Abstract
Purpose
The aim of the current study was to conduct a pooled analysis of studies that have investigated the prognostic value of tumor-infiltrating lymphocytes (TILs) in early-stage triple negative breast cancer (TNBC).
Methods
Participating studies had evaluated the percentage infiltration of stromally located TILs (sTILs) that were quantified in the same manner in patient diagnostic samples of early-stage TNBC treated with anthracycline-based chemotherapy with or without taxanes. Cox proportional hazards regression models stratified by trial were used for invasive disease-free survival (iDFS; primary end point), distant disease-free survival (D-DFS), and overall survival (OS), fitting sTILs as a continuous variable adjusted for clinicopathologic factors.
Results
We collected individual data from 2,148 patients from nine studies. Average age was 50 years (range, 22 to 85 years), and 33% of patients were node negative. The average value of sTILs was 23% (standard deviation, 20%), and 77% of patients had 1% or more sTILs. sTILs were significantly lower with older age (P = .001), larger tumor size (P = .01), more nodal involvement (P = .02), and lower histologic grade (P = .001). A total of 736 iDFS and 548 D-DFS events and 533 deaths were observed. In the multivariable model, sTILs added significant independent prognostic information for all end points (likelihood ratio χ2, 48.9 iDFS; P < .001; χ2, 55.8 D-DFS; P < .001; χ2, 48.5 OS; P < .001). Each 10% increment in sTILs corresponded to an iDFS hazard ratio of 0.87 (95% CI, 0.83 to 0.91) for iDFS, 0.83 (95% CI, 0.79 to 0.88) for D-DFS, and 0.84 (95% CI, 0.79 to 0.89) for OS. In node-negative patients with sTILs ≥ 30%, 3-year iDFS was 92% (95% CI, 89% to 98%), D-DFS was 97% (95% CI, 95% to 99%), and OS was 99% (95% CI, 97% to 100%).
Conclusion
This pooled data analysis confirms the strong prognostic role of sTILs in early-stage TNBC and excellent survival of patients with high sTILs after adjuvant chemotherapy and supports the integration of sTILs in a clinicopathologic prognostic model for patients with TNBC. This model can be found at www.tilsinbreastcancer.org.
INTRODUCTION
The host microenvironment is usually not considered when making prognostic assessment for patients with breast cancer. We and others have previously shown that a higher quantity of immune infiltrate present in both early-stage triple-negative breast cancer (TNBC) and human epidermal growth factor receptor 2 (HER2)–positive breast cancer samples, as quantified using a simple method on standard diagnostic hematoxylin and eosin full section–stained slides, is associated with significantly improved clinical outcomes.1 This simple method of quantifying tumor-infiltrating lymphocytes (TILs) as the percentage infiltration in the tumor and surrounding stroma has been demonstrated to be a reproducible biomarker that could allow for relatively straightforward implementation in standard clinical pathology globally.2,3
Currently, the main prognostic factors in early-stage TNBC represent anatomic tumor burden. In this pooled analysis of patient data obtained mainly from large clinical studies, we sought to establish conclusively the prognostic value of TILs in this breast cancer subtype. Such evidence would strongly support the implementation of a novel biologic biomarker in future breast cancer prognostic staging systems for early-stage TNBC,4 as a clinical trials stratification factor, and potentially guide immunotherapeutic development in breast cancer. With the success of immune checkpoint blockade in other solid tumor types, clarity on the prognostic role of breast cancer immune infiltrate is now warranted.
The primary objective of the current study was therefore to achieve a robust understanding of the prognostic value of the stromal TILs (sTILs) biomarker in early-stage TNBC treated with standard adjuvant chemotherapy. We did this by performing a pooled analysis of data that were derived from more than 2,000 patients from multiple individual studies in which TILs had been evaluated in the same manner on standard diagnostic hematoxylin and eosin–stained slides using prespecified criteria.3 The sTILs variable was chosen a priori to be used in the primary analysis, with intratumoral TILs (iTILs) only in secondary analyses. Large data sets from multiple different types of studies provide new opportunities for the development and validation of prediction models5; therefore, our secondary objective was to develop and validate a prognostic model that incorporated the evaluation of TILs with traditional clinicopathologic factors that would help clinicians to provide an integrated and more accurate estimation of prognosis. We also sought to determine if the prognostic effect of TILs differed quantitatively in patients with early-stage TNBC who were treated with anthracycline with and without taxanes, which may suggest whether the type of chemotherapy matters in their prognostic effects.
METHODS
Patient Data Sets
The study protocol of this collaborative project of selected researchers was approved by the local institutional review committee at Gustave Roussy in November 2014 and authorized by the French National Committee on Computing and Liberty.
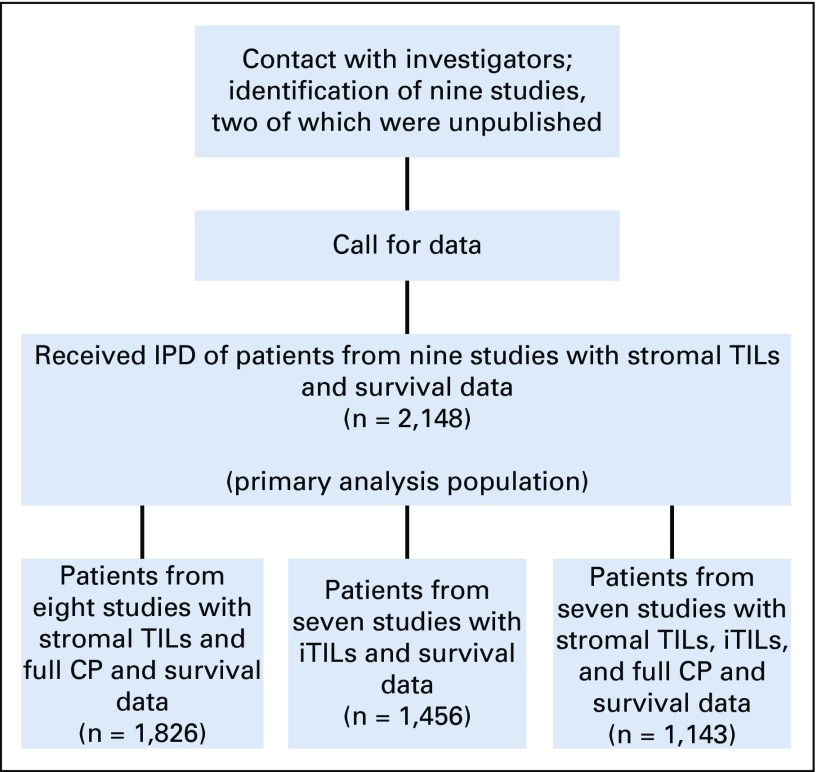
Identified studies were prospective randomized clinical trials or large retrospective hospital series that evaluated the prognostic associations of TILs (stromal and intratumoral) in patients who were diagnosed with early-stage TNBC treated with anthracycline-based chemotherapy with or without taxane in the adjuvant setting using the same prespecified method.6-13 All investigators and groups who had reported their series or who had initiated TIL evaluation were approached and none declined participation. Individual patient-level data were collated, checked, and pooled using individual patient data meta-analysis techniques (Fig 1).
