Abstract
The EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) provides a scientific opinion re‐evaluating the safety of mono‐ and di‐glycerides of fatty acids (E 471) when used as a food additive. The Panel considered that it is very likely that hydrolysis of mono‐ and di‐glycerides of fatty acids by lipases in the gastrointestinal tract would occur, resulting in the release of glycerol and fatty acids. Glycerol (E 422) and fatty acids (E 570) have been re‐evaluated and the Panel concluded that there was no safety concern regarding their use as food additives. Toxicological studies with mono‐ and di‐glycerides rich in unsaturated fatty acids were considered for the re‐evaluation of E 471. No evidence for adverse effects was reported in short‐term, subchronic studies, chronic, reproductive and developmental toxicity studies. Neither carcinogenic potential nor a promotion effect in initiation/promotion was reported. The available studies did not raise any concern with regard to genotoxicity. The refined estimates were based on 31 out of 84 food categories in which E 471 is authorised. The Panel noted that the contribution of E 471 represented at the mean only 0.8–3.5% of the recommended daily fat intake. Based on the approach described in the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 and taking into account the considerations mentioned above, the Panel concluded that there was no need for a numerical acceptable daily intake (ADI) and that the food additive mono‐ and di‐glycerides of fatty acids (E 471) was of no safety concern at the reported uses and use levels. The Panel recommended some modifications of the EU specifications for E 471.
Keywords: mono‐ and di‐glycerides of fatty acids, E 471, monoglycerides, monoacylglycerol, diglycerides, diacylglycerol
Summary
Mono‐ and di‐glycerides of fatty acids (E 471) is authorised as a food additive in the European Union (EU) in accordance with Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/2012. The Scientific Committee on Food (SCF) concluded that the use of mono‐ and di‐glycerides of fatty acids in nutrient preparations for use in infant formulae and follow‐on formulae is acceptable within the direct additive limit of 4 g/L and for use in weaning foods within the direct additive limit of 5 g/kg. The Panel noted that this food additive has not been evaluated for its other authorised uses as a food additive in EU.
The Panel considered that it is very likely that hydrolysis of mono‐ and di‐glycerides of fatty acids by lipases in the gastrointestinal tract would occur, resulting in the release of glycerol and fatty acids. Glycerol (E 422) and fatty acids (E 570) have been re‐evaluated and the Panel concluded that there was no safety concern regarding their use as food additives.
In rats, only traces of cottonseed oil monoglycerides were found in the faeces, indicating that after hydrolysis, the components were well absorbed (97.8 ± 0.4%). In another study, the absorption of hydrolysis products from diglycerides of fatty acids was calculated to be 58.8 ± 14.3%.
The Panel noted that the diacylglycerol (diglyceride) used in several toxicity studies described below was intended to be used for nutritional purposes (as an edible oil substitute) and that it had a composition rich in unsaturated fatty acids (> 95%). The Panel further noted that its composition made this material acceptable with regard to the specifications of E 471. The Panel considered that the results of the toxicological studies with these diacylglycerols can be used for the assessment of E 471.
No study was available to evaluate the acute toxicity of E 471. No evidence for adverse effects were reported in short‐term and subchronic studies in rats and hamsters even at the highest dose tested of 2,500 mg diacylglycerol/kg body weight (bw) per day in the rats and 7,500 mg glyceryl stearate/kg bw per day in hamsters.
The Panel considered that the available studies did not raise any concern with regard to genotoxicity.
No adverse effects were reported in chronic toxicity studies at doses as high as 7,800 and 2,000 mg diacylglycerol/kg bw per day in mice and rats, respectively. In mice and rats, diacylglycerol did neither show carcinogenic potential nor a promotion effect in initiation/promotion studies.
The refined estimates were based on 31 out of 84 food categories in which mono‐ and di‐glycerides of fatty acids (E 471) is authorised. The Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure to mono‐ and di‐glycerides of fatty acids (E 471) as a food additive in European countries for the refined scenario as the food additive may not be used in food categories, for which no usage data have been provided.
However, the Panel noted that considering information from the Mintel's Global New Products Database (GNPD), mono‐ and di‐glycerides of fatty acids (E 471) is used in food categories for which no use levels have been provided to the European Food Safety Authority (EFSA). The main food categories, in terms of amount consumed, for which no use levels reported were: unripened cheese, different kinds of pasta, processed fish and fishery products including molluscs and crustaceans, processed eggs and egg products and salads and savoury‐based sandwich spreads. The Panel further noted that the exposure to mono‐ and di‐glycerides of fatty acids (E 471) from their use according the Annex III to Regulation (EC) No 1333/2008 (Parts 1, 2, 3, 4, and 5 A and B) was not considered in the exposure assessment. Therefore, the exposure to mono‐ and di‐glycerides of fatty acids (E 471) may be underestimated in all scenarios.
The Panel noted that in Annex II of Regulation (EC) No 1333/2008, use levels of mono‐ and di‐glycerides of fatty acids (E 471) in food for infants under the age of 12 weeks are included in category 13.1.1, 13.1.5.1 and 13.1.5.2. The Panel considered that these uses for infants under the age of 12 weeks would require a specific risk assessment in line with the recommendations given by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (1978), the SCF (1998) and EFSA (EFSA Scientific Committee, 2017). Therefore, the current re‐evaluation of mono‐ and di‐glycerides of fatty acids (E 471) as a food additive is not applicable for infants under the age of 12 weeks.
The Panel noted that no specific clinical data addressing the safety of use of mono‐ and di‐glycerides of fatty acids (E 471) in ‘dietary foods for infants for special medical purposes and special formulae for infants’ (food category 13.1.5.1) and in ‘dietary foods for baby and young children for special medical purposes as defined in Directive 1999/21/EC’ (food category 13.1.5.2) considering the defined maximum use levels were available to the Panel.
According to the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA ANS Panel, 2014) and given that:
in the current safety assessment carried out by the Panel, the uses and use levels reported by the food industry in 46 out of 84 food categories in which mono‐ and di‐glycerides of fatty acids (E 471) is authorised were considered. However, only 31 food categories were taken into account for the refined exposure assessment;
mono‐ and di‐glycerides of fatty acids are subjected to hydrolysis by lipases in the gastrointestinal tract to liberate glycerol and fatty acids;
data from the evaluation previously conducted for the food additives glycerol (E 422) and fatty acids (E 570) can be used for the evaluation of the food additive mono‐ and di‐glycerides of fatty acids (E 471);
there was no indication for a genotoxic, carcinogenic or reprotoxic potential from the available data;
the contribution of mono‐ and di‐glycerides of fatty acids (E 471) to the daily diet represented at the mean only 0.8–3.5% of the recommended daily fat intake;
the Panel concluded that there was no need for a numerical acceptable daily intake (ADI) and that the food additive mono‐ and di‐glycerides of fatty acids (E 471) was of no safety concern at the reported uses and use levels.
The Panel recommended that:
the European Commission considers lowering the current limits for toxic elements (arsenic, lead, mercury and cadmium) in the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471) in order to ensure that the food additive will not be a significant source of exposure to these toxic elements in food.
the European Commission considers revising the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471) including maximum limits for impurities currently included in the EU specifications for glycerol (E 422) or recommended by the Panel in the re‐evaluation of glycerol (E 422) (EFSA ANS Panel, 2017b).
the European Commission considers revising the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471) including maximum limits for residual solvents which can be used when manufacturing mono‐ and di‐glycerides of fatty acids (E 471), i.e. tert‐butanol or tert‐pentanol.
the European Commission considers revising the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471) including maximum limits for trans fatty acids because mono‐ and di‐glycerides of fatty acids (E 471) can be manufactured by glycerolysis of hydrogenated fats and/or oils, which contain significant amounts of trans fatty acids.
the European Commission considers revising the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471) including maximum limits for glycidyl esters because refined vegetable oil, which can be used for manufacturing of mono‐ and di‐glycerides of fatty acids (E 471) is the only identified source of glycidyl esters of fatty acids, which are formed during deodorisation.
the European Commission considers revising the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471) including maximum limits for erucic acid because erucic acid can be present among the fatty acids in edible oils which can be used for manufacturing of mono‐ and di‐glycerides of fatty acids (E 471).
more data should be generated to decrease uncertainty arising from the from the occurrence of compounds of toxicological concern (e.g. 3‐monochloropropane‐1,2‐diol (3‐MCPD) or glycidyl esters), which can be produced under certain processing conditions from the food additive mono‐ and di‐glycerides of fatty acids (E 471).
1. Introduction
The present opinion deals with the re‐evaluation of mono‐ and di‐glycerides of fatty acids (E 471) when used as a food additive.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
Regulation (EC) No 1333/20081 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union. In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the European Union before 20 January 2009 has been set up under the Regulation (EU) No 257/20102. This Regulation also foresees that food additives are re‐evaluated whenever necessary in light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU3 of 2001. The report ‘Food additives in Europe 20004’ submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010 the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference
The Commission asks EFSA to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.1.3. Interpretation of Terms of Reference
The ANS Panel described its risk assessment paradigm in its Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012). This Guidance states, that in carrying out its risk assessments, the Panel sought to define a health‐based guidance value e.g. an Acceptable Daily Intake (ADI) (IPCS, 2004) applicable to the general population. According to the definition above, the ADI as established for the general population does not apply to infants below 12 weeks of age (JECFA, 1978; SCF, 1998). In this context, the re‐evaluation of the use of food additives in food for infants below 12 weeks represents a special case for which specific recommendations were given by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (JECFA, 1972, 1978), by the SCF (SCF, 1996, 1998) and EFSA (EFSA Scientific Committee, 2017). The Panel endorsed these recommendations.
In the current EU legislation (Annex II of Regulation (EC) No 1333/2008) use levels of additives in food for infants under the age of 12 weeks are included in categories 13.1.1, 13.1.5.1 and 13.1.5.2.5 The Panel considers that these uses would require a specific risk assessment in line with the recommendations given by JECFA, the SCF and the EFSA Scientific Committee, and endorsed by the Panel in its current Guidance for submission for food additives evaluations (EFSA ANS Panel, 2012). Therefore, a risk assessment as for the general population is not considered to be applicable for infants under the age of 12 weeks and will be performed separately.
Furthermore, this re‐evaluation refers exclusively to the uses of mono‐ and di‐glycerides of fatty acids (E 471) as a food additive in food and does not include a safety assessment of other uses of mono‐ and di‐glycerides of fatty acids.
1.2. Information on existing authorisations and evaluations
Mono‐ and di‐glycerides of fatty acids (E 471) is an authorised food additive in the EU according to Annex II and Annex III of Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/20126.
The food additive mono‐ and di‐glycerides of fatty acids (E 471) has only been evaluated by the SCF for its use in infant formulae, follow‐on formulae, weaning food and food for special medical purposes (FSMPs) for infants and young children. The SCF (1997) concluded that the use of mono‐ and di‐glycerides of fatty acids in nutrient preparations for use in infant formulae and follow‐on formulae is acceptable within the direct additive limit of 4 g/L and within the direct additive limit of 5 g/kg for use in weaning foods. In 1999, the SCF considered its use in FSMPs for infants and young children acceptable at levels up to 5 g/L (SCF, 1999). The Panel noted that E 471 has not been evaluated for its other authorised uses as a food additive in EU.
Mono‐ and di‐glycerides of fatty acids (E 471) were evaluated by JECFA in 1973 (JECFA, 1974) and an ADI ‘not limited’ was established.
2. Data and methodologies
2.1. Data
The Panel was not provided with a newly submitted dossier. EFSA launched public calls for data,7 , 8 to collect relevant information from interested parties.
The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up to September 2017. Attempts were made at retrieving relevant original study reports on which previous evaluations or reviews were based; however, not always these were available to the Panel.
Food consumption data used to estimate the dietary exposure to mono‐ and di‐glycerides of fatty acids (E 471) were derived from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database9).
The Mintel's Global New Products Database (GNPD) was used to check the use of mono‐ and di‐glycerides of fatty acids (E 471) in food products. Mintel's GNPD is an online database that contains the compulsory ingredient information present on the label of numerous food products.
2.2. Methodologies
This opinion was drafted following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and the relevant existing guidance documents from the EFSA Scientific Committee.
The ANS Panel assessed the safety of mono‐ and di‐glycerides of fatty acids (E 471), in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the Scientific Committee on Food (SCF, 2001).
When the test substance was administered in the feed or in the drinking water, but doses were not explicitly reported by the authors as mg/kg body weight (bw) per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012) for studies in rodents or, in the case of other animal species, by JECFA (2000). In these cases, the daily intake is expressed as ‘equivalent’.
Dietary exposure to mono‐ and di‐glycerides of fatty acids (E 471) from their use as food additives was estimated by combining the food consumption data available within the EFSA Comprehensive European Food Consumption Database with the maximum permitted levels (MPLs) and/or reported use levels submitted to EFSA following a call for data. Exposure was estimated according to different scenarios (see Section 3.4). Uncertainties in the exposure assessment were identified and discussed.
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
According to Commission Regulation (EU) No 231/2012, mono‐ and di‐glycerides of fatty acids (E 471) is defined as mono‐ and di‐glycerides of fatty acids consisting of mixtures of glycerol mono‐, di‐ and tri‐esters of fatty acids occurring in food oils and fats. It may contain small amounts of free fatty acids and glycerol. Based on this definition, E 471 is not a discrete chemical substance but a mixture. Depending upon the complexity of the fatty acid sources, E 471 may contain more than 50 different mono‐ and di‐glycerides in combination (EFEMA, 2016a (Documentation provided to EFSA n. 4)). CAS Registration number and EINECS numbers have not been assigned to E 471.
In chemical terms, monoglycerides (or monoacylglycerols) and diglycerides (or diacylglycerols) are defined as esters of the trihydroxy alcohol (glycerol) in which one or two of the hydroxyl groups are esterified with a long‐chain fatty acid. The Panel noted that although the chemical nomenclature of glycerides has been revised to one based on acylglycerols (Christie, 2011), terminology based on glycerides has been retained in regulatory practice.
The Panel noted that to avoid difficulties in the nomenclature of enantiomers of complex mixtures of natural acylglycerols, the IUPAC‐IUB recommended the ‘stereospecific numbering’ (sn)‐system (Christie, 2011). They can exist in three stereochemical forms (Christie, 2011).
The Panel also noted that in a Fischer projection of a natural l‐glycerol derivative, the secondary hydroxyl group is shown to the left of C‐2; the carbon atom above becomes C‐1 and the carbon atom below becomes C‐3 and the prefix ‘sn’ is placed before the root name of the compound (Christie, 2011).
Normally, the 1‐/3‐isomers of the monoacylglycerols are not distinguished from each other and are termed ‘α‐ or ‘α‐monoacylglycerols’, while the 2‐isomers are β‐monoacylglycerols (Christie, 2011). A racemic mixture of sn‐1,2‐ and 2,3‐diacylglycerols are sometimes termed α,β‐diacylglycerols, while sn‐1,3‐diacylglycerols may be designated ‘α,α‐ diacylglycerols (Christie, 2011).
In Figure 1, the general structure of monoacylglycerols and diacylglycerols is shown.
Figure 1.
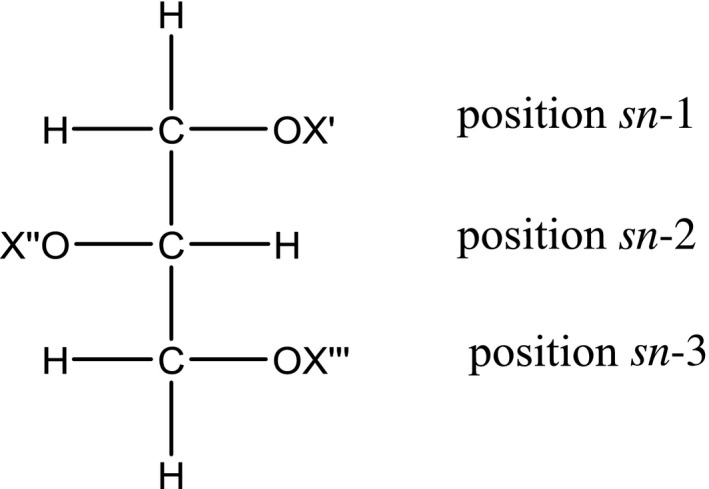
Stereospecific structure of mono‐ and di‐acylglycerols
In Table 1, the specific chemical structure of mono‐ and di‐glycerides of fatty acids (E 471) is listed where X′, X″ and X‴ represents either a hydrogen atom or a fatty acid as acyl moiety (FA).
Table 1.
Specific chemical structure of mono‐ and di‐glycerides of fatty acids (E 471)
| General chemical name | Stereospecific numbering | Specific chemical name | X′ | X″ | X‴ |
|---|---|---|---|---|---|
| Monoglycerides‐ | sn‐1‐monoacyl glycerol | α‐acylglycerol | FA | H | H |
| sn‐2‐monoacylglycerol | β‐acylglycerol | H | FA | H | |
| Diglycerides‐ | sn‐1,2 diacylglycerol | α‐,β‐acylglycerol | FA | H | FA’ |
| sn‐2,3 diacylglycerol | α‐,β‐acylglycerol | H | FA | FA | |
| sn‐1,3 diacylglycerol | α‐, α ‐acylglycerol | FA | FA | H |
FA: fatty acid as acyl.
A racemic mixture of sn‐1,2‐ and 2,3‐diacylglycerols are sometimes termed α,β‐diacylglycerols (Christie, 2011).
According to information provided by the industry, the following fatty acids can be present in the raw materials used for manufacturing the food additive E 471: caprylic (C8:0), capric (C10:0), lauric (C12:0), myristic (C14:0), palmitic (C16:0), stearic (C18:0) and oleic acids (C18:1), which can be present in the food additive E 570. However, also linoleic (C18:2), linolenic (C18:3), and in lesser amounts, palmitoleic (C16:1), margaroleic (C17:1), arachidic (C20:0) and margaric acids (C17:0) can be also present. Hydrogenation of naturally occurring food grade oils allows the double bonds (e.g. found in palmitoleic (C16:1), oleic (C18:1), linoleic (C18:2) and linolenic acids (C18:3)) to become fully saturated and converted into e.g. palmitic or stearic acids (for the three latter fatty acids), respectively (EFEMA, 2016a (Documentation provided to EFSA n. 4)). Although the fatty acid profiles of typical food fats and oils are predominantly in the range C12–C20, fatty acids with shorter (till C6) as well as longer (till C24) chain length are also common in fats and oils (EFEMA, 2016a [Documentation provided to EFSA n. 5]). During hydrogenation of naturally occurring fats and oils, physical properties may also be altered by the conversion of cis double bonds into the isomeric trans configuration (EFSA NDA Panel, 2004).
Source materials can be, among others, coconut, palm, palm kernel, soya, rapeseed (Canola), sunflower, cottonseed, corn, olive, tallow and lard. The Panel noted that the fatty acid moieties may be saturated or (cis‐ or trans‐) unsaturated.
