Abstract
BACKGROUND:
The aim of this study is to investigate the diagnostic and prognostic value of neutrophil CD64 (nCD64) as a novel biomarker in sepsis patients.
METHODS:
One hundred fifty-one adult patients diagnosed with sepsis and 20 age-matched healthy controls were enrolled in the study. Patients with sepsis were further subdivided into a sepsis group and a septic shock group. nCD64 expression, serum procalcitonin (PCT) level, C-reactive protein (CRP) level, and white blood cell (WBC) count were obtained for each patient, and Sequential Organ Failure Assessment (SOFA) scores were calculated.
RESULTS:
nCD64 expression was higher in the sepsis group with confirmed infection than in the control group. The receiver operating characteristic (ROC) curve of nCD64 was higher than those of SOFA score, PCT, CRP and WBC for diagnosing infection. The area under the curve (AUC) of nCD64 combined with SOFA score was the highest for all parameters. The AUC of nCD64 for predicting 28-day mortality in sepsis was significantly higher than those of PCT, CRP, and WBC, but slightly lower than that of SOFA score. The AUC of nCD64 or PCT combined with SOFA score was significantly higher than that of any single parameter for predicting 28-day mortality.
CONCLUSION:
nCD64 expression and SOFA score are valuable parameters for early diagnosis of infection and prognostic evaluation of sepsis patients.
KEY WORDS: Neutrophil CD64, Sepsis, Sequential Organ Failure Assessment score, Procalcitonin, Prognosis, Biomarker
INTRODUCTION
Sepsis is the most common cause of death in critically ill patients and accounts for a large health care burden. Patients who survive from sepsis have a high risk of hospital readmission.[1] However, early diagnosis facilitates immediate and appropriate antibiotic administration, greatly reducing its associated morbidity and mortality.[2,3] Although the Sepsis 3.0 criteria have simplified sepsis recognition using the Sequential Organ Failure Assessment (SOFA) or quick SOFA (qSOFA) score,[4] confirmation of infection still relies on microbiological culture results. Microbiological culture, the gold standard for distinguishing sepsis from noninfectious conditions, requires 24 to 48 hours to complete.[5] Thus, there is an urgent need for a novel biomarker to diagnose bacterial infection more rapidly, which may help to optimize patient prognosis.
Unfortunately, no ideal biomarker has yet been identified. While C-reactive protein (CRP) and procalcitonin (PCT) are highly useful in diagnosing infection, these are over-induced in both inflammatory and infectious diseases and lack sensitivity for differentiating between the two conditions.[6] In recent years, neutrophil CD64 (nCD64), or high-affinity Fcγ receptor I, which is expressed at very low levels in resting neutrophils but is upregulated by inflammatory cytokines during infection,[7] has been proposed as a potential diagnostic and prognostic biomarker for sepsis in hospitalized adults, neonates and children,[8-11] and for distinguishing between bacterial and other types of infection.[12] nCD64 expression has been reported to have higher sensitivity and specificity than PCT and CRP in detecting infection.[13]
Most clinical studies demonstrating that nCD64 level is significantly upregulated in patients with sepsis and is positively correlated with disease severity are constrained by relatively small sample sizes and non-utilization of Sepsis 3.0 criteria, which greatly limit their clinical value.[13] In this investigation, we used a single-center, prospective clinical study design to explore the diagnostic and prognostic significance of nCD64 in sepsis patients.
METHODS
Subject data collection
This study was conducted in the emergency department (ED) of Beijing Chao-Yang Hospital, Capital Medical University, a tertiary teaching hospital with approximately 250,000 ED admissions per year. The study was approved by the Institutional Review Board and Medical Ethics Committee (2013 department 124). Written informed consent was obtained from all enrolled participants. Adult patients who met the Sepsis 3.0 criteria and were admitted in our hospital from September 2016 to September 2017 were enrolled. Sepsis was defined as life-threatening organ dysfunction caused by a host response to infection. For operationalization, organ dysfunction was represented by an increase in the Sequential Organ Failure Assessment (SOFA) score of 2 points or more.[4] A total of 20 age-matched adults who underwent a physical examination at the same hospital were enrolled as healthy controls.
