Abstract
Bovine genital campylobacteriosis has been assessed according to the criteria of the Animal Health Law (AHL), in particular criteria of Article 7 on disease profile and impacts, Article 5 on the eligibility of bovine genital campylobacteriosis to be listed, Article 9 for the categorisation of bovine genital campylobacteriosis according to disease prevention and control rules as in Annex IV and Article 8 on the list of animal species related to bovine genital campylobacteriosis. The assessment has been performed following a methodology composed of information collection and compilation, expert judgement on each criterion at individual and, if no consensus was reached before, also at collective level. The output is composed of the categorical answer, and for the questions where no consensus was reached, the different supporting views are reported. Details on the methodology used for this assessment are explained in a separate opinion. According to the assessment performed, bovine genital campylobacteriosis can be considered eligible to be listed for Union intervention as laid down in Article 5(3) of the AHL. The disease would comply with the criteria as in sections 4 and 5 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in points (d) and (e) of Article 9(1). The assessment here performed on compliance with the criteria as in section 3 of Annex IV referred to in point (c) of Article 9(1) is inconclusive. The animal species to be listed for bovine genital campylobacteriosis according to Article 8(3) criteria is mainly cattle as susceptible and reservoir.
Keywords: Bovine genital campylobacteriosis, BGC, bovine venereal campylobacteriosis, BVC, Campylobacter fetus subsp. venerealis , Animal Health Law, listing, categorisation, impact
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The background and Terms of Reference (ToR) as provided by the European Commission for the present document are reported in Section 1.2 of the scientific opinion on the ad hoc methodology followed for the assessment of the disease to be listed and categorised according to the criteria of Article 5, Annex IV according to Article 9 and 8 within the Animal Health Law (AHL) framework (EFSA AHAW Panel, 2017).
1.2. Interpretation of the Terms of Reference
The interpretation of the ToR is as in Section 1.2 of the scientific opinion on the ad hoc methodology followed for the assessment of the disease to be listed and categorised according to the criteria of Article 5, Annex IV according to Article 9 and 8 within the AHL framework (EFSA AHAW Panel, 2017).
The present document reports the results of assessment on bovine genital campylobacteriosis (BGC) according to the criteria of the AHL articles as follows:
Article 7: bovine genital campylobacteriosis profile and impacts
Article 5: eligibility of bovine genital campylobacteriosis to be listed
Article 9: categorisation of bovine genital campylobacteriosis according to disease prevention and control rules as in Annex IV
Article 8: list of animal species related to bovine genital campylobacteriosis.
2. Data and methodologies
The methodology applied in this opinion is described in detail in a dedicated document about the ad hoc method developed for assessing any animal disease for the listing and categorisation of diseases within the AHL framework (EFSA AHAW Panel, 2017).
3. Assessment
3.1. Assessment according to Article 7 criteria
This section presents the assessment of BGC according to the Article 7 criteria of the AHL and related parameters (see Table 2 of the opinion on methodology (EFSA AHAW Panel, 2017)), based on the information contained in the fact‐sheet as drafted by the selected disease scientist (see Section 2.1 of the scientific opinion on the ad hoc methodology) and amended by the AHAW Panel.
Table 2.
Outcome of the expert judgement on the Article 5 criteria for bovine genital campylobacteriosis
| Criteria to be met by the disease: According to AHL, a disease shall be included in the list referred to in point (b) of paragraph 1 of Article 5 if it has been assessed in accordance with Article 7 and meets all of the following criteria | Final outcome | |
| A(i) | The disease is transmissible | Y |
| A(ii) | Animal species are either susceptible to the disease or vectors and reservoirs thereof exist in the Union | Y |
| A(iii) | The disease causes negative effects on animal health or poses a risk to public health due to its zoonotic character | Y |
| A(iv) | Diagnostic tools are available for the disease | Y |
| A(v) | Risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union | Y |
| At least one criterion to be met by the disease: In addition to the criteria set out above at points A(i)–A(v), the disease needs to fulfil at least one of the following criteria | ||
| B(i) | The disease causes or could cause significant negative effects in the Union on animal health, or poses or could pose a significant risk to public health due to its zoonotic character | Y |
| B(ii) | The disease agent has developed resistance to treatments and poses a significant danger to public and/or animal health in the Union | N |
| B(iii) | The disease causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union | Y |
| B(iv) | The disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism | N |
| B(v) | The disease has or could have a significant negative impact on the environment, including biodiversity, of the Union | N |
Colour code: green = consensus (Yes/No).
3.1.1. Article 7(a) Disease Profile
Campylobacter fetus subsp. venerealis (Cfv) is described as the causative agent of BGC. BGC is a venereal disease, also known as bovine venereal campylobacteriosis (BVC). BGC is sexually transmitted and characterised by infertility, early embryonic death and abortions in bovines (Thompson and Blaser, 2000).
BGC is listed by the World Organization for Animal Health (OIE) since it is deemed to have socioeconomic and public health implications. Several countries have been successful in eradicating BGC, whereas in many countries BGC is still endemic. The incidence of BGC is highest in low‐ and middle‐income countries (LMIC) where natural breeding of cattle is widely practiced, compared to high income countries where cattle are bred through artificial insemination (AI) (Mshelia et al., 2010).
Compared to several other agents causing disease in animals, detailed data on pathogenesis, epidemiology and transmission are lacking due to the fact that there are no reliable serological assays and detection of the causative agent is difficult. It requires specific laboratory equipment to cultivate the agent under microaerobic conditions, very well‐trained technical staff to recognise the bacterial growth among more rapid growing contaminating microflora (expertise) and once a suspected isolate has been cultivated, easy‐to‐perform reliable identification methods are lacking. There are hardly monitoring programs implemented showing numerator and denominator and data are often available from necropsy room findings in aborted fetuses (mostly lacking a denominator to estimate the prevalence). These findings are even more complicated by the fact that in several studies only identification at species level is reported (with an undefined fraction Cfv, the causative agent of BGC) and in some studies the isolates are identified at subspecies level. Finally, in particular in older literature the reliability of the subspecies identification is questionable due to the lack of molecular techniques. The worldwide database on the presence of a disease (OIE) shows positive findings but also their prevalence data are lacking. This results into scarce data on basic aspects of BGC.
3.1.1.1. Article 7(a)(i) Animal species concerned by the disease
Susceptible animal species
Parameter 1 – Naturally susceptible wildlife species (or family/orders)
The naturally susceptible wildlife species of Cfv causing BGC is cattle (Bos taurus) (OIE, 2012).
Parameter 2 – Naturally susceptible domestic species (or family/orders)
The naturally susceptible wildlife species of Cfv causing BGC is cattle (B. taurus) (OIE, 2012).
Parameter 3 ‐ Experimentally susceptible wildlife species (or family/orders)
No experimentally susceptible wildlife species for Cfv causing BGC have been described. It is to be expected that wildlife cattle (B. taurus) is the only wildlife species that is susceptible for BGC.
Parameter 4 – Experimentally susceptible domestic species (or family/orders)
Experimentally susceptible domestic species for Cfv causing BGC are cattle (B. taurus) (Corbeil et al., 1975; Cipolla et al., 1994) and guinea pigs (Cavia porcellus) (Plummer, 2017).
Reservoir animal species
Parameter 5 – Wild reservoir species (or family/orders)
The wild reservoir species for Cfv causing BGC is cattle (B. taurus).
Parameter 6 – Domestic reservoir species (or family/orders)
The domestic reservoir species for Cfv causing BGC is cattle (B. taurus) (Blaser et al., 2008).
3.1.1.2. Article 7(a)(ii) The morbidity and mortality rates of the disease in animal populations
Morbidity
Parameter 1 – Prevalence/incidence
Although BGC is wide‐spread in the world, the lack of monitoring programmes for this disease in many countries makes, it difficult to estimate the prevalence rates of BGC world‐wide. As shown in Table 1, the estimates are based on small studies with highly questionable representability. The prevalence of herds infected with Cfv causing BGC is relatively high in LMIC compared to low prevalence or even eradication of BGC in developed countries (data available of the OIE and published data (Mshelia et al., 2007, 2010)).
Table 1.