The molecular weight of the food additive E 471 will be dependent on the fatty acid sources and the respective proportion of the mono‐ and di‐glycerides.
According to the Commission Regulation (EU) No 231/2012, mono‐ and di‐glycerides of fatty acids (E 471) vary from pale yellow to pale brown oily liquids to white or slightly off‐white hard waxy solids. The solids may be in form of flakes, powders or small beads.
Monoglycerides are polymorphic and can exist in different crystal forms depending on the temperature (Moonen and Bas, 2015). They are not insoluble in water but can form stable hydrated dispersions (Moonen and Bas, 2015) and are soluble in hot organic solvents (EFEMA, 2010a, (Documentation provided to EFSA n. 3)). When distilled monoglycerides are heated to their melting point with water, a gel is formed (Moonen and Bas, 2015).
According to Commission Regulation (EU) No 231/2012, the following glyceryl monostearate, glyceryl monopalmitate, glyceryl monooleate, monostearin, monopalmitin, monoolein and GMS (for glycerol monostearate) are synonyms of mono‐ and di‐glycerides of fatty acids (E 471). The Panel noted that these are not actual synonyms of the food additive E 471.
3.1.2. Specifications
Specifications for mono‐ and di‐glycerides of fatty acids (E 471) have been defined in Commission Regulation (EU) No 231/2012 and by JECFA (2006). The specifications are listed in Table 2.
Table 2.
Specifications of mono‐ and di‐glycerides of fatty acids (E 471) as food additive
| Commission Regulation (EU) No 231/2012a | JECFA (2006) | |
|---|---|---|
| Definition | Mono‐ and di‐glycerides of fatty acids consist of mixtures of glycerol mono‐, di‐ and tri‐esters of fatty acids occurring in food oils and fats. They may contain small amounts of free fatty acids and glycerol | A mixture of mono‐ and di‐glyceryl esters of long chain, saturated and unsaturated fatty acids that occur in food fats; contain not less than 30% of alpha‐monoglycerides and may also contain other isomeric monoglycerides, as well as di‐ and triglycerides, free glycerol, free fatty acids, soap and moisture; usually manufactured by the glycerolysis of edible fats and oils, but may also be prepared by esterification of fatty acids with glycerol, with or without molecular distillation of the product |
| Assay | Content of mono‐ and di‐esters: not less than 70% | – |
| Description | The product varies from a pale yellow to pale brown oily liquid to a white or slightly off‐white hard waxy solid. The solids may be in the form of flakes, powders or small beads | White or cream‐coloured hard fats of waxy appearance, plastic products or viscous liquids |
| Identification | ||
| Infrared absorption spectrum | Characteristic of a partial fatty acid ester of a polyol | The infrared spectrum of the sample is characteristic of a partial fatty acid ester of a polyol |
| Test for glycerol | Passes test | Passes test |
| Test for fatty acids | Passes test | Passes test |
| Solubility | Insoluble in water, soluble in ethanol and toluene at 50°C | Insoluble in water; soluble in ethanol, chloroform and benzene |
| Purity | ||
| Water content | Not more than 2% (Karl Fischer method) | Not more than 2.0% (Karl Fischer Method) |
| Acid value | Not more than 6 | Not more than 6 |
| Free glycerol | Not more than 7% | Not more than 7% |
| Polyglycerols | Not more than 4% diglycerol and not more than 1% higher polyglycerols both based on total glycerol content | – |
| Arsenic | Not more than 3 mg/kg | – |
| Lead | Not more than 2 mg/kg | Not more than 2 mg/kg |
| Mercury | Not more than 1 mg/kg | – |
| Cadmium | Not more than 1 mg/kg | – |
| Total glycerol | Not less than 16% and not more than 33% | – |
| Sulfated ash | Not more than 0.5% determined at 800 ± 25°C | – |
| Soap | – | Not more than 6%, calculated as a sodium oleate |
According to Commission Regulation (EU) No 231/2012, purity criteria apply to the additive free of sodium, potassium and calcium salts of fatty acids; however, these substances may be present up to a maximum level of 6% (expressed as sodium oleate).
The Panel noted that the JECFA definition described a more restricted group of mono‐ and di‐glycerides of fatty acids than the EU specifications for E 471.
According to industry (EFEMA, 2010a, 2016a (Documentation provided to EFSA n. 3 and 4]), there are three principal commercial grades of mono‐ and di‐glycerides which may be nominally described as 40%, 60% and 90% monoglycerides. Other grades with monoglyceride proportions between these limits may also be offered. Their typical composition is shown in Table 3.
Table 3.
Composition (% by weight) of different commercial grades of mono‐ and di‐glycerides of fatty acids (E 471) (EFEMA, 2010a, 2016a (Documentation provided to EFSA no 3 and 4))
| Grade | Glycerol | Others | Monoglycerides | Diglycerides | Triglycerides |
|---|---|---|---|---|---|
| 40% | 4 | 2 | 42 | 44 | 8 |
| 60% | 1 | 2 | 60 | 32 | 5 |
| 90% | 1 | 2 | 93 | 4 | 0 |
The Panel noted that, according to the EU specifications for E 471, impurities of the toxic elements arsenic, cadmium, lead and mercury are accepted up to concentrations of 3, 1, 2 and 1 mg/kg, respectively. Contamination at these levels could have a significant impact on exposure to these toxic elements, which are already close to the health‐based guidance values or benchmark doses (lower confidence limits) established by EFSA (EFSA CONTAM Panel, 2009a,b, 2010, 2012a,b,c, 2014).
According to the available information on the manufacturing process (Section 3.1.3), mono‐ and di‐glycerides of fatty acids (E 471) can be manufactured by direct esterification of glycerol with fatty acids.
Information on the manufacturing processes of glycerol has been considered by the ANS Panel in the re‐evaluation of glycerol (E 422) (EFSA ANS Panel, 2017a). The Panel noted that glycerol (E 422) can be produced by a variety of methods and that many of them lead to the presence or formation of contaminants, which are of toxicological concern. The Panel considered that the manufacturing process for mono‐ and di‐glycerides of fatty acids (E 471) should not allow the presence of residuals of genotoxic or/and carcinogenic concern at a level which would result in a margin of exposure below 10,000. The Panel considered that maximum limits for potential impurities in glycerol as raw material in the manufacturing process of mono‐ and di‐glycerides of fatty acids should also be established for the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471).
The Panel also noted that a maximum residual level for 3‐monochloropropane‐1,2‐diol (3‐MCPD) (not more than 0.1 mg/kg) has been established in EU specifications for glycerol (E 422) (Commission Regulation (EU) No 231/2012); however, there is no limit for 3‐MCPD in the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471).
The Panel noted that epichlorohydrin may be present in mono‐ and di‐glycerides of fatty acids (E 471) from the manufacturing process of glycerol as well as glycidol, which can also be used as starting material for the manufacturing process of monoglycerides of fatty esters. The Panel considered that the presence of epichlorohydrin and/or glycidol in mono‐ and di‐glycerides of fatty acids (E 471) would need further assessment as their presence could raise a safety concern.
According to the available information on the manufacturing process (Section 3.1.3), mono‐ and di‐glycerides of fatty acids (E 471) can be manufactured by glycerolysis of natural or hydrogenated fats and/or oils. According to the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), industrial hydrogenation (used to produce semi‐solid and solid fats that can be used for the production of foods such as margarines, shortenings and biscuits) and deodorisation (a necessary step in refining) of unsaturated vegetable oils high in polyunsaturated fatty acids is one of the three main pathways for the formation of trans fatty acids in food (EFSA NDA Panel, 2004). According to the EFSA NDA Panel, higher intakes of trans fatty acids have consistently been found to be associated with an increased risk of coronary heart disease and it was recommended that trans fatty acids intake should be as low as possible within the context of a nutritionally adequate diet (EFSA NDA Panel, 2010). The Panel noted that there is no limit for trans fatty acids in the specifications for mono‐ and di‐glycerides of fatty acids (E 471).
According to EFSA Panel on Contaminants in Food Chain (CONTAM), refined vegetable oil, which can be used for manufacturing of mono‐ and di‐glycerides of fatty acids (E 471), is the only identified source of glycidyl esters of fatty acids (EFSA CONTAM Panel, 2016). Glycidyl esters of fatty acids are hydrolysed in the gastrointestinal tract to produce free glycidol, which is classified as probably carcinogenic to humans 2A (IARC, 2000; BfR, 2009) and as a carcinogenic and genotoxic compound by the EFSA CONTAM Panel (EFSA CONTAM Panel, 2016). The Panel noted that there is no limit for glycidyl esters in the specifications for mono‐ and di‐glycerides of fatty acids (E 471). The Panel considered that the possible presence of glycidol in mono‐ and di‐glycerides of fatty acids (E 471) would need further assessment as their presence could raise a safety concern.
Rapeseed oil which contains erucic acid can be used for the manufacturing of mono‐ and di‐glycerides of fatty acids (E 471). According to the industry, only rapeseed oil low in erucic acid is used (EFEMA, 2016a (Documentation provided to EFSA n. 4)). Nevertheless, it cannot be excluded that high erucic acid rapeseed oil can be used. Maximum limits for erucic acid have been established in EU according to Commission Regulation (EU) No 696/201410 in edible oils and fats as well as in food containing fats and oils. A tolerable daily intake (TDI) of 7 mg/kg bw per day for erucic acid has been established by the EFSA CONTAM Panel based on a no observed adverse effect level (NOAEL) of 700 mg/kg bw per day for myocardial lipidosis observed in a 7‐day feeding study in young (5–7 weeks) rats and in a 2‐week feeding study in newborn piglets (EFSA CONTAM Panel, 2016). The Panel noted that there are no limits for erucic acid in the current EU specifications for mono‐ and di‐glycerides of fatty acids (E 471).
3.1.3. Manufacturing process
Information provided by the industry
According to information provided by industry (EFEMA, 2010a (Documentation provided to EFSA n. 3), EFEMA, 2016a (Documentation provided to EFSA n. 4)), mono‐ and di‐glycerides (E 471) is generally manufactured in two ways.
-
i
Transesterification process
In the transesterification process, natural or hydrogenated fats/oils react with glycerol. The fats/oils can be derived from one single source or may consist of a blend of fats and oils from different sources in order to achieve the desired fatty acid profile.
-
ii
Direct esterification process
Mono‐ and di‐glycerides of fatty acids (E 471) can also be manufactured by direct esterification of fatty acids with glycerol. The fatty acids used in this process are obtained from food fats and oils by hydrolysis. The edible commercial fatty acids obtained by hydrolysis usually contain associated fatty acids in varying amounts depending on the source of the fatty acids. When direct esterification is used to produce glycerides containing specific fatty acids, the hydrolysed oils are subjected to distillation or fractionation/crystallisation prior to esterification in order to obtain a concentrated fraction of the desired fatty acid.
Trans‐ and direct esterification can be run either batchwise or continuous
According to Fischer (1998 (Documentation provided to EFSA No 10)), it is necessary to deactivate the catalyst (sodium, potassium or calcium salts of fatty acids) used in both main manufacturing methods. The deactivation is performed by adding phosphoric acid. The acid is removed from the mixture together with the insoluble portion of glycerol.
In cases where the increased reaction temperature of the main processes may lead to an oxidation of highly unsaturated fatty acids, methods of synthesis of mono‐ and di‐glycerides by enzyme (lipase) catalysed selective hydrolysis of oils in a suitable solvent have been reported. Typical solvents include short chain hindered alcohols, e.g. tert‐butanol or tert‐pentanol (EFEMA, 2016a, (Documentation provided to EFSA No 4)). The Panel noted that no maximum limits for residual solvents have been established in EU specifications for E 471.
Overview of the literature
Transesterification process
Glycerolysis occurs at high temperature (200–250°C) under alkaline catalysis (Ca(OH)2 or NaOH as catalysts), yielding a mixture of mono‐, di‐ and triglycerides together with a small quantity of unreacted glycerol. Subsequently, the catalyst is neutralised and excess of glycerol is removed (Hasenhuettl, 2008; Moonen and Bas, 2015). Other catalysts (hydrotalcite loaded with K2CO3) can be used to yield mainly monoacylglycerols (Zhang et al., 2015).
In the literature, it is indicated that mono‐ and di‐glycerides of fatty acids (E 471) can also be manufactured by transesterification of oils and fats in supercritical methanol. The final product is purified via supercritical CO2 (Soto et al., 2014). Ethanolysis can also be used as a methanol substitute (Wang et al., 2014). A method to produce monoacylglycerols via glycerolysis by using methyl esters of fatty acids, which are produced during manufacturing of biodiesel coming from linseed oil has also been reported (Schultz et al., 2011).
An enzymatic method to produce structured diacylglycerols containing ω‐3 fatty acids via enzyme‐catalysed glycerolysis of fish oil, using a lipase from Candida antarctica, (Novozym 435) was described by Miranda et al. (2013); the same enzyme was used by Zha et al. (2014) to produce glycerol monolaurate‐enriched monoacylglycerols via a lipase‐catalysed glycerolysis of coconut oil; another enzymatic method to produce diglycerides was described by Chang et al. (2014). However, the Panel noted that it is not clear whether these methods are used to manufacture a substance used as the food additive E 471.
Direct esterification process
In the literature, processes of direct esterification for the manufacturing of mono‐ and di‐glycerides of fatty acids are described. In these processes, fatty acids, glycerol and a catalyst (either acids or bases) are stirred at 100–230°C. Water is continuously removed by distillation. When the reaction is completed, the catalyst is neutralised and excess glycerol is removed by distillation (Hasenhuettl, 2008).
Enzyme‐catalysed esterification methods for the manufacturing of mono‐ and di‐glycerides of fatty acids have been described in the literature (Dordick, 1989; Waldinger and Schneider, 1996; Hari Krishna and Karanth, 2002; Monteiro et al., 2003; Fregolente et al., 2010; Fernandes et al., 2012; Naik et al., 2014; Von der Haar et al., 2015; Vázquez et al., 2016). A lipase is used to catalyse an inverse‐hydrolysis reaction of glycerol and fatty acids yielding selected isomeric configurations of mono‐ and di‐glycerides and less by‐products than in a non‐enzymatic reaction (Akoh, 1993; Rosu et al., 1999). A further solvent extraction can lead to a product of higher purity concerning one constituent, i.e. dicaprin (Sánchez et al., 2014). Alternatively, glycerol can be replaced by 1,2‐acetonide glycerol to yield 1‐monoglyceryl esters of fatty acids (Wang et al., 2013) or the fatty acids (e.g. oleic acid) can be replaced by vinyl fatty acids (vinyloleate) (Wang et al., 2015).
Mostafa et al. (2013) described a method for the use of waste oils from edible oil refinement as source materials for the production of mono‐, di‐ and tri‐glycerides. According to the authors, free fatty acids coming from waste oils can be esterified with glycerol to yield the food additive E 471.
Zlatanos et al. (1985) proposed a manufacturing process for monoglycerides of fatty esters intended to be used either as a food additive or as an emulsifier for polymers. This method appears to yield a product of high purity, without the need of any further purification, where glycidol is used as starting material. No information has been provided to the Panel about the possible use of this method by the manufacturers of food additives.
The strategies currently applied for the manufacturing of the food additive E 471 rich in monoacylglycerols have been described by Zhong et al. (2014).
Purification
After the manufacturing procedure, monoglycerides of fatty acids may be purified by separation from di‐ and tri‐acylglycerides via high‐vacuum distillation and low temperatures (140–170°C) (Moonen and Bas, 2015). Typical distilled monoglycerides produced this way contain 0.5–1% free glycerol, 0.5–1% free fatty acid, 95% monoglycerides and 3–4% diglycerides (EFEMA, 2010a, 2016 (Documentation provided to EFSA n. 3 and 4)). Monoacylglycerides can also be purified by using a combination of flash chromatography with an acetone/hexane binary gradient (Compton et al., 2013).
3.1.4. Methods of analysis in food
In order to determine mono‐ and di‐glycerides of fatty acids (E 471) in final food products must first be extracted. The analytical methods available cannot differentiate between added mono‐ and di‐glycerides (E 471) and the ones of natural occurrence. They can be isolated from fat by a silica gel column chromatography using solvents of different polarity (IUPAC method 2.321) (Paquot and Hautfenne, 1987), from pastry by extraction with butanol (Schmid and Otteneder, 1976), from bakery wares by thin‐layer chromatography (TLC) (isolation and identification) (Kanematsu et al., 1972; Regula, 1975) or from mixtures of flour treatment agents after an extraction with dichloromethane (Regula, 1975). 1‐Monoglycerides can be determined by oxidation with a periodic acid solution applying IUPAC method 2.322 (Paquot and Hautfenne, 1987). Gernert (1968) described the unspecific qualitative analysis of E 471 in dairy products by TLC after extraction from fat with organic solvents (chloroform or dichloromethane) following an alkali digestion of proteins. The separated spots were detected by spraying with 2′,7′‐dichlorofluorescein. Dhara and Singhai (2014) used high‐performance thin‐layer chromatography (HPTLC) to quantify diglycerides of fatty acids in soya oil. The total content of mono‐ and di‐glycerides of fatty acids could be measured by infrared spectroscopy (Murlykina et al., 2015).
Gas chromatography (GC) analysis with flame ionisation detector (FID) accomplished by silylation of the mono‐ and di‐glycerides of fatty acids with trimethylchlorosilane (TMCS) and N,N‐bis(trimethylsilyl)trifluoroacetamide (BSTFA) in the presence of pyridine can be used for the quantification of E 471 (IUPAC method 6.002 (ex 2.326)) (Sahasrabudhe and Legari, 1968; Soe, 1983; Brüschweiler and Dieffenbacher, 1991; Dieffenbacher and Pocklington, 1992). High‐temperature gas–liquid chromatography has also been applied (Pacheco et al., 2014) for measuring mono‐ and di‐glycerides of fatty acids in edible oils, but no information on limits of detection was given. Various gas chromatography–mass spectrometry (GC–MS) methods have been developed for the quantification of diglycerides of fatty acids after derivatisation in edible oils (Zhu et al., 2013) or of monoglycerides of saturated fatty acids in biogas oil with a limit of detection of 8–20 mg/kg (Hirshegger et al., 2014).
Analysis of mono‐ and di‐glycerides in total can be performed by normal phase high‐performance liquid chromatography (HPLC) with an evaporative light scattering detector (ELSD) (Liu et al., 1993; Berner and Dieffenbacher, 1999). Marcato and Cecchin (1996) used HPLC with an ELSD detector to analyse the composition of an antistatic agent similar to the food additive mono‐ and di‐glycerides of fatty acids (E 471). Torres et al. (2005) applied this method and reported a limit of detection of 1 μg per injection. A reversed‐phase HPLC analytical method with UV detection has been reported only for the analysis of a pure food additive called ‘distilled monoglycerides’ (Sudraud et al., 1981).