The following patients were excluded: (i) patients <18 years old; (ii) patients with metastatic tumor, acquired immunodeficiency syndrome, uremia, late-stage liver cirrhosis, active tuberculosis, refractory heart failure, previous transplantation, immunosuppressive therapy, and pregnancy; (iii) patients who refused to participate in this study; and (iv) patients under hospice care or those with a Do-Not-Resuscitate request.
Analysis of laboratory biomarkers
Demographic, clinical (i.e., blood pressure, respiratory rate, and pulse rate) and laboratory (i.e., white blood cell [WBC] count, PCT level and CRP level) information from all healthy controls and enrolled patients were recorded on admission. The presence of infection was presumptively made based on clinical features, laboratory findings and imaging tests recommended by the International Sepsis Forum Consensus Conference on Definitions of Infection.[14] To confirm bacterial infection, samples of blood, urine, sputum or bronchoalveolar lavage fluid were collected for bacteriological culture. Individual SOFA scores were calculated. All patients were followed up for up to 28 days, with the primary endpoint being 28-day mortality.
PCT levels were analyzed by serum electrochemiluminescent immunoassay using the kit and the mini-VIDAS® system (Biomerieux SA, France). Residual blood (100 μL) from routine blood samples was used to determine nCD64 levels (CD45-PC5 and CD64-FITC; Beckman Coulter, Miami, FL, USA), expressed as median fluorescence intensity (MFI), using flow cytometry (FC500; Beckman Coulter) within 24 hours of admission.
Statistical analysis
All analyses were performed using SPSS 22.0 statistical software (SPSS Inc, Chicago, IL, USA). Skewed distribution for nCD64 MFI, PCT, CRP, and SOFA were expressed as the median (25th to 75th percentile). The Mann–Whitney U-test was used for two-group comparisons. A P-value of less than 0.05 was considered statistically significant in all analyses. Receiver operating characteristic (ROC) analyses were performed and areas under the curve (AUC) was calculated using MedCalc 15.0 Software (Acacialaan, Ostend, Belgium) for diagnostic power. A z-test was used to compare AUCs between different curves. Binary logistic regression was performed to analyze the risk factors of 28-day mortality. A Kaplan–Meier survival curve was constructed for nCD64.
RESULTS
Clinical features of patients and controls
A total of 151 patients and 20 healthy controls were enrolled in this study. The patients were further subdivided into a sepsis group and a septic shock group based on the Sepsis 3.0 criteria. No significant difference was found in age or sex ratio between the control and patient (sepsis and septic shock) groups. Baseline characteristics, comorbidity data, related infection sites, and culture results are displayed in Table 1.
Table 1.
Baseline characteristics of patients based on severity of sepsis
Comparison of nCD64, PCT, CRP, WBC, and SOFA score
The nCD64, PCT, CRP and WBC levels of each group are shown in Figure 1. The nCD64, PCT, and CRP levels and SOFA score of the patient groups were significantly higher compared with the control group (P<0.05). The septic shock group had the highest values for these parameters. Significant differences between the sepsis and septic shock groups were noted in all parameters except for CRP and WBC values (Table 1).
Figure 1.
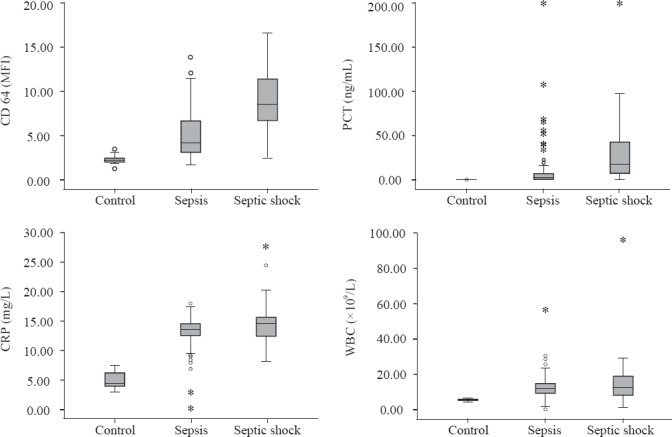
The nCD64, PCT, CRP and WBC levels in each group. The nCD64, PCT, CRP and SOFA score of the patient groups were significantly higher compared with the control (P<0.05). The septic shock group had the highest level of these parameters.
