Abstract
Background:
Major Depressive Disorder (MDD) is an increasingly common and disabling illness. Since the amygdala has been reported to have pathological involvement in mood disorders, we aimed to investigate potential changes to structural connectivity of individual amygdala subnuclei in MDD for the first time, using ultra high-field 7T diffusion MRI.
Methods:
Twenty-four MDD patients (11 female) and twenty-four age-matched healthy control participants (7 female) underwent diffusion-weighted imaging with a 1.05mm isotropic resolution at 7 Tesla. Amygdala nuclei regions of interest were obtained through automated segmentation of 0.69mm resolution T1-weighted images and 0.35mm resolution T2-weighted images. Probabilistic tractography was performed on all subjects, with random seeding at each amygdala nucleus.
Results:
The right lateral, basal, central and centrocortical amygdala nuclei exhibited significantly increased connection density to the rest of the brain, whereas the left medial nucleus demonstrated significantly lower connection density (pFDR < 0.05). Increased connection density in the right lateral and basal nuclei were driven by the stria terminalis and the significant difference in the right central nucleus was driven by the uncinate fasciculus. Decreased connection density at the left medial nucleus did not appear to be driven by any individual white matter tract.
Conclusions:
By exploiting ultra-high resolution MRI, structural hyperconnectivity was demonstrated involving the amygdaloid nuclei in the right hemisphere in MDD. To a lesser extent, impairment of subnuclei connectivity was shown in the left hemisphere.
Keywords: Major Depressive Disorder, Diffusion MRI, Tractography, Amygdala, Ultra high-field, 7T, Neuroimaging
1. Introduction
Major depressive disorder (MDD) is a debilitating condition with significant worldwide prevalence and substantial mortality (1-3). There is an emerging body of literature implicating the amygdala as a particular brain region of interest in the pathology of MDD (4, 5). The amygdala plays an important functional role in emotional processing, fear conditioning and extinction, motivation, social salience and affective state, aberrant regulation of which form the depressive phenotype (6).
Concordant to its functional role, the amygdala has extensive intrinsic and extrinsic structural connections within the brain. Classically, the three main efferent white matter tracts from the amygdala are the anterior commissure, stria terminalis and the amygdalofugal pathway (7). The anterior commissure crosses the midline of the brain, connecting the anterior temporal lobes bilaterally (7). The stria terminalis originates from the central nucleus of the amygdala, projecting to a terminus dorsal to the anterior commissure via the lateral side of the fornix. The amygdalofugal pathway is thought to originate in the anteromedial temporal lobe, passing through the basolateral and central amygdala nuclei towards the midline (8, 9). Ascending branches of the amygdalofugal pathway cross through the nucleus accumbens and conclude in the area of the septal nuclei. Descending amygdalofugal white matter fibers project towards nuclei of the hypothalamus, and medial fibers reach the basal forebrain and olfactory areas. Similarly, the stria terminalis also connects the amygdala to the hypothalamus (7). Functionally, each efferent projection is considered to have a distinct purpose. The anterior commissure plays an important role in coupling the amygdalae and temporal lobe hemispheres and is involved in emotion, olfaction, instinctual behavior and memory consolidation (10, 11). The amygdalofugal tract is thought to exert downstream control over the hypothalamus and septal nuclei, influencing threat reactivity (12). Moreover, reports of amnesia in patients with damage to the amygdalofugal tract suggest an additional role in memory (13). The stria terminalis is theorized to regulate social and motivational conduct (14). Amino acid afferent neurotransmission from the amygdala in non-human primates has been described to reach the frontal, insular, cingulate and temporal cortices. Additionally, hippocampal cornu ammonis (CA) 1 and the entorhinal cortex give rise to significant amygdala input (15). Intrinsically, the lateral nucleus of the amygdala appears to project strongly to the basal nucleus (15). Additionally, there is evidence for a direct connection from the visual pulvinar of the thalamus to the amygdala, possibly enabling emotional salience attribution to simplistic visual information relayed from the visual cortex to facilitate swift and unconscious selective attention switching (16). In fact, this subcortical pathway has been shown to originate in the superior colliculus in the macaque (17) and has been reported to continue efferently from the amygdala to visual cortices 2 and 4 (18).
Given the amygdala’s broad and extensive structural connectivity profile and functional relevance to emotional pathology, it is not surprising that it is a structure frequently associated with MDD pathology. Seed-based resting state fMRI analyses have revealed significantly reduced amygdala connectivity with the cerebellum, occipital cortex, caudate, superior and middle temporal lobes and insula in MDD patients. Additionally, increased amygdala-temporal pole functional connectivity was negatively correlated with depression symptom severity and anxiety ratings (19). In adolescent depression, the amygdala has been reported to exhibit lower positive functional coupling between the amygdala and hippocampus, parahippocampus and brainstem and increased connectivity between the amygdala and precuneus. Where functional connectivity was significantly increased compared to control subjects, blood-oxygenation-level dependent (BOLD) response was inversely correlated with general depression, lassitude and dysphoria scores (20). Longitudinal assessment of high-risk MDD resilient individuals revealed significantly increased functional connectivity between the amygdala and the orbitofrontal cortex, which correlated with positive life events (21). Connectivity between the prefrontal cortices and the limbic system has been cited as frequently altered in MDD (22), and uncinate fasciculus volume, as a proxy for fronto-temporal structural connectivity known to involve the amygdala, has been shown to correlate with both amygdala volume and trait anxiety (23).
