Abstract
The present study used a longitudinal randomized clinical trial to test whether an early intervention has causal effects on children’s autonomic nervous system regulation. When children were infants, parents involved with Child Protective Services received Attachment and Biobehavioral Catch-up (ABC; N = 43), an intervention that promotes sensitive parenting, or a control intervention (N = 53). When children were 9 years old, children whose parents had received ABC exhibited higher respiratory sinus arrhythmia and lower heart rate at rest and during a parent-child interaction than children in the control group. Intervention effects were not detected for children’s average skin conductance levels or for indices of autonomic reactivity. Results suggest that a parenting-focused early intervention impacted the development of children’s autonomic regulation.
Keywords: Autonomic nervous system, Parenting intervention, Longitudinal
1. Introduction
Early experiences with parents are believed to shape children’s autonomic nervous system (ANS) regulation (Kopp, 1982; Sroufe, 1996). Parents who respond sensitively to their children’s needs tend to have children who demonstrate healthier patterns of physiological activity both when at rest and when facing a challenge or stressor (Calkins & Leerkes, 2011; Propper & Holochwost, 2013). However, causal relations between parenting and children’s autonomic regulation are not yet clear. The present study tested the causal impact of early caregiving experiences on autonomic functioning in a randomized clinical trial of the Attachment and Biobehavioral Catch-up (ABC) intervention, a parenting program designed to promote sensitive parenting during infancy. If ABC has an impact on children’s autonomic regulation, it would suggest that alterations in the early caregiving environment have a causal influence on later regulation.
2. Autonomic nervous system
The ANS helps support homeostasis while at rest and facilitates physiological responses to environmental demands. The ANS contains two branches: the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS). Both branches are thought to contribute to coordinated physiological responses to environmental demands; thus, specific behavioral responses to the environment may be supported by the autonomic nervous system (Jänig & Häbler, 2000). Autonomic activity at rest is thought to reflect the overall wear and tear on the body as well as capacity for reactivity (El-Sheikh, Keiley, & Hinnant, 2010), and autonomic reactivity captures the individual’s physiological response to specific environmental stimuli. Several influential theories offer frameworks regarding the psychological significance of specific autonomic indicators, both at rest and in response to environmental demands.
2.1. Motivational theory: heart rate and skin conductance level
Gray’s (1970, 1987) motivational theory posits the presence of a behavioral activation system and a behavioral inhibition system which coordinate a threat response. According to Gray (1970, 1987), the optimal response to a situation may involve either behavioral approach or withdrawal depending on the context (see also: McNaughton & Corr, 2004; McNaughton & Gray, 2000). That is, approach behaviors are warranted when reward is likely, and withdrawal is appropriate when punishment is likely. Fowles (1980) proposed that specific autonomic measures may serve as indicators of each of these motivational systems. Specifically, heart rate, a measure of cardiovascular activity that reflects a combination of PNS and SNS influence, was theorized to index the behavioral activation system. Skin conductance level (SCL), which reflects the electrodermal system and is innervated by the SNS, was theorized to be an index of the behavioral inhibition system.
Empirically, autonomic underarousal (low resting HR and SCL) has been associated with externalizing problems in children and adults (Lorber, 2004; Ortiz & Raine, 2004; Portnoy & Farrington, 2015; Posthumus, Böcker, Raaijmakers, Van Engeland, & Matthys, 2009). In addition, blunted SCL and blunted HR reactivity to orienting stimuli, stressors, and rewards are associated with antisocial behavior and delinquency (Lorber, 2004; Ortiz & Raine, 2004; Raine, 2002). This evidence supports motivational theory in that low behavioral inhibition (i.e., elevated impulsivity) as indexed by blunted SCL is linked to externalizing behaviors, which are characterized by impulsivity. Although the HR findings are somewhat more difficult to interpret than SCL findings in the context of Gray’s theory, low heart rate may be linked to externalizing problems because underarousal exacerbates impulsivity and sensation-seeking (Portnoy et al., 2014).
Notably, elevated HR and SCL at rest and in response to challenge have also been linked to psychopathology, especially in the context of other risk factors. Hyperarousal in the form of high resting HR has been associated with hypervigilance and increased risk for posttraumatic stress disorder following a traumatic experience (Bryant, Salmon, Sinclair, & Davidson, 2007; Kassam-Adams, Garcia-España, Fein, & Winston, 2005), and high resting SCL has been linked to schizophrenia (Dawson, Schell, & Filion, 2007). There is also evidence to suggest that elevated SCL reactivity to a social evaluative stressor exacerbates environmental risk for the development of behavior problems among adolescents (Diamond, Fagunde, s, & Cribbet, 2012). Taken together, it seems that although blunted HR and SCL at rest and in response to challenge have often been linked to elevated risk for externalizing psychopathology, elevated HR and SCL may increase risk for other problems when environmental risk factors are present.
2.2. Polyvagal theory and neurovisceral integration theory: respiratory sinus arrhythmia
Polyvagal theory (Porges, 2011) and neurovisceral integration theory (Thayer, Hansen, Saus-Rose, & Johnsen, 2009) emphasize the role of top-down control in regulating behavior. Indeed, excessive inhibition or approach motivation, in combination with deficient regulation, is thought to correspond to various forms of psychopathology (Beauchaine, 2001). Both theories hypothesize that physiological regulation can be indexed by respiratory sinus arrhythmia (RSA), which reflects parasympathetic control of the heart via the vagus nerve. Therefore, RSA may be useful as a peripheral indicator of an individual’s capacity for top-down control and as a transdiagnostic biomarker of psychopathology (Beauchaine & Thayer, 2015).
RSA levels while at rest are thought to index an individual’s capacity to respond flexibly to environmental demands, which is characteristic of healthy self-regulation (Beauchaine, 2001; Grossman & Taylor, 2007; Holzman & Bridgett, 2017). Indeed, high resting RSA tends to be associated with effective emotion regulation skills and social competence, and low resting RSA has been consistently linked to psychopathology (especially externalizing disorders) in school-age children, adolescents, and adults (2015, Beauchaine, 2001; Eisenberg et al., 1995). Although high RSA reactivity to challenge is often associated with competent emotion regulation (Perry & Calkins, 2018), excessive reactivity has also been linked to dysregulation and psychopathology (Beauchaine, 2015; Boyce et al., 2001), especially in samples exposed to adversity (Calkins, Graziano, & Keane, 2007; Obradović, Bush, Stamperdahl, Adler, & Boyce, 2010; Skowron, Cipriano-Essel, Gatzke-Kopp, Teti, & Ammerman, 2014). Notably, “adaptive” autonomic reactivity may differ by task; for example, it may be more adaptive to suppress negative emotion when trying to complete a frustrating task but to express emotion when disclosing distress and seeking support from a parent. Thus, autonomic responses that are proportional in intensity to the specific contextual challenge appear to be associated with competent developmental outcomes.
3. Early caregiving and the development of the ANS
The early caregiving environment is thought to influence the development of physiological systems involved in behavior and emotions, including the autonomic nervous system (Calkins & Hill, 2007; Loman & Gunnar, 2010; Propper & Holochwost, 2013). During infancy, children are highly dependent on their parents to provide external regulation of their emotional and physiological arousal. Caregiving that is sensitive to infants’ cues helps infants avoid prolonged periods of excessively high or low levels of arousal (Quigley & Moore, 2018). As children become older and assume more of the responsibility for self-regulation, sensitive parenting involves slowly scaffolding children’s abilities to self-regulate (Kopp, 1982). On the other hand, parental behaviors that are frightening, intrusive, or uninvolved are associated with the development of poor emotion regulation skills, behavior problems, and psychopathology (Calkins, 1994; Chang, Schwartz, Dodge, & McBride-Chang, 2003).