FIG 1.
Study flow chart. CP, clinicopathologic; IPD, individual participant data; iTILs, intratumoral tumor-infiltrating lymphocytes; TILs, tumor-infiltrating lymphocytes.
TNBC was defined by estrogen receptor (ER), progesterone receptor (PR), and HER2 negativity, with the exception of the Gustave Roussy (GR) cohort for which PR status was not available. ER and PR status was defined for each study as per protocol definition or by central pathology review by the following percentage of immunohistochemical staining: ER and PR less than 1% for Breast International Group (BIG) 2-98, International Breast Cancer Study group (IBCSG) 22-00 (ClinicalTrials.gov identifier: NCT00022516, and Eastern Cooperative Oncology Group (ECOG) 2197 (ClinicalTrials.gov identifier: NCT00003519), and less than 10% for the others (ECOG 1199 [ClinicalTrials.gov identifier: NCT00004125], the Finland Herceptin [FinHER], European Institute of Oncology [IEO], Protocole Adjuvant dans le Cancer du Sein [PACS] 01, and PACS04). ER and PR staining was performed centrally for all studies except FinHER, IEO, ECOG 2197, and ECOG 1199. For those few patients for which central ER status was missing, local ER was used. Central HER2 evaluation was performed for FinHER, IEO, IBCSG 22-00, ECOG 2197, PACS01, and PACS04 series.
Quantification of TILs
Quantification of sTILs and iTILs was performed according to International Immuno-Oncology Biomarker Working Group guidelines.2,3 A Web site is freely available that describes the method and provides a freely available training tool for TIL assessment by pathologists (www.tilsinbreastcancer.org). TILs data in the PACS01 and PACS04 clinical trials were obtained for the purposes of this study (by R.S. and M.L.) and have not been previously published. For the pooled analysis, the sTILs variable was the primary prespecified biomarker as this has been found to be more reproducible compared with iTILs.2,3
Statistical Analysis
The prespecified primary end point was invasive disease-free survival (iDFS), defined as the date of first invasive recurrence, or second primary or death from any cause.14 Patients still alive without an event of interest were censored at the date of the last visit. Distant disease-free survival (D-DFS) is defined as the date of first distant recurrence or death from any cause. Patients still alive without an event of interest were censored at the date of the last visit. Overall survival (OS) is defined as the date of death from any cause. Patients still alive were censored at the date of the last visit. We used the Kaplan-Meier method to establish survival curves and the log-rank test to compare survival curves across subgroups.
The prespecified primary analysis used sTILs as a continuous variable in the Cox proportional hazards regression model. On the basis of the BIG 2-98 data,8 we assumed a standard deviation of 1.75 on the scale of 10% changes in sTILs in the subset of patients with TNBC. On the basis of this value, a total of 111 invasive events or deaths would provide 85% power at a 5% two-sided significance level to detect a hazard ratio (HR) of 0.85 for a 10% increase in sTILs in a Cox proportional hazards regression model on iDFS.
Univariable and multivariable risk analyses were performed using the Cox proportional hazards regression model with gamma frailty to account for the study heterogeneity. We checked the proportional hazard assumption using trend tests and graphical diagnoses on the basis of Schoenfeld residuals as well as the log-linearity assumption using fitting splines. Associations with clinicopathologic variables were performed using linear regression adjusted for study.
A Cochrane Q-like test was performed and the I2 index was approximated for each end point to characterize the heterogeneity between each study (I2 = 0 indicates no heterogeneity and larger values correspond to increasing heterogeneity). In the multivariable model, we used the likelihood ratio test to evaluate the contribution of sTILs, iTILs, and the clinicopathologic factors (continuous age; categorical tumor size: T1, T2, T3+; continuous number of positive nodes; histologic grade: 1 and 2 v 3; and chemotherapy treatment modality: anthracycline v anthracycline plus taxane). Cox models were weighted to take in account the case-control sampling in the ECOG 2197 study.6
The fully developed multivariable Cox proportional hazards regression model used sTILs and the clinicopathologic factors specified above using gamma frailty for study, together with the Breslow estimator of the baseline hazard. Three such models were developed for the three end points, iDFS, D-DFS, and OS. To estimate the discrimination and calibration of these multivariable prognostic models, we used a leave-one-study-out cross-validation approach. Each study was left out once and the model was rebuilt from the beginning with the other studies. This model was then applied to the omitted study to evaluate discrimination and calibration. We assessed discrimination using time-dependent area under the curve at 5 years,15 whereas calibration was evaluated via calibration plots. During each step of the leave-one-out cross-validation, strata were defined according to deciles of the predicted survival times of the left-out trial. A good calibration corresponds to the absence of large differences between the Kaplan-Meier estimate and the mean prediction value in each of these strata.