Evaluation of nCD64, PCT, CRP, WBC levels and SOFA score in diagnosing bacterial infection
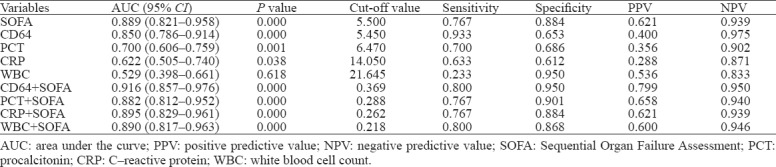
The statistical values of ROC curves for nCD64, PCT, CRP, WBC and SOFA score in differentiating a positive microbial culture from sepsis are shown in Table 2. nCD64 produced the highest AUC (0.879), followed by PCT (0.868), SOFA (0.701), CRP (0.609) and WBC (0.525). The AUC of nCD64 combined with SOFA was higher (0.888) than that of any other parameter alone or in combination.
Table 2.
Analysis of ROC curves in diagnosing positive infection culture in patients with sepsis
Comparison between sepsis patient survivors and non-survivors at the 28-day follow-up
Baseline characteristics are shown in Table 3. Among the patients enrolled, 121 survived and 30 died at the 28-day follow-up. The mortality rate was 19.87% (30/151). The SOFA score and nCD64 and PCT levels were higher in non-survivors. There were significant differences in SOFA score, nCD64, PCT and CRP, but no significant difference in WBC count, between survivors and non-survivors.
Table 3.
Baseline characteristics of patients based on outcome
Prognostic value of nCD64, PCT, CRP, WBC levels, and SOFA score
The ROC curves of nCD64, PCT, CRP, WBC and SOFA score for predicting death are shown in Figure 2. SOFA score had the highest AUC (0.889), followed by nCD64 (0.850), PCT (0.700), CRP (0.622) and WBC (0.529) (Table 4). There were significant differences between SOFA score and CRP or PCT (P<0.001), as well as nCD64 (P<0.001), but there were no significant differences between SOFA score and nCD64 (P=0.358), CRP and PCT (P=0.2637).
Figure 2.
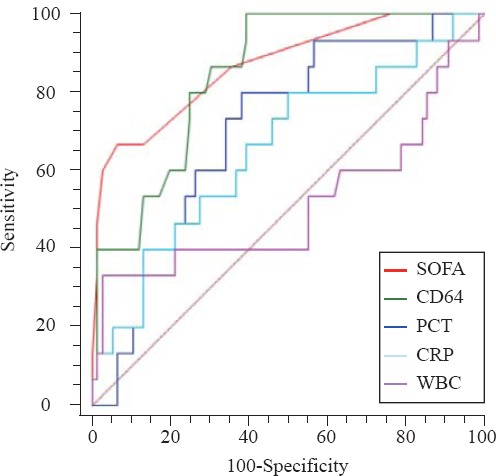
The ROC curves of nCD64, PCT, CRP, WBC and SOFA score for prognosis. The AUC of SOFA was the highest (0.889), followed by nCD64 (0.850), PCT (0.700), CRP (0.622) and WBC (0.529). There were significant differences between SOFA and CRP or PCT (P<0.001), same with the nCD64 (P<0.001), but there were no significant differences between SOFA and nCD64 (P=0.358), CRP and PCT (P=0.2637).
Table 4.
