Abstract
Numerous organizations, including the United States Preventive Services Task Force, recommend annual lung cancer screening (LCS) with low-dose computed tomography (LDCT) for high-risk adults who meet specific criteria. Despite recommendations and national coverage for screening-eligible adults through the Centers for Medicare and Medicaid Services, LCS uptake in the United States remains low (<4%). In recognition of the need to improve and understand LCS across the population, as part of the larger Population-based Research to Optimize the Screening PRocess (PROSPR) consortium, the National Cancer Institute funded the Lung PROSPR Research Consortium consisting of five diverse healthcare systems in Colorado, Hawaii, Michigan, Pennsylvania, and Wisconsin. Using various methods and data sources, the center aims to examine utilization and outcomes of LCS across diverse populations, and assess how variations in the implementation of LCS programs shape outcomes across the screening process. This commentary presents the PROSPR LCS process model, which outlines the interrelated steps needed to complete the screening process from risk assessment to treatment. In addition to guiding planned projects within the Lung PROSPR Research Consortium, this model provides insights on the complex steps needed to implement, evaluate, and improve LCS outcomes in community practice.
Keywords: Lung Cancer, Cancer Screening, Outcomes and Process Assessment, Big Data
INTRODUCTION
Annual lung cancer screening with low-dose computed tomography (LDCT) is recommended for high-risk adults that meet specific age, smoking, and health-status criteria. In 2013, reflective of the potential benefits (estimated 20% reduction in lung cancer death [1]) and harms (false positives and potential radiation exposure [2]), the United States Preventive Services Task Force (USPSTF) provided a Grade “B” recommendation for lung cancer screening with LDCT enabling coverage without cost-sharing [3]. In 2015, the Centers for Medicare and Medicaid Services (CMS) established criteria for national screening coverage [4]. Although lung cancer screening has been implemented in a variety of settings around the country [5, 6], uptake remains low [7]. Early experiences suggest that the outcomes of lung cancer screening in community settings may differ from trial outcomes [8], potentially due to differences in the characteristics of people screened [5, 9] or interpretation of findings [2]. Thus, uncertainties about the benefits, harms, and costs of lung cancer screening in community practice remain.
In recognition of the need to improve lung and other types of cancer screening, in 2018, the National Cancer Institute (NCI) funded the large multisite consortium, Population-based Research to Optimize the Screening PRocess (PROSPR). The overall aim of PROSPR is to conduct multi-site, coordinated, transdisciplinary research to evaluate and improve cervical, colorectal, and lung cancer screening processes. Within the Lung PROPSR Research Consortium, titled Lung cancer screening Optimization in The United States (LOTUS), we aim to assess utilization and outcomes of lung cancer screening across five diverse community-based healthcare systems and to identify key challenges and opportunities to optimize impact. Central to these efforts is understanding how variations in practice and other contextual factors shape the cancer screening process and its outcomes. In this commentary, we provide an overview of the lung cancer screening landscape in the United States including lung cancer burden, evidence and gaps, and implementation challenges. Building upon this landscape, we present our lung cancer screening process model to describe the interrelated steps needed to provide high-quality screening in community settings. Lastly, we describe the five healthcare systems that comprise LOTUS and introduce planned research within the consortium aimed at improving lung cancer screening processes and outcomes, and reducing health disparities.
LUNG CANCER SCREENING LANDSCAPE
Lung Cancer: Burden, Risk, and Disparities
Lung cancer is the largest cause of cancer deaths, and disproportionately affects populations that are already burdened by high poverty rates and low-education levels [10,11]. An estimated 142,670 people in the U.S. will die from lung cancer in 2019, representing nearly 25% of all U.S. cancer deaths [11]. Although modest improvements in incidence and mortality rates have occurred over the last decade, 5-year relative survival for lung cancer remains among the lowest of all cancer types [11].
Despite improvements at the population level, disparities in lung cancer persist across racial, geographic, and socioeconomic groups [10, 11]. Overall, incidence and mortality rates are highest in non-Hispanic Black men, and Black men have a higher proportion of cancer cases diagnosed at distant stages and lower survival rates than non-Hispanic White men [11]. Lung cancer also has the largest geographic variation across cancer types. For example, incidence rates in Kentucky are approximately 3.5 times higher than those in Utah, which corresponds with overall state-level smoking prevalence [11, 12]. Geographic distributions of mortality are closely linked with other social determinants of health including higher levels of socioeconomic deprivation and rurality [10, 11].
