Abstract
Generativity, or concern for and contribution to the well-being of younger generations, plays an important role in successful aging. The purpose of this study was to develop a novel, writing-based intervention to increase feelings of generativity and test the effect of this intervention on well-being and inflammation in a sample of older women. Participants in this study (n=73; mean age = 70.9 years, range 60–86 years) were randomly assigned to a 6-week generativity writing condition (writing about life experiences and sharing advice with others) or a control writing condition (neutral, descriptive writing). Self-reported measures of social well-being, mental health, and physical health, as well as objective measures of systemic and cellular levels of inflammation (plasma pro-inflammatory cytokines interleukin-6 and tumor necrosis factor-α; genome-wide RNA transcriptional profiling), were assessed pre- and post-intervention. The generativity intervention led to significant improvements across multiple domains, including increases in participation in social activities, decreases in psychological distress, more positive expectations regarding aging in the physical health domain, and decreases in pro-inflammatory gene expression. Thus, this study provides preliminary evidence for the ability of a novel, low-cost, low-effort intervention to favorably impact inflammation and well-being in older women.
Clinical Trials Registration
Keywords: generativity, inflammation, aging, intervention
1. Introduction
“I am certain that after the dust of centuries has passed over our cities, we, too, will be remembered not for victories or defeats in battle or in politics, but for our contribution to the human spirit.”
-John F. Kennedy, 1962
The proportion of the world’s population aged 65 and older is growing at a rate unparalleled in history (Population Reference Bureau, 2011), creating an urgent need to study factors relevant to health and well-being in older adults. Generativity is one such factor that appears to play a role in successful aging (Fisher, 1995). Generativity is multi-faceted, involving concern and activity devoted to contributing to others and society, particularly younger generations, and is driven by internal desire and external expectations and opportunities (McAdams & De St Aubin, 1992). The desire to be generative can be motivated by a need to be useful to others or a “need to be needed,” as well as a desire to leave a legacy behind after death (McAdams & De St Aubin, 1992). Essentially, generativity “connects [adults] to other people, institutions, and even societal and global concerns that are deemed worthy of one’s care, investment, and contribution” (McAdams & de St Aubin, 1998).
Some correlational findings have also shed light on the importance of generativity and its related constructs for promoting health and well-being in older adults. For example, generativity is associated with positive psychological well-being (An & Cooney, 2006). Older adults who feel more generative, or feel more socially useful, also have a decreased risk for morbidity and mortality (Gruenewald, Karlamangla, Greendale, Singer, & Seeman, 2007, 2009; Gruenewald, Liao, & Seeman, 2012). Furthermore, engaging in productive activities, which could potentially lead to increases in feelings of generativity, is related to lower markers of inflammation in older adults (S. Kim & Ferraro, 2013). Given that inflammation increases as a function of aging even within older adults (Piber et al., 2019), and also underlies many diseases of aging (e.g., cardiovascular disease, arthritis, cancer; Ferrucci et al., 1999), the impact of generativity on inflammation may be an important contributor to health outcomes in older adult populations.
Despite the relevance of generativity for health and well-being in older adults, generativity is highly understudied in geriatric populations (Schoklitsch & Baumann, 2012), and one area that has been particularly overlooked is the development of interventions to experimentally increase feelings of generativity in older adults, as much of the work on the links between generativity and health has been correlational. Given the relationships between generativity and positive health outcomes, such an intervention may lead to improvements in health and well-being.
The Baltimore Experience Corps Trial, a volunteering intervention in which older adults teach children in elementary schools, provides some preliminary evidence for the impact of generativity interventions on health in older adults. The Experience Corps program, which involves intergenerational contact, was shown to increase feelings of generativity (Gruenewald et al., 2015), suggesting that generativity is a malleable construct, which can be increased by an intervention. The program also led to improvements in both psychological and physical health (Hong & Morrow-Howell, 2010), including reducing depressive symptoms and functional limitations, indicating the potential benefits of a generativity intervention.