RESULTS
Patient Characteristics
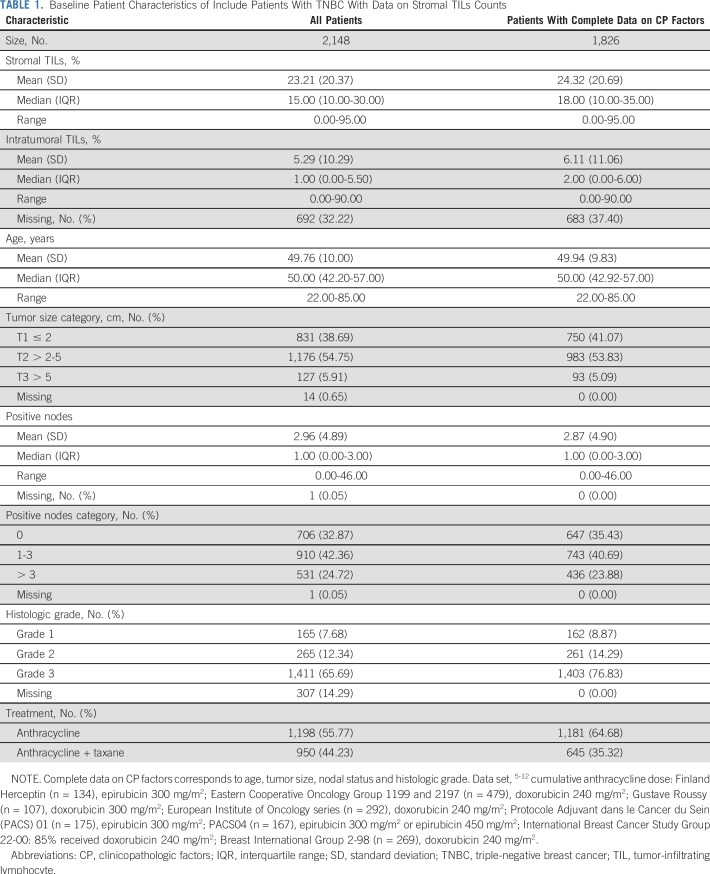
In total, nine studies comprising individual data on 2,148 patients were collected for the assessment of the prognostic value of sTILs in the primary analysis. These studies and the patient characteristics are listed in Table 1 and the Data Supplement. All but one data set10 were prospective clinical trials. For the iDFS end point, median follow-up was 6.58 years (range, 2.71 to 9.12 years) with 736 events (Data Supplement). For the D-DFS end point, median follow-up was 6.99 years (range, 3.50 to 9.54 years) with 584 events. For the OS end point, median follow-up was 7.15 years (range, 4.18 to 9.61 years) with 533 events. Of these 2,148 patients, 1,826 (84.7%) had both sTILs and complete clinicopathologic information available for multivariable modeling (Fig 1 and Table 1).
TABLE 1.
Baseline Patient Characteristics of Include Patients With TNBC With Data on Stromal TILs Counts
Mean sTILs level was 23% (standard deviation, 20%; range 0% to 95%) and median was 15.00% (interquartile range, 10% to 30%). Boxplots are provided from the study and overall in the Data Supplement. Patients with lymph node–negative tumors comprised 32.9% (706 of 2,148 patients) of the cohort (mean sTIL level, 20.2%; standard deviation, 20.3%) and all had received anthracycline alone (55.8%) or anthracycline with taxane (44.2%) adjuvant chemotherapy. In total, 1,592 patients (73.8%) underwent iTILs evaluation, with 1,144 patients (53.1%) having complete clinicopathologic information available (Data Supplement). In this pooled data set, quantities of sTILs and iTILs were strongly correlated (R = 0.63; P < .001).
Association With Clinicopathologic Factors
sTILs quantities were significantly lower with older age (linear model, P = .001). There was a significant inverse relationship between larger tumor burden and the amounts of sTILs present (tumor size P = .01; more nodes P = .02; Data Supplement). Consistent with previous reports, higher grade was associated with larger TILs amounts (P = .001).
Associations With Prognosis
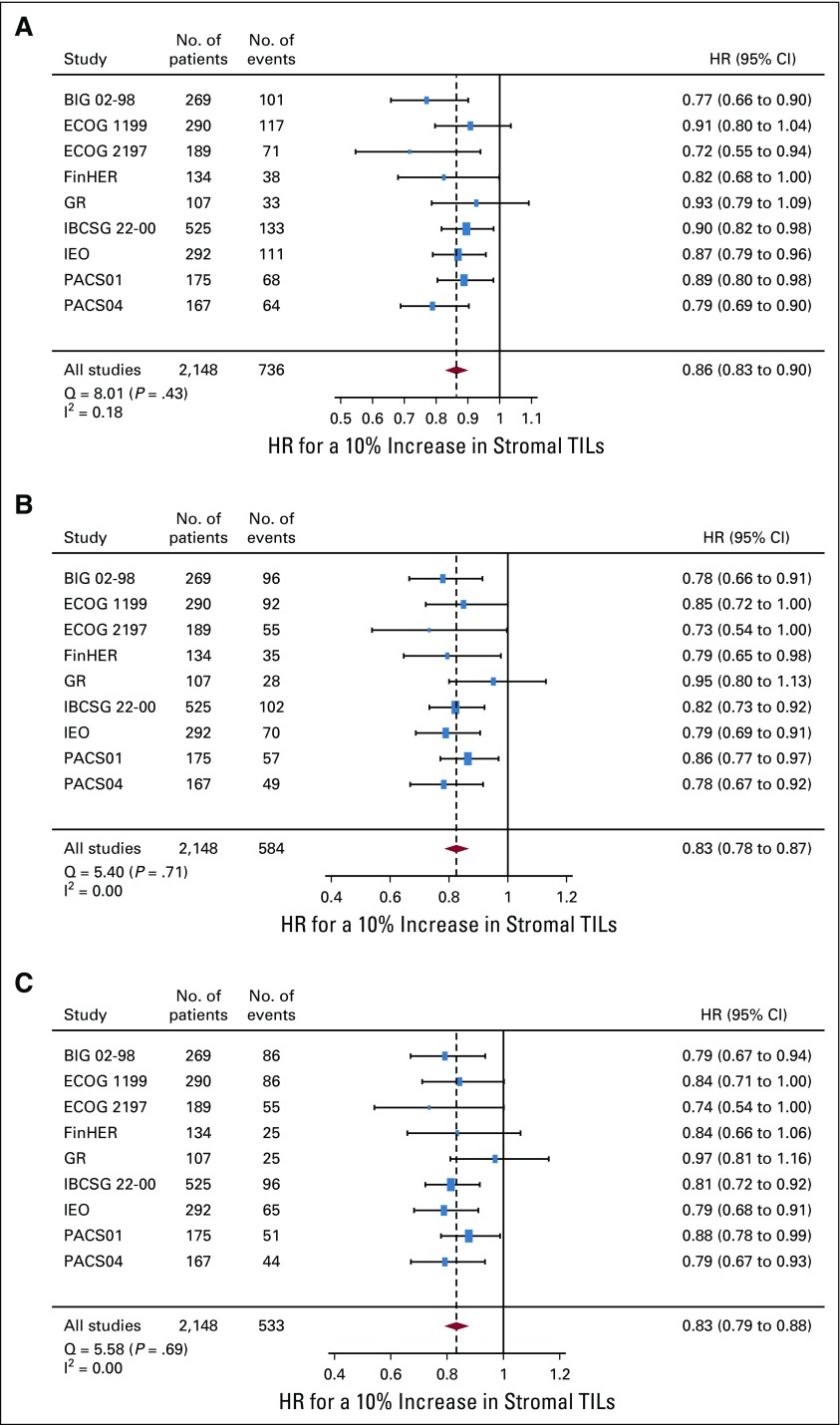
The quantity of sTILs was significantly associated with improved survival outcomes for all end points (Fig 2). For iDFS, HR for a 10% increment in sTILs was equal to 0.86 (95% CI, 0.83 to 0.90) and similar results were observed for D-DFS (0.83 [95% CI, 0.78 to 0.87]) and OS (0.83 [95% CI, 0.79 to 0.88]) with no significant heterogeneity found for each end point. There was no significant interaction between sTILs and chemotherapy treatment (ie, the magnitude of the prognostic effect was similar if patients received anthracycline alone v anthracycline-taxane regimens; P for interaction: iDFS: P = .62; D-DFS: P = .63; OS: P = .54).
