Abstract
Cancer and atrial fibrillation (AF) are common conditions, but for patients affected with both, there is a lack of data about management of anticoagulation and cerebrovascular outcomes. In the first section of this review, we summarize the most relevant studies on stroke risk and management of AF in patients with active cancer, attempting to answer questions of whether to anticoagulate, whom to anticoagulate, and what agents to use. In the second section of the review, we suggest a decision algorithm on the basis of the available evidence and provide practical recommendations for each of the anticoagulant options. In the third section, we discuss the limitations of the available evidence. On the basis of low-quality evidence, we find that patients with cancer and AF have a risk of stroke similar to that of the general population but a substantially higher risk of bleeding regardless of the anticoagulant agent used; this makes anticoagulation-related decisions complex and evidence from the general population not immediately applicable. In general, we suggest stopping anticoagulation in patients with high risk of bleeding and in those with a moderate bleeding risk without a high thromboembolic risk and recommend anticoagulation as in the general population for patients at a low risk for bleeding. However, regardless of initial therapy, we recommend reassessing whether anticoagulation should be given at each point in the clinical course of the disease. High-quality evidence to guide anticoagulation for AF in patients with cancer is needed.
INTRODUCTION
Cancer is one of the leading causes of death worldwide, and its incidence increases as the population ages. Therapeutic decision making must consider not only the heterogeneity of the disease itself but also patient comorbidity and fitness.
Atrial fibrillation (AF) is a common condition in the general population.1 Like cancer, its incidence increases with age. Importantly, AF is often accompanied by a host of cardiovascular comorbidities, such as diabetes, hypertension, or heart disease. These comorbidities are at the same time risk factors for AF development and compound its impact on a patient’s fitness. The most feared complication of AF is cardioembolic stroke, which constitutes 20% to 30% of ischemic strokes and is the subtype of stroke with the highest mortality and functional repercussion. Cardioembolic stroke can be effectively prevented by oral anticoagulants.2
Until recently, the examined relationship between AF and cancer has been limited to epidemiologic data showing that the diagnosis of either one increases the odds of being diagnosed with the other,3 although common risk factors and the increased medical vigilance that comes with either diagnosis might confound the analysis. Yet, the clinical significance and optimal management of AF in patients with active cancer remains uncertain because of a lack of studies and the complexities surrounding them, which hinder obtaining clinically useful data.
In this review, we provide oncologists with a brief overview of the data on the risk of stroke in patients with active cancer and AF and the scarce evidence regarding the management of anticoagulation in these patients. We then describe our personal approach to this clinical scenario. We believe the oncologist needs to take an active part in the choice and follow-up of the anticoagulation, because bleeding and thrombosis are frequent clinical events in these patients, and their optimal management is an important part of successful anticancer treatment.
AF, CARDIOEMBOLIC STROKE, AND ANTICOAGULATION IN THE GENERAL POPULATION AND IN PATIENTS WITH ACTIVE CANCER
Incidence
General population.
The incidence of AF in the general population depends on the presence of cardiovascular comorbidities, particularly age. The prevalence of AF increases from 0.1% in patients younger than 55 years of age to 10% in those older than 85 years.1
Cancer population.
Approximately 2% to 5% of patients diagnosed with cancer have AF at the time of diagnosis, largely associated with the same factors as the general population.4-6 However, the incidence of new-onset AF in patients with a diagnosis of cancer is higher than in the general population3 for many reasons, including shared risk factors (for both cancer and AF) and cancer-induced inflammation.7 In addition, patients with cancer may be more likely to suffer secondary AF (ie, AF triggered by an outside stressor, such as surgery, anemia, sepsis, or hypoxemia).6-8 An increase in incidence has been best documented in the setting of lung and colon cancer surgery (which can be higher than 10% in the former and 5% in the latter),8 but this is likely to be the result of the greater prevalence of these cancers in the general population, because there is no evidence that AF is less likely to occur in patients with other cancers requiring surgery. Finally, certain drugs also promote the development of AF. Some, such as anthracyclines, do so indirectly, by causing heart failure, which increases the risk of AF.7 Other drugs cause AF directly, such as ibrutinib, which increases the risk of AF three to four times through off-target tyrosine kinase inhibition.9
Risk of Stroke
General population.