Together, these findings suggest that generativity is an important factor for healthy aging, and that interventions which increase feelings of generativity, such as the Experience Corps program, can positively impact health and well-being. However, many older adults may have a desire to be more generative but may not have the physical ability or desire to commit to volunteering in this type of “high-intensity” program; for example, the Baltimore Experience Corps Trial involved a commitment of at least 15 hours a week for at least one school year. Thus, an alternative generativity intervention which involves a lower level of physical exertion and time commitment, such as a brief writing-based intervention, may be more accessible to older adults. But, to our knowledge, no research has evaluated whether writing-based interventions might increase feelings of generativity.
Thus, the primary aim of this study was to fill this gap in the literature by testing the effect of a writing-based intervention aimed at increasing feelings of generativity in older adults. Participants in this study were randomly assigned to either a generativity or control condition, both of which involved writing once a week for six weeks. At pre- and post-intervention, participants completed self-report measures of generativity, social well-being, mental health, and physical health. Participants also had blood drawn to measure markers of inflammation pre- and post-intervention, making this the first study to examine the impact of generativity on inflammation. Given the literature linking generativity and positive health outcomes, we hypothesized that the generativity intervention would lead to improvements in self-reported measures of health and well-being, as well as decreases in pro-inflammatory biology from pre- to post-intervention. Thus, our primary aim was to investigate changes in generativity, self-reported well-being (i.e., social, mental, and physical health), and pro-inflammatory biology (i.e., circulating cytokines and pro-inflammatory gene expression) from pre- to post-intervention.
An exploratory, secondary aim of this study was to investigate whether any improvements in health and well-being would be sustained after the intervention ended. Thus, two months after their post-intervention visit, participants completed a follow-up visit, in which they completed an additional blood draw and the same measures of health and well-being as the pre- and post-intervention visits.
2. Material and Methods
2.1. Participants and procedure
2.1.1. Participants
Participants were recruited from multiple sources, including flyers posted in the Los Angeles community (e.g., libraries, senior centers), advertisements in local newspapers, mailers to participants in prior studies and patients from the UCLA Geriatrics Clinic who had consented to learn about future studies. Interested participants were screened for eligibility using a structured telephone interview. Inclusionary criteria included: 1) being a healthy female 60 years of age or older, 2) fluency in English, and 3) access to the Internet and a computer to complete the weekly study sessions.
Given that there are sex differences in generativity (e.g., women generally feel more obligated to assist social institutions and other people; Keyes & Ryff, 1998), generativity interventions may be differentially impactful on women than men. Because it has been proposed that older women may particularly benefit from new outlets to promote generative activity (Carlson, Seeman, & Fried, 2000) and may have the most to gain from a generativity intervention, we decided to solely recruit women for this innovative, exploratory pilot intervention.
Additionally, in order to maximize our ability to detect increases in self-reported generativity in response to the intervention, eligible participants were screened for current perceptions of generativity. Potential participants were asked to answer 7 questions about how generative they wished to be (i.e., generative desire; e.g., “I want to do something that will be valuable to others for a long time”) and 6 questions about how generative they currently felt (i.e., current generative achievement; e.g., “right now, I feel like I do things that will exist for a long time”) using the Generativity Scale (Gruenewald et al., 2015). Answers to items on the scale were measured on a 6-point Likert scale (1 to 6; “disagree strongly” to “agree strongly”) and averaged for each subscale (desire and achievement). Participants were deemed eligible if the difference between their desire and achievement subscale scores (i.e., generative desire – generative achievement) was .20 or higher, indicating that they wished to be more generative than they currently felt.
Prospective participants with the following conditions were excluded: chronic physical or mental health problems that may have impacted the study’s physiological or psychological outcomes (e.g., rheumatoid arthritis, cancer, major depression); regular use of certain prescription medications that may have impacted the study’s physiological outcomes (e.g., immune-modifying drugs, opioids, steroids, psychotropic medications to treat major depression or anxiety); cognitive impairment (Brief Alzheimer Screen less than 26; Mendiondo, Ashford, Kryscio, & Schmitt, 2003); BMI greater than 35; current smoker or excessive caffeine user; or recent nightshift work or time zone shifts (>3 h).