FIG 2.
Forest plot of the prognostic effect of the stromal tumor-infiltrating lymphocytes (TILs) variable for each 10% increment on (A) invasive disease-free survival, (B) distant disease-free survival, and (C) overall survival. Hazard ratios (HR) are derived using Cox proportional hazards regression models and presented with 95% CIs. The size of the boxes is proportional to the inverse of the variance of the estimator in each study. Cochrane Q-like test (Q) and I2 statistics were used to evaluate between-study heterogeneity. BIG, Breast International Group; ECOG, Eastern Cooperative Oncology Group; FinHER, Finland Herceptin; GR, Gustave Roussy; IBCSG, International Breast Cancer Study Group; IEO, European Institute of Oncology; PACS, Protocole Adjuvant dans le Cancer du Sein.
Results of the prognostic analysis of iTILs were similar (Data Supplement), again with no significant heterogeneity between studies for each end point. Overall, a 10% increment in iTILs corresponded to HRs of 0.74 (95% CI, 0.65 to 0.85) for iDFS, 0.73 (95% CI, 0.62 to 0.84) for D-DFS, and 0.76 (95% CI, 0.65 to 0.88) for OS.
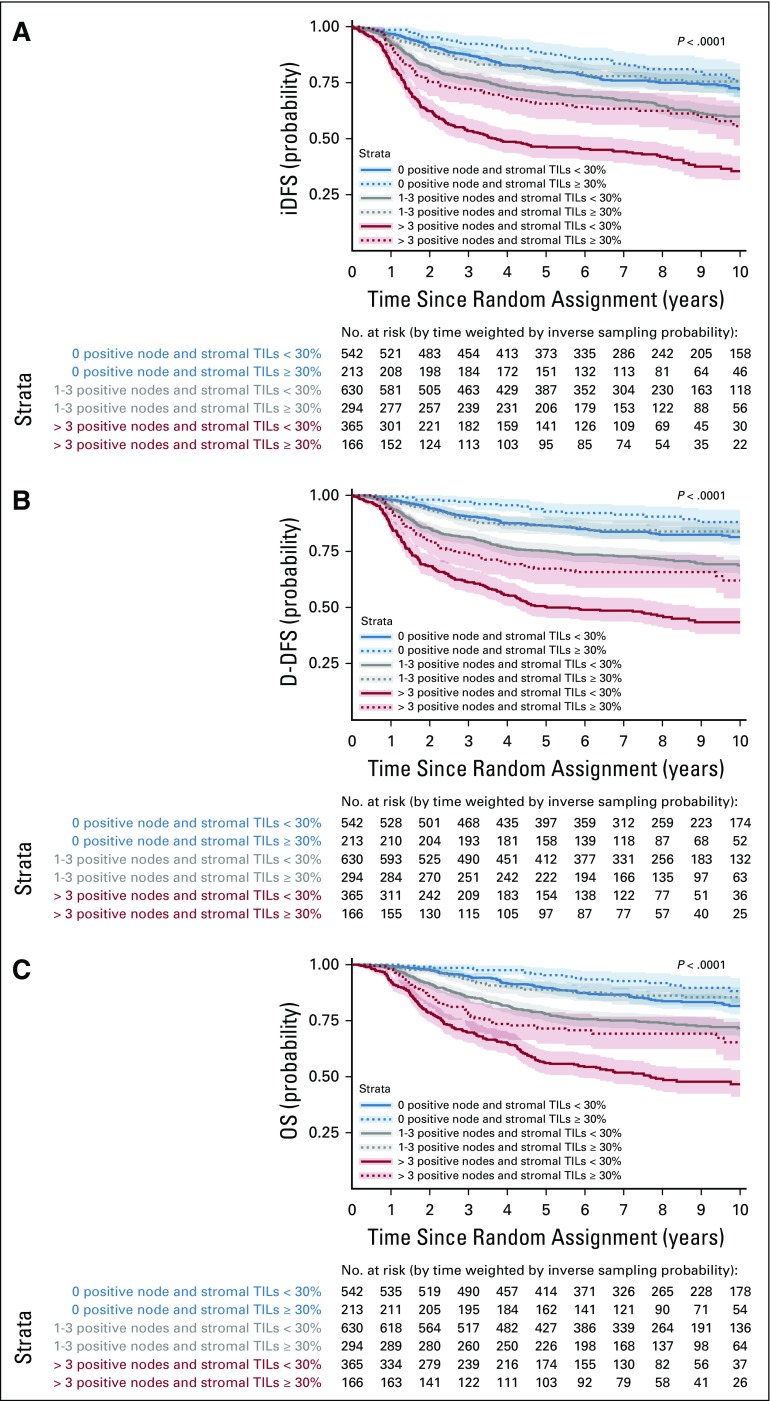
Exploratory Prognostic sTIL Cutoff
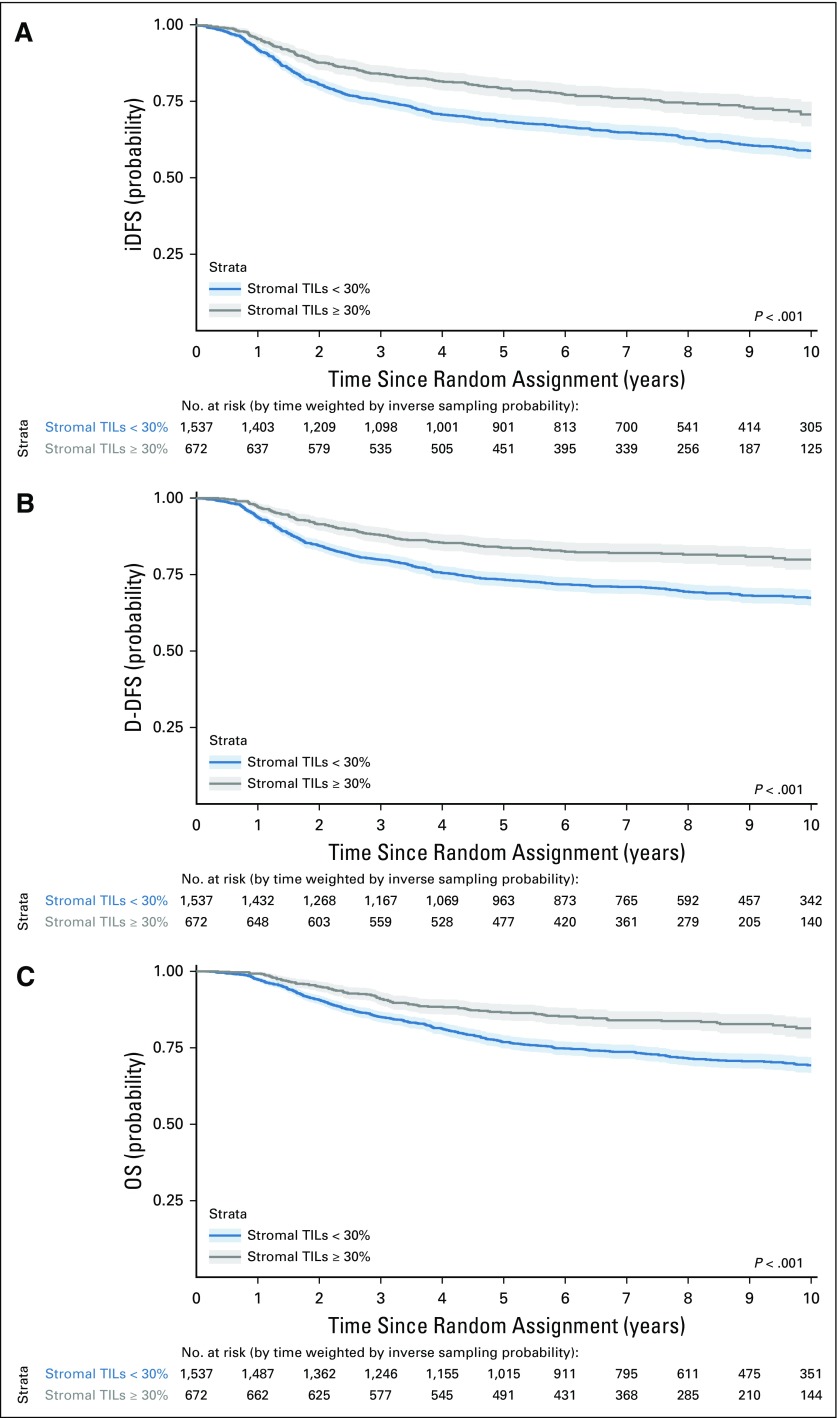
Figure 3 depicts survival curves using the exploratory sTILs level cutoff of 30%, and Figure 4 the survival curves stratified by nodal status and the cutoff. Whereas cutoffs can be difficult to choose for a variable with a linear effect on prognosis in the Cox proportional hazards regression model, this percentage was chosen as it reflected the top quartile of sTILs levels across the cohort for the three clinical end points. Estimated 3-year and 5-year survival probabilities for all three end points across subgroups and for the entire population