Abstract
The Panel on Plant Health performed a pest categorisation of the small spruce bark beetle, Ips amitinus (Eichhoff) (Coleoptera: Curculionidae, Scolytinae), for the EU. I. amitinus is a well‐defined and distinguishable species, native to Europe and attacking mainly spruce (Picea spp.) and pine (Pinus spp.) and sporadically fir (Abies spp.) and larch (Larix spp.). It is distributed in 16 EU Member States and is locally spreading in some. The pest is listed in Annex IIB of Council Directive 2000/29/EC. Protected zones are in place in Ireland, Greece and the United Kingdom. Wood, wood products, bark and wood packaging material are considered as pathways for this pest, which is also able to disperse by flight over tens of kilometres. The insects normally establish on fallen or weakened trees (e.g. after a fire or a drought) but can also occasionally mass‐attack healthy trees, when population densities are high. The males produce pheromones that attract conspecifics of both sexes. Each male attracts one to seven females to establish a brood system; each female produces 1–60 offspring. The insects also inoculate their hosts with pathogenic fungi. There are one or two generations per year. The wide current geographic range of I. amitinus suggests that it is able to establish in most areas in the EU, including the protected zones, where its hosts are present. The damage due to I. amitinus is limited and usually does not require control. Sanitary thinning or clear‐felling is the usual control methods, when necessary. Quarantine measures are implemented to prevent entry in protected zones. All criteria for consideration as a potential protected zone quarantine pest are met. The criteria for considering I. amitinus as a potential regulated non‐quarantine pest are not met since plants for planting are not viewed as a pathway.
Keywords: Curculionidae, European Union, pest risk, plant health, plant pest, quarantine, small spruce bark beetle
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorizations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/2002,3 to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L.. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pests categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L.,Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L.,Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Ips amitinus is one of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest (RNQP)_for the area of the European Union (EU) excluding Ceuta, Melilla and the outermost regions of Member States (MSs) referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
Since I. amitinus is regulated in the protected zones (PZs) only, the scope of the categorisation is the territory of the PZ (Greece, Ireland and the UK); thus, the criteria refer to the PZ instead of the EU territory.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on I. amitinus was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as search term. Relevant papers were reviewed and further references and information were obtained from experts as well as from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, 2017) as well as from the relevant literature.
Data about import of commodity types that could potentially provide a pathway for the pest to enter the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network launched by the Directorate General for Health and Consumers (DG SANCO) and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation as well as notifications of plant pests detected in the territory of the MSs and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for I. amitinus, following guiding principles and steps presented in the EFSA guidance on the harmonised framework for pest risk assessment (EFSA PLH Panel, 2010) and as defined in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
In accordance with the guidance on a harmonised framework for pest risk assessment in the EU (EFSA PLH Panel, 2010), this work was initiated following an evaluation of the EU's plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union RNQP in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants and includes additional information required in accordance with the specific ToR received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a RNQP. If one of the criteria is not met, the pest will not qualify. Note that a pest that does not qualify as a quarantine pest may still qualify as a RNQP that needs to be addressed in the opinion. For the pests regulated in the PZs only, the scope of the categorisation is the territory of the PZ; thus, the criteria refer to the PZ instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pest must be present in the risk assessment area) |
| Regulatory status (Section 3.3 ) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future |
The protected zone system aligns with the pest‐free area system under the International Plant Protection Convention (IPPC) The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone) |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in and spread within the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in and spread within the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6 ) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, whereas addressing social impacts is outside the remit of the Panel, in agreement with EFSA guidance on a harmonised framework for pest risk assessment (EFSA PLH Panel, 2010).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process but, following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? (Yes or No)
Yes, the identity of the pest is established. It can be identified to species using conventional entomological keys.
I. amitinus is an insect of the family Curculionidae, subfamily Scolytinae.4
3.1.2. Biology of the pest
A general description of the biology and ecology of I. amitinus is provided by Chararas (1962), Bakke (1968), Jurc and Bojović (2006), Holuša et al. (2012) and Økland and Skarpaas (2008). The adults overwinter in the bark or in the litter, and disperse in the spring, flying in search of new hosts, sometimes over large distances. In flight mill tests, I. amitinus flew on the average much longer (up to ca 4 h) than I. typographus or I. sexdentatus (Forsse, 1989). These two latter species are known to be able to fly tens of kilometers (Forsse and Solbreck, 1985; Jactel, 1991; Jactel and Gaillard, 1991).
I. amitinus attacks mostly Picea abies and Pinus sylvestris but can attack other spruces and pines as well; it has also been observed on Abies alba and on Larix decidua. I. amitinus attacks felled or weakened trees (e.g. after a fire or a drought) but can also occasionally mass‐attack healthy trees. They tend to oviposit and develop galleries in the higher parts of the trees. Younger hosts are preferred, but Jurc and Bojović (2006) report an outbreak in Slovenia on 70–80 years old trees. The males are the pioneer sex, the first to land on potential hosts. They produce aggregation pheromones (a mixture of ipsenol, ipsdienol and amitinol) (Francke et al., 1980) that attract conspecifics of both sexes. One to seven females join each male on a new host; each female produces 1–60 offspring. From a central nuptial chamber in the phloem, each female excavates a gallery starting first radially (thus giving a star pattern to the gallery system), then extending in parallel to the phloem fibres. The eggs are laid individually at regular intervals in small niches on both sides of the galleries, and each larva then bores its own individual mine, more or less perpendicular to the fibres, ending in a pupal niche. Pupation occurs in the phloem, where the young adults spend several weeks and feed until ready to emerge. There are one or two generations per year; Holuša et al. (2012) observed that, in Central Europe, the species is bivoltine below 600 m but becomes univoltine at higher elevations. Sister broods (produced by adults leaving a first brood system and later on creating another brood) are regularly observed. While several reports mention that the species are only/mostly present at higher elevations (> 1,400 m in France (Chararas, 1962); 1,270 m in Slovenia (Jurc and Bojović, 2006)), Holuša et al. (2012) found it at all elevations sampled, from 290 to 1,000 m in the Czech Republic and in Poland. The beetles carry ophiostomatoid fungi and inoculate their host with them (Kirisits, 2004; Repe et al., 2013). These fungi cause blue staining of the wood and some of them can contribute to tree death.
3.1.3. Intraspecific diversity
Two subspecies have been recorded, namely, I. amitinus helveticus and I. amitinus montanus (EPPO, 2017).
According to Schedl (1932), I. amitinus var. helveticus is synonymous to I. amitinus var. montana. I. amitinus was observed on P. abies, and I. amitinus var. montana was found on Pinus cembra and P. montana. However, using morphometric, behavioural and chemical criteria as well as molecular genetics, Stauffer and Zuber (1998) found no differences between I. amitinus and I. amitinus var. montana.
