Abstract
Purpose
Adjuvant endocrine therapy is a long-term drug therapy prescribed to prevent recurrence of hormone receptor–positive breast cancer. Data on adjuvant endocrine therapy are reported though clinical trials, which may differ from treatment practice and outcomes in the general population of patients with breast cancer. With secondary use of electronic health record (EHR) data, we summarize adjuvant endocrine treatment practice and outcomes in real-world settings.
Methods
We analyzed treatment data derived from EHR data on 1,587 patients with stage I to III breast cancer at a National Cancer Institute–designated comprehensive cancer center to learn the frequencies of real-world adjuvant endocrine drug switches and discontinuation and to explore the potential cause for drug switches and discontinuation from medical records. We measured rates of drug use, drug switches, early drug discontinuation, adverse events, recurrence, and death. We also measured adverse events and change in menopause status as potential causes for drug switch and discontinuation.
Results
Within the study population, approximately 49% of patients were lost to follow-up or did not complete adjuvant treatment through 5 years. Fifty-two percent of patients switched to a different endocrine therapy drug during their treatment. We found that age is correlated with drug switches and that adverse events are correlated with drug switches and discontinuation. We also found that patients who switched to an alternative endocrine therapy during treatment were more likely to complete 5 years of treatment.
Conclusion
This study describes long-term adjuvant endocrine treatment in real-world settings and demonstrates the ability to leverage longitudinal EHR data to characterize oral medication treatment patterns in patients with cancer.
INTRODUCTION
Adjuvant endocrine therapy is prescribed to patients with hormone receptor–positive breast cancer for recurrence prevention after surgery. Clinical guidelines recommend that patients use adjuvant endocrine drugs for a duration of 5 years, with recent guidelines extending the recommendation to 10 years for some high-risk patient populations.1 In this time frame, patients may deviate from their original treatment plan in the form of a drug switch or termination.2 Although clinical trials report rates of drug switches and termination,3 the prevalence and motivations for these events in the general population of patients with breast cancer are unmeasured.
There are several reasons a patient may change her adjuvant endocrine therapy treatment. A drug switch may result from a change in menopausal status, intolerable adverse effects, tumor recurrence, generic drug alternatives, or physician preference. Drug discontinuation may result from intolerable adverse effects, financial factors, or death. Measuring rates of drug switches and termination and determining their possible cause in patients in the general breast cancer population yields an empirical projection for treatment strategies and outcomes.
Electronic health record (EHR) systems store patient medical data, including medications, billing codes, and diagnoses. EHRs allow for mining large quantities of adjuvant endocrine treatment data to determine treatment trends over time. To understand treatment patterns for adjuvant endocrine therapy in the general population of patients with breast cancer, we analyzed Vanderbilt University Medical Center’s (VUMC) EHR data for 1,587 patients with stage I to III breast cancer. Treatment data include adjuvant endocrine therapy drugs taken by the patient, timestamps for each drug, and International Classification of Diseases, Ninth Revision (ICD-9) codes. Our goals were to determine the frequencies of drug switches and discontinuation in the general population of patients with breast cancer and to explore the potential cause for drug switches and discontinuation from medical records.
Knowledge about adjuvant endocrine therapy, including frequencies of drug switches, discontinuation, and adverse events, is derived from clinical trials, which may not be representative of treatment in the general population of patients with breast cancer. This study derives adjuvant endocrine therapy knowledge from real-world treatment by leveraging longitudinal EHR data.
The selective estrogen receptor modulator (SERM) tamoxifen was approved for use in prevention of breast cancer in 1998 and for treatment of noninvasive ductal carcinoma in situ in 2000.4 In 2002, the first aromatase inhibitor (AI), anastrozole, was approved for treatment of breast cancer,3 and in 2004, it demonstrated superiority over tamoxifen for treatment of postmenopausal woman.5 In 2005, two alternative AIs, letrozole and exemestane, were also approved as adjuvant endocrine therapies.
In 1998, clinical guidelines recommended adjuvant endocrine therapy for a duration of 5 years.6 Recent guidelines extended treatment duration to up to 10 years in at-risk populations.1 With extended time frames and a growing number of available drug options, adjuvant endocrine therapy treatment paths can vary across patients. Clinical trials report varying rates of drug termination (31% to 73%),3,7 adverse events,8,9 and drug switches,10 but there is limited information on adjuvant endocrine treatment in the general patient population.
