Abstract
l‐Arginine is considered as a non‐essential amino acid for most adult mammalian species, but it is classified as essential for birds, fish, possibly reptiles and also for strict carnivores. The product subject of this assessment is l‐arginine produced by fermentation with a genetically modified strain of Corynebacterium glutamicum (KCCM 80099). It is intended to be used in feed and water for drinking for all animal species and categories. The following conclusions refer to the additive ‘L‐arginine produced by Corynebacterium glutamicum KCCM 80099’. Neither the genetically modified production strain nor its recombinant DNA were detected in the final product. The additive does not give rise to safety concerns with regard to the genetic modification of the production strain. The use of the additive is safe for target species when supplemented to diets in appropriate amounts, for the consumer and for the environment. The additive is not hazardous by inhalation, is not a skin sensitiser, but is corrosive to skin and eyes. The additive is an effective source of arginine for all species. For the supplemental l‐arginine to be as efficacious in ruminants as in non‐ruminant species, it requires protection against microbial degradation in the rumen.
Keywords: nutritional additive, amino acids, L‐arginine, genetically modified production strain, C. glutamicum KCCM 80099, safety, efficacy
Summary
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on l‐arginine produced by fermentation with Corynebacterium glutamicum KCCM 80099 when used as nutritional additive for all animal species.
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of l‐threonine was in line with the principles laid down in Regulation (EC) No 429/2008 and the relevant EFSA guidance documents. The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts' knowledge, to deliver the present output.
l‐Arginine is considered as a non‐essential amino acid for most adult mammalian species, but it is classified as essential for birds, fish, possibly reptiles and also for strict carnivores.
Neither the genetically modified production strain nor its recombinant DNA were detected in the final product. The additive l‐Arginine, manufactured using the production strain C. glutamicum KCCM 80099, does not give rise to safety concerns with regard to the genetic modification of the production strain.
The use of the additive is safe for target species when supplemented to diets in appropriate amounts, for the consumer and the environment.
The product l‐arginine produced by C. glutamicum KCCM 80099 is corrosive to skin and eyes but is not a skin sensitiser. There is a potential for user exposure by inhalation, however, an acute inhalation toxicity test did not indicate a hazard by inhalation when handling the additive.
The additive is an effective source of arginine for all species. For the supplemental l‐arginine to be as efficacious in ruminants as in non‐ruminant species, it requires protection against microbial degradation in the rumen.
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from CJ Europe GmbH2 for authorisation of the product l‐arginine (l‐arginine feed grade) produced by fermentation with Corynebacterium glutamicum KCCM 80099, when used as a feed additive for all animal species (category: nutritional additives; functional group: amino acids, their salts and analogues).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 2 August 2016.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product l‐arginine (l‐arginine feed grade), when used under the proposed conditions of use (see Section 3.1.7).
1.2. Additional information
The product l‐arginine produced by the genetically modified strain of C. glutamicum KCCM 80099 has not been previously authorised as a feed additive in the European Union (EU). C. glutamicum is considered by EFSA to be suitable for the Qualified Presumption of Safety (QPS) approach to safety assessment when used for amino acid production, provided the susceptibility to antimicrobials has been demonstrated (EFSA, 2007; EFSA BIOHAZ Panel, 2013, 2017).
l‐Arginine (98%) produced by C. glutamicum ATCC 13870 or by C. glutamicum KCTC 10423BP is currently authorised as a nutritional feed additive for all animals without any restrictions by Commission Regulation (EC) No 1139/20073 and Commission implementing Regulation (EU) 2016/9724, respectively.
The EFSA Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) issued two opinions on the safety and efficacy of the product containing l‐arginine produced by fermentation using C. glutamicum (ATCC 13870 and KCTC 10423BP, respectively) for all animal species (EFSA Feedap Panel, 2007, 2016). The FEEDAP Panel issued one opinion on the safety and efficacy of the use of amino acids (chemical group 34) when used as flavourings for all animal species (EFSA FEEDAP Panel, 2014).
The EU Scientific Committee for Food (SCF) found acceptable the use of l‐arginine as a food for particular nutritional purposes (European Commission, 1999). The Joint FAO/Who Expert Committee on Food Additives (JECFA) issued an opinion on the safety evaluation of certain food additives prepared by the sixty‐third meeting of this committee (WHO, 2006) that included l‐arginine.
The EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA Panel) delivered two opinions related to the substantiation of health claims related to l‐arginine (EFSA NDA Panel, 2011a,b).
l‐Arginine like other amino acids and other nitrogen compounds is authorised according to Commission Regulation (EC) No 1243/2008 for infant formulae and follow‐on formulae.5 According to Commission Regulation (EC) No 953/2009 and Commission Directive 2001/15/EC, amino acids such as l‐arginine may be added in all dietary foods for particular nutritional uses including foods for particular nutritional uses intended for special medical purposes.6 l‐Arginine and related compounds are also registered as an ingredient in cosmetic products (Commission Decision 2006/257/EEC).7 l‐Arginine is registered as pharmaceutical grade (for total parenteral nutrition) in many European countries and is described in a monograph of the European Pharmacopoeia (European Pharmacopoeia, 2014). According to Commission Regulation (EEC) 2377/90, l‐arginine is also listed as pharmacologically active substance in veterinary medicinal products and is not subjected to maximum residue levels when used in food producing animals.8
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier9 in support of the authorisation request for the use of l‐arginine produced by C. glutamicum KCCM 80099 as an additive in feed and in water for drinking. The technical dossier was prepared following the provisions of Article 7 of Regulation (EC) No 1831/2003, Regulation (EC) No 429/200810 and the applicable EFSA guidance documents.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts' knowledge, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of l‐arginine in animal feed. The Executive Summary of the EURL report can be found in Annex A.11
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of l‐arginine produced by C. glutamicum KCCM 80099 is in line with the principles laid down in Regulation (EC) No 429/2008 and the relevant guidance documents: Guidance on nutritional additives (EFSA FEEDAP Panel, 2012a), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012b), Technical Guidance: Microbial Studies (EFSA FEEDAP Panel, 2008), Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance (EFSA FEEDAP Panel, 2012c), Guidance for the preparation of dossiers for additives already authorised for use in food (EFSA FEEDAP Panel, 2012d), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012e) and Guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use (EFSA GMO Panel, 2011).
3. Assessment
The applicant has requested authorisation for the product l‐arginine produced by C. glutamicum KCCM 80099 as an additive in feed and water for drinking for all animal species. l‐Arginine is considered as a non‐essential amino acid for most adult mammalian species including humans, but it is classified as essential for birds, fish, possibly reptiles and also for strict carnivores. For mammalian neonates, it is also considered to be essential.
3.1. Characterisation
3.1.1. Characterisation of the active substance
l‐Arginine (International Union of Pure and Applied Chemistry (IUPAC) name: (S)‐2‐amino‐5‐guanidinopentanoic acid; synonym 2‐amino‐5‐guanidinovaleric acid, a compound identified with the Chemical Abstracts Service (CAS) No 74‐79‐3, and the European Inventory of Existing Commercial chemical Substances (EINECS) No 200‐811‐1). It has a molecular mass of 174.2 Da. The molecular formula of l‐arginine is C6H14N4O2. The structural formula is given in Figure 1.
Figure 1.
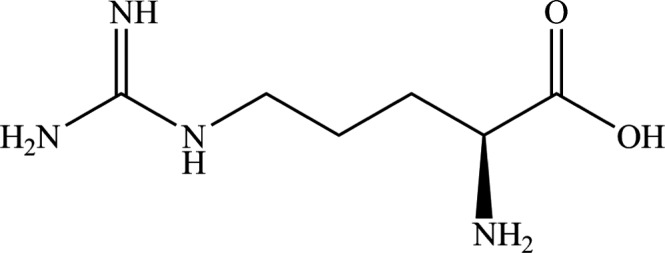
Molecular structure of l‐arginine
3.1.2. Characterisation of the production organism12
The additive is produced by a genetically modified strain of C. glutamicum, which is deposited in the Korean Culture Centre of Microorganisms with accession number KCCM 80099.13 The identity of the strain as belonging to C. glutamicum species was sufficiently demonstrated.14
C. glutamicum KCCM 80099 was tested for antibiotic susceptibility using broth microdilution. The battery of antibiotics tested was that recommended by EFSA (EFSA FEEDAP Panel, 2012c) for ‘Other Gram +’.15 All minimum inhibitory concentration (MIC) values were equal to or below the corresponding cut‐off values defined by the FEEDAP Panel, except for clindamycin which was exceeded by two dilutions (1 mg/L vs 0.25 mg/L). No clindamycin MIC distribution is available in the literature for C. glutamicum that would enable a calculation of a cut‐off value. The limited evidence comes from the literature, in which several Corynebacterium species (but not C. glutamicum) and strains showed a wide MIC range suggesting that resistant strains have a high MIC value. Thus C. glutamicum KCCM 80099 with a slightly elevated MIC (1 mg/L vs 0.25 mg/L) can be considered to represent the susceptible population of this species.
