Abstract
The management options are limited in advanced or recurrent cervical carcinoma. Food Drug Administration has recently approved Programed Cell Death Ligand-1 (PD-L1) blockers in the treatment of advanced PD-L1 positive cervical cancer. We studied PD-L1 expression in Cervical Squamous Cell Carcinoma (CSCC) samples initially on a Tissue Microarray (TMA) and then in full-tissue sections from poorly differentiated cancers. TMA was composed 45 grade II and III tumors. PD-L1 expression was evaluated as categorical data and by obtaining combined positive scores (CPS) of neoplastic and mononuclear inflammatory cells. In TMA samples PD-L1 expression was higher in poorly differentiated cancers compared to grade II tumors by immunohistochemistry. Full-tissue sections from grade III CSCC (n=22) were stained with PD-L1, CD8 and VEGF antibodies. Poorly differentiated CSCC samples had three distinct PD-L1 staining patterns: diffuse, patchy and limited staining within the tumor-stroma interface. The latter pattern was associated with low CPS. Importantly, younger patients (median=32) had tumors with higher PD-L1 expression. The tumor size was positively and lymphovascular invasion negatively associated with PD-L1 expression. In addition, CD8+ tumor infiltrating lymphocyte (TIL) density within the neoplastic tissue matched with PD-L1 levels. The overall survival (OS) rates did not correlate with PD-L1 expression. However, high CD8+ density within the neoplastic tissue was associated with better survival rates in multivariate analysis. PD-L1 expression and CD8+ TIL density may be useful to define a subgroup of patients with relatively better prognosis in poorly differentiated CSCC. It is warranted to validate our results in a larger sample size.
Introduction
Cervical cancer is the third common gynecologic cancer in the United States with an estimated incidence rate of 13 240 in 2018 1. In the last decades, effective screening programs and preventive vaccines facilitated early detection of precursor lesions and decreased cancer rates in the developed countries 2. Currently, recommended therapy for advanced stage cancers, defined as stage IB2 and IVA by International Federation of Gynecology and Obstetrics, is concurrent chemoradiation (CCR) therapy 3. However, recurrent or metastatic disease develops in 15-61% of women within the first two years after completion of the primary therapy 4. The management of recurrent cervical cancer depends on previous treatment modalities. In the presence of prior pelvic irradiation, only curative therapy is pelvic exenteration procedure with high morbidity and mortality rates 5,6. The majority of patients with recurrent or metastatic cervical cancer are treated with palliative platinum-based chemotherapy without significant survival benefits 7,8. The addition of vascular endothelial growth factor (VEGF) inhibitors reduced hazard of disease progression and prolonged overall survival (OS) 9. Epithelial growth factor inhibitors, targeting of PI3K/AKT/mTOR pathway and therapeutic vaccines are other new treatment modalities included in clinical trials for recurrent or metastatic cervical cancer 10-12. Until recently, immunotherapy was emphasized as maintenance therapy for high-risk patients with multiple positive pelvic lymph nodes, uterine corpus extension and positive paraaortic lymph nodes in patients treated with CRR 13. However, Food Drug Administration (FDA) approved Programmed cell death ligand-1 (PD-L1) blocker, pembrolizumab, to treat PD-L1 positive advanced cervical cancer in June 2018.
PD-L1 is a member of B7 family and the receptor of a transmembrane protein, Programmed cell death-1 (PD-1) 14. PD-1 is expressed in effector immune cells and PD-L1 associated with antigen presenting cells such as dendritic and cancer cells 15. The expression of PD-1 is upregulated after T and B cell activation. The immune checkpoint inhibitors maintain tolerance against autoimmunity under physiologic conditions. PD-1/PD-L1 interaction leads to blockage of T cell activation by inhibiting T cell receptor (TCR) signal transduction and C28-CD80- co-stimulation 16. As an immune resistance mechanism PD-L1 is overexpressed in several cancer types 17,18 and immune checkpoint inhibitors are already in use for the treatment of metastatic melanoma, non-small cell lung carcinoma, head and neck, kidney and urothelial carcinomas, Hodgkin lymphoma and mismatched repair deficient cancers 19. Even though FDA has recently approved PD-L1 blockers in advanced cervical cancer it is not well defined which patient subgroup would benefit most from the treatment. We studied PD-L1 expression of cervical squamous cell carcinoma (CSCC) by immunohistochemistry (IHC) in a stepwise approach, first on a tissue microarray (TMA) and then in full-tissue sections from poorly differentiated cancer samples. In addition to PD-L1, immunostains for CD8 and VEGF were performed in the latter group. Results were correlated with clinicopathologic parameters.
