Abstract
The Panel on Plant Health performed a pest categorisation of the strawberry bud weevil, Anthonomus signatus Say, (Coleoptera: Curculionidae), for the EU. A. signatus is a well‐defined and distinguishable species, recognised as a pest of strawberry (Fragaria) fruit production in eastern North America where it is also a pest of Rubus. There are reports of A. signatus associated with non‐rosaceous plants such as Mentha, Nepeta, Rhododendron and Solidago although whether such plants are true hosts is uncertain. This pest categorisation focuses on Fragaria and Rubus as hosts. Anthonomus signatus is not known to occur in the EU. It is listed in Annex IIAI of Council Directive 2000/29/EC. The international trade in Fragaria and Rubus plants for planting provides a potential pathway to introduce A. signatus from North America. Considering climatic similarities between North America and the EU, the thermal biology of A. signatus and host distribution in the EU, A. signatus has the potential to establish within the EU. There would be one generation per year, as in North America. As a pest of field grown Fragaria and Rubus, A. signatus would not be expected to establish in EU glasshouses. In North America, adults clip developing buds, preventing fruit development and reducing yield. Losses are variable and depend on the cultivars attacked. Severe crop losses have been reported. However, some Fragaria cultivars can compensate the loss of buds, e.g. by increasing the weight of fruits developing on remaining buds. Phytosanitary measures are available to reduce the likelihood of introduction of A. signatus from North America. All criteria assessed by EFSA for consideration as a potential Union quarantine pest are met. As A. signatus is not known to occur in the EU, this criterion assessed by EFSA to consider it as a Union regulated non‐quarantine pest is not met.
Keywords: Curculionidae, European Union, pest risk, plant health, plant pest, quarantine, strawberry bud weevil
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/2031 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorizations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/ pest categorisation is not available.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. Slight changes have been made to the template to better align with Regulation 2016/2031.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Pyrus L., Ribes L., Rubus L. and Vitis L. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pests categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under ‘such as’ notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches' broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex IB | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Anthonomus signatus is one of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest (RNQP) for the area of the European Union (EU) excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A search of literature (1997–2017) in Web of Science and Scopus was conducted at the beginning of the categorisation. The search focussed on A. signatus and its geographic distribution, life cycle, host plants and the damage it causes. The following search terms (TS) and combinations were used: TS = (“Anthonomus signatus” OR “Strawberry bud weevil”) AND TS = (geograph* OR distribution OR “life cycle” OR lifecycle OR host OR hosts OR plant* OR damag*)
Further references and information were obtained from experts, from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the EPPO Global Database (EPPO 2017)
Data about import of commodity types that could potentially provide a pathway for the pest to enter the EU were obtained from EUROSTAT and from the EU Seventh Framework Programme project ISEFOR (Increasing Sustainability of European Forests, 2007–2013 KBBE 2009‐3 grant agreement 245268). The ISEFOR project examined the plant nursery trade and, for some EU Member States, collected import data on plants for planting at a much more detailed level than is made publically available via EUROSTAT. While it is recognised that the ISEFOR data is not comprehensive, it does contain some data on imports of plants for planting of A. signatus hosts, such as Fragaria and Rubus.
Statistics about the area of hosts grown in the EU were obtained from EUROSTAT.
2.2. Methodologies
The Panel performed the pest categorisation for A. signatus following guiding principles and steps presented in the EFSA guidance on the harmonised framework for pest risk assessment (EFSA PLH Panel, 2010) and as defined in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
In accordance with the guidance on a harmonised framework for pest risk assessment in the EU (EFSA PLH Panel, 2010), this work was initiated following an evaluation of the EU's plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union RNQP in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required as per the specific terms of reference received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to qualify either as a quarantine pest or as a RNQP. If one of the criteria is not met, the pest will not qualify. In such a case, the working group should consider the possibility to terminate the assessment early and be concise in the sections preceding the question for which the negative answer is reached. Note that a pest that does not qualify as a quarantine pest may still qualify as a regulated non‐quarantine pest which needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone, thus the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism. | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pest must be present in the risk assessment area). |
| Regulatory status (Section 3.3 ) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future. |
The protected zone system aligns with the pest free area system under the International Plant Protection Convention (IPPC). The pest satisfies the IPPC definition of a quarantine pest that is not present in the PRA area (i.e. protected zone). |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests' introduction have an economic or environmental impact on the EU territory? | Would the pests' introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6 ) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met. |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regards to the principle of separation between risk assessment and risk management (EFSA founding regulation1); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, while addressing social impacts is outside the remit of the Panel, in agreement with EFSA guidance on a harmonised framework for pest risk assessment (EFSA PLH Panel, 2010).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but, following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
Yes, the identity of the pest is established.
A. signatus Say, 1831 is an insect in the Order Coleoptera (beetles) and the family Curculionidae (weevils). Clark (1991) provides a key to the Anthonomus‐Curvirostris species group which includes A. signatus.
Three junior synonyms are known, Anthonomus bisignatus Gyllenhal, 1836; Anthonomus pallidus Dietz, 1891; and Anthonomus scutellatus Gyllenhal, 1836.