More recent approaches use bi‐dimensional gas chromatography coupled with time‐of‐flight mass spectrometry (GC x GC‐TOF‐MS) for characterisation of mono‐ and di‐glycerides from fats (Indrasti et al., 2010), or liquid chromatography/atmospheric‐pressure chemical ionisation mass spectrometry (LC–APCI‐MS) for the quantitative determination of food emulsifiers composed of mono‐ and di‐glycerides of fatty acids in complex food matrices (Suman et al., 2009).
Boon‐Seang and Kornél (2013) presented a liquid chromatography–mass spectrometry (LC–MS) method for the determination of monoglycerides of fatty acids in a triglyceride (triolein). Recoveries varied between 76% and 114%. No food samples have been tested. Ultra‐performance convergence chromatography combined with a quadrupole time‐of‐flight mass spectrometry has been applied to measure diglycerides of fatty acids in cow milk fat (Zhou et al., 2014).
Nuclear magnetic resonance (NMR) methods have been published for the determination of molar concentrations of mono‐ and di‐glycerides of fatty acids in the food additive as such (Fernandes et al., 2012), in margarines (Sopelana et al., 2013) or in oils (Nieva‐Echevarría et al., 2014).
3.1.5. Stability of the substance and reaction and fate in food
According to Fischer (1998 (Documentation provided to EFSA n. 10)), during storage, some β‐monoglycerides will be converted into α‐monoglycerides.
In model food heating systems containing water, sodium chloride and glycerol or lipid precursors (mono‐ and di‐glycerides of fatty acids), 3‐MCPD production increased with increasing temperature once above 160°C and with NaCl concentration up to 10% with acylglycerol precursors. The optimum water content was 15–20% for 3‐MCPD. Monoglycerides of fatty acids were significantly better precursors than diglycerides of fatty acids. Baked goods are the major source of 3‐MCPD and the formation of this contaminant in bakery systems has been studied in some detail in model systems (Hamlet et al., 2003, 2004a,b), and mainly, monoglycerides are thought to participate in its formation.
Glycidyl esters are formed mainly from diglycerides of fatty acids on heating vegetable oils to temperatures above 200°C (Masukawa et al., 2010; Hrncirik and van Duijn, 2011; Craft et al., 2012; Destaillats et al., 2012). Glycidyl esters can also be formed by the dehydration of monoglycerides (Craft et al., 2012). The formation mechanism from diglycerides of fatty acids is likely to proceed via an acyloxonium ion or an intramolecular sn‐2 reaction (Hamlet et al., 2002; Weißhaar and Perz, 2010; Rahn and Yaylayan, 2011; Destaillats et al., 2012). Cheng et al. (2016) have confirmed these findings when testing real edible oils, but they noted that the amounts of glycidyl esters measured after heating at temperatures higher than 200°C were lower (1.6 mg/kg at the maximum) compared to the ones measured during the deodorisation process of the oils (3.1 mg/kg at the maximum). They have demonstrated that the higher concentrations correlate mainly with the monoacylglycerol content of the oils.
The food additive E 471 contains esters of a polyol (triol) and hence shows reactions through rearrangement, inter‐ and intramolecular migration of acylic groups and sensitivity to hydrolysis. If unsaturated fatty acids are present in the molecule, the food additive is susceptible to auto‐oxidation (Moonen and Bas, 2015). Auto‐oxidation of fatty acids leads to the formation of hydroperoxides, which decompose to oxygen‐containing products such as aldehydes, ketones and hydroxy compounds. The effect of atmospheric oxygen on fatty acids depends primarily on the temperature, the number of double bonds in the fatty acid and the molecular structure of the fatty acid. Saturated fatty acids show little tendency to undergo autoxidation (Anneken et al., 2012). Kristensen et al. (2006) have demonstrated in a storage study that diacylglycerol oil coming from sunflower oil was oxidatively less stable than sunflower oil having a similar composition of fatty acids. Wang et al. (2010) have compared the oxidative stability of a diacylglycerol oil‐rich soybean oil with palmolein having a completely different composition of fatty acids and demonstrated that palmolein was more stable. Qi et al. (2015) have compared the oxidative stability between diacylglycerol oil and soybean oil having a different composition of fatty acids. They have demonstrated that diacylglycerides isolated from diacylglycerol oil of commerce are more vulnerable to an accelerated oxidation compared to triglycerides isolated from soybean oil. The authors stressed that these results occurred due to the initially different fatty acid composition of the two oils. It has been found that partial hydrogenation to change the fatty acid composition can effectively reduce the oxidation of mono‐ and di‐glycerides of fatty acids coming from corn oil (Zhang et al., 2015).
According to Caponio et al. (2013), the ratio between the total (1,2‐ and 1,3‐)diglycerides of fatty acids and 1,3‐diglycerides of fatty acids is not affected by either oil or storage conditions but only to the storage period. Storage of extra virgin olive oil containing diglycerides of fatty acids at a temperature of 20°C with exposure to light has led to a lower ratio of 1,2/1,3‐diglycerides compared to storage of 4–6°C in the dark for a period of 10–14 months (Ayyad et al., 2015). Nieva‐Echevarría et al. (2015) concluded that after storage of sunflower oil or of minced meat, the main hydrolysis products were 1,2‐diglycerides and 2‐monoglycerides; only small amounts of 1,3‐diglycerides and 1‐monoglycerides were found.
In another study with monopalmitin, it has been demonstrated that monopalmitin is a precursor of the formation of 3‐MCPD esters, when heated for more than 1 h at temperatures between 110°C and 260°C together with 0.5% water and 0.5% chlorides (Hamlet et al., 2014).
3.2. Authorised uses and use levels
Maximum levels of mono‐ and di‐glycerides of fatty acids (E 471) have been defined in Annex II to Regulation (EC) No 1333/2008 on food additives, as amended. In this opinion, these levels are named MPLs.
Currently, mono‐ and di‐glycerides of fatty acids (E 471) is authorised as a food additive in the EU at quantum satis (QS) in 77 food categories (FCs) (Table 4). Seven food categories have a numerical MPL. Mono‐ and di‐glycerides of fatty acids (E 471) is included in the Group I of food additives.
Table 4.
MPLs of mono‐ and di‐glycerides of fatty acids (E 471) in foods according to the Annex II to Regulation (EC) No 1333/2008
| Food category number | Food category name | E‐number/group | Restrictions/exception | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|---|
| 01.3 | Unflavoured fermented milk products, heat‐treated after fermentation | Group I | Quantum satis | |
| 01.4 | Flavoured fermented milk products including heat‐treated products | Group I | Quantum satis | |
| 01.6.1 | Unflavoured pasteurised cream (excluding reduced fat creams) | E 471 | Quantum satis | |
| 01.6.2 | Unflavoured live fermented cream products and substitute products with a fat content of less than 20% | E 471 | Quantum satis | |
| 01.6.3 | Other creams | Group I | Quantum satis | |
| 01.7.1 | Unripened cheese (excluding products falling in category 16) | Group I | Except mozzarella | Quantum satis |
| 01.7.5 | Processed cheese | Group I | Quantum satis | |
| 01.7.6 | Cheese products (excluding products falling in category 16) | Group I | Quantum satis | |
| 01.8 | Dairy analogues, including beverage whiteners | Group I | Quantum satis | |
| 02.1 | Fats and oils essentially free from water (excluding anhydrous milkfat) | E 471 | Except virgin oils and olive oils | 10,000 |
| 02.2.2 | Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions | Group I | Quantum satis | |
| 02.3 | Vegetable oil pan spray | Group I | Quantum satis | |
| 03 | Edible ices | Group I | Quantum satis | |
| 04.2.1 | Dried fruit and vegetables | Group I | Quantum satis | |
| 04.2.2 | Fruit and vegetables in vinegar, oil or brine | Group I | Quantum satis | |
| 04.2.4.1 | Fruit and vegetable preparations excluding compote | Group I | Quantum satis | |
| 04.2.5.1 | Extra jam and extra jelly as defined by Directive 2001/113/EC | E 471 | Quantum satis | |
| 04.2.5.2 | Jam, jellies and marmalades and sweetened chestnut purée as defined by Directive 2001/113/EC | E 471 | Quantum satis | |
| 04.2.5.3 | Other similar fruit or vegetable spreads | E 471 | Quantum satis | |
| 04.2.5.4 | Nut butters and nut spreads | Group I | Quantum satis | |
| 04.2.6 | Processed potato products | Group I | Quantum satis | |
| 05.1 | Cocoa and Chocolate products as covered by Directive 2000/36/EC | E 471 | Quantum satis | |
| 05.2 | Other confectionery including breath freshening microsweets | Group I | Quantum satis | |
| 05.3 | Chewing gum | Group I | Quantum satis | |
| 05.4 | Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | Group I | Quantum satis | |
| 06.2.2 | Starches | Group I | Quantum satis | |
| 06.3 | Breakfast cereals | Group I | Quantum satis | |
| 06.4.1 | Fresh pasta | E 471 | Quantum satis | |
| 06.4.2 | Dry pasta | Group I | Only gluten free and/or pasta intended for hypoproteic diets in accordance with Directive 2009/39/EC | Quantum satis |
| 06.4.3 | Fresh precooked pasta | E 471 | Quantum satis | |
| 06.4.4 | Potato gnocchi | E 471 | Only fresh refrigerated potato gnocchi | Quantum satis |
| 06.4.5 | Fillings of stuffed pasta (ravioli and similar) | Group I | Quantum satis | |
| 06.5 | Noodles | Group I | Quantum satis | |
| 06.6 | Batters | Group I | Quantum satis | |
| 06.7 | Precooked or processed cereals | E 471 | Only quick‐cook rice | Quantum satis |
| 07.1 | Bread and rolls | Group I | Except products in 7.1.1 and 7.1.2 | Quantum satis |
| 07.1.1 | Bread prepared solely with the following ingredients: wheat flour, water, yeast or leaven, salt | E 471 | Quantum satis | |
| 07.1.2 | Pain courant français; Friss búzakenyér, fehér és félbarna kenyerek | E 471 | Quantum satis | |
| 07.2 | Fine bakery wares | Group I | Quantum satis | |
| 08.3.1 | Non‐heat‐treated processed meat | Group I | Quantum satis | |
| 08.3.2 | Heat‐treated processed meat | Group I | Except foie gras, foie gras entier, blocs de foie gras, Libamáj, libamáj egészben, libamáj tömbben | Quantum satis |
| 08.3.3 | Casings and coatings and decorations for meat | Group I | Quantum satis | |
| 09.2 | Processed fish and fishery products including molluscs and crustaceans | Group I | Quantum satis | |
| 09.3 | Fish roe | Group I | Only processed fish roe | Quantum satis |
| 10.2 | Processed eggs and egg products | Group I | Quantum satis | |
| 11.2 | Other sugars and syrups | Group I | Quantum satis | |
| 11.4.3 | Tabletop sweeteners in tablets | E 471 | Quantum satis | |
| 12.1.2 | Salt substitutes | Group I | Quantum satis | |
| 12.2.2 | Seasonings and condiments | Group I | Quantum satis | |
| 12.3 | Vinegars | Group I | Quantum satis | |
| 12.4 | Mustard | Group I | Quantum satis | |
| 12.5 | Soups and broths | Group I | Quantum satis | |
| 12.6 | Sauces | Group I | Quantum satis | |
| 12.7 | Salads and savoury‐based sandwich spreads | Group I | Quantum satis | |
| 12.8 | Yeast and yeast products | Group I | Quantum satis | |
| 12.9 | Protein products, excluding products covered in category 1.8 | Group I | Quantum satis | |
| 13.1.1 | Infant formulae as defined by Directive 2006/141/EC | E 471 | 4,000a | |
| 13.1.2 | Follow‐on formulae as defined by Directive 2006/141/EC | E 471 | 4,000a | |
| 13.1.3 | Processed cereal‐based foods and baby foods for infants and young children as defined by Directive 2006/125/EC | E 471 | Only biscuits and rusks, cereal‐based foods, baby foods | 5,000b |
| 13.1.4 | Other foods for young children | E 471 | Only when sold as powder | 4,000a |
| 13.1.5.1 | Dietary foods for infants for special medical purposes and special formulae for infants | E 471 | From birth onwards in specialised diets, particularly those devoid of proteins | 5,000 |
| 13.1.5.2 | Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC | E 471 | From birth onwards in specialised diets, particularly those devoid of proteins | 5,000 |
| 13.2 | Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5) | Group I | Quantum satis | |
| 13.3 | Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet) | Group I | Quantum satis | |
| 13.4 | Foods suitable for people intolerant to gluten as defined by Regulation (EC) No 41/2009 | Group I | Including dry pasta | Quantum satis |
| 14.1.2 | Fruit juices as defined by Directive 2001/112/EC and vegetable juices | Group I | Only vegetable juices | Quantum satis |
| 14.1.3 | Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products | Group I | Only vegetable nectars | Quantum satis |
| 14.1.4 | Flavoured drinks | Group I | Quantum satis | |
| 14.1.5.2 | Other | Group I | Excluding unflavoured leaf tea; including flavoured instant coffee | Quantum satis |
| 14.2.3 | Cider and perry | Group I | Quantum satis | |
| 14.2.4 | Fruit wine and made wine | Group I | Quantum satis | |
| 14.2.5 | Mead | Group I | Quantum satis | |
| 14.2.6 | Spirit drinks as defined in Regulation (EC) No 110/2008 | Group I | Except whisky or whiskey | Quantum satis |
| 14.2.7.1 | Aromatised wines | Group I | Quantum satis | |
| 14.2.7.2 | Aromatised wine‐based drinks | Group I | Quantum satis | |
| 14.2.7.3 | Aromatised wine‐product cocktails | Group I | Quantum satis | |
| 14.2.8 | Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol | Group I | Quantum satis | |
| 15.1 | Potato‐, cereal‐, flour‐ or starch‐based snacks | Group I | Quantum satis | |
| 15.2 | Processed nuts | Group I | Quantum satis | |
| 16 | Desserts excluding products covered in categories 1, 3 and 4 | Group I | Quantum satis | |
| 17.1c | Food supplements supplied in a solid form including capsules and tablets and similar forms, excluding chewable forms | Group I | Quantum satis | |
| 17.2c | Food supplements supplied in a liquid form | Group I | Quantum satis | |
| 17.3c | Food supplements supplied in a syrup‐type or chewable form | Group I | Quantum satis | |
| 18 | Processed foods not covered by categories 1–17, excluding foods for infants and young children | Group I | Quantum satis |
MPL: maximum permitted level.
If more than one of the substances E 322, E 471, E 472c and E 473 are added to a foodstuff, the maximum level established for that foodstuff for each of those substances is lowered with that relative part as is present of the other substances together in that foodstuff.
E 471, E 472a, E 472b and E 472c are authorised individually or in combination.
FCs 17 refers to food supplements as defined in Directive 2002/46/EC of the European Parliament and of the Council excluding food supplements for infants and young children.
Table 4 summarises the food categories that are permitted to contain mono‐ and di‐glycerides of fatty acids (E 471) and the corresponding MPLs as set by Annex II to Regulation (EC) No 1333/2008.
According to Annex III, Part 1, of Regulation (EC) No 1333/2008, mono‐ and di‐glycerides of fatty acids (E 471) is also authorised as carrier in food additives in colours and fat‐soluble antioxidants and glazing agents for fruit with a maximum level at QS.
According to Annex III, Part 2, Part 3, Part 4 and Part 5 (Section A) of Regulation (EC) No 1333/2008, mono‐ and di‐glycerides of fatty acids (E 471) is also authorised as a food additive in food additives with a maximum level in all food additives preparations at QS, in food enzymes with a maximum level in the products (final food and beverages) at QS, in food flavourings with a maximum level in all flavourings at QS and in all nutrients with a maximum level in the products at QS.
In addition, according to Annex III, Part 5 (Section B) of Regulation (EC) No 1333/2008, mono‐ and di‐glycerides of fatty acids (E 471) is also authorised as food additive in nutrients in foods for infants and young children. It is authorised only for uses in nutrient preparations under the condition that the maximum level in foods mentioned in point 13.1 of Part E of Annex II is not exceeded and the conditions of use specified therein are respected.
3.3. Exposure data
3.3.1. Reported use levels of mono‐ and di‐glycerides of fatty acids (E 471)
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment, especially for those food additives for which no MPL is set and which are authorised according to QS as is the case for mono‐ and di‐glycerides of fatty acids (E 471).
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued a public call,7 , 8 for occurrence data (usage level and/or concentration data) on mono‐ and di‐glycerides of fatty acids (E 471). In response to this public call, information on use levels of mono‐ and di‐glycerides of fatty acids (E 471) in foods was made available to EFSA by industry.
No analytical data on the concentration of mono‐ and di‐glycerides of fatty acids (E 471) in foods were made available by the EU Member States.
Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with data on use levels (n = 1,024) of mono‐ and di‐glycerides of fatty acids (E 471) in foods for 46 out of the 84 food categories in which mono‐ and di‐glycerides of fatty acids (E 471) is authorised. Data were made available by Riemser Arzneimittel AG (2010), Mars (2010), AVIKO (2016), Association of the European Self‐Medication Industry (AESGP, 2016), European Dairy Association (EDA, 2016), European Food Emulsifiers Manufacturers Association (EFEMA, 2016c), European federation of Associations of Health Products Manufacturers (EHPM, 2016), Food Drink Europe (FDE, 2016), International Chewing Gum Association (ICGA, 2016), KRÜGER GmbH & Co. (KRUEGER, 2016) and Specialised Nutrition Europe (SNE, 2016).
The Panel noted that 121 usage levels of 14 food categories referred to niche products. Where other usage levels were available for the given food category, the Panel decided to exclude these use levels from further analysis in the refined exposure scenarios. Only niche products of FC 6.3 Breakfast cereals, FC 13.1.5.1 dietary foods for infants for special medical purposes and special formulae for infants and FC 13.1.5.2 dietary foods for babies and young children for special medical purposes were considered in the refined exposure scenarios and in the specific foods for special medical purposes (FSMP) scenario (see Section 3.4.3) as for these categories, no other data were reported.
The Panel noted that one of the data providers (EFEMA) is not a food industry company using additives in its food products but an association representing food additive producers. Usage levels reported by food additive producers are not considered at the same level as those provided by food industry. Food additive producers might recommend usage levels to the food industry, but the final levels used might, ultimately, be different. Because EFEMA did not confirm that the recommended levels are used by food industry, they were not considered in the refined exposure scenarios. Data from food additive producers were only used in the regulatory maximum level exposure assessment scenario in case of QS authorisation when no data were available from the food industry.
Appendix A provides data on the use levels of mono‐ and di‐glycerides of fatty acids (E 471) in foods as reported by industry.
3.3.2. Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information of over 2 million food and beverage products of which more than 900,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 20 out of its 28 member countries and Norway presented in the Mintel's GNPD.11
For the purpose of this Scientific Opinion, the Mintel's GNPD12 was used for checking the labelling of food and beverage products including food supplements for mono‐ and di‐glycerides of fatty acids (E 471) within the EU's food market as the database contains the compulsory ingredient information on the label.
