Abstract
Acceptance of cancer has long been recognized as playing a critical role in psychological adjustment to the illness, but its associations with distress outcomes have not been quantitatively reviewed. Informed by coping theory and third wave conceptualizations of acceptance, we first propose an integrated model of acceptance of cancer. Then we examine the strength of the relationships between acceptance of cancer and general and cancer-specific distress in cancer patients and potential moderators of these relationships. CINAHL, Embase, MEDLINE, PsycINFO, PsycARTICLES, and Web of Science databases were searched. Random-effects meta-analyses were conducted on 78 records (N=15,448). Small-to-moderate, negative, and significant relationships were found between acceptance of cancer and general distress (r=−.31; 95% CI:−.36 to −.26, k=75); cancer-specific distress (r=−.18; 95% CI:−.21 to −.14, k=13); depressive symptoms (r=−.25; 95% CI:−.31 to −.19, k=41); and anxiety symptoms (r=−.22; 95% CI:−.30 to −.15, k=29). Age, marital status, and stage of cancer were identified as significant moderators. Findings suggest that acceptance of cancer may be important to target in interventions to reduce general and cancer-specific distress in cancer patients. Future research should focus on developing multifaceted measures of acceptance and identifying theory-based psychological and social processes that lead to greater acceptance.
Keywords: cancer, acceptance, depressive symptoms, anxiety, psychological distress, meta-analysis
Introduction
When diagnosed with a life-threatening disease such as cancer, patients are confronted with a range of distressing circumstances (Giese-Davis et al., 2012). Many cancer patients endure aggressive treatments that result in high symptom burden and functional limitations as well as financial difficulties (Cleeland et al., 2013; Peppercorn, 2014). Early-stage cancer patients cope with uncertainties about the future, given their risk of recurrence and metastasis (Dinkel, Kremsreiter, Marten-Mittag, & Lahmann, 2014). In comparison, at the advanced stages of cancer, patients often face the reality of a limited life expectancy, complex medical decision-making, and end-of-life planning (Jaiswal, Alici, & Breitbart, 2014; S. T. Tang et al., 2014). Not surprisingly, as many as 40% of cancer patients suffer from mood disorders or clinically elevated levels of distress, including increased anxiety and depressive symptoms (Caruso, Nanni, Riba, Sabato, & Grassi, 2017; Linden, Vodermaier, MacKenzie, & Greig, 2012; Mitchell et al., 2011), and up to 80% of patients experience symptoms of cancer-specific distress (i.e., cancer-related post-traumatic stress disorder [PTSD] or fear of recurrence) (Abbey, Thompson, Hickish, & Heathcote, 2015; Caruso et al., 2017; Dinkel et al., 2014; van den Beuken-van Everdingen et al., 2008). Among cancer patients, higher levels of distress have been associated with reduced quality of life (L. F. Brown, Kroenke, Theobald, Wu, & Tu, 2010; Koch et al., 2014), poor medication and treatment adherence (Lin, Clark, Tu, Bosworth, & Zullig, 2017; Mausbach, Schwab, & Irwin, 2015), and poor physical health outcomes such as greater symptoms of pain, fatigue, nausea, and sleep difficulties (L. F. Brown et al., 2010; Pinquart & Duberstein, 2010; van den Beuken-van Everdingen et al., 2008). Further, depressive symptoms have been associated with lower survival rates following a cancer diagnosis (Pinquart & Duberstein, 2010; Satin, Linden, & Phillips, 2009).
Acceptance of Cancer
Acceptance of cancer, or making peace with the disease, is one factor that may play an important role in reducing patients’ distress. Acceptance of illness has been conceptualized in multiple ways. Initially, acceptance of illness was defined as a process of value change by which the patient accepts the losses related to the illness while maintaining a sense of self-worth (Wright, 1983). This process may involve exploring new meanings or possibilities in life based on one’s existing values and strengths (Wright, 1983). Later, acceptance was defined as a willingness to be present with one’s illness-related thoughts, feelings, and bodily sensations without judging or making unnecessary attempts to control them (Hayes, Jacobson, Follette, & Dougher, 1994). Similarly, McCracken and Eccleston (2003) described acceptance as a realistic way of living with illness; that is, an accepting patient does not judge, avoid, or deny the illness, but continues feasible engagement in everyday activities.
Acceptance differs from resignation (i.e., fatalism). For cancer patients, resignation refers to considering the illness as fate and believing that there is little or nothing one can do to change or control the illness, its symptoms, and one’s quality of life (Livneh, 2000). In other words, resignation refers to giving up and no longer striving for a fulfilling life—choosing instead to remain helpless, hopeless, and passive. Although some researchers have considered acceptance and resignation as part of the same process (Barata et al., 2018; Wells, Booth-Jones, & Jacobsen, 2009), others have argued that resignation is the opposite of acceptance (J. C. Williams & Lynn, 2010; Wright, 1983). In line with this view, research suggests that acceptance is associated with lower anxiety and depressive symptoms (e.g., Bussell & Naus, 2010; Peters, Goedendorp, Verhagen, van der Graaf, & Bleijenberg, 2014), whereas resignation is associated with higher anxiety and depressive symptoms (Andreu et al., 2012; Hong, Wei, & Wang, 2015). In this review, resignation is excluded, as it appears to be a different process with a distinct relation to psychological distress.
Acceptance also differs from a fighting spirit. In the context of cancer, having a fighting spirit involves viewing the illness as a challenge, maintaining an optimistic outlook, and working towards beating the disease (Livneh, 2000; Watson et al., 1988). Although both acceptance and having a fighting spirit involve taking an active stance, acceptance does not necessarily include efforts of positive reframing or aiming to change the course of the disease. We contend that acceptance of cancer might be closely linked with having a fighting spirit, but only when it is in line with patients’ value systems and the realities of their illness. Further, associations between having a fighting spirit and distress are mixed (e.g., Gillanders, Sinclair, MacLean, & Jardine, 2015; Watson & Homewood, 2008). Among advanced cancer patients in particular, endorsing a fighting spirit has been associated with greater psychological distress (Rand et al., 2016). Thus, the concept of a fighting spirit was excluded from this review.
Theoretical framework.
The acceptance literature is grounded in multiple theories (Carver, Scheier, & Weintraub, 1989; Hayes et al., 1994; Kabat-Zinn, 1990; Lazarus & Folkman, 1984; Linehan, 1993; Park, 2010). In this review, we focus on the most prominent theories: coping theory and third wave cognitive-behavioral therapy approaches. We then propose an integrated model of acceptance based on these theories.
Coping theory.
Coping theory posits that when confronted with significant stressors such as cancer, people evaluate the situation with regard to its impact on their lives (i.e., primary appraisals), and what, if anything, might be done about it (i.e., secondary appraisals) (Lazarus & Folkman, 1984). Based on their appraisals, people employ various cognitive and behavioral efforts (i.e., coping strategies) to manage the demands (Lazarus & Folkman, 1984). In a cancer context, acceptance is emotion-focused coping that involves acknowledging the reality of the illness, learning to live with it, and engaging in attempts to address it (Carver et al., 1989). Acceptance coping may also involve maintaining an empathic attitude toward oneself (i.e., self-compassion) or expressing feelings about the illness experience to others (Sirois, Molnar, & Hirsch, 2015; J. C. Williams, 2007; J. C. Williams & Lynn, 2010).
Another aspect of acceptance coping is developing a sense of meaning in life that broadens one’s focus beyond the illness (Threader & McCormack, 2016). According to Park’s (2010) integrated meaning-making model, acceptance is conceptualized as meanings made, that is, coming to terms with the illness as a result of meaning-making processes. For example, meanings made may include identifying benefits in the illness experience (i.e., benefit finding) or experiencing positive life changes as a result of the illness experience (i.e., post-traumatic growth) (Manne et al., 2018; Park, 2010; Sears, Stanton, & Danoff-Burg, 2003). However, in line with third wave approaches (Fletcher & Hayes, 2005), acceptance can also be conceptualized as a process or a part of ongoing meaning-making efforts.
Third wave approaches.
An alternative conceptualization of acceptance comes from theories underlying third wave cognitive-behavioral therapies, such as Acceptance and Commitment Therapy (ACT: Hayes, Strosahl, & Wilson, 1999), Dialectical Behavioral Therapy (DBT: Linehan, 1993), mindfulness-based interventions (e.g., Mindfulness-Based Stress Reduction [MBSR]: Kabat-Zinn, 1990; Mindfulness-Based Cognitive Therapy [MBCT]: Segal, Williams, & Teasdale, 2002), as well as theories of mindfulness (e.g., Mindfulness-to-Meaning Theory: Garland, Farb, Goldin, & Fredrickson, 2015; Monitor and Acceptance Theory: Lindsay & Creswell, 2017). According to this conceptualization, acceptance involves active awareness of the present moment, including unwanted thoughts, feelings, and bodily sensations, without unnecessary attempts to change these experiences (i.e., “allowing things to be as they already are” Segal et al., 2002, p. 271). From an ACT perspective, acceptance contributes to the development of psychological flexibility, which involves maintaining full awareness of the present moment while persisting in actions aligned with personal values (Hayes, Luoma, Bond, Masuda, & Lillis, 2006).
According to third wave theorists, acceptance of cancer involves a non-judgmental stance and willingness to experience the realities of the disease, such as symptoms, physical decline, and living with uncertainty (Fashler, Weinrib, Azam, & Katz, 2018; Hayes, Follette, & Linehan, 2004; Hulbert-Williams, Storey, & Wilson, 2015). These theorists contend that acceptance is not simply tolerating the existence of cancer, but, rather, embracing the moment-by-moment experience of the illness including the difficult private events (e.g., thoughts, feelings, bodily sensations) as they occur (Hayes et al., 2004). This willingness to experience the events without defense has been referred to as experiential acceptance (Hayes et al., 1994; Karekla & Panayiotou, 2011; Kashdan, Barrios, Forsyth, & Steger, 2006; J. C. Williams & Lynn, 2010). For some patients, acceptance may involve shifting from heightened emotional reactivity to cancer-related thoughts to a stance that reduces this struggle (i.e., cognitive defusion). Third wave theorists also contend that experiential acceptance is the opposite of experiential avoidance, defined as attempts or desires to avoid unwanted private events, such as thoughts, feelings, and symptoms (Hayes et al., 2004; Hulbert-Williams et al., 2015; Kashdan et al., 2006). For example, a cancer patient may withdraw from meaningful social activities in order to avoid exacerbations of pain or fatigue and, as a consequence, may experience increased loneliness and distress. Conversely, experiential acceptance of cancer may lead to growth in self-compassion, courage, and value-based living, which are theoretically linked to increased psychological flexibility and better psychological well-being (Hayes et al., 1994; Lindsay, Chin, et al., 2018; J. C. Williams, 2007).
In third wave theories, acceptance is closely related to mindfulness, defined as an open and nonjudgmental awareness of one’s experiences in the present moment (Kabat-Zinn, 1994). Indeed, some theorists conceptualize acceptance as an aspect of mindfulness (Baer, Smith, & Allen, 2004; Lindsay & Creswell, 2017). For example, in their Monitor and Acceptance Theory, Lindsay and Creswell (2017) posited that acceptance and attention monitoring are the two main components of mindfulness. Thus, acceptance is defined as a “dynamic emotion regulation skill,” which, in the context of cancer, may lead to reduced emotional reactivity and reappraisal of cancer-related stressors (Lindsay & Creswell, 2017, p. 51). Similarly, Mindfulness-to-Meaning Theory (Garland et al., 2015) suggests that acceptance, as a part of mindfulness, is critical to facilitating positive reappraisal which, in turn, improves psychological and existential outcomes (e.g., a sense of meaning in life).
Integrated model of acceptance.
Our integrated model of acceptance of cancer draws upon coping theory (Carver et al., 1989; Lazarus & Folkman, 1984; Park, 2010) and third wave theories (Garland et al., 2015; Hayes et al., 1994; Kabat-Zinn, 1990; Linehan, 1993; Segal et al., 2002). Broadly, we conceptualize acceptance of cancer as an active willingness to be present with cancer-related realities while giving up efforts to judge or control cancer-related appraisals or feelings. In addition, acceptance of cancer involves a behavioral willingness in response to cancer-related stressors, resulting in action aligned with deeply held values (Hayes et al., 1994; J. C. Williams & Lynn, 2010). Thus, acceptance of cancer is a coping strategy that is evidenced by value-based action. For instance, patients accepting early-stage cancer might actively engage in medical decision-making and select treatment options based on personal values. Among patients with terminal cancer, acceptance might lead to diverse behavioral outcomes ranging from clinical trial enrollment to seeking hospice care. In all cases, patients would be showing acceptance if their behavioral responses to cancer are consistent with their values.
According to our integrated model, acceptance of cancer is a nonjudgmental and compassionate way of relating to internal experiences of the illness, such as appraisals, emotional responses, memories, and bodily sensations. Acceptance involves letting go of the struggle to control these internal experiences while simultaneously embracing the reality of the illness. Thus, our definition of acceptance blends coping and third wave theories by acknowledging the central role of appraisals in coping (Lazarus & Folkman, 1984; Park, 2010) while suggesting that the way cancer patients relate to their appraisals (i.e., degree of cognitive fusion) can also lead to distress (Garland et al., 2015; Gillanders et al., 2015; Hayes et al., 2006). In other words, being caught up in the content of distressing thoughts about the illness and viewing them as permanent realities may result in additional suffering. Conversely, adopting an open, accepting posture towards illness-related thoughts and feelings may allow patients to experience this reality with greater ease. We contend along with third wave theorists that acceptance is not an end in itself (Hayes et al., 2006); by accepting the illness to a greater degree, patients are empowered to engage in value-based actions, which might subsequently improve psychological well-being.
Present Study: Acceptance of Cancer and Distress
Consistent with our integrated model of acceptance, studies have shown that greater acceptance of cancer is related to reduced symptoms of cancer-specific distress, depression, and anxiety; however, effect sizes for these relationships vary from small to large (e.g., Dasch et al., 2010; Mack et al., 2008; Peters et al., 2014). Differences in sample characteristics and measures of acceptance across studies may contribute to this variability in effect sizes. Certain demographic and medical subgroups of cancer patients might experience greater mental health benefits from acceptance. In addition, it is possible that only some facets of acceptance captured by certain measures are strongly correlated with distress.
Increasingly, acceptance has been targeted in mindfulness and acceptance-based therapies for cancer patients (e.g., ACT for cancer patients: J. Low et al., 2016). Recent reviews suggest that among cancer patients, mindfulness and acceptance-based interventions lead to significant moderate to large improvement in emotional well-being and quality of life as well as moderate to large reductions in anxiety and depressive symptoms and traumatic stress responses (Fashler et al., 2018; Graham, Gouick, Krahé, & Gillanders, 2016; Haller et al., 2017; Hulbert-Williams et al., 2015; Zhang et al., 2015; Zimmermann, Burrell, & Jordan, 2018). However, few studies have investigated acceptance as a mechanism of change in acceptance-based intervention trials with cancer populations (e.g., Hawkes, Pakenham, Chambers, Patrao, & Courneya, 2014). Determining the strength of the relationship between acceptance of cancer and distress will provide further evidence of its clinical relevance and inform interventions for distressed cancer patients. Therefore, guided by our integrated model of acceptance, we conducted the first meta-analyses to examine the average strength of relationships between acceptance of cancer and (1) general distress; (2) cancer-specific distress (i.e., fear of recurrence and cancer-related PTSD); (3) depressive symptoms; and (4) anxiety symptoms among cancer patients. We also explored potential moderators of these associations, all of which had sufficient variance for meta-regression analyses (i.e., age, gender, marital status, time since cancer diagnosis, and cancer stage). Such analyses inform clinical practice by elucidating whether certain subgroups might derive greater benefit from an acceptance-based intervention. Based on our findings, we then present a number of directions for future research and implications for clinical practice.
Method
Literature Search
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (Moher, Liberati, Tetzlaff, & Altman, 2009). A systematic literature search using CINAHL, Embase, MEDLINE, PsycINFO, PsycARTICLES, and Web of Science databases, using combinations of (a) cancer and (b) acceptance of cancer related search terms was conducted until August 15, 2018 (see Appendix A for the full list of search terms). Reference lists and forward citations of selected eligible articles were examined to identify any studies that may have been missed in the database searches. Authors of the studies that did not include sufficient information to determine study eligibility or conduct statistical analyses were contacted.
Inclusion and Exclusion Criteria
Eligibility criteria were applied in two phases: (1) title and abstract screening, and (2) full-text screening. Eligibility criteria included: (1) examining a sample of adult (18+ years of age) cancer patients/survivors; (2) reporting sufficient information to calculate an effect size representing a relationship between acceptance of cancer and distress (i.e., an effect size or mean, standard deviation, and n); and (3) being written in English. Eligible records included journal articles, books and book chapters, theses and dissertations, brief reports, and conference abstracts and presentations. As part of the inclusion criteria, validated self-report measures of acceptance of cancer were selected a priori. During the database search, additional measures were included if they assessed acceptance in line with our conceptual definition (i.e., recognizing and staying present with the reality of the diagnosis rather than engaging in resignation or fatalism), and if they had acceptable psychometric properties, including reliability (i.e., Cronbach’s alpha ≥ .70) and validity evidence (e.g., strong correlations with other reliable measures of acceptance). Similarly, validated self-report measures of distress were selected a priori, and additional measures found during the database search were included if they had acceptable psychometric properties. When the study sample included multiple disease groups, only information pertaining to cancer patients/survivors was included. When there were multiple records using the same sample, peer-reviewed articles and/or the most relevant studies (e.g., records that examined the acceptance-distress association in greater detail) were chosen. Studies were excluded when sufficient information to compute effect sizes was not provided in the text or could not be received from the authors.