Due to past constraints of MR spatial resolution, imaging time and the availability of technology, studies considering the amygdala in depression have often treated the region as a single, unified structure in analyses. However, both animal and human studies have shown that the amygdala is comprised of at least thirteen anatomically separate and discrete subnuclei (24, 25). Several studies support the notion that these amygdala subregions are functionally distinct during emotional processing (26-28). We therefore set out to utilize a recently developed automated segmentation technique and ultra-high resolution diffusion MRI to investigate the role of amygdala subnuclei connectivity in major depression at a high level of granularity for the first time.
2. Methods
2.1. Participants
Twenty-four participants (mean age = 38.7 years, SD = 9.8 years, 11 females) with a primary diagnosis of MDD were recruited through the Mood and Anxiety Disorders Program at the Icahn School of Medicine at Mount Sinai. Twenty-four age-matched controls were also recruited (mean age = 39.8 years, SD = 12.3 years, 7 females). All participants were English-speaking and between 18 and 65 years of age. Age was not significantly different between groups (p = 1.0). Eligible MDD patients had no psychotic features, and were assessed by the Structured Clinical Interview for DSM-IV disorders (SCID-IV) or the Structured Clinical Interview for DSM-5 Research Version (SCID-5-RV) (29, 30). They were antidepressant free for at least 4 weeks prior to study participation and were currently experiencing a major depressive episode. No depressed participants had previously undergone electroconvulsive therapy. Healthy controls had no current or lifetime psychiatric disorder as determined by the SCID-IV or SCID-5-RV (29, 30). Participants with a current diagnosis of obsessive compulsive disorder (OCD), alcohol or substance abuse in the previous year, or lifetime history of a psychotic illness, bipolar disorder, or neurological disease were excluded. In the MDD group, 33% had co-morbid social phobia, 16% had generalized anxiety, 4% had binge eating disorder, 4% had panic disorder and 4% had post-traumatic stress disorder. Participants with MRI contraindications, unstable medical conditions, or positive urine toxicology on day of scan were also excluded. The mean age of illness onset in the depression sample was 17.5 years (SD = 10.4 years) and the mean duration of depressive episode was 75.0 months (SD = 84.0 months). All participants gave fully-informed written consent prior to investigation. Participant demographics and summary statistics are given in Table 1. This protocol was approved by the local Institutional Review Board.
Table 1.
Participant demographics and summary statistics
| MDD | Control | |
|---|---|---|
| Gender, % Female | 45% | 29% |
| Age (years),Median (Mean) [Minimum, Maximum] | 42.5 (43.2) [24, 63] | 37.3 (33.5) [22, 55] |
| Age (years), 1st Qu. / 3rd Qu. | 31 / 47.5 | 29 / 51.25 |
| Ever treated for a psychiatric illness, % | 87% | 4% |
| Talk therapy only, % | 25% | 0% |
| Ever hospitalized for mental health condition, % | 4% | 0% |
| Received ECT, % | 0% | 0% |
| Age of onset (years), Median (Mean) [Minimum, Maximum] | 15 (16) [7, 47] | - |
| Age of onset (years), 1st Qu. / 3rd Qu. | 10.75 / 21.5 | |
| Recurrent MDD, % | 79% | 0% |
| Duration of current episode (months), Median (Mean) [Minimum, Maximum] | 48 (69.6) [2, 252] | - |
| Duration of current episode (months), 1st Qu./ 3rd Qu. | 5 / 156 | - |
| Marijuana smoked in past month, % | 4% | 0% |
| MADRS rating of MDD symptom severity, Median (Mean) [Minimum, Maximum] | 25.5 (14.25) [13,43] | - |
| MADRS rating of MDD symptom severity, 1st Qu. / 3rd Qu. | 27.5 / 34.5 | - |
2.2. MRI acquisition
Magnetic resonance imaging data was acquired for all participants on a 7 Tesla whole body scanner (Magnetom, Siemens Healthcare, Erlangen, Germany). A SC72CD gradient coil was used with a single coil transmit and a 32-channel receive head coil (Nova Medical, Wilmington, MA, USA). A T1-weighted MP2RAGE sequence (31) was performed on each participant, with a 0.7mm × 0.7mm × 0.7mm voxel resolution. Field of view (FOV) was 225 × 183mm, acquisition matrix was 320 × 260mm, orientation of scan was coronal, repetition time (TR) was 6000ms and echo time (TE) was 3.62ms. Number of slices for a single slab was 240. A coronal-oblique T2-weighted turbo spin echo (T2-TSE) sequence was also obtained for all participants, with a 0.43mm × 0.43mm × 2.0mm voxel resolution. Number of acquired slices was 66. Acquisition matrix was 512 × 408mm, FOV was 222 × 177, TR was 9000ms and TE was 69ms.
A diffusion-weighted imaging sequence was performed in the same scanning session for all participants. TE was 67.6ms and TR was 7200ms. FOV was 210 × 210mm, acquisition matrix was 200 × 200mm, number of slices was 132, flip angle was 90°, spatial resolution of the diffusion data was 1.05mm isotropic and number of gradient directions was 64, with five b=0 s/mm2. The five b=0 acquisitions were interleaved during the acquisition to correct for artefacts, at time points 0.0s, 115.2s, 223.2s, 338.4s and 453.6s. The b-value for the sequence was 1500s/mm2. Two dMRI reverse-direction scans were acquired to correct gradient distortions.