Appendix B lists the number and percentage of the food and beverage products including food supplements labelled with mono‐ and di‐glycerides of fatty acids (E 471) between 2012 and 2017, out of the total number of food products per food subcategory according to the Mintel's GNPD food classification.
In total, mono‐ and di‐glycerides of fatty acids (E 471) was labelled on 33,090 food and beverage products as an ingredient, mainly in chilled desserts, edible ices, bread and bread products, sandwiches/wraps, baking ingredients and mixes, pasta (including fresh and dry pasta, gnocchi and stuffed pasta) and margarines. The percentages of food and beverage products per food subcategory labelled with mono‐ and di‐glycerides of fatty acids (E 471) ranged from less than 0.1% to about 75% in Mintel's GNPD food subcategory ‘dairy based ice cream & frozen yoghurt’. The overall average percentage of foods labelled to contain mono‐ and di‐glycerides of fatty acids (E 471) was 7%.
According to the Mintel's GNPD, mono‐ and di‐glycerides of fatty acids (E 471) is also used in food and beverage products in the following food categories, while for them, no information on usage levels was available:
1.7.1 Unripened cheese excluding products falling in category 16
6.4.1 Fresh pasta
6.4.2 Dry pasta
6.4.3 Fresh precooked pasta
6.4.4 Potato Gnocchi
6.7 Precooked or processed cereals
9.2 Processed fish and fishery products including molluscs and crustaceans
10.2 Processed eggs and egg products
12.7 Salads and savoury‐based sandwich spreads
12.9 Protein products, excluding products covered in category 1.8
12.2.2 Seasonings and condiments
14.2.6 Spirit drinks as defined in Regulation (EC) No 110/2008
14.2.8 Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol
Neglecting foods belonging to these food categories in the exposure assessment may have resulted in an underestimation of the exposure.
3.3.3. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). New consumption surveys added to the comprehensive database were also taken into account in this assessment.9
The food consumption data gathered by EFSA were collected by different methodologies, and thus, direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database represents the best available source of food consumption data across Europe at present.
Food consumption data from the following population groups were used for the exposure assessment: infants, toddlers, children, adolescents, adults and the elderly. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 5).
Table 5.
Population groups considered for the exposure estimates of mono‐ and di‐glycerides of fatty acids (E 471)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlers | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrena | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlya | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Sweden, UK |
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categories as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure estimates.
Food categories considered for the exposure assessment of mono‐ and di‐glycerides of fatty acids (E 471)
The food categories in which the use of mono‐ and di‐glycerides of fatty acids (E 471) is authorised were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
Some food categories or their restrictions/exceptions are not referenced in the EFSA Comprehensive Database and could therefore not be taken into account in the present estimate. This was the case for 10 food categories (Appendix C) and may have resulted in an underestimation of the exposure. The food categories which were not taken into account are (in ascending order of the food category codes):
1.7.6 Cheese products (excluding products falling in category 16); however, these products were reclassified under 01.7.5 Processed cheese
2.3 Vegetable oil pan spray
6.6 Batters
6.7 Precooked or processed cereals
8.3.3 Casings and coatings and decorations for meat
12.1.2 Salt substitutes
14.2.4 Fruit wine and made wine
14.2.5 Mead
14.2.7.2 Aromatised wine‐based drinks
14.2.7.3 Aromatised wine‐product cocktails
For the following food categories, the restrictions/exceptions which apply to the use of mono‐ and di‐glycerides of fatty acids (E 471) were also not referenced, and therefore, the whole food category was considered in the exposure assessment. This was the case for six food categories (Appendix C) and may have resulted in an overestimation of the exposure:
7.1 Bread and rolls, except products in 7.1.1 and 7.1.2
8.3.2 Heat‐treated processed meat, except foie gras, foie gras entier, blocs de foie gras, Libamáj, libamáj egészben, libamáj tömbben
13.1.3 Processed cereal‐based foods and baby foods for infants and young children as defined by Directive 2006/125/EC only biscuits and rusks, cereal‐based foods, baby foods
13.1.4 Other foods for young children, only when sold as powder
13.1.5.1 Dietary foods for infants for special medical purposes and special formulae for infants from birth onwards in specialised diets, particularly those devoid of proteins
13.1.5.2 Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC from birth onwards in specialised diets, particularly those devoid of proteins
In addition, the restrictions which apply to the use of mono‐ and di‐glycerides of fatty acids (E 471) for the food categories, FCs 17.1, 17.2 and 17.3 (food supplements, in solid, liquid and syrup‐type or chewable form) could not be taken into account, and therefore, the whole food category (FC 17) was considered in the specific exposure scenario including food supplements (Section 3.4.3).
Mono‐ and di‐glycerides of fatty acids (E 471) is also authorised in FC 18 (processed foods not covered by categories 1–17, excluding foods for infants and young children). Considering that this food category is extremely unspecific, the foods belonging to this food category (e.g. processed foods, prepared or composite dishes) were reclassified under food categories in accordance to their main component and included as such in the exposure assessment. Also, the food items under FCs 13.2, 13.3 and 13.4, consumed by the population groups: children, adolescents, adults and the elderly, may be very diverse; in addition, there was very limited information on their consumption. Therefore, eating occasions belonging to these food categories were also reclassified under food categories in accordance with their main component. The use levels available for FCs 13.2, 13.3, 13.4 and 18 were not considered in the exposure assessment.
In the refined scenario, 24 additional food categories were not taken into account because no use levels were provided (Appendix C). Furthermore, ten additional food categories were not taken into account in the refined scenario because the use levels were provided by a food additive producer and not deemed suitable for use in this exposure scenario (see Section 3.3.1). These data were included in the regulatory maximum level exposure scenario. For the remaining food categories, the refinements considering the restrictions/exceptions as set in Annex II to Regulation No 1333/2008 were applied.
Overall, for the regulatory maximum level exposure scenario, 41 food categories were included, while for the refined (brand loyal and non‐brand loyal) scenarios, 31 food categories were included in the present exposure assessment to mono‐ and di‐glycerides of fatty acids (E 471) (Appendix C). Compared to the refined scenario, three additional food categories were considered (FC 17.1, 17.2 and 17.3) in the food supplement scenario, while in the FSMP scenario, two additional FCs (FC 13.1.5.1 and 13.1.5.2) were considered resulting in a total number of food categories of 34 and 33, respectively.
3.4. Exposure estimates to mono‐ and di‐glycerides of fatty acids (E 471) from its use as a food additive
The Panel estimated the chronic dietary exposure to mono‐ and di‐glycerides of fatty acids (E 471) for the following population groups: infants, toddlers, children, adolescents, adults and the elderly. Dietary exposure to mono‐ and di‐glycerides of fatty acids (E 471) was calculated by multiplying concentrations of mono‐ and di‐glycerides of fatty acids (E 471) per food category (Appendix C) with their respective consumption amount per kilogram body weight for each individual in the EFSA Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only 1 day per subject were excluded as they are considered as not adequate to assess repeated exposure.
The exposure was estimated for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 5). Based on these distributions, the mean and 95th percentile of exposure were calculated per survey and per population group. The 95th percentile of exposure was only calculated for the population groups with a sufficiently large sample size to allow this calculation (EFSA, 2011a). Therefore, in the present assessment, the 95th percentile of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain were not estimated.
Exposure assessment to mono‐ and di‐glycerides of fatty acids (E 471) was carried out by the ANS Panel based on two different sets of concentration data: (1) MPLs as set down in the EU legislation or the maximum reported use levels as provided to EFSA by industry for categories where mono‐ and di‐glycerides of fatty acids is authorised as QS (defined as the regulatory maximum level exposure assessment scenario) and (2) reported use levels (defined as the refined exposure assessment scenario). These two scenarios are discussed in detail below.
These scenarios did not consider the consumption of food supplements (FC 17.1, 17.2 and 17.3) or FSMP. These exposure sources will be addressed in two additional scenarios, which are described in detail below.
A possible additional exposure from the use of mono‐ and di‐glycerides of fatty acids (E 471) as a carrier in food additives in colours and fat soluble antioxidants and glazing agent in food, as a food additive in food additives, food enzymes, food flavourings, nutrients and nutrients in foods for infants and young children in accordance with Annex III to Regulation (EC) No 1333/2008 (Part 1, 2, 3, 4, 5 (Section A and B)) was not considered in any of the exposure assessment scenarios, due to the absence of information on concentration levels.
3.4.1. Regulatory maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario is based on the MPLs as set in Annex II to Regulation (EC) No 1333/2008 or on the maximum reported use levels provided by industry for food categories in which the food additive is allowed at QS, as described in the EFSA Conceptual framework (EFSA ANS Panel, 2014). As mono‐ and di‐glycerides of fatty acids (E 471) is authorised at QS in the majority of food categories (Table 4), maximum reported use levels were used for the food categories for which use levels were submitted (see Appendix C).
The Panel considered the exposure estimates derived following this scenario as the most conservative possible as it is assumed that the population group will be exposed to mono‐ and di‐glycerides of fatty acids (E 471) present in food at the MPL or maximum reported use levels over a longer period of time.
Appendix C summarises the concentration levels of mono‐ and di‐glycerides of fatty acids (E 471) used in the maximum level exposure assessment scenario.
3.4.2. Refined exposure assessment scenario
The refined exposure assessment scenario is based on use levels reported by industry. This exposure scenario can consider only food categories for which these data were available to the Panel.
Appendix C summarises the concentration levels of mono‐ and di‐glycerides of fatty acids (E 471) used in the refined exposure assessment scenario. Based on the available data set, the Panel calculated two refined exposure estimates based on different model populations:
-
The brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to mono‐ and di‐glycerides of fatty acids (E 471) present at the maximum reported use for one food category. This exposure estimate is calculated as follows:
-
1
– Combining food consumption with the maximum of the reported use levels for the main contributing food category at the individual level.
-
2
– Using the mean of the typical reported use levels, for the remaining food categories.
-
1
The non‐brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to mono‐ and di‐glycerides of fatty acids (E 471) present at the mean reported use in food. This exposure estimate is calculated using the mean of the typical reported use levels for all food categories.
3.4.3. Specific exposure assessment scenarios
‘Food supplement consumers only’ scenario
Mono‐ and di‐glycerides of fatty acids (E 471) is authorised in the FC 17: Food supplements, as defined in Directive 2002/46/EC excluding food supplements for infants and young children. As exposure via food supplements may deviate largely from that via food and the number of food supplement consumers may be low depending on populations and surveys, an additional scenario was calculated in order to reflect additional exposure to mono‐ and di‐glycerides of fatty acids (E 471) from food supplements compared to the exposure to the food additive excluding these sources.
This scenario was estimated assuming that consumers only of food supplements were exposed to mono‐ and di‐glycerides of fatty acids (E 471) present at the maximum reported use level on a daily basis via consumption of food supplements. For the remaining food categories, the mean of the typical reported use levels was used.
As FC 17 does not consider food supplements for infants and toddlers as defined in the legislation, exposure to mono‐ and di‐glycerides of fatty acids (E 471) from food supplements was not estimated for these two population groups.
Appendix C summarises the concentration levels of mono‐ and di‐glycerides of fatty acids (E 471) used in this specific exposure assessment scenario.
FSMP scenario consumers only
As mono‐ and di‐glycerides of fatty acids (E 471) is also authorised in the FC 13.1.5 (13.1.5.1 and 13.1.5.2), an additional exposure assessment scenario taking into account this food category was performed to estimate the exposure of infants and toddlers who may eat and drink these FSMPs. The consumption of these FSMPs is not referenced in the EFSA Comprehensive Database. To consider the potential exposure to food additives via the consumption of these foods FSMPs, the Panel assumed that the amount consumed in infants and toddlers resembles that of comparable foods in infants and toddlers from the general population. Thus, the consumption of FSMP categorised as FC 13.1.5 was assumed to equal that of formulae and food products categorised as FCs 13.1.1, 13.1.2, 13.1.3 and 13.1.4.
Consumers only of FSMP were assumed to be exposed to mono‐ and di‐glycerides of fatty acids (E 471) present at the maximum reported use level on a daily basis via consumption of FCs 13.1.5.1 and 13.1.5.2 (infant formulae, follow‐on formulae and processed cereal‐based foods and baby foods for infants and young children as defined by Commission Directive 2006/125/EC). For the remaining food categories, the mean of the typical reported use levels was used.
Appendix C summarises the concentration levels of mono‐ and di‐glycerides of fatty acids (E 471) used in this specific exposure assessment scenario.
3.4.4. Dietary exposure to mono‐ and di‐glycerides of fatty acids (E 471)
Table 6 summarises the estimated exposure to mono‐ and di‐glycerides of fatty acids (E 471) from its use as a food additive in six population groups (Table 5) according to the different exposure scenarios (Section 3.4.3). Detailed results per population group and survey are presented in Appendix D.
Table 6.
Summary of dietary exposure to mono‐ and di‐glycerides of fatty acids (E 471) from its use as a food additive in the regulatory maximum level exposure assessment scenario and in the refined exposure scenarios, in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants | Toddlers | Children | Adolescents | Adults | The elderly | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (12 weeks–11 months) | (12–35 months) | (3–9 years) | (10–17 years) | (18–64 years) | (≥ 65 years) | |||||||
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | |
| Regulatory maximum level exposure assessment scenario | ||||||||||||
| Mean | 149 | 432 | 118 | 417 | 74 | 376 | 36 | 270 | 53 | 179 | 50 | 185 |
| 95th percentile | 384 | 845 | 291 | 837 | 168 | 761 | 86 | 562 | 110 | 387 | 100 | 360 |
| Refined estimated exposure assessment scenario | ||||||||||||
| Brand‐loyal scenario | ||||||||||||
| Mean | 30 | 179 | 30 | 247 | 45 | 252 | 23 | 184 | 36 | 129 | 37 | 137 |
| 95th percentile | 72 | 519 | 61 | 620 | 124 | 557 | 64 | 416 | 74 | 301 | 70 | 313 |
| Non‐brand‐loyal scenario | ||||||||||||
| Mean | 24 | 58 | 24 | 69 | 21 | 60 | 11 | 42 | 10 | 26 | 9 | 28 |
| 95th percentile | 59 | 111 | 50 | 128 | 52 | 124 | 27 | 89 | 21 | 58 | 18 | 54 |
In the regulatory maximum level exposure assessment scenario, mean exposure to mono‐ and di‐glycerides of fatty acids (E 471) from its use as a food additive ranged from 36 mg/kg bw per day in adolescents to 432 mg/kg bw per day in infants. The 95th percentile of exposure to mono‐ and di‐glycerides of fatty acids (E 471) ranged from 86 mg/kg bw per day in adolescents to 845 mg/kg bw per day in infants.
In the refined estimated exposure scenario in the brand‐loyal scenario, mean exposure to mono‐ and di‐glycerides of fatty acids (E 471) from its use as a food additive ranged from 23 mg/kg bw per day in adolescents to 252 mg/kg bw per day in children. The high exposure to mono‐ and di‐glycerides of fatty acids (E 471) ranged from 61 mg/kg bw per day to 620 mg/kg bw per day in toddlers.
In the non‐brand‐loyal scenario, mean exposure to mono‐ and di‐glycerides of fatty acids (E 471) from its use as a food additive ranged from 9 mg/kg bw per day in adults and elderly to 69 mg/kg bw per day in toddlers. The 95th percentile of exposure to mono‐ and di‐glycerides of fatty acids (E 471) ranged from 18 mg/kg bw per day in elderly to 128 mg/kg bw per day in toddlers.
The main food categories contributing to exposure to mono‐ and di‐glycerides of fatty acids (E 471) are presented in Appendix E.
In all scenarios and population groups except infants, the main contributors were bread and rolls and fine bakery wares.
For the regulatory maximum level and the brand‐loyal exposure scenario, processed fruits and vegetables contributed also largely to the exposure in the groups: toddlers, children, adults and elderly, while for the non‐brand‐loyal scenario, chocolate and chocolate products contributed largely to the total mean exposure estimates in all population groups except infants.
For infants, in all scenarios, the most important contributing food categories were foods for infants and young children and bread and rolls as well as breakfast cereals in the non‐brand‐loyal scenario.
In the refined estimated exposure scenario taking into account the foods for special medical purposes, mean exposure ranged for infants from 42 to 67 mg/kg bw per day and from 22 to 66 mg/kg bw per day for toddlers. The 95th percentile of exposure to mono‐ and di‐glycerides of fatty acids (E 471) ranged from 84 to 123 mg/kg bw per day and from 80 to 123 mg/kg bw per day, respectively.
For the food supplements consumers only, in the brand‐loyal scenario, mean exposure to mono‐ and di‐glycerides of fatty acids (E 471) from their use as food additives ranged for children from 26 to 100 mg/kg bw per day and from 21 to 33 mg/kg bw per day for adults. The 95th percentile of exposure ranged from 64 to 124 mg/kg bw per day and from 52 to 66 mg/kg bw per day in the same two population groups, respectively.
The Panel noted that mono‐ and di‐glycerides of fatty acids are minor constituents of oils and processed oils (Goh and Timms, 1985; Pacheco et al., 2014; Gupta, 2017). Quantification of exposure to mono‐ and di‐glycerides of fatty acids from the natural diet is not precisely known and could therefore not be taken into account in this opinion. However, exposure to mono‐ and di‐glycerides of fatty acids (E 471) can be compared with the fat consumed (EFSA NDA Panel, 2010), due to the similarity in the metabolic breakdown between mono‐, di and tri‐glycerides.
3.4.5. Uncertainty analysis
Uncertainties in the exposure assessment of mono‐ and di‐glycerides of fatty acids (E 471) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 7.
