Abstract
Due to primate adaptations for sociality, captive rhesus macaques have optimal welfare and utility as a biomedical model when they can be maintained in outdoor social groups. As a despotic species, however, aggression can result in costly injuries and may result in temporary or permanent removal of specific individuals from social housing. Enrichment items, such as toys, climbing structures, and foraging material, are employed to keep captive animals occupied. We hypothesized that produce enrichment that requires more processing to extract may reduce socially-derived injuries by keeping animals occupied. We tested the effects of additional weekly produce (corn-in-husk, whole melon, or whole squash) on trauma incidence in an outdoor social group of rhesus macaques across two distinct seasons (mating and birthing seasons) at the California National Primate Research Center. Aggression and status behavioral data, food resource use and proximity, and trauma incidence were collected over two 16-week periods, with eight control and treatment conditions alternating biweekly. Mixed-effects regression modeling was used to determine the best predictors of trauma risk and severe aggression at the group level and at an individual level. We found that food resource use was an important predictor of trauma risk at both group and individual levels; greater use of food resources reduced trauma risk. Produce enrichment did not, however, reduce severe aggression. We suggest that other captive social groups of rhesus macaques with high levels of trauma may benefit from supplemental produce enrichment that increases animal engagement with food resources.
Keywords: Aggression, Environmental Enrichment, Foraging, Welfare, Wounding
INTRODUCTION
Social housing offers the most important form of enrichment for captive primates (Hannibal, Bliss-Moreau, Vandeleest, McCowan, & Capitanio, 2017; Lutz & Novak, 2005), resulting in social buffering during stressful situations (Gilbert & Baker, 2011; Hennessy, Chun, & Capitanio, 2016) and lowered rates of stereotypical and abnormal behaviors (Baker et al., 2012; 2014; Gottlieb, Maier, & Coleman, 2015). Accordingly, primates devoid of social companions can exhibit depression (Perera et al., 2011; Shively & Willard, 2012). Mandatory regulations thus require that primates have social companions (see Hannibal et al., 2017 and Pomerantz & Baker, 2017 for an overview) unless certain conditions preclude social housing (e.g., Baker, 2007). While the vast majority of primates used in biomedical research are housed indoors in pairs or small social groups (Baker, 2007), expansive outdoor enclosures with complex familial social structures offer the best opportunity for naturalistic behavior (Fontenot, Wilkes, & Lynch, 2006; O’Neill, Novak, & Suomi, 1991; Novak, O’Neill, & Suomi, 1992), reduce population density and crowding stress (Dettmer, Novak, Meyer, & Suomi, 2014), and may enhance the reliability and reproducibility of the research conducted on those subjects, as highly standardized and controlled environments have limited external validity (Voelkl & Würbel, 2016). Large outdoor social groups thus represent the “gold standard” for primate research (Hannibal et al., 2017).
Despite the benefits, the housing of social groups of primates comes with unique challenges, such as the need to mitigate aggression and trauma. Rhesus macaques (Macaca mulatta) are the most commonly used primate in biomedical research (Carlsson, Schapiro, Farah, & Hau, 2004). Characterized as highly despotic (Thierry, 2004), rhesus macaques live in large multi-male/multi-female groups and exhibit steep linear dominance hierarchies, which are reinforced through frequent aggression, ranging from mild threats and chases to severe biting and wounding. While high levels of aggression are a normal reflection of hierarchical maintenance, in a captive setting, victims sometimes cannot escape their aggressors, resulting in significant traumas. For example, in some national primate research centers, up to 60% of a breeding group (ranging in size from 100–200 individuals) may be hospitalized within a given year (McCowan, Beisner, & Hannibal, 2018), with costs ranging from $150–3,000 per hospitalization (unpublished raw data). Aside from the financial cost, individual primates may also be temporarily or permanently removed from their social group, which has the potential to perturb social stability (Beisner, Jin, Fushing, & McCowan, 2015; Oates-O’Brien et al., 2010; Wooddell, Kaburu, Dettmer, & Suomi, 2017). Due to these financial and animal welfare concerns, greater scrutiny is being aimed towards reducing aggression and trauma in captive groups of macaques (Hannibal et al., 2017; McCowan, Anderson, Heagerty, & Cameron, 2008; McCowan et al., 2017).
Foraging enrichment is one potential deterrent to socially-derived traumas. Wild monkeys can spend up to 50% of their day foraging (Altmann & Muruthi, 1988; Goldstein & Richard, 1989; Beisner & Isbell, 2008) because natural foods vary in seasonality, distribution and size, and require effort to find and extract. In captive or semi-provisioned settings, foods are easily processed and extracted, and provided in predictable ways, and these primates may spend as little as 20% of their day foraging/feeding (Altmann & Muruthi, 1988; Beisner & Isbell, 2008). This can result in a greater frequency of social hair pulling and hair ingestion (Beisner & Isbell, 2008; Heagerty et al., 2017), aggression (Beisner & Isbell, 2011), and self-directed behaviors and stereotypies (Gottlieb et al., 2011; Lutz & Novak, 2005). As such, common enrichment devices for captive primates focus on foraging based strategies, such as puzzle feeders (Gottlieb et al., 2011; Novak, Kinsey, Jorgensen, & Hazen, 1998), foraging boards (Bayne et al., 1991; Lutz & Novak, 1995), and distribution of food under woodchips and shavings (Boccia & Hijazi, 1998; Byrne & Suomi, 1991; Doane et al., 2013, Lutz & Novak, 1995), which have been shown to alleviate some, but not all, unwanted behaviors. While food has been shown to either elicit or reduce social aggression depending on the study, this likely depends on the size, distribution, and time needed to process and extract food items (Boccia, Laudenslager, & Reite, 1988; Byrne & Suomi, 1991; Doane et al., 2013; Mathy & Isbell, 2001). Furthermore, although foraging enrichment may alter aggression frequencies, the impact on socially inflicted traumas is unclear because the rates of aggression do not necessarily predict rates of trauma (Beisner, Wooddell, Hannibal, Nathman, & McCowan, 2019; Pomerantz & Baker, 2017; Ruehlmann, Bernstein, Gordon, & Balcaen, 1988). Rather, trauma can be mediated by other internal (e.g., policing, sex ratio: Beisner, Jackson, Cameron, & McCowan, 2012; matrilineal fragmentation: Beisner, Jackson, Cameron, & McCowan, 2011) and external (e.g., season: Stavisky, Ramsey, Meeker, Stovall, & Crane, 2018) factors. Therefore, the effect of foraging enrichment on trauma outcomes needs to be directly tested.