Differences in lung cancer rates across groups are largely reflective of underlying differences in the primary risk factor, smoking, which accounts for an estimated 85% of all lung cancer deaths [13]. Among US adults, the prevalence of cigarette smoking decreased from 20.9% in 2005 to 15.5% in 2016, but remains higher among males and adults with lower education levels [12]. American Indians and Alaskan Natives, those living in poverty, and sexual and gender minorities also have higher rates of smoking [12,14]. Besides smoking, differences in quality and timeliness of treatment received [15] and patient and provider awareness of guidelines [16] also contribute to disparities across the care continuum, underscoring the need to identify and target underlying mechanisms across individual, healthcare system, and community levels to reduce inequities.
Lung Cancer Screening: Evidence, Guidelines, and Gaps
Early attempts to develop lung cancer screening date back to the 1950s, beginning with photofluorograms and expanding to chest radiographs [17]. Four screening trials in the 1970s-1980s, and the Prostate, Lung, Colorectal and Ovarian (PLCO) trial in the 1990s found no overall benefit of screening with chest radiography and/or sputum cytology [17, 18]. In 1999, the Early Lung Cancer Action Project (ELCAP), a single arm study, showed the potential of LDCT to detect nodules (23% CT vs 7% chest radiography detection rate) and identify early stage lung cancer suitable for resection [19].
Following these findings, the National Lung Screening Trial (NLST) compared screening outcomes for LDCT to chest radiography among heavy smokers. NLST enrolled individuals at 33 U.S. medical centers and randomized them to three annual rounds of LDCT or chest radiography [1]. LDCT found 20% and 6.7% reductions, respectively, in the risk of lung cancer-specific and all-cause mortality. Two international trials have recently provided additional evidence of the efficacy of LDCT. The Dutch-Belgian Randomized Lung Cancer Screening Trial (NELSON) reported preliminary results indicating similar reductions in mortality as the NLST [20] and the Multicentric Italian Lung Detection (MILD) trial found even greater reductions (39%) in lung cancer mortality [21].
Although there is growing evidence of the efficacy of LDCT, uncertainty regarding the benefits, harms, and costs of screening in community settings exist. Even in controlled settings, serious harms of lung cancer screening have been reported including high false-positive and complication rates [2]. Early evidence indicates that these harms may be higher in community settings [8]. Furthermore, over 75% of NLST participants were evaluated at NCI-designated cancer centers [2], and only 4.5% of all participants were Black [1]. As such, the generalizability and downstream consequences of the NLST findings including differential rates of annual screening eligibility for Blacks [9] have been raised.
Lung Cancer Screening Guidelines
Despite potential uncertainties, based on NLST results [1] and decision modeling [22], several organizations released recommendations for annual LDCT screening beginning in 2012. Notably, in 2013, a Grade B USPSTF recommendation [3] enabled coverage for LDCT without cost-sharing for privately insured people as mandated by the Affordable Care Act (ACA). In 2015, CMS established national screening coverage for high-risk adults, requiring for the first time that shared decision making and tobacco cessation counseling occur prior to cancer screening [4]. However, specific eligibility criteria are complex and vary across guidelines. As such, there is strong need to understand how variations in the screening process across healthcare systems impact utilization, outcomes, and equity of lung cancer screening.
MATERIALS AND METHODS
LUNG CANCER SCREENING PROCESS MODEL
Similar to other cancer screening processes [23], the lung cancer screening process requires the coordination and completion of multiple steps to be effective. Failures at any step undermine the benefits of screening, and if these failures occur more often in medically underserved populations, they may further exacerbate cancer-related disparities. Given the specificity of screening eligibility and variations in guidelines for lung cancer screening, completion and quality of the screening process may be variable across systems. To guide research across the process, we developed the PROSPR lung cancer screening process model (Figure 1) that draws upon previously published PROSPR screening models in breast, cervical, and colorectal cancer screening [23] and prior conceptual work. Below we describe each stage and aligned recommended practices for improving implementation and quality of care [6, 24]. Central to each stage is the need to accurately assess and document relevant data (such as smoking status, shared decision-making, or follow-up recommendations) in the medical record. In LOTUS, this process model will guide efforts to understand how differences across multiple levels—including patients, providers, healthcare systems, and communities—impact outcomes across the continuum of lung cancer care. The model will also help to identify potential targets for interventions to help improve quality, reach, and equity of lung cancer screening.
Figure 1. PROSPR Lung Cancer Screening Process Model.
