Abstract
Dual-energy X-ray absorptiometry (DXA) is widely used in the evaluation of bone fragility in children. Previous recommendations emphasized total body less head and lumbar spine DXA scans for clinical bone health assessment. However, these scan sites may not be possible or optimal for all groups of children with conditions that threaten bone health. The utility of DXA scans of the proximal femur, forearm, and radius were evaluated for adequacy of reference data, precision, ability of predict fracture, and applicability to all, or select groups of children. In addition, the strengths and limitations of vertebral fracture assessment by DXA were evaluated. The new Pediatric Positions provide guidelines on the use of these additional measures in the assessment of skeletal health in children.
Keywords: Children, DXA, Forearm, lateral distal femur, proximal femur, vertebral fracture assessment
Introduction
Dual-energy X-ray absorptiometry (DXA) remains an important component of comprehensive bone health assessment in pediatric and adolescent patients with conditions known or suspected to increase skeletal fragility. Clinically, the assessment of bone mineral content (BMC), areal bone mineral density (aBMD), and bone mineral apparent density (BMAD) by DXA is obtained (1) to assist in the diagnosis of patients presenting with a clinically significant fracture history, (2) as part of the routine assessment of skeletal health in children at high-risk for fractures or poor bone accretion, and (3) to monitor the response to bone-active treatments, medical therapies, or disease processes (1-3).
In the 2013 international society for clinical densitometry (ISCD) Pediatric Official Positions, the Pediatric Task Force reaffirmed the posterior-anterior lumbar spine and total body less head (TBLH) as preferred skeletal sites for DXA assessment in most pediatric patients (4). The position also noted that other skeletal sites may be useful depending on the clinical need, and included a brief discussion about the use of DXA to assess the proximal femur, lateral distal femur (LDF), distal forearm, and for vertebral fracture assessment (VFA). At that time, the strengths and limitations of DXA measurements at these additional skeletal sites were not fully evaluated. Since then, interest in the use of DXA at these alternate skeletal sites in children has grown.
There are many pediatric patients for whom DXA assessments are clinically indicated, yet TBLH or lumbar spine scans are not feasible due to the presence of nonremovable artifacts (orthopedic hardware, tubes), difficulties with positioning, abnormal skeletal morphometry, or severe scoliosis with torsion. For this reason, careful evaluation of DXA assessment at other skeletal sites in children is important for the reasons of accessibility, relevance to fracture prediction, and monitoring. For example, in conditions where the predominant clinical risk factor is reduced ambulation (eg cerebral palsy) a key concern is that of low-energy femur fracture. This raises the question of whether DXA assessment at the proximal or distal femur would be more clinically relevant than TBLH or lumbar spine in these patients. Additionally, many pediatric patients will continue to be at increased risk of fractures once they have reached adulthood. Under the current guidelines, the monitoring of bone health in these patients is disrupted as the recommended site of DXA assessment changes from TBLH to femoral neck at age 18 yr. Finally, the identification of vertebral fractures (VF) continues to be a critical component of bone health monitoring in patients with congenital bone fragility (such as osteogenesis imperfecta, long-standing nonambulation, and those exposed to high dose glucocorticoids). Modern DXA scanners are technically capable of producing high resolution lateral spine images for VFA. The prospect of transitioning from standard radiography to DXA for vertebral fracture assessment (VFA) in pediatric patients to minimize radiation exposure is appealing.
As with the recommended measurement sites of the lumbar spine and TBLH, the alternative sites of the forearm, proximal femur, and LDF require reference ranges derived from data in healthy children to account for the expected sex differences and age-related changes. Furthermore, BMC and aBMD at all skeletal sites are subject to size artifact. The availability of reference ranges for size adjusted outcomes including BMAD (3,5) or height Z-score adjusted aBMD Z-scores (6) should also be considered. The adequacy of the reference ranges is therefore an important factor when considering the utility of other measurement sites for clinical use. An additional consideration in children is that precision may be adversely affected by smaller bone size, skeletal maturation, poor cooperation, and movement. Fracture patterns in healthy children are different from those in adults, and certain groups of children are at extremely high risk for vertebral or lower extremity fractures. The ability of a DXA assessment to predict fracture at all skeletal sites is an important consideration in the clinical evaluation of pediatric bone health.
Key Questions
The Pediatric Task Force was therefore charged with re-evaluating the utility of DXA assessment at the forearm, proximal femur, and LDF, and VFA in the pediatric population. To inform the development of evidence-based position statements, the following sets of questions were developed:
For DXA Assessment at Distal Forearm, Proximal Femur, and LDF
Are there adequate reference data?
What is the precision?
Does it predict fracture or other proxy outcomes?
Should it be used in all children or restricted to special groups?
For VFA
Should VFA be used as a substitute for spine radiography in the identification of symptomatic / asymptomatic osteoporotic VF in children?
When does an abnormal VFA in a child require follow-up spine imaging?
What is the VFA method that should be used to detect an osteoporotic VF in children?
Are there technical and biological factors that limit the accuracy of DXA-based VFA in children (for example DXA model and software, age, sex, pubertal stage, obesity)?
Methodology
Two members of the Pediatric Task Force were assigned to each of the 4 topics (proximal femur, LDF, distal forearm, VFA). Literature searches were performed using electronic databases including PubMed, Ovid/Medline, EmBase, and Scopus. Specific details of the literature search applied to each topic, including search terms, dates of searches, and the number of articles initially identified and ultimately included in the literature review are provided in Appendix 1. The full texts of the manuscripts included in the final literature reviews were obtained and provided to all Task Force members. Key findings of the included manuscripts were initially summarized in tabular form for Task Force review, and then used to develop the position statements put forth in this document. The processes used to review and adopt proposed Official Positions have been previously described (7), and were very similar for the present position development conference (PDC). The 2019 ISCD PDC Executive Summary has been submitted for publication (C. Shuhart, personal communication, May 14, 2019).
Utility of DXA Forearm Measurements
International Society for Clinical Densitometry Official Position
DXA measurements at the 33% radius (also called 1/3 distal radius) may be used clinically in ambulatory children who cannot be scanned at other skeletal sites, provided adequate reference data are available.
Grade: Fair, B, W.
Rationale
The forearm offers several advantages as a site for bone health evaluation. The radius and ulna are the most commonly fractured bones in childhood (8,9). Therefore, forearm assessment may be particularly advantageous for fracture prediction. Cortical and trabecular bone occur in varying proportions along the length of the radius, so forearm measurements can be used to evaluate changes in both compartments. The forearm may be useful for patients who cannot be scanned at standard sites.
Discussion
Are There Adequate Reference Data?
Reference data (Table 1) for forearm BMC and aBMD are available for several large international samples of healthy children using Hologic densitometers (10-13). Some reference data sets include the radius only, whereas others report combined values for the radius and ulna. Commonly used sites include the 1/3 distal region, comprised primarily of cortical bone, and the ultradistal region, comprised of primarily trabecular bone. Forearm aBMD is affected by arm dominance (10-14), and some reference data report nondominant, while others report left forearm measures (10-13). It is important for comparability that the region of interest (ROI) and measurement technique, including measurement of and size-adjustment for forearm length, conform to those used to generate the applicable reference data-set. The largest study generating high-quality reference data is the Bone Mineral Density in Childhood Study (BMDCS), which utilized the 33% radius of the nondominant forearm (13). The BMDCS reference data are accompanied by a method that allows for size adjustment of BMC and aBMD Z-scores by height Z-score.
Table 1.
Reference Data for DXA Bone Mineral Content (BMC), Areal Bone Mineral Density (aBMD), and Bone Mineral Apparent Density (BMAD) in Pediatric Populations
| Reference | Country | Study population | Platform | ROI | Study description |
|---|---|---|---|---|---|
| Forearm | |||||
| Arabi et al, (2004) | Lebanon | n = 363 (179 F) age: 10–17 yr |
Hologic | 33% radius, left arm |
|
| Wu et al, (2005) | China | n = 3919 (3919 F) age: 5–85 yr |
Hologic | 33% radius + ulna, nondominant arm Ultradistal radius + ulna, nondominant arm |
|
| Tang et al, (2007) | China | n = 445 (445 F) age: 6–18 yr |
Hologic | 33% radius + ulna, left arm Ultradistal radius + ulna, left arm |
|
| Zemel et al, (2011) | USA | n = 2014 (1022 F) age: 5–23 yr |
Hologic | 33% radius, nondominant arm |
|
| |||||
| Zanchetta, et al, (1995) | Argentina | n = 778 (433 F) age: 2–20 yr |
Norland | Femoral neck Trochanter |
|
| Faulkner, et al, (1996) | Canada | n = 892 (467 F) age: 8–17 yr |
Hologic | Total hip Femoral neck Trochanter Intertrochanter |
|
| Bachrach, et al, (1999) | USA | n = 423 (230 F) age: 9–26 yr |
Hologic (Pencil Beam) |
Total hip Femoral neck |
|
| Xiaoge, et al, (2000) | China | n = 1488 F age: 15–95 yr (69 between 15-20 yr) |
Hologic | Total hip Femoral neck Trochanter Intertrochanter Wards triangle |
|
| Liao, et al, (2003) | China | n = 2702 F age: 5–96 yr (205 between 5-19 yr |
Hologic | Total hip Femoral neck Trochanter Intertrochanter Wards triangle |
|
| Cromer, et al, (2004) | USA | n = 422 F age: 12–18 |
Hologic | Femoral neck |
|
| Lynn, et al, (2005) | Hong Kong | n = 4274 (2415 F) age: 9–94 yr |
Hologic | Total hip Femoral neck Trochanter |
|
| Kalkwarf, et al, (2007) | USA | n = 2014 (1022 girls) age: 5–20 yr |
Hologic | Total hip Femoral neck |
|
| Ward, et al, (2007) | UK | n = 442 (203 F) age: 6–17 yr |
Hologic | Femoral neck |
|
| Webber, et al, (2007) | Canada | n = 179 (91 F) age: 3–18 yr |
Hologic | Total hip |
|
| Wu, et al, (2008) | China | n = 2433 M age:15–85 yr (123 between 15–19 yr) |
Hologic | Total hip Femoral neck Trochanter Intertrochanter Wards triangle |
|
| Tamayo, et al, (2009) | Mexico | n = 1051 (565 F) age: 7–18 yr |
GE Lunar | Total hip |
|
| Baxter Jones, et al, (2010) | Canada, USA | n = 1875 (1061 F) Asian, Hispanic, Black and Caucasian age: 8–25 yr |
Hologic | Total hip Femoral neck |
|
| Khadilkar, et al, (2011) | India | n = 920 (440 F) age: 5–17 yr |
GE Lunar | Femoral neck |
|
| Zemel et al, (2011) | USA | n = 2014 (1022 F) age: 5–23 yr |
Hologic | Total hip Femoral neck |
|
| Gallo, et al, (2012) | Canada | n = 59 (26 F) age: birth to 1 yr |
Hologic | Femur |
|
| Jeddi, et al, (2013) | Iran | n = 476 (235 F) age: 9–18 yr |
Hologic | Femoral neck |
|
| Kang, et al, (2016) | Korea | n = 1974 (1000 F) age: 10–25 yr |
Hologic | Total femur Femoral neck |
|
| |||||
| Henderson, (2002) | USA | n = 256 age: 3–18 yr |
Hologic (Pencil Beam) |
R1, R2, R3 ROIs |
|
| Zemel, (2009) | USA | n = 821 age: 6–18 yr |
Hologic | R1, R2, R3 ROIs |
|
F, female; ROI, region of interest; SD, standard deviation.