2.3. Structural data processing
The Tl-weighted images were preprocessed using the FreeSurfer version 6.0 “recon-all” pipeline, non-parametric non-uniform intensity correction, intensity normalization, skull stripping and neck removal, automatic segmentation and parcellation steps (32). Multispectral amygdala segmentation was carried out in FreeSurfer development version 6.0, utilizing the T1-weighted and T2-weighted high-resolution images, producing masks for the basal, lateral, accessory basal, central, medial and cortical nuclei (33). The FreeSurfer automated amygdala segmentation was developed using an ex vivo dataset comprised of 10 autopsied human brain hemispheres and 7T field strength MRI scanning with a 0.1mm isotropic resolution. Amygdala nuclei were verified by a neuroanatomist and the automated atlas was built using an algorithm based on Bayesian inference. Validation using publicly available datasets showed that the amygdala automatic atlas significantly outperformed estimations of amygdala volume as a whole by FreeSurfer version 5.1 and performed with 84% accuracy in Alzheimer’s disease discrimination from age-matched controls (33). The nuclei were also grouped into a basolateral complex (accessory basal, basal and lateral nuclei) and a centrocortical complex (central, cortical and medial nuclei) by summating individual nucleus metrics. All outputs were manually inspected to ensure quality of preprocessing and accuracy of segmentation.
2.4. Diffusion data processing
Diffusion data was denoised using MRTrix two-shell phase-reversed processing (34, 35). FreeSurfer segmented and parcellated images were used for whole brain masking in the image processing (32). B1 field inhomogeneity correction was performed for the diffusion images (36). Fiber orientation distributions (FOD) were estimated from the diffusion data using spherical deconvolution (36), and diffusion tensor estimation was carried out using iteratively reweighted linear least squares estimator (37).
Amygdala nuclei regions of interest were co-registered to diffusion space using nearest neighbor interpolation in statistical parametric mapping software (SPM12), with the B0 image as the reference image, the T2-weighted image as the source image and the amygdala nuclei regions of interest as additional images. MRTrix software was used to carry out probabilistic tractography (38), performed using each amygdala nucleus mask as an individual seed region for 250,000 random seeds. Streamlines were thresholded using an FOD amplitude cutoff of 0.15 and at a maximum angle between successive streamline generation steps of 60°. The spherical deconvolution (SIFT2) algorithm was applied to all tracks, to eliminate streamlines that were unlikely to be biologically accurate and allow ground-truth accuracy of streamline count (39, 40). Whole brain fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (AD) maps were created, and using MRTrix’s ‘tcksample’, microstructural measurements of FA, MD, RD and AD were extracted from every voxel along every streamline. Mean measurements per streamline were produced, and averaged to output one mean FA, MD, RD and AD value per amygdala nucleus ROI per participant. Microstructural order is used to refer to the degree of diffusivity and anisotropy with regards to the tissue structure, as factors such as axon diameter, packing density and membrane permeability affect diffusion metrics without being necessarily a correlate of structural integrity (41). Streamline count was used as extracted, using the MRTrix ‘tckstat’ command, applying SIFT2 weightings to improve accuracy to ground truth white matter and to eliminate spurious streamlines. The resultant metric is referred to as connection density.
A secondary analysis was carried out with the aim of elucidating which particular amygdalae-associated white matter tracts contributed to significant differences in connection density in MDD at a subnuclei level. Regions of interest (ROI’s) were manually drawn around the previously produced tracks of the stria terminalis, uncinate fasciculus, amygdalofugal tract, inferior fronto-occipital fasciculus and anterior commissure for each participant. An additional tractography was then performed using the amygdala nuclei that exhibited significant between group differences in streamline density as seeding regions and the individual tract ROI’s as inclusion criteria for streamlines. Again, 250,000 random seeds were used per amygdala nucleus, streamlines were thresholded using an FOD amplitude cutoff of 0.15 and the maximum angle between successive streamline generation steps was 60°. All outputs were visually quality checked to ensure streamlines accurately tracked the correct white matter bundles. Streamline counts of the amygdala nuclei individual tracts were extracted with applied SIFT2 weightings.
2.5. Statistical analysis
Microstructural and streamline count metrics underwent between group analyses using non-parametric Mann-Whitney U testing of the null hypothesis. Testing was carried out both with and without age adjustment. Non-parametric regressions using the R package ‘rfit’ were carried out on the data for the MDD group only, to minimize bias caused by between group differences (42). Non-parametric statistical approaches were used to account for non-normal data distributions. Tractography metrics were regressed against age of illness onset and duration of depressive episode. Statistical results were corrected for multiple comparisons using false-discovery rate (FDR). All statistical analyses were carried out in R, version 3.3.3 (43) and a statistical significance threshold of 0.05 was used.
3. Results
3.1. Qualitative description of anatomical connections of the amygdala nuclei
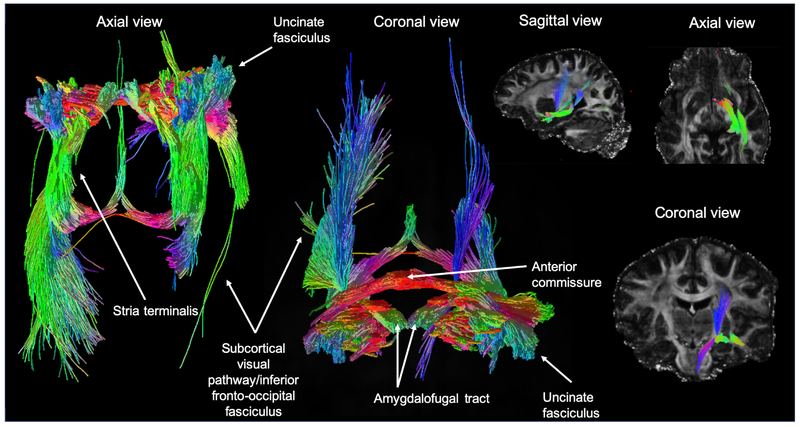
The present ultra-high resolution probabilistic tractography analysis revealed five distinct white matter tracts connecting with the amygdala (Fig. 1). In keeping with prior reports of amygdala structural connectivity, we identified the three main efferent pathways of the amygdala in all participants: the stria terminalis, the anterior commissure and the amygdalofugal tract. A subcortical-visual pathway was also seen in the tractographies, appearing to pass along the inferior fronto-occipital fasciculus. While directionality cannot be assessed using probabilistic tractography, previous literature is suggestive these tracts may represent reciprocal connectivity with the visual areas (17, 18). The uncinate fasciculus was also tracked.
