Abstract
Background:
Incidence of oral tongue squamous cell carcinoma (OTC) is rising among those under age 50. The etiology is unknown.
Methods:
395 cases of OTC diagnosed and/or treated at Vanderbilt University Medical Center between 2000 and 2017 were identified. 113 (28.6%) were early onset (< age 50). Logistic regression was used to identify factors associated with early onset OTC. Cox proportional hazards models evaluated survival and recurrence.
Results:
Compared to typical onset patients, early onset OTC patients were more likely to receive multimodality treatment (surgery & radiation; adjusted odds ratio [aOR]:2.7, 95%CI:1.2–6.3) and report a history of snuff use (aOR:5.4,95% CI:1.8–15.8) and were less likely to report a history of cigarette use (aOR:0.5, 95%CI:0.2–0.9). Early onset patients had better overall survival (adjusted hazard ratio [aHR]: 0.6).
Conclusions:
This is the largest study to evaluate factors associated with early onset OTC and the first to report an association with snuff.
Keywords: oral tongue, squamous cell carcinoma, young, tobacco, snuff
INTRODUCTION
Tobacco use in the US has been declining over the last few decades, and with it, the incidence of many head and neck cancers1,2. However, the incidence of two head and neck cancer subtypes, oropharyngeal squamous cell carcinoma (OPC) and oral tongue squamous cell carcinoma (OTC), are rising3. The increase in OTC incidence is primarily occurring within young (<50 years of age) white males and females3. The rise in incidence of OPC has been attributed to human papillomavirus infection; yet, for OTC, no single cause has been identified4.
Despite the rapid increase in incidence of early onset OTC in the US, few studies have been performed to evaluate risk factors, prognosis, and survival5–12. Previous studies were small (range: 6–79 early onset patients), lacked detailed information on traditional head and neck cancer risk factors, and reported mixed findings. Early reports suggested that early onset OTC was not associated with classical head and neck cancer risk factors such as alcohol and tobacco use and was associated with worse prognosis than typical onset OTC13,14. Yet, several recent studies identified alcohol and tobacco use as the main risk factors for early onset OTC and found no difference in survival between early and typical onset OTC, including in a systematic review of 11 OTC studies by de Morais15–17. Given the limitations of prior studies and the increasing incidence of OTC, larger studies with more detailed clinical information on patient prognosis as well as the traditional head and neck cancer risk factors are needed.
The objective of this study was to compare the distribution of traditional head and neck cancer risk factors and patient prognosis between early (< age 50) and typical onset (age 50 or greater) OTC within a large cohort of patients. We compiled a large retrospective clinical cohort of 395 OTC patients diagnosed and/or treated at Vanderbilt University Medical Center (VUMC) between January 1, 2000 and January 16, 2017 with detailed environmental risk factor information (tobacco [cigarettes, pipe, cigars, snuff, chew; amount and duration of use] and alcohol use) and patient outcome data. To our knowledge, this is the largest US-based study to assess risk factors and prognosis associated with early onset OTC.
MATERIALS AND METHODS
Study Population
Incident, previously untreated cases of OTC were identified though the Vanderbilt Research Derivative (RD), an IRB-approved, identified, searchable database of more than 3.5 million electronic health records (EHRs) from patients seen at VUMC18. The RD contains clinical data collected as part of routine patient care but reorganized to be easily searchable and usable for research purposes. The RD also links with the Vanderbilt Cancer Registry (VCR), which collects detailed clinical information from all reportable neoplasms diagnosed and/or treated at VUMC.
Patients diagnosed with OTC between January 1, 2000 and January 16, 2017 were identified using the following International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) codes: C02.0, C02.1, C02.2, C02.3, C02.8, C02.9. This date range was chosen because the year 2000 was the earliest year in which clinical data was reliably captured in EHRs at VUMC. A complete manual review of each patient’s EHR was conducted to: a) determine whether the patient had a prior history of cancer (other than non-melanoma skin cancer) and b) confirm the OTC diagnosis. Only confirmed incident cases of OTC without a prior history of cancer (other than non-melanoma skin cancer) were included in this study (N=395). Of the 567 OTC patients identified, 17 (3.0%) were excluded due to a prior cancer, 44 (7.8%) were excluded due to misclassification as OTC, and 11 (1.9%) were excluded due to a lack of sufficient records to confirm eligibility (Supplemental Fig. 1).
Clinical Data Abstraction
For the confirmed incident cases of OTC, an additional manual review of the EHR was conducted to obtain variables such as tobacco exposure (cigarette, cigar, pipe, snuff, and chew; amount and duration of use), alcohol exposure (amount and duration of use), treatment course, treatment responses, outcome, and last known follow up. Demographic and tumor characteristics were obtained from the RD (Supplemental Table 1). Pathologic and molecular characteristics of the tumors, such as HPV testing, were beyond the scope of this analysis. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Vanderbilt University19.
Statistical Analyses
Patient characteristics were evaluated overall and by age of onset of OTC (early [<age 50] or typical [age 50+). These cutoffs were chosen based on a recent study of SEER data that demonstrated that OTC incidence was increasing nationally among those less than age 503. Determinants for early onset OTC were first evaluated in univariate models. To evaluate the independent determinants of early onset OTC, factors significantly associated with early onset OTC in the univariate analysis or considered important based on the literature (i.e., gender, race, clinical stage), were included in the multivariate model as covariates to estimate an adjusted odds ratio (aOR). Additional sensitivity analyses were performed by modifying the age definition of early onset OTC.