What is the Precision?
The BMDCS reported that the precision for the 33% radius was the poorest of the 4 skeletal sites examined (Table 2) (15), but this and other studies (11,16,17) reported coefficient of variation values ≤1.9%, the minimum acceptable precision for the lumbar spine for adults [https://www.iscd.org/official-positions/2015-iscd-official-positions-adult/]. The monitoring time interval was determined to be about 1 yr for children under 18 yr, supporting the conclusion that the precision is reasonable for clinical use. Positioning challenges and motion artifact are likely to be greater in children with medical illnesses and disabilities.
Table 2.
Precision Estimates for DXA Areal Bone Mineral Density (aBMD) and Bone Mineral Content (BMC) in Pediatric Populations
| Reference | Country | Study population | Platform | ROI | Precision estimates1 |
|
|---|---|---|---|---|---|---|
| aBMD | BMC | |||||
| Forearm | ||||||
| Shepherd, et al, 2011 | USA | n = 155 (70 F) age: 6–16 yr2 |
Hologic | 33% radius, nondominant arm | Overall: 1.65% 6–9 yr: 1.91% 10–13 yr: 1.39% 14–16 yr: 1.66% |
|
| Tang, et al, (2007) | China | n = 33 age: not specified |
Hologic | 33% radius + ulna, left arm UD radius + ulna, left arm |
33% radius + ulna: 1.03% UD radius + ulna: 1.35% |
|
| Soderpalm, et al, (2007) | Sweden | n = 20 (0 F) age: 6–37 yr |
GE Lunar | Not specified | 1.3% | |
| Maimoun, et al, (2013) | France | Not specified | Hologic | Not specified, dominant arm | <1% | |
| Proximal Femur | ||||||
| Zanchetta, et al, (1995) | Argentina | n= 40 age = 2–20 yr |
Norland | Total hip | 1.5% | |
| Faulkner, et al, (1996) | Canada | Not specified | Hologic | Femral neck | 0.9% | |
| Cromer, et al, (2004) | USA | Not specified | Hologic | Total hip | 1.4% | |
| Leonard, et al, (2009) | Canada | n: 15(9 F) age 10 yr; 17 (9 F) age 10–18 yr |
Hologic | Total hip | <10 yr: 1.6% 10–18 yr: 1.0 % |
|
| Baxter Jones, et al, (2010) | Canada | n: not specified age: “young adults” |
Hologic | Total hip Femoral neck |
Total hip: 1.4% Femoral neck: 3.5% |
|
| Khadilkar, et al, (2011) | India | n = 31 (17 F) age: 11.6 ± 4 yr |
GE Lunar (Pencil beam) |
Femoral neck | 4.2% | |
| Jeddi, et al, (2013) | Iran | n = 10 age: “children” |
Hologic | Femoral neck | 2.4% | |
| Liu, et al, (2013) | China | n = 15 each at 7 different centers age: not specified |
Hologic | Hip Joint Femoral neck |
Hip joint: 1.02%–1.79% Femoral neck: 1.39%–2.33% |
|
| Shepherd, et al, (2013) | USA | n = 155 (70 F) age: 6–16 yr |
Hologic | Total Hip Femoral neck |
Total hip:
Femoral neck:
|
|
| Lateral Distal Femur | ||||||
| Henderson, (2002) | USA | n=5 age: “children” |
Hologic (Pencil Beam) |
R1, R2, R3 | Mean Absolute ± SE R1: 2.9 ± 0.8% R2: 2.3 ± 0.7% R3: 2.6 ± 1.2% |
|
| Modelesky, (2008) | USA | n=8 age: 8–12 yr Cerebral palsy |
Hologic | Not specified | 3.6% | 4.0% |
| Henderson, (2010) | USA | n = 30 age: 5–17 yr Limited mobility |
Hologic (Pencil Beam) |
R1, R2, R3 | R1: 2.6% R2: 2.0% R3: 2.1% |
|
| Hobby, (2013) | USA | not specified | Hologic | Not specified | 5%–7% | |
| Damcott, (2013) | USA | n = 14 age: 4–9 yr Nonambulatory |
GE Lunar | Not specified | 4.5% | |
| Fan, (2018)3 | USA | n = 51 age: 1–4 yr |
Hologic | R1, R2, R3 | R1: 2.3% R2: 1.9% R3:1.9% |
R1: 4.11% R2:1.7% R3:1.9% |
F, females; ROI, region of interest; UD, ultra-distal; yr, years.
Percent coefficient of variation, unless otherwise specified.
Precision studies conducted in healthy children, unless otherwise specified.
Abstract only.
Does it Predict Fracture or Other Proxy Outcomes?
Retrospective, cross-sectional, case-control, and prospective studies in healthy children report associations between forearm DXA measurements and fractures of the forearm and other long bones (18-25). However, a recent prospective study found that forearm aBMD in boys was less predictive of fractures than lumbar spine and total hip aBMD (26). Forearm aBMD is responsive to the positive effects of upper limb impact-loading activities (16,27). The ability of forearm aBMD to identify children with underlying skeletal disorders or who are at-risk for fragility fractures or fractures in locations associated with significant morbidity (such as vertebral compression and femoral fractures) is unknown.
Should it be Used in all Children or Restricted to Special Groups?
Few studies have examined the association of forearm aBMD with skeletal outcomes in children with medical illnesses, limited mobility, and physical disabilities, and results are conflicting. Radius aBMD correlated with deficits at other skeletal sites in children with Duchenne muscular dystrophy (17) and juvenile idiopathic arthritis (28), and was lower in nonambulatory girls with Rett syndrome (29), but not in children with anorexia nervosa (30), bone marrow failure syndromes (31), and neurofibromatosis type 1 (32). However, the forearm may be measured under circumstances when other skeletal sites cannot be measured safely, accurately, or without artifact interference.
Future Directions
Further research is needed to determine if forearm DXA assessment is useful for predicting fractures, monitoring bone accrual, and assessing the response to medical interventions, especially in children with limited ambulation, physical disabilities, or who have conditions where cortical and trabecular bone may be differentially affected. Reference ranges are needed for: forearm measures in children <5 yr of age; for the ultra-distal radius ROI for all ages; and for general electric (GE) Lunar densitometers. The need for size adjusted measures to improve fracture prediction also requires investigation. Precision data for the forearm in children have been derived from healthy populations, and may differ from precision estimates in children with disorders affecting the skeleton. To what extent younger age, lower bone density, underlying disease, and technician experience influence precision measurements of the proximal femur in children should be further explored.
Utility of DXA Proximal Femur Measurements
International Society for Clinical Densitometry Official Position
Proximal femur DXA measurements may be used, if reference data are available, for assessing children with reduced weight bearing and mechanical loading of the lower extremities or in children at-risk for bone fragility who would benefit from continuity of DXA measurements through the transition into adulthood.
Grade: Fair, B, W
Rationale
The total body DXA scan primarily quantifies cortical bone mass in children, but the reported Z-score is an average of the entire skeleton, so clinically significant deficits in weight-bearing bones may be masked by normal upper extremity bone mass (33).The proximal femur previously was not considered an optimal site for DXA assessment because of concerns related to variability in skeletal maturation of the hip (4). However, pediatric sex- and race-specific normative BMC, aBMD, and BMAD (for femoral neck) data are available. DXA assessment at the proximal femur allows for the evaluation of both cortical (total hip) and trabecular (femoral neck) skeletal compartments of the lower extremity. The ability to assess lower extremity bone mass may be important in children with limited ambulation or weight-bearing activity. Precision of proximal femur scans in children may be affected by scan acquisition issues (positioning and movement), and analysis issues (presence of open growth plates, ability to image the lesser trochanter, and placement of ROI). Older children with, or at-risk for future skeletal fragility may benefit from having proximal femur measurements obtained to allow for better longitudinal assessment of bone mass across the transition to young adulthood.
Discussion
Are There Adequate Reference Data?
Cross-sectional and longitudinal reference data (Table 1) for DXA measurements of the proximal femur have been established in numerous geographic regions (13,34-54). Most reference data were obtained on Hologic densitometers. Published reference data from GE Lunar densitometers are available from children living in India for the femoral neck and Mexico for the total hip. Pediatric reference data are limited to children 5 yr and older, with the exception of 1 study that reported aBMD of the entire femur in a sample of Canadian children from 0 to 12 mo (48). Based on comparisons between DXA manufacturers and models for the spine and total body, manufacturer specific reference data should be used (4).
What is the Precision?
Few studies provide details regarding the number and age of children assessed for precision estimates (see Table 2). There is measurement error in each DXA measurement due to machine error and patient positioning. When following individuals over time, knowledge of precision helps to determine when a difference between measurements exceeds that of measurement error. Precision estimates combined with expected rates of change are needed to inform monitoring time interval, the time interval within which a significant change (ie, one that exceeds measurement error) in aBMD is expected to occur due to growth, or a pharmaceutical agent or intervention. In the largest pediatric precision study (n = 155, ages 5–16) (15), precision estimates for the total hip were similar to those of the whole body and spine. In this study, the monitoring time interval for total hip and proximal femur aBMD was at or below 1 yr, depending on age.
Does it Predict Fracture or Other Proxy Outcomes?