C. fetus prevalence world‐wide
| Country | Samples | Result | Reference |
|---|---|---|---|
| Argentina | Aborted bovine fetuses | 26 of 354 tested fetuses (7%) were C. fetus positive | Campero et al. (2003) |
| Australia | Aborted bovine fetuses | 11% of 265 tested fetuses were C. fetus positive | Jerrett et al. (1984) |
| Brazil | Preputial washings of bulls | 170 of 327 tested bulls (52.3%) and 17 of 19 tested farms (89.5%) were C. fetus positive | Pellegrin et al. (2002) |
| Brazil (Goiás) | Vaginal mucus samples of cows | 22.4% of 1,685 cows were C. fetus positive | Andrade et al. (1986) |
| USA (California) | Blood samples of cows | 189 of 400 (47%) tested cows were C. fetus positive | Akhtar et al. (1990) |
| USA (California) | Blood samples of dairy cows | 22.2% of 790 tested cows were C. fetus positive | Akhtar et al. (1990) |
| Canada | Preputial washings of bulls | 18 of 529 (3%) bulls tested were C. fetus positive | Devenish et al. (2005) |
| Colombia | Preputial washings of bulls | 103 farms tested, 15% of the farms had C. fetus positive bulls | Griffiths et al. (1984) |
| Egypt | BGC prevalence of 10% in buffalo cows | Mshelia et al. (2010) | |
| India (Calcutta) | Fecal samples from cattle | No C. fetus found in 120 samples | Chattopadhay et al. (2001) |
| India (West Bengal) | Estimated BGC prevalence of 6% in cattle | Mshelia et al. (2010) | |
| Japan | Fecal samples from cattle | 26.5% of 94 tested samples were Cff positive. ‘A few’ samples were Cfv positive | Giacoboni et al. (1993) |
| Japan | Fecal samples from healthy cattle | 13 of 338 (4%) samples were C. fetus positive | Ishihara et al. (2004) |
| New Zealand | Vaginal mucus samples from cows and preputial washings from bulls |
1.230 mucus samples from 125 beef cow herds were tested, 70% of herds had > 1 C. fetus positive CVM sample All 54 preputial washings from 9 herds were C. fetus negative |
McFadden et al. (2005) |
| Nigeria | Vaginal mucus samples from cows and preputial washings from bulls |
15 of 585 (3%) tested bulls were C. fetus positive 5 of 104 (5%) tested cows were C. fetus positive |
Bawa et al. (1991) |
| Nigeria | Vaginal mucus samples from cows and preputial washings from bulls |
3.7% of vaginal mucus samples of cows were C. fetus positive 11% of preputial washings of bulls were C. fetus positive |
Mshelia et al. (2010) |
| Nigeria | Vaginal mucus samples from cows and preputial washings from bulls |
Total; 270 bovine samples tested, consisting of 170 preputial washings from bulls and 100 vaginal mucus samples of cows. Of these 270 samples, 2.2% were Cfv positive and 1.5% were Cff positive |
Mshelia et al. (2012) |
| North America | Fecal samples from dairy cows cattle | 5% of 720 cows were Campylobacter spp. positive | Harvey et al. (2004) |
| Malawi | Vaginal mucus samples from cows and preputial washings from bulls |
1 bull was tested positive for vibriosis Vaginal mucus samples gave no clear result |
Klastrup and Halliwell (1977) |
| Scotland | Preputial washings of bulls | 0% of 109 tested bulls were C. fetus positive | McGowan and Murray (1999) |
| South Africa (Republic of Transkei) | Preputial washings of bulls | 10 of 14 (71%) tested sites were C. fetus positive | Pefanis et al. (1988) |
| South Africa (Gauteng province) | Preputial washings of bulls | 2.1% of 143 tested bulls were C. fetus positive | Njiro et al. (2011) |
| Tanzania | Preputial washings of bulls | 3 of 58 (5.1%) tested bulls were Cfv positive | Swai et al. (2005) |
| Turkey | Preputial washings of bulls and aborted bovine fetuses | Cfv is isolated from both bulls and aborted fetuses | Mshelia et al. (2010) |
| United Kingdom | Aborted bovine fetuses | 28 of 161 (17%) tested samples were C. fetus positive | Devenish et al. (2005) |
| Zimbabwe | Aborted bovine fetuses |
9.5% of 21 tested fetuses were C. fetus positive Estimated; BGC prevalence is 33% in cows in Zimbabwe |
Mshelia et al. (2010) |
Cff: Campylobacter fetus subsp. fetus; Cfv: Campylobacter fetus subsp. venerealis.
Parameter 2 – Case‐morbidity rate (% clinically diseased animals out of infected ones)
Bulls are asymptomatic carriers of Cfv, so by definition the case‐morbidity rate is 0% in these. The case‐morbidity rate in cows is unknown, since infection in naturally served animals is mainly detected through the BGC disease symptoms, such as abortion as most clear symptom, and there are no data of the total population of infected animals.
Mortality
Parameter 3 – Case‐fatality rate
Infection with Cfv will not cause death of the infected bull and/or cow, but can result in embryo mortality and abortion. The disease can spread rapidly through a herd and abortions and/or infertility due to BGC can reduce the annual weaning rate by 10% (Mshelia et al., 2007).
3.1.1.3. Article 7(a)(iii) The zoonotic character of the disease
Cfv is restricted to the genital tract of cattle and no human cases are reported, except for one isolate from a woman with bacterial vaginosis in Sweden in 1987 (Holst et al., 1987).
3.1.1.4. Article 7(a)(iv) The resistance to treatments, including antimicrobial resistance
Parameter 1 – Resistant strain to any treatment even at laboratory level
All C. fetus strains and most Cfv strains are resistant to naladixic acid and all C. fetus strains are sensitive to cephalothin (On, 1996). In a field study, 1,084 C. fetus strains were isolated from bovines in Alberta and 95% of the isolates showed to be resistant to naladixic acid, 60% of the isolates was resistant for doxycycline, 57% was resistant to tetracycline and 1% was resistant to ciprofloxacin and enrofloxacin (Inglis et al., 2006). The subspecies, however, was not reported and given that the isolates were obtained from faeces, it might be C. fetus subsp. fetus only. There is one study from Germany specifically reporting on susceptibility (Hanel et al., 2011). They report full susceptibility of 50 investigated strains to gentamicin. In 14% of the strains, there was reduced susceptibility to one or more antimicrobials, mostly to lincomycin and spectinomycin.
3.1.1.5. Article 7(a)(v) The persistence of the disease in an animal population or the environment
Animal population
Parameter 1 – Duration of infectious period in animals
BGC infections in cows are usually self‐limiting and most cows usually regain fertility within 5 months following elimination of the infection from the uterus (Timoney et al., 1988). However, in an experimental study, the infection persisted in cows for several months, possibly more than a year (Cipolla et al., 1994). Bulls can be life‐long carriers of the pathogen (Blaser et al., 2008).
Parameter 2 – Presence and duration of latent infection period
For bulls, the latent infection period of Cfv causing BGC is from the moment of infection as they can act as a vector to transmit the agent to the next animal. For cows, this period is unknown.
Parameter 3 – Presence and duration of the pathogen in healthy carriers
It has been estimated that up to 10% of infected animals remain life‐long carriers of Cfv causing BGC (Irons et al., 2004), whereas cows can become permanent vaginal carriers (Dekeyser, 1984) and older bulls can be life‐long carriers in the crypts of the prepuce (García et al., 1983).
Environment
Parameter 4 – Length of survival (dpi) of the agent and/or detection of DNA in selected matrices (soil, water, air) from the environment (scenarios: high and low T)
Soil and water in cattle fields can be contaminated with C. fetus, however data about the length of survival of C. fetus in the environment is lacking. Campylobacter coli and Campylobacter jejuni can survive up to 10 months in cattle manure; however, the survival of these Campylobacter spp. is apparently quite different from C. fetus and this must be extrapolated with care (Wagenaar et al., 2014).
3.1.1.6. Article 7(a)(vi) The routes and speed of transmission of the disease between animals, and, when relevant, between animals and humans
Routes of transmission
Parameter 1 – Types of routes of transmission from animal to animal (horizontal, vertical)
The route of transmission from animal to animal of Cfv is venereal with mainly asymptomatic bulls spreading the infection. Cows become infected through natural service or AI with contaminated semen. Bulls can become infected by serving an infected cow and transmission may occur between bulls during mounting. Vertical transmission has never been reported.
Parameter 2 – Types of routes of transmission between animals and humans (direct, indirect, including food‐borne)
Not applicable – humans are not susceptible to Cfv.
Speed of transmission
Parameter 3 – Incidence between animals and, when relevant, between animals and humans
The transmission of Cfv between animals within a herd depends on the presence of a ‘vector’; an infected bull that spreads the infection between animals, because BGC is a venereally transmitted infection. However, no quantitative estimates are available in bibliography.
Parameter 4 – Transmission rate (beta) (from R0 and infectious period) between animals and, when relevant, between animals and humans
No data available about the transmission rate of BGC between animals.
3.1.1.7. Article 7(a)(vii) The absence or presence and distribution of the disease in the Union, where the disease is not present in the Union, the risk of its introduction into the Union
Presence and distribution
Parameter 1 – Map where the disease is present in the EU
The map where BGC is present in the European Union (EU) is depending on the self‐reporting of the country and this will certainly not show a full picture. The BGC distribution in the EU in 2016 as reported to OIE is presented in Figure 1.
Figure 1.
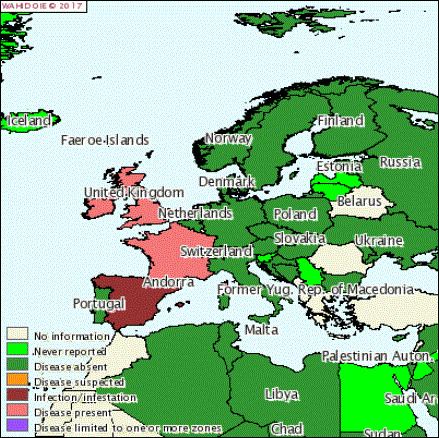
BGC distribution in the EU in 2016 (obtained from OIE (online))
Parameter 2 – Type of epidemiological occurrence (sporadic, epidemic, endemic) at MS level
The use of AI with BGC‐free semen in Europe has greatly reduced the incidence of BGC. In countries from which BGC is reported, the type of epidemiological occurrence is only sporadic cases. For the sporadic cases, there are no studies on risk factors. Furthermore, the notification of BGC cases in Europe to OIE may be affected by underreporting (OIE, online).