3.1.2.1. Information relating to the genetically modified microorganism
The parental microorganism and the donor organism are sufficiently characterised. The genetic modification process is adequately explained. Uncertainty remains on the possible presence of an antimicrobial resistance gene in the genome of the production strain, although its absence can be reasonably expected.16 , 17 , 18 , 19
3.1.3. Manufacturing process20
l‐Arginine is produced by fermentation using C. glutamicum KCCM 80099. After fermentation, the resulting broth is inactivated, the biomass is separated and the arginine is purified.21 , 22
3.1.4. Characterisation of the additive23
According to the specification, the additive contains ≥ 98% l‐arginine on dry matter basis, ≤ 0.5% water and ≤ 0.1% ash. The analysis of five batches showed an average value of l‐arginine of 99.6% on dry matter basis (range 99.4–99.7%).24 Moisture average was 0.48% (range 0.42–0.54%) and residue on ignition 0.04% (range 0.02–0.06%). Consequently, the amount of unidentified material is below 0.1% on a dry matter basis. Analytical data on specific optical rotation of five batches showed an average value of + 27.4° (range + 27.4° to + 27.5°), which is within the range described in the European Pharmacopoeia for this amino acid.25
3.1.4.1. Impurities
Three batches were analysed for impurities.26 Cadmium, lead and arsenic were below the limit of detection (LOD),27 whereas mercury ranged from 0.034 to 0.048 mg/kg. Dioxins and dioxin‐like PCBs were < LOD.28 Regarding the microbial contamination, Salmonella spp. (tested in 25 g sample)29 were negative and total bacterial counts, Escherichia coli, yeasts and filamentous fungi were < 102 CFU/g. With respect to the mycotoxins, aflatoxins ranged from < 0.05 (LOD) to 0.5 μg/kg, ochratoxin was < 1 μg/kg, deoxynivalenol < 100 μg/kg and zearalenone < 17 μg/kg. The concentrations of these impurities do not represent a safety concern.
No viable cells of the production strain were found in three batches of the product (each tested in triplicate).30 , 31
No recombinant DNA was detected in three batches of l‐Arginine.32 , 33
3.1.4.2. Physicochemical characteristics
The additive is a white odourless crystalline powder,34 with a bulk density of 400–600 kg/m3 and a solubility in water (at 25°C) of 50–60 g/L.35 pH value (10% solution) ranged from 11.7 to 11.8 in five batches tested.22
The particle size distribution (three batches of the additive) was analysed by laser‐diffraction technique. The fraction < 10 μm ranged from 2.6% to 3.1% (v/v), the fraction < 50 μm ranged from 11.4% to 13.9% (v/v) and the fraction < 100 μm ranged from 25.9% to 30.2% (v/v).36 The dusting potential of three batches of the final product (Stauber–Heubach method) ranged from 2.1 to 2.6 g/m3.37
3.1.5. Stability and homogeneity
The shelf life of the additive (three batches) was studied when stored in sealed brown glass containers at 25 for 12 months or 40°C for 6 months. The losses detected at the end of the respective storage periods ranged from 0% to 0.8% at 25°C and from 0.2% to 0.7% at 40°C.38
The stability of the additive (three batches) was studied in a vitamin/mineral premixture (with choline chloride (80 g/kg)) when stored in sealed brown glass containers at 25°C or 40°C for 6 months.39 l‐Arginine was added at a concentration of 5%. The losses observed at the end of the storage period ranged from 15% to 17% at 25°C and from 25% to 33% at 40°C.
The stability of the additive (three batches) was studied in three different compound feeds (mash or pelleted feed for pigs for fattening, laying hens and chickens for fattening) when supplemented at 0.2%.40 The basal diets consisted of maize, wheat and soybean meal in the case of laying hens and chickens for fattening; and of barley, maize and soybean meal in the case of the pigs for fattening. The diets contained about 1,000 mg choline chloride/kg feed. Pelleting was performed at 60°C and 2.35 bar for 8 s and the only loss observed was 1% in one of the three batches studied. The compound feeds were stored in sealed brown glass containers at 25°C or 40°C for 3 months. No losses were observed in mash feed or pelleted feed at the end of the storage period.
The stability of the final product (three batches) was studied in water for drinking when used at a concentration of 0.05% and stored at 25°C or 40°C for 48 h. No losses were observed.41
The capacity of the additive to distribute homogeneously in feed (mash and pelleted compound feeds described above) was studied analysing sets of 10 subsamples. In both cases, mash and pelleted compound feed, the coefficients of variation ranged from 2% to 4%.42
3.1.6. Physicochemical incompatibilities in feed
No physicochemical incompatibilities in feed are expected with medicinal products or feed materials. High losses in premixtures indicate incompatibility with one or more constituents of the feed premixture.
3.1.7. Conditions of use
The current application for l‐arginine is as a nutritional additive to feed for all animal species and categories without maximum content in feed or time of administration. According to the applicant, the additive can be added directly to compound feedingstuffs or via premixtures or water for drinking.43 No inclusion levels are proposed as the requirements in quantitative terms depend on the species, the physiological state of the animal, the performance level and the environmental conditions, as well as the amino acid composition of the unsupplemented diet.
3.2. Safety
3.2.1. Safety of the genetic modifications44
The recipient organism is considered to be safe. The slightly elevated MIC (1 mg/L vs 0.25 mg/L) for clindamycin can be considered to represent the susceptible population of this species. The introduced gene does not trigger a safety concern. No vector sequences are expected to remain in the final production strain.
Although uncertainty remains on the possible presence of an antimicrobial resistance gene in the production strain, neither the genetically modified production strain nor its recombinant DNA was detected in the final product. Therefore, the additive does not give rise to safety concerns with regard to the genetic modification of the production strain.
