Abstract
Recent studies indicated that intramammary administration of active vitamin D3 hormone (1,25D3) inhibits the inflammatory process associated with mastitis. We hypothesized that attenuation of endoplasmic reticulum (ER) stress by 1,25D3 in mammary epithelial cells (MECs) is an important cellular mechanism contributing to this beneficial effect of intramammary treatment with 1,25D3. To test this hypothesis, the effect of 1,25D3 was studied on induction of ER stress in a transformed human MEC line, MCF-7 cells. Treatment with two different ER stress inducers, thapsigargin (TG) and tunicamycin (TM), caused a dose-dependent induction of ER stress as evident from up-regulation of protein kinase RNA-like ER kinase (PERK), heat shock protein family A (Hsp70) member 5 (HSPA5), activating transcription factor (ATF4), ATF6, DNA damage inducible transcript 3 (DDIT3) and spliced X-box binding protein 1 (sXBP1) and impaired cell viability and decreased expression of vitamin D receptor (VDR) in MCF-7 cells (P < 0.05). Treatment with 1,25D3 (100 nM) inhibited TG (10 nM)- and TM (1 μg/mL)-induced mRNA and/or protein levels of ATF4, ATF6, DDIT3 and HSPA5 in MCF-7 cells (P < 0.05). In addition, 1,25D3 (100 nM) antagonized the effect of TG (10 nM) and TM (1 μg/mL) on mRNA and protein levels of VDR and mRNA levels of genes involved in production and degradation of 1,25D3 in MCF-7 cells (P < 0.05). Moreover, 1,25D3 (100 nM) inhibited nuclear factor-κB (NF-κB) activation in response to TM (10 nM) and TG (1 μg/mL) in MCF-7 cells. In conclusion, the present findings show that 1,25D3 is effective in attenuating ER stress and the NF-κB-driven inflammatory response in MCF-7 cells. This indicates that attenuation of ER stress by 1,25D3 in MECs may contribute to the recently observed inhibitory effect of intramammary treatment of dairy cows with 1,25D3 on the inflammatory process associated with mastitis.
Introduction
Mastitis refers to an inflammation of mammary tissue mostly caused by infections with different pathogenic bacteria. Mastitis in dairy cattle has great economic impact due to milk production loss, costs for veterinary treatment and potentially fatal outcome [1]. Despite antibiotics are widely used for the treatment of mastitis [2], this treatment strategy is increasingly considered critically due to limited effectiveness owing to the occurrence of antibiotic-resistant strains. Against this background, dietary interventions may be a reasonable strategy in the prevention and treatment of the inflammatory response associated with mastitis.
Previous in vitro-studies revealed that the active vitamin D3 hormone, 1,25-hydroxyvitamin D3 (1,25D3), improves bactericidal capacity of human and bovine monocytes against common bacterial pathogens involved in mastitis development [3–5]. This effect likely contributes to a decreased bacterial growth in mammary glands experimentally infected with Streptococcus uberis [6] and an increased expression of host-defense genes in mammary immune cells [7, 8] of dairy cows subjected to intramammary treatment with 1,25D3 or its metabolite 25D3. Apart from monocytes and lymphocytes, mammary epithelial cells (MECs) surrounding alveoli in the milk parenchyma in the mammary gland act as important innate immunocompetent cells by producing a significant amount of pro-inflammatory cytokines upon pathogen contact [9–11]. Pathogen-dependent induction of immune functions in MECs is mediated by toll-like receptor (TLR2, TLR4)-dependent sensing of pathogen-associated molecular patterns (PAMPs) like lipopolysaccharide (LPS) and lipoteichoic acid [12]. Sensing of PAMPs leads to the activation of the key regulator of inflammation nuclear factor-kappa B (NF-κB), thereby, stimulating the production of pro-inflammatory cytokines, chemokines, reactive oxygen species (ROS) and other host-defense molecules via inducing more than hundred immune relevant genes [13]. Despite the NF-κB-regulated acute inflammatory response is important to effectively combat the infectious bacteria causing mastitis, it is important that the inflammatory process is rapidly attenuated because prolonged production of ROS, cytokines and other inflammatory molecules causes structural damage of the mammary gland through injurious action on cellular components (lipids, proteins, DNA), thereby, decreasing cell viability, and ultimately inducing cell death [14].
Recently, it was shown in a mouse model of mastitis that the inflammatory process induced by LPS administration in the mammary gland is also closely related to induction of endoplasmic reticulum (ER) stress in mammary tissue and that attenuation of ER stress by a secondary plant metabolite protects from LPS-induced mastitis by inhibiting the pro-inflammatory NF-κB signaling pathway [15]. Thus, inhibition of ER stress is likely a suitable strategy in the prevention and therapy of the inflammatory process associated with mastitis. ER stress describes a state characterized by the accumulation of misfolded proteins owing to an imbalance in ER quality control pathways, such as folding, trafficking and degradation [16]. As a consequence of ER stress, the unfolded protein response (UPR), which involves three different transmembrane ER stress sensors, namely ATF6 (activating transcription factor 6), IRE1 (inositol-requiring protein 1a) and PERK (protein kinase RNA-like ER kinase), is initiated in order to restore normal ER function by different mechanisms including transient attenuation of new protein synthesis, stimulation of IRE1-dependent mRNA degradation and induction of molecular chaperones [17–19]. The close link between NF-κB-driven inflammation and ER stress during mastitis is likely explained by their mutual interaction; activation of all ER stress sensors causes downstream activation of NF-κB, and ER stress-inducing stimuli, such as ROS and cytokines, are produced from immunocompetent cells during the course of the inflammatory process [20, 21].
Owing to their high metabolic and secretory capacity, MECs are particularly susceptible to environmental conditions that cause ER stress and thus the UPR pathway is critical in maintaining ER homeostasis in MECs [22–24]. Interestingly, several indications exist that 1,25D3 inhibits ER stress in different cell types [25, 26]. Whether this is also the case in MECs remains to be established. In light of the above-described beneficial effects of 1,25D3 on mechanisms involved in mastitis development, we hypothesized that attenuation of ER stress by 1,25D3 in MECs is an important cellular mechanism leading to inhibition of NF-κB-driven inflammation. To test this hypothesis, the effect of 1,25D3 was studied on induction of ER stress in a transformed human MEC line. Despite ER stress is known to be indirectly induced by several inflammatory mediators like LPS [27], ER stress was induced in the present study by more selective ER stress inducers, such as tunicamycin (TM), which causes ER stress by inhibiting protein glycosylation [28], and thapsigargin (TG), which causes ER stress by inhibiting the sarco-/endoplasmic reticulum calcium ATPase [29]. Because 1,25D3 exerts many of its effects via the vitamin D receptor (VDR) [30] and ER stress was found to suppress transcriptional activity of the VDR gene in human epithelial cells [31], the effect of ER stress and 1,25D3 on expression of VDR and of genes encoding hydroxylases involved in local 1,25D3 production and degradation was also studied.
Materials and methods
Chemicals
1,25D3 and tunicamycin (TM) were purchased from Sigma-Aldrich (Steinheim, Germany) and thapsigargin (TG) was purchased from Biomol (Hamburg Germany). From all test compounds, stock solutions in dimethylsulfoxide (DMSO; Sigma-Aldrich) were prepared (1,25D3: 10 mM; TM: 5 mg/mL; TG: 5 mM).
Cell culture
MCF-7 cells were obtained from Cell Lines Service (Eppelheim, Germany) and grown in Dulbecco´s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS; both from Gibco/Life Technologies, Darmstadt, Germany) and 0.05 mg/mL gentamicin (Invitrogen, Karlsruhe) in a cell incubator at 37°C in a humidified atmosphere of 95% air and 5% CO2. Growth medium was changed every 2 days. After reaching a confluence of 70–80%, the cells were either sub-cultivated or used for experiments. Aliquots from stock solutions were directly added to the growth medium and control cells were incubated with the same vehicle concentration (DMSO) at the concentrations indicated in figure legends. All experiments were performed at three times from a different cell passage number (= independent experiments).
