Abstract
In chronic obstructive pulmonary disease (COPD), acute exacerbation of COPD requiring hospital admission is associated with mortality and healthcare costs. The ERICA study assessed multiple clinical measures in people with COPD, including the short physical performance battery (SPPB), a simple test of physical function with 3 components (gait speed, balance and sit-to-stand). We tested the hypothesis that SPPB score would relate to risk of hospital admissions and length of hospital stay. Data were analysed from 714 of the total 729 participants (434 men and 280 women) with COPD. Data from this prospective observational longitudinal study were obtained from 4 secondary and 1 tertiary centres from England, Scotland, and Wales. The main outcome measures were to estimate the risk of hospitalisation with acute exacerbation of COPD (AECOPD and length of hospital stay derived from hospital episode statistics (HES). In total, 291 of 714 individuals experienced 762 hospitalised AECOPD during five-year follow up. Poorer performance of SPPB was associated with both higher rate (IRR 1.08 per 1 point decrease, 95% CI 1.01 to 1.14) and increased length of stay (IRR 1.18 per 1 point decrease, 95% CI 1.10 to 1.27) for hospitalised AECOPD. For the individual sit-to-stand component of the SPPB, the association was even stronger (IRR 1.14, 95% CI 1.02 to 1.26 for rate and IRR 1.32, 95% CI 1.16 to 1.49 for length of stay for hospitalised AECOPD). The SPPB, and in particular the sit-to-stand component can both evaluate the risk of H-AECOPD and length of hospital stay in COPD. The SPPB can aid in clinical decision making and when prioritising healthcare resources.
Introduction
In the United Kingdom (UK), chronic obstructive pulmonary disease (COPD) related mortality and healthcare costs are projected to increase significantly, with annual direct healthcare costs in England alone expected to increase from £1.5 billion in 2011 to £2.32 billion by 2030.[1] Most of the cost of treating COPD arises from inpatient care,[2] specifically for hospitalised acute exacerbations (i.e. episodic worsening of symptoms) of COPD (H-AECOPD).
According to 2016–17 statistics of the National Health Services (NHS) Digital, more than 128,000 individuals with a specific code for COPD exacerbation in the United Kingdom were admitted to hospital, of which 97% were emergency admissions with a median hospital length of stay of three days.[3] Leaving aside cost, admission with AECOPD is a major event in the lifetime of a COPD patient. National UK audit data shows that, while outcomes have improved, in-patient mortality remains high (4.3%) with a further 2.8% of those discharged dying within 30 days. Importantly, longer length of stay was associated with increased mortality at both 30 and 90 days (9.9 and 22.6% respectively).[4]
The risk of having an acute exacerbation of COPD is determined by the patient’s contact with an infectious (i.e. bacterial or viral) or environmental (e.g. air pollution) triggers. However, the need for hospitalisation for AECOPD is also determined by the patient’s physical capacity. In a prior analysis of data from the COPD Biomarker Qualification Consortium,[5] we showed that an integrative test of physical capacity, the six minute walk test (6MWT) distance, was not at all predictive of the likelihood of acute exacerbation but was strongly predictive of one year mortality and to a lesser extent of hospitalisation.
The 6MWT is cumbersome in general practice and to some extent in secondary care; this is acknowledged in the recent update of the National Institute for Health and Care Excellence 2018 guidelines that recommended against the use of the BODE Index for prognostication since the components (which include 6MWT) are time-intensive and seldom feasible in primary care.[6] In particular, the 6MWT requires a minimum 30m flat, uninterrupted track in an unhurried setting, which is often unavailable, and when completed properly should be undertaken twice to minimise learning effect. Thus, a proper evaluation may take 30 minutes. In contrast, the short physical performance battery (SPPB) can be undertaken with no special facilities in a routine clinic and is used in clinical practice by geriatricians and those in pulmonary rehabilitation.[7]
We therefore hypothesised in this study that SPPB score could serve both to identify patients who are more likely to be hospitalised or, when admitted, to have a longer length of stay. Data from the UK multicentre Evaluating the Role of Inflammation in Chronic Airways disease (ERICA) cohort study, which has been described in more detail elsewhere[8,9] were used with the aim being to evaluate the relationship between functional measures, specifically SPPB and risk of H-AECOPD. Further, we aimed to determine a relationship with length of hospital stay for initial (i.e. first hospital admission after assessment) AECOPD. To address these questions, we combined routinely collected hospital electronic health record data with baseline ERICA data.
Methods
Study design and participants
Observational data is reported according to the STROBE statement.[10] Data were used from the ERICA cohort, a multi-centre observational, non-interventional, epidemiological study with a sample size of 729 individuals with stable COPD (Global Initiative for Chronic Obstructive Lung Disease grade II-IV). The study was originally designed and powered on the basis of a tertile analysis of variables pulse wave velocity and QMVC, based on an estimated sample size of 800 individuals with COPD. Full study design and participant details are available in the published ERICA cohort protocol.[8] The study is registered with the UK Clinical Research Network Study Portfolio (StudyID 11101). Baseline data were collected between December 2011 and January 2014. Patient level cohort data were linked to hospital admission data obtained from the NHS admitted patient care dataset, Hospital Episodes Statistics (HES) in England, Scotland and Wales, which captures all H-AECOPD, since cohort baseline visit until November 2017. Analyses were limited to a maximum five years of follow-up. See S1 Text for further details.
Study outcomes
The primary outcome measure was H-AECOPD. These data were first cleaned for episode status and inpatient (i.e. hospitalised) AECOPD episodes were identified using validated criteria (S1 Table).[11] Acute exacerbations of COPD were extracted from both primary and secondary positions of international classification of diseases and related health problems 10th revision coding. Only so-called definite and possible H-AECOPD were considered for this analysis. Only episodes during the study follow-up were evaluated. Admission and discharge dates were used to determine hospital length of stay (i.e. number of days) for initial H-AECOPD after ERICA assessment.