3.2.2. Safety for the target species
Tolerance studies with essential and conditionally essential amino acids cannot be designed in accordance with the protocols of conventional toxicity experiments because high dietary concentrations of a certain amino acid will result in amino acid imbalances and depression of feed intake and, hence, impaired performance. This statement is, in principle, also applicable to non‐essential amino acids since a well‐balanced dietary protein should have a certain ratio between essential and non essential amino acids for optimal performance and low nitrogen emissions per product (Baker, 2004). Nevertheless, for nutritional additives produced by fermentation, the risks associated with the residues of the fermentation process in the final product need to be assessed. In this specific product, the amount of identified material represents > 99.9% on a dry matter basis. Therefore, the FEEDAP Panel considers that safety concerns for target species are highly unlikely to arise from l‐arginine produced by C. glutamicum KCCM 80099.
The classification of l‐arginine as a dispensable or an indispensable amino acid, its dietary requirements, the adverse effects of excess of arginine in the diets and the lysine‐arginine antagonism have been discussed in a previous opinion (EFSA FEEDAP Panel, 2016). In that opinion, it was reported that feeding weaned piglets (age: 3–4 weeks; live weight 7 kg) in short‐term experiments (3–4 weeks) with 0.67%, 1.6% and 2.0% supplemental l‐arginine decreased weight gain and feed intake, but had variable effects on feed/gain and no effect on the nitrogen balance (Southern and Baker, 1982; Anderson et al., 1984), whereas a moderate l‐arginine supplementation (0.22%) did not affect performance of growing piglets (Rosell and Zimmerman, 1985). More recent research (Hu et al., 2015; Wu et al., 2016), however, revealed a higher tolerance to l‐arginine in pig diets. The authors assessed the safety of long‐term l‐arginine supplementation (0%, 1%, 1.5% and 2%) to a typical maize‐soybean meal diet (1.35% arginine as background) in pigs between 30 and 121 days of age, based on general observations (e.g. behaviour, skin health, and hair appearance), feed intake, growth, body composition, as well as haematological and blood chemistry measurements. Results of all of the measured variables in the pigs were within physiological ranges and were not adversely affected by the l‐arginine supplementation.
The initial products of l‐arginine degradation by ruminal microorganisms are ornithine, δ‐aminovaleric acid and putrescine (Lewis and Emery, 1962), which are then either converted to volatile fatty acids or incorporated into microbial cell biomass. As these products have no recorded deleterious effects to the host animal, there are no safety concerns arising from ruminal l‐arginine metabolism when supplemented to diets in appropriate amounts.
The FEEDAP Panel, in its previous statement (EFSA FEEDAP Panel, 2010), identified risks of nutritional imbalances and hygienic concerns in amino acids when administered in water for drinking.
3.2.2.1. Conclusions on safety for the target species
The use of l‐arginine produced by C. glutamicum KCCM 80099 is safe for target species when supplemented to diets in appropriate amounts. There are no safety concerns arising from ruminal l‐arginine metabolism.
3.2.3. Safety for the consumer
The absorption and metabolic fate of arginine were described in a previous opinion (EFSA, 2007).
As a general principle, conventional toxicology studies are considered to be inappropriate for amino acids.
The product under assessment is produced by fermentation. Concerns for the consumer would derive not from the amino acid itself, which will be incorporated into animal protein, but from possible residues from the fermentation. Considering that the additive is highly purified (≥ 99.9% l‐arginine on dry matter basis), no additional toxicological data are required (EFSA FEEDAP Panel, 2012).
Amino acids supplemented to feed will be incorporated into proteins of tissues and/or products of target animal species and any of their potential excess will be metabolised and excreted. Therefore, the composition of tissues and products of animal origin will not be changed by the use of l‐arginine in animal nutrition.
3.2.3.1. Conclusions on safety for the consumer
The composition of edible tissues and products of animal origin will not be changed by the use of l‐arginine in animal nutrition. Considering the high purity of the product under assessment, no risks are expected for the consumer from the use of l‐arginine produced by C. glutamicum KCCM 80099 as a feed additive.
3.2.4. Safety for the user
The test item tested in the submitted toxicological studies was the product under assessment (99.1% purity on ‘as is’ basis).
3.2.4.1. Effects on the respiratory system
The portion of particles having a diameter smaller than 100, 50 and 10 μm is about 30%, 14% and 3% (v/v), respectively. The dusting potential is up to 2.6 g/m3, indicating that exposure by inhalation of the user is likely (see Section 3.1.4).
An acute inhalation test was carried out on RccHan™:WIST rats (five males and five females) in accordance with OECD guideline 403.45 The adverse effects seen after 4 h exposure at the dose of 5.1 g l‐arginine/m3 were decreased respiratory rate, hunched posture, piloerection and wet fur, but the animals recovered to normality from days 2 to 4 post exposure. No mortality occurred in the following 2 weeks and no pathological findings were noted at necropsy. Although transient adverse effects were observed at an exposure twofold the maximum dusting potential measured in the additive, they do not indicate a hazard by inhalation when handling the additive.