Material and Methods:
Study Design
Step 1: (Tissue Microarray):
The TMA was composed of 45 CSCC and 37 matching benign squamous epithelium from cancer patients and 8 normal squamous mucosa from healthy individuals. There were 21 Grade II and 24 Grade III samples. TMA was stained with PD-L1 and p16 INK4 antibodies. PD-L1 immunostaining results were evaluated as categorical data (<1% membranous staining: negative; 1-49% positivity: expressed; 50-100%: diffusely expressed) and continuous data. In categorical analysis, total percentage of partial and complete membranous staining of only neoplastic cells was recorded. The latter reading included combined percentage of positively stained neoplastic and mononuclear inflammatory cells and was called Combined Positive Score (CPS). In cervical cancer, CPS is the recommended method of evaluation for the FDA-approved commercial PD-L1 assays. P16INK4 staining was considered positive when there is at least 5% nuclear and/or cytoplasmic block staining in the neoplastic tissue.
Step 2: poorly differentiated CSCC samples:
Twenty-two out of 24 poorly differentiated CSCC from TMA samples were available for additional immunohistochemical studies. Representative full-tissue sections were stained with PD-L1, CD8 and VEGF antibodies. PD-L1 expression was evaluated in a similar way to TMA samples and included both categorical data and CPS. Even though there was an in situ carcinoma component in some samples only invasive carcinoma was graded in all readings. CD8+ Tumor Infiltrating Lymphocytes (TIL) were evaluated separately in the neoplastic tissue and surrounding stroma. The number of CD8+ TIL was counted in 15-20 high power fields and a mean was obtained for neoplastic cells and surrounding stroma. The density of TIL was divided into 3 groups: (1+): less than 5 TIL, (2+): (6-19 TIL) and (3+): 20 or more CD8+ TIL 20. VEGF staining was recorded as percent of positively stained neoplastic cells.
Immunohistochemistry
Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ) was used as per manufacturer's protocol with proprietary reagents for all antibodies. Briefly, 4 micron-thick tissue sections were deparaffinized on the automated system with EZ Prep solution. Heat-induced antigen retrieval method was used in Cell Conditioning for 1 hour. Sections were stained with rabbit PD-L1 monoclonal antibody (CST 13684, Cell Signaling Technology, Danvers, MA) at a concentration of 1:50 with appropriate positive and negative controls. A mouse monoclonal CD8 antibody (#760-4250, Ventana, Oro Valley, AZ) was used at a prediluted concentration and incubated for 32 minutes. A rabbit VEGF (#ab52917, Abcam, Cambridge, MA) and p16 Ink4a (Proteintech Group; Rosemont, IL) antibodies were diluted to 1:200 and 1:500 concentrations respectively. The Ventana ChromoMap kit was used as a detection system. Slides were counterstained with Hematoxylin which was followed by dehydration and cover slipping.
Statistical Analyses
Patient characteristics were summarized using descriptive statistics including mean, median and range for continuous measures and proportions and frequencies for categorical measures. The association between continuous variables and PD-L1 Status and CD8 were assessed using Kruskal-Wallis tests or Student T test. The associations between categorical variables and PD-L1 Status and CD8 were evaluated using Fisher’s exact tests. Results from the overlapping TMA and full-tissue samples were compared by Pearson Correlation test. Log-rank tests were used to determine if clinical variables were independently associated with OS. Cox proportional hazards models were used to incorporate clinical variables into a multivariate survival model. Model development was completed by first including any clinical variable with p < 0.05 into an initial model, followed by backward elimination to remove variables with p > 0.05 from the final model. All analyses were performed with SAS version 9.4.