Study Selection
Figure 1 shows a flowchart of study selection. The first author (ES) screened all of the titles and abstracts and, when found eligible, the full-texts; the second author (DBT) screened 20% of the titles and abstracts and 20% of the full-texts. Any discrepancies were resolved through consensus meetings. The electronic database search identified 8,449 records. After excluding duplicates and adding 73 records found from other sources (e.g., backwards and forwards citation searches), 3,983 records were extracted for title and abstract screening. A total of 3,507 records were excluded with 95.3% agreement between raters (Cohen’s kappa = .72). Thus, 476 records were selected for full-text screening of which 342 records were excluded, and 134 were selected for meta-analyses. There was 92.6% interrater agreement for full-text screening (Cohen’s kappa = .79). Of the 134 records examined for coding, 36 records included sufficient information for analyses. We contacted authors of 98 records and received sufficient data for 42 records. Overall, 78 records with sufficient information were included in effect size calculations, and the remaining 56 records were excluded due to insufficient information for these calculations. When records reported effect sizes individually for each sex or subsample (e.g., individual samples from different sites), these were treated as individual samples, resulting in effect sizes from 86 independent samples. Each independent sample was coded separately.
Fig 1.
Flow chart of record selection
From 75 separate records, we included 83 independent samples in the analysis examining the relationship between acceptance of cancer and general distress. We included 13 independent samples from 13 records in the analysis examining the relationship between acceptance of cancer and cancer-specific distress. From 41 records, we included 46 independent samples in the analysis examining the relationship between acceptance of cancer and depressive symptoms, and from 29 records, we included 31 independent samples in the analysis examining the relationship between acceptance of cancer and anxiety.
Coding
A systematic coding frame was created based on a modified version of Lipsey and Wilson’s (2001) example codebook. For each record, the following information was coded: type of record (e.g., journal article, dissertation); year of publication; year of data collection; publication status (i.e., published vs. not published); and study design (i.e., cross-sectional, longitudinal, case-control, or intervention). For each sample, the following descriptive information was coded: sample size, ethnic composition (i.e., percent White), mean years of education, cancer type (e.g., breast, prostate), and country (e.g., country where the data were collected). The following potential moderators were coded: mean age of the sample; gender (i.e., percent female); marital status (i.e., percent married/partnered); mean time since diagnosis (in years); and advanced stage (i.e., percent with advanced-stage cancer). Advanced-stage cancer included: stage III, IV, and metastatic breast cancer, colorectal cancer, gynecological cancer, Hodgkin’s lymphoma, non-small cell lung cancer, and prostate cancer; stage III and metastatic testicular cancer; and extensive-stage small cell lung cancer (National Cancer Institute, 2015). The first author (ES) conducted the initial coding, and the second author (DBT) coded the information reported in 20% of the selected full-texts. There was 94.8% interrater agreement for coding (Cohen’s kappa = .89). Cases of disagreement were resolved during consensus meetings.
Pearson’s product-moment correlation coefficient (r) was used as the main outcome metric. For cross-sectional and case-control studies, acceptance of cancer and general and/or cancer-specific distress correlation coefficients were extracted. For longitudinal and intervention studies, only the initial (or baseline) acceptance of cancer and general and/or cancer-specific distress correlation coefficients were extracted in order to include the largest possible sample and minimize the effects of extraneous factors (e.g., intervention, practice effects). All of the extracted correlation coefficients were weighted by sample size to compute the overall study effect sizes.
Quality Assessment
The quality of study reporting was assessed using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (von Elm, Altman, & Egger, 2008). The first author (ES) assessed reporting quality for all of the records, and the second author (DBT) assessed the reporting quality of 20% of the records. The interrater agreement for study reporting quality was 91.4% (Cohen’s kappa = .81). Disagreements were resolved during consensus meetings.
Meta-analytic Method
Four separate meta-analyses were conducted examining the relationships between acceptance of cancer and (1) general distress (i.e., distress, depressive and anxiety symptoms, reduced mental health and emotional well-being); (2) cancer-specific distress (i.e., fear of recurrence and cancer-related PTSD); (3) depressive symptoms; and (4) anxiety symptoms. General distress was examined separately because some measures of distress do not differentiate between depressive and anxiety symptoms and only provide information about global distress or general emotional/mental health. Separate meta-analyses for depressive and anxiety symptoms were conducted due to their prevalence in cancer patients and associations with important health outcomes (L. F. Brown et al., 2010; Lin et al., 2017; Pinquart & Duberstein, 2010). When studies reported multiple effect sizes from the same sample, these effect sizes were aggregated using the MAc package available in R, which accounts for the dependencies among within-study effect sizes (Hunter & Schimdt, 2004).
All data were coded in SPSS (version 24.0) and analyzed using MAc and metafor packages in R v.3.4.0 (R Core Team, 2013). Raw correlations were converted to the Fisher’s Z scale to stabilize the variance, and analyses were performed using the transformed values (Hedges & Olkin, 1985). Summary statistics of Fisher’s Z-transformed values and their 95% confidence intervals (CIs) were converted back to the r metric (Schulze, 2004) using an integral z-to-r transformation for ease of interpretation.
Meta-analyses were conducted using random-effects models with restricted maximum likelihood estimators (Viechtbauer, 2005). Random-effects models were chosen as they compute less biased and more conservative estimated effect sizes compared to fixed-effects models (Card, 2011). In accordance with Hunter and Schmidt (2004), meta-analyses were conducted with raw correlations (r) and correlations corrected for measurement reliability (ρ). When reliability coefficients were not reported in the study, the median reliability coefficients of the measures were imputed based on those reported in the sample. For single-item measures, a reliability coefficient of .60 was imputed (Hunter & Schimdt, 2004; Sharma & Yetton, 2007). Effect sizes were considered statistically significant for p-values < .05.
Following the computation of the mean effect size, heterogeneity of effect sizes was described using Cochrane Q, I,2 and Tau2 (τ2) statistics. Potential moderators were examined when significant heterogeneity was present, as demonstrated by an I2 ≥ 25%, a τ2 > 0, and a significant Q-statistic (Borenstein, Hedges, Higgins, & Rothstein, 2011). Effects of potential moderators, such as age, gender, marital status, time since diagnosis, and stage of cancer, were examined with meta-regression analyses using a restricted maximum likelihood model. Each moderator was examined independently in order to maximize the number of studies included in the analyses.
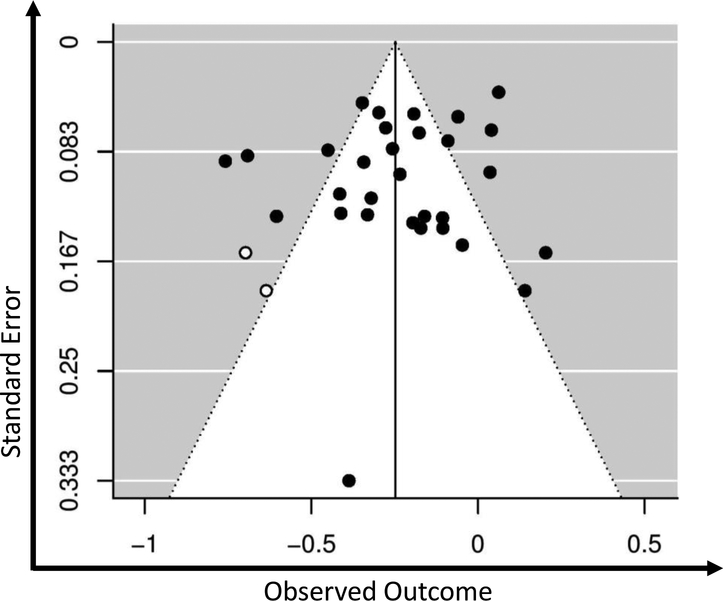
To identify potential publication bias, Begg’s funnel plots (Begg & Mazumdar, 1994) with Duval and Tweedie’s (2000) trim-and-fill adjustment were created and inspected visually as well as with Egger’s test of asymmetry and Begg’s rank test (Begg & Mazumdar, 1994; Sterne & Egger, 2006). In addition, Orwin’s Fail-safe N was calculated to estimate the number of studies with an effect size of zero (r = .00) that would be required to reduce the mean effect size across studies to a specified inconsequential level (i.e., a non-significant effect size) (Lipsey & Wilson, 2001). Finally, meta-regression analyses were conducted examining publication status (i.e., published vs. not published), year of data collection, and study reporting quality as potential moderators.
Results
Characteristics of the Studies
Of the 78 records included, 43 were cross-sectional, 26 were longitudinal, two were case-control, and seven were intervention studies. The mean sample size of the included studies was 179.62 (SD = 211.20; range = 12–1,280). The mean age of the samples was 55.75 (SD = 8.52; range = 25.00–73.30; k = 68). Concerning gender, 32 studies included a mixed sample; 36 studies included a female sample; six studies included a male sample; and four studies did not report the gender of the sample. The mean percentage of females across studies was 71.13% (SD = 33.43%; k = 74). The mean percentage of Caucasians across studies was 78.09% (SD = 27.84%; range = 0.00–100.00; k = 51). The mean percentage of participants who were married or partnered was 71.61% (SD =15.92%; range = 31.37–100.00; k = 56). The mean time since diagnosis was 2.16 years (SD = 1.78; range = 0.01–8.46; k = 28). The mean percentage of participants with advanced-stage cancer (e.g., stage III, IV, or metastatic) was 25.87% (SD = 25.40%; range = 0.00–100.00; k = 41). The majority of studies included samples of mixed cancer patients (29.49%, 23/78) or breast cancer patients (41.03%, 32/78). Appendix B provides additional information about the studies included in the meta-analyses.
Study Reporting Quality
The mean study reporting quality rating based on the STROBE criteria was 73.29% (SD = 8.99%; range = 57.14–93.10). The majority of studies fulfilled over 60% of the STROBE criteria. Almost all studies provided adequate information about the scientific background, specified their hypotheses, described measures and methods of main analyses, and discussed study limitations. However, several prevalent weaknesses in reporting methods and results were identified. For example, a small minority of studies reported how sample size was determined (reported in 5.13%, 4/78), reasons for non-participation (23.08%, 18/78), and methods of dealing with missing data (17.95%, 14/78). Appendix C provides additional information about study reporting quality.
Mean Effect Sizes
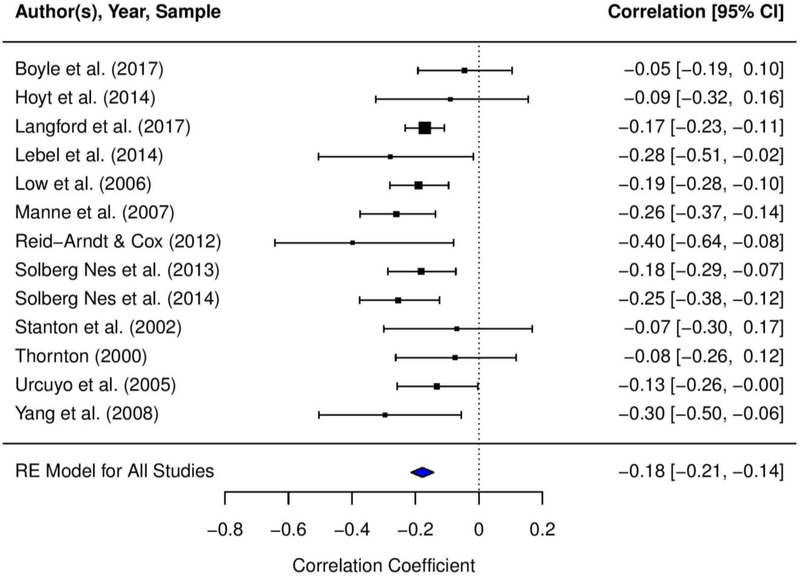
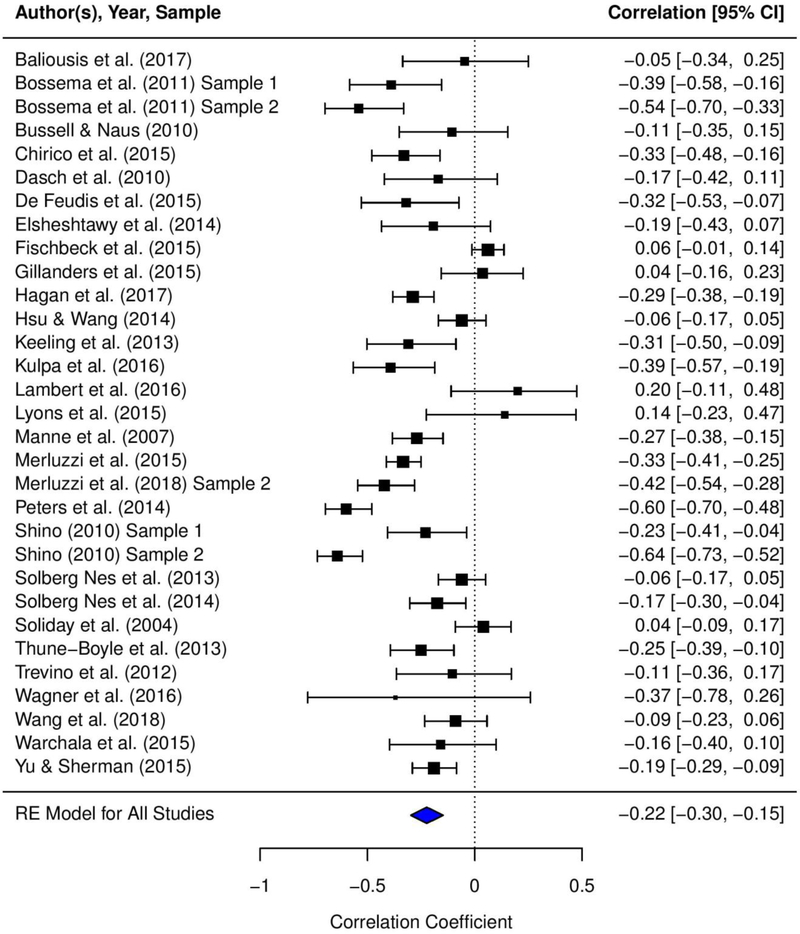
Table 1 presents the mean effect sizes that were calculated for the relationships between acceptance of cancer and (1) general distress, (2) cancer-specific distress, (3) depressive symptoms, and (4) anxiety symptoms. Appendix D presents the forest plots depicting the mean effect sizes for each study and the 95% CIs for the effect sizes. We found small to moderate, negative, and statistically significant mean effect sizes for the relationships between acceptance of cancer and general distress (r = −.31, p < .0001), cancer-specific distress (r = −.18, p < .0001), depressive symptoms (r = −.25, p < .0001), and anxiety symptoms (r = −.22, p < .0001). Based on stem-and-leaf plots, one study (Lyons et al., 2015) was identified as an outlier for the association between acceptance of cancer and depressive symptoms. Sensitivity analyses conducted excluding this study did not yield statistically significant differences in the results; thus, the study was included in the final analyses (Lipsey & Wilson, 2001).
Table 1.
Mean Effect Sizes for Associations between Acceptance of Cancer and Distress Outcomes
| Association | k | N | r | SE | 95% CI for r | Z for r | τ2 | Q | I2 | Fail-safe N | ρ | 80% CR for ρ | Z for ρ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acceptance of cancer - general distress | 75 | 14,368 | −.31 | .03 | −.36, −.26 | −10.79*** | .06 | 873.24*** | .91 | 183 | −.41 | −.84, .33 | −9.68*** |
| Acceptance of cancer - cancer-specific distress | 13 | 2,943 | −.18 | .02 | −.21, −.14 | −9.70*** | .00 | 12.76 | .00 | 13 | −.22 | −.31, −.13 | −9.23*** |
| Acceptance of cancer - depressive symptoms | 41 | 8,606 | −.25 | .03 | −.31, −.19 | −7.94*** | .04 | 370.98*** | .87 | 73 | −.33 | −.73, .22 | −7.58*** |
| Acceptance of cancer - anxiety symptoms | 29 | 4,974 | −.22 | .04 | −.30, −.15 | −5.63*** | .04 | 206.82*** | .86 | 40 | −.29 | −.69, .25 | −5.59*** |
p < .001.
Note. CI = confidence interval. CR = credibility interval. Fail-safe N = Orwin’s Fail-safe N. I2 = percentage of between-study variability. k = number of studies. N = total number of participants included in the analysis. ρ = reliability corrected mean effect size (correlation). Q = Cochran’s (1954) Q-statistic of heterogeneity. r = mean effect size (correlation). SE = standard error. τ2 = estimated variance of the population effect sizes. Z = z-test for statistical significance of the mean effect size. A z-score greater than the absolute value of 1.96 indicates statistical significance (Higgins, Thompson, Deeks, & Altman, 2003).
Moderator Analyses
Heterogeneity analyses demonstrated significant between-study variance in the relationships between acceptance of cancer and general distress (Q = 873.24; I2 = .91; τ2 = .06), depressive symptoms (Q = 370.98; I2 = .87; τ2 = .04), and anxiety symptoms (Q = 206.82; I2 = .86; τ2 = .04); thus, we conducted moderation analyses for these associations. However, there was minimal heterogeneity in the relationship between acceptance of cancer and cancer-specific distress (Q = 12.76; I2 = .00; τ2 = .00); therefore, we did not conduct moderation analyses for this association. Table 2 presents the results of the meta-regression analyses examining the five moderators.
Table 2.