Table 7.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/− |
| Use of data from food consumption survey of a few days to estimate long‐term (chronic) exposure for high percentiles (95th percentile) | + |
| Correspondence of reported use levels to the food items in the EFSA Comprehensive Food Consumption Database: uncertainties to which types of food the levels refer to | +/− |
| Uncertainty in possible national differences in use levels of food categories | +/− |
|
Concentration data:
|
+ +/− + |
| Consumption data considered in the refined exposure assessment: 34–95% of the amount of food consumed (grams per kg body weight) corresponded to 31 food categories (out of 84 authorised food categories) taken into account | − |
| Food categories selected for the exposure assessment: exclusion of food categories due to missing FoodEx linkage (n = 14/84) | − |
| Food categories selected for the exposure assessment: inclusion of food categories without considering the restriction/exception (n = 5 MPL scenario/n= 4 refined scenarios out of 84 food categories) | + |
| Food categories included in the exposure assessment: data not available for certain food categories which were excluded from the exposure estimates (n = 46/84) | − |
| Foods which may contain E 471 according to Annex III to Regulation (EC) No 1333/2008 not taken into account | − |
|
Regulatory maximum level exposure assessment scenario:
|
+ +/− − |
|
Refined exposure assessment scenarios:
|
+/− − |
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
Mono‐ and di‐glycerides of fatty acids (E 471) is authorised as a Group I food additive in 64 food categories and has a specific authorised use in 20 food categories (Table 4) from which in total 31 were taken into account in the refined exposure scenarios. Data on usage levels in foodstuffs in other food categories were not reported by the food industry or have been excluded due to missing connection with the foods referenced in the EFSA Comprehensive Database. Since the majority of food categories correspond to the general Group I food additives authorisation (Table 4), mono‐ and di‐glycerides of fatty acids (E 471) may not necessarily be used in some of these food categories. This may explain why reported use levels of mono‐ and di‐glycerides of fatty acids (E 471) were only available for 46 food categories. However, the Panel noted that information from the Mintel's GNPD (Appendix B) showed that food belonging to some of the remaining 38 food categories were labelled with mono‐ and di‐glycerides of fatty acids (E 471), such as unripened cheese, different kinds of pasta, processed fish and fishery products including molluscs and crustaceans, processed eggs and egg products and salads and savoury‐based sandwich spreads, etc. This may indicate an underestimation of exposure. In none of the exposure scenarios, the use of mono‐ and di‐glycerides of fatty acids (E 471) according to Annex III to Regulation No 1333/2008 was considered. Neglecting this source of exposure may also have resulted in an underestimation of exposure to mono‐ and di‐glycerides of fatty acids (E 471) in all scenarios.
Overall, based on the assumption that the food additive is not used in the food categories in which it is permitted but for which no usage data were provided by the stakeholders, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure to mono‐ and di‐glycerides of fatty acids (E 471) as a food additive in all exposure scenarios.
However, considering information from the Mintel's GNPD that mono‐ and di‐glycerides of fatty acids (E 471) is labelled in a number of foods for which no use levels were provided, and the potential use of mono‐ and di‐glycerides of fatty acids (E 471) according to Annex III which could not be considered in the exposure assessment, the exposure to mono‐ and di‐glycerides of fatty acids (E 471) may have been underestimated in all scenarios.
3.5. Biological and Toxicological studies
The Panel noted that the diacylglycerol (diglyceride) used in several of the toxicity studies described below was intended to be used for nutritional purposes (as an edible oil substitute) and it had a composition rich in unsaturated fatty acids (> 95%). The Panel further noted that its composition made this material acceptable with regard to the specifications of E 471. The Panel considered that the results of the toxicological studies with these diacylglycerols can be used for the assessment of E 471.
3.5.1. Absorption, distribution, metabolism and excretion
Hydrolysis of the diglyceride of fatty acids (fatty acid composition: oleic acid 80.5%, total saturated fatty acids 7.9%, total polyunsaturated fatty acids 4.9%, monounsaturated fatty acids other than oleic acid 6.6%) was examined in a model digestive system in vitro (Martin et al., 2014) by using a model of simulated bile secretion. After 60 min at 37°C, 1,3‐diolein was shown to be nearly completely hydrolysed to free fatty acids with only low levels of monoglycerides present.
Ames et al. (1951) estimated the absorption of naturally produced cottonseed oil and monoglycerides of the fatty esters contained in cottonseed oil in eight male albino rats per group. Fat intake was 25% cottonseed oil or monoglycerides. Intake and fat content in the faeces were determined for 1 week. The absorption was determined by measuring the digestibility factor (e.g. the percentage of ingested fat that was not excreted in the faeces). Naturally produced cottonseed oil was absorbed to 96.8 ± 0.5% and monoglycerides of the fatty esters contained in cottonseed oil were absorbed to 97.8 ± 0.4%.
Absorption of diacylgycerol was examined in nine 5‐week‐old male Sprague–Dawley rats (Chen et al., 2013). The tested diacylglycerol consisted of 87% diacylglycerols but contained also monoacylglycerols (2%) and triacylglycerols (11%). The total fatty acid composition was primarily C18:2, C18:1, C18:3 (n–3) and C16:0 present at 53.6%, 32.9%, 8.8% and 3%, respectively. Diacylglycerol was administered by oral gavage at 2.5 mL diacylglycerol/kg bw plus 7.5 mL/kg bw in distilled water with blood samples collected at 0, 6 and 24 h. The serum fatty acid concentrations was not different at the time points examined compared to the concentration at time 0. Faeces were collected from the last study day. The digestibility coefficient of the diacylglcerol was calculated by the authors to be 58.8 ± 14.33% and equals the absorption.
Overall, in rats, only traces of cottonseed oil monoglycerides were found in the faeces, indicating that after hydrolysis the components were well absorbed (97.8 ± 0.4%). In another study, the absorption of hydrolysis products diglycerides of fatty acids was calculated to be 58.8 ± 14.3%. In a study to elucidate the mechanism of absorption of diglycerides, the hydrolysis of the diglyceride 1,3‐diolein was examined in a model digestive system in vitro. 1,3‐diolein was shown to be nearly completely hydrolysed to free fatty acids with low levels of monoglycerides present.
3.5.2. Acute toxicity
No data available.
3.5.3. Short‐term and subchronic toxicity
Rats
In the study by Morita et al. (2008a), heated di‐ and tri‐acylglycerol were prepared by deep‐frying potato slices at 180°C for 8 h per day for three consecutive days. Sprague–Dawley rats (10/sex per group) were fed diets containing different ratio of heated to unheated oils for 90 days, and two experiments were designed. In the first one, rats were exposed to various ratio of heated to unheated diacylglycerol: 0%/5.5% (control 1; Group 1), 1.0%/4.5% (Group 2), 2.75%/2.75% (Group 3) and 5.5%/0% (Group 4). In the second one, two additional groups received the feed containing 5.5% unheated (Group 5, control) or 5.5% heated triacylglycerol oil (Group 6). These doses were settled out from two preliminary 14‐day studies, which according to the authors did not result in any adverse effects on body weights, feed consumption, clinical pathology (haematology, serum chemistry and urinalysis), organ weights and macroscopic observations or microscopic examinations of a limited number of organs. Some statistically significant differences were observed on clinical observation, body weights, consumption and functional observational battery (FOB) tests, but they were considered not dose related. Clinical pathology, ophthalmic examination and macroscopic and microscopic examinations of rats also showed some changes that were considered not dose related or not treatment related. The authors considered the observed changes as consistent with normal background observations in normal rats of the same age and strain. The authors identified a no observed effect levels of heated diacylglycerol or triacylglycerol oil of 5.5% in the diet, equivalent to 3,178–4,120 mg/kg per day for male and female rats, respectively, the highest dose tested. The Panel agreed with this conclusion.
Hamsters
In a study with male Golden Syrian hamsters, two groups of 15 animals were dosed for 8 weeks with 0% or 5% glyceryl monostearate (equivalent to 0 or 2,500 mg glyceryl monostearate/kg bw per day) (Orten and Dajani, 1957). At this time point, the treated group was subdivided: 5 hamsters were continued and 10 were changed to a 15% concentration in the diet (equivalent to 7,500 mg glyceryl monostearate/kg bw per day). Body weights of all animals were taken weekly, food consumption data recorded and the animals observed carefully for any gross symptoms. At the end of the 28‐week period, the remaining animals were autopsied and sections of liver, kidney, bladder and testis were taken for histopathological examination. Apart from a slight decrease in body weight gain at a dose level of 15%, no other significant adverse effects were reported. In a second trial lasting 22 weeks, two groups of 14 hamsters were dosed with 15% glyceryl monostearate via diet and one group was supplemented with agar, which resulted in a body weight gain comparable with the controls. The Panel noted that this study had a number of limitations, a limited number of organs were examined and there were no information on clinical chemistry and haematological parameters with diacylglycerol or glyceryl stearate; however, no adverse effects were reported.
Overall, no evidence for adverse effects were reported in short‐term and subchronic studies in rats and hamsters even at 2,500 diacylglycerol/kg bw per day in rats and 7,500 mg glyceryl stearate/kg bw per day in hamsters, the highest doses tested.
3.5.4. Genotoxicity
In vitro
In the study by Kasamatsu et al. (2005), diacylglycerol having a composition rich in unsaturated fatty acids (> 95%) was assessed for its mutagenicity in the reverse mutation assay using Salmonella Typhimurium strains TA1535, TA1537, TA98 and TA100, and a tryptophan‐dependent auxotrophic mutant of Escherichia coli, strain WP2uvrA up to a maximum concentration of 5,000 μg/plate in dimethyl sulfoxide, both in the absence and presence of rat liver S9 metabolic activation in two independent experiments. No mutagenicity was observed. The Panel noted that the study was performed in compliance with good laboratory practice (GLP) and following the current OECD Guideline E 471.
Diacylglycerol was tested for its potential to induce chromosomal aberrations in a Chinese hamster female lung cell line both in the absence and presence of S9 metabolic activation (Kasamatsu et al., 2005). The cells were exposed to concentrations of 1,250, 2,500 and 5,000 μg/mL in a 0.5% carboxymethyl cellulose sodium aqueous solution for 6 h (short‐term treatment) both in the absence and presence of S9 metabolic activation and for 24 and 48 h (continuous treatment), only in the absence of metabolic activation. Fixation of cells was performed in all cases at 24 and 48 h from the beginning of treatment, respectively. Despite the test concentrations of 5,000 and 2,500 μg/mL exceeded the highest test concentration of 2,000 μg/mL recommended by the current OECD guideline No. 473, no toxicity occurred as measured by the cell growth inhibition of the test compound treated cells compared to the concurrent vehicle control values. The results obtained indicated that diacylglycerol did not induce any statistically significant increase for both structural chromosomal aberrations and polyploid cells at any concentration assayed in any treatment condition. The Panel noted that the study, except for the use of higher concentrations than the one recommended by the guideline was in line with the current OECD Guideline 473.
In vivo
In the study by Kasamatsu et al. (2005), diacylglycerol, having a composition rich in unsaturated fatty acids (> 95%), was assessed for its genotoxicity in an in vivo micronucleus test. ICR male mice (no number of animals reported) were treated once daily for 2 days at an interval of 24 h by oral gavage with 500, 1,000 and 2,000 mg/kg bw of diacylglycerol suspended in olive oil, the latter dose level being the limit dose for this test. The animals were sacrificed 24 h after the beginning of treatment. Bone marrow smears were prepared, stained with acridine orange and 2,000 polychromatic erythrocytes (PCE) were analysed per animal for the presence of micronuclei. Results obtained indicated that the frequencies of micronucleated PCE did not increase significantly in any test compound treatment group compared to the concurrent vehicle control. The Panel noted that the study was adequately performed and essentially complied with the requirements of the current OECD guideline TG 474 with the exception that 2,000 PCE per animal, instead of the recommended 4,000, were scored and no number of animals was reported. Furthermore, the Panel noted that a significant reduction (p < 0.05) of the ratio of immature (PCE) to mature normochromatic erythrocytes was observed at the highest (2,000 mg/kg bw) dose level used indicating that the target tissue (bone marrow) was exposed, thus corroborating the negative genotoxicity findings.
No genotoxicity of diacylglycerol (rich in unsaturated fatty acids (> 95%)) was observed in adequately conducted studies which included a bacterial reverse mutation assay (Ames test), an in vitro test for the induction of chromosomal aberrations and an in vivo bone marrow micronucleus test. This combination of tests fulfilled the basic requirements to cover the three genetic endpoints (i.e. gene mutations and structural and numerical chromosome aberrations) required for the assessment of genotoxicity (EFSA Scientific Committee, 2011).
Overall, the Panel considered that the diacylglycerol tested in these studies was of no concern for genotoxicity.
3.5.5. Chronic toxicity and carcinogenicity
Mice
Male and female Crl:CD‐1 (ICR) BR mice (50 animals/group) were fed a diet containing diacylglycerol (rich in unsaturated fatty acids (> 95%)) for 24 months (Chengelis et al., 2006a). Dietary concentrations (% diacylglycerol/% triacylglycerol) were 0%/6.0% (control), 1.5%/4.5%, 3.0%/3.0% and 6.0%/0%. An additional control group received the standard diet (fat content 4.5%). Both triacylglycerol and/or diacylglycerol, when presented separately or together in the diet at a total fat level of 6.0%, resulted in some differences relative to the basal diet control (lower survival, higher body weights, lower food consumption and higher incidences of macroscopic and microscopic findings), presumably related to the higher dietary fat content and/or the semi‐purified diet. However, these parameters were similar in groups fed a diet with 6.0% dietary fat, which was either diacylglycerol or TG. The authors concluded that diacylglycerol at dietary concentrations up to 6.0% (equivalent to 7,800 and 10,020 mg/kg bw per day in males and females, respectively), for 24 months produced no signs of systemic toxicity and had no effect on the incidence of neoplastic findings.
Rats
In a chronic toxicity study, groups of 60 male and female Crj:CD(SD) strain SPF rats were fed diets containing 2.65% or 5.3% diacylglycerol oil (equal to 1,770 and 2,350 mg/kg bw per day) for up to 105 weeks (Soni et al., 2001). The total fat content in the diets was 7% resulting from a fat content of 1.7% in the basal feed and, in the case of the low dose diet, from addition of 2.65% vegetable oil mixture with a fatty acid composition comparable to that of diacylglycerol oil. Control group 1 received 5.3% of the same vegetable oil mixture and control group 2 received 5.3% of a mixture of rapeseed and soybean oils. Haematology, clinical chemistry and urine examinations, organ weights and macroscopic and histological examinations were performed at the end of 30 and 77 weeks with 10 animals/group. The remaining animals were euthanised at the end of 105 weeks. The cumulative survival rate, occurrence of clinical signs, body weights and food consumption measurements were similar in all groups and did not reveal any adverse effects of diacylglycerol oil. Urinalysis did not reveal any treatment‐related effects. Occasional treatment‐related differences in various parameters such as haematology (prothrombin time, number of platelets), clinical chemistry (aminotransferases, lactate dehydrogenase, high‐density lipoprotein (HDL)‐cholesterol) and organ weights (pituitary, thyroids and spleen) were noted. However, these changes were noted at one time point only without any dose‐related effects and were not associated with any histopathological alterations. In female rats exposed to 5.3% diacylglycerol oil, a significantly higher incidence of mammary gland tumours was noted; however, because it was within the range of tumours in historical controls, it was not considered related to diacylglycerol oil treatment. According to the authors, the NOAEL was the highest dose tested, 1,770 and 2,350 mg/kg bw per day for males and females, respectively. The Panel agreed with this conclusion.
Male and female Crl:CD_(SD)‐IGS BR rat (50 animals/group) were exposed to diacylglycerol oil (rich in unsaturated fatty acids (> 95%)), administered in the diet for 24 months (Chengelis et al., 2006b). All dietary fat (consistently 5.5%) was provided by diacylglycerol and/or the control article, triacylglycerol oil. Dietary concentrations (% diacylglycerol/% triacylglycerol) were 0%/5.5%; 1%/4.5%; 2.75%/2.75% and 5.5%/0%. Separate groups were fed the 0%/5.5% and 5.5%/0% diets ad libitum. Another group received the standard rodent diet (fat content 4.5%) on the restricted feeding regimen. There were no diacylglycerol‐related effects on haematology or serum chemistry parameters. There were no diacylglycerol‐related ophthalmic findings. No effects on the incidence of preneoplastic or neoplastic lesions were noted. Changes attributed to the increased dietary fat content of the groups fed diet with 5.5% dietary fat (groups restricted feeding regimen) when compared to the standard control diet (Group 4.5% total fat content, restricted feeding regimen) included lower survival in both males and females, and higher body weights with proportional increases in organ weights and body fat. Higher serum cholesterol, triglycerides, free fatty acids and β‐hydroxybutyrate, as well as changes in urine total volume and specific gravity, were attributed to the higher fat content. Microscopic changes included increased incidences of myocardial degeneration in the heart, nephropathy and tubular vacuolation in the kidneys, hepatocyte vacuolation, necrosis, spongiosis hepatis, congestion and sinus dilation of the liver, bone marrow hyperplasia and congestion in the lungs for both males and females. According to the authors, the NOAELs for systemic toxicity of diacylglycerol administered in the diet for 24 months to rats was equal to 1,982 and 2,645 mg/kg bw per day for the restricted diet males and females, respectively, and 1,946 and 2,507 mg/kg bw per day for the ad libitum diet males and females, respectively. The authors also concluded that diacylglycerol‐treated animals had no higher risk of carcinogenic effects than rats fed on similar feeding regimens with a diet in which all dietary fat came from triacylglycerol. The Panel agreed with these conclusions.
Initiation–Promotion studies
The Panel considered that the results of the two initiation–promotion studies identified did neither show an enhancing effect of triacylglycerol or diacylglycerol on dimethylbenz(a)anthracene‐induced mammary gland tumorigenesis in Sprague–Dawley rats (Sugano et al., 2002) nor of diacylglycerol on tumour development in a medium‐term multiorgan carcinogenesis bioassay using F344 rats.
Overall, the Panel noted that no adverse effects were reported in chronic toxicity studies at doses as high as 7,800 and 2,000 mg diacylglycerol/kg bw per day in mice and rats, respectively. In rats and mice, diacylglycerol did neither show a carcinogenic potential nor a promotion effect in initiation/promotion studies.
3.5.6. Reproductive and developmental toxicity
Reproductive toxicity
In a study with albino rats, groups of 10 males and 10 female animals were placed on diets containing 15% and 25% (equivalent to 7,500 or 12,500 mg/kg bw per day) cottonseed oil or cottonseed oil monoglycerides (Ames et al., 1951). The Panel noted that this study was performed with a very low number of animals and cannot be used for hazard characterisation.
An oral two‐generation reproduction toxicity study in rats (30/sex per group) was performed with diacylglycerol oil (rich in unsaturated fatty acids (> 95%)), having a composition rich in unsaturated fatty acids (> 95%), according to GLP and following the OECD guideline (Morita et al., 2008b). Dose volume was 5 mL/kg bw per day; vehicle was corn oil. Diacylglycerol oil was administered via gavage for at least 70 days prior to mating at dose levels of 0, 1.25, 2.5 or 5.0 mL diacylglycerol/kg per day (equal to 0, 1,160, 2,320 and 4,630 mg/kg bw per day). An additional group received a triacylglycerol oil with a similar fatty acid composition to diacylglycerol oil. The rats were treated throughout the mating, gestation and lactation periods during two generations. Administration of diacylglycerol or triacylglycerol oil did not reveal any dose‐related effect on the parental generations. Reproductive and developmental effects of diacylglycerol oil were not observed. According to the authors, the NOAEL of this study was 4,630 mg diacylglycerol/kg bw per day, the highest dose tested. The Panel agreed with this NOAEL.