Potential predictor variables
All significant variables reported by Hurst et al.[12] and functional measures assessed in the ERICA cohort were considered. A full list of predictor variables is shown in S2 Table including demographics, lung function measurements, blood markers, health-related quality of life and respiratory symptoms questionnaire data, and functional assessments. Measures of particular interest were those measuring physical capacity including SPPB and its components (i.e. 4-metre gait speed (4MGS), balance, and 5 repetition sit-to-stand), quadriceps maximum voluntary contraction (QMVC), and 6MWT. The SPPB is a battery of tests and used to evaluate the physical performance of the lower extremities. Its three components score 0–4. Total SPPB score is the sum of points of each component, with a maximum of twelve denoting no functional limitation. QMVC is a surrogate marker of functional activity (i.e. quadriceps muscle strength) where the best effort of six contractions was recorded, and the QMVC%predicted was derived from the Seymour equation.[13] The 6MWT was used to assess exercise intolerance and to evaluate functional exercise capacity by recording the distance walked as quickly as possible for six minutes. The exacerbation history in the twelve months prior to study baseline was dichotomised (i.e. 0 vs. ≥ 1). Prior exacerbation history was self-reported and defined as requiring treatment with oral steroids or antibiotics.
Statistical analysis
Missing values are described in S1 and S2 Figs, S2 Text; only complete cases were considered. Relationships between baseline variables were quantified using Spearman’s pair-wise correlations; values < 0.30 were considered weak, 0.30–0.50 as moderate, and > 0.50 as strong (S3 Fig).[14] Negative binomial regression was used to examine the association between functional measures and (i) the rate of H-AECOPD within the study period, and (ii) length of hospital stay (per day). Analyses were adjusted for exposure times (time between baseline visit date and earliest of death, or end of study period). Regression estimates are presented as incidence-rate ratios (IRR). Incidence risk ratios for log-transformed biomarkers represent a twofold increase in the biomarker.
All analyses were stratified by recruitment site and adjusted for age and sex. Further analyses were adjusted for body mass index, smoking status, and covariates found to be of significance in the main multivariate model by Hurst et al, namely exacerbation history (previous year), forced expiratory volume in one second (FEV1) measured in litres, and productive cough (defined using questionnaire data: “If you cough, do you produce phlegm (sputum)?”).[12] Covariates were tested for collinearity resulting in the omission of Medical Research Council dyspnoea score and white cell count. Predictors for the final analyses were derived sequentially, firstly estimating the association of each individual variable fully adjusted, following stepwise regression including the significant variables only, whilst considering collinearity and clinical utility.
In stepwise regression analysis, only predictors with a significance level above α 0.1 for backward selection and α 0.05 for forward stepwise selection were considered. For each stepwise regression, likelihood ratio tests were conducted to determine if independent variables should remain in the analysis or not, and the maximum number of variables considered in each regression analysis were based on the least number of events.[15] As sex and exacerbation history can act as effect modifiers, in sensitivity analyses, we explored analysis stratified by these factors and tested for interactions.
To evaluate the ability of SPPB, or its sit-to-stand component, to predict time to H-AECOPD, we used Cox-regression with significance assessed using log-rank test for trend. Estimates are displayed using Kaplan-Meier plots.
All tests were two-sided and of statistical significance level of p = 0.05. Our analyses were performed using Stata version 13 (College Station, Texas) and R (R Foundation).
Results
Descriptive statistics
In total, 714 individuals with stable COPD and complete data were included in the analysis. At baseline, the mean ± standard deviation age of the cohort was 67 ± 8 years with 61% of participants being male. A third of the cohort was overweight, another third obese; and a third were current smokers. Exacerbations during the year prior to baseline were self-reported by 67% individuals with a corresponding mean of 2 ± 2 events per person-year. Mean FEV1% predicted was 52 ± 16%. About half of the cohort (51%) experienced breathlessness that limited daily activities (Medical Research Council dyspnoea score ≥ 3) and 46% had productive cough on most mornings (Table 1 and S3 Table, S2 Text). Individuals with a history of AECOPD at baseline were more likely to be younger, female, have worse lung function, shorter walking distance, and lower SPPB scores.
Table 1. Baseline characteristics.