At the California National Primate Research Center (CNPRC), most macaques live in expansive outdoor 0.5 acre field corrals consisting of 100–200 monkeys from different matrilineal family structures. While field corrals provide both environmental and social complexity, and those with grass substrate offer additional foraging opportunities which has been associated with reduced aggression (Beisner & Isbell, 2008; 2011), trauma remains a significant issue. We therefore examined whether a weekly supplement of long-lasting produce would reduce the occurrence of socially inflicted trauma. While all groups at the CNPRC receive a once-weekly supply of fresh produce (e.g., apples, cucumbers), the foods are easily consumed, few foods require the animals to break open a tough outer peel, husk or shell, and the animals finish eating the produce in 1–2 hours. Since enrichment is ineffective once depleted (Bennett, Perkins, Tenpas, Reinebach, & Pierre, 2016), we hypothesized that providing large, intact sources of produce that require greater time investment (i.e., days) to breakdown, such as large melons or corn-in-husks, would occupy animals’ time and result in a reduction in traumas. In addition, we analyzed whether providing produce enrichment requiring processing reduced the frequency of severe aggression to test whether a change in severe aggression may underlie any observed changes in trauma.
METHODS
Subjects and materials
Two experimental studies of produce enrichment were conducted on a single social group (formed in 2002) of rhesus macaques (Macaca mulatta) housed in a 0.5 acre outdoor corral at the CNPRC in Davis, California. The first study was conducted from March to June 2017, during the birth season, and the second was conducted from September to December 2018, during the mating season. The corral contained multiple A-frame structures, hanging barrels, and swings. Monkeys were exposed to ambient light and temperature and were fed commercial monkey chow and seed mixture (sunflower seeds, oats) twice daily. All procedures adhered to the American Society of Primatologists Principles for the Ethical Treatment of Non-Human Primates and were approved by the UC Davis Institutional Animal Care and Use Committee.
We selected a group with a relatively high rate of trauma. Data from a previous study of seven rhesus macaque groups at the CNPRC showed a rate of moderate-severe trauma per animal ranging from 0 – 0.21/week (mean ± SD: 0.04 ± 0.04) whereas this rate ranged from 0.03 – 0.21/week (mean± SD: 0.11 ± 0.05) for the study group in 2017–2018. For the purposes of this study, we focused on subjects 3 years and older, when macaques enter maturity and begin to have adult-like social interactions (Smith, Crummett, & Brandt, 1994). Across the two studies, we observed a total of 99 subjects (69 females (3–22 years old), 5 adult males (6–19 years old), 25 subadult males (3–5 years old)). The first study began with 90 subjects, with 10 subjects (9 male, 1 female) permanently removed from the group for management, research or health reasons, and the second study began with 69 subjects, with 3 subjects permanently removed (2 male, 1 female). Due to removal of subadult males, the sex ratio changed from 2.6 females per male in spring 2017 to 6.6 females per male in fall 2018.
Study design
Data were collected for 16 continuous weeks for each study. The study design involved a 2-week alternating schedule of control and treatment periods. During both treatment and control weeks, the group received its regular produce enrichment of three standard produce boxes either Thursday or Friday morning (e.g., apples, broccoli, celery, cucumbers, oranges, or zucchinis). During treatment weeks, the study group received approximately 40 pieces of long-lasting produce (4–5 boxes) every Monday morning, which included corn-in-husk, whole melons, or whole squash. We determined the amount of produce necessary via pilot testing on a different social group (mean ± SD weekly rate of moderate-severe trauma per animal: 0.068 ± 0.04) and found that this volume of produce was enough to measurably reduce the incidence of hospitalized trauma in a similarly sized social group, whereas half that amount had the opposite effect. During the second study, behavioral data collection was cancelled during weeks 10 and 11 due to poor air quality from nearby wildfires; trauma data were still collected.
Food resource use data collection
Behavior and food-use data were collected 3–4 days per week from 900–1200 hr and 1300–1600 hr each day by three observers (inter-rater reliability, Krippendorff’s alpha ≥ 0.85). To measure the impact of the produce, one observer recorded food use on four days per week (Monday – Friday, except Wednesday) using 5-minute continuous focal-area samples. The cage was divided into six sections of approximately equal size, and each section was sampled twice in the AM and twice in the PM. During a focal-area sample, the observer rated the dispersal of each food resource (monkey chow, seed mixture, colony produce, study produce) in each section on a scale of 1–3. We quantified dispersal as the distance between the food resources in the section by dividing the section into four equal quadrants and counting how many quadrants contained food resource (1: one quadrant, 2: two quadrants, 3: three or four quadrants). Additionally, the intactness (how much of the original produce was left) and coverage (quantity of food in the section) of each food resource were also recorded during the first study. However, preliminary analyses showed that coverage and dispersal were highly correlated, and none of the variables (coverage, dispersal, intactness) produced minimally good models or showed an association to trauma or aggression outcomes. Therefore, observer effort was adjusted in the second study to record only one of these variables (dispersal) for further examination.
Each focal-area sample began and ended with a demographic scan of the number of subjects present in each section and the number actively using a food resource. During scans, observers did not distinguish between resource types (e.g., colony vs. study produce). Total subjects using a resource measured the direct impact of providing extra produce. Total subjects present in the section near a food resource measured its potential indirect impact, as there may be animals interested in the food resources even if not currently using them (e.g., subordinates waiting for a dominant animal to leave a resource). For each demographic scan, the number of subjects using a resource or present in the section was rated on a scale from 0–4: (0) zero animals using a resource (or present in the section), (1) 1–3, (2) 4–6, (3) 7–9, and (4) 10 or more animals. A scale was used instead of actual counts because animal movement and changes in behavior often occurred rapidly, making inter-rater reliabilities of actual counts unrealistic. If no food resource was present in the section, the observer marked ‘no resource present’ and recorded no other variables (95 of 1494 scans had no resources present).
Food resource use variables
To examine the effects of food resource use, we generated several variables summarizing the dispersal of food resources (coverage and intactness were not examined in the analysis of both studies combined). For group level analyses, we calculated the mean, median, and maximum value for each week (Table 1). For individual-level analyses, these measures were aggregated for each biweekly period to match the biweekly experimental design. Group-level variables were summarized on a weekly scale, rather than biweekly, because group-level trauma was sufficiently frequent and variable to analyze at this scale, and sample size across both studies is increased from 16 bi-weeks to 32 weeks (minus the 2 weeks lost due to wildfires).
Table 1.