Developmental toxicity
Diacylglycerol oil (rich in unsaturated fatty acids (> 95%)) was administered via gavage to four groups of mated female Crl:CD(SD)IGS BR rats (25/group) once daily from gestation day 6 through 17, at dose levels of 0, 1.25, 2.5 or 5.0 mL/kg per day (equal 0, 1,160, 2,320 and 4,630 mg/kg bw per day) (Morita et al., 2008c). The study was performed according to GLP and following the ICH Guideline (1994). Apart from the slightly different dosing period, the study was performed following the OECD guideline TG 414. Dose volume was 5 mL/kg bw per day; vehicle was corn oil. No mortality or treatment‐related clinical or internal findings were noted in any of the groups. Compared to animals in control group, mean maternal body weights, body weight gains, net body weights, net body weight gains, gravid uterine weights and food consumption were not affected by diacylglycerol oil administration. Similarly, the reproductive and fetal parameters were not affected by diacylglycerol oil administration. No diacylglycerol oil‐related fetal malformations or developmental variations were noted. According to the authors, the NOAEL of this study for both maternal and developmental toxicity was 4,630 mg diacylglycerol/kg bw per day, the highest dose tested. The Panel agreed with this NOAEL.
Overall, a two‐generation reproduction toxicity study and a prenatal developmental toxicity study in rats with diacylglycerol oil, rich in unsaturated fatty acids (> 95%), showed no parental, reproductive and developmental effects up to the highest dose tested (4,630 mg diacylglycerol/kg bw per day).
3.5.7. Other studies
Studies in mice
Tove (1964) administered for 3 weeks diets supplemented with increasing doses of palmitate and stearate (given as glycerylmonopalmitate and glycerylmonostearate) to groups of five or six male weanling mice (no data about strain). No effects on weight gain and survival were detected at doses of 5%, 10% and 20% stearate (equivalent to 8,450, 16,900 and 33,800 mg/kg bw per day glycerylmonostearate) or 5% and 10% palmitate (equivalent to and 8,400 and 16,900 mg/kg bw per day glycerylmonopalmitate). Reduced weight gain was reported at levels above 30% stearate and above 20% palmitate; mortality occurred at levels above 50% stearate and above 20% palmitate. In animals found dead necropsy revealed emaciation although food consumption was normal. The Panel considered that this study cannot be used for the safety evaluation of E471 as only effects at very high levels of saturated fat on growth and mortality were studied.
Studies in rats
Meguro et al. (2006) compared the effects of dietary diacylglycerol (rich in unsaturated fatty acids (90–95%)) with triacylglycerol with a similar fatty acid composition on protein kinase C (PKC) activation and on 1,2‐diacylglycerol levels. Using male Wistar rats, after 1 month of feeding, no differences in cytosolic and membrane PKC activities in the lingual, oesophageal, gastric, small intestinal, caecal, proximal colonic and distal colonic mucosa were found between the 5% diacylglycerol and triacylglycerol oil groups or between the 23% diacylglycerol and triacylglycerol oil groups. In rats fed a diet containing either 10% diacylglycerol or triacylglycerol oil, the 1,2‐diacylglycerol levels in the caecum and colon contents and in the faeces and serum were similar. Exposure of Caco‐2 cells to diacylglycerol and triacylglycerol oils had no effect on PKC activity in the membrane fraction suggesting the absence of an influence on PKC activity in diacylglycerol and triacylglycerol oils, which are composed of long‐chain fatty acids.
Studies in humans
Morita and Soni (2009) reviewed 14 studies, performed between 1996 and 2008, in which effects of diacyl glycerol oil have been evaluated. The doses of diacyl glycerol oil estimated in four of the studies varied between 300 and 760 mg/kg bw per day; in five other studies, the intake of diacylglycerol oil was ad libitum, and in the remaining studies, the intake could not be estimated. The duration of the studies varied between 1 month and 1 year. The total number of participants in all 14 studies was 650; among them 11 children, 10 uremic patients and 59 patients were with type 2 diabetes. No adverse effects were noted.
Telle‐Hansen et al. (2012) published a 12‐week study in 10 subjects in which triacyl glycerol‐based food items were replaced with diacyl glycerol‐based food items. The daily dose was 11,200 mg diacylglycerol. No adverse effects were observed.
3.5.8. Studies with other emulsifiers
Mono‐ and di‐glycerides of fatty acids (E 471) is included in the list of EFEMA index of food emulsifiers (EFEMA, 2015).
In several recent studies, some other emulsifiers have been reported to alter the gut microbiota, to promote gut inflammation, obesity and to impair glycaemic control (Swidsinski et al., 2009a,b; Renz et al., 2012; Merga et al., 2014; Cani and Everard, 2015; Chassaing et al., 2015, 2017; Romano‐Keeler and Weitkamp, 2015; Lecomte et al., 2016; Nejrup et al., 2017; Shah et al., 2017). The Panel noted that, even though some of these effects are not systematically studied in toxicity studies performed according to toxicity testing guidelines, they would be investigated on a case‐by‐case basis if indicated by the results of the general toxicity testing as recommended in the Guidance for submission of food additives (EFSA ANS Panel, 2012).
3.6. Discussion
Glycerol is used in the manufacturing process of mono‐ and di‐glycerides of fatty acids (E 471) and, therefore, the recommendations made by the Panel for the specifications of glycerol (E 422) used as a food additive (EFSA ANS Panel, 2017a) should apply to the EU specifications for E 471.
As mono‐ and di‐glycerides of fatty acids (E 471) can be manufactured by glycerolysis of hydrogenated fats and or oils, which contain significant amounts of trans fatty acids, the Panel noted that a maximum limit for trans fatty acids should be included in the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471).
Some edible oils, such as rapeseed, which can be used for manufacturing mono‐ and di‐glycerides of fatty acids (E 471) may contain erucic acid. A TDI of 7 mg/kg bw per day for erucic acid has been established by the EFSA CONTAM Panel (2016); therefore, the Panel considered that a maximum limit for erucic acid should be included in the specifications.
When food containing monoglycerides of fatty acids are heat treated at temperatures above 160°C in the presence of NaCl, 3‐MCPD can be formed, and when food containing diglycerides of fatty acids is heat treated at temperatures above 200°C, glycidyl esters of fatty acids can be formed (EFSA CONTAM Panel, 2016).
The diglyceride 1,3‐diolein was shown to be nearly completely hydrolysed to free fatty acids in a model of simulated bile secretion. Accordingly, the Panel considered that it is very likely that hydrolysis of mono‐ and di‐glycerides of fatty acids by lipases in the gastrointestinal tract would occur, resulting in the release of glycerol and fatty acids. The Panel re‐evaluated glycerol (E 422) and fatty acids (E 570) as food additives and concluded that there was no safety concern regarding their use as food additives (EFSA ANS Panel, 2017a,b).
In rats, only traces of cottonseed oil monoglycerides were found in the faeces, indicating that after hydrolysis, the components were well absorbed (97.8 ± 0.4%). In another study, the absorption of hydrolysis products of diglycerides of fatty acids was calculated to be 58.8 ± 14.3%.
The Panel noted that the diacylglycerol (diglyceride) used in several of the toxicity studies described below was intended to be used for nutritional purposes (as an edible oil substitute) and it had a composition rich in unsaturated fatty acids (> 95%). The Panel further noted that its composition made this material acceptable with regard to the specifications of E 471. The Panel considered that the results of the toxicological studies with these diacylglycerols can be used for the assessment of E 471.
No study was available for the acute toxicity of E 471. No evidence for adverse effects were reported in short‐term and subchronic studies in rats and hamsters even at the highest dose tested of 2,500 mg diacylglycerol/kg bw per day in the rats and 7,500 mg glyceryl stearate/kg bw per day in hamsters.
The Panel considered that the available studies did not raise any concern with regard to genotoxicity.
No adverse effects were reported in chronic toxicity studies at doses as high as 7,800 and 2,000 mg diacylglycerol/kg bw per day in mice and rats, respectively. In mice and rats, diacylglycerol did neither show carcinogenic potential nor a promotion effect in initiation/promotion studies.
No parental, reproductive or developmental toxicity was observed in a two‐generation reproduction toxicity study and a prenatal developmental toxicity study in rats with diacylglycerol oil‐rich in unsaturated fatty acids (> 95%) up to the highest dose tested (4,630 mg diacylglycerol/kg bw per day).
The Panel noted that recent studies with other emulsifiers had demonstrated effects on the microbiota, which might also be relevant to emulsifiers in general; however, there were no specific studies on mono‐ and di‐glycerides of fatty acids and effects on the microbiota itself.
To assess the dietary exposure to mono‐ and di‐glycerides of fatty acids (E 471) from its use as a food additive, the exposure was calculated based on (1) MPLs and maximum levels of data provided to EFSA (defined as the regulatory maximum level exposure assessment scenario) and (2) reported use levels (defined as the refined exposure assessment scenario brand‐loyal and non‐brand‐loyal consumer scenario).
Mono‐ and di‐glycerides of fatty acids (E 471) is authorised in a wide range of foods. The Panel did not identify brand loyalty to a specific food category, and therefore, considered that the non‐brand‐loyal scenario covering the general population was the most appropriate and realistic scenario for risk characterisation of E 471 because it is assumed that the population is most likely to be exposed long term to the food additive E 471 present at the mean reported use in processed food.
The refined estimates of mono‐ and di‐glycerides of fatty acids (E 471) were based on 31 out of 84 food categories in which the food additive is authorised. The Panel considered that the uncertainties identified have resulted in an overestimation of the exposure to mono‐ and di‐glycerides of fatty acids (E 471) as a food additive in European countries for the refined scenario, if the food additive is not used in food categories, for which no usage data were provided.
However, the Panel noted that, considering information from the Mintel's GNPD, mono‐ and di‐glycerides of fatty acids (E 471) is used in food categories for which no use levels were provided to EFSA. The main food categories, in terms of amount consumed, for which no use levels were reported were unripened cheese, different kinds of pasta, processed fish and fishery products including molluscs and crustaceans, processed eggs and egg products and salads and savoury‐based sandwich spreads. The Panel further noted that the exposure to mono‐ and di‐glycerides of fatty acids (E 471) from their use according the Annex III to Regulation (EC) No 1333/2008 (Parts 1, 2, 3, 4 and 5 A and B) was not considered in the exposure assessment. Therefore, the exposure to mono‐ and di‐glycerides of fatty acids (E 471) may also have been underestimated in all scenarios, if also considering possible exposure via the consumption of foods via authorised food categories, for which no use levels were provided by the food industry. However, the possible underestimation of the exposure may have been negated by the assumption in all exposure scenarios that all foods belonging to an authorised food category contain the food additive, whereas according to the Mintel's GNPD, on average only 7% of the authorised food items contained mono‐ and di‐glycerides of fatty acids (E 471).
The Panel also noted that the refined exposure estimates are based on information provided on the reported levels of use of mono‐ and di‐glycerides of fatty acids (E 471). If actual practice changes, these refined estimates may no longer be representative and should be updated.
The Panel noted that in Annex II of Regulation (EC) No 1333/2008 use levels of mono‐ and di‐glycerides of fatty acids (E 471) in food for infants under the age of 12 weeks are included in category 13.1.1, 13.1.5.1 and 13.1.5.2. The Panel considered that these uses for infants under the age of 12 weeks would require a specific risk assessment in line with the recommendations given by JECFA (1978), the SCF (1998) and EFSA (EFSA Scientific Committee, 2017). Therefore, the current re‐evaluation of mono‐ and di‐glycerides of fatty acids (E 471) as a food additive is not applicable for infants under the age of 12 weeks.
The Panel noted that no specific clinical data addressing the safety of use of mono‐ and di‐glycerides of fatty acids (E 471) in ‘dietary foods for infants for special medical purposes and special formulae for infants’ (FC 13.1.5.1) and in ‘dietary foods for baby and young children for special medical purposes as defined in Directive 1999/21/EC’ (FC 13.1.5.2) considering the defined maximum use levels were available to the Panel.
Exposure to mono‐ and di‐glycerides of fatty acids (E 471) can be compared with the fat consumed, due to the similarity in the metabolic breakdown between mono‐, di and tri‐glycerides. A lower bound of the reference intake for total fat of 20 energy % (E%) and an upper bound of 35 E% are proposed in the Scientific Opinion on Dietary Reference Values for fats, including saturated, polyunsaturated, monounsaturated and trans fatty acids and cholesterol (EFSA NDA Panel, 2010). Based on these recommendations, the intake of fat would range from 741 to 1,296 mg/kg bw, assuming 2,000 kcal/day as the reference energy intake for adults (Regulation (EU) 1169/2011) and a body weight of 60 kg.
Exposure estimates to the food additive E 471 (mono‐ and di‐glycerides of fatty acids) ranged from 10 to 26 mg/kg bw per day, at the mean, and from 21 to 58 mg/kg bw per day for the 95th percentile in the adult population group considering the non‐brand‐loyal exposure assessment scenario. At the mean intake level of mono‐ and di‐glycerides (E 471), the contribution of this food additive to the daily fatty acid intake ranged from 0.8% to 3.5%, whereas at the highest 95th percentile of exposure, the contribution could be up to 7.8% of the recommended for adults daily fat intake.
4. Conclusion
According to the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA ANS Panel, 2014) and given that:
in the current safety assessment carried out by the Panel, the uses and use levels reported by the food industry in 46 out of 84 food categories in which mono‐ and di‐glycerides of fatty acids (E 471) is authorised were considered. However, only 31 food categories were taken into account for the refined exposure assessment;
mono‐ and di‐glycerides of fatty acids are subjected to hydrolysis by lipases in the gastrointestinal tract to liberate glycerol and fatty acids;
data from the evaluation previously conducted for the food additives glycerol (E 422) and fatty acids (E 570) can be used for the evaluation of the food additive mono‐ and di‐glycerides of fatty acids (E 471);
there was no indication for a genotoxic, carcinogenic or reprotoxic potential from the available data;
the contribution of mono‐ and di‐glycerides of fatty acids (E 471) represented at the mean only 0.8–3.5% of the recommended daily fat intake;
the Panel concluded that there was no need for a numerical ADI and that the food additive mono‐ and di‐glycerides of fatty acids (E 471) was of no safety concern at the reported uses and use levels.
5. Recommendations
The Panel recommended that:
the European Commission considers lowering the current limits for toxic elements (arsenic, lead, mercury and cadmium) in the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471) in order to ensure that the food additive will not be a significant source of exposure to these toxic elements in food.
the European Commission considers revising the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471) including maximum limits for impurities currently included in the EU specifications for glycerol (E 422) or recommended by the Panel in the re‐evaluation of glycerol (E 422) (EFSA ANS Panel, 2017b).
the European Commission considers revising the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471) including maximum limits for residual solvents which can be used when manufacturing mono‐ and di‐glycerides of fatty acids (E 471), i.e. tert‐butanol or tert‐pentanol.
the European Commission considers revising the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471) including maximum limits for trans fatty acids because mono‐ and di‐glycerides of fatty acids (E 471) can be manufactured by glycerolysis of hydrogenated fats and/or oils, which contain significant amounts of trans fatty acids.
the European Commission considers revising the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471) including maximum limits for glycidyl esters because refined vegetable oil, which can be used for manufacturing of mono‐ and di‐glycerides of fatty acids (E 471) is the only identified source of glycidyl esters of fatty acids, which are formed during deodorisation.
the European Commission considers revising the EU specifications for mono‐ and di‐glycerides of fatty acids (E 471) including maximum limits for erucic acid because as erucic acid can be present among the fatty acids in edible oils, which can be used for manufacturing of mono‐ and di‐glycerides of fatty acids (E 471).
more data should be generated to decrease uncertainty arising from the occurrence of compounds of toxicological concern (e.g. 3‐MCPD or glycidyl esters), which can be produced under certain processing conditions from the food additive mono‐ and di‐glycerides of fatty acids (E 471).
Documentation provided to EFSA
Association of the European Self‐Medication Industry (AESGP, 2016). Data on usage levels of mono‐ and di‐glycerides of fatty acids (E 471) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 4), Published 12 October 2015. Submitted to EFSA on 27 May 2016.
AVIKO, 2016. Data on usage levels of mono‐ and di‐glycerides of fatty acids (E 471) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 4), Published 12 October 2015. Submitted to EFSA on 10 May 2016.
EFEMA (European Food Emulsifier Manufacturer's Association), 2010a. Mono and Diglycerides of fatty acids E 471. Specifications, manufacturing methods and chemistry. Austen Business Solutions Ltd. Submitted by EFEMA on the 31 January 2011.
EFEMA (European Food Emulsifiers Manufacturers Association), 2016a. Document on mono‐ and di‐glycerides of fatty acid E 471. Specifications, manufacturing methods and chemistry. 3/3/2010. Submitted by EFEMA on 30 September 2016.
EFEMA (European Food Emulsifiers Manufacturers Association), 2016b. EFEMA response to EFSA's request for information on the chemical identity of each individual fatty acid including their percentage in the sources used for each food additive listed in the call for technical data. Submitted by EFEMA on 30 September 2016.
EFEMA (European Food Emulsifiers Manufacturers Association), 2016c. Data on usage levels of mono‐ and di‐glycerides of fatty acids (E 471) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 4), Published 12 October 2015. Submitted to EFSA on 31 May 2016.
EDA (European Dairy Association), 2016. Data on usage levels of mono‐ and di‐glycerides of fatty acids (E 471) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 4), Published 12 October 2015. Submitted to EFSA on 30 May 2016.
EHPM (European federation of Associations of Health Products Manufacturers), 2016. Data on usage levels of mono‐ and di‐glycerides of fatty acids (E 471) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 4), Published 12 October 2015. Submitted to EFSA on 31 May 2016.
FDE (Food Drink Europe), 2016. Data on usage levels of mono‐ and di‐glycerides of fatty acids (E 471) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 4), Published 12 October 2015. Submitted to EFSA on 31 May 2016.
Fischer W, 1998. Production of high concentrated monoglyceride. Lecture given on occasion of the DGF‐ Symposium in Magdeburg/Germany in October 1998. Unpublished document. Submitted by EFEMA on 30 September 2016.
ICGA (International Chewing Gum Association), 2016. Data on usage levels of mono‐ and di‐glycerides of fatty acids (E 471) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 4), Published 12 October 2015. Submitted to EFSA on 31 May 2016.
KRÜGER GmbH & Co. KG, 2016. Data on usage levels of mono‐ and di‐glycerides of fatty acids (E 471) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 4), Published 12 October 2015. Submitted to EFSA on 25 May 2016.
Mars, 2010. Data on usage levels of mono‐ and di‐glycerides of fatty acids (E 471) in foods in response to the EFSA call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents (2009). Submitted to EFSA on 19 May 2010.
Palsgaard, 2011. Data on mono‐ and di‐glycerides of fatty acids (E 471) in response to the EFSA call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents (2009). Submitted by Riemser Arzneimittel AG, May 2010. Submitted by Palsgaard, February 2011.
Pre‐evaluation document prepared by Fraunhofer, September 2013.
Riemser Arzneimittel AG, 2010. Data on usage levels of mono‐ and di‐glycerides of fatty acids (E 471) in foods in response to the EFSA call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents (2009). Submitted by Riemser Arzneimittel AG on 31 May 2010.