The risk of stroke in patients with AF is assessed by means of the CHADS2 (congestive heart failure, hypertension, age ≥ 75, diabetes mellitus [1 point each] and previous stroke/transient ischemic attack [2 points]) and CHA2DS2VASc scores (congestive heart failure, hypertension, age 65-74, diabetes mellitus, vascular disease, female sex [1 point each] and age ≥ 75, previous stroke/transient ischemic attack/thromboembolism [2 points each]) (Data Supplement). For CHA2DS2VASc, the risk of stroke ranges from 0.2%/y for those with 0 points to 12%/y to 14%/y for those with 9 points.10
Cancer population.
Similarly to the risk of venous thromboembolic disease (VTE), the risk of arterial thromboembolism, including stroke, is increased in most cancer types, particularly in the few months after diagnosis.11,12 In an analysis of more than 250,000 patients with cancer paired with as many without cancer, the cumulative incidence of stroke at 6 months was 3% and 1.6%, respectively. Although this presents a two-fold increase in stroke risk over the general population, this is strongly dependent on cancer type (eg, patients with lung cancer had four times the risk, and those with breast or prostate cancer did not seem to have an increased risk of stroke).12 This increase in the incidence of stroke in patients with cancer is the result of an increase in the frequency of unexplained strokes, believed to be secondary to hypercoagulability or noninfectious endocarditis.11 However, cardioembolic stroke is not increased in patients with cancer.11,13 This is of major practical consequence, given the increased risk of bleeding in cancer,14-16 and renders the assessment of the net clinical benefit of anticoagulation for AF even more complex.
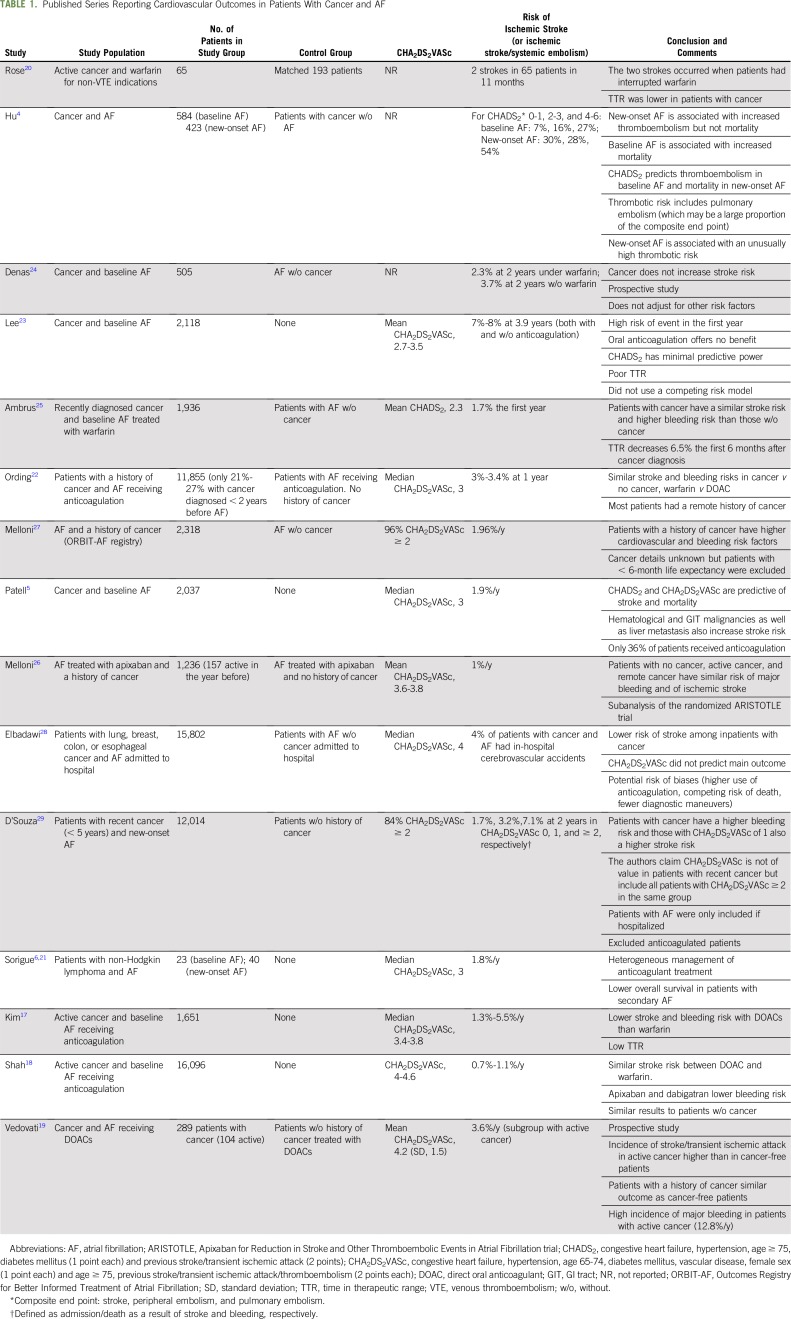
Table 1 lists the data from published series assessing the risk of stroke in patients with cancer and AF.4-6,17-29 Although these studies are heterogeneous, a distinction that seems consistently relevant in terms of risk of stroke is that between baseline AF and new-onset AF.