List of variables
| Variable | Level of Measure |
|---|---|
| season (spring vs. fall); sex ratio (low vs. high) | per study (season and sex ratio confounded) |
| sex (male vs. female) | per individual |
| age | per individual |
| dominance rank | per individual |
| dominance certainty | per individual |
| submission received (SBTs & displacements) | per individual, group-level sum |
| submission given (SBTs & displacements) | per individual, group-level sum |
| severe aggression received | per individual, group-level sum |
| severe aggression given | per individual, group-level sum |
| impartial policing rate | group-level: weekly, individual-level: biweekly |
| enrichment condition (control vs. enrichment) | group-level: weekly, individual-level: biweekly |
| mean level of dispersal of monkey chow | group-level: weekly, individual-level: biweekly |
| mean level of dispersal of colony produce | group-level: weekly, individual-level: biweekly |
| mean level of dispersal of study produce | group-level: weekly, individual-level: biweekly |
| mean level of dispersal of seed mixture | group-level: weekly, individual-level: biweekly |
| mean level of dispersal of all food resources | group-level: weekly, individual-level: biweekly |
| median level of dispersal of monkey chow | group-level: weekly, individual-level: biweekly |
| median level of dispersal of colony produce | group-level: weekly, individual-level: biweekly |
| median level of dispersal of study produce | group-level: weekly, individual-level: biweekly |
| median level of dispersal of seed mixture | group-level: weekly, individual-level: biweekly |
| median level of dispersal of all food resources | group-level: weekly, individual-level: biweekly |
| maximum level of dispersal of monkey chow | group-level: weekly, individual-level: biweekly |
| maximum level of dispersal of colony produce | group-level: weekly, individual-level: biweekly |
| maximum level of dispersal of study produce | group-level: weekly, individual-level: biweekly |
| maximum level of dispersal of seed mixture | group-level: weekly, individual-level: biweekly |
| mean score (0–4) of animals using a resource | group-level: weekly, individual-level: biweekly |
| mean score (0–4) of animals near a resource | group-level: weekly, individual-level: biweekly |
| median score (0–4) of animals using a resource | group-level: weekly, individual-level: biweekly |
| median score (0–4) of animals near a resource | group-level: weekly, individual-level: biweekly |
| proportion of scans scored 1–3 animals using a resource | group-level: weekly, individual-level: biweekly |
| proportion of scans scored 4–6 animals using a resource | group-level: weekly, individual-level: biweekly |
| proportion of scans scored 7–9 animals using a resource | group-level: weekly, individual-level: biweekly |
| proportion of scans scored 10+ animals using a resource | group-level: weekly, individual-level: biweekly |
| proportion of scans with at least 1 animal using a resource | group-level: weekly, individual-level: biweekly |
| proportion of scans scored 1–3 animals near a resource | group-level: weekly, individual-level: biweekly |
| proportion of scans scored 4–6 animals near a resource | group-level: weekly, individual-level: biweekly |
| proportion of scans scored 7–9 animals near a resource | group-level: weekly, individual-level: biweekly |
| proportion of scans scored 10+ animals near a resource | group-level: weekly, individual-level: biweekly |
| proportion of scans with at least 1 animal near a resource | group-level: weekly, individual-level: biweekly |
Similarly, we generated several variables summarizing the number of animals actively using (or in proximity to) a resource. We calculated mean and median values for each weekly (group level analyses) and biweekly period (individual level analyses) for both the number of animals using a resource and the number present in the section. Additionally, we summarized animals’ use of (or proximity to) food resources by tallying how often each numerical level of the scale was recorded across all demographic scans per weekly or biweekly period (e.g.,’1–3 animals’ was recorded for animals using a resource in 20% of all scans during week 1). These tallies generated five additional variables reflecting the number of animals using a resource during the scans (i.e., one for each level from 0 to 4), and five reflecting the number of animals present in the section near a resource (Table 1). Finally, we tallied the number of demographic scans in which at least one animal was recorded using a resource (or present in the resource section).
Behavioral data collection
We used an event sampling design to record all aggressive and submissive interactions. On four days per week (Monday – Friday, except Wednesday) one observer recorded aggressive interactions, including the identities of all initiators and recipients, the levels of aggression and submission, and interventions from third parties. Aggression was categorized as mild (e.g., open mouth threat, short chase <6 m), moderate (e.g., grapple/ wrestling, chase >6 m), and severe (bite or pin to the ground). Submission included freeze/turn away, move away, run away a short distance (< 6m), run away a long distance (> 6m), and crouch (recipient is cornered or stops resisting aggression). Interventions in which the intervening animal approached the conflict or directed aggression to both combatants were considered to be impartial policing. On three days per week (Monday, Thursday, & Friday) the second observer recorded status signaling interactions, i.e., peaceful approaches that elicited submissive behaviors by the recipient including silent-bared teeth displays (SBTs), rump present, freeze/turn away, move away, and run away.
Social status variables
Multiple aspects of social status were calculated to control for their influence on individual-level risk of receiving trauma, including dominance rank, dominance certainty, and counts of submission and subordination signals given and received (Table 1). To calculate dominance rank and dominance certainty, we used all instances of dyadic aggression (dyadic wins and bidirectional or sparring aggression) to generate a win-loss matrix. We then used the R package Perc to calculate the best rank ordering of the matrix (Fujii et al., 2015). From Perc’s dyadic dominance probability matrix, we then calculated dominance certainty for each subject, which measures the average level of certainty versus ambiguity of a subject’s dominance relationships (Vandeleest et al., 2016). From status signaling data, we calculated for each subject the total number of status signals given and received and totals for each sub-category of status (i.e., SBT, displacement).
Aggression and policing variables
Multiple measures of aggressive behavior were calculated to examine the influence of aggression rates on group-level trauma frequencies, to examine individual-level participation in aggression with respect to trauma risk, and to test the impact of produce enrichment on aggressive behavior. We focused on severe aggression, since nearly all socially-derived traumas are inflicted by biting behavior. For group-level analyses, we calculated the rate of severe aggression per group member for each week of study. For individual-level analyses, we calculated the total severe aggression received and given for each subject. Finally, we also calculated the rate of impartial policing per group member for each week of study (group-level analyses) and for each biweekly period (individual-level analyses) to control for its potential influence on trauma and aggression outcomes.
Trauma data collection
Trauma was documented on all subjects once per week on Monday mornings by one observer (the same observer for both studies: ACN). Each subject was visually inspected from outside the corral for evidence of trauma, including: crush/bruising trauma (bruising without piercing of the skin), digit trauma (any trauma to the fingers or toes), lacerations (tears that rip the skin), punctures (straight holes into the skin with no tearing), and tail tip trauma (trauma to the end of the tail). Traumas were assigned to the previous week if they were already healing at the time of observation and assigned to the current week if they were fresh and likely occurred that morning. In addition, hospital records were used to record additional traumas that required veterinary treatment that happened during the week.