Cell viability assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, Steinheim, Germany) assay was used to assess cell viability in response to TM, TG and 1,25D3. For the MTT assay, MCF-7 cells were seeded in 96-well culture plates at a density of 1.2 x 104 cells per well and incubated as indicated in figure legends. The MTT assay was carried out exactly as described recently [32].
RNA isolation and quantitative real-time polymerase-chain reaction (qPCR) analysis
For qPCR analysis, MCF-7 cells were seeded in 24-well culture plates at a cell density of 6 x 104 per well and incubated as indicated in figure legends. Total RNA extraction, cDNA synthesis and qPCR were performed as described recently [32]. Gene-specific primers were synthesized by Eurofins MWG Operon (Ebersberg, Germany). Characteristics of primers are listed in Table 1. Normalization was carried out using multiple reference genes (ATP5B, GAPDH, SDHA, YWHAZ) as described recently [32].
Table 1. Sequences of gene-specific primers used for qPCR analysis.
| Gene name | Primer sequence (forward, reverse) | Product size (bp) | NCBI GenBank |
|---|---|---|---|
| Reference genes | |||
| ATP5B | TCGCGTGCCATTGCTGAGCT CGTGCACGGGACACGGTCAA | 218 | NM_001686 |
| GAPDH | GCCTTCCGTGTCCCCACTGC CAATGCCAGCCCCAGCGTCA | 211 | XR_002046 |
| SDHA | CCAAGCCCATCCAGGGGCAAC TCCAGAGTGACCTTCCCAGTGCCAA | 100 | NM_004168 |
| YWHAZ | TGGGGACTACGACGTCCCTCAA CATATCGCTCAGCCTGCTCGG | 115 | NM_003406 |
| Target genes | |||
| ATF4 | GTTCTCCAGCGACAAGGCT GCATCCAAGTCGAACTCCTT | 150 | NM_001675 |
| ATF6 | GTCTCCCCTTTCCTTATATGG AAGGCTTGGGCTGAATTGAAG | 164 | NM_007348 |
| CEBPA | GTGGACAAGAACAGCAACGAG CATTGTCACTGGTCAGCTCCA | 133 | NM_001287435 |
| CYP2R1 | CTCAGTGGGTGAACTCATCAT CGTACAACTGCATCTTCAGAG | 264 | NM_024514 |
| CYP24A1 | GCTTGTATCGACAACCGGTT CAGACAGGCTTTTAAATACGG | 294 | NM_000782 |
| CYP27B1 | GTTTGCATTTGCTCAGAGGCA GCTCATACAGAGCCCAAGAG | 218 | NM_000785 |
| DDIT3 | GGAGAACCAGGAAACGGAAAC GCTTGAGCCGTTCATTCTCTT | 129 | NM_001195053 |
| FASN | GGCCGTGGTCTTGAGAGATG TAGTTGCTCTGTCCCGCATTG | 189 | NM_004104 |
| HMGCR | GACAGGATGCAGCACAGAATG TTGAACACCTAGCATCTGCAAAC | 179 | NM_000859 |
| HSPA5 | GAGGAGGAGGACAAGAAGGA CAGGAGTGAAGGCGACATAG | 157 | NM_005347 |
| LDLR | GTCAGCTCCACAGCCGTAAG CCCAGAGCTTGGTGAGACATTG | 128 | NM_000527 |
| NFKB1 | GCAGATGGCCCATACCTTCAA CACCATGTCCTTGGGTCCAG | 285 | NM_003998 |
| PERK | GTCGCCAATGGGATAGTGACG GCTCTCGTTTCCATGTCTGG | 166 | NM_004836 |
| RXRA | TTCGCTAAGCTCTTGCTC ATAAGGAAGGTGTCAATGGG | 113 | NM_0012319020 |
| VDR | CCAGTTCGTGTGAATGATGG GTCGTCCATGGTGAAGGA | 384 | NM_000376 |
| sXBP1 | TGCTGAGTCCGCAGCAGGTG GCTGGCAGGCTCTGGGGAAG | 169 | NM_005080 |
Immunoblotting
For immunoblotting, MCF-7 cells were seeded in 6-well culture plates at a cell density of 1.8 x 105 per well and treated as indicated in figure legends. For detection of HSPA5 and DDIT3 cell lysates were prepared with radioimmunoprecipitation (RIPA) assay lysis buffer [50 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, and 1% sodium deoxycholate] containing protease inhibitor cocktail (Sigma-Aldrich). For detection of phosphorylated NF-κB (p-NF-κB), cell lysates were prepared with RIPA lysis buffer containing the phosphatase inhibitors sodium fluoride (5 mM) and sodium-orthovanadat (1 mM) (both from Sigma-Aldrich) and protease inhibitor cocktail. For detection of VDR and NF-κB nuclear extracts were prepared with the Nuclear Extract Kit (Active Motif, La Hulpe, Belgium) according to the manufacturer’s protocol. Protein concentrations of lysates and nuclear extracts were determined by the BCA protein assay kit (Interchim, Montlucon, France) and BSA as standard. A total amount of 10–15 μg protein was separated by SDS-PAGE and electrotransferred to nitrocellulose membranes. The membranes were incubated with primary antibodies [rabbit anti-HSPA5 (dilution 1:5000) and mouse anti-DDIT3 (dilution 1:2000) (both from Thermo Fisher Scientific, Darmstadt, Germany), rabbit anti-VDR (dilution 1:300), rabbit anti-NF-κB (dilution 1:500) and mouse anti-p-NF-κB (dilution 1:500; all from Santa Cruz, Heidelberg, Germany)] at 4°C overnight. The primary antibodies against mouse anti-β-actin (dilution 1:40.000, Abcam, Cambridge, UK) and rabbit anti-vinculin (dilution 1:10.000, Thermo Fisher Scientific) were incubated as reference proteins for normalization at room temperature for 2 h. The membranes were washed and then incubated with horseradish peroxidase-conjugated secondary antibodies anti-rabbit-IgG (dilution 1:10.000, Sigma-Aldrich) and anti-mouse-IgG (dilution 1:10.000, Santa Cruz) at room temperature for 2 h. Afterward, blots were developed using enhanced chemiluminescence (ECL) Plus (GE Healthcare, München, Germany). The signal intensities of specific bands were detected with a Bio-Imaging system (Syngene, Cambridge, UK) and quantified using Syngene GeneTools software (nonlinear dynamics; Syngene). For calculation of protein levels, the band intensity of the target protein was normalized by that of the reference protein.
Statistical analysis
All data represent means and SD. The means and SD were calculated from all replicates for the same treatments of all independent experiments. In each independent experiment, all treatments were represented in 1–8 wells (= technical replicates per treatment: immunoblotting, one; qPCR, three; MTT assay, eight) depending on the plate format. Statistical analysis was performed using the Minitab statistical software (Rel. 13.0, State College, PA, USA). Data from qPCR and MTT assay were subjected to 2-factorial ANOVA with classification factors being treatment (T), experiment (E) and the interaction of both factors (T x E). Because data from immunoblotting included only one replicate per treatment within each independent experiment, treatment effects were analyzed by 1-factorial ANOVA. For statistically significant F values, individual means of the treatment groups were compared by Fisher´s multiple range test. Effects were considered significant if P < 0.05.