EHRs contain patient medical information including medication events and diagnoses. VUMC’s Synthetic Derivative is a data source that contains deidentified health records from more than two million patients.11 MedEx, a natural language-processing method, identified more than 400 million medication events from medication lists and clinical notes in Synthetic Derivative.12 From these resources, patient diagnoses, medication events, and ICD-9 codes, along with their respective timestamps, form a basis for analyzing adjuvant endocrine therapy in patients with breast cancer.
METHODS
Data Collection
Our data source is deidentified EHRs collected at VUMC as part of the Synthetic Derivative and the EHR-linked Vanderbilt Tumor Registry. The Tumor Registry is considered a gold standard for VUMC’s data on patients with cancer, although death may be under-reported as a result of failure to report back to care facilities. The patient cohort met the following criteria: patients were diagnosed with stage I to III breast cancer, determined from the Vanderbilt Tumor Registry; patients received one or more of the following adjuvant endocrine therapy drugs: anastrozole, exemestane, letrozole, or tamoxifen; and patient’s adjuvant endocrine therapy began between 1998 and 2011. These date restrictions enforce that patients were recommended 5 years of treatment and will have at least 5 years of follow-up data. This study was done with the approval of Vanderbilt’s Institutional Review Board (140691, type exempt).
Data used in the study are adjuvant endocrine therapy medication events, patient ICD-9 codes, and their respective timestamps. Medication events are mentions of the patient’s currently prescribed drugs extracted from clinical notes, and the timestamps for medication events are the time at which they were documented in the note. Death and recurrence data were collected from Vanderbilt’s Tumor Registry. We used the earliest medication event date for start time. Stop times, which are undocumented in the EHR, are estimated at 6 months after the maximum medication event date. These estimates are based on an expected patient follow-up of every 6 months in the first 5 years after diagnosis. Patients do not take more than one adjuvant endocrine therapy drug simultaneously, so we interpreted the presence of a new endocrine therapy drug in a record as a switch to the new drug.
Population Overview
To review our patient population, we calculated statistics on patients grouped by 5-year treatment completion status and switching status. We calculated outcomes of completion, death, and recurrence to determine differences among the patient groups.
Treatment Trends
Changes in the adjuvant endocrine therapy drug market and new adjuvant endocrine therapy knowledge lead to changes in treatment switch and stop frequencies over time. To determine drug prescription trends, we extracted prescriptions for the selected adjuvant endocrine therapy drugs and calculated prescription frequencies over time. We graphed the results along with the percentage of patients who switched or stopped drugs by year. We hypothesized that as drug options increase, drug switch frequency increases and drug stop frequency decreases.
Exploring Causes for Treatment Change
We hypothesized that the two adjuvant endocrine drug classes, SERMs and AIs, incite different stop and switch patterns as a result of the properties of the drugs. We hypothesized that switches from a SERM to an AI are most likely concomitant with changes in pre- or perimenopausal to postmenopausal status because AIs are only effective in postmenopausal women. Alternatively, we hypothesized that a switch from an AI is most likely concomitant with drug toxicity because patients beginning on AIs are postmenopausal and their menopausal status will not change.
We explored causes for drug switches or discontinuation with computational chart reviews. We performed a text search for the stem words “stop,” “switch,” “complain,” and “discontinue” in health plan clinical notes written within a month (before or after) of a patient’s treatment change. Next, we manually reviewed a random sample of notes containing a search word for direct references to changes in adjuvant endocrine therapy.
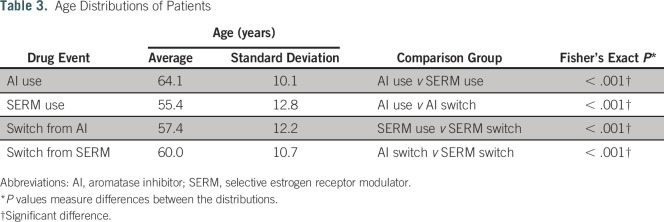
To explore the likelihood that switches from SERMs are correlated with a change in menopause status, we calculated the age distribution and P value of patients on SERMs and patients switching from SERMs. For comparison, we calculated the age distribution and P values for patients on AIs and patients switching from AIs. We hypothesized that the age of patients switching from a SERM is greater than the age of patients taking SERMs, indicating that their age and menopause progression are correlated with switching. We also checked for evidence of artificial postmenopause progression through Current Procedural Terminology codes for oophorectomies and by medication events for the estrogen-suppressing drugs goserelin and leuprorelin before a switch from an SERM. Patients undergoing oophorectomies or taking estrogen-suppressing drugs become postmenopausal and will likely switch medications.
