CAPSULE SUMMARY:
IgG Fc glycans were characterized in 225 healthy children and 40 children with unexplained recurrent respiratory infections, revealing age-dependent variation and suggesting a role for IgG glycosylation in immunocompetence.
Keywords: IgG, Fc, glycosylation, humoral immunity, recurrent infection, immunodeficiency, pediatric immunity, IgA deficiency
To the Editor
In children, antibody levels and repertoire diversity mature with age. A further determinant of humoral immunocompetence, still poorly characterized in the pediatric population, is IgG Fc glycosylation. Paired biantennary glycans at asparagine 297 in each heavy chain modulate the ability of IgG to bind complement, Fcγ receptors, and other ligands. The availability of more than 30 glycoforms provides the opportunity to “fine tune” IgG effector capacity (1). Variation in IgG Fc glycan utilization with age is recognized but small sample sizes and contrasting reports leave the pediatric end of this spectrum poorly defined (2-6). We sought to test the hypothesis that IgG Fc glycans vary predictably with age in childhood, and to explore the possibility that altered IgG Fc glycosylation may contribute to otherwise unexplained respiratory infections in children.
We obtained blood samples from 267 children aged 9 months - 18 years: 145 healthy children from Boston Children’s Hospital; 82 healthy children from Erasmus Medical Center, Rotterdam; and 40 children evaluated at the Immunology Clinic at Boston Children’s Hospital for unexplained recurrent respiratory infections (RRI; see Online Repository for recruitment procedures). Unexplained RRI was defined as recurrent infections of the middle ear, sinuses, and/or lung sufficient to trigger immunological consultation during which the attending immunologist elected to perform quantitative assessment of humoral immunity, but for which no cause was ultimately determined. Patients were excluded if RRI was attributed to an environmental cause, a predisposing condition such as cystic fibrosis, or a diagnosable immunodeficiency such as hypogammaglobulinemia, IgG subclass deficiency, specific antibody deficiency, or a defect in complement or cellular immunity (Table E1 in the Online Repository). Patients with IgA below the lower limit of normal in the absence of other immune abnormality were termed “low-IgA” and evaluated as a distinct subgroup (Table E2). Subjects receiving immunoglobulin supplementation at any point before sampling were excluded.
IgG Fc glycans were quantitated by liquid chromatography and electrospray ionization mass spectrometry (LC-ESI-MS). Glycopeptide fragments from IgG2 and IgG3 are indistinguishable using this method and are reported together. Methods and statistical strategies are provided in the Online Repository.
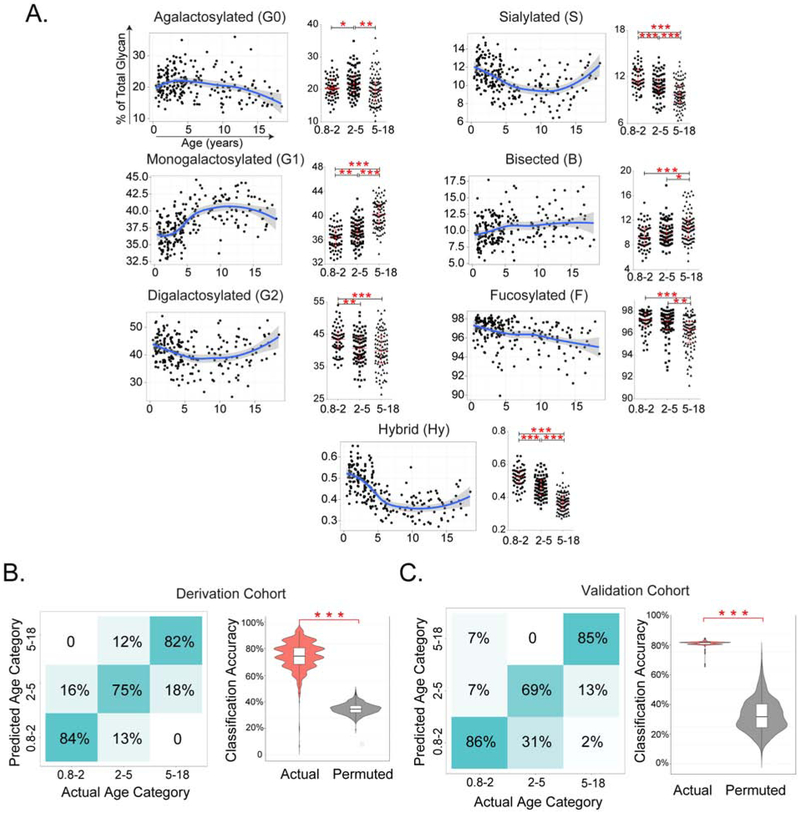
Individual glycoforms as well as summary measurements of galactosylated, sialylated, bisected, and fucosylated glycans were plotted as continuous variables and by age group: 9 months-2 years (n=59), 2-5 years (n=76), and 5-18 years (n=92). Galactosylation exhibited age-dependent variability consistent across IgG subclasses (Fig 1A and Fig E1-E4). Digalactosylated (G2) forms were overrepresented in younger children, largely at the expense of monogalactosylated (G1) glycans. The agalactosylated (G0) fraction was highest in children aged 2-5 years. Younger children exhibited more sialylated and core-fucosylated glycans (Fig 1A), typically considered anti-inflammatory, as well as alteration in glycans of unclear functional impact including those featuring a bisecting N-acetylglucosamine or variant hybrid structures (IgG1 shown in Fig 1A; Fig E1&E4 for other isotypes). Thus, compared with older children, younger children exhibited a distinct pattern of IgG Fc glycosylation.