3.1.4. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, the organism can be detected by visual searching, often after damage symptoms are seen. The species can be identified by examining morphological features, for which conventional entomological keys exist, e.g. Balachowsky (1949); Grüne (1979); Schedl (1981) and Wood (1982).
During the attacks of I. amitinus, brown sawdust is expelled from the entry holes and, when the broods and the young adults start feeding on the phloem around the galleries, the bark can flake off. This phenomenon can be amplified by the action of woodpeckers. Within and behind the phloem, maternal galleries, parallel to the fibres, and transversal larval galleries can be seen. The sapwood shows blue staining due to the fungi introduced by the beetles. The adult beetles are dark brown or black in colour, cylindrical, 3.5–4.5 mm long. The larvae are apodous, with a dark amber cephalic capsule. Pheromone trapping (see Section 3.1.2) could allow catching the beetles but would not indicate establishment.
Although I. typographus, I. amitinus and I. cembrae are sometimes considered as sibling species (Stauffer, 1997), the adults can be distinguished by morphological traits (e.g. Balachowsky, 1949) or molecular features (Stauffer, 1997). Based on the differences in polygamy between I. typographus and I. amitinus (1–4 females per familial system in I. typographus vs 3–7 females/system in I. amitinus), the gallery systems of both species can also be distinguished (1–4 branches for I. typographus; 3–7 branches for I. amitinus; as there are many galleries on each attacked tree, the two species can be distinguished based on the average number of branches). As the two species often occur together on the same trees (with I. amitinus often favouring the higher parts of the trees), there could be a certain level of confusion in case of superficial monitoring.
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
I. amitinus is present in Europe and in Tunisia (restricted distribution). In non‐EU Europe, the insect has been reported from Bosnia and Herzegovina, Macedonia (FYROM), Montenegro, Russia, Serbia, Switzerland and Ukraine (Figure 1).
Figure 1.
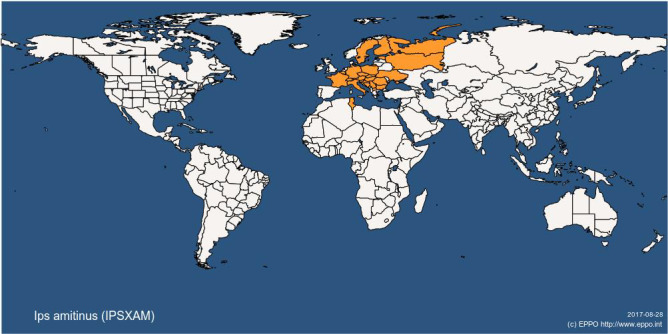
Global distribution map for Ips amitinus (extracted from EPPO global database accessed on 28 August 2017)
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
YES, I. amitinus is present and widely distributed in the EU; it has been reported from 16 MS. The pest is absent in the protected zones (Greece, Ireland and the UK).
Table 2.
Current distribution of Ips amitinus in the 28 EU MS based on information from the EPPO Global Database
| Country | EPPO GD (Last updated: 12/7/2017 Last accessed: 28/8/2017) |
|---|---|
| Austria | Present, restricted distribution |
| Belgium | Present, restricted distribution |
| Bulgaria | Present, widespread |
| Croatia | Present, restricted distribution |
| Cyprus | No information |
| Czech Republic | Present, widespread |
| Denmark | No information |
| Estonia | Present, restricted distribution |
| Finland | Present, widespread |
| France | Present, restricted distribution (Corse: absent, confirmed by survey) |
| Germany | Present, widespread |
| Greece | Absent, confirmed by survey |
| Hungary | Present, restricted distribution |
| Ireland | Absent, confirmed by survey |
| Italy | Present, restricted distribution |
| Latvia | No information |
| Lithuania | Absent, pest no longer present |
| Luxembourg | No information |
| Malta | No information |
| Netherlands | Absent, pest no longer present |
| Poland | Present, widespread |
| Portugal | Absent, confirmed by survey |
| Romania | Present, widespread |
| Slovak Republic | Present, restricted distribution |
| Slovenia | Present, restricted distribution |
| Spain | Absent, invalid record |
| Sweden | Present, restricted distribution |
| United Kingdom | Absent, confirmed by survey |
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
I. amitinus is listed in Council Directive 2000/29/EC. Details are presented in Tables 3 and 4.
Table 3.
Ips amitinus in Council Directive 2000/29/EC
| Annex II, Part B | Harmful organisms whose introduction into, and whose spread within, certain protected zones shall be banned if they are present on certain plants or plant products | ||
|---|---|---|---|
| (a) | Insects, mites and nematodes, at all stages of their development | ||
| Species | Subject of contamination | Protected zones | |
| 6 (a) | Ips amitinus | Plants of Abies Mill., Larix Mill., Picea A. Dietr., Pinus L. over 3 m in height, other than fruit and seeds, wood of conifers (Coniferales) with bark, isolated bark of conifers | EL, IRL, UK |
Table 4.
