Abstract
Background:
Rates of cannabis use among patients receiving methadone maintenance therapy are high, and cannabis use may be associated with outcomes of methadone maintenance therapy. We examined the effect of cannabis use on opioid use in patients receiving methadone maintenance therapy to test the hypothesis that cannabis use is associated with a reduction in opioid use.
Methods:
In this systematic review, we searched MEDLINE/PubMed, Embase, PsycINFO, CINAHL and ProQuest Dissertations and Theses Global from inception to July 12, 2018. We summarized the effects of cannabis use on opioid use during methadone maintenance therapy and treatment retention. We conducted meta-analyses using a random effects model.
Results:
We included 23 studies in our review. We performed a meta-analysis of 6 studies, with a total number of participants of 3676, examining use of cannabis and opioids during methadone maintenance therapy. Owing to high heterogeneity, we described the studies qualitatively but provide the forest plots as supplemental material. The overall quality of evidence was very low, with a high risk of bias, owing to the nature of observational studies.
Interpretation:
We found no consensus among studies that cannabis use is associated with reduced opioid use or longer treatment retention when used during methadone maintenance therapy in patients with opioid use disorder. PROSPERO Registration: CRD42015029372
The opioid epidemic related to an increase in prescriptions1–4 has escalated in the past 2 decades. Opioid use disorder is a fundamental component of this crisis.5,6 Despite the morbidity and mortality due to opioid use disorder, high rates of HIV infection and hepatitis C among patients with the disorder and high unemployment rates, treatment options are limited in scope and effectiveness.7,8 Methadone maintenance therapy is the most common treatment for opioid use disorder,9,10 although other treatments are available.11 Despite the reported benefits of methadone maintenance therapy in managing opioid use disorder, the number of patients receiving this therapy who continue to use illicit opioids is high.12 The rates of cannabis use among patients receiving methadone maintenance therapy are higher than those in the general population: about a third of Canadians have used cannabis once in their lifetime,13 whereas 59.7% of males and 43.5% of females receiving methadone maintenance therapy reported having used cannabis.14–16 Research suggests that polydrug use (aside from cannabis) is prevalent in the methadone maintenance therapy population.17,18 There is limited evidence to suggest that cannabis use may reduce opioid use in pain management19 but not in opioid use disorder.20 Studies have shown that, in US states with dispensary-based medical cannabis laws, fewer prescription opioids are dispensed, 21 and there are fewer opioid-related deaths.22 The US Department of Veterans Affairs has suggested that cannabis should be legalized not only as a mechanism to lower prescription opioid use but also to manage opioid withdrawal. 23 This report made headlines labelling cannabis as an “exit drug.”23
However, the “exit hypothesis” — the idea that cannabis can be used to manage withdrawal symptoms and therefore help patients with opioid use disorder to stop using opioids – has not been examined systematically. With the legalization of cannabis in Canada, this question is relevant. We examined the relation between cannabis use and opioid use during methadone maintenance therapy. We focused our question on methadone maintenance therapy as it is the most commonly used treatment for opioid use disorder. We sought to determine 1) whether patients using cannabis during methadone maintenance therapy have lower rates of opioid use during therapy and 2) whether cannabis use improves treatment retention in opioid use disorder.
Methods
This review is presented according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.24 It is registered with PROSPERO (no. CRD42015029372). The protocol with detailed methods was published elsewhere.15 The protocol included our target population, intervention, comparison, outcomes, search terms and a detailed search strategy (Supplemental Table S1, Appendix 1, available at www.cmajopen.ca/content/7/4/E665/suppl/DC1). We provide a summary of the methods below.
Information sources and study selection
We searched MEDLINE/PubMed, Embase, PsycINFO, CINAHL and ProQuest Dissertations and Theses Global from inception to July 12, 2018 for relevant studies. We applied no language or demographic restrictions. We formulated our study question using the PICO (Patient, Intervention, Comparison, Outcome) model: In patients with opioid use disorder receiving methadone maintenance treatment, is cannabis use associated with illicit opioid use, treatment retention, polydrug use, criminal activity or risk behaviours for HIV infection (injection drug use, needle sharing and unprotected sex)? We included both studies with observational designs and those with randomized controlled trial designs.
Data collection
Included studies looked at the association between cannabis use and outcomes of methadone maintenance therapy. To be included, a study had to measure methadone maintenance therapy outcomes by reporting participants’ continued opioid use during treatment or treatment retention rates by cannabis use. We excluded studies in which other treatments for opioid use disorder such as buprenorphine were used. We included only methadone maintenance therapy because it was the most commonly used treatment and to avoid the heterogeneity that would have resulted from including different treatment interventions. All articles were screened independently in duplicate at all stages, including data extraction (H.M., C.L., L.Z., M.B. and X.M.Z.). We measured interrater agreement with the κ statistic. Risk of bias was assessed independently in duplicate (H.M., C.L., L.Z., M.B. and X.M.Z.) with the use of the modified Newcastle–Ottawa Scale, as all our included studies were observational by design.25 We measured the overall quality of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework.26
Statistical analysis
As per our protocol, the intent was to meta-analyze the primary studies and provide quantitative summary estimates. However, when we performed meta-analyses, using random effects taking into account heterogeneity expected in observational studies, the high heterogeneity precluded meaningful conclusions, and therefore we provide a description of the included studies and their findings qualitatively. We determined heterogeneity using the I2 statistic; a value greater than 40% indicated high heterogeneity.27
We used RevMan version 5.3 software (Cochrane Community) for the meta-analyses.28 Some studies measured cannabis use both before and during treatment, and we chose to use the in-treatment cannabis measurement, as we were interested in how current cannabis use affects outcomes of current methadone maintenance therapy. In studies that included multiple follow-up time points for the outcome measurement, we included the latest follow-up point in the meta-analysis. We did not assess publication bias, as it has been shown that funnel plots do not accurately depict publication bias for meta-analyses that have fewer than 10 studies.29 However, we provide funnel plots of publication bias for illicit opioid use and treatment retention in Supplemental Figures S1 and S2, respectively, Appendix 1.
