Abstract
The EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) provides a scientific opinion re‐evaluating the safety of polyglycerol esters of fatty acids (PEFA) (E 475) when used as a food additive. In 1978, the Scientific Committee on Food (SCF) endorsed an acceptable daily intake (ADI) of 25 mg/kg body weight (bw) per day previously established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Absorption of intact PEFA in the gastrointestinal tract was extremely low. PEFA was rapidly and almost fully hydrolysed to polyglycerols and fatty acids in the gastrointestinal tract. The safety of polyglycerols and specific fatty acids has recently been assessed and no adverse effects were identified in the available studies. No adverse effects of PEFA at any dose have been observed in short‐term, subchronic or chronic toxicity studies. A no observed adverse effect level (NOAEL) of 9,000 mg/kg bw per day was identified from subchronic studies and of 2,500 mg/kg bw per day from chronic studies, the highest doses tested. No genotoxic potential of PEFA was identified from the limited information available. The reproductive toxicity studies showed no adverse effects of PEFA but had major limitations. Clinical chemistry and urinalysis, from a clinical study with limited information, did not reveal any adverse effects in volunteers receiving up to 300 mg/kg bw per day for 3 weeks. The highest exposure to PEFA used as a food additive was 2.6 and 6.4 mg/kg bw per day in children at the mean and the 95th percentile, respectively, for the non‐brand loyal scenario. Considering all the above, the Panel concluded that the food additive PEFA (E 475) was not of safety concern at the reported uses and use levels and that there was no need for a numerical ADI. The Panel recommended some modifications of the EU specifications for E 475.
Keywords: polyglycerol esters of fatty acids, polyglyceryl fatty acid esters, PEFA, E 475
Summary
Polyglycerol esters of fatty acids (PEFA) (E 475) is authorised as a food additive in the European Union (EU) in accordance with Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/2012.
In 1978, the Scientific Committee on Food (SCF) endorsed an acceptable daily intake (ADI) of 25 mg/kg body weight (bw) per day previously established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1974 based on a long‐term feeding study in rats showing no adverse effects at 5% PEFA in the diet (corresponding to 2,500 mg/kg bw per day). In addition, JECFA stated that alterations in the fatty acids distribution or the polyglycerol content of the food additive PEFA would have no toxicological bearing but requested proper biochemical studies on other members of PEFA, particularly those containing short‐chain fatty acids. In 1990, JECFA discussed a request to alter the specifications of PEFA and to increase the average polyglycerol chain lengths permitted from three to ten glycerol units. However, the Committee was unable to accept this request since no data supporting this request were submitted. Finally, JECFA decided to maintain the ADI of 0–25 mg/kg bw per day for PEFA having an average chain length of up to three glycerol units.
According to the EU specifications for E 475, PEFA (E 475) is a mixture of reaction products formed by the esterification of polyglycerols with food fats and oils or with fatty acids occurring in foods, fats and oils. The polyglycerol moiety is predominantly di‐, tri‐ and tetraglycerol and contains not more than 10% of polyglycerols equal to or higher than heptaglycerol. According to the information provided by industry on fatty acid composition of vegetable fats and oils used for the manufacturing process of E 475, palmitic acid, stearic acid, oleic acid and linoleic acid are the main fatty acids present in the raw materials.
Depending on manufacturing processes and starting materials, toxic and potentially carcinogenic impurities such as epichlorohydrin, glycidol, erucic acid and trans‐fatty acids may be present in PEFA (E 475). There is thus a need to include maximal levels for these impurities in the specifications of PEFA (E 475).
The dietary exposure to PEFA (E 475) from its use as a food additive was calculated according to different scenarios. PEFA (E 475) is authorised in 16 food categories; seven data on use levels were provided for only three food categories. The majority of these data (six out of seven) were related to niche products. Furthermore, the Panel noted that, considering information from the Mintel's Global New Products Database (GNPD), PEFA (E 475) is used in some food categories for which no use levels were provided to the European Food Safety Authority (EFSA), such as dietary supplements, eggs and egg products, desserts and dairy analogues, including beverage whiteners. The Panel further noted that the exposure to PEFA (E 475) from its use according the Annex III to Regulation (EC) No 1333/2008 (Part 1, 2, 5) was not considered in the exposure assessment. Therefore, the exposure to PEFA (E 475) in the refined exposure scenarios may have been underestimated. However, the possible underestimation of the exposure may have been negated by the assumption that all foods belonging to one of the three included food categories contain the food additive, whereas according to the Mintel's GNPD on average only 1.2% of the authorised food items contained PEFA (E 475). The use of use levels in niche products in the refined exposure estimation may also have contributed to an overestimation of the exposure. Niche products are often specific products that contain different, often higher, levels of a food additive than the other foods belonging to the same food category. Applying these levels to all foods may have resulted in an overestimation of the exposure. The exposure in the regulatory maximum level exposure scenario is very likely overestimated; 11 food categories were included assuming that all foods belonging to a food category contain the additive at a level equal to the maximum permitted level (MPL).
Based on the considerations described above, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure to PEFA (E 475) as a food additive in all exposure scenarios assuming that the food additive is not used in the food categories in which it is permitted but for which no usage data were provided.
The Panel did not identify brand loyalty to a specific food category for the exposure to PEFA (E 475), and therefore considered the non‐brand‐loyal scenario covering the general population as the most appropriate and realistic scenario for risk characterisation of the food additive. It is assumed that the population is most likely exposed long‐term to PEFA (E 475) present at the mean reported use in processed food. Based on these exposure estimates, it is not likely that the exposure to PEFA (E 475) will exceed the current ADI of 25 mg/kg bw per day set by JECFA and endorsed by the SCF.
PEFA were hydrolysed in the gastrointestinal tract followed by absorption of polyglycerol and the fatty acid moiety. Although the polyglycerol appears to be excreted unchanged, the fatty acids were either rapidly metabolised to carbon dioxide or incorporated in the body.
The Panel noted that the available data showed a very low acute oral toxicity of PEFA. There were several short‐term or subchronic toxicity studies with PEFA. The studies have limitations; however, no adverse effects were observed up to 10% in the diet (equivalent to 9,000 mg/kg bw per day), the highest dose tested. The available limited information on the genotoxicity of PEFA did not indicate a genotoxic potential. The available chronic toxicity and carcinogenicity studies with PEFA in mice and rats also had limitations. However, none of these studies gave any indication of a carcinogenic potential of PEFA. No maternal, reproductive or developmental toxicity were reported from two old dietary three‐generation reproductive toxicity studies. Prenatal developmental toxicity studies were not available.
In a clinical study with limited information, 37 volunteers were exposed to 2–20 g PEFA per day (up to 300 mg/kg bw per day) for 3 weeks. Clinical chemistry and urinalysis did not reveal any adverse effects.
The Panel noted that very few relevant studies on the biological effects of PEFA have been published since the JECFA and the SCF evaluations. The Panel also noted that no adverse effects of PEFA at any dose have been observed in short‐term, subchronic or chronic toxicity studies. A no observed adverse effect level (NOAEL) of 9,000 mg/kg bw per day was identified from subchronic studies and of 2,500 mg/kg bw per day from chronic studies, the highest doses tested. The limited information on genetic toxicity did not identify any genotoxic potential of PEFA. The available reproductive toxicity studies showed no adverse effects of PEFA but had major limitations.
Furthermore, the Panel noted that the absorption of intact PEFA before hydrolysis to polyglycerols and fatty acids in the gastrointestinal tract is extremely low. The safety of polyglycerols and fatty acids has recently been assessed in the opinions on the re‐evaluation of polyglycerol polyricinoleate (E 476) and of fatty acids (E 570). No adverse effects of polyglycerols or fatty acids were identified in studies reported in these opinions.
Considering that:
absorption of intact PEFA in the gastrointestinal tract was extremely low;
PEFA was rapidly and almost fully hydrolysed to polyglycerols and fatty acids in the gastrointestinal tract;
The safety of polyglycerols and specific fatty acids has recently been assessed and no adverse effects were identified in the available studies even at the highest doses tested;
Although limited, the available database on PEFA did not give any indication of adverse effects in short‐term, subchronic, chronic, or reproductive toxicity studies neither of any genotoxic potential;
in a clinical study with limited information, volunteers exposed to PEFA (up to 300 mg/kg bw per day for 3 weeks), clinical chemistry and urinalysis did not reveal any adverse effects;
the highest exposure to PEFA used as a food additive was 2.6 and 6.4 mg/kg bw per day in children at the mean and the 95th percentile, respectively, at the non‐brand loyal scenario.
The Panel concluded that the food additive PEFA (E 475) was not of safety concern at the reported uses and use levels and that there was no need for a numerical ADI.
The Panel recommended that:
the European Commission considers lowering the current limits for toxic elements (arsenic, lead, mercury and cadmium) in the EU specifications for PEFA (E 475 in order to ensure that the food additive will not be a significant source of exposure to these toxic elements in food.
the European Commission considers revising the EU specifications for PEFA (E 475) including maximum limits for epichlorohydrin and glycidol, given that during the manufacturing processes of polyglycerols these genotoxic impurities could be present.
the European Commission considers revising the EU specifications for PEFA (E 475) including maximum limits for trans fatty acids because PEFA (E 475) can be manufactured by glycerolysis of hydrogenated fats and/or oils, which contain significant amounts of trans fatty acids.
the European Commission considers revising the EU specifications for PEFA (E 475) including maximum limits for glycidyl esters/glycidol and 3‐monochloropropane‐1,2‐diol (3‐MCPD) esters, because it is likely that residues of those substances occur in the food additive PEFA (E 475), if they were present in the raw materials used in the manufacturing of the food additive by transesterification.
the European Commission considers revising the EU specifications for PEFA (E 475) including maximum limits for erucic acid since erucic acid can be present among the fatty acids in edible oils, which can be used for manufacturing of PEFA (E 475).
the European Commission considers revising the EU specifications for PEFA (E 475) including maximum limits for impurities currently included in the EU specifications for glycerol (E 422) or recommended by the Panel in the re‐evaluation of glycerol (E 422) (EFSA ANS Panel, 2017a,b,c).
1. Introduction
The present opinion deals with the re‐evaluation of the safety of polyglycerol esters of fatty acids (E 475) when used as a food additive.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union. In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the European Union before 20 January 2009 has been set up under the Regulation (EU) No 257/20101. This Regulation also foresees that food additives are re‐evaluated whenever necessary in light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU of 2001. The report “Food additives in Europe 2000” submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010 the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference
The Commission asks the European Food Safety Authority to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.2. Information on existing evaluations and authorisations
Polyglycerol esters of fatty acids (E 475) is a food additive authorised according to Annex II and Annex III of Regulation (EC) No 1333/2008. Specific purity criteria have been defined in Commission Regulation (EU) No 231/20122.
The latest toxicological evaluation for polyglycerol esters of fatty acids (PEFA) presented by JECFA was published in 1974 (JECFA, 1974a). Based on a long‐term feeding study in rats showing no adverse effects at 5% PEFA in the diet (corresponding to 2,500 mg/kg body weight (bw) per day), an ADI of 0–25 mg/kg bw per day was established by applying an uncertainty factor of 100. In addition, JECFA stated that alterations in the fatty acids distribution or the polyglycerol content of the food additive PEFA would have no toxicological bearing but requested proper biochemical studies on other members of PEFA, particularly those containing short‐chain fatty acids (JECFA, 1974b).