3.1.2. Biology of the pest
A. signatus has one generation per year. Adults can overwinter beneath strawberry plants and plant debris within fields, although most literature reports adults overwintering within the vegetation of adjacent field boundaries and under plant debris in wooded areas (Metcalf and Metcalf, 1993; Smith et al., 1997; Kovach et al., 1999). Adults emerge in the spring and can be found when daytime temperatures are consistently over 16°C, typically from mid‐March in the southern USA to mid‐May in Canada, coinciding with those hosts that have early season bud development (Schaefers, 1978 – cited by Mailloux and Bostanian, 1993; Metcalf and Metcalf, 1993; McPhie and Burrack, 2016). The weevils emerge in spring and feed on young leaves of Fragaria and Rubus and possibly other plants, but most extensively on their flower buds, which provide them the necessary pollen to reach sexual maturity. Afterwards, adults mate and females search for Fragaria and Rubus plants to oviposit and continue feeding.
An adult gravid female will chew a hole in the side of an unopened host flower bud then deposit an egg in the hole. She will then eat about halfway through the pedicel, between 3 and 6 mm below the bud, partially severing the bud from the stem, creating the bud clip effect. This prevents the bud from further development and provides a protected environment for the egg to hatch and for a larva to develop within. The bud can hang from the plant for a few days but will eventually wilt and drop to the ground (Clarke & Howitt, 1975; Mailloux and Bostanian, 1993; Kovach et al., 1999). Each female can lay about 80 eggs, depending on the temperature and they can be seen mating throughout the oviposition period (Baerg, 1923; cited in Smith et al., 1997).
Most feeding occurs at field edges (Campbell et al., 1989; Kovach et al., 1993 in Kovach et al., 1999) up to 15 m in from the field edge (Handley and Dill, 2009) suggesting that adults do not move far or quickly. Foord et al. (2017) reported that A. signatus rarely fly or walk more than 30 feet (approx. 10 m) while looking for food or places to lay eggs.
The egg hatches in 6–14 days and the larva feeds on the immature pollen within the bud for 3–4 weeks. There are three larval instars (Mailloux and Bostanian, 1993). Even when the buds are completely severed from the plant, the buds contain enough nutrients for larvae to complete their development. Pupation occurs within the dry bud and takes 5–8 days. Adult weevils emerge during May and June and feed for a few weeks on pollen and flower petals before searching for overwintering sites in late July and August (Baerg, 1923; cited in Sorensen, 1993; Smith et al., 1997).
On cool, cloudy days, the adults are slow‐moving but in bright warm conditions they can fly short distances (Smith et al., 1997; CSL, 1998).
3.1.3. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, the organism can be detected by visual searching, often after damage symptoms are seen. The species can be identified by examining morphological features, for which keys exist, e.g. Ahmad and Burke (1972); Burke (1968) and Clark (1991).
In North America, there are no generally accepted sampling techniques to monitor for A. signatus (Bostanian et al., 1999). Methods, such as the sticky traps, pheromone traps, sweep nets and beat cloths, to monitor A. signatus are not effective at predicting population size or bud damage (Howard, 2007). In regions with a history of A. signatus damage, monitoring for bud damage should begin at the time of bud emergence and continue once or twice weekly throughout the period of flowering (Handley and Dill, 2009).
A. signatus is noticed when the damage symptoms (clipped buds) are detected, either hanging from the plants or laying on the ground.
Eggs are 0.5 mm long, glassy‐white.
Larvae are 3–4 mm long, off‐white becoming greyish with age. They remain in the bud to pupate.
Pupae are yellowish‐white (2–3 mm × 1–2 mm).
Larva and pupa of A. signatus are well described, keyed by Ahmad and Burke (1972). Adults are about 2.5 mm long. The head is brownish black with an elongated curved rostrum. The elytrae are pale brown to dark reddish brown. A large black spot is often present on each elytron although some adults do not have these spots. Legs are brown. Careful examination is required to reliably distinguish adult and immature stages of these species from those of Anthonomus rubi, the strawberry blossom weevil, which occurs in Europe (CSL, 1998).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
A. signatus is only present in North America and has not been reported in the EU (Smith et al., 1997; EPPO Global database, 2017; Figure 1 and Table 2).
Figure 1.
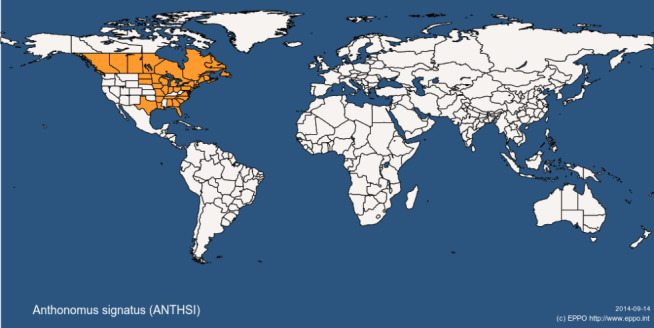
Global distribution map for Anthonomus signatus (extracted from EPPO global database accessed on 20 March 2017)
Table 2.