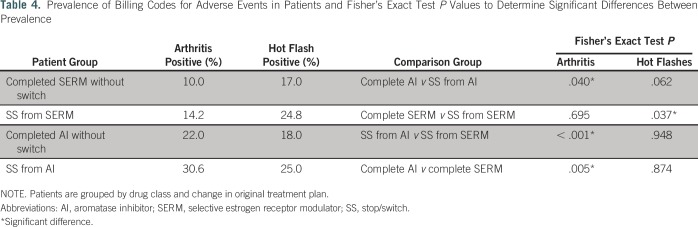
To explore the likelihood that switches or discontinuations from AIs are correlated with toxicity, we probed for a correlation between switching and discontinuation and ICD codes for hot flashes and arthritis pain. The targeted ICD-9 codes were 714.*, 715.*, 716.*, 729.*, 627.2, and 782.62. We compared the rate of ICD codes in patients who switched or stopped medication during treatment to the rate of ICD codes in patients who completed treatment with no change. ICD codes must have occurred within a year before treatment change or completion to ensure that we compared an equal time frame across groups and that ICD codes were relevant to the current treatment plan. We hypothesized that ICD prevalence for hot flashes and arthritis would be higher in patients who switched or stopped treatment, indicating adverse events as a reason for change. Furthermore, we hypothesized that patients who stopped or switched from AIs will have higher ICD rates than patients who stopped or switched from SERMs because stopping or switching from an SERM may result from a different cause, such as menopause status. To determine significant differences between ICD prevalence, we calculated Fisher’s exact test P values.
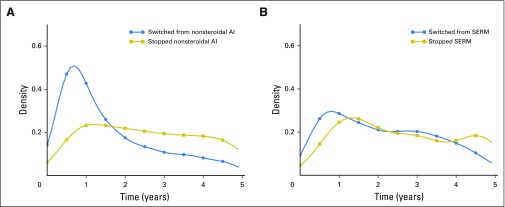
SERMs and AIs have different mechanisms of action, resulting in disparate rates of toxicity, and disparate efficacy among age groups. Consequently, age-related and toxicity-related reasons for change exhibit different distributions across the two drug classes. To explore whether changes in SERM and AI treatment occur at different times, we examined the time until treatment change in both drug classes. Switching from or stopping an AI as a result of adverse events may be localized to a certain time of toxicity onset. Conversely, changes in treatment as a result of menopause progression are not localized to a certain time because the change is dependent on a patient’s age and estrogen levels. We tested these hypotheses by graphing time until drug switch or discontinuation for patients on SERMs and AIs.
RESULTS
Population Overview
Our study includes 1,587 patients with stage I to III breast cancer taking adjuvant endocrine therapy drugs. The average age of patients at treatment start was 56.9 years (standard deviation, 12.3 years). The average number of unique endocrine therapy drugs per patient is 1.5 (standard deviation, 0.7 drugs). Approximately 64% of patients continued care at Vanderbilt after 5 years of adjuvant endocrine therapy treatment.
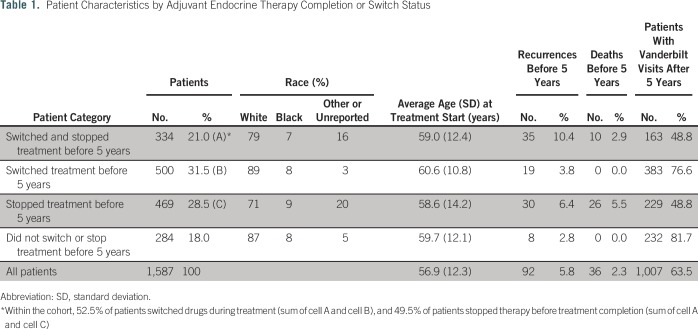
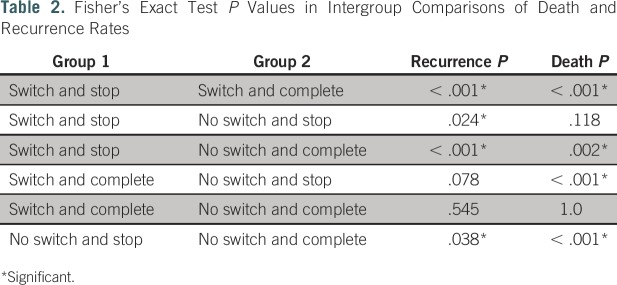
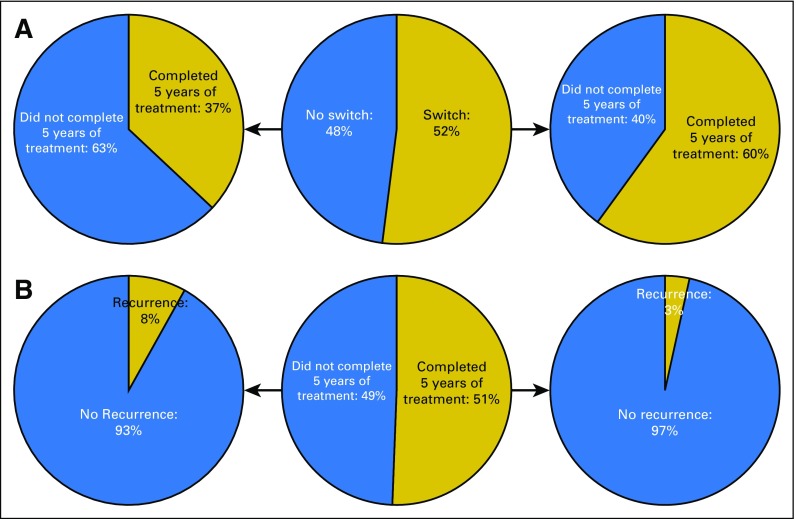