Fig. 1.
An axial, coronal and sagittal view of probabilistic tractography seeded at all amygdala nuclei combined. White matter tracts reconstructed include: the stria terminalis, the uncinate fasciculus, a subcortical visual pathway passing along the inferior fronto-occipital fasciculus, the amygdalofugal tract and the anterior commissure. The color of streamlines is determined by their directionality.
Our analyses did not consider discrete end point regions of interest, and anatomical connections were not investigated in a quantitative manner. Qualitatively however, virtual rendering of the amygdala subnuclei structural connectivity shows that a) nuclei exhibit differing profiles of streamlines and b) the basal and lateral nuclei exhibit shorter range connections than the medial, central and cortical nuclei.
3.2. Tissue microstructure
Several differences in the depression group compared to controls indicated an increase in microstructural order. In the left hemisphere, there was evidence of decreased microstructural order in the MDD group. However, when non-parametric between group testing was performed with an additional adjustment for age, no significant differences in tissue microstructure in the MDD group compared to controls survived. These findings are presented as supplemental information.
3.3. Connection density
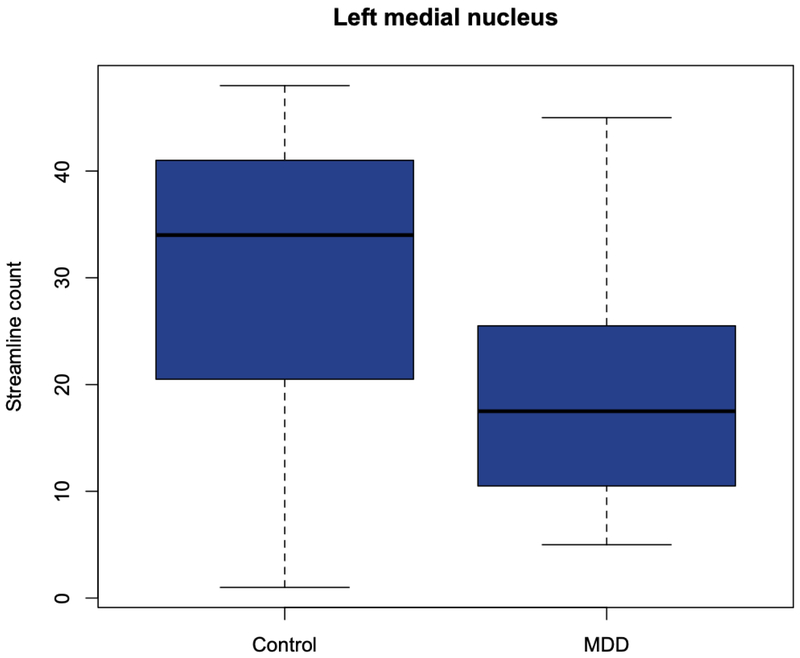
Streamline count was significantly increased in the MDD group compared to controls in tractography seeded at the right lateral nucleus (pFDR = 0.03), the right basal nucleus (pFDR = 0.03), the right central nucleus (pFDR = 0.02) and the right centrocortical complex (pFDR = 0.03) (Fig. 2). In the left hemisphere, there was a significant decrease in streamline count originating from the left medial nucleus (pFDR = 0.03) in the MDD group (Fig. 3). All results remained significant with statistical adjustment for age.
Fig. 2.
Significantly increased streamline density of tracks seeded at the right lateral nucleus, right basal nucleus, right central nucleus and right centrocortical complex in the MDD group compared to controls.
Fig. 3.
Significant decrease in streamline density in the MDD group compared to controls in streamlines emanating from the left medial nucleus.