In several cross-sectional studies, it has been demonstrated that infants who experience sensitive, responsive caregiving have lower average heart rates and greater RSA reactivity during the emotionally distressing still-face paradigm and Strange Situation procedure than infants who receive less sensitive care (Haley & Stansbury, 2003; Moore et al., 2009; Propper et al., 2008). Higher levels of maternal sensitivity have also been positively associated with greater increases in infants’ RSA reactivity during non-interpersonal stressful contexts, such as an arm restraint, than lower levels of sensitivity in a short-term longitudinal study (N. B. Perry, Calkins, & Bell, 2016). In addition, infants who are reared in stressful caregiving environments (e.g., hostile parenting, abuse or neglect) are at risk for high HR and low RSA at rest and low RSA reactivity to a variety of tasks (for reviews, see Propper & Holochwost, 2013; Quigley & Moore, 2018). Taken together, it appears that among infants, sensitive parenting is linked to lower resting HR, higher resting RSA, and greater RSA reactivity during emotionally challenging events than insensitive or harsh parenting. To our knowledge, no studies have examined the potential association between sensitive parenting and infant SCL.
Early caregiving may also have long-lasting effects on the functioning of children’s autonomic nervous systems. For example, maltreatment has been linked to low resting RSA in early childhood (Skowron et al., 2011). Similarly, experiencing more supportive and less negative parenting in early childhood is associated with lower resting HR in middle childhood (Bell & Belsky, 2008) than experiencing less supportive and more negative parenting. With regard to autonomic reactivity, toddlers who experienced hostile parenting exhibited blunted HR and RSA reactivity to a battery of collaborative and independent challenge tasks during early childhood than children who did not experience parental hostility (Calkins, Graziano, Berdan, Keane, & Degnan, 2008). In addition, experiencing institutional care in early life has been associated with blunted HR reactivity to social stress in middle childhood (McLaughlin et al., 2015). These childhood effects parallel those observed in infancy in that higher quality parenting is associated with lower resting HR and higher resting RSA, as well as greater HR and RSA reactivity to challenge, than lower quality parenting.
Findings regarding the effect of early parenting on SCL reactivity at later ages are more mixed. Several studies suggest that positive early caregiving experiences (maternal sensitivity, high-quality parent child relationships) are linked to low SCL reactivity to challenges such as viewing frightening videos in early childhood (Gilissen, Koolstra, van IJzendoorn, Bakermans-Kranenburg, & van der Veer, 2007) and engaging in marital conflict discussions in adulthood (Raby, Roisman, Simpson, Collins, & Steele, 2015). However, low SCL reactivity has also been associated with harsh early caregiving experiences. Specifically, abused children exhibit blunted SCL reactivity to cognitive challenge (Carrey, Butter, Persinger, & Bialik, 1995) and when listening to an angry argument between adult strangers (Pollak, Vardi, Bechner, & Curtin, 2005).
In summary, experiencing sensitive, responsive parenting has been most consistently linked to lower resting HR, higher resting RSA, and greater HR and RSA reactivity to a variety of challenges. The effect of early caregiving on SCL is less clear. It may be that moderately harsh caregiving is linked to greater SCL reactivity but extreme levels of negative caregiving (i.e., abuse) are linked to blunted SCL reactivity. The potential effects of early caregiving on resting SCL among children are currently unknown. Taken together, the literature suggests that caregiving experiences during infancy and early childhood appear to have a particularly potent effect on developing stress response systems, and that effects may differ across autonomic indicators.
3.1. Parenting-focused interventions
Given that the development of the autonomic nervous system is associated with experiences within the early caregiving environment (Beauchaine, Gatzke-Kopp, & Mead, 2007), early interventions that enhance parenting quality have the potential to lead to lasting alterations in the functioning of children’s autonomic nervous systems. Although several studies have found that parenting-focused interventions have an effect on children’s cortisol regulation (Bernard, Dozier, Bick, & Gordon, 2015; Slopen, McLaughlin, & Shonkoff, 2014), very few studies have considered intervention effects on children’s autonomic outcomes.
That said, preliminary evidence suggests that early intervention with parents can impact children’s developing autonomic nervous systems. Specifically, a randomized clinical trial of the Incredible Years program for preschoolers diagnosed with ADHD indicated that the parenting-focused intervention increased children’s resting RSA and RSA reactivity to a frustrating block-building task (Z. Bell, Shader, Webster-Stratton, Reid, & Beauchaine, 2018). The effect of the intervention on children’s resting RSA levels was partially mediated by decreases in negative parenting behaviors. In addition, a single-case study on the effects of Parent-Child Interaction Therapy, which focuses on improving child behavior through parenting training, demonstrated that the child’s resting RSA levels increased from pre-treatment to posttreatment (Bagner et al., 2009). Finally, an intervention encouraging maternal-newborn skin-to-skin contact among premature infants, which could be considered a form of sensitive caregiving for newborn infants, resulted in higher resting RSA in infancy and at age 10 as well as less RSA reactivity to a social stressor at age 10 than seen among children who did not receive the intervention (Feldman, Rosenthal, & Eidelman, 2014).
Taken together, these findings suggest that early interventions that target parenting behaviors may alter the development of children’s autonomic nervous systems. However, it remains unclear whether an intervention that focuses on parental sensitivity could also affect children’s autonomic regulation in the long term. Further, it is unknown what impact a parenting intervention would have on children’s physiology in a sample of children who have experienced early adversity in the form of involvement with Child Protective Services.
4. The present study
The present study investigated whether a parenting intervention, Attachment and Biobehavioral Catch-up (ABC), implemented when children were infants, has effects on children’s HR, RSA, and SCL at 9 years old. Previous studies have demonstrated that ABC increases parental sensitivity and child behavioral regulation in early childhood (Bernard, Meade, & Dozier, 2013; Bernard, Simons, & Dozier, 2015; Lind, Bernard, Yarger, & Dozier, 2019 in press). ABC has also been associated with normalizing diurnal cortisol production among children exposed to early adversity, with gains persisting over several years (Bernard, Dozier et al., 2015, Bernard, Hostinar, & Dozier, 2015). The current study is the first to test ABC’s effects on children’s autonomic regulation.
Measures of children’s SCL, HR, and RSA activity were collected during a resting baseline and during an ecologically valid parent-child discussion task. Because all families were recruited by referral from Child Protective Services due to risk for child maltreatment, children in the control group were expected to exhibit elevated HR at rest, low RSA at rest, and a blunted pattern of HR and RSA reactivity consistent with a history of abuse and/or neglect (Carrey et al., 1995; McLaughlin et al., 2015). In contrast, children whose parents received ABC were expected to exhibit lower resting HR and higher resting RSA than children who received a control intervention. In addition, ABC was expected to be associated with greater HR and RSA reactivity (i.e., a greater increase in HR and a greater decrease in RSA from baseline to the discussion task) than the control intervention. Because early caregiving effects on SCL are ambiguous, specific hypotheses were not generated regarding intervention effects on resting SCL or SCL reactivity.