SNE (Specialised Nutrition Europe), 2016. Data on usage levels of mono‐ and di‐glycerides of fatty acids (E 471) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 4), Published 12 October 2015. Submitted to EFSA on 30 May 2016.
Abbreviations
- 3‐MCPD
3‐monochloropropane‐1,2‐diol
- ADI
acceptable daily intake
- AESGP
Association of the European Self‐Medication Industry
- ANS
EFSA Scientific Panel on Food Additives and Nutrient Sources added to Food
- BfR
Bundesinstitut für Risikobewertung
- BSTFA
N,N‐bis(trimethylsilyl)trifluoroacetamide
- bw
body weight
- CAS
Chemical Abstracts Service
- CONTAM
EFSA Panel on Contaminants in Food Chain
- EDA
European Dairy Association
- EFEMA
European Food Emulsifiers Manufacturers Association
- EINECS
European Inventory of Existing Chemical Substances
- EHPM
European federation of Associations of Health Products Manufacturers
- ELSD
evaporative light scattering detector
- FAO
Food and Agriculture Organization of the United Nations
- FCs
food categories
- FDE
Food Drink Europe
- FID
flame ionisation detection
- FOB
functional observational battery
- FSMP
foods for special medical purposes
- GC
gas chromatography
- GC x GC‐TOF‐MS
gas chromatography coupled with time‐of‐flight mass spectrometry
- GLP
good laboratory practice
- GMS
glycerol monostearate
- GNPD
Global New Products Database
- HDL
high‐density lipoprotein
- HPLC
high‐performance liquid chromatography
- HPTLC
high‐performance thin layer chromatography
- IARC
International Agency for Research on Cancer
- ICGA
International Chewing Gum Association
- IUPAC
International Union of Pure and Applied Chemistry
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LC–APCI‐MS
liquid chromatography/atmospheric‐pressure chemical ionisation mass spectrometry
- LC–MS
liquid chromatography–mass spectrometry
- MPL
maximum permitted level
- MS
mass spectrometry
- NDA
EFSA Panel on Dietetic Products, Nutrition and Allergies
- NMR
nuclear magnetic resonance
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- PCE
polychromatic erythrocytes
- PKC
protein kinase C
- QS
quantum satis
- SCF
Scientific Committee on Food
- sn
stereospecific numbering
- SNE
Specialised Nutrition Europe
- TemaNord
is a publishing series for results of the often research‐based work that working groups or projects under Nordic Council of Ministers have put in motion
- TDI
tolerable daily intake
- TLC
thin‐layer chromatography
- TMCS
trimethylchlorosilane
- UV
ultravisible
- WHO
World Health Organization
Appendix A – Summary of the reported use levels in food (mg/kg or mg/L) of mono‐ and di‐glycerides of fatty acids (E 471) provided by industry
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.5045
Appendix B – Number and percentage of food products labelled with mono‐ and di‐glycerides of fatty acids (E 471) out of the total number of food products present in the Mintel GNPD per food subcategory between 2012 and 2017
1.
Appendix B can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.5045
Appendix C – Concentration levels of mono‐ and di‐glycerides of fatty acids (E 471) used in the exposure scenarios (mg/kg or mg/L as appropriate)
1.
Appendix C can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.5045
Appendix D – Summary of total estimated exposure of mono‐ and di‐glycerides of fatty acids (E 471) per population group and survey: mean and 95th percentile (mg/kg bw per day)
1.
Appendix D can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.5045
Appendix E – Main food categories contributing to exposure to mono‐ and di‐glycerides of fatty acids (E 471) (> 5% to the total mean exposure)
1.
Appendix E can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.5045
Supporting information
Summary of the reported use levels in food (mg/kg or mg/L) of mono‐ and di‐glycerides of fatty acids (E 471) provided by industry
Number and percentage of food products labelled with mono‐ and di‐glycerides of fatty acids (E 471) out of the total number of food products present in the Mintel GNPD per food subcategory between 2012 and 2017
Concentration levels of mono‐ and di‐glycerides of fatty acids (E 471) used in the exposure scenarios (mg/kg or mg/L as appropriate)
Summary of total estimated exposure of mono‐ and di‐glycerides of fatty acids (E 471) per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to mono‐ and di‐glycerides of fatty acids (E 471) (> 5% to the total mean exposure)
Suggested citation: EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Boon P, Chrysafidis D, Gürtler R, Mosesso P, Tobback P, Rincon AM, Horvath Zs and Lambré C, 2017. Scientific opinion on the re‐evaluation of mono‐ and di‐glycerides of fatty acids (E 471) as food additives. EFSA Journal 2017;15(11):5045, 43 pp. 10.2903/j.efsa.2017.5045
Requestor: European Commission
Question number: EFSA‐Q‐2011‐00557
Panel members: Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipič, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Gunter Georg Kuhnle, Claude Lambré, Jean‐Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The ANS Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 26 September 2017
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
COM(2001) 542 final.
Food Additives in Europe 2000, Status of safety assessments of food additives presently permitted in the EU, Nordic Council of Ministers, TemaNord, 2002, 560.
Food of category 13.1.1: Infant formulae as defined by Directive 2006/141/EC; Food of category 13.1.5.1: Dietary foods for infants for special medical purposes and special formulae for infants. This interpretation also applies to those food additives in food category 13.1.5.2 (Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC) for which exceptional uses in food for infants under the age of 12 weeks are indicated.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p 1.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents. Published: 22 November 2009. Available online: http://www.efsa.europa.eu/en/dataclosed/call/ans091123
Call for food additives usage level and/or concentration data in food and beverages intended for human consumption Published 12 October 2015. Available online: http://www.efsa.europa.eu/en/data/call/151012
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
Commission Regulation (EU) No 694/2014 of 24 June 2014 amending Regulation (EC) No 1881/2006 as regards maximum levels of erucic acid in vegetable oils and fats and foods containing vegetable oils and fats. OJ L 184, 25.6.2014, p 1–2.
Missing Bulgaria, Cyprus, Estonia, Latvia, Lithuania, Luxembourg, Malta and Slovenia.
http://www.gnpd.com/sinatra/home/ accessed on 23/11/2016 (up to the date if the work done on the opinion).
References
- Akoh CC, 1993. Lipase‐catalyzed synthesis of partial glyceride. Biotechnology Letters, 15, 949–954. [Google Scholar]
- Ames S, O'grady MP, Embree N and Harris P, 1951. Molecularly distilled monoglycerides. III. Nutritional studies on monoglycerides derived from cottonseed oil. Journal of the American Oil Chemists Society, 28, 31–33. [Google Scholar]
- Anneken DJ, Both S, Christoph R, Fieg G, Steinberner U and Westfechtel A, 2012. Fatty Acids. Ullmann's Encyclopedia of Industrial Chemistry, 14, 73–116. [Google Scholar]
- Ayyad Z, Valli E, Bendini A, Adrover‐Obrador S, Femenia A and Gallina Toschi T, 2015. Extra virgin olive oil stored in different conditions: focus on diglycerides. Italian Journal of Food Science, 27, 166–172. [Google Scholar]
- Berner D and Dieffenbacher A, 1999. Determination of mono‐ and diacylglycerols in edible oils and fats by high performance liquid chromatography and evaporative light scattering detector: results of collaborative studies and the standardized method (Technical Report). Pure and Applied Chemistry, 71, 1983–1991. [Google Scholar]
- BfR (Bundesinstitut für Risikobewertung), 2009. Initial evaluation of the assessment of levels of glycidol fatty acids esters detected in refined vegetable fats. BfR Opinion No 007/2009, 10 March 2009.
- Boon‐Seang C and Kornél N, 2013. Enrichment and quantification of monoacylglycerols and free fatty acids by solid phase extraction and liquid chromatography–mass spectrometry. Journal of Chromatography B, 932, 50–58. 10.1016/j.jchromb.2013.05.026 [DOI] [PubMed] [Google Scholar]
- Brüschweiler H and Dieffenbacher A, 1991. Determination of mono‐and diglycerides by capillary gas chromatography. Pure and Applied Chemistry, 63, 1153–1162. [Google Scholar]
- Cani PD and Everard A, 2015. Keeping gut lining at bay: impact of emulsifiers. Trends in Endocrinology and Metabolism, 26, 273–274. [DOI] [PubMed] [Google Scholar]
- Caponio F, Paradiso VM, Bilancia MT, Summo C, Pasqualone A and Gomes T, 2013. Diacylglycerol isomers in extra virgin olive oil: effect of different storage conditions. Food Chemistry, 140, 772–776. [DOI] [PubMed] [Google Scholar]
- Chang SW, Huang M, Hsieh YH, Luo YT, Wu TT, Tsai CW, Chen CS and Shaw JF, 2014. Simultaneous production of fatty acid methyl esters and diglycerides by four recombinant Candida rugosa lipase's isozymes. Food Chemistry, 155, 140–145. [DOI] [PubMed] [Google Scholar]
- Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE and Gewirtz AT, 2015. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature, 519, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, de Wiele TV, De Bodt J, Marzorati M and Gewirtz , 2017. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Shou TX, Zhang QY and Li D, 2013. Bioavailability of diacylglycerol microemulsion. Journal of Food Biochemistry, 37, 144–150. [Google Scholar]
- Cheng W, Liu G and Liu X, 2016. Formation of glycidyl fatty acid esters both in real edible oils during laboratory‐scale refining and in chemical model during high temperature exposure. Journal of Agricultural and Food Chemistry, 64, 5919–5927. 10.1021/acs.jafc.6b01520 [DOI] [PubMed] [Google Scholar]
- Chengelis CP, Kirkpatrick JB, Bruner RH, Freshwater L, Moritac O, Tamaki Y and Suzuki H, 2006a. A 24‐month dietary carcinogenicity study of DAG in mice. Food and Chemical Toxicology, 44, 122–137. 10.1016/j.fct.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Chengelis CP, Kirkpatrick JB, Bruner RH, Freshwater L, Moritac O, Tamaki Y and Suzuki H, 2006b. A 24‐month dietary carcinogenicity study of DAG (diaglycerol) in rats. Food and Chemical Toxicology, 44, 98–121. 10.1016/j.fct.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Christie W, 2011. Lipid structure and function: simple lipids. AOAC library.
- Compton DL, Laszlo JA and Evans KO, 2013. Influence of solid supports on acyl migration in 2‐monoacylglycerols: purification of 2‐MAG via flash chromatography. Journal of American oil chemists’ society, 90, 1397–1403. [Google Scholar]
- Craft BD, Nagy K, Seefelder W, Dubois M and Destaillats F, 2012. Glycidyl esters in refined palm (Elaeis guineensis) oil and related fractions. Part II: practical recommendations for effective mitigation. Food Chemistry, 132, 73–79. [DOI] [PubMed] [Google Scholar]
- Destaillats F, Craft BD, Dubois M and Nagy K, 2012. Glycidyl esters in refined palm (Elaeis guineensis) oil and related fractions, Part I: Formation Mechanism. Food Chemistry, 131, 1391–1398. [DOI] [PubMed] [Google Scholar]
- Dhara R and Singhai RS, 2014. Process optimization of enzyme catalyzed production of dietary diagilglycerol (DAG) using TLIM as biocatalyst. Journal of Oleo Science, 63, 169–176. [DOI] [PubMed] [Google Scholar]
- Dieffenbacher A and Pocklington WD, 1992. Standard Methods for the Analysis of Oils, Fats and Derivatives ‐ 1st Supplement to the, 7th Edition Blackwell Science, Oxford. [Google Scholar]
- Dordick JS, 1989. Enzymatic catalysis in monophasic organic solvents. Enzyme and Microbial Technology, 11, 194–211. [Google Scholar]
- EFEMA , 2015. Available online: http://www.emulsifiers.org/files/Index_of_food_emulsifiers_7th%20Edition_FINAL.pdf
- EFSA (European Food Safety Authority), 2007. Scientific opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760 [60 pp.] 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources), 2014. Statement on a conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010. EFSA Journal 2014;12(6):3697, 11 pp. 10.2903/j.efsa.2014.3697 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2017a. Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Dusemund B, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Parent‐Massin D, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Younes M, Boon P, Chrysafidis D, G€urtler R, Tobback P, Rincon AM, Tard A and Lambre C, 2016. Scientific opinion on the re‐evaluation of glycerol (E 422) as a food additive. EFSA Journal 2017;15(3):4720, 64 pp. 10.2903/j.efsa.2017.4720 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Dusemund B, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Parent‐Massin D, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Younes M, Boon P, Chrysafidis D, Gurtler R, Tobback P, Gergelova P, Rincon AM and Lambre C, 2017b. Scientific Opinion on the re‐evaluation of fatty acids (E 570) as a food additive. EFSA Journal 2017;15(5):4785, 48 pp. 10.2903/j.efsa.2017.4785 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009a. Scientific opinion on cadmium in food. EFSA Journal 2009;7(10):980, 139 pp. 10.2903/j.efsa.2009.98 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009b. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351, 199 pp. 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012a. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 241 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012b. Scientific Opinion on lead dietary exposure in the European population. EFSA Journal 2012;10(7):2831, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012c. Scientific Opinion on cadmium dietary exposure in the European population. EFSA Journal 2012;10(1):2551, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on dietary exposure to inorganic arsenic in the European population. EFSA Journal 2014;12(3):3597, 68 pp. 10.2903/j.efsa.2014.3597 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2016. Risks for human health related to the presence of 3‐ and 2‐monochloropropanediol (MCPD), and their fatty acid esters, and glycidyl fatty acid esters in food. EFSA Journal 2016;14(5):4426, 159 pp. 10.2903/j.efsa.2016.4426 [DOI] [Google Scholar]
- EFSA NDA Panel (Scientific Panel on Dietetic Products, Nutrition and Allergies), 2004. Opinion on a request from the Commission related to the presence of trans fatty acids in foods and the effect on human health of the consumption of trans fatty acids. FSA Journal, 81, 1–4. 10.2903/j.efsa.2004.81 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2010. Scientific Opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA Journal 2010;8(3):1461, 107 pp. 10.2903/j.efsa.2010.1461 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Bresson J‐L, Dusemund B, Gundert‐Remy U, Kersting M, Lambre C, Penninks A, Tritscher A, Waalkens‐Berendsen I, Woutersen R, Arcella D, Court Marques D, Dorne J‐L, Kass GEN and Mortensen A, 2017. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. EFSA Journal 2017;15(5):4849, 58 pp. 10.2903/j.efsa.2017.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes JLN, de Souza ROMA and Azeredo RBdV, 2012. 13C NMR quantification of mono and diacylglycerols obtained through the solvent‐free lipase‐catalyzed esterification of saturated fatty acids. Magnetic Redonance in Chemistry, 50, 424–428. 10.1002/mrc.3814 [DOI] [PubMed] [Google Scholar]
- Fregolente PB, Pinto GM, Wolf‐Maciel MR and Filho RM, 2010. Monoglyceride and diglyceride production through lipase‐catalyzed glycerolysis and molecular distillation. Applied Biochemistry and Biotechnology, 160, 1879–1887. [DOI] [PubMed] [Google Scholar]
- Gernert F, 1968. Beitrag zum Nachweis von Emulgatoren in Lebensmitteln. Zeitschrift für Lebensmittel‐Untersuchung und Forschung, 138, 216–220. [Google Scholar]
- Goh EM and Timms RE, 1985. Determination of mono‐ and diglycerides in palm oil, olein and stearin. JAOCS, 62, 730–734. [Google Scholar]
- Gupta MK, 2017. Practical Guide to Vegetable Oil Processing, 2nd Edition AOCS Press, Published by Elsevier Inc. [Google Scholar]
- Hamlet CG, Sadd PA, Crews C, Velisek J and Baxter DE, 2002. Occurrence of 3‐chloro‐propane‐1,2‐diol (3‐MCPD) and related compounds in foods: a review. Food Additives and Contaminants, 19, 619–631. [DOI] [PubMed] [Google Scholar]
- Hamlet CG, Sadd PA and Gray DA, 2003. Influence of composition, moisture, pH and temperature on the formation and decay kinetics of monochloropropanediols in wheat flour dough. European Food Research and Technology, 216, 122–128. [Google Scholar]
- Hamlet CG, Sadd PA and Gray DA, 2004a. Generation of monochloropropanediols (MCPDs) in model dough systems. 1 Leavened doughs. Journal of Agricultural and Food Chemistry, 52, 2059–2066. [DOI] [PubMed] [Google Scholar]
- Hamlet CG, Sadd PA and Gray DA, 2004b. Generation of monochloropropanediols (MCPDs) in model dough systems. 2 Unleavened doughs. Journal of Agricultural and Food Chemistry, 52, 2067–2072. [DOI] [PubMed] [Google Scholar]
- Hamlet CG, Asuncion L, Valisek J, Dolezal M, Zelinková Z, Crews C, Anderson W and Pye C, 2014. Investigation of the formation of 3‐chloropropane‐1‐2‐diol (3‐MCPD) from mono‐ and di‐esters of its fatty acids in foods (FS231006, FS231074, FS231075). Available online: https://www.food.gov.uk/sites/default/files/C04072_Final%20report_July%202014_151014.pdf
- Hari Krishna S and Karanth NG, 2002. Lipases and lipase‐catalysed esterfication reactions in nonaqueous media. Catalysis Reviews: Science and Engineering, 44, 499–591. [Google Scholar]
- Hasenhuettl GL, 2008. Synthesis and Commercial Preparation of Food Emulsifiers. Springer, New York: pp. 11–38. [Google Scholar]
- Hirshegger L, Shoeber S and Mittelbach M, 2014. Efficient and sensitive method for the quantification of saturated monoacylglycerols in biodiesel by gas chromatography‐mass spectrometry. European Journal of Lipid Science and Technology, 116, 89–96. [Google Scholar]
- Hrncirik K and van Duijn G, 2011. An initial study on the formation of 3‐MCPD esters during oil refining. European Journal of Lipid Science and Technology, 113, 374–379. [Google Scholar]