Table 1 lists patient population statistics broken down by 5-year completion status and switch status, and Table 2 lists P values for intergroup comparisons of recurrence and death rates. Data comparisons of patients grouped by switch status and completion status are illustrated in Figures 1A and 1B. We show that approximately 48% of our patient population did not complete at least 5 years of endocrine therapy for a reason other than death and, therefore, may not have achieved the lowest possible risk of recurrence. The probability of a patient completing 5 years of therapy if they switched drugs was 60%, whereas the probability of a patient completing 5 years if they did not make a drug switch was 37%. Patients in our population who completed at least 5 years of treatment experienced recurrence at a rate of 3.4%, and patients who did not complete 5 years of treatment experienced recurrence at a rate of 8.0%.
Table 1.
Patient Characteristics by Adjuvant Endocrine Therapy Completion or Switch Status
Table 2.
Fisher’s Exact Test P Values in Intergroup Comparisons of Death and Recurrence Rates
Fig 1.
(A) Rates of treatment completion in patients grouped by presence of a drug switch. The center circle represents the full patient cohort, and the marginal circles represent completion rates of the switch and no-switch subcohorts. (B) Rates of recurrence in patients grouped by 5-year treatment completion. The center circle represents the full patient cohort, and the marginal circles represent recurrence rates of the patients who did and did not complete 5 years of treatment.
Treatment Trends
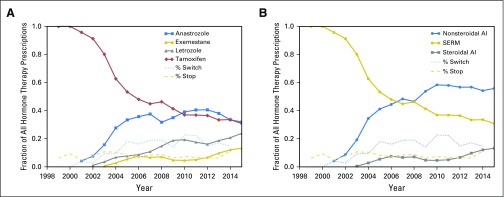
Figure 2 shows the endocrine therapy prescription frequencies at VUMC with the percentage of patients stopping or switching their drugs each year. The graphs reflect an increase in AIs after 2004. The percentage of patients stopping treatment decreased over time, and the percentage of patients switching drugs increased.
Fig 2.
(A) Changes in adjuvant endocrine therapy prescription frequencies and percentage of patients stopping or switching at Vanderbilt University Medical Center over time. (B) Changes in adjuvant endocrine therapy class prescription frequencies and percentage of patients stopping or switching at Vanderbilt University Medical Center over time. AI, aromatase inhibitor; SERM, selective estrogen receptor modulator.
Exploring Causes for Treatment Change
Of the 1,303 patients who switched or stopped adjuvant endocrine therapy before 5 years, 383 (29%) had an instance of a selected stem word in their clinical notes near the time of change. The clinical notes of 120 patients (9%) possessed “stop,” 73 (6%) possessed “switch,” 298 (23%) possessed “complain,” and 59 (5%) possessed “discontinue.” In a random selection of 100 patients with stem word–positive notes, 16% possessed a documented cause for changes to adjuvant endocrine therapy, 20% possessed documented stopping or switching without an explicit cause, and the remaining 64% had stem words that did not reference adjuvant endocrine therapy.
Table 3 shows that the average age of patients switching from an SERM was higher than the average age of patients on SERMs. In contrast, the average age of patients switching from AIs was lower than the average age of patients on AIs. The distributions support that patients switching from SERMs to AIs may be attributed to change in postmenopausal status.