| Total | Without AECOPD history at baselinea | With AECOPD history at baseline a | P value | |
|---|---|---|---|---|
| Characteristic | Mean ± SD or n (%) | |||
| Description | ||||
| Age (years) | 67 ± 8 | 68 ± 8 | 67 ± 7 | 0.022 |
| Sex, n (%) | < 0.001 | |||
| Male | 434 (61) | 171 (72) | 261 (55) | |
| Female | 280 (39) | 65 (28) | 212 (45) | |
| Body mass index (kg/m2) | 27 ± 6 | 27 ± 5 | 27 ± 6 | 0.389 |
| Musculoskeletal measures | ||||
| 6MWT distance (metre) | 346 ± 130 | 384 ± 122 | 326 ± 130 | < 0.001 |
| SPPB (0–12) | 10 ± 2 | 10 ± 2 | 9 ± 3 | 0.015 |
| 4MGS score (0–4) | 4 ± 1 | 4 ± 1 | 3 ± 1 | < 0.001 |
| Balance points (0–4) | 4 ± 1 | 4 ± 1 | 4 ± 1 | 0.719 |
| Sit-to-stand score (0–4) | 2 ± 1 | 3 ± 1 | 2 ± 1 | 0.004 |
| QMVC peak (kg) | 31 ± 11 | 29 ± 12 | 33 ± 11 | < 0.001 |
| QMVC % predicted | 44 ± 8 | 46 ± 8 | 44 ± 8 | 0.005 |
| Lung function | ||||
| FEV1% predicted | 52 ± 16 | 57 ± 14 | 50 ± 16 | < 0.001 |
| Smoking status, n (%) | 0.032 | |||
| Current | 218 (31) | 85 (36) | 133 (28) | |
| Former | 492 (69) | 151 (64) | 340 (72) | |
| GOLD, n (%) | < 0.001 | |||
| Grade II | 406 (57) | 166 (70) | 237 (50) | |
| Grade III | 240 (34) | 56 (24) | 183 (39) | |
| Grade IV | 68 (10) | 14 (6) | 53 (11) | |
| Productive cough, n (%) | < 0.001 | |||
| Never | 46 (7) | 32 (14) | 14 (3) | |
| Other | 662 (94) | 200 (86) | 459 (97) | |
| Biochemical measures | ||||
| log Glucose (mmol/L) | 1.60 ± 0.15 | 1.61 ± 0.16 | 1.61 ± 0.15 | 0.945 |
| log Fibrinogen (g/dL) | 1.22 ± 0.23 | 1.18 ± 0.23 | 1.24 ± 0.23 | 0.002 |
| log C-reactive protein (mg/L) | 1.26 ± 1.08 | 1.11 ± 1.03 | 1.32 ± 1.09 | 0.016 |
| GFR (mL/min/1.73 m2) | 88 ± 18 | 87 ± 19 | 89 ± 18 | 0.443 |
| Neutrophil count (mm3) | 4.75 ± 1.70 | 4.57 ± 1.65 | 4.81 ± 1.67 | 0.015 |
| Cardiovascular status | ||||
| Heart rate (bpm) | 75 ± 13 | 74 ± 12 | 75 ± 12 | 0.546 |
| Questionnaires | ||||
| SGRQ-C (0–100) | 50 ± 21 | 40 ± 20 | 54 ± 20 | < 0.001 |
| CAT (0–40) | 20 ± 8 | 17 ± 8 | 21 ± 8 | < 0.001 |
Values are given as the mean and standard deviation, or No. of cases (%). Baseline data of study participants are included.
aSelf-reported prior to study.
SD, standard deviation. 6MWT, six-minute walk test. SPPB, short physical performance battery. 4MGS, four-metre gait speed. QMVC, quadriceps maximum voluntary contraction. FEV1, forced expiratory volume in one second. GOLD, global initiative for obstructive lung disease. GFR, glomerular filtration rate. SGRQ-C, St George's respiratory questionnaire for COPD. CAT, COPD assessment test.
In total, 291 (41%) experienced at least one H-AECOPD during the study follow-up; 159 (22%) had multiple events (S4–S6 Figs). Overall, 127 (18%) individuals died and of these, the majority 103 (81%) died following discharge having been hospitalised for AECOPD during the study period. Median (interquartile range (IQR)) length of hospital stay for initial (i.e. first hospital admission after assessment) H-AECOPD was 3 (1–7) days. For the 159 readmitted, the median time to hospital readmission was 179 (54–421) days, of whom 65 individuals (41%) were readmitted to hospital within 90 days after initial admission and had a median length of stay of 3 (2–7) days.
Factors associated with rate of H-AECOPD
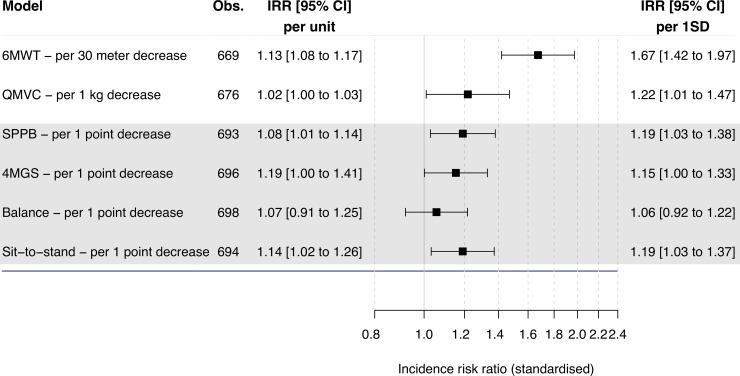
Adjusted analysis showed 6MWT, SPPB and its 4MGS, and sit-to-stand components, and QMVC were all associated with a higher risk of H-AECOPD (Fig 1 and S7 Fig, S4 Table). The 6MWT (IRR 1.13 per 30 metre decrease, 95% CI 1.08 to 1.17, p < 0.001), FEV1 (IRR 0.84 per 100 ml increase, 95% CI 0.81 to 0.86, p < 0.001) or disease severity measured by Global Initiative for Chronic Obstructive Lung Disease (IRR 2.51 per increase to next stage, 95% CI 2.04 to 3.10, p < 0.001), and males (IRR 2.41, 95% CI 1.77 to 3.29, p < 0.001) had the strongest associated IRRs.
Fig 1. Associations of baseline musculoskeletal measures and rate of hospitalised acute exacerbation of chronic obstructive pulmonary disease in the ERICA cohort.
Risk indicated as incidence risk ratios. Estimates derived using negative binomial regression. Analyses adjusted for recruitment site. Age, sex, body mass index, smoking status, forces expiratory volume in one second, phlegm, and exacerbation history were included as covariates. Figure displays standardised IRRs, allowing comparison of measurements on different scales. Obs, number of observations included in analysis. IRR, incidence risk ratios. CI, confidence intervals. SD, standard deviation. 6MWT, six-minute walk test. SPPB, short physical performance battery. 4MGS, four-metre gait speed. QMVC, quadriceps maximum voluntary contraction.
Fully adjusted multivariable stepwise regression retained the following significant predictors: male gender (IRR 2.14, 95% CI 1.55 to 2.96, p < 0.001), FEV1, (IRR 0.88 per 100 ml increase, 95% CI 0.85 to 0.91, p < 0.001), exacerbation history ≥ 1 (IRR 1.96, 95% CI 1.39 to 2.76, p < 0.001), COPD assessment test score (IRR 1.03 per 1 point increase, 95% CI 1.01 to 1.05, p = 0.010), resting heart rate (IRR 1.01 per 1 bpm increase, 95% CI 1.00 to 1.03, p = 0.025), and 6MWT (IRR 1.08 per 30 metre decrease, 95% CI 1.04 to 1.12, p < 0.001; Table 2).