Many (19,22,23,25,55-57), but not all (58,59) case-control studies report lower proximal femur aBMD in those with a history of fracture compared to those with no such history. Results from prospective studies are also variable. In a preliminary report from the prospective population-based Generation R Cohort study (n = 1848), femoral neck aBMD and hip structural analysis at 6 yr of age predicted future fractures (60). In another smaller prospective study, each standard deviation decrease in femoral neck aBMD was associated with an odds ratio for fracture of 1.8 in healthy males aged 6.5–8.5 yr followed for 7.4 yr (60,61). However, further follow-up to a mean age of 22.6 yr showed no differences in aBMD measurements at any skeletal site among those who did or did not have fractures (26). In contrast, baseline proximal femur measurements did not predict fractures in 125 healthy girls who completed 8.5 yr of follow-up, but trochanteric aBMD was lower in the group with fractures at follow-up (18). Other prospective studies have shown no association between proximal femur measurements and subsequent fracture (24,62). A limitation of all studies is that they examined the predictive value of proximal femur measurements for upper limb or all fractures, but not specifically hip or other lower extremity fractures. Hip fractures are rare in youth, especially among otherwise healthy individuals, and associations are thus difficult to detect.
Can it be Used in all Children or Restricted to Special Groups?
Children with limited physical activity are at significant risk for low proximal femur bone mass. Generally, this encompasses individuals with substantially reduced mobility from neurological, neuromuscular or muscular disorders, immobilization subsequent to trauma or burns, activity restriction due to fear of fractures, hypotonia, or difficulty engaging with peers. Children in the latter group include those with autism spectrum disorder (63-65) who have a greater risk of hip (66), but not upper limb fractures, and limited engagement in sports and other physical activities (66,67) The increased risk of hip fracture in this population persists into adult life. A cohort of steroid-naïve patients with Duchenne muscular dystrophy was found to have aBMD deficits of the proximal femur that were greater in magnitude and apparent at a younger age compared to the lumbar spine (68). In contrast to what is observed in healthy children, the femur was the most common site of fracture (40% of all fractures) in these participants. Greater deficits in proximal femur aBMD compared to other skeletal sites have also been identified in cohorts of children with osteogenesis imperfecta (69) and childhood survivors of acute lymphoblastic leukemia (70). In the latter study, proximal femur aBMD deficits persisted over 12 mo despite normalization of bone mass at other sites. While these studies are limited by small sample sizes, they serve as examples of clinical situations where proximal femur DXA assessment may yield additional clinically relevant information.
Studies of children with other conditions at risk for skeletal fragility did not find associations of proximal femur BMC or aBMD with fractures (eg, anorexia nervosa (71,72), exercise induced amenorrhea (72,73), inflammatory bowel disease (74), Turner syndrome (75), suspected primary osteoporosis (76), transfusion dependent thalassemia (77), and childhood survivors of solid tumors (78)). However, many of these studies were not powered to detect fractures.
Importantly, many children with chronic conditions that threaten bone health and are at increased risk of fracture are likely to require ongoing monitoring of bone density into adulthood. Proximal femur measurements have good precision and a monitoring time interval at or below 1 yr of age, so it is a suitable choice for monitoring changes over time, especially in groups such as survivors of acute lymphoblastic leukemia. Importantly, the use of proximal femur measurements would allow for continuity of DXA measurements through the adult transition. While there are no studies to support the specific age at which proximal femur measurements should be acquired for continuity of care, as a general rule they should be considered once statural growth is completed.
Future Directions
Further research is needed to determine the utility of proximal femur scans and ROI (ie, total hip vs femoral neck) in predicting hip and other fracture risk, and monitoring treatment or disease progression. It is unknown if adjustment of proximal femur DXA measurements for height Z-score, pubertal stage, bone age, or other factors improves fracture prediction in high-risk children. The optimal age ranges and specific patient populations that would benefit need to be further delineated. Manufacturers should partner worldwide with pediatric bone researchers to develop and optimize proximal femur reference databases.
Proximal femur precision data in children have been derived from healthy populations, and may differ from precision estimates in children with disorders affecting the skeleton. To what extent younger age, lower bone density, underlying disease, and technician experience influence precision measurements of the proximal femur in children should be further explored.
It is unknown whether children with low proximal femur bone mass in childhood retain residual deficits into adulthood, or whether a catch-up effect occurs over time. Large, long-term prospective studies that follow children through adulthood are needed to determine whether proximal femur measurements at various time points (prepuberty, puberty, and postpuberty), as well as other skeletal sites, predict future fracture risk at 1 or more sites.
Utility of DXA LDF Measurements
International Society for Clinical Densitometry Official Position
LDF DXA measurements correlate well with increased lower extremity fragility fracture risk in nonambulatory children. LDF DXA may be used, if reference data are available, to:
Assess BMD in children when the presence of nonremovable artifacts (orthopedic hardware, tubes), positioning difficulties, abnormal skeletal morphometry, or severe scoliosis with torsion interfere with DXA acquisition at other anatomical sites.
Monitor the effects of changes of weight-bearing in nonambulatory children.
Grade: Fair, B, W
Rationale
LDF measurements assess bone density at an easily accessible, weight-bearing skeletal site that is responsive to mechanical loading and is also a clinically relevant fracture location (79,80) for patients with conditions that limit mobility, such as cerebral palsy, Rett syndrome, spinal muscular atrophy, and Duchenne muscular dystrophy. These children are at high risk for low energy fractures of the lower extremities, particularly of the distal femur (80-86). This skeletal site may be important for children with limited ambulation or weight-bearing physical activity to avoid underestimating their degree of skeletal compromise and to optimize the timing of necessary therapeutic interventions. Discordance between lumbar spine aBMD and LDF aBMD has been described in patients with limited mobility (33,87-92). Thus, relevant information regarding bone health in these patients might be missed if only the lumbar scan is obtained (89,91).
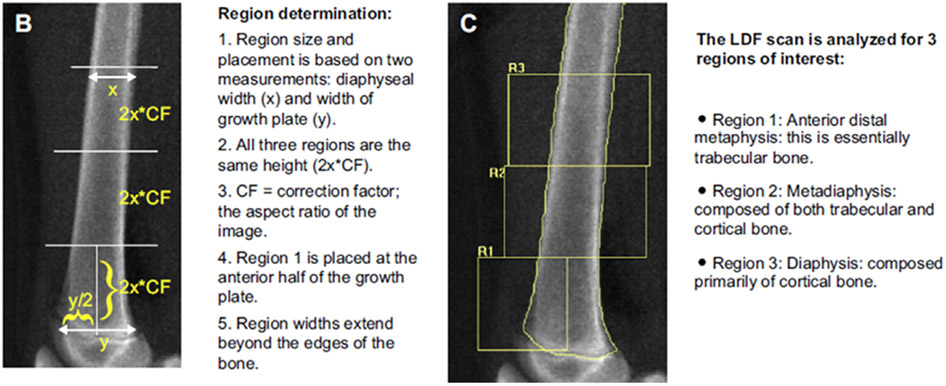
The LDF scan provides information about 3 types of bone: Region (R)1 is metaphyseal and is comprised primarily of trabecular bone, R2 is metadiaphyseal and comprised of a mixture of cortical and trabecular bone, and R3 is diaphyseal which is primarily cortical bone (103) (see Fig. 1). These regions can respond differently to various medical conditions and/or treatments, as shown with decreasing ambulation in children with spinal muscular atrophy (86), functional motor status in boys with Duchenne muscular dystrophy (33), immobilization following orthopedic surgery (93), and with bisphosphonate use (82,94,95). The total body DXA scan primarily quantifies cortical aBMD in children, but the reported Z-score is an average of the entire skeleton, so clinically significant deficits in weight-bearing bones may be masked by normal upper extremity aBMD (33). The LDF site may be important to identify those at increased risk for fracture, to optimize the timing of necessary therapeutic interventions, to monitor treatment effect for children with limited ambulation or weight-bearing physical activity, and to better delineate the effect of weight-bearing activity as a crucial modifier of cortical bone mass.
Fig. 1.
Lateral distal femur scan showing the 3 regions of interest. Region (R)1 is metaphyseal and is comprised primarily of trabecular bone, R2 is metadiaphyseal and comprised of a mixture of cortical and trabecular bone, and R3 is diaphyseal which is primarily cortical bone. Reproduced from Zemel et al, (97).
Discussion
Are There Adequate Reference Data?
Reference data (Table 1) for the LDF are available for scans acquired on Hologic pencil beam (96) and fan beam (97) densitometers. Few centers are using pencil beam densitometers. The reference data for fan beam densitometers were generated from a robust sample allowing for age, sex, and race-specific ranges for children aged 6–18 yr. However, measurements were obtained only at a single clinical center. The LDF aBMD results are not as strongly influenced by height compared to lumbar spine and total body aBMD (13,96,97).
Clinical utility of the LDF scan is hampered by the lack of a broad age range of normative data. Young children with chronic medical conditions known to negatively impact bone can experience fragility fractures in the first 1–2 yr of life (86,98). Pediatric patients with complex medical needs are living longer and an increased fracture may risk persist through young adulthood (99-101).
What is the Precision?
Precision estimates for the LDF scan are approx 3% (range 1.2%–7.1%) (Table 2). In general, precision estimates are better for aBMD than BMC, and at cortical regions compared to trabecular regions. Precision estimates for repeat analyses (0.7%–1.8%) are better than for repeat scans (100,102). Compared to other ISCD recommended skeletal sites, precision estimates are slightly worse than average precision error for the lumbar spine (<2%–3%) and total body (1%–2%) (103).
Does it Predict Fracture or Other Proxy Outcomes?
Lower extremity fractures, specifically at the distal femur, are the most common type of fracture in non-weight-bearing children (84,104). In a study of 619 children with cerebral palsy and Duchenne muscular dystrophy (80), a strong correlation between LDF aBMD and prior fracture was demonstrated: 35%–42% of those with LDF aBMD Z-scores <5.0 had fractured compared with 13%–15% of those with aBMD Z-scores >1.0. There are no large, longitudinal studies demonstrating a direct association between LDF aBMD and subsequent fracture.
The responsiveness of LDF aBMD to loss of weight-bearing activity has been shown (33,86,105) in patients with acute immobilization (93,95) and spinal cord injury (106). LDF aBMD also associates with indicators of ambulation (gross motor function classification system level, phenotype severity, activity level), therapeutic interventions (87,98), nutritional status, severity of medical comorbidities, and medication use (91,101). Collectively, these studies demonstrate that LDF aBMD has utility to monitor the effects of disease or treatment.
