Approximately 70,000 adolescents and young adults (AYAs) between the ages of 15 and 39 years are diagnosed with cancer each year in the United States, which represents 6% of the cancer population.1,2 Diagnoses mostly commonly include leukemia, lymphoma, sarcoma, melanoma, breast cancer, colorectal cancer, thyroid cancer, testicular cancer, and brain tumors, among others.3 Relatively good overall survival (ie, 5-year survival rate of 83% across disease sites) has resulted in a growing population of AYA cancer survivors.4 AYA survivors face unique challenges, many of which stem from the interruption of diagnosis and treatment during a key phase of psychosocial growth and development. AYA patients must cope with cancer at a time of identity formation and the establishment of the independence necessary for adulthood. AYAs report challenges with emotional well-being, body image, and health management.5 Social interactions often are strained because families can be overprotective and friendships difficult to maintain.5 Romantic relationships can be challenging because survivors often are uncomfortable about discussing cancer with others.5 AYAs also may struggle to meet educational goals and sustain employment.6 Despite a growing awareness of their unique needs,7 comparatively little attention has focused on cognitive impairment in AYAs diagnosed with non-CNS cancers. This state of affairs is surprising in light of data that suggest an important role for cognition in resuming normal social activities and returning to education and employment after cancer in non-AYA survivors (ie, children, adults).6,8,9
Cognition in patients with cancer typically is assessed in three ways. The first is to administer self-report questionnaires that directly ask patients about changes in their memory, concentration, word-finding, and other cognitive abilities. Patients’ own perceptions of cognition arguably are the most clinically meaningful because the goal of the provider is to improve how the patient feels.10 The second way is through objective neuropsychological testing. Neuropsychological testing typically is administered under optimal conditions (eg, in a quiet office) rather than in situations in which cognitive lapses might be expected (eg, when patient is tired, distressed, or distracted). Thus, neuropsychological testing captures patients’ best performance rather than their typical functioning, which may be better reflected by subjective cognition. The third way is to conduct brain imaging to evaluate structural changes associated with brain regions known to be important for cognitive functioning or to examine patterns of activation at the same time cognition is being assessed. Although time consuming and burdensome for patients, brain imaging can provide important information. Imaging has demonstrated reductions in brain volumes as well as differential patterns of activation among patients with cancer compared with healthy individuals.11 These changes are associated with worse performance on neuropsychological tests, but the brain often is able to compensate to some degree, which obscures abnormalities.12
To our knowledge, no studies have examined objective neuropsychological performance or conducted brain imaging in samples that comprise exclusively cancer survivors diagnosed as AYAs; only two studies have focused on self-reported subjective cognitive impairment. Prasad et al9 found that cancer survivors diagnosed between the ages of 15 and 24 years often experienced cognitive impairment, with 22% reporting problems with memory, 14% with task efficiency, and 13% with organization. Self-reported impairments in task efficiency were associated with unemployment and depression. Rey et al13 found that 58% of AYAs with breast cancer report problems with concentration or memory in the first 28 months after diagnosis. These findings are consistent with studies of cognition in cancer survivors diagnosed as children who demonstrated impairment on neuropsychological tests of attention, memory, processing speed, and cognitive fluency despite IQ scores in the normal range.14-17 Moreover, neurocognitive deficits in childhood cancer survivors are associated with structural abnormalities observed through brain imaging.14,18 Thus, available data suggest that cognitive impairment is likely a significant problem in cancer survivors diagnosed as AYAs.