Table 2. Factors associated with rate of H-AECOPD in the stepwise multivariable model.
| Multivariable analysisa | Stepwise regression (n = 610) | ||
|---|---|---|---|
| Factors | IRR (95% CI) | IRR (95% CI) | P value |
| Sex–male | 2.41 (1.77 to 3.29) | 2.14 (1.55 to 2.96) | < 0.001 |
| 6MWT distance–per 30 metre decrease | 1.13 (1.08 to 1.17) | 1.08 (1.04 to 1.12) | < 0.001 |
| SPPB score–per 1 point decrease | 1.08 (1.01 to 1.14) | Omitted | N/A |
| QMVC peak–per 1 kg decrease | 1.02 (1.00 to 1.03) | Omitted | N/A |
| FEV1 –per 100 ml increase | 0.84 (0.81 to 0.86) | 0.88 (0.85 to 0.91) | < 0.001 |
| Exacerbation history (1 year), ≥ 1b | 1.94 (1.40 to 2.67) | 1.96 (1.39 to 2.76) | < 0.001 |
| Fibrinogen–per 1 log unit increase | 1.95 (1.03 to 3.68) | Omitted | N/A |
| Neutrophils–per 1 unit increase | 1.14 (1.05 to 1.24) | Omitted | N/A |
| Resting heart rate–per 1 bpm increase | 1.02 (1.01 to 1.03) | 1.01 (1.00 to 1.03) | 0.025 |
| SGRQ-C–per 4 point increase | 1.07 (1.03 to 1.10) | Omitted | N/A |
| CAT–per 1 point increase | 1.05 (1.03 to 1.07) | 1.03 (1.01 to 1.05) | 0.010 |
Factors significantly associated with rate of H-AECOPD were included in the stepwise regression. Analyses were adjusted for recruitment site.
aAdjusted for age, sex, body mass index, smoking status, forced expiratory volume in one second, phlegm, and exacerbation history.
bSelf-reported prior to study.
IRR, incidence risk ratios. CI, confidence intervals. 6MWT, six-minute walk test. SPPB, short physical performance battery. QMVC, quadriceps maximum voluntary contraction. FEV1, forced expiratory volume in one second. SGRQ-C, St George's respiratory questionnaire for COPD. CAT, COPD assessment test.
Factors associated with H-AECOPD length of stay
Including data from individuals admitted to hospital only (n = 291), multivariable analysis identified multiple measures to be associated with H-AECOPD length of stay (Fig 2 and S8 Fig, S5 Table). All functional measures, except for QMVC were associated with a higher risk of H-AECOPD stay. Age (IRR 1.83 per 10-year increase, 95% CI 1.48 to 2.26, p < 0.001), 6MWT (IRR 1.14 per 30-metre decrease, 95% CI 1.08 to 1.20, p < 0.001), and SPPB (IRR 1.18 per 1 point decrease, 95% 1.10 to 1.27, p < 0.001) were the strongest associated variables.
Fig 2. Associations of baseline musculoskeletal measures and hospital length of stay after admission for acute exacerbation of chronic obstructive pulmonary disease in the ERICA cohort.
Risk indicated as incidence risk ratios. Estimates derived using negative binomial regression. Analyses adjusted for recruitment site. Age, sex, body mass index, smoking status, forces expiratory volume in one second, phlegm, and exacerbation history were included as covariates. Figure displays standardised IRRs, allowing comparison of measurements on different scales. Obs, number of observations included in analysis. IRR, incidence risk ratios. CI, confidence intervals. SD, standard deviation. 6MWT, six-minute walk test. SPPB, short physical performance battery. 4MGS, four-metre gait speed. QMVC, quadriceps maximum voluntary contraction.
Fully adjusted multivariable stepwise regression retained the following significant predictors: age (IRR 1.53 per 10-year increase, 95% CI 1.18 to 1.98, p = 0.001), body mass index (IRR 0.93 per 1 point increase, 95% CI 0.90 to 0.96, p < 0.001), glucose (IRR 2.89 per twofold increase, 95% CI 1.18 to 7.05, p = 0.020), and SPPB (IRR 1.19 per 1 point decrease, 95% CI 1.10 to 1.30, p < 0.001; Table 3).
Table 3. Factors associated with H-AECOPD length of stay in the stepwise multivariable model.
| Multivariable analysisa | Stepwise regression (n = 233) | ||
|---|---|---|---|
| Factor | IRR (95% CI) | IRR (95% CI) | P value |
| Age–per 10 year increase | 1.83 (1.48 to 2.26) | 1.53 (1.18 to 1.98) | 0.001 |
| BMI–per 1 point increase | 0.96 (0.93 to 0.99) | 0.93 (0.90 to 0.96) | < 0.001 |
| 6MWT distance–per 30 metre decrease | 1.14 (1.08 to 1.20) | Omitted | N/A |
| SPPB–per 1 point decreaseb | 1.18 (1.10 to 1.27) | 1.19 (1.10 to 1.30) | < 0.001 |
| Exacerbation history (1 year), ≥ 1c | 0.62 (0.39 to 0.97) | Omitted | N/A |
| Glucose–per 1 log unit increase | 8.78 (2.81 to 27.49) | 2.89 (1.18 to 7.05) | 0.020 |
| Fibrinogen–per 1 log unit increase | 3.14 (1.37 to 7.18) | Omitted | N/A |
| GFR–per 1 unit increase | 0.98 (0.97 to 1.00) | Omitted | N/A |
Factors significantly associated with H-AECOPD length of stay were included in the stepwise regression. Analyses were adjusted for recruitment site.
aAdjusted for age, sex, body mass index, smoking status, forced expiratory volume in one second, phlegm, and exacerbation history.
bWhen replacing SPPB with the sit-to-stand component both the sit-to-stand component and the 6MWT remain, but 6MWT is insignificant.
cSelf-reported prior to study.