Should it be Used in all Children or Restricted to Special Groups?
LDF aBMD measurements respond positively to exercise in healthy, typically developing children (105,107), and is sensitive to disease processes and treatments affecting bone strength such as changes in weight-bearing over time in children with chronic conditions that affect ambulation (33,86); temporary immobilization; and bisphosphonate treatment in children with cerebral palsy, osteogenesis imperfecta, and other chronic conditions associated with low aBMD (82,94,95,98). LDF is a clinically valid technique in patients with skeletal dysplasia (108,109) in whom abnormal skeletal morphometry limits aBMD assessment at standard sites.
Future Directions
The broader use of LDF scans in children requires improvements in reference data, with expanded age ranges for younger children and into adulthood, and reference ranges for GE Lunar devices. It is unknown whether fracture prediction using LDF aBMD is improved with size adjustment. The assessment of LDF aBMD has been validated in adults with neuromuscular impairment, although it requires a slightly different analysis technique since the growth plate landmark used in the pediatric bone analysis is no longer present (100). Importantly, the need for standardized scan acquisition protocols, software analysis and reporting programs supported by DXA manufacturers for LDF scans is paramount.
Longitudinal studies in typically developing healthy children are needed to establish the expected change in LDF aBMD for longitudinal monitoring of chronically ill children. This change, plus the least significant change of the clinical center acquiring the scans, must be known to interpret serial changes. It is unknown if precision estimates at the LDF are different for ambulatory children compared to nonambulatory children. This is clinically relevant given the less favorable precision estimates noted in some studies involving medically complex children and importance of DXA technician training for this technique. To what extent younger age, lower bone density, underlying disease, and technician experience influence precision measurements of the proximal femur in children should be further explored.
Larger, prospective, longitudinal studies are needed to evaluate the predictive value of LDF aBMD for fracture in children with medical conditions that negatively impact bone, especially those at increased risk for lower extremity fragility fractures and impaired weight-bearing ability. Further studies are necessary to elucidate the sensitivity of the 3 LDF ROIs to treatment interventions and the recommended interval for repeat monitoring to assess for therapeutic response.
International Society for Clinical Densitometry Official Position
Precision assessment at each skeletal measurement site should be calculated in a sample representative of the patient population being evaluated.
Grade: Fair, A, W
Rationale and Discussion
It is widely recognized that ascertainment of precision is necessary for determining least significant change when monitoring individuals over time. It is also helpful for interpreting DXA measurements at a single time point, since it provides a frame of reference for the potential range of measurement error for a single measurement. In the pediatric population, precision may be influenced by factors contributing to movement, positioning, and ability to cooperate such as developmental age, cognitive ability, and movement disorders, or technical factors such as bone edge detection in small bones. Many published studies of precision have been based on healthy children who are able to cooperate. These values may not reflect precision in patient groups, such as children with limited mobility, for whom results of DXA scans may affect clinical care, nor do they reflect the skill level of DXA operators at individual centers. Precision assessment in samples representative of the patient populations being evaluated requires time, effort, and parental consent, and must be performed within local regulations for radiation safety. However, such information would potentially benefit these pediatric populations.
Utility of VFA in Children
International Society for Clinical Densitometry Official Position
DXA VFA may be used as a substitute for spine radiography in the identification of symptomatic and asymptomatic VF, provided the evaluator has experience in the assessment of pediatric VF
Grade Fair, B, W
International Society for Clinical Densitometry Official Position
Following VFA, additional spine imaging should be considered in the following circumstances:
Vertebrae that are technically unevaluable by VFA (ie not sufficiently visible), provided the detection of a VF would change clinical management.
Assessment of a single, Genant Grade 1 VF, if confirmation of a Grade 1 VF alone would change clinical management.
Radiographic findings that are not typical for an osteoporotic VF (eg suspected destructive inflammatory or malignant processes, congenital malformations, acquired misalignments, or dislocations)
Grade: Fair, B, W
International Society for Clinical Densitometry Official Position
The Genant semi-quantitative method should be used for VFA in children.
Grade: Fair, C, W
Rationale
Low-trauma VFs are a frequent manifestation of osteoporosis in high-risk pediatric populations, including children with osteogenesis imperfecta (110) and glucocorticoid-treated disorders (Fig. 2) (111-113). Recent studies in children with glucocorticoid-treated illnesses have shown that moderate and severe prevalent VFs are the strongest independent predictors of incident VF, even more so than aBMD. Even mild VFs are independent predictors of future VF in children (111,114).
Fig. 2.
Five year-old child with osteogenesis imperfecta type III. (A) Lateral spine radiograph. (B) Lateral spine DXA VFA. Both show multiple vertebral fractures at T5-11. From Diacinti D, et al, (125).
The Steroid-induced Osteoporosis in the Paediatric Population research program showed that VF can occur with aBMD Z-scores greater than −2.0 (111,114,115), the historic cut-off to define osteoporosis in children. This fact, along with the observation that significantly different lumbar spine aBMD Z-scores are generated depending on the normative database used (115-117), aligns with the ISCD’s 2013 recommendation that aBMD Z-score cut-off is not required in a child with a low-trauma VF (118).
Pediatric VFs are frequently asymptomatic (119), and go undetected in the absence of routine surveillance (112,120-122). These observations have led to recommendations which promote periodic screening for VF by spine radiographs in at-risk children, and heighten the need for a VF detection method that is efficient and involves low radiation. VFA by DXA is one such method, with additional advantages that the entire spine can be visualized on a single cassette, and that parallax is eliminated, both of which facilitate a radiologist’s assessment of vertebral morphology.
Discussion
Should VFA be Used as a Substitute for Spine Radiography in the Identification of Symptomatic/Asymptomatic Osteoporotic VF in Children?
Six pediatric studies (Table 3) have evaluated the sensitivity and specificity of DXA-based VFA in comparison to spine radiographs (123-128). Studies vary in manufacturer/models, analysis software, VF detection techniques, study design, and morphometric definitions of fractures. VF occur most frequently in children in the mid-thoracic region (120,129), and this area was not evaluated in 1 study (126). Nevertheless, these studies showed that: (1) sensitivities and specificities for VF identification were highest for expert reader appraisal using the Genant semi-quantitative method rather than automated or human 6-point morphometric analysis (123-125,128); and (2) assessment by expert readers was the most important determinant of high sensitivities and specificities on both Hologic (Discovery) (125) and GE Lunar (iDXA) (124) devices (there were no published studies using the Hologic Horizon instrument, Lunar models earlier than the iDXA, or Norland machines). These results emphasize the importance of VFA assessments by expert readers (ie radiologists with expertise in pediatric vertebral morphology). Importantly, 1 study showed a 3–5-fold lower radiation exposure by VFA compared to radiography (123,124).
Table 3.
Summary of DXA Vertebral Fracture Assessment (VFA) Validation Studies
| Reference | Patient population | DXA machine and software |
Methods | Main findings1 | Comments |
|---|---|---|---|---|---|
| Adiotomre et al Eur Radiol (2017) | 250 children Mean age 11.5 yr (range 5–16 yr) 44% OI, 36% secondary OP or unexplained fractures |
GE Lunar iDXA Software NA | Three experienced radiologists VF were visually classified as >10% height ratio reduction The simplified algorithm-based qualitative (ABQ) score (Ref 1) was used to classify a treatment-worthy VF as >25% height ratio reduction |
VF Prevalence2: 36% % Vertebrae Evaluable3: Radiography: 84%–91% VFA: 90%–93% Reader Agreement (kappa): For >10% height ratio reduction: Radiography: 72%–77% (64% all agreed) VFA: 73%–91% (66% all agreed) Radiography vs VFA: 0.42 Sensitivity: 70% (for VF>25%) Specificity: 97% (for VF>25%) |
10% vertebral height ratio overlaps with normal physiological rounding of vertebrae in children (see text) Average effective radiation dose was 41.9 μSv for iDXA and 232.7 μSv for radiographs. |
| Crabtree et al Bone (2017) | 80 children Mean age 12.0 yr (range 5.1–18.8 yr) 26% inflammatory bowel disease, 19% OI, 10% acute lymphoblastic leukaemia, other <10% |
GE Lunar iDXA Software version 13.6 | One experienced radiologist, two pediatricians with expertise in bone disease VF were visually classified as >10% to <25% (mild), >25% to <50% (moderate) and >50% (severe) vertebral height ratio reduction Moderate & severe VF were considered clinically significant |
VF Prevalence: 49% % Vertebrae Evaluable (T3 to L5): Radiography: 92.8%–94.8% VFA: 98.4% Reader Agreement (kappa, for VF >25% height reduction): Radiography: 0.81–0.86 VFA: 0.78–0.89 Radiography vs VFA: 0.83 Sensitivity: 92.3% Specificity: 98.5% PPV: 92.3% NPV: 98.5% |
10% vertebral height ratio reduction overlaps with normal physiological rounding of vertebrae in children (see text) Additional assessment by 6-point automated morphometric analysis performed consistently and substantially worse than VFA. Sensitivity and specificity for any VF (including 10-25% height ratio reduction) were lower than VF >25% height ratio reduction alone. The average effective radiation dose was 42 μSv for iDXA and 97 μSv for radiographs |
| Diacinti et al Calcif Tissue Int (2015) | 58 children, mean ± SD age 9.7 ± 8.5 yr (range 1–18 yr) All with osteogenesis imperfecta (76% with OI type I) |
Hologic Discovery A Software Version 4.3 | Two experienced radiologists Visual assessment by the Genant SQ method (Ref 2) Grade 0 (normal), ≤ 20%; grade 1 VF (mild), >20% to 25%; grade 2 VF (moderate), >25% to 40%; grade 3 VF (severe), >40% Discrepancies resolved by consensus |
VF Prevalence: 62% %Vertebrae Evaluable: Radiography 97.9% VFA: 90.9% Reader Agreement (kappa): Radiography: NA VFA: 0.96 Radiography vs VFA: 0.81 / vertebra Sensitivity: 95.6% / patient Specificity: 100% / patient PPV: 100% NPV: 97.2% |
Additional analyses using the Hologic QDR Physician’s Viewer (version 7.02) for automated vertebral morphometry showed good diagnostic performance (sensitivity 94%, specificity 90.6%), though not as high as for VFA. The lower specificity for automated vertebral morphometry was due to 10 vertebrae classified as fractured, that were normal by radiography. |