In 1990, JECFA discussed a request to alter the specifications of PEFA and to increase the average polyglycerol chain lengths permitted from three to ten glycerol units. However, JECFA was unable to accept this request since no data supporting this request were submitted. Finally, JECFA decided to maintain the ADI of 0–25 mg/kg bw per day for PEFA having an average chain length of up to three glycerol units; the specifications were not changed (JECFA, 1990) and a new toxicological monograph was not prepared.
Toxicological data for PEFA were also evaluated by the SCF in 1978 (SCF, 1978). The Committee endorsed the ADI of 0–25 mg/kg bw established by the JECFA Committee (1974a,b) and considered the database to be acceptable. No details on the toxicological evaluation were given.
PEFA has also been reviewed by the Nordic Council of Ministers (TemaNord, 2002). It was concluded that PEFA have not been fully tested according to modern standards but accepted that PEFA is hydrolysed to its constituents, fatty acids and polyglycerols after ingestion. A re‐evaluation was considered not to be necessary because of the metabolic fate of PEFA.
2. Data and methodologies
2.1. Data
The Panel on Food Additives and Nutrient Sources added to Food (ANS) was not provided with a newly submitted dossier. EFSA launched public calls for data,3 , 4 , 5 , 6 to collect relevant information from interested parties.
The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up to November 2017. Attempts were made at retrieving relevant original study reports on which previous evaluations or reviews were based, however not always these were available to the Panel.
Food consumption data used to estimate the dietary exposure to PEFA (E 475) were derived from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database7).
The Mintel's Global New Products Database (GNPD) was used to verify the use of PEFA (E 475) in food and beverage products and food supplements. The Mintel's GNPD is an online database that contains the compulsory ingredient information present on the label of numerous products.
2.2. Methodologies
The assessment was conducted in line with the principles described in the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing Guidances from the EFSA Scientific Committee.
The ANS Panel assessed the safety of PEFA (E 475) as a food additive in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the Scientific Committee on Food (SCF, 2001).
When the test substance was administered in the feed or in the drinking water, but doses were not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee (2012) for studies in rodents or, in the case of other animal species, by JECFA (2000).
Dietary exposure to PEFA (E 475) from its use as a food additive was estimated by combining the food consumption data available within the EFSA Comprehensive Database with the maximum permitted levels and/or reported use levels and analytical data submitted to EFSA following a call for data. The exposure was estimated according to different exposure scenarios (see Section 3.4). Uncertainties in the exposure assessment were identified and discussed with regard to their impact on the final exposure estimates.
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
PEFA (E 475) is a mixture of reaction products formed by the esterification of polyglycerols with food fats and oils or with fatty acids occurring in foods, fats and oils. The polyglycerol moiety is predominantly di‐, tri‐ and tetraglycerol and contains not more than 10% of polyglycerols equal to or higher than heptaglycerol (Commission Regulation (EU) No 231/2012).
No EINECS number has been assigned in the Commission Regulation (EU) No 231/2012 for this food additive. The CAS Registry number for E 475 is 503590‐90‐7; however, no EINECS number correlated to this CAS Registry number in the EC inventory.
PEFA is also known by the synonyms polyglycerol fatty acid esters and polyglycerin esters of fatty acid esters (Commission Regulation (EU) No 231/2012).
PEFA includes a large group of closely related compounds (e.g. diglycerol monolaureate, triglycerol distearate, tetraglycerol tetraoleate) and therefore no single structure, molecular formula and molecular weight can be assigned. A general structural formula is given in Figure 1.
Figure 1.
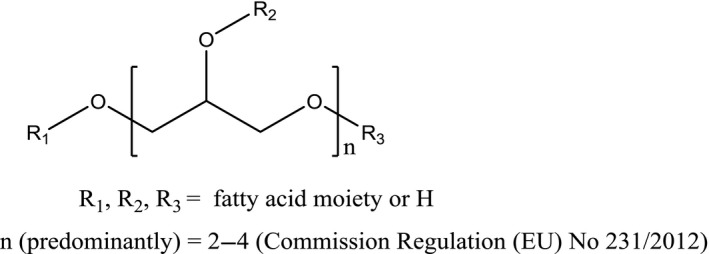
General structural formula of PEFA (E 475)
The composition of polyglycerol esters in PEFA depends on the carbon chain length of the fatty acid moieties and the number of glycerol monomers in the polyglycerol group. The variety of components can be explained by the composition of the starting materials and by the manufacturing process (see Section 3.1.3).
The polyglycerol moiety of PEFA is a linear condensation product of glycerol, where primary hydroxyl groups react to form an ether linkage. Some cyclic condensation products may be present (Behrens and Mieth, 1984). The composition of the alcoholic component was examined by gas chromatographic analysis in 11 commercial PEFA products (Gross, 1980). The content was: glycol 0.7–2.6% (average 1.3%), glycerol 13.6–36.2% (average 26.2%), cyclic diglycerol 0.6–8.8% (average 4.9%), diglycerol 17.4–29.1% (average 22.7%), triglycerol 12.7–22.8% (average 15.6%), tetraglycerol 7.0–11.5% (average 9.1%), pentaglycerol 4.1–8.1% (average 6.0%), hexaglycerol 2.1–6.6% (average 3.8%), hepta‐ and higher glycerols 1.9–6.6% (average 3.6%) and non‐linear polyglycerols or not identified compounds 3.1–12.8% (average 6.8%) (Gross, 1980).
PEFA (E 475) may contain minor amounts of mono‐, di‐ and triglycerides together with free glycerol and polyglycerols and free fatty acids. Salts of fatty acids may also be present (EFEMA, 2009 (Documentation provided to EFSA n. 1)).
PEFA (E 475) being not a single compound, there are no well‐defined values for its physicochemical properties. Only limited information about these properties is available in the literature.
According to Cosmetics Ingredient Review (CIR, 2016), the physical properties and appearance of polyglycerol esters of fatty acids mainly depend on their molecular structure; those with a higher degree of polymerisation and shorter or unsaturated fatty acid chains are viscous to plastic pastes, and the polyglycerol esters with a lower degree of polymerisation and longer, saturated fatty acid chains are generally processed in the form of powders, flakes or small beads. The colour of the esters is dependent on the source of the fatty acids, but the polyglycerol will also add to the colour of the ester. The solubility of polyglycerol esters in organic solvents depends on the nature of the solvent and on the polarity of the ester, but generally the esters will show best solubility in protic and dipolar aprotic solvents, such as lower alcohols and dimethyl sulfoxide (DMSO) (CIR, 2016).
The water solubility depends on the carbon chain length of the fatty acids and the degree of esterification (Behrens and Mieth, 1984). Polyglycerol esters of fatty acids with short‐ or medium‐chain length are soluble in water, while long‐chain compounds are dispersible. The polyglycerol esters are more hydrophilic with increasing molecular weight of the polyol, and more hydrophobic with increasing chain length of the fatty acids (Behrens and Mieth, 1984).
3.1.2. Specifications
Specifications for PEFA (E 475) have been defined in Commission Regulation No 231/2012 and by JECFA (JECFA, 2006), as described in Table 1.
Table 1.
Specifications for PEFA (E 475) according to Commission Regulation (EU) No 231/2012 and JECFA (2006)
| Commission Regulation No 231/2012 | JECFA (2006) | |
|---|---|---|
| Definition |
Polyglycerol esters of fatty acids are produced by the esterification of polyglycerol with food fats and oils or with fatty acids occurring in foods fats and oils The polyglycerol moiety is predominantly di‐, tri‐ and tetraglycerol and contains not more than 10% of polyglycerols equal to or higher than heptaglycerol |
Mixed partial esters formed by reacting polymerised glycerols with edible fats, oils or fatty acids; minor amounts of mono‐, di‐ and triglycerides, free glycerol and polyglycerols, free fatty acids and sodium salts of fatty acids may be present; degree of polymerisation varies, and is specified by a number (such as tri‐) that is related to the average number of glycerol residues per polyglycerol molecule. A specified polyglycerol consists of a distribution of molecular species characteristic of its nominal degree of polymerisation. By varying the proportions as well as the nature of the fats or fatty acids to be reacted with the polyglycerols, a large and diverse class of products may be obtained The article of commerce may be further specified as to saponification value, solidification point of the free fatty acids, iodine value, hydroxyl value and ash content |
| Assay | Content of total fatty acid ester not less than 90% | |
| Description | Light yellow to amber, oily to very viscous liquids; light tan to medium brown, plastic or soft solids; and light tan to brown, hard, waxy solids | Light yellow to amber, oily to very viscous liquids; light tan to medium brown, plastic or soft solids; and light tan to brown, hard, waxy solids |
| Identification | ||
| Tests for glycerol and polyglycerols | Passes test | Passes test |
| Tests for fatty acids | Passes test | Passes test |
| Solubility | The esters range from very hydrophilic to very lipophilic, but as a class tend to be dispersible in water and soluble in organic solvents and oils | From very hydrophilic to very lipophilic, but as a class tend to be dispersible in water and soluble in organic solvents and oils |
| Purity a | ||
| Sulfated ash | Not more than 0.5% (800 ± 25°C) | – |
| Acids other than fatty acids | Less than 1% | Acids other than fatty acids should not be detectable |
| Free fatty acids | Not more than 6% estimated as oleic acid | – |
| Total glycerol and polyglycerol | Not less than 18% and not more than 60% | – |
| Free glycerol and polyglycerol | Not more than 7% | – |
| Arsenic | Not more than 3 mg/kg | – |
| Lead | Not more than 2 mg/kg | Not more than 2 mg/kg |
| Mercury | Not more than 1 mg/kg | – |
| Cadmium | Not more than 1 mg/kg | – |
| Polyglycerols | The polyglycerol moiety should be composed of not less than 70% of di‐, tri‐ and tetraglycerols and should contain no more than 10% of polyglycerols equal to or higher than heptaglycerol | |
Purity criteria apply to the additive free of sodium, potassium and calcium salts of fatty acids, however, these substances may be present up to a maximum level of 6% (expressed as sodium oleate).
The Panel noted that, according to the EU specifications for PEFA (E 475), impurities of the toxic elements arsenic, cadmium, lead and mercury are accepted up concentrations of 3, 1, 2 and 1 mg/kg, respectively. Contamination at those levels could have a significant impact on the exposure already are close to the health based guidance values or benchmark doses (lower confidence limits) established by EFSA (EFSA CONTAM Panel, 2009a,b, 2010, 2012a,b,c, 2014).
The Panel noted that according to EU specifications there is no value (only predominantly) for the content of di‐, tri‐ and tetraglycerols, but in JECFA > 70% is given.
The Panel noted that epichlorohydrin and glycidol may be present in PEFA (E 475) from the manufacturing processes of polyglycerols (Bastida‐Rodriguez, 2013). Epichlorohydrin and glycidol are classified as a carcinogen (2A) according to IARC (1999) and probably carcinogenic to humans (2A) according to IARC (2000), respectively. The EFSA Panel on Contaminants in Food Chain (CONTAM) has characterised glycidol as genotoxic and carcinogenic (EFSA CONTAM Panel, 2016). The Panel considered that the presence of epichlorohydrin and/or glycidol in PEFA (E 475) would need further assessment as their presence could raise a safety concern.
The Panel noted that a maximum residual level for 3‐monochloropropane‐1,2‐diol (3‐MCPD) (not more than 0.1 mg/kg) has been established in EU specifications for glycerol (E 422) (Commission Regulation (EU) No 231/2012), which can be used for manufacturing of polyglycerol. Esters of 3‐MCPD are also contaminants of processed vegetable oils. Esters of 3‐ MCPD were found at the highest levels in palm oil/fat, which can be used as raw material for PEFA (E 475). It has been confirmed that the toxicity of 3‐MCPD fatty acid esters should be considered equivalent (on a molar basis) to that of the parent compound (3‐MCPD) (EFSA CONTAM Panel, 2016). The Panel noted that there is no limit for 3‐MCPD/3‐MCPD esters in the specifications for PEFA (E 475). The Panel considered that the possible presence of 3‐MCPD esters in PEFA (E 475) would need further assessment, as their presence could raise a safety concern.