are reported in the Data Supplement. Large absolute differences in survival outcomes are observed across all nodal categories. For all patients with node-negative TNBC with at least 30% sTILs present at diagnosis (n = 213), excellent survival can be observed with an estimated 3-year iDFS of 92% (95% CI, 89% to 98%), 3-year D-DFS of 97% (95% CI, 95% to 99%), and 3-year OS of 99% (95% CI, 97% to 100%).
FIG 3.
Kaplan-Meier curves of (A) invasive disease-free survival (iDFS), (B) distant disease-free survival (D-DFS), and (C) overall survival (OS) according to stromal tumor-infiltrating lymphocytes (TILs) dichotomized at the value of 30% in the entire population. Shaded areas correspond to pointwise 95% CIs. P values correspond to log-rank tests.
FIG 4.
Kaplan-Meier curves of (A) invasive disease-free survival (iDFS), (B) distant disease-free survival (D-DFS), and (C) overall survival (OS) according to nodal status and stromal tumor-infiltrating lymphocytes (TILs) dichotomized at the value of 30%. Shaded areas correspond to pointwise 95% CIs. P values correspond to log-rank tests. iDFS estimated 3-year survival for stromal TILs ≥ 30% v < 30% for node negative disease: 92% (95% CI, 0.89 to 0.96) v 88% (95% CI, 0.85 to 0.90); estimated 5-year survival: 88% (95% CI, 0.84 to 0.93) v 81% (95% CI, 0.77 to 0.84). D-DFS estimated 3-year survival for stromal TILs ≥ 30% v < 30% for node-negative disease: 97% (95% CI, 0.95 to 0.99) v 91% (95% CI, 0.88 to 0.83); estimated 5-year survival: 93% (95% CI, 0.89 to 0.96) v 87% (95% CI, 0.84 to 0.90). OS estimated 3-year survival for stromal TILs ≥ 30% v < 30% for node-negative disease: 99% (95% CI, 0.97 to 1.00) v 95% (95% CI, 0.93 to 0.97); estimated 5-year survival: 95% (95% CI, 0.92 to 0.98) v 90% (95% CI, 0.87 to 0.92).
