Abstract
Objective
Ladostigil reduces oxidative stress and microglial activation in aging rats. We assessed its safety and potential efficacy in a 3-year, randomized, double-blind, placebo-controlled phase 2 clinical trial in patients with mild cognitive impairment (MCI) and medial temporal lobe atrophy.
Methods
Patients 55 to 85 years of age with MCI, Clinical Dementia Rating (CDR) score of 0.5, Mini-Mental State Examination (MMSE) score >24, Wechsler Memory Scale–Revised Verbal Paired Associates I score ≤18, and Medial Temporal Lobe Atrophy Scale score >1 were stratified by APOE ε4 genotype and randomly assigned (1:1) to ladostigil 10 mg/d or placebo. Primary outcomes were safety and onset of Alzheimer disease dementia. Secondary endpoints were Neuropsychological Test Battery (NTB) composite, Disability Assessment in Dementia (DAD), and Geriatric Depression Scale (GDS) scores. Exploratory outcomes were NTB component, CDR, and MMSE scores. Biomarkers included MRI-derived whole-brain, hippocampus, and entorhinal cortex volumes.
Results
Two hundred ten patients from 15 sites in Austria, Germany, and Israel were randomly allocated to placebo (107 patients) or ladostigil (103 patients). After 36 months, 21 of 103 patients on placebo and 14 of 99 patients receiving ladostigil progressed to Alzheimer disease (log-rank test p = 0.162). There were no significant effects on the NTB composite, DAD, or GDS score. Whole-brain and hippocampus volumes decreased more in the placebo than in the ladostigil group (whole brain, p = 0.025, Cohen d = 0.43; hippocampus, p = 0.043, d = 0.43). Serious adverse events were reported by 28 of 107 patients treated with placebo and 26 of 103 with ladostigil.
Conclusion
Ladostigil was safe and well tolerated but did not delay progression to dementia. Its association with reduced brain and hippocampus volume loss suggests a potential effect on atrophy.
ClinicalTrials.gov identifier
Classification of evidence
This study provides Class II evidence that for patients with MCI and medial temporal lobe atrophy, ladostigil did not significantly decrease the risk of the development of Alzheimer disease.
Ladostigil is a monoamine oxidase (MAO) and acetylcholinesterase inhibitor originally designed for the treatment of Alzheimer disease (AD) with comorbid depression.1 In a 12-month placebo-controlled trial in 200 patients with mild to moderate AD, no statistically significant cognitive effects for ladostigil 80 mg twice daily were observed, possibly because acetylcholinesterase inhibition averaged only 21.3% (clinicaltrials.gov/ct2/show/NCT01354691), and 40% to 50% is needed for significant effects.2 The only adverse events greater than with placebo were vomiting in 3.94% and insomnia 5.94%. No added benefit was found from its brain-selective MAO inhibition.
At 20-fold lower concentrations than those inhibiting either MAO or acetylcholinesterase, ladostigil prevents the fall in the mitochondrial potential resulting from oxidative stress3 and the release of proinflammatory cytokines from activated microglia.4,5 Glial activation, together with increases in reactive oxygen species and proinflammatory cytokines,6–8 may contribute to neurodegeneration9,10 and is found in the brains of aged rats that exhibit deficits in spatial memory4,11 and humans with AD12 and mild cognitive impairment (MCI).13
Six-month treatment of middle-aged rats with a low dose of ladostigil (1 mg/kg/d, equivalent to 10 mg/d in humans) prevented deficits in episodic and spatial memory and reduced age-associated glial cell changes in parietal cortex and hippocampus.10,14 A dose of 8.5 mg/kg/d, which significantly inhibited both MAO and acetylcholinesterase, was much less effective in preventing memory impairment.10 This is reminiscent of the lack of efficacy of acetylcholinesterase inhibitors in people with MCI,15 probably because of too great an increase in acetylcholine.16 Treatment with ladostigil in MCI may reduce reactive oxygen species and proinflammatory changes and slow progression.
Methods
Primary research question
The primary objective for this clinical trial was to assess the safety of ladostigil (10 mg/d) and to explore its effect on ameliorating progression from MCI to AD. Other objectives included assessing the effect of ladostigil on cognition, daily functioning, and biomarkers. This study provides Class II evidence because of the risk for attrition bias, in that <80% of randomized participants completed the randomized trial.
Study design and participants
For this double-blind, randomized, fixed-dose, placebo-controlled parallel-group trial, we enrolled patients from 16 outpatient clinical sites in Austria, Germany, and Israel. Patients were ambulatory, male and female, and 55 to 85 years of age; met provisional research core clinical criteria for MCI (i.e., having a cognitive concern, impairment in at least 1 cognitive domain, independence in functional abilities, and no dementia)17; had a Clinical Dementia Rating (CDR)18 score of 0.5, memory box score 0.5 or 1, no other box score >1, Mini-Mental State Examination (MMSE) score >24, Wechsler Memory Scale–Revised (WMS-R) Verbal Paired Associates score ≤18,19 and Medial Temporal Lobe Atrophy Scale20 score >1 as evaluated by a central rater (R.S., Medical University of Graz). An informant or study partner with at least 10 h/wk of contact with the participant who agreed to monitor medication also was required.
Major exclusion criteria were prior use of marketed medications approved for AD (e.g., acetylcholinesterase inhibitors, memantine, and ginkgo biloba), 15-item Geriatric Depression Scale (GDS)21 score >5, any significant neurologic illness other than MCI, and clinically significant, advanced, or unstable illnesses that might bias assessment or put a participant at risk.
Randomization and masking
We randomly assigned patients by a 1:1 allocation to double-blind treatment with ladostigil 10 mg/d or placebo after stratification by APOE ε4 genotype and site determined at the screening visit. The randomization process was managed and controlled centrally by a study-independent project manager. Block sizes of 4 within each stratum were used to ensure that at the conclusion of the trial treatment groups would be of nearly equal size. The study drug, ladostigil 10 mg, or placebo was supplied as capsules of identical appearance and taste packaged in blister packs. All study personnel and participants were blinded to treatment assignment.