TABLE 1.
Published Series Reporting Cardiovascular Outcomes in Patients With Cancer and AF
Baseline AF.
Baseline AF is any AF already diagnosed at the time of cancer diagnosis. One of the most relevant studies in this setting is a cohort of more than 2,000 patients with recently diagnosed cancer and AF reported by Patell et al,5 who, along with a relatively low risk of stroke (1.9%/y), found that CHADS2 and CHA2DS2VASc scores were predictive of stroke. Denas et al24 and Ambrus et al25 reported no difference in stroke risk in patients with AF treated with warfarin on the basis of the presence or absence of cancer. Overall, the risk of stroke in patients with cancer and baseline AF seems to be similar to that of the general population, with CHADS2 and CHA2DS2VASc being predictive of stroke risk.5 However, the heterogeneity of the studies, particularly regarding anticoagulant treatment and the population included, still calls for more evidence, preferably gathered prospectively.
New-onset AF.
New-onset AF is that diagnosed at the time of, or at any point after, the diagnosis of cancer. Contrary to baseline AF, some studies indicate that the CHADS2 and CHA2DS2VASc scores may not predict stroke risk in new-onset AF.4,29
Although the distinction between baseline and new-onset AF needs additional support, it would align with the idea that AF in patients with cancer is frequently secondary. According to this idea, resolution of the trigger could resolve secondary AF, leading to a lower risk of stroke.30 Importantly, new-onset AF in patients with cancer may reveal a low homeostatic reserve and a high risk of death.6,31,32
Anticoagulation
General population.
The approach to a patient with AF requires many considerations that fall outside the scope of this review, but an essential question is whether the patient should receive anticoagulation. In most clinical scenarios, patients should receive anticoagulation because the benefits clearly outweigh the risks (ie, bleeding).1,2 CHA2DS2VASc scores of 2 or greater are generally an indication to anticoagulate.1,33 Bleeding risk should not factor into the equation for most patients, because most risk factors for bleeding are also risk factors for stroke, so that patients with the highest bleeding risk are often the ones who benefit the most from anticoagulation.1,34
Cancer population.
When considering anticoagulation for AF, patients with cancer have two differential features: the risks of VTE and of noncardioembolic stroke are higher than in the general population and may have to factor into the decision for some cancer subtypes,12,35 and bleeding risk is much higher than in the general population14,15,17,18,23,25,36 (particularly in patients with GI cancers) because of local barrier disruption, invasive procedures, treatment- or disease-related thrombocytopenia, and disseminated intravascular coagulation.36 Of note, bleeding in patients with cancer may be more severe than in the general population.16,37
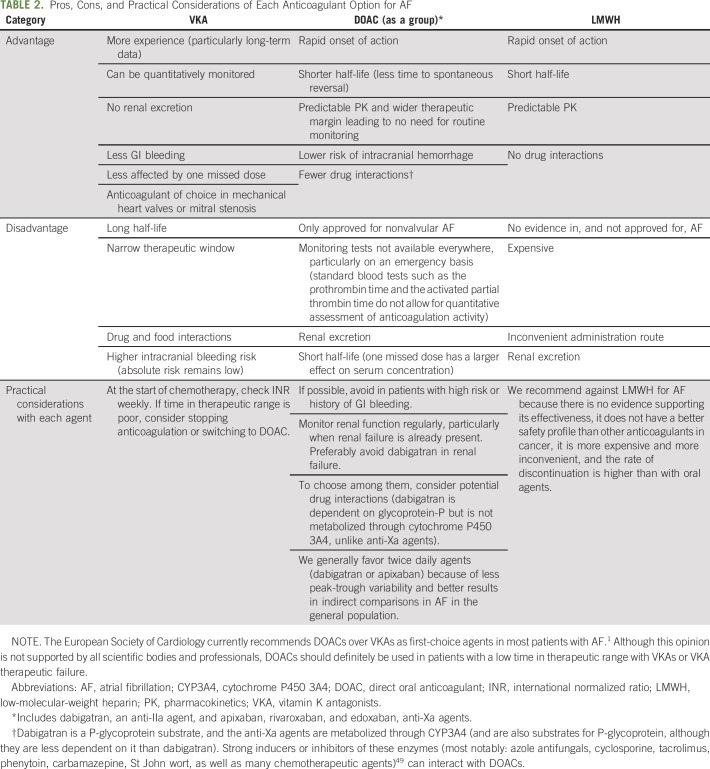
This uncertainty over the risk-benefit of anticoagulation in AF and cancer is reflected in the recent European Society of Cardiology guidelines, which do not make specific recommendations but rather suggest that each patient be assessed individually.35 If the decision is made to anticoagulate, the immediate question is which agent to use. Table 2 lists the basic characteristics of the available options in the general population.
