Overview
More than 80% of men with prostate cancer undergo active treatment, which can be associated with significant morbidity. Outcomes of surgical treatment vary widely depending on where and by whom the patient was treated, implying that there is room for improvement. Factors influencing outcomes include patient characteristics as well as some measure of procedure volume. While relationships between volume and outcomes for prostatectomy can most likely be explained by differences between surgeons (e.g., experience, technical skill), the hospital environment (e.g., team communication, safety culture) has the potential to either amplify or dampen the effects.
While most patient factors are immutable, these other aspects of surgical care and the delivery environment may afford opportunities for quality improvement. Collaborative quality improvement initiatives may prove to be an important vehicle for achieving better prostate cancer care. These grass roots organizations, driven largely by urologists dedicated to providing prostate cancer care, have had initial successes in improving some aspects of prostate cancer quality, including reducing unwarranted use of imaging and perioperative morbidity. However, much of the variation in functional outcomes after prostate cancer surgery arises from differences in technical skill. Evaluating and improving intraoperative surgeon performance will inevitably be challenging, as it requires acquisition and interpretation of data collected in the operating room. To this end, several methods have been described to objectively assess what happens in the operating room.
Introduction
Prostate cancer is the most common cancer in men with an incidence of more than 240,000 cases annually.1 More than 80% of these men undergo active treatment with either surgery or radiotherapy,2 which can be associated with significant morbidity.3 This morbidity weighed heavily in the United States Preventive Services Task Force’s recommendation against prostate specific antigen (PSA)-based prostate cancer screening.4 Nevertheless, a certain segment of the population carries a disproportionate share of this burden. For example, older age and a reduced preoperative level of function are strong determinants of the impact of treatment on urinary and sexual quality of life.3,5 However, even when accounting for patient factors, these outcomes vary widely depending on where and by whom the patient was treated, suggesting that surgical quality plays an important role.
In this review, we will first describe important factors influencing outcomes of surgical prostate cancer treatment. While some of these are immutable (e.g., age, baseline function), others may afford opportunities for improvement. Next, we will examine potential strategies for improving quality. Finally, we will discuss areas of future research on surgical quality of prostate cancer care.
Important determinants of patient outcomes
Patient factors
Ideally, one would like to achieve cancer control without impairment of urinary and sexual quality of life for all patients undergoing surgical prostate cancer treatment. However, both cancer control rates and morbidity vary widely, and are partly determined by characteristics of the patients and their disease. For instance, successful cancer control is largely determined by the biological propensity for metastases, with high-grade disease having a much greater propensity for this.6 It is also well established that postoperative morbidity, which typically manifests as urinary incontinence and erectile dysfunction, is substantially affected by patient age and preoperative functional status. Based on data from large cohort studies, 27% of men in their late seventies had frequent urinary leakage two years after radical prostatectomy, compared to only 5% of men younger than sixty.3 Similarly, younger age is among the most important predictors of recovery of postoperative erectile function.5 Patients with better baseline urinary and sexual function also have significantly better outcomes after prostatectomy. For example, men with unimpaired preoperative function had a two to three times higher likelihood of achieving erections suitable for intercourse than men whose preoperative function was in the lowest quartile.5 In addition, other patient factors such as preoperative PSA, body mass index, and educational level are also associated with postoperative outcomes (Figure).3,7 While most of these patient factors are not modifiable, they can inform preoperative counseling and assist the patient with treatment decision-making.
Figure.
