Abstract
Background.
Despite recent efforts, disagreement remains amongst front-line clinicians regarding the operational definition of a syndrome commonly referred to as Posterior Fossa Syndrome (PFS) and/or Cerebellar Mutism Syndrome (CMS).
Methods.
This study surveyed experts in the clinical care of children with posterior fossa tumors to identify trends and discrepancies in diagnosing PFS.
Results.
All surveyed professionals conceptualized PFS as a spectrum diagnosis. The majority agreed mutism is the most important symptom for diagnosis. However, results highlighted ongoing discrepancies related to important features of PFS.
Conclusions.
Greater PFS conceptual alignment amongst providers is needed to formulate specific diagnostic criteria that would further research and clinical care. The authors propose preliminary diagnostic criteria for PFS that require refinement through careful clinical characterization and targeted empirical investigation.
Keywords: Posterior Fossa Syndrome, Cerebellar Mutism Syndrome, medulloblastoma, pediatric brain tumor
1. Introduction
Contemporary treatment for medulloblastoma, the most common malignant pediatric brain tumor1, involves surgical resection, cranial radiation therapy, and chemotherapy.2 Recent treatment advances have resulted in significant improvements in survival rates (70–85%),2 shifting the focus of research towards improving quality of life. Following surgical resection, up to 29% of medulloblastoma patients develop symptoms most commonly referred to as Posterior Fossa Syndrome (PFS) and/or Cerebellar Mutism Syndrome (CMS).3,4 Patients with PFS experience speech and language changes, motor impairments, and emotional lability.5 Typically, symptom onset is delayed (1–6 days post-surgery) and duration is limited (1 day-4 months).6 However, many patients experience persistent cognitive, motor, and emotion regulation difficulties.6–8 Patients treated for medulloblastoma with PFS evidenced poorer intellectual ability, processing speed, attention, working memory, and visual-spatial skills at 1- 3- and 5-years post-diagnosis compared to similarly treated medulloblastoma patients without PFS.10
There is disagreement among experts regarding the operational definition of PFS. Inconsistencies include the diagnostic label (PFS, CMS, cerebellar cognitive affective syndrome), a required period of mutism, and broader theoretical conceptualization (categorical or continuous). A diagnosis of PFS is typically made by an attending physician’s subjective yes/no report, without demonstration of inter-rater reliability and disagreement regarding diagnostic criteria among clinicians. Formulation of a universal definition has been proposed.3,5–7,10 The Posterior Fossa Society, a group of international experts in pediatric neuro-oncology, neurosurgery, radiology, and neuropsychology, constructed a definition of the syndrome using the nominal group technique. A consensus committee generated 10 diagnostic consensus statements based on published reviews. Group members (27–30 respondents) completed Delphi questionnaires that assessed opinions of PFS and level of agreement with proposed consensus statements. Subsequently, results were evaluated at a consensus conference. The final working definition identified delayed onset mutism or reduced speech accompanied by emotional lability after cerebellar tumor resection in children as fundamental features of the syndrome.5 Mutism was noted to always be transient, though syndrome recovery may be prolonged.5 Notably, the Posterior Fossa Society used “post-operative pediatric CMS” as the diagnostic label.
Given remaining diagnostic ambiguities surrounding PFS, the present study aimed to gather information from clinicians who specialize in treating children with posterior fossa tumors about their conceptualization of PFS. The goal in ascertaining information from clinical experts was to identify trends in diagnostic practice and reveal any potential remaining controversies.
2. Methods
2.1. Participants
Potential participants included 56 surveyed professionals associated with the SJMB12 protocol initiated at St. Jude Children’s Research Hospital considered PFS experts based on regular clinical care of this population. SJMB12, an ongoing, prospective, frontline treatment protocol for medulloblastoma, includes 19 sites across multiple continents.
2.2. Materials and Procedures
A brief online survey, depicted in Supplemental Table 1, was developed based on the extant research literature and consisted of 10 questions. Participants provided demographic information and were asked about their conceptualization of PFS. Lastly, they were asked to rank order the importance of symptoms related to speech/language, motor, and mood/affect impairments in diagnosing PFS.
3. Results
3.1. Participant Characteristics
Of the 56 professionals contacted, 32 (57%) responded to the survey. As shown in Supplemental Table 2, participants primarily consisted of neuro-oncologists (56%) and neuropsychologists (34%), with an average of 13.89 years of clinical experience with the PFS population. The majority of respondents were from the United States, with an even distribution among different geographic regions. The remainder resided in Australia, New Zealand, and Canada.
3.2. Examination of Survey Responses
All participants conceptualized PFS as continuous (on a spectrum from mild to severe) as opposed to categorical (present/not present). Twenty-one respondents (66%) stated that there is a pathognomonic symptom of PFS, with 16 of those respondents (76%) identifying mutism. However, 66% of participants reported that a period of mutism is not necessary to diagnose PFS. Alternate pathognomonic symptom responses included other speech/language difficulties (limited phrase length, disordered speech production), apraxia, and emotional lability. Seventy-two percent reported using PFS and CMS interchangeably.