Results
PD-L1 expression in cervical squamous cell cancer does not correlate with overall survival
In TMA samples (n=45), median patient age was 42 (24-83). Primary surgeries performed between 1991 and 2006. Only one patient received preoperative radiation therapy. Locoregional lymph node dissection was performed in 44 patients and 14 of them had positive regional lymph nodes. The majority of patients were diagnosed at stage I (n=34) and stage II (n=5) diseases. Only one patient had stage III and 2 patients had stage IV disease. PD-L1 expression was significantly different between tumor and normal tissue controls (p<0.001) and also between moderately (grade II) and poorly differentiated (grade III) cancer samples. The difference was borderline significant in categorical analysis (p=0.04) between grade II and III tumors. Mean CPS was 3% for moderately differentiated CSCC and 14% for poorly differentiated CSCC. PD-L1 expression difference was more pronounced in CPS analysis (p=0.027). Patients with stage I disease (n=34) had higher PD-L1 expressing tumors compared to patient with stage II, III and IV disease (n=8) (p=0.026). All tumor samples expressed p16 INK4. There was no association between PD-L1 and p16 INK4 expression. Clinicopathologic parameters such as patient’s age, lymphovascular space invasion (LVSI), tumor size, lymph node status and OS did not correlate with PD-L1 expression in TMA cohort.
Younger patients with poorly differentiated CSCC had higher PD-L1 expressing tumors
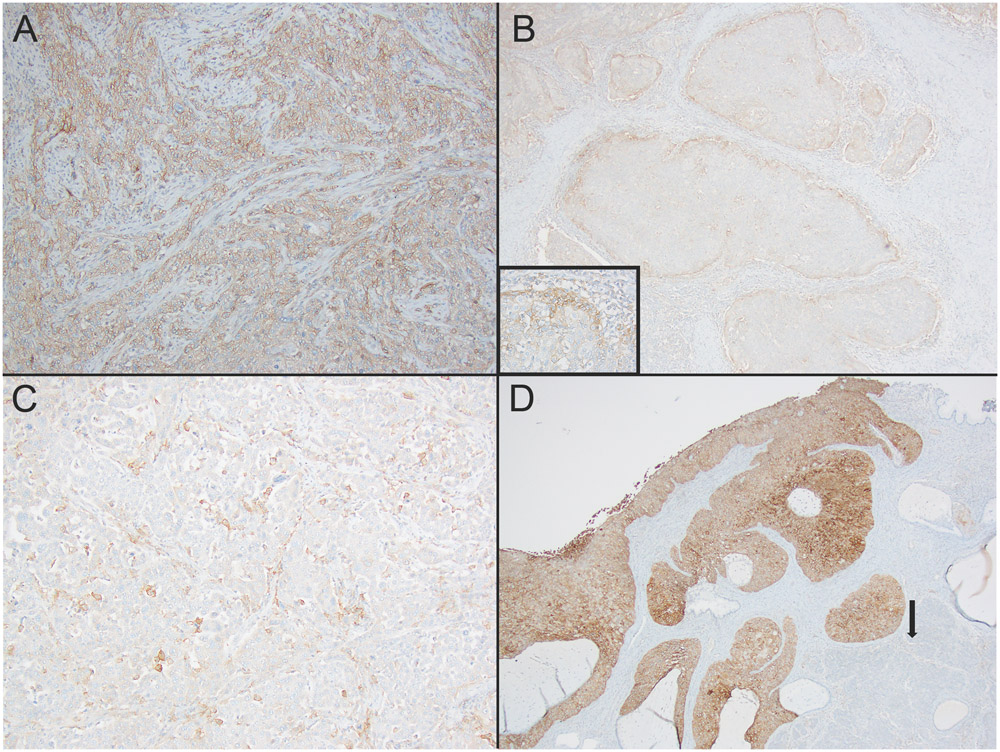
In poorly differentiated CSCC (n=22), the median patient age was 47 (24-73). There were 16 stage I, four stage II and two stage IV disease. In categorical analyses, 8 samples (36%) had less than 1% PD-L1 expression and negative result. Fourteen samples expressed or diffusely expressed PD-L1 antibody (≥1% PD-L1 expression). There was diffuse PD-L1 expression (50% or more membranous positivity) in 5 cases (23%) (Figure 1A). CPS for PD-L1 varied from 0 to 80% (median: 5%). There were 4 cases with 0% CPS. PD-L1 expression was localized around tumor-stroma interface in 5 samples (figure 1B). All five samples had low total PD-L1 scores (5-20%). Remaining twelve cases with less than 50% PD-L1 expression had patchy staining pattern (Figure 1C). Twelve samples out of 22 poorly differentiated CSCC samples had matching positive or negative PD-L1 categorical results with TMA samples. The combined PD-L1 score in tumor and mononuclear inflammatory cells did not correlate between TMA and overlapping full-tissue sections from poorly differentiated CSCC samples. However, there was a positive trend in 22 poorly differentiated carcinoma samples (p=0.07). In one poorly differentiated CSCC sample, diffuse and strong PD-L expression was limited to in-situ carcinoma. Invasive carcinoma component of the sample had 0% PD-L1 expression score (figure 1D). Final result was recorded as 0% for the sample.