5. Method
5.1. Participants
Participants were 96 children (52% male) enrolled in a longitudinal study on the efficacy of the ABC intervention. Families were originally recruited from a major Mid-Atlantic city when children were infants, by referral from Child Protective Services due to risk for abuse or neglect. At the time of recruitment, these families were randomly assigned to receive either ABC or a control intervention (described below in more detail). Families were not informed about whether they had been assigned to ABC or the control intervention. At pre-intervention, children assigned to ABC did not differ from children assigned to the control intervention in age, race, or diurnal cortisol levels (Bernard, Dozier et al., 2015), and caregivers did not differ in parent age, educational attainment, race (Bernard et al., 2012), parenting sensitivity or attachment-related representations (Zajac, Raby, & Dozier, 2019). Families were invited to participate in a follow-up assessment when the children were in middle childhood, and the present data were collected during laboratory visits when children were 9 years old (M = 9.45 years, SD = 0.34; visits were completed from 2015 to 2017). Parents reported on the race and ethnicity of their children. Most children were reported to be Black or African American (66.9%) or multiracial (11.7%), and approximately one fifth were reported to be White (21.4%). In addition, about one fifth of the children were reported to be Hispanic/Latino (18.6%). At the time of the 9-year assessment, about one third (34.9%) of the parents had not completed high school, about half had completed high school or received their GED (50.9%), and less than one sixth had completed some college or more (14.4%). About half of the parents reported personal employment as a main source of income (47%), and all parents reported receiving additional financial support from family or government programs. Whereas financial need at the 9-year-old assessment was not associated with intervention condition (χ2(1) = 0.00, p = 1.00), a marginally larger proportion of parents who received ABC reported completing high school or receiving a GED at the 9-year-old assessment than parents who received the control intervention (χ2(1) = 3.47, p = .06). Thus, parent educational attainment was considered as a possible covariate.
5.2. Procedures
5.2.1. Experimental intervention
Attachment and Biobehavioral Catch-up (ABC) is a 10-session, home-based parenting intervention that promotes sensitive caregiving for parents of infants. ABC focuses on three main targets for parents: 1) increasing sensitivity to child signals, 2) increasing nurturance to child distress, and 3) decreasing frightening and harsh behaviors. During intervention sessions, parent coaches provide “in the moment” feedback to support parents in recognizing their children’s signals and providing responsive care (Bernard et al., 2013). Parent coaches also use video feedback to enhance parents’ understanding of intervention targets.
5.2.2. Control intervention
Developmental Education for Families (DEF) was designed to match ABC in mode of delivery (home-based with a parent coach) and dosage (10 sessions of one hour each), but included content about children’s development rather than targeting sensitive caregiving. Parent coaches taught parents about developmental milestones in various domains and engaged parents and children in activities that were designed to promote cognitive and language development. As in ABC, video feedback was used to demonstrate to parents how such activities benefited their children.
5.2.3. Parent-child interaction task
As part of the laboratory visit completed when children were 9 years old, parent-child dyads participated in a video-recorded parent-child discussion. Research assistants first attached physiological sensors to the children and gave them general instructions, including instructions to sit as still as possible during the recording session. During this time (approximately 10 min), children were able to adjust to the temperature of the room and to having the sensors attached. This procedure helps reduce the effect of ambient temperature on sweating during skin conductance collection. In addition, ambient temperature was measured immediately prior to baseline using an infrared thermometer. After sensor attachment, parents and children were seated across from each other at a small table, and baseline autonomic data were collected during a three minute paced breathing task (Butler, Wilhelm, & Gross, 2006; Grossman & Taylor, 2007). After the paced breathing, parents and children engaged in two discussions. During the first discussion, the children were instructed to talk to their parents for 8 min about an event that had upset them recently. This discussion was designed to elicit negative emotions in the children and is thus referred to as the distress discussion. Immediately prior to sensor attachment, children were privately interviewed by a member of the research staff and prompted to select an interpersonally distressing event or situation that was primarily peer-related (rather than sibling-related) and was not a routine source of conflict with their parents. For the second discussion, dyads were instructed to plan the perfect day for the child for five minutes. This discussion is referred to as the positive event discussion. The order of the tasks was held constant rather than counterbalanced. Because of the potential for children to become distressed during the first discussion, the second discussion was intended to allow for emotional recovery. Researchers observed the parent-child interactions from behind a one-way mirror.
5.2.4. Physiological data collection, cleaning, and reduction
Software and hardware from the James Long Company were used for data acquisition, cleaning, and processing (James Long Company, Caroga Lake, NY, USA).
5.2.4.1. Heart rate
Heart rate data were collected using two disposable electrocardiography (ECG) electrodes on the rib cage (one on the left and one on the right) and one grounding electrode on the chest (a bipolar configuration). Data were collected at a sampling rate of 1000 readings per second, using James Long equipment for amplification and digitization. Software provided by the James Long Company was used to process and clean ECG data for each subject. The software algorithm identified heartbeats, calculated IBIs as the difference in milliseconds between the beats and identified “suspicious” IBIs for visual verification or correction. Mis-identified heartbeats were manually corrected. Consistent with previous work with children in middle childhood (Woody, Feurer, Sosoo, Hastings, & Gibb, 2016), children’s ECG data were excluded from analyses for all tasks if 10% or more of the heart beats required manual correction. Consequently, heart rate data were unavailable for 15 children, but missingness was not significantly related to intervention group (6 ABC, 9 DEF; χ2(1) = 0.17, p = .68). For all other children, the average heart rate during each of the three tasks was calculated. Heart rate is expressed in beats per minute (BPM).
5.2.4.2. RSA
RSA was calculated using the cleaned heart rate data, as described above. In addition, respiration data were collected using a pneumatic bellows belt fastened around the mid-section. RSA was estimated using the peak-to-valley method, which quantifies the difference in IBIs during respiratory inspiration and expiration. Average RSA levels during each of the three tasks were calculated. In addition to cases for which ECG data were not available (described above), RSA data were excluded for one case in all three tasks due to a physically impossible respiration value (greater than 240 breaths per minute), and the RSA value during the positive event task was excluded for another case because the value was over six standard deviations higher than the mean. As average RSA values across tasks were positively skewed (skewness statistics: Baseline = 3.66, Distress = 3.17, Positive = 2.51), a natural log transformation was applied to reduce skew and prepare the data for analyses (Lewis, Furman, McCool, & Porges, 2012; skewness statistics after transformation: Baseline = −1.56, Distress = −0.62, Positive = −1.97). None of the decisions about the statistical significance of the associations differed when analyses were performed on non-transformed RSA data. In accordance with methodological recommendations, the paced breathing activity was selected as the baseline task to experimentally control for the effect of respiration on the calculation of RSA (Grossman & Taylor, 2007).
5.2.4.3. Skin conductance
Two Ag-AgCl electrodes were attached to the inside tip of the pointer and ring fingers of each participant’s non-dominant hand, and a constant voltage was passed between the electrodes. Double-sided adhesive collars were used to secure the electrodes to the fingers, and a small amount of Biogel conductive paste was used to create a connection between the skin and electrode. SCL was collected at a sampling rate of 1000 samples per second, using James Long Company equipment for amplification and digitization. The average SCL was calculated during the baseline, the distress discussion, and the positive event discussion. SCL is expressed in microsiemens (μS). Each participant’s data were screened for impossible (i.e., negative) values and values that exceeded the maximum measurable value. Ultimately, all cases were retained in the present analyses.
6. Results
6.1. Preliminary analyses
Several covariates were considered in preliminary analyses. In keeping with previous research and recommendations (El-Sheikh et al., 2010; Laborde, Mosley, & Thayer, 2017), the demographic variables of age, gender, race, ethnicity, height, weight, and body mass index (BMI) were considered as possible covariates. Gender, race, and ethnicity were coded as dichotomous variables (Gender: Female = 1, Male = 0; Race: White = 1, non-White = 0; Ethnicity: Hispanic/Latino = 1, non-Hispanic/Latino = 0). Height and weight were measured by a research assistant during the 9-year laboratory visit, and BMI was calculated using the formula recommended by the Centers for Disease Control and Prevention (2014). In addition, time of day and ambient room temperature at the time of autonomic data collection were considered as possible covariates.