When focusing on the T1-2, N0 subpopulation with at least 30% TILs—one third of the node-negative population (206 of 684 patients)—3-year estimates for iDFS, D-DFS, and OS were 93% (95% CI, 90% to 97%), 98% (95% CI, 95% to 100%), and 99% (95% CI, 98% to 100%), respectively.
Integrated Clinicopathologic Prognostic Model
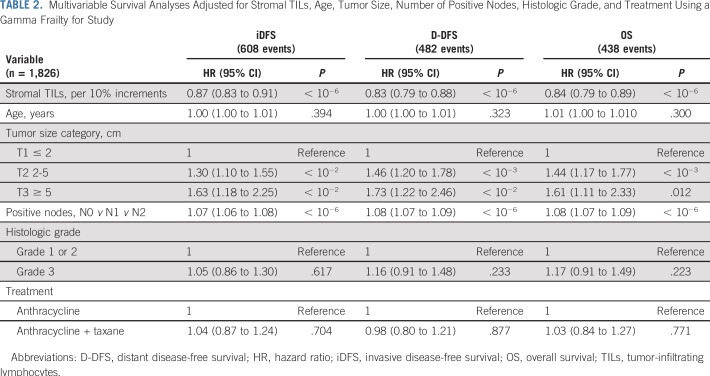
Univariable and multivariable analyses confirmed the significant and independent prognostic value of both sTILs and iTILs in early-stage TNBC as a continuous variable (Table 2 and Data Supplement). Using a likelihood ratio test, we investigated the added prognostic information of TILs to standard clinical prognostic factors. sTILs added significant prognostic information beyond that provided by conventional clinicopathologic factors (likelihood ratio test χ2, 48.9 for iDFS; χ2, 55.8 for D-DFS; and χ2, 48.5 for OS; all P < .001). In contrast, iTILs did not add additional information to the model with sTILs (Data Supplement).
TABLE 2.
Multivariable Survival Analyses Adjusted for Stromal TILs, Age, Tumor Size, Number of Positive Nodes, Histologic Grade, and Treatment Using a Gamma Frailty for Study
An integrated prognostic model that combined standard prognostic clinicopathologic factors with continuous sTIL levels was created to provide improved estimates of survival outcomes in early-stage TNBC. Addition of the sTILs variable increased the discrimination of the model as estimated using leave-one-study-out cross-validation from a 5-year area under the curve of 0.65 (0.55; 0.71) to 0.68 (0.56; 0.76) for IDFS, from 0.67 (0.55; 0.76) to 0.69 (0.58; 0.78) for D-DFS, and from 0.68 (0.56; 0.81) to 0.69 (0.58; 0.78) for OS (Data Supplement). This prognostic model was well calibrated on all three end points in the leave-one-study-out cross-validation (Data Supplement). The model can be used by clinicians to estimate survival incorporating the sTIL biomarker and is freely accessible at the following Web site: www.tilsinbreastcancer.org.
DISCUSSION
In this study, by pooling individual patient-level data from nine large studies, we have comprehensively demonstrated that the quantity of stromal TILs at the time of diagnosis is a robust and independent prognostic factor for early-stage TNBC and adds important prognostic information to the estimations of survival when combined with standard clinicopathologic factors. Hence, despite the complexities of the antitumor immune response, these data strongly suggest that the quantity of preexisting immune infiltrate present at diagnosis strongly correlates with outcomes in patients with early-stage TNBC treated with anthracycline-based chemotherapy. Evaluation of the stromal TILs method has been previously published and reproducibility demonstrated3,16; therefore, our data suggest that this new biologic biomarker is ready for clinical use and could be implemented globally for patient prognostication and clinical trial stratification. Our prognostic model is freely available online and this should thus facilitate its uptake by clinicians and pathologists world-wide, as well as for investigators who wish to externally validate the model on their TNBC data sets.17
In the current study, it is the quantity of baseline TILs infiltrate observed at diagnosis that is prognostic. As all patients in this study had breast surgery followed by adjuvant chemotherapy, this suggests that, despite the generation of high TILs, the primary tumor cannot be completely eradicated by the host immune system, but the effective antitumor immunity memory must still have been initiated. Immune infiltrates in primary breast cancers have been demonstrated to be predominantly T cells.18 In support of this, the quantity of CD8+ T cells when evaluated by immunohistochemistry has also been shown to be prognostic.16,19 Biologic mechanisms that underlie the heterogeneity observed in the quantity of the immune infiltrate and that drive the T-cell response in breast cancers are largely unknown. Somatic and germline mutations, genomic instability, intracellular antigens, and such host factors as the microbiome have all been hypothesized to contribute.20-22 Of interest, we observed inverse relationships between TILs quantity with tumor size and nodal burden, which supports oncogenic-mediated immune evasion with larger disease burden, but increased levels with high histologic grade, which supports genomic instability as a possible trigger.23 In general, breast cancers have low mutational burden, perhaps with the exception of BRCA germline mutated tumors.24 It is currently unknown whether qualitative T-cell differences exist in high TIL TNBCs or if simply quantitative differences dictate prognosis. Additional research into the molecular basis of TIL heterogeneity is likely to be informative.
We observed strong prognostic effects of stromal TIL values on all survival end points above provided by important clinicopathologic factors. One could speculate that generating an effective antitumor immune response may result in significant survival improvements for patients with low TIL levels, though our data do not directly support this. Given that the vast majority of early-stage TNBCs contained at least 1% TILs, it would seem that immunotherapeutic agents that require a preexisting response are likely to be efficacious in this setting.25 More research into the duration and type of chemotherapy that can enhance or promote an immune response in combination to optimize with immune therapies, such as checkpoint blockade, is warranted.