Procedures
The study included a screening period followed by a 36-month double-blind treatment period. Eligible patients were given blinded ladostigil or placebo. Participants were seen for 8 full study assessment visits (baseline and months 3, 6, 12, 18, 24, 30, and 36) and for 5 safety assessment visits in between (months 9, 15, 21, 27, and 33). Participants who withdrew were to be seen after their decisions to withdraw. Those who progressed to dementia were discontinued.
Screening visits included obtaining informed consent; assessing eligibility criteria; and collecting demographic data, medical history, and vital signs. The following tests or ratings were obtained during screening: brain MRI, ECG, modified Hachinski Ischemic Scale,22 WMS-R Verbal Paired Associates I,19 MMSE, GDS,21 CDR,18 APOE genotyping, clinical laboratories for hematology, biochemistry (including thyroid stimulating hormone, free T4, folate, vitamin B12, and Treponema pallidum particle agglutination assay for syphilis), urine analysis, and research diagnostic assessment.
Postscreening visits included recording concomitant medication, administering study medication, assessing compliance by pill count (except baseline), obtaining vital signs, and assessing adverse events. The full study assessment visits also included assessing physical and cognitive status and safety laboratories for hematology, biochemistry, and urine analysis and administering the CDR, Neuropsychological Test Battery (NTB),23 Disability Assessment in Dementia (DAD),24 GDS,21 Neurotrax Mindstreams battery,25 and research diagnostic assessment. The NTB23 included the Rey Auditory Verbal Learning Test (RAVLT), Controlled Word Association Test, Category Fluency Test, WMS-R Digit Span,19 and Trail Making Test Parts A and B.
Physical and neurologic examinations were performed at baseline and months 12, 24, and 36. MRI scans were obtained at screening and months 12, 24, and 36. MMSE was done at baseline and month 36. ECGs were done at baseline and months 6, 12, 18, 24, 30, and 36, and adverse events were assessed at each visit using the lowest-level Medical Dictionary for Regulatory Activities term (version 14.0, meddra.org/). All raters underwent formal training in the administration and scoring conventions for the scales and cognitive tests, and only personnel trained as raters were allowed to rate participants.
High-resolution T1-weighted 3D MRI scans with 1-mm isotropic resolution were acquired on 1.5T (6 sites) and 3T scanners (9 sites). Brain volume loss was estimated with SIENA,26 which is part of the FSL software package (FMRIB Centre, Oxford UK). In brief, SIENA starts by extracting brain and skull images from the baseline and follow-up T1 data.26,27 The 2 brain images are aligned to each other using the skull images to constrain the registration scaling; both brain images are resampled into the space halfway between the 2 images. Tissue-type segmentation is carried out28 to find brain/nonbrain edge points, and then perpendicular edge displacement (between baseline and follow-up) is estimated at these edge points. Finally, the mean edge displacement is converted into a (global) estimate of percentage brain volume change between the 2 time points. Loss of hippocampal volume and entorhinal cortex volume was estimated fully automated with the longitudinal stream of the FreeSurfer software package.29 After interparticipant registration of baseline and follow-up scans, FreeSurfer tools were applied to obtain topologically and geometrically accurate masks for the hippocampus and entorhinal cortex bilaterally.
Because the hippocampus and entorhinal cortex segmentation with FreeSurfer is particularly sensitive to motion and susceptibility artifacts, we reviewed the segmentation of scans that showed volume changes ±4% and excluded those with motion or technically induced artifacts (a previous scan-rescan study reported a coefficient of variation for hippocampus volumes of ≈3% for 3T MRI data from young patients in the absence of atrophy).30 An experienced reader blinded to treatment allocation and clinical data assessed the quality of the scans.
Outcomes
Safety outcomes included adverse events and vital signs, clinical laboratory tests, weight, body mass index, ECGs, and physical examination findings. The primary clinical outcome was conversion to AD dementia based on National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association31 criteria determined by the site investigator. Secondary outcomes compared the effect of ladostigil with placebo on the NTB composite, DAD, and GDS scores. Exploratory outcomes were the neuropsychological scale scores of the NTB, CDR global score >0.5, CDR sum-of-boxes score, MMSE score, and NeuroTrax Mindstreams battery score (reported separately). Biomarkers included whole-brain, hippocampus, and entorhinal cortex volumes on MRI.
Statistical analysis
All analyses were conducted as specified in the statistical analysis plan. Safety analyses included all participants who were randomly allocated and took at least 1 dose of study drug. The incidence and number of adverse events and serious adverse events were summarized by placebo and ladostigil treatment groups for the safety population. Per protocol, efficacy analyses included all patients treated with study drug or placebo who had at least 1 valid postbaseline assessment of the efficacy variable.
Planned sample size for the primary outcome was calculated assuming that 18% would progress to dementia over 3 years in the ladostigil group and 30% in the placebo group. We estimated that 200 participants (100 per treatment arm) would be needed to obtain a power of 86% at 5% (2 tailed) significance level, assuming that 20% would discontinue the trial. Estimates were based on a previous trial in which 16% of patients per year progressed to dementia32 and an observational study of patients with medial temporal atrophy of whom 45.7% converted over 3 years.33,34 Differences between conversion to AD in the 2 groups were examined with survival analysis, log-rank test supported with a Kaplan-Meier plot, and a multivariate Cox regression survival model to control for APOE ε4 carrier status and country.
Per protocol, interim analyses for efficacy were performed after all participants completed years 1 and 2 early on the basis of the primary outcome. To preserve an overall α error of p < 0.05, the threshold for the first interim analysis was set at p = 0.0005 and the second at p = 0.014, allowing the significance threshold at year 3 to be set at p = 0.045 (O'Brien-Fleming method).35
For secondary and exploratory analyses, cognitive and clinical rating scores were examined with mixed model repeated measures assuming a first-order autoregressive covariance structure to account for the shorter interval to the first follow-up visit (3 months) compared to the others (6 months) and modeling time as a categorical measure. Change in CDR global score from 0.5 at baseline was analyzed by Kaplan-Meier plot and log-rank test.