Current distribution of Anthonomus signatus in North America based on the information from the EPPO Global Database and other sources
| Country | Subnational distribution (e.g. States/Provinces) | References |
|---|---|---|
| Canada |
Alberta, British Columbia, Manitoba, New Brunswick, Newfoundland, Nova Scotia, Ontario Prince Edward Island, Quebec, Saskatchewan |
Bousquet (1991); EPPO GD (Last update: 23/1/2013 Last accessed: 20/3/2017) |
| United States of America | Alabama, Arkansas, Connecticut, Delaware, District of Columbia, Florida, Georgia, Illinois, Iowa, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Missouri, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Virginia, Wisconsin | EPPO GD (Last update : 23/1/2013 Last accessed: 20/3/2017) |
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
No, Anthonomus signatus is not known to occur in the EU.
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
A. signatus is listed in Council Directive 2000/29/EC. Details are shown in Tables 3 and 4.
Table 3.
The listing of Anthonomus signatus within Council Directive 2000/29/EC
| Annex II, Part A | Harmful organisms whose introduction into, and spread within, all Member States shall be banned if they are present on certain plants or plant products | |
| Section I | Harmful organisms not known to occur in the community and relevant for the entire community | |
| (a) | Insects, mites and nematodes, at all stages of their development | |
| Species | Subject of contamination | |
| 4. | Anthonomus signatus | Plants of Fragaria L., intended for planting, other than seeds |
3.3.2. Legislation addressing plants and plant parts on which Anthonomus signatus is regulated
Table 4.
Regulated hosts and commodities that may involve Anthonomus signatus in Annexes III, IV and V of Council Directive 2000/29/EC
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all Member States | |
| 18 | Plants of Cydonia Mill., Malus Mill., Prunus L. and Pyrus L. and their hybrids, and Fragaria L., intended for planting, other than seeds | Without prejudice to the prohibitions applicable to the plants listed in Annex III A (9), where appropriate, non‐ European countries, other than Mediterranean countries, Australia, New Zealand, Canada, the continental states of the USA |
| Annex IV, Part A | Special requirements which must be laid down by all Member States for the introduction and movement of plants, plant products and other objects into and within all Member States | |
| Section I | Plants, plant products and other objects originating outside the community | |
| Plants, plant products and other objects | Special requirements | |
| 21.3. | Plants of Fragaria L., intended for planting other than seeds | Without prejudice to the provisions applicable to the plants listed in Annex III(A)(18), and Annex IV(A)(I)(19.2), (21.1) and (21.2), official statement that the plants originate in an area known to be free from Anthonomus signatus Say and Anthonomus bisignifer (Schenkling) |
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the Community, before being moved within the Community – in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community | |
| Part A | Plants, plant products and other objects originating in the Community | |
| Section II | Plants, plant products and other objects produced by producers whose production and sale is authorised to persons professionally engaged in plant production, other than those plants, plant products and other objects which are prepared and ready for sale to the final consumer, and for which it is ensured by the responsible official bodies of the Member States, that the production thereof is clearly separate from that of other products | |
| 2.1 | Plants intended for planting other than seeds of the genera Abies Mill., Apium graveolens L., Argyranthemum spp., Aster spp., Brassica spp., Castanea Mill., Cucumis spp., Dendranthema (DC) Des Moul., Dianthus L. and hybrids Exacum spp., Fragaria L., Gerbera Cass., Gypsophila L., all varieties of New Guinea hybrids of Impatiens L., Lactuca spp., Larix Mill., Leucanthemum L., Lupinus L., Pelargonium l'Hérit. ex Ait., Picea A. Dietr., Pinus L., Platanus L., Populus L., Prunus laurocerasus L., Prunus lusitanica L., Pseudotsuga Carr., Quercus L., Rubus L., Spinacia L., Tanacetum L., Tsuga Carr. and Verbena L. | |
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
Much North American literature refers to A. signatus as a pest primarily of strawberry (Fragaria) but it is also known as a pest of Rubus fruit such as blackberry (Rubus fruticosus), black raspberry (Rubus occidentalis), dewberry (a group of species in the genus Rubus, closely related to the blackberries) and raspberry (Rubus idaeus).
Campbell et al. (1989) records earlier literature that reports A. signatus occurring on redbud (Cercis sp.), rhododendron (Rhododendron sp.), rambler rose (Rosa multiflora), cinquefoil (Potentilla sp.), apple (Malus sp.), milkweed (Asclepias sp.), goldenrod (Solidago sp.), basswood (Tilia americana), wild bergamot (Monarda fistulosa), mint (Mentha sp.), catnip (Nepeta cataria) and heal‐all (Prunella vulgaris). Whether these are true hosts on which the organism can complete its life cycle is unknown. CABI (2017) regard such reports as incidental occurrences. This categorisation will focus on Fragaria and Rubus because these are the hosts which the US and Canadian literature reports feeding damage and life stage development on.