Risk of introduction
Parameter 3 – Routes of possible introduction
As some countries report on a more regular basis cases (e.g. the UK), the pathogen is frequently detected in some European countries but the disease is sporadic (few outbreaks). In countries that do not report cases and are supposed to be free of BGC, there is a risk for introduction of BGC. The routes of possible introduction of BGC are import of infected cattle or contaminated bovine products, like semen and embryos.
Parameter 4 – Number of animal moving and/or shipment size
In 2014, the EU has imported around 9.7 million doses of bovine semen (Eurostat; The European Platform of Exporters of Bovine Genetics (ExPla)).
Parameter 5 – Duration of infectious period in animal and/or commodity
The duration of the infectious period in animals is mentioned in Section 3.1.1.5 of this fact‐sheet.
Parameter 6 – List of control measures at border (testing, quarantine, etc.)
The general animal health requirements governing the intra‐EU trade and import of bovine semen are laid down in Council Directive 88/407/EEC1 and for bovine embryos in Council Directive 89/556/EEC.2 These directives harmonise the animal health conditions for the trade within the EU and import to the EU from third countries, as well as the conditions of collection and storage. According to Council Directive 88/407/EEC, bulls whose semen is used for intra‐community trade must be kept in quarantine before being admitted to an AI station. During the quarantine, bulls younger than 6 months are tested once for BGC and bulls older than 6 months are tested three times with 1 week intervals. Bulls that are in production must be tested annually. Bulls that are on hold are excluded with the proviso that when they are longer than 6 months on hold, they should be tested at the earliest 30 days prior to the resumption of the semen production. Bulls in non‐EU countries are mainly tested twice per year.
Bulls are screened for BGC as described in the Terrestrial Animal Health Code by the OIE (2013).
If an animal is tested positive for BGC in an AI station, the AI station is closed and all semen obtained in the period from the latest negative test will be destroyed, according to Council Directive 88/407/EEC. All bulls will be treated with antibiotics and must be tested negative for BGC for 3 times with 2 weeks interval, before the AI centre is allowed to continue their production.
Parameter 7 – Presence and duration of latent infection and/or carrier status
The presence and duration of the latent infection and/or carrier status of BGC are mentioned in Section 3.1.1.5 of this fact‐sheet.
Parameter 8 – Risk of introduction
If animals are infected or products are contaminated, the risk of introduction of BGC to the EU is high, but spread can be prevented by the control measures of AI centres and treatment of animals.
3.1.1.8. Article 7(a)(viii) The existence of diagnostic and disease control tools
Diagnostic tools
Parameter 1 – Existence of diagnostic tools
BGC is diagnosed by diagnostic tools prescribed by the OIE (2012). The immunofluorescence antibody test (IFAT) is suitable to detect if a sample contains suspected C. fetus bacteria, but for definite diagnosis confirmation has to be done by isolating C. fetus from the sample. The isolation of the pathogen causing BGC can be challenging, since C. fetus is slow‐growing and requires specific microaerobic conditions. It is critical that collected samples are sent immediately to the laboratory and cultured. If transport takes long, transport medium should be used. It is recommended to use selective Skirrow medium to isolate C. fetus. Alternatively, the filtration‐technique can be used, where the sample is brought onto a 0.65 μm filter, allowing the Campylobacter bacteria to pass to a non‐selective blood‐based (5–7% blood) medium. Identification of C. fetus can be done with biochemical tests or molecular tests, as described in the OIE manual (OIE, 2012). Serological assays are not suitable for diagnosis due to cross‐reaction between C. fetus subsp. fetus and C. fetus subsp. venerealis.
Control tools
Parameter 2 – Existence of control tools
BGC can be controlled by vaccination (Section 3.1.4.2), antimicrobials (Section 3.1.4.3), the separation of infected from non‐infected animals and control measurements for the prevention of introduction to a herd by infected animals or their products (Section 3.1.1.7 Parameter 6), including quarantine measurements. Artificial insemination is considered to be the most effective for controlling BGC, as is evidenced by farms that have changed from natural breeding to controlled AI programmes (Figueiredo et al., 2002).
3.1.2. Article 7(b) The impact of diseases
3.1.2.1. Article 7(b)(i) The impact of the disease on agricultural and aquaculture production and other parts of the economy
The level of presence of the disease in the Union
Parameter 1 – Number of MSs where the disease is present
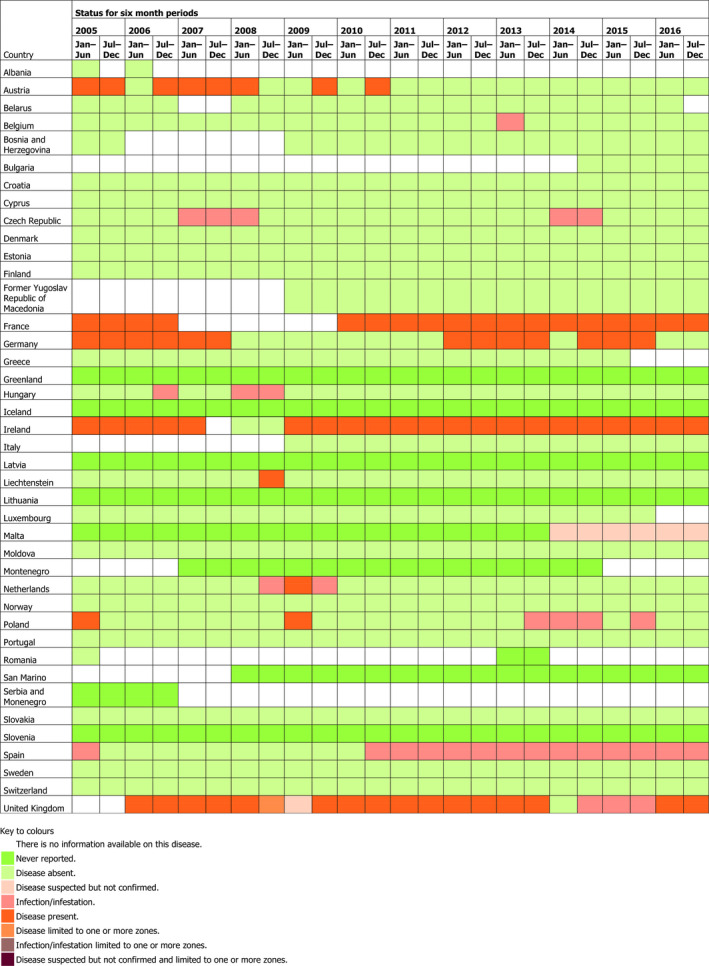
See Section 3.1.1.7, BGC is sporadic in several MSs. In 2016, sporadic cases of BGC were reported in four MSs: the United Kingdom, Ireland, France and Spain. The presence of the disease in EU countries from 2005 to 2016 is presented in Table A.1 in Appendix A.
The loss of production due to the disease
Parameter 2 – Proportion of production losses (%) by epidemic/endemic situation
Control measures prevent the spread of BGC within the EU. Data about production losses in the EU are not available; however, it was estimated that during the first year of infection, the gross profit margins may be reduced by 66% and when the disease becomes established within a herd, gross profit margins are 36% lower than those of uninfected herds (Hum et al., 1994).
In Argentina, weaning rates in BGC‐infected herds decrease by 10%, which accounts for an annual loss of $165 million (Jimenez et al., 2011).
In the modern dairy industry, it appears that more than 90% of dairy calves are born to AI. For example, in Denmark in 2015, around 17% of first parity dairy cows and 7% of older dairy cows were bred using natural service, with the balance using AI and overall for dairy cattle, 90% are inseminated using AI (SEGES, 2016). Similarly in France, 79% of births in dairy herds are by AI (IDELE, 2017). In Ireland, extrapolated data from the Irish Cattle Breeding Federation indicate approximately 45–60% of calves from the dairy herds are sired by AI (ICBF, 2017). According to the German Cattle Breeders' Federation, in Germany around 25% of cattle farms use AI (ADR, 2016), most probably those are almost all farms keeping dairy cows. In the Netherlands, in 2016 approximately 225,000 natural matings were registered as compared to 1.6 million first AI. In Finland, dairy herds are bred using AI even if in some larger herds also natural mating is used.
Therefore, the economic losses due to BCG in the EU are mostly linked to the beef cattle sector where natural mating is used. Most of the meat bovine herd in Europe is located in four EU Member States: France (34.4%), Spain (15.2%), the United Kingdom (12.8%) and Ireland (8.7%). Together, they host more than 70% of the European meat herd (EUROSTAT, online). In France, only 13% of beef cattle are bred from AI (IDELE, 2017). In the UK, the majority of beef herds use natural service. AI in beef cows is still uncommon in the UK due to the problems of heat detection and handling for AI (Penny, 2017). In Ireland, only about 23% of calves in beef herds are bred by AI (Agriland, online) and around 80,000 beef stock bulls were present during 2016 (ICBF, 2017). In Italy, data from the National Data Bank on farm animals indicate 17% ‘linea vacca‐vitello’ beef cows representing 900,000 animals are bred by natural service (BDN, online). In Spain, around 95% of beef cattle use natural services (UGAVAN, 2017).
3.1.2.2. Article 7(b)(ii) The impact of the disease on human health
Not applicable – humans are not susceptible to infection with BGC.
