Abstract
Purpose
Eastern Africa was recently described as a high-incidence geographic area for esophageal cancer. Mozambique is included in this region. This study aimed to characterize this malignant disease at Maputo Central Hospital (MCH) to develop a global program for esophageal cancer management in Mozambique.
Methods
MCH records from between 2012 and 2016 were used to assess the clinical, pathologic, and outcome profiles of esophageal tumors. A descriptive analysis of data collected was performed. Overall survival was evaluated using Kaplan-Meier curves.
Results
In the study, 522 consecutive patient cases of esophageal cancer were recorded. The median patient age was 56.1 years (range, 27 to 97 years); 291 (55.7%) patients were women, and 230 (44.1%) were men. Regarding tumor site, 113 patients (21.6%) had a tumor in the lower third, 154 (29.5%) in the middle, and 50 (9.6%) in the upper third of the esophagus; in the remaining 196 (37.5%), tumor site was unknown. Squamous cell carcinoma comprised 94.4% of cases with documented histopathology (74.9% of the sample). Surgical treatment was possible in 32 patients (6.1%). Disease stage was documented only in these 32 surgical patients; 28.1%, 53.1%, and 18.8% had stage I, II, and III disease, respectively. The remaining patient cases seemed to involve clinically advanced tumors. The median follow-up time was of 1.6 months. The median survival time was of 3.5 months for all patients; for patients treated with curative intent, it was of 8.7 months.
Conclusion
Esophageal carcinoma is a common malignant tumor at MCH and is diagnosed in the advanced stages resulting in poor prognosis. Therefore, implementation of an Esophageal Cancer Program in Mozambique is essential.
INTRODUCTION
Esophageal cancer is the eighth most frequent cancer worldwide and sixth most common cause of death resulting from cancer.1 The distribution of esophageal cancer varies geographically, with 80% of cases occurring in developing countries.2 The highest incidences of esophageal cancer in the world have been observed in China, northeastern Iran, southeastern United States, and Southern Africa.3 Among both sexes, there are more than 20-fold differences in incidence in world regions, with rates ranging from 0.8 per 100,000 in West Africa to 17.0 per 100,000 in East Asia in men and 0.2 per 100,000 in Micronesia/Polynesia to 7.8 per 100,000 in East Africa in women.4 Mortality rates are elevated in East Asia (14.1 per 100,000) and Southern Africa (12.8 per 100,000) in men and in East Africa (7.3 per 100,000) and Southern Africa (6.2 per 100,000) in women.4 The high incidence rates of esophageal cancer (esophageal squamous cell carcinoma) in Central, Southern, and East Africa, together with diagnosis at advanced stages, lead to high mortality rates.1,2,5-7 The reasons underlying the high frequency of this malignancy on that continent remain largely unknown, and investigation to evaluate potential etiologic effects of dietary, lifestyle, environmental, and other factors affecting incidence in this region, including genetics, is needed.2,8-10 In their study, Liu et al11 demonstrated discrete subtypes of esophageal squamous cell carcinoma in sub-Saharan Africa and suggested that the endemic nature of this disease reflects exposure to a carcinogen other than tobacco or oncogenic viruses.
In Mozambique, data on the basis of the Maputo Central Hospital (MCH) registry reveal that esophageal cancer is the fourth most frequent tumor for both sexes, and it is the most frequent occurring in the digestive tract.12,13 Dysphagia related to esophageal cancer has been a major cause of hospitalization. Many of these patients are admitted with severe malnutrition and dehydration resulting from difficulties in swallowing, which are related to poor prognosis.14 With the aims of creating a global program to fight this disease, promoting good practices, and empowering disease management, MCH records were used to evaluate the burden of esophageal cancer in the hospital; assess the clinical and pathologic profiles of such tumors, treatment approach; and barriers in collection of data in this context; and define a plan of action that can contribute to the improvement of care management for these patients. The study was performed at MCH, located in Maputo, the capital of Mozambique. It is a 1,500-bed quaternary hospital and serves the Eduardo Mondlane University Medical School as a teaching hospital. MCH is the national reference hospital of the country.