We performed a sensitivity analysis by excluding all studies with Newcastle–Ottawa Scale scores of 0 or 1 on individual questions. We performed further subgroup by country and method of cannabis use measurement (subjective v. objective) to further explain any significant heterogeneity found. We were unable to perform subgroup analyses by length of follow-up owing to the limited number of studies.
Ethics approval
As this study was solely literature based, it was not eligible for institutional ethics approval, and none was sought.
Results
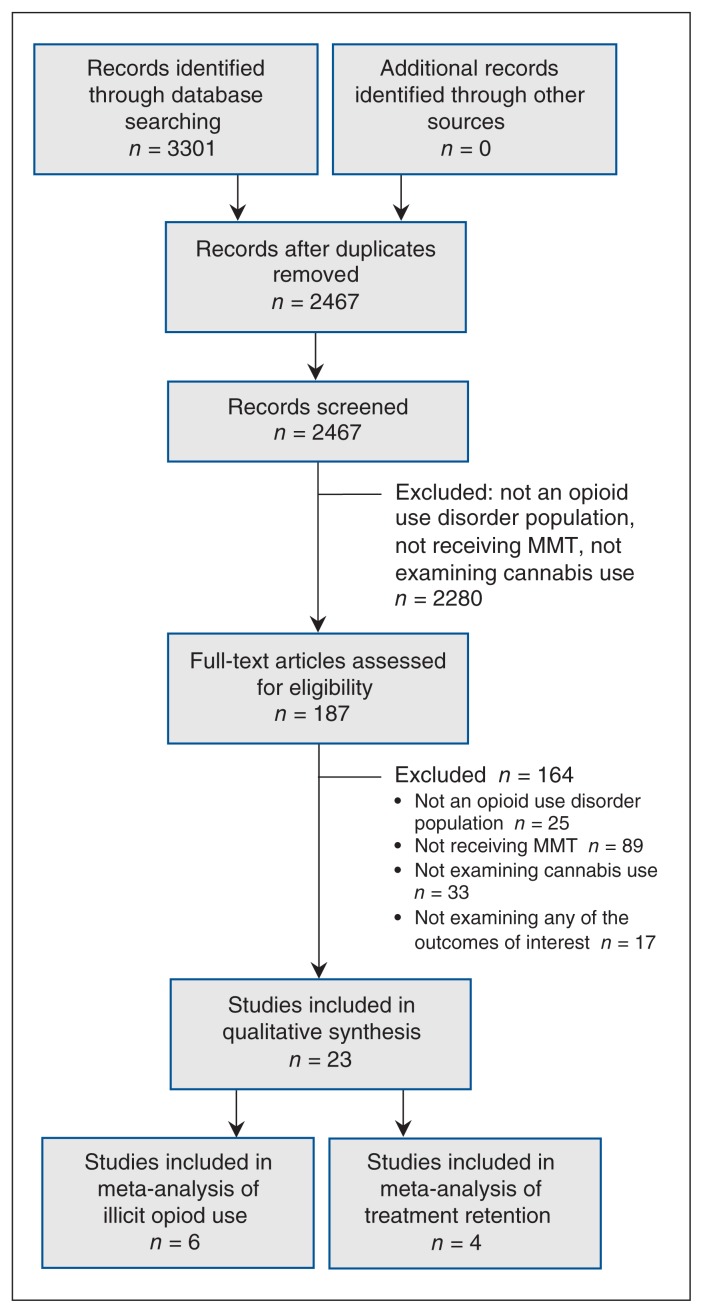
Among the 2467 unique citations screened, 23 studies were included in the qualitative synthesis (Figure 1).13,14,17,20,30–48 Interrater agreement was acceptable for both title/abstract (κ = 0.63, 95% confidence interval 0.57–0.69) and full-text screening (κ = 0.60, 95% confidence interval 0.45–0.74). Although we did not apply any age restrictions, all studies were of adult populations. In studies that reported the proportion of participants with any recent or current (i.e., not lifetime measurements) cannabis use, the prevalence varied from 11.2% to 78.6%.35,41 As the meta-analyses had high heterogeneity, we present forest plots of illicit opioid use during treatment by cannabis use and treatment retention in Supplemental Figures S3 and S4, respectively, Appendix 1.
Figure 1:
Flow diagram showing selection of included studies. Note: MMT = methadone maintenance treatment.
Of the 23 studies, 12 looked at continued opioid use,12–14,30–38 11 examined treatment retention in relation to using cannabis during methadone maintenance therapy,13,17,31,32,34,39–43,46 10 looked at polydrug use,13,30,31,35,37,43–47 2 looked at criminal activity,31,48 and 1 investigated risk behaviours for HIV infection. 43 Additional details, including which variables each study controlled for, are provided in Table 1. We attempted to obtain data necessary to calculate odds ratios when such data were not included in a study. We contacted 12 authors for missing data, but our requests were unmet. Additional details by outcome are presented in Supplemental Table S2, Appendix 1.