Statistical analyses
We used generalized linear models (GLMs) to examine five outcome variables: (1) group-level weekly counts of all trauma (N=30 weeks; recall, 2 weeks were lost due to wildfires), (2) group-level weekly counts of hospitalized trauma, (3) group-level weekly counts of severe aggression, (4) individual-level biweekly incidence of trauma (measured as 0/1; N=1218 subject bi-weeks), and (5) individual-level biweekly counts of severe aggression received. A negative binomial distribution was used for all analyses of count data (as counts of trauma and severe aggression were over-dispersed) and a binomial distribution for analyses of individual-level incidence of trauma. Data from both studies were combined for each analysis. We used an information theoretic (I-T) approach for model fitting and model selection (Anderson & Burnham, 2002; Burnham, Anderson, & Huyvaert, 2011). Predictors in our models included variables reflecting the efficacy of our foraging enrichment treatment (categorical treatment vs. control condition; variables assessing the number of animals using and near food resources; variables assessing the dispersal of the food resources), variables to gauge the role of aggression in trauma risk (severe aggression given and received), and variables to control for factors that might mask the ability to detect a treatment effect (policing rates, for all analyses; sex, age, and social status variables, for individual-level analyses only). Finally, the number of days present in the group (due to possible hospitalization) was set as an exposure variable for individual-level analyses, and the number of subjects present in the group was set as an exposure variable for group-level analyses.
We fit all univariate and multivariate combinations of our predictors that were consistent with our hypotheses, avoiding the inclusion of correlated variables in the same model. Group-level analyses were limited by the sample size (N = 30 weeks), and we therefore fit models with a maximum of two predictors for these analyses. For each model, we calculated AIC scores corrected for sample size (AICc). To select a candidate set of the most plausible models, we chose all models with ΔAICc < 5 and further reduced this list of models by applying the concept of parsimony and removing models if we found a simpler model in the candidate set that had a lower AICc score. In other words, if adding a term to a model increased the AICc score rather than decreased, it was removed from the candidate set. For all models in this final candidate set, we calculated model likelihoods, model weights and evidence ratios to help select the final models with strongest evidence. Finally, we only considered models with AICc better than the empty model with no predictors. Statistical analyses were conducted using RStudio using the packages glmmADMB (function glmmadmb; Fournier et al., 2012) and MASS (function glm.nb; Venables & Ripley, 2002).
RESULTS
Summary Statistics
A total of 277 traumas (179 in spring 2017, 98 in fall 2018) were documented across the two studies, and 50.5% required veterinary treatment (43.5% in spring 2017, 63.2% in fall 2018). The mean ± SD count of total trauma during enrichment weeks was 6.6 ± 3.7, compared to 10.6 ± 3.9 during control. The mean ± SD count of hospitalized trauma during enrichment weeks was = 3.1 ± 2.3 compared to 5.7 ± 2.6 during control. The mean ± SD rate of severe aggression per subject was 0.59 ± 0.15 during spring 2017 and 0.65 ± 0.21 during fall 2018. During control weeks the rate of severe aggression was 0.59 ± 0.15 compared to 0.64 ± 0.20 during enrichment weeks.
Group level trauma & severe aggression
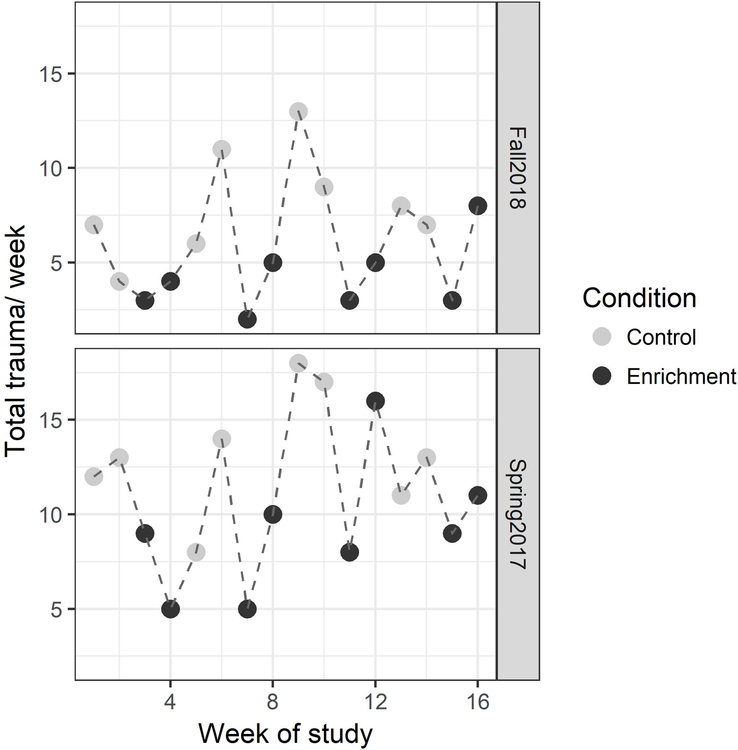
Analyses of the weekly count of traumas showed that the produce enrichment significantly reduced both total trauma (Figure 1) and hospitalized trauma. First, there were four best-fit models of total trauma per week (Model 1: AICc = 155.5, weight = 0.41; Model 2: AICc = 155.8, weight = 0.35; Model 3: AICc = 157.1, weight = 0.18; Model 4: AICc = 159.6, weight = 0.05), and the precise variable describing the effect of produce enrichment on trauma differed across the models. Models 1 and 4 showed that trauma was lower during the enrichment condition than control (Table 2), while Models 2 and 3 showed that trauma was lower during weeks when more animals were actively using food resources, measured as the mean score (0–4) of animals using food resources (Table 2: Model 2) and the proportion of demographic scans in which 4–6 animals were using food resources (Table 2: Model 3). According to Model 1, total trauma was reduced by 38% during the produce enrichment condition compared to control (e.g., point estimates for traumas/week based on the model were 6.2 for enrichment vs. 9.9 for control conditions). In addition, trauma was higher when the rate of severe aggression was higher (Table 2: Models 3 and 4) and unexpectedly higher during spring compared to fall (Table 2: Models 1 and 2). Second, there were three best-fit models of hospitalized trauma (Model 1: AICc = 139.8, weight = 0.47; Model 2: AICc = 140.0, weight = 0.41; Model 3: AICc = 142.4, weight = 0.12) again with different variables describing the impact of produce enrichment. These models showed that hospitalized trauma was lower during the enrichment condition than control (Model 1: β = −0.647, p = 0.002), when a higher proportion of demographic scans showed 4–6 animals using food resources (Model 2: β = −8.39, p = 0.002), and during weeks when there was a higher mean score (0–4) of animals actively using food resources (Model 3: β = −1.50, p = 0.008). According to Model 1, hospitalized trauma was reduced by 47% during the produce enrichment condition compared to control (e.g., point estimates for hospitalized traumas/ week were 3.6 for enrichment vs. 6.8 for control conditions).
Figure 1.
Plot of the count of total trauma per week across all 16 weeks of study for both Spring 2017 and Fall 2018 studies. Light gray dots represent control weeks and black dots represent produce enrichment weeks.
Table 2.
