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents Published: 23 May 2010. Available from: http://www.efsa.europa.eu/en/dataclosed/call/ans091123
Call for food additives usage level and/or concentration data in food and beverages intended for human consumption – Extended deadline: 30 September 2014 Available online: http://www.efsa.europa.eu/sites/default/files/consultation/140310.pdf
Call for technical data on certain thickening agents permitted as food additives in the EU ‐ Extended Deadline: 31 December 2015. Available online: http://www.efsa.europa.eu/it/data/call/141219
12–13/10/2016.
Available online: http://www.efsa.europa.eu/en/food-consumption/comprehensive-database
Commission Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16.
EUROPEAN PARLIAMENT AND COUNCIL DIRECTIVE No 95/2/EC of 20 February 1995 on food additives other than colours and sweeteners.
Missing Bulgaria, Cyprus, Estonia, Latvia, Lithuania, Luxembourg, Malta and Slovenia.
http://www.gnpd.com/sinatra/home/ accessed on 5/8/2016.
Available online: http://www.efsa.europa.eu/en/press/news/150428.htm
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
Digestibility was defined by the authors as = (total intake of test material − increase in faecal weight)/total intake of test material.
Homeostasis model assessment for insulin resistance (HOMA‐IR).
The method consists of so‐called ‘gastric and intestinal phases’. During ‘gastric phase’, the sample pH is adjusted to pH 4.0 and pepsin solution is added under incubation. During the ‘intestinal phase’ done in an Amicon stirred cell, a small dialysis bag containing NaHCO3 is added to neutralise the gastric phase. After 30 min, a mixture of pancreatin/bile acid is added and dialysis continued for another 2 h.
References
- Adiotomre J, Easiwood MA, Edwards CA and Brydon WG, 1990. Dietary fiber: in vitro methods that anticipate nutrition and metabolic activity in humans. The American Journal of Clinical Nutrition, 52, 128–134. [DOI] [PubMed] [Google Scholar]
- Ahrens F, Pfeuffer M, Hagemeister H and Barth CA, 1991. The hypercholesterolemic effect of guar gum depends on dietary sucrose‐studies in minipigs. Zeitschrift fur Ernahrungswissenschaft, 30, 109–117. [DOI] [PubMed] [Google Scholar]
- Anonymous , 1964. Evaluation of Jaguar A‐20 and Karaya gum. Assay report No. 3110860 and 3110861. Unpublished report from the Wisconsin Alumni Research Foundation submitted to the World Health Organization, cited in JECFA 1975b.
- AOAC (Association of Official Agricultural Chemists), 2002. AOAC Official Methods 937.12 and 935.61. In: Horwitz W, (eds.). Chapter 43 – Spices and Other Contaminants. Food Compensition; Additives; Natural Contaminants, 17th Edition, Vol. II. Official Methods of Analysis of AOC International. 14 pp. [Google Scholar]
- Aronson JK, 2009. Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier LTD, Oxford. pp. 387–388. [Google Scholar]
- den Besten G, Havinga R, Bleeker A, Rao S, Gerding A, van Eunen K, Groen AK, Reijngoud DJ and Bakker BM, 2014. The short‐chain fatty acid uptake fluxes by mice on a guar gum supplemented diet associate with amelioration of major biomarkers of the metabolic syndrome. PLoS ONE, 9, e107392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behall KM, Scholfield DJ, Lee K, Powell AS and Moser PB, 1987. Mineral balance in adult men: effect of four refined fibers. American Journal of Clinical Nutrition, 46, 307–314. [DOI] [PubMed] [Google Scholar]
- Booth AN, Hendrickson AP and Deeds F, 1963. Physiologic effects of three microbial polysaccharides on rats. Toxicology and Applied Pharmacology, 5, 478–484. [DOI] [PubMed] [Google Scholar]
- Bosscher D, Van Caillie‐Bertrand M and Deelstra H, 2001. Effect of thickening agents, based on soluble dietary fiber, on the availability of calcium, iron and zinc from infant formulas. Nutrition, 17, 614–618. [DOI] [PubMed] [Google Scholar]
- Brillantino A, Iacobellis F, Izzo G, Di Martino N, Grassi R and Renzi A, 2014. Maintenance therapy with partially hydrolyzed guar gum in the conservative treatment of chronic anal fissure: results of a prospective, randomized study. Biomed Research International, 2014, 64942, 5 pp. doi: 10.1155/2014/964942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier A, L'Archevệque J, Soucy R, Malo JL and Dolovich J, 1992. Rate of sensitization to guar gum in a carpet manufacturing plant. The Journal of Allergy and Clinical Immunology 89(1 Part 2), 170. [DOI] [PubMed] [Google Scholar]
- Casas JA, Mohedano AF and Garcıa‐Ochoa F, 2000. Viscosity of guar gum and xanthan/guar gum mixture solutions. Journal of the Science of Food and Agriculture, 80, 1722–1727. [Google Scholar]