Alcohol use was categorized separately by gender according to CDC guidelines20: no alcohol use (<12 drinks in lifetime); light use (12+ drinks in lifetime, but <1 drink/week); mild to moderate use (1–7 drinks/week [females], 1–14 drinks/week [males]); heavy use (8+ alcoholic drinks/week [females], 15+ drinks/week [males]). Tobacco use was determined at the time of diagnosis. One product-year was defined as one pack of cigarettes, one cigar, one pipe, one can of snuff, or one pouch of chewing tobacco per day per year.
The association between onset of OTC (early vs. typical) and a) overall survival; and b) time to recurrence (local, regional, and distant combined) were evaluated by estimating hazard ratios (HRs) and 95% confidence intervals (CIs) from Cox proportional hazards models; years since cancer diagnosis was used as the time variable. To assess risk of death from any cause, the only censoring event was at time of last known follow-up. For overall recurrence, the analysis was restricted to those who had a complete response at the end of treatment, which was determined at the patient’s 3-month post-treatment oncologic visit and was defined as no clinical and/or radiographic evidence of disease. Follow up ended at time of first recurrence (local, regional, or distant) with censoring events consisting of death or last oncologic follow-up. To account for differences in baseline patient characteristics and treatment, Cox proportional hazards models for survival and recurrence were adjusted for sex, race, overall clinical stage, treatment, alcohol use at diagnosis, and ever vs. never tobacco use. Survival and recurrence analyses were also stratified by never versus ever tobacco use status.
A predictive model for overall survival was generated using recursive partitioning. The resulting model was then pruned to the size resulting in optimal cross validation error. Observations with missing values or whose tumor classification was classified as TX were classified using surrogate variables. Analyses were performed by STATA IC version 15 and R version 3.3.2. P-values less than 0.05 (2-tailed) were considered statistically significant.
RESULTS
Patient Characteristics
Among the 395 patients with incident OTC, median age was 58 (interquartile range [IQR]: 48–67). The majority of patients were white (95.2%), male (60.2%), stage I-II (59.5%; AJCC 6th and 7th Editions), treated with surgical resection alone (58.2%), never or light alcohol users (50.6%), and current or past tobacco users (64.0%) (Supplemental Table 2).
Risk Factors Analysis
Compared to typical onset OTC, early onset patients were significantly less likely to report a history of cigarette smoking and heavy alcohol use at the time of diagnosis. Early onset OTC patients were also significantly more likely to receive surgical resection plus radiation therapy, and to be never tobacco users. Yet, early onset patients were significantly more likely to report snuff use (Table 1). Forty-six percent of early onset OTC patients were never tobacco users (Table 1). More importantly, 37% of early onset OTC patients had no history of traditional head and neck cancer risk factors (tobacco use and/or heavy alcohol use). In the multivariate analysis, snuff use (aOR: 5.4, 95% CI: 1.8–15.8), multimodality treatment (aOR: 2.7, 95% CI: 1.2–6.3 [surgery plus radiation]), and former cigarette smoking (aOR: 0.5, 95% CI: 0.2–0.9) remained significant (Table 1). In sensitivity analyses, the association between snuff use and early onset OTC strengthened as the age cutoff for defining early onset OTC was decreased (Supplemental Table 3).
Table 1.
Univariate and multivariate analyses of risk factors for early onset OTC.
| Characteristic | Typical onset | Early onset | ||||
|---|---|---|---|---|---|---|
| No. patients = 282 | No. patients = 113 | |||||
| No. patients (% of typical) | No. patients (% of early) | OR (95% CI) | P value | aOR (95% CI) | Adjusted p value | |
| Age at diagnosis (yrs) | ||||||