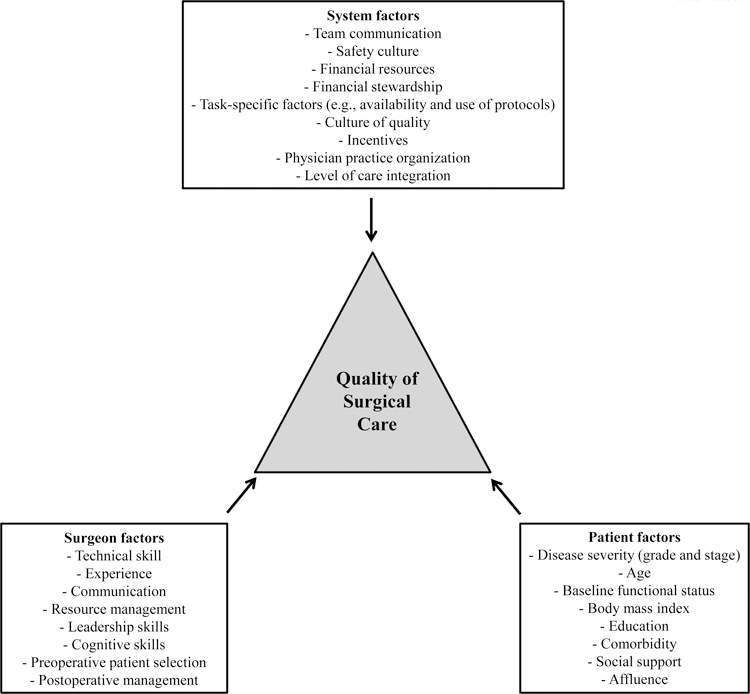
Factors influencing surgical quality of care.
Surgeon and hospital factors
Some outcomes of prostate cancer surgery are affected by whom the patient saw and where he got his care. For example, patients undergoing surgery in high-volume hospitals had an 8% absolute reduction in late urinary complications compared to those treated in a low-volume setting.8 Surgery in high-volume hospitals was also associated with significant decreases in 30-day mortality (relative risk 0.66) and readmission (relative risk 0.77).9 While most of these differences are likely explained by the kind of surgeons that operate in these hospitals, hospital-level factors such as team communication and safety culture may help surgeons with achieving better outcomes.10,11
It is well accepted that high-volume surgeons achieve better cancer control with less morbidity. In a multi-institutional analysis, only 11% of patients treated by high-volume surgeons experienced a biochemical recurrence at five years, compared to 18% of patients treated by low-volume surgeons.12 Regarding postoperative morbidity, patients who received care from high-volume surgeons had a 6% absolute reduction in postoperative complication rates (26% vs. 32%), an 8% absolute reduction in late urinary complications (20% vs. 28%), and a 4% absolute reduction in long-term incontinence (16% vs. 20%), compared to those treated by low-volume surgeons.8
While relationships between surgeon volume and outcomes are well established, one may contemplate why they exist. Based on the premise that “practice makes perfect”, surgeon skill almost certainly plays a central role, particularly with respect to achieving ideal urinary and sexual outcomes. Conceptually, surgeon technical skill in the operating room encompasses three domains: surgeon judgment, knowledge, and dexterity.13 Surgeon judgment comprises the decision making that takes place during a surgical procedure (e.g., the extent of the nerve-sparing that can be safely performed) while knowledge refers to the surgeon’s knowledge base that informs this decision making.13 Manual dexterity relates to the psychomotor tasks at hand during the procedure, for example accurately tying a knot or appropriately handling the tissue.13 A relationship between manual dexterity and outcomes in surgery has been shown in the laboratory setting. For example, surgeons with higher performance scores in motion analysis had lower leakage rates and better patency of vascular anastomosis.14 Nonetheless, it is hard to directly link technical skill to real-life outcomes, in part because technical skill is only very rarely formally assessed during actual surgical procedures.15
There are also non-technical skills that may affect surgical outcomes. These include the surgeon’s ability to work with a team and his cognitive skills (e.g., situation awareness, anticipating problems, and appropriate strategies for workload distribution).16 In addition to these intraoperative technical and non-technical skills, surgeon skill extends to preoperative patient selection and postoperative management (Figure). While we do not understand which of these surgeon skills are most important for the relationship between volume and outcomes, all of them likely contribute. Most importantly, cultivating these skill sets may afford a valuable opportunity for surgical quality improvement.