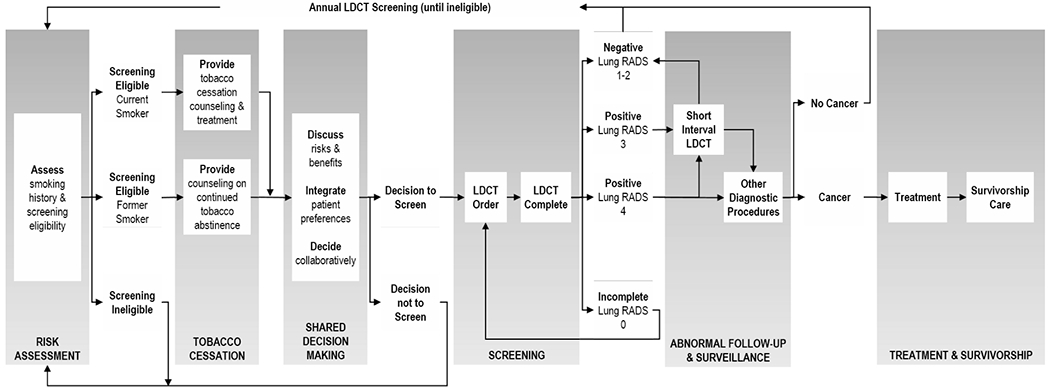
Visual model depicting interrelated steps across the lung cancer screening process
RESULTS
Risk Assessment.
Lung cancer screening begins with accurate risk assessment. Ideally all patients are routinely assessed for smoking status and history (including smoking frequency, pack-years and quit date) at all points of care. Risk assessment for lung cancer screening may also include an evaluation of overall health status, family history of lung cancer, and high-risk occupational exposures.
Tobacco Cessation Counseling & Treatment.
Primary prevention through tobacco cessation among current smokers is a cornerstone to help reduce lung cancer incidence, improve quality and life expectancy, and reduce health costs [13]. As such, tobacco cessation is recommended to be incorporated into all lung cancer screening programs. All patients who are current smokers should be offered personalized treatment options, and former smokers provided counseling on the importance of continued smoking abstinence.
Shared Decision Making.
Shared decision making is a collaborative process where providers and patients discuss, deliberate, and decide upon the best approach for care [25, 26]. CMS requires shared decision making for baseline lung cancer screening and also provides reimbursement for shared decision making counseling. High-quality shared decision making requires clear communication of the potential benefits and risks of screening, support for patients to communicate their goals, and collaborative decision-making by which patients and providers come together to make an informed decision concordant with patients’ values [25, 26]. There are several decision support tools available for lung cancer screening that may help guide shared decision making, but alone do not constitute shared decision making [26].
LDCT Screening.
Once a patient is identified as eligible and interested in screening, an order should be placed and tracked to ensure that the patient receives a timely LDCT at an accredited location. Prior to screening, eligibility, documentation of shared decision making, and tobacco cessation counseling should be confirmed. To minimize harms, lung cancer screening scans must be performed with a low-dose technique [3]. The ACR recommends that LDCT scans have a volume CT dose index of 3mGy or lower and an effective dose of 1 mSv or lower [24]. Following completion of LDCT, results should be communicated directly to patients and to their provider team in a timely manner. In accordance with the American College of Radiology (ACR), LDCT findings should be categorized by the Lung-RADS system [incomplete (0), negative (1), benign appearance or behavior (2), probably benign (3), and suspicious (4-A/B/X)] and include any modifiers for clinically significant non-lung cancer findings (“S”) [24]. Lung-RADS, nodule characteristics, follow-up recommendations, and incidental findings should be documented systematically in a radiology report. At present, Lung-RADS employs a categorization strategy based on baseline nodule diameter and change over time [24]; however, there is emerging evidence regarding the use of volumetric measurement [27]. In LOTUS, we will leverage our established consortium to track and assess any changes to evaluation of nodules, cancer staging, or any other aspects of lung cancer care that may occur in the future.
Diagnostic Follow-Up. Treatment, and Survivorship.
Coordinated, multilevel approaches should be used to ensure that all patients with positive results (defined as Lung-RADS 3 or greater) receive appropriate follow-up or diagnostic care as recommended by professional guidelines, and that all patients with negative results return for annual screening until no longer eligible [6, 24]. Patients with significant incidental findings, such as coronary arterial calcification, pulmonary fibrosis, aortic aneurysm, and non-pulmonary nodules and masses (e.g. thyroid, kidney), should receive appropriate care in a timely manner. For patients who are diagnosed with lung cancer, care teams and healthcare systems should coordinate care and track outcomes. Once patients have completed treatment, comprehensive survivorship care should be provided and coordinated between patients’ primary care and oncology teams.
DISCUSSION
Implementation of lung cancer screening into community practice is complex and challenging, accentuated by the need to interface and coordinate with different provider teams to complete the process. A substantial challenge is lack of accurate documentation of smoking status and history. Although commonly used EHRs allow for discrete capture of smoking status, lack of detailed capture (e.g. pack-years) remains common [28]. Additionally, some patients may be hesitant to disclose smoking status, reducing the accuracy and completeness of documented data [28]. Another key challenge is integration of tobacco cessation counseling into the process [25]. Smoking cessation on its own can be challenging to implement. Integrating it within lung cancer screening adds challenges [25], but also offers opportunities to deliver behavioral counseling and initiate pharmacotherapeutic approaches to achieve cessation [26]. Personalized approaches to tobacco treatment may help to support smoking cessation in the context of lung cancer screening [26].