Information on the manufacturing processes of glycerol has been considered by the ANS Panel in the re‐evaluation of glycerol (E 422) (EFSA ANS Panel, 2017a). The Panel noted that glycerol (E 422) can be produced by a variety of methods and that many of them lead to the presence or formation of contaminants, which are of toxicological concern. The Panel considered that the manufacturing process for PEFA (E 475) should not allow the presence of residuals of genotoxic or/and carcinogenic concern at a level which would result in a margin of exposure (MOE) below 10,000. The Panel considered that maximum limits for potential impurities in glycerol as raw material in the manufacturing process of PEFA should also be established for the EU specifications for PEFA (E 475).
PEFA (E 475) can be manufactured by glycerolysis of natural or hydrogenated fats and oils. According to EFSA (EFSA NDA Panel, 2004), industrial hydrogenation (used to produce semi‐solid and solid fats that can be used for the production of foods such as margarines, shortenings and biscuits) and deodorisation (a necessary step in refining) of unsaturated vegetable oils high in polyunsaturated fatty acids is one of the three main pathways for the formation of trans fatty acids in food. According to EFSA (EFSA NDA Panel, 2010), higher intakes of trans fatty acids have consistently been found to be associated with an increased risk of coronary heart disease and it was recommended that trans‐fatty acids intake should be as low as possible within the context of a nutritionally adequate diet. The Panel noted that there is no limit for trans fatty acids in the specifications for PEFA (E 475).
According to the EFSA CONTAM Panel, refined vegetable oil, which can be used for manufacturing of PEFA (E 475), is the only identified source of glycidyl esters of fatty acids (EFSA CONTAM Panel, 2016). Glycidyl esters of fatty acids are hydrolysed in the gastrointestinal tract to produce free glycidol, which is recognised as probably carcinogenic to humans 2A (IARC, 2000; BfR, 2009) and as a carcinogenic and genotoxic compound by the EFSA CONTAM Panel (2016). The Panel noted that there is no limit for glycidyl esters in the specifications for PEFA (E 475). The Panel considered that the possible presence of glycidol/glycidyl esters in PEFA (E 475) would need further assessment, as their presence could raise a safety concern.
Rapeseed oil which contains erucic acid could be used for the manufacturing of PEFA (E 475) (EFEMA, 2016, (Documentation provided to EFSA n. 2)). According to the industry, only rapeseed oil low in erucic acid is used, nevertheless it cannot be excluded that high erucic acid rapeseed oil can be used. Maximum levels for erucic acid have been established in EU according to Commission Regulation (EU) No 696/2014 in edible oils and fats as well as in food containing fats and oils. A tolerable daily intake (TDI) of 7 mg/kg bw per day for erucic acid has been established by the EFSA CONTAM Panel based on a no observed adverse effect level (NOAEL) of 700 mg/kg bw per day for myocardial lipidosis observed in a 7‐day feeding study in young (5–7 weeks) rats and in a 2‐week feeding study in newborn piglets (EFSA CONTAM Panel, 2016). The Panel noted that there are no limits for erucic acid in the current EU specifications for PEFA (E 475).
The Panel noted that there is a maximum limit set for sum of dioxins in Regulation (EC) 1881/2006 (Section 5) for marine oils (5.7), fat of bovine animals and sheep, poultry and pigs (5.10), mixed animal fat (5.11) and vegetable oils and fats (5.12).
3.1.3. Manufacturing process
The preparation of polyglycerols was reported by the Panel in the re‐evaluation of polyglycerol polyricinoleate (E 476) (EFSA ANS Panel, 2017b). According to Bastida‐Rodriguez (2013), the polyglycerol portion can be prepared by three routes: (1) polymerisation of glycerol using a strong base as a catalyst, (2) polymerisation of glycidol, which leads to linear polyglycerols or (3) polymerisation of epichlorohydrin, followed by hydrolysis, which also leads to linear polyglycerols. Polyglycerols produced by polymerisation of epichlorohydrin contain reduced proportions of cyclic components. The Panel noted that epichlorohydrin, glycidol, and the contaminants expected to occur in glycerol, could also be present in polyglycerols.
According to information from industry (EFEMA, 2016 (Documentation provided to EFSA n. 2)), PEFA (E 475) can be produced by one of the two following procedures:
-
i
Transesterification process
In this process, a transesterification of refined edible fats and/or oils (triglycerides) with polyglycerol. In this process, natural or hydrogenated fats/oils react with polyglycerol. The fat/oil can be derived from one single source or may consist of a blend of fats and oils from different sources in order to achieve the desired fatty acid profile.
-
ii
Direct esterification process
In this process, fatty acids are esterified with polyglycerol. The fatty acids used in this process are obtained from food fats and oils by hydrolysis. The edible commercial fatty acids obtained by hydrolysis usually contain associated fatty acids in a varying amount depending on the source of the fatty acid. When direct esterification is used to produce polyglycerides containing specific fatty acids, the hydrolysed oils are subjected to distillation or fractionation/crystallisation prior to esterification, in order to obtain a concentrated fraction of the desired fatty acid.
The fatty acid composition of the raw materials (EFEMA, 2016 (Documentation provided to EFSA n. 2))
The oils and fats used as sources of the fatty acids suitable for the production of the food additive are foodstuffs of vegetable, animal or marine origin qualified for human consumption. The most common sources are palm, palm kernel, sunflower, rapeseed, soy, coconut, cottonseed, corn and mustard but oils from other crops recognised to be safe for use as a vegetable oil for consumption are also suitable for the manufacturing process. Likewise, animal‐based fats, e.g. lard and tallow, can be used. Each of these oils/fats show a certain fatty acid profile; the typical profile is predominantly in the range of C12–C20; however, fatty acids with shorter (till C6) or longer (till C24) chain length are also commonly used.
The purpose of refining the edible fats and oils is to remove free fatty acids and to ensure the quality and stability. Two main refining processes are used on crude oils, chemical refining and physical refining.
The fats and oils are composed predominantly of triacylglycerides showing a variation in the distribution of carbon chain length and number of unsaturated bonds. Minor constituents are mono‐ and diacylglycerides, fatty acids and minor organic compounds that are inherently part of the vegetable oil composition. The minor constituents are eliminated by refining process.
Commercially available fatty acids are obtained by hydrolysis (‘splitting’) of edible fat/oil. The effect of the ‘split’ is to produce a mixed group of fatty acids, varying in chain length and in number of double bonds and in the position of the double bonds. The fatty acids produced are often a mixture of stearic, palmitic and oleic acids with minor amounts of other fatty acids that are natural constituents of the source oil. These commercial fatty acids represent wide range compositions depending on the source and on the manufacturing process. The content of stearic acid could be approximately 80% if the fatty acid is produced fully hydrogenated soybean oil or rapeseed oil, while the content of stearic acid may be equal to palmitic acid when the fatty acid is produced by fully hydrogenated palm oil.
According to the information provided by industry on fatty acid composition of vegetable fats and oils used for the manufacturing process of E 475, palmitic acid, stearic acid, oleic acid and linoleic acid are the main fatty acids present in the raw material ‘food fats and oils’. Nevertheless, as according to the EU specifications, fatty acids present in food fats and oils can be used as a raw material, minor constituents (fatty acids) of fats and oils may also be used, meaning that the fatty acid composition of the food additive does not necessarily correspond with the fatty acid composition of the food fats and oils, used as the source of the fatty acids. The Panel noted that only information on the fatty acid composition of the raw material used for the manufacturing of the food additive (E 475) was submitted.
3.1.4. Methods of analysis in food
No specific method for the determination of PEFA in food was identified. A number of methods have been published for the fractionation followed by an identification of the various components of the food additive. Various techniques have been used such as thin‐layer chromatography (TLC), gas chromatography (GC) and high‐performance liquid chromatography (HPLC)) as reported in Behrens and Mieth (1984). The European Food Emulsifier Manufacturer's Association (EFEMA, 2009 (Documentation provided to EFSA n. 1)) recommended a method for a compliance check with specifications of the food additive, which is described in the JECFA specifications (2006). This method is based on saponification of the fatty acid esters followed by silylation of the formed polyglycerols, which are then analysed by GC, in order to check compliance with the specifications regarding the content of di‐, tri‐ and tetra‐polyglycerols as well as of the polyglycerols equal to or higher than heptaglycerol. In order to identify the particular constituents of the food additive, only three fractions of the food additive (di‐esters of di‐glycerol, di‐esters of tri‐glycerol and mono‐esters of tri‐glycerol) could be separated and analysed in details for their composition according to De Meulenaer et al. (2000). Cassel et al. (2001) have separated 11 polyglycerol esters of lauric acid, which can be present in PEFA, by using liquid chromatography coupled with evaporative light scattering detection.
3.1.5. Stability of the substance and reaction and fate in food
Baichwal and Lalla (1973) studied the stability of PEFA either alone (pure PEFA) or in the presence of pharmaceutical adjuvants: (i) four oil‐soluble colours; (ii) sodium cyclamate and saccharine; (iii) sodium lauryl sulfate and cetrimide as surfactants; and (iv) diethylphthalate and triacetin as plasticisers. The different samples were stored for 2 months at two different degrees of relative humidity (RH 60% and 80%) in various conditions. The degree of PEFA degradation was measured by the change in acid value (AV).8 The degradation of pure PEFA was 0.34% and 1.7% at 40°C and 50°C, respectively, independently of the humidity. When stored under visible or solar light at 55–60°C, degradation was 3.75%. The results indicated a high degree of stability of PEFA up to 50°C under 80% RH.
According to Norn (2015), the thermal stability of polyglycerol esters under neutral conditions was relatively good and the esters were considered as stable also when in liquid form at temperatures below 100°C. The intramolecular and intermolecular migrations were stated to be limited under these conditions. Alkaline substances (e.g. soaps) showing catalytic effect will decrease the stability of the esters. The instability was further enhanced by temperatures exceeding 100°C, and a migration towards di‐ or higher esters as well as the formation of free polyglycerol can take place. In the presence of alkali, or acids or by the action of lipases polyglycerol esters were hydrolysed (Norn, 2015; CIR, 2016). When the esters are present under the form of unsaturated fatty acids, oxidation occurs.
3.2. Authorised uses and use levels
Maximum levels of PEFA (E 475) have been defined in accordance with Annex II to Regulation (EC) No 1333/2008 on food additives, as amended. In this opinion, these levels are named maximum permitted levels (MPLs).
Table 2 summarises food categories (FCs) that are permitted to contain PEFA (E 475) and the corresponding MPLs as set by Annex II to Regulation (EC) No 1333/2008.
Table 2.