Results and discussion
Treatment with ER stress inducers cause induction of ER stress-induced UPR and an impairment of cell viability in MCF-7 cells
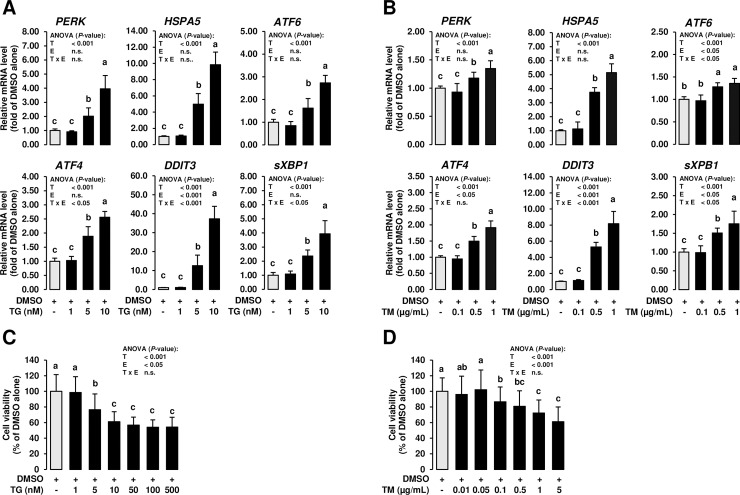
To investigate the induction of ER stress in MCF-7 cells, MCF-7 cells were treated for 24 h with TG and TM at different concentrations (TG: 1, 5 and 10 nM; TM: 0.1, 0.5 and 1 μg/mL), which were recently reported to induce ER stress in other cell types [33, 34]. Despite TG and TM induce ER stress via distinct mechanisms [28, 29], both ER stress inducers caused a quite similar response in MCF-7 cells. Treatment with TG and TM dose-dependently induced ER stress as evident from marked upregulation of known ER stress-sensitive genes such as PERK, heat shock protein family A (Hsp70) member 5 (HSPA5), ATF6, ATF4, DNA damage inducible transcript 3 (DDIT3) and spliced X-box binding protein 1 (sXBP1) (P < 0.05; Fig 1A and 1B) at 5 and 10 nM TG and 0.5 and 1 μg/mL TM. In line with the fact that sustained ER stress causes cell death via apoptosis, 24 h-treatment with both ER stress inducers impaired cell viability at concentrations ≥ 5 nM TG (P < 0.05; Fig 1C) and ≥ 0.1 μg/mL TM (P < 0.05; Fig 1D). At TG concentrations of 5 and 10 nmol/L, MCF-7 cell viability was reduced by 23 and 39%, respectively, while TM concentrations of 0.1 and 1 μg/mL decreased cell viability by 13 and 28%, respectively.
Fig 1. Effect of ER stress inducers on expression of UPR target genes and cell viability in mammary epithelial cells.
MCF-7 cells were incubated in DMEM with 1% FBS with either vehicle alone (DMSO, 0.1% v/v) or increasing concentrations of TG (dissolved in DMSO; A: 1 to 10 nM; C: 1 to 500 nM) or TM (dissolved in DMSO; B: 0.1 to 1 μg/mL; D: 0.01 to 5 μg/mL) for 24 h. A, B: Bars represent relative mRNA levels expressed as fold of vehicle alone and are means ± SD from three independent experiments. C, D: Bars represent relative cell viability expressed as percent of vehicle alone and are means ± SD from three independent experiments. A-D: Bars with unlike letters are significantly different (P < 0.05). 2-factorial ANOVA classification factors: treatment (T), experiment (E), interaction (T x E). Abbreviations: ATF4/6, activating transcription factor 4; DDIT3, DNA damage inducible transcript 3; HSPA5, heat shock protein family A (Hsp70) member 5; PERK, protein kinase RNA-like ER kinase; sXBP1, spliced X-box binding protein 1.
Treatment with 1,25D3 inhibits ER stress induced by TM and TG in MCF-7 cells
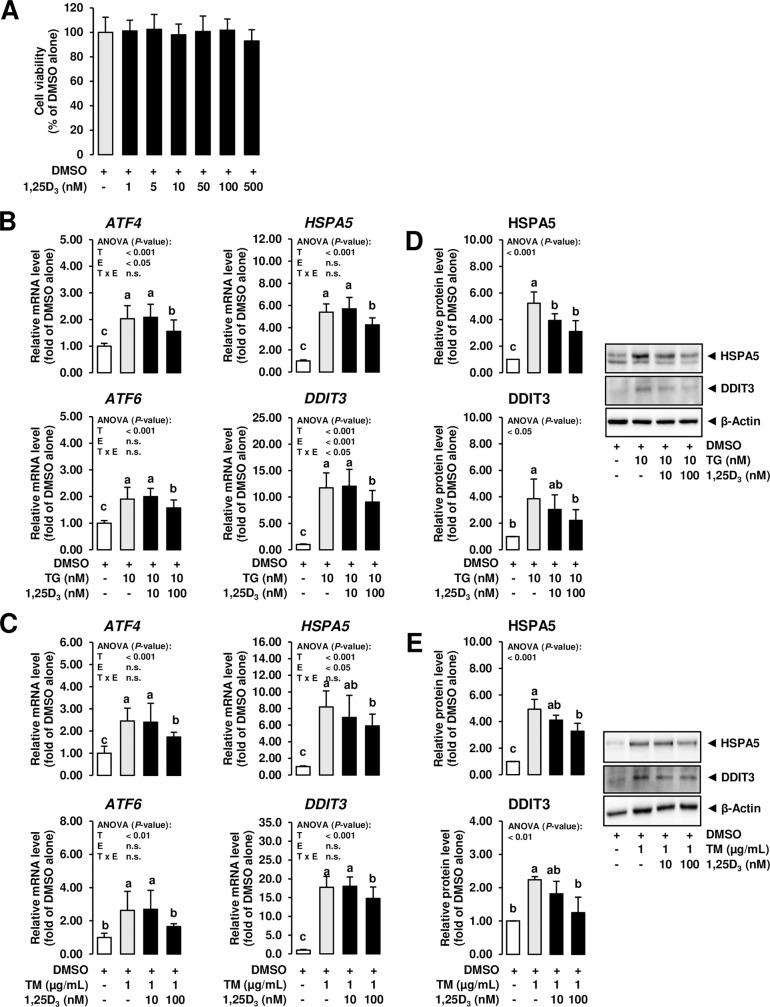
In order to investigate the potential of 1,25D3 to modulate ER stress induced by ER stress inducers in MCF-7 cells, the ER stress inducers were used at 10 nM (TG) and 1 μg/mL (TM). At these concentrations of TG and TM, ER stress was clearly induced but cell viability was only moderately impaired thus enabling us to avoid strong bias of impaired cell viability on cellular effects caused by ER stress. Treatment of MCF-7 cells with 1,25D3 alone at a wide concentration range (1 to 500 nmol/L), which has been shown to exert biological effects in other cell culture studies [35], for 24 h did not impair cell viability compared to vehicle control cells (Fig 2A). Thus, the effect of 1,25D3 on ER stress signaling induced by TG and TM was studied at a low (10 nmol/L) and a high (100 nmol/L) concentration. In this experiment, cells treated with 1,25D3 and ER stress inducers were pre-treated for 24 h with 1,25D3 alone and subsequently co-treated for 24 h with 1,25D3 and ER stress inducers. As shown in Fig 2B and 2C, ER stress induced by TG (10 nM) and TM (1 μg/ml) was attenuated by the high concentration (100 nmol/L) of 1,25D3 as evident from decreased mRNA levels of the ER stress-sensitive genes ATF4, HSPA5, ATF6, DDIT3, PERK and sXBP1 (the latter two are not shown; P < 0.05). In addition, treatment with 100 nmol/L 1,25D3 attenuated TG- and TM-induced protein levels of the ER stress markers HSPA5 and DDIT3 in MCF-7 cells (P < 0.05; Fig 2D and 2E). These results clearly indicated that 1,25D3 inhibits ER stress induction by TG and TM in MCF-7 cells.
Fig 2. Effect of 1,25D3 on ER stress-induced expression of UPR target genes in mammary epithelial cells.