3.1.2.3. Article 7(b)(iii) The impact of the disease on animal welfare
Parameter 1 – Severity of clinical signs at case level and related level and duration of impairment
Infection of BGC in bulls is asymptomatic. Infection in cows can result in moderate endometritis and salpingitis and cows can become infertile for several months, but usually not life‐long. Since infection is often not detected for a long time or only when fertility rates drop, clinical symptoms seem to be very weak, if any, suggesting a rather minor impact on animal welfare.
3.1.2.4. Article 7(b)(iv) The impact of the disease on biodiversity and the environment
Biodiversity
Parameter 1 – Endangered wild species affected: listed species as in CITES and/or IUCN list
Only cattle (B. taurus) are reported to be infected with BGC; however, it cannot be excluded that rare bovine species on the CITES and/or IUCN list can also be infected with BGC; studies are, however, not available.
Parameter 2 – Mortality in wild species
BGC can cause embryonic death in wild bovines.
Environment
Parameter 3 – Capacity of the pathogen to persist in the environment and cause mortality in wildlife
Soil and water can be contaminated with C. fetus, however data about the length of survival of C. fetus in the environment is lacking.
3.1.3. Article 7(c) Its potential to generate a crisis situation and its potential use in bioterrorism
Parameter 1 – Listed in OIE/CFSPH classification of pathogens
BGC is an OIE‐listed disease but not listed by the CFSPH.
Parameter 2 – Listed in the Encyclopaedia of Bioterrorism Defence of Australia Group
BGC is not listed in the Encyclopaedia of Bioterrorism Defence of Australia Group.
Parameter 3 – Included in any other list of potential bio‐ agro‐terrorism agents
BGC is not included in any other list of potential bio‐ agro‐terrorism agents.
3.1.4. Article 7(d) The feasibility, availability and effectiveness of the following disease prevention and control measures
3.1.4.1. Article 7(d)(i) Diagnostic tools and capacities
Availability
Parameter 1 – Officially/internationally recognised diagnostic tool, OIE certified
Diagnostic tests for BGC are prescribed by the OIE (2012) and include immunofluorescence and an antigen‐enzyme‐linked immunosorbent assay (ELISA) assay to detect C. fetus as well as isolation and identification methods of the agent.
Effectiveness
Parameter 2 – Se and Sp of diagnostic test
IFAT to detect C. fetus subsp. venerealis has a reported sensitivity and specificity of 92.6% and 88.9% and the detection limit ranges between 102 and 104 CFU/mL (Figueiredo et al., 2002). The OIE recommended tests to diagnose BGC are the detection of the antigen (bacterium) by culture or the combination of an antigen catching ELISA to detect the presence of C. fetus species cultured in transport medium followed by isolation. This ELISA has a specificity of up to 98.5% (Brooks et al., 2004; Devenish et al., 2005). The sensitivity of the culturing method is not determined but the number of C. fetus in preputial samples of infected bulls range from < 102 to > 2 × 105 organisms per millilitre (Clark, 1971), which can be below the detection limit of the recommended methods. Once the bacterium has been isolated, subspecies identification is very challenging and no molecular diagnostic test is available with 100% sensitivity and 100% specificity to identify C. fetus subsp. venerealis (OIE, 2012; van der Graaf‐van Bloois et al., 2013). Whole genome sequencing can be used to identify C. fetus subspecies with 100% specificity. Alternatively, an ELISA can be used to detect antibodies. This technique, however, is not recommended for individual cases and this assay lacks the specificity in differentiating the two subspecies and can therefore not be used to diagnose BGC.
Feasibility
Parameter 3 – Type of sample matrix to be tested (blood, tissue, etc.)
In bulls, smegma samples may be obtained by scraping, suction or by preputial washing (OIE, 2012). The amount of C. fetus bacteria recovered from scraping samples will be greater than with the suction or preputial washing methods. Furthermore, less contamination from background microflora was observed in scraping samples compared with the samples collected using the other two methods (Tedesco et al., 1977).
Cows or heifers should be sampled when the animals are close to oestrus or are in oestrus. Cervico‐vaginal mucus samples may be obtained by swabbing, suction, or by washing the vaginal cavity (OIE, 2012).
Aborted bovine fetuses, including the placenta, can be tested to detect an infection with C. fetus. The stomach contents (abomasal fluid), lungs and liver have been shown to be the best samples for the recovery of the bacterium (OIE, 2012).
3.1.4.2. Article 7(d)(ii) Vaccination
Availability
Parameter 1 – Types of vaccines available on the market (live, inactivated, DIVA, etc.)
Several commercial vaccines are available for BGC consisting of inactivated C. fetus cells, including Vibrin® (Pfizer), Vibrio Leptoferm 5 (Pfizer) and BioAbortogen H (San Jorge Bago, Argentina). Both male and female cattle can be vaccinated against C. fetus.
Parameter 2 – Availability/production capacity (per year)
Unknown.
Effectiveness
Parameter 3 – Field protection as reduced morbidity (as reduced susceptibility to infection and/or to disease)
According to information of the producer, the use of Vibrin can increase pregnancy rates in vaccinated heifers up to 44% compared to non‐vaccinated control heifers. Both male and female cattle can be vaccinated against C. fetus. Vaccination of bulls might help to control the spread of infection but the effect is limited.
Parameter 4 – Duration of protection
It is recommended to vaccinate against BGC by annual revaccination with a single dose between 30 days and 7 months before breeding (Pfizer, online).
Feasibility
Parameter 5 – Way of administration
The way of vaccine administration is subcutaneously.
3.1.4.3. Article 7(d)(iii) Medical treatments
Availability
Parameter 1 – Types of drugs available on the market
Types of drugs against BGC available on the market are antibiotics, for example, streptomycin or oxytetracycline.
Parameter 2 – Availability/production capacity (per year)
The availability and production capacity of drugs against BGC are unknown.
Effectiveness
Parameter 3 – Therapeutic effects on the field (effectiveness)
In bulls, antibiotic treatment can be successful if the bulls are less than 3 years old, while antibiotic treatment of older bulls is often not sufficient to clear the infection, and the older bulls remain life‐long carriers of the bacterium (Blaser et al., 2008).
The effectiveness of antibiotic treatment in cows and heifers is unknown, since female cattle are mainly not treated, because treatment results are poor and most females develop protective immunity enabling them to resist re‐infection (Taylor, 2002; Mshelia et al., 2007).
Feasibility
Parameter 4 – Way of administration
The way of administration of antibiotics against BGC is local in bulls.
3.1.4.4. Article 7(d)(iv) Biosecurity measures
Availability
Parameter 1 – Available biosecurity measures
Proper husbandry practices (e.g. careful selection of replacement cows and bulls) reduce the risk of introducing C. fetus into a herd. Only animals that are tested negative for C. fetus should be allowed to enter the herd. The risk of disease transmission can be substantially reduced or eliminated by applying sanitary protocols recommended by the International Embryo Transfer Society (IETS) and the OIE. The basic principle to ensure such a high level of biosecurity for semen relies on the concept of pathogen‐free semen collection centres. In the case of embryos, practical guidelines have been published in the manual of IETS in order to provide risk management procedures ensuring the safety of herds using embryo transfer.
In the EU, Council Directive 88/407/EEC sets the measure for the animal health conditions of intra‐Community trade and imports from third countries of frozen bovine semen. Among those testing of C. fetus infection should be carried out (either by IFAT or by culture) in the animals in approved semen collection centres.
Effectiveness
Parameter 2 – Effectiveness of biosecurity measures in preventing the pathogen introduction
The major biosecurity measure against BGC is the use of BGC‐free semen or embryos and this will ensure that no BGC is introduced into a herd.
Feasibility
Parameter 3 – Feasibility of biosecurity measures
The diagnostic tests for BGC described in the OIE Manual to test if animals or materials are BGC‐free are suitable to perform world‐wide, even in LMIC.
3.1.4.5. Article 7(d)(v) Restrictions on the movement of animals and products
Availability
Parameter 1 – Available movement restriction measures
According to Council Directive 88/407/EEC, if an animal of an AI station is tested positive for BGC, the AI station will be closed and production and trade or animals and their products are prohibited. The animals of the closed AI station are treated with antibiotics and must be tested negative for BGC for three times with 2 weeks interval, before the AI centre is allowed to continue the production and trade.
Effectiveness
Parameter 2 – Effectiveness of restriction of animal movement in preventing the between farm spread
The restriction of movement of BGC‐infected animals to another farm will prevent the spread of BGC.
Feasibility
Parameter 3 – Feasibility of restriction of animal movement
If artificial insemination is used, the restriction of movement of animals from one farm to another is suitable for BGC.
3.1.4.6. Article 7(d)(vi) Killing of animals
Availability
Parameter 1 – Available methods for killing animals
For BGC, killing of animal measures are available.
Effectiveness
Parameter 2 – Effectiveness of killing animals (at farm level or within the farm) for reducing/stopping spread of the disease
If a BGC‐infected animal is killed, the disease will not spread further from this animal.
Feasibility
Parameter 3 – Feasibility of killing animals
The economic loss by killing highly productive bulls can be very high. If the bull can be effectively treated with antibiotics, killing the infected bull is not necessary. If the antibiotic treatment is not effective and the bull remains BGC positive, it cannot be used for production in AI stations, and killing will be an option.