MPLs of PEFA (E 475) in foods according to the Annex II to Regulation (EC) No 1333/2008
| FCS category number | Food categories | E‐number | Restrictions/exception | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|---|
| 01.4 | Flavoured fermented milk products including heat‐treated products | E 475 | 2,000 | |
| 01.8 | Dairy analogues, including beverage whiteners | E 475 | Only milk and cream analogues | 5,000 |
| Only beverage whiteners | 500 | |||
| 02.2.2 | Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions | E 475 | 5,000 | |
| 05.2 | Other confectionery including breath freshening microsweets | E 475 | Only sugar confectionery | 2,000 |
| 05.3 | Chewing gum | E 475 | 5,000 | |
| 05.4 | Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | E 475 | 2,000 | |
| 06.3 | Breakfast cereals | E 475 | Only granola type breakfast cereal | 10,000 |
| 07.2 | Fine bakery wares | E 475 | 10,000 | |
| 10.2 | Processed eggs and egg products | E 475 | 1,000 | |
| 13.2 | Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5) | E 475 | 5,000 | |
| 13.3 | Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet) | E 475 | 5,000 | |
| 14.2.6 | Spirit drinks as defined in Regulation (EC) No 110/2008 | E 475 | Only emulsified liqueurs | 5,000 |
| 16 | Desserts excluding products covered in category 1, 3 and 4 | E 475 | 2,000 | |
| 17.1a | Food supplements supplied in a solid form including capsules and tablets and similar forms, excluding chewable forms | E 475 | quantum satis | |
| 17.2a | Food supplements supplied in a liquid form | E 475 | quantum satis | |
| 17.3a | Food supplements supplied in a syrup‐type or chewable form | E 475 | quantum satis |
MPL: Maximum permitted level.
FC 17 refers to food supplements as defined in Directive 2002/46/EC of the European Parliament and of the Council excluding food supplements for infants and young children.
According to Annex III, Part 1, of Regulation (EC) No 1333/2008, PEFA (E 475) is also authorised as carrier in colours and fat‐soluble antioxidants with a maximum level at quantum satis (QS).
In addition, according to Annex III, Part 2, PEFA (E 475) is authorised as a food additive other than carrier in preparations of colours and fat‐soluble antioxidants with a maximum level at QS.
According to Annex III Part 5 (section A), PEFA (E 475) is furthermore authorised as a carrier and food additive in beta‐carotene, lutein, lycopene and vitamin E preparations at QS, as well as in vitamin A and D preparations with a maximum level of 2 mg/kg in the final food (except nutrients intended to be used in foodstuffs for infants and young children listed in point 13.1 of Part E of Annex II).
3.3. Exposure data
3.3.1. Reported use levels or data on analytical levels of PEFA (E 475)
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment, especially for those food additives for which no MPL is set and which are authorised according to QS.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued public calls6 , 9 for occurrence data (usage level and/or analytical data) on PEFA (E 475). In response to these calls, information on the actual use levels of PEFA (E 475) in foods was made available to EFSA by industry.
No analytical data on the concentration of PEFA (E 475) in foods were made available by the Member States.
Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with seven data on use levels of PEFA (E 475) in foods for three out of the 16 food categories in which PEFA (E 475) is authorised. These data were made available to EFSA by FoodDrinkEurope (FDE, 2013) and the International Chewing Gum Association (ICGA, 2014).
The Panel noted that out of the seven data provided, six referred to niche products: three data on fillings of various sponge cakes, fine bakery wares and chocolate eggs in FC 5.4 Decorations, coatings and fillings, except fruit based fillings covered by FC 4.2.4, and three data on different kinds of sponge cakes and fine bakery wares in FC 7.2 Fine bakery wares. Since no other usage levels were available for these food categories, the Panel included these niche products in the exposure assessment.
Appendix A reports data on the use levels of PEFA (E 475) in foods as provided by industry.
3.3.2. Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information of over 2 million food and beverage products of which more than 900,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 20 out of its 28 member countries and Norway presented in the Mintel's GNPD.10
For the purpose of this Scientific Opinion, the Mintel's GNPD11 was used for checking the labelling of food and beverage products including food supplements containing PEFA (E 475) within the EU's food market as the database contains the compulsory ingredient information on the label.
Appendix B lists the number and percentage of the food and beverage products labelled with PEFA (E 475) between 2012 and 2017, out of the total number of food products per food subcategory according to the Mintel's GNPD food classification.
In total, PEFA (E 475) was labelled on 2,230 food and beverage products as an ingredient, mainly in cakes, pastries and sweet goods, sweet biscuits/cookies, baking ingredients and mixes, and frozen and chilled desserts. The percentages of food and beverage products per food subcategory labelled with PEFA (E 475) ranged from less than 0.1% to about 9.5% in the Mintel's GNPD food subcategory ‘cakes, pastries & sweet goods’. The overall average percentage of foods labelled to contain PEFA (E 475) was 1.2%.
According to the Mintel's GNPD, PEFA (E 475) is used in a few food products of the following authorised food categories for which no use levels were provided:
17 Dietary supplements
10.2 Eggs and egg products
16 Desserts excluding products covered in category 1, 3 and 4
1.8 Dairy analogues, including beverage whiteners
Neglecting foods belonging to these food categories in the exposure assessment may have resulted in an underestimation of the exposure.
3.3.3. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). New consumption surveys added to the EFSA Comprehensive database in 2015 were also taken into account in this assessment.7
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database represents the best available source of food consumption data across Europe at present.
Food consumption data from the following population groups were used in the exposure assessment: infants, toddlers, children, adolescents, adults and the elderly. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 3).
Table 3.
Population groups considered for the exposure estimates of PEFA (E 475)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlersa | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrenb | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Spain, Sweden, UK, Netherlands |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlya , b | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Sweden, UK, Netherlands |
‘Toddlers’ in the EFSA Comprehensive Database corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013.
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure estimates. In practice, the FoodEx food codes were matched to the FCS food categories.
Food categories considered for the exposure assessment of PEFA (E 475)
The food categories in which the use of PEFA (E 475) is authorised were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system) at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
For the remaining food categories, the refinements considering the restrictions/exceptions as set in Annex II to Regulation No 1333/2008 were applied (Appendix C).
For the refined scenario, 13 food categories were not taken into account because no use level were provided to EFSA (Appendix C).
Considering that FC 18 (Processed foods not covered by categories 1–17, excluding foods for infants and young children) is extremely unspecific, the foods belonging to this food category in the EFSA Comprehensive Database (e.g. processed foods, prepared or composite dishes) were reclassified under food categories in accordance to their main ingredient and included as such in the exposure assessment.
Also, the food items belonging to FCs 13.2, 13.3 and 13.4, consumed by the population groups children, adolescents, adults, and the elderly, may be very diverse; in addition, there was very limited information on their consumption. Therefore, eating occasions belonging to these food categories were also reclassified under food categories in accordance to their main ingredient. The MPLs available for FCs 13.2 and 13.3 were not considered in the exposure assessment.
Overall, for the regulatory maximum level exposure scenario, 11 food categories were included, after exclusion of FCs 13.2 and 13.3 and of the food categories related to food supplements (FCs 17.1, 17.2 and 17.3). In the refined exposure scenarios, only three food categories were included (Appendix C).
3.4. Exposure estimates PEFA (E 475) from its use as a food additive
The Panel estimated the chronic dietary exposure to PEFA (E 475) for the following population groups: infants, toddlers, children, adolescents, adults and the elderly. Dietary exposure to PEFA (E 475) was calculated by multiplying PEFA (E 475) concentrations per food category (Appendix C) with their respective consumption amount per kilogram body weight for each individual in the EFSA Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only one day per subject were excluded as they are considered as not adequate to assess repeated exposure.
This was carried out for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 3). Based on these distributions, the mean and 95th percentile of exposure were calculated per survey and per population group. The 95th percentile of exposure was only calculated for those population groups with a sufficiently large sample size to allow this calculation (EFSA, 2011a). Therefore, in the present assessment, 95th percentiles of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain were not estimated.
Exposure assessment of PEFA (E 475) was carried out by the ANS Panel based on: (1) MPLs as set down in the EU legislation (defined as the regulatory maximum level exposure assessment scenario); and (2) reported use levels (defined as the refined exposure assessment scenario). These two scenarios are discussed in detail below.
These scenarios did not consider exposure to PEFA (E 475) via the intake of food supplements. Use of PEFA (E 475) in food supplements is authorised as QS, but no use levels were available to EFSA for a separate food supplements consumers only exposure scenario.
A possible additional exposure from the use of PEFA (E 475) according to Annex III to Regulation (EC) No 1333/2008 (Part 1, 2, 5) was also not considered in any of the exposure assessment scenarios due to lack of concentration data.
Regulatory maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario is based on the MPLs as set in Annex II to Regulation (EC) No 1333/2008 and listed in Table 2 and Appendix C.
The Panel considers the exposure estimates derived following this scenario as the most conservative, since it is assumed that that the population will be exposed to PEFA (E 475) present in food at the MPL over a longer period of time.
Refined exposure assessment scenario
The refined exposure assessment scenario is based on use levels reported by food categories. This exposure scenario can consider only food categories for which these data were available to the Panel.
Appendix C summarises the use levels of PEFA (E 475) used in the refined exposure assessment scenario. Based on the available data set, the Panel calculated two refined exposure estimates based on different model populations:
-
The brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to PEFA (E 475) present at the maximum reported use level for one food category. This exposure estimate was calculated as follows:
-
1
— Combining food consumption with the maximum of the reported use levels for the main contributing food category at the individual level.
-
2
— Using the mean of the typical reported use levels, for the remaining food categories.
-
1
The non‐brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to PEFA (E 475) present at the mean reported use levels in food. This exposure estimate was calculated using the mean of the typical reported use levels for all food categories.
3.4.1. Dietary exposure to PEFA (E 475)
Table 4 summarises the estimated exposure to PEFA (E 475) from its use as a food additive in six population groups (Table 3) according to the different exposure scenarios. Detailed results per population group and survey are presented in Appendix D.
Table 4.
Summary of dietary exposure to PEFA (E 475) from its use as a food additive in the regulatory maximum level exposure assessment scenario and in the refined exposure scenarios, in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants | Toddlers | Children | Adolescents | Adults | The elderly | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (12 weeks–11 months) | (12–35 months) | (3–9 years) | (10–17 years) | (18–64 years) | (≥ 65 years) | |||||||
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | |
| Regulatory maximum level exposure assessment scenario | ||||||||||||
| Mean | 0.7 | 26.1 | 27.1 | 65.1 | 18.4 | 54.7 | 6.9 | 26.0 | 2.5 | 21.2 | 2.2 | 28.5 |
| 95th percentile | 29.5 | 104.6 | 65.0 | 127.4 | 38.9 | 125.0 | 15.9 | 60.6 | 8.8 | 59.2 | 6.4 | 69.7 |
| Refined estimated exposure assessment scenario | ||||||||||||
| Brand‐loyal scenario | ||||||||||||
| Mean | 0 | 9.3 | 1.1 | 25.6 | 0.2 | 26.2 | 0.3 | 13.0 | 1.0 | 9.3 | 1.0 | 8.9 |
| 95th percentile | 0 | 40.0 | 5.5 | 63.5 | 1.0 | 65.6 | 1.1 | 35.0 | 4.1 | 27.9 | 3.7 | 24.4 |
| Non‐brand‐loyal scenario | ||||||||||||
| Mean | 0 | 0.9 | 0.1 | 2.5 | 0.1 | 2.6 | 0.1 | 1.3 | 0.1 | 0.9 | 0.1 | 0.9 |
| 95th percentile | 0 | 3.9 | 0.5 | 6.2 | 0.4 | 6.4 | 0.3 | 3.4 | 0.4 | 2.7 | 0.4 | 2.4 |
In the regulatory maximum level exposure assessment scenario, mean exposure to PEFA (E 475) from its use as a food additive ranged from 0.7 mg/kg bw per day in infants to 65.1 mg/kg bw per day in toddlers. The 95th percentile of exposure to PEFA (E 475) ranged from 6.4 mg/kg bw per day in the elderly to 127.4 mg/kg bw per day in toddlers.