Healthcare reform and its potential impact on quality of care
Surgeons’ decision-making has the potential to be affected by the system in which they work in and the incentives that exist within that system. While the system is unlikely to change the technical quality of prostatectomy, it may very well affect pre- and postoperative patient care as well as other factors influencing quality of care (e.g., team work, safety culture). There has been a significant shift in practice organization of urologists, away from the solo or small group practice towards organization into large single-specialty groups.17 Physicians practicing in these large groups may have more direct financial incentives to utilize certain services (e.g., preoperative imaging, robotic prostatectomy), which could lead to overuse of these services among patients who are unlikely to benefit.18 However, alternatively, these large groups function like “focused factories”, with resources dedicated to a few service lines, which ultimately may translate into more effective care with better outcomes and less complications.18 Physicians organized in large groups also place a stronger emphasis on quality and are more likely to implement quality improvement processes.19
With reforms to the delivery system underway, changes in physician organization, care integration, and incentives, however, may have implications for the quality of prostate cancer care. The implementation of accountable care organizations (ACOs) created a push towards more care coordination as part of a broader goal of improving quality and constraining growth spending. These ACOs also have significant financial incentives to deter outsourcing of expensive care, however, which may discourage referral to highly specialized centers, such as comprehensive cancer centers.20 Thus, their implementation could indirectly encourage a shifting of surgical prostate cancer care away from these dedicated centers, which typically have high volumes, to the local delivery environment.
Strategies for improving surgical quality of care
Framework for measuring quality of care
A prerequisite for improving the surgical quality of care is being able to measure it. To this end, Avedis Donabedian pioneered a structure-process-outcomes framework in the 1960s, which is still widely applicable today.21
Measures of structure include assessment of the adequacy of facilities or equipment, and of the qualifications of medical staff (Table 1). Such measures might also include the training, experience, or skill of individual surgeons. The underlying assumption for these measures is that given an appropriate setting, good medical care will follow.21
Process measures focus on whether “good” medical care is appropriately applied and therefore require the specification of appropriate standards of care (Table 2).21 Examples of process measures include obtaining the appropriate preoperative work-up, while avoiding overuse of unnecessary tests among patients who are unlikely to benefit from them (e.g., bone scan for low risk patients).
Lastly, the evaluation of outcomes is an important component of assessing surgical quality of care. Morbidity, mortality, and cancer control all have face validity; however, many factors other than quality have the potential to affect these outcomes, complicating their measurement.21 In addition, because of its protracted clinical course, measuring prostate cancer mortality requires very long follow-up. For these reasons, most studies of surgical quality of care have focused on structure, process, and short-term outcome measures (Table 2).
Table 1.
Examples of structure of surgical prostate cancer care, according to Donabedian’s framework.21
| Domain | Example relevant to surgical prostate cancer care |
|---|---|
| Assessment of the adequacy of facilities | Number of hospital beds Number of operating rooms Availability of intensive care unit Financial resources |
| Assessment of the adequacy of equipment | Availability of surgical robot |
| Qualifications of medical staff | Board certification of urologists Training, experience, and skill of individual surgeons Communication skill of surgical team Safety culture within surgical teams |
Table 2.
Endorsed measures of surgical quality of prostate cancer care. The endorsing body and known compliance rates are shown for each measure. DRE = digital rectal exam; PSA = prostate specific antigen; PCPI = Physician Consortium for Performance Improvement; NQF = National Quality Forum.
| Measure | Endorsement | Compliance rate, % |
|---|---|---|
| Structure | ||
| Hospital with one or more board certified urologists | RAND | 92.6† |
| Process | ||
| DRE by treating physician | RAND | 80.6† |
| PSA by treating physician | RAND | 90.9† |
| Gleason grade by treating physician | RAND | 92.0† |
| Clinical stage by treating physician | RAND | 76.5† |
| Family history by treating physician | RAND | 76.7† |
| Comorbid disease by treating physician | RAND | 84.9† |
| Baseline urinary function by treating physician | RAND | 78.4† |
| Baseline sexual function by treating physician | RAND | 46.4† |
| Baseline bowel function by treating physician | RAND | 52.1† |
| Avoiding overuse of a bone scan for staging low risk prostate cancer | PCPI, NQF | 73.5‡ |
| Percentage of radical prostatectomy pathology reports that include the pT category, the pN category, the Gleason score and a statement about margin status. | NQF | 52.0* |
| At least 2 follow-up visits with treating physician within 1 year after completion of therapy | RAND | 55.0† |
| Outcomes | ||
| Not needing a procedure related to bladder neck contracture or urethral stricture | RAND | 74.3# |
Using Donabedian’s framework, RAND developed a comprehensive set of objective quality measures for prostate cancer care based on a step-wise process. Only indicators with high validity and feasibility were retained in the final list.22 Several of these indicators are measures of quality of care for the surgical prostate cancer patient (Table 2). More recently, the American Urological Association and the American Medical Association convened the Physician Consortium for Performance Improvement to produce a set of quality measures relevant to prostate cancer, incorporating some of the RAND indicators and an up-to-date evidence base. The National Quality Forum, a nonprofit organization consisting of a wide variety of stakeholders with the goal of improving the quality of the United States’ health care system, has also endorsed and developed several measures. The overall compliance with these measures of surgical quality is summarized in Table 2. Compliance was lowest for documentation of pretreatment functional status and pathology reporting, while pretreatment assessment of disease severity had higher compliance rates. However, given that for many measures compliance was less than 80%, there is room for quality improvement.