Current EU legislation (2000/29/EC) only regulates A. signatus on Fragaria plants for planting, other than seed. However, as noted above, A. signatus does have other hosts, at least within the genus Rubus. Given that imports of Rubus plants for planting are permitted from the USA and Canada, A. signatus could potentially be carried into the EU via pathways other than Fragaria plants for planting.
3.4.2. Entry
Is the pest able to enter into the EU territory?
Yes, A. signatus could potentially enter the EU at least via Fragaria and Rubus plants for planting.
The EU can import A. signatus host plants for planting from North America, including from regions where the pest occurs. ‘Green’ strawberry plants are field grown plants grown to be transplanted as plants for planting for fruit production. Rooted plants can be traded in bags with or without growing medium. Due to the growing and shipping conditions, such plants could potentially be contaminated by a range of pests, including A. signatus (EPPO, 2008).
International trade statistics describing the trade in plants for planting are not sufficiently detailed as to allow for the amount of trade in individual plant species to be determined. The CN code that includes strawberry plants for planting is 0602 90 30 (vegetable and strawberry plants). A search of EUROSTAT indicated that there were no EU imports of CN 0602 90 30 materials from Canada during the period 2010–2015; however, there were imports from the USA, shown below in Table 5:
Table 5.
EU imports of vegetable and strawberry plants (CN 0602 90 30) from the USA 2010–2015 (hundreds of kilograms) (Source: EUROSTAT accessed 20/3/2017)
| Year | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Total |
|---|---|---|---|---|---|---|---|
| Sum | 6,875 | 8,373 | 8,031 | 8,408 | 8,449 | 6,188 | 46,324 |
Although EUROSTAT cannot be used to distinguish strawberry plants from vegetable plants for planting, data collected during the ISEFOR project indicate that there was international movement of strawberry plants for planting from the USA into the EU between 2000 and 2012 hence a possible pathway for entry exists (Table 6).
Table 6.
EU MS imports of CN 0602 2090 (trees, shrubs and bushes, grafted or not, of kinds which bear edible fruit or nuts) from the USA and Canada. An unknown proportion of these imports could be Rubus (hundreds of kilograms)
| Source | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Total |
|---|---|---|---|---|---|---|---|
| United States | 858 | 602 | 688 | 632 | 1,055 | 508 | 9,598 |
| Canada | 29 | 2 | 1 | 33 |
Rubus spp. plants for planting can also be imported from North America into the EU. Rubus plants for planting are categorised within CN imports code CN 0602 2090 (trees, shrubs and bushes, grafted or not, of kinds which bear edible fruit or nuts (excl. vine slips).
ISEFOR data indicate that there have been imports of Rubus plants for planting from the USA into the EU. Field grown Rubus plants grown in the USA or Canada to be shipped to the EU and transplanted for fruit production could potentially be contaminated by a range of pests, including A. signatus (Smith et al., 1997).
A. signatus is not known to have spread or been introduced anywhere outside of its native distribution within the eastern USA and Canada.
Up to March 2017, there were zero records of interception of A. signatus in the Europhyt database.
3.4.3. Establishment
Is the pest able to become established in the EU territory?
Yes, biotic factors (host availability) and abiotic factors (climate suitability) suggest that A. signatus would find large parts of the EU suitable for establishment.
3.4.3.1. EU distribution of main host plants
A global distribution map of the area of strawberry harvest is provided as Figure 2, available at CAPRA project website. The map is based on Monfreda et al. (2008) with additional data handling conducted during the EU funded project PRATIQUE (Baker, 2012). The maximum in the legend (dark green) represents the top 5% of the world strawberry distribution (the 95% quantile).
Figure 2.
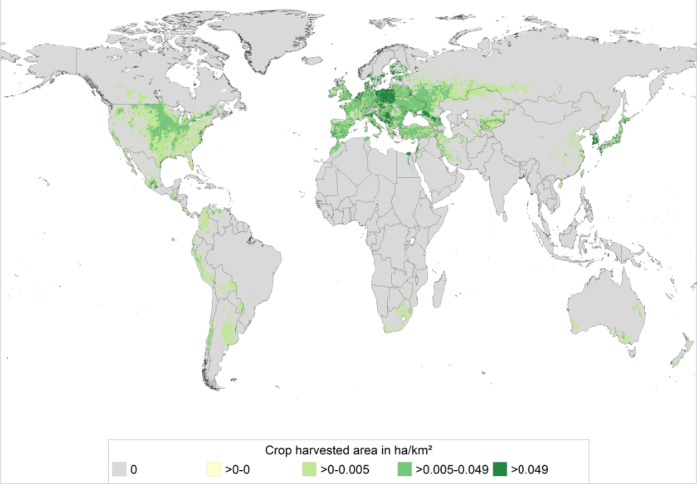
Global distribution of the density of harvested strawberry (ha crop/km2) (source CAPRA database accessed on 20 March 2017)
As indicated in Figure 2, some of the worlds' greatest density of strawberry production occurs in Europe. Table 7 indicates the top three EU countries of strawberry production by area harvested, together with total EU area. Poland grows the largest area of strawberries of any other EU MS. Approximately 70% of the annual area of EU strawberry production occurs in Poland, Germany and Spain. Typically, approximately half of the total EU strawberry area is usually grown in Poland.