Table 1:
Study characteristics
| Investigator, year | Country | Study design | Sample size (% female) | Definition of cannabis use* | Outcome(s) of interest | Statistical analysis | Findings |
|---|---|---|---|---|---|---|---|
| Best et al.,30 1999 | UK | Cross-sectional | 200 (30) | Categorical: daily users, occasional users (not every day in previous month), nonusers | Illicit opioid use, polydrug use | ANOVA, post hoc Scheffe test, linear regression Factors adjusted for: use of various substances, appetite, overall health, depression, anxiety |
Cannabis nonusers had more occasions of heroin use than occasional and daily users Cannabis nonusers consumed significantly more alcohol and crack cocaine than occasional and daily users |
| Epstein et al.,31 2003 | US | Secondary analysis (3 separate analyses), 12 mo | 408 (40.4) | Dichotomized cannabis use and cannabis abuse/dependence diagnosis | Illicit opioid use, treatment retention, polydrug use, criminal activity | Cox proportional hazards regression Factors adjusted for: not stated Confounders in the regression |
No significant association between cannabis use and illicit opioid use No significant association between cannabis use and treatment retention Suggests an association between cocaine abstinence and cannabis use Cannabis use category not associated with any differences in criminal activity |
| Levine et al.,32 2015 | US | Retrospective cohort, 1 yr | 290 (40.3) | Dichotomized cannabis use | Illicit opioid use, treatment retention | Logistic regression Factors adjusted for: total years of use of various substances |
Not significant, but statistics not reported Cannabis-negative urine associated with treatment retention in both men and women |
| Lions et al.,33 2014 | France | Secondary RCT analysis, 45 wk | 158 (15.2) | Dichotomized: daily users v. nondaily users | Illicit opioid use | Univariate logistic regression Factors adjusted for: not stated |
Pretreatment daily cannabis: OR 1.46 (95% CI 0.61–3.77), NS In-treatment daily cannabis: OR 2.81 (95% CI 1.22–6.48) |
| Nava et al.,34 2007 | Italy | Prospective cohort, 12 mo | 121 (14) | Dichotomized: long-term users (> 6 mo) and currently smoking at least 7 times per week v. nonusers never exposed to marijuana smoking | Illicit opioid use, treatment retention | Hierarchical linear modelling Factors adjusted for: not stated |
Significant association between cannabis use and illicit opioid use No significant association between cannabis use and treatment retention |
| Nirenberg et al.,35 1996 | US | Prospective cohort, 6 mo | 70 (1.4) | Dichotomized cannabis use and categorical (4 groups) | Illicit opioid use, polydrug use | ANOVA Factors adjusted for: not stated |
No significant association between cannabis use and illicit opioid use No significant difference in use of cocaine or benzodiazepine between 4 cannabis groups |
| Proctor et al.,36 2016† | US | Retrospective cohort, 12 mo | 2410 (40.4) | Dichotomized cannabis use | Illicit opioid use | Logistic regression Factors adjusted for: age, gender, employment status, ethnicity, marital status, average daily methadone dosage |
Intake cannabis values in relation to opioid use at 4 time points: 3 mo: OR 1.17 (95% CI 0.83–1.63); 6 mo: OR 0.59 (95% CI 0.32–1.10); 9 mo: OR 0.63 (95% CI 0.24–1.66); 12 mo: OR 0.23 (95% CI 0.05–1.16) |
| Saxon et al.,37 1996 | US | Prospective cohort, 18 mo | 353 (38.2) | Categorical: 7-point scale ranging from 0 (never) to 6 (≥ 4 times per day) | Illicit opioid use, treatment retention, polydrug use | Cox regression model Factors adjusted for: age, gender, previous methadone treatment, substance use |
No significant association between cannabis use, and illicit opioid use or treatment retention Significant association between cannabis use and cocaine use |
| Scavone et al.,13 2013 | US | Retrospective cohort, 9 mo | 91 (36.6) | Dichotomized cannabis use | Illicit opioid use, treatment retention, polydrug use | ANCOVA, parallel ANCOVA Factors adjusted for: daily opioid expenditure |
No significant relation between frequency of cannabis use in treatment and opiate use No significant association between cannabis use and treatment retention Correlation between rates of cannabis use and illicit benzodiazepine use |
| Somers et al.,38 2012 | Ireland | Retrospective cohort, 15 mo | 123 (NR) | Dichotomized cannabis use | Illicit opioid use | Logistic regression Factors adjusted for: variables that were significant from univariate analysis, did not explicitly state which ones |
Baseline: OR 0.88 (95% CI 0.67–1.15); 3 mo: OR 0.79 (95% CI 0.58–1.10); 9 mo: OR 0.78 (95% CI 0.55–1.20); 15 mo: OR 1.45 (95% CI 0.82–2.50); Total: adjusted OR 0.32 (95% CI 0.06–1.66) |
| Wasserman et al.,20 1998 | US | Prospective cohort, 6 mo | 74 (40.5) | Dichotomized cannabis use | Illicit opioid use | Cox proportional hazards regression Factors adjusted for: abstinence goals, positive moods, pleasant events, negative moods, life events, perceived stress, opioid withdrawal symptoms |