- Clifton PM, Galbraith C and Coles L, 2014. Effect of a low dose whey/guar preload on glycemic control in people with type 2 diabetes–a randomised controlled trial. Nutrition Journal, 13, 103. doi: 10.1186/1475-2891-13-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins TF, Welsh JJ, Black TN, Graham SL and O'Donnell MW Jr, 1987. Study of the teratogenic potential of guar gum. Food and Chemical Toxicology, 25, 807–814. [DOI] [PubMed] [Google Scholar]
- CRC , 1972. CRC Handbook of Food Additives. 2nd Edition, ed. by Furia TE. The Chemical Rubber Co., Cleveland. pp. 319–320. [Google Scholar]
- Crociani F, Alessandrini A, Mucci MM and Biavati B, 1994. Degradation of complex carbohydrates by Bifidobacterium spp. International Journal of Food Microbiology 24, 199–210. [DOI] [PubMed] [Google Scholar]
- Cox GE, Baily DE and Morgareidge K, 1974. Subacute feeding in dogs with a pre‐cooked gum blend. Unpublished report from the Food and Drug Labs, Inc. submitted to the World Health Organization by Hercules BV.
- Cummings JH and Englyst HN, 1987. Fermentation in the human large intestine and the available substrates. American Journal of Clinical Nutrition, 45, 1243–1255. [DOI] [PubMed] [Google Scholar]
- Eberendu AR, Booth C, Luta G, Edwards JA and McAnalley BH, 2005. Quantitative determination of saccharides in dietary glyconutritional products by anion‐exchange liquid chromatography with integrated pulsed amperometric detection. Journal of AOAC International, 88, 998–1007. [PubMed] [Google Scholar]
- EC (European Commission) , 2004. Commission decision of 13 April 2004 suspending the placing on the market and import of jelly mini‐cups containing the food additives E 400, E 401, E 402, E 403, E 404, E 405, E 406, E 407, E 407a, E 410, E 412, E 413, E 414, E 415, E 417 and/or E 418. C(2004) 1401. OJ EU L 118/70, 23.4.2004.
- Edwards CA and Eastwood MA, 1995. Caecal and faecal short‐chain fatty acids and stool output in rats fed on diets containing non‐starch polysaccharides. British Journal of Nutrition, 73, 773–781. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. doi: 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal, 2011;9(3):2097, 34 pp. doi: 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9 (3):1970, 27 pp. doi: 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA AFC Panel (EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food), 2004. Opinion on a request from the Commission related to the use of certain food additives in jelly mini cups. EFSA Journal 2004;82(2):8, 11 pp. doi: 10.2903/j.efsa.2004.82 [DOI] [Google Scholar]
- EFSA ANS Panel (European Food Safety Authority Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. doi: 10.2903/j.efsa.2012.2760. Available online: http://www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA ANS Panel on Food Additives and Nutrient Sources added to Food), 2014. Statement on aconceptual framework for the risk assessment of certain food additives re‐evaluated under CommissionRegulation (EU) No 257/2010. EFSA Journal 2014;12(6):3697, 11 pp. doi: 10.2903/j.efsa.2014.3697 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009a. Scientific Opinion on cadmium in food. EFSA Journal 2009;7(10):980, 139 pp. doi: 10.2903/j.efsa.2009.980 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009b. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351, 199 pp. doi: 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. doi: 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 241 pp. doi: 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2010. Scientific Opinion on the substantiation of health claims related to guar gum and maintenance of normal blood glucose concentrations (ID 794), increase in satiety (ID 795) and maintenance of normal blood cholesterol concentrations (ID 808) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2010;8(2):1464, 17 pp. doi: 10.2903/j.efsa.2010.1464 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2011. Scientific Opinion on the substantiation of health claims related to partially hydrolysed guar gum (PHGG) and decreasing potentially pathogenic gastro‐intestinal microorganisms (ID 788), changes in short chain fatty acid (SCFA) production and/or pH in the gastro‐intestinal tract (ID 787, 813), changes in bowel function (ID 813, 853, 1902, 1903, 1904, 2929, 2930, 2931), and reduction of gastro‐intestinal discomfort (ID 813, 1902, 1903, 1904, 2929, 2930, 2931) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2011;9(6):2254, 20 pp. doi: 10.2903/j.efsa.2011.2254 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspectsof Risk Assessments carried out by EFSA. Part 2: General Principles. EFSA Journal 2009;7(7):1051, 22 pp. doi: 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA ScientificCommittee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. doi: 10.2903/j.efsa.2012.2579. Available online: www.efsa.europa.eu [DOI] [Google Scholar]
- Europ. Pharm. Comment. , 2005. European Pharmacopeia Commentary, Arzneibuch‐Kommentar, Wissenschaftliche Verlagsgesellschaft, Stuttgart, Germany.