The economic loss of killing a cow will be much lower, but the feasibility of killing infected cows is questionable since recovery usually occurs spontaneously within 5 months and the acquired immunity protects the cows from re‐infection.
Culling infected animals in a herd has proven to be effective to control the disease (Truyers et al., 2014).
3.1.4.7. Article 7(d)(vii) Disposal of carcasses and other relevant animal by‐products
Availability
Parameter 1 – Available disposal option
Semen or embryos contaminated with BGC can be destroyed.
Effectiveness
Parameter 2 – Effectiveness of disposal option
Disposal of the semen or embryos will prevent BGC spread.
Feasibility
Parameter 3 – Feasibility of disposal option
Disposal of BGC contaminated semen or embryos can be expensive, but is feasible world‐wide.
3.1.5. Article 7(e) The impact of disease prevention and control measures
3.1.5.1. Article 7(e)(i) The direct and indirect costs for the affected sectors and the economy as a whole
Parameter 1 – Cost of control (e.g. treatment/vaccine, biosecurity)
The cost to vaccinate a herd against BGC can vary significantly. Prices can be affected by number of cattle to be vaccinated, regional pricing and prices set by the vaccine supplier. A cost calculation is made in 2010 by the Government of Queensland (Queensland Government, online).
Parameter 2 – Cost of eradication (culling, compensation)
Within the EU, no compensation is given for the eradication of BGC‐infected cattle or products thereof. The economic loss cost of the eradication is strongly dependent on the local situation.
Parameter 3 – Cost of surveillance and monitoring
Both preputial washings and vaginal mucus can be screened for the presence of Campylobacter. The total costs depend on the number of animals to be tested and are strongly dependent on the local situation. Bulls in AI centres are tested at least once per year, according to Council Directive 88/407/EEC.
Parameter 4 – Trade loss (bans, embargos, sanctions) by animal product
No data available about trade loss by animal product caused by BGC.
Parameter 5 – Importance of the disease for the affected sector (% loss or € lost compared to business amount of the sector)
In 2003, it was estimated that with approximately 20 million cows producing 11 million calves world‐wide, a reduced weaning rate of 10% due to BGC results in the loss of 1.1 million calves yearly with a trade value of approximately 165 million $ (Campero et al., 2003).
3.1.5.2. Article 7(e)(ii) The societal acceptance of disease prevention and control measures
The disease prevention and control measures, like testing the materials and treat the animals with antibiotics, are fully acceptable. Killing of animals to restrict spread meets societal problems.
3.1.5.3. Article 7(e)(iii) The welfare of affected subpopulations of kept and wild animals
Parameter 1 – Welfare impact of control measures on domestic animals
The control measures for BGC on domestic animals have no impact on their welfare.
Parameter 2 – Wildlife depopulation as control measure
Wild life depopulation is not required as control measure for BGC.
3.1.5.4. Article 7(e)(iv) The environment and biodiversity
Environment
Parameter 1 – Use and potential residuals of biocides or medical drugs in environmental compartments (soil, water, feed, manure)
The use and potential residuals of biocides or medical drugs in environmental compartments is hardly applicable for BGC.
Biodiversity
Parameter 2 – Mortality in wild species
BGC can possible cause embryonic death in feral cattle, but this disease is primarily a problem in domestic animals.
3.2. Assessment according to Article 5 criteria
This section presents the results of the expert judgement on the criteria of Article 5 of the AHL about BGC (Table 2). The expert judgement was based on Individual and Collective Behavioural Aggregation (ICBA) approach described in detail in the opinion on the methodology (EFSA AHAW Panel, 2017). Experts have been provided with information of the disease fact‐sheet mapped into Article 5 criteria (see supporting information, Annex A), based on that the experts indicate their Y/N or ‘na’ judgement on each criterion of Article 5, and the reasoning supporting their judgement.
The minimum number of judges in the judgement was 12. The expert judgement was conducted as described in the methodological opinion (EFSA AHAW Panel, 2017). For details on the interpretation of the questions see Appendix B of the methodological opinion (EFSA AHAW Panel, 2017).
3.2.1. Outcome of the assessment of bovine genital campylobacteriosis according to criteria of Article 5(3) of the AHL on its eligibility to be listed
As from the legal text of the AHL, a disease is considered eligible to be listed as laid down in Article 5 if it fulfils all criteria of the first set from A(i) to A(v) and at least one of the second set of criteria from B(i) to B(v). According to the assessment methodology (EFSA AHAW Panel, 2017), a criterion is considered fulfilled when the outcome is ‘Yes’. According to the results shown in Table 2, BGC complies with all criteria of the first set and with two criteria of the second set; therefore, it is considered eligible to be listed for Union intervention as laid down in Article 5(3) of the AHL.
3.3. Assessment according to Article 9 criteria
This section presents the results of the expert judgement on the criteria of Annex IV referring to categories as in Article 9 of the AHL about BGC (Tables 3–7). The expert judgement was based on ICBA approach described in detail in the opinion on the methodology. Experts have been provided with information of the disease fact‐sheet mapped into Article 9 criteria (see supporting information, Annex A), based on that the experts indicate their Y/N or ‘na’ judgement on each criterion of Article 9, and the reasoning supporting their judgement.
Table 3.
Outcome of the expert judgement related to the criteria of section 1 of Annex IV (category A of Article 9) for bovine genital campylobacteriosis (CI: current impact; PI: potential impact)
| Criteria to be met by the disease: The disease needs to fulfil all of the following criteria | Final outcome | |
| 1 | The disease is not present in the territory of the Union OR present only in exceptional cases (irregular introductions) OR present only in a very limited part of the territory of the Union | NC |
| 2.1 | The disease is highly transmissible | N |
| 2.2 | There are possibilities of airborne or waterborne or vector‐borne spread | N |
| 2.3 | The disease affects multiple species of kept and wild animals OR single species of kept animals of economic importance | Y |
| 2.4 | The disease may result in high morbidity and significant mortality rates | N |
| At least one criterion to be met by the disease: In addition to the criteria set out above at points 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, including epidemic or pandemic potential OR possible significant threats to food safety | N |
| 4(CI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 4(PI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | Y |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b)(CI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(b)(PI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(c)(CI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(c)(PI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(d)(CI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
| 5(d)(PI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
Colour code: green = consensus (Yes/No), yellow = non‐consensus (NC).
Table 7.
Outcome of the expert judgement related to the criteria of section 5 of Annex IV (category E of Article 9) for bovine genital campylobacteriosis
| Diseases in category E need to fulfil criteria of Sections 1, 2 or 3 of Annex IV of AHL and/or the following: | Final outcome | |
| E | Surveillance of the disease is necessary for reasons relating to animal health, animal welfare, human health, the economy, society or the environment (If a disease fulfils the criteria as in Article 5, thus being eligible to be listed, consequently category E would apply) | Y |
Colour code: green = consensus (Yes/No).
The minimum number of judges in the judgement was 12. The expert judgement was conducted as described in the methodological opinion (EFSA AHAW Panel, 2017). For details on the interpretation of the questions, see Appendix B of the methodological opinion (EFSA AHAW Panel, 2017).
Table 4.
Outcome of the expert judgement related to the criteria of section 2 of Annex IV (category B of Article 9) for bovine genital campylobacteriosis (CI: current impact; PI: potential impact)
| Criteria to be met by the disease: The disease needs to fulfil all of the following criteria | Final outcome | |
| 1 | The disease is present in the whole OR part of the Union territory with an endemic character AND (at the same time) several Member States or zones of the Union are free of the disease | NC |
| 2.1 | The disease is moderately to highly transmissible | Y |
| 2.2 | There are possibilities of airborne or waterborne or vector‐borne spread | N |
| 2.3 | The disease affects single or multiple species | Y |
| 2.4 | The disease may result in high morbidity with in general low mortality | N |
| At least one criterion to be met by the disease: In addition to the criteria set out above at points 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, including epidemic potential OR possible significant threats to food safety | N |
| 4(CI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | N |
| 4(PI) | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | Y |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b)(CI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(b)(PI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(c)(CI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(c)(PI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(d)(CI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
| 5(d)(PI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
Colour code: green = consensus (Yes/No), yellow = non‐consensus (NC).
Table 6.
Outcome of the expert judgement related to the criteria of Section 4 of Annex IV (category D of Article 9) for bovine genital campylobacteriosis
| Criteria to be met by the disease: The disease needs to fulfil all of the following criteria | Final outcome | |
| D | The risk posed by the disease in question can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread | Y |
| The disease fulfils criteria of sections 1, 2, 3 or 5 of Annex IV of AHL | Y | |
Colour code: green = consensus (Yes/No).
3.3.1. Non‐consensus‐questions
This section displays the assessment related to each criterion of Annex IV referring to the categories of Article 9 of the AHL where no consensus was achieved in form of tables (Tables 8 and 9). The proportion of Y, N or ‘na’ answers is reported, followed by the list of different supporting views for each answer.
Table 8.
Outcome of the expert judgement related to criterion 1 of Article 9
| Question | Final outcome | Response | |||
|---|---|---|---|---|---|
| Y (%) | N (%) | Na (%) | |||
| 1 (cat. A) | The disease is not present in the territory of the Union OR present only in exceptional cases (irregular introductions) OR present only in a very limited part of the territory of the Union | NC | 33 | 67 | 0 |
| 1 (cat. B) | The disease is present in the whole OR part of the Union territory with an endemic character AND (at the same time) several Member States or zones of the Union are free of the disease | NC | 33 | 67 | 0 |
| 1 (cat. C) | The disease is present in the whole OR part of the Union territory with an endemic character | NC | 42 | 58 | 0 |
NC: non‐consensus; number of judges: 12.