Significant association between cannabis use and illicit opioid use |
| Zielinski et al.,14 2017 | Canada | Cross-sectional | 777 (46.7) | Dichotomized cannabis use in previous 30 d | Illicit opioid use | Multivariable logistic regression analysis Factors adjusted for: age, gender, methadone dosage, treatment duration |
No significant association between cannabis use and illicit opioid use |
| Joe et al.,39 1998 | US | Prospective cohort, 360 d | 981 (39) | Dichotomized: at least weekly marijuana use or not | Treatment retention | Hierarchical linear regression model Factors adjusted for: age, ethnicity, marital status, legal status, employment status, number of lifetime arrests |
No significant association between cannabis use and treatment retention |
| Peles et al.,40 2006 | Israel | Prospective cohort, 11 yr | 492 (27.2) | Dichotomized cannabis use | Treatment retention | Fisher exact test, Cox regression analysis Factors adjusted for: age, children, methadone dosage, use of various substances |
No significant association between cannabis use and treatment retention |
| Peles et al.,41 2008 | US, Israel | Prospective cohort, 12 mo | 794 (31.0) | Dichotomized cannabis use | Treatment retention | Kaplan–Meier survival analysis with log rank for cumulative retention, Cox regression Factors adjusted for: methadone dosage, age |
No significant association between cannabis use and treatment retention |
| Schiff et al.,42 2007 | Israel | Retrospective cohort, 13 mo | 2683 (14.1) | Dichotomized cannabis use | Treatment retention | Logistic regression Factors adjusted for: age, gender |
Significant relation between cannabis use and increased treatment retention |
| Weizman et al.,43 2004 | Israel | Prospective cohort, 12 mo | 283 (NR) | Dichotomized: cannabis abuse v. not | Treatment retention, polydrug use, risk behaviours for HIV infection | Cox regression survival analysis Factors adjusted for: heroin, cocaine and benzodiazepine abuse |
No significant association between cannabis use and treatment retention Significant association between cannabis use and use of benzodiazepine, amphetamine and cocaine Cannabis use was not related to any risk behaviours (statistics not reported) |
| White et al., 201417 | US | Retrospective cohort, 15–17 mo | 604 (39.4) | Dichotomized cannabis use | Treatment retention | χ2 test, Fisher exact test Factors adjusted for: not stated |
At baseline, cannabis use was significantly associated with treatment retention |
| Bleich et al.,44 1999 | Israel | Prospective cohort, 12 mo | 148 (29.8) | Positive result of urine test for cannabis during 12th mo of treatment‡ | Polydrug use | χ2 test Factors adjusted for: not stated |
Benzodiazepine abusers were more likely to currently abuse cannabis than nonabusers of benzodiazepine |
| Peirce et al.,45 2009 | US | Secondary RCT analysis, 12 wk | 386 (44) | Cannabis use, defined as positive result of testing of urine/breath sample obtained at study intake | Polydrug use | Mixed-model regression Factors adjusted for: age, gender, ethnicity, employment status, criminal activity, additional demographic factors |
Significant association between cannabis use and stimulant use |
| Saxon et al.,46 1993 | US | Cross-sectional | 98 (0) | Dichotomized cannabis use | Polydrug use | Mann–Whitney test Factors adjusted for: not stated |
Significant association between cannabis use and other drug use |
| Strain et al.,47 1991 | US | Cross-sectional | 66 (45) | Dichotomized: those with v. without history of cannabis use diagnosis | Polydrug use |
Z-test Factors adjusted for: not stated |
No significant association between cannabis use and use of alcohol, sedatives and cocaine |
| Bell et al.,48 1997 | Australia | Prospective cohort, 12 mo | 304 (43.1) | Continuous: average daily use of cannabis in previous month | Criminal activity | Multiple linear regression Factors adjusted for: age, gender, employment, benzodiazepine use, cost of drugs |
Cannabis use was significant predictor of criminal activity at 12 mo |
Note: ANCOVA = analysis of covariance, ANOVA = analysis of variance, CI = confidence interval, NR = not reported, NS = not significant, OR = odds ratio, RCT = randomized controlled trial.
“Dichotomized cannabis use” means users versus nonusers or at least 1 positive urine screen result versus none, unless specified otherwise.
This study had too many results to present in this table, so we included only intake cannabis values in relation to opioid use at all time points. See study for more results.
An abuser of any substance of abuse was defined as having a positive urine test result for that substance during the 12th month of treatment.
All the studies had a moderate or high risk of bias on at least 1 Newcastle–Ottawa Scale criterion. Eight studies did not adjust for any confounding variables.17,31,33–35,44,46,47 Complete details of the Newcastle–Ottawa Scale ratings are provided in Table 2.