A: MCF-7 cells were incubated in DMEM with 1% FBS with either vehicle alone (DMSO, 0.1% v/v) or increasing concentrations of 1,25D3 (dissolved in DMSO; 1 to 500 nM) for 24 h. Bars represent relative cell viability expressed as percent of vehicle alone and are means ± SD from three independent experiments. B, C: MCF-7 cells were pre-incubated in DMEM with 1% FBS and with either vehicle alone (DMSO, 0.1% v/v) or 1,25D3 (10 or 100 nM) alone for 24 h and subsequently co-incubated in DMEM with 1% FBS and with either vehicle alone (DMSO, 0.1% v/v), TG (10 nm) or TM (1 mg/mL) alone or 1,25D3 (10 or 100 nM) together with TG (10 nM) or TM (1 μg/mL) for additional 24 h. Bars represent relative mRNA levels (B, C) and relative protein levels (D, E) expressed as fold of vehicle alone and are means ± SD from three independent experiments. A-E: Bars with unlike letters are significantly different (P < 0.05). 2-factorial ANOVA classification factors: treatment (T), experiment (E), interaction (T x E). Abbreviations: ATF4/6, activating transcription factor 4; DDIT3, DNA damage inducible transcript 3; HSPA5, heat shock protein family A (Hsp70) member 5.
Treatment with 1,25D3 antagonizes the effect of ER stress inducers on expression of VDR in MCF-7 cells
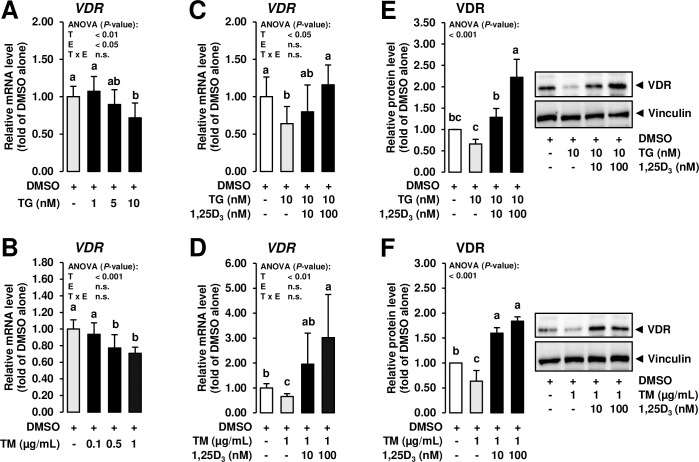
Apart from non-genomic effects, most biological effects of 1,25D3 are mediated by the nuclear VDR which is present in many cell types including MCF-7 cells [36, 37]. In cultured human keratinocytes, treatment with TG was found to decrease transactivation of the VDR gene [31] indicating that the response of epithelial cells to 1,25D3 is impaired under conditions of ER stress. In addition, siRNA-mediated knockdown of VDR was reported to inhibit the ability of 1,25D3 to repress ER stress in human umbilical vein endothelial cells [26]. This indicated that inhibition of ER stress by 1,25D3 is VDR-dependent in endothelial cells and that this mechanism may also play a role in the inhibition of ER stress in MCF-7 cells. To clarify if ER stress affects expression of VDR in MCF-7 cells, the effect of increasing concentrations of TG and TM were investigated on the mRNA level of VDR. Treatment with TG and TM decreased VDR mRNA level at a concentration ≥ 10 nM and ≥ 0.5 μg/mL, respectively (P < 0.05; Fig 3A and 3B), which clearly indicates that VDR expression is decreased in the presence of ER stress in this MEC line—an observation which is in contrast to the effect of ER stress in an immortalized kidney tubule cell line [33].
Fig 3. Effect of ER stress inducers alone and combined effect of ER stress inducers and 1,25D3 on expression of VDR in mammary epithelial cells.
A, B: MCF-7 cells were incubated in DMEM with 1% FBS with either vehicle alone (DMSO, 0.1% v/v) or increasing concentrations of TG (dissolved in DMSO; 1 to 10 nM) or TM (dissolved in DMSO; 0.1 to 1 μg/mL) for 24 h. C-F: MCF-7 cells were pre-incubated in DMEM with 1% FBS and with either vehicle alone (DMSO, 0.1% v/v) or 1,25D3 (10 or 100 nM) alone for 24 h and subsequently co-incubated in DMEM with 1% FBS and with either vehicle alone (DMSO, 0.1% v/v), TG (10 nm) or TM (1 mg/mL) alone or 1,25D3 (10 or 100 nM) together with TG (10 nM) or TM (1 μg/mL) for additional 24 h. Bars represent relative mRNA levels (A, B, C, D) and relative protein levels (E, F) expressed as fold of vehicle alone and are means ± SD from three independent experiments. A-H: Bars with unlike letters are significantly different (P < 0.05). 2-factorial ANOVA classification factors: treatment (T), experiment (E), interaction (T x E). Abbreviations: VDR, vitamin D receptor.
It has long been known that VDR expression is regulated by 1,25D3 at least in the intestine, i.e., 1,25D3 increases VDR mRNA level and newly synthesized VDR [38]. Thus, to next study whether treatment with 1,25D3 is capable of increasing VDR expression in MCF-7 cells exposed to ER stress, the expression of VDR was studied at both the mRNA and the protein level in MCF-7 cells treated with both ER stress inducers. While treatment of MCF-7 cells with TG (10 nM) and TM (1 μg/mL) decreased mRNA and protein levels of VDR compared to vehicle control cells (P < 0.05), treatment with 1,25D3 of MCF-7 cells co-incubated with TG and TM dose-dependently increased mRNA and protein levels of VDR above levels of vehicle control cells (P < 0.05; Fig 3C–3F). This finding indicated that 1,25D3 counter-regulates the inhibitory effect of ER stress on VDR expression in MCF-7 cells.
Attenuation of new protein synthesis by PERK-dependent phosphorylation/inactivation of eukaryotic initiation factor 2α and IRE1-dependent decay of mRNAs during ER stress are important mechanisms of the UPR which decreases the load of ER folding and degradation pathways, thereby, allowing the ER to better cope with misfolded proteins accumulating during ER stress [18]. It is thus possible that this mechanism is responsible for the downregulation of VDR in MCF-7 cells. In cultured adipocytes, which are highly specialized cells for the synthesis and storage of neutral lipids, it has been found that lipogenesis, which takes place in the ER, and expression of lipogenic genes is decreased by treatment with TG and TM [39], likely as a result of adaptive attenuation of new protein synthesis during ER stress. De novo-synthesis of lipids, such as fatty acids and cholesterol, is also an important metabolic function of MECs, thereby, providing sufficient amounts of lipids to be secreted into the milk. In order to evaluate if the expression of lipogenic and cholesterogenic genes is also reduced in MCF-7 cells under conditions of ER stress as a consequence of an impaired metabolic capacity of the ER, the mRNA levels of key lipogenic and cholesterogenic genes, fatty acid synthase (FASN), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) and low density lipoprotein receptor (LDLR), were determined. Unlike VDR gene expression, gene expression of FASN, HMGCR and LDLR was not reduced in MCF-7 cells treated with ER stress inducers (Fig 4A and 4B), which might indicate that the metabolic capacity of the ER was not severely impaired by ER stress induction. The observation that treatment with TG even induced FASN, HMGCR and LDLR in MCF-7 cells is in line with findings in hepatocytes, where TG and TM were reported to stimulate proteolytic activation of the master regulator of lipid synthesis sterol regulatory element-binding protein-1 (SREBP-1) via splicing of XBP1 [40–42]. This ER stress-dependent mechanism aims to provide lipids required to facilitate expansion of the ER during the UPR. The disparate regulation of genes involved in lipid synthesis in response to ER stress in adipocytes [39] and MCF-7 is hard to explain from our data, but it shows that the decrease of VDR expression in response to TG and TM in MCF-7 cells cannot be simply attributed to an attenuation of global protein synthesis. It is well-known that regulation of human and mouse VDR gene expression is exceedingly complex and is mediated by multiple enhancers located both upstream of the VDR gene transcription start site and within downstream enhancers, all of which contain multiple binding sites for different transcription factors, including RUNX family transcription factor 2, CCAAT/enhancer binding protein (CEBP), cAMP response element binding protein and even retinoid X receptor alpha (RXRA) [43–46], which is known as the heterodimerization partner of VDR [47]. The contribution of one or more of these transcription factors to the basal expression of the VDR gene might be critical considering recent observations that siRNA-mediated suppression of these transcription factors reduced the basal level of VDR gene expression [45]. Thus, it is not unlikely that the induction of ER stress in MCF-7 cells may have decreased, by whatever mechanism, the expression of one or more of these transcription factors required for the transcription of the VDR gene. To address this issue, we have determined the expression of two of these transcription factors, RXRA and CEBPA. While the mRNA level of RXRA was not decreased by treatment of MCF-7 cells with either TG or TM, the mRNA level of CEBPA was decreased or tended (P < 0.1) to be decreased in MCF-7 cells treated with TM and TG, respectively (Fig 4C and 4D). However, treatment with 1,25D3 failed to counter-regulate the inhibitory effect of ER stress on CEBPA expression. Thus, these data may help explain the down-regulation of VDR gene transcription in MCF-7 cells exposed to ER stress, but cannot provide an explanation for the counter-regulatory role of 1,25D3. Future studies are warranted to clarify this issue.