| Median (IQR) | 63 (58–70) | 42 (36–45) | ||||
| Gender | ||||||
| Male | 171 (60.6) | 67 (59.3) | ref | ref | ||
| Female | 111 (39.4) | 46 (40.7) | 1.1 (0.7–1.7) | 0.805 | 1.0 (0.5–1.7) | 0.898 |
| Race | ||||||
| White | 265 (94.0) | 111 (98.2) | ref | ref | ||
| African American or Black | 10 (3.6) | 1 (0.9) | 0.2 (0.1–1.9) | 0.175 | - | - |
| Asian | 6 (2.1) | 1 (0.9) | 0.4 (0.1–3.3) | 0.396 | 0.2 (0.1–2.2) | 0.207 |
| American Indian/AK native | 1 (0.4) | 0 | - | - | - | - |
| Native Hawaiian | 0 | 0 | - | - | - | - |
| Clinical Stage | ||||||
| T | ||||||
| T1 | 110 (39.0) | 44 (38.9) | ref | |||
| T2 | 90 (31.9) | 41 (36.3) | 1.1 (0.7–1.9) | 0.616 | ||
| T3 | 41 (14.5) | 14 (12.4) | 0.9 (0.4–1.7) | 0.658 | ||
| T4a | 18 (6.4) | 4 (3.5) | 0.6 (0.2–1.7) | 0.312 | ||
| TX | 18 (6.4) | 10 (8.9) | 1.4 (0.6–3.2) | 0.448 | ||
| Missing | 5 (1.8) | 0 | - | - | ||
| N | ||||||
| N0 | 193 (68.4) | 74 (65.5) | ref | |||
| N1 | 35 (12.4) | 14 (12.4) | 1.0 (0.5–2.0) | 0.902 | ||
| N2a | 2 (0.7) | 1 (0.9) | 1.3 (0.1–14.6) | 0.829 | ||
| N2b | 22 (7.8) | 9 (8.0) | 1.1 (0.5–2.4) | 0.877 | ||
| N2c | 11 (3.9) | 4 (3.5) | 1.0 (0.3–3.1) | 0.930 | ||
| NX | 14 (5.0) | 11 (9.7) | 2.0 (0.9–4.7) | 0.092 | ||
| Missing | 5 (1.8) | 0 | - | - | ||
| M | ||||||
| M0 | 258 (91.5) | 99 (87.6) | ref | |||
| MX | 19 (6.7) | 13 (11.5) | 1.8 (0.9–3.8) | 0.127 | ||
| Missing | 5 (1.8) | 1 (0.9) | - | - | ||
| Overallb | ||||||
| I | 102 (36.2) | 41 (36.3) | ref | ref | ||
| II | 67 (23.8) | 25 (22.1) | 0.9 (0.5–1.7) | 0.803 | 0.9 (0.4–1.9) | 0.771 |
| III | 45 (16.0) | 20 (17.7) | 1.1 (0.6–2.1) | 0.758 | 1.0 (0.4–2.2) | 0.908 |
| IVA | 45 (16.0) | 16 (14.2) | 0.9 (0.5–1.7) | 0.722 | 0.7 (0.3–1.8) | 0.473 |
| IVB | 1 (0.4) | 0 | - | - | - | - |
| Missing | 22 (7.8) | 11 (9.7) | - | - | - | - |
| Grade | ||||||
| I: Well differentiated | 58 (20.6) | 25 (22.1) | 1.1 (0.6–1.9) | 0.691 | ||
| II: Moderately differentiated | 166 (58.9) | 64 (56.6) | ref | |||
| III: Poorly differentiated | 33 (11.7) | 13 (11.5) | 1.0 (0.5–2.1) | 0.952 | ||
| Non-high grade | 25 (8.9) | 11 (9.7) | 1.1 (0.5–2.5) | 0.735 | ||
| Treatment | ||||||
| Surgery only | 171 (60.6) | 59 (52.2) | ref | ref | ||
| Surgery + chemoradiation | 54 (19.2) | 24 (21.2) | 1.3 (0.7–2.3) | 0.379 | 1.2 (0.5–2.6) | 0.661 |
| Surgery + radiation | 30 (10.6) | 21 (18.6) | 2.0 (1.1–3.8) | 0.028 | 2.7 (1.2–6.3) | 0.019 |
| Otherc | 27 (9.6) | 9 (8.0) | 1.0 (0.4–2.2) | 0.934 | 1.4 (0.5–4.0) | 0.498 |
| Alcohol Use at Diagnosisd | ||||||
| None | 74 (26.2) | 33 (29.2) | ref | ref | ||
| Light | 67 (23.8) | 26 (23.0) | 0.9 (0.5–1.6) | 0.656 | 1.0 (0.5–2.2) | 0.980 |
| Mild to moderate | 70 (24.8) | 37 (32.7) | 1.2 (0.7–2.1) | 0.560 | 1.3 (0.6–2.8) | 0.452 |
| Heavyg | 37 (13.1) | 5 (4.4) | 0.3 (0.1–0.8) | 0.022 | 0.4 (0.1–1.2) | 0.100 |
| Missing | 34 (12.1) | 12 (10.6) | - | - | - | - |
| Drinks per week, Median (IQR) | 8 (2–20)f | 4 (2–6)g | ||||
| Past Alcohol Used | ||||||
| None | 72 (25.5) | 32 (28.3) | ref | |||
| Light | 32 (11.4) | 13 (11.5) | 0.9 (0.4–2.0) | 0.818 | ||
| Mild to moderate | 56 (19.9) | 28 (24.8) | 1.1 (0.6–2.1) | 0.708 | ||
| Heavyg | 61 (21.6) | 20 (17.7) | 0.7 (0.4–1.4) | 0.362 | ||
| Missing | 61 (21.6) | 20 (17.7) | - | - | ||
| Drinks per week, Median (IQR) | 24 (10–40)h | 18 (9–36)i | ||||
| Reported Tobacco Use | ||||||
| Never | 90 (31.9) | 52 (46.0) | 1.8 (1.2–2.8) | 0.009 | ||
| Everj | 192 (68.1) | 61 (54.0) | ref | |||
| Reported Smoking Tobacco Usek | ||||||
| No | 100 (35.5) | 61 (54.0) | 2.1 (1.4–3.3) | 0.001 | ||
| Yes | 182 (64.5) | 52 (46.0) | ref | |||
| Cigarette Use | ||||||
| Never | 107 (37.9) | 63 (55.8) | ref | ref | ||
| Former | 95 (33.7) | 23 (20.4) | 0.4 (0.2–0.7) | 0.002 | 0.5 (0.2–0.9) | 0.034 |
| Current | 80 (28.4) | 27 (23.9) | 0.6 (0.3–0.9) | 0.042 | 0.7 (0.4–1.4) | 0.350 |
| Pack-years, Median (IQR) | 42 (30–63)l | 20 (11.5–36)m | ||||
| Reported Cigar Use | ||||||
| No | 250 (88.7) | 103 (91.2) | ref | |||
| Yes | 32 (11.4) | 10 (8.9) | 0.8 (0.4–1.6) | 0.468 | ||