Zero-order correlations of study variables and covariates are presented in Table 1. Time of day, ambient temperature at the time of data collection, child height, child gender, and child age were not significantly correlated with any study variables (see Table 1). Although children’s race, ethnicity, weight, and BMI were significantly correlated with several autonomic variables, there were no significant intervention differences for any of these or any other candidate covariates (Table 1; p-values ranged from .29 to .95). Therefore, random assignment to the intervention conditions was considered successful, and covariates were excluded in the subsequent analyses.
Table 1:
Correlations of study variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Intervention | – | ||||||||||||||||||
| 2. Temperature | .01 | – | |||||||||||||||||
| 3. Time | .10 | .11 | – | ||||||||||||||||
| 4. Gender | −.03 | −.18† | .03 | – | |||||||||||||||
| 5. Age | −.03 | −.18† | .12 | −.24* | – | ||||||||||||||
| 6. Race | .00 | .02 | .15 | −.14 | .02 | – | |||||||||||||
| 7. Hispanic | .01 | −.01 | .16 | −.06 | −.02 | .45*** | – | ||||||||||||
| 8. Height (inches) | −.12 | −.03 | −.09 | .02 | .36** | −.21† | −.25* | – | |||||||||||
| 9. Weight (pounds) | .02 | .00 | .03 | −.04 | .30** | −.05 | −.09 | .69*** | – | ||||||||||
| 10. BMI | .06 | −.01 | .04 | −.04 | .22* | .02 | −.02 | .49*** | .96*** | – | |||||||||
| 11. HR Baseline | −.23* | −.13 | .15 | −.06 | .12 | .29* | .10 | .15 | .31* | .30* | – | ||||||||
| 12. HR Distress Task | −.27* | −.18 | .10 | .00 | .17 | .24* | .13 | .11 | .26* | .26* | .92*** | – | |||||||
| 13. HR Positive Task | −.33** | −.10 | .07 | .07 | .11 | .08 | .13 | .20† | .29* | .26* | .78*** | .87*** | – | ||||||
| 14. RSA Baseline | .35** | −.18 | −.04 | .04 | .16 | −.14 | .01 | .00 | −.15 | −.14 | −.53*** | −.42*** | −.37** | – | |||||
| 15. RSA Distress Task | .35** | −.06 | −.04 | .08 | −.07 | −.21† | −.03 | .00 | −.18 | −.18 | −.58*** | −.61*** | −.57*** | .74*** | – | ||||
| 16. RSA Positive Task | .31** | −.08 | −.04 | .02 | −.07 | −.03 | .04 | −.08 | −.29* | −.28* | −.41*** | −.46*** | −.54*** | .62*** | .79*** | – | |||
| 17. SCL Baseline | −.12 | −.05 | −.01 | −.00 | −.06 | .36** | .24* | −.05 | .03 | .07 | .31** | .23* | .11 | −.24* | −.26* | .02 | – | ||
| 18. SCL Distress Task | −.03 | .02 | .06 | −.03 | .00 | .36** | .25* | −.07 | .05 | .10 | .27* | .20† | .05 | −.16 | −.25* | .01 | .91*** | – | |
| 19. SCL Positive Task | −.08 | .07 | .06 | −.12 | .03 | .28** | .13 | −.11 | .04 | .10 | .31* | .27* | .12 | −.18 | −.25* | −.01 | .71*** | .79*** | – |
p < .1
p < .05
p < .01
p < .001
Note: Intervention was dummy-coded with Attachment and Biobehavioral Catch-up (ABC; treatment condition) coded as 1 and Developmental Education for Families (DEF; control condition) coded as 0. For the Gender variable, female was coded as 1 and male was coded as 0. For the Race variable, White was coded as 1 and non-White was coded as 0. For the Hispanic variable, Hispanic was coded as 1 and non-Hispanic was coded as 0. Correlations with RSA are presented with the log-transformed variables.
Parent educational attainment was also considered as a possible covariate. Having a parent who reported completing high school or obtaining a GED was associated with higher RSA (r = .28, p = .02) and higher SCL (r = .22, p = .04) during the positive event discussion. However, controlling for educational attainment did not change the pattern of results; therefore, educational attainment was not included in final models testing intervention effects.
The current sample contained two twin pairs and one pair of non-twin siblings. In addition, nine children completed the 9-year lab visit with a caregiver other than the one who had received ABC (or the control intervention) for a variety of reasons. Including only one child per family or excluding the children who completed the 9-year visit with a different caregiver did not alter any of the decisions about statistical significance. As a result, the full sample was retained in the analyses reported below.
6.2. Intervention effects on physiological regulation
Separate repeated measures analyses of variance (ANOVAs) were conducted with each autonomic variable. In each model, intervention was included as a between-subjects factor (ABC vs. DEF) and task (baseline, distress, and positive tasks) was included as a within-subjects factor. Lower-bound results are reported in all models because the assumption of sphericity was violated. All post hoc analyses were Bonferroni-corrected for multiple comparisons.
This analysis strategy allowed us to simultaneously test our hypotheses regarding levels of autonomic activity while at rest and in response to the two parent-child discussion tasks. To evaluate that our results were not due to the selection of this analytic approach, we also ran regression models predicting baseline level and reactivity for each autonomic indicator (reactivity was calculated as the difference in average activity between baseline and the first discussion task), and these results did not differ from the ANOVA results. Therefore, we present only the ANOVA results.
6.2.1. Heart rate
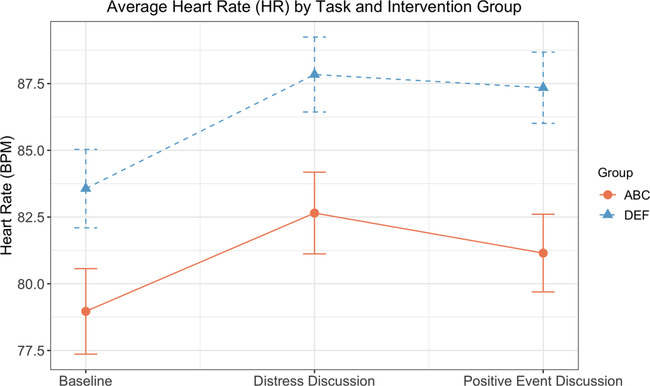
Fig. 1 depicts the effects of intervention group and task on heart rate. In the 2 × 3 (intervention group × task) repeated measures ANOVA predicting heart rate, there was a main effect of intervention (F (1,79) = 7.34, p = .008, ηp2 = .09) and a main effect of task (F (1,79) = 25.62, p < .001, ηp2 = .25). Children in the ABC group had significantly lower heart rates (M = 80.92, SE = 1.45) than children in the DEF group (M = 86.25, SE = 1.33, p = .008, Cohen’s d = −.60). In addition, for the full sample, heart rate during the baseline task (M = 81.27, SE = 1.09) was significantly lower than during the two discussion tasks (Distress: M = 85.25, SE = 1.04, p < .001, Cohen’s d = −.41; Positive: M = 84.25, SE = .99, p < .001, Cohen’s d = −.32). Average heart rate was not significantly different between the two discussion tasks (p = .21, Cohen’s d = .10). The intervention × task interaction was not statistically significant (F(1,79) = .97, p = .33, ηp2 = .01).
Fig. 1.
Average heart rate (HR) in beats per minute (BPM) by task and intervention group. Data depicted are estimated marginal means and standard errors from repeated measures ANOVAs. ABC = Attachment and Biobehavioral Catch-up (treatment condition). DEF = Developmental Education for Families (control condition).