Regulated hosts and commodities that may involve Ips amitinus in Annexes III, IV and V of Council Directive 2000/29/EC
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all Member States | ||
|---|---|---|---|
| Description | Country of origin | ||
| 1 | Plants of Abies Mill., […] Larix Mill., Picea A. Dietr., Pinus L., […], other than fruit and seeds | Non‐European Countries | |
| Annex IV, Part B | Special requirements which shall be laid down by all member states for the introduction and movement of plants, plant products and other objects into and within certain protected zones | ||
| Plants, plant products and other objects | Special requirements | Protected zone(s) | |
| 4. | Wood of conifers (Coniferales) | Without prejudice to the requirements applicable to the wood listed in Annex IV(A)(I)(1.1), (1.2), (1.3), (1.4), (1.5), (1.6), (1.7), where appropriate, and Annex IV(B)(1), (2), (3):the wood shall be stripped of its bark;or(b) official statement that the wood originates in areas known to be free from Ips amitinus Eichhof;or(c) there shall be evidence by a mark ‘Kiln‐dried’, ‘KD’ or another internationally recognised mark, put on the wood or on its packaging in accordance with current commercial usage, that it has undergone kiln‐drying to below 20% moisture content, expressed as a percentage of dry matter, at time of manufacture, achieved through an appropriate time/temperature schedule. | EL, IRL, UK |
| 10. | Plants of Abies Mill., Larix Mill., Picea A. Dietr. and Pinus L. over 3 m in height, other than fruit and seeds | Without prejudice to the provisions applicable to the plants listed in Annex III(A)(1), Annex IV(A)(I)(8.1), (8.2), (9), (10), Annex IV(A)(II)(4), (5), and Annex IV(B)(7), (8), (9), where appropriate, official statement that the place of production is free from Ips amitinus Eichhof. | EL, IRL, UK |
| 14.2 | Isolated bark of conifers (Coniferales) | Without prejudice to the provisions applicable to the bark listed in Annex IV(B)(14.1), official statement that the consignment:has been subjected to fumigation or other appropriate treatments against bark beetles;ororiginates in areas known to be free from Ips amitinus Eischhof. | EL, IRL, UK |
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the Community, before being moved within the Community—in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community | ||
| Part A | Plants, plant products and other objects originating in the Community | ||
| Section II | Plants, plant products and other objects produced by producers whose production and sale is authorised to persons professionally engaged in plant production, other than those plants, plant products and other objects which are prepared and ready for sale to the final consumer, and for which it is ensured by the responsible official bodies of the Member States, that the production thereof is clearly separate from that of other products | ||
| 2.1 | Plants intended for planting other than seeds of the genera Abies Mill., […] Larix Mill., […], Picea A. Dietr., Pinus L., […] | ||
3.3.2. Legislation addressing plants and plant parts on which Ips amitinus is regulated
3.3.3. Legislation addressing the organisms vectored by Ips amitinus (Directive 2000/29/EC)
According to Kirisits (2004), I. amitinus often carries Ceratocystis polonica, Graphium fimbriisporum, Ophiostoma bicolor, Ophiostoma brunneo‐ciliatum and Ophiostoma penicillatum, but is also found associated with Ceratocystiopsis cf. alba; Ceratocystiopsis minuta; Graphium (Pesotum ?) spp.; Leptographium lundbergii; Leptographium spp.; Ophiostoma cucullatum; Ophiostoma minus; Ophiostoma piceae; Ophiostoma piceaperdum; Ophiostoma cf. piceaperdum and Ophiostoma piliferum.
Repe et al. (2013) often found Grosmannia piceaperda and C. minuta with I. amitinus in Slovenian forests, but they also recorded O. bicolor; O. brunneo‐ciliatum; G. cucullata; O. piceae; G. penicillata; Ceratocystis polonica; Graphium fimbriisporum and O. fuscum.
Some of these ophiostomatoid fungi are pathogenic; none of them are regulated.
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
According to the EPPO Global Database (accessed on 29 June 2017), and the literature relevant to I. amitinus, the pest mostly attacks Picea abies, Picea pungens, Pinus sylvestris; Pinus cembra; Pinus mugo; Pinus heldreichii and Pinus peuce but also settles on other Picea spp.; Pinus spp.; Abies alba; Abies spp.; Larix decidua.
The hosts for which I. amitinus is regulated are comprehensive of the host range: the pest is regulated on four genera: Abies, Larix, Picea and Pinus.
3.4.2. Entry
Is the pest able to enter into the protected zones? If yes, identify and list the pathways
Yes, the pest is already established in 16 MSs and can enter the protected zones by human assisted spread or by natural spread from EU areas where the pest is present.
The main pathways of entry are:
wood of Picea, Pinus, Abies and Larix spp. from countries where the pest occurs;
wood chips of conifers from countries where the pest occurs;
bark of conifers from countries where the pest occurs;
wood packaging material and dunnage from countries where the pest occurs.
Ips species are regularly intercepted on wood, wood packaging material and dunnage. I. amitinus was repeatedly found in imported timber in Norway (Økland and Skarpaas, 2008), Sweden (Lundberg, 1988 and Lindelöw, 2013), USA (Haack, 2001) and New Zealand (Brockerhoff et al., 2006). In the Europhyt database, between 1994 and 2017, there are, in total, 66 records of Ips species (39 of which are at species level), all on coniferous wood or packaging material, but no records for I. amitinus.
There are no records of interception that indicate that plants for planting can be a pathway for I. amitinus. Plants for planting are not considered a pathway for I. amitinus since young plants for trade are not attacked by the pest.
There is a large overlap in the host range and geographical distribution of I. amitinus and I. sexdentatus. For I. sexdentatus, (EFSA PLH Panel, in press) analysed the volume of coniferous wood imported into PZs from countries where the pest is present, based on data from Eurostat. The vast majority (> 99%) of imported coniferous wood originates from EU countries. Based on these data, it can be concluded that there is trade of coniferous wood from countries where I. amitinus is present to PZs (~ 0.45 million tonnes/year).
3.4.3. Establishment
Is the pest able to become established in the EU territory?
Yes, the pest is already established in 16 MSs. The climate of the EU protected zones is similar to that of the other MS where Ips amitinus is established, and the pest's main host plants are present (Figure 2).
3.4.3.1. EU distribution of main host plants
Figure 2.
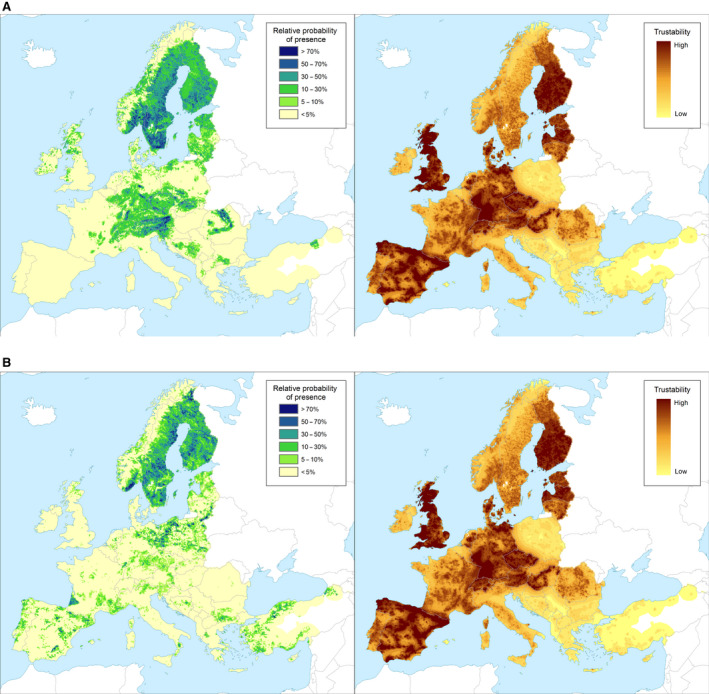
Left panel: Relative probability of presence (RPP) of the genera Picea and Pinus in Europe, mapped at 100 km2 resolution. The underlying data are from European‐wide forest monitoring data sets and from national forestry inventories based on standard observation plots measuring in the order of hundreds m2. RPP represents the probability of finding at least one individual of the taxon in a standard plot placed randomly within the grid cell. For details, see Appendix A (courtesy of JRC, 2017). Right panel: Trustability of RPP. This metric expresses the strength of the underlying information in each grid cell and varies according to the spatial variability in forestry inventories. The colour scale of the trustability map is obtained by plotting the cumulative probabilities (0–1) of the underlying index (for details see Appendix A).