Table 9.
Outcome of the expert judgement related to criterion 4(CI) of Article 9
| Question | Final outcome | Response | |||
|---|---|---|---|---|---|
| Y (%) | N (%) | na (%) | |||
| 4 (cat. C) | The disease has a significant impact on the economy of parts of the Union, mainly related to its direct impact on certain types of animal production systems | NC | 25 | 75 | 0 |
NC: non‐consensus; number of judges: 12.
Reasoning supporting the judgement
Supporting Yes for 1 (cat. A):
The disease is mostly not present and only rare sporadic cases have been reported in a limited part of the Union (few MSs).
There is no definition of a free status, neither at EU nor at OIE level.
Supporting Yes for 1 (cat. B):
Only sporadic cases of the disease are reported in Scotland, Ireland, France, Spain, and the UK, but the microorganism is continuously present in the EU.
The disease is sporadic in some countries with free‐ranging extensive beef breeding systems using natural service.
Supporting Yes for 1 (cat. C):
Cases of disease have been reported sporadically in several countries. However, the disease is asymptomatic and there are no testing measures currently in place and variable reporting can be an issue. Therefore, it is likely that the prevalence is underestimated and the disease may be more widespread across MSs, especially in those with free‐ranging extensive beef breeding systems using natural service.
It would appear to be endemic in a number of MSs, e.g. the UK, Ireland, France and Spain, where cases occur without any links to importation from elsewhere. The reporting of cases of BGC is sporadic rather than the presence of C. fetus.
Reasoning supporting the judgement
Supporting Yes:
Losses are confined to beef breeding using natural service in extensive systems – i.e. no AI – the losses then occur in a particular type of production system in more than one MSs.
Supporting No:
The infection causes low morbidity and no mortality due to control measures and the nature of the pathogen. So far, no significant losses in the EU have been reported.
3.3.2. Outcome of the assessment of criteria in Annex IV for bovine genital campylobacteriosis for the purpose of categorisation as in Article 9 of the AHL
As from the legal text of the AHL, a disease is considered fitting in a certain category (A, B, C, D or E corresponding to point (a) to point (e) of Article 9(1) of the AHL) if it is eligible to be listed for Union intervention as laid down in Article 5(3) and fulfils all criteria of the first set from 1 to 2.4 and at least one of the second set of criteria from 3 to 5(d) as shown in Tables 3–7. According to the assessment methodology (EFSA AHAW Panel, 2017), a criterion is considered fulfilled when the outcome is ‘Yes’. With respect to different type of impact where the assessment is divided into current and potential impact, a criterion will be considered fulfilled if at least one of the two outcomes is ‘Y’ and, in case of no ‘Y’, the assessment is inconclusive if at least one outcome is ‘NC’.
A description of the outcome of the assessment of criteria in Annex IV for BGC for the purpose of categorisation as in Article 9 of the AHL is presented in Table 10.
Table 10.
Outcome of the assessment of criteria in Annex IV for bovine genital campylobacteriosis for the purpose of categorisation as in Article 9 of the AHL
| Category | Article 9 criteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1° set of criteria | 2° set of criteria | ||||||||||
| 1 | 2.1 | 2.2 | 2.3 | 2.4 | 3 | 4 | 5a | 5b | 5c | 5d | |
| Geographical distribution | Transmissibility | Routes of transmission | Multiple species | Morbidity and mortality | Zoonotic potential | Impact on economy | Impact on society | Impact on animal welfare | Impact on environment | Impact on biodiversity | |
| A | NC | N | N | Y | N | N | Y | N | N | N | N |
| B | NC | Y | N | Y | N | N | Y | N | N | N | N |
| C | NC | Y | Y | Y | Y | N | Y | N | N | N | N |
| D | Y | ||||||||||
| E | Y | ||||||||||
According to the assessment here performed, BGC complies with the following criteria of the sections 1–5 of Annex IV of the AHL for the application of the disease prevention and control rules referred to in points (a)–(e) of Article 9(1):
To be assigned to category A, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment BGC complies with criterion 2.3, but not with criteria 2.1, 2.2 and 2.4 and the assessment is inconclusive on compliance with criterion 1. To be eligible for category A, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and BGC complies with criterion 4.
To be assigned to category B, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment BGC complies with criteria 2.1 and 2.3, but not with criteria 2.2 and 2.4 and the assessment is inconclusive on compliance with criterion 1. To be eligible for category B, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a–d) and BGC complies with criterion 4.
To be assigned to category C, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and according to the assessment BGC complies with criteria 2.1, 2.2, 2.3 and 2.4 and the assessment is inconclusive on compliance with criterion 1. To be eligible for category C, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5a‐d) and BGC complies with criterion 4.
To be assigned to category D, a disease needs to comply with criteria of Sections 1, 2, 3 or 5 of Annex IV of the AHL and with the specific criterion D of Section 4, with which BGC complies.
To be assigned to category E, a disease needs to comply with criteria of Sections 1, 2 or 3 of Annex IV of the AHL and/or the surveillance of the disease is necessary for reasons relating to animal health, animal welfare, human health, the economy, society or the environment. The latter is applicable if a disease fulfils the criteria as in Article 5, with which BGC complies.
3.4. Assessment of Article 8
This section presents the results of the assessment on the criteria of Article 8(3) of the AHL about BGC. The Article 8(3) criteria are about animal species to be listed, as it reads below:
‘3. Animal species or groups of animal species shall be added to this list if they are affected or if they pose a risk for the spread of a specific listed disease because:
they are susceptible for a specific listed disease or scientific evidence indicates that such susceptibility is likely; or
they are vector species or reservoirs for that disease, or scientific evidence indicates that such role is likely'.
For this reason, the assessment on Article 8 criteria is based on the evidence as extrapolated from the relevant criteria of Article 7, i.e. the ones related to susceptible and reservoir species or routes of transmission, which cover also possible role of biological or mechanical vectors.3 According to the mapping, as presented in Table 5, Section 3.2of the scientific opinion on the ad hoc methodology (EFSA AHAW Panel, 2017), the main animal species to be listed for BGC according to the criteria of Article 8(3) of the AHL are as displayed in Table 11.
Table 5.
Outcome of the expert judgement related to the criteria of section 3 of Annex IV (category C of Article 9) for bovine genital campylobacteriosis (CI: current impact; PI: potential impact)
| Criteria to be met by the disease: The disease needs to fulfil all of the following criteria | Final outcome | |
| 1 | The disease is present in the whole OR part of the Union territory with an endemic character | NC |
| 2.1 | The disease is moderately to highly transmissible | Y |
| 2.2 | The disease is transmitted mainly by direct or indirect transmission | Y |
| 2.3 | The disease affects single or multiple species | Y |
| 2.4 | The disease usually does not result in high morbidity and has negligible or no mortality AND often the most observed effect of the disease is production loss | Y |
| At least one criterion to be met by the disease: In addition to the criteria set out above at points 1–2.4, the disease needs to fulfil at least one of the following criteria | ||
| 3 | The disease has a zoonotic potential with significant consequences on public health, or possible significant threats to food safety | N |
| 4(CI) | The disease has a significant impact on the economy of parts of the Union, mainly related to its direct impact on certain types of animal production systems | NC |
| 4(PI) | The disease has a significant impact on the economy of parts of the Union, mainly related to its direct impact on certain types of animal production systems | Y |
| 5(a)(CI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(a)(PI) | The disease has a significant impact on society, with in particular an impact on labour markets | N |
| 5(b)(CI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(b)(PI) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | N |
| 5(c)(CI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(c)(PI) | The disease has a significant impact on the environment, due to the direct impact of the disease OR due to the measures taken to control it | N |
| 5(d)(CI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
| 5(d)(PI) | The disease has a significant impact on a long‐term effect on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | N |
Colour code: green = consensus (Yes/No), yellow = non‐consensus (NC).
Table 11.
Main animal species to be listed for bovine genital campylobacteriosis according to criteria of Article 8 (source: data reported in Section 3.1.1.1)
| Class | Order | Family | Genus/species | |
|---|---|---|---|---|
| Susceptible | Mammalia | Artiodactyla | Bovidae | Cattle (Bos taurus) |
| Rodentia | Caviidae | Guinea pig (Cavia porcellus) | ||
| Reservoir | Mammalia | Artiodactyla | Bovidae | Cattle (Bos taurus) |
| Vectors | None | |||
4. Conclusions
TOR 1: for each of those diseases an assessment, following the criteria laid down in Article 7 of the AHL, on its eligibility of being listed for Union intervention as laid down in Article 5(3) of the AHL;
According to the assessment here performed, BGC complies with all criteria of the first set and with two criteria of the second set, and therefore, can be considered eligible to be listed for Union intervention as laid down in Article 5(3) of the AHL.
TOR 2a: for each of the diseases which was found eligible to be listed for Union intervention, an assessment of its compliance with each of the criteria in Annex IV to the AHL for the purpose of categorisation of diseases in accordance with Article 9 of the AHL;
According to the assessment here performed, BGC meets the criteria as in Sections 4 and 5 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in points (d) and (e) of Article 9(1) of the AHL. According to the assessment here performed, it is inconclusive whether BGC complies with the criteria as in Section 3 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (c) of Article 9(1) of the AHL. Compliance of BGC with the criteria as in Section 3 is dependent on a decision on criterion 1.