- Fǻk F, Jakobsdottir G, Kulcinskaja E, Marungruang N, Matziouridou C, Nilsson U, Stǻlbrand H and Nyman M, 2015. The physico‐chemical properties of dietary fibre determine metabolic responses, short‐chain fatty acids profiles and gut microbiota composition in rats fed low‐ and high‐fat diets. PLoS ONE, 10, e0127252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (Food and Drug Administration), 2016. CFR Code of Federal Regulations Title 21, Volume 4, Revised 1 April 2016. Specific labeling requirements for specific drug products. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=201.319.
- FDRL , (Food and Drug Research Laboratories) 1972. Teratologic evaluation of FDA 71‐16 (guar gum) in mice, rats, hamsters. PB‐221 800. Final report prepared under DHEW contract No. FDA 71‐260. Maspeth, NY.
- FDRL (Food and Drug Research Laboratories), 1973. Teratologic evaluation of FDA 71‐16 (Guar Gum) in rabbits. PB‐223 819. Final report prepared under DHEW contract No. FDA 71‐260. Maspeth, NY.
- Glicksman M, 1969. Gum Technology in the Food Industry. Academic Press, New York. 590 pp. [Google Scholar]
- Graham SL, Arnold A, Kasza L, Ruffin GE, Jackson RC, Watkins TL and Graham CH, 1981. Subchronic effects of guar gum in rats. Food and Cosmetics Toxicology, 19, 287–290. [DOI] [PubMed] [Google Scholar]
- Greim H, Hartwig A, Reuter U, Richter‐Reichhelm HB and Thielmann HW, 2009. Chemically induced pheochromocytomas in rats: mechanisms and relevance for human risk assessment. Critical Reviews in Toxicology, 39, 695–718. [DOI] [PubMed] [Google Scholar]
- Gruenwald J, Brendler T and Jaenicke C (eds.), 2007. PDR for Herbal Medicines, 4th Edition. Thomson Healthcare Inc., Montvale NJ, USA. [Google Scholar]
- GSFA (General Standard for Food Additives), 2011. Codex General Standard for Food Additives, Online database: Guar gum (412). Updated up to the 34th Session of the Codex Alimentarius Commission (2011). Available online: http://www.codexalimentarius.net/gsfaonline/additives/details.htmLmL?id=9
- Halama WH and Mauldin JL, 1992. Distal esophageal obstruction due to a guar gum preparation (Cal‐Ban 3000). Southern Medical Journal, 85, 642–645. [DOI] [PubMed] [Google Scholar]
- Harmuth‐Hoene A and Schelenz R, 1980. Effect of dietary fiber on mineral absorption in growing rats. Journal of Nutrition, 110, 1774–1784. [DOI] [PubMed] [Google Scholar]
- Harmuth‐Hoene AE, Jakubick V and Schelenz R, 1978. Der Einfluss von Guarmehl in der Nahrung auf die Stickstoffbilanz, den Proteinstoffwechsel und die Transitzeit der Nahrung in Ratten. Nutrition and Metabolism, 22, 32–43. [PubMed] [Google Scholar]
- Huth K, 1985. Die Rolle der Ballaststoffe in der Ernährung—diätetische Möglichkeiten mit Guar. In: Grundlagen und Klinik der enteralen Ernährung (pp. 134‐141). Springer Berlin Heidelberg. [Google Scholar]
- ILDIS , 2013. International Legume Database & Information Service. Available online: http://www.ildis.org/LegumeWeb 26.11.2014.
