Abstract
PURPOSE:
Reducing acute care use is an important strategy for improving value in cancer care. However, little information is available to describe and compare population-level hospital use across cancer types. Our aim was to estimate unplanned hospitalization rates and to describe the reasons for hospitalization in a population-based cohort recently diagnosed with cancer.
MATERIALS AND METHODS:
California Cancer Registry data linked with administrative inpatient data were used to examine unplanned hospitalization among individuals diagnosed with cancer between 2009 and 2012 (n = 412,850). Hospitalizations for maintenance chemotherapy, radiotherapy, or planned surgery were excluded. Multistate models were used to estimate age-adjusted unplanned hospitalization rates, accounting for survival.
RESULTS:
Approximately 67% of hospitalizations in the year after diagnosis were unplanned, 35% of newly diagnosed individuals experienced an unplanned hospitalization, and 67% of unplanned hospitalizations originated in the emergency department (ED). Nonmalignancy principal diagnoses most frequently associated with unplanned hospitalization included infection (15.8%) and complications of a medical device or care (6.5%). Unplanned hospitalization rates were highest for individuals with hepatobiliary or pancreatic cancer (2.08 unplanned hospitalizations per person-year at risk), lung cancer (1.58 unplanned hospitalizations), and brain or CNS cancer (1.47 unplanned hospitalizations), and were lowest among individuals with prostate cancer (0.18 unplanned hospitalizations) and melanoma (0.25 unplanned hospitalizations).
CONCLUSION:
The population burden of unplanned hospitalization among individuals newly diagnosed with cancer is substantial. Many unplanned hospitalizations originate in the ED and are associated with potentially preventable admission diagnoses. Efforts to reduce unplanned hospitalization might target subgroups at higher risk and focus on the ED as a source of admission.
INTRODUCTION
Hospitalization is a primary driver of cancer-related health care spending.1-4 Outpatient treatment modalities are increasingly common, and the cost of cancer drugs and imaging continues to rise; nonetheless, hospitalizations for cancer, which recent data suggest last longer and cost more than those for other conditions,5 make up more than one half of total cancer-related treatment costs in the year after diagnosis as well as in the last year of life.4 A growing body of evidence suggests that cancer-related acute care use can be reduced with timely outpatient management enhanced access and early palliative care.6-8 Consequently, reducing potentially avoidable hospitalizations has been increasingly viewed as a promising target for improving the quality and reducing the costs of cancer care.
Despite growing attention to acute care use among individuals with cancer, population estimates of hospitalization rates are lacking. Many studies of cancer hospitalizations have been restricted to small subgroups of patients with cancer in clinical trials9,10 or Medicare beneficiaries 65 years of age and older,4,11,12 limiting generalizability. Other studies examining acute care use have focused heavily on costs4 rather than on patterns of admission5,12 and have not accounted for differences in time at risk (eg, because of competing risk of mortality and time hospitalized)5,11 or have examined use only within a narrow window of time during cancer treatment, such as among decedents near the end of life13,14 or within 30 days after cancer surgery.15-17 Recent recommendations suggest that a key strategy for reducing unplanned acute care use is to identify subgroups of and individual patients with cancer at high risk.18 Studies that examine hospitalization patterns broadly (across cancer types and throughout the trajectory of care) would help inform these efforts.
In this study, we sought to address this gap by producing population-based estimates of unplanned hospitalization across all cancer types during the 1-year period after initial diagnosis, using a multistate modeling approach to account for the competing risk of mortality. Our results shed light on the population burden of unplanned hospitalization among individuals with cancer, and may provide benchmarks against which to examine future trends, as well as to guide targeted interventions to reduce unplanned hospitalization rates.