Table 2:
Risk of bias assessment using the modified Newcastle–Ottawa Scale
| Investigator | Selection bias: is source population representative? | Performance bias | Detection bias | Information bias | Total score* | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Is sample size sufficient, is there sufficient power? | Did study adjust for confounders? | Did study use appropriate statistical analysis? | Are there few missing data, was this handled appropriately? | Outcome measurement appropriate? | Objective assessment of outcome of interest? | |||
| Bell et al.48 | 2 | 2 | 3 | 3 | 2 | 2 | 2 | 16 |
|
| ||||||||
| Best et al.30 | 2 | 3 | 2 | 1 | 1 | 2 | 0 | 11 |
|
| ||||||||
| Bleich et al.44 | 1 | 1 | 0 | 1 | 1 | 1 | 3 | 8 |
|
| ||||||||
| Epstein et al.31 | 0 | 1 | 0 | 3 | 2 | 3 | 3 | 12 |
|
| ||||||||
| Joe et al.39 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 20 |
|
| ||||||||
| Levine et al.32 | 2 | 2 | 2 | 2 | 1 | 3 | 3 | 15 |
|
| ||||||||
| Lions et al.33 | 1 | 2 | 0 | 2 | 1 | 3 | 2 | 11 |
|
| ||||||||
| Nava et al.34 | 0 | 1 | 0 | 2 | 1 | 2 | 3 | 9 |
|
| ||||||||
| Nirenberg et al.35 | 2 | 1 | 0 | 1 | 1 | 3 | 3 | 11 |
|
| ||||||||
| Peirce et al.45 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 19 |
|
| ||||||||
| Peles et al.40 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 21 |
|
| ||||||||
| Peles et al.41 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 21 |
|
| ||||||||
| Proctor et al.36 | 3 | 3 | 3 | 1 | 1 | 3 | 3 | 17 |
|
| ||||||||
| Saxon et al.46 | 1 | 1 | 0 | 1 | 1 | 2 | 3 | 9 |
|
| ||||||||
| Saxon et al.37 | 2 | 2 | 3 | 2 | 2 | 2 | 3 | 16 |
|
| ||||||||
| Scavone et al.13 | 1 | 1 | 3 | 1 | 2 | 1 | 2 | 11 |
|
| ||||||||
| Schiff et al.42 | 3 | 3 | 2 | 3 | 2 | 3 | 3 | 19 |
|
| ||||||||
| Somers et al.38 | 2 | 1 | 1 | 1 | 2 | 1 | 3 | 11 |
|
| ||||||||
| Strain et al.47 | 2 | 0 | 0 | 1 | 2 | 2 | 1 | 8 |
|
| ||||||||
| Wasserman et al.43 | 2 | 0 | 3 | 3 | 3 | 3 | 3 | 17 |
|
| ||||||||
| Weizman et al.43 | 2 | 2 | 2 | 1 | 1 | 1 | 3 | 12 |
|
| ||||||||
| White et al.17 | 2 | 3 | 0 | 1 | 2 | 2 | 3 | 13 |
|
| ||||||||
| Zielinski et al.14 | 3 | 3 | 3 | 3 | 1 | 2 | 3 | 18 |
0 = definitely no (high risk of bias), 1 = mostly no (met a little of the criterion), 2 = mostly yes (met most of the criterion), 3 = definitely yes (low risk of bias). Maximum total score 21.
Continued opioid use
Twelve studies examined the relation between cannabis use and continued opioid use, with a total sample size of 3676.13,14,20,30–36,38,46 The majority of the studies showed no association between cannabis use and opioid use (Table 1).
There was significant heterogeneity among the studies. These results did not change when we excluded studies with a high risk of bias. Subgroup analyses by country and objective v. subjective measurement of cannabis did not reduce the heterogeneity. We present additional information on these studies in Supplemental Figures S3 and S4, Appendix 1.
The overall quality of evidence was very low, with critical issues of inconsistency and imprecision (Table 3), in addition to a moderate risk of bias. Owing to the nature of observational study designs, GRADE ratings of quality start at low, and any additional concerns in quality assessment make the quality very low.
Table 3:
Grading of Recommendations Assessment, Development and Evaluation evidence profile for primary outcomes
| Outcome, no. of studies | Quality assessment | Quality | Importance | |||||
|---|---|---|---|---|---|---|---|---|
| Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | |||
| Illicit opioid use, 7 | Observational studies | Serious* | Very serious†‡ | Not serious | Very serious§ | None | ⊕○○○ VERY LOW |
CRITICAL |
| Retention, 4 | Observational studies | Not serious | Serious† | Not serious | Very serious§ | None | ⊕○○○ VERY LOW |
CRITICAL |
Moderate risk of bias across studies.
Point estimates vary widely across studies, little overlap between individual confidence intervals.
Heterogeneity not explained by subgroup analyses.
Small samples, wide pooled 95% confidence interval.
Treatment retention
Eleven studies investigated cannabis use and retention in methadone maintenance therapy.13,17,31,32,34,39–43,46 Significant heterogeneity was seen (I2 = 90%). A sensitivity analysis conducted by excluding studies with high risk of bias to explain heterogeneity did not change the result. In the subgroup analysis by country, we found that studies conducted in the United States showed cannabis use to be significantly associated with decreased retention rates, whereas those conducted in Israel showed the opposite direction. Subgroup analyses by country had an I2 of 0%, indicating no heterogeneity. The overall quality of evidence was low, with quality issues related to inconsistency and imprecision. The funnel plot is presented in Supplemental Figure S2, Appendix 1.
Secondary outcome measures
Originally, we had aimed to further investigate the association between cannabis use and polydrug use, criminal activity, and risk behaviours for HIV infection and viral hepatitis. For polydrug use, we included 10 studies that investigated the association between cannabis use and use of cocaine, benzodiazepines, alcohol and various forms of cannabis.13,30,31,35,37,43–48 Owing to the various substances included and outcome measurements, we were unable to combine results for a metaanalysis. Two studies reporting on criminal activity could not be quantitatively analyzed.31,48 One study reported on cannabis use and HIV infection.43
Interpretation
We included 23 studies that examined the association between cannabis use and opioid use and retention in methadone maintenance therapy. Meta-analysis of 6 of these studies showed high heterogeneity that affected the interpretation of the results. The overall quality of evidence was low, with high risk of bias. The results from individual studies suggest that cannabis use may potentially have no effect on opioid use in patients receiving methadone maintenance therapy. The results for treatment retention are inconclusive. We observed a difference in the association between cannabis use and retention in methadone maintenance therapy in a subgroup analysis by country, specifically between studies conducted in the US and Israel. A previous study showed differences in retention in opioid use disorder treatment between Israel and the US, arguably due to various sociodemographic factors.41 However, given the conflicting findings by region, the overall effect of cannabis use on treatment retention in opioid use disorder is unknown.