Figure 1:
Patterns of PD-L1 expression in Cervical Squamous Cell Carcinoma (CSCC). 1A: diffuse membranous expression (100x). 1B: Localized staining between tumor and stroma (100x). Inset: higher power (200x). 1C: Patchy staining in neoplastic cells (100x). 1D: PD-L1 expression was lost in the invasive component of CSCC (arrow), diffuse and strong expression in squamous carcinoma in situ component (100x).
The median patient age was 56 (34-73) for negative PD-L1 expressing tumors and 36 (31-41) for high PD-L1 (50% or more) expressing tumors. PD-L1 expression levels were correlated with diagnosis at young age when PD-L1 was categorical variable (p=0.028) and also in also CPS analysis (p=0.016). The median tumor size was 2.2 cm for PD-L1 negative tumors and 3.5 cm for PD-L1 expression tumors. The tumor size was correlated with high PD-L1 expression with a borderline significance (p=0.048) in the categorical analysis. The association was stronger in CPS result (p=0.014). Five out of 8 lymph node positive patients had tumor with no PD-L1 expression. None of the patients with diffuse PD-L1 expressing tumors had positive regional lymph nodes. However, negative trend between PD-L1 expression and positive lymph node status was not significant (p=0.07). Out of 22 samples 14 had LVSI. PD-L1 expression was inversely associated with LVSI in both categorical and CPS analysis (p=0.03). Other clinicopathologic parameters such as disease stage and OS did not correlate with PD-L1 expression levels. Table 1 shows summary of clinicopathologic parameters and PD-L1 expression in poorly differentiated CSCC.
Table 1:
Summary of clinicopathologic parameters and PD-L1 expression in poorly differentiated Cervical Squamous Cell Carcinoma.
| No | Age | Stage | Margin Status |
LIV | PD-L1 Tumor |
CPS |
|---|---|---|---|---|---|---|
| 1 | 34 | I | N | Y | N | 5% |
| 2 | 53 | II | N | Y | N | 5% |
| 3 | 36 | I | N | N | E | 40% |
| 4 | 57 | I | N | Y | E | 5% |
| 5 | 38 | I | N | Y | DE | 60% |
| 6 | 36 | I | N | N | DE | 70% |
| 7 | 54 | I | N | Y | E | 5% |
| 8 | 41 | I | N | N | DE | 70% |
| 9 | 61 | IV | N | Y | N | 1% |
| 10 | 62 | II | N | Y | E | 20% |
| 11 | 31 | I | N | N | DE | 60% |
| 12 | 42 | I | N | Y | E | 20% |
| 13 | 61 | IV | N | Y | N | 0% |
| 14 | 42 | I | N | Y | N | 0% |
| 15 | 46 | II | N | Y | E | 15% |
| 16 | 72 | II | Y | Y | E | 5% |
| 17 | 33 | I | N | N | DE | 80% |
| 18 | 24 | I | N | Y | E | 1% |
| 19 | 50 | I | N | Y | N | 0% |
| 20 | 33 | I | N | N | E | 10% |
| 21 | 73 | I | N | N | N | 0% |
| 22 | 59 | I | N | N | N | 5% |
LIV: Lymphovascular Involvement. CPS: Combined positive score.
N: Negative, E: expressed, DE: diffusely expressed. Y: yes, N: no.