3.4. Connection density of individual white matter bundles
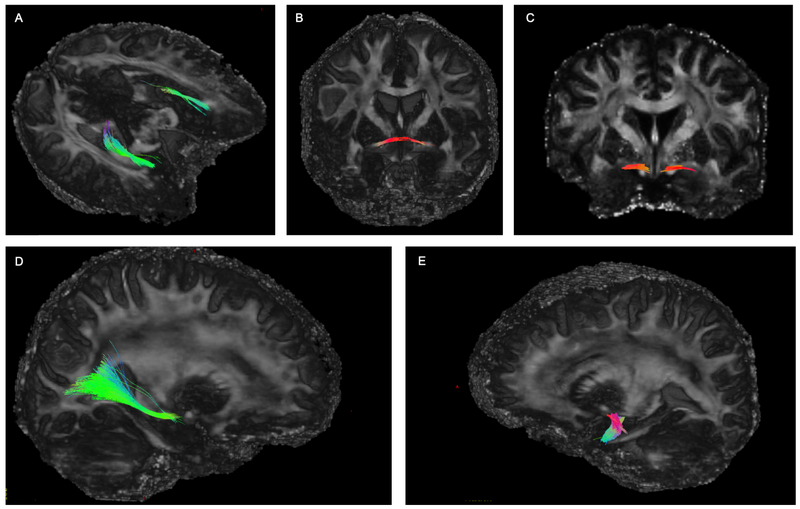
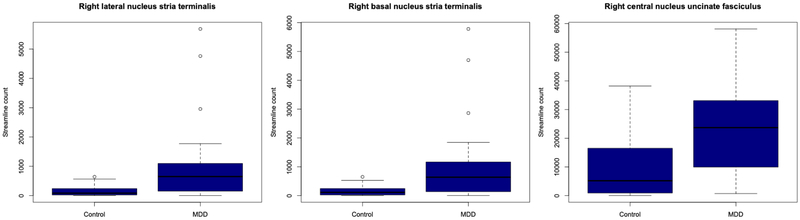
Our findings of significant differences in connection density in white matter tracks seeded at the right lateral, right basal, right central and left medial nuclei in MDD were robust to both statistical adjustment for age and correction for multiple comparisons. For this reason, a secondary analysis was carried out on these nuclei to determine which individual white matter tracts contributed to depression-related changes in structural connectivity. The stria terminalis, anterior commissure, inferior fronto-occipital fasciculus, uncinate fasciculus and amygdalofugal pathway were isolated for each nucleus in each participant (Fig. 4). False discovery rate corrected non-parametric between group testing with adjustment for age revealed that the stria terminalis seeded from both the right lateral and basal nuclei had significantly increased connection density in the MDD group (pFDR = 0.01, pFDR = 0.02 respectively) (Fig. 5). The anterior commissure, inferior fronto-occipital fasciculus, uncinate and amygdalofugal pathway showed no significant differences in the major depression group when seeded from the right lateral and right basal nuclei. Significantly increased general connection density in streamlines seeded at the right central nucleus in MDD was driven by the uncinate fasciculus (pFDR = 0.01) (Fig. 5). No other white matter tract showed significant between group differences when seeded at the right central nucleus. Lower general connection strength in streamlines seeded at the left medial nucleus did not appear to be driven by any of the single white matter tracts isolated in this study, with no significant differences identified in the MDD group in the stria, uncinate, anterior commissure, amygdalofugal pathway or inferior fronto-occipital fasciculus.
Fig. 4.
Isolated white-matter tracts seeded from the amygdala nuclei: a) the stria terminalis b) anterior commissure c) amygdalofugal tract d) inferior fronto-occipital fasciculus and e) uncinate fasciculus. The color of streamlines is determined by their directionality.
Fig. 5.
Significantly increased connection density of the stria terminalis in streamlines seeded at the right lateral and right basal nuclei and significantly increased connection density of the uncinate fasciculus seeded from the right central nucleus.
3.5. Association with clinical variables
Non-parametric regression in the depression group with covariation for age revealed that RD of streamlines was positively correlated with age of onset at the left basolateral nucleus (p = 0.01) and left accessory basal nucleus (p = 0.01). MD of streamlines seeded at the left basolateral complex were also significantly positively associated with age of illness onset (p = 0.01). However, none of these associations survived correction for multiple comparisons. No association was identified between diffusion imaging metrics and duration of depressive episode. Similarly, no significant relationship was identified using non-parametric age-controlled regression between connection density metrics of the individual white matter tracts and depression onset age or episode duration.
4. Discussion
In prior analyses of limbic structures in MDD, the amygdala has commonly been treated as a unified structure at conventional MRI field strengths (44-47). However, histologically and anatomically, reports suggest that over thirteen distinct subnuclei make up the amygdala (48). We present here a study that utilizes 7 Tesla field strength, ultra-high resolution imaging and a recently developed segmentation technique to carry out an analysis more closely resembling ground-truth biology. We show that three of the right amygdala nuclei display an increased connection density, suggesting structural hyperconnectivity of the right amygdala. Furthermore, we show that significantly increased connection density in MDD is driven by the stria terminalis in tracks seeded at the right lateral and basal nuclei, and the uncinate fasciculus in tracks seeded at the right central nucleus. Left medial amygdala nucleus tracks showed changes in count suggestive of hypoconnectivity. Importantly, we show differential changes in the amygdala lateral, basal, central and medial substructures that are specific to MDD, indicating that the amygdala nuclei do not react uniformly to MDD status. Furthermore, we provide here both a recommendation for the consideration of the amygdala nuclei as distinct entities and further characterization of structural brain changes in depression.
Our findings of increasing microstructural order without age adjustment in streamlines seeded at the right basal nucleus, right lateral nucleus, right central nucleus, right centrocortical complex and left lateral nucleus, combined with statistically robust increases in streamline count, suggest that these nuclei are hyperconnected with the rest of the brain in MDD. Previous studies of the structural connectivity of the amygdala in depression are concordant with these results (49, 50). Decreased MD and increased FA within the left amygdala was identified in remitted depressed individuals, suggesting a greater cell density and number of fibers. Additional tractography analyses in the same cohort revealed an increase in structural connectivity between the left amygdala and the hippocampus, cerebellum and brainstem (49). Increased correlation strength between grey matter volume of the amygdala and angular gyrus was also identified in MDD compared to controls (50). In adolescent depression, significantly lower FA and increased RD was reported in the bilateral uncinate fasciculus, a major white matter tract connecting the amygdala to frontal regions (51). Furthermore, healthy adolescents with high familial risk for MDD revealed significantly reduced FA in a tract-based spatial statistics analysis in the uncinate and inferior fronto-occipital fasciculi, both of which structurally involve the amygdalae (52). A meta-analysis of seven voxel-based diffusion imaging studies confirmed FA alterations compared to controls in the inferior fronto-occipital fasciculus in major depression (53).