The differences between our results and those of other investigators likely reflect how cannabis use was measured. For instance, studies in our review dichotomized cannabis use in some way, categorizing people based on any cannabis use versus no use or grouping those who used more than a certain amount of cannabis versus those who used less. This choice of measurement was likely made because estimating the amount and type/strength/concentration of cannabis is challenging, 49 and it makes the establishment of a dose–response relation in any of these studies impossible.50 This choice could obscure an association in either direction. Heavy use of cannabis has been reported to be associated with adverse health effects.51,52 The studies in our systematic review did not distinguish between cannabis use disorder and recreational cannabis use. Compared to recreational users, patients with cannabis use disorder have high rates of comorbid psychiatric disorders,53 which are associated with poorer treatment outcomes.54 Peirce and colleagues45 suggested that a diagnosis of cannabis use disorder in the previous 12 months is associated with less stimulant drug use during methadone maintenance therapy, whereas recent (in the previous month) cannabis use is associated with more stimulant use. Their study shows that the recency of cannabis use may affect the use of other drugs during methadone maintenance therapy. It is possible that patients in that study who used cannabis had more severe opioid-related problems or other factors driving their use, such as pain, which would have created a confounding effect; hence, the differences seen in the association of cannabis use with opioid use may have varied by the population of interest (e.g., pain or addiction cohorts).
Our findings suggest that cannabis use does not affect treatment outcomes for patients receiving methadone maintenance therapy. In these cases, it was not an exit drug. The broad negative health effects of heavy cannabis use have been well documented. 51,52 We should continue to counsel patients on the potential risks of cannabis use while emphasizing that we have no evidence to support the use of cannabis in opioid use disorder treatment. Previous studies showed that, in jurisdictions with medical cannabis laws, fewer prescription opioids were issued.21,22 Even in populations with pain conditions and not opioid addiction, those observations do not show that a reduction in opioid prescription was solely due to cannabis as a replacement for opioids.21,22 A national cohort study investigating cannabis use in patients prescribed opioids for pain showed that cannabis use did not reduce opioid use and was associated with worse pain control and psychiatric symptoms.55
Further studies are needed to address the notion of cannabis use as a substitute for opioid use, whether for pain or for opioid use disorder. We need more research to understand the relations between opioid use, treatment outcomes and cannabis use in opioid use disorder. More investigation is needed to reconcile the findings of the prescription pattern studies with those of the patient population studies. The observational nature of the studies means that confounders such as the association of cannabis use with severity of opioid addiction or pain could obscure significant relations. Further research should include detailed definitions of cannabis use.
Limitations
There are limitations to be taken into consideration when interpreting our findings. The results come from small to medium-sized observational studies with limited data on confounding variables. For example, cannabis is seldom used alone; it is associated with polydrug use and with comorbid substance use disorders in patients receiving methadone maintenance therapy.43,56,57 Polydrug use and substance use disorders are associated with poorer outcomes in opioid use disorder.58 There were also methodological limitations. Our meta-analyses had substantial heterogeneity, partly because of methodological variability: there were differences in methadone maintenance therapy duration, associated psychosocial interventions, definition of cannabis use and outcome measures. Our subgroup analyses did not explain this heterogeneity for continued opioid use. The limited number of studies included in the meta-analyses precluded further subgroup analyses to identify other possible sources of heterogeneity, such as length of follow-up. Given the nature of observational studies, there are likely to be other sources of heterogeneity that cannot be detected because unknown confounding variables affect the outcomes. Finally, we were unable to do a complete grey literature search.
Conclusion
We found no consensus among studies that cannabis use is associated with reduced opioid use or longer treatment retention when used during methadone maintenance therapy in patients with opioid use disorder. The study limitations must be taken into account when interpreting these results. Further studies are needed to address and examine the notion of cannabis use and its effect on treatment outcomes in patients with opioid use disorder.
Supplementary Material
Footnotes
Competing interests: Laura Zielinski reports personal fees from Canadian Cannibis Clinics. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Heather McBrien and Zainab Samaan contributed to the study conception. Heather McBrien developed the search strategy. Heather McBrien, Candice Luo, and Zainab Samaan analyzed and interpreted the data. Heather McBrien, Candice Luo, Nitika Sanger, Laura Zielinski, Meha Bhatt, Xi Ming Zhu, David Marsh and Lehana Thabane contributed to drafting the manuscript. Candice Luo, Nitika Sanger, Laura Zielinski, Meha Bhatt, Xi Ming Zhu, David Marsh, Lehana Thabane and Zainab Samaan critically revised the manuscript for important intellectual content. All of the authors contributed to the study design, approved the final version to be published and agreed to act as guarantors of the work.
Funding: This work was funded by Catalyst Grant 155404 from the Canadian Institutes of Health Research.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/7/4/E665/suppl/DC1.