High CD8+ T cell density in both tumor and stroma correlates with low-stage disease
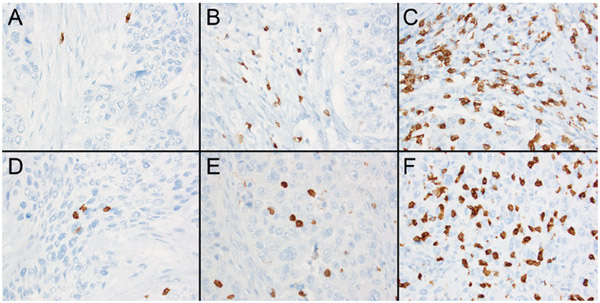
CD8+ TIL density was high (3+) within the neoplastic tissue in 9 Stage I disease. High-CD8 density in surrounding stroma was observed in 16 stage I and 4 stage II diseases. Two stage IV diseases had (1+) tumor and (2+) stromal TIL density (figure 2). CD8+ TIL density both in neoplastic tissue and stroma was positively correlated with low-stage disease. CPS for PD-L1 was associated with CD8+ TIL density in tumor (p=0.04) and stroma (p=0.035). There was no correlation between CD8+ TIL and the patient age. OS rates varied from 9 to 245 months in patients with poorly differentiated CSCC (mean: 113 months). Patients with (1+), (2+) and (3+) CD8 expressing tumors had different OS rates in univariate analysis. In multivariate analysis high CD8+ TIL density in the neoplastic tissue (3+ TIL) was associated with better OS rates after the disease stage was adjusted (p=0.004) (figure 3).
Figure 2:
CD8+ Tumor Infiltrating Lymphocytes. 2A: (1+) staining within the neoplastic tissue (200x) 2B: (2+) staining (200x). 2C: (3+) staining (200x).
FIG. 3.
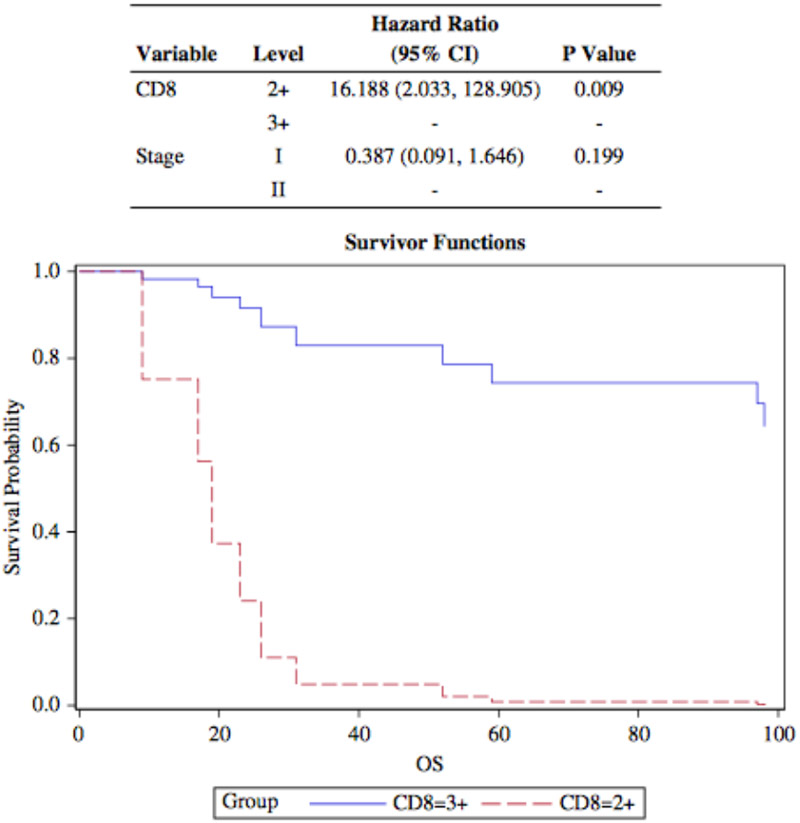
Overall survival (OS) rate and CD8+ tumor-infiltrating lymphocytes (TIL) density in periturmoral stroma. Multivariable analysis shows thay high CD8+ TIL density is associated with better OS rates compared with intermediate TIL levels. CI indicates confidence interval.
VEGF expression was localized to neoplastic tissue and surrounding vasculature. Only marker expression in the neoplastic tissue was evaluated. VEGF expression varied from 0% to 20% (median: 1%). Even though there was a negative trend between VEGF and PD-L1 expression, the expression levels did not correlate in our limited sample size. There was no association between VEGF expression and clinicopathologic parameters.