- A. Distribution map of the genus Picea in the European Union territory (based on data from the species: P. abies, P. sitchensis, P. glauca, P. engelmannii, P. pungens, P. omorika, P. orientalis).
- B. Distribution map of the genus Pinus in the European Union territory (based on data from the species: P. sylvestris, P. pinaster, P. halepensis, P. nigra, P. pinea, P. contorta, P. cembra, P. mugo, P. radiata, P. canariensis, P. strobus, P. brutia, P. banksiana, P. ponderosa, P. heldreichii, P. leucodermis, P. wallichiana).
3.4.3.2. Climatic conditions affecting establishment
According to the Köppen–Geiger climate classification (Kottek et al., 2006) and given the current distribution of I. amitinus, most of the EU area (including the PZs) is suitable for establishment (Figure 3).
Figure 3.
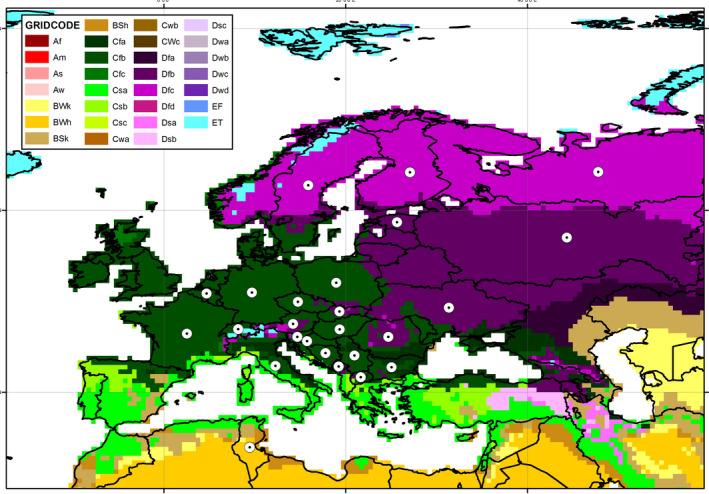
The current distribution of Ips amitinus presented by white dots on the Köppen‐Geiger climate classification map (Kottek et al., 2006) of Eurasia
3.4.4. Spread
Is the pest able to spread within the EU territory following establishment? How?
Yes, adults can disperse naturally or with human assistance.
RNQPs: Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects?
No, plants for planting are not considered to be a pathway.
In flight mill tests, I. amitinus flew on the average much longer (up to ca 4 h) than I. typographus or I. sexdentatus (Forsse, 1989), two species known to fly tens of kilometres. The pest can also spread by human assistance, for example, with the transportation of wood, wood chips, bark and wood packaging material and dunnage of conifers. In Finland, a spread rate of 20 km/year has been observed (Koponen, 1980). In north‐west Russia (Saint Petersburg), Mandelshtam (1999) reported a continuous range expansion.
3.5. Impacts
Would the pests' introduction have an economic or environmental impact on the EU territory?
Yes. Although I. amitinus is most often a secondary pest species on weakened or dead trees, it may kill trees under certain conditions, after triggering events such as storms, forest fires or droughts.
RNQPs: Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? 5
No, plants for planting are not considered to be a pathway.
So far, I. amitinus has rarely been reported as a very noticeable pest, and EPPO removed it from its A2 list in 1996 (EPPO, 1996). However, Jurc and Bojović (2006) report an outbreak over 25 ha in Slovenia, and Økland and Skarpaas (2008 and refs. therein) calculated that it could increase the likelihood of I. typographus outbreaks.
I. amitinus may inoculate its hosts with pathogenic ophiostomatoid fungi which blue stain the wood and may contribute to tree death (Kirisits, 2004; Repe et al., 2013).
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
Yes.
Is it possible to eradicate the pest in a restricted area within 24 months after the presence of the pest was confirmed in the PZ?
Yes.
RNQPs: Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated?
Plants for planting are not a major pathway, probably even not a pathway at all.
In isolated areas (e.g. islands) that cannot be reached by natural spread, measures can be put in place to prevent the introduction with wood and bark. Striping wood of its bark and heat treatment of wood, bark and chips is effective as specified in Annex IVB of 2000/29/EC. When such geographical barriers do not exist, the pest will eventually be able to enter new territories by natural dispersal.
Eradication is possible as the pest mainly attacks fallen or weakened trees in the EU territory. Provided incipient populations are localised very early (i.e. preferably before the new brood has emerged), the attacked material can be removed and destroyed. However, eradication is difficult because all suitable host material (fallen or weakened trees) in the surrounding area within a radius of several kilometres should be localised and removed.
3.6.1. Biological or technical factors limiting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
In spite of quarantine regulations bearing on round wood, wood packaging material and wood products other than paper, Ips spp. are regularly intercepted at ports.
It is difficult to successfully eradicate the pest from forest areas after an introduction. All infested trees and tree parts (including pieces of fallen or broken material) have to be detected and removed within a suitable radius of several kilometres.
3.6.2. Control methods
As with other bark beetle species, visual monitoring allows attacked trees to be located.
Silvicultural methods are the usual control methods. They include sanitation thinning and clear‐felling with rapid removal of the infested material (Stadelmann et al., 2013; Fettig and Hilszczanski, 2015 and Grégoire et al., 2015).
3.7. Uncertainty
Although Økland and Skarpaas (2008 and refs. therein) predict possible interactions between I. amitinus and I. typographus that could increase the overall frequency of bark beetle outbreaks, such interactions have never been reported so far. However, as both species often coexist on the same trees and are not always distinguished from each other, there is some limited uncertainty regarding the occurrence of these interactions in the past.
4. Conclusions
Ips amitinus meets the criteria assessed by EFSA for consideration as a potential PZ quarantine pest for the territory of the PZs: Greece, Ireland and the UK (Table 5).