TABLE 2.
Pros, Cons, and Practical Considerations of Each Anticoagulant Option for AF
Vitamin K antagonists (VKAs) have several downsides that particularly affect patients with cancer. These include interaction with chemotherapeutic agents or other drugs, oral intolerance as a result of nausea, unsteady diet, and treatment discontinuation due to invasive procedures. Furthermore, they are less efficacious than low-molecular-weight heparin (LMWH) for the treatment of cancer-associated VTE.38 However, most of the available evidence does not suggest a notably higher incidence of stroke than the general population with AF24,25 or a higher risk of bleeding than either LMWH or direct oral anticoagulants (DOACs).16,36,39
Until recently, because of lack of evidence and fear of potential drug interaction, DOACs were not recommended in patients with cancer. However, recent trials in the prevention and treatment of cancer-associated VTE40,41 indicate that DOACs are effective and generally safe. The international society on Thrombosis and Haemostasis now accepts them as a valid option for the treatment of VTE in patients with cancer.42 Although there are no trials in patients with AF and cancer, and the retrospective evidence available is more limited, a large retrospective comparative analysis for AF18 suggested that the efficacy and safety of DOACs compared with warfarin are similar to those in the general population (incidence of ischemic stroke and severe bleeding of 0.8%/y and 2%/y with DOACs and 1%/y and 3%/y with warfarin, respectively). A relevant nuance is that trials in patients with VTE have shown that GI bleeding is increased in patients receiving full-dose DOACs, so particular caution is needed in patients at risk for GI bleeding (largely those with GI cancers and those with previous GI bleeding).42,43 LMWH has not been studied for stroke prevention in AF and is not approved for that indication.
Periprocedural Management
General population.
A plethora of retrospective trials and a subsequent randomized trial44 have shown that LMWH increases major bleeding without decreasing stroke risk in patients with AF receiving VKAs undergoing ambulatory procedures. Therefore, most patients should interrupt VKA without bridging, at least for ambulatory procedures. Given the short half-life of DOACs and lack of evidence of benefit, bridging with LMWH is generally not advised.
Cancer population.
Prospective data are lacking in patients with cancer. A large retrospective series showed that bridging with LMWH in patients with cancer (anticoagulated for any indication, not only AF) increased bleeding without decreasing thrombotic risk.45
Management of Bleeding
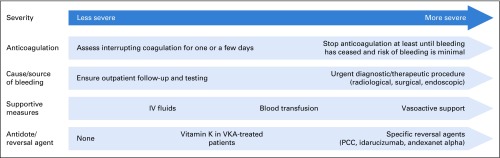
Severe bleeding is not common in the general population, but any-grade bleeding is a frequent complication of anticoagulant treatment. The therapeutic approach to bleeding should be step based, with more aggressive measures added to more basic ones as the severity of the bleeding episode increases46,47 (Appendix Fig A1, online only).