IRR, incidence risk ratios. CI, confidence intervals. BMI, body mass index. 6MWT, six-minute walk test. SPPB = short physical performance battery. GFR, glomerular filtration rate.
Sensitivity analysis for rate of H-AECOPD
Overall, IRRs were higher for men and 6MWT for those with no exacerbation history (Table 2 and S6 Table). Incidence risk ratios of exacerbation history were higher for women when stratifying by gender (Table 2 and S7 Table). When testing for interactions, both prior exacerbation history and sex were significant.
Kaplan–Meier curves (Fig 3) according to SPPB tertiles demonstrated that reduced time to first H-AECOPD was associated with higher SPPB or sit-to-stand scores (log-rank test for trend: p = 0.032 and 0.008 respectively).
Fig 3. Time to first H-AECOPD estimates.
(A) Estimates by SPPB tertiles. (B) Estimates by sit-to-stand tertiles.
Discussion
In patients with stable COPD, we show that assessments of physical function including the SPPB, and in particular the sit-to-stand component as a standalone, identify patients at risk of H-AECOPD. Furthermore, barring age, the sit-to-stand is the strongest associated measure that predicts length of stay for H-AECOPD. We additionally confirmed our prior findings, that 6MWT predicts H-AECOPD in individuals with COPD after adjusting for common and known predictive covariates. The only equipment required for the sit-to-stand test is a chair and a stopwatch (an integral function on most smartphones) it can be performed virtually anywhere, and the test can be performed in less than five minutes. Therefore we propose that it may be a useful tool in both primary and secondary care for identification of patients at risk of H-AECOPD as well as those that are likely to have a prolonged length of stay.
Strengths of this study
This study has many strengths. Firstly, multiple functional measures were used simultaneously in a large cohort of individuals clinically stable at assessment. Thus, although the importance of physical performance has been previously reported for some of these assessments, this is the first study to compare several of these tests and also comprehensively control for all other clinical aspects of COPD. In addition, measurements were performed at five different sites across the UK.
Secondly, event rates were stable throughout the study period, which is not only encouraging but also rates were comparable to those in large cohort studies including ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points)[16] suggesting that the findings are generalisable outside the UK. Hospital admissions were identified using validated criteria, and only definite and possible episodes were included in the analysis. In contrast to self-reported hospital admission, which may suffer from under-reporting,[17,18] H-AECOPD episodes were captured objectively using electronic health record data. Individuals had different observation periods as a result of different entry times into the study. The use of study inclusion and admission (i.e. event) dates allowed to adjust for exposure time and therefore used the correct probability distributions.
Third, while previous exacerbation is an established predictor of exacerbation that can be easily assessed, we also investigated factors associated with hospitalisation in patients without exacerbation history. This is of interest since in patients with no previous exacerbation, the predictors of an exacerbation are less well established. Lastly, collection of a comprehensive data set permitted better adjustment for covariates than prior single centre studies.
Limitations of this study
Naturally this study has several limitations. Hospital Episode Statistics were obtained from the NHS Digital (England), NHS Scotland and NHS Wales. Apart from admission and discharge dates, we did not have spell data (i.e. total continuous stay and use of a hospital bed) available for individuals registered with the NHS Scotland and NHS Wales. The study period covered the time from study enrolment until the end of study, or death, and therefore the pre-enrolment hospital use history could not be obtained from HES. The final number of individuals included in the analysis were limited to those followed up by the NHS, slightly reducing the statistical power.
The study size precluded stratification by gender to assess the association between baseline measures and H-AECOPD stay (i.e. duration). Also, we explored for non-linearity of variables considered but lacked statistical power to identify any difference. There were differences in study populations between recruitment sites. For example, individuals from Cardiff were more symptomatic (based on COPD impact scores); however we caution that the departments at each of the five participating hospitals had variations in practice making analysis of difference in prognosis between sites of doubtful value. Nevertheless, we addressed this by adjusting for recruitment site in our analyses. There were missing data; in order to optimise the analysis, we included as many observations as possible and reported the number of observations included in each analysis.
Analyses were also adjusted for productive cough, believed to be an indicator of inflammation. A large proportion had productive cough on most mornings but there was no significant association with the outcomes in our cohort (see supplementary tables).
Quadriceps maximum voluntary contraction was included in the study both because we have previously found it to predict survival in COPD[19] and also because mechanistically it could explain SPPB score.[20] The study did not confirm QMVC to be a measure that could have widespread uses despite being associated with a higher risk of H-AECOPD, since it was not related to length of hospital stay. In addition, the test requires specialist equipment that is bulky and not currently commercially available. However, the effect of the SPPB, and therefore also QMVC, is potentially underestimated due to the relatively high SPPB mean score (10 points), with scores above ten indicating no functional limitation.