Fig 4. Effect of ER stress inducers alone and combined effect of ER stress inducers and 1,25D3 on expression of genes involved in lipid synthesis and transcriptional regulators of VDR in mammary epithelial cells.
MCF-7 cells were pre-incubated in DMEM with 1% FBS and with either vehicle alone (DMSO, 0.1% v/v) or 1,25D3 (10 or 100 nM) alone for 24 h and subsequently co-incubated in DMEM with 1% FBS and with either vehicle alone (DMSO, 0.1% v/v), TG (10 nm; A, C) or TM (1 mg/mL; B, D) alone or 1,25D3 (10 or 100 nM) together with TG (10 nM; A, C) or TM (1 μg/mL; B, D) for additional 24 h. Bars represent relative mRNA levels expressed as fold of vehicle alone and are means ± SD from three independent experiments. Bars with unlike letters are significantly different (P < 0.05). 2-factorial ANOVA classification factors: treatment (T), experiment (E), interaction (T x E). Abbreviations: CEBPA, CCAAT enhancer binding protein alpha; FASN, fatty acid synthase; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; LDLR, low density lipoprotein receptor; RXRA, retinoid X receptor alpha.
Treatment with 1,25D3 antagonizes the effect of ER stress inducers on expression of genes involved in production and degradation of 1,25D3 in MCF-7 cells
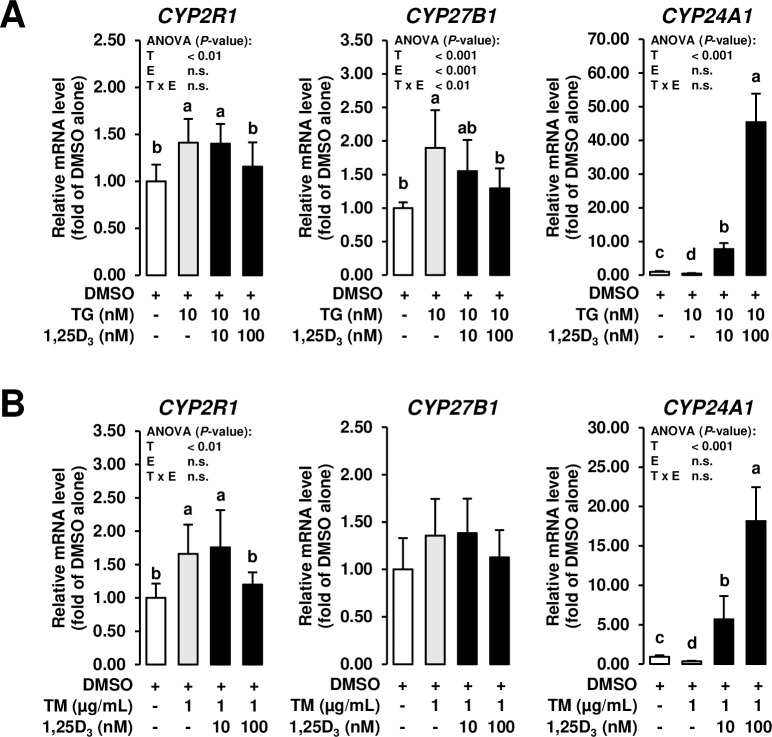
Because intramammary levels of 1,25D3 are known to be affected by mammary epithelial cell production and degradation of 1,25D3 via specific hydroxylases, the effect of ER stress inducers on the mRNA levels of 25-hydroxylase (encoded by CYP2R1), 1α-hydroxylase (encoded by CYP27B1), and 24-hydroxylase (encoded by CYP24A1) was also studied. While the mRNA levels of CYP2R1 and CYP27B1, both of which are involved in stepwise hydroxylation of vitamin D3 into 1,25D3, were increased by TG and TM alone compared to vehicle control cells, the mRNA levels of these genes were decreased by the high concentration of 1,25D3 in MCF-7 cells co-incubated with ER stress inducers to levels observed in vehicle control cells (P < 0.05; Fig 5A and 5B). At least CYP27B1 expression has been recently demonstrated to be upregulated in monocytes and macrophages in response to TLR activation [3]. This mechanism likely explains upregulation of CYP27B1 mRNA in innate immune cells of the bovine mammary gland during mastitis [48], because mastitis-inducing bacteria are sensed by TLRs. Since TLR activation is also known to induce ER stress, upregulation of CYP27B1 might be also indicative of ER stress induction and the observed downregulation of CYP27B1 in MCF-7 cells treated with 1,25D3 might indicate that ER stress was attenuated in MCF-7 cells. Apart from this, it is also possible that the cellular 1,25D3 status of MCF-7 cells was impaired due to ER stress induction and the decreased mRNA levels of CYP27B1 and CYP2R1 in response to 1,25D3 supplementation reflect a feedback regulatory mechanism signaling sufficient cellular 1,25D3 levels. Supportive of such a feedback regulatory mechanism is also the observation that the mRNA level of CYP24A1, which catalyzes the breakdown of 1,25D3 and which is known to be highly upregulated by 1,25D3 [49, 50], was decreased by treatment of MCF-7 cells with both ER stress inducers, whereas CYP24A1 mRNA level was strongly increased by 1,25D3 in a dose-dependent manner in MCF-7 cells co-incubated with TG and TM (P < 0.05; Fig 5A and 5B).
Fig 5. Effect of ER stress inducers alone and combined effect of ER stress inducers and 1,25D3 on expression of genes involved in production and degradation of 1,25D3 in mammary epithelial cells.
MCF-7 cells were pre-incubated in DMEM with 1% FBS and with either vehicle alone (DMSO, 0.1% v/v) or 1,25D3 (10 or 100 nM) alone for 24 h and subsequently co-incubated in DMEM with 1% FBS and with either vehicle alone (DMSO, 0.1% v/v), TG (10 nm; A) or TM (1 mg/mL; B) alone or 1,25D3 (10 or 100 nM) together with TG (10 nM; A) or TM (1 μg/mL; B) for additional 24 h. Bars represent relative mRNA levels expressed as fold of vehicle alone and are means ± SD from three independent experiments. Bars with unlike letters are significantly different (P < 0.05). 2-factorial ANOVA classification factors: treatment (T), experiment (E), interaction (T x E). Abbreviations: CYP2R1, cytochrome P450 family 2 subfamily R member 1; CYP24A1, cytochrome P450 family 24 subfamily A member 1; CYP27B1, cytochrome P450 family 27 subfamily B member 1.