As a general principle, it is important to keep in mind that anticoagulation does not cause bleeding on its own (it prevents clotting in the presence of bleeding). Therefore, a cause or trigger needs to be looked for and, if possible, treated (eg, endoscopic or angiographic examination). It should also be remembered that these patients have a high thrombotic risk (up to 6% to 8% of patients with major bleeding develop VTE48), both because of the indication for which they receive anticoagulation and because acute bleeding is a prothrombotic environment. Therefore, it is essential to restart anticoagulation (most often prophylactic-dose LMWH) as soon as the bleeding has subsided and the risk of rebleeding is low.46,47
MY APPROACH: ASSESSMENT AND MANAGEMENT OF AF IN PATIENTS WITH CANCER
Risk Assessment
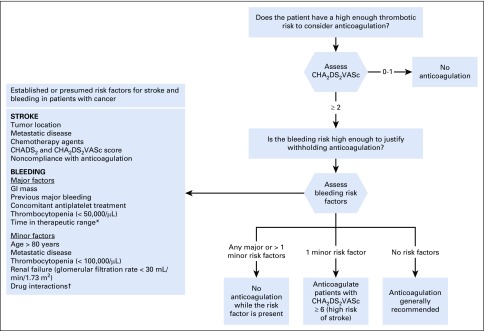
Given the lack of prospective evidence and the many factors involved in decision-making (Fig 1), an individualized approach is essential, in line with the European Society of Cardiology guidelines recommendations.35 Unfortunately, most prothrombotic factors also increase bleeding risk, and it is uncertain how much weight, if any, each risk factor should have.
Fig 1.
Established or presumed risk factors for stroke and bleeding in patients with cancer: proposed algorithm to decide whether patients with active cancer should receive anticoagulation for atrial fibrillation. (*) If time in therapeutic range is poor, consider switching to a direct oral anticoagulant. (†) See Short and Connors49 and Kraajipoel and Carrier.50 CHADS2, congestive heart failure, hypertension, age ≥ 75, diabetes mellitus (1 point each) and previous stroke/transient ischemic attack (2 points); CHA2DS2VASc, congestive heart failure, hypertension, age 65-74, diabetes mellitus, vascular disease, female sex (1 point each) and age ≥ 75, previous stroke/transient ischemic attack/thromboembolism (2 points each).
Although some data indicate that there are no truly low-risk patients with cancer,29 there is insufficient evidence that patients with cancer and AF with a CHA2DS2VASc 0 to 1 obtain a net benefit from anticoagulation. In patients with CHA2DS2VASc of 2 or more, in the general population, anticoagulation is generally recommended (even at the expense of a higher bleeding risk with treatment), because cardioembolic strokes generally have worse functional outcomes than most bleedings (except intracranial, which are rare), and this is often used to justify treatment even in patients with high bleeding risk.51 However, major bleeding increases mortality52 and thrombotic risk in the acute phase and decreases adherence to antithrombotic medication. In the general population, a 1%/y risk of stroke is the threshold to recommend anticoagulation,33 knowing that anticoagulation carries a risk of major bleeding between 2% and 5%/y.2 Similar data from patients with active cancer are difficult to obtain, given the differences between studies, but most of them show a consistently higher risk of bleeding: 4% in the first 3 months of anticoagulation, 4% to 6% at 6 months, and up to 10%/y to 15%/y.14,15,19,36,38 Assuming that the mortality of bleeding in patients with cancer is the same, although it may be higher,16,37 a consistent stroke risk of at least 4%/y to 5%/y without anticoagulation (which does not seem to be the case for a majority of patients; Table 1) would be required to justify anticoagulation. It has been argued that, because anticoagulation may also offer protection against VTE, one may have a lower threshold for anticoagulation in patients with a high risk of VTE.35 Although worth investigating, at present we would generally not recommend anticoagulation in a patient with AF on the basis of VTE risk, given that many patients with a high risk of VTE also have high bleeding risks. Ultimately, given how variable and dynamic bleeding risk is in patients with cancer, an in-depth discussion with the patient, including periodic assessment of the need to anticoagulate, is warranted.
Initiating Anticoagulation
Primary AF.
In patients with a CHA2DS2VASc of 0 or 1, we would recommend against anticoagulation for AF. For CHA2DS2VASc of 2 or more, we would first gauge bleeding risk. This was already suggested by Farmakis et al8 in 2014, and all evidence published subsequently supports their position. In patients with a high bleeding risk (any major or more than one minor risk factor from Fig 1), we would generally suggest stopping anticoagulation during chemotherapy and restarting later, when tumor burden is lower and risk of thrombocytopenia is back to baseline. Conversely, we would tend to treat patients with a low bleeding risk (no bleeding risk factors) as one would the general population. For patients with a moderate risk (only one minor risk factor), we would generally favor anticoagulation for patients with a previous stroke or a CHA2DS2VASc of 6 or more (stroke risk approximately 10%/y10; Fig 1).