- IPCS , 2004. IPCS Risk Assessment Terminology (Part 1: IPCS/OECD Key Generic terms used in Chemical Hazard/Risk Assessment. Available online: http://www.inchem.org/documents/harmproj/harmproj/harmproj1.pdf
- Jang AL, Hwang SK and Kim DU, 2015. Effects of guar gum ingestion on postprandial blood pressure in older adults. The Journal of Nutrition, Health and Aging, 19, 299–304. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1970. Thirteenth report of the Joint FAO/WHO Expert Committee on Food Additives. Available online: http://whqlibdoc.who.int/trs/WHO_TRS_445.pdf
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1972. WHO Technical Report Series, Evaluation of certain food additives and the contaminants mercury, lead, and cadmium. Sixteenth report of the Joint FAO/WHO Expert Committee on Food Additives. No. 505. World Health Organization, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1974. Seventeenth Report of the Joint FAO/WHO Expert Committee on Food Additives. Available online: http://whqlibdoc.who.int/trs/WHO_TRS_539.pdf
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1975a. In: Toxicological Evaluation of Certain Food Additives. 19th Report of the Joint FAO/WHO Expert Committee on Food Additives, World Health Organisation (WHO), tech. Rep. Ser. 1975, No 576; FAO Nutrition Meetings report Series, 1975, No 55. Available online: http://whqlibdoc.who.int/trs/WHO_TRS_576.pdf
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1975b. Toxicological evaluation of some food colours, thickening agents, and certain other substances. WHO Food Additives Series No. 8.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1978. Evaluation of certain food additives. Twenty‐first report of the Joint FAO/WHO Expert Committee on Food Additives. No. 617. World Health Organization, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. 976. Lead. WHO Food Additives Series 44, 39 pp.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2008a. Guar gum. Published in FAO JECFA Monographs 5. Joint FAO/WHO Expert Committee on Food Additives.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2008b. Guar gum (clarified). Published in FAO JECFA Monographs 5. Joint FAO/WHO Expert Committee on Food Additives.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2008c. Guar Gum Chemical and Technical assessment Prepared by Yoko Kawamura, PhD, for the 69th JECFA. Available online: http://www.fao.org/fileadmin/templates/agns/pdf/jecfa/cta/69/Guar_gum.pdf
- Kanerva L, Tupasela O, Jolanki R, Vaheri E, Estlander T and Keskinen H, 1988. Occupational allergic rhinitis from guar gum. Journal of Clinical Allergy, 18, 245–252. [DOI] [PubMed] [Google Scholar]
- Keane KW, Smutko CJ, Krieger CH and Denton AE, 1962. The addition of water to purified diets and its effect upon growth and protein efficiency ratio in the rat. The Journal of Nutrition, 77, 18–22. [DOI] [PubMed] [Google Scholar]
- Koepp P and Hegewisch S, 1981. Effects of guar on plasma viscosity and related parameters in diabetic children. European Journal of Pediatrics, 137, 31–33. [DOI] [PubMed] [Google Scholar]
- Krantz JC, 1947. Unpublished report from General Mills, Inc. submitted to the World Health Organization, cited in JECFA 1975b.
- Krantz JC, 1948. The feeding of guar gum to rats (lifespan) and to monkeys. Unpublished report from the University of Maryland, School of Medicine submitted to the World Health Organization by General Mills Chemicals, Inc., cited in JECFA 1975b.