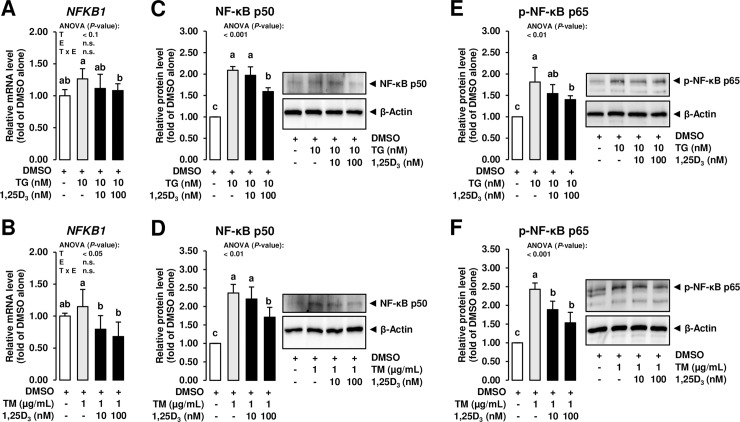
Treatment with 1,25D3 inhibits NF-κB activation in response to TM and TG in MCF-7 cells
To finally study whether the inhibitory effect of 1,25D3 on ER stress induction affects the inflammatory response of MECs or not, the mRNA level of NF-κB p50 subunit (encoded by NFKB1) and the protein concentration of NF-κB p50 in nuclear extracts of MCF-7 cells was determined. NF-κB acts as the key regulator of the inflammatory process associated with mastitis development and is typically activated in the mammary gland epithelium via TLRs which sense specific PAMPs from pathogenic bacteria such as LPS. Upon activation of this transcription factor, a large set of genes encoding cytokines, chemokines, adhesion molecules and other pro-inflammatory products are induced and contribute to a pronounced burst of inflammatory mediator secretion from MECs [9–12]. Despite the rapid NF-κB-driven inflammatory response is important to effectively combat the infectious bacteria, the inflammatory process must be controlled to protect MECs from severe cellular damage and cell death, because production of milk components depends on the number and activity of vital MECs [51], whereas cellular death of MECs and parenchymal fibrosis after infection reduces the synthetic capacity of the mammary gland epithelium [52]. While treatment with both ER stress inducers increased the nuclear protein level of NF-κB p50 subunit compared to treatment with DMSO alone, the mRNA level of NFKB1 and the protein level of NF-κB p50 subunit were decreased by the high concentration of 1,25(OH)2D3 compared to cells treated with either TG or TM alone (P < 0.05; Fig 6A–6D). While the major step in the initiation of NF-κB-dependent gene transcription involves phosphorylation, ubiquitination, and degradation of inhibitor of κB (IκB) proteins, which sequester NF-κB in the cytosol in resting cells, thereby allowing NF-κB to enter the nucleus where it can bind to regulatory sequences of target genes [53], it has been demonstrated that this step alone is often not sufficient to initiate gene expression. Inducible post-translational modification of NF-κB subunits by phosphorylation of multiple phosphor-acceptor sites has been shown to be equally important for initiation of NF-κB-dependent gene transcription [54]. In order to address this, the protein level of p-NF-κB p65 was determined in MCF-7 cells exposed to ER stress inducers and 1,25D3. Like unphosphorylated NF-κB, the protein level of p-NF-κB was increased by both ER stress inducers compared to treatment with DMSO alone, but decreased by the high concentration of 1,25D3 compared to cells treated with either TG or TM alone (P < 0.05; Fig 6E and 6F). These findings clearly indicated that the NF-κB-regulated inflammatory process induced by ER stress is inhibited by of 1,25D3 in MCF-7 cells. Considering that prolonged ER stress causes a persistent inflammatory process and leads to cell death via apoptosis, the observed inhibition of ER stress by 1,25D3 in MCF-7 cells can be interpreted as beneficial with regard to prevention and treatment of the inflammatory response associated with mastitis.
Fig 6. Effect of ER stress inducers alone and combined effect of ER stress inducers and 1,25D3 on expression of NF-κB in mammary epithelial cells.
MCF-7 cells were pre-incubated in DMEM with 1% FBS and with either vehicle alone (DMSO, 0.1% v/v) or 1,25D3 (10 or 100 nM) alone for 24 h and subsequently co-incubated in DMEM with 1% FBS and with either vehicle alone (DMSO, 0.1% v/v), TG (10 nm; A, C, E) or TM (1 mg/mL; B, D, F) alone or 1,25D3 (10 or 100 nM) together with TG (10 nM; A, C, E) or TM (1 μg/mL; B, D, F) for additional 24 h. Bars represent relative mRNA levels (A, B) and relative protein levels (C-F) expressed as fold of vehicle alone and are means ± SD from three independent experiments. Bars with unlike letters are significantly different (P < 0.05). 2-factorial ANOVA classification factors: treatment (T), experiment (E), interaction (T x E). Abbreviations: NF-κB, nuclear factor-κB.
Conclusion
Although a general limitation of this study is the use of a transformed human breast cancer cell line, which displays differences from normal MECs with regard to the abundance of certain receptors, such as estrogen receptors [55], and the response to non-physiological (e.g. exogenous retinoic acid) stimuli [56], both MCF-7 cells and normal MECs cells exhibit a similar regulation by important lactogenic hormones including oxytocin and prolactin [57–59]. In addition, MCF-7 cells like normal MECs are VDR positive cells and exposure to 1,25D3 causes a marked induction of CYP24A1 and several immune response genes [37]. Moreover, cellular stress signaling, such as ER stress-induced activation of the UPR via the ER stress signaling proteins ATF6, IRE1 and PERK and ER stress-mediated activation of NF-κB, occurs largely identical in MCF-7 cells [60, 61] like in non-cancer MECs, thus, allowing to use the MCF-7 cell line specifically for studying the potential of 1,25D3 to modulate ER stress-induced NF-κB-driven inflammatory response in MECs. The present findings show that 1,25D3 is effective in attenuating ER stress and the NF-κB-driven inflammatory response in MCF-7 cells. This indicates that attenuation of ER stress by 1,25D3 in MECs may contribute to the recently observed inhibitory effect of intramammary treatment of dairy cows with 1,25D3 on the inflammatory process associated with mastitis [6–8]. In addition, the observation that the expression of VDR decreased upon induction of ER stress and 1,25D3 increased VDR expression in MCF-7 cells exposed to ER stress demonstrates that 1,25D3 counter-regulates the inhibitory effect of ER stress on VDR expression in MECs. Moreover, ER stress altered the expression of MEC hydroxylases involved in regulating 1,25D3 levels in a way which favors an increase of 1,25D3 levels, whereas 1,25D3 during ER stress modulated the expression of hydroxylases regulating 1,25D3 levels in a way which promotes a decrease of 1,25D3 levels. Albeit being speculative, it appears that the protective effect of 1,25D3 against ER stress in MCF-7 cells involves an improved responsiveness to 1,25D3 through induction of VDR expression, while stimulation of 1,25D3 production during ER stress may be interpreted as an adaptive response to the impaired responsiveness to 1,25D3 of MCF-7 cells exposed to ER stress.