Analysis of ROC curves in predicting 28-day mortality in patients with sepsis
The combination of nCD64 and SOFA score achieved an AUC of 0.916, followed by the combination of PCT and SOFA (0.882; Table 4 and Figure 3). A significant difference in AUC was found between PCT+SOFA and PCT (P=0.0015), and between nCD64+SOFA and nCD64 (P=0.0160). There was no significant difference between nCD64+SOFA and PCT+SOFA (P=0.2028), nCD64+SOFA and SOFA (P=0.2366), PCT+SOFA and SOFA (P=0.5201), PCT+SOFA and CD64 (P=0.4804).
Figure 3.
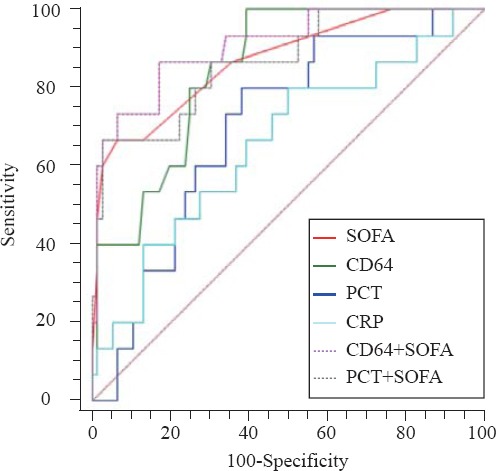
The ROC curves of combination. The combination of nCD64 and SOFA achieved an AUC of 0.916, followed by the combination of PCT and SOFA (0.882). A significant difference of AUC was found between PCT+SOFA and PCT (P=0.0015), nCD64+SOFA and nCD64 (P=0.0160). There was no significant difference between nCD64+SOFA and PCT+SOFA (P=0.2028), nCD64+SOFA and SOFA (P=0.2366), PCT+SOFA and SOFA (P=0.5201), PCT+SOFA and CD64 (P=0.4804).
Binary logistic regression analysis revealed that nCD64 and SOFA were independent risk factors of 28-day mortality in patients with sepsis (Table 5). A Kaplan–Meier survival analysis showed that patients with a higher nCD64 than the cut-off value of 5.45 at baseline had a lower chance of survival at 28 days (P<0.001; Figure 4).
Table 5.
Independent predictive variables analysis by multivariate logistic regression

Figure 4.
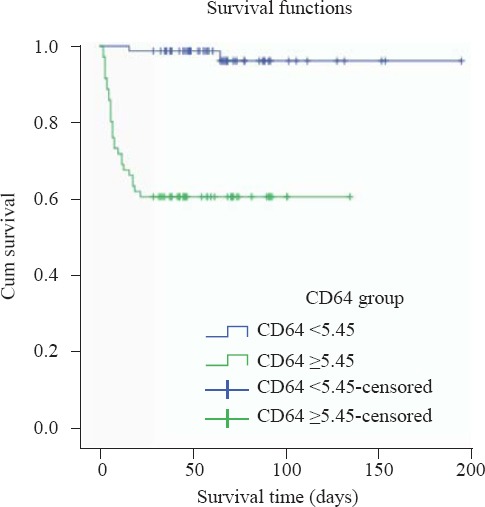
Kaplan–Meier survival curves. A Kaplan–Meier survival analysis showed that patients with a higher nCD64 than the cut-off value of 5.45 at baseline had a lower chance of survival at 28 days.
DISCUSSION
Timely and accurate diagnosis and proper risk stratification are extremely vital in reducing sepsis-related mortality in the ED.[15] Scoring systems have been widely used for risk stratification and prognostic assessment in sepsis patients. Sepsis 3.0 criteria confirmed the superiority of SOFA in diagnosing and predicting sepsis. Several large-scale clinical trials have validated the advantage of SOFA in predicting mortality with suspected infection.[16] In the present study, we investigated the SOFA score with other inflammatory markers such as CD64, PCT, and CRP in the assessment of sepsis and infection.