- Krantz JC, Carr CJ and Farson DB, 1948. Guar polysaccharide as a precursor of glycogen. Journal of the American Dietetic Association, 24, 212. [PubMed] [Google Scholar]
- Lagier F, Cartier A, Somer J, Dolovich J and Malo JL, 1990. Occupational asthma due to guar gum. Journal of Allergy and Clinical Immunology, 85, 785–790. [DOI] [PubMed] [Google Scholar]
- Leegwater DC, de Groot P and van Kalmthout‐Kuyper M, 1974. The aetiology of caecal enlargement in the rat. Food and Cosmetics Toxicology, 12, 687–697. [DOI] [PubMed] [Google Scholar]
- Leung AY and Foster S, 1996. Guar gum. In: John Wiley and Sons, Inc. (ed.). Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics. John Wiley & Sons Inc., New York, NY. pp. 289–291. [Google Scholar]
- Lewis JH, 1992. Esophageal and small bowel obstruction from guar gum‐containing “diet pills”: analysis of 26 cases reported to the Food and Drug Administration. American Journal of Gastroenterology, 87, 1424–1428. [PubMed] [Google Scholar]
- Lewis RJ Sr, (ed.), 2007. Hawley's Condensed Chemical Dictionary. 15th Edition. John Wiley & Sons, Inc., New York, NY. 625 pp. [Google Scholar]
- Leznoff A, Haight JS and Hoffstein V, 1986. Reversible obstructive sleep apnea caused by occupational exposure to guar gum dust. The American Review Respiratory Disease, 133, 935–936. [PubMed] [Google Scholar]
- Licht TR, Hansen M, Poulsen M and Dragsted LO, 2006. Dietary carbohydrate source influences molecular fingerprints of the rat faecal microbiota. BMC Microbiology, 6, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo LJ, Cartier A, L'Archeveque J, Ghezzo H, Soucy F, Somers J and Dolovich J, 1990. Prevalence of occupational asthma and immunologic sensitization to guar gum among employees at a carpet‐manufacturing plant. Journal of Allergy Clinical and Immunology 86(4 Pt. 1), 562–569. [DOI] [PubMed] [Google Scholar]
- Martindale , 2014. The Complete Drug Reference, 38th Edition. Pharmaceutical Press, London, UK. [Google Scholar]
- McIvor ME, Cummings CC and Mendeloff AI, 1985. Long‐term ingestion of guar gum is not toxic in patients with noninsulin‐dependent diabetes mellitus. American Journal of Clinical Nutrition, 41, 891–894. [DOI] [PubMed] [Google Scholar]
- Melnick RL, Huff J, Haseman JK, Dieter MP, Grieshaber CK, Wyand DS, Russfield AB, Murthy ASK, Fleischmann RW and Lilja HS, 1983. Chronic effects of agar, guar gum, gum arabic, locust bean gum, or tara gum in F344 rats and B6C3F1 mice. Food and Chemical Toxicology, 21, 305–311. [DOI] [PubMed] [Google Scholar]
- Merck Index , 2006. Guaran. In: The Merck Index, an Encyclopedia of Chemicals, Drugs, and Biologicals. Available online: http://themerckindex.cambridgesoft.com/themerckindex/Forms/Search/ContentArea/C
- Meyer K, Rosa C, Hischenhuber C and Meyer R, 2001. Determination of locust bean gum and guar gum by polymerase chain reaction and restriction fragment length polymorphism analysis. Journal of AOAC International, 84, 89–99. [PubMed] [Google Scholar]
- Moriceau S, Besson C, Levrat MA, Moundras C, Rémésy C, Morand C and Demigné C, 2000. Cholesterol‐lowering effects of guar gum: changes in bile acid pools and intestinal reabsorption. Lipids, 35, 437–444. [DOI] [PubMed] [Google Scholar]
- Morse JM and Malloy WX, 1990. Esophageal obstruction caused by Cal‐Ban. Gastroenterology, 98, 805–806. [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program), 1982. Carcinogenesis bioassay of guar gum (CAS No. 9000‐30‐0) in F344 rats and B6C3F1 mice (feed study). Natlional Toxicology Program Technical Report Series 229, 1–132. [PubMed] [Google Scholar]
- Ochuba GU and Von Riesen VL, 1980. Fermentation of polysaccharides by Klebsielleae and other facultative bacilli. Applied and Environmental Microbiology, 39, 988–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opper FH, Isaacs KL and Warshauer DM, 1990. Esophageal obstruction with a dietary fiber product designed for weight reduction. Journal of Clinical Gastroenterology, 12, 667–669. [DOI] [PubMed] [Google Scholar]
- Owusu‐Asiedu A, Patience JF, Laarveld B, Van Kessel AG, Simmins PH and Zijlstra RT, 2006. Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. Journal of Animal Science, 84, 843–852. [DOI] [PubMed] [Google Scholar]