6.2.2. Respiratory sinus arrhythmia
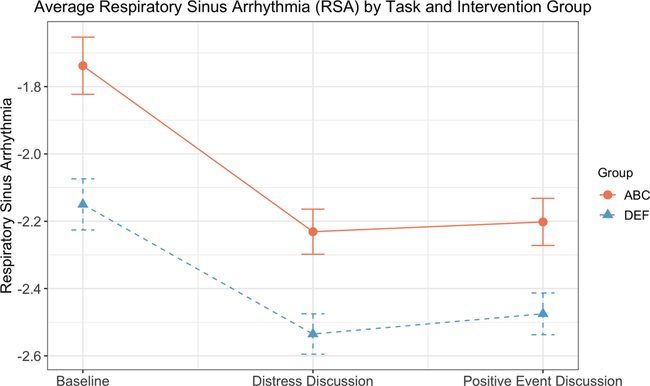
Fig. 2 depicts effects of intervention group and task on RSA. Analyses were conducted as described above, but with RSA included as the dependent variable. There were significant main effects of intervention (F(1,77) = 14.14, p < .001, ηp2 = .16) and task (F(1,77) = 68.38, p < .001, ηp2 = .47). Children who received ABC had significantly higher RSA (M = −2.08, SE = 0.06) than children who received DEF (M = −2.39, SE = 0.06, p = .001, Cohen’s d = .80). In addition, RSA during the baseline task (M = −1.98, SE = 0.06) was higher than RSA during the two discussion tasks (Distress: M = −2.40, SE = 0.05, p < .001, Cohen’s d = .87; Positive: M = −2.35, SE = 0.05, p < .001, Cohen’s d = .76). RSA was not significantly different between the discussion tasks (p = .14). The intervention × task interaction was not statistically significant (F(1,79) = .97, p = .33, ηp2 = .01).
Fig. 2.
Average respiratory sinus arrhythmia (RSA) by task and intervention group. Data depicted are estimated marginal means and standard errors from repeated measures ANOVAs, with natural log transformed RSA data. ABC = Attachment and Biobehavioral Catch-up (treatment condition). DEF = Developmental Education for Families (control condition).
Finally, to check whether RSA results are driven by respiration rate, three separate multiple regressions were run predicting RSA during each task from intervention, controlling for respiration rate during that task (see Table 2). This method was selected because respiration rate is thought to be a possible confound in RSA measurement, especially during discussion tasks (Grossman & Taylor, 2007). Even when controlling for respiration, intervention remained a significant predictor of RSA during all three tasks. Children who received ABC had higher average RSA levels during all three tasks than children who received the control intervention. Respiration rate was a significant predictor of baseline RSA and RSA during the positive discussion task, but only a marginally significant predictor of RSA in the distress discussion task.
Table 2:
Effects of Attachment and Biobehavioral Catch-up and Respiration on Children’s Respiratory Sinus Arrythmia.
| Model 1: Baseline |
Model 2: Distress Task |
Model 3: Positive Task |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | B | SE | β | B | SE | β | |
| (Constant) | −3.06*** | .26 | −2.93*** | .24 | −2.84*** | .18 | |||
| Intervention | .29 | .11 | .26* | .30 | .09 | .35** | .28 | .09 | .33** |
| Respiration rate | .21 | .06 | .37*** | .13 | .08 | .18† | .12 | .06 | .22* |
| R2 | .25*** | .15** | .15** | ||||||
| F | 12.87 | 6.94 | 6.63 | ||||||
p < .1
p < .05
p < .01
p < .001.
Note: Intervention was dummy-coded with the ABC intervention coded as 1 and the control intervention coded as 0.
6.2.3. Skin conductance level
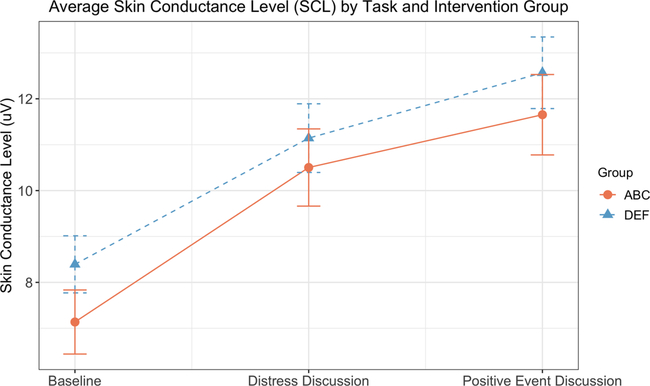
Fig. 3 depicts intervention group and task effects on skin conductance. There was a significant main effect of task (F(1,93) = 80.22, p < .001, ηp2 = .46). Average skin conductance levels increased from baseline (M = 7.77, SE 0= .47) to the distress discussion (M = 10.82, SE = 0.56, p < .001, Cohen’s d = −.60), and from the distress discussion to the positive event discussion (M = 12.11, SE = 0.59, p = .003, Cohen’s d = −.23). Neither the main effect of the intervention nor the intervention × task interaction was statistically significant (F (1,93) = 0.87, p = .35, ηp2 = .009 and F(1,93) = 0.39, p = .54, ηp2 = .004, respectively).
Fig. 3.
Average skin conductance level (SCL) by task and intervention group. Data depicted are estimated marginal means and standard errors from repeated measures ANOVAs. ABC = Attachment and Biobehavioral Catch-up (treatment condition). DEF = Developmental Education for Families (control condition).
7. Discussion
The present study evaluated whether an intervention implemented in infancy to enhance parental sensitivity altered children’s autonomic regulation in middle childhood. Results indicated that children whose parents received ABC exhibited lower average heart rates and higher average RSA during baseline and parent-child discussions than children whose parents received a control intervention. As children’s HR and RSA levels during the discussion tasks reflect a combination of baseline activity and reactivity to the challenge, this pattern of results may reflect a particularly robust effect of the intervention on children’s baseline physiological activity. Within motivational and polyvagal theories, this pattern of results suggests that the ABC intervention may be associated with reduced behavioral activation (indexed by HR) and enhanced physiological regulation (indexed by RSA) at rest. Intervention effects were not observed for children’s overall SCL or for children’s autonomic reactivity to the parent-child discussion tasks. Because children’s parents were randomly assigned to receive one of the two interventions when the children were infants, these results suggest that receiving a parenting intervention early in life that enhances parental responsiveness to child cues has long-term, causal effects on the functioning of children’s autonomic nervous systems.
Because the families who participated in this study were recruited by referral from Child Protective Services due to allegations of child maltreatment, the children were at risk for physiological dysregulation (Gunnar, 2000; Watts-English, Fortson, Gibler, Hooper, & De Bellis, 2006). The results of the present study indicate that receiving a parenting-focused intervention such as ABC early in life helps promote healthy autonomic regulation among children exposed to adversity. Although the current study did not collect information about children’s autonomic regulation prior to the intervention, the groups were equivalent on demographic and other important variables prior to receiving the intervention as well as family financial need at the time the autonomic data were collected. This increases our confidence that the group differences in children’s autonomic activity are due to the effects of the ABC intervention.