Meta-Regression Analyses Examining Moderators of Associations between Acceptance of Cancer and Distress Outcomes
| Moderator | Outcome | k | N | I2 | R2 | B | SE | 95% CI | Z | Q(w) |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | General Distress | 65 | 11,622 | .91 | .00 | −.001 | .004 | −.009, .006 | −.31 | 724.21*** (df = 69) |
| Depressive Symptoms | 34 | 6,099 | .83 | .11 | −.010 | .005 | −.019, −.001 | −2.10* | 216.60*** (df = 36) | |
| Anxiety Symptoms | 26 | 3,709 | .82 | .04 | −.007 | .005 | −.017, .003 | −1.30 | 130.81*** (df = 26) | |
| Gender | General Distress | 71 | 13,306 | .91 | .00 | −.001 | .001 | −.003, .001 | −1.32 | 832.24*** (df = 76) |
| Depressive Symptoms | 39 | 7,752 | .87 | .03 | −.002 | .001 | −.004, .001 | −1.36 | 344.16*** (df = 42) | |
| Anxiety Symptoms | 28 | 4,503 | .85 | .00 | −.001 | .001 | −.004, .002 | −.85 | 185.19*** (df = 28) | |
| Marital Status | General Distress | 53 | 10,499 | .91 | .04 | .003 | .002 | −.001, .008 | 1.55 | 677.57*** (df = 56) |
| Depressive Symptoms | 26 | 5,735 | .89 | .20 | .007 | .003 | .001, .012 | 2.43* | 239.84*** (df = 27) | |
| Anxiety Symptoms | 20 | 3,184 | .85 | .18 | .006 | .003 | .000, .011 | 2.08* | 126.78*** (df = 19) | |
| Time Since Diagnosis | General Distress | 28 | 5,328 | .87 | .08 | .036 | .023 | −.010, .082 | 1.55 | 182.55*** (df = 28) |
| Depressive Symptoms | 13 | 2,874 | .82 | .21 | .047 | .025 | −.002, .097 | 1.87† | 53.49*** (df = 12) | |
| Anxiety Symptoms | 7 | 1,531 | .85 | .13 | .054 | .040 | −.023, .131 | 1.37 | 42.28*** (df = 6) | |
| Cancer Stage | General Distress | 39 | 5,818 | .90 | .00 | .000 | .002 | −.004, .003 | −.21 | 363.50*** (df = 39) |
| Depressive Symptoms | 18 | 2,885 | .54 | .63 | .004 | .002 | .001, .007 | 2.59** | 40.91** (df = 16) | |
| Anxiety Symptoms | 13 | 1,598 | .00 | 1.00 | .004 | .001 | .002, .006 | 3.40*** | 12.18 (df = 11) |
p < .10;
p < .05;
p < .01;
p < .001.
Note. I2 = percentage of between-study variability. k = number of records included the analysis. N = total number of participants included in the analysis. Q(w) = the variance within group means. R2 = percentage of between-study variability explained by the moderator. SE = standard error. 95% CI = 95% confidence interval for B. Z = z-test for statistical significance of B. Age = the average age of the sample. Gender = percent female. Marital status = percent married/partnered. Time since diagnosis = mean time since diagnosis in years. Cancer stage = percent with advanced-stage cancer (e.g., stage III, IV, or metastatic).
Age.
Patients’ age was a significant moderator of the relationship between acceptance of cancer and depressive symptoms. We found that for every one year increase in age, the relationship between acceptance of cancer and depressive symptoms strengthened by 0.010 (b = −.010, SE = .005, z = −2.10, 95% CI −.019 to −.001; k = 34). However, age did not significantly moderate associations between acceptance of cancer and general distress (b = −.001, SE = .004, z = −.31, 95% CI −.009 to .006; k = 65) or anxiety symptoms (b = −.007, SE = .005, z = −1.30, 95% CI −.017 to .003; k = 26).
Gender.
Gender did not significantly moderate relationships between acceptance of cancer and general distress (b = −.001, SE = .001, z = −1.32, 95% CI −.003 to .001; k = 71), depressive symptoms (b = −.002, SE = .001, z = −1.36, 95% CI −.004 to .001; k = 39), or anxiety symptoms (b = −.001, SE = .001, z = −.85, 95% CI −.004 to .002; k = 28).
Marital status.
Marital status (i.e., percent of patients married/partnered) was a significant moderator of the relationships between acceptance of cancer and depressive symptoms (b = .007, SE = .003, z = 2.43, 95% CI .001 to .012; k = 26) as well as anxiety symptoms (b = .006, SE = .003, z = 2.08, 95% CI .000 to .011; k = 20). For each percent increase in married/partnered patients in the sample, the relationship between acceptance of cancer and depressive symptoms weakened by 0.007, and the relationship between acceptance of cancer and anxiety symptoms weakened by 0.006. However, the relationship between acceptance of cancer and general distress was not significantly moderated by marital status (b = .003, SE = .002, z = 1.55, 95% CI −.001 to .008; k = 53).
Time since diagnosis.
Time since diagnosis did not significantly moderate the relationships between acceptance of cancer and general distress (b = .036, SE = .023, z = 1.55, 95% CI −.010 to .082; k = 28), depressive symptoms (b = .047, SE = .025, z = 1.87, 95% CI −.002 to .097; k = 13), or anxiety symptoms (b = .054, SE = .040, z = 1.37, 95% CI −.023 to .131; k = 7).
Cancer stage.
Cancer stage was a significant moderator of the relationships between acceptance of cancer and depressive symptoms (b = .004, SE = .002, z = 2.59, 95% CI .001 to .007; k = 18) as well as anxiety symptoms (b = .004, SE = .001, z = 3.40, 95% CI .002 to .006; k = 13). For each percent increase in advanced-stage cancer patients in the sample, associations between acceptance of cancer and both depressive and anxiety symptoms weakened by 0.004. However, the relationship between acceptance of cancer and general distress was not significantly moderated by cancer stage (b = .000, SE = .002, z = −.21, 95% CI −.004 to .003; k = 39).
Publication Bias
Publication bias was an unlikely explanation for the relationships between acceptance of cancer and general distress (fail-safe N = 183), depressive symptoms (fail-safe N = 73), and anxiety (fail-safe N = 40), given the high number of studies with null effects that would be required to reduce the effect sizes to inconsequential levels. Further support for a low likelihood of publication bias included symmetrical distribution of the effect sizes in trim-filled funnel plots (see Appendix E) and non-significant results for Egger’s regression and Begg’s rank correlation tests. For the acceptance of cancer and cancer-specific distress association, fail-safe N indicated that only thirteen studies with null effects would be required to bring this relationship below a significant level (r = −.10, p > .05). However, publication bias was determined to be unlikely, as the trim-filled funnel plot showed symmetrical distribution of the effect sizes and Egger’s regression and Begg’s rank correlation tests were non-significant.
Additionally, publication status (i.e., published vs. not published), year of data collection, and study reporting quality did not significantly moderate the associations between acceptance of cancer and general distress (ps = .429, .544, and .059, respectively), cancer-specific distress (ps = .279, .748, and .667, respectively), depressive symptoms (ps = .235, .773, and .342, respectively), or anxiety (ps = .335, .322, and .338, respectively).
Discussion
The aims of the current meta-analysis were to estimate associations between acceptance of cancer and distress outcomes and examine whether the strength of these associations differed by demographic or medical factors. We found significant, small to moderate, negative relationships between acceptance of cancer and general distress, cancer-specific distress, depressive symptoms, and anxiety symptoms. These results are consistent with our integrated model of acceptance of cancer, which is grounded in coping theory (Lazarus & Folkman, 1984) and third wave theories (Hayes et al., 1999; Kabat-Zinn, 1990; Linehan, 1993; Segal et al., 2002). Additionally, age, marital status, and stage of cancer were identified as significant moderators of associations between acceptance of cancer and certain types of distress.
Overall, our findings suggest that acceptance may play a role in reducing cancer patients’ psychological distress. As stated earlier, we conceptualize acceptance of cancer as an active process that changes how patients relate to their internal experiences of the illness such as appraisals, emotional responses, and bodily sensations. Specifically, acceptance involves taking a nonjudgmental and compassionate stance toward internal experiences, thereby reducing the struggle with the realities of the illness. In addition, we conceptualize acceptance as behavioral willingness in response to cancer-related stressors, resulting in action consistent with deeply held values. For example, cancer patients employing acceptance-based coping strategies may focus on learning to live with the disease, take an active role in treatment decision-making, or express their thoughts and feelings about the disease to loved ones, all of which may reduce distress (Mack et al., 2008).
Although all of our results were small to moderate effect sizes, acceptance of cancer had the smallest association with cancer-specific distress, the largest association with general distress, and similar small associations with anxiety and depressive symptoms. Several explanations for these findings warrant mention. First, most of the included measures of cancer-specific distress assessed cancer-related post-traumatic stress symptoms, which tend to be endorsed at low levels compared to fear of recurrence and general distress (Abbey et al., 2015). Thus, limited variance in cancer-specific distress may have resulted in a small correlation with acceptance. In addition, fewer studies assessed cancer-specific distress (k=13) relative to other distress outcomes (ks=29 to 75). These findings also might be partially explained by limitations of acceptance measures (e.g., unitary construct). For instance, current measures do not assess acceptance of cancer-specific stressors, such as the prospect of death, changes in physical appearance, and role changes, which may be more strongly associated with cancer-specific distress.
Moderators of the acceptance of cancer and distress relationship
The current results suggest that acceptance confers greater psychological benefits for certain subgroups of cancer patients.
Age.
Although the relationship between acceptance of cancer and depressive symptoms was stronger for older cancer patients, this moderation effect was not significant when examining general distress or anxiety. These findings should be cautiously interpreted, as both younger and older adults were underrepresented in the studies. Acceptance may be an important process for older adults as they attain a developmental stage that involves reflection on their lives, including fulfilled life goals and regrets. Alternatively, acceptance may be equally important for adults across the age spectrum as they face a medical reality that interferes with important life goals or activities.
Gender.
Gender did not significantly moderate the relationships between acceptance of cancer and general distress, anxiety, or depressive symptoms, suggesting that acceptance may be an equally important coping strategy for men and women. Gender differences in acceptance coping have not been consistent across studies (Czerw, Religioni, & Deptala, 2016; Nowicki et al., 2015). It is possible that acceptance of different facets of cancer (e.g., reduced self-sufficiency, changes in body image) may confer greater mental health benefits for certain gender groups. However, current measures of acceptance are not multidimensional, thereby limiting our ability to identify possible gender differences in these associations. Alternatively, our null moderation findings for gender may be due to the limited representation of male cancer patients in the analyses.
Marital status.
Acceptance of cancer was more strongly correlated with depressive and anxiety symptoms among cancer patients without a spouse or partner. Marital status can be considered a proxy variable for enhanced social support, which appears to influence patients’ choice of coping strategies (Holland & Holahan, 2003; Kim, Han, Shaw, McTavish, & Gustafson, 2010) and, in turn, influence distress. When partner support is absent, coping strategies such as acceptance might be more important for reducing distress. However, the present findings were not entirely consistent, as marital status did not moderate the acceptance of cancer-general distress relationship. Our mixed findings might reflect the variable quality of marital relationships, as being in an unsatisfying marriage might undermine mental health to a greater extent than being single or divorced (Thomas, Liu, & Umberson, 2017; K. Williams, 2003).
Time since diagnosis.
Time since diagnosis did not significantly moderate the relationships between acceptance of cancer and general distress, anxiety, or depressive symptoms, suggesting that acceptance may be equally important at different phases of the cancer trajectory. As patients cope with initial diagnosis, or different phases of treatment or end-of-life care, acceptance may play a critical role in regulating emotions. Future longitudinal research should examined this hypothesis at different phases of the cancer trajectory. Additionally, information regarding time since diagnosis was limited and long-term cancer survivors were underrepresented in our analyses.
Stage of cancer.
Acceptance of cancer was more strongly correlated with depressive and anxiety symptoms among cancer patients with early-stage disease; however, this finding was not replicated when examining general distress. As advanced-stage cancer patients were underrepresented in the analyses, the results should be interpreted with caution. Additionally, given that many cancer patients do not have an accurate perception of their disease stage (Applebaum et al., 2014), stage of cancer as identified from medical charts might not be an appropriate proxy variable for patients’ understanding of their prognosis.
Limitations
Limitations of included studies should be noted. First, most of the studies were conducted in the United States, and the majority of participants were married middle-aged Caucasian women. In addition, most participants were diagnosed with early-stage cancers within the past 5 years. Given the current variance in sample characteristics, we only examined five demographic and medical variables as potential moderators. Future studies should explore other possible moderators (e.g., cancer type, comorbid medical conditions) in more diverse samples. Also, certain demographics or medical information were not reported in some studies, which led to reduced statistical power for a subset of the moderation analyses. The measurement of acceptance is another limitation, with all studies assessing it as a unitary construct that does not include all aspects of our conceptual definition. We contend that acceptance of cancer may be a multifaceted construct (e.g., acceptance of death, changes in physical appearance, reduced self-sufficiency). Therefore, measure development is required in order to identify potentially distinct associations of acceptance facets with distress and determine if these associations differ by gender or other moderating factors. Additionally, few authors using the COPE or Brief COPE reported whether the instructions were revised to refer to acceptance of cancer-specific stressors. However, it is likely that many patients considered their cancer experience when completing these measures, as the original instructions focus on coping with stressful life events.
Other study design and reporting issues warrant attention. First, in order to maximize the sample sizes and reduce the impact of third variables, all of our analyses were based on baseline correlational data. Thus, we cannot infer directionality and change over time in the acceptance of cancer-distress relationships. It is possible that increased acceptance is a result of reductions in distress, a hypothesis that may be tested in future longitudinal investigations. Additionally, as in prior reviews, our analysis of study reporting quality showed that many researchers did not report information such as the determination of sample size or reasons for non-participation, thereby lowering their reporting quality and limiting our ability to evaluate their research procedures. Even though moderation analyses for study reporting quality did not suggest that this was a significant concern, greater transparency in study reporting would improve readers’ ability to evaluate findings. Finally, as in previous reviews, our meta-analytic results may be affected by publication bias (i.e., the file drawer problem). However, we attempted to minimize this limitation by including unpublished results (e.g., dissertations, conference abstracts), and analyses of publication bias suggested that this was not a significant concern.
Directions for Future Research
Measurement of acceptance of cancer.
Current measures do not assess certain aspects of acceptance (e.g., behavioral willingness) and acceptance of specific cancer-related stressors (e.g., role changes). Multifaceted measures of acceptance are needed that incorporate patients’ perspectives through cognitive interviewing. Developing behavioral indices of acceptance focused on value-based action related to the cancer experience (e.g., adherence to goal-concordant medical care) would advance intervention research.
Acceptance in relation to attention monitoring and cognitive appraisal.
According to Monitor and Acceptance Theory (MAT) (Lindsay & Creswell, 2017), acceptance and attention monitoring skills together explain how mindfulness leads to reduced distress, raising the question as to whether acceptance without attention monitoring is sufficient for improving psychological outcomes. As none of the studies included in our meta-analyses measured attention monitoring, future research should test this hypothesis.
Additionally, some theorists contend that as part of mindfulness, acceptance has a critical role in promoting positive reappraisal (i.e., mentally reconstructing the illness and related stressors as meaningful or growth promoting), which confers mental health benefits (Garland et al., 2015; Garland et al., 2010). Although greater acceptance is correlated with more positive reappraisal in cancer patients (e.g., Bright & Stanton, 2018), the directionality of this relationship should be tested. Alternatively, acceptance might initially require cognitive control or nonappraisal in order to overcome habitual emotional reactivity (Y. Y. Tang, Hölzel, & Posner, 2015), and over time, cognitive control efforts may not be required to facilitate acceptance. Research is needed to test these hypotheses.
Acceptance and positive psychological outcomes.
Positive psychological outcomes (e.g., meaning in life, benefit finding, posttraumatic growth, courage, self-compassion, value clarification, positive emotions) in relation to acceptance of cancer have received less attention (Butts & Gutierrez, 2018; Garland et al., 2015; Garland et al., 2010; Gilbert & Choden, 2014; Hayes et al., 2006; Park, 2010). While Park (2010) contended that acceptance of a stressor is an outcome of the meaning-making process, acceptance may also be part of an active meaning-making process (Garland et al., 2017), leading to more effective meanings-made or growth, and, in turn, reducing distress. Acceptance can also be conceptualized as a profound act of courage and self-compassion (Gilbert & Choden, 2014; Neff, 2003). Research is needed to test these competing conceptualizations of acceptance.
Acceptance of cancer in a medical context.
In a cancer context, acceptance involves acknowledging one’s disease stage, prognosis, and available treatment options. However, up to 75% of cancer patients do not have an accurate understanding of their disease stage or prognosis (Applebaum et al., 2014), which may, in part, reflect a lack of acceptance.
Additionally, acceptance of cancer might impact patients’ engagement in medical decision-making. For example, patients with greater acceptance of their advanced cancer might be more willing to have end-of-life discussions, which may result in higher rates of advance care planning and enrollment in hospice compared to patients with less acceptance. Longitudinal studies are needed to investigate the potential predictive value of acceptance in medical decision-making while taking into account patients’ prognostic awareness and their values.
Acceptance of cancer in a social and cultural context.
Most cancer patients have a primary family caregiver who shares the psychosocial burden of the illness. Caregiver support may facilitate patient acceptance through enhanced emotional and cognitive processing of cancer information and meaning-making. Additionally, the majority of studies on acceptance of cancer have been conducted in the United States, and few studies have included information on patients’ and caregivers’ religious or spiritual beliefs and practices. Future cross-cultural studies are needed, as the meaning of acceptance of cancer for patients and caregivers depends on cultural and religious worldviews.
Implications for Clinical Practice and Intervention Research
Our findings have a number of implications for clinical practice and intervention research. First, our results contribute to a growing body of research suggesting that interventions fostering acceptance in cancer patients might improve mental health outcomes (Hulbert-Williams et al., 2015; Zhang et al., 2015). Reviews examining the effects of MBCT and MBSR in cancer patients showed moderate to large effects on anxiety and depressive symptoms (ds = .28 to .90) (Haller et al., 2017; Zhang et al., 2015). Additionally, pilot findings suggest that ACT leads to significant improvement in acceptance and reductions in distress outcomes in cancer patients (Fashler et al., 2018; Hulbert-Williams et al., 2015). Overall, our findings in combination with these trial results suggest that acceptance of cancer-related experiences might be targeted in interventions aiming to improve mental health in cancer patients. As the evidence for mindfulness-based interventions grows, clinicians have effectively disseminated them in cancer centers and cancer support groups (Shennan, Payne, & Fenlon, 2011).
Our findings also tentatively suggest that acceptance of cancer may benefit a number of patient subgroups (e.g., men and women, patients at different periods since diagnosis). However, a number of our moderation findings were mixed and limited by the small sample sizes for certain subgroups. Thus, recommendations regarding the targeting or tailoring of acceptance-based interventions to cancer patient subgroups are premature.