Supporting information
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Zhao X, Lacasse P. Mammary tissue damage during bovine mastitis: causes and control. J Anim Sci. 2008; 86(13 Suppl): 57–65. 10.2527/jas.2007-0302 [DOI] [PubMed] [Google Scholar]
- 2.Doehring C, Sundrum A. The informative value of an overview on antibiotic consumption, treatment efficacy and cost of clinical mastitis at farm level. Prev Vet Med. 2019; 165: 63–70. 10.1016/j.prevetmed.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 3.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006; 311: 1770–1773. 10.1126/science.1123933 [DOI] [PubMed] [Google Scholar]
- 4.Nelson CD, Reinhardt TA, Thacker TC, Beitz DC, Lippolis JD. Modulation of the bovine innate immune response by production of 1alpha,25-dihydroxyvitamin D(3) in bovine monocytes. J Dairy Sci. 2010; 93: 1041–1049. 10.3168/jds.2009-2663 [DOI] [PubMed] [Google Scholar]
- 5.Merriman KE, Kweh MF, Powell JL, Lippolis JD, Nelson CD. Multiple β-defensin genes are upregulated by the vitamin D pathway in cattle. J Steroid Biochem Mol Biol. 2015; 154: 120–129. 10.1016/j.jsbmb.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 6.Lippolis JD, Reinhardt TA, Sacco RA, Nonnecke BJ, Nelson CD. Treatment of an intramammary bacterial infection with 25-hydroxyvitamin D(3). PLoS One. 2011; 6: e25479 10.1371/journal.pone.0025479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merriman KE, Powell JL, Santos JEP, Nelson CD. Intramammary 25-hydroxyvitamin D(3) treatment modulates innate immune responses to endotoxin-induced mastitis. J Dairy Sci. 2018; 101: 7593–7607. 10.3168/jds.2017-14143 [DOI] [PubMed] [Google Scholar]
- 8.Merriman KE, Poindexter MB, Kweh MF, Santos JEP, Nelson CD. Intramammary 1,25-dihydroxyvitamin D(3) treatment increases expression of host-defense genes in mammary immune cells of lactating dairy cattle. J Steroid Biochem Mol Biol. 2017; 173: 33–41. 10.1016/j.jsbmb.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 9.Günther J, Liu S, Esch K, Schuberth HJ, Seyfert HM. Stimulated expression of TNF-alpha and IL-8, but not of lingual antimicrobial peptide reflects the concentration of pathogens contacting bovine mammary epithelial cells. Vet Immunol Immunopathol. 2010; 135: 152–157. 10.1016/j.vetimm.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 10.Günther J, Koczan D, Yang W, Nürnberg G, Repsilber D, Schuberth HJ, et al. Assessment of the immune capacity of mammary epithelial cells: comparison with mammary tissue after challenge with Escherichia coli. Vet Res. 2009; 40: 31 10.1051/vetres/2009014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griesbeck-Zilch B, Meyer HH, Kühn CH, Schwerin M, Wellnitz O. Staphylococcus aureus and Escherichia coli cause deviating expression profiles of cytokines and lactoferrin messenger ribonucleic acid in mammary epithelial cells. J Dairy Sci. 2008; 91: 2215–2224. 10.3168/jds.2007-0752 [DOI] [PubMed] [Google Scholar]
- 12.Strandberg Y, Gray C, Vuocolo T, Donaldson L, Broadway M, Tellam R. Lipopolysaccharide and lipoteichoic acid induce different innate immune responses in bovine mammary epithelial cells. Cytokine. 2005; 31: 72–86. 10.1016/j.cyto.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 13.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999; 18: 6853–6866. 10.1038/sj.onc.1203239 [DOI] [PubMed] [Google Scholar]
- 14.Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018; 80: 50–64. 10.1016/j.semcdb.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 15.Su S, Li X, Li S, Ming P, Huang Y, Dong Y, et al. Rutin protects against lipopolysaccharide-induced mastitis by inhibiting the activation of the NF-κB signaling pathway and attenuating endoplasmic reticulum stress. Inflammopharmacology. 2019; 27: 77–88. 10.1007/s10787-018-0521-x [DOI] [PubMed] [Google Scholar]
- 16.Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev. 2012; 92: 537–576. 10.1152/physrev.00027.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004; 279: 25935–25938. 10.1074/jbc.R400008200 [DOI] [PubMed] [Google Scholar]
- 18.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8: 519–529. 10.1038/nrm2199 [DOI] [PubMed] [Google Scholar]
- 19.Maurel M, Chevet E, Tavernier J, Gerlo S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem Sci. 2014; 39: 245–254. 10.1016/j.tibs.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 20.Leonardi A, Vito P, Mauro C, Pacifico F, Ulianich L, Consiglio E, et al. Endoplasmic reticulum stress causes thyroglobulin retention in this organelle and triggers activation of nuclear factor-kappa B via tumor necrosis factor receptor-associated factor 2. Endocrinology. 2002; 143: 2169–2177. 10.1210/endo.143.6.8825 [DOI] [PubMed] [Google Scholar]
- 21.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010; 140: 900–917. 10.1016/j.cell.2010.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobrovnikova-Marjon E, Hatzivassiliou G, Grigoriadou C, Romero M, Cavener DR, Thompson CB, et al. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc Natl Acad Sci U S A. 2008; 105: 16314–16319. 10.1073/pnas.0808517105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Invernizzi G, Thering BJ, McGuire MA, Savoini G, Loor JJ. Sustained upregulation of stearoyl-CoA desaturase in bovine mammary tissue with contrasting changes in milk fat synthesis and lipogenic gene networks caused by lipid supplements. Funct Integr Genomics. 2010; 10: 561–575. 10.1007/s10142-010-0179-y [DOI] [PubMed] [Google Scholar]
- 24.Invernizzi G, Naeem A, Loor JJ. Short communication: Endoplasmic reticulum stress gene network expression in bovine mammary tissue during the lactation cycle. J Dairy Sci. 2012; 95: 2562–2566. 10.3168/jds.2011-4806 [DOI] [PubMed] [Google Scholar]
- 25.Riek AE, Oh J, Sprague JE, Timpson A, de las Fuentes L, Bernal-Mizrachi L, et al. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients, J Biol Chem. 2012; 287: 38482–38494. 10.1074/jbc.M112.386912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haas MJ, Jafri M, Wehmeier KR, Onstead-Haas LM, Mooradian AD. Inhibition of endoplasmic reticulum stress and oxidative stress by vitamin D in endothelial cells. Free Radic Biol Med. 2016; 99: 1–10. 10.1016/j.freeradbiomed.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Zhang M, Zhu M, Gu J, Song J, Cui L, et al. Paeoniflorin prevents endoplasmic reticulum stress-associated inflammation in lipopolysaccharide-stimulated human umbilical vein endothelial cells via the IRE1α/NF-κB signaling pathway. Food Funct. 2018; 9: 2386–2397. 10.1039/c7fo01406f [DOI] [PubMed] [Google Scholar]
- 28.de Virgilio M, Kitzmüller C, Schwaiger E, Klein M, Kreibich G, Ivessa NE. Degradation of a short-lived glycoprotein from the lumen of the endoplasmic reticulum: the role of N-linked glycans and the unfolded protein response. Mol Biol Cell. 1999; 10: 4059–4073. 10.1091/mbc.10.12.4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mengesdorf T, Althausen S, Oberndorfer I, Paschen W. Response of neurons to an irreversible inhibition of endoplasmic reticulum Ca(2+)-ATPase: relationship between global protein synthesis and expression and translation of individual genes. Biochem J. 2001; 356: 805–812. 10.1042/0264-6021:3560805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014; 21: 319–329. 10.1016/j.chembiol.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, et al. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J Biol Chem. 2011; 286: 34121–34130. 10.1074/jbc.M111.250431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen G, Fischer J, Most E, Eder K, Ringseis R. Decreased all-trans retinoic acid-induced expression of sodium-iodide transporter in mammary epithelial cells caused by conjugated linoleic acid isomers. J Agric Food Chem. 2019; 67: 4493–4504. 10.1021/acs.jafc.9b00673 [DOI] [PubMed] [Google Scholar]