| Hoyer-Kuhn et al J Clin Densitometry (2015) | 18 children median age 10.3 yr (range 5–17 yr) OI or other osteoporotic conditions |
GE Lunar iDXA Software version 13.6 | Two independent investigators T11 to L5 were analysed, by Smith-Bindman 6-point vertebral morphometry (Ref 3) Intraclass correlation coefficient used to assess agreement between investigators or modalities (VFA vs radiography) Vertebral morphometry was defined as fish-shape, wedge-shape or compression; fracture defined according to Genant SQ (>20% height ratio loss, Ref 2) |
VF Prevalence: NA %Vertebrae Evaluable: Radiography: 85.7% VFA: 87.3% Reader Agreement (kappa): Radiography: 0.89 VFA: 0.96 Radiography vs VFA: 0.7 Sensitivity: NA Specificity: NA |
Thoracic region largely not assessed in this study; most VF in children will occur in this region. Results given are for “compression fracture” morphology, which showed intermediate agreement between “fish-shaped” and “wedge-shaped”. Small sample size. |
| Kyriakou et al Bone (2015) | 165 children underwent VFA; 20 of these children underwent comparison of VFA to radiography. Median age (for the 165 children): 13.4 yr (range 3.6–18.0). Median age for the 20 children who underwent comparison to radiograph: NA. Underlying diagnoses: OI, chronic illness, osteonecrosis, or history of recurrent fractures. |
Lunar Prodigy Software Version: NA | Two nonradiologist reviewers Manual re-positioning of six point placement to generate vertebral heights Genant SQ method (Ref 2) for VF definition and categorization Grade 0 (normal), ≤20%; grade 1 VF (mild), >20% to 25%; grade 2 VF (moderate), >25% to 40%; grade 3 VF (severe), >40%. |
VF Prevalence: NA % Vertebrae Evaluable: Radiography: NA VFA: 84%, 93% of unevaluable vertebrae were between T6 and T9 Reader Agreement (kappa): Radiography: NA VFA: 0.85 Radiography vs VFA: 0.79 Sensitivity: 75% Specificity: 98% VFA PPV: 90% VFA NPV: 95% |
Radiographs were carried out within 2 mo of the DXA VFA. Most of the children who had both VFA and radiography had no evidence of VF by either method. Only 20 children underwent comparison of DXA VFA to radiography. |
| Mäyränpää et al Bone (2007) | 65 children Median age 12.1 yr (range 4.6 to 17.1 yr) Suspected osteoporosis based on a low BMD (< −2.0 SD) and low trauma fractures, including: OI, secondary osteoporosis and otherwise healthy children with low trauma fractures |
Hologic Discovery A Software version 12.4 | Four readers: 2 experienced clinicians, one orthopedic surgeon, one radiologist Discrepancies resolved by consensus Two visual VF methods (both methods defined a VF as >20% loss in vertebral height ratio): 1. Genant SQ method (Ref 2) 2. Makitie method (Ref 4) |
VF Prevalence: 20% % Vertebrae Evaluable: Radiography: T4 to T6: 87.7% T7 to L4: 99.8% VFA: T5 to T7: 90% T8 to L4: 96% Reader Agreement (kappa): Radiography: 0.98 VFA: NA Radiography vs VFA: 0.34 Sensitivity: NA Specificity: NA |
QDR Physician’s Viewer (version 4.0) for automated vertebral morphometry correctly identified the shape of lumbar vertebrae in most children, but was limited in shape detection in the thoracic region. |
GE, General Electric; NA, not available; OI, osteogenesis imperfecta; OP, osteoporosis; SQ, semi-quantitative.
Additional References Cited in the Table:
Ferrar et al, 2003, J Bone Miner Res, 18: 933–938
Genant et al, 1993, J Bone Miner Res, 8 (9): 1137–48
Smith-Binden et al, 1991, J Bone Miner Res, 6 (1): 25–34
Makitie et al, J Ped 2005, 146: 395–401
All results are expressed at the per vertebrae level; see original articles for per subject results, where available.
VF prevalence is based on results from gold standard conventional radiography at the subject level.
From T4 to L4, unless otherwise specified.
When Does an Abnormal VFA in a Child Require Follow-up Imaging?
In patients with a high risk of VF, especially in those where management will be influenced by the presence of VF, unevaluable vertebrae by VFA should be reimaged by conventional radiography or MRI. Conventional radiography should be considered first due to its ease, rapid availability, and low cost. However, mid- and upper thoracic vertebrae may also be poorly visible on conventional radiographs (123,124), so MRI may be required following conventional radiography. MRI will also be useful in identifying additional clinical signs of VF, such as edema, that may facilitate diagnosis.
The thoracic region is the most frequent site of VF in children and adults (130), yet it is also the most frequent site of unevaluable vertebrae by VFA, particularly in the upper thoracic area (T4–7) (124,125). Vertebrae in the thoracic region (124,125) may be unevaluable due to obscuration by overlying lung tissue and scapulae. Anterior wedging due to normal physiology in the mid-thoracic region can also occur. Thoracic vertebra evaluation is further complicated in younger children because vertebrae have a rounded appearance due to lack of or emerging ossification of the ring apophysis. This appearance can be mistaken for a VF by an inexperienced reader (124,125,129). In borderline cases, an expert reader with the ability to distinguish the radiographic features of the developing spine from the signs of VF (including loss in vertebral height ratio plus other discrete signs such as endplate interruption, anterior cortical buckling, and loss of endplate parallelism) is particularly important. Incidental findings of deformities that are not typical for osteoporotic fractures (eg, Schmorl’s nodes, fibrous dysplasia, osteomyelitis, congenital vertebral abnormalities, endplate infarcts due to sickle cell disease, infection, or malignancy) should be further evaluated by conventional radiography or MRI as clinically indicated.
It is well-established in both children and adults that agreement is lowest for Grade 1 VF, whether by DXA VFA or conventional radiography (124,125,131,132). Incident Genant Grade 1 VF in patients with minimal or equivocal risk factors for osteoporotic VF and without previous fractures, should undergo follow-up radiography or MRI for accurate VF identification, particularly if identification of a Grade 1 VF would alter patient management.
What is the VFA Method That Should be Used to Detect an Osteoporotic VF in Children?
The Genant semi-quantitative method is the most widely used, standardized method to diagnose VF in children and adults alike (118,133,134). Vertebral bodies are graded according to the extent of the reduction in height ratios when the anterior vertebral height is compared with the posterior height (anterior wedge fracture), middle height to the posterior height (biconcave fracture), and posterior height to the posterior height of adjacent vertebral bodies (crush fracture). The definition of a VF is >20% loss in vertebral height ratio.
The Steroid-induced Osteoporosis in the Paediatric Population research program demonstrated that VF were more common than fractures at other skeletal sites as manifestations of osteoporosis in children with glucocorticoid-treated leukemia (135). This program provided convincing evidence for the validity of the Genant semi-quantitative method in the young (111,114,121). Genant-defined VF in children (1–18 yr of age) were associated with clinical predictors including back pain, low and/or declining spine aBMD Z-scores, and GC exposure (111,114,121). Moderate and severe VF were the strongest predictors of incident VF, although mild VF also independently predicted incident VF. Prevalent Genant-defined VF further predicted incident non-VF (135).
Are There Technical and Biological Factors that Limit the Accuracy of DXA-Based VFA in Children?
Cooperation is required to minimize motion artifact. Children under 5 yr and those with developmental delay may pose challenges in image acquisition. Likewise, positioning can be challenging in those with neuromuscular disorders. These issues can be mitigated by a DXA technologist with experience in imaging these special groups. Obesity limits edge detection in all DXA methodology, but specific limitations to VFA evaluability have not been identified. Spinal implants and moderate to severe scoliosis also limit VFA, but in DXA-based techniques and lateral spine radiography, alike. The published literature does not suggest an effect of sex or pubertal stage on image quality. Technical factors limiting the accuracy of DXA-based VFA in children across different manufacturers and models have not been fully evaluated.
Future Directions
Further research is required to determine the full spectrum of diseases that would benefit from routine VFA for detection and monitoring of VF. Low and/or declining spine aBMD Z-scores, back pain, GC exposure, and increases in body mass index are independent predictors of VF to varying extents. However, the sensitivity and specificity of these variables, in isolation or in combination, in predicting VF among children with diseases at particular risk for VF (eg, GC-treated Duchenne muscular dystrophy, leukemia, rheumatic disorders, nephrotic syndrome, and motor disorders (111,113,121,136,137)) remains unexplored.
Children with VF have the potential to undergo vertebral body reshaping following transient GC exposure, provided there is sufficient residual linear growth to restore normal vertebral dimensions through the process of bone modeling (135). “Spontaneous” (medication-unassisted) vertebral body reshaping may obviate the need for therapy, in the absence of additional clinically significant indicators of bone fragility. The utility of VFA in assessing vertebral body reshaping following VF has not been explored.
The full impact of DXA make, model and operator experience on image quality requires further study, particularly in younger patients. Comparative studies assessing the validity of DXA VFA in younger vs older children, and in different osteoporotic conditions are warranted. Further assessment of the natural variability in vertebral height ratios at different ages and at different locations along the developing spine would also help facilitate validation and further implementation of automated vertebral morphometry into pediatric clinical practice.
Summary and Overall Future Directions
There are now sufficient data to support the technical validity of DXA assessment at the distal forearm, proximal femur, and LDF, and also for VFA in children. The ability to utilize these alternate DXA sites will afford clinicians greater flexibility when evaluating bone health in pediatric populations. This is particularly relevant when whole body or lumbar spine scans cannot be obtained in certain patients, or when the underlying condition necessitates evaluation of fracture risk or monitoring of specific skeletal site. Despite the growing experience with the use of DXA at these alternate sites, further studies are needed to better define which skeletal site(s) and regions of interest provide the most relevant information for a given patient group or clinical scenario. More robust reference data are needed for both technological platforms (Hologic and GE Lunar devices) to provide complete representation according to age, sex, and population ancestry. Additionally, in order to adequately monitor bone health in at-risk groups of children and adolescents, precision data are needed in samples representative of the patients for whom DXA scans are clinically utilized. As these knowledge gaps are addressed, improvements can be expected in earlier identification of risk for fracture, monitoring practices, and the clinical care of children and adolescents with a variety of medical conditions who are at risk for compromised bone accretion and low energy fractures.