Regarding intervention research, identifying mechanisms of change in mindfulness and acceptance-based interventions for cancer patients is an important future direction. Recent reviews of studies with primarily healthy adults found promising evidence that changes in acceptance, mindfulness, rumination, worry, and self-compassion are potential mechanisms underlying the mental health benefits of mindfulness- and acceptance-based interventions (Alsubaie et al., 2017; Gu, Strauss, Bond, & Cavanagh, 2015). In addition, it is not clear whether acceptance, mindfulness more broadly (including acceptance and attention monitoring), or a combination of the aforementioned variables is the critical mechanism of change leading to psychological improvement. Two recent studies comparing attention monitoring training to acceptance and attention monitoring training in healthy adults found that the latter group had greater reductions in distress reactivity (Lindsay, Chin, et al., 2018; Lindsay, Young, Smyth, Brown, & Creswell, 2018). However, the pain literature has produced mixed evidence of the mediating role of acceptance in acceptance-based interventions (Elvery, Jensen, Ehde, & Day, 2017; McCracken & Gutierrez-Martinez, 2011). Future research may examine other potential mechanisms of change in acceptance-based interventions with cancer and other medical populations, such as meaning-making, value clarification, and value-based action. Finally, future research could focus on identifying therapist behaviors that may promote acceptance of cancer such as processing patients’ understanding of their diagnosis and its impact on their life or guiding patients through mindfulness exercises.
Conclusion
Consistent with our integrated model of acceptance based on coping theory (Lazarus & Folkman, 1984) and third wave theories (Hayes et al., 1999; Kabat-Zinn, 1990; Linehan, 1993; Segal et al., 2002), we found that greater acceptance of cancer was related to reduced distress. We also found preliminary evidence that acceptance of cancer might have a greater impact on the mental health of older age groups, singles, and those with early-stage disease. Findings suggest that links between acceptance of cancer and psychological outcomes warrant further study in mindfulness and acceptance-based intervention trials. Critical next steps include developing multifaceted measures of acceptance of cancer, clarifying the directionality of the acceptance-distress relationship, and identifying psychological and social processes that facilitate acceptance.
Highlights.
Integrated model of acceptance of cancer is presented
A meta-analytic review of acceptance of cancer and distress relationships
Results revealed significant relationships with small-to-moderate effect sizes
Age, marital status, and cancer stage were significant moderators
Theoretical and clinical implications with future directions are discussed
Acknowledgments
We would like to thank Adam T. Hirsh, Ph.D. and John McGrew, Ph.D. for their assistance with this project.
Appendix A. Syntax for search terms
Table A.1.
Syntax for Search Terms
| “cancer” | |
| (cancer OR tumor OR tumour OR oncolog* OR neoplasm) | |
| “acceptance of cancer” | |
| (“accept* of illness” OR “accept* of diagnosis” OR “accept* of cancer” OR “accept* of disease” OR “accept* illness” OR “accept* diagnosis” OR “accept* cancer” OR “accept* disease” OR “illness accept*” OR “diagnosis accept*” OR “cancer accept*” OR “disease accept*” OR “adjust* to illness” OR “adjust* to diagnosis” OR “adjust* to cancer” OR “adjust* to disease” OR “illness adjust*” OR “diagnosis adjust*” OR “cancer adjust*” OR “disease adjust*” OR “adapt* to illness” OR “adapt* to diagnosis” OR “adapt* to cancer” OR “adapt* to disease” OR “illness adapt*” OR “diagnosis adapt*” OR “cancer adapt*” OR “disease adapt*” OR “mak* peace” OR “peace with illness” OR “peace with diagnosis” OR “peace with cancer” OR “peace with disease” OR “peace* accept*”). | |
Appendix B. Studies included in the meta-analyses
Table B.1.
Studies Included in the Meta-Analyses
| Study (Country) | N | Design | Sample | Mean Age (SD) | % Female | % Adv. Stage | Mean Time Since Dx (SD) in years | % Married | % Quality | Acceptance Measure [α] | General Distress Measure [α] | ESa | Agg. ESb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acceptance of Cancer - General Distress: | |||||||||||||
| Aarstad, Lode, Larsen, Bru, and Aarstad (2011) (Norway) | 96 | C-S | Head and Neck | 61.00 (11.00) | 21.88 | - | 4.00 (2.00) | - | 67.86 | COPE-A [.63] | BDI | −0.140 | −0.140 |
| Aguado Loi et al. (2013) (USA) | 68 | C-S | Breast | 55.40 (10.40) | 100.00 | 14.71 | 2.80 (1.50) | 55.88 | 93.10 | BCOPE-A | PHQ-9 [.91] | −0.320 | −0.320 |
| Asuzu and Elumelu (2013) (Nigeria) | 237 | C-S | Mixed | 49.91 (13.48) | 83.54 | - | - | 72.57 | 68.97 | BCOPE-A | FACT-EWB | −0.065 | −0.065 |
| Avis et al. (2012) ** (USA) | 653 | C-S | Breast | - | 100.00 | 8.12 | 0.35 (0.10) | 71.67 | 73.33 | BCOPE-A | BDI | −0.280 | −0.280 |
| Baliousis, Rennoldson, Dawson, Mills, and das Nair (2017) ** (UK) | 45 | Long. | Stem Cell | 59.50 (11.70) | 31.11 | - | 2.40 (3.47) | 75.56 | 89.66 | BCOPE-A | −0.111 | ||
| DASS-A | −0.047 | ||||||||||||
| Bonnaud-Antignac, Bourdon, Dréno, and Quéreux (2017) (France) | 78 | Long. | Melanoma | 53.26 (12.70) | 42.31 | 0.00 | 1.92 | 70.51 | 61.29 | BCOPE-A | QLQ-C30 EWB | −0.040* | −0.040 |
| Bossema et al. (2011) Sample 1 (Netherlands) | 62 | C-C | Rectal | 68.60 (9.60) | 25.81 | - | - | - | 63.33 | ICQ-A [.85] | −0.425 | ||
| QLQ-C30 EWB [.82] | −0.315* | ||||||||||||
| Bossema et al. (2011) Sample 2 (Netherlands) | 60 | C-C | Rectal | 67.90 (10.30) | 36.67 | - | - | - | 63.33 | ICQ-A [.85] | −0.671 | ||
| QLQ-C30 EWB [.82] | −0.551* | ||||||||||||
| Boyle, Stanton, Ganz, and Bower (2017) (USA) | 175 | Long. | Breast | 53.00 (8.02) | 100.00 | 9.71 | 1.43 (0.22) | 78.20 | 83.33 | BCOPE-A | −0.056 | ||
| PANAS-N [.88] | −0.023 | ||||||||||||
| Bright and Stanton (2018) (USA) | 130 | Long. | Breast | 54.20 (11.70) | 100.00 | 8.46 | 0.50 (0.21) | 66.15 | 81.25 | COPE-A [.62] | −0.149 | ||
| CES-D-INT [.49] | −0.010 | ||||||||||||
| Brunault et al. (2016) (France) | 120 | C-S | Breast | 56.98 (10.80) | 100.00 | 5.83 | 0.54 | 69.17 | 86.67 | BCOPE-A | QLQ-C30 EWB | −0.530 | −0.530 |
| Bussell and Naus (2010) (USA) | 59 | Long. | Breast | 50.00 | 100.00 | 30.50 | - | - | 68.75 | BCOPE-A | −0.245 | ||
| POMS [.94] | −0.281 | ||||||||||||
| Cameron (1999)1 Sample 1 (USA) | 25 | Long. | Colorectal | 63.00 (12.30) | 100.00 | 24.00 | 1.62 (0.69) | 68.00 | 83.33 | COPE-A [.50] | POMS [.96] | 0.200 | 0.200 |
| Cameron (1999)1 Sample 2 (USA) | 19 | Long. | Colorectal | 69.10 (10.60) | 0.00 | 36.90 | 1.72 (1.20) | 57.90 | 83.33 | COPE-A [.50] | POMS [.96] | 0.250 | 0.250 |
| Carver et al. (1993) (USA) | 59 | Long. | Breast | 58.02 (10.83) | 100.00 | 0.00 | - | 71.19 | 70.97 | COPE-A [.68] | POMS [.87] | −0.680 | −0.680 |
| Chabowski et al. (2017) (Poland) | 155 | C-S | Lung | 62.23 (9.86) | 48.40 | 29.68 | - | - | 64.29 | AIS | SF-8 MH | −0.747* | −0.747 |
| Chirico, Lucidi, Mallia, D’Aiuto, and Merluzzi (2015) (Italy) | 123 | C-S | Breast | 45.69 (10.01) | 100.00 | 23.60 | - | - | 67.86 | CBI-2-A [.78] | −0.370 | ||
| CES-D [.88] | −0.310 | ||||||||||||
| Cieślak and Golusiński (2017) Sample 1 (Poland) | 71 | C-S | Breast | - | 100.00 | - | - | - | 57.14 | AIS | CWLA-D | −0.475 | −0.475 |
| Cieślak and Golusiński (2017) Sample 2 (Poland) | 19 | C-S | Breast | - | 100.00 | - | - | - | 57.14 | AIS | CWLA-D | −0.178 | −0.178 |
| Culver, Arena, Antoni, and Carver (2002) (USA) | 131 | Long. | Breast | 54.82 (10.77) | 100.00 | 0.00 | - | 55.73 | 70.97 | BCOPE-A | DI [.77] | −0.230 | −0.230 |
| Dasch et al. (2010) (USA) | 53 | Long. | Breast | 53.34 (9.99) | 100.00 | 6.00 | - | 88.68 | 71.88 | BCOPE-A [.67] | HADS-A [.87] | −0.170 | −0.170 |
| David, Montgomery, and Bovbjerg (2006) (USA) | 60 | Long. | Breast | 52.00 (12.21) | 100.00 | - | - | 55.00 | 66.67 | BCOPE-A | POMS | −0.140 | −0.140 |
| De Feudis, Lanciano, and Rinaldi (2015) (Italy) | 61 | C-S | Breast | 54.02 (11.92) | 100.00 | - | - | 75.41 | 71.43 | BCOPE-A [.57] | −0.364 | ||
| HADS-D [.65] | −0.310 | ||||||||||||
| Durá-Ferrandis et al. (2017) (USA) ** | 1,280 | Long. | Breast | 72.70 (5.90) | 100.00 | - | - | 55.31 | 81.25 | BCOPE-A [.67] | −0.068 | ||
| SF-12 MH [.87] | −0.071 | ||||||||||||
| Elsheshtawy, Abo-Elez, Ashour, Farouk, and El Zaafarany (2014) (Egypt) | 56 | C-S | Breast | 52.00 (13.30) | 100.00 | 12.50 | - | 62.50 | 57.14 | BCOPE-A | −0.259 | ||
| HADS-D | −0.255 | ||||||||||||
| Fischbeck et al. (2015) ** (Germany) | 689 | C-S | Melanoma | - | 51.38 | - | 8.46 | 82.73 | 73.33 | BCOPE-A | 0.016 | ||
| QLQ-C30 EWB [.89] | −0.085* | ||||||||||||
| Fischer et al. (2013) ** (Netherands) | 57 | Inter. | Breast | 50.70 (6.90) | 100.00 | - | - | 85.96 | 78.13 | BCOPE-A [.70] | HSCL-25 [.90] | −0.353 | −0.353 |
| Gillanders, Sinclair, MacLean, and Jardine (2015) (Scotland) | 105 | C-S | Mixed | - | 45.71 | - | 3.59 (4.61) | 76.19 | 93.10 | BCOPE-A | −0.005 | ||
| FACT-EWB | −0.158 | ||||||||||||
| Green, Pakenham, Headley, and Gardiner (2002) ** (Australia) | 56 | Inter. | Prostate | 73.30 (6.40) | 0.00 | - | - | - | 87.50 | COPE-A | DASS [.92] | 0.373 | 0.373 |
| Hagan et al. (2017) (USA) | 350 | C-S | Mixed | 64.86 (10.86) | 46.00 | - | - | 70.00 | 71.43 | BCOPE-A [.81] | −0.410 | ||
| HADS-D | −0.368 | ||||||||||||
| Hamilton et al. (2014) ** (USA) | 351 | Long. | Hematologic Malignancy | 49.01 | 47.86 | - | 1.52 (2.35) | - | 75.00 | BCOPE-A | −0.244 | ||
| POMS-F | −0.013 | ||||||||||||
| Heitzmann et al. (2011) ** (USA) | 31 | C-S | Mixed | - | - | - | - | - | 67.86 | CBI-A | FACT-EWB | −0.710* | −0.710 |
| Hoyt, Gamarel, Saigal, and Stanton (2016) ** (USA) | 171 | Long. | Testicular | 25.00 (3.30) | 0.00 | - | 2.46 (1.48) | 44.00 | 75.00 | BCOPE-A | FACT-EWB | −0.330* | −0.330 |
| Hsu and Wang (2014)2 ** (Taiwan) | 311 | Long. | Breast | 47.54 (8.79) | 100.00 | - | - | 89.40 | - | BCOPE-A | −0.150 | ||
| HADS-D | −0.200 | ||||||||||||
| Ichikura, Yamashita, Sugimoto, Kishimoto, and Matsushima (2018) ** (Japan) | 116 | C-S | Head and Neck | - | 9.48 | - | - | - | 85.71 | BCOPE-A [.68] | BDI [.88] | −0.017 | −0.017 |
| Karademas, Tsagaraki, and Lambrou (2009) ** (Greece) | 40 | C-S | Mixed | - | - | - | - | - | 64.29 | AIS [.80] | GHQ [.92] | −0.430 | −0.430 |
| Keeling, Bambrough, and Simpson (2013) (UK) | 74 | C-S | Brain | 38.30 (10.67) | 52.70 | 0.00 | 2.13 (1.52) | - | 76.67 | BCOPE-A [.75] | −0.347 | ||
| PANAS-P [.95] | −0.330* | ||||||||||||
| Kulpa, Ziętalewicz, Kosowicz, Stypuła-Ciuba, and Ziółkowska (2016) (Poland) | 78 | C-S | Gynecological | 54.00 | 100.00 | - | - | - | 64.29 | CERQ-A [.72] | −0.398 | ||
| HADS-D [.75] | −0.393 | ||||||||||||
| Lambert et al. (2016) ** (Australia) | 42 | Inter. | Prostate | 63.76 (6.75) | 0.00 | - | - | 100.00 | 80.65 | BCOPE-A | 0.161 | ||
| HADS-D | 0.078 | ||||||||||||
| Li et al. (2015) (China) | 665 | C-C | Breast | 45.55 (6.43) | 100.00 | 0.00 | 0.01 (0.02) | 94.10 | 87.10 | CERQ-A | FACT-EWB | −0.550* | −0.550 |
| Low, Stanton, Thompson, Kwan, and Ganz (2006) (USA) | 417 | Long. | Breast | 58.10 | 100.00 | - | - | 69.00 | 65.52 | BCOPE-A [.74] | CES-D [.70] | −0.250 | −0.250 |
| Lyons et al. (2015) ** (USA) | 31 | Inter. | Breast | 48.90 (6.52) | 100.00 | 22.58 | - | 87.10 | 63.33 | BCOPE-A | 0.184 | ||
| FACT-EWB | −0.200* | ||||||||||||
| Manne, Ostroff, and Winkel (2007) (USA) | 238 | Inter. | Breast | 49,50 (10.40) | 100.00 | 12.00 | - | - | 84.38 | COPE-A [.74] | −0.351 | ||
| MHI-WB [.87] | −0.310* | ||||||||||||
| Mazanec, Daly, Douglas, and Musil (2011) ** (USA) | 80 | Long. | Mixed | 62.28 (12.92) | 58.75 | 12.5 | - | 63.75 | 76.67 | CBI-A | PAIS | −0.591 | −0.591 |
| Merluzzi and Martinez Sanchez (1997) (USA) | 124 | C-S | Mixed | 60.50 | 66.00 | 36.00 | - | 70.00 | 60.71 | CBI-A [.87] | MHI | −0.320* | −0.320 |
| Merluzzi and Martinez Sanchez (2018) (USA) ** | 72 | C-S | Breast | 55.00 | 100.00 | 32.40 | 3.01 | 100.00 | 72.41 | CBI-A | −0.330 | ||
| PAIS [.97] | −0.221* | ||||||||||||
| Merluzzi, Philip, Yang, Heitzmann, and Conley (2015) ** (USA) | 471 | C-S | Mixed | - | - | - | - | - | 64.29 | −0.440 | |||
| HADS-D | −0.504 | ||||||||||||
| Merluzzi et al. (2018) Sample 1 (USA) | 560 | C-S | Mixed | 63.10 | 66.61 | - | - | 60.54 | 67.86 | CBI-3-A [.81] | CES-D | −0.590 | −0.590 |
| Merluzzi et al. (2018) Sample 2 (USA) | 151 | C-S | Mixed | 63.00 | 62.91 | - | - | 66.89 | 67.86 | CBI-3-A [.81] | −0.553 | ||
| HADS-D | −0.535 | ||||||||||||
| Merluzzi et al. (2018) Sample 3 (USA) | 287 | C-S | Mixed | 64.10 | 64.46 | - | - | 60.28 | 67.86 | CBI-3-A [.81] | CES-D | −0.296 | −0.296 |
| Milbury et al. (2016)2 ** (USA) | 158 | C-S | Lung | 62.90 (10.10) | 37.30 | 69.20 | 0.18 (0.13) | 97.60 | - | COPE-A | BSI | −0.094 | −0.094 |
| Mosher, Duhamel, Egert, and Smith (2010) ** (USA) | 87 | C-S | Breast | 50.00 (10.00) | 100.00 | 49.00 | 3.00 (4.00) | 50.57 | 85.71 | CBI-A [.57] | MHI [.93] | −0.536* | −0.536 |