METHODS
MCH records were retrospectively assessed from the Pathology and Surgery Services database to obtain the clinical and pathologic characteristics of patients with esophageal cancer admitted to and treated at MCH between January 1, 2012, and April 30, 2014. From May 2014 to December 2016, data were obtained from the MCH hospital-based cancer registry. This registry was implemented in May 2014 and includes information about patient cases of esophageal cancer identified in the departments of pathology and oncology, as well as data on cancer-related deaths from the Intrahospital Death Registration System.13 In addition, surgery department files were also assessed to complete information on patients diagnosed on the basis of clinical data only. Demographic variables collected for each patient included sex, age, race, and origin of the patient; clinical and pathologic data included anatomic site, histologic subtype, grade, basis of diagnosis, and treatments performed for each patient (surgery and chemotherapy). For patients undergoing surgery with curative intent, information on stage (American Joint Committee on Cancer seventh edition15), tumor size, resection status, and occurrence of lymphatic, vascular, muscular, perineural, adventitia, and/or lymph node invasion was also collected. A descriptive analysis of data collected was performed to describe the clinicopathologic profile of esophageal cancer at MCH. Overall survival was evaluated using Kaplan-Meier curves. The study was approved by the Mozambican National Bioethics Committee (reference No. 432/CNBS/2017).
RESULTS
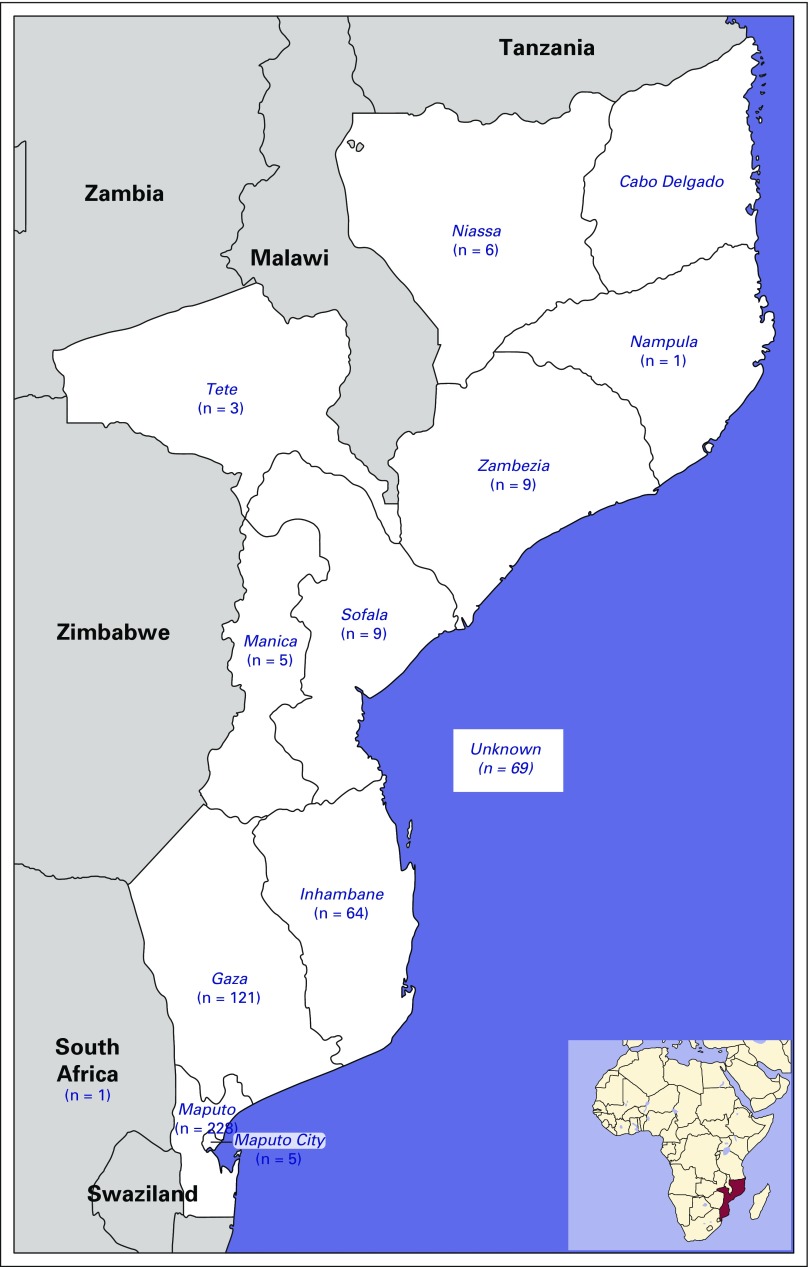
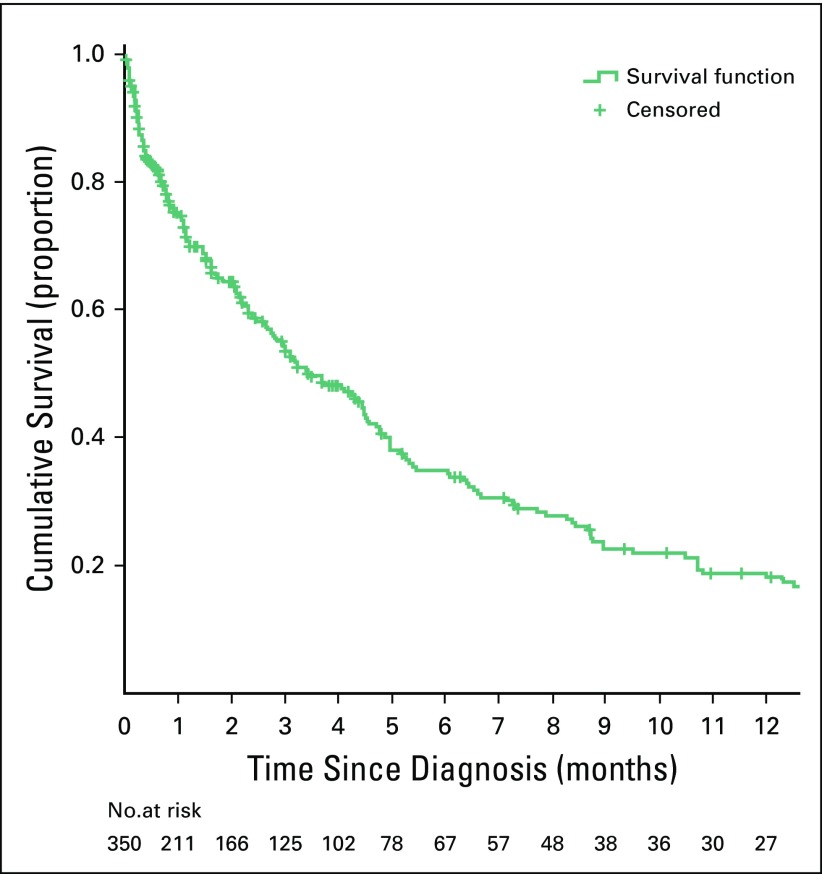
Over the study period, 522 consecutive patient cases of esophageal cancer were recorded (Table 1). The mean age at diagnosis was 56.1 years (standard deviation, 13.23 years; range, 23 to 97 years); 53 patients (10.2%) were age < 40 years; 14 patients (2.6%) were age ≤ 30 years. Most patients were women (n = 291; 55.7%), black (n = 520; 99.6%), and born in southern regions of the country (n = 418; 80.1%; Fig 1). The male-to-female ratio was between 0.7 and 0.9 in all 10-year age groups (20 to 29, 30 to 39, 40 to 49, 50 to 59, 60 to 69, and ≥ 70 years). Dysphagia to solids was documented in all patients and required nutritional support. However, nutritional management with gastrostomy is unsuccessful in a large number of patients. Information on the anatomic site of the tumor was not available for 196 patients (37.5%); among the remaining patients, 154 (47.2%) had a tumor located in the middle third of the esophagus, 113 (34.7%) in the lower third, 50 (15.3%) in the upper third, and nine (2.8%) in the esophagogastric junction (Table 1). Diagnosis was histologically confirmed for 391 patient cases (74.9%; Table 1). Among those, most cases were squamous cell carcinomas (n = 369; 94.4%); 10 (2.6%) were adenocarcinomas, and three (0.8%) were adenosquamous carcinomas. Among the 192 