TOR 2b: for each of the diseases which was found eligible to be listed for Union intervention, a list of animal species that should be considered candidates for listing in accordance with Article 8 of the AHL.
Abbreviations
- AHAW
EFSA Panel on Animal Health and Welfare
- AHL
Animal Health Law
- AI
artificial insemination
- BCG
Bovine genital campylobacteriosis
- BVC
Bovine venereal campylobacteriosis
- Cff
Campylobacter fetus subsp. fetus
- CFU
colony forming unit
- Cfv
Campylobacter fetus subsp. venerealis
- ELISA
enzyme‐linked immunosorbent assay
- IETS
International Embryo Transfer Society
- ICBA
Individual and Collective Behavioural Aggregation
- IFAT
immunofluorescence antibody test
- LMIC
low‐ and middle‐income countries
- OIE
World Organization for Animal Health
- ToR
Terms of Reference
Appendix A – Presence/absence of BGC in EU
Table A.1.
Presence/absence of BGC in domestic animals countries in Europe from 2005 to 2016, obtained from OIE WAHIS (OIE, online)
Annex A – Mapped fact‐sheet used in the individual judgement on bovine genital campylobacteriosis
Annex A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.4990
Supporting information
Mapped fact‐sheet used in the individual judgement on bovine genital campylobacteriosis
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Candiani D, Beltrán‐Beck B, Kohnle L and Bicout D, 2017. Scientific Opinion on the assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): bovine genital campylobacteriosis. EFSA Journal 2017;15(10):4990, 30 pp. 10.2903/j.efsa.2017.4990
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00038
Panel members: Dominique Bicout, Anette Bøtner, Andrew Butterworth, Paolo Calistri, Klaus Depner, Sandra Edwards, Bruno Garin‐Bastuji, Margaret Good, Christian Gortázar Schmidt, Virginie Michel, Miguel Angel Miranda, Simon More, Søren Saxmose Nielsen, Mohan Raj, Liisa Sihvonen, Hans Spoolder, Jan Arend Stegeman, Hans‐Hermann Thulke, Antonio Velarde, Preben Willeberg and Christoph Winckler.
Acknowledgements: The Panel wishes to thank Jaap Wagenaar for the support provided to this scientific output.
Adopted: 14 September 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1 and Figure 1 (Annex): © World Organization for Animal Health (OIE); Table A1 (Appendix) and Table 2 (Annex): © World Organization for Animal Health (OIE).
Notes
Council Directive 88/407/EEC of 14 June 1988 laying down the animal health requirements applicable to intra‐ Community trade in and imports of deep‐frozen semen of domestic animals of the bovine species. OJ L 194, 22.7.1988, p. 10–23.
Council Directive 89/556/EEC of 25 September 1989 on animal health conditions governing intra‐Community trade in and importation from third countries of embryos of domestic animals of the bovine species. OJ L 302, 19.10.1989, p. 1–11.
A vector is a living organism that transmits an infectious agent from an infected animal to a human or another animal. Vectors are frequently arthropods. Biological vectors may carry pathogens that can multiply within their bodies and be delivered to new hosts, usually by biting. In mechanical vectors the pathogens do not multiply within the vector, which usually remains infected for shorter time than in biological vectors.
References
- ADR (Arbeitsgemeinschaft Deutscher Rinderzüchter e.V.), 2016. Das Wichtigste in Kürze 2015. Available online: http://www.adr-web.de/services/files/jahresbericht/jahresbericht-2016/Das%20Wichtigste%20in%20K%C3%BCrze%202015.pdf
- Agriland , online. Only 23% of beef calves are born to an AI sire. Available online: http://www.agriland.ie/farming-news/only-23-of-beef-calves-are-born-to-an-ai-sire/ [Accessed: 18 September 2017]
- Akhtar S, Riemann HP, Thurmond MC, Farver TB and Franti CE, 1990. The use of loglinear model to evaluate factors associated with sero‐prevalence of Campylobacter fetus in dairy cattle. Theriogenology, 34, 989–1001. [DOI] [PubMed] [Google Scholar]
- Andrade JRA, Silveira W, Oliveira VCDE and Viana HA, 1986. Prevalência de Campylobacter fetus (vibriose) em bovinos no Estado de Goiás. A Hora Veterinaria, 33, 31–37. [Google Scholar]
- Bawa EK, Adekeye JO, Oyedipe EO and Omoh JU, 1991. Prevalence of bovine campylobacteriosis in indigenous cattle of three states in Nigeria. Tropical Animal Health and Production, 23, 157–160. [DOI] [PubMed] [Google Scholar]
- BDN (National Data Bank), online. BDN dell'Anagrafe Zootecnica istituita dal Ministero della Salute presso il CSN dell'Istituto “G. Caporale” di Teramo. Available online: http://statistiche.izs.it/portal/page?_pageid=73,12918&dad=portal&_schema=PORTAL&op=elenco_rep&p_report=plet_rep_bov&p_titolo=Bovini%20e%20Bufalin [Accessed: 21 September 2017]
- Blaser MJ, Newell DG, Thompson SA and Zechner EL, 2008. Pathogenesis of Campylobacter fetus . In: Nachamkin I, Szymanski CM and Blaser MJ (eds.). Campylobacter, 3rd Edition. ASM Press, Washington, DC. pp. 401–428. [Google Scholar]
- Brooks BW, Devenish J, Lutze‐Wallace CL, Milnes D, Robertson RH and Berlie‐Surujballi G, 2004. Evaluation of a monoclonal antibody‐based enzyme‐linked immunosorbent assay for detection of Campylobacter fetus in bovine preputial washing and vaginal mucus samples. Veterinary Microbiology, 103, 77–84. [DOI] [PubMed] [Google Scholar]
- Campero CM, Moore DP, Odeon AC, Cipolla AL and Odriozola E, 2003. Aetiology of bovine abortion in Argentina. Veterinary Research Communications, 27, 359–369. [DOI] [PubMed] [Google Scholar]
- Chattopadhay UK, Rasheed M, Sur SK and Pal D, 2001. The occurrence of campylobacteriosis in animals and their handlers in and around Calcutta. Journal of Medical Microbiology, 50, 933–934. [DOI] [PubMed] [Google Scholar]
- Cipolla AL, Casaro AP, Terzolo HR, Estela ES, Brooks BW and Garcia MM, 1994. Persistence of Campylobacter fetus subspecies venerealis in experimentally infected heifers. Veterinary Record, 134, 628. [DOI] [PubMed] [Google Scholar]
- Clark BL, 1971. Review of bovine vibriosis. Australian Veterinary Journal, 47, 103–107. [DOI] [PubMed] [Google Scholar]
- Corbeil LB, Schurig GG, Bier PJ and Winter AJ, 1975. Bovine veneral vibriosis: antigenic variation of the bacterium during infection. Infection and Immunity, 11, 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekeyser J, 1984. Campylobacter Infections in Man and Animals. CRC Press, Boca Raton, Florida. 246 pp. [Google Scholar]
- Devenish J, Brooks B, Perry K, Milnes D, Burke T, McCabe D, Duff S and Lutze‐Wallace CL, 2005. Validation of a monoclonal antibody‐based capture enzyme‐linked immunosorbent assay for detection of Campylobacter fetus . Clinical and Diagnostic Laboratory Immunology, 12, 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Candiani D, Gervelmeyer A, Zancanaro G, Kohnle L, Morgado J and Bicout D, 2017. Scientific opinion on an ad hoc method for the assessment on listing and categorisation of animal diseases within the framework of the Animal Health Law. EFSA Journal 2017;15(5):4783, 42 pp. 10.2903/j.efsa.2017.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUROSTAT , online. Meat production statistics. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Meat_production_statistics#Further_Eurostat_information [Accessed: 18 September 2017]
- Figueiredo JF, Pellegrin AO, Fóscolo CD, Machada RP, Miranda KL and Lage AP, 2002. Evaluation of the direct fluorescent antibody test for the diagnosis of bovine genital Campylobacteriosis. Revista Latinoamericana de Microbiología, 44, 118–123. [PubMed] [Google Scholar]
- García MM, Eaglesome MD and Rigby C, 1983. Campylobacters important in veterinary medicine. Veterinary Bulletin, 53, 793–818. [Google Scholar]
- Giacoboni GI, Itoh K, Hirayama K, Takahashi E and Mitsuoka T, 1993. Comparison of fecal Campylobacter in calves and cattle of different ages and areas in Japan. The Journal of Veterinary Medical Science, 55, 555–559. [DOI] [PubMed] [Google Scholar]
- van der Graaf‐van Bloois L, van Bergen MA, van der Wal FJ, de Boer AG, Duim B, Schmidt T and Wagenaar JA, 2013. Evaluation of molecular assays for identification Campylobacter fetus species and subspecies and development of a C. fetus specific real‐time PCR assay. Journal of Microbiological Methods, 95, 93–97. [DOI] [PubMed] [Google Scholar]