Meaning of this study
Our data suggest that the SPPB performed almost as well as the 6MWT for risk of H-AECOPD while being more practical; moreover the sit-to-stand component in particular could be useful as a standalone test in time-limited settings such as primary care with confidence since it was not inferior to the SPPB. The good performance of SPPB was not entirely unexpected. In the geriatrics literature, the SPPB is a well-established tool for predicting risk of admission to nursing home facilities. In a prior study, Kon et al. showed that one component of SPPB, the 4MGS, when measured at point of discharge for H-AECOPD, had a strong predictive value for 90-day readmission however that population has a strong pre-test probability of admission.[21] In a similar population, Barker et al. reported that overall SPPB measured at point of discharge following AECOPD was predictive of mortality risk.[22] In a study of 50 COPD patients the sit-to-stand performance related to other prognostic scoring systems in COPD, including the BODE score.[23]. In addition, the sit-to-stand component of the SPPB battery has some similarities with the one minute sit-to-stand test which is predictive of mortality in COPD.[24,25] However, the present study extends knowledge by showing that that the sit-to-stand and the SPPB are associated both with admission risk and length of stay in stable COPD outpatients, which is important for primary and secondary care. Importantly, no other study provides data suggesting that in patients with stable COPD, SPPB or the sit-to-stand are associated with H-AECOPD incidence as well as related length of stay, and this information further adds support for SPPB being used as a drug development tool and endpoint for clinical trials addressing AECOPD, especially since the European Medicines Agency favours the SPPB as the measure of choice in the assessment of frailty.[26] More recently, Hopkinson et al. emphasised the importance of considering individual risk factors such as exercise capacity, which influences long term prognosis including hospital admission.[27]
Some treatments are presently available that can reduce the risk of exacerbation including vaccination and optimisation of inhaled therapies, which it is increasingly recognised, should be tailored to the individuals eosinophil status.[28] In addition, exercise-based treatments, most notably pulmonary rehabilitation, can increase physical capacity.[29,30] Lastly although the available data are mixed, some reports suggest that, at least in subsets of COPD patients, novel strategies to deliver pulmonary rehabilitation can reduce re-admission rates and length of stay by early application of telemedicine techniques.[31,32] All of these interventions have costs and thus in terms of prioritising patients who will derive most benefit there is a need for a stratification tool. Based on our findings we propose that the sit-to-stand component be adopted as a routine measure in the care pathway for COPD patients, as its use can aid in identification of at-risk patients and thus aid resource planning.
Future studies
Future studies, using larger cohorts and/or different geographical populations, should replicate our findings. In particular, when developing or evaluating a multivariable prognostic model, the sample size should be estimated based on the D or C-statistic in order to sufficiently capture the significance of prognostic of specific biomarkers and produce robust estimates.[33] In addition, evaluating these measures longitudinally would allow better estimation of the association between H-AECOPD rate, duration, and readmission at different time points, as well as estimation of a minimally important difference in COPD, the latter of which is currently lacking in literature.
Conclusions
Findings indicate that physical function including the SPPB, and in particular the sit-to-stand component as a standalone test can both evaluate the risk of H-AECOPD and length of hospital stay in individuals with COPD. Moreover since sit-to-stand and SPPB can be performed almost anywhere and without special equipment. Our data support the use of sit-to-stand or SPPB to aid in clinical decision making at an individual level and when prioritising healthcare resources, and support the incorporation of this tool into the annual COPD patient clinical review.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
GFR = glomerular filtration rate. 6MW = six-minute walk. QMVC = quadriceps maximum voluntary contraction. CRP = C-reactive protein. HR = heart rate. CAT = COPD assessment test. SPPB = short physical performance battery. BMI = body mass index. FEV1 = forced expiratory volume in one second. GOLD = global initiative for obstructive lung disease.
(PDF)
SGRQ = St. George respiratory questionnaire for COPD. QMVC = quadriceps maximum voluntary contraction. 6MW = six-minute walk. GFR = glomerular filtration rate.
(PDF)
EXAC = exacerbation history. MRC = Medical Research Council dyspnoea score. SGRQ = St. George respiratory questionnaire for COPD. CAT = COPD assessment test. HR = heart rate. WBC = white cell count. NEUT = neutrophils. FIB = fibrinogen. CRP = C-reactive protein. CHOL = total cholesterol. SMOKE = smoking status. PHL = phlegm. GRF = glomerular filtration rate. HB = haemoglobin. WD = six-minute walk. SPPB = short physical performance battery. FEV = forced expiratory volume in one second. QMVC = quadriceps maximum voluntary contraction. BMI = body mass index. BG = glucose. Correlation coefficients with a values <0.30 were considered weak, 0.30–0.50 as moderate, and >0.50 as strong.
(PDF)
Total number of H-AECOPD (n = 291). FEV1 = forced expiratory volume in one second. FVC = forced vital capacity. NHS = National Health Services. H-AECOPD = hospitalised acute exacerbation of COPD. FUP = follow-up period.
(DOCX)
Hospital admission data obtained from the National Health Service (NHS) Digital, NHS Wales, and NHS Scotland.
(PDF)
(A) Mean event rates with 95% confidence intervals per 100 per-years during study period, (B) H-AECOPD frequency, and (C) H-AECOPD duration. Depth of blue indicates the cumulative number of individuals with first H-AECOPD during the study period. Red dashed line indicates the median number of hospital admissions for H-AECOPD amongst those experienced an H-AECOPD.
(PDF)
(PDF)
(PDF)
Acknowledgments
The views and opinions expressed are those of the authors and do not necessarily reflect those of the University of Cambridge, the NHS, the NIHR, the Department of Health and Social Care, or other parent institutions of the authors. The corresponding author and co-authors had full access to the data in the study and take responsibility for the integrity of the data, the accuracy of the analyses, and the decision to submit for publication.
Data Availability
The dataset underlying these findings, with de-identified participant data (including the data dictionary), are available to interested and qualified researchers upon request and can be obtained from the Cambridge Clinical Trials Unit. Access to hospital episode statistics requires a data sharing agreement with the National Health Services. For data access, please contact cctu@addenbrookes.nhs.uk.