Discussion
Immunotherapy can potentially be a significant treatment modality in cervical cancer since its oncogenesis involves presence of human papilloma virus (HPV) infection as an etiologic factor 2. Currently clinical trials are in progress to determine efficacy of PD-1/PD-L1 blockers 21 in the advanced cervical cancer. PD-L1 expression by immunohistochemistry has been tested as a biomarker to predict treatment response to anti PD-1/PD-L1 therapy with conflicting results in the literature. Patients with no PD-L1 expressing tumors in other anatomical sites had clinical benefit from anti PD-L1 treatment 22. The diagnostic challenges involved in interpretation of PD-L1 immunostaining include tumor heterogeneity, transient marker expression and lack of standard PD-L1 readings 15. In cervical cancer, CPS evaluation is recommended for the FDA-approved assays. We used both CPS and categorical analysis of PD-L1 expression in the neoplastic tissue. The evaluation of full-tissue sections showed three distinct staining patterns in poorly differentiated carcinoma: diffuse (more than 50% membranous positivity), focal/patchy and interface pattern with low CPS. The tumor-stroma interface staining pattern was recognized by prior investigators 23 and associated with better prognosis compared to diffuse PD-L1 expression in patients with CSCC 24. Only five out of 22 poorly differentiated CSCC cases had diffuse CPS in our samples. The overlapping poorly differentiated CSCC samples from TMA and full-tissue sections did not have matching PD-L1 results. The spatial heterogeneity of marker expression can potentially give positive or negative results depending on the area tested. PD-L1 expression can also be a dynamic or transient process and affected from prior radiation treatment 25. Surgery was the first-line treatment modality in our cohort. Only one patient received radiation therapy before surgical treatment.
The tumor subtype was correlated with the level of PD-L1 expression in the literature. CSCC samples expressed higher PD-L1 levels compared to adenocarcinoma and adenosquamous carcinoma of the cervix 26. We showed poorly differentiated SCCC had higher PD-L1 expression compared to grade II tumors. The result is comparable with poorly differentiated cancers involving bladder 27, endometrium 28, ovary 29 and the lung 30. However, lack of well-differentiated CSCC samples in the TMA is one of the shortcomings of our study. In addition to tumor grade, the disease stage was also correlated with PD-L1 expression in TMA samples. There was a difference in PD-L1 expression between early stage (stage I) and higher stage cancers. The limited sample size of advanced cancers (only 8 patients) hampers our result and a cautious interpretation is required. Another interesting but limited finding involved a sample. There was a loss PD-L1 expression in the invasive carcinoma even though diffuse and strong PD-L1 positivity was noted in carcinoma in situ component. In contrast to our observation a proportional increase in the marker expression with the increasing grade of cervical intraepithelial neoplasia and invasive SCC was reported in a prior study 31.
Among the clinicopathologic parameters, young- age was associated with high PD-L1 expression in poorly differentiated CSCC. Immune-senescence can be a plausible explanation for the finding. In later life, thymic involution and lower amounts of T cell progenitors from bone marrow result in very few naïve T cell production. Elderly people have marked expression of CD27 and CD28-negative CD8+ senescent T cells 32. In a recent metanalysis the efficacy of PD-1/PD-L1 blockers had comparable results in adult patients younger and older than 65 years in current clinical trials involving head and neck, lung and kidney cancers 33. On the other hand, hyper-progressive disease was associated with old age and worse OS in patients with nonsmall cell carcinoma treated with PD1/PD-L1 blockers 34. Other pathologic parameters associated with PD-L1 expressions were tumor size and LVSI. LVSI was positively associated with PD-L1 expression in several cancer types including endometrial 35 and lung cancers 36. The negative correlation with LVSI and PD-L1 expression in our study contradicts prior results. However, the analysis included only poorly differentiated CSCC samples. PD-L1 expression was not correlated with LVSI in TMA samples.
When marker expression levels were compared PD-L1 expression was associated with CD8 levels both in tumor and surrounding stroma of poorly differentiated CSSC samples. In another HPV associated cancer, squamous cell carcinoma of head and neck (SCCHN), CD8+ T cells were found to express higher levels of PD-1 in tumor microenvironment 23. In addition, aggressive tumor morphology was associated with high PD-L1 levels and high CD3 and CD8 positive lymphocytic infiltrates in SCCHN 37. In our poorly differentiated CSSC group, high-CD8+ TIL levels were correlated with low-stage disease and better OS rates even after disease stage was adjusted. In cervical carcinoma, high CD8 levels were reportedly associated with absence of lymph node metastasis 38 and low CD8/treg ratio was associated with poor survival rates 39. CD8+ TIL density likewise was correlated with better survival outcomes in SCCHN 37,40 and nonsmall cell lung cancer 41.