Strengths of this study include the large number of patients pooled from multiple different studies, including large prospective clinical trials. Using an exploratory stromal TILs cutoff of 30% or more, the observed excellent survival supports the clinical validity of the TILs biomarker. This suggests that the biomarker could be incorporated into future prognostic staging systems for early-stage, node-negative TNBC, similar to the recent integration of the prognostic 21-gene assay for patients with hormone receptor–positive, HER2-negative, node-negative disease.26 This stromal TIL score has been demonstrated to be reproducible between pathologists after adequate training.2 A limitation is that our study does not include patients who were diagnosed in the past 10 years, which may affect survival estimates. In addition, the exact immune subset of the TILs biomarker responsible for good outcomes is not known. Whereas this study does not directly address the question of de-escalation for the subgroup of node negative TNBC patients with high sTILs that have good prognosis when treated by anthracycline-based chemotherapy (with or without taxane), the identification of this subgroup with good prognosis can still be useful. Excluding this subgroup of high sTILs and node-negative patients may also be useful in future clinical trials of new therapeutics for early-stage TNBC through the reduction of toxicity and cost for patients with a good outcome, as well as in the required number of patients. Future prospective validation of the proposed sTIL cutoff could also investigate whether this can aid clinicians in the identification of patients with TNBC with a good prognosis who may require a less intensive type and duration of traditional cytotoxic chemotherapies.
In conclusion, we have shown that baseline stromal TIL quantities provide important and independent prognostic information in early-stage TNBC. A subgroup of patients with high-TIL, early-stage, node-negative TNBC have low rates of recurrence and death from disease at 3 years after standard anthracycline-based adjuvant chemotherapy
ACKNOWLEDGMENT
The authors thank the patients, physicians, nurses, and data managers who participated in these clinical trials.
Footnotes
Presented at the San Antonio Breast Cancer Symposium, San Antonio, TX, December 8-12, 2015.
Supported by the Ligue Nationale Contre le Cancer as well as by the International Breast Cancer Study Group and participating centers. Support for central coordination, data management, and statistics was provided by the Swedish Cancer League, the Cancer Council Australia, the Australia and New Zealand Breast Cancer Trials Group, the Frontier Science and Technology Research Foundation, the Swiss Group for Clinical Cancer Research, the Swiss Cancer League/Oncosuisse, and the US National Institutes of Health (Grant No. CA75362). M.L.-T., J.L., F.A., and S.M. are funded by grants from ANR and CGI (RHU MyPROBE and ANR-17-RHUS-0008). S.L. is supported by a Cancer Council Victoria John Colebatch Fellowship, the Breast Cancer Research Foundation, NY, and the National Breast Cancer Foundation of Australia.
AUTHOR CONTRIBUTIONS
Conception and design: Sherene Loi, Damian Drubay, Sylvia Adams, Giancarlo Pruneri, Sunil Badve, Sandra Demaria, Roberto Salgado, Stefan Michiels
Administrative support: Sherene Loi, Sunil Badve
Provision of study materials or patients: Sherene Loi, Sylvia Adams, Giancarlo Pruneri, Prudence A. Francis, Heikki Joensuu, Maria Vittoria Dieci, Sunil Badve, Robert Gray, Jerome Lemonnier, Christos Sotiriou, Pirkko-Liisa Kellokumpu-Lehtinen
Collection and assembly of data: Sherene Loi, Damian Drubay, Sylvia Adams, Giancarlo Pruneri, Prudence A. Francis, Magali Lacroix-Triki, Heikki Joensuu, Maria Vittoria Dieci, Sunil Badve, Robert Gray, Elisabetta Munzone, Jerome Lemonnier, Pirkko-Liisa Kellokumpu-Lehtinen, Kathryn Gray, Fabrice Andre, Stefan Michiels
Data analysis and interpretation: Sherene Loi, Damian Drubay, Sylvia Adams, Giancarlo Pruneri, Prudence A. Francis, Heikki Joensuu, Sunil Badve, Elisabetta Munzone, Christos Sotiriou, Martine J. Piccart, Andrea Vingiani, Kathryn Gray, Fabrice Andre, Carsten Denkert, Roberto Salgado, Stefan Michiels
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Sherene Loi
Consulting or Advisory Role: AstraZeneca (Inst), MedImmune (Inst), Seattle Genetics (Inst), Bristol-Myers Squibb (Inst), Pfizer (Inst), Novartis (Inst), Genentech (Inst), Merck Sharp & Dohme (Inst)
Research Funding: Genentech (Inst), Pfizer (Inst), Novartis (Inst), Merck (Inst), Puma Biotechnology (Inst), Bristol-Myers Squibb (Inst)
Sylvia Adams
Consulting or Advisory Role: GlaxoSmithKline, Merck, Genentech, Bristol-Myers Squibb
Research Funding: Merck (Inst), Genentech (Inst), Celgene (Inst), Bristol-Myers Squibb (Inst), Amgen (Inst), Novartis (Inst)
Giancarlo Pruneri
Expert Testimony: Roche Molecular Diagnostics
Travel, Accommodations, Expenses: Roche
Prudence A. Francis
Honoraria: AstraZeneca, Novartis
Travel, Accommodations, Expenses: Pfizer
Magali Lacroix-Triki
Leadership: MyPL
Stock and Other Ownership Interests: MyPL
Honoraria: Myriad Genetics, Genomic Health
Consulting or Advisory Role: Roche
Travel, Accommodations, Expenses: Agendia
Heikki Joensuu
Employment: Orion Corporation, Neutron Therapeutics
Leadership: Sartar Therapeutics
Stock and Other Ownership Interests: Orion Corporation, Faron Pharmaceuticals
Consulting or Advisory Role: Neutron Therapeutics
Patents, Royalties, Other Intellectual Property: Sartar Therapeutics
Maria Vittoria Dieci
Consulting or Advisory Role: Eli Lilly, Genomic Health, Celgene
Travel, Accommodations, Expenses: Pfizer, Celgene
Sunil Badve
Stock and Other Ownership Interests: YeSSGenomics
Speakers' Bureau: Genomic Health
Sandra Demaria
Consulting or Advisory Role: Eisai, Lytix Biopharma, EMD Serono, AstraZeneca, AbbVie
Research Funding: Lytix Biopharma (Inst)
Robert Gray
Research Funding: Abbott Molecular, Agios, Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Genentech, Genomic Health, Genzyme, GlaxoSmithKline, Janssen-Ortho, Millennium Pharmaceuticals, Onyx, Pfizer, Sanofi, Sequenta, Syndax, Novartis
Elisabetta Munzone
Consulting or Advisory Role: Pierre Fabre
Christos Sotiriou
Consulting or Advisory Role: Astellas Pharma, Cepheid, Vertex, Puma Biotechnology, Seattle Genetics, Amgen
Speakers' Bureau: Eisai, Prime Oncology, Teva, Foundation Medicine