Funding Statement
This was an investigator sponsored study. The study was funded by a grant (9157-61188) from Innovate UK (formerly known as Technology Strategy Board) with contributory funding in kind (e.g. scientific expertise and meeting rooms) from GSK, a consortium partner, who also funded the corresponding author’s PhD. As a consortium partner, GSK was involved in the study design, data analysis, decision to publish, and preparation of the manuscript. The specific roles of all authors are articulated in the ‘author contributions’ section. RTS was a co-investigator on the grant and as a consortium member was involved in the decision to publish, and preparation of the manuscript. IBW, JC and CMM acknowledge funding support from the NIHR Cambridge Comprehensive Biomedical Research Centre. CEB is supported by the NIHR Nottingham BRC respiratory theme. This work was supported by core funding from: the UK Medical Research Council (MR/L003120/1), the British Heart Foundation (RG/13/13/30194; RG/18/13/33946) and the National Institute for Health Research [Cambridge Biomedical Research Centre at the Cambridge University Hospitals NHS Foundation Trust] [*]. This work was also supported by Health Data Research UK, which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation and Wellcome. *The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
References
- 1.McLean S, Hoogendoorn M, Hoogenveen RT, Feenstra TL, Wild S, Simpson CR, et al. Projecting the COPD population and costs in England and Scotland: 2011 to 2030. Sci Rep. 2016;6: 31893 Available: 10.1038/srep31893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and State-Specific Medical and Absenteeism Costs of COPD Among Adults Aged 18 Years in the United States for 2010 and Projections Through 2020. Chest. 2015;147: 31–45. 10.1378/chest.14-0972 [DOI] [PubMed] [Google Scholar]
- 3.National Health Services Digital. Hospital Admitted Patient Care Activity, 2016–17. All diagnoses: Chronic obstructive pulmonary disease [Internet]. NHS Digital; Available: https://files.digital.nhs.uk/publication/7/d/hosp-epis-stat-admi-diag-2016-17-tab.xlsx
- 4.Stone RA, Holzhauer-Barrie J, Lowe D, McMillan V, Saleem Khan M, Searle L, et al. Who cares when it matters most? National Chronic Obstructive Pulmonary Disease (COPD) Audit Programme: Outcomes from the clinical audit of COPD exacerbations admitted to acute units in England 2014. Results and data analysis. [Internet]. London; 2017. Available: https://www.rcplondon.ac.uk/projects/outputs/copd-who-cares-when-it-matters-most-outcomes-report-2014 [Google Scholar]
- 5.Celli B, Tetzlaff K, Criner G, Polkey MI, Sciurba F, Casaburi R, et al. The 6-minute Walk Test as a COPD Stratification Tool: Insights From the COPD Biomarker Qualification Consortium. Am J Respir Crit Care Med. 2016; 10.1164/rccm.201508-1653OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NICE. Chronic obstructive pulmonary disease in over 16s: diagnosis and management (NG115) [Internet]. 2018. Available: www.nice.org.uk/guidance/ng115
- 7.Sapey E, Bafadhel M, Bolton CE, Wilkinson T, Hurst JR, Quint JK. Building toolkits for COPD exacerbations: lessons from the past and present. Thorax. 2019; thoraxjnl-2018–213035. 10.1136/thoraxjnl-2018-213035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan D, Gale NS, McEniery CM, Bolton CE, Cockcroft JR, MacNee W, et al. Evaluating the role of inflammation in chronic airways disease: the ERICA study. COPD. 2014;11: 552–559. 10.3109/15412555.2014.898031 [DOI] [PubMed] [Google Scholar]
- 9.Mohan D, Forman JR, Allinder M, McEniery CM, Bolton CE, Cockcroft JR, et al. Fibrinogen does not relate to cardiovascular or muscle manifestations in COPD: cross-sectional data from the ERICA study. Thorax. 2018; 10.1136/thoraxjnl-2018-211556 [DOI] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61: 344–349. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 11.Rothnie KJ, Mullerova H, Thomas SL, Chandan JS, Smeeth L, Hurst JR, et al. Recording of hospitalizations for acute exacerbations of COPD in UK electronic health care records. Clin Epidemiol. 2016;8: 771–782. 10.2147/CLEP.S117867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363: 1128–1138. 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 13.Seymour JM, Spruit MA, Hopkinson NS, Natanek SA, Man WD, Jackson A, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36: 81–88. 10.1183/09031936.00104909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J. Statistical Power Analysis for the Behavioral Sciences [Internet]. Elsevier Science; 2013. Available: https://books.google.co.uk/books?id=rEe0BQAAQBAJ
- 15.Van Belle G. Statistical rules of thumb. John Wiley & Sons; 2011. [Google Scholar]
- 16.Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, Edwards L, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J. 2008;31: 869–873. 10.1183/09031936.00111707 [DOI] [PubMed] [Google Scholar]
- 17.Norrish A, North D, Kirkman P, Jackson R. Validity of self-reported hospital admission in a prospective study. Am J Epidemiol. 1994;140: 938–942. Available: https://www.ncbi.nlm.nih.gov/pubmed/7977281 10.1093/oxfordjournals.aje.a117182 [DOI] [PubMed] [Google Scholar]
- 18.Seidl H, Meisinger C, Kirchberger I, Burkhardt K, Kuch B, Holle R. Validity of self-reported hospital admissions in clinical trials depends on recall period length and individual characteristics. J Eval Clin Pr. 2016;22: 446–454. 10.1111/jep.12506 [DOI] [PubMed] [Google Scholar]
- 19.Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62: 115–120. Available: http://ovidsp.ovid.com/athens/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med5&AN=17090575 10.1136/thx.2006.062026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canavan JL, Maddocks M, Nolan CM, Jones SE, Kon SSC, Clark AL, et al. Functionally Relevant Cut Point for Isometric Quadriceps Muscle Strength in Chronic Respiratory Disease. Am J Respir Crit Care Med. 2015;192: 395–397. 10.1164/rccm.201501-0082LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kon SS, Jones SE, Schofield SJ, Banya W, Dickson MJ, Canavan JL, et al. Gait speed and readmission following hospitalisation for acute exacerbations of COPD: a prospective study. Thorax. 2015;70: 1131–1137. 10.1136/thoraxjnl-2015-207046 [DOI] [PubMed] [Google Scholar]