MATERIALS AND METHODS
Study Design and Data Sources
We examined hospitalization among patients with cancer, using a retrospective cohort design and two linked data sources: (1) the California Department of Public Health’s California Cancer Registry (CCR), a population-based cancer surveillance system that collects clinical and sociodemographic information about all individuals diagnosed with cancer in California19; and (2) the California Office of Statewide Health Planning and Development (OSHPD) death registry–linked Patient Discharge Data files, which include coded patient discharge information for all acute care hospitals licensed in the state of California (excluding Veterans Administration and military hospitals).20 OSHPD and CCR records are linked by CCR using probabilistic matching that is based on patient demographic variables such as social security number (SSN) and date of birth.21-23
Cohort
Our cohort included all adults identified in the CCR as having been diagnosed with cancer (other than nonmelanoma skin cancer) between 2009 and 2012 (n = 520,838). We excluded individuals who were younger than 18 years of age at diagnosis (1.1%), who were diagnosed with in situ disease (9.7%), who did not have an OSHPD ID number for linkage (either because they had never had an OSHPD encounter or because they lacked a valid SSN; 9.5%), or who had multiple values of the variables needed for uniquely identifying individuals and hospital encounters (< 1%). The final eligible sample included n = 412,850 adults with cancer.
Variable Definitions
We obtained sociodemographic variables from CCR data. These included: age (in years); sex (female or male); race/ethnicity (white, non-Hispanic; black, non-Hispanic; Hispanic or Latino of any race; Asian or Pacific Islander, non-Hispanic; or other); marital status (married or not married [single, widowed, separated, divorced]); and socioeconomic status (SES) quintiles that were based on composite scores developed for use by the CCR.24 Composites were derived using census tract–level SES indicators (eg, education, income). Individuals were classified into quintiles from lowest to highest (highest, upper middle, middle, lower middle, or lowest) and insurance type (private, public [Medicare and Medicaid], uninsured, or other).
Using SEER site recode variables,25 we created 15 broad categories of cancer site, accounting for all malignancies (breast, prostate, lung, colorectal, hepatobiliary and pancreatic, melanoma, thyroid, brain and CNS, other digestive, urinary, head and neck, bone and soft tissue, other female reproductive, hematologic, or other). Cancer stage (I, II, III, or IV) was classified using the SEER-modified American Joint Commission on Cancer staging manual. A full description of cancer site and stage categories is available in the Data Supplement.
All-cause hospitalization was defined as any inpatient admission to an acute care hospital that began on or after the day of cancer diagnosis (recorded in CCR) to 365 days after diagnosis. We considered multiple admission records for an individual patient within an overlapping timeframe (ie, admission records with a zero-day difference between the discharge date and subsequent admission date) as a single episode and obtained information about that episode from the last record. In our analysis of hospitalization rates and reasons for hospitalization, we focused on a subset of hospitalizations that excluded admissions for maintenance chemotherapy or radiotherapy, as well as admissions for planned surgery (defined as a scheduled admission with a primary surgical procedure and a primary diagnosis of cancer). Hereafter, we refer to this subset as unplanned hospitalizations.
Principal diagnoses associated with each unplanned hospitalization were classified using International Classification of Diseases (9th revision, clinical modification) codes derived from the hospitalization record in OSHPD. Principal diagnosis codes were grouped using the single-level Clinical Classification Software program downloaded from the Healthcare Cost and Utilization Project Web site.26 We further collapsed procedures and principal diagnoses into relevant categories selected on the basis of common procedures and reasons for admission among patients with cancer in previous studies,5,11 including (1) cancer related, (2) infection and/or fever, (3) cardiovascular (including heart attack, stroke, embolism, and dysrhythmias), (4) complications of a medical device or medical care, (5) GI, and (6) respiratory. A full list of Clinical Classification Software groups included in each category can be found in the Data Supplement.
Statistical Analysis
All analyses were conducted using Stata 14 MP (StataCorp, College Station, TX), with descriptive statistics calculated for all variables. Statistical significance was set at P < .05.
We calculated the percentage of patients with cancer with any all-cause hospitalization or unplanned hospitalization stratified by cancer type, stage, and sociodemographic characteristics. All hospitalizations beginning on or after the day of diagnosis were included in this analysis.