| Nairn and Merluzzi (2003) ** Sample 1 (USA) | 137 | C-S | Mixed | - | - | - | - | - | 64.29 | CBI-A | PAIS | −0.566 | −0.566 |
| Nairn and Merluzzi (2003) ** Sample 2 (USA) | 383 | C-S | Mixed | - | - | - | - | - | 64.29 | CBI-A | −0.252 | ||
| CES-D | −0.247 | ||||||||||||
| Northouse et al. (2013) ** (USA) | 484 | Inter. | Mixed | 60.50 (10.90) | 61.40 | - | 3.62 | - | 83.87 | BCOPE-A | FACT-EWB | −0.163* | −0.163 |
| Perczek (1999)1 (USA) | 31 | Long. | Prostate | - | - | 0.00 | 0.04 | - | 63.33 | BCOPE-A | POMS [.93] | −0.150 | −0.150 |
| Peters, Goedendorp, Verhagen, van der Graaf, and Bleijenberg (2014) ** (Netherlands) | 137 | C-S | Mixed | 59.00 | 61.31 | - | - | 81.02 | 75.00 | ICQ-A | −0.597 | ||
| HADS-D | −0.435 | ||||||||||||
| Polanski, Chabowski, Jankowska-Polanska, Janczak, and Rosinczuk (2018) Sample 1 (Poland) | 72 | C-S | Lung | 62.80 (9.60) | 43.06 | 51.39 | - | 62.50 | 65.52 | AIS | QLQ-C30 EWB | −0.593 | −0.593 |
| Polanski et al. (2018) Sample 2 (Poland) | 185 | C-S | Lung | 64.10 (8.80) | 45.41 | 34.59 | - | 58.92 | 65.52 | AIS | QLQ-C30 EWB | −0.742 | −0.742 |
| Pucheu et al. (2010)2 ** (France) | 64 | Long. | Mixed | 55.40 (10.40) | 75.00 | - | 0.23 | - | - | ICQ-A | QLQ-C30 EWB | −0.230* | −0.230 |
| Rees-McGee (1991)1 (USA) | 101 | C-S | Mixed | 67.00 (8.30) | 45.54 | 0.00 | 0.31 (0.10) | 86.00 | 78.57 | COPE-A [.84] | MHI [.94] | −0.560* | −0.560 |
| Reid-Arndt and Cox (2012) (USA) ** | 36 | C-S | Breast | 55.81 (10.45) | 100.00 | 8.00 | - | 66.67 | 73.33 | BCOPE-A | −0.427 | ||
| POMS-T | −0.323 | ||||||||||||
| Roussi, Krikeli, Hatzidimitriou, and Koutri (2007) (Greece) | 72 | Long. | Breast | 54.13 | 100.00 | - | - | 83.00 | 65.63 | COPE-A | POMS | −0.430 | −0.430 |
| Schlegel, Talley, Molix, and Bettencourt (2009) (USA) | 223 | Long. | Breast | 59.19 (12.72) | 100.00 | 16.59 | - | 72.65 | 70.97 | BCOPE-A | CES-D [.87] | −0.110 | −0.110 |
| Schnoll, Knowles, and Harlow (2002) ** (USA) | 109 | C-S | Mixed | 60.30 (11.00) | 76.00 | - | 4.69 (5.28) | 71.00 | 71.43 | COPE-A | PAIS [.86] | −0.102 | −0.102 |
| Shino (2010)1 Sample 1 (Namibia) | 102 | C-S | Mixed | 56.77 (14.27) | 100.00 | - | 0.97 (0.97) | 31.37 | 74.07 | CBI-A [.65] | −0.348 | ||
| HADS-D | −0.330 | ||||||||||||
| Shino (2010)1 Sample 2 (South Africa) | 125 | C-S | Mixed | 52.21 (12.56) | 100.00 | - | 2.43 (2.52) | 35.20 | 74.07 | CBI-A [.91] | −0.724 | ||
| HADS-D | −0.660 | ||||||||||||
| Siwik et al. (2016)2 ** (USA) | 45 | C-S | Gynecological | 55.16 (1284) | 100.00 | - | 1.77 | - | - | COPE-A | BDI | −0.160 | −0.160 |
| Solberg Nes, Ehlers, Patten, and Gastineau (2013) ** (USA) | 314 | C-S | Hematologic Malignancy | 58.00 | 37.00 | - | - | - | 64.29 | BCOPE-A | −0.136 | ||
| BDI [.92] | −0.027 | ||||||||||||
| Solberg Nes, Ehlers, Patten, and Gastineau (2014) ** (USA) | 213 | Long. | Hematologic Malignancy | 57.00 (12.03) | 48.36 | - | - | 81.20 | 64.52 | BCOPE-A | −0.278 | ||
| BDI [.92] | −0.260 | ||||||||||||
| Soliday, Garofalo, Smith, Prostko, and Warner (2004) ** (USA) | 226 | C-S | Mixed | 52.40 (9.30) | 72.12 | 71.00 | - | 81.00 | 65.52 | BCOPE-A [.57] | 0.087 | ||
| HADS-D [.79] | 0.110 | ||||||||||||
| Stanton, Danoff-Burg, and Huggins (2002) (USA) | 70 | Long. | Breast | 52.63 (11.94) | 100.00 | 0.00 | - | 70.00 | 80.65 | COPE-A [.69] | POMS [.90] | 0.030 | 0.030 |
| Thornton (2000)1 (USA) | 106 | Long. | Prostate | 61.16 (7.22) | 0.00 | 18.00 | - | 100.00 | 80.00 | BCOPE-A | −0.202 | ||
| PSS | −0.110 | ||||||||||||
| Thuné-Boyle, Stygall, Keshtgar, Davidson, and Newman (2013) (UK) | 155 | C-S | Breast | 55.70 (13.50) | 100.00 | 8.00 | - | 50.97 | 80.00 | BCOPE-A [.65] | −0.288 | ||
| HADS-D [.79] | −0.247 | ||||||||||||
| Trevino, Fasciano, and Prigerson (2013) ** (USA) | 95 | C-S | Mixed | 33.40 (5.51) | 68.42 | 52.63 | 3.40 (2.99) | 56.84 | 72.41 | PEACE-A [.74] | MQOL-PWB [.86] | −0.474* | −0.474 |
| Trevino et al. (2012) ** (USA) | 53 | C-S | Mixed | 33.89 (5.70) | 66.04 | 52.83 | 3.72 (3.05) | 49.06 | 82.76 | BCOPE-A [.80] | −0.124 | ||
| MQOL-A [.70] | −0.105 | ||||||||||||
| Urcuyo, Boyers, Carver, and Antoni (2005) ** (USA) | 230 | C-S | Breast | 53.45 (12.34) | 100.00 | 0.00 | - | 70.87 | 75.00 | BCOPE-A | −0.341 | ||
| DI [.89] | −0.290 | ||||||||||||
| Wagner, Johns, Brown, Hanna, and Bigatti (2016) ** (USA) | 12 | Inter. | Mixed | 59.10 | 66.67 | 90.91 | - | 100.00 | 80.65 | S-PEACE | −0.637 | ||
| HADS-D | −0.534 | ||||||||||||
| Wang et al. (2018) (Taiwan) | 180 | C-S | Breast | 49.33 (8.11) | 100.00 | 18.40 | - | 87.80 | 75.00 | BCOPE-A | −0.250 | ||
| BABS-N [.63] | −0.140 | ||||||||||||
| Warchala, Wojtyna, and Krysta (2015) ** (Poland) | 60 | Long. | Leukemia | 39.62 (12.80) | 43.33 | - | - | 68.33 | 61.29 | AIS [.82] | −0.099 | ||
| RSC-P [.83] | −0.264* | ||||||||||||
| Yang, Brothers, and Andersen (2008) ** (USA) | 65 | Long. | Breast | 54.00 (11.00) | 100.00 | 100.00 | - | 72.00 | 80.65 | BCOPE-A | SF-36-MCS [.86] | −0.487* | −0.487 |
| Yeung, Lu, and Lin (2014) (China) | 238 | C-S | Mixed | 55.70 (9.10) | 74.37 | 17.65 | - | 90.34 | 79.31 | CBI-A [.84] | QOL-CS PWB [.90] | −0.500* | −0.500 |
| Yu and Sherman (2015) ** (Australia) | 338 | C-S | Breast | 53.50 (9.22) | 100.00 | 28.70 | 2.76 (1.86) | 100.00 | 75.86 | BCOPE-A [.67] | −0.274 | ||
| DASS-A [.81] | −0.190 | ||||||||||||
| Acceptance of Cancer - Cancer-Specific Distress: | |||||||||||||
| Boyle et al. (2017) ** (USA) | 175 | Long. | Breast | 53.00 (8.02) | 100.00 | 9.71 | 1.43 (0.22) | 78.29 | 83.33 | BCOPE-A | IOC-W [.94] | −0.045 | −0.045 |
| Hoyt et al. (2014) ** (USA) | 66 | C-S | Prostate | 65.76 (9.04) | 0.00 | - | - | 89.40 | 64.52 | BCOPE-A | IES-I | −0.090 | −0.090 |
| Langford et al. (2017) ** (USA) | 957 | C-S | Mixed | 57.47 (11.87) | 78.89 | 67.92 | - | 64.68 | 83.33 | BCOPE-A [.68] | IES [.92] | −0.171 | −0.171 |
| Lebel et al. (2014) ** (Canada) | 56 | Inter. | Mixed | 54.80 (9.00) | 100.00 | 32.20 | - | 58.90 | 93.10 | BCOPE-A [.81] | −0.279 | ||
| IES [.79] | −0.260 | ||||||||||||
| Low et al. (2006) (USA) | 417 | Long. | Breast | 58.10 | 100.00 | - | - | 69.00 | 65.52 | BCOPE-A [.74] | IES-R-C [.89] | −0.190 | −0.190 |
| Manne et al. (2007) (USA) | 238 | Inter. | Breast | 49,50 (10.40) | 100.00 | 12.00 | - | - | 84.38 | COPE-A [.74] | IES [.89] | −0.260 | −0.260 |
| Reid-Arndt and Cox (2012) ** | 36 | C-S | Breast | 55.81 (10.45) | 100.00 | 8.00 | - | 66.67 | 73.33 | BCOPE-A | IES-R-C | −0.398 | −0.398 |
| Solberg Nes et al. (2013) ** (USA) | 314 | C-S | Hematologic Malignancy | 58.00 | 37.00 | - | - | - | 64.29 | BCOPE-A | IES [.92] | −0.182 | −0.182 |
| Solberg Nes et al. (2014) ** (USA) | 213 | Long. | Hematologic Malignancy | 57.00 (12.03) | 48.36 | - | - | 81.20 | 64.52 | BCOPE-A | IES [.92] | −0.254 | −0.254 |
| Stanton et al. (2002) (USA) | 70 | Long. | Breast | 52.63 (11.94) | 100.00 | 0.00 | - | 70.00 | 80.65 | COPE-A [.69] | FORS [.76] | −0.070 | −0.070 |
| Thornton (2000)1 (USA) | 106 | Long. | Prostate | 61.16 (7.22) | 0.00 | 18.00 | - | 100.00 | 80.00 | BCOPE-A | −0.075 | ||
| IES-A [.82] | −0.090 | ||||||||||||
| Urcuyo et al. (2005) ** (USA) | 230 | C-S | Breast | 53.45 (12.34) | 100.00 | 0.00 | - | 70.87 | 75.00 | BCOPE-A | −0.133 | ||
| PCBC-R | −0.100 | ||||||||||||
| Yang et al. (2008) ** (USA) | 65 | Long. | Breast | 54.00 (11.00) | 100.00 | 100.00 | - | 72.00 | 80.65 | BCOPE-A | IES-C [.89] | −0.296 | −0.296 |
Pearson’s r.
The aggregated effect sizes.
Denotes doctoral dissertation
Denotes conference presentation
Denotes ES that was reverse-coded.
Denotes a record that included additional information from the author.
Note. N = Sample size for the effect size reported; α = Cronbach’s alpha for the measure; ES = Effect size; Adv. stage = Advanced-stage cancer (e.g., stage III or IV); Mean Time Since Dx = Mean time since cancer diagnosis (in years); SD = Standard Deviation; Study Designs are listed as C-C= Case-control, C-S= Cross-Sectional, Inter.= Intervention, and Long.= Longitudinal; Quality = Study reporting quality as measured by the Strengthening the reporting of observational studies in epidemiology [STROBE Checklist] (von Elm, Altman, & Egger, 2008). Acceptance of Cancer Measures: AIS = Acceptance of Illness Scale; BCOPE-A = Brief COPE - Acceptance Subscale; CBI-A = Cancer Behavior Inventory - Accepting Cancer/Maintaining Positive Attitude Subscale; CBI-2-A = Cancer Behavior Inventory version 2.0 - Accepting Cancer/Maintaining Positive Attitude Subscale; CBI-3-A = Cancer Behavior Inventory version 3.0 - Accepting Cancer/Maintaining Positive Attitude Subscale; CCCS-A = Coping with Colorectal Cancer Scale - Acceptance Subscale; CERQ-A = Cognitive Emotion Regulation Questionnaire - Acceptance Subscale; COPE-A = COPE Acceptance Subscale; ICQ-A = Illness Cognitions Questionnaire - Acceptance Subscale; PEACE-A = Peace, Equanimity and Acceptance in the Cancer Experience - Acceptance Subscale; S-PEACE = Single item peaceful acceptance of cancer. General Distress Measures: BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory; BSI-A = Brief Symptom Inventory- Anxiety Subscale; CES-D = Center for Epidemiologic Studies Depression Scale; CES-D-INT = Center for Epidemiologic Studies Depression Scale - Interpersonal Subscale; CES-D-NA = Center for Epidemiologic Studies Depression Scale - Negative Affect Subscale; CES-D-PA = Center for Epidemiologic Studies Depression Scale - Positive Affect Subscale; CES-D-SOM = Center for Epidemiologic Studies Depression Scale - Somaticizing Subscale; BSI-D = Brief Symptom Inventory - Depression Subscale; BSI-GSI = Brief Symptom Inventory - Global Severity Index; CWLA-D = Coping with Loss of Ability Scale - Depression Subscale; DASS = Depression Anxiety Distress Scales-21 Total Score; DASS-A = Depression Anxiety Distress Scales-21 - Anxiety Subscale; DASS-Dep = Depression Anxiety Distress Scales-21 - Depression Subscale; DASS-Dis = Depression Anxiety Distress Scales-21 - Distress Subscale; DI = Distress Index; DS = Demoralization Scale; DT = Distress Thermometer; FACT-EWB = Functional Assessment of Cancer Therapy - Emotional Well-being Subscale; GAD-7 = Generalized Anxiety Disorder-7; GHQ = General Health Questionnaire; HADS = Hospital Anxiety and Depression Scale - Total Score; HADS-A = Hospital Anxiety and Depression Scale - Anxiety Subscale; HADS-D = Hospital Anxiety and Depression Scale - Depression Subscale; HSCL-25 = Hopkins Symptom Checklist 25; MHI = Mental Health Inventory; MHI-A = Mental Health Inventory-Anxiety Subscale; MHI-D = Mental Health Inventory-Depression Subscale; MHI-L = Mental Health Inventory - Loss of Behavioral and Emotional Control Subscale; MHI-WB = Mental Health Inventory - Well-being Subscale; MQOL-A = McGill Quality of Life Questionnaire - Anxiety Subscale; MQOL-D = McGill Quality of Life Questionnaire - Depression Subscale; MQOL-PWB = McGill Quality of Life Questionnaire - Psychological Quality of Life Subscale; PAIS = Psychological Adjustment to Physical Illness Scale: Psychological Distress Subscale; PANAS = Positive and Negative Affect Schedule - Negative Affect; PANAS = Positive and Negative Affect Schedule - Positive Affect; PHQ-8 = Patient Health Questionnaire-8; PHQ-9 = Patient Health Questionnaire-9; POMS = Profile of Mood States: Total Mood Disturbance; POMS-A = Profile of Mood States - Anger Subscale; POMS-C = Profile of Mood States - Confusion Subscale; POMS-D = Profile of Mood States - Depression Subscale; POMS-F = Profile of Mood States - Fatigue Subscale; POMS-T = Profile of Mood States - Tension Subscale; PROMIS-A = Patient-Reported Outcomes Measurement Information System - Anxiety Scale; PROMIS-D = Patient-Reported Outcomes Measurement Information System - Depression Scale; PSS = Perceived Stress Scale; QLQ-C30 EWB = EORTC Quality of Life Questionnaire - Emotional Well-being Subscale; QOL-CS PWB = Quality of Life-Cancer Survivors Instrument - Psychological Well-being Subscale; SCL-90 = Symptom Distress Checklist-90 - Global Severity Index; SF-12-MCS = Short Form 12 - Mental Component Score; SF-36-MCS = Short Form 36 - Mental Component Score; STAI-S = State-Trait Anxiety Inventory - State Anxiety Subscale; STAI-T = State-Trait Anxiety Inventory - Trait Anxiety Subscale; ZDS = Zung’s Depression Scale; WHO-P = World Health Organization Quality of Life Instrument - Psychological Health Subscale. Cancer-Specific Distress Measures: IES-A = Impact of Events Scale - Avoidance Subscale; IES-C = Impact of Events Scale - Combined Score; IES-I = Impact of Events Scale - Intrusions Subscale; IES-R-A = Impact of Events Scale Revised - Avoidance Subscale; IES-R-C = Impact of Events Scale Revised - Combined Score; IES-R-H = Impact of Events Scale Revised – Hyperarousal Subscale; IES-R-I = Impact of Events Scale Revised - Intrusions Subscale; IOC-W = Impact of Cancer Scale - Worry Subscale; FORS = Fear of Recurrence Scale; PCBC-L = Profile of Concerns About Breast Cancer - Life and Pain Issues Subscale; PCBC-R = Profile of Concerns About Breast Cancer - Rejection Issues Subscale; PCL-C = PTSD Checklist: Civilian Version.