patient cases with available information on histologic grade, 83 (43.2%) were well differentiated, 88 (45.8%) were moderately differentiated, and 21 (10.9%) were poorly differentiated. In the studied series, 55.8% of patients underwent no staging imaging. Surgical treatment with curative intent (Ivor-Lewis procedure) was only possible in 32 patients (6.1%); surgical feeding gastrostomy was performed using a urinary catheter tube in 199 patients (38.1%), and the remaining 291 patients (55.7%) received best support care. For patients undergoing surgery with curative intent, the median tumor size was 40 mm (interquartile range, 25.5 mm; range, 15 to 100 mm); 28.1%, 53.1%, and 18.8% of patients had stage I, II, and III disease, respectively; 21 patients (65.6%) had negative margins; and lymphatic, vascular, perineural, muscular propria, adventitia, and lymph node invasion were found in 14 (43.8%), 14 (43.8%), 11 (34.4%), 31 (96.9%), 20 (64.5%), and eight patients (25.0%), respectively. The neoadjuvant chemotherapy protocol used (cisplatin 75-100 mg/m2 intravenously on day 1 plus fluorouracil 1,000 mg/m2 continuous infusion over 24 hours on days 1 to 5. Cycled every 28 days), included four cycles before operation and two after the operation. In adjuvant/palliative context the same protocol was used during six cycles. Chemotherapy was administered to 18 patients (3.4%).Only one patient received neoadjuvant chemotherapy; for the remaining 17 patients, chemotherapy was adjuvant or palliative. Radiotherapy was not available. In survival analyses, 172 patients (32.9%) were excluded (127 patients were lost to follow-up, 26 had as date of diagnosis the date of death, and 19 had only the year of diagnosis and/or follow-up available.). The median follow-up time was of 1.6 months (1.3 v 2.1 months for alive and dead patients, respectively, at the date of last contact). The median survival time was of 3.5 months for all patients (Fig 2), whereas for patients treated with curative intent (either surgery alone or with neoadjuvant chemotherapy, according to stage), it was of 8.7 months.
Table 1.
Descriptive Analysis of Demographic and Clinical Characteristics of Patients With Esophageal Cancer Registered at MCH Over Study Period (N = 522)
Fig 1.
Geographic distribution of place of birth of patients by provinces in the country.
Fig 2.
Overall survival of patients with esophageal cancer since date of diagnosis.
DISCUSSION
This study reveals that esophageal cancer is a common condition at MCH. The surgery, gastroenterology, medical oncology, and pathology departments in the hospital were the sources of information used in this study. However, a multidisciplinary approach toward treating esophageal cancer was nonexistent. In our study sample, most patients were black women. The sex ratio found in our study was different from that in other African countries; however, it was closer to values found in Ethiopia, Kenya, and Sudan; furthermore, a decrease