- IARC (World health organization international agency for research on cancer), 2000. Glycidol. IARC monographs on the evaluation of carcinogenic risks to humans. 77, 469‐486. Available online: https://monographs.iarc.fr/ENG/Monographs/vol77/mono77.pdf
- Indrasti D, Che Man YB, Chin ST, Mustafa S, Mat Hashim D and Abdul Manaf M, 2010. Regiospecific analysis of mono‐ and di‐glycerides in glycerolysis products by GC × GC‐TOF‐MS. Journal of the American Oil Chemists’ Society, 87, 1255–1262. [Google Scholar]
- IPCS , 2004. IPCS Risk Assessment Terminology (Part 1: IPCS/OECD Key Generic terms used in Chemical Hazard/Risk Assessment. Available online: http://www.inchem.org/documents/harmproj/harmproj/harmproj1.pdf
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1972. WHO Technical Report Series, Evaluation of certain food additives and the contaminants mercury, lead, and cadmium. Sixteenth report of the Joint FAO/WHO Expert Committee on Food Additives. No. 505. World Health Organization, Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1974. Toxicological evaluation of certain food additives with a review of general principles and of specifications. Seventeenth Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, No.539 & FAO Nutrition Meetings Report Series, No. 53. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1978. Evaluation of certain food additives. Twenty‐first report of the Joint FAO/WHO Expert Committee on Food Additives. No. 617. World Health Organization, Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Monograph 1. Combined compendium of food additive specifications. All specifications monographs from the 1st to the 65th meeting (1956–2005). Volume 4, Available online: http://www.fao.org/ag/agn/jecfa-additives/search.html
- Kanematsu H, Maruyama T, Niiya I, Imamura M and Kawakita H, 1972. Separation and determination of mono‐, diglycerides and propylene glycol mono fatty acids esters in margarine and shortening. Journal of the Japanese Society for Food Science and Nutrition, 25, 46–50. [Google Scholar]
- Kasamatsu T, Ogura R, Ikeda N, Morita O, Saigo K, Watabe H, Saito Y and Suzuki H, 2005. Genotoxicity studies on dietary diacylglycerol (DAG) oil. Food and Chemical Toxicology, 43, 253–260. 10.1016/j.fct.2004.10.001 [DOI] [PubMed] [Google Scholar]
- Kristensen JB, Nielsen NS, Javobsen C and Huiling Mu, 2006. Oxidative stability of diacylglycerol oil and butter blends containing diacylglycerols. European Journal of Lipid Science and Technology, 108, 336–350. 10.1002/ejlt.200500232 [DOI] [Google Scholar]
- Lecomte M, Couedelo Leslie, Meugnier E, Plaisancie P, Letisse M, Bérengère B, Gabert L, Penhoat A, Durand A, Pineau G, Joffre F, Géloën A, Vaysse C, Laugerette F and Michalski MC, 2016. Dietary emulsifiers from milk and soybean differently impact adiposity and inflammation in association with modulation of colonic goblet in high‐fat fed mice. Molecular Nutrition and Food Research, 60, 606–620. [DOI] [PubMed] [Google Scholar]
- Liu J, Lee T, Bobik E Jr, Guzman‐Harty M and Hastillow C, 1993. Quantitative determination of monoglycerides and diglycerides by high‐performance liquid chromatography and evaporative light‐scattering detection. Journal of the American Oil Chemists’ Society, 70, 343–347. [Google Scholar]
- Marcato B and Cecchin G, 1996. Analysis of mixtures containing free fatty acids and mono‐, di‐ and triglycerides by high‐performance liquid chromatography coupled with evaporative light‐scattering detection. Journal of Chromatography A, 730, 83–90. [Google Scholar]
- Martin D, Moran‐Valero MI, Vázquez L, Reglero G and Torres CF, 2014. Comparative in vitro intestinal digestion of 1,3‐diglyceride and 1‐monoglyceride rich oils and their mixtures. Food Research International, 64, 603–609. [DOI] [PubMed] [Google Scholar]
- Masukawa Y, Shiro H, Nakamura S, Kondo N, Jin N, Suzuki N, Ooi N and Kudo N, 2010. A new analytical method for the quantification of glycidol fatty acid esters in edible oils. Journal of Oleo Science, 59, 81–88. [DOI] [PubMed] [Google Scholar]
- Meguro S, Osaki N, Onizawa K, Yajima N, Hase T, Matsuo N and Tokimitsu I, 2006. Comparison of dietary triacylglycerol oil and diacylglycerol oil in protein kinase C activation. Food and Chemical Toxicology, 45, 1165–1172. 10.1016/j.fct.2006.12.024 [DOI] [PubMed] [Google Scholar]
- Merga Y, Campbell BJ and Rhodes JM, 2014. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Digestive Diseases, 32, 475–483. [DOI] [PubMed] [Google Scholar]
- Miranda K, Baeza‐Jimenez R, Noriega‐Rodriguez JA, Garcia HS and Otero C, 2013. Optimization of structured diacylglycerols production containing x‐3 fatty acids via enzyme‐catalysed glycerolysis of fish oil. European Food Research and Technology, 236, 435–440. 10.1007/s00217-012-1889-2 [DOI] [Google Scholar]
- Monteiro JB, Nascimento MG and Ninow JL, 2003. Lipase‐catalyzed synthesis of monoacylglycerol in a homogeneous system. Biotechnology Letters, 25, 641–644. [DOI] [PubMed] [Google Scholar]
- Moonen H and Bas H, 2015. Emulsifiers in food technology In: Nord V. (ed.). Mono‐ and Diglycerides, 2nd Edition John Wiley & sons Ltd, West Sussex, UK: pp. 73–90. [Google Scholar]
- Morita O and Soni MG, 2009. Safety assessment of diacylglycerol oil as an edible oil: a review of the published literature. Food and Chemical Toxicology, 47, 9–21. 10.1016/j.fct.2008.09.044 [DOI] [PubMed] [Google Scholar]
- Morita O, Tamaki Y, Kirkpatrick JB and Chengelis CP, 2008a. Safety assessment of heated diacylglycerol oil: subchronic toxicity study in rats. Food and Chemical Toxicology, 46, 2748–2757. 10.1016/j.fct.2008.04.031 [DOI] [PubMed] [Google Scholar]
- Morita O, Knapp JF, Tamaki Y, Nemec MD, Varsho BJ and Stump DG, 2008b. Safety assessment of dietary diacylglycerol oil: a two‐generation reproductive toxicity study in rats. Food and Chemical Toxicology, 46, 3059–3068. 10.1016/j.fct.2008.06.008 [DOI] [PubMed] [Google Scholar]
- Morita O, Knapp JF, Tamaki Y, Varsho BJ, Stump DG and Nemec MD, 2008c. Effects of dietary diacylglycerol oil on embryo/fetal development in rats. Food and Chemical Toxicology, 46, 2510–2516. 10.1016/j.fct.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Mostafa NA, Maher A and Abdelmoez W, 2013. Production of mono‐, di‐, and triglycerides from waste fatty acids through esterification with glycerol. Advances in Bioscience and Biotechnology, 4, 900–907. [Google Scholar]
- Murlykina N, Upatova O, Yancheva M, Ukleina O and Murlykina M, 2015. Application of infrared spectroscopy for quantitative analysis of new food emulsifiers. Ukrainian Food Journal, 4, 299–309. [Google Scholar]
- Naik MK, Naik SN and Mohanty S, 2014. Enzymatic glycerolysis for conversion of sunflower oil to food based emulsifiers. Catalysis Today, 237, 145–149. [Google Scholar]
- Nejrup RG, Licht TR and Hellgren LI, 2017. Fatty acid composition and phospholipid types used in infant formulas modifies the establishment of human gut bacteria in germ‐free mice. Scientific Reports, 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieva‐Echevarría B, Goicoechea E, Manzanos MJ and Guillén MD, 2014. A method based on 1H NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures. Food Research International, 66, 379–387. [Google Scholar]
- Nieva‐Echevarría B, Goicoechea E, Manzanos MJ and Guillén MD, 2015. Usefulness of 1H NMR in assessing the extent of lipid digestion. Food Chemistry, 179, 182–190. [DOI] [PubMed] [Google Scholar]
- Orten JM and Dajani RM, 1957. A study of the effects of certain food emulsifiers in hamsters. Journal of Food Science, 22, 529–541. [Google Scholar]
- Pacheco C, Palla C, Crapiste GH and Carrin ME, 2014. Simultaneous quantitation of FFA, MAG, DAG, and TAG in enzymatically modified vegetable oils and fats. Food Analytical Methods, 7, 2013–2022. [Google Scholar]
- Paquot C and Hautfenne A, 1987. Standard Methods for the Analysis of Oils, Fats and Derivatives (IUPAC). Blackwell Science, Oxford. [Google Scholar]
- Qi JF, Wang XY, Shin J‐A, Lee Y‐H, Jang Y‐S, Lee JH, Hong S‐T and Lee K‐T, 2015. Relative oxidative stability of diacylglycerol and triacylglycerol oils. Journal of Food Science, 80, C510–C514. 10.1111/1750-3841.12792 [DOI] [PubMed] [Google Scholar]
- Rahn AKK and Yaylayan VA, 2011. What do we know about the molecular mechanism of 3‐MCPD ester formation? European Journal of Lipid Science and Technology, 113, 323–329. [Google Scholar]
- Regula E, 1975. Dünnschichtchromatographischer Nachweis von Calcium‐ und Natrium‐stearoyllactylat neben anderen Emulgatoren in Lebensmitteln. Journal of Chromatography A, 115, 639–644. [DOI] [PubMed] [Google Scholar]
- Renz H, Brandtzaeg P and Hornef M, 2012. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nature Reviews‐Immunology, 12, 9–23. [DOI] [PubMed] [Google Scholar]
- Romano‐Keeler J and Weitkamp JH, 2015. Maternal influences on fetal microbial colonization and immune development. Pediatric Research, 77, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosu R, Yasui M, Iwasaki Y and Yamane T, 1999. Enzymatic synthesis of symmetrical 1,3‐diacylglycerols by direct esterification of glycerol in solvent‐free system. Journal of American Oil Chemists’ Society, 76, 839–843. [Google Scholar]
- Sahasrabudhe MR and Legari JJ, 1968. A chromatographic method for the analysis of propylene glycol fatty acid esters in shortenings containing mono‐ and diglycerides. Journal of the American Oil Chemists Society, 45, 148–151. [DOI] [PubMed] [Google Scholar]
- Sánchez DA, Tonetto GM and Ferreira ML, 2014. Separation of acylglycerides obtained by enzymatic esterification using solvent extraction. Journal of American Oil Chemists’ Society, 76, 839–843 91, 261–270. [Google Scholar]
- SCF (Scientific Committee for Food), 1996. Opinion on additives in nutrient preparations for use in infant formulae, follow‐on formulae and weaning foods. Available online: http://ec.europa.eu/food/fs/sc/scf/reports/scf_reports_40.pdf [Accessed: 7 June 1996]
- SCF (Scientific Committee for Food), 1997. Food science and techniques. Reports of the Scientific Committee for Food, 40th Series. Opinion on additives in nutrient preparations for use in infant formulae, follow‐on formulae and weaning food, expressed on 7 June 1996, pp 13–30.
- SCF (Scientific Committee for Food), 1998. Opinion of the Scientific Committee of Food on the applicability of the ADI (Acceptable Daily Intake) for food additives to infants. Available online: http://ec.europa.eu/food/fs/sc/scf/out13_en.html [Accessed: 17 September 1998].
- SCF (Scientific Committee for Food), 1999. Food science and techniques. Reports of the Scientific Committee for Food, 43rd Series.
- SCF (Scientific Committee for Food), 2001. Guidance on submissions for food additive evaluations by the Scientific Committee on Food. SCF/CS/ADD/GEN/26 Final. 12 July 2001.
- Schmid MJ and Otteneder H, 1976. Nachweis und Bestimmung von Monoglyceridzusatzes zu Teigwaren. Getreide Brot Mehl, 30, 62–64. [Google Scholar]
- Schultz GAS, da Silveira KC, Libardi DB, Peralba MdCR and Samios D, 2011. Synthesis and characterization of mono‐acylglycerols through the glycerolysis of methyl esters obtained from linseed oil. European Journal of Lipid Science and Technology, 113, 1533–1540. 10.1002/ejlt.201100079 [DOI] [Google Scholar]
- Shah R, Kolanos R, DiNovi MJ, Mattia A and Kaneko KJ, 2017. Dietary exposures for the safety assessment of seven emulsifiers commonly added to foods in the United States and implications for safety. Food additives and Contaminants: Part A, 34, 905–917. [DOI] [PubMed] [Google Scholar]
- Soe JB, 1983. Analyses of monoglycerides and other emulsifiers by gas chromatography. Fette, Seifen, Anstrichmittel, 85, 72–76. [Google Scholar]
- Soni MG, Kimura H and Burdock GA, 2001. Chronic study of diaglycerol oil in rats. Food and Chemical Technology, 39, 317–329. [DOI] [PubMed] [Google Scholar]
- Sopelana P, Arizabaleta I, Ibargoitia ML and Guillén MD, 2013. Characterisation of the lipidic components of margarines by 1H nuclear magnetic resonance. Food Chemistry, 141, 3357–3364. [DOI] [PubMed] [Google Scholar]
- Soto G, Hegel P and Pereda S, 2014. Supercritical production and fractionation of fatty acid esters and acylglycerols. The Journal of Supercritical Fluids, 93, 74–81. [Google Scholar]
- Sudraud G, Coustard JM and Retho C, 1981. Analytical and structural study of some food emulsifiers by high‐performance liquid chromatography and off‐line mass spectrometry. Journal of Chromatography, 204, 397–406. [Google Scholar]
- Sugano M, Akahoshi A, Nishida E, Shibuta A and Ohkawa Y, 2002. Dimethylbenz(a)anthracene‐induced mammary tumorigenesis in sprague‐dawley rats fed saturated and polyunsaturated triacyglycerols and diaglycerols. Journal of Oleo Science, 51, 535–588. [Google Scholar]
- Suman M, Silva G, Catellani D, Bersellini U, Caffarra V and Careri M, 2009. Determination of food emulsifiers in commercial additives and food products by liquid chromatography/atmospheric‐pressure chemical ionisation mass spectrometry. Journal of Chromatography A, 1216, 3758–3766. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Loening‐Baucke V and Herber A, 2009a. Mucosal flora in Crohn's disease and ulcerative colitis ‐ an overview. Journal of Physiology and Pharmacology, 60(supplement 6), 61–71. [PubMed] [Google Scholar]
- Swidsinski A, Ung V, Sydora BC, Loening‐Baucke V, Doerffel Y, Verstraelen H and Fedorak RN, 2009b. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflammatory Bowel Diseases, 15, 359–364. [DOI] [PubMed] [Google Scholar]
- Telle‐Hansen VH, Narverud I, Retterstøl K, Wesseltoft‐Rao N, Mosdøl A, Granlund L, Christiansen KF, Lamglait A, Halvorsen B, Holven KB and Ulven SM, 2012. Substitution of TAG oil with diacylglycerol oil in food items improves the predicted 10 years cardiovascular risk score in healthy, overweight subjects. Journal of Nutritional Science, 1, e17 10.1017/jns.2012.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres CF, Vasquez L, Senorans FJ and Reglero G, 2005. Study of the analysis of alkoxyglycerols and other non‐polar lipids by liquid chromatography coupled with evaporative light scattering detector. Journal of Chromatography A, 1078, 28–34. 10.1016/j.chroma.2005.04.015 [DOI] [PubMed] [Google Scholar]
- Tove SB, 1964. Toxicity of saturated fat. Journal of Nutrition, 84, 234–243. [DOI] [PubMed] [Google Scholar]
- Vázquez L, Jordán A, Reglero G and Torres CF, 2016. A first attempt into the production of acylglycerol mixtures from echium oil. Frontiers in Bioengineering and Biotechnology, 3, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von der Haar D, Stäbler A, Wichmann R and Schweiggert‐Weisz U, 2015. Enzyme‐assisted process for DAG synthesis in edible oils. Food Chemistry, 176, 263–270. [DOI] [PubMed] [Google Scholar]
- Waldinger C and Schneider M, 1996. Enzymatic esterification of glycerol III. Lipase‐catalyzed synthesis of regioisomerically pure 1,3‐sn‐diacylglycerols and 1 (3)‐rac‐monoacylglycerols derived from unsaturated fatty acids. Journal of the American Oil Chemists’ Society, 73, 1513–1519. [Google Scholar]
- Wang Y, Zhao M, Tang S, Song K, Han X and Ou S, 2010. Evaluation of the oxidative stability of diacylglycerol‐enriched soybean oil and palm olein under rancimat‐accelerated oxidation conditions. Journal of the American Oil Chemists’ Society, 87, 483–491. 10.1007/s11746-009-1521-1 [DOI] [Google Scholar]
- Wang X, Jin Q, Wang T, Huang J and Wang X, 2013. An improved method for the synthesis of 1‐monoolein. Journal of Molecular Catalysis B: Enzymatic, 97, 130–136. [Google Scholar]
- Wang X, Liang L, Yu Z, Rui L, And Jin Q and Wang X, 2014. Scalable synthesis of highly pure 2‐monoolein by enzymatic ethanolysis. European Journal of Lipid Science and Technology, 116, 627–634. [Google Scholar]
- Wang X, Zou W, Yang W, Jin Q and Wang X, 2015. Preparation of 1,3‐diolein by irreversible acylation. Journal of American Oil Chemists’ Society, 92, 185–191. [Google Scholar]
- Weißhaar R and Perz R, 2010. Fatty acid esters of glycidol in refined fats and oils. European Journal of Lipid Science and Technology, 112, 158–165. [Google Scholar]
- Zha B, Chen Z, Wang L, Wang R, Chen Z and Zheng L, 2014. Production of glycerol monolaurate‐enriched monoacylglycerols by lipase‐catalyzed glycerolysis from coconut oil. European Journal of Lipid Science and Technology, 116, 328–335. [Google Scholar]
- Zhang Z, Wang Y, Ma X, Wang E, Liu M and Yan R, 2015. Characterisation and oxidation stability of monoacylglycerols from partially hydrogenated corn oil. Food Chemistry, 173, 70–79. [DOI] [PubMed] [Google Scholar]
- Zhong N, Cheong LZ and Xu X, 2014. Strategies to obtain high content of monoacylglycerols. European Journal of Lipid Science, 116, 97–107. [Google Scholar]
- Zhou Q, Gao B, Zhang X, Xu Y, Shi H and Yu L, 2014. Chemical profiling of triacylglycerols and diacylglycerols in cow milk fat by ultra‐performance convergence chromatography combined with a quadrupole time‐of‐flight mass spectrometry. Food Chemistry, 143, 199–204. [DOI] [PubMed] [Google Scholar]
- Zhu H, Clegg MS, Shoemaker CF and Wang SC, 2013. Characterization of diacylglycerol isomers in edible oils using gas chromatography–ion trap electron ionization mass spectrometry. Journal of chromatography A, 1304, 194–202. [DOI] [PubMed] [Google Scholar]
- Zlatanos SN, Sagredos AN and Papageorgiou VP, 1985. High yield monoglycerides preparation from glycidol and carboxylic acids. JAOCS, 62, 1575–1576. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the reported use levels in food (mg/kg or mg/L) of mono‐ and di‐glycerides of fatty acids (E 471) provided by industry
Number and percentage of food products labelled with mono‐ and di‐glycerides of fatty acids (E 471) out of the total number of food products present in the Mintel GNPD per food subcategory between 2012 and 2017
Concentration levels of mono‐ and di‐glycerides of fatty acids (E 471) used in the exposure scenarios (mg/kg or mg/L as appropriate)
Summary of total estimated exposure of mono‐ and di‐glycerides of fatty acids (E 471) per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to mono‐ and di‐glycerides of fatty acids (E 471) (> 5% to the total mean exposure)