In the brand‐loyal exposure assessment scenario, mean exposure to PEFA (E 475) from its use as a food additive ranged from 0 mg/kg bw per day in infants to 26.2 mg/kg bw per day in children. The high exposure to PEFA (E 475) ranged from 0 to 65.6 mg/kg bw per day the same two population groups.
In the non‐brand‐loyal exposure assessment scenario, the corresponding ranges of exposure were from 0 to 2.6 mg/kg bw per day at the mean level, and 0 to 6.4 mg/kg bw per day at the high level, in the same two populations. Note that the exposure in this scenario was about a factor 10 lower than in the brand‐loyal scenario for all population groups.
The main food categories contributing to the exposure to PEFA (E 475) are presented in Appendix E.
In all scenarios and population groups, one of the main contributors was FC 7.2 ‘fine bakery wares’. In the regulatory maximum level exposure assessment scenario, also FCs 6.3 ‘breakfast cereals’, 1.4 ‘flavoured fermented milk products’ and 2.2 ‘fat and oil emulsions mainly of type water‐in‐oil’ contributed largely to the exposure in all population groups.
3.4.2. Uncertainty analysis
Uncertainties in the exposure assessment of PEFA (E 475) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 5.
Table 5.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/− |
| Use of data from food consumption survey covering only a few days to estimate high percentile (95th) of long‐term (chronic) exposure | + |
| Correspondence of reported use levels to the food items in the EFSA Comprehensive Food Consumption Database: uncertainties to which types of food the levels refer | +/− |
| Uncertainty in possible national differences in use levels of food categories | +/− |
|
Concentration data:
|
+ +/− + |
| Food categories included in the exposure assessment: data not available for certain food categories which were excluded from the exposure estimates (n = 13/16) | − |
| Foods which may contain E 475 according to Annex III to Regulation (EC) No 1333/2008 not taken into account | − |
|
Regulatory maximum level exposure assessment scenario:
|
+ − |
|
Refined exposure assessment scenarios:
|
+/− − |
+, uncertainty with potential to cause overestimation of exposure; −, uncertainty with potential to cause underestimation of exposure.
PEFA (E 475) is authorised in 16 food categories (Table 2) of which three are authorised at QS. Food industry provided usage data on only three food categories. However, the Panel noted that information from the Mintel's GNPD (Appendix B) showed that foods belonging to some of the remaining 13 food categories were labelled with PEFA (E 475), such as dietary supplements, desserts, dairy analogues, eggs and egg products. However, the use levels provided by industry referred to the majority of the foods labelled with PEFA (E 475) available in the Mintel's GNPD (Appendix B).
In none of the exposure scenarios, the use of PEFA (E 475) according to Annex III to Regulation No 1333/2008 was considered. Neglecting this source of exposure may have resulted in an underestimation of exposure to PEFA (E 475) in all scenarios.
Overall, based on the assumption that the food additive is not used in the food categories in which it is permitted but for which no usage data were provided, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure to PEFA (E 475) as a food additive in all exposure scenarios, taking into account that according to the Mintel's GNPD on average only 1.2% of the authorised food items contained PEFA (E 475) and that use levels provided by industry referred to the majority of the foods labelled with PEFA (E 475) in the Mintel's GNPD.
3.5. Biological and Toxicological data
The Panel noted that the test material used in most of the toxicological studies (report from Unilever Research Laboratory (1966)) was stated to be ‘glyceran esters (PGE19)’, an ester of fatty acids and polyglycerol, described as ‘intended for use in the bakery industry as an additive’. Therefore, the Panel considered that this compound could be used as an example of PEFA used as a food additive (E 475).
3.5.1. Absorption, distribution, metabolism and excretion
In vitro
The enzymatic hydrolysis of fatty acid‐labelled tri‐ and decaglycerol esters (triglycerol monooleate, triglycerol tetraoleate, decaglycerol monooelate, decaglycerol decaoleate) as well as fatty acid‐labelled decaglycerol monoeicosanoate was investigated in vitro using fresh pancreatic juice plus bile as a mixture of digestive enzymes (no details about concentration and duration) (Michael and Coots, 1971). The distribution of 14C among the hydrolysis products was determined by TLC and radioassay procedures. The extent of hydrolysis of polyglycerol esters with oleic acids was 97% for triglycerol monooleate, 98% for triglycerol tetraoleate, 89% for decaglycerol monooleate and 92% for decaglycerol decaoleate; indicating that pancreatic enzymes can hydrolyse the ester linkages between the polyglycerols and the fatty acids. A lower extent of hydrolysis, 21%, was reported for decaglycerol monoeicosanoate.
In vivo
Michael and Coots (1971) studied the metabolism of tri‐ and decaglycerol esters of fatty acids in male Sprague‐Dawley rats. Different polyglycerol esters (Table 6) were labelled with 14C either in the fatty acids or the polyglycerol moiety. The esters were administered by gavage as 7–8 g of liquid diet containing 1% labelled ester (corresponding to 14–20 mg/kg bw of compound). The cumulative excretion of administered radioactivity was measured in metabolism cages as well as the radioactivity remaining in the carcass and in the gastrointestinal content at termination; 51 h after administration (Table 6). The total recovery of radioactivity ranged from 88% to 98% of the administered amount.
Table 6.
Metabolic fate of 14C‐labelled polyglycerol esters of fatty acids (Michael and Coots, 1971)
| Labelled compound | % of recovered radioactivity (means) | Absorption in %a | ||||
|---|---|---|---|---|---|---|
| CO2 | Urine | Faeces | GI content | Carcass | ||
| Polyglycerol moiety labelled | ||||||
| Decaglycerol*monooleate | 2.1 | 36.8 | 9.5 | 46.5 | 5.3 | 44.2 |
| Decaglycerol*decaoleate | 3.5 | 33.5 | 15.5 | 44.6 | 3.0 | 40.0 |
| Fatty acid moiety labelled | ||||||
| Triglycerol monooleate* | 68.2 | 1.3 | 0.1 | 2.8 | 27.7 | 97.2 |
| Triglycerol tetraoleate* | 70.4 | 1.4 | 1.5 | 3.0 | 23.6 | 95.4 |
| Decaglycerol monooelate* | 68.5 | 2.2 | 0.6 | 4.0 | 24.7 | 95.4 |
| Decaglycerol decaoleate* | 66.0 | 1.7 | 0.9 | 2.8 | 28.7 | 96.4 |
| Decaglycerol monoeicosanoate* | 55.5 | 1.6 | 9.9 | 12.2 | 20.8 | 77.9 |
*14C‐labelled moiety; GI: gastrointestinal.
Total absorption in % of recovered radioactivity assuming no excretion via the bile: total recovery (100%) minus unabsorbed radioactivity in faeces and GI content.
Results with decaglycerol monooleate and decaglycerol decaoleate 14C‐labelled in the decaglycerol moiety indicated absorption of the polyglycerol part from the gastrointestinal tract of around 40% apparently independent of the fatty acid moiety. The absorbed radioactivity was mainly excreted via urine as unchanged decaglycerol. Only minor amounts were exhaled as 14CO2 suggesting no or minimal metabolism of decaglycerol. The authors stated that this effect might be related to un‐polymerised glycerol present as an impurity.
In comparison, polyglycerol esters of fatty acids 14C‐labelled at the oleic acid moiety (Table 6) showed almost complete absorption of radioactivity (> 95% for tested esters). The absorption was also independent of the polyglycerol moiety of the ester. Radioactivity was incorporated in carcass (23.6–28.7% of recovered radioactivity) or exhaled as 14CO2 (66–70.4% of recovered radioactivity) indicating that the fatty acids were mainly metabolised. The eicosanoic acid moiety (saturated C20 fatty acid) was not absorbed as well as the oleic acid (unsaturated C18 fatty acid). The authors suggested that this difference may reflect a slower rate of hydrolysis of eicosanoic ester (Michael and Coots, 1971).
Another study was performed to investigate the possible accumulation of polyglycerol (Ostertag and Wurziger, 1965). Wistar rats (no data about sex), received a diet containing 10% polyglycerol ester with a high melting point (no further details about the test substance) for 8 months. At termination, body tissues were analysed for polyglycerol. No polyglycerol was detected in depot fat, or in fat of muscle, liver, kidney, spleen or carcass, suggesting no accumulation.
Overall, the Panel considered that in vitro and in vivo data indicated that polyglycerol esters of fatty acids are hydrolysed in the gastrointestinal tract, resulting in the production of polyglycerol and fatty acids.
3.5.2. Acute toxicity
Rats given single doses of 7, 14 or 29 g/kg bw of a polyglycerol ester via gavage did not show any signs of toxic effects. Similarly, rabbits given single doses of 10–29 g/kg bw via gavage showed no toxic effects (as described by JECFA, 1974a). The available data thus indicated a low acute oral toxicity of PEFA.
3.5.3. Short‐term and subchronic toxicity
A number of short‐term and subchronic toxicity studies with polyglycerol esters of fatty acids are available. None of these studies were performed according to current guidelines.
Nine different esters of diglycerol, triglycerol, hexaglycerol, nonaglycerol, decaglycerol or triacontaglycerol with fatty acids from cottonseed and peanut oils were administered to male Sherman rats (n = 8 per group) at a concentration of 8% in the diet (equivalent to 6,480 mg/kg bw per day) for 11 weeks (Babayan et al., 1964). No differences were found between the different esters concerning body weight gain, and histopathology of liver, kidneys and ileum. The body weight gain in treatment groups was similar to the lard control but increased compared with the basic diet controls. No clinical signs were observed except diarrhoea in all treatment groups during the ad libitum feeding period.
A 90‐day study performed under conditions close to the current guideline was reported by King et al. (1971). Groups of 10 male and 10 female Sprague–Dawley rats were maintained on: (a) basal diet with oleic acid and glycerol at a level corresponding to the ester in the 5% level in the basal diet, and (b) 16% soybean oil in basal diet or diets containing 2.5%, 5% or 10% of the decaglycerol decaoleate. All diets were isocaloric. Urinalysis was performed at week 3 and week 9 of the exposure period and blood sampled for haematology at week 5 and 11 and at necropsy. At the end of the exposure period, rats were killed, necropsy was performed, the organ weights were determined and tissues were examined by histopathology. The highest dose of the test substance induced an increase in total nitrogen in the urine of females compared with the soybean oil control. No histopathological effects were however detected in the kidney or the urinary bladder. In males, food consumption was increased in the high‐dose group; however, the body weight gain was not altered. The Panel noted that no biologically significant adverse effects were reported in this study up to 10% in the diet (equivalent to 9,000 mg/kg bw per day), the highest dose tested. The Panel further noted that no untreated control group was included in this study.
There are further short‐term and subchronic studies available (Unilever Research Laboratory, 1966 (Documentation provided to EFSA n. 8); Briski, 1970; as referred to by JECFA, 1974a; Baichwal and Lalla, 1973). These studies have limitations and methodological weaknesses, therefore, they are not described in detail in this evaluation; however, the Panel noted that no toxic effects were detected in these studies at dose levels ≥ 9% in the diet (equivalent to 7,290 mg/kg bw per day).
Overall, the Panel noted that the available data have limitations, however no adverse effects were observed up to 10% in the diet (equivalent to 9,000 mg/kg bw per day), the highest dose tested.