Secondary AF.
For patients with secondary AF, anticoagulation may be a less-relevant concern. The trigger should be dealt with, and attention must be paid to a potential low homeostatic reserve that indicates high risk of an unfavorable clinical outcome. A cardiology consultation to rule out underlying cardiac damage and to aid with management of the acute episode is recommended. These patients may have a higher risk of developing permanent AF in the long term, but if the triggering event is resolved, the risk of stroke and the benefits of anticoagulation seem uncertain.30 We would therefore argue for clinical follow-up (with or without temporary anticoagulation until the end of the AF episode) rather than up-front indefinite anticoagulation.
Periprocedural bridging and bleeding.
We recommend against periprocedural bridging for most patients receiving anticoagulation for AF. In patients who suffer a major bleed, the risk of rebleeding is high, so we would favor stopping anticoagulation, at least until the trigger (eg, GI mass) has been resolved. However, in the acute phase of the bleeding episode, the risk of VTE is high, so prophylactic-dose LMWH should be administered as soon as the bleeding has subsided and until hospital discharge.46,47
Choices of agents.
Concerning specific anticoagulant agents, we generally continue with the drug (VKA or DOAC) the patient is taking if AF was previous to the diagnosis of cancer and anticoagulation is warranted. If the patient is diagnosed with new-onset AF, there is no evidence to choose one over another, and we would consider each of them on a case-by-case basis, generally following the considerations in Table 2.
SHORTCOMINGS OF THE EXISTING DATA AND FUTURE CHALLENGES
Data are insufficient for us to have a firm grasp on the risk of ischemic stroke in patients with AF and cancer. A summary of what we perceive are the most relevant limitations can be found in the Data Supplement. However, the major limitation with the available data is that patients with cancer are considered a single population. Different cancer sites and histological subtypes have widely different clinical courses, which should probably make physicians consider AF differently in these patients. Similarly, the risk-benefit balance of anticoagulation for AF likely changes during the course of the disease, particularly bleeding risk, which is higher when tumor burden is largest and treatment is administered.
CONCLUSION
AF in patients with cancer remains an underexamined topic. Given the thrombotic and hemorrhagic risk of these patients, evidence cannot be extrapolated from the general population. The available evidence, severely limited in amount and quality, points toward a risk of ischemic stroke that is not much higher than in the general population. Bleeding risk is higher, and the optimal anticoagulation strategy remains uncertain. Therefore, and at least until higher-grade evidence becomes available, an individualized and dynamic approach is essential and arguably more important than the initial strategy.
Appendix
Fig A1.
Stepwise management of bleeding in patients under anticoagulation. PCC, prothrombin complex concentrate; VKA, Vitamin K antagonist.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Marc Sorigue
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Atrial Fibrillation and Stroke Risk in Patients With Cancer: A Primer for Oncologists
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
No potential conflicts of interest were reported.
REFERENCES
- 1.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 2.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 3.Conen D, Wong JA, Sandhu RK, et al. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. 2016;1:389–396. doi: 10.1001/jamacardio.2016.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu YF, Liu CJ, Chang PM, et al. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int J Cardiol. 2013;165:355–357. doi: 10.1016/j.ijcard.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 5.Patell R, Gutierrez A, Rybicki L, et al. Usefulness of CHADS2 and CHA2DS2-VASc scores for stroke prediction in patients with cancer and atrial fibrillation. Am J Cardiol. 2017;120:2182–2186. doi: 10.1016/j.amjcard.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 6.Sorigue M, Gual-Capllonch F, Garcia O, et al. Incidence, predictive factors, management, and survival impact of atrial fibrillation in non-Hodgkin lymphoma. Ann Hematol. 2018;97:1633–1640. doi: 10.1007/s00277-018-3346-1. [DOI] [PubMed] [Google Scholar]
- 7.Mery B, Guichard J-B, Guy J-B, et al. Atrial fibrillation in cancer patients: Hindsight, insight and foresight. Int J Cardiol. 2017;240:196–202. doi: 10.1016/j.ijcard.2017.03.132. [DOI] [PubMed] [Google Scholar]