Multistate modeling is a flexible approach capable of modeling multiple changes in a patient’s condition over a period of time, including both absorbing events (eg, death) and events that are not absorbing but may occur multiple times per subject (eg, hospitalization).27 Because many individuals with cancer die or spend prolonged periods of time in the hospital, we sought to estimate rates of unplanned hospitalization using multistate models that accounted for the duration each individual was at risk of being hospitalized within the observation period. To this end, we created a person-period data set by partitioning each person’s follow-up data into nonoverlapping periods, with each person-period classified into one of three states in a multistate model. The three possible states included: (1) at risk (alive, not hospitalized, and therefore at risk of hospitalization), (2) hospitalized (alive, but temporarily not at risk of hospitalization), or (3) dead (and therefore not at risk of hospitalization for the remainder of the observation period). Persons could transition in and out of the hospitalized or at risk states until either transitioning to the dead state, an absorbing event, or until the end of the 365-day period after cancer diagnosis (Data Supplement).
We modeled the unplanned hospitalization incidence rate (ie, transition from the at-risk to the hospitalized state) as a function of cancer type using a log-linear Poisson regression model for clustered data. Lung cancer was selected as the reference category because it was the most prevalent cancer type that affected both men and women. We adjusted rates for patient age but did not include other individual sociodemographic or clinical characteristics in our models because our goal was to estimate unplanned hospitalization rates that accounted for time spent at risk, rather than to identify individual predictors of unplanned hospitalization. Clustering at the individual level (ie, multiple person-periods at risk of hospitalization per individual) was accounted for using a robust sandwich estimator of standard errors. To assess whether age-adjusted between-site differences were modified by patient age, we fit models with and without site × age interaction terms. Postestimation predictive margins were calculated to estimate the predicted age-adjusted unplanned hospitalization rate in the year after diagnosis adjusted for time spent at risk, for each cancer type (see Data Supplement for additional details about our modeling approach). Because unplanned hospitalization rates were calculated on the basis of transitions from the at-risk to the hospitalized state, they do not include the first hospitalization for individuals who were diagnosed with cancer while in the hospital.
RESULTS
Cohort Characteristics
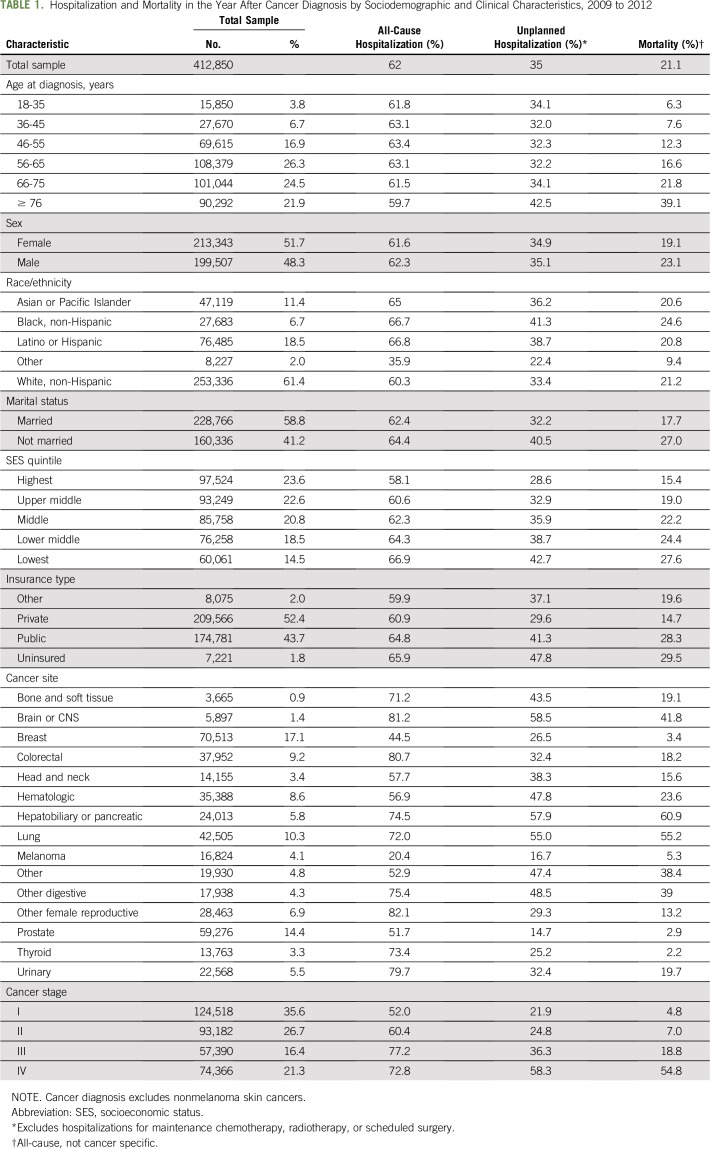
In this cohort of 412,850 adults diagnosed with cancer between 2009 and 2012, just over one half were female (51.7%), and a majority were white, non-Hispanic (61.4%). The most common cancer site was breast (17.1%), followed by prostate (14.4%), lung (10.3%), and colorectal (9.2%) cancers. More than one third were diagnosed with advanced cancer, defined as either stage III (16.4%) or stage IV (21.3%) disease, and 21.1% died within a year after diagnosis (Table 1).