3.5.4. Genotoxicity
In vitro
The Panel noted that in the NICNAS report (2013), two in vitro chromosomal aberration studies on 1,2,3‐propanetriol, homopolymer, dodecanoate, structurally relevant for polyglycerol esters of fatty acids (E 475), are described. NICNAS (2013) reported that 1,2,3‐propanetriol, homopolymer, dodecanoate was positive in an in vitro chromosome aberration study in Chinese hamster V79 cells in the presence of metabolic activation and further stated that ‘given that in vitro chromosomal aberration studies can give a high frequency of false positives’, 1,2,3‐propanetriol, homopolymer, dodecanoate was retested for induction of chromosomal aberrations in human peripheral blood lymphocytes. In the second in vitro chromosome aberration study, 1,2,3‐propanetriol, homopolymer, dodecanoate did not show any induction of chromosomal aberrations both in the absence and presence of S9 metabolic activation. The unpublished studies described in the NICNAS (2013) report were not available to EFSA and, therefore, the Panel could not assess these studies.
In silico
The evaluation of structural alerts for genotoxicity in polyglycerol esters of fatty acids (E 475) with palmitic acid as fatty acid moiety (Figure 2) was performed with the OECD QSAR Tool box (2.6.13) using the following profilers: ‘Alerts for in vitro mutagenicity (Ames test) by ISS’, ‘DNA alerts for Ames, MN, and CA by OASIS v.1.3’, ‘Alerts for DNA binding (by OASIS v.1.3 and OECD)’ and ‘Alerts for in vivo mutagenicity (Micronucleus) by ISS’.
Figure 2.
Triglycerol tripalmitate and triglycerol dipalmitate used as representative substances for the ‘in silico calculation’
No relevant structural alerts for genotoxicity were identified with the exception of the alert ‘H‐acceptor‐path3‐H‐acceptor’ detected by the profiler ‘Structure Alerts for the in vivo micronucleus assay’. The ‘H‐acceptor‐path3‐H‐acceptor’ refers to the possibility of non‐covalent binding to DNA or proteins as a result of the presence of two bonded atoms connecting two hydrogen bond acceptors. However, the Panel noted that the positive predictivity of such alerts for in vivo genotoxicity is quite low, ranging from ‘none’ (34%) to 63% depending on the database, with a high incidence of false positives (Benigni et al., 2010, 2012).
Overall, the Panel considered that the available information of genotoxicity of PEFA did not indicate a genotoxic potential.
3.5.5. Chronic toxicity and carcinogenicity
Mice
The carcinogenic potential of PEFA in ‘street’ mice (2 months old at initiation, no data about sex) were studied by Bichel et al. (1964). This strain had a high spontaneous tumour rate concerning mammary adenocarcinoma and leucosis. Mice (n = 175 per dose) received 0% or 1% PEFA in the diet (equivalent to 0 and 1,500 mg/kg bw per day) for 15.5 months. No effects on body weight gain and survival were reported. The Panel noted that the study was only briefly reported and did not present sufficient details of the protocol. However, histopathology of liver, lung, kidney and spleen gave no indication of carcinogenicity. In addition, the incidences of mammary adenocarcinoma were not altered compared to controls and the latency period was not shortened.
A long‐term feeding study was performed in Colworth C57BL mice (Unilever Research Laboratory, 1966 (Documentation provided to EFSA n. 8)). Twenty‐five mice per sex were exposed for 80 weeks via the diet containing 5% (equivalent to 7,500 mg/kg bw per day) polyglycerol ester (PGE 19) or ground‐nut oil (control). No adverse effects on body weight, food consumption, haematological parameters and survival rate were noted. The organ weights (heart, liver, spleen, kidneys, testes measured) were similar in the test and control groups, except for the liver and kidney weights of treated female, which were significantly increased. However, histopathology did not show any remarkable findings (no further data). The Panel considered that although no clinical chemistry data on liver and kidney function were available, the increased weights of liver and kidney were probably due to an adaptive response since histopathology revealed no adverse effects in these organs. The Panel considered the only dose tested of 5% in the diet equivalent to 7,500 mg/kg bw per day to be a NOAEL.
Rats
In a long‐term feeding study (Unilever Research Laboratory, 1966 (Documentation provided to EFSA n. 8)), Colworth Wistar rats (n = 28 per sex per group) were fed 5% polyglycerol ester (PGE 19) (equivalent to 2,500 mg/kg bw per day) or 5% groundnut oil (control) in their diet for 2 years. Body weight, food consumption, haematological parameters, and survival rate were not affected by treatment with the test substance. At weeks 59 and 104, clinical chemistry parameters on liver and kidney function (no further details) were comparable between controls and animals from the treatment group. The carcass fat contained no polyglycerol and the levels of free fatty acid, unsaponifiable residue and fatty acid composition of carcass fat were not different from control values. Furthermore, organ weights (liver, kidneys, spleen, heart, testes, thyroid, adrenals, pituitary), tumour incidences and tumour distribution were similar in controls and treated rats. No effects were detected in histopathology (no further data). From this study, the JECFA committee (JECFA, 1974a) derived a NOAEL of 5% in the diet equivalent to 2,500 mg/kg bw per day, the only dose tested. The Panel agreed with this NOAEL.
Overall, the available data on chronic toxicity have limitations. However, none of the chronic studies on PEFAs gave any indication of a carcinogenic potential.
3.5.6. Reproductive and developmental toxicity
Reproductive toxicity
Groups of Wistar rats (sex and number of animals per group not stated) were maintained up to 14 months on a diet containing either 0%, 5% or 10% polyglycerol ester (the fatty acids of the polyglycerol preparations used were chiefly stearic and oleic acids), or 5% or 10% lard through 3 generations. The diets were continued without interruption and included the periods of gestation and lactation. The rate of growth was normal. Histopathology revealed no effects. Reproduction and lactation were not affected (no further details; Bodansky et al., 1938). The reported data were very limited and cannot be used for hazard characterisation.
A test group of 22 rats and a control group of 28 rats (strain and sex not stated) were kept on diets containing 0% or 1.5% of polyglycerol ester (PGE 19), respectively, for three generations. The groups were maintained for over 1 year without significant variation in fertility and reproductive performance. Gross and histological examination of the third generation revealed no consistent treatment‐related abnormality (no further data; Unilever Research Laboratory, 1966 (Documentation provided to EFSA n. 8), abstract of study in an unpublished report; as referred to by JECFA, 1974a). The reported data were very limited and cannot be used for hazard characterisation.
Developmental toxicity
No prenatal developmental toxicity studies were available.
Overall, in two old dietary three‐generation reproductive toxicity studies with PEFA, no maternal, reproductive or developmental toxicity was reported. The reporting of both studies was very limited. The Panel noted that these studies were inadequate for the hazard identification of these endpoints.
3.5.7. Other studies
Humans studies
Thirty‐seven volunteers (19–24 years old no information about sex) ingested a diet containing polyglycerol ester (PGE 19) which resulted in exposures of 2–20 g polyglycerol ester per day for three weeks. No clinical symptoms were observed (Unilever Research Laboratory, 1966 (Documentation provided to EFSA n. 8)). No abnormalities were detected in clinical chemistry concerning the following parameters: plasma proteins, serum amino acids, thymol turbidity, serum bilirubin, total and free serum cholesterol, serum alkaline phosphatase, serum aspartate aminotransferase, serum alanine aminotransferase, cholinesterase and cholesterol esterase. No effects were found on 24‐h urine volume, urinary creatinine or urea output. The total, split faecal fat and total faecal nitrogen were not altered.
A case report from a single woman who reacted positive to glyceryl laurate patch test was described by Washizaki et al. (2008). The Panel noted that, as stated by the authors, this was the first time ever reported of such a reaction; in addition, lauric acid has a known irritant effect, therefore the Panel concluded that there is no indication for an allergenic potential of PEFA (E 475) used as a food additive.
3.5.8. Studies with other emulsifiers
PEFA (E 475) is included in the list of EFEMA index of food emulsifiers (EFEMA, 2015).
In several recent studies, some other emulsifiers have been reported to alter the gut microbiota, to promote gut inflammation, obesity and to impair glycaemic control (Swidsinski et al., 2009a,b; Renz et al., 2012; Merga et al., 2014; Cani and Everard, 2015; Chassaing et al., 2015; Romano‐Keeler and Weitkamp, 2015; Lecomte et al., 2016; Chassaing et al., 2017; Nejrup et al., 2017; Shah et al., 2017). The Panel noted that, even though some of these effects are not systematically studied in toxicity studies performed according to toxicity testing guidelines, they would be investigated on a case‐by‐case basis if indicated by the results of the general toxicity testing as recommended in the Guidance for submission of food additives (EFSA ANS Panel, 2012). The Panel considered that additional studies would be needed to show the relevance of the effects seen in mice for human health and if PEFA can induce such effects.
3.6. Discussion
According to the EU specifications for E 475, PEFA (E 475) is a mixture of reaction products formed by the esterification of polyglycerols with food fats and oils or with fatty acids occurring in foods, fats and oils. The polyglycerol moiety is predominantly di‐, tri‐ and tetraglycerol and contains not more than 10% of polyglycerols equal to or higher than heptaglycerol. According to the information provided by industry on fatty acid composition of vegetable fats and oils used for the manufacturing process of E 475, palmitic acid, stearic acid, oleic acid and linoleic acid are the main fatty acids present in the raw material.
Depending on manufacturing processes and starting materials, toxic and potentially carcinogenic impurities such as epichlorohydrin, glycidol, erucic acid and trans fatty acids may be present in PEFA (E 475). There is thus a need to include maximal levels for these impurities in the specifications of PEFA (E 475).
The Panel noted that recent studies with other emulsifiers had demonstrated effects on the microbiota, which might also be relevant to emulsifiers in general; however, there were no specific studies on PEFA and effects on the microbiota itself.
The dietary exposure to PEFA (E 475) from its use as a food additive was calculated according to different scenarios, as described in Section 3.4. A specific exposure scenario addressing the exposure to PEFA (E 475) via food supplements was not included: PEFA (E 475) is authorised in food supplements at QS and no usage level data were provided.
PEFA (E 475) is authorised in 16 food categories, seven data on use levels were provided for only three food categories. The majority of these data (six out of seven) were related to niche products. Furthermore, the Panel noted that, considering information from the Mintel's GNPD, PEFA (E 475) is used in some food categories for which no use levels were provided to EFSA, such as dietary supplements, eggs and egg products, desserts and dairy analogues, including beverage whiteners. The Panel further noted that the exposure to PEFA (E 475) from its use according the Annex III to Regulation (EC) No 1333/2008 (Part 1, 2, 5) was not considered in the exposure assessment. Therefore, the exposure to PEFA (E 475) in the refined exposure scenarios may have been underestimated. However, the possible underestimation of the exposure may have been negated by the assumption that all foods belonging to one of the three included food categories contain the food additive, whereas according to the Mintel's GNPD on average only 1.2% of the authorised food items contained PEFA (E 475). The use of use levels in niche products in the refined exposure estimation may also have contributed to an overestimation of the exposure. Niche products are often specific products that contain different, often higher, levels of a food additive than the other foods belonging to the same food category. Applying these levels to all foods may have resulted in an overestimation of the exposure. The exposure in the regulatory maximum level exposure scenario is very likely overestimated; 11 food categories were included assuming that all foods belonging to a food category contain the additive at a level equal to the MPL.
Overall, based on the considerations described above, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure to PEFA (E 475) as a food additive in all exposure scenarios assuming that the food additive is not used in the food categories in which it is permitted but for which no usage data were provided.
The Panel did not identify brand loyalty to a specific food category for the exposure to PEFA (E 475), and therefore considered the non‐brand‐loyal scenario covering the general population as the most appropriate and realistic scenario for risk characterisation of the food additive. It is assumed that the population is most likely exposed long‐term to PEFA (E 475) present at the mean reported use in processed food. Based on the non‐brand‐loyal scenario, it is not likely that the exposure to PEFA (E 475) will exceed the current ADI of 25 mg/kg bw per day set by JECFA (1974 and endorsed by the SCF (1978); the highest P95 of exposure was 6.4 mg/kg bw per day in children (Table 4).