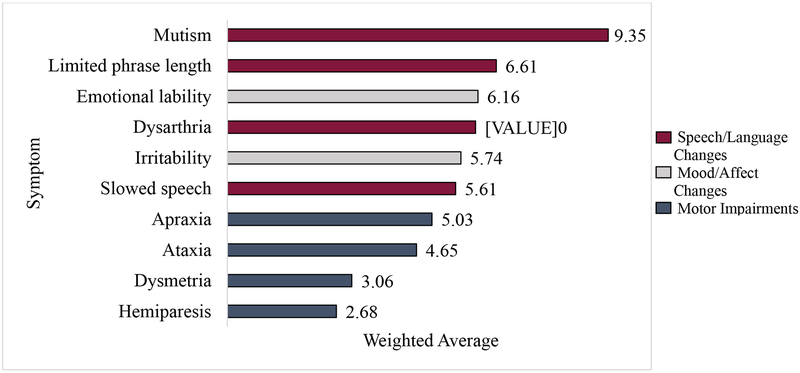
Participants were asked to rank order 10 symptoms associated with PFS from most to least important. Each item was given a weighted value (1–10) based on level of importance reported by participants. The analysis was conducted using data from 31 respondents. Figure 1 depicts the weighted average of the 10 items. Generally, the presence of mutism was the most important symptom when diagnosing PFS. This was followed by other speech/language symptoms and emotion regulation symptoms. Motor impairments were ranked least important.
Figure 1.
Rank order of importance (N = 31)
3.3. “Junior” and “Senior” Experts
Due to the wide range in years of experience amongst respondents, further analyses examined differences between “junior” and “senior” experts, using the median years working with PFS patients (15) to divide the sample. Junior experts included professionals with 1–13 years of PFS experience (n=15), and senior experts included those with 15–30 years (n=17). Both groups ranked mutism as most important in diagnosing PFS. Senior experts rated all speech/language symptoms (mutism, dysarthria, limited phrase length, slowed speech, in order) as most important, followed by emotional lability and irritability. Motor impairments (ataxia, apraxia, dysmetria, and hemiparesis, in order) were ranked least important. Conversely, junior experts’ rankings intermixed speech/language and emotion regulation symptoms with respect to symptom importance, with emotional lability as the second most important.
4. Discussion
4.1. Review of Findings
The current findings clarify particular aspects of the conceptualization of PFS, while also demonstrating ongoing diagnostic disagreement. All experts conceptualized PFS as continuous with presentations that range from mild to severe, despite research that routinely uses a dichotomous categorization (present/not present).8,10 Mutism was overwhelmingly ranked the most important feature when considering a diagnosis. However, most reported a period of mutism as not necessary to diagnose PFS. Variability among all other responses was noted, and junior and senior experts seem to conceptualize PFS differently. While both experts largely concentrate on speech/language changes when diagnosing PFS, junior experts seem to equally consider emotion regulation symptoms that may include irritability and emotional lability, dysphoria, apathy, distress, inconsolability, tearfulness, and distractibility.11,12 Taken together, these results may reflect the impact of the Posterior Fossa Society’s definition of CMS5 for junior experts, as they may be more inclusive of other important features as opposed to primarily speech/language changes.
Consistent with the goals of the Posterior Fossa Society, elucidating areas of disagreement among experts in the conceptualization of PFS can help work towards greater alignment. However, future research is required to establish specific diagnostic criteria that can be used reliably across assessors. Given the current study’s findings, including “mutism” in the diagnostic label is counter to the syndrome definition, as mutism does not appear to be required for diagnosis. PFS is a broader term than CMS that reduces confusion surrounding inclusion of patients in this group that display significant speech/language changes but not complete mutism. Likewise, movement towards a dimensional diagnostic approach with severity ratings that better reflects conceptualization of PFS is needed, such as those used for Intellectual Disabilities (Mild, Moderate, Severe, Profound).13 Dichotomous methods result in a loss of information related to individual differences,14 which can hamper research progress. Dimensional diagnostic approaches would accelerate research investigating the etiology of PFS, improving prediction of lasting neuropsychological impairment, and identifying targeted interventions. Additionally, diagnostic agreement would allow for examining associations between PFS and neuroimaging findings in a uniform clinical population that might guide surgical approaches to minimizing the occurrence of this syndrome.
4.2. Proposed Diagnostic Criteria for Posterior Fossa Syndrome
Based on survey results, the authors propose a set of “working” diagnostic criteria for PFS below, with the expectation that future clinical characterization and research will help evaluate and refine these criteria. The criteria can be found in Table 1.