References
- 1.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–20. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 2.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulozzi LJ, Ryan GW. Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006;31:506–11. doi: 10.1016/j.amepre.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Emergency department visits involving nonmedical use of selected prescription drugs, United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2010;59:705–9. [PubMed] [Google Scholar]
- 5.Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–74. doi: 10.1146/annurev-publhealth-031914-122957. [DOI] [PubMed] [Google Scholar]
- 6.Han B, Compton WM, Jones CM, et al. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. JAMA. 2015;314:1468–78. doi: 10.1001/jama.2015.11859. [DOI] [PubMed] [Google Scholar]
- 7.Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23:63–75. doi: 10.1097/HRP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 8.Socías ME, Ahamad K. An urgent call to increase access to evidence-based opioid agonist therapy for prescription opioid use disorders. CMAJ. 2016;188:1208–9. doi: 10.1503/cmaj.160554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farré M, Mas A, Torrens M, et al. Retention rate and illicit opioid use during methadone maintenance interventions: a meta-analysis. Drug Alcohol Depend. 2002;65:283–90. doi: 10.1016/s0376-8716(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 10.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and metaanalysis. BMJ. 2012;345:e5945. doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruneau J, Ahamad K, Goyer MÈ, et al. Management of opioid use disorders: a national clinical practice guideline. CMAJ. 2018;190:E247–57. doi: 10.1503/cmaj.170958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahid H, Bhatt M, Sanger N, et al. Association between family factors and illicit polysubstance use amongst methadone maintenance patients with opioid use disorder. Int J High Risk Behav Addict. 2018;7:e58786. [Google Scholar]
- 13.Scavone JL, Sterling RC, Weinstein SP, et al. Impact of cannabis use during stabilization on methadone maintenance treatment. Am J Addict. 2013;22:344–51. doi: 10.1111/j.1521-0391.2013.12044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zielinski L, Bhatt M, Sanger N, et al. Association between cannabis use and methadone maintenance treatment outcomes: an investigation into sex differences. Biol Sex Differ. 2017;8:8. doi: 10.1186/s13293-017-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zielinski L, Bhatt M, Eisen RB, et al. Association between cannabis use and treatment outcomes in patients receiving methadone maintenance treatment: a systematic review protocol. Syst Rev. 2016;5:139. doi: 10.1186/s13643-016-0317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timko C, Schultz NR, Cucciare MA, et al. Retention in medication-assisted treatment for opiate dependence: a systematic review. J Addict Dis. 2016;35:22–35. doi: 10.1080/10550887.2016.1100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White WL, Campbell MD, Spencer RD, et al. Patterns of abstinence or continued drug use among methadone maintenance patients and their relation to treatment retention. J Psychoactive Drugs. 2014;46:114–22. doi: 10.1080/02791072.2014.901587. [DOI] [PubMed] [Google Scholar]
- 18.Taylor OD. Poly substance use in methadone maintenance therapy (MMT) patients. J Hum Behav Soc Environ. 2015;25:822–9. [Google Scholar]
- 19.Vowles KE, McEnteea ML, Julnes TF, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569–76. doi: 10.1097/01.j.pain.0000460357.01998.f1. [DOI] [PubMed] [Google Scholar]
- 20.Wasserman DA, Weinstein MG, Havassy BE, et al. Factors associated with lapses to heroin use during methadone maintenance. Drug Alcohol Depend. 1998;52:183–92. doi: 10.1016/s0376-8716(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 21.Bradford AC, Bradford WD, Abraham A, et al. Association between US state medical cannabis laws and opioid prescribing in the Medicare part D population. JAMA Intern Med. 2018;178:667–72. doi: 10.1001/jamainternmed.2018.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachhuber MA, Saloner B, Cunningham CO, et al. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med. 2014;174:1668–73. doi: 10.1001/jamainternmed.2014.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angell TVA. issues new medical marijuana policy for military veterans. [accessed 2019 Oct 29];Forbes. 2017 Dec 19; Available: https://www.forbes.com/sites/tomangell/2017/12/19/v-a-issues-new-medical-marijuana-policy-for-military-veterans/#6d1ff4215b90. [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bawor M, Dennis BB, Anglin R, et al. Sex differences in outcomes of methadone maintenance treatment for opioid addiction: a systematic review protocol. Syst Rev. 2014;3:45. doi: 10.1186/2046-4053-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–2. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Oxford (UK): Cochrane Collaboration; 2011. [updated March 2011] [Google Scholar]
- 28.Stang A. Critical evaluation of the Newcastle–Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 29.Lau J, Ioannidis JPA, Terrin N, et al. Evidence based medicine: the case of the misleading funnel plot. BMJ. 2006;333:597. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Best D, Gossop M, Greenwood J, et al. Cannabis use in relation to illicit drug use and health problems among opiate misusers in treatment. Drug Alcohol Rev. 1999;18:31–8. [Google Scholar]