Model outputs for the top four models of group-level total trauma
| Model Parameters | Model 1 β coefficients | Model 2 β coefficients | Model 3 β coefficients | Model 4 β coefficients |
|---|---|---|---|---|
| Enrichment vs. control | −0.472** | -- | -- | −0.514** |
| Animals using food resources, average score | -- | −1.229** | -- | -- |
| Scans with 4–6 animals using food resources, proportion | -- | -- | −7.225** | -- |
| Fall vs. spring | 0.374** | 0.405** | -- | -- |
| Severe aggression/ animal | -- | 1.019** | 0.754* |
p < 0.05
p < 0.01
Finally, analyses of group-level rates of severe aggression found no effect of produce enrichment. The best-fit model was the empty model.
Individual level trauma and severe aggression
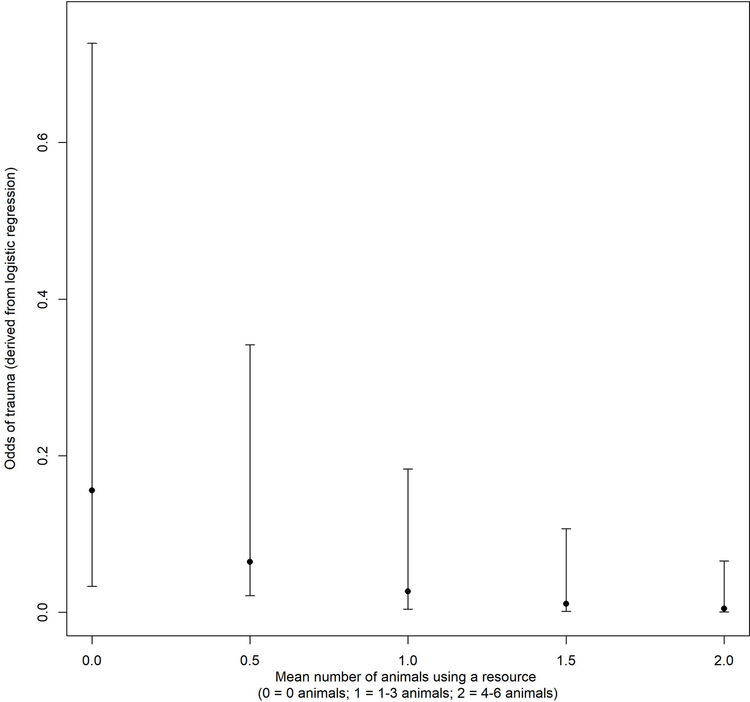
There was a single best-fit model of individual-level presence or absence of trauma per biweekly period across the two studies (AICc = 1057.8, ΔAICc = 5.7 compared to the second best model) which showed that the odds of receiving trauma was lower when more animals were actively using food resources (β = −1.73, p = 0.0008; Figure 2). According to this model, increasing the mean number of animals using a resource from a score of 0 (none using a resource) to a score of 1 (1–3 animals using a resource), the probability of receiving trauma drops by a factor of five. In addition, older males (sex [male]: β = 2.23, p < 0.0001, age: β = 0.01, p = 0.59, age × sex [male]: β = −0.26, p = 0.003) and high-ranking individuals (β = −0.75, p = 0.05) were less likely to receive trauma.
Figure 2.
Plot of model-predicted odds of receiving trauma for individuals given different levels of resource use by the animals (based on the best-fit model of individual-level trauma). The number of animals using a resource during each scan was coded as 0, 1, 2, 3 or 4, which represent ordered categories zero, 1–3, 4–6, 7–9, and 10+ animals; weekly mean values were calculated using the numerical codes.
There were two best-fit models for analyses of individual-level severe aggression received per biweekly period across the two studies (Model 1: AICc = 3378.5, weight = 0.64; Model 2: AICc = 3379.7, weight = 0.36). According to both models, the strongest predictors of receipt of severe aggression were demographic variables – males, high-ranking individuals, and older individuals were less likely to receive severe aggression than females, low-ranking, and younger individuals, respectively (Table 3). Further, Model 1 showed that the produce enrichment may have increased the risk of receiving severe aggression, showing a near-significant trend that receipt of severe aggression was more likely when there was a higher mean dispersion of all food resources per section (β = 0.173, p = 0.07). According to this model, an individual was 1.2 times more likely to receive severe aggression if the mean dispersion of all food resources increased from 1 quadrant of the section containing food resources to 2 quadrants.
Table 3.
Model outputs for the top two models of individual-level receipt of severe aggression
| Model Parameters | Model 1 β coefficients | Model 2 β coefficients |
|---|---|---|
| Dispersion of all food resources, average code | 0.173 | -- |
| Percent rank | −1.616** | −1.626** |
| Sex [male vs. female] | −0.443** | −0.448** |
| Age | −0.038** | −0.038** |
p < 0.05
p < 0.01
DISCUSSION
We investigated whether providing supplemental, long-lasting produce enrichment would reduce the occurrence of socially-derived traumas in a large social group of rhesus macaques. Although wounding is a common and costly byproduct of group living for species that use frequent aggression to maintain and reinforce dominance relationships, the consequences of such wounding can extend far beyond the individual, possibly affecting group level stability (e.g., Beisner, et al., 2015; Oates-O’Brien et al., 2010), making it a critical issue for animal welfare. Both group-level and individual-level analyses showed that providing produce enrichment reduced trauma incidence and risk. At the group level, total trauma was reduced by 38% and hospitalized trauma was reduced by 47%, while group-level rates of severe aggression were unaffected by the produce enrichment. Individual-level results were similar, indicating that the risk of receiving trauma was reduced when more animals were actively using food resources, however the risk of receiving severe aggression may have increased due to the produce enrichment when the dispersion of food resources was greater. We discuss these findings in detail below.
Supplemental produce effects on trauma
At the group level, supplemental long-lasting produce reduced total trauma and hospitalized trauma by 38% and 47%, respectively. Both group-level and individual-level analyses suggest that the produce enrichment may have reduced trauma by engaging animals’ interest in foraging and occupying their time, because best-fit models for both group-level and individual-level analyses showed that trauma risk was lower when more animals were actively using a food resource. Although we suspect that long-lasting produce more effectively engages animals’ interest than produce that is more easily processed and eaten, our study design did not separate the effects of increased produce from increased processing time, and further, not all types of long-lasting produce will be equally engaging. For example, both celery and corn-in-husk last longer than apples, suggesting both foods may be effective at engaging animals in foraging activity. However, remaining celery leaves will often remain untouched, whereas corn may be heavily processed for several days or a week, until the husks are scattered about and eventually ignored. To better assess the mechanism by which supplemental produce impacts trauma, future studies should compare supplemental produce with different processing times (e.g., whole squash vs. apples) and measure individuals’ activity budgets, as it is also possible that produce could increase resting times due to the extra time required for digestion, which could reduce trauma.