- Paganus A, Mäenpää J, Åkerblom HK, Stenman U‐H, Knip M and Simell O, 1987. Beneficial effects of palatable guar and guar plus fructose diets in diabetic children. Acta Paediatrica Scandinavica, 76, 76–81. [DOI] [PubMed] [Google Scholar]
- Papanikolaou I, Stenger R, Bessot JC, de Blay F and Pauli G, 2007. Anaphylactic shock to guar gum (food additive E412) contained in a meal substitute. Allergy 62, 822. [DOI] [PubMed] [Google Scholar]
- Papathanasopoulos A and Camilleri M, 2010. Dietary fiber supplements: effects on obesity and metabolic syndrome and relationship to gastrointestinal functions. Gastroenterology, 138, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittler MH and Ernst E, 2001. Guar gum for body weight reduction: meta‐analysis of randomized trials. American Journal of Medicine, 110, 724–730. [DOI] [PubMed] [Google Scholar]
- Platt SR and Clydesdale FM, 1987. Mineral binding characteristics of lignin, guar gum, cellulose, pectin and neutral detergent fiber under simulated duodenal pH conditions. Journal of Food Science, 52, 1414–1419. [Google Scholar]
- Prado BM, Kim S, Özen BF and Mauer LJ, 2005. Differentiation of carbohydrate gums and mixtures using fourier transform infrared spectroscopy and chemometrics. Journal of Agriculture and Food Chemistry, 53, 2823–2829. [DOI] [PubMed] [Google Scholar]
- Ramis‐Ramos G, Simo‐Alfonso EF, Mongay‐Fernandez C and Ruiz‐Angel MJ, 2003. Leguminosae gum labelling and guar evaluation in carob gum by capillary electrophoresis. Laboratory of Journal, 7, 66–68. [Google Scholar]
- Ranft K and Imhof W, 1983. Bolusobstruktion des distalen Ösophagus durch pflanzliche Quellstoffe (Guarmehl). DMW‐Deutsche Medizinische Wochenschrift, 108, 1968–1969. [DOI] [PubMed] [Google Scholar]
- Rideout TC, Harding SV, Jones PJH and Fan MZ, 2008. Guar gum and similar soluble fibers in the regulation of cholesterol metabolism: current understandings and future research priorities. Vascular Health and Risk Management, 4, 1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl J, Linseisen J, Hoffman J and Wolfram G, 1999. Some dietary fibers reduce the absorption of carotenoids in women. Journal of Nutrition, 129, 2170–2176. [DOI] [PubMed] [Google Scholar]
- Russo L, Andreozzi P, Zito FP, Vozzella L, Savino IG, Sarnelli G and Cuomo R, 2015. Partially hydrolyzed guar gum in the treatment of irritable bowel syndrome with constipation: effects of gender, age, and body mass index. The Saudi Journal of Gastroenterology, 21, 104–110. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4392570/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers AA, Vercellotti JR, West SHE and Wilkins TD. 1977a. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Applied and Environment Microbiology 33, 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers AA, West SHE, Vercellotti JR and Wilkins TD, 1977b. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Applied and Environment Microbiology, 33, 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper RB, Eisenberg DM and Phillips RS, 2004. Common dietary supplements for weight loss. American Family Physician, 70, 1731–1738. [PubMed] [Google Scholar]
- Sax NI, 1984. Guar gum. Dangerous Properties of Industrial Materials. 6th Edition. New York, NY: Van Nostrand Reinhold. 1490 pp. [Google Scholar]
- SCF (Scientific Committee for Food), 1978. Reports from the Scientific Committee for Food (7th series). Opinion expressed 1977. Food science and techniques, 1978.
- SCF (Scientific Committee for Food), 1996. Opinion on additives in nutrient preparations for use in infant formulae, follow‐on formulae and weaning foods. 7 June 1996. Available online: http://ec.europa.eu/food/fs/sc/scf/reports/scf_reports_40.pdf
- SCF (Scientific Committee for Food), 1998. Opinion of the Scientific Committee of Food on the applicability of the ADI (Acceptable Daily Intake) for food additives to infants. 17 September 1998. Available online: http://ec.europa.eu/food/fs/sc/scf/out13_en.htmLmL
- SCF (Scientific Committee for Food), 1999. Reports from the Scientific Committee for Food (43rd series). Opinion expressed 1997. Food science and techniques, 1999.
- SCF (Scientific Committee on Food), 2001. Guidance on submissions for food additive evaluations by the scientific committee on food. Opinion expressed on 11 July 2001.