Change from baseline on MRI-derived brain volume measures was analyzed with analysis of covariance controlling for country and for baseline.36 Two scans from month 12 and 6 scans from month 24 were excluded from the analysis for whole-brain volume because of global artifacts, and after visual assessment of the masks and image quality, 9 scans were excluded from all visits: 6 from month 12, 13 from month 24, and 16 from month 36. Scans available for analysis for whole-brain volume and for hippocampus and entorhinal cortex were at baseline 202 for each: year 1, 167 and 163; year 2, 132, and 116; and at year 3, 116 and 94, respectively.
We calculated standardized z scores for the individual component tests of the NTB (i.e., the RAVLT Delayed [sum of recognition and delayed recall], Controlled Word Association Test total, Digit Span total, Category Fluency Test, Trail Making Test Parts A and B) and calculated a composite NTB z score by taking the mean of the z scores across the tests at each assessment for each participant. As per the statistical analysis plan, specified subgroup analyses were also conducted of APOE ε4 carrier and noncarriers. We used SAS version 9.2 (SAS Institute Inc, Cary, NC) or R (R Foundation for Statistical Computing, Vienna, Austria) for all statistical analysis.
Standard protocol approvals, registrations, and patient consents
The trial was conducted in accordance with the principles of Good Clinical Practice37 and the Declaration of Helsinki.38 National central ethics committees in Austria and Germany and the Israel Ministry of Health gave approvals; and institutional review boards gave approval at each site in Israel. Eligible patients or their legal representatives provided written informed consent before participating. The trial was registered on the European Clinical Trials Database (EudraCT) No. 2011-004187-30, and ClinicalTrials.gov, NCT01429623.
Data availability
Individual participant data will not be shared; the statistical analysis plan is available on request; further summary data are available at ClinicalTrials.gov; and individual data tables from the clinical study report will be shared on request from any qualified investigator.
Results
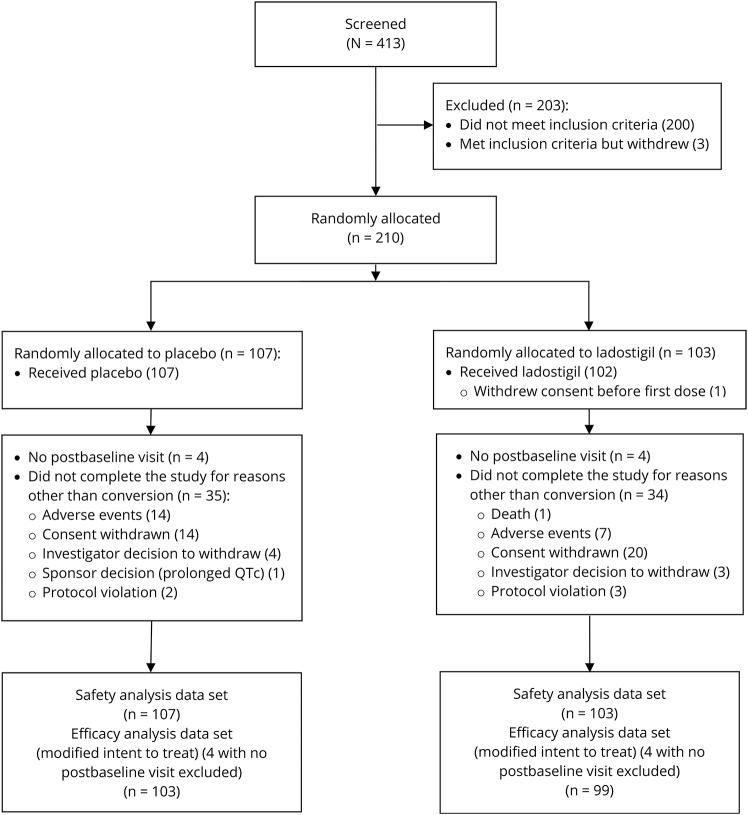
Between February 17, 2012, and August 1, 2013, 210 patients were randomly allocated to placebo (107 patients) or ladostigil (103 patients). One patient assigned to ladostigil withdrew before taking study medication. Four patients in each group lacked postbaseline assessments; thus, 103 and 99 patients receiving placebo and ladostigil, respectively, were included in the modified intent-to-treat population. Baseline demographic and clinical characteristics were similar between the treatment groups (table 1). Thirty-five (34.0%) patients receiving placebo and 34 (34.3%) receiving ladostigil withdrew from the trial before 3 years before developing dementia. The most frequent primary reasons for discontinuation were withdrawal of consent, 14 (40.0%) in the placebo group and 20 (58.8%) in the ladostigil group, and adverse events, 14 (40.0%) in the placebo group and 7 (20.6%) in the ladostigil group (figure 1). About 80% of the patients in each treatment group received study drug for at least 1 year. The exposure to study drug was 223.2 person-years in the ladostigil group and 224.8 person-years in the placebo group.
Table 1.