Results from in vivo studies on tri‐ and decaglycerol esters of fatty acids demonstrated an almost complete enzymatic hydrolysis of the ester bond in the gastrointestinal tract. Decaglycerol was absorbed to around 40% and excreted unchanged primarily via the urine thus indicating no metabolism of the polyglycerol in vivo. On the other hand, absorption of the oleic acid moiety of the various esters of tri‐ and decaglycerol appeared to be nearly complete (absorption > 95%). The fatty acids and/or their metabolites were incorporated into the body but mainly metabolised to 14CO2 which was exhaled. The esters of eicosanoid acid, a saturated C20 fatty acid, were not absorbed as well as those with oleic acid (unsaturated C18 fatty acid). The authors stated that this difference may reflect a slower rate of hydrolysis (Michael and Coots, 1971).
Overall the Panel noted that PEFA were hydrolysed in the gastrointestinal tract followed by absorption of polyglycerol and the fatty acid moiety. Although the polyglycerol appears to be excreted unchanged, the fatty acids were either rapidly metabolised to carbon dioxide or incorporated in the body.
The Panel noted that the available data showed a very low acute oral toxicity of PEFA.
There were several short‐term or subchronic toxicity studies with PEFA. The studies have limitations, however no adverse effects were observed up to 10% in the diet (equivalent to 9,000 mg/kg bw per day), the highest dose tested.
The available limited information on the genotoxicity of PEFA did not indicate a genotoxic potential.
The available chronic toxicity and carcinogenicity studies with PEFA in mice and rats had also limitations. However, none of these studies gave any indication of a carcinogenic potential of PEFA.
There were only two old dietary three‐generation reproductive toxicity studies with PEFA available. No maternal, reproductive or developmental toxicity were reported in these studies. Prenatal developmental toxicity studies were not available.
In a clinical study with limited information, 37 volunteers were exposed to 2–20 g PEFA per day (up to 300 mg/kg bw per day) for 3 weeks. Clinical chemistry and urinalysis did not reveal any adverse effects.
In summary, the Panel noted that very few relevant studies on the biological effects of PEFA have been published since the JECFA evaluation in 1974 and the SCF in 1978. The Panel also noted that no adverse effects of PEFA at any dose have been observed in short‐term, subchronic or chronic toxicity studies. A NOAEL of 9,000 mg/kg bw per day was identified from subchronic studies and of 2,500 mg/kg bw per day from chronic studies, the highest doses tested. The limited information on genetic toxicity did not identify any genotoxic potential of PEFA. The available reproductive toxicity studies showed no adverse effects of PEFA but had major limitations.
Furthermore, the Panel noted that the absorption of intact PEFA before hydrolysis to polyglycerols and fatty acids in the gastrointestinal tract is extremely low. The safety of polyglycerols and specific fatty acids has recently been assessed in the opinions on the re‐evaluation of polyglycerol polyricinoleate (E 476) (EFSA ANS Panel, 2017a) and of fatty acids (E 570) (EFSA ANS Panel, 2017c). No adverse effects of polyglycerols or specific fatty acids were identified in studies reported in those opinions.
4. Conclusions
Considering that:
absorption of intact PEFA in the gastrointestinal tract was extremely low;
PEFA was rapidly and almost fully hydrolysed to polyglycerols and fatty acids in the gastrointestinal tract;
The safety of polyglycerols and specific fatty acids has recently been assessed and no adverse effects were identified in the available studies even at the highest doses tested;
Although limited, the available database on PEFA did not give any indication of adverse effects in short‐term, subchronic, chronic or reproductive toxicity studies neither of any genotoxic potential;
in a clinical study with limited information, volunteers exposed to PEFA (up to 300 mg/kg bw per day for 3 weeks), clinical chemistry and urinalysis did not reveal any adverse effects;
the highest exposure to PEFA used as a food additive was 2.6 and 6.4 mg/kg bw per day in children at the mean and the 95th percentile, respectively, at the non‐brand loyal scenario.
The Panel concluded that the food additive PEFA (E 475) was not of safety concern at the reported uses and use levels and that there was no need for a numerical ADI.
Recommendations
The Panel recommended that:
the European Commission considers lowering the current limits for toxic elements (arsenic, lead, mercury and cadmium) in the EU specifications for PEFA (E 475 in order to ensure that the food additive will not be a significant source of exposure to these toxic elements in food.
the European Commission considers revising the EU specifications for PEFA (E 475) including maximum limits for epichlorohydrin and glycidol, given that during the manufacturing processes of polyglycerols these genotoxic impurities could be present.
the European Commission considers revising the EU specifications for PEFA (E 475) including maximum limits for trans fatty acids because PEFA (E 475) can be manufactured by glycerolysis of hydrogenated fats and/or oils, which contain significant amounts of trans fatty acids.
the European Commission considers revising the EU specifications for PEFA (E 475) including maximum limits for glycidyl esters/glycidol and 3‐MCPD esters, because it is likely that residues of those substances occur in the food additive PEFA (E 475), if they were present in the raw materials used in the manufacturing of the food additive by transesterification.
the European Commission considers revising the EU specifications for PEFA (E 475) including maximum limits for erucic acid since erucic acid can be present among the fatty acids in edible oils, which can be used for manufacturing of PEFA (E 475).
the European Commission considers revising the EU specifications for PEFA (E 475) including maximum limits for impurities currently included in the EU specifications for glycerol (E 422) or recommended by the Panel in the re‐evaluation of glycerol (E 422) (EFSA ANS Panel, 2017a,b,c)
Documentation provided to EFSA
EFEMA (European Food Emulsifier Manufacturers’ Association), 2009. EFEMA index of food emulsifiers. September 2009, 5th Edition. Submitted by EFEMA, January 2011,
EFEMA (European Food Emulsifiers Manufacturers Association), 2016. EFEMA response to EFSA's request for information on the chemical identity of each individual fatty acid including their percentage in the sources used for each food additive listed in the call for technical data. Submitted by EFEMA on 30 September 2016.
FDE (Food Drink Europe), 2013. Data on usage levels of polyglycerol esters of fatty acids (PEFA) (E 475) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 2), Published 26 March 2013. Submitted to EFSA on 29 November 2013.
ICGA (International Chewing Gum Association), 2014. Data on usage levels of polyglycerol esters of fatty acids (PEFA) (E 475) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 2), Published 26 March 2013. Submitted to EFSA on 30 September 2014.
Philp JM, 1963. Food additive testing. Some practical consideration as exemplified bij test with the emulsifiers, glyceranan ester PGE, 10. Submitted by EFEMA, January 2011.
Philp JM, 1967. A symposium on Chemical Additives in Food. 3. Biological evaluation of food additives. General considerations as exemplified by work on the emulsifier glyceran ester PGE 19. Ed: Goodwin RW. J&A Churchill LTD, London, 1967, 43–62. Submitted by EFEMA, January 2011.
Pre‐evaluation document prepared by Fraunhofer, December 2012.
Unilever Research Laboratory, 1966. Biological effects of glyceran ester PGE 19. Synopsis and contents. Submitted by EFEMA, January 2011.
Abbreviations
- 3‐MCPD
3‐monochloropropane‐1,2‐diol
- ADI
acceptable daily intake
- ANS
EFSA Scientific Panel on Food Additives and Nutrient Sources added to Food
- AV
acid value
- BfR
Bundesinstitut für Risikobewertung
- bw
body weight
- CAS
Chemical Abstracts Service
- CIR
Cosmetics Ingredient Review
- CONTAM
EFSA Panel on Contaminants in Food Chain
- DMSO
dimethyl sulfoxide
- EFEMA
European Food Emulsifiers Manufacturers Association
- EINECS
European Inventory of Existing Chemical Substances
- FAO
Food and Agriculture Organization of the United Nations
- FCs
food categories
- FCS
food categorisation system
- FDE
Food Drink Europe
- GC
gas chromatography
- GNPD
Global New Products Database
- HPLC
high‐performance liquid chromatography
- IARC
International Agency for Research on Cancer
- ICGA
International Chewing Gum Association
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- MOE
margin of exposure
- MPL
maximum permitted level
- MS
mass spectrometry
- NICNAS
National Industrial Chemicals Notification and Assessment Scheme
- NDA
EFSA Panel on Dietetic Products, Nutrition and Allergies
- NMR
nuclear magnetic resonance
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- PGE
polyglycerol ester
- PEFA
polyglycerol esters of fatty acids
- QS
quantum satis
- QSAR
quantitative structure–activity relationship
- RH
relative humidity
- SCF
Scientific Committee on Food
- TemaNord
is a publishing series for results of the often research‐based work that working groups or projects under Nordic Council of Ministers have put in motion
- TDI
tolerable daily intake
- TLC
thin‐layer chromatography
- WHO
World Health Organization
Appendix A – Summary of the reported use levels in food (mg/kg or mg/L) of PEFA (E 475) provided by industry
Appendix B – Number and percentage of food products labelled with PEFA (E 475) out of the total number of food products present in the Mintel GNPD per food subcategory between 2012 and 2017
Appendix C – Concentration levels of PEFA (E 475) used in the exposure scenarios (mg/kg or mg/L as appropriate)
Appendix D – Summary of total estimated exposure of PEFA (E 475) per population group and survey: mean and 95th percentile (mg/kg bw per day)
Appendix E – Main food categories contributing to exposure to PEFA (E 475) (> 5% to the total mean exposure)
Appendix A–E can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2017.5089
Supporting information
Summary of the reported use levels in food (mg/kg or mg/L) of PEFA (E 475) provided by industry
Number and percentage of food products labelled with PEFA (E 475) out of the total number of food products present in the Mintel GNPD per food subcategory between 2012 and 2017
Concentration levels of PEFA (E 475) used in the exposure scenarios (mg/kg or mg/L as appropriate)
Summary of total estimated exposure of PEFA (E 475) per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to PEFA (E 475) (> 5% to the total mean exposure)
Suggested citation: EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Boon P, Chrysafidis D, Gürtler R, Mosesso P, Parent‐Massin D, Tobback P, Rincon AM, Horvath Zs and Lambré C, 2017. Scientific Opinion on the re‐evaluation of polyglycerol esters of fatty acids (E 475) as a food additive. EFSA Journal 2017;15(12):5089, 32 pp. 10.2903/j.efsa.2017.5089
Requestor: European Commission
Question number: EFSA‐Q‐2011‐00564
Panel members: Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipič, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Gunter Georg Kuhnle, Claude Lambré, Jean‐Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The ANS Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 23 November 2017
Notes
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents. Published: 22 November 2009. Available online: http://www.efsa.europa.eu/en/dataclosed/call/ans091123
Call for food additives usages level and/or concentration data in food and beverages intended to human consumption. Published: 26 March 2013. Available online: https://www.efsa.europa.eu/sites/default/files/consultation/130327%2C0.pdf
Call for scientific data on selected food additives permitted in the EU. Published: 7 July 2014. Available online: http://www.efsa.europa.eu/en/dataclosed/call/140324.htm
Call for technical data on salts of fatty acids (E470a,b) and related food additives authorised in the EU. Published: 19 January 2016. Available online: http://www.efsa.europa.eu/sites/default/files/consultation/160119.pdf
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
Acid value: mg of KOH needed to neutralize the organic acids I 1 g of fatty material.