A recent RCT of the Incredible Years intervention with preschool-aged children with ADHD demonstrated that the intervention increased children’s resting RSA from pre-intervention to post-intervention and that this effect was partially mediated by reductions in harsh parenting (Bell et al., 2018). The present study complements these findings by showing that receiving ABC in infancy is associated with increased resting RSA in middle childhood as well as increased average RSA during parent-child interaction. In addition, although children exposed to early adversity are at risk for hyperarousal at rest (Propper & Holochwost, 2013), sensitive and responsive caregiving is thought to contribute to the development of parasympathetic dominance and cardiac autonomic balance (Quigley & Moore, 2018). The present study supports this idea by providing evidence that an intervention designed to promote sensitive parenting leads to higher resting RSA than a control intervention, which may be especially important for a sample of children at risk for hyperarousal. In addition, the development of hyperarousal among children exposed to adversity is thought to be an adaptation to a threatening environment (Perry, Pollard, Blakley, Baker, & Vigilante, 1995). Increased resting RSA and decreased resting HR suggests that children whose parents received ABC may have experienced a less threatening early environment following the intervention than children whose parents received the control intervention.
Although the ABC intervention affected average levels of cardiac activity throughout the tasks, intervention effects were not detected for autonomic reactivity (i.e., change in autonomic activity from baseline to discussion tasks). These null effects contrast with other studies that have reported associations between early caregiving environment and later autonomic reactivity (Z. Bell et al., 2018; McLaughlin et al., 2015; Raby et al., 2015). There are several possible reasons for these discrepant findings, including the different samples (CPS-referred children versus adults, preschoolers with ADHD). Although there are many differences between these samples, perhaps the most notable difference is that the CPS-referred children are at high risk for abuse or neglect. Abuse and neglect are more extreme forms of insensitive caregiving than are typically observed in community samples and may result in overpowering effects on autonomic reactivity that are not easily resolved by a brief parenting intervention. Although McLaughlin et al. (2015) did observe an effect of intervention on autonomic reactivity for institutionalized children, the intervention was a dramatic change of the entire rearing environment (moving from institutional care to foster care) rather than a brief parenting intervention. In addition, as patterns of autonomic reactivity do not seem to stabilize until later in childhood (Quigley & Moore, 2018), a ten-session intervention that is delivered in infancy may have a lower likelihood of impacting autonomic reactivity than an intervention that is more prolonged.
Another potential explanation is the context-dependent nature of RSA reactivity. In the current study, we assessed children’s autonomic activity during a parent-child interaction task that was designed to be emotionally challenging for the child. In particular, the task, discussing a personally upsetting event with the parent, was intended to elicit feelings of moderate anxiety. In contrast, Bell et al. (2018) assessed children’s autonomic functioning in the context of a potentially frustrating block-building interaction with the parent, McLaughlin et al. (2015) reported effects of a foster care intervention on heart rate reactivity to the Trier Social Stress Test (although they did not observe effects on RSA reactivity during this task), and Raby et al. (2015) observed associations between childhood parental sensitivity and adults’ electrodermal reactivity during a marital conflict discussion. Individuals’ caregiving histories may have a larger influence on physiological responses under conditions that were perceived as more threatening than the parent-child discussions used in the present study. For example, children whose parents received ABC may interpret a parent-child conflict discussion as less threatening than children whose parents received the control intervention, and so differences in autonomic activity may emerge during such a task. A task for future intervention studies will be to collect children’s autonomic data at regular intervals throughout childhood within diverse emotionally challenging situations. Finally, the pre-discussion interviews that children completed with research assistants to select topics for the parent-child distress discussion may have unintentionally resulted in moderate arousal for children. Because the nature of the first discussion task was explained to children during these interviews, children may have anticipated the upcoming discussion during the baseline, potentially resulting in a rise above their true resting state.
No significant effects of the ABC intervention on children’s SCL were observed. Notably, children’s SCL increased from the distress discussion to the positive discussion. Although the positive event discussion was intended as an emotional recovery period, the task still demanded children’s attention and may have resulted in higher levels of engagement from children than the first discussion because it was enjoyable. Indeed, in addition to its role in reflecting behavioral inhibition (Fowles, 1980), SCL is thought to reflect general arousal as well as task engagement and attention allocation (Dawson, Schell, & Filion, 2000). Thus, SCL may not be as sensitive to nuanced differences in social interaction tasks as RSA. Measures of electrodermal activity have profitably been used in interpersonal contexts that involve conflict (Levenson & Gottman, 1983; Raby et al., 2015), suggesting that SCL may best represent behavioral inhibition when it is measured in conflict or frustration context.
Methodologically, although it is common practice to calculate the average skin conductance level across the full task, examining skin conductance peaks or average level in shorter epochs to investigate more fine-grained changes in skin conductance level across the session may be better suited for detecting effects. In addition, SCL may contain more measurement error than cardiac data, as SCL measurement is highly susceptible to movement artifacts. Although children were instructed to keep their hands as still as possible, the recording session took approximately twenty minutes and most children were unable to remain still for the entire period. Finally, although children’s cardiac pre-ejection period (PEP) data were not collected in the current study, future studies should consider including PEP as an additional measure of SNS activity.
These results have potentially important implications for children’s functioning. Specifically, low HR and high RSA observed during these tasks may correspond to effective emotional and behavioral regulation, especially during parent-child discussions. Conversely, high resting HR and low resting RSA may indicate risk for psychopathology or impaired social functioning in general. Indeed, Beauchaine et al. (Beauchaine, 2001; Beauchaine et al., 2007) have suggested that emotional lability and dysregulation may result from high sympathetic activation that is left unfettered by an ineffective parasympathetic nervous system. Future research is needed to extend the current findings by examining how the two branches of the ANS work together to contribute to children’s physiological regulation and to emotional, behavioral, and social functioning across time.
Although low resting HR has previously been associated with aggression and conduct problems (Lorber, 2004; Ortiz & Raine, 2004; Portnoy & Farrington, 2015), many of the prior studies were conducted with individuals from low-risk communities, and these findings may not consistently apply to samples exposed to adversity (Raine, 2002). In addition, elevated heart rate among individuals with histories of trauma may indicate hypervigilance, as it is associated with a greater likelihood of developing post-traumatic stress disorder (Bryant et al., 2007). Finally, maltreated boys with high resting RSA exhibit lower levels of aggressive behaviors than maltreated boys with low resting RSA (Gordis, Feres, Olezeski, Rabkin, & Trickett, 2010). These data raise the possibility that higher resting RSA and lower resting HR are likely to correspond to less problematic behavioral functioning among samples exposed to adversity.
The present study demonstrated that receiving ABC, a parenting intervention implemented in infancy, was associated with healthier patterns of physiological regulation in children during middle childhood than receiving a control intervention. Specifically, children who received ABC had lower average heart rates and higher average RSA than children in the control condition. Because these results are part of a longitudinal randomized clinical trial, our findings support the notion that the ABC intervention implemented during infancy has causal effects on children’s physiological regulation in middle childhood. Results may provide insights into the physiological mechanisms by which early parenting-focused interventions such as ABC may have long-term benefits for children’s emotional, behavioral, and social functioning.