PCT and CRP are widely used as specific biomarkers for diagnosing bacterial infection or guiding antibiotic therapy in hospitalized patients, but are limited in their ability to distinguish sepsis from other inflammatory conditions.[17] PCT was reported to be a better biomarker than CRP for diagnosing sepsis.[18] However, its results may lead to incorrect antibiotic use in critically ill patients.[19]
Porges et al[20] reported that nCD64 is an early participant in Fc-dependent cell activation and the development of immune responses. Upregulation of nCD64 occurs within 4 to 6 hours after infection or inflammatory cytokine invasion, and dramatically decreases within 2 days after removal of the stimulus, returning to be normal in 7 days.[11,21] This suggests that nCD64 is a potential biomarker for early diagnosis of infection and sepsis. In recent years, nCD64 has been proposed as a promising potential biomarker for diagnosing sepsis in both pediatric and adult patients.[22-25] In our study, nCD64 in sepsis patients was significantly upregulated compared with the control group (5.1 vs. 2.2, P<0.001) and increased with the disease severity, which indicated that bacterial infection activated nCD64 expression in neutrophils. This strongly suggests the value of this biomarker in the differential diagnosis of sepsis. nCD64, PCT, and SOFA were also significantly higher in septic shock compared with sepsis. Similar to previous studies,[11,26,27] our findings indicate that nCD64, PCT and SOFA can be valuable biomarkers in estimating the severity of sepsis.
Because nCD64 is rapidly upregulated in the early stages of pathogen invasion, it may be particularly valuable in narrowing down the differential diagnosis in emergent situations. De Jong et al[28] demonstrated that nCD64 distinguished between critically ill patients with culture-positive or -negative sepsis and correlated with the disease severity. nCD64 combines high sensitivity and specificity in both adults and children. It also performs well in differentiating infection from flares in autoimmune inflammatory diseases.[29] Consistent with previous studies, our research demonstrated that nCD64 has a higher AUC compared with PCT, SOFA, CRP, and WBC in diagnosing a positive blood culture, suggesting that clinicians may be able to identify infectious sepsis using nCD64 before obtaining the pathogen culture results. nCD64 showed a higher specificity (0.75) compared with PCT, SOFA, CRP, and WBC in diagnosing infection. The sensitivity (0.89) of nCD64 was higher than SOFA, CRP, and WBC but slightly lower than PCT.
The potential role of nCD64 in predicting the outcome of sepsis has been widely investigated. Several studies found that high nCD64 expression during early sepsis might be associated with a better prognosis.[30,31] A recent study demonstrated that nCD64 has high diagnostic accuracy in recognizing ventilator-associated pneumonia (VAP). When accompanied by an identified pathogen, nCD64 can predict survival in ICU patients. nCD64 has also been shown to be a fairly good predictor of death in the ICU, in contrast to CRP, interleukin (IL)-6, and lipopolysaccharide-binding protein (LBP).[32] We found that there was also a significant difference in SOFA, nCD64, PCT and CRP values between survivors and non-survivors, which is consistent with previous studies.[8,33] The AUC of the ROC curve showed that SOFA score had the best predictive ability, followed by nCD64, PCT, CRP, and WBC. These results suggest a possible prognostic role for SOFA and nCD64 in predicting in-hospital mortality.
We conducted a univariate logistic regression analysis to explore the effect of each biomarker alone or in combination with nCD64 expression and SOFA score for predicting mortality. Among all parameters, only SOFA and nCD64 entered the logistic model as independent risk factors of 28-day mortality. A Kaplan–Meier curve using the cut-off value of 5.45 for nCD64 also showed that higher nCD64 expression might be associated with a shorter survival time.