TABLE 1.
Hospitalization and Mortality in the Year After Cancer Diagnosis by Sociodemographic and Clinical Characteristics, 2009 to 2012
Unplanned Hospitalization
Among individuals diagnosed with cancer between 2009 and 2012, a majority (62%) had at least one all-cause hospitalization in the year after diagnosis, and more than one third (35%) had an unplanned hospitalization (Table 1). By sociodemographic characteristics, the proportion with an unplanned hospitalization was highest in the age group older than 76 years (42.5%), among individuals of non-Hispanic black race/ethnicity (41.3%), who were not married (40.5%), and who were uninsured (47.8%). The proportion with an unplanned hospitalization increased as SES decreased, ranging from 28.6% of individuals in the highest SES quintile to 42.7% of individuals in the lowest quintile. The proportion with an unplanned hospitalization also differed substantially by cancer site and stage, ranging from 14.7% for prostate cancer to 58.5% for brain or CNS cancers. Unplanned hospitalization increased together with stage at diagnosis, ranging from 21.9% for stage I disease to 58.3% for stage IV disease (Table 1).
Unplanned Hospitalization Rates
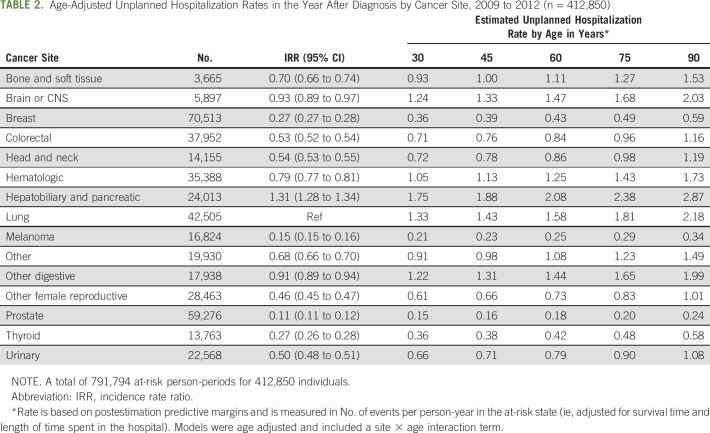
In our multistate modeling of unplanned hospitalization rates, all cancer types had significantly lower rates of unplanned hospitalization than lung cancer (the reference category), with the exception of hepatobiliary and pancreatic cancer (incidence rate ratio [IRR], 1.31; 95% CI, 1.28 to 1.34; Table 2). We found that age modified the between-site differences. Therefore, we included an interaction term of cancer site by age in our model and used postestimation predictive margins to estimate rates for each site separately at ages 30, 45, 60, 75, and 90 years. For a 60-year-old patient, estimated unplanned hospitalization rates were highest for hepatobiliary or pancreatic cancer (2.08 unplanned hospitalizations per person-year at risk), and lung cancer (1.58 unplanned hospitalizations), and lowest for prostate cancer (0.18 unplanned hospitalizations) and melanoma (0.25 unplanned hospitalizations; Table 2).
TABLE 2.