Call for food additives usages level and/or concentration data in food and beverages intended to human consumption. Published: 27 March 2013. Available online: http://www.efsa.europa.eu/en/dataclosed/call/130327.htm
Missing Bulgaria, Cyprus, Estonia, Latvia, Lithuania, Luxembourg, Malta and Slovenia.
http://www.gnpd.com/sinatra/home/ accessed on 24/7/2017.
References
- Babayan VK, Kaunitz H and Slanetz CA, 1964. Nutritional studies of polyglycerol esters. Journal of the American Oil Chemists’ Society, 41, 434–437. [Google Scholar]
- Baichwal MR and Lalla JK, 1973. Studies in polyglycerol esters – part III. Stability, toxicity and compatibility of polyglycerol stearate with pharmaceutical adjuvants. The Indian Journal of Pharmacy, 35, 140–145. [Google Scholar]
- Bastida‐Rodriguez J, 2013. The food additive polyglycerol polyricinoleate (E‐476): structure, applications, and production methods. ISRN chemical engineering, 21 pp. [Google Scholar]
- Behrens H and Mieth G, 1984. Synthese, charakterisierung und applikation von polyglycerolen und polyglycerolfettsaureestern. Die Nahrung, 28, 815–835. [DOI] [PubMed] [Google Scholar]
- Benigni R, Bossa C and Worth A, 2010. Structural analysis and predictive value of the rodent in vivo micronucleus assay results. Mutagenesis, 25, 335–341. [DOI] [PubMed] [Google Scholar]
- Benigni R, Bossa C, Tcheremenskaia O, Battistelli CL and Crettaz P, 2012. The new ISSMIC database on in vivo micronucleus and its role in assessing genotoxicity testing strategies. Mutagenesis, 27, 87–92. [DOI] [PubMed] [Google Scholar]
- BfR (Bundesinstitut für Risikobewertung), 2009. Intitial evaluation of the assessment of levels of glycidol fatty acids esters detected in refined vegetable fats. BfR Opinion No 007/2009, 10 March 2009. [Google Scholar]
- Bichel J, Therkelsen AJ and Stenderup A, 1964. Untersuchungen über die mögliche cancerogenität eines polyglycerinesters. Arzneimittel Forschung, 14, 238–239. [PubMed] [Google Scholar]
- Bodansky M, Herrmann CL and Campbell K, 1938. Utilization of polyglycerol esters. Biochemical Journal, 32, 1938–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briski B, 1970. Unpublished report, Institute of Public Health of Croatia, Yugoslavia, as referred to by JECFA, 1974a.
- Cani PD and Everard A, 2015. Keeping gut lining at bay: impact of emulsifiers. Trends in Endocrinology and Metabolism, 26, 273–274. [DOI] [PubMed] [Google Scholar]
- Cassel S, Chaimbault P, Debaig C, Benvegnu T, Claude S, Plusquellec D, Rollin P and Lafosse M, 2001. Liquid chromatography of polyglycerol fatty esters and fatty ethers on porous graphitic carbon and octadecyl silica by using evaporative light scattering detection and mass spectrometry. Journal of Chromatography A, 919, 95–106. [DOI] [PubMed] [Google Scholar]
- Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE and Gewirtz AT, 2015. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature, 519, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, de Wiele TV, De Bodt J, Marzorati M and Gewirtz, AT , 2017. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut, 66, 1414–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIR (Cosmetic Ingredient Review), 2016. Safety assessment of polyglyceryl fatty acid esters as used in cosmetics. Available online: https://www.cir-safety.org/sites/default/files/polyglyceryl%20fatty%20acid.pdf [DOI] [PubMed]
- De Meulenaer B, Van Royen G, Vanhoutte B and Huyghebaert A, 2000. Combined liquid and gas chromatographic characterisation of polyglycerol fatty acid esters. Journal of Chromatography A, 896, 239–251. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Scientific opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Dusemund B, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Parent‐Massin D, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Younes M, Boon P, Chrysafidis D, Gürtler R, Tobback P, Rincon AM, Tard A and Lambre C, 2017a. Scientific opinion on the re‐evaluation of glycerol (E 422) as a food additive. EFSA Journal 2017;15(3):4720, 64 pp. 10.2903/j.efsa.2017.4720 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Dusemund B, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Parent‐Massin D, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Younes M, Boon P, Chrysafidis D, Gürtler R, Tobback P, Rincon AM, Tard A and Lambre C, 2017b. Scientific opinion on the re‐evaluation of polyglycerol polyricinoleate (E 476) as a food additive. EFSA Journal 2017;15(4):4743, 39 pp. 10.2903/j.efsa.2017.4743 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Dusemund B, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Parent‐Massin D, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Younes M, Boon P, Chrysafidis D, Gürtler R, Tobback P, Gergelova P, Rincon AM and Lambre C, 2017c. Scientific Opinion on the re‐evaluation of fatty acids (E 570) as a food additive. EFSA Journal 2017;15(5):4785, 48 pp. 10.2903/j.efsa.2017.4785 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009a. Scientific opinion on cadmium in food. EFSA Journal 2009;7(10):980, 139 pp. 10.2903/j.efsa.2009.98 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009b. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351, 199 pp. 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012a. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 241 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012b. Scientific Opinion on lead dietary exposure in the European population. EFSA Journal 2012;10(7):2831, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012c. Scientific Opinion on cadmium dietary exposure in the European population. EFSA Journal 2012;10(1):2551, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on dietary exposure to inorganic arsenic in the European population. EFSA Journal 2014;12(3):3597, 68 pp. 10.2903/j.efsa.2014.3597 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2016. Risks for human health related to the presence of 3‐ and 2‐monochloropropanediol (MCPD), and their fatty acid esters, and glycidyl fatty acid esters in food. EFSA Journal 2016;14(5):4426, 159 pp. 10.2903/j.efsa.2016.4426 [DOI] [Google Scholar]
- EFSA NDA Panel (Scientific Panel on Dietetic Products, Nutrition and Allergies), 2004. Opinion on a request from the Commission related to the presence of trans fatty acids in foods and the effect on human health of the consumption of trans fatty acids. EFSA Journal 2004;81, 1–4, 10.2903/j.efsa.2004.81 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2010. Scientific Opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA Journal 2010;8(3):1461, 107 pp. 10.2903/j.efsa.2010.1461 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- Gross M, 1980. Der polyglyzerinanteil international handelsüblicher polyglyzerinester. Lebensmittelindustrie, 27, 117–120. [Google Scholar]
- IARC (International Agency for Research Cancer), 1999. Epichlorohydrin. Mongraph, 71, 603–629. Available online: https://monograph.iarc.fr/ENG/Monograph/vol71/mono71.pdf [Google Scholar]
- IARC (International Agency for Research Cancer), 2000. Glycidol. Mongraph, 77, 469–487. Available online: https://monograph.iarc.fr/ENG/Monograph/vol77/mono77.pdf [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1974a. Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. Polyglycerol esters of fatty acids. WHO Food Additives Series, No. 5. World Health Organization, Geneva, Switzerland. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1974b. Toxicological evaluation of certain food additives with a review of general principles and of specifications. Seventeenth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 539. World Health Organization, Geneva, Switzerland. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1990. Evaluation of certain food additives and contaminants. Twenty‐fifth report of the Joint FAO/WHO Expert Committee on Food Additives. No. 789. World Health Organization, Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Monograph 1. Combined compendium of food additive specifications. All specifications monographs from the 1st to the 65th meeting (1956–2005). Volume 4, Available online: http://www.fao.org/ag/agn/jecfa-additives/search.html
- King WR, Michael WR and Coots RK, 1971. Subacute oral toxicity of polyglycerol ester. Toxicology and Applied Pharmacology, 20, 327–333. [DOI] [PubMed] [Google Scholar]
- Lecomte M, Couedelo Leslie, Meugnier E, Plaisancie P, Letisse M, Bérengère B, Gabert L, Penhoat A, Durand A, Pineau G, Joffre F, Géloën A, Vaysse C, Laugerette F and Michalski MC, 2016. Dietary emulsifiers from milk and soybean differently impact adiposity and inflammation in association with modulation of colonic goblet in high‐fat fed mice. Molecular Nutrition and Food Research, 60, 606–620. [DOI] [PubMed] [Google Scholar]
- Merga Y, Campbell BJ and Rhodes JM, 2014. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Digestive Diseases, 32, 475–483. [DOI] [PubMed] [Google Scholar]
- Michael WR and Coots RH, 1971. Metabolism of polyglycerol and polyglycerol esters. Toxicology and Applied Pharmacology, 20, 334–345. [DOI] [PubMed] [Google Scholar]
- Nejrup RG, Licht TR and Hellgren LI, 2017. Fatty acid composition and phospholipid types used in infant formulas modifies the establishment of human gut bacteria in germ‐free mice. Scientific Reports, 7(1), 3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICNAS (National Industrial Chemicals Notification and Assessment Scheme), 2013. File No. STD/1453. Public report: 1,2,3‐Propanetriol, homopolymer, dodecanoate.
- Norn V, 2015. Emulsifiers in food technology In: Nord V. (ed.). Mono‐ and Diglycerides, 2nd Edition John Wiley & sons Ltd, West Sussex, UK: pp. 73–90. [Google Scholar]
- Ostertag H and Wurziger J, 1965. Beiträge zum biologischen Verhalten von Polyglycerin‐Verbindungen. Arzneimittel‐Forschung, 15, 869–872. [PubMed] [Google Scholar]
- Renz H, Brandtzaeg P and Hornef M, 2012. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nature Reviews‐Immunology, 12, 9–23. [DOI] [PubMed] [Google Scholar]
- Romano‐Keeler J and Weitkamp JH, 2015. Maternal influences on fetal microbial colonization and immune development. Pediatric Research, 77, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food), 1978. Report of the Scientific Committee for Food on emulsifiers, stabilizers, thickeners and gelling agents (Opinion expressed 30 November 1978). Seventh series.
- SCF (Scientific Committee for Food), 2001. Guidance on submissions for food additive evaluations by the Scientific Committee on Food. SCF/CS/ADD/GEN/26 Final. 12 July 2001.
- Shah R, Kolanos R, DiNovi MJ, Mattia A and Kaneko KJ, 2017. Dietary exposures for the safety assessment of seven emulsifiers commonly added to foods in the United States and implications for safety. Food Additives and Contaminants: Part A, 34, 905–917. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Loening‐Baucke V and Herber A, 2009a. Mucosal flora in Crohn's disease and ulcerative colitis ‐ an overview. Journal of Physiology and Pharmacology, 60(supplement 6), 61–71. [PubMed] [Google Scholar]
- Swidsinski A, Ung V, Sydora BC, Loening‐Baucke V, Doerffel Y, Verstraelen H and Fedorak RN, 2009b. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflammatory Bowel Diseases, 15, 359–364. [DOI] [PubMed] [Google Scholar]
- TemaNord (Nordic Council of Ministers), 2002. E 475 Polyglycerol esters of fatty acids. Food Additives in Europe 2000 ‐ Status of safety assessments of food additives presently permitted in the EU, 479–481.
- Washizaki K, Kanto H, Yazaki S and Ito M, 2008. A case of allergic contact dermatitis to polyglyceryl laurate. Contact Dermatitis, 58, 187–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the reported use levels in food (mg/kg or mg/L) of PEFA (E 475) provided by industry
Number and percentage of food products labelled with PEFA (E 475) out of the total number of food products present in the Mintel GNPD per food subcategory between 2012 and 2017
Concentration levels of PEFA (E 475) used in the exposure scenarios (mg/kg or mg/L as appropriate)
Summary of total estimated exposure of PEFA (E 475) per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to PEFA (E 475) (> 5% to the total mean exposure)