Although the nCD64 index was specific for bacterial infection in ICU patients, it had a relatively low sensitivity and specificity for predicting death. Combining nCD64 with other high-specificity or -sensitivity parameters such as PCT can significantly improve its diagnostic accuracy.[34] This was demonstrated in a case-control study which employed a combination of CRP, PCT, and nCD64 in the diagnosis of sepsis patients with confirmed infection.[35] A previous study likewise showed that the combination of PCT and SOFA score can improve the performance of single predictors.[36] Measurement of nCD64 expression combined with CRP is also advisable when diagnosing sepsis.[37] The high performance of bioscore that combines nCD64 with PCT and sTREM-1 serum levels for diagnosing sepsis in critically ill patients in the ICU has also been confirmed.[38] We found that compared with single parameters, either a combination of nCD64 and SOFA or PCT and SOFA can improve the AUC for predicting 28-day mortality or diagnosing infection in sepsis. AUC was highest (0.888) when SOFA and nCD64 were combined, which was better than either single parameter for diagnosing infection. The combination of nCD64 and SOFA showed the highest AUC (0.916) compared with the combination of PCT and SOFA for predicting mortality. Thus, the combination of nCD64+SOFA may be a more promising biomarker for identifying infectious etiologies and for predicting mortality. All of these findings highlight the superiority of nCD64 compared with other parameters in diagnosing and predicting sepsis.
There are several limitations in our study. The relatively small sample size and the non-randomized single-center design with a short observational period may have resulted in selection bias for clinical data analysis. Further randomized, multicenter studies with larger sample size and long-term follow-up are needed to validate our results. In addition, we only detected relative nCD64 expression in neutrophils, which could vary between different flow cytometers. Quantitative flow cytometry may be needed to validate and improve our findings.
CONCLUSIONS
nCD64 expression, particularly combined with the SOFA score, is a valuable marker for early diagnosis of infection in sepsis, risk stratification and evaluation of prognosis in sepsis patients in the ED.
Footnotes
Funding: None.
Ethical approval: The study was approved by the Institutional Review Board and Medical Ethics Committee (2013 department 124).
Conflicts of interest: The authors have no conflict of interest to declare.
Contributors: WY proposed and wrote the paper. All authors read and approved the final version.
REFERENCES
- 1.Meyer N, Harhay MO, Small DS, Prescott HC, Bowles KH, Gaieski DF, et al. Temporal trends in incidence, sepsis-related mortality, and hospital-based acute care after sepsis. Crit Care Med. 2018;46(3):354–60. doi: 10.1097/CCM.0000000000002872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136(5):1237–48. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 4.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters RP, van Agtmael MA, Danner SA, Savelkoul PH, Vandenbroucke-Grauls CM. New developments in the diagnosis of blood stream infections. Lancet Infect Dis. 2004;4(12):751–60. doi: 10.1016/S1473-3099(04)01205-8. [DOI] [PubMed] [Google Scholar]
- 6.van Engelen TSR, Wiersinga WJ, Scicluna BP, van der Poll T. Biomarkers in sepsis. Crit Care Clin. 2018;34(1):139–52. doi: 10.1016/j.ccc.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Repp R, Valerius T, Sendler A, Gramatzki M, Iro H, Kalden JR, et al. Neutrophils express the high affinity receptor for IgG (Fc gamma RI, CD64) after in vivo application of recombinant human granulocyte colony-stimulating factor. Blood. 1991;78(4):885–9. [PubMed] [Google Scholar]
- 8.Davis BH, Olsen SH, Ahmad E, Bigelow NC. Neutrophil CD64 is an improved indicator of infection or sepsis in emergency department patients. Arch Pathol Lab Med. 2006;130(5):654–61. doi: 10.5858/2006-130-654-NCIAII. [DOI] [PubMed] [Google Scholar]