- 22.Barker R, Kon SSC, Jones SE, Maddocks M, Gao W, Nolan CM, et al. Short Physical Performance Battery and long term prognosis following severe acute exacerbation of COPD: a prospective cohort study. Eur Respir J. 2018;52: PA3850 10.1183/13993003.congress-2018.PA3850 [DOI] [Google Scholar]
- 23.Jones SE, Kon SSC, Canavan JL, Patel MS, Clark AL, Nolan CM, et al. The five-repetition sit-to-stand test as a functional outcome measure in COPD. Thorax. 2013;68: 1015 LP– 1020. 10.1136/thoraxjnl-2013-203576 [DOI] [PubMed] [Google Scholar]
- 24.Crook S, Frei A, Ter Riet G, Puhan MA. Prediction of long-term clinical outcomes using simple functional exercise performance tests in patients with COPD: a 5-year prospective cohort study. Respir Res. 2017;18: 112 10.1186/s12931-017-0598-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puhan MA, Siebeling L, Zoller M, Muggensturm P, ter Riet G. Simple functional performance tests and mortality in COPD. Eur Respir J. 2013/03/21. 2013;42: 956–963. 10.1183/09031936.00131612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Committee for Medicinal Products for Human Use. Points to consider on frailty: Evaluation instruments for baseline characterisation of clinical trial populations. Draft [Internet]. London; 2015. Available: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-points-consider-frailty-evaluation-instruments-baseline-characterisation-clinical-trial_en.pdf
- 27.Hopkinson NS, Molyneux A, Pink J, Harrisingh MC. Chronic obstructive pulmonary disease: diagnosis and management: summary of updated NICE guidance. BMJ. 2019;366: l4486 10.1136/bmj.l4486 [DOI] [PubMed] [Google Scholar]
- 28.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Pocket guide to COPD diagnosis, management, and prevention. A guide for health care professionals, 2019 report. [Internet]. 2019. Available: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-POCKET-GUIDE-FINAL_WMS.pdf
- 29.Troosters T, Bourbeau J, Maltais F, Leidy N, Erzen D, De Sousa D, et al. Enhancing exercise tolerance and physical activity in COPD with combined pharmacological and non-pharmacological interventions: PHYSACTO randomised, placebo-controlled study design. BMJ Open. 2016;6: e010106–e010106. 10.1136/bmjopen-2015-010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med. 2000;109: 207–212. 10.1016/s0002-9343(00)00472-1 [DOI] [PubMed] [Google Scholar]
- 31.Bhatt SP, Patel SB, Anderson EM, Baugh D, Givens T, Schumann C, et al. Video Telehealth Pulmonary Rehabilitation Intervention In COPD Reduces 30-day Readmissions. Am J Respir Crit Care Med. 2019; 10.1164/rccm.201902-0314LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker PP, Pompilio PP, Zanaboni P, Bergmo TS, Prikk K, Malinovschi A, et al. Telemonitoring in Chronic Obstructive Pulmonary Disease (CHROMED). A Randomized Clinical Trial. Am J Respir Crit Care Med. 2018;198: 620–628. 10.1164/rccm.201712-2404OC [DOI] [PubMed] [Google Scholar]
- 33.Jinks RC, Royston P, Parmar MKB. Discrimination-based sample size calculations for multivariable prognostic models for time-to-event data. BMC Med Res Methodol. 2015;15: 82 10.1186/s12874-015-0078-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
GFR = glomerular filtration rate. 6MW = six-minute walk. QMVC = quadriceps maximum voluntary contraction. CRP = C-reactive protein. HR = heart rate. CAT = COPD assessment test. SPPB = short physical performance battery. BMI = body mass index. FEV1 = forced expiratory volume in one second. GOLD = global initiative for obstructive lung disease.
(PDF)
SGRQ = St. George respiratory questionnaire for COPD. QMVC = quadriceps maximum voluntary contraction. 6MW = six-minute walk. GFR = glomerular filtration rate.
(PDF)
EXAC = exacerbation history. MRC = Medical Research Council dyspnoea score. SGRQ = St. George respiratory questionnaire for COPD. CAT = COPD assessment test. HR = heart rate. WBC = white cell count. NEUT = neutrophils. FIB = fibrinogen. CRP = C-reactive protein. CHOL = total cholesterol. SMOKE = smoking status. PHL = phlegm. GRF = glomerular filtration rate. HB = haemoglobin. WD = six-minute walk. SPPB = short physical performance battery. FEV = forced expiratory volume in one second. QMVC = quadriceps maximum voluntary contraction. BMI = body mass index. BG = glucose. Correlation coefficients with a values <0.30 were considered weak, 0.30–0.50 as moderate, and >0.50 as strong.
(PDF)
Total number of H-AECOPD (n = 291). FEV1 = forced expiratory volume in one second. FVC = forced vital capacity. NHS = National Health Services. H-AECOPD = hospitalised acute exacerbation of COPD. FUP = follow-up period.
(DOCX)
Hospital admission data obtained from the National Health Service (NHS) Digital, NHS Wales, and NHS Scotland.
(PDF)
(A) Mean event rates with 95% confidence intervals per 100 per-years during study period, (B) H-AECOPD frequency, and (C) H-AECOPD duration. Depth of blue indicates the cumulative number of individuals with first H-AECOPD during the study period. Red dashed line indicates the median number of hospital admissions for H-AECOPD amongst those experienced an H-AECOPD.
(PDF)
(PDF)
(PDF)
Data Availability Statement
The dataset underlying these findings, with de-identified participant data (including the data dictionary), are available to interested and qualified researchers upon request and can be obtained from the Cambridge Clinical Trials Unit. Access to hospital episode statistics requires a data sharing agreement with the National Health Services. For data access, please contact cctu@addenbrookes.nhs.uk.