Table 5.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of the pest is established. It can be identified to the species level using conventional entomological keys | The identity of the pest is established. It can be identified to the species level using conventional entomological keys | When I. amitinus coexists with I. typographus on the same trees, there can be some confusion when examination is not thorough |
| Absence/presence of the pest in the EU territory (Section 3.2 ) | I. amitinus is present and widely distributed in the EU; it has been reported from 16 EU MS. The protected zones, Ireland, Greece and the United Kingdom, are free from the pest | I. amitinus is present and widely distributed in the EU; it has been reported from 16 EU MS. The protected zones, Ireland, Greece and the United Kingdom, are free from the pest | Apparent absence from some MS could be due to difficulty in distinguishing I. amitinus from I. typographus |
| Regulatory status (Section 3.3 ) |
The pest is currently officially regulated by 2000/29/EC on plants of Abies, Larix, Picea and Pinus over 3 m in height, other than fruit and seeds, wood of conifers (Coniferales) with bark, isolated bark of conifers I. amitinus is regulated as a quarantine pest in protected zones (Annex IIB): Ireland, Greece and the United Kingdom |
The pest is currently officially regulated by 2000/29/EC on plants of Abies, Larix, Picea and Pinus over 3 m in height, other than fruit and seeds, wood of conifers (Coniferales) with bark, isolated bark of conifers I. amitinus is regulated as a quarantine pest in protected zones (Annex IIB): Ireland, Greece and the United Kingdom |
Although there are some scattered reports of galleries being seen on Abies sp. and Larix decidua, full development of I. amitinus on these genera has not been described in the available literature |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) |
Entry: the pest is already established in 16 MSs. Since entry by natural spread from EU areas where the pest is present is possible, only isolated areas (e.g. islands) can be long‐term protected zones.Establishment: the climate of the EU protected zones is similar to that of MSs where I. amitinus is established, and the pest's main host plants are present Spread: adults can disperse naturally. They can fly over tens of kilometres. The pest can also spread by human assistance, e.g. with the transportation of wood, wood chips, bark, wood packaging material and dunnage of conifers |
Plants for planting are not a pathway for the spread of I. amitinus | None |
| Potential for consequences in the EU territory (Section 3.5 ) | The pest is very secondary | Young trees are not attacked by I. amitinus; therefore impacts in nurseries are not expected | Possible interactions with Ips typographus have been described and modelled but so far not supported by observations |
| Available measures (Section 3.6 ) |
In isolated areas (e.g. islands) that cannot be reached by natural spread, measures can be put in place to prevent the introduction of the pest. For wood, wood products, wood chips and bark, this can be achieved by debarking wood and heat treatment of wood, bark and chips When such geographical barriers do not exist, there is no possibility to prevent the entry, establishment and spread of I. amitinus by natural dispersal |
Young plants are not attacked by I. amitinus | Inspections of large shipments at entry are difficult to perform with complete accuracy |
| Conclusion on pest categorisation (Section 4 ) | All criteria assessed by EFSA above for consideration as potential protected zone quarantine pest are met | The criteria for considering I. amitinus as a potential regulated non‐quarantine pest are not met since plants for planting are not a pathway | See above |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | Considering the reportedly low impact of the pest, no further assessment is deemed necessary | ||
Abbreviations
- CLC
Corine Land Cover
- EPPO
European and Mediterranean Plant Protection Organization
- EUFGIS
European Information System on Forest Genetic Resources
- EU MS
European Union Member State
- FAO
Food and Agriculture Organization
- GD2
Georeferenced Data on Genetic Diversity
- IPPC
International Plant Protection Convention
- JRC
Joint Research Centre of the European Commission
- PLH
EFSA Panel on Plant Health
- PZ
protected zone
- RNQP
regulated non‐quarantine pest
- RPP
relative probability of presence
- RRO
risk reduction option
- SMFA
spatial multiscale frequency analysis
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Appendix A – Methodological notes on Figure 2
1.
The relative probability of presence (RPP) reported here for Picea and Pinus spp. in Figure 2 and in the European Atlas of Forest Tree Species (de Rigo et al., 2016; San‐Miguel‐Ayanz et al., 2016) is the probability of that genus to occur in a given spatial unit (de Rigo et al., 2017). In forestry, such a probability for a single taxon is called ‘relative’. The maps of RPP are produced by means of the constrained spatial multiscale frequency analysis (C‐SMFA) (de Rigo et al., 2014, 2017) of species presence data reported in geolocated plots by different forest inventories.
A.1. Geolocated plot databases
The RPP models rely on five geodatabases that provide presence/absence data for tree species and genera: four European‐wide forest monitoring data sets and a harmonised collection of records from national forest inventories (de Rigo et al., 2014, 2016, 2017). The databases report observations made inside geolocalised sample plots positioned in a forested area, but do not provide information about the plot size or consistent quantitative information about the recorded species beyond presence/absence.
The harmonisation of these data sets was performed within the research project at the origin of the European Atlas of Forest Tree Species (de Rigo et al., 2016; San‐Miguel‐Ayanz, 2016; San‐Miguel‐Ayanz et al., 2016). Given the heterogeneity of strategies of field sampling design and establishment of sampling plots in the various national forest inventories (Chirici et al. 2011a,b), and also given legal constraints, the information from the original data sources was harmonised to refer to an INSPIRE compliant geospatial grid, with a spatial resolution of 1 km2 pixel size, using the ETRS89 Lambert Azimuthal Equal‐Area as geospatial projection (EPSG: 3035, http://spatialreference.org/ref/epsg/etrs89-etrs-laea/).
A.1.1. European National Forestry Inventories database
This data set was derived from National Forest Inventory data and provides information on the presence/absence of forest tree species in approximately 375,000 sample points with a spatial resolution of 1 km2/pixel, covering 21 European countries (de Rigo et al., 2014, 2016).
A.1.2. Forest Focus/Monitoring data set
This project is a Community scheme for harmonised long‐term monitoring of air pollution effects in European forest ecosystems, normed by EC Regulation No. 2152/20036. Under this scheme, the monitoring is carried out by participating countries on the basis of a systematic network of observation points (Level I) and a network of observation plots for intensive and continuous monitoring (Level II). For managing the data, the JRC implemented a Forest Focus Monitoring Database System, from which the data used in this project were taken (Hiederer et al., 2007; Houston Durrant and Hiederer, 2009). The complete Forest Focus data set covers 30 European Countries with more than 8,600 sample points.
A.1.3. BioSoil data set
This data set was produced by one of a number of demonstration studies performed in response to the ‘Forest Focus’ Regulation (EC) No. 2152/2003 mentioned above. The aim of the BioSoil project was to provide harmonised soil and forest biodiversity data. It comprised two modules: a Soil Module (Hiederer et al., 2011) and a Biodiversity Module (Houston Durrant et al., 2011). The data set used in the C‐SMFA RPP model came from the Biodiversity module, in which plant species from both the tree layer and the ground vegetation layer were recorded for more than 3,300 sample points in 19 European Countries.