- Griffiths IB, Gallego MI and De Leon LS, 1984. Levels of some reproductive diseases in the dairy cattle of Colombia. Tropical Animal Health and Production, 16, 219–223. [DOI] [PubMed] [Google Scholar]
- Hanel I, Hotzel H, Muller W and Tomaso H, 2011. Antimicrobial susceptibility testing of German Campylobacter fetus subsp. venerealis isolates by agar disk diffusion method. Berliner und Münchener Tierärztliche Wochenschrift, 124, 198–202. [PubMed] [Google Scholar]
- Harvey RB, Droleskey RE, Sheffield CL, Edrington TS, Callaway TR, Anderson RC, Drinnon DL, Ziprin RL, Scott HM and Nisbet DJ, 2004. Campylobacter prevalence in lactating dairy cows in the United States. Journal of Food Protection, 67, 1476–1479. [DOI] [PubMed] [Google Scholar]
- Holst E, Wathne B, Hovelius B and Mardh PA, 1987. Bacterial vaginosis: microbiological and clinical findings. European Journal of Clinical Microbiology, 6, 536–541. [DOI] [PubMed] [Google Scholar]
- Hum S, Brunner J, McInnes A, Mendoza G and Stephens J, 1994. Evaluation of cultural methods and selective media for the isolation of Campylobacter fetus subsp venerealis from cattle. Australian Veterinary Journal, 71, 184–186. [DOI] [PubMed] [Google Scholar]
- ICBF (Irish Cattle Breeding Federation), 2017. Beef & Dairy Breed Statistics 2017. ICBF Web Editor, 44 pp. Available online: https://www.icbf.com/wp/?p=9153
- IDELE (Insistut de l'Elevage), 2017. Stratégies de conduit de la reproduction des bovins. Vu dans Reproscope, La letter de l'observatoire de la reproduction des bovins en France. IDELE, 2 pp. Available online: http://idele.fr/?eID=cmis_download&oID=workspace://SpacesStore/4f8d06b1-cfbf-43fd-94dc-d824d9a66efc
- Inglis GD, Morck DW, McAllister TA, Entz T, Olson ME, Yanke LJ and Read RR, 2006. Temporal prevalence of antimicrobial resistance in Campylobacter spp. from beef cattle in Alberta feedlots. Applied and Environmental Microbiology, 72, 4088–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons PC, Schutte AP, van der Walt ML and Bishop GC, 2004. Genital campylobacteriosis in cattle. In: Coetzer JAW and Trustin RC (eds.). Infectious Diseases of Livestock, 2nd Edition. Oxford University Press, Oxford, UK. pp. 1359–1468. [Google Scholar]
- Ishihara K, Kira T, Ogikubo K, Morioka A, Kojima A, Kijima‐Tanaka M, Takahashi T and Tamura Y, 2004. Antimicrobial susceptibilities of Campylobacter isolated from food‐producing animals on farms (1999–2001): results from the Japanese Veterinary Antimicrobial Resistance Monitoring Program. International Journal of Antimicrobial Agents, 24, 261–267. [DOI] [PubMed] [Google Scholar]
- Jerrett IV, McOrist S, Waddington J, Browning JW, Malecki JC and McCausland IP, 1984. Diagnostic studies of the fetus, placenta and maternal blood from 265 bovine abortions. The Cornell veterinarian, 74, 8–20. [PubMed] [Google Scholar]
- Jimenez DF, Perez AM, Carpenter TE and Martinez A, 2011. Factors associated with infection by Campylobacter fetus in beef herds in the Province of Buenos Aires, Argentina. Preventive Veterinary Medicine, 101, 157–162. [DOI] [PubMed] [Google Scholar]
- Klastrup NO and Halliwell RW, 1977. Infectious causes of infertility/abortion of cattle in Malawi. Nordisk Veterinaermedicin, 29, 325–330. [PubMed] [Google Scholar]
- McFadden AM, Heuer C, Jackson R, West DM and Parkinson TJ, 2005. Investigation of bovine venereal campyloacteriosis in beef cow herds in New Zealand. New Zealand Veterinary Journal, 53, 45–52. [DOI] [PubMed] [Google Scholar]
- McGowan AC and Murray RD, 1999. Health status of bulls used for natural breeding on farms in south west Scotland. Zentralblatt für Veterinärmedizin. Reihe B, 46, 311–321. [DOI] [PubMed] [Google Scholar]
- Mshelia GD, Singh J, Admin JD, Woldehiwet Z, Egwu GO and Murray RD, 2007. Bovine venereal campylobacteriosis: an overview. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, 2, 14. [Google Scholar]
- Mshelia GD, Amin JD, Woldehiwet Z, Murray RD and Egwu GO, 2010. Epidemiology of bovine venereal campylobacteriosis: geographic distribution and recent advances in molecular diagnostic techniques. Reproduction in Domestic Animals, 45, e221–e230. [DOI] [PubMed] [Google Scholar]
- Mshelia GD, Amin JD, Egwu GO, Woldehiwet Z and Murray RD, 2012. The prevalence of bovine venereal campylobacteriosis in cattle herds in the Lake Chad basin of Nigeria. Tropical Animal Health and Production, 44, 1487–1489. [DOI] [PubMed] [Google Scholar]
- Njiro SM, Kidanemariam AG, Tsotetsi AM, Katsande TC, Mnisi M, Lubisi BA, Potts AD, Baloyi F, Moyo G, Mpofu J, Kalake A and Williams R, 2011. A study of some infectious causes of reproductive disorders in cattle owned by resource‐poor farmers in Gauteng Province, South Africa. Journal of the South African Veterinary Association, 82, 213–218. [DOI] [PubMed] [Google Scholar]
- OIE (World Organization for Animal Health), 2012. Bovine genital campylobacteriosis. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 7th Edition. France, Paris. pp. 651–662. [Google Scholar]
- OIE (World Organization for Animal Health), 2013. Terrestrial Animal Health Code, 22nd Edition. France, Paris. p. 306. [Google Scholar]
- OIE , online. WAHIS Interface. Available online: http://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home
- On SL, 1996. Identification methods for campylobacters, helicobacters, and related organisms. Clinical Microbiology Reviews, 9, 405–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pefanis SM, Herr S, Venter CG, Kruger LP, Queiroga CC and Amaral L, 1988. Trichomoniasis and campylobacteriosis in bulls in the Republic of Transkei. Journal of the South African Veterinary Association, 59, 139–140. [PubMed] [Google Scholar]
- Pellegrin AO, Lage AP, Sereno JRB, Ravaglia E, Costa MS and Leite RC, 2002. Bovine Genital Campylobacteriosis in Pantanal, State of Mato Grosso do Sul, Brazil. Revue d'élevage et de médecine vétérinaire des pays tropicaux, 55, 169–173. [Google Scholar]
- Penny CD, 2017. Synchronisation for Artificial Insemination. Beef Herd Fertility. National Animal Disease Information Service. Available online: http://www.nadis.org.uk/bulletins/beef-herd-fertility/synchronised-breeding-in-beef-herds.aspx
- Pfizer , online. Available online: http://www.pfizer.com/
- Plummer PJ, 2017, personal communication.
- Queensland Government , online. Available online: https://www.daf.qld.gov.au/
- SEGES Avlsværdivurdering Kvæg Team, 2016. Årsstatistik Avl 2015/16. SEGES, Skejby, 158 pp. Available online: https://www.landbrugsinfo.dk/Kvaeg/Avl/Avlsstatistik/Sider/aarsstat_2016.pdf?download=true
- Swai ES, Hulsebosch J and Van der Heijden W, 2005. Prevalence of genital campylobacteriosis and trichomonosis in crossbred breeding bulls kept on zero‐grazed smallholder dairy farms in the Tanga region of Tanzania. Journal of the South African Veterinary Association, 76, 224–227. [DOI] [PubMed] [Google Scholar]
- Taylor JH, 2002. Registration and use of veterinary medicines in New Zealand. New Zealand Veterinary Journal, 50, 64–66. [DOI] [PubMed] [Google Scholar]
- Tedesco LF, Errico F and Del Baglivi LP, 1977. Comparison of three sampling methods for the diagnosis of genital vibriosis in the bull. Australian Veterinary Journal, 53, 470–472. [DOI] [PubMed] [Google Scholar]
- Thompson SA and Blaser MJ, 2000. Pathogenesis of Campylobacter fetus infections. In: Nachamkin I, Szymanski CM and Blaser MJ (eds.). Campylobacter. ASM Press, Washington, DC. pp. 321–347. [Google Scholar]
- Timoney JF, Gillespie JH, Scott FW and Barlough JE, 1988. Hagan and Bruner's Microbiology and Infectious Diseases of Domestic Animals, 8th edition. Cornell University Press, Ithaca, US. 925 pp. [Google Scholar]
- Truyers I, Luke T, Wilson D and Sargison N, 2014. Diagnosis and management of venereal campylobacteriosis in beef cattle. BMC Veterinary Research, 10, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UGAVAN (Unión de Ganaderos de Vacas Nodrizas), 2017. La Unión de Ganaderos de Enfermería Vacas, frente a su realidad: Una de cada dos fincas no es rentable. Personal communication to EFSA, September 2017.
- Wagenaar JA, van Bergen MA, Blaser MJ, Tauxe RV, Newell DG and van Putten JP, 2014. Campylobacter fetus infections in humans: exposure and disease. Clinical Infectious Diseases, 58, 1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mapped fact‐sheet used in the individual judgement on bovine genital campylobacteriosis