Table 3.
Age Distributions of Patients
Of the patients who switched from an SERM to an AI, 16.5% had a reported oophorectomy through either a Current Procedural Terminology code 58940 or 58720 or an ICD-9 parent code of 65 before their switch date. In addition, 11.5% of patients switching from an SERM to an AI received either goserelin or leuprorelin (estrogen-suppressing drugs that place a patient in a postmenopausal state). Altogether, 28% of the patients who switched from an SERM to an AI underwent an artificial change to postmenopausal status.
Arthritis ICD rates in patients who stopped or switched drugs were 42% and 72% greater than arthritis ICD rates for patients who completed treatment without a switch from SERMs and AIs, respectively (14.2% and 30.6% v 10.0% and 22.0%, respectively). Hot flash ICD rates in patients who stopped or switched drugs were 46% and 39% greater than hot flash ICD rates for patients who completed treatment without a switch from SERMs and AIs, respectively (24.8% and 25.0% v 17.0% and 18.0%, respectively). Furthermore, patients who stopped or switched from an AI had approximately double the rate of arthritis ICD codes of patients who stopped or switched from an SERM (Table 4).
Table 4.
Prevalence of Billing Codes for Adverse Events in Patients and Fisher’s Exact Test P Values to Determine Significant Differences Between Prevalence
Figures 3A and 3B show the time distributions for stopping and switching from AIs and SERMs. All drug change distributions peaked within the first year of treatment. Switching from and stopping an AI occurred within a localized time. Switching from or stopping an SERM had a broad distribution.
Fig 3.
(A) Density plot of time patients spent on aromatase inhibitors (AIs) before stopping or switching treatment. (B) Density plot of time patients spent on selective estrogen receptor modulators (SERMs) before stopping or switching treatment.
DISCUSSION
Using EHR data from a cohort of 1,587 patients with stage I to III breast cancer receiving adjuvant endocrine therapy, we found that approximately 48% of patients did not complete the recommended minimum of 5 years of treatment and 52% of patients switched to a different endocrine therapy drug during their treatment. Using ICD codes, we found that patients who changed their adjuvant endocrine treatment experienced higher rates of arthritis and hot flashes than other patients. Changes in treatment in patients on SERMs followed menopause progression inferred through age, administration of estrogen-suppressing drugs, or surgeries. In addition, switching treatment from an AI is likely to occur at the beginning of treatment, whereas switching from an SERM is not localized to a treatment time.
Patients who switched drugs at some point during their treatment were more likely to complete at least 5 years of adjuvant endocrine therapy (Fig 1A). Switching drugs may be an expedient that encourages patients to continue treatment, and the additional treatment time as a result of the switch may benefit the patients. Patients who completed at least 5 years of adjuvant endocrine therapy had a lower rate of recurrence than patients who did not complete 5 years of treatment (3.4% v 8.0%, respectively; Fig 1B). The recurrence rates fall within the CI of population recurrence rates reported for stage I breast cancer (95% CI, 3% to 15%).13
Endocrine therapy prescription changes at VUMC reflect an increase in options for patients, and as a result, the percentage of patients who switched adjuvant endocrine drugs exceeded the percentage of patients who stopped adjuvant endocrine therapy in 2004 (Fig 2). The option to switch between endocrine therapy drugs may encourage adherence to the recommended treatment duration.
From our computational and manual chart review, we estimate that 5% of patients had a clearly documented cause for adjuvant endocrine therapy treatment change. When documentation regarding the cause of treatment change is sparse, inferring potential reasons for change with EHR data-applied informatics methods is an alternative way to retrieve such information but presents many challenges.
The age distributions for patients at the time of a drug switch (Table 3) support that switching from an SERM is related to age. The average age of patients at the time of switching from an SERM was higher than the average age of patients who were being treated with SERMs. In contrast, patient age at the switch from AIs was lower than patient age during AI use. A switch from an SERM is more likely related to age than a switch from an AI. In addition, Figures 3A and 3B show that switching from an SERM was not localized to a specific duration of treatment, whereas switching from an AI was. Switches from an AI are more likely a result of adverse events that manifest within a year of treatment. For example, Henry et al8 reported that musculoskeletal symptoms from AI use peaked within 6 months. Switches from an SERM are likely dependent on a patient’s individual menopause progression and can appear at any time during treatment.