Supplementary Material
Acknowledgments
DRW was supported by K23 DK114477 from the National Institutes of Health.
Footnotes
Supplementary Materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jocd.2019.07.002.
References
- 1.Bishop N, Braillon P, Burnham J, et al. 2008. Dual-energy X-ray aborptiometry assessment in children and adolescents with diseases that may affect the skeleton: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 11 (1):29–42. [DOI] [PubMed] [Google Scholar]
- 2.Crabtree NJ, Shaw NJ, Bishop NJ, et al. 2017. Amalgamated reference data for size-adjusted bone densitometry measurements in 3598 children and young adults-the ALPHABET Study. J Bone Miner Res 32(1):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kindler JM, Lappe JM, Gilsanz V, et al. 2019. Lumbar spine bone mineral apparent density in children: results from the bone mineral density in childhood study. J Clin Endocrinol Metab 104(4):1283–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crabtree NJ, Arabi A, Bachrach LK, et al. 2014. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom 17(2):225–242. [DOI] [PubMed] [Google Scholar]
- 5.Crabtree NJ, Hogler W, Cooper MS, et al. 2013. Diagnostic evaluation of bone densitometric size adjustment techniques in children with and without low trauma fractures. Osteoporos Int 24(7):2015–2024. [DOI] [PubMed] [Google Scholar]
- 6.Zemel BS, Leonard MB, Kelly A, et al. 2010. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab 95(3):1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shepherd JA, Schousboe JT, Broy SB, et al. 2015. Executive summary of the 2015 ISCD position development conference on advanced measures from DXA and QCT: fracture prediction beyond BMD. J Clin Densitom 18 (3):274–286. [DOI] [PubMed] [Google Scholar]
- 8.Cooper C, Dennison EM, Leufkens HG, et al. 2004. Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res 19 (12):1976–1981. [DOI] [PubMed] [Google Scholar]
- 9.Hedstrom EM, Svensson O, Bergstrom U, Michno P. 2010. Epidemiology of fractures in children and adolescents. Acta Orthop 81(1):148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arabi A, Nabulsi M, Maalouf J, et al. 2004. Bone mineral density by age, gender, pubertal stages, and socioeconomic status in healthy Lebanese children and adolescents. Bone 35(5):1169–1179. [DOI] [PubMed] [Google Scholar]
- 11.Tang SY, Shan PF, Xie H, et al. 2007. Bone mineral content and bone mineral density at lumbar spine and forearm in Chinese girls aged 6-18 years. J Endocrinol Invest 30 (3):205–209. [DOI] [PubMed] [Google Scholar]
- 12.Wu XP, Dai RC, Shan PF, et al. 2005. Establishment of BMD reference curves at different skeletal sites in women, using a Cartesian coordinate numeration system. Osteoporos Int 16(12):1655–1662. [DOI] [PubMed] [Google Scholar]
- 13.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. 2011. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab 96(10):3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karjalainen P, Alhava EM. 1977. Bone mineral content of the forearm in a healthy population. Acta Radiol Ther Phys Biol 16(2):199–208. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd JA, Wang L, Fan B, et al. 2011. Optimal monitoring time interval between DXA measures in children. J Bone Miner Res 26(11):2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maimoun L, Coste O, Philibert P, et al. 2013. Peripubertal female athletes in high-impact sports show improved bone mass acquisition and bone geometry. Metabolism 62 (8):1088–1098. [DOI] [PubMed] [Google Scholar]
- 17.Soderpalm AC, Magnusson P, Ahlander AC, et al. 2007. Low bone mineral density and decreased bone turnover in Duchenne muscular dystrophy. Neuromuscul Disord 17 (11–12):919–928. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari SL, Chevalley T, Bonjour JP, Rizzoli R. 2006. Childhood fractures are associated with decreased bone mass gain during puberty: an early marker of persistent bone fragility? J Bone Miner Res 21(4):501–507. [DOI] [PubMed] [Google Scholar]
- 19.Goulding A, Grant AM, Williams SM. 2005. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res 20 (12):2090–2096. [DOI] [PubMed] [Google Scholar]
- 20.Goulding A, Jones IE, Taylor RW, et al. 2000. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. J Bone Miner Res 15 (10):2011–2018. [DOI] [PubMed] [Google Scholar]
- 21.Jones G, Boon P. 2008. Which bone mass measures discriminate adolescents who have fractured from those who have not? Osteoporos Int 19(2):251–255. [DOI] [PubMed] [Google Scholar]
- 22.Jones IE, Taylor RW, Williams SM, et al. 2002. Four-year gain in bone mineral in girls with and without past forearm fractures: a DXA study. Dual energy X-ray absorptiometry. J Bone Miner Res 17(6):1065–1072. [DOI] [PubMed] [Google Scholar]
- 23.Kalkwarf HJ, Laor T, Bean JA. 2011. Fracture risk in children with a forearm injury is associated with volumetric bone density and cortical area (by peripheral QCT) and areal bone density (by DXA). Osteoporos Int 22 (2):607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wren TAL, Shepherd JA, Kalkwarf HJ, et al. 2012. Racial disparity in fracture risk between white and nonwhite children in the United States. J Pediatr 161(6):1035–1040 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goulding A, Jones IE, Taylor RW, et al. 2001. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study. J Pediatr 139(4):509–515. [DOI] [PubMed] [Google Scholar]
- 26.Chevalley T, Bonjour JP, Audet MC, et al. 2017. Fracture prospectively recorded from prepuberty to young adulthood: are they markers of peak bone mass and strength in males? J Bone Miner Res 32(9):1963–1969. [DOI] [PubMed] [Google Scholar]
- 27.Dowthwaite JN, Dunsmore KA, Gero NM, et al. 2015. Arm bone loading index predicts DXA musculoskeletal outcomes in two samples of post-menarcheal girls. J Musculoskelet Neuronal Interact 15(4):358–371. [PMC free article] [PubMed] [Google Scholar]
- 28.Dey S, Jahan A, Yadav TP, et al. 2014. Measurement of bone mineral density by dual energy X-ray absorptiometry in juvenile idiopathic arthritis. Indian J Pediatr 81(2):126–132. [DOI] [PubMed] [Google Scholar]
- 29.Cepollaro C, Gonnelli S, Bruni D, et al. 2001. Dual X-ray absorptiometry and bone ultrasonography in patients with Rett syndrome. Calcif Tissue Int 69(5):259–262. [DOI] [PubMed] [Google Scholar]
- 30.Faje AT, Karim L, Taylor A, et al. 2013. Adolescent girls with anorexia nervosa have impaired cortical and trabecular microarchitecture and lower estimated bone strength at the distal radius. J Clin Endocrinol Metab 98(5):1923–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shankar RK, Giri N, Lodish MB, et al. 2017. Bone mineral density in patients with inherited bone marrow failure syndromes. Pediatr Res 82(3):458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yilmaz K, Ozmen M, Bora Goksan S, Eskiyurt N. 2007. Bone mineral density in children with neurofibromatosis 1. Acta Paediatr 96(8):1220–1222. [DOI] [PubMed] [Google Scholar]
- 33.Tian C, Wong BL, Hornung L, et al. 2016. Bone health measures in glucocorticoid-treated ambulatory boys with Duchenne muscular dystrophy. Neuromuscul Disord 26 (11):760–767. [DOI] [PubMed] [Google Scholar]
- 34.Bachrach LK, Hastie T, Wang MC, et al. 1999. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clinic Endocrinol Metab 84(12):4702–4712. [DOI] [PubMed] [Google Scholar]
- 35.Cromer BA, Binkovitz L, Ziegler J, et al. 2004. Reference values for bone mineral density in 12- to 18-year-old girls categorized by weight, race, and age. Pediatr Radiol 34 (10):787–792. [DOI] [PubMed] [Google Scholar]
- 36.Faulkner RA, Bailey DA, Drinkwater DT. 1996. Bone densitometry in Canadian children 8-17 years of Age. Calcif Tissue Int 59(5):344–351. [DOI] [PubMed] [Google Scholar]
- 37.Liao E-Y, Wu X-P, Luo X-H, et al. 2003. Establishment and evaluation of bone mineral density reference databases appropriate for diagnosis and evaluation of osteoporosis in Chinese women. J Bone Miner Metab 21(3):184–192. [DOI] [PubMed] [Google Scholar]
- 38.Lynn HS, Lau EM, Au B, Leung PC. 2005. Bone mineral density reference norms for Hong Kong Chinese. Osteoporos Int 16(12):1663–1668. [DOI] [PubMed] [Google Scholar]
- 39.Tamayo J, Diaz R, Lazcano-Ponce E, et al. 2009. Reference values for areal bone mineral density among a healthy Mexican population. Salud Publica de Mexico 51(Suppl 1): S56–S83. [DOI] [PubMed] [Google Scholar]
- 40.Ward KA, Ashby RL, Roberts SA, et al. 2007. UK reference data for the Hologic QDR Discovery dual-energy x ray absorptiometry scanner in healthy children and young adults aged 6-17 years. Arch Dis Child 92(1):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webber CE, Beaumont LF, Morrison J, et al. 2007. Age-predicted values for lumbar spine, proximal femur, and whole-body bone mineral density: results from a population of normal children aged 3 to 18 years. Can Assoc Radiol J 58(1):37–45. [PubMed] [Google Scholar]
- 42.Wu XP, Hou YL, Zhang H, et al. 2008. Establishment of BMD reference databases for the diagnosis and evaluation of osteoporosis in central southern Chinese men. J Bone Miner Metab 26(6):586–594. [DOI] [PubMed] [Google Scholar]
- 43.Wu XP, Liao EY, Huang G, et al. 2003. A comparison study of the reference curves of bone mineral density at different skeletal sites in native Chinese, Japanese, and American Caucasian women. Calcif Tissue Int 73(2):122–132. [DOI] [PubMed] [Google Scholar]
- 44.Xiaoge D, Eryuan L, Xianping W, et al. 2000. Bone mineral density differences at the femoral neck and Ward’s traingle: a comparison study on the reference data between Chinese and Caucasian women. Calcif Tissue Int 67(3):195–198. [DOI] [PubMed] [Google Scholar]
- 45.Zanchetta JR, Plotkin H, Alvarez Filgueira ML. 1995. Bone mass in children: normative values for the 2-20-year-old population. Bone 16(4 Suppl):393S–399S. [DOI] [PubMed] [Google Scholar]