Age-Adjusted Unplanned Hospitalization Rates in the Year After Diagnosis by Cancer Site, 2009 to 2012 (n = 412,850)
Characteristics of Unplanned Hospitalizations
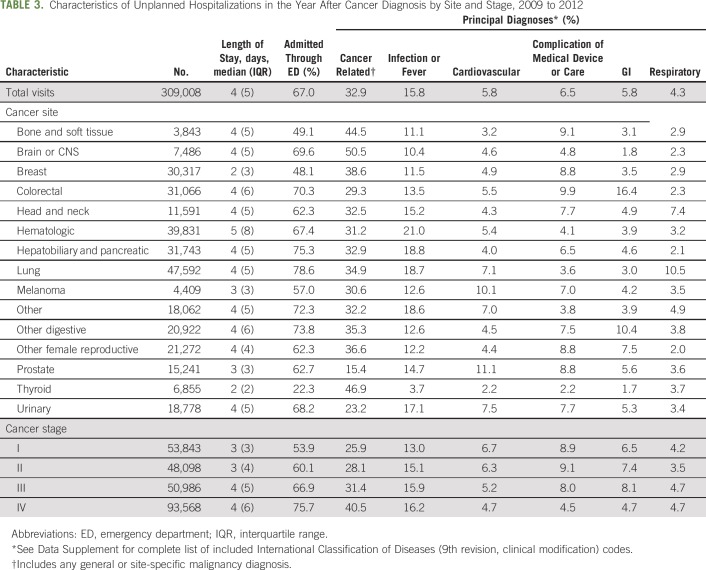
Of the 458,573 all-cause hospitalizations in the cohort, approximately 67% (n = 309,008) met our definition of an unplanned hospitalization (not listed in tables).
Emergency department admissions
Approximately two thirds of unplanned hospitalizations in the year after diagnosis originated in the emergency department (ED; 67.0%; Table 3). The percentage of ED admissions varied widely by cancer type. Rates were lowest among unplanned hospitalizations for thyroid (22.3%), breast (48.1%), and bone and soft tissue (49.1%) cancer, and highest among unplanned hospitalizations for lung (78.6%), hepatobiliary or pancreatic (75.3%), and other digestive cancers (73.8%). The percentage of unplanned hospitalizations originating in the ED also increased together with cancer stage, from 53.9% for stage I cancer to 75.7% for stage IV cancer (Table 3).
TABLE 3.
Characteristics of Unplanned Hospitalizations in the Year After Cancer Diagnosis by Site and Stage, 2009 to 2012
Principal diagnoses
Among unplanned hospitalizations in the year after diagnosis, one third listed cancer as the principal diagnosis or reason for admission (32.9%). The most common noncancer principal diagnosis was infection or fever, associated with 15.8% of hospitalizations, followed by complication of medical device or care (6.5%), GI (5.8%), cardiovascular (5.8%), and respiratory (4.3%) diagnoses (Table 3). Cancer types with the highest incidence of unplanned hospitalization for each primary diagnosis were hematologic cancer for infection or fever hospitalizations (21.0%); prostate cancer for cardiovascular hospitalizations (11.1%); colorectal cancer for complications of a medical device or care (9.9%) and GI-related hospitalizations (16.4%); and lung cancer for respiratory hospitalizations (10.5%).
DISCUSSION
In this population-based cohort of individuals newly diagnosed with cancer, we found that more than one third experienced an unplanned hospitalization during the year after diagnosis, with substantial variation by cancer site and stage. There was a higher incidence of unplanned hospitalization among groups known to have higher rates of acute care service use, including individuals of black, non-Hispanic race/ethnicity and lower SES.28,29 These findings are consistent with results of other studies using multivariable models to identify predictors of acute care use in patients with cancer16,30-33 and point to the need for a greater understanding of the predictors of unplanned hospitalization by cancer type, particularly among sociodemographic subgroups with higher rates of use.
The higher incidence of unplanned hospitalization among those with advanced-stage cancer suggests that inpatient use may, to some extent, be driven by disease severity. At the same time, other work suggests that there is an overuse of inpatient care among patients with cancer with incurable late-stage disease, with the result that many individuals with cancer spend their last weeks of life receiving aggressive hospital care that may not be consistent with their preferences.34-37 Additional investigation of variation in hospitalization among patients with cancer with incurable, advanced-stage disease is warranted to identify opportunities to reduce unnecessary inpatient use and promote end-of-life care that is consistent with patients’ expressed wishes.