Demographic and clinical characteristics of participants (modified intent-to-treat population)
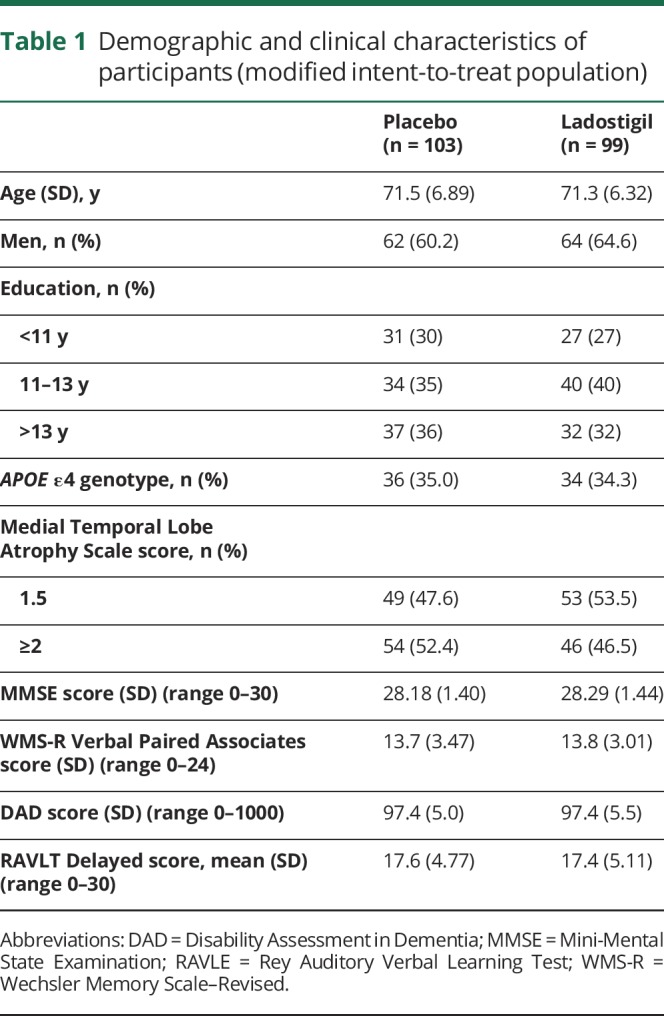
Figure 1. Trial profile.
On the primary outcome, over 3 years, 20.4% (21 of 103 patients) of the placebo group converted to dementia compared to 14.1% (14 of 99 patients) of the ladostigil group (log-rank test, χ2 = 1.955, df = 1, p = 0.162; Cox regression −0.463, standard error [SE] 0.402; 95% confidence interval [CI] −1.250 to 0.324, p = 0.249, figure 2). In a planned exploratory subgroup analysis, among the APOE 4ε noncarriers (n = 128), 18% (12 of 67) of the placebo group vs 8% (5 of 65) of the ladostigil group converted (log-rank test, χ2 = 3.85, df = 1, p = 0.047, Cox regression −1.455, SE 0.662; 95% CI −2.75 to −0.16, p = 0.028), while the APOE 4ε carrier group showed no difference in conversions: 25% (9 of 36) vs 26% (9 of 34) (log rank, χ2 = 0.35, df = 1, p = 0.85, Cox regression 0.765, SE 0.784; 95% CI −0.77 to 2.301, p = 0.329).
Figure 2. Progression from mild cognitive impairment to dementia over 3 years.
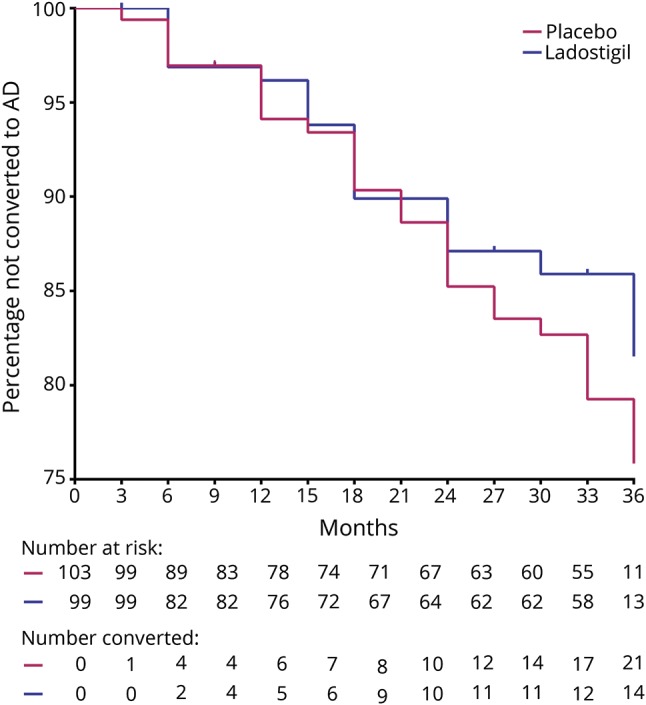
Compared to 14.1% of ladostigil-treated participants, 20.4% of placebo-treated participants progressed to dementia (log rank, χ2 =1.955, df = 1, p = 0.162). AD = Alzheimer disease.
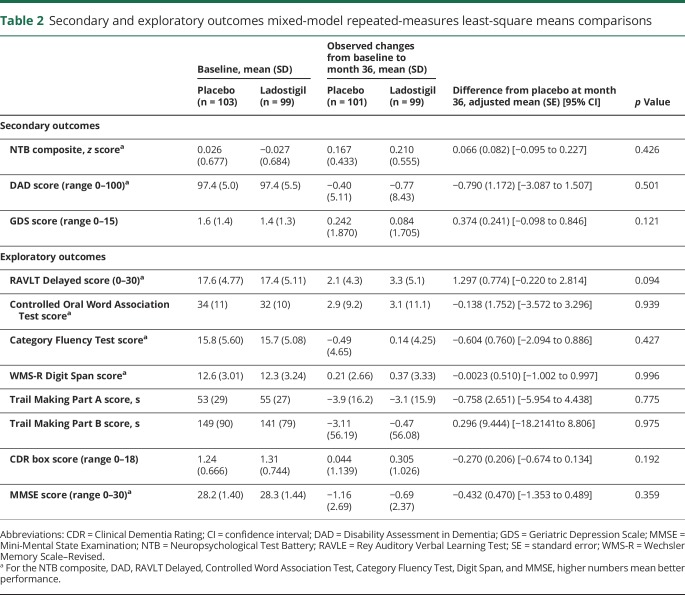
There were no statistically significant differences between placebo and ladostigil treatment on the secondary outcomes (NTB composite, GDS, and DAD scores) or on exploratory outcomes (6 NTB neuropsychological tests, assessing recognition and delayed memory, executive function and attention; table 2) and no significant differences on the MMSE score, CDR box score, or the proportion having CDR global scores >0.5 (log-rank test, df = 1, p = 0.374).