3.2.4.2. Effects on skin and eyes
The product under assessment has a high pH (11.8) in aqueous solution.
An in vitro study (skin corrosion: transcutaneous electrical resistance test) was carried out according to OECD guideline 430.46 The mean transcutaneous electrical resistance value recorded for the 24 h contact period was 3.3 kΩ. This result indicates that the l‐arginine under assessment has the potential to cause skin corrosion in vivo.
As the product is corrosive for the skin, it is also considered corrosive for the eye.
A local lymph node assay (LLNA) was performed using female CBA/Ca mice, in accordance with OECD Guideline 429.47 The concentration at which no toxic signs were observed (10%) was selected in a preliminary screening test and used as the highest dose investigated in the LLNA. Three groups (4 mice/group) were treated with 50 μL (25 μL per ear) of the test item as a solution 1% in distilled water at concentrations of 10%, 5% and 2.5% (w/w). A further group of four animals (control) was treated with the diluent alone (1% test item in distilled water). The proliferation response of lymph node cells was expressed as the ratio of 3H‐thymidine incorporation into lymph node cells of test nodes relative to that recorded for the control nodes (stimulation index). No proliferation response was elicited by the treatment. No clinical signs, mortality or changes in body weight were observed. The product under assessment is not considered a skin sensitiser.
3.2.4.3. Conclusions on safety for the user
The product l‐arginine produced by C. glutamicum KCCM 80099 is corrosive to skin and eyes but is not a skin sensitiser. There is a potential for user exposure by inhalation, however, an acute inhalation toxicity test did not indicate a hazard by inhalation when handling the additive.
3.2.5. Safety for the environment48
l‐Arginine is a natural component of animals and plants whose use in animal nutrition would not lead to any localised increase of its concentration in the environment. It is mainly not excreted as such, but as urea or uric acid and carbon dioxide. The FEEDAP Panel concludes that the use of the product l‐arginine produced by C. glutamicum KCCM 80099 in animal nutrition would not pose a risk to the environment.
Although uncertainty remains on the possible presence of an antimicrobial resistance gene in the production strain, neither the genetically modified production strain nor its recombinant DNA was detected in the final product. Therefore, the additive does not give rise to environmental safety concerns with regard to the genetic modification of the production strain.
3.3. Efficacy
Efficacy studies are not required for amino acids naturally occurring in proteins of plants and animals. The nutritional role of the amino acid l‐arginine is well established in the scientific literature (Schuhmacher, 2002).
In beef or dairy cattle fed a variety of diets, l‐arginine has not been identified to be limiting (Schwab et al., 2005). The rapid degradation of l‐arginine by ruminal microorganisms has been described in a previous opinion (EFSA FEEDAP Panel, 2016). Consequently, for the supplemental l‐arginine to be as efficacious in ruminants as in non‐ruminant species, it requires protection against degradation in the rumen.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation49 and Good Manufacturing Practice.
4. Conclusions
Neither the genetically modified production strain nor its recombinant DNA were detected in the final product. The additive l‐Arginine, manufactured using the production strain C. glutamicum KCCM 80099, does not give rise to safety concerns with regard to the genetic modification of the production strain.
The use of the additive is safe for target species when supplemented to diets in appropriate amounts, for the consumer and the environment.
The additive is not hazardous by inhalation, is not a skin sensitiser, but is corrosive to skin and eyes.
The additive is an effective source of arginine for all species. For the supplemental l‐arginine to be as efficacious in ruminants as in non‐ruminant species, it requires protection against microbial degradation in the rumen.
5. Recommendations
The description of the additive should contain the statement ‘l‐arginine produced by Corynebacterium glutamicum KCCM 80099′.
Documentation provided to EFSA
L‐Arginine, feed grade, produced by C. glutamicum KCCM 80099. August 2016. Submitted by CJ Europe GmbH.
L‐Arginine, feed grade, produced by C. glutamicum KCCM 80099. Supplementary information. December 2016. Submitted by CJ Europe GmbH.
Evaluation report of the European Union Reference Laboratory for Feed Additives on the Methods(s) of Analysis for L‐Arginine, feed grade, produced by C. glutamicum KCCM 80099.
Comments from Member States.
Abbreviations
- ATCC
American type culture collection
- CAS
Chemical Abstracts Service
- CFU
colony forming unit
- CJ
Cheil Jedang
- DNA
deoxyribonucleic acid
- EINECS
European Inventory of Existing Commercial chemical Substances
- EURL
European Union Reference Laboratory
- FEEDAP
Panel on additives and products or substances used in animal feed
- GMO
genetically modified microorganism
- IUPAC
International Union of Pure and Applied Chemistry
- JECFA
Joint FAO/Who Expert Committee on Food Additives
- KCCM
Korean Culture Centre of Microorganisms
- LLNA
local lymph node assay
- LOD
limit of detection
- MIC
minimum inhibitory concentration
- NDA
Dietetic products, nutrition and allergies
- OECD
Organisation for Economic Co‐operation and Development
- PCR
polymerase chain reaction assay
- pH
hydrogen potential
- QPS
Qualified Presumption of Safety
- SCF
Scientific Committee of Food
- WHO
World Health Organization
Annex A – Evaluation report of the European Reference Laboratory on the analytical methods submitted in connexion with the application for authorisation of l‐arginine produced by Corynebacterium glutamicum KCCM 80099
1.