Acknowledgments
Funding
This work was supported by funding from the National Institute of Mental Health (NIMH), grant number R01MH074374.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- Bagner DM, Sheinkopf SJ, Miller-Loncar CL, Vohr BR, Hinckley M, Eyberg SM, … Lester BM (2009). Parent-Child Interaction Therapy for children born premature: A case study and illustration of vagal tone as a physiological measure of treatment outcome. Cognitive and Behavioral Practice, 16(4), 468–477. 10.1016/j.cbpra.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13, 183–214. 10.1017/S095457940 1002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology, 3, 43–47. 10.1016/j.copsyc.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, & Thayer JF (2015). Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology, 98(2), 338–350. 10.1016/j.ijpsycho.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, & Mead HK (2007). Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology, 74(2), 174–184. 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell BG, & Belsky J (2008). Parenting and children’s cardiovascular functioning. Child: Care, Health and Development, 34(2), 194–203. 10.1111/j.1365-2214.2007.00788.x. [DOI] [PubMed] [Google Scholar]
- Bell Z, Shader T, Webster-Stratton C, Reid MJ, & Beauchaine TP (2018). Improvements in negative parenting mediate changes in children’s autonomic responding following a preschool intervention for ADHD. Clinical Psychological Science, 6(1), 216770261772755 10.1177/2167702617727559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Dozier M, Bick J, Lewis-Morrarty E, Lindhiem O, & Carlson E (2012). Enhancing attachment organization among maltreated children: Results of a randomized clinical trial. Child Development, 83(2), 623–636. 10.1111/j.1467-8624.2011.01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Meade E, & Dozier M (2013). Parental synchrony and nurturance as targets in an attachment based intervention: Building upon Mary Ainsworth’s insights about mother–Infant interaction. Attachment & Human Development, 15(5–6), 507–523. 10.1080/14616734.2013.820920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Dozier M, Bick J, & Gordon MK (2015). Intervening to enhance cortisol regulation among children at risk for neglect: Results of a randomized clinical trial. Development and Psychopathology, 27(3), 829–841. 10.1017/S095457941400073X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Hostinar CE, & Dozier M (2015). Intervention effects on diurnal cortisol rhythms of child protective services–referred infants in early childhood. JAMA Pediatrics, 169(2), 112 10.1001/jamapediatrics.2014.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Simons R, & Dozier M (2015). Effects of an attachment-based intervention on child protective services-referred mothers’ event-related potentials to children’s emotions. Child Development, 86(6), 1673–1684. 10.1111/cdev.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ, … Steinberg L (2001). Autonomic reactivity and psychopathology in middle childhood. The British Journal of Psychiatry, 179(2), 144–150. 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Salmon K, Sinclair E, & Davidson P (2007). Heart rate as a predictor of posttraumatic stress disorder in children. General Hospital Psychiatry, 29(1), 66–68. 10.1016/J.GENHOSPPSYCH.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, & Gross JJ (2006). Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology, 43(6), 612–622. 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD (1994). Origins and outcomes of individual differences in emotion regulation. Monographs of the Society for Research in Child Development, 59, 53–72. [PubMed] [Google Scholar]
- Calkins SD, & Hill A (2007). Caregiver influences on emerging emotion regulation In Gross JJ (Ed.). Handbook of emotion regulation (pp. 229–247). New York: The Guilford Press. [Google Scholar]
- Calkins SD, & Leerkes EM (2011). Early attachment processes and the development of emotional self-regulation In Vohs KD, & Baumeister RF (Eds.). Handbook of self-regulation: Research, theory, and applications (pp. 355–373). (2nd ed.). New York: The Guilford Press. [Google Scholar]
- Calkins SD, Graziano PA, Berdan LE, Keane SP, & Degnan KA (2008). Predicting cardiac vagal regulation in early childhood from maternal-child relationship quality during toddlerhood. Developmental Psychobiology, 50(8), 751–766. 10.1002/dev.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, & Keane SP (2007). Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology, 74(2), 144–153. 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrey NJ, Butter HJ, Persinger MA, & Bialik RJ (1995). Physiological and cognitive correlates of child abuse. Journal of the American Academy of Child and Adolescent Psychiatry, 34(8), 1067–1075. 10.1097/00004583-199508000-00017. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2014). Calculating BMI using the english system. Retrieved fromhttps://www.cdc.gov/nccdphp/dnpao/growthcharts/training/bmiage/page5_2.html.
- Chang L, Schwartz D, Dodge KA, & McBride-Chang C (2003). Harsh parenting in relation to child emotion regulation and aggression. Journal of Family Psychology, 17(4), 598–606. 10.1037/0893-3200.17.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, & Filion DL (2000). In Cacioppo JT, Tassinary LG, & Berntson GG (Eds.). The electrodermal system (pp. 200–223). (2nd ed.). New York: Cambridge University Press. [Google Scholar]
- Dawson ME, Schell AM, & Filion DL (2007). The electrodermal system. Handbook of Psychophysiology, 2, 200–223. [Google Scholar]
- Diamond LM, Fagundes CP, & Cribbet MR (2012). Individual differences in adolescents’ sympathetic and parasympathetic functioning moderate associations between family environment and psychosocial adjustment. Developmental Psychology, 48(4), 918–931. 10.1037/a0026901. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Maszk P, Smith M, & Karbon M (1995). The role of emotionality and regulation in children’s social functioning: A longitudinal study. Child Development, 66(5), 1360–1384. 10.1111/j.1467-8624.1995.tb00940.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Keiley M, & Hinnant JB (2010). Developmental trajectories of skin conductance level in middle childhood: Sex, race, and externalizing behavior problems as predictors of growth. Biological Psychology, 83(2), 116–124. 10.1016/J.BIOPSYCHO.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Rosenthal Z, & Eidelman AI (2014). Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biological Psychiatry, 75(1), 56–64. 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Fowles DC (1980). The three arousal model: Implications of gray’s two-factor learning theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology, 17(2), 87–104. 10.1111/j.1469-8986.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Gilissen R, Koolstra CM, van IJzendoorn MH, Bakermans-Kranenburg MJ, & van der Veer R (2007). Physiological reactions of preschoolers to fear-inducing film clips: Effects of temperamental fearfulness and quality of the parent–child relationship. Developmental Psychobiology, 49(2), 187–195. 10.1002/dev.20188. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Feres N, Olezeski CL, Rabkin AN, & Trickett PK (2010). Skin conductance reactivity and respiratory sinus arrhythmia among maltreated and comparison youth: Relations with aggressive behavior. Journal of Pediatric Psychology, 35(5), 547–558. 10.1093/jpepsy/jsp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA (1970). The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy, 8(3), 249–266. 10.1016/0005-7967(70)90069-0. [DOI] [PubMed] [Google Scholar]
- Gray JA (1987). (2nd ed.). The psychology of fear and stress Problems in the behavioural sciences5. Cambridge University Press. [Google Scholar]
- Grossman P, & Taylor EW (2007). Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology, 74(2), 263–285. 10.1016/J.BIOPSYCHO.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Gunnar MR (2000). Early adversity and the development of stress reactivity and regulation In Nelson CA (Vol. Ed.), The effects of early adversity on neurobehavioral development: Vol. 31, (pp. 163–200). New York, NY: Psychology Press; 10.1016/S0028-3932(01)00018-5. [DOI] [Google Scholar]
- Haley DW, & Stansbury K (2003). Infant stress and parent responsiveness: Regulation of physiology and behavior during still-face and reunion. Child Development, 74(5), 1534–1546. 10.1111/1467-8624.00621. [DOI] [PubMed] [Google Scholar]
- Holzman JB, & Bridgett DJ (2017). Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: A meta-analytic review. Neuroscience and Biobehavioral Reviews, 74, 233–255. 10.1016/j.neubiorev.2016.12.032. [DOI] [PubMed] [Google Scholar]