| Cigar-years, Median (IQR) | 45 (12.9–294)n | 6 (1–15)o | ||||
| Reported Pipe Use | ||||||
| No | 265 (94.0) | 111 (98.2) | ref | |||
| Yes | 17 (6.0) | 2 (1.8) | 0.3 (0.1–1.2) | 0.093 | ||
| Pipe-years, Median (IQR) | 4.5 (3–30)p | 5.5 (5–6)q | ||||
| Reported Smokeless Tobacco User | ||||||
| No | 250 (88.7) | 93 (82.3) | ref | |||
| Yes | 32 (11.4) | 20 (17.7) | 1.7 (0.9–3.1) | 0.094 | ||
| Reported Snuff Use | ||||||
| No | 274 (97.2) | 99 (87.6) | ref | ref | ||
| Yes | 8 (2.8) | 14 (12.4) | 4.8 (2.0–11.9) | 0.001 | 5.4 (1.8–15.8) | 0.002 |
| Can-years, Median (IQR) | 18 (5–30)s | 9.5 (2–20)t | ||||
| Reported Chewing Tobacco Use | ||||||
| No | 257 (91.1) | 99 (87.6) | ref | |||
| Yes | 25 (8.9) | 14 (12.4) | 1.5 (0.7–2.9) | 0.291 | ||
| Pouch-years, Median (IQR) | 6.75 (1.4–20)u | 11 (2–14)v | ||||
| Reported Illicit Drug Use | ||||||
| No | 201 (71.0) | 76 (67.3) | ref | |||
| Yes | 17 (6.0)w | 12 (10.6)x | 1.9 (0.9–4.1) | 0.119 | ||
| Missing | 65 (23.0) | 25 (22.1) | - | - | ||
| Highest Education Level | ||||||
| < HS | 29 (10.3) | 4 (3.5) | 0.4 (0.1–1.1) | 0.061 | ||
| HS | 98 (34.8) | 39 (34.5) | ref | |||
| College | 57 (20.2) | 30 (26.6) | 1.3 (0.7–2.4) | 0.342 | ||
| Graduate | 15 (5.3) | 11 (9.7) | 1.8 (0.8–4.4) | 0.165 | ||
| Post-Grad | 15 (5.3) | 4 (3.5) | 0.7 (0.2–2.2) | 0.500 | ||
| Missing | 68 (24.1) | 25 (22.1) | - | - |
Italicized variables were included in the multivariate analysis
Abbreviations: OR – odds ratio; aOR – adjusted odds ratio; CI – confidence interval; IQR – interquartile range
Contains 2 patients listed as “T4”, 19 patients listed as “T4a”, and 1 patient listed as “T4b”
13 patients (8 typical, 5 early) with only T and N stages were given an overall classification assuming no distant metastases (M0)
Surgery and induction chemotherapy followed by chemoradiation (N=8), chemoradiation (N=6), induction chemotherapy followed by chemoradiation (N=5), palliative care (N=3), radiation only (N=3), surgery with induction chemotherapy (N=3), chemotherapy only (N=2), surgery with chemotherapy (N=2), chemotherapy followed by chemoradiation (N=1), chemotherapy followed by radiation (N=1), induction chemotherapy (N=1), surgery with induction chemotherapy and radiation (N=1),
None: <12 drinks lifetime; Light: >12 weeks lifetime but 0 drinks/week; Mild to moderate: <8 drinks/week (F) or <15 drinks/week (M); Heavy: >=8 drinks/week (F) or >=15 drinks/week (M)
Patient labeled as heavy alcohol use if specified 'heavy use', 'significant use', 'a loť, 'alcohol abuse', or 'alcoholism'
82 typical onset mild to moderate and heavy alcohol users
37 early onset mild to moderate and heavy alcohol users
49 typical onset mild to moderate and heavy alcohol users
25 early onset mild to moderate and heavy alcohol users
Defined as former/current cigarette smoker or reported user of cigars, pipes, snuff, or chewing tobacco
Cigarette, cigar, or pipe use
138 typical onset former or current cigarette users
42 early onset former or current cigarette users
21 typical onset cigar users
5 early onset cigar users
9 typical onset pipe users
2 early onset pipe users
Snuff or chewing tobacco use
5 typical onset snuff users
10 early onset snuff users
10 typical onset chewing tobacco users
6 early onset chewing tobacco users
Marijuana (N=15), cocaine (N=5), LSD (N=2)
Marijuana (N=9), cocaine (N=4), methamphetamine (N=1)
Cumulative Survival All-Cause Mortality
Median follow-up for our study was 27.2 months (IQR:11.9–64.2 months). Five and 10-year survival rates for early onset OTC were 75.8% and 64.1%, respectively compared to 71.0% and 46.7% for typical onset OTC (Fig. 1). Compared to typical onset, early onset OTC was associated with an almost 50 percent reduction in hazard of death (adjusted hazard ratio [aHR]:0.6, 95% CI: 0.3–0.9) (Table 2). In analyses stratified by tobacco use, the association of early onset OTC with improved survival was strongest among patients who were tobacco users (aHR:0.3, 95% CI:0.1–0.8) (Supplemental Table 4).
Fig. 1.