in sex ratio to increasing incidence of women (used as an indicator of underlying incidence in the general population) was described in a recent report.16 In the same study, a sex ratio of 1.4 was reported by another cancer registry in Mozambique (Beira Cancer Registry), but only 30 patients were included in the analyses, hindering extrapolation to the general population in that country. Although no information on migration or place of residence could be obtained for the patients included in our study, a report related to the study of migration in Mozambique reveals that there are low internal migration rates in Mozambique, so this lack of information is not expected to have greatly influenced our results.17 Diagnosis was usually determined at an advanced stage of disease, precluding the curative approach, and nutritional status at diagnosis was usually poor according to clinical data. In a South African study involving 1,868 patients, similar stage, performance status, weight loss, and long history of dysphagia were described; furthermore, only 19.8% of those patients could be treated with curative intent, and squamous cell carcinoma was also the most frequent histologic type.7 In our study, most patients required nutritional support, as observed in South African reports.7,18 Gastrostomy was the most frequently performed palliative surgery, but it is often responsible for complex skin lesions and additional suffering.19,20 Stent palliation represents a more efficient approach.21 However, the retail price for the imported stent is a barrier.22 Therefore, we have performed palliative surgical procedures, such as bypass. The clinical and pathologic profiles observed in our study are similar to those observed in other African countries in the region.21-23 This study shows that it is necessary to implement a global esophageal cancer program in Mozambique and at MCH, aiming to increase the imaging stage and treatment of esophageal cancer with radical surgery, chemoradiotherapy, neoadjuvant and palliative chemotherapy, and endoscopic palliation of dysphagia (Table 2). Soon, the use of radiotherapy will also be available in this hospital, which, together with the nutrition and clinical psychology departments, will have an important role in cancer treatment and prehabilitation of surgical patients.24 The African Esophageal Cancer Consortium, the call to action in esophageal cancer by von Loon et al,22 could be a useful platform to implement multisite investigation in this field and capacity building and to share research in treatment and palliative care, including the palliation of dysphagia. However, risk factors associated with esophageal carcinogenesis in Mozambique and East Africa are largely unknown.25 In sub-Saharan Africa, case-control studies have shown that low economic level, consumption of local fermented beverages and foods cooked with charcoal, and smoking may be associated with a high risk of esophageal cancer.8,24-29 Deficits