In the current application, authorisation is sought under Article 4(1) for l‐arginine produced by fermentation with Corynebacterium glutamicum KCCM 80099, under the category/functional group 3(c) ‘nutritional additives’/‘amino acids, their salts and analogues’, according to Annex I of Regulation (EC) No 1831/2003. Authorisation is sought for all animal species. l‐Arginine is already authorised as feed additive under Commission Regulation (EC) No 1139/2007 and Commission Implementing Regulation (EU) 2016/972.
For the quantification of l‐arginine in feed additive, premixtures and feedingstuffs, the Applicant submitted the ring‐trial validated community method (Commission Regulation (EC) No 152/2009). The method was further ring‐trial validated by CEN resulting in EN ISO 13903:2005. The method is based on ion exchange chromatography coupled with post‐column derivatisation and photometric detection (IEC‐VIS). This method does not distinguish between the salts and the amino acid enantiomers, and it is designed for feedingstuffs and premixtures. The following performance characteristics were reported for the quantification of total arginine: a relative standard deviation for repeatability (RSDr) ranging from 2.3% to 3.3% and a relative standard deviation for reproducibility (RSDR) ranging from 7.2% to 9.7%.
In addition, the EURL identified the ‘l‐arginine monograph’ of the Food Chemical Codex (FCC) for the characterisation of the feed additive. Since the Applicant provided no experimental data to determine arginine in water, the EURL is neither able to evaluate nor to recommend a method for official control to determine arginine in water.
Based on the performance characteristics available, the EURL recommends for official control the community method or the equivalent EN ISO 13903:2005 method based on IEC‐VIS for the quantification of arginine in the feed additive, premixtures and feedingstuffs together with the ‘l‐arginine monograph’ of the FCC for the characterisation of the feed additive.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Suggested citation: EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wester P, Costa L, Dierick N, Leng L, Glandorf B, Herman L, Kärenlampi S and Wallace RJ, 2017. Scientific Opinion on the safety and efficacy of l‐arginine produced by Corynebacterium glutamicum KCCM 80099 for all animal species. EFSA Journal 2017;15(6):4858, 15 pp. 10.2903/j.efsa.2017.4858
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00405
Panel members: Gabriele Aquilina, Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Georges Bories, Andrew Chesson, Pier Sandro Cocconcelli, Gerhard Flachowsky, Jürgen Gropp, Boris Kolar, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Alberto Mantovani, Baltasar Mayo, Fernando Ramos, Guido Rychen, Maria Saarela, Roberto Edoardo Villa, Robert John Wallace and Pieter Wester.
Adopted: 17 May 2017
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
CJ Europe GmbH, Ober der Roeth 4, 65824, Schwalbach am Taunus. Germany.
Commission Regulation (EC) No 1139/2007 of 1 October 2007 concerning the authorisation of l‐arginine as a feed additive. OJ L 256, 11, 2.10.2007, p. 3.
Commission Implementing regulation (EU) 2016/972 of 17 June 2016 concerning the authorisation of L‐arginine produced by Corynebacterium glutamicum KCTC 10423BP as feed additive for all animal species. OJ L 161/18, 18.6.2016, p. 3.
Commission Regulation (EC) No 1243/2008 of 12 December 2008 amending Annexes III and VI to Directive 2006/141/EC as regards compositional requirements for certain infant formulae. OJ L 335 25, 13.12.2008, p. 18.
Commission Directive 2001/15/EC of 15 February 2001 on substances that may be added for specific nutritional purposes in foods for particular nutritional uses. OJ L 52, 22.2.2001, pp. 19–25. Commission Regulation (EC) No 953/2009 of 13 October 2009 on substances that may be added for specific nutritional purposes in foods for particular nutritional uses. OJ L 269 9, 14.10.2009, 11 pp.
Commission Decision of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products. OJ L 97, 5.4.2006, pp. 1–528.
Commission Regulation (EC) No 1931/1999, amending Annexes I, II and III of Council Regulation (EEC) No 2377/90 laying down a Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. OJ L 240, 10.9.1999, pp. 3–10.
FEED dossier reference: FAD‐2016‐0037.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep_fad-2016-0037_arginine.pdf
This section has been amended following the provisions of Article 8(6) and Article 18 of Regulation (EC) No 1831/2003.
Technical dossier/Section II/Annex_CONFID_II_2_06.
Technical dossier/Section II/Annex_CONFID_II_2_02.
Technical dossier/Section II/Supplementary information October 2016/Annex Q.1.
Technical dossier/Section II/Annex_CONFID_II_2_04.