Seventy-eight older women (mean age 70.9 ± 6.3 years) were enrolled in the study and randomized into either a 6-week generativity (n=40) or control (n=38) condition. Five participants (n=2 in the generativity condition, n=3 in the control condition) did not complete the study (see CONSORT diagram in Supplemental Material). Two of these participants were removed by the study investigators for not meeting study eligibility criteria; two participants dropped out before completing the post-intervention assessment due to scheduling conflicts; and one participant did not receive all required components of the study due to technical issues. Thus, the final sample that was analyzed pre- to post-intervention consisted of 73 participants, described in further detail below (Demographics Table provided in Supplemental Material). Note that one subject was unable to complete the blood draw at the post-intervention visit, and is thus missing data for inflammatory outcomes for both the primary aim, and was removed from the study at this point and has no data for the exploratory aim. Additionally, note that for the exploratory aim (pre- to 2-month-follow up visit), one additional subject did not complete a blood draw and thus does not have data for inflammatory outcomes for the follow-up visit.
Participants in both groups were told that the study was examining how writing about experiences relates to health and biological outcomes. All participants provided written consent before participating. All procedures were approved by the UCLA Human Subjects Protection Committee.
2.1.2. Pre-intervention assessment
The study was conducted between January 2016 and March 2017 (when the intended sample size was reached; see Sample Size Determination in Supplementary Material) using a randomized, double-blind design. The random allocation sequence was generated by a consultant who did not interact with participants. Randomization was done using a computerized uniform random number generator in blocks of 4. Participants were enrolled in the study by the study coordinator (S.O.), who was blind to study condition and interacted with participants at all in-person study visits. Another member of the study team (M.M.) did not meet the participants at either pre- or post-intervention visits (i.e., M.M. met participants only at the final 2-month follow-up visit) and was responsible for administering the online interventions via e-mail to the participants.
Participants began the study at the UCLA Clinical and Translational Research Center (CTRC) where a phlebotomist, who was blind to condition, drew blood in order to assess inflammatory outcomes. Participants then completed self-report measures of generativity, social well-being, and mental and physical health. Finally, the study coordinator, who was blind to condition throughout the entirety of the study, gave participants general instructions for the writing portion of the study and broadly familiarized them with the online survey and writing format.
2.1.3. Intervention
2.1.3.1. General procedures
Beginning the week after the pre-intervention assessment, all participants received an email, once weekly for six weeks, with a link to log in to an online system (SurveyMonkey) to receive their instructions and complete their writing. Participants in both conditions were asked to write once weekly and to write about various topics each week based on recommendations for maximizing efficacy of positive psychological interventions (Layous, Nelson, & Lyubomirsky, 2012; Lyubomirsky & Layous, 2013). All prompts from both conditions, as well as further details of the intervention, are included in the Supplementary Material.
Across both conditions, participants were instructed not to begin their weekly session until they were able to sit quietly, alone, without distraction and complete the writing in one, uninterrupted session each week. Participants were asked to write for however long they desired, as long as they spent at least ten minutes writing for each session. They were reminded each week that the writing portion of the study was important and that they should “really try to get into the writing experience.” All participants were told not to worry about grammar, spelling, or sentence structure in order to allow them to fully immerse themselves in the writing experience. Participants were also told that their writing would be confidential and only identifiable by an anonymous study identifier, not their personal information.
Each week, immediately after the writing portion of their session was complete, participants were asked to respond to questions assessing their feelings post-writing, as detailed below under “weekly assessments.”
2.1.3.2. Generativity condition
Participants in the generativity condition were asked to respond to prompts asking them to share their experiences and advice with others. Pilot testing of the generativity prompts revealed that some older adults found it hard to connect with a much younger generation (e.g., people in their twenties), both because of age and generational differences. In response to this pilot testing, the target audience to receive the wisdom and advice from the generativity participants was middle-aged adults. Participants in the generativity condition were asked to provide responses to prompts such as, “What are some of the most important lessons you feel you have learned over the course of your life? If a middle-aged person asked you ‘what have you learned in your ____ years in this world,’ what would you tell him or her? You can think and write about any aspect of life you think would be important to share with middle-aged adults looking for advice. You can also focus on one lesson or several lessons.”