Figure 1. IgG1 Fc glycans in healthy children.
(A) Level of galactosylated, sialylated, bisected, fucosylated and hybrid IgG1 glycans in 145 healthy children, as defined by LC-ESI-MS, plotted by age. Please see Online Supplement for other IgG isotypes. (B) Accuracy of age classification of Boston cohort by Fc glycosylation profiles in the derivation Boston cohort (left, n=145). (C) Validation of age classification by Fc glycosylation profile in the Dutch cohort (right, n=82) using actual vs. permuted data. P values (adjusted in A for multiple comparisons): * <0.05, **<0.01, ***, <0.001
To test whether IgG Fc glycans varied in a regular manner, we assessed the ability of combinations of glycoforms to differentiate children by age. An elastic net multinomial classifier was trained in the Boston healthy cohort, yielding a classification accuracy of ~80% across age categories (Fig1B). Validation in the Dutch cohort confirmed 80% accuracy (Fig1C), demonstrating a robust and generalizable relationship between IgG Fc glycosylation and age in children. Females older than the average age of menarche (≥12 years) showed enhanced Fc galactosylation compared to their similarly-aged male counterparts, consistent with the known effect of estrogen on IgG glycans (Fig E5A,B) (7). These findings link age and sex to predictable changes in IgG Fc glycosylation in childhood.
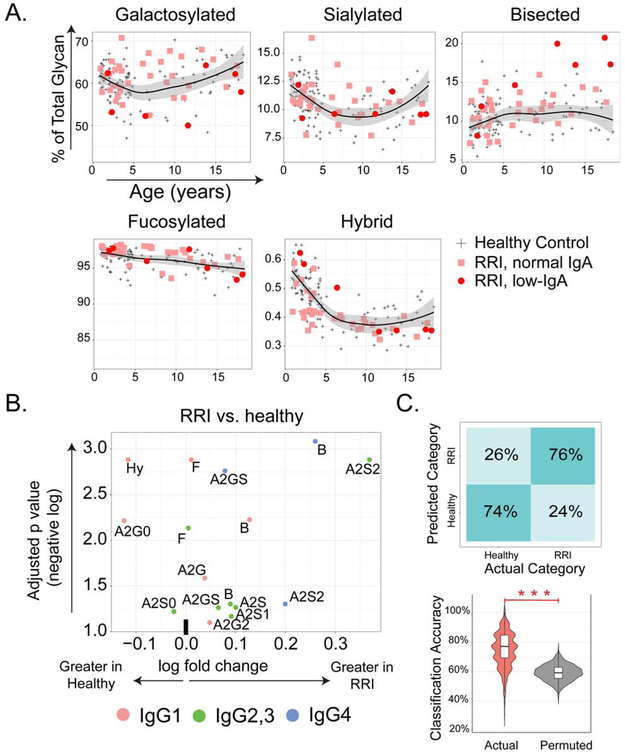
To explore the role of IgG Fc glycosylation in susceptibility to infection, we performed LC-ESI-MS in 40 patients with unexplained RRI, together with 3 age-matched healthy controls per case to eliminate batch effects (n=120). Cases exhibited elevated levels of bisected glycans across all subclasses, as well as enhanced sialylation of IgG2/3 and IgG4 (Fig 2A,B, and Fig E2). Binomial classifiers using glycan data categorized 74% of RRI and healthy subjects correctly (p<0.001 when compared to permuted data, Fig 2C). Although the number of low-IgA patients was limited, an especially marked abundance of bisected forms was noted in this subpopulation (Fig E6). Thus, otherwise unexplained RRI in children is associated with IgG Fc glycan aberrancy.