- 33.Chiappisi E, Ringseis R, Eder K, Gessner DK. Effect of endoplasmic reticulum stress on metabolic and stress signaling and kidney-specific functions in Madin-Darby bovine kidney cells. J. Dairy Sci. 2017; 100: 6689–6706. 10.3168/jds.2016-12406 [DOI] [PubMed] [Google Scholar]
- 34.Wen G, Ringseis R, Eder K. Endoplasmic reticulum stress inhibits expression of genes involved in thyroid hormone synthesis and their key transcriptional regulators in FRTL-5 thyrocytes. PLoS One. 2017; 12: e0187561 10.1371/journal.pone.0187561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abu El Maaty MA, Dabiri Y, Almouhanna F, Blagojevic B, Theobald J, Büttner M, et al. Activation of pro-survival metabolic networks by 1,25(OH)(2)D(3) does not hamper the sensitivity of breast cancer cells to chemotherapeutics. Cancer Metab. 2018; 6: 11 10.1186/s40170-018-0183-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diesing D, Cordes T, Fischer D, Diedrich K, Friedrich M. Vitamin D-metabolism in the human breast cancer cell line MCF-7. Anticancer Res. 2006; 26(4A): 2755–2759. [PubMed] [Google Scholar]
- 37.Simmons KM, Beaudin SG, Narvaez CJ, Welsh J. Gene signatures of 1,25-dihydroxyvitamin D3 exposure in normal and transformed mammary cells. J Cell Biochem. 2015; 116: 1693–1711. 10.1002/jcb.25129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strom M, Sandgren ME, Brown TA, DeLuca HF. 1,25-Dihydroxyvitamin D3 up-regulates the 1,25-dihydroxyvitamin D3 receptor in vivo. Proc Natl Acad Sci U S A. 1989; 86: 9770–9773. 10.1073/pnas.86.24.9770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koc M, Mayerová V, Kračmerová J, Mairal A, Mališová L, Štich V, et al. Stress of endoplasmic reticulum modulates differentiation and lipogenesis of human adipocytes. Biochem Biophys Res Commun. 2015; 460: 684–690. 10.1016/j.bbrc.2015.03.090 [DOI] [PubMed] [Google Scholar]
- 40.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 2008; 320: 1492–1496. 10.1126/science.1158042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009; 119: 1201–115. 10.1172/JCI37007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Damiano F, Alemanno S, Gnoni GV, Siculella L. Translational control of the sterol-regulatory transcription factor SREBP-1 mRNA in response to serum starvation or ER stress is mediated by an internal ribosome entry site. Biochem J. 2010; 429: 603–612. 10.1042/BJ20091827 [DOI] [PubMed] [Google Scholar]
- 43.Dhawan P, Peng X, Sutton AL, MacDonald PN, Croniger CM, Trautwein C, et al. Functional cooperation between CCAAT/enhancer-binding proteins and the vitamin D receptor in regulation of 25-hydroxyvitamin D3 24-hydroxylase. Mol Cell Biol. 2005; 25: 472–487. 10.1128/MCB.25.1.472-487.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zella LA, Kim S, Shevde NK, Pike JW. Enhancers located within two introns of the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D3. Mol Endocrinol. 2006; 20: 1231–1247. 10.1210/me.2006-0015 [DOI] [PubMed] [Google Scholar]
- 45.Zella LA, Meyer MB, Nerenz RD, Lee SM, Martowicz ML, Pike JW. Multifunctional enhancers regulate mouse and human vitamin D receptor gene transcription. Mol Endocrinol. 2010; 24: 128–147. 10.1210/me.2009-0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SM, Meyer MB, Benkusky NA, O'Brien CA, Pike JW. Mechanisms of Enhancer-mediated Hormonal Control of Vitamin D Receptor Gene Expression in Target Cells. J Biol Chem. 2015; 290: 30573–30586. 10.1074/jbc.M115.693614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orlov I, Rochel N, Moras D, Klaholz BP. Structure of the full human RXR/VDR nuclear receptor heterodimer complex with its DR3 target DNA. EMBO J. 2012; 31: 291–300. 10.1038/emboj.2011.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson CD, Reinhardt TA, Beitz DC, Lippolis JD. In vivo activation of the intracrine vitamin D pathway in innate immune cells and mammary tissue during a bacterial infection. PLoS One. 2010; 5: e15469 10.1371/journal.pone.0015469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reinhardt TA, Koszewski NJ, Omdahl J, Horst RL. 1,25-Dihydroxyvitamin D(3) and 9-cis-retinoids are synergistic regulators of 24-hydroxylase activity in the rat and 1, 25-dihydroxyvitamin D(3) alters retinoic acid metabolism in vivo. Arch Biochem Biophys. 1999; 368: 244–248. 10.1006/abbi.1999.1335 [DOI] [PubMed] [Google Scholar]
- 50.Väisänen S, Dunlop TW, Sinkkonen L, Frank C, Carlberg C. Spatio-temporal activation of chromatin on the human CYP24 gene promoter in the presence of 1alpha,25-Dihydroxyvitamin D3. J Mol Biol. 2005; 350: 65–77. 10.1016/j.jmb.2005.04.057 [DOI] [PubMed] [Google Scholar]
- 51.Singh K, Erdman RA, Swanson KM, Molenaar AJ, Maqbool NJ, Wheeler TT, et al. Epigenetic regulation of milk production in dairy cows. J Mammary Gland Biol Neoplasia. 2010; 15: 101–112. 10.1007/s10911-010-9164-2 [DOI] [PubMed] [Google Scholar]
- 52.Akers RM, Nickerson SC. Mastitis and its impact on structure and function in the ruminant mammary gland. J Mammary Gland Biol Neoplasia. 2011; 16: 275–289. 10.1007/s10911-011-9231-3 [DOI] [PubMed] [Google Scholar]
- 53.Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995; 14: 2876–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang B, Yang XD, Lamb A, Chen LF. Posttranslational modifications of NF-kappaB: another layer of regulation for NF-kappaB signaling pathway. Cell Signal. 2010; 22: 1282–1290. 10.1016/j.cellsig.2010.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao C, Pan Y, Li Y, Xu X, Lin Y, Wang W, et al. Effect of sodium/iodide symporter (NIS)-mediated radioiodine therapy on estrogen receptor-negative breast cancer. Oncol Rep. 2015; 34: 59–66. 10.3892/or.2015.3946 [DOI] [PubMed] [Google Scholar]
- 56.Kogai T, Schultz JJ, Johnson LS, Huang M, Brent GA. Retinoic acid induces sodium/iodide symporter gene expression and radioiodide uptake in the MCF-7 breast cancer cell line. Proc Natl Acad Sci U S A. 2000; 97: 8519–8524. 10.1073/pnas.140217197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rillema JA, Yu TX, Jhiang SM. Effect of prolactin on sodium iodide symporter expression in mouse mammary gland explants. Am J Physiol Endocrinol Metab. 2000; 279: E769–E772. 10.1152/ajpendo.2000.279.4.E769 [DOI] [PubMed] [Google Scholar]
- 58.Tazebay UH, Wapnir IL, Levy O, Dohan O, Zuckier LS, Zhao QH, et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med. 2000; 6: 871–878. 10.1038/78630 [DOI] [PubMed] [Google Scholar]
- 59.Arturi F, Ferretti E, Presta I, Mattei T, Scipioni A, Scarpelli D, et al. Regulation of iodide uptake and sodium/iodide symporter expression in the MCF-7 human breast cancer cell line. J Clin Endocrinol Metab. 2005; 90: 2321–2326. 10.1210/jc.2004-1562 [DOI] [PubMed] [Google Scholar]
- 60.Fan P, Tyagi AK, Agboke FA, Mathur R, Pokharel N, Jordan VC. Modulation of nuclear factor-kappa B activation by the endoplasmic reticulum stress sensor PERK to mediate estrogen-induced apoptosis in breast cancer cells. Cell Death Discov. 2018; 4: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vo DH, Hartig R, Weinert S, Haybaeck J, Nass N. G-Protein-Coupled Estrogen Receptor (GPER)-Specific Agonist G1 Induces ER Stress Leading to Cell Death in MCF-7 Cells. Biomolecules 2019; 9: pii: E503 10.3390/biom9090503 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.