A.1.4. European Information System on Forest Genetic Resources (EUFGIS)
EUFGIS (http://portal.eufgis.org) is a smaller geodatabase providing information on tree species composition in over 3,200 forest plots in 34 European countries. The plots are part of a network of forest stands managed for the genetic conservation of one or more target tree species. Hence, the plots represent the natural environment to which the target tree species are adapted.
A.1.5. Georeferenced Data on Genetic Diversity (GD2)
GD2 (http://gd2.pierroton.inra.fr) provides information about 63 species of interest for genetic conservation. The database covers 6,254 forest plots located in stands of natural populations that are traditionally analysed in genetic surveys. While this database covers fewer species than the others, it covers 66 countries in Europe, North Africa and the Middle East, making it the data set with the largest geographic extent.
A.2. Modelling methodology
For modelling, the data were harmonised in order to have the same spatial resolution (1 km2) and filtered to a study area comprising 36 countries in the European continent. The density of field observations varies greatly throughout the study area and large areas are poorly covered by the plot databases. A low density of field plots is particularly problematic in heterogeneous landscapes, such as mountainous regions and areas with many different land use and cover types, where a plot in one location is not representative of many nearby locations (de Rigo et al., 2014). To account for the spatial variation in plot density, the model used here (C‐SMFA) considers multiple spatial scales when estimating RPP. Furthermore, statistical resampling is systematically applied to mitigate the cumulated data‐driven uncertainty.
The presence or absence of a given forest tree species then refers to an idealised standard field sample of negligible size compared with the 1 km2 pixel size of the harmonised grid. The modelling methodology considered these presence/absence measures as if they were random samples of a binary quantity (the punctual presence/absence, not the pixel one). This binary quantity is a random variable having its own probability distribution which is a function of the unknown average probability of finding the given tree species within a plot of negligible area belonging to the considered 1 km2 pixel (de Rigo et al., 2014). This unknown statistic is denoted hereinafter with the name of ‘probability of presence’.
C‐SMFA preforms spatial frequency analysis of the geolocated plot data to create preliminary RPP maps (de Rigo et al., 2014). For each 1 km2 grid cell, the model estimates kernel densities over a range of kernel sizes to estimate the probability that a given species is present in that cell. The entire array of multiscale spatial kernels is aggregated with adaptive weights based on the local pattern of data density. Thus, in areas where plot data are scarce or inconsistent, the method tends to put weight on larger kernels. Wherever denser local data are available, they are privileged ensuring a more detailed local RPP estimation. Therefore, a smooth multi‐scale aggregation of the entire arrays of kernels and data sets is applied instead of selecting a local ‘best performing’ one and discarding the remaining information. This array‐based processing, and the entire data harmonisation procedure, are made possible thanks to the semantic modularisation which defines the Semantic Array Programming modelling paradigm (de Rigo, 2012).
The probability to find a single species (e.g. a particular coniferous tree species) in a 1 km2 grid cell cannot be higher than the probability of presence of all the coniferous species combined. The same logical constraints applied to the case of single broadleaved species with respect to the probability of presence of all the broadleaved species combined. Thus, to improve the accuracy of the maps, the preliminary RPP values were constrained so as not to exceed the local forest‐type cover fraction with an iterative refinement (de Rigo et al., 2014). The forest‐type cover fraction was estimated from the classes of the Corine Land Cover (CLC) maps which contain a component of forest trees (Bossard et al., 2000; Büttner et al. 2012).
The resulting probability of presence is relative to the specific tree taxon, irrespective of the potential co‐occurrence of other tree taxa with the measured plots, and should not be confused with the absolute abundance or proportion of each taxon in the plots. RPP represents the probability of finding at least one individual of the taxon in a plot placed randomly within the grid cell, assuming that the plot has negligible area compared with the cell. As a consequence, the sum of the RPP associated with different taxa in the same area is not constrained to be 100%. For example, in a forest with two co‐dominant tree species which are homogeneously mixed, the RPP of both may be 100% (see e.g. the Glossary in San‐Miguel‐Ayanz et al. (2016), http://forest.jrc.ec.europa.eu/media/atlas/Glossary.pdf).
The robustness of RPP maps depends strongly on sample plot density, as areas with few field observations are mapped with greater uncertainty. This uncertainty is shown qualitatively in maps of ‘RPP trustability’. RPP trustability is computed on the basis of the aggregated equivalent number of sample plots in each grid cell (equivalent local density of plot data). The trustability map scale is relative, ranging from 0 to 1, as it is based on the quantiles of the local plot density map obtained using all field observations for the species. Thus, trustability maps may vary among species based on the number of databases that report a particular species (de Rigo et al., 2014, 2016).
The RPP and relative trustability range from 0 to 1 and are mapped at a 1 km spatial resolution. To improve visualisation, these maps can be aggregated to coarser scales (i.e. 10 × 10 pixels or 25 × 25 pixels, respectively, summarising the information for aggregated spatial cells of 100 km2 and 625 km2) by averaging the values in larger grid cells.
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Kertész V, Aukhojee M and Grégoire J‐C, 2017. Scientific Opoinion on pest categorisation of Ips amitinus . EFSA Journal 2017;15(10):5038, 26 pp. 10.2903/j.efsa.2017.5038
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00196
Panel members: Claude Bragard, David Caffier, Thierry Candresse, Elisavet Chatzivassiliou, Katharina Dehnen‐Schmutz, Gianni Gilioli, Jean‐Claude Gregoire, Josep Anton Jaques Miret, Michael Jeger, Alan MacLeod, Maria Navajas Navarro, Björn Niere, Stephen Parnell, Roel Potting, Trond Rafoss, Vittorio Rossi, Gregor Urek, Ariena Van Bruggen, Wopke Van der Werf, Jonathan West and Stephan Winter.
Acknowledgements: The Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 28 September 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © EPPO
Figure 2: © European Union, 2017. Reuse is authorised, provided the source is acknowledged
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
Although the leading taxonomists in the 2000s (Wood, 1982; Bright and Skidmore, 2002) still considered the Scolytidae to be a family distinct from the Curculionidae according to morphological criteria, modern phylogenetics supports the position of scolytine beetles (Scolytinae) within the family Curculionidae (Knížek and Beaver, 2004; Hulcr et al., 2015). This is reflected by the growing number of citations in Scopus (2017) referring to Scolytinae (18 in 1990 vs 177 in 2016), as opposed to citations referring to Scolytidae (50 in 1990 vs 15 in 2016). The Scolytinae includes two subcategories, the ‘bark beetles’ which live in the phloem and the ‘ambrosia beetles’ which live in the sapwood.
See Section 2.1 on what falls outside EFSA's remit.