References
Aarstad, A. K., Lode, K., Larsen, J. P., Bru, E., & Aarstad, H. J. (2011). Choice of psychological coping in laryngectomized, head and neck squamous cell carcinoma patients versus multiple sclerosis patients. European Archives of Oto-Rhino-Laryngology, 268, 907–915. doi:10.1007/s00405-010-1417-6
Aguado Loi, C. X., Baldwin, J. A., McDermott, R. J., McMillan, S., Martinez Tyson, D., Yampolskaya, S., & Vandeweerd, C. (2013). Risk factors associated with increased depressive symptoms among Latinas diagnosed with breast cancer within 5 years of survivorship. Psycho-Oncology, 22, 2779–2788. doi:10.1002/pon.3357
Asuzu, C. C., & Elumelu, T. N. (2013). Assessing cancer patients’ quality of life and coping mechanisms in radiotherapy department of the University College Hospital, Ibadan. Psycho-Oncology, 22, 2306–2312. doi:10.1002/pon.3290
Avis, N. E., Levine, B., Naughton, M. J., Case, D. L., Naftalis, E., & Van Zee, K. J. (2012). Explaining age-related differences in depression following breast cancer diagnosis and treatment. Breast Cancer Research and Treatment, 136, 581–591. doi:10.1007/s10549-012-2277-0
Baliousis, M., Rennoldson, M., Dawson, D. L., Mills, J., & das Nair, R. (2017). Perceptions of hematopoietic stem cell transplantation and coping predict emotional distress during the acute phase after transplantation. Oncology Nursing Forum, 44, 96–107. doi:10.1188/17.ONF.96–107
Bonnaud-Antignac, A., Bourdon, M., Dréno, B., & Quéreux, G. (2017). Coping strategies at the time of diagnosis and quality of life 2 years later. Cancer Nursing, 40, E45-E53. doi:10.1097/NCC.0000000000000337
Bossema, E. R., Seuntiëns, M. W. M., Marijnen, C. A. M., Baas-Thijssen, M. C. M., van de Velde, C. J. H., & Stiggelbout, A. M. (2011). The relation between illness cognitions and quality of life in people with and without a stoma following rectal cancer treatment. Psycho-Oncology, 20, 428–434. doi:10.1002/pon.1758
Boyle, C. C., Stanton, A. L., Ganz, P. A., & Bower, J. E. (2017). Posttraumatic growth in breast cancer survivors: Does age matter? Psycho-Oncology, 26, 800–807. doi:10.1002/pon.4091
Bright, E. E., & Stanton, A. L. (2018). Prospective investigation of social support, coping, and depressive symptoms: A model of adherence to endocrine therapy among women with breast cancer. Journal of Consulting and Clinical Psychology, 86, 242–253. doi:10.1037/ccp0000272
Brunault, P., Champagne, A. L., Huguet, G., Suzanne, I., Senon, J. L., Body, G., … Camus, V. (2016). Major depressive disorder, personality disorders, and coping strategies are independent risk factors for lower quality of life in non-metastatic breast cancer patients. Psycho-Oncology, 25, 513–520. doi:10.1002/pon.3947
Bussell, V. A., & Naus, M. J. (2010). A longitudinal investigation of coping and posttraumatic growth in breast cancer survivors. Journal of Psychosocial Oncology, 28, 61–78. doi:10.1080/07347330903438958
Cameron, C. L. (1999). Coping and adjustment to colorectal cancer (Doctoral dissertation). Retrieved from ProQuest Dissertations & Theses database. (9941617)
Carver, C. S., Pozo, C., Harris, S. D., Noriega, V., Scheier, M. F., Robinson, D. S., … Clark, K. C. (1993). How coping mediates the effect of optimism on distress: A study of women with early stage breast cancer. Journal of Personality and Social Psychology, 65, 375–390. doi:10.1037/0022–3514.65.2.375
Chabowski, M., Polański, J., Jankowska-Polanska, B., Lomper, K., Janczak, D., & Rosinczuk, J. (2017). The acceptance of illness, the intensity of pain and the quality of life in patients with lung cancer. Journal of Thoracic Disease, 9, 2952–2958. doi:10.21037/jtd.2017.08.70
Chirico, A., Lucidi, F., Mallia, L., D’Aiuto, M., & Merluzzi, T. V. (2015). Indicators of distress in newly diagnosed breast cancer patients. PeerJ, 3, 1–17. doi:10.7717/peerj.1107
Cieślak, K., & Golusiński, W. (2017). Coping with loss of ability vs. acceptance of disease in women after breast cancer treatment. Reports of Practical Oncology & Radiotherapy, 22, 231–236. doi:10.1016/j.rpor.2017.01.001
Culver, J. L., Arena, P. L., Antoni, M. H., & Carver, C. S. (2002). Coping and distress among women under treatment for early stage breast cancer: Comparing African Americans, Hispanics and non-Hispanic whites. Psycho-Oncology, 11, 495–504. doi:10.1002/pon.615
Dasch, K. B., Cohen, L. H., Belcher, A., Laurenceau, J. P., Kendall, J., Siegel, S., … Graber, E. (2010). Affective differentiation in breast cancer patients. Journal of Behavioral Medicine, 33, 441–453. doi:10.1007/s10865-010-9274-8
David, D., Montgomery, G. H., & Bovbjerg, D. H. (2006). Relations between coping responses and optimism-pessimism in predicting anticipatory psychological distress in surgical breast cancer patients. Personality and Individual Differences, 40, 203–213. doi:10.1016/j.paid.2005.05.018
De Feudis, R., Lanciano, T., & Rinaldi, S. (2015). Coping strategies of southern Italian women predict distress following breast cancer surgery. Europe’s Journal of Psychology, 11, 280–294. doi:10.5964/ejop.v11i2.908
Durá-Ferrandis, E., Mandelblatt, J. S., Clapp, J., Luta, G., Faul, L., Kimmick, G., … Hurria, A. (2017). Personality, coping, and social support as predictors of long-term quality-of-life trajectories in older breast cancer survivors: CALGB protocol 369901 (Alliance). Psycho-Oncology, 26, 1914–1921. doi:10.1002/pon.4404
Elsheshtawy, E. A., Abo-Elez, W. F., Ashour, H. S., Farouk, O., & El Zaafarany, M. I. (2014). Coping strategies in Egyptian ladies with breast cancer. Breast Cancer: Basic & Clinical Research, 8, 97–102. doi:10.4137/bcbcr.s14755
Fischbeck, S., Imruck, B. H., Blettner, M., Weyer, V., Binder, H., Zeissig, S. R., … Beutel, M. E. (2015). Psychosocial care needs of melanoma survivors: Are they being met? PloS One, 10, 1–13. doi:10.1371/journal.pone.0132754
Fischer, M. J., Wiesenhaan, M. E., Heijer, A. D., Kleijn, W. C., Nortier, J. W. R., & Kaptein, A. A. (2013). From despair to hope: A longitudinal study of illness perceptions and coping in a psycho-educational group intervention for women with breast cancer. British Journal of Health Psychology, 18, 526–545. doi:10.1111/j.2044–8287.2012.02100.x
Gillanders, D. T., Sinclair, A. K., MacLean, M., & Jardine, K. (2015). Illness cognitions, cognitive fusion, avoidance and self-compassion as predictors of distress and quality of life in a heterogeneous sample of adults, after cancer. Journal of Contextual Behavioral Science, 4, 300–311. doi:10.1016/j.jcbs.2015.07.003
Green, H. J., Pakenham, K. I., Headley, B. C., & Gardiner, R. A. (2002). Coping and health-related quality of life in men with prostate cancer randomly assigned to hormonal medication or close monitoring. Psycho-Oncology, 11, 401–414. doi:10.1002/pon.599
Hagan, T. L., Fishbein, J. N., Nipp, R. D., Jacobs, J. M., Traeger, L., Irwin, K. E., … Temel, J. S. (2017). Coping in patients with incurable lung and gastrointestinal cancers: A validation study of the Brief COPE. Journal of Pain and Symptom Management, 53, 131–138. doi:10.1016/j.jpainsymman.2016.06.005
Hamilton, B. K., Rybicki, L., Dabney, J., McLellan, L., Haddad, H., Foster, L., … Copelan, E. A. (2014). Quality of life and outcomes in patients ⩾ 60 years of age after allogeneic hematopoietic cell transplantation. Bone Marrow Transplantation, 49, 1426–1431. doi:10.1038/bmt.2014.166
Heitzmann, C. A., Merluzzi, T. V., Jean-Pierre, P., Roscoe, J. A., Kirsh, K. L., & Passik, S. D. (2011). Assessing self-efficacy for coping with cancer: Development and psychometric analysis of the brief version of the Cancer Behavior Inventory (CBI-B). Psycho-Oncology, 20, 302–312. doi:10.1002/pon.1735
Hoyt, M. A., Gamarel, K. E., Saigal, C. S., & Stanton, A. L. (2016). Goal navigation, approach-oriented coping, and adjustment in young men with testicular cancer. Annals of Behavioral Medicine, 50, 572–581. doi:10.1007/s12160-016-9785-9
Hoyt, M. A., Marin-Chollom, A. M., Bower, J. E., Thomas, K. S., Irwin, M. R., & Stanton, A. L. (2014). Approach and avoidance coping: Diurnal cortisol rhythm in prostate cancer survivors. Psychoneuroendocrinology, 49, 182–186. doi:10.1016/j.psyneuen.2014.07.007
Hsu, W. Y., & Wang, A. W. T. (2014, October). Different effects of reflective pondering and brooding on depression among breast cancer patients-the mediating role of active coping. Psycho-Oncology, 23 (Suppl. 3), 339–340. Abstract for poster presentation at the IPOS 16th World Congress of Psycho-Oncology and Psychosocial Academy in Lisbon, Portugal.
Ichikura, K., Yamashita, A., Sugimoto, T., Kishimoto, S., & Matsushima, E. (2018). Patterns of stress coping and depression among patients with head and neck cancer: A Japanese cross-sectional study. Psycho-Oncology, 27, 556–562. doi:10.1002/pon.4549
Karademas, E. C., Tsagaraki, A., & Lambrou, N. (2009). Illness acceptance, hospitalization stress and subjective health in a sample of chronic patients admitted to hospital. Journal of Health Psychology, 14, 1243–1250. doi:10.1177/1359105309345169
Keeling, M., Bambrough, J., & Simpson, J. (2013). Depression, anxiety and positive affect in people diagnosed with low-grade tumours: The role of illness perceptions. Psycho-Oncology, 22, 1421–1427. doi:10.1002/pon.3158
Kulpa, M., Ziętalewicz, U., Kosowicz, M., Stypuła-Ciuba, B., & Ziółkowska, P. (2016). Anxiety and depression and cognitive coping strategies and health locus of control in patients with ovary and uterus cancer during anticancer therapy. Contemporary Oncology, 20, 171–175. doi:10.5114/wo.2016.60074
Lambert, S. D., McElduff, P., Girgis, A., Levesque, J. V., Regan, T. W., Turner, J., … Chong, P. (2016). A pilot, multisite, randomized controlled trial of a self-directed coping skills training intervention for couples facing prostate cancer: Accrual, retention, and data collection issues. Supportive Care in Cancer, 24, 711–722. doi:10.1007/s00520-015-2833-3
Langford, D. J., Cooper, B., Paul, S., Humphreys, J., Keagy, C., Conley, Y. P., … Dunn, L. B. (2017). Evaluation of coping as a mediator of the relationship between stressful life events and cancer-related distress. Health Psychology, 36, 1147–1160. doi:10.1037/hea0000524
Lebel, S., Maheu, C., Lefebvre, M., Secord, S., Courbasson, C., Singh, M., … Catton, P. (2014). Addressing fear of cancer recurrence among women with cancer: A feasibility and preliminary outcome study. Journal of Cancer Survivorship: Research & Practice, 8, 485–496. doi:10.1007/s11764014-03573
Li, L., Zhu, X., Yang, Y., He, J., Yi, J., Wang, Y., & Zhang, J. (2015). Cognitive emotion regulation: Characteristics and effect on quality of life in women with breast cancer. Health & Quality of Life Outcomes, 13, 1–10. doi:10.1186/s12955-015-0242-4
Low, C. A., Stanton, A. L., Thompson, N., Kwan, L., & Ganz, P. A. (2006). Contextual life stress and coping strategies as predictors of adjustment to breast cancer survivorship. Annals of Behavioral Medicine, 32, 235–244. doi:10.1207/s15324796abm3203_10
Lyons, K. D., Hull, J. G., Kaufman, P. A., Li, Z., Seville, J. L., Ahles, T. A., … Hegel, M. T. (2015). Development and initial evaluation of a telephone-delivered, behavioral activation, and problem-solving treatment program to address functional goals of breast cancer survivors. Journal of Psychosocial Oncology, 33, 199–218. doi:10.1080/07347332.2014.1002659
Manne, S., Ostroff, J. S., & Winkel, G. (2007). Social-cognitive processes as moderators of a couple-focused group intervention for women with early stage breast cancer. Health Psychology, 26, 735–744. doi:10.1037/0278–6133.26.6.735
Mazanec, S. R., Daly, B. J., Douglas, S., & Musil, C. (2011). Predictors of psychosocial adjustment during the postradiation treatment transition. Western Journal of Nursing Research, 33, 540–559. doi:10.1177/0193945910382241
Merluzzi, T. V., & Martinez Sanchez, M. A. (1997). Assessment of self-efficacy and coping with cancer: Development and validation of the Cancer Behavior Inventory. Health Psychology, 16, 163–170. doi:10.1037/0278–6133.16.2.163
Merluzzi, T. V., & Martinez Sanchez, M. A. (2018). Husbands’ perceptions of their wives’ breast cancer coping efficacy: Testing congruence models of adjustment. Cancer Management and Research, 10, 297–304. doi:10.2147/CMAR.S157124
Merluzzi, T. V., Philip, E., Yang, M., Heitzmann, C., & Conley, C. (2015). Assessing self-efficacy for coping with cancer: Exploratory (EFA) and confirmatory factor analyses (CFA) of version 3 of the Cancer Behavior Inventory (CBI). Psycho-Oncology, 24, 106–107. doi:10.1002/pon.3874
Merluzzi, T. V., Philip, E. J., Heitzmann Ruhf, C. A., Liu, H., Yang, M., & Conley, C. C. (2018). Self-efficacy for coping with cancer: Revision of the cancer behavior inventory (version 3.0). Psychological Assessment, 30, 486–499. doi:10.1037/pas0000483
Milbury, K., Shinn, E., Fosella, F., Pisters, K., Bruera, E., & Carmack, C. (2016, March). Coping processes predict survival in patients with lung cancer. Psychosomatic Medicine, 78 (Suppl. 3), A74. Abstract for poster presentation at the American Psychosomatic Society 74th Annual Meeting in Denver, Colorado.
Mosher, C. E., Duhamel, K. N., Egert, J., & Smith, M. Y. (2010). Self-efficacy for coping with cancer in a multiethnic sample of breast cancer patients: Associations with barriers to pain management and distress. The Clinical Journal of Pain, 26, 227–234. doi:10.1097/AJP.0b013e3181bed0e3
Nairn, R. C., & Merluzzi, T. V. (2003). The role of religious coping in adjustment to cancer. Psycho-Oncology, 12, 428–441. doi:10.1002/pon.654
Northouse, L. L., Mood, D. W., Schafenacker, A., Kalemkerian, G., Zalupski, M., LoRusso, P., … Kershaw, T. (2013). Randomized clinical trial of a brief and extensive dyadic intervention for advanced cancer patients and their family caregivers. Psycho-Oncology, 22, 555–563. doi:10.1002/pon.3036
Perczek, R. E. (1999). The role of optimism, coping, and distress: A prospective study of men facing a prostate cancer diagnosis (Doctoral dissertation). Retrieved from ProQuest Dissertations & Theses database. (9938332)
Peters, M., Goedendorp, M., Verhagen, S., van der Graaf, W. T. A., & Bleijenberg, G. (2014). Exploring the contribution of psychosocial factors to fatigue in patients with advanced incurable cancer. Psycho-Oncology, 23, 773–779. doi:10.1002/pon.3481
Polanski, J., Chabowski, M., Jankowska-Polanska, B., Janczak, D., & Rosinczuk, J. (2018). Histological subtype of lung cancer affects acceptance of illness, severity of pain, and quality of life. Journal of Pain Research, 11, 727–733. doi:10.2147/jpr.S155121
Pucheu, S., Oumima, N., You-Harada, C., Susbielle, D. D., Roygnan, C., Brasselet, V., … Consoli, S. M. (2010, May). Existential meaning and adaptation to cancer. Psycho-Oncology, 19 (Suppl. 2), S81-S82. Abstract for poster presentation at the IPOS 12th World Congress of Psycho-Oncology in Quebec, Canada.
Rees-McGee, S. A. (1991). Religiosity, control beliefs, coping strategies, and psychological adjustment to cancer (Doctoral dissertation). Retrieved from ProQuest Dissertations & Theses database. (9134879)
Reid-Arndt, S. A., & Cox, C. R. (2012). Stress, coping and cognitive deficits in women after surgery for breast cancer. Journal of Clinical Psychology in Medical Settings, 19, 127–137. doi:10.1007/s10880-011-9274-z
Roussi, P., Krikeli, V., Hatzidimitriou, C., & Koutri, I. (2007). Patterns of coping, flexibility in coping and psychological distress in women diagnosed with breast cancer. Cognitive Therapy and Research, 31, 97–109. doi:10.1007/s10608-006-9110-1
Schlegel, R. J., Talley, A. E., Molix, L. A., & Bettencourt, B. A. (2009). Rural breast cancer patients, coping and depressive symptoms: A prospective comparison study. Psychology & Health, 24, 933–948. doi:10.1080/08870440802254613
Schnoll, R. A., Knowles, J. C., & Harlow, L. (2002). Correlates of adjustment among cancer survivors. Journal of Psychosocial Oncology, 20, 37–59. doi:10.1300/J077v20n01_03
Shino, E. N. (2010). Psychological distress, quality of life, coping and adjustment: A comparison of Oshiwambo-speaking and Sesotho-speaking patients with breast and/or cervical cancer (Doctoral dissertation). Retrieved from http://hdl.handle.net/11660/2741.
Siwik, C. J., Phillips, K., Zimmaro, L. A., Bayley-Veloso, R., Hicks, A., Cash, E., … Sephton, S. E. (2016, March). Psychological and physiological effects of problem-focused and emotional approach to coping styles in gynecological cancer patients. Psychosomatic Medicine, 78 (Suppl. 3), A60. Abstract for poster presentation at the American Psychosomatic Society 74th Annual Meeting in Denver, Colorado.