of selenium and zinc in foods also seem to have an important role in malignant transformation of esophageal epithelium in this region.30 In Kenya, endoscopic evaluation of asymptomatic individuals living in regions with high esophageal cancer rates revealed that 14.4% of patients had dysplastic lesions.31 In this study, the identification of risk areas was performed using Lugol’s solution. However, endoscopy using narrow band imaging seems more effective for those with an early diagnosis of esophageal cancer.32 In Mozambique, there is currently no information on these issues; therefore, it is necessary to study the local risk factors and promote early diagnosis in high-risk areas (in which the provinces of Gaza, Inhambane, and Maputo seem to be included according to the present data), possibly using endoscopy through narrow band imaging, because this technical resource is available at the MCH gastroenterology department, with Lugol staining improving diagnosis. However, large tumors that prevent endoscopic stent placement, as well as the difficulty of managing the reported feeding gastrostomies, suggest that the role of palliative surgery associated with chemotherapy or radiotherapy should be evaluated in a scientific way in this context.33
Table 2.
Synopsis of Esophageal Cancer Program in Mozambique
This study has several limitations that need to be addressed. For 25% of our patients, the diagnosis was on the basis of clinical diagnosis only, endoscopy, or death certificate. The data obtained are from those patients for whom clinical records were available. As a retrospective study, there is a lack of information on some variables (eg, staging, time of follow-up, and outcomes for some patients). In order to overcome these limitations, MCH is currently implementing a hospital-based cancer registry comprising data from 2014 onward that integrates relevant sources of information. 13 However, the quality of information produced by the registry is dependent not only on the organization and availability of the hospital records but also importantly on the quality of the information included in such records. For example, information about disease stage was not present in almost all clinical records and was only available for patients undergoing surgery with curative intent, when this information referred to pathologic stage. A recent study conducted in Tanzania34 reported a clinical profile and problems similar to those described in our study, which reinforces the need for a regional intervention in this disease.
Esophageal cancer is a commonly diagnosed and treated condition at MCH, but diagnosis is made at an advanced stage of disease, which is related to poor prognosis. This study reveals the necessity for the development of a comprehensive program to combat esophageal cancer in Mozambique.
ACKNOWLEDGMENT
We thank the ESTIMA project for its scientific support and the Mozambique Central Hospital for making their data available.