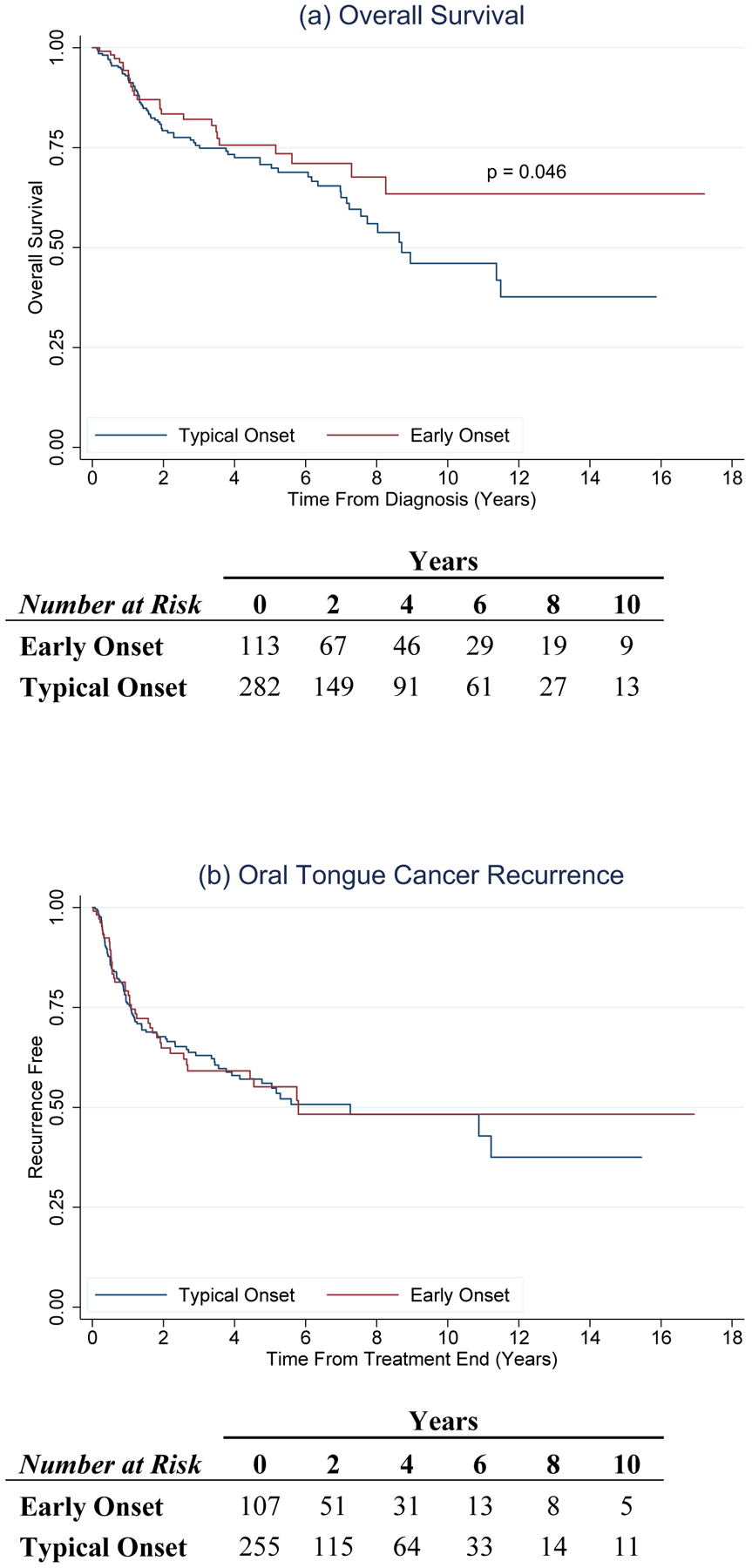
Overall survival and recurrence for typical onset (blue) and early onset (red) OTC. Figure 1(a) shows a significantly improved overall survival (p = 0.046) for patients with early onset OTC. Figure 1(b) shows no difference in OTC recurrence between patients with typical and early onset OTC.
Table 2.
Cox proportional hazards model, overall survival and recurrence.
| Survival, all-cause mortality | ||||
|---|---|---|---|---|
| Onset | Hazard ratio (95% CI) | P value | Adjusted hazard ratioa (95% CI) | P value |
| Typical | ref | - | - | - |
| Early | 0.7 (0.5–1.1) | 0.133 | 0.6 (0.3–0.9) | 0.046 |
| OTC recurrenceb | ||||
| Onset | Hazard ratio (95% CI) | P value | Adjusted hazard ratioa (95% CI) | P value |
| Typical | ref | - | - | - |
| Early | 1.0 (0.7–1.4) | 0.962 | 0.8 (0.5–1.3) | 0.307 |
Abbreviations: OTC – oral tongue squamous cell carcinoma; CI – confidence interval
Adjusted for sex, race, overall clinical stage, treatment, alcohol use at diagnosis, and ever v. never tobacco use
Three patients with diffuse distant recurrence were assessed radiographically only. If no exact end of treatment date was found in the EHR, the treatment end date was assumed to be the 1st of month (N=7). If no exact recurrence date was found in the EHR, the date of recurrence was assumed to be the 1st of month (N=1). If there was unclear treatment response, OTC recurrence was assumed if specified as such in the physician note.
Risk of Recurrence
Three hundred and sixty-two (92%) of the 395 patients were assessed for recurrence; 33 patients either had residual disease following treatment completion or were lost to follow-up. One hundred and thirty-six 136 (37.6%) patients recurred (75 local; 66 regional; 13 distant). Two and 5-year recurrence rates for early versus typical onset OTC were 35.2% and 45.5%, and 32.5% and 44.3%, respectively (Fig. 1). No association between age of OTC onset and risk of recurrence was observed (Table 2). Additionally, no associations were observed in analyses stratified by tobacco use (Supplemental Table 4).