Our estimates of noncancer principal diagnoses associated with unplanned hospitalization were similar to results from previous work using administrative data.5 Common reasons for unplanned hospitalization in our study have been suggested to be potentially preventable or amenable to outpatient management, including certain infections such as pneumonia and urinary tract infections.11,38-40 One of the most common noncancer principal diagnoses in our cohort was complication of a medical device or care. Recent work examining adverse events experienced by patients with cancer in the year after treatment initiation found that nearly one third of adverse events could have been prevented or mitigated in severity.41
More than two thirds of the unplanned hospitalizations in our study originated in the ED. A recent study of visits by patients with cancer to the ED found that nearly one half were associated with potentially preventable diagnoses.42 In addition, medical home models, patient navigation, and related interventions to improve outpatient support (eg, community-based palliative care, after-hours telephone support, evidence-based treatment guidelines, out-of-hospital emergency teams) have been shown to address patients’ urgent needs while reducing acute care use and improving quality of care.43-45 One oncology practice found that implementing an oncology nurse practitioner–led supportive care clinic to address urgent care needs reduced symptom-related care admissions by 31%.46 Another found that establishing a specialized cancer ED reduced all-cause hospitalizations by 51%.47 Taken together, the large percentage of ED admissions among unplanned hospitalizations in our study and recent evidence that enhanced support for urgent care needs can effectively reduce acute care use suggest that targeting cancer-related ED visits may be a promising approach to reduce unplanned hospitalization. These findings support additional examination of potentially unmet needs among patients with cancer who experience unplanned admission through the ED.
Our study has several important limitations. As with any inpatient use study using an observational design and inpatient administrative data, we did not have available many clinical details (eg, outpatient treatment information, comorbidities performance status, laboratory data) that influence hospitalization and that could shed additional light on the potential preventability of the hospitalizations. For instance, as has been noted in other work, the primary diagnosis in cancer hospitalizations is often malignancy related, which can be a barrier to understanding other factors underlying hospitalization and their potential preventability.11 In addition, our reliance on SSNs for the linkage procedures used to combine CCR, OSHPD, and vital statistics data may have resulted in underrepresentation of some minority racial/ethnic groups as well as individuals of lower SES.48 The exclusion of individuals in CCR who were unable to be linked with the OSHPD inpatient data may have led to overestimation of rates if a large proportion of excluded individuals never experienced a hospitalization. Because our data were limited to individuals who were diagnosed and received treatment in California, we may have underestimated hospitalization rates for individuals who were diagnosed in California but who received inpatient care in other states.
Reducing potentially avoidable cancer hospitalizations remains an important target for improving the quality and reducing the costs of cancer care. Our study has provided valuable baseline population estimates of unplanned hospitalization among individuals with cancer in the year after diagnosis, a period that coincides with active treatment of many patients. Although we examined hospitalization broadly by cancer type, such information is critical given the urgent need to reduce the costs of cancer care. Efforts to target subgroups of newly diagnosed individuals at a high risk of unplanned hospitalization might focus on hepatobiliary or pancreatic, lung, and advanced cancers, and on the ED as a point of entry for a majority of unplanned hospitalizations.
ACKNOWLEDGMENT
Supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the National Cancer Institute’s SEER Program under contract HHSN261201000140C awarded to the Center Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and grants from the Oncology Nursing Society, Jonas Nurse Leaders Scholars Program, and the Larry L. Hillblom Foundation (R.L.W.). Presented in part at the 2017 ASCO Quality Care in Oncology Symposium, March 3-4, 2017, Orlando, FL. The ideas and opinions expressed herein are those of the authors and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors or subcontractors. We thank Ann Brunson, MS, of the Center for Oncology Hematology Outcomes Research and Training (COHORT), Division of Hematology Oncology, UC Davis School of Medicine, for assistance with data acquisition for this study.