AUTHOR CONTRIBUTIONS
Conception and design: Jotamo Come, Clara Castro, Carla Carrilho, Lúcio Lara Santos
Administrative support: Lúcio Lara Santos
Collection and assembly of data: Jotamo Come, Atílio Morais, Matchecane Cossa, Prassad Modcoicar, Satish Tulsidâs, Lina Cunha, Vitória Lobo, Alberto Gudo Morais, Lúcio Lara Santos
Data analysis and interpretation: Jotamo Come, Clara Castro, Nuno Lunet, Sofia Cotton, Carla Carrilho, Lúcio Lara Santos
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Jotamo Come
No relationship to disclose
Clara Castro
No relationship to disclose
Atílio Morais
No relationship to disclose
Matchecane Cossa
No relationship to disclose
Prassad Modcoicar
No relationship to disclose
Satish Tulsidâs
No relationship to disclose
Lina Cunha
No relationship to disclose
Vitória Lobo
No relationship to disclose
Alberto Gudo Morais
No relationship to disclose
Sofia Cotton
No relationship to disclose
Nuno Lunet
No relationship to disclose
Carla Carrilho
No relationship to disclose
Lúcio Lara Santos
No relationship to disclose
REFERENCES
- 1.Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–387. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 2.Cheng ML, Zhang L, Borok M, et al. The incidence of oesophageal cancer in Eastern Africa: Identification of a new geographic hot spot? Cancer Epidemiol. 2015;39:143–149. doi: 10.1016/j.canep.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 5.Hendricks D, Parker MI. Oesophageal cancer in Africa. IUBMB Life. 2002;53:263–268. doi: 10.1080/15216540212643. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Bray F, Ferlay J, et al. Cancer in Africa 2012. Cancer Epidemiol Biomarkers Prev. 2014;23:953–966. doi: 10.1158/1055-9965.EPI-14-0281. [DOI] [PubMed] [Google Scholar]
- 7.Dandara C, Robertson B, Dzobo K, et al. Patient and tumour characteristics as prognostic markers for oesophageal cancer: A retrospective analysis of a cohort of patients at Groote Schuur Hospital. Eur J Cardiothorac Surg. 2014;49:629–634. doi: 10.1093/ejcts/ezv135. [DOI] [PubMed] [Google Scholar]
- 8.McCormack VA, Menya D, Munishi MO, et al. Informing etiologic research priorities for squamous cell esophageal cancer in Africa: A review of setting-specific exposures to known and putative risk factors. Int J Cancer. 2017;140:259–271. doi: 10.1002/ijc.30292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell JD, Yau C, Bowlby R, et al. Genomic, pathway network, and immunologic features distinguishing squamous carcinomas. Cell Rep. 2018;23:194–212.e6. doi: 10.1016/j.celrep.2018.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Costa AM, Fregnani JHTG, Pastrez PRA, et al. HPV infection and p53 and p16 expression in esophageal cancer: Are they prognostic factors? Infect Agent Cancer. 2017;12:54. doi: 10.1186/s13027-017-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Snell JM, Jeck WR, et al. Subtyping sub-Saharan esophageal squamous cell carcinoma by comprehensive molecular analysis. JCI Insight. 2016;1:e88755. doi: 10.1172/jci.insight.88755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenzoni C, Vilajeliu A, Carrilho C, et al. Trends in cancer incidence in Maputo, Mozambique, 1991-2008. PLoS One. 2015;10:e0130469. doi: 10.1371/journal.pone.0130469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrilho C, Fontes F, Tulsidás S, et al. Cancer incidence in Mozambique in 2015-2016: Data from the Maputo Central Hospital Cancer Registry. Eur J Cancer Prev. doi: 10.1097/CEJ.0000000000000457. epub ahead of print on June 22, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Snyder E, Amado V, Jacobe M, et al. General surgical services at an urban teaching hospital in Mozambique. J Surg Res. 2015;198:340–345. doi: 10.1016/j.jss.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edge SB, Byrd D, Compton CC, et al., editors. AJCC Cancer Staging Manual. ed 7. New York, NY: Springer; 2010. [Google Scholar]
- 16.Middleton DRS, Bouaoun L, Hanisch R, et al. Esophageal cancer male to female incidence ratios in Africa: A systematic review and meta-analysis of geographic, time and age trends. Cancer Epidemiol. 2018;53:119–128. doi: 10.1016/j.canep.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Vletter F. Migration and development in Mozambique: Poverty, inequality and survival. Devel Sou Afr. 2006;24:137–153. [Google Scholar]