In order to create a concrete target of generativity for the participants, so that the exercise was not merely a journaling intervention, participants in the generativity condition were told prior to the first writing assignment that their responses for the next 6 weeks would be compiled (anonymously, with all names and identifying information removed) into a book or website dedicated to helping middle-aged adults gain valuable insights and advice from older adults. Several additional procedures were enacted to convince participants of the value, importance, and relevance of their writings; these are detailed in the Supplementary Material.
2.1.3.3. Control condition
Participants in the control group were asked to write about topics that were intended to be neutral and descriptive in nature. They were instructed not to think of or describe social features or psychological thoughts linked to the topics. For example, one prompt read, in part: “In the space provided below, please describe what you had for lunch today—what it looked like, how it tasted… please try to focus on the details of what you ate, how it looked, and how it tasted, rather than on who you were with or what you were thinking about during this time.” Participants in the control condition were also never told their writing would be shared with others.
2.1.4. Post-intervention assessment
After completing the 6 weeks of writing, participants returned to the UCLA CTRC for the post-intervention assessment. Similarly to the pre-intervention assessment, participants had blood drawn and completed self-report measures.
2.1.5. Two-month follow-up assessment
Two months after their post-intervention assessment, participants returned to the UCLA CTRC, where they had blood drawn and completed self-report measures. Participants were then debriefed and paid for participation.
2.2. Self-report measures
2.2.1. Overview of measures
At the pre- and post-intervention assessments (as well as exploratory 2-month follow-up assessments), self-report measures of global feelings of generativity, social well-being, mental health, and physical health were taken. In addition, each week immediately post-writing, participants completed a measure of momentary feelings of generativity. Further details on these measures are included in the Supplemental Material. In sum, all measures other than momentary feelings of generativity were taken at the pre-intervention, post-intervention, and follow-up assessment timepoints.
2.2.2. Weekly assessments
2.2.2.1. Post-writing measure of momentary generativity
To assess participants’ momentary feelings of generativity post-writing, they were asked immediately post-writing to indicate how they “feel right now” in response to three words reflective of generativity (i.e., “helpful,” “caring,” and “useful”) among other distractor words. Responses were on a scale of 0 (“not at all”) to 4 (“extremely”) and means were taken across these three items to create a momentary generativity scale (α = .79, assessed at the first week). Higher scores indicate greater feelings of generativity post-writing.
2.2.3. Self-report assessments taken at pre-intervention, post-intervention, and follow-up
2.2.3.1. Global feelings of generativity
Participants completed a standardized, reliable measure of generativity (Loyola Generativity Scale; McAdams & De St Aubin, 1992), which includes items assessing key components of generativity, such as feeling needed by others and contributing to society.
2.2.3.2. Social well-being
In order to measure participation in social activities, participants were asked to complete the Lifestyle Activities Questionnaire (Carlson et al., 2011; Parisi et al., 2015), a measure used to measure lifestyle activities in previous interventions in older adults (i.e., Baltimore Experience Corps Trial; Parisi et al., 2015; Parisi et al., 2012). As another measure of social well-being, participants completed the UCLA Loneliness Scale, a valid, reliable scale measuring subjective feelings of social isolation (Russell, 1996). Finally, as a measure of perceived social support, participants completed the Social Provisions Scale, a valid, reliable scale (Cutrona, 1984; Cutrona & Russell, 1987).
2.2.3.3. Mental health
First, in order to measure participants’ expectations regarding aging in the mental health domain, the Expectations Regarding Mental Health Scale (of the 12-item Expectations Regarding Aging Survey) was used (Sarkisian, Steers, Hays, & Mangione, 2005)1.