Technical dossier/Section II/Supplementary information December 2016.
Technical dossier/Section II/Annex_CONFID_II_2_07.
Technical dossier/Section II/Annex_CONFID_II_2_05.
This section has been amended following the provisions of Article 8(6) and Article 18 of Regulation (EC) No 1831/2003.
Technical dossier/Section II.3.1.
Technical dossier/Section II/Annex II.3.12.
This section has been amended following the provisions of Article 8(6) and Article 18 of Regulation (EC) No 1831/2003.
Technical dossier/Section II/Annex II.1.4. Method according to the EU official method (Regulation (EC) 152/2009) for determination of amino acids.
Technical dossier/Section II/Annex II.1.2.
Technical dossier/Section II/Annex II.1.5.
LOD in mg/kg was 1 for arsenic and lead and 0.1 for cadmium.
LOD of PCDD/F (in ng/kg) ranged from 0.02 to 1 and that of PCBs ranged from 0.5 to 20 depending on the substance analysed.
Technical dossier/Supplementary information December 2016/Response to question 2.
Technical dossier/Section II/Annex_CONFID_II_2_08.
Technical dossier/Section II/Supplementary information December 2016 /Annex Q5.
Technical dossier/Section II/Annex_CONFID_II_2_09.
Technical dossier/Section II/Supplementary information December 2016 /Annex Q6.
Technical dossier/Section II/Annex II.3.1.
Technical dossier/Section II.1.5.
Technical dossier/Section II/Annex II.1.6.
Technical dossier/Section II/Annex II.1.7.
Technical dossier/Section II/Annex II.4.1. Method according to the EU official method (Reg EC 152/2009).
Technical dossier/Section II/Annexes II.4.2 and II.4.4. Method according to the EU official method (Reg EC 152/2009).
Technical dossier/Section II/Annex II.4.3 and Supplementary information December 2016/Annex Q3 and Response to question 3. Method according to the EU official method (Reg EC 152/2009).
Technical dossier/Section II/Annex II.4.5.
Technical dossier/Section II/Annex II.4.6.
Technical dossier/Section II.5.1.
This section has been amended following the provisions of Article 8(6) and Article 18 of Regulation (EC) No 1831/2003.
Technical dossier/Section III/Annex III.3.2.
Technical dossier/Section III/Annex III.3.3.
Technical dossier/Section III/Annex III.3.4.
This section has been amended following the provisions of Article 8(6) and Article 18 of Regulation (EC) No 1831/2003.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- Anderson LC, Lewis AJ, Peo ER Jr and Crenshaw JD, 1984. Effects of excess arginine with and without supplemental lysine on performance, plasma amino acid concentrations and nitrogen balance of young swine. Journal of Animal Science, 58, 369–377. [DOI] [PubMed] [Google Scholar]
- Baker DH, 2004. Animal models for human amino acid responses. Journal of Nutrition, 134, 1646–1650. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2007. Opinion of the Scientific Committee on a request from EFSA on the introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. EFSA Journal 2007;5(8):587, 16 pp. 10.2903/j.efsa.2007.587 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013. Scientific Opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2013 update). EFSA Journal 2013;11(11):3449, 105 pp. 10.2903/j.efsa.2013.3449 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Girones R, Herma NL, Koutsoumanis K, Lindqvist R, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlström H, Cocconcelli PS, Klein G (deceased), Prieto Maradona M, Querol A, Peixe L, Suarez JE, Sundh I, Vlak JM, Aguilera‐Gomez M, Barizzone F, Brozzi R, Correia S, Heng L, Istace F, Lythgo C and Fernandez Escamez PS, 2017. Scientific Opinion on the update of the list of QPS‐recommended biological agents intentionally added to food or feed as notified to EFSA. EFSA Journal 2017;15(3):4664, 177 pp. 10.2903/j.efsa.2017.4664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Feedap Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2007. Scientific Opinion of the Panel on Additives and Products or Substances used in Animal feed on the safety and efficacy of the product containing L‐arginine produced by fermentation from Corynebacterium glutamicum (ATCC 13870) for all animal species. EFSA Journal 2007;5(5):473, 19 pp. 10.2903/j.efsa.2007.473 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2008. Technical Guidance: microbial studies. EFSA Journal 2008;6(10), 836, 3 pp. 10.2903/j.efsa.2008.836 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2010. Statement on the use of feed additives authorised/applied for use in feed when supplied via water. EFSA Journal 2010;8(12);1956 10.2903/j.efsa.2010.1956 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for the preparation of dossiers for nutritional additives. EFSA Journal 2012;10(1):2535, 14 pp. 10.2903/j.efsa.2012.2535 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA Journal 2012;10(6):2740, 10 pp. 10.2903/j.efsa.2012.2740 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012d. Guidance for the preparation of dossiers for additives already