- 46.Adams J, Mughal Z, Roberts S, Ward K. 2012. The manchester children’s bone densitometry (hologic DXA) reference database: an update. Osteoporos Int 5:S554. [Google Scholar]
- 47.Baxter-Jones ADG, Burrows M, Bachrach LK, et al. 2010. International longitudinal pediatric reference standards for bone mineral content. Bone 46(1):208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallo S, Vanstone CA, Weiler HA. 2012. Normative data for bone mass in healthy term infants from birth to 1 year of age. J Osteoporos 2012:672403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeddi M, Roosta MJ, Dabbaghmanesh MH, et al. 2013. Normative data and percentile curves of bone mineral density in healthy Iranian children aged 9-18 years. Arch Osteoporos 8:114. [DOI] [PubMed] [Google Scholar]
- 50.Kalkwarf HJ, Zemel BS, Yolton K, et al. 2013. Bone mineral content and density of the lumbar spine of infants and toddlers: influence of age, sex, race, growth, and human milk feeding. J Bone Miner Res 28(1):206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang MJ, Hong HS, Chung SJ, et al. 2016. Body composition and bone density reference data for Korean children, adolescents, and young adults according to age and sex: results of the 2009-2010 Korean National Health and Nutrition Examination Survey (KNHANES). J Bone Miner Metab 34(4):429–439. [DOI] [PubMed] [Google Scholar]
- 52.Khadilkar AV, Sanwalka NJ, Chiplonkar SA, et al. 2011. Normative data and percentile curves for Dual Energy X-ray Absorptiometry in healthy Indian girls and boys aged 5-17 years. Bone 48(4):810–819. [DOI] [PubMed] [Google Scholar]
- 53.Kim SH, Park MJ, Kim DH. 2014. Age-and gender-specific reference values of bone mineral density in Korean adolescents and young adults. Horm Res Paediatr 1:194. [Google Scholar]
- 54.Yi KH, Hwang JS, Kim EY, et al. 2014. Reference values for bone mineral density according to age with body size adjustment in Korean children and adolescents. J Bone Miner Metab 32(3):281–289. [DOI] [PubMed] [Google Scholar]
- 55.Christoffersen T, Emaus N, Dennison E, et al. 2018. The association between childhood fractures and adolescence bone outcomes: a population-based study, the Tromso Study, Fit Futures. Osteoporos Int 29:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goulding A, Cannan R, Williams SM, et al. 1998. Bone mineral density in girls with forearm fractures. J Bone Miner Res 13(1):143–148. [DOI] [PubMed] [Google Scholar]
- 57.Jones G, Ma D, Cameron F. 2006. Bone density interpretation and relevance in Caucasian children aged 9-17 years of age: insights from a population-based fracture study. J Clinic Densitom 9(2):202–209. [DOI] [PubMed] [Google Scholar]
- 58.Farr JN, Tomas R, Chen Z, et al. 2011. Lower trabecular volumetric BMD at metaphyseal regions of weight-bearing bones is associated with prior fracture in young girls. J Bone Miner Res 26(2):380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suuriniemi M, Mahonen A, Kovanen V, et al. 2003. Relation of PvuII site polymorphism in the COL1A2 gene to the risk of fractures in prepubertal Finnish girls. Physiol Genomics 14(3):217–224. [DOI] [PubMed] [Google Scholar]
- 60.Grgic O, Trajanoska K, Heppe D, et al. 2018. Structural geometry of bones is prominently associated with risk of fracture in children. Calcif Tissue Int 102(1 Suppl 1):S23. [Google Scholar]
- 61.Chevalley T, Bonjour JP, van Rietbergen B, et al. 2011. Fractures during childhood and adolescence in healthy boys: relation with bone mass, microstructure, and strength. J Clin Endocrinol Metab 96(10):3134–3142. [DOI] [PubMed] [Google Scholar]
- 62.Flynn J, Foley S, Jones G. 2007. Can BMD assessed by DXA at age 8 predict fracture risk in boys and girls during puberty?: an eight-year prospective study. J Bone Miner Res 22(9):1463–1467. [DOI] [PubMed] [Google Scholar]
- 63.Ekhlaspour L, Baskaran C, Campoverde KJ, et al. 2016. Bone density in adolescents and young adults with autism spectrum disorders. J Autism Dev Disord 46(11):3387–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neumeyer AM, Cano Sokoloff N, McDonnell EI, et al. 2018. Nutrition and bone density in boys with Autism spectrum disorder. J Acad Nutr Diet 118(5):865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neumeyer AM, Gates A, Ferrone C, et al. 2013. Bone density in peripubertal boys with autism spectrum disorders. J Autism Dev Disord 43(7):1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neumeyer AM, O’Rourke JA, Massa A, et al. 2015. Brief report: bone fractures in children and adults with autism spectrum disorders. J Autism Dev Disord 45(3):881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furlano RI, Bloechliger M, Jick H, Meier CR. 2014. Bone fractures in children with autistic spectrum disorder. J Dev Behav Pediatr 35(6):353–359. [DOI] [PubMed] [Google Scholar]
- 68.Larson CM, Henderson RC. 2000. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Pediatr Orthop 20(1):71–74. [PubMed] [Google Scholar]
- 69.Davie MW, Haddaway MJ. 1994. Bone mineral content and density in healthy subjects and in osteogenesis imperfecta. Arch Dis Child 70(4):331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mostoufi-Moab S, Kelly A, Mitchell JA, et al. 2018. Changes in pediatric DXA measures of musculoskeletal outcomes and correlation with quantitative CT following treatment of acute lymphoblastic leukemia. Bone 112: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Faje AT, Fazeli PK, Miller KK, et al. 2014. Fracture risk and areal bone mineral density in adolescent females with anorexia nervosa. Int J Eat Disord 47(5):458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kandemir N, Slattery M, Ackerman KE, et al. 2018. Bone parameters in anorexia nervosa and athletic amenorrhea: comparison of two hypothalamic amenorrhea States. J Clinic Endocrinol Metab 103(6):2392–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ackerman KE, Cano Sokoloff N, De Nardo Maffazioli G, et al. 2015. Fractures in relation to menstrual status and bone parameters in young athletes. Med Sci Sports Exerc 47(8):1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pepe J, Zawadynski S, Herrmann FR, et al. 2017. Structural basis of bone fragility in young subjects with inflammatory bowel disease: a high-resolution pQCT study of the SWISS IBD Cohort (SIBDC). Inflamm Bowel Dis 23(8):1410–1417. [DOI] [PubMed] [Google Scholar]
- 75.Landin-Wilhelmsen K, Bryman I, Windh M, Wilhelmsen L. 1999. Osteoporosis and fractures in Turner syndrome-importance of growth promoting and oestrogen therapy. Clinic Endocrinol 51(4):497–502. [DOI] [PubMed] [Google Scholar]
- 76.Mayranpaa MK, Tamminen IS, Kroger H, Makitie O. 2011. Bone biopsy findings and correlation with clinical, radiological, and biochemical parameters in children with fractures. J Bone Miner Res 26(8):1748–1758. [DOI] [PubMed] [Google Scholar]
- 77.Wong P, Fuller PJ, Gillespie MT. 2013. Thalassemia bone disease: the association between nephrolithiasis, bone mineral density and fractures. Osteoporos Int 24(7):1965–1971. [DOI] [PubMed] [Google Scholar]
- 78.Bilariki K, Anagnostou E, Masse V, et al. 2010. Low bone mineral density and high incidences of fractures and vitamin D deficiency in 52 pediatric cancer survivors. Horm Res Paediatr 74(5):319–327. [DOI] [PubMed] [Google Scholar]
- 79.Harcke HT, Taylor A, Bachrach S, et al. 1998. Lateral femoral scan: an alternative method for assessing bone mineral density in children with cerebral palsy. Pediatr Radiol 28(4):241–246. [DOI] [PubMed] [Google Scholar]
- 80.Henderson RC, Berglund LM, May R, et al. 2010. The relationship between fractures and DXA measures of BMD in the distal femur of children and adolescents with cerebral palsy or muscular dystrophy. J Bone Miner Res 25(3):520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Downs J, Bebbington A, Woodhead H, et al. 2008. Early determinants of fractures in Rett syndrome. Pediatrics 121 (3):540–546. [DOI] [PubMed] [Google Scholar]
- 82.Henderson RC, Lark RK, Kecskemethy HH, et al. 2002. Bisphosphonates to treat osteopenia in children with quadriplegic cerebral palsy: a randomized, placebo-controlled clinical trial. J Pediatr 141(5):644–651. [DOI] [PubMed] [Google Scholar]
- 83.Joseph S, Wang C, Di Marco M, et al. 2019. Fractures and bone health monitoring in boys with Duchenne muscular dystrophy managed within the Scottish Muscle Network. Neuromuscul Disord 29(1):59–66. [DOI] [PubMed] [Google Scholar]
- 84.Khoury DJ, Szalay EA. 2007. Bone mineral density correlation with fractures in nonambulatory pediatric patients. J Pediatr Orthop 27(5):562–566. [DOI] [PubMed] [Google Scholar]
- 85.Presedo A, Dabney KW, Miller F. 2007. Fractures in patients with cerebral palsy. J Pediatr Orthop 27(2): 147–153. [DOI] [PubMed] [Google Scholar]
- 86.Wasserman HM, Hornung LN, Stenger PJ, et al. 2017. Low bone mineral density and fractures are highly prevalent in pediatric patients with spinal muscular atrophy regardless of disease severity. Neuromuscul Disord 27(4):331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Afzal SY, Wender AR, Jones MD, et al. 2014. The effect of low magnitude mechanical stimulation (LMMS) on bone density in patients with Rett syndrome: a pilot and feasibility study. J Pediatr Rehabil Med 7(2):167–178. [DOI] [PubMed] [Google Scholar]
- 88.Chen CL, Ke JY, Wang CJ, et al. 2011. Factors associated with bone density in different skeletal regions in children with cerebral palsy of various motor severities. Dev Med Child Neurol 53(2):131–136. [DOI] [PubMed] [Google Scholar]
- 89.Finbraten AK, Syversen U, Skranes J, et al. 2015. Bone mineral density and vitamin D status in ambulatory and non-ambulatory children with cerebral palsy. Osteoporos Int 26(1):141–150. [DOI] [PubMed] [Google Scholar]
- 90.Henderson RC, Kairalla JA, Barrington JW, et al. 2005. Longitudinal changes in bone density in children and adolescents with moderate to severe cerebral palsy. J Pediatr 146(6):769–775. [DOI] [PubMed] [Google Scholar]