Implementation of shared decision making is an often cited barrier to lung cancer screening [16, 26]. Evidence suggests that the quality and frequency of shared decision making discussions are limited [29]. Limited time to engage in the process is reported as a barrier among providers, even in fee-for-service settings where reimbursement for counseling may be available [16, 29]. Other challenges include lack of familiarity with guidelines, competing priorities, and integration into clinical workflows [16]. As such, there is great need to develop effective strategies for integrating high-quality shared decision making into lung cancer screening that ensures patient-centered care while aligning with clinical workflows and demands.
There is concern that the evaluation and management of patients with screen-detected lung nodules will vary considerably across providers and healthcare systems, resulting in outcomes that differ from the remarkably low rate of invasive procedures and downstream complications observed in the NLST [5, 8, 9]. Variation in the detection and management of screen-detected nodules has the potential to lead to higher rates of harms including false-positives, subsequent imaging, inappropriate use of functional imaging (e.g. FDG-PET), and an increased number of invasive procedures, with inherent risk of harm [2,8]. Therefore, documenting and evaluating the variation in management of patients and subsequent outcomes in community practices is key to ensuring screening guidelines are informed by and adequately communicate the potential harms and benefits of screening.
A related challenge is managing costs associated with incidental findings, false positives, and complications resulting from lung cancer screening [5, 8, 9]. Although lung cancer screening itself may be covered, downstream care may result in high costs to both the patient and the healthcare system [8, 30]. These costs serve as potential barriers to care, particularly among underserved communities. To address cost and other barriers, tailored and collaborative approaches between healthcare systems and the communities they serve are needed to ensure that lung cancer screening reduces, rather than increases, persistent disparities in lung cancer—an overarching goal of LOTUS.
Lung PROSPR Research Consortium: LOTUS
The goal of the Lung PROSPR Research Consortium—LOTUS—is to address lung cancer disparities by evaluating utilization and outcomes of the LDCT screening across diverse populations. Drawing upon a network of interdisciplinary scientists with expertise in lung cancer screening, tobacco cessation, healthcare delivery, and disparities research, LOTUS aims to conduct observational and interventional studies across five heterogeneous healthcare systems (Table 1). The healthcare systems in LOTUS include Henry Ford Health System (HFHS), Kaiser Permanente Colorado (KPCO), Kaiser Permanente Hawaii (KPHI), Marshfield Clinic Health System (MCHS), and University of Pennsylvania Health System (UPHS). Diversity and size of our patient populations (Table 2) and variations in the implementation of lung cancer screening across our systems (Table 1) will enable understanding of how patient, provider, and system-level differences shape utilization and outcomes. Drawing from feedback from key stakeholders, screening policies and processes will be tracked over time to understand how local and national changes impact outcomes across community settings.
Table 1.
LOTUS Healthcare System Characteristics and Lung Cancer Screening Programs
| HFHS† | KPCO† | KPHI† | MCHS† | UPHS† | |
|---|---|---|---|---|---|
| Healthcare System Characteristics | |||||
| Region | Metropolitan Detroit | Denver/Boulder Front Range | Oahu, Hawaii Island, Maui, Kauai | Central, North Central, and Northwestern Wisconsin | Philadelphia, Greater Delaware Valley |