Patients who stop or switch their treatment experience higher rates of arthritis and hot flashes than patients who complete their treatment without changes. The highest rates of arthritis occurred in patients who stopped or switched AI treatment. Patients who stopped or switched either AI treatment or SERM treatment experienced similar rates of hot flashes, but both of these patient groups experienced more hot flashes than patients who did not switch treatment. Previous studies have reported musculoskeletal adverse effect rates of 44%,8 44% to 47%,14 and 23%15 and hot flash rates of 30%15 and 35% with AI use.3 Our lower rates are a result of the source of our data—ICD codes. ICD codes appear in the patient record if the provider considers the symptom severe enough to bill for it or document it. A symptom may not be documented, but that does not guarantee the symptom’s absence.
This study is limited by the completeness of EHR data. Recurrence rates and death may be underdocumented as a result of lack of follow-up with patients, and ICD codes for adverse symptoms may be underdocumented because providers do not always bill for them. Although we can make inferences about reasons for stopping or switching adjuvant endocrine therapy treatment, the cause of treatment change is infrequently documented in physician notes, making validation difficult. In addition to change in menopause status, adverse events, and recurrence, changes in treatment can be a result of financial factors16 or physician preference, neither of which are documented in the EHR. Last, we omitted factors like body mass index and drug interactions that may influence the results from this study.
In conclusion, this study demonstrates the ability to leverage longitudinal EHRs to characterize treatment trends in a cohort of patients taking adjuvant endocrine therapy. EHR data are a source for real-world frequencies for these patients regarding tumor recurrence, drug switch, and treatment cessation, as well as a source for exploratory analyses on causes for treatment change.
Footnotes
Supported by National Library of Medicine Training Grant No. 5T15LM007450 and National Center for Advancing Translational Sciences Grant No. UL1 TR000445.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Morgan Harrell
Administrative support: Morgan Harrell
Provision of study material or patients: Morgan Harrell
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Morgan Harrell
No relationship to disclose
Daniel Fabbri
Stock and Other Ownership Interests: Maize Analytics
Patents, Royalties, Other Intellectual Property: System for Explanation-Based Auditing of Medical Records Data; US 20130046786 A1; AUDITING OF SQL QUERIES USING SELECT TRIGGERS. Publication number: 20140230070
Mia Levy
Leadership: Personalis, GenomOncology, SechTechDx
Stock and Other Ownership Interests: Personalis, GenomOncology, SechTechDx
Consulting or Advisory Role: Personalis, GenomOncology, SechTechDx
Research Funding: GenomOncology
Patents, Royalties, Other Intellectual Property: GenomOncology
Travel, Accommodations, Expenses: Personalis, GenomOncology
REFERENCES
- 1.Rugo HS, Rumble RB, Macrae E, et al. : Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 34:3069-3103, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Hershman DL: Perfecting breast-cancer treatment: Incremental gains and musculoskeletal pains. N Engl J Med 372:477-478, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Howell A, Cuzick J, Baum M, et al. : Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365:60-62, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Costantino J, Redmond C, et al. : A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med 320:479-484, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Mouridsen H, Chaudri-Ross HA: Efficacy of first-line letrozole versus tamoxifen as a function of age in postmenopausal women with advanced breast cancer. Oncologist 9:497-506, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Early Breast Cancer Trialists’ Collaborative Group: Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet 351:1451-1467, 1998. [PubMed]
- 7.Murphy CC, Bartholomew LK, Carpentier MY, et al. : Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res Treat 134:459-478, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry NL, Giles JT, Ang D, et al. : Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 111:365-372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kligman L, Younus J: Management of hot flashes in women with breast cancer. Curr Oncol 17:81-86, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Güth U, Myrick ME, Schötzau A, et al. : Drug switch because of treatment-related adverse side effects in endocrine adjuvant breast cancer therapy: How often and how often does it work? Breast Cancer Res Treat 129:799-807, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Roden DM, Pulley JM, Basford MA, et al. : Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther 84:362-369, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H, Stenner SP, Doan S, et al. : MedEx: A medication information extraction system for clinical narratives. J Am Med Inform Assoc 17:19-24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brewster AM, Hortobagyi GN, Broglio KR, et al. : Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst 100:1179-1183, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crew KD, Greenlee H, Capodice J, et al. : Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 25:3877-3883, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Garreau JR, Delamelena T, Walts D, et al. : Side effects of aromatase inhibitors versus tamoxifen: The patients’ perspective. Am J Surg 192:496-498, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Zafar SY, Peppercorn JM, Schrag D, et al. : The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist 18:381-390, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]