- SCF (Scientific Committee for Food), 2003. Report of the Scientific Committee on Food on the Revision of Essential Requirements of Infant Formulae and Follow‐on Formulae. Adopted on 4 April 2003. SCF/CS/NUT/IF/65 Final. 18 May 2003.
- Scherz H and Mergentaler E, 1980. Analytik der als Lebensmittelzusatzstoffe verwendeten Polysaccharide. Z. Lebensm. Unters. Forsch., 170, 280–286. [DOI] [PubMed] [Google Scholar]
- Seebach AV, 1987. Nahrungsmittelallergie auf Guarmehl: Zungenbrennen als Leitsymptom. Allergologie, 4, 155. [Google Scholar]
- Seidner DL, Roberts IM and Smith MS, 1990. Esophageal obstruction after ingestion of a fiber‐containing diet pill. Gastroenterology, 99, 1820–1822. [DOI] [PubMed] [Google Scholar]
- Semenza G, 1975. Report on the possible digestion of locust bean gum in the stomach and/or in the small intestine in an in vitro study. Unpublished report from the Eidgenössische Technische Hochschule Zürich submitted to the World Health Organization by the Institut Européen des Industries de la Gomme de Caroube, cited in JECFA 1975b.
- Slavin J and Green H, 2007. Dietary fibre and satiety. British Nutrition Foundation Nutrition Bulletin, 32, 32–42. [Google Scholar]
- Stanford Research Inst. , 1972. Study of the mutagenic effects of guar gum (FDA‐71‐16). Maxwell WA, Newell GW, Stanford Research Institute, PB‐221 815.
- Sunvold GD, Fahey GC, Merchen NR, Titgemeyer EC, Bourquin LD, Bauer LL and Reinhart GA, 1995a. Dietary fiber for dogs: IV. In vitro fermentation of selected fiber sources by dog fecal inoculum and in vivo digestion and metabolism of fiber‐supplemented diets. Journal of Animal Sciences, 73, 1099–1109. [DOI] [PubMed] [Google Scholar]
- Sunvold GD, Fahey GC, Merchen NR, Bourquin LD, Titgemeyer EC, Bauer LL and Reinhart GA, 1995b. Dietary fiber for cats: in vitro fermentation of selected fiber sources by cat fecal inoculum and in vivo utilization of diets containing selected fiber sources and their blends. Journal of Animal Sciences, 73, 2329–2339. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Sung IY, Mujo K and Takehiko Y, 1994. Protein and energy utilization of growing rats fed on the diets containing intact or partially hydrolyzed guar gum. Comparative Biochemistry and Physiology, 107A, 255–260. [Google Scholar]
- Tako M, 1992. Synergistic interaction between xanthan and konjac glucomannan in aqueous media. Bioscience Biotechnology abd Biochemistry, 56, 1188–1192. [Google Scholar]
- Taylor JR, Streetman DS and Castle SS, 1998. Medication bezoars: a literature review and report of a case. The annals of pharmacotherapy, 32, 940–946. [DOI] [PubMed] [Google Scholar]
- TemaNord , 2002. Nordic Council of Ministers. E 412 Guar gum. In: Food Additives in Europe 2000 – Status of safety assessments of food additives presently permitted in the EU, 4 p.
- Thazhath SS, Wu T, Bound MJ, Checklin HL, Jones KL, Willoughby S, Horowitz M and Rayner CK, 2014. Changes in meal composition and duration affect postprandial endothelial function in healthy humans. American Journal of Physiology: Gastrointestinal and Liver Physiology, 307, G1191–G1197. [DOI] [PubMed] [Google Scholar]
- Til HP, Spanjers MT and de Groot AP, 1974. Sub‐chronic toxicity study with locust bean gum in rats. Unpublished report from Centraal Instituut voor Voedingsonderzoek TNO submitted to the World Health Organization by Hercules BV and Institut Européen des Industries de la Gomme de Caroube.
- Tischler AS, Powers JF and Alroy J, 2004. Animal models of pheochromocytoma. Histology and Histopathology, 19, 883–895. [DOI] [PubMed] [Google Scholar]
- Todd PA, Benfield P and Goa KL, 1990. Guar gum. A review of its pharmacological properties and use as a dietary adjunct in hypercholesterolemia. Drugs, 39, 917–928. [DOI] [PubMed] [Google Scholar]
- TomLin J, Read NW, Edwards CA and Duerden BI, 1988. The digestion of guar gum by individual strains of colonic bacteria. Microbial Ecology in Health and Disease 1, 163–167. [Google Scholar]
- Topping DL and Clifton PM, 2001. Short‐Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiological Reviews, 81, 1031–1064. [DOI] [PubMed] [Google Scholar]
- Towle GA and Schranz RE, 1975. The action of rat microflora on carob bean gum solutions in vitro. Unpublished report from Hercules Research Center submitted to the World Health Organization by Hercules Incorporated, cited in JECFA 1975b.