- 8.Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: Atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63:945–953. doi: 10.1016/j.jacc.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: A systematic review and meta-analysis. Blood. 2016;128:138–140. doi: 10.1182/blood-2016-05-712828. [DOI] [PubMed] [Google Scholar]
- 10.Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: The Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 11.Navi BB, Iadecola C. Ischemic stroke in cancer patients: A review of an underappreciated pathology. Ann Neurol. 2018;83:873–883. doi: 10.1002/ana.25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navi BB, Reiner AS, Kamel H, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grazioli S, Paciaroni M, Agnelli G, et al. Cancer-associated ischemic stroke: A retrospective multicentre cohort study. Thromb Res. 2018;165:33–37. doi: 10.1016/j.thromres.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Prandoni P, Lensing AWA, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 15.Trujillo-Santos J, Nieto JA, Tiberio G, et al. Predicting recurrences or major bleeding in cancer patients with venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100:435–439. [PubMed] [Google Scholar]
- 16.Prandoni P, Trujillo-Santos J, Sanchez-Cantalejo E, et al. Major bleeding as a predictor of mortality in patients with venous thromboembolism: Findings from the RIETE Registry. J Thromb Haemost. 2010;8:2575–2577. doi: 10.1111/j.1538-7836.2010.04039.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim K, Lee Y-J, Kim T-H, et al. Effect of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with newly diagnosed cancer. Korean Circ J. 2018;48:406–417. doi: 10.4070/kcj.2017.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah S, Norby FL, Datta YH, et al. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2018;2:200–209. doi: 10.1182/bloodadvances.2017010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vedovati MC, Giustozzi M, Verdecchia P, et al. Patients with cancer and atrial fibrillation treated with doacs: A prospective cohort study. Int J Cardiol. 2018;269:152–157. doi: 10.1016/j.ijcard.2018.07.138. [DOI] [PubMed] [Google Scholar]
- 20.Rose AJ, Sharman JP, Ozonoff A, et al. Effectiveness of warfarin among patients with cancer. J Gen Intern Med. 2007;22:997–1002. doi: 10.1007/s11606-007-0228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorigue M, Sarrate E, Franch-Sarto M, et al. Risk of cardioembolic stroke in patients with cancer and atrial fibrillation. Am J Cardiol. 2018;121:1656–1657. doi: 10.1016/j.amjcard.2018.02.060. [DOI] [PubMed] [Google Scholar]
- 22.Ording AG, Horváth-Puhó E, Adelborg K, et al. Thromboembolic and bleeding complications during oral anticoagulation therapy in cancer patients with atrial fibrillation: A Danish nationwide population-based cohort study. Cancer Med. 2017;6:1165–1172. doi: 10.1002/cam4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee Y-J, Park JK, Uhm J-S, et al. Bleeding risk and major adverse events in patients with cancer on oral anticoagulation therapy. Int J Cardiol. 2016;203:372–378. doi: 10.1016/j.ijcard.2015.10.166. [DOI] [PubMed] [Google Scholar]
- 24.Denas G, Pengo V, Joppi R, et al. Cancer as a risk factor for stroke in atrial fibrillation patients receiving long-term oral anticoagulant therapy. Thromb Res. 2015;136:488. doi: 10.1016/j.thromres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Ambrus DB, Reisman JI, Rose AJ. The impact of new-onset cancer among veterans who are receiving warfarin for atrial fibrillation and venous thromboembolism. Thromb Res. 2016;144:21–26. doi: 10.1016/j.thromres.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 26.Melloni C, Dunning A, Granger CB, et al. Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and a history of cancer: Insights from the ARISTOTLE trial. Am J Med. 2017;130:1440–1448.e1. doi: 10.1016/j.amjmed.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Melloni C, Shrader P, Carver J, et al. Management and outcomes of patients with atrial fibrillation and a history of cancer: The ORBIT-AF registry. Eur Hear J Qual Care Clin Outcomes. 2017;3:192–197. doi: 10.1093/ehjqcco/qcx004. [DOI] [PubMed] [Google Scholar]
- 28.Elbadawi A, Elgendy IY, Ha LD, et al. In-hospital cerebrovascular outcomes of patients with atrial fibrillation and cancer (from the National Inpatient Sample Database) Am J Cardiol. 2018;121:590–595. doi: 10.1016/j.amjcard.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 29.D’Souza M, Carlson N, Fosbøl E, et al. CHA2DS2-VASc score and risk of thromboembolism and bleeding in patients with atrial fibrillation and recent cancer. Eur J Prev Cardiol. 2018;25:651–658. doi: 10.1177/2047487318759858. [DOI] [PubMed] [Google Scholar]