AUTHOR CONTRIBUTIONS
Conception and design: Robin L. Whitney, Janice F. Bell, Daniel J. Tancredi, Patrick S. Romano, Ted Wun, Jill G. Joseph
Provision of study material or patients: Ted Wun
Collection and assembly of data: Robin L. Whitney, Janice F. Bell
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Unplanned Hospitalization Among Individuals With Cancer in the Year After Diagnosis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Robin L. Whitney
Research Funding: Amgen
Ted Wun
Consulting or Advisory Role: Janssen Pharmaceuticals, Pfizer
Research Funding: Janssen Pharmaceuticals (Inst), Pfizer (Inst), Daiichi Sankyo (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Warren JL, Yabroff KR, Meekins A, et al. : Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst 100:888-897, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yabroff KR, Lund J, Kepka D, et al. : Economic burden of cancer in the United States: Estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev 20:2006-2014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang S, Long SR, Kutikova L, et al. : Estimating the cost of cancer: Results on the basis of claims data analyses for cancer patients diagnosed with seven types of cancer during 1999 to 2000. J Clin Oncol 22:3524-3530, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Yabroff KR, Lamont EB, Mariotto A, et al. : Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst 100:630-641, 2008 [DOI] [PubMed] [Google Scholar]
- 5.https://www.hcup-us.ahrq.gov/reports/statbriefs/sb125.pdf Price RA, Stranges E, Elixhauser A: Healthcare Cost and Utilization Project. Statistical Brief No. 125: Cancer hospitalizations for adults, 2009. Rockville, MD, Agency for Healthcare Research and Quality, 2012. [PubMed]
- 6.Obermeyer Z, Makar M, Abujaber S, et al. : Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA 312:1888-1896, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733-742, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Hoverman JR, Klein I, Harrison DW, et al. : Opening the black box: The impact of an oncology management program consisting of level I pathways and an outbound nurse call system. J Oncol Pract 10:63-67, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Brunetto AT, Ang JE, Olmos D, et al. : A study of the pattern of hospital admissions in a specialist phase I oncology trials unit: Unplanned admissions as an early indicator of patient attrition. Eur J Cancer 46:2739-2745, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Du XL, Osborne C, Goodwin JS: Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J Clin Oncol 20:4636-4642, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manzano J-GM, Luo R, Elting LS, et al. : Patterns and predictors of unplanned hospitalization in a population-based cohort of elderly patients with GI cancer. J Clin Oncol 32:3527-3533, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clough JD, Patel K, Riley GF, et al. : Wide variation in payments for Medicare beneficiary oncology services suggests room for practice-level improvement. Health Aff (Millwood) 34:601-608, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Brooks GA, Cronin AM, Uno H, et al. : Intensity of medical interventions between diagnosis and death in patients with advanced lung and colorectal cancer: A CanCORS analysis. J Palliat Med 19:42-50, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morden NE, Chang C-H, Jacobson JO, et al. : End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood) 31:786-796, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown EG, Burgess D, Li CS, et al. : Hospital readmissions: necessary evil or preventable target for quality improvement. Ann Surg 260:583-589, discussion 589-591, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stitzenberg KB, Chang Y, Smith AB, et al: Exploring the burden of inpatient readmissions after major cancer surgery. J Clin Oncol 33:455-464, 2015. [DOI] [PMC free article] [PubMed]
- 17.Yermilov I, Bentrem D, Sekeris E, et al. : Readmissions following pancreaticoduodenectomy for pancreas cancer: A population-based appraisal. Ann Surg Oncol 16:554-561, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Handley NR, Schuchter LM, Bekelman JE: Best practices for reducing unplanned acute care for patients with cancer. J Oncol Pract 14:306-313, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.California Cancer Registry : California Cancer Registry overview. http://www.ccrcal.org/Inside_CCR/FAQ.shtml
- 20.OSHPD : Patient discharge data by principal diagnosis group 2009-2014. https://www.oshpd.ca.gov/HID/Data_Request_Center/Data_Documentation.html
- 21.Chew HK, Wun T, Harvey D, et al. : Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 166:458-464, 2006 [DOI] [PubMed] [Google Scholar]
- 22.California Cancer Registry : Cancer data for research. http://www.ccrcal.org/Data_and_Statistics/Cancer_Data_for_Research.shtml
- 23.Zingmond DS, Ye Z, Ettner SL, et al. : Linking hospital discharge and death records--accuracy and sources of bias. J Clin Epidemiol 57:21-29, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Schupp C, Harrati A, et al. : Developing an Area-Based Socioeconomic Measure from American Community Survey Data. Fremont, CA, Cancer Prevention Institute of California, 2014. [Google Scholar]
- 25. https://seer.cancer.gov/siterecode/ National Cancer Institute SEER: Site recode.