| State(s) | Michigan | Colorado | Hawaii | Wisconsin Michigan | Pennsylvania New Jersey Delaware |
| Type of Care | Mixed Model | Managed Care | Managed Care | Mixed Model | Mixed Model |
| NCI-Designated4 Cancer Center | No | No | No | No | Yes |
| Teaching intensity4 | Major | Minor | Minor | Non-Teaching | Major |
| State-level smoking prevalence1 | 20.4% | 15.6% | 13.1% | 17.1% | 18.0% |
| State-level lung cancer incidence2 (per 100,000) | 65.6 | 43.3 | 46.2 | 60.0 (WI) | 64.7 (PA) |
| State-level lung cancer mortality3 (per 100,000) | 48.5 | 30.2 | 31.6 | 43.0 (WI) | 45.2 (PA) |
| Lung Cancer Screening Program | |||||
| Program Launch | 4/1/2012 | 1/1/2014 | 1/9/2015 | 4/1/2014 | 5/1/2014 |
| Risk Assessment and Screening Eligibility | Centralized assessment | Centralized assessment | Provider-driven assessment | Provider-driven assessment | Provider-driven assessment |
| Ordering provider documents eligibility at order | Ordering provider documents eligibility at order | Navigator confirms eligibility at order | Ordering provider documents eligibility at order | Navigator confirms eligibility at scheduling | |
| Tobacco Cessation Counseling | Performed by the ordering provider or navigator | Performed by the ordering provider | Performed by the ordering provider or navigator | Performed by the ordering provider | Performed by the ordering provider |
| Shared Decision Making (SDM) | Centralized SDM clinic | Performed by the ordering provider | Performed by navigator | Performed by the ordering provider | Performed by the ordering provider |
| LDCT Order | Primary care or specialty provider can place order | Provider referral to navigator, who places order | Provider referral to navigator, who places order | Primary care or specialty provider can place order | Primary care or specialty provider can place order |
| Reporting Results | Sent by navigator to patient and primary care provider | Sent by navigator to patient and primary care provider | Sent by navigator to patient and primary care provider | Sent to ordering provider to be discussed with patient | Sent to ordering provider to be discussed with patient |
| Monitoring Follow-Up & Treatment | Outreach and tracking by navigator | Decentralized | Outreach and tracking by navigator | Outreach and tracking by radiology | Decentralized |
Henry Ford Health System (HFHS); Kaiser Permanente Colorado (KPCO); Kaiser Permanente Hawaii (KPHI); Marshfield Clinic Health System (MCHS); and University of Pennsylvania Health System (UPHS).
Data Sources.
Centers for Disease Control and Prevention (CDC). 2011-2016 State Tobacco Activities Tracking and Evaluation (STATE) System. BRFSS Survey Data; 2017.
U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. State Cancer Profiles, Incidence Rate Report by State, Lung & Bronchus, 2011-2015; 2018.
U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. State Cancer Profiles, Death Rate Report by State, Lung & Bronchus, 2011-2015; 2018.
Agency for Healthcare Research and Quality (AHRQ). Compendium of U.S. Health Systems, 2016. AHRQ Pub. No. 17-0046-1-EF: September 2017.
Table 2.
LOTUS Primary Care Patient Characteristics by Healthcare System, 2010-2017
| HFHS† | KPCO† | KPHI† | MCHS† | UPHS† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| Patient Population | 532,286 | 100.0 | 575,206 | 100.0 | 253,700 | 100.0 | 173,061 | 100.0 | 279,949 | 100.0 |
| Age | ||||||||||
| 35-44 years | 185,484 | 34.9 | 213,809 | 37.2 | 98,561 | 38.9 | 52,302 | 30.2 | 84,919 | 30.3 |
| 45-54 years | 139,475 | 26.2 | 148,246 | 25.8 | 66,225 | 26.1 | 40,242 | 23.3 | 70,330 | 25.1 |
| 55-64 years | 107,799 | 20.3 | 126,682 | 22.0 | 53,210 | 21.0 | 38,892 | 22.5 | 62,715 | 22.4 |
| 65-79 years | 76,980 | 14.5 | 70,572 | 12.3 | 28,275 | 11.2 | 31,380 | 18.1 | 49,098 | 17.5 |