- 31.Epstein DH, Preston KL. Does cannabis use predict poor outcome for heroin-dependent patients on maintenance treatment? Past findings and more evidence against. Addiction. 2003;98:269–79. doi: 10.1046/j.1360-0443.2003.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine AR, Lundahl LH, Ledgerwood DM, et al. Gender-specific predictors of retention and opioid abstinence during methadone maintenance treatment. J Subst Abuse Treat. 2015;54:37–43. doi: 10.1016/j.jsat.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Lions C, Carrieri MP, Michel L, et al. Predictors of non-prescribed opioid use after one year of methadone treatment: an attributable-risk approach (ANRS–Methaville trial) Drug Alcohol Depend. 2014;135:1–8. doi: 10.1016/j.drugalcdep.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Nava F, Manzato E, Lucchini A. Chronic cannabis use does not affect the normalization of hypothalamic–pituitary–adrenal (HPA) axis induced by methadone in heroin addicts. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1089–94. doi: 10.1016/j.pnpbp.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Nirenberg TD, Cellucci T, Liepman MR, et al. Cannabis versus other illicit drug use among methadone maintenance patients. Psychol Addict Behav. 1996;10:222–7. [Google Scholar]
- 36.Proctor SL, Copeland AL, Kopak AM, et al. Outcome predictors for patients receiving methadone maintenance treatment: findings from a retrospective multi-site study. J Subst Use. 2016;21:601–13. [Google Scholar]
- 37.Saxon AJ, Wells EA, Fleming C, et al. Pre-treatment characteristics, program philosophy and level of ancillary services as predictors of methadone maintenance treatment outcome. Addiction. 1996;91:1197–209. doi: 10.1046/j.1360-0443.1996.918119711.x. [DOI] [PubMed] [Google Scholar]
- 38.Somers CJ, O’Connor J. Retrospective study of outcomes, for patients admitted to a drug treatment centre board. Ir Med J. 2012;105:295–8. [PubMed] [Google Scholar]
- 39.Joe GW, Simpson DD, Broome KM. Effects of readiness for drug abuse treatment on client retention and assessment of process. Addiction. 1998;93:1177–90. doi: 10.1080/09652149835008. [DOI] [PubMed] [Google Scholar]
- 40.Peles E, Schreiber S, Adelson M. Factors predicting retention in treatment: 10-year experience of a methadone maintenance treatment (MMT) clinic in Israel. Drug Alcohol Depend. 2006;82:211–7. doi: 10.1016/j.drugalcdep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Peles E, Linzy S, Kreek MJ, et al. One-year and cumulative retention as predictors of success in methadone maintenance treatment: a comparison of two clinics in the United States and Israel. J Addict Dis. 2008;27:11–25. doi: 10.1080/10550880802324382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schiff M, Levit S, Moreno RC. Retention and illicit drug use among methadone patients in Israel: a gender comparison. Addict Behav. 2007;32:2108–19. doi: 10.1016/j.addbeh.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Weizman T, Gelkopf M, Melamed Y, et al. Cannabis abuse is not a risk factor for treatment outcome in methadone maintenance treatment: a 1-year prospective study in an Israeli clinic. Aust N Z J Psychiatry. 2004;38:42–6. doi: 10.1046/j.1440-1614.2003.01296.x. [DOI] [PubMed] [Google Scholar]
- 44.Bleich AVI, Gelkopf M, Schmidt V, et al. Correlates of benzodiazepine abuse in methadone maintenance treatment: a 1-year prospective study in an Israeli clinic. Addiction. 1999;94:1533–40. doi: 10.1046/j.1360-0443.1999.941015339.x. [DOI] [PubMed] [Google Scholar]
- 45.Peirce JM, Petry NM, Roll JM, et al. Correlates of stimulant treatment outcome across treatment modalities. Am J Drug Alcohol Abuse. 2009;35:48–53. doi: 10.1080/00952990802455444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saxon AJ, Calsyn DA, Greenberg D, et al. Urine screening for marijuana among methadone-maintained patients. Am J Addict. 1993;2:207–11. [Google Scholar]
- 47.Strain EC, Brooner RK, Bigelow GE. Clustering of multiple substance use and psychiatric diagnoses in opiate addicts. Drug Alcohol Depend. 1991;27:127–34. doi: 10.1016/0376-8716(91)90031-s. [DOI] [PubMed] [Google Scholar]
- 48.Bell J, Mattick R, Hay A, et al. Methadone maintenance and drug-related crime. J Subst Abuse. 1997;9:15–25. doi: 10.1016/s0899-3289(97)90003-1. [DOI] [PubMed] [Google Scholar]
- 49.van der Pol P, Liebregts N, de Graaf R, et al. Validation of self-reported cannabis dose and potency: an ecological study. Addiction. 2013;108:1801–8. doi: 10.1111/add.12226. [DOI] [PubMed] [Google Scholar]
- 50.Scavone JL, Sterling RC, Van Bockstaele EJ. Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neuroscience. 2013;248:637–54. doi: 10.1016/j.neuroscience.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–27. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volkow ND, Swanson JM, Evins AE, et al. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. 2016;73:292–7. doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- 53.Khan SS, Secades-Villa R, Okuda M, et al. Gender differences in cannabis use disorders: results from the national epidemiologic survey of alcohol and related conditions. Drug Alcohol Depend. 2013;130:101–8. doi: 10.1016/j.drugalcdep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosic T, Naji L, Bawor M, et al. The impact of comorbid psychiatric disorders on methadone maintenance treatment in opioid use disorder: a prospective cohort study. Neuropsychiatr Dis Treat. 2017;13:1399–408. doi: 10.2147/NDT.S129480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell G, Hall WD, Peacock A, et al. Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: findings from a 4-year prospective cohort study. Lancet Public Health. 2018;3:e341–50. doi: 10.1016/S2468-2667(18)30110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Degenhardt L, Hall W, Lynskey M. The relationship between cannabis use and other substance use in the general population. Drug Alcohol Depend. 2001;64:319–27. doi: 10.1016/s0376-8716(01)00130-2. [DOI] [PubMed] [Google Scholar]
- 57.Blanco C, Hasin DS, Wall MM, et al. Cannabis use and risk of psychiatric disorders: prospective evidence from a US national longitudinal study. JAMA Psychiatry. 2016;73:388–95. doi: 10.1001/jamapsychiatry.2015.3229. [DOI] [PubMed] [Google Scholar]
- 58.Kelly SM, O’Grady KE, Mitchell SG, et al. Predictors of methadone treatment retention from a multi-site study: a survival analysis. Drug Alcohol Depend. 2011;117:170–5. doi: 10.1016/j.drugalcdep.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.