- Jänig W, & Häbler HJ (2000). Specificity in the organization of the autonomic nervous system: A basis for precise neural regulation of homeostatic and protective body functions. Progress in Brain Research, 122, 351–367. [DOI] [PubMed] [Google Scholar]
- Kassam-Adams N, Garcia-España JF, Fein JA, & Winston FK (2005). Heart rate and posttraumatic stress in injured children. Archives of General Psychiatry, 62(3), 335–340. [DOI] [PubMed] [Google Scholar]
- Kopp CB (1982). Antecedents of self-regulation: A developmental perspective. Developmental Psychology, 18(2), 199–214. 10.1037/0012-1649.18.2.199. [DOI] [Google Scholar]
- Laborde S, Mosley E, & Thayer JF (2017). Heart rate variability and cardiac vagal tone in psychophysiological research – recommendations for experiment planning, data analysis, and data reporting. Frontiers in Psychology, 8(213), 1–18. 10.3389/fpsyg.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson RW, & Gottman JM (1983). Marital interaction: Physiological linkage and affective exchange. Journal of Personality and Social Psychology, 45(3), 587–597. 10.1037/0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- Lewis GF, Furman SA, McCool MF, & Porges SW (2012). Statistical strategies to quantify respiratory sinus arrhythmia: Are commonly used metrics equivalent? Biological Psychology, 89(2), 349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind T, Bernard K, Yarger H, & Dozier M (2019). Promoting compliance in children referred to Child Protective Services (CPS): A randomized clinical trial (n.d.) Child Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman MM, & Gunnar MR (2010). Early experience and the development of stress reactivity and regulation in children. Neuroscience and Biobehavioral Reviews, 34, 867–876. 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber MF (2004). Psychophysiology of aggression, psychopathy, and conduct problems: A meta-analysis. Psychological Bulletin, 130(4), 531–552. 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, & Nelson CA (2015). Causal effects of the early caregiving environment on development of stress response systems in children. Proceedings of the National Academy of Sciences of the United States of America, 112(18), 5637–5642. 10.1073/pnas.1423363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton N, & Corr PJ (2004). A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neuroscience and Biobehavioral Reviews, 28(3), 285–305. 10.1016/J.NEUBIOREV.2004.03.005. [DOI] [PubMed] [Google Scholar]
- McNaughton N, & Gray JA (2000). Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. Journal of Affective Disorders, 61(3), 161–176. 10.1016/S0165-0327(00)00344-X. [DOI] [PubMed] [Google Scholar]
- Moore GA, Hill-Soderlund AL, Propper CB, Calkins SD, Mills-Koonce WR, & Cox MJ (2009). Mother-infant vagal regulation in the face-to-face still-face paradigm is moderated by maternal sensitivity. Child Development, 80(1), 209–223. 10.1111/j.1467-8624.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- Obradović J, Bush NR, Stamperdahl J, Adler NE, & Boyce WT (2010). Biological Sensitivity to Context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development, 81(1), 270–289. 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, & Raine A (2004). Heart rate level and antisocial behavior in children and adolescents: A meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 43(2), 154–162. 10.1097/01.chi.0000101373.03068.5c. [DOI] [PubMed] [Google Scholar]
- Perry NB, & Calkins SD (2018). A biopsychosocial perspective on the development of emotion regulation across childhood In Cole PM, & Hollenstein T (Eds.). Emotion regulation: A matter of time New York: Routledge; 10.4324/9781351001328-1. [DOI] [Google Scholar]
- Perry BD, Pollard RA, Blakley TL, Baker WL, & Vigilante D (1995). Childhood trauma, the neurobiology of adaptation, and “use‐dependent” development of the brain: How “states” become “traits.”. Infant Mental Health Journal, 16(4), 271–291. . [DOI] [Google Scholar]
- Perry NB, Calkins SD, & Bell MA (2016). Indirect effects of maternal sensitivity on infant emotion regulation behaviors: The role of vagal withdrawal. Infancy, 21(2), 128–153. 10.1111/infa.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Vardi S, Bechner AMP, & Curtin JJ (2005). Physically abused children’s regulation of attention in response to hostility. Child Development, 76(5), 968–977. 10.1111/j.1467-8624.2005.00890.x. [DOI] [PubMed] [Google Scholar]
- Porges SW (2011). The polyvagal theory: Neurophysiological foundations of emotions, attachment, communication and self-regulation. New York: Norton. [Google Scholar]
- Portnoy J, & Farrington DP (2015). Resting heart rate and antisocial behavior: An updated systematic review and meta-analysis. Aggression and Violent Behavior, 22, 33–45. 10.1016/J.AVB.2015.02.004. [DOI] [Google Scholar]
- Portnoy J, Raine A, Chen FR, Pardini D, Loeber R, & Jennings JR (2014). Heart rate and antisocial behavior: The mediating role of impulsive sensation seeking. Criminology, 52(2), 292–311. 10.1111/1745-9125.12038. [DOI] [Google Scholar]
- Posthumus JA, Böcker KBE, Raaijmakers MAJ, Van Engeland H, & Matthys W (2009). Heart rate and skin conductance in four-year-old children with aggressive behavior. Biological Psychology, 82(2), 164–168. [DOI] [PubMed] [Google Scholar]
- Propper CB, & Holochwost SJ (2013). The influence of proximal risk on the early development of the autonomic nervous system. Developmental Review, 33(3), 151–167. 10.1016/J.DR.2013.05.001. [DOI] [Google Scholar]
- Propper CB, Moore GA, Mills-Koonce WR, Halpern CT, Hill-Soderlund AL, Calkins SD, … Cox M (2008). Gene-environment contributions to the development of infant vagal reactivity: The interaction of dopamine and maternal sensitivity. Child Development, 79(5), 1377–1394. 10.1111/j.1467-8624.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- Quigley KM, & Moore GA (2018). Development of cardiac autonomic balance in infancy and early childhood: A possible pathway to mental and physical health outcomes. Developmental Review, 49, 41–61. 10.1016/J.DR.2018.06.004. [DOI] [Google Scholar]
- Raby KL, Roisman GI, Simpson JA, Collins WA, & Steele RD (2015). Greater maternal insensitivity in childhood predicts greater electrodermal reactivity during conflict discussions with romantic partners in adulthood. Psychological Science, 26(3), 348–353. 10.1177/0956797614563340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A (2002). Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology, 30(4), 311–326. 10.1023/A:1015754122318. [DOI] [PubMed] [Google Scholar]
- Skowron EA, Cipriano-Essel E, Gatzke-Kopp LM, Teti DM, & Ammerman RT (2014). Early adversity, RSA, and inhibitory control: Evidence of children’s neurobiological sensitivity to social context. Developmental Psychobiology, 56(5), 964–978. 10.1002/dev.21175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowron EA, Loken E, Gatzke-Kopp LM, Cipriano-Essel EA, Woehrle PL, Van Epps JJ, … Ammerman RT (2011). Mapping cardiac physiology and parenting processes in maltreating mother-child dyads. Journal of Family Psychology, 25(5), 663–674. 10.1037/a0024528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, McLaughlin KA, & Shonkoff JP (2014). Interventions to improve cortisol regulation in children: A systematic review. Pediatrics, 133(2), 312–326. 10.1542/peds.2013-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe LA (1996). Emotional development: The organization of emotional life in the early years. New York: Cambridge University Press. [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, & Johnsen BH (2009). Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine, 37(2), 141–153. [DOI] [PubMed] [Google Scholar]
- Watts-English T, Fortson BL, Gibler N, Hooper SR, & De Bellis MD (2006). The psychobiology of maltreatment in childhood. The Journal of Social Issues, 62(4), 717–736. 10.1111/j.1540-4560.2006.00484.x. [DOI] [Google Scholar]
- Woody ML, Feurer C, Sosoo EE, Hastings PD, & Gibb BE (2016). Synchrony of physiological activity during mother–child interaction: Moderation by maternal history of major depressive disorder. Journal of Child Psychology and Psychiatry, 57(7), 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac L, Raby KL, & Dozier M (2019). Attachment state of mind and childhood experiences of maltreatment as predictors of sensitive care from infancy through middle childhood: Results from a longitudinal study of parents involved with Child Protective Services. Development and Psychopathology. [DOI] [PubMed] [Google Scholar]