- 26.Healthcare Cost and Utilization Project : Clinical classifications software (CCS) for ICD-9-CM. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
- 27.Andersen PK, Keiding N: Multi-state models for event history analysis. Stat Methods Med Res 11:91-115, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Andersen RM: Revisiting the behavioral model and access to medical care: Does it matter? J Health Soc Behav 36:1-10, 1995 [PubMed] [Google Scholar]
- 29.Calvillo-King L, Arnold D, Eubank KJ, et al. : Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: Systematic review. J Gen Intern Med 28:269-282, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenblatt DY, Weber SM, O’Connor ES, et al. : Readmission after colectomy for cancer predicts one-year mortality. Ann Surg 251:659-669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendren S, Morris AM, Zhang W, et al. : Early discharge and hospital readmission after colectomy for cancer. Dis Colon Rectum 54:1362-1367, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Stimson CJ, Chang SS, Barocas DA, et al. : Early and late perioperative outcomes following radical cystectomy: 90-day readmissions, morbidity and mortality in a contemporary series. J Urol 184:1296-1300, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Reddy DM, Townsend CM Jr, Kuo YF, et al: Readmission after pancreatectomy for pancreatic cancer in Medicare patients. J Gastrointest Surg 13:1963-1974; discussion 1974-1975, 2009. [DOI] [PMC free article] [PubMed]
- 34.Mack JW, Weeks JC, Wright AA, et al. : End-of-life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol 28:1203-1208, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Earle CC, Neville BA, Landrum MB, et al. : Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 22:315-321, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Earle CC, Neville BA, Landrum MB, et al. : Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care 17:505-509, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Whitney RL, Bell JF, Tancredi DJ, et al. : Hospitalization rates and predictors of rehospitalization among individuals with advanced cancer in the year after diagnosis. J Clin Oncol 35:3610-3617, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brooks GA, Abrams TA, Meyerhardt JA, et al. : Identification of potentially avoidable hospitalizations in patients with GI cancer. J Clin Oncol 32:496-503, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huntley AL, Thomas R, Mann M, et al. : Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta-analysis. Fam Pract 30:266-275, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Kruzikas DT. Preventable Hospitalizations: A Window into Primary and Preventive Care, 2000, Publication No. 04-0056, Rockville, MD, Agency for Healthcare Research and Quality, 2004. [Google Scholar]
- 41.Lipitz-Snyderman A, Pfister D, Classen D, et al. : Preventable and mitigable adverse events in cancer care: Measuring risk and harm across the continuum. Cancer 123:4728-4736, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panattoni L, Fedorenko C, Greenwood-Hickman MA, et al. : Characterizing potentially preventable cancer- and chronic disease-related emergency department use in the year after treatment initiation: A regional study. J Oncol Pract 14:e176-e185, 2018 [DOI] [PubMed] [Google Scholar]
- 43.Kuntz G, Tozer JM, Snegosky J, et al. : Michigan Oncology Medical Home Demonstration Project: first-year results. J Oncol Pract 10:294-297, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Elsayem AF, Elzubeir HE, Brock PA, et al. : Integrating palliative care in oncologic emergency departments: Challenges and opportunities. World J Clin Oncol 7:227-233, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colligan EM, Ewald E, Ruiz S, et al. : Innovative oncology care models improve end-of-life quality, Reduce utilization and spending. Health Aff (Millwood) 36:433-440, 2017 [DOI] [PubMed] [Google Scholar]
- 46.Meisenberg BR, Graze L, Brady-Copertino CJ: A supportive care clinic for cancer patients embedded within an oncology practice. J Community Support Oncol 12:205-208, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Ahn S, Lee YS, Lim KS, et al. : Emergency department cancer unit and management of oncologic emergencies: Experience in Asan Medical Center. Support Care Cancer 20:2205-2210, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Bohensky MA, Jolley D, Sundararajan V, et al. : Data linkage: A powerful research tool with potential problems. BMC Health Serv Res 10:346, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]