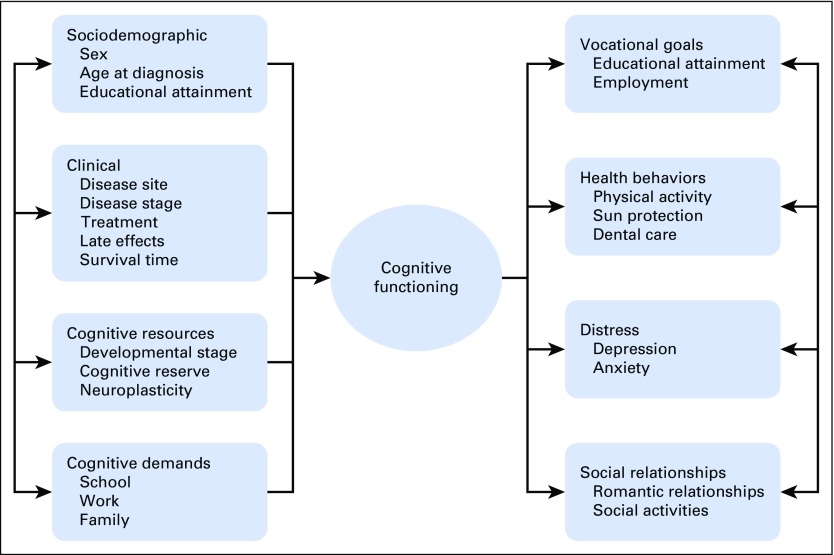
Cancer-related cognitive impairment is likely to affect survivorship among AYAs (Fig 1). AYA survivors often must juggle multiple responsibilities at work and home in addition to cancer follow-up care.19,20 Cognitive demands likely differ between younger AYAs who may be focused on educational attainment and development of social and romantic relationships compared with older AYAs who may be focused on work and family. Competing demands at school, work, and home are particularly relevant to cognition because problems in executive functions, such as multitasking, have been reported in patients with cancer diagnosed as adults.21 In addition, the extent to which cancer-related cognitive impairment affects AYAs’ ability to adhere to medical recommendations is unclear, but data from childhood cancer survivors suggest that self-reported cognitive problems are a risk factor for poor health behaviors (eg, reduced physical activity, sun protection, dental care).22 Anecdotally, AYA survivors express difficulty with focusing and carrying on a complex conversation in situations wherein distraction is high, such as in a crowd. Embarrassment about cognitive issues may increase social isolation.
Fig 1.
Putative factors that contribute to and result from cognitive functioning in cancer survivors diagnosed as adolescents or young adults.
Studies that focus specifically on survivors diagnosed as AYAs are important because various cognitive abilities peak at different points in the life span.23-25 Moreover, significant age-related differences may exist in cognition within AYAs. For example, an analysis of age differences in standardized IQ scores in individuals without cancer indicated that short-term memory is highest around high school graduation; attention and visuospatial skills are greatest in early adulthood; and learned knowledge, such as vocabulary and arithmetic, are highest among individuals ≥ 50 years of age.23 Thus, although cancer and its treatment are believed to contribute to cognitive aging,24,26 various patterns of deficits may depend on when in life diagnosis and treatment occur. Of note, the Prasad et al9 study found that cancer survivors diagnosed as AYAs were less likely than those diagnosed as children to report impairment in task efficiency and memory, abilities that peak in early adulthood.23 Thus, AYA survivors may demonstrate greater impairments relative to older survivors in cognitive abilities that peak later in life, such as vocabulary and arithmetic. In addition, differences may exist between younger AYAs and older AYAs with regard to the cognitive effect of cancer and its treatment. Adolescents may be treated with different protocols compared with older AYAs, despite having the same cancer, classically acute lymphoblastic leukemia.
Conversely, AYA survivors may demonstrate greater resilience to cognitive deficits than adult cancer survivors as a function of greater cognitive reserve and neuroplasticity. Cognitive reserve refers to compensatory ability in which performance on cognitive tests is better than would be expected on the basis of the degree of brain pathology.27,28 Cognitive reserve has been associated with less-severe cognitive impairment after chemotherapy in adult patients with cancer.29 AYA patients may have greater cognitive reserve than older patients because of neuroplasticity. Neuroplasticity, or the brain’s ability to form new neural connections, is higher during the critical and sensitive periods of brain development in AYAs30,31 and may facilitate the establishment of compensatory pathways, which maintain performance on neurocognitive testing and provide recovery from cognitive impairment.30 However, these changes must be evaluated within the broader context of developmental changes in brain maturation.32,33 By young adulthood (eg, 18 to 20 years of age), the neural regions associated with higher-order cognitive abilities, including the prefrontal cortex and portions of the temporal cortex, have matured, whereas the brain regions associated with sensory and motor processes develop earlier. The timing of these maturational changes may be affected negatively by cancer treatment as well as by the stress associated with the cancer diagnosis.34 Although cancer and its treatment likely negatively affect both cognitive reserve and neuroplasticity relative to individuals without cancer, they may be protective factors among AYA survivors. The roles cognitive reserve and neuroplasticity play in cognition in AYA survivors are speculative and should be evaluated empirically.