To improve quality, we need an infrastructure for identifying differences in patterns of care and treatment outcomes with the goal of leveraging these differences for quality improvement. Local or regional quality collaboratives are set up to provide such an infrastructure.23 For example, a regional collaborative reduced variation in the use of unwarranted imaging tests significantly by feedback of baseline data and review of guidelines for staging of localized prostate cancer.23 Similarly, audit and feedback improved the quality of radical prostatectomy pathology reports in a statewide intervention.24 A statewide collaborative of hospitals in Michigan was able to reduce perioperative morbidity by 2.6% through similar audit and feedback processes, which translated into 2,500 fewer Michigan patients with surgical complications each year.25
Getting into the operating room
While these examples illustrate how collaboratives can improve some aspects of quality, to achieve bigger gains, particularly in urinary and sexual function, “getting under the hood” in the operating room will be needed. Evaluating and improving intraoperative surgeon performance is a challenging task and requires acquisition and interpretation of data collected in the operating room. Surgeon performance can be evaluated with a variety of objective measures, such as procedure-specific checklists, global rating scales, and motion analysis. The Objective Structured Assessment of Technical Skills (OSATS) combines procedure-specific checklists and a global rating scale and is the only measure that has been used in multiple studies assessing performance during real-life surgery as opposed to a laboratory setting.26 Although the OSATS is currently the best standard for assessment of operative skills, high-level evidence only supports its use with gynecological bench tasks in a laboratory setting. The evidence underlying its use in the operating room setting is significantly weaker. A recent systematic review of the literature concluded that the OSATS can be used for the assessment of real-life performance with a goal of performance feedback and discussion, but should not be used for important examination or licensing decisions.26 Based on these data, the OSATS may be an appropriate tool to assess surgeon performance of radical prostatectomy for quality improvement.
More than two-thirds of radical prostatectomies are currently performed laparoscopically with robotic assistance. This allows for the additional possibility to videotape surgeries and later assess surgeon performance. However, rating surgeon skill based on videotapes has uncertain reliability and validity.26 Therefore, although assessment of videotaped surgeries would significantly decrease the logistical effort of assessing surgeon performance, significant methodological issues remain.
Many factors other than surgeon skill may affect what happens in an operating room. Researchers from the Imperial College in London have argued to describe an “operation profile” including all aspects of perioperative care (Table 3).15 Methods to capture these data would include video and audio recording, participant observers in the operating room, and brief postoperative questionnaires and interviews. Some operating rooms may even have sophisticated data recording systems installed, which capture visual and audio recordings of movements and communications within the operating room in addition to real-time recordings of physiological outputs as well as therapeutic equipment settings and performance.15
Table 3.