In non-human primates, the amygdala nuclei are structurally connected to a larger number of regions in juveniles, and connectivity is pruned and refined over time (54). Developmental white matter changes are particularly protracted in the uncinate fasciculus (54) and perhaps for this reason, the uncinate is particularly vulnerable to psychiatric illness status. Functional MRI studies have shown replicable alterations in temporal lobe – prefrontal coupling in major depression (55), the principal structural correlate of which is the uncinate fasciculus (56). Uncinate microstructure has previously been associated with apathy in humans, and is considered to be important in episodic memory, social ability and emotional function (57). The central nucleus is a major site for efferent projections from the amygdala, and it is highly involved in the mediation of behavioral responses to stress (58). Our results show for the first time evidence of central subnucleus-specific involvement of the uncinate fasciculus in MDD. The stria terminalis is another major amygdala efferent, projecting dorsally from multiple subnuclei and branching to the hypothalamic and septal nuclei (59). Given its terminus at the hypothalamus, it is unsurprising that the stria is involved in motivation and reactivity to stress, both of which are foci of dysregulation in MDD (7). The present methodology allows us to implicate connection density of the stria terminalis streamlines seeded specifically at the right basal and lateral amygdala nuclei in depression, which is a unprecedented level of detail in humans to date. Interestingly, the basal, lateral and central nuclei are the three amygdala subregions that have been implicated in significant developmental pruning of their connections (54). It is possible that the maturation and plasticity required at these nuclei during development make them liable to deviations which associate with depression status.
In agreement with a portion of previously published results in MDD (19, 46), we also report a finding of significantly decreased connection density in depressed patients, localized in streamlines seeded at the medial nucleus. This finding did not appear to be driven by any individual white matter tract, but was an effect observed in medial nucleus streamlines generally to the whole brain. Left lateral nucleus MD, left basolateral complex RD and left lateral nucleus RD along emanating streamlines were also significantly increased when analysis was carried out without age-adjustment, suggesting a possible loss in microstructural order. Taken together, the present results illustrate an important feature concerning amygdala connectivity in MDD: the amygdala’s structural connectivity appears to be differentially enhanced or weakened based on the particular nuclei and hemisphere. Interestingly, we also reveal a significant positive association with three microstructural measures and age at illness onset, although the results did not survive correction for multiple comparisons. The range of onset ages in the sample may therefore be potential confounding factor in the dataset, showing that our finding of significantly decreased structural connectivity in MDD is sensitive to the age at which individuals develop MDD, and as such should be interpreted with caution.
Our study is a promising demonstration of how ultra high-field MRI may enable differential interrogation of connectivity among amygdala subnuclei in MDD, there are several limitations to the study which should be noted. Firstly, duration of illness, participant age and age at onset of the first episode of MDD were varied within the sample, which may be considered confounding factors. Secondly, although a sizable sample for a 7T investigation, the sample size used here was relatively small, due in part to more cautious MRI screening protocols at 7T than at conventional field strengths. A significant limitation of the methodology is also that probabilistic tractography cannot differentiate between fibers emanating from each nucleus and fibers passing through the nuclei. Given that the amygdala subnuclei are extensively interconnected in a manner well-defined by the non-human primate literature (56, 60), efferent, afferent or origination status of streamlines cannot be accurately identified in the present study. Additionally, longitudinal study of the participants was not carried out, and therefore the significant differences in structural connectivity in MDD could either pre-exist onset of depression and represent a possible vulnerability, or be a consequence of the illness. Nevertheless, the presented findings are a useful pilot demonstration of the advantages of high-resolution imaging in MDD and segmentation of heterogenous brain regions.
Overall, we report significant changes in white matter connection density in MDD compared to controls that imply both increased and decreased structural connection strength. Increases in connection density in the right hemisphere were driven specifically by the uncinate fasciculus and stria terminalis. Importantly, we show results that differ between amygdala subnuclei, demonstrating the importance and clinical significance of the separate consideration of amygdala substructures in major depression.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | ||||
| Bacterial or Viral Strain | ||||
| Biological Sample | ||||
| Cell Line | ||||
| Chemical Compound or Drug | ||||
| Commercial Assay Or Kit | ||||
| Deposited Data; Public Database | ||||
| Genetic Reagent | ||||
| Organism/Strain | ||||
| Peptide, Recombinant Protein | ||||
| Recombinant DNA | ||||
| Sequence-Based Reagent | ||||
| Software; Algorithm | MRTrix | http://www.brain.org.au/software/mrtrix/ | RRID:SCR_006971 | |
| Software; Algorithm | FreeSurfer | http://surfer.nmr.mgh.harvard.edu/ | RRID:SCR_001847 | |
| Transfected Construct | ||||
| Other |
6. Acknowledgements
NIH R01 MH109544
NIH R01 CA202911
NARSAD Young Investigator Grant
Icahn School of Medicine Capital Campaign, Translational and Molecular Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, Siemens Healthcare
Footnotes
Conflict of interest
Dr. Balchandani (the Principal Investigator in this study) is a named inventor on patents relating to magnetic resonance imaging (MRI) and RF pulse design. The patents have been licensed to GE Healthcare, Siemens AG, and Philips international. Dr. Balchandani receives royalty payments relating to these patents.
In the past 5 years, Dr. Murrough has provided consultation services to Boehreinger Ingelheim, Sage Therapeutics, FSV7, Novartis, Allergan, Fortress Biotech, Janssen Research and Development, Medavante-Prophase and Global Medical Education (GME) and has received research support from Avanir Pharmaceuticals, Inc.