Council of the European Union, 2003. Regulation (EC) No 2152/2003 of the European Parliament and of the Council of 17 November 2003 concerning monitoring of forests and environmental interactions in the Community (Forest Focus). Official Journal of the European Union 46 (L 324), 1–8.
References
- Bakke A, 1968. Ecological studies on bark beetles (Coleoptera: Scolytidae) associated with Scots pine (Pinus sylvestris L.) in Norway with particular reference to the influence of temperature. Meddelelser fra det Norske Skogsforsoksvesen, 21, 441–602. [Google Scholar]
- Balachowsky A, 1949. Faune de France. 50. Coleoptères Scolytides. Lechevalier, Paris. 320 pp. [Google Scholar]
- Bossard M, Feranec J and Otahel J, 2000. CORINE land cover technical guide ‐ Addendum 2000. Tech. Rep. 40, European Environment Agency. Availale online: https://www.eea.europa.eu/ds_resolveuid/032TFUPGVR, INRMM‐MiD:13106045.
- Bright DE and Skidmore RE, 2002. A catalogue of Scolytidae and Platypodidae (Coleoptera), Supplement 2 (1995–1999). NRC Research Press, Ottawa, Canada. 523 pp. [Google Scholar]
- Brockerhoff EG, Bain J, Kimberley M and Knížek M, 2006. Interception frequency of exotic bark and ambrosia beetles (Coleoptera: Scolytinae) and relationship with establishment in New Zealand and worldwide. Canadian Journal of Forest Research, 36, 289–298. [Google Scholar]
- Büttner G, Kosztra B, Maucha G and Pataki R, 2012. Implementation and achievements of CLC2006. Tech. rep., European Environment Agency. Available online: http://www.eea.europa.eu/ds_resolveuid/GQ4JECM8TB, INRMM‐MiD:14284151.
- Chararas C, 1962. Etude biologique des scolytides des conifères. Encyclopedie Entomologique, Ser. A, Nr. 38, Paul Lechevalier, Paris.
- Chirici G, Bertini R, Travaglini D, Puletti N and Chiavetta U, 2011a. The common NFI database. In: Chirici G, Winter S and McRoberts RE (eds.). National forest inventories: contributions to forest biodiversity assessments. Springer, Berlin. pp. 99–119. [Google Scholar]
- Chirici G, McRoberts RE, Winter S, Barbati A, Brändli U‐B, Abegg M, Beranova J, Rondeux J, Bertini R, Alberdi Asensio I and Condés S, 2011b. Harmonization tests. In: Chirici G, Winter S and McRoberts RE (eds.). National forest inventories: contributions to forest biodiversity assessments. Springer, Berlin. pp. 121–190. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2010. PLH Guidance on a harmonised framework for pest risk assessment and the identification and evaluation of pest risk management options by EFSA. EFSA Journal 2010;8(2):1495, 66 pp. 10.2903/j.efsa.2010.1495 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), in press. Pest categorisation of Ips sexdentatus. EFSA Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 1996. Data sheets on quarantine pests. Ips amitinus. Available online: https://www.eppo.int/QUARANTINE/data.../IPSXAM_ds.pdf
- EPPO (European and Mediterranean Plant Protection Organization), 2017. EPPO Global Database. Available online: https://gd.eppo.in [Accessed: 28 August 2017].
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- Fettig CJ and Hilszczanski J, 2015. Management strategies for bark beetles in conifer forests. In: Vega FE and Hofstetter RW (eds.). Bark Beetles. Biology and Ecology of Native and Invasive Species. Elsevier. Pp. 555–584. [Google Scholar]
- Forsse E, 1989. Migration in bark beetles with special reference to the spruce bark beetle Ips typographus. PhD dissertation, Sveriges Lantbruksuniversitet, Uppsala.
- Forsse E and Solbreck C, 1985. Migration in the bark beetle Ips typographus L.: duration, timing and height of flight. Zeitschrift Für Angewandte Entomologie, 100, 47–57. [Google Scholar]
- Francke W, Sauerwein P, Vité JP and Klimetzek D, 1980. The pheromone bouquet of Ips amitinus . Naturwissenschaften, 67, 147. [Google Scholar]
- Grégoire JC, Raffa KF and Lindgren BS, 2015. Economics and politics of bark beetles. In: Vega FE and Hofstetter RW(eds.). Bark Beetles. Biology and Ecology of Native and Invasive Species. Elsevier. pp. 585–613 [Google Scholar]
- Grüne S, 1979. Brief Illustrated Key to European Bark Beetles. M. & H. Schaper, Hannover. 182 pp. [Google Scholar]
- Haack RA, 2001. Intercepted Scolytidae (Coleoptera) at US ports of entry: 1985–2000. Integrated Pest Management Reviews, 6, 253–282. [Google Scholar]
- Hiederer R, Houston Durrant T, Granke O, Lambotte M, Lorenz M, Mignon B and Mues V, 2007. Forest focus monitoring database system ‐ validation methodology. Vol. EUR 23020 EN of EUR – Scientific and Technical Research. Office for Official Publications of the European Communities. 10.2788/51364 [DOI]
- Hiederer R, Houston Durrant T and Micheli E, 2011. Evaluation of BioSoil demonstration project ‐ soil data analysis. Vol. 24729 of EUR ‐ Scientific and Technical Research. Publications Office of the European Union. 10.2788/56105 [DOI]
- Holuša J, Lukášová K, Grodzki W, Kula E and Matoušek P, 2012. Is Ips amitinus (Coleoptera: Curculionidae) abundant in wide range of altitudes. Acta Zoologica Bulgarica, 64, 219–228. [Google Scholar]
- Houston Durrant T and Hiederer R, 2009. Applying quality assurance procedures to environmental monitoring data: a case study. Journal of Environmental Monitoring, 11, 774–781. [DOI] [PubMed] [Google Scholar]
- Houston Durrant T, San‐Miguel‐Ayanz J, Schulte E and Suarez Meyer A, 2011. Evaluation of BioSoil demonstration project: forest biodiversity ‐ analysis of biodiversity module. Vol. 24777 of EUR – Scientific and Technical Research. Publications Office of the European Union. 10.2788/84823 [DOI]
- Hulcr J, Atkinson T, Cognato AI, Jordal BH and McKenna DD, 2015. Morphology, taxonomy and phylogenetics of bark beetles. In: Vega FE and Hofstetter RW (eds.). Bark Beetles. Elsevier, Biology and Ecology of Native and Invasive Species. pp. 41–84. [Google Scholar]