Recursive Partitioning Analysis
Using recursive partitioning analysis, patient age at OTC diagnosis (≤ age 65 vs. > age 65) and N classification (N0 vs. N1+) were identified as the major factors influencing survival. Tobacco use was not identified as a predictor for risk of death. Patients were classified into three groups with respect to risk of death from all causes (Fig. 2). The group with the lowest risk of death (HR: 0.6) was patients aged 65 or less without nodal disease. The group with the highest risk of death (HR: 1.6) was patients older than 65. Interestingly, risk of death for those less than age 65 varied greatly based on the presence of nodal metastases (HR: 0.6 for N0 and 1.4 for N1+).
Fig. 2.
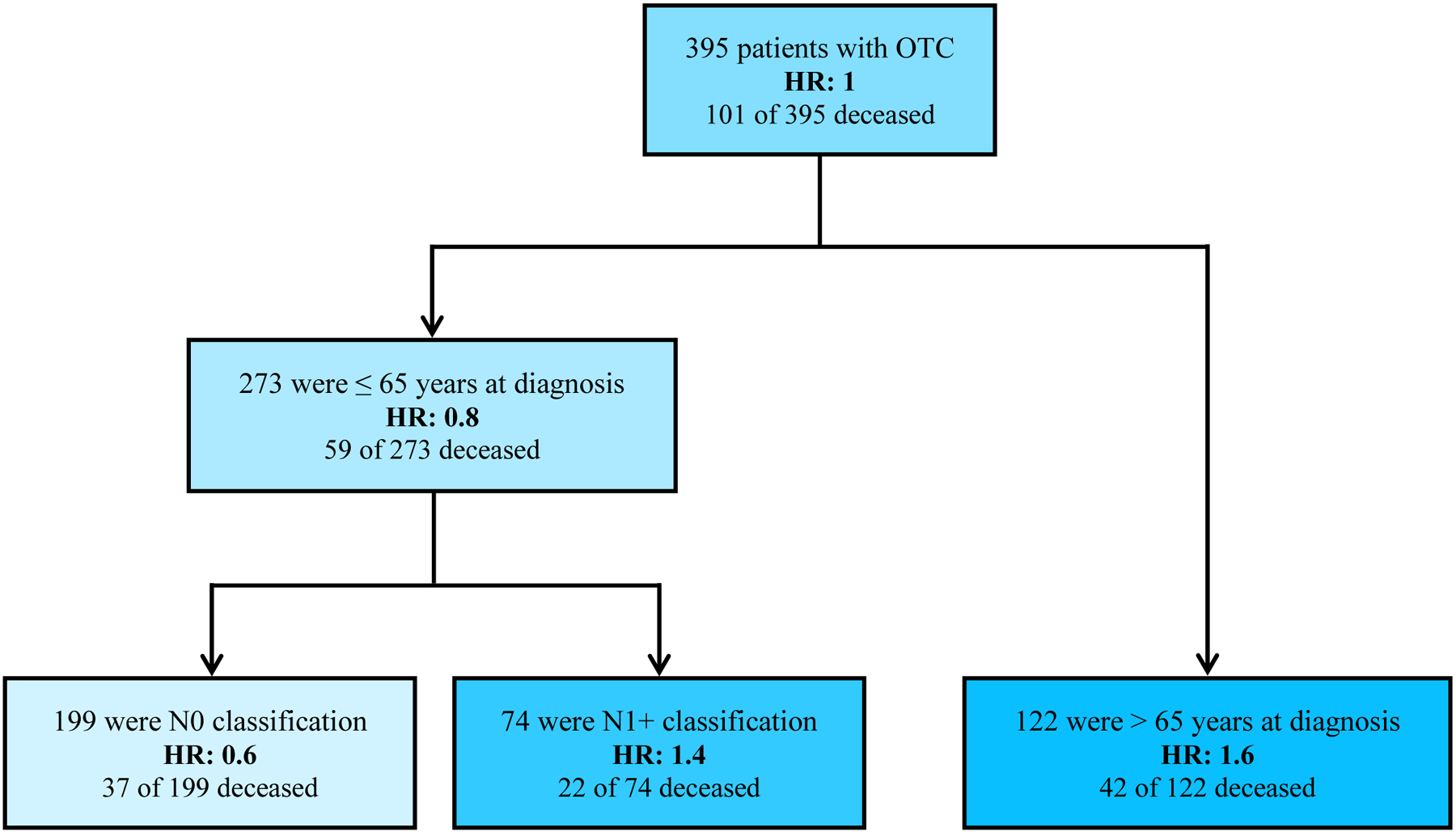
Predictive model of overall survival using recursive portioning analysis. Three subsets of OTC patients were identified and age at OTC diagnosis and N classification were predictive of overall survival. Abbreviations: OTC – oral tongue squamous cell carcinoma; HR – hazard ratio
DISCUSSION
This is the largest study to date to evaluate risk factors associated with early onset OTC and the first study to report an association between early onset OTC and snuff use – a type of smokeless tobacco that is becoming increasingly popular with younger birth cohorts21. Use of other smokeless tobacco products such as chewing tobacco have also been on the rise22. However, no associations between early onset OTC and chewing tobacco use nor with any other tobacco products (cigarette, cigar, or pipe) were observed. Early onset OTC was also associated with improved overall survival, particularly among ever tobacco users. No significant difference in recurrence was observed. In the recursive partitioning analysis, patient age at diagnosis and N classification were the main factors influencing survival.