No other authors reported biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- 1.Conwell Y, Duberstein PR, Cox C, Herrmann JH, Forbes NT, Caine ED (1996): Relationships of age and axis I diagnoses in victims of completed suicide: a psychological autopsy study. Am J Psychiatry. 153:1001–1008. [DOI] [PubMed] [Google Scholar]
- 2.Cuijpers P, Smit F (2002): Excess mortality in depression: a meta-analysis of community studies. J Affect Disord. 72:227–236. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Birnbaum HG, Shahly V, Bromet E, Hwang I, McLaughlin KA, et al. (2010): Age differences in the prevalence and co-morbidity of DSM-IV major depressive episodes: results from the WHO World Mental Health Survey Initiative. Depress Anxiety. 27:351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang Y, Wang F, Xie G, Liu J, Li L, Su L, et al. (2007): Reduced ventral anterior cingulate and amygdala volumes in medication-naive females with major depressive disorder: A voxel-based morphometric magnetic resonance imaging study. Psychiatry Res. 156:83–86. [DOI] [PubMed] [Google Scholar]
- 5.Bora E, Fornito A, Pantelis C, Yucel M (2012): Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 138:9–18. [DOI] [PubMed] [Google Scholar]
- 6.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF (2012): The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 35:121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamali A, Sair HI, Blitz AM, Riascos RF, Mirbagheri S, Keser Z, et al. (2016): Revealing the ventral amygdalofugal pathway of the human limbic system using high spatial resolution diffusion tensor tractography. Brain Struct Funct. 221:3561–3569. [DOI] [PubMed] [Google Scholar]
- 8.Nolte J (2002): The human brain. An introduction to its functional anatomy. 5th edition ed. New Jersey: Humana Press. [Google Scholar]
- 9.Hutchins T, Herrod HC, Quigley E, Anerson J & Salzman K Dissecting the white matter tracts: Interactive diffusion tensor imaging teaching atlas. University of Utah Department of Neuroradiology. [Google Scholar]
- 10.Bamiou DE, Sisodiya S, Musiek FE, Luxon LM (2007): The role of the interhemispheric pathway in hearing. Brain Res Rev. 56:170–182. [DOI] [PubMed] [Google Scholar]
- 11.Allen LS, Gorski RA (1991): Sexual dimorphism of the anterior commissure and massa intermedia of the human brain. J Comp Neurol. 312:97–104. [DOI] [PubMed] [Google Scholar]
- 12.Usunoff KG, Schmitt O, Itzev DE, Haas SJ, Lazarov NE, Rolfs A, et al. (2009): Efferent projections of the anterior and posterodorsal regions of the medial nucleus of the amygdala in the mouse. Cells Tissues Organs. 190:256–285. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Miyazawa Y, Akaoka F, Yamada T (1997): Amnesia following damage to the mammillary bodies. Neurology. 48:160–165. [DOI] [PubMed] [Google Scholar]
- 14.Wood RI, Swann JM (2005): The bed nucleus of the stria terminalis in the Syrian hamster: subnuclei and connections of the posterior division. Neuroscience. 135:155–179. [DOI] [PubMed] [Google Scholar]
- 15.Amaral DG, Insausti R (1992): Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Exp Brain Res. 88:375–388. [DOI] [PubMed] [Google Scholar]
- 16.Abivardi A, Bach DR (2017): Deconstructing white matter connectivity of human amygdala nuclei with thalamus and cortex subdivisions in vivo. Hum Brain Mapp. 38:3927–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rafal RD, Koller K, Bultitude JH, Mullins P, Ward R, Mitchell AS, et al. (2015): Connectivity between the superior colliculus and the amygdala in humans and macaque monkeys: virtual dissection with probabilistic DTI tractography. J Neurophysiol. 114:1947–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catani M, Jones DK, Donato R, Ffytche DH (2003): Occipito-temporal connections in the human brain. Brain. 126:2093–2107. [DOI] [PubMed] [Google Scholar]
- 19.Ramasubbu R, Konduru N, Cortese F, Bray S, Gaxiola-Valdez I, Goodyear B (2014): Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front Psychiatry. 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, et al. (2014): Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 71:1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer AS, Camacho MC, Ho TC, Whitfield-Gabrieli S, Gotlib IH (2018): Neural Markers of Resilience in Adolescent Females at Familial Risk for Major Depressive Disorder. JAMA Psychiatry. 75:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeberg I, Kjaerstad HL, Miskowiak KW (2018): Neural and Behavioral Predictors of Treatment Efficacy on Mood Symptoms and Cognition in Mood Disorders: A Systematic Review. Front Psychiatry. 9:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baur V, Hanggi J, Jancke L (2012): Volumetric associations between uncinate fasciculus, amygdala, and trait anxiety. BMC Neurosci. 13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding SL, Royall JJ, Sunkin SM, Ng L, Facer BA, Lesnar P, et al. (2016): Comprehensive cellular-resolution atlas of the adult human brain. J Comp Neurol. 524:3127–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janak PH, Tye KM (2015): From circuits to behaviour in the amygdala. Nature. 517:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, et al. (2004): Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 16:1730–1745. [DOI] [PubMed] [Google Scholar]
- 27.Hrybouski S, Aghamohammadi-Sereshki A, Madan CR, Shafer AT, Baron CA, Seres P, et al. (2016): Amygdala subnuclei response and connectivity during emotional processing. Neuroimage. 133:98–110. [DOI] [PubMed] [Google Scholar]
- 28.Balderston NL, Schultz DH, Hopkins L, Helmstetter FJ (2015): Functionally distinct amygdala subregions identified using DTI and high-resolution fMRI. Soc Cogn Affect Neurosci. 10:1615–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.First MD, Spitzer RL, Williams JBW, Gibbon M (1995): Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition.: New York: New York Psychiatric Institute. [Google Scholar]
- 30.First MB WJBW, Karg RS, Spitzer RL (2015): Structured Clinical Interview for DSM-5 Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). Arlington, VA: American Psychiatric Association. [Google Scholar]