Table 2.
Secondary and exploratory outcomes mixed-model repeated-measures least-square means comparisons
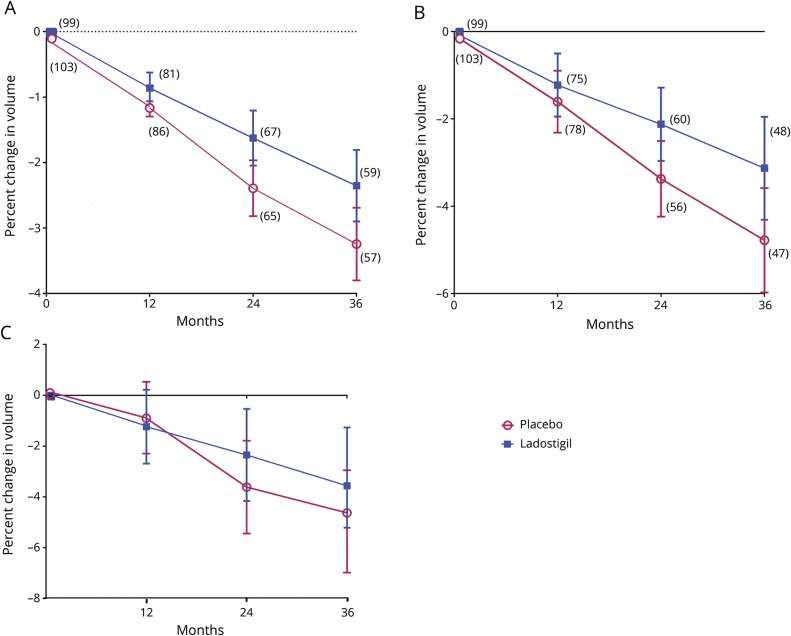
There was significantly less loss of whole-brain and hippocampal volume in the ladostigil-treated patients than in placebo patients, but no significant difference was seen between groups in volume loss for entorhinal cortex (figure 3).
Figure 3. Changes in (A) whole-brain, (B) total hippocampus, and (C) total entorhinal cortex MRI volumes over 3 years.
(A) Estimated marginal mean analysis of covariance (ANCOVA) with 95% confidence intervals (CIs) controlling for baseline to each time point: 12 months p = 0.23, d = 0.19; 24 months p = 0.013, d = 0.44; 36 months p = 0.025, d = 0.43. Number of patients is given in parentheses. Baseline values: placebo 1,324.9 (76.92) mL; ladostigil 1,334.1 (76.10) mL. Annualized decreases in volumes for whole brain are −1.09% and −0.74% for placebo and ladostigil, respectively. (B) Estimated marginal mean ANCOVA with 95% CIs controlling for baseline to each time point: 12 months p = 0.46, d = 0.13; 24 months p = 0.043, d = 0.39; 36 months p = 0.043, d = 0.43. Number of patients is given in parentheses. Baseline: placebo 6.843 (1.177) mL; ladostigil 6.648 (1.286) mL. Annualized decreases in volumes for hippocampus are −1.55% and −1.12% for placebo and ladostigil, respectively. (C) Estimated marginal mean ANCOVA with 95% CIs controlling for baseline to each time point: 12 months p = 0.73, d = 0.06; 24 months p = 0.33, d = 0.18; 36 months p = 0.23, d = 0.26. The number of patients is as in panel B. Baseline: placebo 3.250 (0.748) mL; ladostigil 3.121 (0.783) mL.
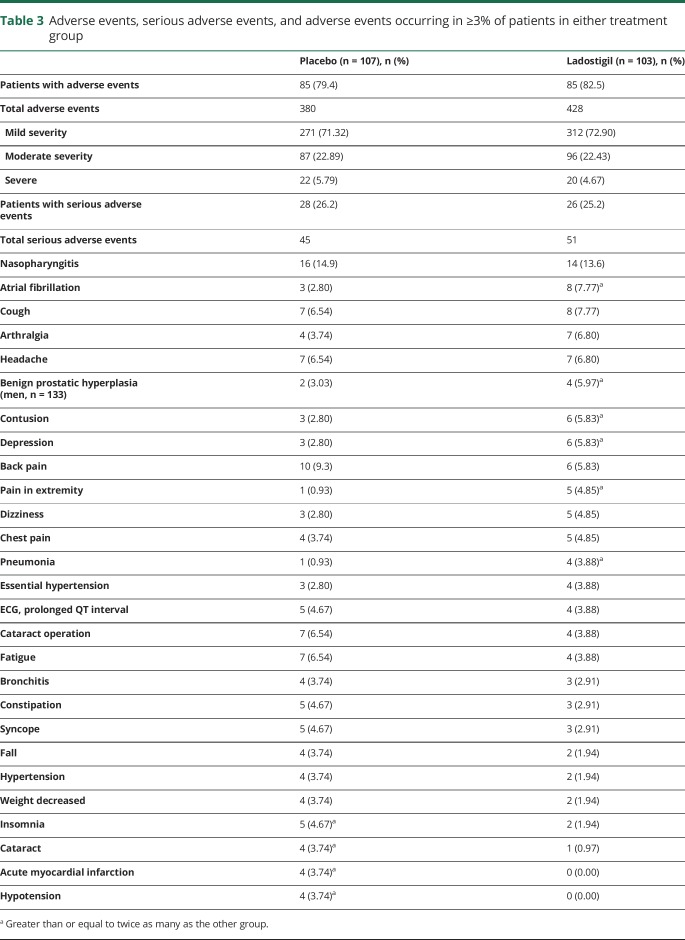
Differences between placebo and ladostigil treatment-emergent adverse events occurring more frequently in the ladostigil group were as follows: atrial fibrillation in 2.8% (n = 3) vs 7.8% (n = 8), depression in 2.8% (n = 3) vs 5.8% (n = 6), prostatic hypertrophy in 3.0% (n = 2) vs 6.0% (n = 4) of men, and extremity pain in 0.93% (n = 1) vs 4.85% (n = 5) (table 3). Acute myocardial infarction and hypotension each occurred in 3.74% (n = 4) of placebo-treated patients compared to no occurrences in the ladostigil group. There were no differences in severity of adverse events; 72.2% overall were mild and 5.2% were severe. A total of 45 serious adverse events occurred in 26.2% (28 of 107) of participants of the placebo group and 51 occurred in 25.2% (26 of 103) of the ladostigil group of the safety population and were judged as unlikely related (32.1%) or not related (67.9%) to treatment. A 73-year-old woman in the ladostigil group died suddenly at home, attributed to cardiac arrest. Her death was judged unlikely related to study medication.