Characterizing the “operation profile” of a surgical procedure. Modified from Vincent et al.15
| Factor | Components measured |
|---|---|
| Patient factors | Cancer stage and grade Age Preoperative functional status Body mass index Education level |
| Surgical team | Familiarity with procedure Experience of previous work together Fatigue Stress |
| Processes and procedures | Adequacy of notes and management plan Consent and patient preparation Anesthetic procedures |
| Key operative events | Blood loss Minor and major complications Error compensation and recovery |
| Flow of information following patient | Handover |
| Surgeon technical skills | Surgeon judgement Surgeon knowledge informing intraoperative decision making Manual dexterity |
| Surgeon non-technical skills | Surgeon’s ability to work with a team Cognitive skills (situation awareness, anticipating problems) Strategies for workload distribution |
| Team performance | Team communication Leadership Safety culture Responsiveness and flexibility |
| Decision-making and situation awareness | Patient limitations Operation limitations Surgeon’s limitations Team limitations |
| Operative environment | Availability and adequacy of facilities Availability and adequacy of equipment Noise and lighting Distractions |
| Interruptions | Phone calls, messages, events outside the operating room |
Conclusions
The large amount of variation in prostate cancer outcomes and quality of care suggests that there is significant room for improvement. Some factors influencing quality of surgical prostate cancer care are modifiable, such as the technical quality with which the operation is performed. While preoperative processes of care have been successfully improved through collaborative quality improvement initiatives, assessing and improving surgeon skill is significantly more challenging, but potentially even more important. This would involve getting into the operating room to collect data on patients, surgeon performance, and operating room teams. Future research should then focus on finding ways to successfully use these data to broadly improve the technical aspects of care that are almost certainly tightly linked with patient reported outcomes.
Key points:
Outcomes of surgical treatment of prostate cancer vary widely depending on where and by whom the patient was treated, which implies that there is room for improvement.
Some drivers of variation in outcomes of prostatectomy are immutable (e.g., patient age and preoperative level of functioning), but others may afford opportunities for quality improvement (e.g., surgeon knowledge and skill, team coordination within the hospital).
Collaboratives had initial success in reducing use of unwarranted imaging and perioperative morbidity.
Evaluating and improving intraoperative surgeon performance will be challenging, because it requires acquisition and interpretation of data collected in the operating room.
Further research is needed on how to develop a successful quality improvement intervention in the operating room.
Acknowledgments
Grant support - FRS: T32 DK07782 from the NIH/NIDDK and Postdoctoral Fellowship PF-12-118-01-CPPB from the American Cancer Society. BLJ: Postdoctoral Fellowship 121805-PF-12-008-01-CPHPS from the American Cancer Society.
References:
- 1.American Cancer Society: Cancer Facts & Figures. 2011. Available at: http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-figures-2011, accessed August 24, 2011.
- 2.Cooperberg MR, Broering JM and Carroll PR: Time Trends and Local Variation in Primary Treatment of Localized Prostate Cancer. Journal of Clinical Oncology 2010; 28: 1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanford JL, Feng Z, Hamilton AS, et al. : Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA 2000; 283: 354–360. [DOI] [PubMed] [Google Scholar]
- 4.Moyer VA: Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of internal medicine 2012; 157: 120–134. [DOI] [PubMed] [Google Scholar]
- 5.Alemozaffar M, Regan MM, Cooperberg MR, et al. : Prediction of Erectile Function Following Treatment for Prostate Cancer. JAMA 2011; 306: 1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooperberg MR, Pasta DJ, Elkin EP, et al. : The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J. Urol 2005; 173: 1938–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanda MG, Dunn RL, Michalski J, et al. : Quality of life and satisfaction with outcome among prostate-cancer survivors. New England Journal of Medicine 2008; 358: 1250–1261. [DOI] [PubMed] [Google Scholar]