Table 7.
EU area of strawberry production 2011–2015 (thousands of hectares) ranked by area
| 2011 | 2012 | 2013 | 2014 | 2015 | 5‐year mean | Mean % | |
|---|---|---|---|---|---|---|---|
| European Union sum | 101.07 | 103.00 | 97.10 | 109.48 | 107.43 | 103.62 | 100.0 |
| Poland | 50.60 | 50.60 | 40.20 | 52.90 | 52.30 | 49.32 | 47.6 |
| Germany | 13.49 | 15.00 | 15.58 | 15.35 | 14.72 | 14.83 | 14.2 |
| Spain | 6.86 | 7.65 | 7.97 | 7.79 | 7.21 | 7.50 | 7.2 |
| Other EU MS | 30.14 | 29.75 | 33.37 | 33.46 | 33.20 | 31.98 | 30.9 |
Table 8 indicates the top three EU countries of raspberry production by area harvested, together with the total EU area. Poland grows more raspberries than all other EU Member States combined. Typically over 80% of the total EU raspberry area occurs in Poland, Bulgaria and Spain.
Table 8.
EU area of raspberry production 2011–2015 (thousands of hectares)
| 2011 | 2012 | 2013 | 2014 | 2015 | 5‐year mean | Mean % | |
|---|---|---|---|---|---|---|---|
| European Union sum | 35.32 | 37.09 | 37.63 | 37.07 | 38.73 | 37.17 | 100.0 |
| Poland | 27.10 | 28.40 | 28.80 | 28.30 | 27.40 | 28.00 | 75.3 |
| Bulgaria | 1.60 | 1.37 | 1.33 | 1.19 | 1.52 | 1.40 | 3.8 |
| Spain | 1.04 | 1.44 | 1.35 | 1.49 | 1.85 | 1.43 | 3.9 |
| Other EU MS | 5.58 | 5.88 | 6.15 | 6.09 | 7.96 | 6.33 | 17.0 |
3.4.3.2. Climatic conditions affecting establishment
A. signatus is distributed in the eastern USA and across Canada (See Figure 1) within a variety of Köppen–Geiger climate zones. The global Köppen–Geiger climate zones (Kottek et al., 2006) describe terrestrial climate in terms of average minimum winter temperatures and summer maxima, amount of precipitation and seasonality (rainfall pattern). In North America, A. signatus occurs in zones Cfa (warm temperate, fully humid hot summer), Cfb (warm temperate, fully humid, warm summer) and Dfb (snow, fully humid, warm summer); climate zones that also cover large portions of the EU where Fragaria and/or Rubus are grown.
Development and phenology studies suggest a temperature threshold for development of A. signatus to be around 8.3°C (Clarke & Howitt, 1975); thresholds below 10°C are typical for species in northern Europe.
Considering its distribution in North America, availability of hosts outdoors in Europe and its threshold temperature for development, A. signatus has the potential to establish in many parts of the EU.
As a pest of field grown strawberries and Rubus, A. signatus would not be expected to establish in glasshouses.
3.4.4. Spread
Is the pest able to spread within the EU territory following establishment? (Yes or No) How?
Yes, as a free living organism, adults can disperse naturally, e.g. by walking and, or flying.
Foord et al. (2017) reported that adult A. signatus rarely fly or walk more than 10 m while looking for food or places to lay eggs, hence spread is likely to be relatively slow. However, if accidentally transported with plants for planting, or as a hitchhiker, the pest could spread over greater distances in a short time.
3.5. Potential or observed impacts in the EU
Would the pests' introduction have an economic or environmental impact in the EU territory?
Yes, the introduction of A. signatus could cause yield losses to susceptible Fragaria and / or Rubus crops.
3.5.1. Potential pest impacts
3.5.1.1. Direct impacts of the pest
Clarke & Howitt (1975) described A. signatus as one of the most important insect pests in Michigan strawberries between 1972 and 1975, after the withdrawal of organochlorine pesticides. A. signatus remained a major pest (Bostanian et al., 1999) and is still regarded as one of key insect pests of strawberries in north eastern North America (McPhie and Burrack, 2016, 2017). Yield loss is quite variable and depends on which buds are cut and which cultivars are fed upon (Kovach et al., 1999). Severe crop loss can be caused by high A. signatus infestations (Pritts et al., 1999). Early cultivars are more susceptible than late cultivars (Dorval, 1938; cited by Mailloux and Bostanian, 1993). Yield losses can have a ‘devastating effect’ on fruit growers because the loss occurs to the early season fruit which are the most valuable (Mailloux and Bostanian, 1993). Studies in the early 20th century cited in later literature quantify losses and note that reductions of 75% were not uncommon (Headlee, 1918; cited by Smith et al., 1997). Other quantified losses in strawberry are reported in Table 9.
Table 9.