Table 3.
Adverse events, serious adverse events, and adverse events occurring in ≥3% of patients in either treatment group
Discussion
Low-dose ladostigil was well tolerated and safe over an exposure of 223.2 person-years in 103 participants or an average of 2.16 years per participant. Discontinuation was similar in the 2 treatment arms. There were slightly more adverse events in total with ladostigil compared to placebo; slightly fewer serious adverse events occurred with ladostigil; and fewer patients receiving ladostigil discontinued because of adverse events than placebo-treated patients. The 5 more cases of atrial fibrillation in the ladostigil group than with placebo were mild, not clearly of new onset, and unexplained, and all participants remained in the trial. There were 4 more episodes of myocardial infarction in the placebo group, also considered not due to study treatment. Other treatment-emergent adverse events were balanced between treatments and were mild. There was, however, no significant difference between ladostigil and placebo in progression to dementia: only 14.1% (n = 14) of ladostigil-treated and 20.4% (n = 21) of placebo-treated participants.
In comparison, differences in whole-brain and hippocampal volumes favoring ladostigil were observed over the 3 years of treatment. The decline in the placebo group was consistent with that observed in the Alzheimer's Disease Neuroimaging Initiative,39 wherein the annualized decreases in volumes fall between the patients with late amnestic MCI and the unimpaired participants, and with the placebo groups in randomized trials of galantamine,40 donepezil,41 and Souvenaid.42 The differences between ladostigil and placebo were comparable to the drug-placebo differences in these 3 trials as well. Notably, the participants with MCI in those trials were more cognitively impaired than in this ladostigil trial. These studies and ours support previous observations that decreases in brain volumes may precede cognitive impairment and dementia onset.43,44 Any potential disease-modifying effect of ladostigil in an early MCI population may require up to 2 years to become evident by whole-brain and hippocampal volume changes and 3 years to be reflected by progression to dementia (figure 3, A and B).
Unique features and strengths of the study design included requiring MCI core provisional research diagnostic criteria supported by requiring medial temporal atrophy using a validated visual rating scale20,33,45 and by a blinded central rater for a neuronal injury biomarker17 as inclusion criteria, long-term treatment over 3 years, and the use of dementia onset as the primary outcome. Thus, trial participants fulfilled National Institute on Aging/Alzheimer's Association consensus-based diagnostic criteria for MCI due to AD with intermediate likelihood for progressing to AD dementia.17
Double-blinded, placebo-controlled treatment over 3 years allowed potential treatment effects to be detected later in patients with MCI, who generally decline very slowly. Indeed, half the dementia diagnoses in the placebo group were made in the third year. In addition, the use of dementia onset as the primary outcome is itself a clinically meaningful endpoint accepted by the Food and Drug Administration that does not require support from measures of cognition, activities of daily living, or global ratings.46
Limitations to the trial design and conduct included more discontinuations than expected, potential variations in the dementia outcome diagnoses across clinical sites, and fewer participants than anticipated who progressed to dementia. Because participants who withdrew from the trial or reached dementia diagnoses were discontinued from follow-up, we lost the opportunity to assess their clinical and neuropsychological progression over the full 3 years of the trial. Participants withdrew mainly because of adverse events (twice as many in the placebo group) and withdrawal of consent (more in the ladostigil group). Some participants lost to follow-up could have been deteriorating more rapidly, which is another consideration for an overall lack of cognitive decline. This could have reduced any treatment differences if the placebo group had been declining at a greater rate than the ladostigil group. We did not use central adjudication for the MCI core diagnosis at study entry or for the dementia endpoint; this may have diminished the precision in determining the primary clinical outcome and could have acted against detecting a treatment benefit. Not requiring a CSF or β-amyloid PET biomarker—the latter not widely available in 2012 and 2013—as part of eligibility criteria also could be considered a limitation.
The WMS-R Verbal Paired Associates I threshold score of 18 for inclusion in the study is comparable to the mean of 16.8 (SD 4.0) for a population sample of 70- to 74-year-olds (WMS-R manual),19 and memory impairment was required to be only mild at baseline overall. Mean memory scores at baseline for paired associates were ≈0.80 SD below the mean and about the 25th percentile for the RAVLT Delayed.
Visually determined medial temporal atrophy with an established, reliable technique20 is reported as the best validated biomarker for AD,45 although its specificity is limited and may identify non–Alzheimer-related cognitive impairment as well45 or may be related to age alone.47 Prospective studies however, have not been done despite its ease of use.45,47 Notably, about half the participants had baseline medial temporal atrophy scores of 1.5, the minimum allowable for entry. Thus, a substantial proportion of participants had only slight memory impairment and minimal medial temporal atrophy and may not have been expected to worsen over 3 years.
In planning the trial, we expected at least 30% of the placebo group to progress to dementia over 3 years on the basis of previous clinical trials and cohort studies of from 3% to 15.3% per year.32,34 We observed, however, only a 20% progression for the placebo-treated group and a nonsignificant risk difference of 7% compared to ladostigil, although the trial was powered at 0.86 to detect an 18% risk difference and would have required substantially more participants to detect a statistically significant 7% difference.