Figure 2. IgG Fc glycans in healthy children and RRI subjects.
(A) Scatterplots of galactosylated, sialylated, bisected, fucosylated and hybrid glycans by age in IgG1 from RRI with normal IgA (n=33), RRI with low IgA (n=7) and age-matched healthy children (n=120, black LOESS line, 95% confidence interval indicated in gray). (B) Volcano plot depicting differences between healthy and RRI subjects. A2=biantennary, G = galactosylation (0, 1 or 2), S=sialylated (0, 1 or 2), B=bisected, Hy=hybrid. (C) Accuracy of classification model distinguishing healthy from RRI patients trained using actual data (pink) as compared to permuted data (gray), *** p<0.001.
Fc glycans modulate IgG effector capacity, and it is likely that variation in healthy children and in those with RRI will have implications for immune function. Contrasting reports of the effect of individual glycoforms on IgG function, as well as simultaneous variation across a wide spectrum of glycoforms, complicate prediction of the net functional impact of the changes observed here (1). In healthy children, the abundance of digalactosylated and sialylated forms, as well as enhanced core fucosylation, could attenuate the effector potency of the IgG pool, especially before the age of 2 years. Whether the glycan changes noted here in children with unexplained RRI represent a predisposing factor or an effect of frequent infections cannot be determined from this dataset. However, chronic immune stimulation is typically associated with lower levels of sialylation and/or galactosylation, a pattern distinct from and even opposite that observed here (3, 8, 9). If in fact IgG Fc glycan aberrancy predisposes to RRI in some children, then an intriguing implication is that immunoglobulin supplementation addresses not only abundance and repertoire but also the deficit in IgG bearing normal Fc glycans and therefore with normal effector function.
Assessment of IgG Fc glycans is not part of the routine immunologic evaluation. Our data suggest that IgG Fc glycosylation represents a potential axis of humoral immune regulation in childhood and raise the intriguing possibility that IgG glycan aberrancy could contribute to otherwise unexplained respiratory infections in some patients. These findings highlight the need to consider the impact of IgG Fc glycans on immunocompetence, in childhood and beyond.
MATERIALS AND METHODS
Study Approval.
Boston healthy control and immunodeficient samples were collected under Boston Children’s Hospital Institutional Review Board-approved protocols NS10-11-0578 and P00007449 (discard samples) and 04-04-051 (written informed consent from legal guardians). Dutch healthy control samples were collected with approval of the Medical Ethics Committee of the Erasmus Medical Center, Rotterdam (MEC-2005-137) with written informed consent from legal guardians.
Healthy children.
Plasma from healthy children was obtained from (a) healthy patients recruited from Boston Children’s Hospital (n=119) (1); (b) laboratory discards (n=26) (1); and (c) consented healthy children from Erasmus Medical Center, Rotterdam (n=82) (2, 3).
Immunodeficient children.
Discard plasma was collected from children in whom quantitative IgG subtype testing had been ordered from the Immunology Clinic at Boston Children’s Hospital. Medical record review was performed within 1 month to identify subjects meeting criteria for unexplained RRI (see main text) and to collect non-identifying demographic, laboratory and clinical data. All identifiers were then destroyed.
Liquid chromatography and electrospray ionization mass spectrometry (LC-ESI-MS).
Total IgG was isolated from human plasma as described previously (4). Dried antibodies were dissolved in 40 μL of 25 mM ammonium bicarbonate buffer (pH 7.9), containing 0.025 mg/mL TPCK-treated trypsin and incubated overnight at 37 °C. The tryptic IgG digests were separated by reverse phase liquid chromatography (LC) and analyzed by mass spectrometry (MS) as described previously (4).
Data processing, including analyte peak integration and data curation, was performed using LaCyTools v1.0 (5). The data were converted to mzXML files and the runs were aligned based on the exact mass and the average retention time over all runs of the three most abundant glycoforms of each IgG subclass (Table E3). Using the described separation methods, glycopeptides were separated in three glycopeptide clusters, one for each of the IgG subclasses IgG1 and IgG4 and one for the combination of IgG2 and IgG3 as these result in indistinguishable glycopeptides. After alignment, sum spectra were calibrated based on at least four glycopeptides per cluster with a signal to noise ratio (S/N) above 9 (Table E4). Spectra were excluded from further analysis when the total intensity of their analytes did not exceed three times the average intensity of the negative controls. Analytes were included in the final data analysis when their average S/N (calculated per biological class) was above 9. This inclusion criteria resulted in the extraction of 23 IgG1, 12 IgG2,3 and 10 IgG4 glycoforms for all spectra (Table E5). For further analysis of compositional features, derived traits reflecting groups of similar glycoforms were calculated as defined (Table E6).