Despite the increasing incidence of OTC among younger birth cohorts in the US, few studies have evaluated risk factors associated with early onset OTC. Of the few studies conducted to date, results have been conflicting. A study by Friedlander et al. showed no significant difference in alcohol use between younger and older patients with OTC8, while a study by Siegelmann-Danieli et al. found that there was significantly less heavy alcohol and tobacco (cigarette, cigar, and chewing tobacco) use in early versus typical onset OTC patients9. Although snuff was first hypothesized to be a cause of increased incidence and mortality of early onset OTC in the 1980s23,24, our study is the first to report an association. Previous studies have not been powered to detect an association and/or limited their analyses to patients with tobacco use or cigarette smoking history. Only three prior studies have explicitly included non-cigarette tobacco products, such as cigars and chewing tobacco, in their analyses5,7,9. Schantz et al. and Sarkaria & Harari found that over half of patients with early onset OTC either smoked cigarettes or used chewing tobacco, but they did not perform separate analyses for each tobacco product5,7. While, Siegelmann-Danieli et al. observed significantly less combined cigarette, cigar, chewing tobacco, and heavy alcohol use in early onset OTC patients than in patients with typical onset OTC9.
Reports of survival among early versus late onset OTC have also been conflicting. The first reports of early onset OTC in the US indicated a worse overall patient prognosis with increased mortality13,14,25. Yet, more recent studies, such as our own, suggest that early onset OTC is associated with better overall survival than typical onset OTC11,12. The discrepancy between earlier and more recent reports could be due to better recognition of the changing epidemiologic trends in this disease resulting in better recognition of OTC in younger patients. Additionally, the earlier reports of poorer prognosis among early onset patients may have influenced how younger patients were subsequently treated. We did find that early onset patients received more aggressive treatment than their typical onset counterparts, despite no difference in overall clinical staging. Contrary to our observation, current guidelines issued by the National Comprehensive Cancer Network do not specify a change in treatment approach based on age26; yet, our findings suggest that age-stratified treatment approaches should potentially be evaluated in the future. Additionally, while some studies have reported higher locoregional recurrence rates in patients with early onset OTC8,10, we did not observe a significant difference in recurrence rates among those with early versus typical onset OTC. Results of the recursive partitioning analysis showed age of diagnosis and N classification were the strongest predictors of prognosis. Older patients (> age 65) had the worst overall survival, while younger patients (≤ age 65) with N0 classification had the best prognosis.
Our study has several strengths. With a cohort size of 395 OTC patients, 113 of which were early onset, this study is the largest study conducted in the United States to evaluate risk factors and prognosis among patients with early onset OTC. We had access to detailed alcohol and tobacco use information (product, amount and duration of use), through the Vanderbilt Head and Neck Clinic Patient Intake form which was routinely given to all cancer patients from mid-2003 onward. We also had high quality clinical data on the patients included in this study due to VUMC being an early adopter of electronic health records.
Our study also had several limitations. We did not include patients without OTC in our analyses; thus, the lack of an unaffected control group limited our ability to delineate whether the factors associated with early onset OTC were specific to OTC patients or were solely due to differences in patient age. However, the proportion of snuff use in our early onset OTC patients (12.4%) is greater than the reported national snuff use rates for similar age groups (5–6%), suggesting that snuff use may have some causal role in the development of early onset OTC, yet we would expect that snuff use rates are higher than the national average among young people in the “tobacco belt”27. This study was conducted retrospectively and relied on patient-reported risk factor data. Therefore, the data may be affected by underreporting due to patient-perceived guilt over engaging in cancer-associated behaviors or over reporting due to recall bias. Nevertheless, both early and typical onset groups should be affected equally, limiting the impact of these potential biases on our analyses.
One limitation of our survival models was that we did not address confounders such as baseline functional status and underlying comorbidities, which could be a cause of the worse survival seen in older patients. Due to the constraints of the EHR system, we were unable to determine disease-specific mortality. Disease-specific survival would allow for better assessment of the observed differences in 10-year survival rates, which may be better in younger people simply because of their age. The similarities in 5-year survival rates (76 vs. 71% for early and typical onset, respectively) may indicate that these two disease entities have similar prognoses. In fact, a study using SEER data found no difference in disease-specific mortality between early and typical onset OTC patients28. Another possible reason for better survival in early onset patients is that some of the cancers may be caused by HPV due to the inclusion of overlapping and unspecified tongue cancers (C02.8 and C02.9). However, we carefully reviewed each case to confirm the anatomic site of the cancer and excluded all cases with possible overlap with anatomic sites within the oropharynx (Supplemental Fig. 1). Our study was limited to a single tertiary care center, albeit one with a large catchment area in the middle of the “tobacco belt.” Finally, we relied on patient records to determine vital status, which may have resulted in an under reporting of mortality outcomes.
Our results suggest that a portion of early onset OTC may be a result of smokeless tobacco product use, particularly snuff. However, only a small proportion of patients with early onset OTC were snuff users (12%), suggesting the presence of additional, unknown risk factors contributing to the rise of early onset OTC among younger individuals. Future work is needed to more comprehensively evaluate early onset OTC risk factors with prospective studies able to obtain more detailed information on potential risk factors prior to cancer diagnosis. Additionally, given the parallels between OPC and OTC in younger patients, larger molecular studies of OTC tumors are needed to evaluate the possibility of an infectious etiology.
Supplementary Material
Acknowledgements:
This work was supported by Vanderbilt Institute for Clinical and Translational Research (UL1 TR000445 from NCATS/NIH) and Vanderbilt University Medical Center institutional funding.