Table 1.
Proposed Diagnostic Criteria for Posterior Fossa Syndrome
| Criterion A. | Acquired cerebellar injury (e.g., post-surgical or stroke-related), with symptoms in Criteria B, and C or D, emerging within two weeks of injury. |
| Criterion B. | Presence of one of the following speech and/or language deficits:
|
| Criterion C. | Presence of notable changes in mood/affect characterized by irritability (excessive tearfulness, crying, agitation, or anger), emotional lability (rapid changes in mood), and/or flat affect. |
| Criterion D. | Presence of motor dysfunction defined as: apraxia (inability to execute purposeful movements on command, despite having the physical capacity to perform the movement), ataxia (difficulty coordinating muscle movements), dysmetria (undershoot or overshoot of intended position with the hand, arm, or leg), hypokinesia (abnormally diminished motor activity), and/or hemiparesis (weakness one side of the body). |
Note: *Criteria A and B1 (mutism) are sufficient for a diagnosis of PFS. In the absence of B1 (mutism), Criteria A, B2, and C or D must be met for diagnosis of PFS.
4.3. Limitations and Future Directions
The current study had several strengths, including many years of experience amongst respondents with respect to frontline care of the target population, and a rank-order approach of symptoms identified in the extant literature. However, limitations include sampling issues, as respondents were largely neuro-oncologists or neuropsychologists, with less representation of other rehabilitation professionals. Further, the sample size in the present study, though similar to that of the Posterior Fossa Society’s consensus study,5 was small. This line of research could greatly benefit from future work that explores conceptualization practices in a larger sample that includes a broader range of disciplines. Additionally, the proposed diagnostic criteria are a starting place requiring refinement based on careful clinical characterization (e.g., to establish severity indicators and subtypes, such as PFS with or without emotion dysregulation, given different weight assigned to these symptoms as a function of years of clinical experience), as well as empirical investigation to establish reliability and predictive validity.
Supplementary Material
Acknowledgements:
The authors thank St. Jude Children’s Research Hospital employees and experts who participated in this study.
Funding: This work was supported, in part, by the National Cancer Institute (St. Jude Cancer Center Support [CORE] Grant [P30-CA21765]) and the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Northcott PA, Jones DW, Kool M, et al. Medulloblastomics: the end of the beginning. Nat Rev Cancer. 2012;12(12):818–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 3.De Smet HJ, Baillieux H, Catsman-Berrevoets C, De Deyn PP, Mariën P, Paquier PF. Postoperative motor speech production in children with the syndrome of ‘cerebellar’ mutism and subsequent dysarthria: A critical review of the literature. Eur J Paediatr Neurol. 2007;11(4):193–207. [DOI] [PubMed] [Google Scholar]
- 4.Korah MP, Esiashvili N, Mazewski CM, et al. Incidence, risks, and sequelae of posterior fossa syndrome in pediatric medulloblastoma. Int J Radiat Oncol Biol Phys. 2010;77(1):106–112. [DOI] [PubMed] [Google Scholar]
- 5.Gudrunardottir T, Morgan AT, Lux AL, et al. Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv Syst. 2016;32(7):1195–1203. [DOI] [PubMed] [Google Scholar]
- 6.Gudrunardottir T, Sehested A, Juhler M, Schmiegelow K. Cerebellar mutism: review of the literature. Childs Nerv Syst. 2011;27(3):355–363. [DOI] [PubMed] [Google Scholar]
- 7.Robertson PL, Muraszko KM, Holmes EJ, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children’s Oncology Group. J Neurosurg. 2006;105(6 Suppl):444–451. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe-Christensen C, Mullins L, Scott J, McNall-Knapp R. Persistent psychosocial problems in children who develop posterior fossa syndrome after medulloblastoma resection. Pediatr Blood Cancer. 2007;49(5):723–726. [DOI] [PubMed] [Google Scholar]
- 9.Patay Z. Postoperative posterior fossa syndrome: unraveling the etiology and underlying pathophysiology by using magnetic resonance imaging. Childs Nerv Syst. 2015;31(10):1853–1858. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber JE, Palmer SL, Conklin HM, et al. Posterior fossa syndrome and long-term neuropsychological outcomes among children treated for medulloblastoma on a multi-institutional, prospective study. Neuro Oncol. 2017;19(12):1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanier JC, Abrams AN. Posterior fossa syndrome: Review of the behavioral and emotional aspects in pediatric cancer patients. Cancer. 2017;123(4):551–559. [DOI] [PubMed] [Google Scholar]
- 12.Turkel SB, Shu Chen L, Nelson MD, et al. Case series: Acute mood symptoms associated with posterior fossa lesions in children. J Neuropsychiatry Clin Neurosci. 2004;16(4):443–445. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 14.MacCallum RC, Zhang S, Preacher KJ, et al. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7(1):19–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.