Patents, Royalties, Other Intellectual Property: Epigenetic portraits of human breast cancer (PCT/EP2012/050836, WO2012/098215); a companion diagnostic for CDK4/CDK6 inhibitory drugs that is based on CDK4 phosphorylation, which patient to be treated and how (PCT/EP2017/061780)
Travel, Accommodations, Expenses: Roche, Genentech
Martine J. Piccart
Consulting or Advisory Role: AstraZeneca, Eli Lilly, MSD, Novartis, Pfizer, Genentech, Crescendo Biologics, Periphagen, Huya Bioscience International, Debiopharm Group, PharmaMar, Odonate Therapeutics, G1 Therapeutics, Menarini, Seattle Genetics
Research Funding: AstraZeneca (Inst), Eli Lilly (Inst), MSD (Inst), Novartis (Inst), Pfizer (Inst), Genentech (Inst), Radius Health (Inst), Synthon (Inst), Servier (Inst)
Other Relationship: Radius Health
Pirkko-Liisa Kellokumpu-Lehtinen
Consulting or Advisory Role: Novartis, Pfizer (Inst), Bristol-Myers Squibb
Travel, Accommodations, Expenses: Sanofi, Pierre Fabre, Roche
Kathryn Gray
Stock and Other Ownership Interests: Dvax
Fabrice Andre
Research Funding: AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Eli Lilly (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca
Carsten Denkert
Stock and Other Ownership Interests: Sividon Diagnostics
Honoraria: Teva Pharmaceuticals, Novartis, Pfizer, Roche, Amgen
Consulting or Advisory Role: MSD Oncology, Amgen
Patents, Royalties, Other Intellectual Property: VMScope digital pathology software, predictive gene expression signature for therapy response
Roberto Salgado
Consulting or Advisory Role: Roche, AstraZeneca
Research Funding: Merck, Puma Biotechnology
Travel, Accommodations, Expenses: Roche, Merck
Stefan Michiels
Consulting or Advisory Role: International Drug Development Institute, Hexal, Johnson & Johnson, Genticel, Mabxience, Roche, QuintilesIMS
Patents, Royalties, Other Intellectual Property: Patent pending of a prognostic gene score in early breast cancer (WO2017EP66533)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Savas P, Salgado R, Denkert C, et al. Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nat Rev Clin Oncol. 2016;13:228–241. doi: 10.1038/nrclinonc.2015.215. [DOI] [PubMed] [Google Scholar]
- 2.Denkert C, Wienert S, Poterie A, et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: Results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29:1155–1164. doi: 10.1038/modpathol.2016.109. [DOI] [PubMed] [Google Scholar]
- 3.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Joint Committee on Cancer BreastinHortobagyi GN, Connolly JL, D’Orsi CJ.(eds)AJCC Cancer Staging Manual ed 8New York, NY: Springer; 2017 [Google Scholar]
- 5.Riley RD, Ensor J, Snell KI, et al. External validation of clinical prediction models using big datasets from e-health records or IPD meta-analysis: Opportunities and challenges. BMJ. 2016;353:i3140. doi: 10.1136/bmj.i3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams S, Gray R, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes (TILs) in triple negative breast cancers (TNBC) from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann Oncol. 2014;25:1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 8.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 9.Pruneri G, Gray KP, Vingiani A, et al. Tumor-infiltrating lymphocytes (TILs) are a powerful prognostic marker in patients with triple-negative breast cancer enrolled in the IBCSG phase III randomized clinical trial 22-00. Breast Cancer Res Treat. 2016;158:323–331. doi: 10.1007/s10549-016-3863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pruneri G, Vingiani A, Bagnardi V, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. 2016;27:249–256. doi: 10.1093/annonc/mdv571. [DOI] [PubMed] [Google Scholar]
- 11.Roche H, Allouache D, Romieu G, et al. Five-year analysis of the FNCLCC-PACS04 trial: FEC100 vs ED75 for the adjuvant treatment of node positive breast cancer. Cancer Res. 2009;69 abstr nr602. [Google Scholar]
- 12.Roché H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: The FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24:5664–5671. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 13.Dieci MV, Mathieu MC, Guarneri V, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26:1698–1704. doi: 10.1093/annonc/mdv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gourgou-Bourgade S, Cameron D, Poortmans P, et al. Guidelines for time-to-event end point definitions in breast cancer trials: Results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials) Ann Oncol. 2015;26:873–879. doi: 10.1093/annonc/mdv106. [DOI] [PubMed] [Google Scholar]
- 15.Uno H, Cai T, Tian L, et al. Evaluating prediction rules for t-year survivors with censored regression models. J Am Stat Assoc. 2007;102:527–537. [Google Scholar]
- 16.Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 17.Leon-Ferre RA, Polley MY, Liu H, et al. Impact of histopathology, tumor-infiltrating lymphocytes, and adjuvant chemotherapy on prognosis of triple-negative breast cancer. Breast Cancer Res Treat. 2018;167:89–99. doi: 10.1007/s10549-017-4499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruffell B, Au A, Rugo HS, et al. Leukocyte composition of human breast cancer. Proc Natl Acad Sci USA. 2012;109:2796–2801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25:1536–1543. doi: 10.1093/annonc/mdu191. [DOI] [PubMed] [Google Scholar]
- 20.Spranger S, Luke JJ, Bao R, et al. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proc Natl Acad Sci USA. 2016;113:E7759–E7768. doi: 10.1073/pnas.1609376113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zitvogel L, Ayyoub M, Routy B, et al. Microbiome and anticancer immunosurveillance. Cell. 2016;165:276–287. doi: 10.1016/j.cell.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 23.Woo SR, Fuertes MB, Corrales L, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. Erratum: Immunity 42:199, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nolan E, Savas P, Policheni AN, et al. Combined immune checkpoint blockade as a therapeutic strategy for BRCA1-mutated breast cancer. Sci Transl Med. 2017;9:eaal4922. doi: 10.1126/scitranslmed.aal4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loi S, Adams S, Schmid P, et al. Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): Results from KEYNOTE-086. Ann Oncol. 2017;28(suppl 5; abstr LBA13) [Google Scholar]
- 26.Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]