- 8.Begg CB, Riedel ER, Bach PB, et al. : Variations in morbidity after radical prostatectomy. N. Engl. J. Med 2002; 346: 1138–1144. [DOI] [PubMed] [Google Scholar]
- 9.Yao SL and Lu-Yao G: Population-based study of relationships between hospital volume of prostatectomies, patient outcomes, and length of hospital stay. J. Natl. Cancer Inst 1999; 91: 1950–1956. [DOI] [PubMed] [Google Scholar]
- 10.Haynes AB, Weiser TG, Berry WR, et al. : A surgical safety checklist to reduce morbidity and mortality in a global population. N. Engl. J. Med 2009; 360: 491–499. [DOI] [PubMed] [Google Scholar]
- 11.Birkmeyer NJO, Finks JF, Greenberg CK, et al. : Safety Culture and Complications After Bariatric Surgery. Ann. Surg 2012. [DOI] [PubMed]
- 12.Barocas DA, Mitchell R, Chang SS, et al. : Impact of surgeon and hospital volume on outcomes of radical prostatectomy. Urol. Oncol 2010; 28: 243–250. [DOI] [PubMed] [Google Scholar]
- 13.Darzi A and Mackay S: Assessment of surgical competence. Quality and Safety in Health Care 2001; 10: ii64–ii69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta V, Mandalia M, Mackay S, et al. : Relationship between skill and outcome in the laboratory-based model. Surgery 2002; 131: 318–323. [DOI] [PubMed] [Google Scholar]
- 15.Vincent C, Moorthy K, Sarker SK, et al. : Systems approaches to surgical quality and safety: from concept to measurement. Annals of surgery 2004; 239: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yule S, Flin R, Paterson-Brown S, et al. : Non-technical skills for surgeons in the operating room: a review of the literature. Surgery 2006; 139: 140–149. [DOI] [PubMed] [Google Scholar]
- 17.Schlossberg S: Supergroups and Economies of Scale. Urologic Clinics of North America 2009; 36: 95–100. [DOI] [PubMed] [Google Scholar]
- 18.Casalino LP, Devers KJ and Brewster LR: Focused factories? Physician-owned specialty facilities. Health Affairs 2003; 22: 56–67. [DOI] [PubMed] [Google Scholar]
- 19.Audet A-MJ, Doty MM, Shamasdin J, et al. : Measure, Learn, And Improve: Physicians’ Involvement In Quality Improvement. Health Aff 2005; 24: 843–853. [DOI] [PubMed] [Google Scholar]
- 20.Hohn DC: Health Care Reform and Its Implications for the Academic Cancer Center. Journal of the National Comprehensive Cancer Network 2012; 10: 130–132. [DOI] [PubMed] [Google Scholar]
- 21.Donabedian A: Evaluating the quality of medical care. Milbank Mem Fund Q 1966; 44: Suppl:166–206. [PubMed] [Google Scholar]
- 22.Spencer BA, Steinberg M, Malin J, et al. : Quality-of-care indicators for early-stage prostate cancer. J. Clin. Oncol 2003; 21: 1928–1936. [DOI] [PubMed] [Google Scholar]
- 23.Miller DC, Murtagh DS, Suh RS, et al. : Regional Collaboration to Improve Radiographic Staging Practices Among Men With Early Stage Prostate Cancer. J. Urol 2011; 186: 844–849. [DOI] [PubMed] [Google Scholar]
- 24.Imperato PJ, Waisman J, Wallen M, et al. : Results of a cooperative educational program to improve prostate pathology reports among patients undergoing radical prostatectomy. J Community Health 2002; 27: 1–13. [DOI] [PubMed] [Google Scholar]
- 25.Share DA, Campbell DA, Birkmeyer N, et al. : How A Regional Collaborative Of Hospitals And Physicians In Michigan Cut Costs And Improved The Quality Of Care. Health Aff 2011; 30: 636–645. [DOI] [PubMed] [Google Scholar]
- 26.Van Hove PD, Tuijthof GJM, Verdaasdonk EGG, et al. : Objective assessment of technical surgical skills. British Journal of Surgery 2010; 97: 972–987. [DOI] [PubMed] [Google Scholar]
- 27.Spencer BA, Miller DC, Litwin MS, et al. : Variations in Quality of Care for Men With Early-Stage Prostate Cancer. Journal of Clinical Oncology 2008; 26: 3735–3742. [DOI] [PubMed] [Google Scholar]
- 28.Choi WW, Williams SB, Gu X, et al. : Overuse of Imaging for Staging Low Risk Prostate Cancer. J. Urol 2011; 185: 1645–1649. [DOI] [PubMed] [Google Scholar]
- 29.Miller DC, Spencer BA, Shah RB, et al. : The quality of surgical pathology care for men undergoing radical prostatectomy in the U.S. Cancer 2007; 109: 2445–2453. [DOI] [PubMed] [Google Scholar]
- 30.Hu JC, Gold KF, Pashos CL, et al. : Role of surgeon volume in radical prostatectomy outcomes. J. Clin. Oncol 2003; 21: 401–405. [DOI] [PubMed] [Google Scholar]