- 9.Pourmand A, Pyle M, Yamane D, Sumon K, Frasure SE. The utility of point-of-care ultrasound in the assessment of volume status in acute and critically ill patients. World J Emerg Med. 2019;10(4):232–8. doi: 10.5847/wjem.j.1920-8642.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates:neutrophil CD64 as a diagnostic marker. Pediatrics. 2008;121(1):129–34. doi: 10.1542/peds.2007-1308. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Li ZY, Zeng L, Zhang AQ, Pan W, Gu W, et al. Neutrophil CD64 expression as a diagnostic marker for sepsis in adult patients:a meta-analysis. Crit Care. 2015;19:245. doi: 10.1186/s13054-015-0972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gros A, Roussel M, Sauvadet E, Gacouin A, Marque S, Chimot L, et al. The sensitivity of neutrophil CD64 expression as a biomarker of bacterial infection is low in critically ill patients. Intensive Care Med. 2012;38(3):445–52. doi: 10.1007/s00134-012-2483-6. [DOI] [PubMed] [Google Scholar]
- 13.Yeh CF, Wu CC, Liu SH, Chen KF. Comparison of the accuracy of neutrophil CD64, procalcitonin, and C-reactive protein for sepsis identification:a systematic review and meta-analysis. Ann Intensive Care. 2019;9(1):5. doi: 10.1186/s13613-018-0479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers KA, Park AY, Banuelos RC, Darger BF, Akkanti BH, Macaluso A, et al. Outcomes of severe sepsis and septic shock patients after stratification by initial lactate value. World J Emerg Med. 2018;9(2):113–7. doi: 10.5847/wjem.j.1920-8642.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign:International Guidelines for Management of Sepsis and Septic Shock 2016. Intensive Care Med. 2017;43(3):304–77. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 16.Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, et al. Prognostic accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317(3):290–300. doi: 10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]
- 17.Au-Yong A. Towards evidence-based emergency medicine:best BETs from the Manchester Royal Infirmary BET 2:C-reactive protein in the diagnosis of bacteraemia. Emerg Med J. 2012;29(5):423–4. doi: 10.1136/emermed-2012-201302.3. [DOI] [PubMed] [Google Scholar]
- 18.Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med. 2003;31(6):1737–41. doi: 10.1097/01.CCM.0000063440.19188.ED. [DOI] [PubMed] [Google Scholar]
- 19.Koeze J, Hendrix MG, van den Bergh FA, Brouwer RM, Zijlstra JG. In critically ill patients the procalcitonin level can be misleading. Crit Care. 2011;15(2):422. doi: 10.1186/cc10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porges AJ, Redecha PB, Doebele R, Pan LC, Salmon JE, Kimberly RP. Novel Fc gamma receptor I family gene products in human mononuclear cells. J Clin Invest. 1992;90(5):2102–9. doi: 10.1172/JCI116094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen JM, Seed B. Isolation and expression of functional high-affinity Fc receptor complementary DNAs. Science. 1989;243(4889):378–81. doi: 10.1126/science.2911749. [DOI] [PubMed] [Google Scholar]
- 22.Yang AP, Liu J, Yue LH, Wang HQ, Yang WJ, Yang GH. Neutrophil CD64 combined with PCT, CRP and WBC improves the sensitivity for the early diagnosis of neonatal sepsis. Clin Chem Lab Med. 2016;54(2):345–51. doi: 10.1515/cclm-2015-0277. [DOI] [PubMed] [Google Scholar]
- 23.Kipfmueller F, Schneider J, Prusseit J, Dimitriou I, Zur B, Franz AR, et al. Role of neutrophil CD64 index as a screening marker for late-onset sepsis in very low birth weight infants. PLoS One. 2015;10(4):e0124634. doi: 10.1371/journal.pone.0124634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groselj-Grenc M, Ihan A, Pavcnik-Arnol M, Kopitar AN, Gmeiner-Stopar T, Derganc M. Neutrophil and monocyte CD64 indexes, lipopolysaccharide-binding protein, procalcitonin and C-reactive protein in sepsis of critically ill neonates and children. Intensive Care Med. 2009;35(11):1950–8. doi: 10.1007/s00134-009-1637-7. [DOI] [PubMed] [Google Scholar]