Impacts of A. signatus on strawberry fruit production reported in US and Canadian literature
| Impact | Location | References |
|---|---|---|
| Damaging up to 60% of the buds | Manitoba | MacNay (1950) in Campbell et al. (1989) |
| Damaged 39% of the buds | South‐western Quebec | Paradis et al. (1977) in Campbell et al. (1989) |
| 10–64% of 800 inflorescences surveyed in 4 strawberry fields had injury from strawberry bud weevil | Maine | Handley et al. (2000) |
| Yield losses of 10–70% | Quebec | Paradis (1979) in Mailloux and Bostanian (1993) |
| Yield reduction 50–100% | New York | Schaefers (1978) in Mailloux and Bostanian (1993) |
Later literature reports strawberry cultivars compensating the loss of buds by increasing fruit weight from remaining buds and increasing the number of higher order buds matured (English‐Loeb et al., 1999; McPhie and Burrack, 2016, 2017). However, whether compensating growth sufficiently recovers losses from impacts on early season fruit is doubtful. Harvest timing may be affected by A. signatus damage (McPhie and Burrack, 2017) and additional impact, e.g. on quality, cannot be discarded.
As insecticides are the only management tool available against A. signatus (McPhie and Burrack, 2016), and the insect activity period coincides with strawberry bloom, pesticide applications targeting the weevil would coincide with bees and other pollinators visiting Fragaria (as they have entomophilous pollination). As a consequence, negative impacts on pollination services could occur.
3.5.1.2. Indirect pest impacts (e.g. by bacteria or viruses transmitted by the pest)
Whilst a comprehensive literature review was not conducted, of the literature that was used for this categorisation, there was no suggestion that A. signatus transmits any plant pathogens.
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
Yes. The likelihood of pest entry can be mitigated if host plants for planting are sourced from pest free areas. Host plants for planting, such as strawberry plants, should be inspected prior to export to the EU and found free from A. signatus. In places where the pest occurs, insecticides are the only management tool available against A. signatus (McPhie and Burrack, 2016).
3.6.1. Biological or technical factors affecting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
Factors favouring the feasibility and effectiveness of measures to prevent entry, establishment and spread:
Symptoms of the pest, i.e. cut buds, are visible during field inspections.
Adults can be detected by visual inspection.
Strawberry cuttings (stolons) traded as plants for planting without soil or growing media reduces the likelihood that overwintering stages will be present at pathway origin.
Biological factors (one generation per year, sexual reproduction) hinder the likelihood of A. signatus establishing; sluggish movement would hinder its rate of spread in the EU.
Factors disfavouring the feasibility and effectiveness of measures to prevent entry, establishment and spread:
If whole plants for planting are harvested with a little soil, rather than taking aerial runners (stolons), overwintering adults sheltering under older plants could be transported along the pathway.
Larvae develop and pupate inside buds where they are protected from contact insecticides and natural enemies.
3.6.2. Control methods
Growers use chemical insecticides to control A. signatus populations (Bostanian et al., 1999; McPhie and Burrack, 2016, 2017).
Populations are suppressed by removing strawberry plants at the end of the season, to remove in field overwintering sites.
Fields are kept weeds free and plant debris removed.
Tolerant varieties are used in areas where the pest has a history of causing damage.
Mixing staminate varieties (i.e. those with only male flowers) with pistillate varieties (only female flowers) in a ratio of 1:5 can lessen impacts (Metcalf and Metcalf, 1993).
3.7. Uncertainty
Although there are uncertainties, for example regarding the possible impacts that would result from A. signatus establishing within the EU, the uncertainties are not sufficient as to cast doubt as to whether A. signatus satisfies the criteria necessary for it to be regarded as a Union quarantine pest (Table 10).
Table 10.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of the pest is well established; it can be identified to species using conventional entomological keys | The identity of the pest is well established; it can be identified to species using conventional entomological keys | Species identity is key for validity of the categorisation. Although the species may be renamed, its identity would not change |
| Absence/presence of the pest in the EU territory (Section 3.2 ) | The pest is not known to occur in the EU | The pest is not known to occur in the EU and therefore does not meet this criterion to qualify as a Union RNQP | Pest may be present in the EU at non damaging densities and therefore remain undetected |
| Regulatory status (Section 3.3 ) |
For Union quarantine pests, regulatory status is not an explicit criterion to consider within 2016/2031 (but see below regarding feasible measures) The pest is currently officially regulated on Fragaria plants for planting by Dir 2000/29/ EC |
The criteria on pest presence, to be considered as potential regulated non ‐ quarantine pest, is not met, therefore other criteria for consideration as RNQP do not need to be assessed | NONE |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) |
The pest could potentially enter, establish and spread in the EU At least two pathways, Rubus and Fragaria are possible for the pest to enter the EU |