In summary, clinicopathologic features can be important in selecting patients for pembroluzimab treatment in CSCC. PD-L1 expression was higher in grade III CSCC compared to grade II samples. Even though PD-L1 expression was not directly associated with OS tumor size, patient age and LVSI were correlated with PD-L1 expression in poorly differentiated CSCC. CD8+ TIL levels in neoplastic tissue and its surrounding stroma were positively correlated with PD-L1 expression. In poorly differentiated CSCC, PD-L1 expression and CD8+ TIL density may define a subgroup of patients with relatively better survival outcomes. Our results should be validated in a larger patient cohort especially in patients treated with prior CCR therapy.
Acknowledgements
Support for Shared Resources was provided by Cancer Center Support Grant (CCSG) CA076292 to H. Lee Moffitt Cancer Center. This study was supported by R01CA157664, R01CA124515 and U01CA232758.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Yang A, Wu TC, Hung CF. Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. Journal of gynecologic oncology. 2016;27(5):e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma J, Monk BJ, Wolfson AH. New Strategies for Multimodality Therapy in Treating Locally Advanced Cervix Cancer. Seminars in radiation oncology. 2016;26(4):344–348. [DOI] [PubMed] [Google Scholar]
- 4.Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. American journal of obstetrics and gynecology. 2016;214(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marnitz S, Kohler C, Muller M, Behrens K, Hasenbein K, Schneider A. Indications for primary and secondary exenterations in patients with cervical cancer. Gynecologic oncology. 2006;103(3):1023–1030. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg GL, Sukumvanich P, Einstein MH, Smith HO, Anderson PS, Fields AL. Total pelvic exenteration: the Albert Einstein College of Medicine/Montefiore Medical Center Experience (1987 to 2003). Gynecologic oncology. 2006;101(2):261–268. [DOI] [PubMed] [Google Scholar]
- 7.Boussios S, Seraj E, Zarkavelis G, et al. Management of patients with recurrent/advanced cervical cancer beyond first line platinum regimens: Where do we stand? A literature review. Critical reviews in oncology/hematology. 2016;108:164–174. [DOI] [PubMed] [Google Scholar]
- 8.Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(28):4649–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tewari KS, Sill MW, Long HJ 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. The New England journal of medicine. 2014;370(8):734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monk BJ, Mas Lopez L, Zarba JJ, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(22):3562–3569. [DOI] [PubMed] [Google Scholar]
- 11.Tinker AV, Ellard S, Welch S, et al. Phase II study of temsirolimus (CCI-779) in women with recurrent, unresectable, locally advanced or metastatic carcinoma of the cervix. A trial of the NCIC Clinical Trials Group (NCIC CTG IND 199). Gynecologic oncology. 2013;130(2):269–274. [DOI] [PubMed] [Google Scholar]
- 12.Yang A, Farmer E, Lin J, Wu TC, Hung CF. The current state of therapeutic and T cell-based vaccines against human papillomaviruses. Virus research. 2017;231:148–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagae S, Monk BJ, Pujade-Lauraine E, et al. Advances and Concepts in Cervical Cancer Trials: A Road Map for the Future. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2016;26(1):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nature immunology. 2007;8(3):239–245. [DOI] [PubMed] [Google Scholar]
- 15.Xu-Monette ZY, Zhang M, Li J, Young KH. PD-1/PD-L1 Blockade: Have We Found the Key to Unleash the Antitumor Immune Response? Frontiers in immunology. 2017;8:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riley JL. PD-1 signaling in primary T cells. Immunological reviews. 2009;229(1):114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England journal of medicine. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 18.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England). 2016;387(10027):1540–1550. [DOI] [PubMed] [Google Scholar]
- 19.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. Journal for immunotherapy of cancer. 2018;6(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England journal of medicine. 2003;348(3):203–213. [DOI] [PubMed] [Google Scholar]