- Track NS, Cawkwell ME, Chin BC, Chiu SS, Haberer SA and Honey CR, 1985. Guar gum consumption in adolescent and adult rats: short‐ and long‐term metabolic effects. Canadian Journal of Pharmacology, 63, 1113–1121. [DOI] [PubMed] [Google Scholar]
- Tsai LB and Whistler RL, 1975. Digestibility of galactomannans. Unpublished report submitted to the World Health Organization by Professor H. Neukom, Chairman of the Technical Committee of Inst. Europ. des Industries de la Gomme de Caroube, cited in JECFA 1975b.
- Turner PR, Tuomilehto J, Happonen P, La Ville AR, Shaikh M and Lewis B, 1990. Metabolic studies on the hyperlipidaemic effect of guar gum. Artherosclerosis, 81, 145–150. [DOI] [PubMed] [Google Scholar]
- Ullmann , 2007. Polysaccharides. Ullmann's Encyclopedia of Industrial Chemistry, Online version. Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim.
- Urdiain M, Doménech‐Sánchez A, Albertí S, Benedí VJ and Rosselló JA, 2004. Identification of two additives, locust bean gum (E‐410) and guar gum (E‐412), in food products by DNA‐based methods. Food Additives and Contaminants, 21, 619–625. [DOI] [PubMed] [Google Scholar]
- Urdiain M, Doménech‐Sánchez A, Albertí S, Benedí VJ and Rosselló JA, 2005. New method of DNA isolation from two food additives suitable for authentication in polymerase chain reaction assays. Journal of Agriculture and Food Chemistry, 53, 3345–3347. [DOI] [PubMed] [Google Scholar]
- Vandenplas Y, Hauser B, Devreker T, Mahler T, Degreef E and Wauters GV, 2011. Gastro‐esophageal reflux in children: symptoms, diagnosis and treatment. Journal of Pediatric Sciences, 3, e101. [Google Scholar]
- Vohra P, Shariff G and Kratzer FH, 1979. Growth inhibition of some gums and pectin for tribolium castaneum larvae, chickens and Japanese quail. Nutrition Reports International, 19, 463–469. [Google Scholar]
- Wahl K, Kotz A, Hädrich J, Malisch R, Anastassiades M and Sigalova I, 2007. The guar gum case: contamination with PCP and dioxins and analytical problems. Available online: http://www.dioxin20xx.org/pdfs/2008/08-301.pdf
- Wielinga W, 2010. Seed gums. In: Imeson A (ed.). Food Stabilizers, Thickeners and Gelling Agents. Wiley‐Blackwell, Chichester. pp. 167–179. [Google Scholar]
- Wobling RH, Becker G and Forth W, 1980. Inhibition of the intestinal absorption of iron by sodium alginate and guar gum in rat. Digestion, 20, 403–409. [DOI] [PubMed] [Google Scholar]
- Yoon SJ, Chu DC and Juneja LR, 2008. Chemical and physical properties, safety and application of partially hydrolysed guar gum as dietary fiber. Journal of Clinical Biochemistry and Nutrition, 42, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E, Anderson B, Haworth S, Lawlor T and Mortelmans K, 1992. Salmonella mutagenicity tests: V. Results from testing of 311 chemicals. Environmental and Molecular Mutagenesis 19, 2–141. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wong JW, Jia Z, Vaclavikova M, Trucksess MW and Begley TH, 2014. Screening multimycotoxins in food‐grade gums by stable isotope dilution and liquid chromatography/tandem mass spectrometry. Journal of AOAC International, 97, 889–895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of reported use levels of guar gum (E 412) provided by industry (mg/L or mg/kg as appropriate)
Concentration levels of guar gum (E 412) used in the refined exposure scenarios (mg/L or mg/kg as appropriate)
Summary of total estimated exposure of guar gum (E 412) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and high level (mg/kg bw per day)