Foraging enrichment (such as foraging boards, woodchips, or foods that require active manipulation) has historically been employed as a strategy to reduce unwanted behaviors, including aggression, and increase positive species-typical behaviors (Boinski, Swing, Gross, & Davis, 1999; Young, 2003). Unlike previous studies that have found that foraging enrichment can reduce aggression (Bayne et al., 1991; Boccia & Hijazi, 1998; but see Boinski et al., 1999; Byrne & Suomi, 1991), even by up to 50% in adults and 90% in juveniles (Anderson & Chamove, 1984; Chamove & Anderson, 1979; Chamove, Anderson, Morgan-Jones, & Jones, 1982) our analyses suggest that the decrease in trauma was not due to a reduction in severe aggression. The produce enrichment did not reduce rates of severe aggression. At the group-level, enrichment did not influence rates of severe aggression, and at the individual-level, the near-significant trend suggesting that the risk of receiving severe aggression may have increased when food resources were dispersed across more quadrants per section showed a small effect size (i.e., a subject was 1.2 times more likely to receive severe aggression if dispersion increased from 1 to 2 quadrants) in the unexpected direction. Rather, we found that the receipt of severe aggression was best explained by demographic variables (sex, age, dominance rank), as young males were less likely to receive severe aggression than females (but more likely to receive traumas).
Since trauma rates were reduced by 38% (or 47% for hospitalized trauma), yet severe aggression rates were unaffected (or perhaps increased) by the produce, it seems that the risk of trauma per instance of severe aggression was lower during the produce enrichment condition. Perhaps during the produce enrichment condition, aggressors were more likely to moderate the intensity of their bites. The existing literature suggests that the social dynamics underlying trauma appear to be more complex than a simple positive correlation with biting aggression and trauma (Beisner et al. 2019), and a number of studies point to the possibility that aggressors moderate their bites to influence the likelihood of causing injury (Beisner et al. 2019; Owens 1975; Ruehlmann et al. 1988). For instance, in a recent study following group formation and instability in rhesus macaques, males inflicted more severe trauma on other males than they did females (Linden et al., 2018), suggesting that males inhibited their bites towards females. Incidentally, our individual-level analyses provide further evidence for bite inhibition based on sex. Although sex was included as a control variable, rather than a variable of interest, we found that young males were far more likely to receive trauma than females, yet males were less likely to receive severe aggression than females. This suggests that aggressors may have intentionally inflicted trauma when biting male victims, or conversely, may have moderated their bites towards females. Further research is needed to determine whether, and under what circumstances, bite moderation occurs.
The enrichment appeared to be equally effective in both the fall mating season and in spring, however, our group-level analyses had too few data points to examine an interaction between season and treatment, preventing us from formally comparing the treatment effectiveness across seasons. Further, because sex ratio changed (from 2.6 to 6.6 females per male) between the two studies, the effects of season and sex ratio are confounded in these analyses. We suspect, however, that the change in sex ratio may have had a larger effect on trauma than season because our group-level analyses showed that total trauma was more frequent during the spring birth season than the fall mating season, which is in contrast to the expected increase in aggression and/or trauma due to mating season competition (Stavisky et al. 2018; Wilson & Boelkins 1970).
Foraging as a management strategy to reduce social trauma
Foraging based strategies have historically been implemented as environmental enrichment for captive primates. To our knowledge though, this is the first study to systematically examine the effect of food enrichment on trauma in a large-outdoor group of rhesus macaques. Given that the treatment reduced total trauma by 38% (47% reduction in hospitalized trauma) compared to control weeks, long-lasting produce may be an effective behavioral management strategy to reduce trauma. However, given that we studied only one social group which had a relatively high rate of trauma, the potential for such enrichment to reduce trauma in other captive groups may depend on baseline levels of trauma. For instance, Boccia & Hijazi (1998) found that, for two groups of pigtailed macaques (Macaca nemestrina), scattering sunflower seeds in a woodchip substrate (which increased foraging time) resulted in a reduction of aggressive behaviors in one group with a high baseline level of aggression but not in the other group that already had a low baseline level of aggression. In sum, this suggests that the beneficial effects of reducing trauma via foraging enrichment not only depend on the unique group dynamics (e.g., baseline level of aggression or trauma), but may also be species-dependent, and perhaps especially beneficial for despotic species (Thierry, 2004), which are characterized by high levels of baseline aggression and trauma. Management strategies should thus take into consideration the frequency and severity of traumas, which will vary across groups and across species, in order to assess whether supplemental produce will potentially be an effective regimen to reduce socially inflicted traumas. In addition, future research should investigate the cost effectiveness of implementing additional produce enrichment (e.g., Bennett et al., 2016), comparing the cost savings due to reduced hospitalizations (as a result of reduced trauma) against the costs of the supplemental produce. This type of financial assessment would provide valuable information to behavioral and colony managers, which will enhance the well-being of captive nonhuman primates.
Acknowledgements
DH, BB, AN, and BM developed the research questions and study design. LJW and AN collected the data. AN supervised the data collectors thanked below. LJW and BB wrote the manuscript. BB and DH analyzed the data and prepared graphs. The authors have no conflict of interests to declare. We thank the Primate Behavioral Health Services and animal care staff of the California National Primate Research Center for their contribution in time to this project. We also thank our data collection team: Alyssa Maness, Sasha Winkler, Nicole Lin, and Anh-Thu Le. This research was supported by NIH R24 OD011136 and NIH P51-OD01107–53.