- 21.Borcoman E, Le Tourneau C. Pembrolizumab in cervical cancer: latest evidence and clinical usefulness. Therapeutic advances in medical oncology. 2017;9(6):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. The Lancet Oncology. 2016;17(12):e542–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer research. 2013;73(6):1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heeren AM, Punt S, Bleeker MC, et al. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2016;29(7):753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen MJ, Xu LJ, Yang L, et al. Radiation alters PD-L1/NKG2D ligand levels in lung cancer cells and leads to immune escape from NK cell cytotoxicity via IL-6-MEK/Erk signaling pathway. Oncotarget. 2017;8(46):80506–80520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy OL, Shintaku PI, Moatamed NA. Programmed death-ligand 1 (PD-L1) is expressed in a significant number of the uterine cervical carcinomas. Diagnostic pathology. 2017;12(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawahara T, Ishiguro Y, Ohtake S, et al. PD-1 and PD-L1 are more highly expressed in high-grade bladder cancer than in low-grade cases: PD-L1 might function as a mediator of stage progression in bladder cancer. BMC urology. 2018;18(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Joehlin-Price AS, Rhoades J, et al. Programmed Death Ligand 1 Expression Among 700 Consecutive Endometrial Cancers: Strong Association With Mismatch Repair Protein Deficiency. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2018;28(1):59–68. [DOI] [PubMed] [Google Scholar]
- 29.Wieser V, Gaugg I, Fleischer M, et al. BRCA1/2 and TP53 mutation status associates with PD-1 and PD-L1 expression in ovarian cancer. Oncotarget. 2018;9(25):17501–17511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Driver BR, Miller RA, Miller T, et al. Programmed Death Ligand-1 (PD-L1) Expression in Either Tumor Cells or Tumor-Infiltrating Immune Cells Correlates With Solid and High-Grade Lung Adenocarcinomas. Archives of pathology & laboratory medicine. 2017;141(11):1529–1532. [DOI] [PubMed] [Google Scholar]
- 31.Yang W, Lu YP, Yang YZ, Kang JR, Jin YD, Wang HW. Expressions of programmed death (PD)-1 and PD-1 ligand (PD-L1) in cervical intraepithelial neoplasia and cervical squamous cell carcinomas are of prognostic value and associated with human papillomavirus status. The journal of obstetrics and gynaecology research. 2017;43(10):1602–1612. [DOI] [PubMed] [Google Scholar]
- 32.Henson SM, Riddell NE, Akbar AN. Properties of end-stage human T cells defined by CD45RA re-expression. Current opinion in immunology. 2012;24(4):476–481. [DOI] [PubMed] [Google Scholar]
- 33.Elias R, Giobbie-Hurder A, McCleary NJ, Ott P, Hodi FS, Rahma O. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. Journal for immunotherapy of cancer. 2018;6(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA oncology. 2018;4(11):1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crumley S, Kurnit K, Hudgens C, Fellman B, Tetzlaff MT, Broaddus R. Identification of a subset of microsatellite-stable endometrial carcinoma with high PD-L1 and CD8+ lymphocytes. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi X, Wu S, Sun J, Liu Y, Zeng X, Liang Z. PD-L1 expression in lung adenosquamous carcinomas compared with the more common variants of non-small cell lung cancer. Scientific reports. 2017;7:46209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karpathiou G, Casteillo F, Giroult JB, et al. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: Immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget. 2017;8(12):19310–19322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piersma SJ, Jordanova ES, van Poelgeest MI, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer research. 2007;67(1):354–361. [DOI] [PubMed] [Google Scholar]
- 39.Jordanova ES, Gorter A, Ayachi O, et al. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(7):2028–2035. [DOI] [PubMed] [Google Scholar]
- 40.Ou D, Adam J, Garberis I, et al. Clinical relevance of tumor infiltrating lymphocytes, PD-L1 expression and correlation with HPV/p16 in head and neck cancer treated with bio- or chemo-radiotherapy. Oncoimmunology. 2017;6(9):e1341030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Guindy DM, Helal DS, Sabry NM, Abo El-Nasr M. Programmed cell death ligand-1 (PD-L1) expression combined with CD8 tumor infiltrating lymphocytes density in non-small cell lung cancer patients. Journal of the Egyptian National Cancer Institute. 2018;30(4):125–131. [DOI] [PubMed] [Google Scholar]