- 91.Henderson RC, Lark RK, Gurka MJ, et al. 2002. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics 110(1 Pt 1):e5. [DOI] [PubMed] [Google Scholar]
- 92.Szalay EA, Harriman D. 2006. Adapting pediatric DXA scanning to clinical orthopaedics. J Pediatr Orthop 26 (5):686–690. [DOI] [PubMed] [Google Scholar]
- 93.Szalay EA, Harriman D, Eastlund B, Mercer D. 2008. Quantifying postoperative bone loss in children. J Pediatr Orthop 28(3):320–323. [DOI] [PubMed] [Google Scholar]
- 94.Grissom LE, Kecskemethy HH, Bachrach SJ, et al. 2005. Bone densitometry in pediatric patients treated with pamidronate. Pediatr Radiol 35(5):511–517. [DOI] [PubMed] [Google Scholar]
- 95.Hobby BD, Dominguez-Bartmess S, Szalay EA. 2013. Prevention of postoperative osteopenia using IV pamidronate: a Pilot Study. J Pediatr Orthop 33(7):763–767. [DOI] [PubMed] [Google Scholar]
- 96.Henderson RC, Lark RK, Newman JE, et al. 2002. Pediatric reference data for dual X-ray absorptiometric measures of normal bone density in the distal femur. AJR Am J Roentgenol 178(2):439–443. [DOI] [PubMed] [Google Scholar]
- 97.Zemel BS, Stallings VA, Leonard MB, et al. 2009. Revised pediatric reference data for the lateral distal femur measured by Hologic Discovery/Delphi dual-energy X-ray absorptiometry. J Clin Densitom 12(2):207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bachrach SJ, Kecskemethy HH, Harcke HT, et al. 2006. Pamidronate treatment and posttreatment bone density in children with spastic quadriplegic cerebral palsy. J Clin Densitom 9(2):167–174. [DOI] [PubMed] [Google Scholar]
- 99.Grossberg R, Blackford MG, Kecskemethy HH, et al. 2015. Longitudinal assessment of bone growth and development in a facility-based population of young adults with cerebral palsy. Dev Med child Neurol 57(11):1064–1069. [DOI] [PubMed] [Google Scholar]
- 100.Henderson RC, Henderson BA, Kecskemethy HH, et al. 2015. Adaptation of the lateral distal femur DXA scan technique to adults with disabilities. J Clin Densitom 18 (1):102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Henderson RC, Kairalla J, Abbas A, Stevenson RD. 2004. Predicting low bone density in children and young adults with quadriplegic cerebral palsy. Dev Med Child Neurol 46(6):416–419. [DOI] [PubMed] [Google Scholar]
- 102.Mueske NM, Chan LS, Wren TA. 2014. Reliability of lateral distal femur dual-energy X-ray absorptiometry measures. J Clin Densitom 17(4):522–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gordon CM, Bachrach LK, Carpenter TO, et al. 2008. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 11(1):43–58. [DOI] [PubMed] [Google Scholar]
- 104.Szalay EA, Cheema A. 2011. Children with spina bifida are at risk for low bone density. Clin Orthop Relat Res 469 (5):1253–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pettersson U, Nordstrom P, Alfredson H, et al. 2000. Effect of high impact activity on bone mass and size in adolescent females: a comparative study between two different types of sports. Calcif Tissue Int 67(3):207–214. [DOI] [PubMed] [Google Scholar]
- 106.Lauer R, Johnston TE, Smith BT, Mulcahey MJ, Betz RR, Maurer AH. 2007. Bone mineral density of the hip and knee in children with spinal cord injury. J Spinal Cord Med 30(Suppl 1):S10–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nordstrom P, Pettersson U, Lorentzon R. 1998. Type of physical activity, muscle strength, and pubertal stage as determinants of bone mineral density and bone area in adolescent boys. J Bone Miner Res 13(7):1141–1148. [DOI] [PubMed] [Google Scholar]
- 108.Kecskemethy HH, Kubaski F, Harcke HT, Tomatsu S. 2016. Bone mineral density in MPS IV A (Morquio syndrome type A). Mol Genet Metab 117(2):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kubaski F, Kecskemethy HH, Harcke HT, Tomatsu S. 2016. Bone mineral density in mucopolysaccharidosis IVB. Mol Genet Metab Rep 8:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ben Amor IM, Roughley P, Glorieux FH, Rauch F. 2013. Skeletal clinical characteristics of osteogenesis imperfecta caused by haploinsufficiency mutations in COL1A1. J Bone Miner Res 28(9):2001–2007. [DOI] [PubMed] [Google Scholar]
- 111.Cummings EA, Ma J, Fernandez CV, et al. 2015. Incident vertebral fractures in children with leukemia during the four years following diagnosis. J Clin Endocrinol Metab 100(9):3408–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ma J, McMillan HJ, Karaguzel G, et al. 2017. The time to and determinants of first fractures in boys with Duchenne muscular dystrophy. Osteoporos Int 28(2):597–608. [DOI] [PubMed] [Google Scholar]
- 113.Singh A, Schaeffer EK, Reilly CW. 2018. Vertebral fractures in Duchenne muscular dystrophy patients managed with deflazacort. J Pediatr Orthop 38(6):320–324. [DOI] [PubMed] [Google Scholar]
- 114.Alos N, Grant RM, Ramsay T, et al. 2012. High incidence of vertebral fractures in children with acute lymphoblastic leukemia 12 months after the initiation of therapy. J Clin Oncol 30(22):2760–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ma J, Siminoski K, Alos N, et al. 2015. The choice of normative pediatric reference database changes spine bone mineral density Z-scores but not the relationship between bone mineral density and prevalent vertebral fractures. J Clin Endocrinol Metab 100(3):1018–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kocks J, Ward K, Mughal Z, et al. 2010. Z-score comparability of bone mineral density reference databases for children. J Clinic Endocrinol Metab 95(10):4652–4659. [DOI] [PubMed] [Google Scholar]
- 117.Leonard MB, Propert KJ, Zemel BS, et al. 1999. Discrepancies in pediatric bone mineral density reference data: potential for misdiagnosis of osteopenia. J Pediatr 135(2 Pt 1):182–188. [DOI] [PubMed] [Google Scholar]
- 118.Bishop N, Arundel P, Clark E, et al. 2014. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 pediatric official positions. J Clin Densitom 17(2):275–280. [DOI] [PubMed] [Google Scholar]
- 119.Fink HA, Milavetz DL, Palermo L, et al. 2005. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res 20 (7):1216–1222. [DOI] [PubMed] [Google Scholar]
- 120.Halton J, Gaboury I, Grant R, et al. 2009. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res 24(7):1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.LeBlanc CM, Ma J, Taljaard M, et al. 2015. Incident vertebral fractures and risk factors in the first three years following glucocorticoid initiation among pediatric patients with rheumatic disorders. J Bone Miner Res 30(9):1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rodd C, Lang B, Ramsay T, et al. 2012. Incident vertebral fractures among children with rheumatic disorders 12 months after glucocorticoid initiation: a national observational study. Arthritis Care Res 64(1):122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Adiotomre E, Summers L, Allison A, et al. 2017. Diagnostic accuracy of DXA compared to conventional spine radiographs for the detection of vertebral fractures in children. Eur Radiol 27(5):2188–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Crabtree NJ, Chapman S, Hogler W, et al. 2017. Vertebral fractures assessment in children: evaluation of DXA imaging versus conventional spine radiography. Bone 97:168–174. [DOI] [PubMed] [Google Scholar]
- 125.Diacinti D, Pisani D, D’Avanzo M, et al. 2015. Reliability of vertebral fractures assessment (VFA) in children with osteogenesis imperfecta. Calcif Tissue Int 96(4):307–312. [DOI] [PubMed] [Google Scholar]
- 126.Hoyer-Kuhn H, Knoop K, Semler O, et al. 2016. Comparison of DXA scans and conventional X-rays for spine morphometry and bone age determination in children. J Clin Densitom 19(2):208–215. [DOI] [PubMed] [Google Scholar]
- 127.Kyriakou A, Shepherd S, Mason A, et al. 2015. A critical appraisal of vertebral fracture assessment in paediatrics. Bone 81:255–259. [DOI] [PubMed] [Google Scholar]
- 128.Mayranpaa MK, Helenius I, Valta H, et al. 2007. Bone densitometry in the diagnosis of vertebral fractures in children: accuracy of vertebral fracture assessment. Bone 41(3):353–359. [DOI] [PubMed] [Google Scholar]
- 129.Jaremko JL, Siminoski K, Firth GB, et al. 2015. Common normal variants of pediatric vertebral development that mimic fractures: a pictorial review from a national longitudinal bone health study. Pediatr Radiol 45(4):593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Siminoski K, Lee KC, Jen H, et al. 2012. Anatomical distribution of vertebral fractures: comparison of pediatric and adult spines. Osteoporos Int 23(7):1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schousboe JT, Debold CR. 2006. Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos Int 17(2):281–289. [DOI] [PubMed] [Google Scholar]
- 132.Siminoski K, Lentle B, Matzinger MA, et al. 2014. Observer agreement in pediatric semiquantitative vertebral fracture diagnosis. Pediatr Radiol 44(4):457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Genant HK, Wu CY, van Kuijk C, Nevitt MC. 1993. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8(9):1137–1148. [DOI] [PubMed] [Google Scholar]
- 134.Rosen HN, Vokes TJ, Malabanan AO, et al. 2013. The official positions of the International Society for clinical densitometry: vertebral fracture assessment. J Clin Densitom 16 (4):482–488. [DOI] [PubMed] [Google Scholar]
- 135.Ward LM, Ma J, Lang B, et al. 2018. Bone morbidity and recovery in children with acute lymphoblastic leukemia: results of a six-year prospective Cohort Study. J Bone Miner Res 33(8):1435–1443. [DOI] [PubMed] [Google Scholar]
- 136.Kilpinen-Loisa P, Paasio T, Soiva M, et al. 2010. Low bone mass in patients with motor disability: prevalence and risk factors in 59 Finnish children. Dev Med Child Neurol 52 (3):276–282. [DOI] [PubMed] [Google Scholar]
- 137.Phan V, Blydt-Hansen T, Feber J, et al. 2014. Skeletal findings in the first 12 months following initiation of glucocorticoid therapy for pediatric nephrotic syndrome. Osteoporos Int 25(2):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.