In summary, increased research and clinical attention to cancer-related cognitive impairment in AYAs are critical to understanding and addressing their unique challenges. Future research should seek to understand through neurocognitive testing and brain imaging, respectively, which cognitive processes and underlying neural structures are affected. In addition, an increased understanding of patient-reported cognitive lapses in everyday life may contribute to behavioral and educational interventions to mitigate the effects of cancer-related cognitive impairment on daily functioning. Sex differences in cognition in AYAs should be explored because studies of pediatric cancer survivors have indicated that girls are at greater risk of cognitive impairment than boys.35 Moreover, evidence has demonstrated differences in brain structure between healthy males and healthy females,36 which may affect the cognitive effects of cancer and its treatment. Additional research into associations between cancer-related cognitive impairment and social, emotional, and functional difficulties is needed. Behavioral interventions can improve perceived and objective cognition in adult patients with cancer.37 Computerized cognitive training to increase working memory may improve cognitive performance and changes in brain activation in pediatric cancer survivors.38,39 These types of interventions also should be tested in AYAs. AYA survivors should be offered ongoing support services and provided with specialized resources for education and career placement to build on their existing strengths. Health care professionals also should monitor potential neurocognitive issues that emerge over time. Such measures could greatly improve overall quality of life among AYA cancer survivors.
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cognition in Adolescent and Young Adults Diagnosed With Cancer: An Understudied Problem
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Heather S.L. Jim
Consulting or Advisory Role: RedHill Biopharma, Janssen Scientific Affairs
Sarah L. Jennewein
No relationship to disclose
Gwendolyn P. Quinn
Research Funding: Boehringer Ingelheim (Inst)
Damon R. Reed
Consulting or Advisory Role: Loxo Oncology, Shire, Janssen Pharmaceuticals
Brent J. Small
No relationship to disclose
REFERENCES
- 1. National Cancer Institute: Adolescents and young adults with cancer, 2015. https://www.cancer.gov/types/aya.
- 2.Sender L, Zabokrtsky KB. Adolescent and young adult patients with cancer: A milieu of unique features. Nat Rev Clin Oncol. 2015;12:465–480. doi: 10.1038/nrclinonc.2015.92. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Furlong W, Rae C, Greenberg ML, et al. Surveillance and survival among adolescents and young adults with cancer in Ontario, Canada. Int J Cancer. 2012;131:2660–2667. doi: 10.1002/ijc.27523. [DOI] [PubMed] [Google Scholar]
- 5.Wong AWK, Chang TT, Christopher K, et al. Patterns of unmet needs in adolescent and young adult (AYA) cancer survivors: In their own words. J Cancer Surviv. 2017;11:751–764. doi: 10.1007/s11764-017-0613-4. [DOI] [PubMed] [Google Scholar]
- 6.Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: An in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv. 2009;3:223–232. doi: 10.1007/s11764-009-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: Summary of an Institute of Medicine workshop. Oncologist. 2015;20:186–195. doi: 10.1634/theoncologist.2014-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: A report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad PK, Hardy KK, Zhang N, et al. Psychosocial and neurocognitive outcomes in adult survivors of adolescent and early young adult cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2015;33:2545–2552. doi: 10.1200/JCO.2014.57.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savard J, Ganz PA. Subjective or objective measures of cognitive functioning-what’s more important? JAMA Oncol. 2016;2:1263–1264. doi: 10.1001/jamaoncol.2016.2047. [DOI] [PubMed] [Google Scholar]
- 11.Simó M, Rifà-Ros X, Rodriguez-Fornells A, et al. Chemobrain: A systematic review of structural and functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:1311–1321. doi: 10.1016/j.neubiorev.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser J, Bledowski C, Dietrich J. Neural correlates of chemotherapy-related cognitive impairment. Cortex. 2014;54:33–50. doi: 10.1016/j.cortex.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Rey D, Bouhnik AD, Mancini J, et al. Self-reported cognitive impairment after breast cancer treatment in young women from the ELIPPSE40 cohort: The long-term impact of chemotherapy. Breast J. 2012;18:406–414. doi: 10.1111/j.1524-4741.2012.01275.x. [DOI] [PubMed] [Google Scholar]