| 80+ years | 22,548 | 4.2 | 15,897 | 2.8 | 7,429 | 2.9 | 10,245 | 5.9 | 12,887 | 4.6 |
| Sex | ||||||||||
| Female | 297,572 | 55.9 | 301,500 | 52.4 | 125,771 | 49.6 | 92,091 | 53.2 | 159,597 | 57.0 |
| Male | 234,697 | 44.1 | 273,700 | 47.6 | 127,929 | 50.4 | 80,970 | 46.8 | 120,352 | 43.0 |
| Race/Ethnicity | ||||||||||
| Asian/Pacific Islander | 16,353 | 3.1 | 20,170 | 3.5 | 128,548 | 50.7 | 1,632 | 0.9 | 11,222 | 4.0 |
| Non-Hispanic Black | 141,606 | 26.6 | 23,096 | 4.0 | 2,861 | 1.1 | 556 | 0.3 | 66,536 | 23.8 |
| Hispanic | 11,983 | 2.3 | 77,428 | 13.5 | 8,475 | 3.3 | 2,635 | 1.5 | 8,065 | 2.9 |
| Native American | 2,004 | 0.4 | 3,731 | 0.7 | 3,067 | 1.2 | 723 | 0.4 | 256 | 0.1 |
| Non-Hispanic White | 302,382 | 56.8 | 348,632 | 60.6 | 68,435 | 27.0 | 148,024 | 85.5 | 176,950 | 63.2 |
| Multiple/Other | 8,323 | 1.6 | 18,137 | 3.2 | 0 | 0.0 | 485 | 0.3 | 8,615 | 3.1 |
| Unknown | 49,635 | 9.3 | 84,012 | 14.6 | 42,314 | 16.7 | 19,006 | 11.0 | 8,305 | 3.0 |
| Insurance Type | ||||||||||
| Commercial | 393,608 | 73.9 | 366,663 | 63.7 | 188,963 | 74.5 | 109,929 | 63.5 | 169,938 | 60.7 |
| Medicaid | 29,322 | 5.5 | 19,747 | 3.4 | 11,808 | 4.7 | 22,001 | 12.7 | 16,482 | 5.9 |
| Medicare | 99,019 | 18.6 | 65,327 | 11.4 | 22,684 | 8.9 | 37,420 | 21.6 | 86,224 | 30.8 |
| Other insurance | 8,629 | 1.6 | 118,696 | 20.6 | 30,245 | 11.9 | 3,711 | 2.1 | 7,296 | 2.6 |
| Smoking Status | ||||||||||
| Never Smoker | 222,826 | 41.9 | 303,769 | 52.8 | 130,352 | 51.4 | 87,860 | 51 | 181,279 | 58.1 |
| Former Smoker | 120,141 | 22.6 | 148,060 | 25.7 | 59,576 | 23.5 | 38,978 | 22.5 | 90,245 | 28.9 |
| Current Smoker | 66,765 | 12.5 | 59,623 | 10.4 | 28,478 | 11.2 | 29,202 | 16.9 | 33,363 | 10.7 |
| Unknown | 122,554 | 23.0 | 63,754 | 11.1 | 35,294 | 13.9 | 17,021 | 9.8 | 7,043 | 2.3 |
| LDCT Scans | ||||||||||
| 2014 | 277 | 0.1 | 536 | 0.1 | 0 | 0.0 | 0 | 0.0 | 70 | 0.0 |
| 2015 | 261 | 0.1 | 1,176 | 0.2 | 0 | 0.0 | 7 | 0.0 | 164 | 0.1 |
| 2016 | 837 | 0.2 | 1,892 | 0.3 | 393 | 0.2 | 278 | 0.2 | 327 | 0.1 |
| 2017 | 1,595 | 0.3 | 2,623 | 0.5 | 484 | 0.2 | 353 | 0.2 | 501 | 0.2 |
| Rural Urban Commuting Area‡ | ||||||||||
| Metropolitan | 513,859 | 96.5 | 571,851 | 99.4 | 188,890 | 74.5 | 39,871 | 23.0 | 276,749 | 98.9 |
| Micropolitan | 1,255 | 0.2 | 1,319 | 0.2 | 51,716 | 20.4 | 56,933 | 32.9 | 919 | 0.3 |
| Low density | 1,774 | 0.3 | 1,958 | 0.4 | 13,015 | 5.1 | 73,952 | 42.7 | 395 | 0.1 |
| Median Household Income (Census Tract)‡ | ||||||||||
| $24,500 or less | 45,068 | 8.5 | 85 | 0.0 | 3,662 | 1.4 | 2,305 | 1.3 | 7,202 | 2.6 |
| $24,501-$50,000 | 188,075 | 35.3 | 2,372 | 0.4 | 1,810 | 0.7 | 305 | 0.2 | 14,202 | 5.1 |
| $50,001-$100,000 | 244,609 | 46.0 | 132,693 | 23.1 | 40,670 | 16.0 | 89,629 | 51.8 | 56,281 | 20.1 |
| $100,001-$200,000 | 39,062 | 7.3 | 342,591 | 59.6 | 186,677 | 73.6 | 80,756 | 46.7 | 124,930 | 44.6 |
| $200,000 or more | 0 | 0.0 | 96,719 | 16.8 | 20,881 | 8.2 | 66 | 0.0 | 75,986 | 27.1 |
| Proportion of Households at or below the Federal Poverty Limit (Census Tract)‡ | ||||||||||
| 9.99% or less | 289,333 | 54.3 | 549,402 | 95.5 | 187,479 | 73.9 | 75,425 | 43.6 | 194,318 | 69.4 |
| 10-24.99% | 130,278 | 24.5 | 25,719 | 4.5 | 53,011 | 20.9 | 91,827 | 53.1 | 47,982 | 17.1 |
| 25% or more | 97,169 | 18.3 | 1 | 0.0 | 9,554 | 3.8 | 3,504 | 2.0 | 30,448 | 10.9 |
| Proportion of Adults with a 4-yr college degree or higher (Census Tract)‡ | ||||||||||
| 9.99% or less | 95,335 | 17.9 | 22,807 | 4.0 | 8,029 | 3.2 | 6,473 | 3.7 | 16,426 | 5.9 |
| 10-24.99% | 194,880 | 36.6 | 107,048 | 18.6 | 98,329 | 38.8 | 119,396 | 69.0 | 61,627 | 22.0 |
| 25% or more | 226,616 | 42.6 | 445,269 | 77.4 | 143,708 | 56.6 | 44,887 | 25.9 | 194,769 | 69.6 |
Henry Ford Health System (HFHS); Kaiser Permanente Colorado (KPCO); Kaiser Permanente Hawaii (KPHI); Marshfield Clinic Health System (MCHS); and University of Pennsylvania Health System (UPHS).