- Jactel H, 1991. Dispersal and flight behaviour of lps sexdentatus (Coleoptera: Scolytidae) in pine forest. Annales des Sciences forestières, 48, 417–428. [Google Scholar]
- Jactel H and Gaillard J, 1991. A preliminary study of the dispersal potential of Ips sexdentatus (Boern) (Col., Scolytidae) with an automatically recording flight mill. Journal of Apllied Entomology, 112, 138–145. [Google Scholar]
- Jurc M and Bojović S, 2006. Bark beetle outbreaks during the last decade with special regard to the eight‐toothed bark beetle (Ips amitinus Eichh.) outbreak in the Alpine region of Slovenia. In: Csóka Gy, Hirka A and Koltay A (eds.). Biotic damage in forests. Proceedings of the IUFRO (WP 7.03.10) Symposium held in Mátrafüred, Hungary, September 12‐16, 2004. Pp. 12–16. [Google Scholar]
- Kirisits T, 2004. Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi. In: Lieutier F, Day KR, Battisti A, Grégoire JC and Evans HF (eds.). Bark and wood boring insects in living trees in Europe, a synthesis. Springer, Netherlands. pp. 181–236. [Google Scholar]
- Knížek M and Beaver R, 2004. Taxonomy and systematics of bark and ambrosia beetles. In: Lieutier F, Day K, Battisti A, Grégoire JC and Evans H (eds.). Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis. Kluwer, Dordrecht. pp. 41–54. 10.1007/978-1-4020-2241-8_11 [DOI] [Google Scholar]
- Koponen M, 1980. Distribution of Ips amitinus Coleoptera Scolytidae in Finland in 1974–1979. Notulae Entomologicae, 60, 223–225. [Google Scholar]
- Kottek M, Grieser J, Beck C, Rudolf B and Rubel F, 2006. World map of the Köppen‐Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. [Google Scholar]
- Lindelöw Å, 2013. Väntad barkborre funnen i Sverige ‐ fynd av Ips amitinus (Coleoptera, Scolytinae). [Ips amitinus (Coleoptera, Scolytinae) expected and found in Sweden.] –. Entomologisk Tidskrift, 134, 203–206. [Google Scholar]
- Lundberg S, 1988. Some interesting beetle finds in pine wood imported to Norrbotten Sweden. Entomologisk Tidskrift, 109, 49–50. [Google Scholar]
- Mandelshtam MJ, 1999. Current status of Ips amitinus Eichh. (Coleoptera, Scolytidae) in North‐West Russia. Entomologica Fennica, 10, 29–34. [Google Scholar]
- Økland B and Skarpaas O, 2008. Draft pest risk assessment report on the small spruce bark beetle, Ips amitinus. Commissioned report from Norwegian Forest and Landscape Institute. 25 pp. [Google Scholar]
- Repe A, Kirisits T, Piškur B, De Groot M, Kump B and Jurc M, 2013. Ophiostomatoid fungi associated with three spruce‐infesting bark beetles in Slovenia. Annals of Forest Science, 70, 717–727. [Google Scholar]
- de Rigo D, 2012. Semantic Array Programming for environmental modelling: application of the Mastrave library. In: Seppelt R, Voinov AA, Lange S and Bankamp D (eds.). International Environmental Modelling and Software Society (iEMSs) 2012 International Congress on Environmental Modelling and Software ‐ Managing Resources of a Limited Planet: pathways and Visions under Uncertainty, Sixth Biennial Meeting. pp. 1167–1176. [Google Scholar]
- de Rigo D, Caudullo G, Busetto L and San‐Miguel‐Ayanz J, 2014. Supporting EFSA assessment of the EU environmental suitability for exotic forestry pests: final report. EFSA Supporting Publications 11 (3), EN‐434+. 10.2903/sp.efsa.2014.en-434, INRMM‐MiD:13114000 [DOI]
- de Rigo D, Caudullo G, Houston Durrant T and San‐Miguel‐Ayanz J, 2016. The European Atlas of Forest Tree Species: modelling, data and information on forest tree species. In: San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T and Mauri A (eds.). European Atlas of Forest Tree Species. Publ. Off. EU, Luxembourg, pp. e01aa69+. Available online: https://w3id.org/mtv/FISE-Comm/v01/e01aa69 [Google Scholar]
- de Rigo D, Caudullo G, San‐Miguel‐Ayanz J and Barredo JI, 2017. Robust modelling of the impacts of climate change on the habitat suitability of forest tree species. Publication Office of the European Union, 58 pp. ISBN:978‐92‐79‐66704‐6, 10.2760/296501, INRMM‐MiD:14314400 [DOI]
- San‐Miguel‐Ayanz J, 2016. The European Union Forest Strategy and the Forest Information System for Europe. In: San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T and Mauri A (eds.). European Atlas of Forest Tree Species. Publ. Off. EU, Luxembourg, pp. e012228+. [Google Scholar]
- San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A (eds.), 2016. European Atlas of Forest Tree Species. Publication Office of the European Union, Luxembourg. ISBN: 978‐92‐79‐36740‐3, 10.2788/4251, Avilable online: https://w3id.org/mtv/FISE-Comm/v01/ [DOI] [Google Scholar]
- Schedl KE, 1932. Scolytidae, platypodidae. In Winkler A (ed.) Catalogus Regionis Paelearctica, Wien. pp. 1632–1647. [Google Scholar]
- Schedl KE, 1981. 91. Familie: Scolytidae (Borken‐ und Ambrosiakäfer). In: Freude H, Harde KW and Lohse GA (eds.), Die Käfer Mitteleuropas. Goecke & Evers, Krefeld. pp. 34–99. [Google Scholar]
- Scopus , 2017. Available online: https://www.scopus.com/home.uri. [Accessed: 28 March 2017]
- Stadelmann G, Bugmann H, Meier F, Wermelinger B and Bigler C, 2013. Effects of salvage logging and sanitation felling on bark beetle (Ips typographus L.) infestations. Forest Ecology and Management, 305, 273–281. 10.1016/j.foreco.2013.06.003 [DOI] [Google Scholar]
- Stauffer C, 1997. A molecular method for differentiating sibling species within the genus Ips . In: Grégoire JC, Liebhold AM, Stephen FM, Day KR and Salom SM (eds.). Proceedings: Integrating cultural tactics into the management of bark beetle and reforestation pests. USDA Forest Service General Technical Report NE‐236. pp. 87–91. [Google Scholar]
- Stauffer C and Zuber M, 1998. Ips amitinus var. montana (Coleoptera, Scolytidae) is synonymous to Ips amitinus : a morphological, behavioural and genetic re‐examination. Biochemical Systematics and Ecology, 26, 171–183. [Google Scholar]
- Wood SL, 1982. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Brigham Young University, Provo, Utah. 1359 pp. [Google Scholar]