Solberg Nes, L., Ehlers, S. L., Patten, C. A., & Gastineau, D. A. (2013). Self-regulatory fatigue in hematologic malignancies: Impact on quality of life, coping, and adherence to medical recommendations. International Journal of Behavioral Medicine, 20, 13–21. doi:10.1007/s12529-011-9194-1
Solberg Nes, L., Ehlers, S. L., Patten, C. A., & Gastineau, D. A. (2014). Self-regulatory fatigue, quality of life, health behaviors, and coping in patients with hematologic malignancies. Annals of Behavioral Medicine, 48, 411–423. doi:10.1007/s12160-014-9621-z
Soliday, E., Garofalo, J. P., Smith, S. R., Prostko, R. A., & Warner, R. R. P. (2004). Psychosocial functioning of carcinoid cancer patients: Test of a stress and coping mediated model. Journal of Applied Biobehavioral Research, 9, 156–171. doi:10.1111/j.1751–9861.2004.tb00098.x
Stanton, A. L., Danoff-Burg, S., & Huggins, M. E. (2002). The first year after breast cancer diagnosis: Hope and coping strategies as predictors of adjustment. Psycho-Oncology, 11, 93–102. doi:10.1002/pon.574
Thornton, A. A. (2000). Psychosocial adjustment and benefit-finding in prostate cancer patients and their partners (Doctoral dissertation). Retrieved from ProQuest Dissertations & Theses database. (3041537)
Thuné-Boyle, I. C. V., Stygall, J., Keshtgar, M. R. S., Davidson, T. I., & Newman, S. P. (2013). Religious/spiritual coping resources and their relationship with adjustment in patients newly diagnosed with breast cancer in the UK. Psycho-Oncology, 22, 646–658. doi:10.1002/pon.3048
Trevino, K. M., Fasciano, K., & Prigerson, H. G. (2013). Patient-oncologist alliance, psychosocial well-being, and treatment adherence among young adults with advanced cancer. Journal of Clinical Oncology, 31, 1683–1689. doi:10.1200/jco.2012.46.7993
Trevino, K. M., Maciejewski, P. K., Fasciano, K., Greer, J., Partridge, A., Kacel, E. L., … Prigerson, H. G. (2012). Coping and psychological distress in young adults with advanced cancer. Journal of Supportive Oncology, 10, 124–130. doi:10.1016/j.suponc.2011.08.005
Urcuyo, K. R., Boyers, A. E., Carver, C. S., & Antoni, M. H. (2005). Finding benefit in breast cancer: Relations with personality, coping, and concurrent well-being. Psychology & Health, 20, 175–192. doi:10.1080/08870440512331317634
von Elm, E., Altman, D. G., & Egger, M. (2008). The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Journal of Clinical Epidemiology, 61, 344–349. doi:10.1016/j.jclinepi.2007.11.008
Wagner, C. D., Johns, S., Brown, L. F., Hanna, N., & Bigatti, S. M. (2016). Acceptability and feasibility of a meaning-based intervention for patients with advanced cancer and their spouses: A pilot study. American Journal of Hospice & Palliative Medicine, 33, 546–554. doi:10.1177/1049909115575709
Wang, A. W.-T., Cheng, C.-P., Chang, C.-S., Chen, D.-R., Chen, S.-T., Shieh, V., … Hsu, W.-Y. (2018). Does the factor structure of the Brief COPE fit different types of traumatic events? A test of measurement invariance. European Journal of Psychological Assessment, 34, 162–173. doi:10.1027/1015–5759/a000321
Warchala, A., Wojtyna, E., & Krysta, K. (2015). Mental state and its psychophysical conditions in patients with acute leukaemia treated with bone marrow transplantation. Psychiatria Danubina, 27, S415-S422.
Yang, H. C., Brothers, B. M., & Andersen, B. L. (2008). Stress and quality of life in breast cancer recurrence: moderation or mediation of coping? Annals of Behavioral Medicine, 35, 188–197. doi:10.1007/s12160-008-9016-0
Yeung, N. C. Y., Lu, Q., & Lin, W. (2014). Specificity may count: not every aspect of coping self-efficacy is beneficial to quality of life among Chinese cancer survivors in China. International Journal of Behavioral Medicine, 21, 629–637. doi:10.1007/s12529-014-9394-6
Yu, Y., & Sherman, K. A. (2015). Communication avoidance, coping and psychological distress of women with breast cancer. Journal of Behavioral Medicine, 38, 565–577. doi:10.1007/s10865-015-9636-3
Appendix C. Study reporting quality assessment overview
Table C.1.
STROBE Study Reporting Quality Assessment (N = 78)
| Section | Item no. | Recommendation | No. of studies |
|---|---|---|---|
| Title and Abstract | |||
| 1 (a) | Indicate the study’s design with a commonly used term in the title or the abstract. | 26 | |
| (b) | Provide in the abstract an informative and balanced summary of what was done and what was found. | 74 | |
| Introduction | |||
| 2 | Explain the scientific background and rationale for the investigation being reported. | 74 | |
| 3 | State specific objectives, including any prespecified hypotheses. | 71 | |
| Methods | |||
| 4 | Present key elements of study design early in the paper. | 70 | |
| 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection. | 51 | |
| 6 (a) | Cohort study—Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up. | 58 | |
| Case-control study—Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls. | |||
| Cross-sectional study—Give the eligibility criteria, and the sources and methods of selection of participants. | |||
| 6 (b) | Cohort study—For matched studies, give matching criteria and number of exposed and unexposed. | 4 (NA for 70) | |
| Case-control study—For matched studies, give matching criteria and the number of controls per case. | |||
| 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable. | 74 | |
| 8 | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group. | 74 | |
| 9 | Describe any efforts to address potential sources of bias. | 52 | |
| 10 | Explain how the study size was arrived at. | 4 | |
| 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why. | 74 | |
| 12 (a) | Describe all statistical methods, including those used to control for confounding. | 73 | |
| 12 (b) | Describe any methods used to examine subgroups and interactions. | 70 (NA for 4) | |
| 12 (c) | Explain how missing data were addressed. | 14 | |
| 12 (d) | Cohort study—If applicable, explain how loss to follow-up was addressed. | 17 (NA for 39) | |
| Case-control study—If applicable, explain how matching of cases and controls was addressed. | |||
| Cross-sectional study—If applicable, describe analytical methods taking account of sampling strategy. | |||
| 12 (e) | Describe any sensitivity analyses. | 33 | |
| Section | Item no. | Recommendation | No. of studies |
| Results | |||
| 13 (a) | Report numbers of individuals at each stage of study—e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed. | 33 | |
| 13 (b) | Give reasons for non-participation at each stage. | 18 | |
| 13 (c) | Consider use of a flow diagram. | 7 (NA for 43) | |
| 14 (a) | Give characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders. | 73 | |
| 14 (b) | Indicate number of participants with missing data for each variable of interest. | 1 | |
| 14 (c) | Cohort study—Summarize follow-up time (e.g., average and total amount). | 26 (NA for 47) | |
| 15 | Cohort study—Report numbers of outcome events or summary measures over time. | 74 | |
| Case-control study—Report numbers in each exposure category, or summary measures of exposure. | |||
| Cross-sectional study—Report numbers of outcome events or summary measures. | |||
| 16 (a) | Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Make clear which confounders were adjusted for and why they were included. | 71 | |
| 16 (b) | Report category boundaries when continuous variables were categorized. | 25 (NA for 48) | |
| 16 (c) | If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period. | 2 (NA for 71) | |
| 17 | Report other analyses done—e.g., analyses of subgroups and interactions, and sensitivity analyses. | 73 | |
| Discussion | |||
| 18 | Summarize key results with reference to study objectives. | 74 | |
| 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias. | 65 | |
| 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence. | 70 | |
| 21 | Discuss the generalizability (external validity) of the study results. | 47 | |
| Other information | |||
| 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based. | 45 (NA for 5) |
Note. NA = Not applicable.
Adapted from “The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies,” by E. von Elm, D. G. Altman, and M. Egger, 2008, Journal of Clinical Epidemiology, 61, p. 346. Copyright 2008 by Elsevier.
Appendix D. Forest plots
Fig D.1.
Forest plot for the association between acceptance of cancer and general distress
Fig D.2.
Forest plot for the association between acceptance of cancer and cancer-specific distress
Fig D.3.
Forest plot for the association between acceptance of cancer and depressive symptoms
Fig D.4.
Forest plot for the association between acceptance of cancer and anxiety symptoms
Appendix E. Funnel plots
Fig E.1.
Trim-filled funnel plot for the association between acceptance of cancer and general distress
Fig E.2.
Trim-filled funnel plot for the association between acceptance of cancer and cancer-specific distress
Fig E.3.
Trim-filled funnel plot for the association between acceptance of cancer and depressive symptoms
Fig E.4.
Trim-filled funnel plot for the association between acceptance of cancer and anxiety symptoms
Footnotes
Declarations of interest: None.
References
- Abbey G, Thompson SBN, Hickish T, & Heathcote D (2015). A meta-analysis of prevalence rates and moderating factors for cancer-related post-traumatic stress disorder. Psycho-Oncology, 24, 371–381. doi: 10.1002/pon.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsubaie M, Abbott R, Dunn B, Dickens C, Keil TF, Henley W, & Kuyken W (2017). Mechanisms of action in mindfulness-based cognitive therapy (MBCT) and mindfulness-based stress reduction (MBSR) in people with physical and/or psychological conditions: A systematic review. Clinical Psychology Review, 55, 74–91. doi: 10.1016/j.cpr.2017.04.008 [DOI] [PubMed] [Google Scholar]
- Andreu Y, Galdón MJ, Durá E, Martínez P, Pérez S, & Murgui S (2012). A longitudinal study of psychosocial distress in breast cancer: Prevalence and risk factors. Psychology & Health, 27, 72–87. doi: 10.1080/08870446.2010.542814 [DOI] [PubMed] [Google Scholar]
- Applebaum AJ, Kolva EA, Kulikowski JR, Jacobs JD, DeRosa A, Lichtenthal WG, … Breitbart W (2014). Conceptualizing prognostic awareness in advanced cancer: A systematic review. Journal of Health Psychology, 19, 1103–1119. doi: 10.1177/1359105313484782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer RA (2010). Self-compassion as a mechanism of change in mindfulness- and acceptance-based treatments In Baer RA (Ed.), Assessing mindfulness and acceptance processes in clients: Illuminating the theory and practice of change (pp. 135–153). Oakland, CA: New Harbinger. [Google Scholar]
- Baer RA, Smith GT, & Allen KB (2004). Assessment of mindfulness by self-report: The Kentucky Inventory of Mindfulness Skills. Assessment, 11, 191–206. doi: 10.1177/1073191104268029 [DOI] [PubMed] [Google Scholar]
- Baliousis M, Rennoldson M, Dawson DL, Mills J, & das Nair R (2017). Perceptions of hematopoietic stem cell transplantation and coping predict emotional distress during the acute phase after transplantation. Oncology Nursing Forum, 44, 96–107. doi: 10.1188/17.ONF.96-107 [DOI] [PubMed] [Google Scholar]
- Barata A, Gonzalez BD, Sutton SK, Small BJ, Jacobsen PB, Field T, … Jim HS (2018). Coping strategies modify risk of depression associated with hematopoietic cell transplant symptomatology. Journal of Health Psychology, 23, 1028–1037. doi: 10.1177/1359105316642004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg CB, & Mazumdar M (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 50, 1088–1101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, & Rothstein HR (2011). Introduction to meta-analysis. West Sussex, UK: John Wiley & Sons. [Google Scholar]
- Bright EE, & Stanton AL (2018). Prospective investigation of social support, coping, and depressive symptoms: A model of adherence to endocrine therapy among women with breast cancer. Journal of Consulting and Clinical Psychology, 86, 242–253. doi: 10.1037/ccp0000272 [DOI] [PubMed] [Google Scholar]
- Brown AJ, Shen MJ, Urbauer D, Taylor J, Parker PA, Carmack C, … Bodurka DC (2017). The advance care planning readiness scale: Development and validation of a measure of willingness to discuss and acceptance of end-of-life care in gynecologic cancer patients. International Journal of Gynecological Cancer, 27, 838–846. doi: 10.1097/igc.0000000000000953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LF, Kroenke K, Theobald DE, Wu J, & Tu W (2010). The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psycho-Oncology, 19, 734–741. doi: 10.1002/pon.1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussell VA, & Naus MJ (2010). A longitudinal investigation of coping and posttraumatic growth in breast cancer survivors. Journal of Psychosocial Oncology, 28, 61–78. doi: 10.1080/07347330903438958 [DOI] [PubMed] [Google Scholar]
- Butts MC, & Gutierrez D (2018). Using acceptance and commitment therapy to (re)conceptualize stress appraisal. Journal of Mental Health Counseling, 40, 95–112. doi: 10.17744/mehc.40.2.01 [DOI] [Google Scholar]
- Card NA (2011). Applied meta-analysis for social science research. New York, NY: Guilford Press. [Google Scholar]
- Caruso R, Nanni MG, Riba MB, Sabato S, & Grassi L (2017). The burden of psychosocial morbidity related to cancer: Patient and family issues. International Review of Psychiatry, 29, 389–402. doi: 10.1080/09540261.2017.1288090 [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF, & Weintraub JK (1989). Assessing coping strategies: A theoretically based approach. Journal of Personality and Social Psychology, 56, 267–283. doi: 10.1037/0022-3514.56.2.267 [DOI] [PubMed] [Google Scholar]
- Chen Hsiu C, Su Ching K, & Siew Tzuh T (2017). Current status of accurate prognostic awareness in advanced/terminally ill cancer patients: Systematic review and meta-regression analysis. Palliative Medicine, 31, 406–418. doi: 10.1177/0269216316663976 [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Zhao F, Chang VT, Sloan JA, O’Mara AM, Gilman PB, … Fisch MJ (2013). The symptom burden of cancer: Evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group’s symptom outcomes and practice patterns study. Cancer, 119, 4333–4340. doi: 10.1002/cncr.28376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran WG (1954). The combination of estimates from different experiments. Biometrics, 10, 101–129. doi: 10.2307/3001666 [DOI] [Google Scholar]
- Craigs CL, Bennett MI, Hurlow A, West RM, & Ziegler LE (2018). Older age is associated with less cancer treatment: A longitudinal study of English cancer patients. Age and Ageing. 10.1093/ageing/afy094 [DOI] [PubMed] [Google Scholar]
- Czerw AI, Bilińska M, & Deptała A (2016). The assessment of the impact of socio-economic factors in accepting cancer using the Acceptance of Illness Scale (AIS). Contemporary Oncology, 20, 261–265. doi: 10.5114/wo.2015.54901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerw AI, Religioni U, & Deptala A (2016). Adjustment to Life with Lung Cancer. Advances in Clinical and Experimental Medicine, 25, 733–740. doi: 10.17219/acem/61014 [DOI] [PubMed] [Google Scholar]
- Dasch KB, Cohen LH, Belcher A, Laurenceau JP, Kendall J, Siegel S, … Graber E (2010). Affective differentiation in breast cancer patients. Journal of Behavioral Medicine, 33, 441–453. doi: 10.1007/s10865-010-9274-8 [DOI] [PubMed] [Google Scholar]
- Dinkel A, Kremsreiter K, Marten-Mittag B, & Lahmann C (2014). Comorbidity of fear of progression and anxiety disorders in cancer patients. General Hospital Psychiatry, 36, 613–619. doi: 10.1016/j.genhosppsych.2014.08.006 [DOI] [PubMed] [Google Scholar]
- Duval S, & Tweedie R (2000). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56, 455–463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- Elvery N, Jensen MP, Ehde DM, & Day MA (2017). Pain catastrophizing, mindfulness, and pain acceptance: What’s the difference? Clinical Journal of Pain, 33, 485–495. doi: 10.1097/AJP.0000000000000430 [DOI] [PubMed] [Google Scholar]
- Fashler SR, Weinrib AZ, Azam MA, & Katz J (2018). The use of acceptance and commitment therapy in oncology settings: A narrative review. Psychological Reports, 121, 229–252. doi: 10.1177/0033294117726061 [DOI] [PubMed] [Google Scholar]
- Fletcher L, & Hayes SC (2005). Relational frame theory, acceptance and commitment therapy, and a functional analytic definition of mindfulness. Journal of Rational Emotive & Cognitive Behavior Therapy, 23, 315–336. doi: 10.1007/s10942-005-0017-7 [DOI] [Google Scholar]
- Garland EL, Farb NA, Goldin P, & Fredrickson BL (2015). Mindfulness broadens awareness and builds eudaimonic meaning: A process model of mindful positive emotion regulation. Psychological Inquiry, 26, 293–314. doi: 10.1080/1047840X.2015.1064294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Fredrickson B, Kring AM, Johnson DP, Meyer PS, & Penn DL (2010). Upward spirals of positive emotions counter downward spirals of negativity: Insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clinical Psychology Review, 30, 849–864. doi: 10.1016/j.cpr.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Thielking P, Thomas EA, Coombs M, White S, Lombardi J, & Beck A (2017). Linking dispositional mindfulness and positive psychological processes in cancer survivorship: A multivariate path analytic test of the mindfulness-to-meaning theory. Psycho-Oncology, 26, 686–692. doi: 10.1002/pon.4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese-Davis J, Waller A, Carlson LE, Groff S, Zhong L, Neri E, … Bultz BD (2012). Screening for distress, the 6th vital sign: common problems in cancer outpatients over one year in usual care: associations with marital status, sex, and age. BMC Cancer, 12, 441–452. doi: 10.1186/1471-2407-12-441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P, & Choden. (2014). Mindful compassion: How the science of compassion can help you understand your emotions, live in the present, and connect deeply with others. Oakland, CA: New Harbinger. [Google Scholar]
- Gillanders DT, Sinclair AK, MacLean M, & Jardine K (2015). Illness cognitions, cognitive fusion, avoidance and self-compassion as predictors of distress and quality of life in a heterogeneous sample of adults, after cancer. Journal of Contextual Behavioral Science, 4, 300–311. doi: 10.1016/j.jcbs.2015.07.003 [DOI] [Google Scholar]