Note. Unless indicated by an double dagger (‡), data are from patient medical record. Census tract indicators (‡) use 2011-2015 American Community Survey Data and are based on patient’s most recent listed address. Entry into the healthcare system is defined by first year of healthcare plan enrollment (HFHS, KPCO, KPHI, MCHS) or primary care visit within the healthcare system (UPHS, HFHS). LDCT scans are limited to patients who have entered the system. Age is at the time of heatlhcare system entry, and patients younger than age 35 and older than age 90 are excluded. Insurance status is determined at time of healthcare system entry (KPCO, KPHI, MCHS) or most recent visit (UPHS, HFHS). Smoking status is based on most recent visit for all systems. Percentages of census data or insurance do not total 100% due to missing data.
To achieve our goals, LOTUS will conduct analyses across four interrelated research projects designed to: 1) characterize utilization and patterns of care across the screening process, 2) estimate the benefits, harms, and costs of lung cancer screening, 3) evaluate the uptake and impact of smoking cessation counseling within lung cancer screening, and 4) advance the precision of lung cancer screening risk assessment and treatment. Analyses within these projects will help to inform development and testing of pilot interventions across our systems. To support these analyses, EHR data across our systems will be pooled and harmonized to build a state-of-the-art data repository that captures lung cancer screening and treatment outcomes, and the multilevel factors that influence the screening process. Multilevel data will include characteristics of patients (e.g. demographics, comorbidities, smoking behaviors, and social determinants of health), providers (e.g. demographics, clinical specialties, and patient panels), and healthcare systems (e.g. payer structure and screening policies). Utilization of subsequent imaging, diagnostic tests, and invasive procedures after screening, and details on surgical resection, chemotherapy, and radiation for patients with lung cancer will be evaluated. For active smokers, we will assess provision of tobacco cessation counseling and use of pharmacotherapy. We will also examine the prevalence and impact of process factors—such as shared decision making and patient outreach—on screening utilization and outcomes. The combination of observational data analysis, contextual data, and intervention development will enable LOTUS to advance scientific understanding and implementation of lung cancer screening in community practice that can be extended beyond our five healthcare systems.
Conclusion
Lung cancer screening programs have the potential to substantially decrease lung cancer mortality through tobacco cessation, early detection, and treatment, yet uncertainties regarding outcomes in community settings remain. Due to the complexity of screening eligibility and other factors, there are challenges to implementing lung cancer screening in community practices and a pressing need to identify effectives strategies for overcoming these barriers. The PROSPR lung cancer screening process model presented in this paper serves to conceptualize the components necessary to support high-quality lung cancer screening, and to encourage others to use this framework to understand and improve lung cancer screening outcomes across diverse settings.
ACKNOWLEDGMENTS
We are grateful to all members of the consortium, particularly research staff and programmers who make this work possible.
Funding: Research reported in this publication was supported by the NCI/NIH under Award Numbers UM1CA221939 and U24CA221936.
Footnotes
Disclosures: Portions of this manuscript were presented at the 2019 American Thoracic Society International Conference and 2019 International Cancer Screening Network Conference. Dr. Doubeni is a member of the US Preventive Services Task Force (USPSTF). All other authors have no disclosures. The contents are solely the responsibility of the authors and do not represent the official views of the NIH or the USPSTF.
REFERENCES
- 1.National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry D, Brawley OW, et al. Benefits and Harms of CT Screening for Lung Cancer: A Systematic Review. JAMA. 2012;307(22):2418–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyer VA, U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Medicare & Medicaid Services. Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT). 2015. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274 Accessed February 25, 2019.