Statistics.
The ggplot2 R software package, version 3.1.2 (RStudio inc., Boston, MA) was used to graph age dependence scatter plots with locally weighted scatterplot smoothing (LOESS) illustrated in blue line and 95% confidence interval indicated in gray area. Both the glycan and the microsphere array measurements were compared under the non-paired, non-equal variance and two-sided hypothesis with a confidence interval of 0.95. Calculated p values were adjusted by the FDR (Benjamini-Hochberg) method and reported as adjusted p values (10). Magnitude effect size is evaluated by the Cliff’s delta method, thresholds indicated as follows: ∣d∣<0.147 "negligible", ∣d∣<0.33 "small", ∣d∣<0.474 "medium", ∣d∣ ⩾ 0.474 “large”. Binomial and Multinomial classification models were built using the Glmnet R package (11). To reduce the impact of age, each immunodeficient subject (RRI or low-IgA) was age matched to three healthy control subjects. Classifiers were trained using either IgG LC-ESI-MS, UPLC glycan, or microsphere array data with the elastic net mixing parameter at 0.4. Coefficient weights of each measurements contributing to the model were evaluated. Predictive robustness was evaluated by permutation tests.
Supplementary Material
Acknowledgments
LDN was supported by the Division of Intramural Research, NIAID, NIH. RS was supported by the funding from the Science Foundation Ireland Starting Investigator Research Grant (SFI SIRG) under Grant Number 13/SIRG/2164. HS and ROF was supported by the funding from EU FP7 Program HighGlycan, Grant No.278535. MEA was supported by NIH grants P20GM104416, R01AI131975, P01AI120756 and an Innovative Research Award from the Rheumatology Research Foundation. PAN was supported by NIH grants R21AI099435, R01AR065538, R01AR073201 and P30AR070253, an Innovative Research Award from the Rheumatology Research Foundation, the Cogan Family Foundation, the Fundacion Bechara, and the Arbuckle Family.
Disclosure of potential interest: The authors report no related conflict of interest. LDN was supported by the Division of Intramural Research, NIAID, NIH. MEA was supported by NIH grants P20GM104416, R01AI131975, P01AI120756 and an Innovative Research Award from the Rheumatology Research Foundation. PAN was supported by NIH grants R21AI099435, R01AR065538, R01AR073201, and P30AR070253, an Innovative Research Award from the Rheumatology Research Foundation, the Arbuckle Family Fund for Arthritis Research, and the Fundación Bechara.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dekkers G, Rispens T, Vidarsson G. Novel Concepts of Altered Immunoglobulin G Galactosylation in Autoimmune Diseases. Front Immunol. 2018;9:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parekh R, Roitt I, Isenberg D, Dwek R, Rademacher T. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. The Journal of experimental medicine. 1988;167(5):1731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ercan A, Barnes MG, Hazen M, Tory H, Henderson L, Dedeoglu F, et al. Multiple juvenile idiopathic arthritis subtypes demonstrate pro-inflammatory IgG glycosylation. Arthritis and rheumatism. 2012;64(9):3025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pucic M, Muzinic A, Novokmet M, Skledar M, Pivac N, Lauc G, et al. Changes in plasma and IgG N-glycome during childhood and adolescence. Glycobiology. 2012;22(7):975–82. [DOI] [PubMed] [Google Scholar]
- 5.Kristic J, Vuckovic F, Menni C, Klaric L, Keser T, Beceheli I, et al. Glycans are a novel biomarker of chronological and biological ages. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(7):779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Haan N, Reiding KR, Driessen G, van der Burg M, Wuhrer M. Changes in Healthy Human IgG Fc-Glycosylation after Birth and during Early Childhood. J Proteome Res. 2016;15(6):1853–61. [DOI] [PubMed] [Google Scholar]
- 7.Ercan A, Kohrt WM, Cui J, Deane KD, Pezer M, Yu EW, et al. Estrogens regulate glycosylation of IgG in women and men. JCI insight. 2017;2(4):e89703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science (New York, NY. 2006;313(5787):670–3. [DOI] [PubMed] [Google Scholar]
- 9.Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. The Journal of clinical investigation. 2013;123(5):2183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.