- 31.Marques JP, Gruetter R (2013): New developments and applications of the MP2RAGE sequence--focusing the contrast and high spatial resolution R1 mapping. PLoS One. 8:e69294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. (2002): Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 33:341–355. [DOI] [PubMed] [Google Scholar]
- 33.Saygin ZM, Kliemann D, Iglesias JE, van der Kouwe AJW, Boyd E, Reuter M, et al. (2017): High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage. 155:370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E (2016): Denoising of diffusion MRI using random matrix theory. Neuroimage. 142:394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veraart J, Fieremans E, Novikov DS (2016): Diffusion MRI noise mapping using random matrix theory. Magn Reson Med. 76:1582–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. (2010): N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 29:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veraart J, Sijbers J, Sunaert S, Leemans A, Jeurissen B (2013): Weighted linear least squares estimation of diffusion MRI parameters: strengths, limitations, and pitfalls. Neuroimage. 81:335–346. [DOI] [PubMed] [Google Scholar]
- 38.Smith RE, Tournier JD, Calamante F, Connelly A (2012): Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage. 62:1924–1938. [DOI] [PubMed] [Google Scholar]
- 39.Dell'Acqua F, Tournier JD (2018): Modelling white matter with spherical deconvolution: How and why? NMR Biomed.e3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith RE, Tournier JD, Calamante F, Connelly A (2015): SIFT2: Enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. Neuroimage. 119:338–351. [DOI] [PubMed] [Google Scholar]
- 41.Jones DK, Knosche TR, Turner R (2013): White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 73:239–254. [DOI] [PubMed] [Google Scholar]
- 42.Kloke JDaM, J.W. (2012): (Rfit: Rank-based estimation for linear models. The R Journal. 4:57–64. [Google Scholar]
- 43.Team RC R: A Language and Environment for Statistical Computing. 3.3.3 ed. Vienna, Austria. [Google Scholar]
- 44.Kang SG, Na KS, Choi JW, Kim JH, Son YD, Lee YJ (2017): Resting-state functional connectivity of the amygdala in suicide attempters with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 77:222–227. [DOI] [PubMed] [Google Scholar]
- 45.Murphy ER, Barch DM, Pagliaccio D, Luby JL, Belden AC (2016): Functional connectivity of the amygdala and subgenual cingulate during cognitive reappraisal of emotions in children with MDD history is associated with rumination. Dev Cogn Neurosci. 18:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye X, Feng T, Tammineni P, Chang Q, Jeong YY, Margolis DJ, et al. (2017): Regulation of Synaptic Amyloid-beta Generation through BACE1 Retrograde Transport in a Mouse Model of Alzheimer's Disease. J Neurosci. 37:2639–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Connolly CG, Ho TC, Blom EH, LeWinn KZ, Sacchet MD, Tymofiyeva O, et al. (2017): Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J Affect Disord. 207:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding SL, Royall JJ, Sunkin SM, Ng L, Facer BA, Lesnar P, et al. (2017): Comprehensive cellular-resolution atlas of the adult human brain. J Comp Neurol. 525:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnold JF, Zwiers MP, Fitzgerald DA, van Eijndhoven P, Becker ES, Rinck M, et al. (2012): Fronto-limbic microstructure and structural connectivity in remission from major depression. Psychiatry Res. 204:40–48. [DOI] [PubMed] [Google Scholar]
- 50.Wu H, Sun H, Wang C, Yu L, Li Y, Peng H, et al. (2017): Abnormalities in the structural covariance of emotion regulation networks in major depressive disorder. J Psychiatr Res. 84:237–242. [DOI] [PubMed] [Google Scholar]
- 51.LeWinn KZ, Connolly CG, Wu J, Drahos M, Hoeft F, Ho TC, et al. (2014): White matter correlates of adolescent depression: structural evidence for frontolimbic disconnectivity. J Am Acad Child Adolesc Psychiatry. 53:899–909, 909 e891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang H, Fan X, Williamson DE, Rao U (2011): White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology. 36:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy ML, Frodl T (2011): Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol Mood Anxiety Disord. 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saygin ZM, Osher DE, Koldewyn K, Martin RE, Finn A, Saxe R, et al. (2015): Structural connectivity of the developing human amygdala. PLoS One. 10:e0125170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwabuchi SJ, Krishnadas R, Li C, Auer DP, Radua J, Palaniyappan L (2015): Localized connectivity in depression: a meta-analysis of resting state functional imaging studies. Neurosci Biobehav Rev. 51:77–86. [DOI] [PubMed] [Google Scholar]
- 56.Webster MJ, Ungerleider LG, Bachevalier J (1991): Connections of inferior temporal areas TE and TEO with medial temporal-lobe structures in infant and adult monkeys. J Neurosci. 11:1095–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hollocks MJ, Lawrence AJ, Brookes RL, Barrick TR, Morris RG, Husain M, et al. (2015): Differential relationships between apathy and depression with white matter microstructural changes and functional outcomes. Brain. 138:3803–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalin NH, Shelton SE, Davidson RJ (2004): The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 24:5506–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oler JA, Tromp DP, Fox AS, Kovner R, Davidson RJ, Alexander AL, et al. (2017): Connectivity between the central nucleus of the amygdala and the bed nucleus of the stria terminalis in the non-human primate: neuronal tract tracing and developmental neuroimaging studies. Brain Struct Funct. 222:21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LeDoux J (1998): Fear and the brain: where have we been, and where are we going? Biol Psychiatry. 44:1229–1238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.