The proportion of APOE ε4 carriers (34%) was lower than in previous, larger MCI trials, in which it ranged from 41% to 63%.32,42,48,49 Patients with MCI who are APOE ε4 carriers are more likely than noncarriers to progress to dementia in clinical trials and in this trial were twice as likely to progress as noncarriers (26% vs 13%). There was, however, no difference in progression between placebo- and ladostigil-treated individuals in the APOE ε4 carrier group (25% vs 26%). In comparison, within the larger and more slowly progressing APOE ε4 noncarriers, the placebo group progressed at twice the rate of ladostigil-treated group (18% vs 8%, p = 0.028). The APOE ε4 carriers were ≈1 year older and had lower WMS-R Verbal Paired Associates I and RAVLT Delayed scores than the noncarriers (data not shown). Age, neurodegeneration, and APOE ε4 carriage are associated with greater amyloid pathology.44,50 Among the converters with an APOE ε4 allele, the 9 in the ladostigil group had an average age of 76.11 ±3.35 years compared to 69.44 ± 4.55 years (p < 0.005) in the 9 converters in the placebo group, suggesting that carriers in the former group had more advanced pathology and may have been more clinically severe for ladostigil to be effective. Taken together, the study participants who had early MCI showing milder medial temporal atrophy, less memory impairment, and slower clinical progression may have more likely responded to ladostigil.
In light of the uncertainty that MCI diagnoses substantially predict dementia over a 3-year period, future trials might include patients diagnosed with early MCI supported by MRI or molecular biomarkers for neurodegeneration to enhance the likelihood for neurodegenerative pathology.
Acknowledgment
The authors acknowledge the members of the data safety monitoring committee: Prof. Michael Davidson, Prof. Lutz Frölich, and Prof. Bengt Winblad; and members of the Clinical Advisory Board: Lon S. Schneider, MD, Mary Sano, PhD, Bengt Winblad, MD, PhD, Philip Scheltens, MD, PhD, Lutz Frolich, MD, Ronald G. Thomas, PhD, John Harrison, PhD, and Jonathan Rabinowitz, PhD.
Glossary
- AD
Alzheimer disease
- CDR
Clinical Dementia Rating
- CI
confidence interval
- DAD
Disability Assessment in Dementia
- GDS
Geriatric Depression Scale
- MAO
monoamine oxidase
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- NTB
Neuropsychological Test Battery
- RAVLT
Rey Auditory Verbal Learning Test
- SE
standard error
- WMS-R
Wechsler Memory Scale–Revised
Appendix 1. Authors
Appendix 2. Coinvestigators
Footnotes
Class of Evidence: NPub.org/coe
Study funding
The trial was sponsored by Avraham Pharmaceuticals, Ltd, Yavne, Israel.
Disclosure
L. Schneider reports grants from the National Institute on Aging and the state of California and other funding from University of Southern California, Los Angeles; grants from Baxter, Eli Lilly, Forum, Lundbeck, Merck, Novartis, Roche/Genentech, Biogen, and TauRx; and personal fees from AC Immune, Accera, Avraham, Boehringer Ingelheim, Cerespir, Cognition, Corium, Eli Lilly, Forum, Genentech, Merck, Neurim, Neuronix, Roche, Stemedica, Takeda, TauRx, vTv, and Toyama. Y. Geffen was an employee of Avraham Pharmaceuticals, Ltd. J. Rabinowitz reports research grant support, travel support, and/or speaker or consultancy fees from Avraham Pharmaceuticals, Amgen, Janssen, JNJ, Eli Lilly, Lundbeck, Pfizer, Pierre Fabre, F. Hoffmann-La Roche, Minerva, Intra-Cellular Therapies, and Takeda. R. Thomas reports grants from National Institute on Aging and personal fees from Avraham Pharmaceuticals and Toyama/FujiFilm. R. Schmidt reports grants from the Austrian Science Fund. He received personal fees from QPS for scan evaluation in the study and from Merz Austria, Pfizer, Novartis, Dr. Peitner Austria, Axon Neuroscience, and Neuroscios. S. Ropele reports grants from the Austrian Science Fund and personal fees from QPS for image analysis, Axon Neuroscience, and Neuroscios. M. Weinstock is an inventor on patents involving ladostigil and a board member of Avraham Pharmaceuticals, Ltd. Go to Neurology.org/N for full disclosures.