The pest is not present in the EU and therefore other criteria for consideration as an RNQP do not need to be assessed |
The following uncertainties affect the likelihood of entry, establishment or spread but do not change the conclusion that entry, establishment and spread is possible: a) Are plants other than Fragaria and Rubus true hosts? b) Will industry practices change the way plants for planting are handled or the quality of imported plants change and facilitate entry? c) Is entry with fruit possible? d) What is the rate of natural spread? e) Will biotic factors inhibit establishment – e.g. competition from A. rubi? |
| Potential for consequences in the EU territory (Section 3.5 ) | The establishment of the pest in the EU could cause yield losses to strawberry and Rubus fruit grown outdoors. The related European species Anthonomus rubi is regarded as a serious pest of strawberries and A. signatus could cause similar impacts | The pest is not present in the EU; therefore, other criteria for consideration as an RNQP do not need to be assessed |
Much of the literature reporting yield losses is many years old, but it is still relevant today Some US Fragaria cultivars compensate damage to buds by increasing the size of remaining fruit. Would EU cultivars react in the same way? |
| Available measures (Section 3.6 ) | Phytosanitary measures are available to inhibit the likelihood of entry into the EU, e.g. sourcing host plants for planting from pest free areas; prohibiting soil from being carried with host plants for planting | The pest is not present in the EU; therefore, other criteria for consideration as an RNQP do not need to be assessed | The possibility that the pest is actually present in wider areas than thought may hinder the validity of the strategies including measures such as PFA, or PFPP |
| Conclusion on pest categorisation | Anthonomus signatus meets all of the criteria assessed by EFSA above for consideration as a potential Union quarantine pest | Anthonomus signatus is not known to occur in the EU and therefore does not meet at least one of the criteria assessed by EFSA for consideration as a Union regulated non quarantine pest | NONE |
| Aspects of assessment to focus on/scenarios to address in future if appropriate |
While Fragaria and Rubus are recognised hosts, there is uncertainty over the status of other plants on which A. signatus has been found. Research is required to confirm whether such plants are truly hosts There is little uncertainty about the suitability of the abiotic environment in Europe for establishment but work is necessary to better understand the likelihood of entry. Of lesser priority is the clarification of the susceptibility of European host varieties to this pest |
||
4. Conclusions
A. signatus meets the criteria assessed by EFSA for consideration as a potential Union quarantine pest.
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization of the United Nations
- IPPC
International Plant Protection Convention
- ISEFOR
Increasing Sustainability of European Forests
- MS
Member State
- PLH
EFSA Panel on Plant Health
- RNQP
regulated non‐quarantine pest
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Suggested citation: Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Gregoire J‐C, Jaques Miret JA, Navarro MN, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Czwienczek E, Aukhojee M and MacLeod A, 2017. Scientific Opinion on the pest categorisation of Anthonomus signatus . EFSA Journal 2017;15(7):4882, 22 pp. 10.2903/j.efsa.2017.4882
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00197
Panel members: Claude Bragard, David Caffier, Thierry Candresse, Elisavet Chatzivassiliou, Katharina Dehnen‐Schmutz, Gianni Gilioli, Jean‐Claude Gregoire, Josep Anton Jaques Miret, Michael Jeger, Alan MacLeod, Maria Navajas Navarro, Björn Niere, Stephen Parnell, Roel Potting, Trond Rafoss, Vittorio Rossi, Gregor Urek, Ariena Van Bruggen, Wopke Van der Werf, Jonathan West and Stephan Winter.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © EPPO Figure 2: © CAPRA
Adopted: 24 May 2017
Note
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
References
- Ahmad M and Burke HR, 1972. Larvae of the weevil tribe Anthonomini (Coleoptera, Curculionidae). Miscellaneous Publications of the Entomological Society of America, 8, 31–81. [Google Scholar]
- Baerg WJ, 1923. The strawberry weevil. Arkansas Agricultural Experiment Station Bulletin, 185, 3–29. [Google Scholar]
- Baker RHA, 2012. An introduction to the PRATIQUE Research Project. EPPO Bulletin, 42, 1–2. 10.1111/j.1365-2338.2011.02522.x [DOI] [Google Scholar]
- Bostanian NJ, Binns M, Kovach J, Racette G and Mailloux G, 1999. Predictive model for strawberry bud weevil (Coleoptera: Curculionidae) adults in strawberry fields. Environmental Entomology, 28, 398–406. [Google Scholar]
- Bousquet Y, 1991. Checklist of beetles of Canada and Alaska. Research Branch Agriculture Canada Publication, Ottawa, Canada. [Google Scholar]
- Burke HR, 1968. Pupae of the weevil tribe Anthonomini (Coleoptera: Curculionidae). Technical Monographs, Texas Agricultural Experiment Station, 5, 1–92. [Google Scholar]
- CABI , 2017. Anthonomus signatus (strawberry bud weevil). Cabi Crop Protection Compendium Datasheet, CABI Wallingford. Available online: http://www.cabi.org/cpc/datasheet/5731 [Accessed: 27 March 2017]
- Campbell JM, Sarazin MJ and Lyons DB, 1989. Canadian beetles (Coleoptera) injurious to crops, ornamentals, stored products and buildings. Research Branch Agriculture Canada, Publicn No. 1826. 491 p.