REFERENCES
- 1.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. [DOI] [PubMed] [Google Scholar]
- 2.Jamal AKB, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current Cigarette Smoking Among Adults — United States, 2005–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1205–1211. [DOI] [PubMed] [Google Scholar]
- 3.Tota JE, Anderson WF, Coffey C, et al. Rising incidence of oral tongue cancer among white men and women in the United States, 1973–2012. Oral Oncol. 2017;67:146–152. [DOI] [PubMed] [Google Scholar]
- 4.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J Clin Oncol. 2015;33(29):3235–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schantz SP, Byers RM, Goepfert H, Shallenberger RC, Beddingfield N. The implication of tobacco use in the young adult with head and neck cancer. Cancer. 1988;62(7):1374–1380. [DOI] [PubMed] [Google Scholar]
- 6.Schantz SP, Byers RM, Goepfert H. Tobacco and cancer of the tongue in young adults. JAMA. 1988;259(13):1943–1944. [DOI] [PubMed] [Google Scholar]
- 7.Sarkaria JN, Harari PM. Oral tongue cancer in young adults less than 40 years of age: rationale for aggressive therapy. Head Neck. 1994;16(2):107–111. [DOI] [PubMed] [Google Scholar]
- 8.Friedlander PL, Schantz SP, Shaha AR, Yu G, Shah JP. Squamous cell carcinoma of the tongue in young patients: a matched-pair analysis. Head Neck. 1998;20(5):363–368. [DOI] [PubMed] [Google Scholar]
- 9.Siegelmann-Danieli N, Hanlon A, Ridge JA, Padmore R, Fein DA, Langer CJ. Oral tongue cancer in patients less than 45 years old: institutional experience and comparison with older patients. J Clin Oncol. 1998;16(2):745–753. [DOI] [PubMed] [Google Scholar]
- 10.Vargas H, Pitman KT, Johnson JT, Galati LT. More aggressive behavior of squamous cell carcinoma of the anterior tongue in young women. Laryngoscope. 2000;110(10 Pt 1):1623–1626. [DOI] [PubMed] [Google Scholar]
- 11.Davidson BJ, Root WA, Trock BJ. Age and survival from squamous cell carcinoma of the oral tongue. Head Neck. 2001;23(4):273–279. [DOI] [PubMed] [Google Scholar]
- 12.Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973–1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg. 2002;128(3):268–274. [DOI] [PubMed] [Google Scholar]
- 13.Byers RM. Squamous cell carcinoma of the oral tongue in patients less than thirty years of age. Am J Surg. 1975;130(4):475–478. [DOI] [PubMed] [Google Scholar]
- 14.Amsterdam JT, Strawitz JG. Squamous cell carcinoma of the oral cavity in young adults. J Surg Oncol. 1982;19(2):65–68. [DOI] [PubMed] [Google Scholar]
- 15.Pytynia KB, Grant JR, Etzel CJ, Roberts D, Wei Q, Sturgis EM. Matched analysis of survival in patients with squamous cell carcinoma of the head and neck diagnosed before and after 40 years of age. Arch Otolaryngol Head Neck Surg. 2004;130(7):869–873. [DOI] [PubMed] [Google Scholar]
- 16.Blanchard P, Belkhir F, Temam S, et al. Outcomes and prognostic factors for squamous cell carcinoma of the oral tongue in young adults: a single-institution case-matched analysis. Eur Arch Otorhinolaryngol. 2017;274(3):1683–1690. [DOI] [PubMed] [Google Scholar]
- 17.de Morais EF, Mafra RP, Gonzaga AKG, de Souza DLB, Pinto LP, da Silveira EJD. Prognostic Factors of Oral Squamous Cell Carcinoma in Young Patients: A Systematic Review. J Oral Maxillofac Surg. 2017;75(7):1555–1566. [DOI] [PubMed] [Google Scholar]
- 18.Danciu I, Cowan JD, Basford M, et al. Secondary use of clinical data: the Vanderbilt approach. J Biomed Inform. 2014;52:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcohol and Public Health: Frequently Asked Questions. https://www.cdc.gov/alcohol/faqs.htm. Accessed Jan 28, 2018.
- 21.Delnevo CD, Wackowski OA, Giovenco DP, Manderski MT, Hrywna M, Ling PM. Examining market trends in the United States smokeless tobacco use: 2005–2011. Tob Control. 2014;23(2):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smokeless Tobacco Use in the United States. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/smokeless/use_us/index.htm. Accessed Feb 12, 2018.
- 23.Depue RH. Rising mortality from cancer of the tongue in young white males. N Engl J Med. 1986;315(10):647. [DOI] [PubMed] [Google Scholar]
- 24.Davis S, Severson RK. Increasing incidence of cancer of the tongue in the United States among young adults. Lancet. 1987;2(8564):910–911. [DOI] [PubMed] [Google Scholar]
- 25.Son YH, Kapp DS. Oral cavity and oropharyngeal cancer in a younger population. Review of literature and experience at Yale. Cancer. 1985;55(2):441–444. [DOI] [PubMed] [Google Scholar]
- 26.NCCN Clinical Practice Guidelines in Oncology (National Comprehensive Cancer Network Guidelines): Head and Neck Cancer (Version I.2016). 2016; https://oralcancerfoundation.org/wp-content/uploads/2016/09/head-and-neck.pdf. Accessed April 8, 2018. [Google Scholar]
- 27.Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2007;89 http://monographs.iarc.fr/ENG/Monographs/vol89/mono89.pdf. [PMC free article] [PubMed] [Google Scholar]
- 28.Patel SC, Carpenter WR, Tyree S, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29(11):1488–1494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