References
- 1.Weinstock M, Gorodetsky E, Poltyrev T, Gross A, Sagi Y, Youdim M. A novel cholinesterase and brain-selective monoamine oxidase inhibitor for the treatment of dementia comorbid with depression and Parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry 2003;27:555–561. [DOI] [PubMed] [Google Scholar]
- 2.Darreh-Shori T, Almkvist O, Guan ZZ, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology 2002;59:563–572. [DOI] [PubMed] [Google Scholar]
- 3.Maruyama W, Weinstock M, Youdim MB, Nagai M, Naoi M. Anti-apoptotic action of anti-Alzheimer drug, TV3326 [(N-propargyl)-(3R)-aminoindan-5-yl]-ethyl methyl carbamate, a novel cholinesterase-monoamine oxidase inhibitor. Neurosci Lett 2003;341:233–236. [DOI] [PubMed] [Google Scholar]
- 4.Weinstock M, Luques L, Poltyrev T, Bejar C, Shoham S. Ladostigil prevents age-related glial activation and spatial memory deficits in rats. Neurobiol Aging 2011;32:1069–1078. [DOI] [PubMed] [Google Scholar]
- 5.Panarsky R, Luques L, Weinstock M. Anti-inflammatory effects of ladostigil and its metabolites in aged rat brain and in microglial cells. J Neuroimmune Pharmacol 2012;7:488–498. [DOI] [PubMed] [Google Scholar]
- 6.Nichols NR, Day JR, Laping NJ, Johnson SA, Finch CE. GFAP mRNA increases with age in rat and human brain. Neurobiol Aging 1993;14:421–429. [DOI] [PubMed] [Google Scholar]
- 7.Nolan Y, Maher FO, Martin DS, et al. Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. J Biol Chem 2005;280:9354–9362. [DOI] [PubMed] [Google Scholar]
- 8.Morgan TE, Xie Z, Goldsmith S, et al. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience 1999;89:687–699. [DOI] [PubMed] [Google Scholar]
- 9.Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol 2005;37:289–305. [DOI] [PubMed] [Google Scholar]
- 10.Weinstock M, Bejar C, Schorer-Apelbaum D, Panarsky R, Luques L, Shoham S. Dose-dependent effects of ladostigil on microglial activation and cognition in aged rats. J Neuroimmune Pharmacol 2013;8:345–355. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher M, Nicolle MM. Animal models of normal aging: relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res 1993;57:155–162. [DOI] [PubMed] [Google Scholar]
- 12.Okello A, Edison P, Archer HA, et al. Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology 2009;72:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parbo P, Ismail R, Hansen KV, et al. Brain inflammation accompanies amyloid in the majority of mild cognitive impairment cases due to Alzheimer's disease. Brain 2017;140:2002–2011. [DOI] [PubMed] [Google Scholar]
- 14.Shoham S, Linial M, Weinstock M. Age-induced spatial memory deficits in rats are correlated with specific brain region alterations in microglial morphology and gene expression. J Neuroimmune Pharmacol 2019;14:251–262. [DOI] [PubMed] [Google Scholar]
- 15.Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev 2012:CD009132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haam J, Yakel JL. Cholinergic modulation of the hippocampal region and memory function. J Neurochem 2017;142(suppl 2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. WMS-R: Wechsler Memory Scale–Revised: Manual. San Antonio: Psychological Corp, Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 20.Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 1992;55:967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yesavage DA, Sheikh JI. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1983;17:37–49. [DOI] [PubMed] [Google Scholar]
- 22.Pantoni L, Inzitari D. Hachinski's ischemic score and the diagnosis of vascular dementia: a review. Ital J Neurol Sci 1993;14:539–546. [DOI] [PubMed] [Google Scholar]
- 23.Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol 2007;64:1323–1329. [DOI] [PubMed] [Google Scholar]
- 24.Feldman H, Sauter A, Donald A, et al. The Disability Assessment for Dementia scale: a 12-month study of functional ability in mild to moderate severity Alzheimer disease. Alzheimer Dis Assoc Disord 2001;15:89–95. [DOI] [PubMed] [Google Scholar]
- 25.Doniger GM, Simon ES. Construct Validity of NeuroTrax™: Comparison with Paper-Based Tests. 2014. Available at: portal.neurotrax.com/docs/construct_validity.pdf. Accessed August 27, 2019. [Google Scholar]
- 26.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002;17:479–489. [DOI] [PubMed] [Google Scholar]
- 27.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 2001;20:45–57. [DOI] [PubMed] [Google Scholar]
- 29.Fischl B. Freesurfer Neuroimage 2012;62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maclaren J, Han Z, Vos SB, Fischbein N, Bammer R. Reliability of brain volume measurements: a test-retest dataset. Sci Data 2014;1:140037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 32.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005;352:2379–2388. [DOI] [PubMed] [Google Scholar]
- 33.DeCarli C, Frisoni GB, Clark CM, et al. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch Neurol 2007;64:108–115. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell J, Arnold R, Dawson K, Nestor PJ, Hodges JR. Outcome in subgroups of mild cognitive impairment (MCI) is highly predictable using a simple algorithm. J Neurol 2009;256:1500–1509. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549–556. [PubMed] [Google Scholar]
- 36.Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med 2002;21:2917–2930. [DOI] [PubMed] [Google Scholar]
- 37.Food and Drug Administration. ICH harmonised tripartite guideline E6: guideline for Good Clinical Practice. 1996. Available at: fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073122.pdf. Accessed September 19, 2011.
- 38.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 39.Leung KK, Bartlett JW, Barnes J, Manning EN, Ourselin S, Fox NC. Cerebral atrophy in mild cognitive impairment and Alzheimer disease: rates and acceleration. Neurology 2013;80:648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prins ND, van der Flier WA, Knol DL, et al. The effect of galantamine on brain atrophy rate in subjects with mild cognitive impairment is modified by apolipoprotein E genotype: post-hoc analysis of data from a randomized controlled trial. Alzheimer's Res Ther 2014;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubois B, Chupin M, Hampel H, et al. Donepezil decreases annual rate of hippocampal atrophy in suspected prodromal Alzheimer's disease. Alzheimers Dement 2015;11:1041–1049. [DOI] [PubMed] [Google Scholar]
- 42.Soininen H, Solomon A, Visser PJ, et al. 24-Month intervention with a specific multinutrient in people with prodromal Alzheimer's disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol 2017;16:965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen RC, Aisen P, Boeve BF, et al. Mild cognitive impairment due to Alzheimer disease in the community. Ann Neurol 2013;74:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frisoni GB, Boccardi M, Barkhof F, et al. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. Lancet Neurol 2017;16:661–676. [DOI] [PubMed] [Google Scholar]
- 46.Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer's disease. N Engl J Med 2013;368:1169–1171. [DOI] [PubMed] [Google Scholar]
- 47.Ten Kate M, Barkhof F, Boccardi M, et al. Clinical validity of medial temporal atrophy as a biomarker for Alzheimer's disease in the context of a structured 5-phase development framework. Neurobiol Aging 2017;52:167–182 e1. [DOI] [PubMed] [Google Scholar]
- 48.Winblad B, Gauthier S, Scinto L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 2008;70:2024–2035. [DOI] [PubMed] [Google Scholar]
- 49.Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer's disease from mild cognitive impairment: the InDDEx study. Lancet Neurol 2007;6:501–512. [DOI] [PubMed] [Google Scholar]
- 50.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 2015;313:1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data will not be shared; the statistical analysis plan is available on request; further summary data are available at ClinicalTrials.gov; and individual data tables from the clinical study report will be shared on request from any qualified investigator.