- 18.Akateh C, Zhao L, Orringer MB, et al. Racial disparities in the surgical outcomes after esophagectomy for esophageal cancer. Cancer Res. 2012;72 suppl 8; abstr 2662. [Google Scholar]
- 19.Min YW, Jang EY, Jung JH, et al. Comparison between gastrostomy feeding and self-expandable metal stent insertion for patients with esophageal cancer and dysphagia. PLoS One. 2017;12:e0179522. doi: 10.1371/journal.pone.0179522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vizhi K, Rao HB, Venu RP. Percutaneous endoscopic gastrostomy site infections: Incidence and risk factors. Indian J Gastroenterol. 2018;37:103–107. doi: 10.1007/s12664-018-0822-4. [DOI] [PubMed] [Google Scholar]
- 21.Loots E, Sartorius B, Madiba TE, et al. Is clinical research in oesophageal cancer in South Africa in crisis? A systematic review. World J Surg. 2017;41:810–816. doi: 10.1007/s00268-016-3778-5. [DOI] [PubMed] [Google Scholar]
- 22.Loon KV, Mwachiro MM, Abnet CC, et al. The African Esophageal Cancer Consortium: A call to action. J Glob Oncol. 2018;4:1–9. doi: 10.1200/JGO.17.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabel JV, Chamberlain RM, Ngoma T, et al. Clinical and epidemiologic variations of esophageal cancer in Tanzania. World J Gastrointest Oncol. 2016;8:314–320. doi: 10.4251/wjgo.v8.i3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Roy B, Pereira B, Bouteloup C, et al. Effect of prehabilitation in gastro-oesophageal adenocarcinoma: Study protocol of a multicentric, randomised, control trial-the PREHAB study. BMJ Open. 2016;6:e012876. doi: 10.1136/bmjopen-2016-012876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munishi MO, Hanisch R, Mapunda O, et al. Africa’s oesophageal cancer corridor: Do hot beverages contribute? Cancer Causes Control. 2015;26:1477–1486. doi: 10.1007/s10552-015-0646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kigen G, Busakhala N, Kamuren Z, et al. Factors associated with the high prevalence of oesophageal cancer in Western Kenya: A review. Infect Agent Cancer. 2017;12:59. doi: 10.1186/s13027-017-0169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mlombe YB, Rosenberg NE, Wolf LL, Dzamalala CP, Chalulu K, Chisi J, et al. Environmental risk factors for oesophageal cancer in Malawi: A case-control study. Malawi Med J. 2015;27:88–92. doi: 10.4314/mmj.v27i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sewram V, Sitas F, O’Connell D, et al. Tobacco and alcohol as risk factors for oesophageal cancer in a high incidence area in South Africa. Cancer Epidemiol. 2016;41:113–121. doi: 10.1016/j.canep.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Kachala R. Systematic review: Epidemiology of oesophageal cancer in Sub-Saharan Africa. Malawi Med J. 2010;22:65–70. doi: 10.4314/mmj.v22i3.62190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaafsma T, Wakefield J, Hanisch R, et al. Correction: Africa’s oesophageal cancer corridor—Geographic variations in incidence correlate with certain micronutrient deficiencies. PLoS One. 2015;10:e0142648. doi: 10.1371/journal.pone.0142648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mwachiro MM, Burgert SL, Lando J, et al. Esophageal squamous dysplasia is common in asymptomatic Kenyans: A prospective, community-based, cross-sectional study. Am J Gastroenterol. 2016;111:500–507. doi: 10.1038/ajg.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CT, Chang CY, Lee YC, et al. Narrow-band imaging with magnifying endoscopy for the screening of esophageal cancer in patients with primary head and neck cancers. Endoscopy. 2010;42:613–619. doi: 10.1055/s-0030-1255514. [DOI] [PubMed] [Google Scholar]
- 33.Hihara J, Hamai Y, Emi M, et al. Esophageal bypass operation prior to definitive chemoradiotherapy in advanced esophageal cancer with tracheobronchial invasion. Ann Thorac Surg. 2014;97:290–295. doi: 10.1016/j.athoracsur.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 34.Mmbaga EJ, Deardorff KV, Mushi B, et al. Characteristics of esophageal cancer cases in Tanzania. J Glob Oncol. 2018;4:1–10. doi: 10.1200/JGO.2016.006619. [DOI] [PMC free article] [PubMed] [Google Scholar]