Footnotes
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Altmann J, & Muruthi P (1988). Differences in the daily life between semi-provisioned and wild-feeding baboons. American Journal of Primatology, 15, 213–221. 10.1002/ajp.1350150304 [DOI] [PubMed] [Google Scholar]
- Anderson DR, & Burnham KP (2002). Avoiding pitfalls when using information-theoretic methods. The Journal of Wildlife Management, 66(3), 912–918. 10.2307/3803155 [DOI] [Google Scholar]
- Anderson JR, & Chamove AS (1984). Allowing captive primates to forage. In: Standards in Laboratory Animal Management, Part 2, The Universities Federation for Animal Welfare, UFAW, Potters Bar, UK. [Google Scholar]
- Baker K (2007). Enrichment and primate centers: closing the gap between research and practice. Journal of Applied Animal Welfare Science, 10, 49–54. 10.1080/10888700701277618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, Neu K, Griffis C, & Schoof VAM (2014). Comparing options for pair housing rhesus macaques using behavioral welfare measures. American Journal of Primatology, 76(1), 30–42. 10.1002/ajp.22190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, Neu K, Griffis C, Schoof V, & Maloney M (2012). Benefits of pair housing are consistent across a diverse population of rhesus macaques. Applied Animal Behaviour Science, 137(3–4), 148–156. 10.1016/j.applanim.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne K, Mainzer H, Dexter S, Campbell G, Yamada F, & Suomi SJ (1991). The reduction of abnormal behaviors in individually housed rhesus monkeys (Macaca mulatta) with a foraging/grooming board. American Journal of Primatology, 23(1), 23–35. 10.1002/ajp.1350230104 [DOI] [PubMed] [Google Scholar]
- Beisner BA, & Isbell LA (2008). Ground substrate affects activity budgets and hair loss in outdoor groups of rhesus macaques (Macaca mulatta). American Journal of Primatology, 70, 1160–1168. 10.1002/ajp.20615 [DOI] [PubMed] [Google Scholar]
- Beisner BA, & Isbell LA (2011). Factors affecting aggression among females in captive groups of rhesus macaques (Macaca mulatta). American Journal of Primatology, 73(11), 1152–1159. 10.1002/ajp.20982 [DOI] [PubMed] [Google Scholar]
- Beisner BA, Jackson ME, Cameron AN, & McCowan B (2011). Detecting instability in animal social networks: genetic fragmentation is association with social instability in rhesus macaques. PLoS One, 6(1), e16365 10.1371/journal.pone.0016365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner BA, Jackson ME, Cameron A, & McCowan B (2012). Sex ratio, conflict dynamics, and wounding in rhesus macaques. Applied Animal Behaviour Science, 137(3–4), 137–147. 10.1016/j.applanim.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner BA, Jin J, Fushing H, & McCowan B (2015). Detection of social group instability among captive rhesus macaques using joint network modeling. Current Zoology, 61, 70–84. 10.1093/czoolo/61.1.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner BA, Wooddell LJ, Hannibal DL, Nathman A, & McCowan B (2019). High rates of aggression do not predict high rates of trauma in captive groups of macaques. Applied Animal Behaviour Science, 212: 82–89. 10.1016/j.applanim.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AJ, Perkins CM, Tenpas PD, Reinebach AL, & Pierre PJ (2016). Moving evidence into practice: cost analysis and assessment of macaques’ sustained behavioral engagement with videogames and foraging devices. American Journal of Primatology, 78(12), 1250–1264. 10.1002/ajp.22579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia ML, & Hijazi AS (1998). A foraging task reduces agonistic and stereotypic behaviors in pigtail macaque social groups. Laboratory Primate Newsletter, 37(3). [Google Scholar]
- Boccia ML, Laudenslager M, & Reite M (1988). Food distribution, dominance, and aggressive behaviors in bonnet macaques. American Journal of Primatology, 16(2), 123–130. 10.1002/ajp.1350160203 [DOI] [PubMed] [Google Scholar]
- Boinski S, Swing SP, Gross TS, & Davis JK (1999). Environmental enrichment of brown capuchins (Cebus apella): behavioral and plasma and fecal cortisol measures of effectiveness. American Journal of Primatology, 48(1), 46–68. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR, & Huyvaert KP (2011). AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behavioral Ecology and Sociobiology, 65(1), 23–35. 10.1007/s00265-010-1029-6 [DOI] [Google Scholar]
- Byrne GD, & Suomi SJ (1991). Effects of woodchips and buried food on behavior patterns and psychological well-being of captive rhesus monkeys. American Journal of Primatology, 23(3), 141–151. 10.1002/ajp.1350230302 [DOI] [PubMed] [Google Scholar]
- Carlsson HE, Schapiro SJ, Farah I, & Hau J (2004). Use of primates in research: a global overview. American Journal of Primatology, 63(4), 225–237. 10.1002/ajp.20054 [DOI] [PubMed] [Google Scholar]
- Chamove AS, Anderson JR, Morgan-Jones SC, & Hones SP (1982). Deep woodchip litter: hygiene, feeding, and behavioural enhancement in eight primate species. International Journal for the Study of Animal Problems, 3, 308–318. [Google Scholar]
- Chamove AS, & Anderson JR (1979). Woodchip litter in macaque groups. Journal of the Institute of Animal Technicians, 30, 69–74. [Google Scholar]
- Dettmer AM, Novak MA, Meyer JS, & Suomi SJ (2014). Population density-dependent hair cortisol concentrations in rhesus monkeys (Macaca mulatta). Psychoneuroendocrinology, 42, 59–67. 10.1016/j.psyneuen.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane CJ, Andrews K, Schaefer LJ, Morelli N, McAllister S, & Coleman K (2013). Dry bedding provides cost-effective enrichment for group-housed rhesus macaques (Macaca mulatta). Journal of the American Association for Laboratory Animal Science, 52(3), 247–252. [PMC free article] [PubMed] [Google Scholar]
- Fontenot BM, Wilkes MN, & Lynch CS (2006). Effects of outdoor housing on self-injurious and stereotypic behavior in adult male rhesus macaques (Macaca mulatta). Journal of the American Association for Laboratory Animal Science, 45(5), 35–43. [PubMed] [Google Scholar]
- Fournier D, Skaug H, Ancheta J, Ianelli J, Magnussen A, Maunder M, … Sibert J (2012). AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optimization Methods & Software, 27, 233–249. 10.1080/10556788.2011.597854 [DOI] [Google Scholar]
- Fujii K, Jin J, Shev A, Beisner BA, McCowan B, & Fushing H (2015). Perc: Using Percolation and Conductance to find information flow certainty in direct network. R package version 0.1.3. https://cran.r-project.org/web/packages/Perc/index.html.