- 5.Gould MK, Sakoda LC, Ritzwoller DP, Simoff M, Neslund-Dudas C, Kushi LH, et al. Monitoring lung cancer screening use and outcomes at four Cancer Research Network sites. Ann Am Thorac Soc. 2017;14(12):1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson CC, Mckee AB. American Thoracic Society/American Lung Association Lung Cancer Screening Implementation Guide. Am J Respir Crit Care Med. 2018;198(9):1120–1121. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Fedewa SA. Lung Cancer Screening With Low-Dose Computed Tomography in the United States-2010 to 2015. JAMA Oncol. 2017;3(9):1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huo J, Xu Y, Sheu T, Volk RJ, Shih Y-CT. Complication Rates and Downstream Medical Costs Associated With Invasive Diagnostic Procedures for Lung Abnormalities in the Community Setting. JAMA Intern Med. January 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iaccarino JM, Steiling KA, Wiener RS. Lung Cancer Screening in a Safety-Net Hospital: Implications of Screening a Real-World Population versus the National Lung Screening Trial. Ann Am Thorac Soc. 2018;15(12):1493–1495. [DOI] [PubMed] [Google Scholar]
- 10.Finke I, Behrens G, Weisser L, Brenner H, Jansen L. Socioeconomic Differences and Lung Cancer Survival—Systematic Review and Meta-Analysis. Front Oncol. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 12.Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, et al. Current Cigarette Smoking Among Adults - United States, 2016. MMWR. 2018;67(2):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chyou PH, Nomura AM, Stemmermann GN. A prospective study of the attributable risk of cancer due to cigarette smoking. Am J Public Health. 1992;82(1):37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drope J, Liber AC, Cahn Z, Stoklosa M, Kennedy R, Douglas C, et al. Who’s still smoking? Disparities in adult cigarette smoking prevalence in the United States. CA Cancer J Clin. 2018;68(2):106–115. [DOI] [PubMed] [Google Scholar]
- 15.Wolf A, Alpert N, Tran BV, Liu B, Flores R, Taioli E. Persistence of racial disparities in early-stage lung cancer treatment. J Thorac Cardiovasc Surg. December 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberth JM, McDonnell KK, Sercy E, Khan S, Strayer SM, Dievendorf AC, et al. A national survey of primary care physicians: Perceptions and practices of low-dose CT lung cancer screening. Prev Med Rep. 2018;11:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruparel M, Quaife SL, Navani N, Wardle J, Janes SM, Baldwin DR. Pulmonary nodules and CT screening: the past, present and future. Thorax. 2016;71(4):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306(17):1865–1873. [DOI] [PubMed] [Google Scholar]
- 19.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. The Lancet. 1999;354(9173):99–105. [DOI] [PubMed] [Google Scholar]
- 20.de Koning H, Van Der Aalst C, Ten Haaf K, Oudkerk. PL02.05 Effects of volume CT lung cancer screening: Mortality results of the NELSON randomized-controlled population based trial. J Thorac Oncol. 2018;13(10):S185. [Google Scholar]
- 21.Pastorino U, Silva M, Sestini S, Sabia F, Boeri M, Cantarutti A, et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol. 2019. July 1;30(7):1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Koning HJ, Meza R, Plevritis SK, ten Haaf K, Munshi VN, Jeon J, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(5):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaber EF, Kim JJ, Schapira MM, Tosteson ANA, Zauber AG, Geiger AM, Kamineni A, et al. Unifying Screening Processes Within the PROSPR Consortium: A Conceptual Model for Breast, Cervical, and Colorectal Cancer Screening. J Natl Cancer Inst. 2015;107(6):djv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American College of Radiology. Lung CT Screening Reporting and Data System (Lung-RADS): Version 1.1 Assessment Categories, 2019. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads Accessed November 5, 2019.
- 25.Elwyn G, Durand MA, Song J, Aarts J, Barr PJ, Berger Z, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowenstein LM, Deyter GMR, Nishi S, Wang T, Volk RJ. Shared decision-making conversations and smoking cessation interventions: critical components of low-dose CT lung cancer screening programs. Transl Lung Cancer Res. 2018;7(3):254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han D, Heuvelmans MA, Oudkerk M. Volume versus diameter assessment of small pulmonary nodules in CT lung cancer screening. Transl Lung Cancer Res. 2017;6(1):52–61. doi: 10.21037/tlcr.2017.01.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modin HE, Fathi JT, Gilbert CR, Wilshire CL, Wilson AK, Aye RW, et al. Pack-Year Cigarette Smoking History for Determination of Lung Cancer Screening Eligibility. Comparison of the Electronic Medical Record versus a Shared Decision-making Conversation. Ann Am Thorac Soc. 2017;14(8):1320–1325. [DOI] [PubMed] [Google Scholar]
- 29.Brenner AT, Malo TL, Margolis M, Elston Lafata J, James S, Vu MB, et al. Evaluating Shared Decision Making for Lung Cancer Screening. JAMA Intern Med. 2018;178(10):1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rai A, Doria-Rose VP, Silvestri GA, Yabroff KR. Evaluating Lung Cancer Screening Uptake, Outcomes, and Costs in the United States: Challenges with Existing Data and Recommendations for Improvement. J Natl Cancer Inst. January 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