- 14.Krull KR, Sabin ND, Reddick WE, et al. Neurocognitive function and CNS integrity in adult survivors of childhood Hodgkin lymphoma. J Clin Oncol. 2012;30:3618–3624. doi: 10.1200/JCO.2012.42.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edelmann MN, Daryani VM, Bishop MW, et al. Neurocognitive and patient-reported outcomes in adult survivors of childhood osteosarcoma. JAMA Oncol. 2016;2:201–208. doi: 10.1001/jamaoncol.2015.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lofstad GE, Reinfjell T, Hestad K, et al. Cognitive outcome in children and adolescents treated for acute lymphoblastic leukaemia with chemotherapy only. Acta Paediatr. 2009;98:180–186. doi: 10.1111/j.1651-2227.2008.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102:881–893. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddick WE, Taghipour DJ, Glass JO, et al. Prognostic factors that increase the risk for reduced white matter volumes and deficits in attention and learning for survivors of childhood cancers. Pediatr Blood Cancer. 2014;61:1074–1079. doi: 10.1002/pbc.24947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zebrack B, Kent EE, Keegan TH, et al. “Cancer sucks,” and other ponderings by adolescent and young adult cancer survivors. J Psychosoc Oncol. 2014;32:1–15. doi: 10.1080/07347332.2013.855959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belpame N, Kars MC, Beeckman D, et al. “The AYA Director”: A synthesizing concept to understand psychosocial experiences of adolescents and young adults with cancer. Cancer Nurs. 2016;39:292–302. doi: 10.1097/NCC.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 21.Deprez S, Vandenbulcke M, Peeters R, et al. Longitudinal assessment of chemotherapy-induced alterations in brain activation during multitasking and its relation with cognitive complaints. J Clin Oncol. 2014;32:2031–2038. doi: 10.1200/JCO.2013.53.6219. [DOI] [PubMed] [Google Scholar]
- 22.Krull KR, Annett RD, Pan Z, et al. Neurocognitive functioning and health-related behaviours in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Eur J Cancer. 2011;47:1380–1388. doi: 10.1016/j.ejca.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartshorne JK, Germine LT. When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol Sci. 2015;26:433–443. doi: 10.1177/0956797614567339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J Clin Oncol. 2012;30:3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McArdle JJ, Grimm KJ, Hamagami F, et al. Modeling life-span growth curves of cognition using longitudinal data with multiple samples and changing scales of measurement. Psychol Methods. 2009;14:126–149. doi: 10.1037/a0015857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: A cause of early onset frailty? Med Hypotheses. 2006;67:212–215. doi: 10.1016/j.mehy.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 27.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ismail FY, Fatemi A, Johnston MV. Cerebral plasticity: Windows of opportunity in the developing brain. Eur J Paediatr Neurol. 2017;21:23–48. doi: 10.1016/j.ejpn.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Kolb B, Gibb R. Brain plasticity and behaviour in the developing brain. J Can Acad Child Adolesc Psychiatry. 2011;20:265–276. [PMC free article] [PubMed] [Google Scholar]
- 32.Casey BJ, Tottenham N, Liston C, et al. Imaging the developing brain: What have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 34.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 35.von der Weid N, Mosimann I, Hirt A, et al. Intellectual outcome in children and adolescents with acute lymphoblastic leukaemia treated with chemotherapy alone: Age- and sex-related differences. Eur J Cancer. 2003;39:359–365. doi: 10.1016/s0959-8049(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 36.Ruigrok AN, Salimi-Khorshidi G, Lai MC, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferguson RJ, Sigmon ST, Pritchard AJ, et al. A randomized trial of videoconference-delivered cognitive behavioral therapy for survivors of breast cancer with self-reported cognitive dysfunction. Cancer. 2016;122:1782–1791. doi: 10.1002/cncr.29891. [DOI] [PubMed] [Google Scholar]
- 38.Conklin HM, Ogg RJ, Ashford JM, et al. Computerized cognitive training for amelioration of cognitive late effects among childhood cancer survivors: A randomized controlled trial. J Clin Oncol. 2015;33:3894–3902. doi: 10.1200/JCO.2015.61.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou P, Li Y, Conklin HM, et al. Evidence of change in brain activity among childhood cancer survivors participating in a cognitive remediation program. Arch Clin Neuropsychol. 2012;27:915–929. doi: 10.1093/arclin/acs095. [DOI] [PMC free article] [PubMed] [Google Scholar]