- Gilbert MH, & Baker KC (2011) Social buffering in adult male rhesus macaques (Macaca mulatta): effects of stressful events in single vs pair housing. Journal of Medical Primatology, 40(2), 71–78. 10.1111/j.1600-0684.2010.00447.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SJ, & Richard AF (1989). Ecology of rhesus macaques (Macaca mulatta) in northwest Pakistan. International Journal of Primatology, 10, 531–567. 10.1007/bf02739364 [DOI] [Google Scholar]
- Gottlieb DH, Ghirardo S, Minier DE, Sharpe N, Tatum L, & McCowan B (2011). Efficacy of 3 types of foraging enrichment for rhesus macaques (Macaca mulatta). Journal of the American Association for Laboratory Animal Science, 50(6), 888–894. [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Maier A, & Coleman K (2015). Evaluation of environmental and intrinsic factors that contribute to stereotypic behavior in captive rhesus macaques (Macaca mulatta). Applied Animal Behaviour Science, 171, 184–191. 10.1016/j.applanim.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal DL, Bliss-Moreau E, Vandeleest J, McCowan B, & Capitanio J (2017). Laboratory rhesus macaque social housing and social changes: implications for research. American Journal of Primatology, 79, e22528 10.1002/ajp.22528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heagerty A, Wales RA, Prongay K, Gottlieb DH, & Coleman K (2017). Social hair pulling in captive rhesus macaques (Macaca mulatta). American Journal of Primatology, 79(12), e22720 10.1002/ajp.22720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Chun K, & Capitanio JP (2016). Depressive-like behavior, its sensitization, social buffering, and altered cytokine responses in rhesus macaques moved from outdoor social groups to indoor housing. Social Neuroscience, 12(1), 65–75. 10.1080/17470919.2016.1145595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DA (1998). Effects of provisioning on the social behaviour of Japanese and rhesus macaques” Implications for socioecology. Primates, 40(1), 187–198. 10.1007/bf02557710 [DOI] [PubMed] [Google Scholar]
- Linden JB, McCowan B, Capitanio JP, & Isbell LA (2018). Male-inflicted wounds have opposite effects on hair cortisol for captive male and female rhesus macaques (Macaca mulatta) following new group formation. Primates, 10.1007/s10329-018-0703-6 [DOI] [PMC free article] [PubMed]
- Lutz CK, & Novak MA (1995). Use of foraging racks and shavings as enrichment tools for groups of rhesus monkeys (Macaca mulatta). Zoo Biology, 14(5), 463–474. 10.1002/zoo.1430140508 [DOI] [Google Scholar]
- Lutz CK, & Novak MA (2005). Environmental enrichment for nonhuman primates: theory and application. Institute for Laboratory Animal Research Journal, 46(2), 178–191. 10.1093/ilar.46.2.178 [DOI] [PubMed] [Google Scholar]
- Mathy JW, & Isbell LA (2001). The relative importance of size of food and interfood distance in eliciting aggression in captive rhesus macaques (Macaca mulatta). Folia Primatologica, 72, 268–277. 10.1159/000049948 [DOI] [PubMed] [Google Scholar]
- McCowan B, Anderson K, Heagerty A, & Cameron A (2008). Utility of social network analysis for primate behavioral management and well-being. Applied Animal Behaviour Science, 109(2–4), 396–405. 10.1016/j.applanim.2007.02.009 [DOI] [Google Scholar]
- McCowan B, Beisner B, & Hannibal D (2018). Social management of laboratory rhesus macaques housed in large groups using a network approach: a review. Behavioural Processes, 156: 77–82. 10.1016/j.beproc.2017.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak MA, Kinsey JH, Jorgensen, & Hazen TJ (1998). Effects of puzzle feeders on pathological behavior in individually housed rhesus monkeys. American Journal of Primatology, 46(3), 213–227. [DOI] [PubMed] [Google Scholar]
- Novak MA, O’Neill P, & Suomi SJ (1992). Adjustments and adaptations to indoor and outdoor environments: continuity and change in young adult rhesus monkeys. American Journal of Primatology, 28, 125–138. 10.1002/ajp.1350280205 [DOI] [PubMed] [Google Scholar]
- Oates-O’Brien RS, Farver TB, Anderson-Vicino KC, McCowan B, & Lerche NW (2010). Predictors of matrilineal overthrows in large captive breeding groups of rhesus macaques (Macaca mulatta). Journal of the American Association for Laboratory Animal Science, 49(2), 196–201. [PMC free article] [PubMed] [Google Scholar]
- O’Neill PL, Novak MA, & Suomi SJ (1991). Normalizing laboratory-reared rhesus macaque (Macaca mulatta) behavior with exposure to complex outdoor enclosures. Zoo Biology, 10, 237–245. 10.1002/zoo.1430100307 [DOI] [Google Scholar]
- Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, Lange C, Higley JD, Rosoklija G, Hen R, Sackeim HA, & Coplan JD (2011). Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One, 64, e17600 10.1371/journal.pone.0017600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz O, & Baker KC (2017). Higher levels of submissive behaviors at the onset of the pairing process of rhesus macaques (Macaca mulatta) are associated with lower risk of wounding following introduction. American Journal of Primatology, 79(8), e22671 10.1002/ajp.22671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehlmann TE, Bernstein IS, Gordon TP, & Balcaen P (1988). Wounding patterns in three species of captive primates. American Journal of Primatology, 14(2), 125–134. 10.1002/ajp.1350140203 [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Porter LM, & Suarez SA (1996). Enrichment effects on rhesus monkeys successively housed singly, in pairs, and in groups. Applied Animal Behaviour Science, 48(3–4), 159–171. 10.1016/0168-1591(96)01038-6 [DOI] [Google Scholar]
- Shively CA, & Willard SL (2012). Behavioral and neurobiological characteristics of social stress versus depression in nonhuman primates. Experimental Neurology, 233(1), 87–94. 10.1016/j.expneurol.2011.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Crummett TL, & Brandt KL (1994). Ages of eruption of primate teeth: a compendium of aging individuals and comparing life histories. Yearbook of Physical Anthropology, 37: 177–231. 10.1002/ajpa.1330370608 [DOI] [Google Scholar]
- Stavisky RC, Ramsey JK, Meeker T, Stovall M, & Crane MM (2018). Trauma rates and patterns in specific pathogen free (SPF) rhesus macaque (Macaca mulatta) groups. American Journal of Primatology, 80(3), e22742 10.1002/ajp.22742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry BR (2004). Social epigenesis. In: Thierry BR, Singh M, Kaumanns W, editors. Macaque societies: a model for the study of social organization. New York: Cambridge University Press; P 267–290. 10.1007/s10764-005-6467-z [DOI] [Google Scholar]
- Vandeleest JJ, Beisner BA, Hannibal DL, Nathman AC, Capitanio JP, Hsieh F, Atwill ER, & McCowan B (2016). Decoupling social status and status certainty effects on health in macaques: a network approach. PeerJ, 4:e2394 10.7717/peerj.2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables WN & Ripley BD (2002). Modern Applied Statistics with S. Fourth Edition. Springer, New York: 10.1007/978-0-387-21706-2 [DOI] [Google Scholar]
- Voelkl B & Würbel H (2016). Reproducibility crisis? Are we ignoring reaction norms? Trends in Pharmacological Sciences, 37(7), 509–510. 10.1016/j.tips.2016.05.003 [DOI] [PubMed] [Google Scholar]
- Wooddell LJ, Kaburu SSK, Suomi SJ, & Dettmer AM (2017). Elo-rating for tracking rank fluctuations after demographic changes involving semi-free-ranging rhesus macaques (Macaca mulatta). Journal of the American Association for Laboratory Animal Science, 56(3), 260–268. [PMC free article] [PubMed] [Google Scholar]
- Wilson AP, & Boelkins RC (1970). Evidence for seasonal variation in aggressive behaviour by Macaca mulatta. Animal Behaviour, 18, 719–724. 10.1016/0003-3472(70)90017-5 [DOI] [PubMed] [Google Scholar]
- Young RJ (2003). Environmental Enrichment for Captive Animals. UFAW Animal Welfare Series, Blackwell Science, Oxford, UK: 10.1002/9780470751046 [DOI] [Google Scholar]