- Given BA, Given CW, & Kozachik S (2008). Family support in advanced cancer. CA: A Cancer Journal for Clinicians, 51, 213–231. doi: 10.3322/canjclin.51.4.213 [DOI] [PubMed] [Google Scholar]
- Graham CD, Gouick J, Krahé C, & Gillanders D (2016). A systematic review of the use of acceptance and commitment therapy (ACT) in chronic disease and long-term conditions. Clinical Psychology Review, 46, 46–58. doi: 10.1016/j.cpr.2016.04.009 [DOI] [PubMed] [Google Scholar]
- Gu J, Strauss C, Bond R, & Cavanagh K (2015). How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? A systematic review and meta-analysis of mediation studies. Clinical Psychology Review, 37, 1–12. doi: 10.1016/j.cpr.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Hagerty RG, Butow PN, Ellis PM, Dimitry S, & Tattersall MHN (2005). Communicating prognosis in cancer care: A systematic review of the literature. Annals of Oncology, 16, 1005–1053. doi: 10.1093/annonc/mdi211 [DOI] [PubMed] [Google Scholar]
- Haller H, Winkler MM, Klose P, Dobos G, Kümmel S, & Cramer H (2017). Mindfulness-based interventions for women with breast cancer: An updated systematic review and meta-analysis. Acta Oncologica, 56, 1–12. doi: 10.1080/0284186X.2017.1342862 [DOI] [PubMed] [Google Scholar]
- Hawkes AL, Pakenham KI, Chambers SK, Patrao TA, & Courneya KS (2014). Effects of a multiple health behavior change intervention for colorectal cancer survivors on psychosocial outcomes and quality of life: A randomized controlled trial. Annals of Behavioral Medicine, 48, 359–370. doi: 10.1007/s12160-014-9610-2 [DOI] [PubMed] [Google Scholar]
- Hayes SC, Follette VM, & Linehan MM (2004). Mindfulness and acceptance: Expanding the cognitive-behavioral tradition. New York, NY: Guilford Press. [Google Scholar]
- Hayes SC, Jacobson NS, Follette VM, & Dougher MJ (1994). Acceptance and change: Content and context in psychotherapy. Reno, NV: Context Press. [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, Masuda A, & Lillis J (2006). Acceptance and commitment therapy: Model, processes and outcomes. Behavior Research & Therapy, 44, 1–25. doi: 10.1016/j.brat.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Hayes SC, Strosahl K, & Wilson KG (1999). Acceptance and commitment therapy: An experiential approach to behavior change. New York, NY: Guilford Press. [Google Scholar]
- Hedges LV, & Olkin I (1985). Statistical methods for meta-analysis. Orlando, FL: Academic Press. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ, 327, 557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland KD, & Holahan CK (2003). The relation of social support and coping to positive adaptation to breast cancer. Psychology & Health, 18, 15–29. doi: 10.1080/0887044031000080656 [DOI] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, & Ott U (2011). How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science, 6, 537–559. doi: 10.1177/1745691611419671 [DOI] [PubMed] [Google Scholar]
- Hong J, Wei Z, & Wang W (2015). Preoperative psychological distress, coping and quality of life in Chinese patients with newly diagnosed gastric cancer. Journal of Clinical Nursing, 24, 2439–2447. doi: 10.1111/jocn.12816 [DOI] [PubMed] [Google Scholar]
- Hulbert-Williams NJ, Storey L, & Wilson KG (2015). Psychological interventions for patients with cancer: Psychological flexibility and the potential utility of acceptance and commitment therapy. European Journal of Cancer Care, 24, 15–27. doi: 10.1111/ecc.12223 [DOI] [PubMed] [Google Scholar]
- Hunter JE, & Schimdt FL (2004). Methods of meta-analysis: Correcting error and bias in research findings (2nd ed.). Thousand Oaks, CA: Sage. [Google Scholar]
- IBM. (2016). IBM SPSS Statistics for Windows. IBM Corp., Armonk, NY. [Google Scholar]
- Jaiswal R, Alici Y, & Breitbart W (2014). A comprehensive review of palliative care in patients with cancer. International Review of Psychiatry, 26, 87–101. doi: 10.3109/09540261.2013.868788 [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J (1990). Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. New York, NY: Dell. [Google Scholar]
- Kabat-Zinn J (1994). Wherever you go, there you are: Mindfulness meditation in everyday life. New York, NY: Hyperion. [Google Scholar]
- Kaliampos A, & Roussi P (2018). Quality of partner support moderates positive affect in patients with cancer. Psycho-Oncology, 27, 1298–1304. doi: 10.1002/pon.4672 [DOI] [PubMed] [Google Scholar]
- Karekla M, & Constantinou M (2010). Religious coping and cancer: Proposing an acceptance and commitment therapy approach. Cognitive and Behavioral Practice, 17, 371–381. doi: 10.1016/j.cbpra.2009.08.003 [DOI] [Google Scholar]
- Karekla M, & Panayiotou G (2011). Coping and experiential avoidance: Unique or overlapping constructs? Journal of Behavior Therapy and Experimental Psychiatry, 42, 163–170. doi: 10.1016/j.jbtep.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Barrios V, Forsyth JP, & Steger MF (2006). Experiential avoidance as a generalized psychological vulnerability: Comparisons with coping and emotion regulation strategies. Behavior Research & Therapy, 44, 1301–1320. doi: 10.1016/j.brat.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Kim J, Han JY, Shaw B, McTavish F, & Gustafson D (2010). The roles of social support and coping strategies in predicting breast cancer patients’ emotional well-being: Testing mediation and moderation models. Journal of Health Psychology, 15, 543–552. doi: 10.1177/1359105309355338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch L, Bertram H, Eberle A, Holleczek B, Schmid-Hopfner S, Waldmann A, … Arndt V (2014). Fear of recurrence in long-term breast cancer survivors-still an issue: Results on prevalence, determinants, and the association with quality of life and depression from the cancer survivorship-a multi-regional population-based study. Psycho-Oncology, 23, 547–554. doi: 10.1002/pon.3452 [DOI] [PubMed] [Google Scholar]
- Lazarus RS, & Folkman S (1984). Stress, appraisal, and coping. New York, NY: Springer. [Google Scholar]
- Lin C, Clark R, Tu P, Bosworth HB, & Zullig LL (2017). Breast cancer oral anti-cancer medication adherence: A systematic review of psychosocial motivators and barriers. Breast Cancer Research and Treatment, 165, 247–260. doi: 10.1007/s10549-017-4317-2 [DOI] [PubMed] [Google Scholar]
- Linden W, Vodermaier A, MacKenzie R, & Greig D (2012). Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. Journal of Affective Disorders, 141, 343–351. doi: 10.1016/j.jad.2012.03.025 [DOI] [PubMed] [Google Scholar]
- Lindsay EK, Chin B, Greco CM, Young S, Brown KW, Wright AGC, … Creswell JD (2018). How mindfulness training promotes positive emotions: Dismantling acceptance skills training in two randomized controlled trials. Journal of Personality and Social Psychology, 115, 944–973. doi: 10.1037/pspa0000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay EK, & Creswell JD (2017). Mechanisms of mindfulness training: Monitor and Acceptance Theory (MAT). Clinical Psychology Review, 51, 48–59. doi: 10.1016/j.cpr.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay EK, Young S, Smyth JM, Brown KW, & Creswell JD (2018). Acceptance lowers stress reactivity: Dismantling mindfulness training in a randomized controlled trial. Psychoneuroendocrinology, 87, 63–73. doi: 10.1016/j.psyneuen.2017.09.015 [DOI] [PubMed] [Google Scholar]
- Linehan MM (1993). Cognitive-behavioral treatment of borderline personality disorder. New York, NY: Guilford Press. [Google Scholar]
- Lipsey MW, & Wilson DB (2001). Practical meta-analysis. Thousand Oaks, CA: Sage. [Google Scholar]
- Livneh H (2000). Psychosocial adaptation to cancer: The role of coping strategies. Journal of Rehabilitation, 66, 40–49. [Google Scholar]
- Low J, Serfaty M, Davis S, Vickerstaff V, Gola A, Omar RZ, … Austen JSJ (2016). Acceptance and commitment therapy for adults with advanced cancer (CanACT): Study protocol for a feasibility randomised controlled trial. Trials, 17, 1–8. doi: 10.1186/s13063-016-1169-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons KD, Hull JG, Kaufman PA, Li Z, Seville JL, Ahles TA, … Hegel MT (2015). Development and initial evaluation of a telephone-delivered, behavioral activation, and problem-solving treatment program to address functional goals of breast cancer survivors. Journal of Psychosocial Oncology, 33, 199–218. doi: 10.1080/07347332.2014.1002659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack JW, Nilsson M, Balboni T, Friedlander RJ, Block SD, Trice E, & Prigerson HG (2008). Peace, Equanimity, and Acceptance in the Cancer Experience (PEACE): Validation of a scale to assess acceptance and struggle with terminal illness. Cancer, 112, 2509–2517. doi: 10.1002/cncr.23476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne SL, & Badr H (2008). Intimacy and relationship processes in couples’ psychosocial adaptation to cancer. Cancer, 112, 2541–2555. doi: 10.1002/cncr.23450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne SL, Kashy DA, Virtue S, Criswell KR, Kissane DW, Ozga M, … Rodriguez L (2018). Acceptance, social support, benefit-finding, and depression in women with gynecological cancer. Quality of Life Research, 27, 2991–3002. doi: 10.1007/s11136-018-1953-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Schwab RB, & Irwin SA (2015). Depression as a predictor of adherence to adjuvant endocrine therapy (AET) in women with breast cancer: a systematic review and meta-analysis. Breast Cancer Research and Treatment, 152, 239–246. doi: 10.1007/s10549-015-3471-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, & Eccleston C (2003). Coping or acceptance: What to do about chronic pain? Pain, 105, 197–204. doi: 10.1016/S0304-3959(03)00202-1 [DOI] [PubMed] [Google Scholar]
- McCracken LM, & Gutierrez-Martinez O (2011). Processes of change in psychological flexibility in an interdisciplinary group-based treatment for chronic pain based on Acceptance and Commitment Therapy. Behavior Research & Therapy, 49, 267–274. doi: 10.1016/j.brat.2011.02.004 [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, & Meader N (2011). Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncology, 12, 160–174. doi: 10.1016/S1470-2045(11)70002-X [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6, 1–6. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. (2015). Cancer staging fact sheet. Retrieved from https://www.cancer.gov/about-cancer/diagnosis-staging/staging
- Neff K (2003). Self-compassion: An alternative conceptualization of a healthy attitude toward oneself. Self & Identity, 2, 223–250. doi: 10.1080/15298860390129863 [DOI] [Google Scholar]
- Nowicki A, Marciniak J, Farbicka P, & Banaszkiewicz Z (2015). Satisfaction with life and disease acceptance by patients with a stomy related to surgical treatment of the rectal cancer - determinants of quality of life? Polish Journal of Surgery, 87, 434–442. doi: 10.1515/pjs-2015-0085 [DOI] [PubMed] [Google Scholar]
- Park CL (2010). Making sense of the meaning literature: An integrative review of meaning making and its effects on adjustment to stressful life events. Psychological Bulletin, 136, 257–301. doi: 10.1037/a0018301 [DOI] [PubMed] [Google Scholar]
- Peppercorn J (2014). The financial burden of cancer care: Do patients in the US know what to expect? Expert Review of Pharmacoeconomics & Outcomes Research, 14, 835–842. doi: 10.1586/14737167.2014.963558 [DOI] [PubMed] [Google Scholar]
- Peters M, Goedendorp M, Verhagen S, van der Graaf WTA, & Bleijenberg G (2014). Exploring the contribution of psychosocial factors to fatigue in patients with advanced incurable cancer. Psycho-Oncology, 23, 773–779. doi: 10.1002/pon.3481 [DOI] [PubMed] [Google Scholar]
- Pinquart M, & Duberstein PR (2010). Depression and cancer mortality: A meta-analysis. Psychological Medicine, 40, 1797–1810. doi: 10.1017/S0033291709992285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M, Frohlich C, Silbereisen RK, & Wedding U (2005). Death acceptance in cancer patients. Omega: Journal of Death & Dying, 52, 217–235. doi: 10.2190/E5F0-GJUX-AM68-4777 [DOI] [Google Scholar]
- R Core Team. (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: Retrieved from http://www.R-project.org/ [Google Scholar]
- Rand K, Banno D, Shea A, Cripe L, Rand KL, Banno DA, … Cripe LD (2016). Life and treatment goals of patients with advanced, incurable cancer. Supportive Care in Cancer, 24, 2953–2962. doi: 10.1007/s00520-016-3113-6 [DOI] [PubMed] [Google Scholar]
- Satin JR, Linden W, & Phillips MJ (2009). Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer, 115, 5349–5361. doi: 10.1002/cncr.24561 [DOI] [PubMed] [Google Scholar]
- Schulze R (2004). Meta-analysis: A comparison of approaches. Cambridge, MA: Hogrefe & Huber. [Google Scholar]
- Scrignaro M, Barni S, & Magrin ME (2011). The combined contribution of social support and coping strategies in predicting post-traumatic growth: A longitudinal study on cancer patients. Psycho-Oncology, 20, 823–831. doi: 10.1002/pon.1782 [DOI] [PubMed] [Google Scholar]
- Sears SR, Stanton AL, & Danoff-Burg S (2003). The yellow brick road and the emerald city: Benefit finding, positive reappraisal coping and posttraumatic growth in women with early-stage breast cancer. Health Psychology, 22, 487–497. doi: 10.1037/0278-6133.22.5.487 [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, & Teasdale JD (2002). Mindfulness-based cognitive therapy for depression: A new approach to preventing relapse. New York, NY: Guildford Press. [Google Scholar]
- Sharma R, & Yetton P (2007). The contingent effects of training, technical complexity, and task interdependence on successful information systems implementation. MIS Quarterly, 31, 219–238. doi: 10.2307/25148789 [DOI] [Google Scholar]
- Shennan C, Payne S, & Fenlon D (2011). What is the evidence for the use of mindfulness-based interventions in cancer care? A review. Psycho-Oncology, 20, 681–697. doi: 10.1002/pon.1819 [DOI] [PubMed] [Google Scholar]
- Sirois FM, Molnar DS, & Hirsch JK (2015). Self-compassion, stress, and coping in the context of chronic illness. Self & Identity, 14, 334–347. doi: 10.1080/15298868.2014.996249 [DOI] [Google Scholar]
- Sterne JAC, & Egger M (2006). Regression methods to detect publication and other bias in meta-analysis In Rothstein HR, Sutton AJ, & Borenstein M (Eds.), Publication bias in meta-analysis (pp. 99–110). Chichester, UK: John Wiley & Sons. [Google Scholar]
- Tang ST, Liu LN, Lin K-C, Chung J-H, Hsieh C-H, Chou W-C, & Su P-J (2014). Trajectories of the multidimensional dying experience for terminally ill cancer patients. Journal of Pain and Symptom Management, 48, 863–874. doi: 10.1016/j.jpainsymman.2014.01.011 [DOI] [PubMed] [Google Scholar]
- Tang YY, Hölzel BK, & Posner MI (2015). The neuroscience of mindfulness meditation. Nature Reviews Neuroscience, 16, 213–225. doi: 10.1038/nrn3916 [DOI] [PubMed] [Google Scholar]
- Thomas PA, Liu H, & Umberson D (2017). Family relationships and well-being. Innovation in Aging, 1, 1–11. doi: 10.1093/geroni/igx025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threader J, & McCormack L (2016). Cancer-related trauma, stigma and growth: The ‘lived’ experience of head and neck cancer. European Journal of Cancer Care, 25, 157–169. doi: 10.1111/ecc.12320 [DOI] [PubMed] [Google Scholar]
- Tirch DD (2010). Mindfulness as a context for the cultivation of compassion. International Journal of Cognitive Therapy, 3, 113–123. doi: 10.1521/ijct.2010.3.2.113 [DOI] [Google Scholar]
- van den Beuken-van Everdingen MHJ, Peters ML, de Rijke JM, Schouten HC, van Kleef M, & Patijn J (2008). Concerns of former breast cancer patients about disease recurrence: A validation and prevalence study. Psycho-Oncology, 17, 1137–1145. doi: 10.1002/pon.1340 [DOI] [PubMed] [Google Scholar]
- Viechtbauer W (2005). Bias and efficiency of meta-analytic variance estimators in the random-effects model. Journal of Educational and Behavioral Statistics, 30, 261–293. doi: 10.3102/10769986030003261 [DOI] [Google Scholar]
- von Elm E, Altman DG, & Egger M (2008). The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Journal of Clinical Epidemiology, 61, 344–349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- Watson M, Greer S, Young J, Inayat Q, Burgess C, & Robertson B (1988). Development of a questionnaire measure of adjustment to cancer: the MAC scale. Psychological Medicine, 18, 203–209. doi: 10.1017/S0033291700002026 [DOI] [PubMed] [Google Scholar]
- Watson M, & Homewood J (2008). Mental Adjustment to Cancer Scale: Psychometric properties in a large cancer cohort. Psycho-Oncology, 17, 1146–1151. doi: 10.1002/pon.1345 [DOI] [PubMed] [Google Scholar]
- Wells KJ, Booth-Jones M, & Jacobsen PB (2009). Do coping and social support predict depression and anxiety in patients undergoing hematopoietic stem cell transplantation? Journal of Psychosocial Oncology, 27, 297–315. doi: 10.1080/07347330902978947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR, Deal AM, Lund JL, Chang Y, Muss HB, Pergolotti M, … Sanoff HK (2018). Patient-reported comorbidity and survival in older adults with cancer. Oncologist, 23, 433–439. doi: 10.1634/theoncologist.2017-0404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JC (2007). Development of a multifaceted acceptance scale: Construction and initial validation (Master’s thesis). Retrieved from ProQuest Dissertations & Theses database. (1447639)
- Williams JC, & Lynn SJ (2010). Acceptance: An historical and conceptual review. Imagination, Cognition and Personality, 30, 5–56. doi: 10.2190/IC.30.1.c [DOI] [Google Scholar]
- Williams K (2003). Has the future of marriage arrived? A contemporary examination of gender, marriage, and psychological well-being. Journal of Health and Social Behavior, 44, 470–487. [PMC free article] [PubMed] [Google Scholar]
- Wright BA (1983). Physical disability: A psychosocial approach (2nd ed.). New York, NY: HarperCollins Publishers. [Google Scholar]
- Zhang M-F, Wen Y-S, Liu W-Y, Peng L-F, Wu X-D, & Liu Q-W (2015). Effectiveness of mindfulness-based therapy for reducing anxiety and depression in patients with cancer: A meta-analysis. Medicine, 94, 1–9. doi: 10.1097/MD.0000000000000897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann FF, Burrell B, & Jordan J (2018). The acceptability and potential benefits of mindfulness-based interventions in improving psychological well-being for adults with advanced cancer: A systematic review. Complementary Therapies in Clinical Practice, 30, 68–78. doi: 10.1016/j.ctcp.2017.12.014 [DOI] [PubMed] [Google Scholar]