authorised for use in food. EFSA Journal 2012;10(1):2538, 4 pp. 10.2903/j.efsa.2012.2538 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012e. Guidance for establishing the safety of additives for the consumer. EFSA Journal 2012;10(1):2537, 12 pp. 10.2903/j.efsa.2012.2537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014. Scientific Opinion on the safety and efficacy of the use of amino acids (chemical group 34) when used as flavourings for all animal species. EFSA Journal 2014;12(5):3670, 19 pp. 10.2903/j.efsa.2014.3670 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016. Scientific Opinion on the safety and efficacy of L‐arginine produced by Corynebacterium glutamicum KCTC 10423BP for all animal species. EFSA Journal 2016;14(1):4345, 17 pp. 10.2903/j.efsa.2016.4345 [DOI] [Google Scholar]
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms), 2011. Scientific Opinion on Guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use. EFSA Journal 2011;9(6):2193, 54 pp. 10.2903/j.efsa.2011.2193 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2011a. Scientific Opinion on the substantiation of health claims related to: anthocyanidins and proanthocyanidins (ID 1787, 1788, 1789, 1790, 1791); sodium alginate and ulva (ID 1873); vitamins, minerals, trace elements and standardised ginseng G115 extract (ID 8, 1673, 1674); vitamins, minerals, lysine and/or arginine and/or taurine (ID 6, 1676, 1677); plant‐based preparation for use in beverages (ID 4210, 4211); Carica papaya L. (ID 2007); ―fish protein‖ (ID 651); acidic water‐based, non‐alcoholic flavoured beverages containing calcium in the range of 0.3 to 0.8 mol per mol of acid with a pH not lower than 3.7 (ID 1170); royal jelly (ID 1225, 1226, 1227, 1228, 1230, 1231, 1326, 1328, 1329, 1982, 4696, 4697); foods low in cholesterol (ID 624); and foods low in trans‐fatty acids (ID 672, 4333) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2011;9(4):2083, 34 pp. 10.2903/j.efsa.2011.2083 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2011b. Scientific Opinion on the substantiation of health claims related to L‐arginine and ‘immune system functions’ (ID 455, 1713), growth or maintenance of muscle mass (ID 456, 1712, 4681), normal red blood cell formation (ID 456, 664, 1443, 1712), maintenance of normal blood pressure (ID 664, 1443), improvement of endothelium‐dependent vasodilation (ID 664, 1443, 4680), ‘physical performance and condition’ (ID 1820), ‘système nerveux’ (ID 608), maintenance of normal erectile function (ID 649, 4682), contribution to normal spermatogenesis (ID 650, 4682), ‘function of the intestinal tract’ (ID 740), and maintenance of normal ammonia clearance (ID 4683) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2011;9(4):2051, 30 pp. 10.2903/j.efsa.2011.2051 [DOI] [Google Scholar]
- European Commission , 1999. Opinion on substances for nutritional purposes which have been proposed for use in the manufacture of foods for particular nutritional purposes. Available online https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out31_en.pdf
- European Pharmacopoeia (PhEur), 2014. Arginine, Monograph 07/2014:0806, 8th Edition Council of Europe. (COE)—European Directorate for the Quality of Medicines, Strasbourg, France. [Google Scholar]
- Hu S, Li X, Rezaei R, Meininger CJ, McNeal CJ and Wu G, 2015. Safety of long‐term dietary supplementation with L‐arginine in pigs. Amino Acids, 47, 925–936. [DOI] [PubMed] [Google Scholar]
- Lewis TR and Emery RS, 1962. Metabolism of amino acids in the bovine rumen. Journal of Dairy Science, 45, 1487–1492. [Google Scholar]
- Rosell VL and Zimmerman DR, 1985. Threonine requirement of pigs weighing 5 to 15 kg and the effect of excess methionine in diets marginal in threonine. Journal of Animal Science, 60, 480–486. [DOI] [PubMed] [Google Scholar]
- Schuhmacher A, 2002. Limitierende Aminosäuren im Futter für wachsende Schweine [in German]; Limiting amino acids in diets for growing pigs. Postdoctoral thesis, Faculty of Veterinary Medicine, University of Leipzig, 307 pp.
- Schwab CG, Huhtanen P, Hunt CW and Hvelplund T, 2005. Nitrogen requirements of cattle In: Pfeffer E, Hristov AN. (eds.). Nitrogen and Phosphorus Nutrition of Cattle. CABI International, Wallingford, UK: pp. 13–70. [Google Scholar]
- Southern LL and Baker DH, 1982. Performance and concentration of amino acids in plasma and urine of young pigs fed diets with excesses of either arginine or lysine. Journal of Animal Science, 55, 857–866. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2006. Safety evaluation of certain food additives. Joint FAO/WHO Expert Committee on Food Additives (JECFA), WHO Food Additives Series: 54. International Programme on Chemical Safety, WHO, Geneva, Switzerland. [Google Scholar]
- Wu Z, Hou Y, Hu S, Bazer F, Meininger C, McNeal C and Wu G, 2016. Catabolism and safety of supplemental L‐arginine in animals. Amino Acids, 48, 1541–1552. [DOI] [PubMed] [Google Scholar]