- Clark WE, 1991. The Anthonomus‐Curvirostris species group (Coleoptera, Curculionidae). Transactions of the American Entomological Society, 117, 39–66. [Google Scholar]
- Clarke RG and Howitt AJ, 1975. Development of the strawberry weevil under laboratory and field conditions. Annals of the Entomological Society of America 68, 715–718. [Google Scholar]
- CSL , 1998. Strawberry weevils. Quarantine Identification Card No. 33. Central Science Laboratory, York, UK. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2010. PLH Guidance on a harmonised framework for pest risk assessment and the identification and evaluation of pest risk management options by EFSA. EFSA Journal 2010;8(2):1495, 66 pp. 10.2093/j.efsa.2010.1495 [DOI] [Google Scholar]
- English‐Loeb G, Pritts M, Kovach J, Rieckenberg R and Kelly MJ, 1999. Compensatory ability of strawberries to bud and flower removal: Implications for managing the strawberry bud weevil (Coleoptera: Curculionidae). Journal of Economic Entomology, 92, 915–921. [Google Scholar]
- EPPO , 2008. Draft commodity‐specific phytosanitary procedure: consignment inspection of Fragaria plants for planting. Bulletin OEPP/EPPO Bulletin, 38, 396–406. [Google Scholar]
- EPPO , 2017. EPPO Global Database. Available online: https://gd.eppo.int
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- Foord K, Hahn J and Grabowski M, 2017. Strawberry bud weevil. Pest management for the home strawberry patch. University of Minnesota Extension. Available online: http://www.extension.umn.edu/garden/yard-garden/fruit/pest-management-in-the-home-strawberry-patch/strawberry-bud-weevil/ [Accessed: 28 March 2017].
- Handley DT and Dill JF, 2009. A strawberry integrated pest management program for a cold climate, direct‐marketing region. Acta Horticulturae, 842, 645–648. [Google Scholar]
- Handley DT, Wheeler A and Dill JF, 2000. A Survey of Strawberry Inflorescence Injury Caused by Strawberry Bud Weevil, Anthonomus signatus. Abstracts. 97th Annual International Conference of the American Society for Horticultural Science, Disney's Coronado Spring Resort, Lake Buena Vista, Florida, USA 23–26 July 2000.
- Howard CS, 2007. The Impact of the Strawberry Bud Weevil (Anthonomus signatus) on Raspberry (Rubus idaeus) in Maine. University of Maine MSc Thesis, Electronic Theses and Dissertations. Paper 623.
- ISEFOR . Available online: http://www.isefor.com/index.php
- Kottek M, Grieser J, Beck C, Rudolf B and Rubel F, 2006. World map of Köppen‐ Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. [Google Scholar]
- Kovach J, Wilcox W, Agnello A and Pritts M, 1993. Strawberry IPM scouting procedures: a guide to sampling for common pests in New York State. NY IPM No. 203b. Cornell Cooperative Extension, Cornell University, Ithaca, NY. [Google Scholar]
- Kovach J, Rieckenberg R, English‐Loeb G and Pritts M, 1999. Oviposition patterns of the strawberry bud weevil (Coleoptera : Curculionidae) at two spatial scales and implications for management. Journal of Economic Entomology, 92, 1358–1363. [Google Scholar]
- Mailloux G and Bostanian NJ, 1993. Development of the strawberry bud weevil (Coleoptera: Curculionidae) in strawberry fields. Annals of the Entomological Society of America, 86:384–393. [Google Scholar]
- McPhie D and Burrack HJ, 2016. Effects of microbial, organically acceptable, and reduced risk insecticides on Anthonomus signatus (Curculionidae: Coleoptera) in strawberries (Fragaria ananassa). Crop Protection, 89, 255–258. 10.1016/j.cropro.2016.07.034 [DOI] [Google Scholar]
- McPhie D and Burrack HJ, 2017. Effect of Simulated Anthonomus signatus (Coleoptera: Curculionidae) Injury on Strawberries (Fragaria × ananassa) Grown in Southeastern Plasticulture Production. Journal of Economic Entomology, 110, 208–212. 10.1093/jee/tow266 [DOI] [PubMed] [Google Scholar]
- Metcalf RL and Metcalf RA, 1993. Destructive & Useful Insects, 5th Edition. McGraw‐Hill, New York. [Google Scholar]
- Monfreda C, Ramankutty N. and Foley JA, 2008. Farming the planet: 2. Geographic distribution of crop areas, yields, physiological types, and net primary production in the year 2000. Global Biogeochemical Cycles, 22, 1–19. [Google Scholar]
- Pritts MP, Kelly MG and English‐Loeb G, 1999. Strawberry Cultivars Compensate for Simulated Bud Weevil (Anthonomus signatus Say) Damage in Matted Row Plantings', HortScience, 34, 109–111. [Google Scholar]
- Schaefers G, 1978. Efficacy of Lorsban brand insecticides for the reduction of strawberry ‘bud’ weevil, Anthonomus signatus Say, damage in strawberries. Down to Earth, 35, 1–3. [Google Scholar]
- Smith IM, McNamara DG, Scott PR and Holderness M, 1997. Anthonomus signatus. In Quarantine Pests for Europe, 2nd Edition. CABI / EPPO, Wallingford, 1425 pp.
- Sorensen KA, 1993. The strawberry weevil. Available online: http://ipm.ncsu.edu/small_fruit/Strawberry_Weevil.html [Accessed: 28 March 2017]