- 25.Rogina P, Stubljar D, Lejko-Zupanc T, Osredkar J, Skvarc M. Expression of CD64 on neutrophils (CD64 index):diagnostic accuracy of CD64 index to predict sepsis in critically ill patients. Clin Chem Lab Med. 2015;53(4):e89–91. doi: 10.1515/cclm-2014-0814. [DOI] [PubMed] [Google Scholar]
- 26.Cardelli P, Ferraironi M, Amodeo R, Tabacco F, De Blasi RA, Nicoletti M, et al. Evaluation of neutrophil CD64 expression and procalcitonin as useful markers in early diagnosis of sepsis. Int J Immunopathol Pharmacol. 2008;21(1):43–9. doi: 10.1177/039463200802100106. [DOI] [PubMed] [Google Scholar]
- 27.Ma Y, Yu XY, Wang Y. Dose-related effects of dexmedetomidine on immunomodulation and mortality to septic shock in rats. World J Emerg Med. 2018;9(1):56–63. doi: 10.5847/wjem.j.1920-8642.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jong E, de Lange DW, Beishuizen A, van de Ven PM, Girbes AR, Huisman A. Neutrophil CD64 expression as a longitudinal biomarker for severe disease and acute infection in critically ill patients. Int J Lab Hematol. 2016;38(5):576–84. doi: 10.1111/ijlh.12545. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann JJ. Neutrophil CD64:a diagnostic marker for infection and sepsis. Clin Chem Lab Med. 2009;47(8):903–16. doi: 10.1515/CCLM.2009.224. [DOI] [PubMed] [Google Scholar]
- 30.Bae MH, Park SH, Park CJ, Cho EJ, Lee BR, Kim YJ, et al. Flow cytometric measurement of respiratory burst activity and surface expression of neutrophils for septic patient prognosis. Cytometry B Clin Cytom. 2016;90(4):368–75. doi: 10.1002/cyto.b.21274. [DOI] [PubMed] [Google Scholar]
- 31.Danikas DD, Karakantza M, Theodorou GL, Sakellaropoulos GC, Gogos CA. Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis Correlation to CD64 and CD14 antigen expression. Clin Exp Immunol. 2008;154(1):87–97. doi: 10.1111/j.1365-2249.2008.03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Djordjevic D, Pejovic J, Surbatovic M, Jevdjic J, Radakovic S, Veljovic M, et al. Prognostic value and daily trend of interleukin-6, neutrophil CD64 expression, c-reactive protein and lipopolysaccharide-binding protein in critically ill patients:reliable predictors of outcome or not? J Med Biochem. 2015;34(4):431–9. doi: 10.1515/jomb-2015-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu KH, Chan MC, Wang JM, Lin LY, Wu CL. Comparison of Fcgamma receptor expression on neutrophils with procalcitonin for the diagnosis of sepsis in critically ill patients. Respirology. 2011;16(1):152–60. doi: 10.1111/j.1440-1843.2010.01876.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Hou JH, Li Q, Chen KJ, Wang SN, Wang JM. Biomarkers for diagnosis of sepsis in patients with systemic inflammatory response syndrome:a systematic review and meta-analysis. Springerplus. 2016;5(1):2091. doi: 10.1186/s40064-016-3591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauer PR, Kashyap R, League SC, Park JG, Block DR, Baumann NA, et al. Diagnostic accuracy and clinical relevance of an inflammatory biomarker panel for sepsis in adult critically ill patients. Diagn Microbiol Infect Dis. 2016;84(2):175–80. doi: 10.1016/j.diagmicrobio.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou H, Guo S, Lan T, Ma S, Zhang F, Zhao Z. Risk stratification and prediction value of procalcitonin and clinical severity scores for community-acquired pneumonia in ED. Am J Emerg Med. 2018;36(12):2155–60. doi: 10.1016/j.ajem.2018.03.050. [DOI] [PubMed] [Google Scholar]
- 37.Dimoula A, Pradier O, Kassengera Z, Dalcomune D, Turkan H, Vincent JL. Serial determinations of neutrophil CD64 expression for the diagnosis and monitoring of sepsis in critically ill patients. Clin Infect Dis. 2014;58(6):820–9. doi: 10.1093/cid/cit936. [DOI] [PubMed] [Google Scholar]
- 38.Gibot S, Bene MC, Noel R, Massin F, Guy J, Cravoisy A, et al. Combination biomarkers to diagnose sepsis in the critically ill patient. Am J Respir Crit Care Med. 2012;186(1):65–71. doi: 10.1164/rccm.201201-0037OC. [DOI] [PubMed] [Google Scholar]