- 30.Quon MJ, Behlouli H, Pilote L. Anticoagulant use and risk of ischemic stroke and bleeding in patients with secondary atrial fibrillation associated with acute coronary syndromes, acute pulmonary disease, or sepsis. JACC Clin Electrophysiol. 2018;4:386–393. doi: 10.1016/j.jacep.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Imperatori A, Mariscalco G, Riganti G, et al. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg. 2012;7:4. doi: 10.1186/1749-8090-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lardaro T, Self WH, Barrett TW. Thirty-day mortality in ED patients with new onset atrial fibrillation and actively treated cancer. Am J Emerg Med. 2015;33:1483–1488. doi: 10.1016/j.ajem.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer DE, Ezekowitz MD. Adding rigor to stroke risk prediction in atrial fibrillation. J Am Coll Cardiol. 2015;65:233–235. doi: 10.1016/j.jacc.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Kittelson JM, Steg PG, Halperin JL, et al. Bivariate evaluation of thromboembolism and bleeding in clinical trials of anticoagulants in patients with atrial fibrillation. Thromb Haemost. 2016;116:544–553. doi: 10.1160/TH15-12-1000. [DOI] [PubMed] [Google Scholar]
- 35.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 36.Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49–S55. doi: 10.1016/S0049-3848(14)50009-6. [DOI] [PubMed] [Google Scholar]
- 37.Brenner B, Bikdeli B, Tzoran I, et al. Arterial ischemic events are a major complication in cancer patients with venous thromboembolism. Am J Med. 2018;131:1095–1103. doi: 10.1016/j.amjmed.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 38.Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582–589. doi: 10.1016/j.thromres.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ay C, Kamphuisen PW, Agnelli G. Antithrombotic therapy for prophylaxis and treatment of venous thromboembolism in patients with cancer: Review of the literature on current practice and emerging options. ESMO Open. 2017;2:e000188. doi: 10.1136/esmoopen-2017-000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 41.Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711–719. doi: 10.1056/NEJMoa1814468. [DOI] [PubMed] [Google Scholar]
- 42.Khorana AA, Noble S, Lee AYY, et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16:1891–1894. doi: 10.1111/jth.14219. [DOI] [PubMed] [Google Scholar]
- 43.Li A, Garcia DA, Lyman GH, et al. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb Res. 2019;173:158–163. doi: 10.1016/j.thromres.2018.02.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823–833. doi: 10.1056/NEJMoa1501035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tafur AJ, Wysokinski WE, McBane RD, et al. Cancer effect on periprocedural thromboembolism and bleeding in anticoagulated patients. Ann Oncol. 2012;23:1998–2005. doi: 10.1093/annonc/mds058. [DOI] [PubMed] [Google Scholar]
- 46.Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: A report of the American College of Cardiology task force on expert consensus decision pathways. J Am Coll Cardiol. 2017;70:3042–3067. doi: 10.1016/j.jacc.2017.09.1085. [DOI] [PubMed] [Google Scholar]
- 47.Ageno W, Büller HR, Falanga A, et al. Managing reversal of direct oral anticoagulants in emergency situations. Anticoagulation Education Task Force white paper. Thromb Haemost. 2016;116:1003–1010. doi: 10.1160/TH16-05-0363. [DOI] [PubMed] [Google Scholar]
- 48.Sarode R, Milling TJ, Jr, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: A randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128:1234–1243. doi: 10.1161/CIRCULATIONAHA.113.002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Short NJ, Connors JM. New oral anticoagulants and the cancer patient. Oncologist. 2014;19:82–93. doi: 10.1634/theoncologist.2013-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kraaijpoel N, Carrier M. How I treat cancer-associated venous thromboembolism. Blood. 2019;133:291–298. doi: 10.1182/blood-2018-08-835595. [DOI] [PubMed] [Google Scholar]
- 51.Rechenmacher SJ, Fang JC. Bridging anticoagulation: Primum non nocere. J Am Coll Cardiol. 2015;66:1392–1403. doi: 10.1016/j.jacc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Marijon E, Le Heuzey J-Y, Connolly S, et al. Causes of death and influencing factors in patients with atrial fibrillation: A competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013;128:2192–2201. doi: 10.1161/CIRCULATIONAHA.112.000491. [DOI] [PubMed] [Google Scholar]