Second, psychological distress was also measured. We created a composite for psychological distress by standardizing and summing three widely-used, reliable measures used to assess anxiety (Spielberger Trait Anxiety; Spielberger, 2010), depression (Beck Depression Inventory; Beck, Steer, & Carbin, 1988), and perceived stress (Perceived Stress Scale; Cohen, Kamarck, & Mermelstein, 1983). These three scales were significantly correlated with each other (r’s = .6 - .7, p’s < .0001), and the results of a principal components analysis revealed that the composite of these three scales reflects a single factor or component, which explained 76% of the variance in the indicator variables. Further details of the psychological distress composite are included in the Supplemental Material.
2.2.3.4. Physical health
The Lifestyle Activities Questionnaire, mentioned above, was also used to measure participation in physical activities (Carlson et al., 2011; Parisi et al., 2015). Participants’ expectations regarding aging in the physical health domain were also measured, using the Expectations Regarding Physical Health Scale (of the 12-item Expectations Regarding Aging Survey; Sarkisian et al., 2005).
2.3. Inflammatory measures
Circulating levels of markers of systemic inflammation and pro-inflammatory gene expression in peripheral blood mononuclear cells (PBMC) were both measured at pre-intervention, post-intervention, and the follow-up assessment, providing multiple levels of analysis for inflammatory outcomes.
2.3.1. Plasma levels of cytokines
Venous whole blood was collected using EDTA, held on wet ice until centrifuged at 4°C, and plasma aliquots prepared and frozen at −80°C until performance of immunoassays. Plasma concentrations of interleukin(IL)-6 and tumor necrosis factor (TNF)-α were determined by high-sensitivity ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol; the lower limits of the assays were 0.2 and 0.5 pg/mL, respectively. All samples were assayed in duplicate, and pre- and post-intervention samples from each participant were assayed on the same plate. For plasma samples with TNF-α concentrations below the limit of detection, a value of 0.25 pg/mL was assigned (one-half the lower limit of the assay). Intra- and inter-assay coefficients of variation (CVs) for IL6 were <5%. Intra- and inter-assay CVs for TNF-α were 9.1% and 19.6%, respectively, due to the low concentration of TNF-α in the internal laboratory control sample utilized on all assay plates.
2.3.2. Gene expression and bioinformatics
Genome-wide transcriptional profiling was conducted on PBMC isolated by density gradient centrifugation from heparinized whole blood, preserved in RLT lysis buffer (Qiagen), and frozen at −80°C until RNA extraction was performed. RNA was extracted from preserved frozen PBMC samples (Qiagen RNeasy) and checked for suitable mass (> 100 ng by NanoDrop 1000) and integrity (RNA integrity number > 8 by Agilent TapeStation capillary electrophoresis). All samples meeting quality criteria were assayed by RNA sequencing in the UCLA Neuroscience Genomics Core Laboratory using Illumina TruSeq cDNA library synthesis and multiplex DNA sequencing on an Illumina HiSeq 4000 instrument with single strand 65 bp sequence reads. Each sample yielded >10 million sequence reads, each of which was mapped to the RefSeq human genome sequence using HISAT2 software (D. Kim, Langmead, & Salzberg, 2015) and quantified as transcript counts per million total transcripts using StringTie software (Pertea, Kim, Pertea, Leek, & Salzberg, 2016).
2.4. Statistical analyses
2.4.1. General analytic strategy
All analyses were done using a standard statistical program (SPSS 25.0). When testing between-group effects for the primary aim, analyses of covariance (ANCOVA) were conducted, testing the effect of condition (generativity vs. control) at post-intervention, controlling for baseline (pre-intervention) values. Similarly, for the exploratory aim (i.e., to test whether any effects at post-intervention were sustained at follow-up), ANCOVAs testing the effect of condition at the 2-month follow-up, controlling for baseline values, were conducted. For analyses examining weekly outcomes (note: only momentary feelings of generativity were measured weekly), scores were averaged across all 6 weeks of the intervention; ANCOVAs were then performed on these averaged scores. ANCOVA was chosen as the analytic strategy as it increases statistical power and is the recommended strategy for randomized studies (Van Breukelen, 2006).
Due to known influences of demographic factors (age and white/non-white race) on physical and mental health outcomes, all analyses initially controlled for these factors as covariates but were dropped if not significant (p > .1). Additionally, due to known effects of body mass index (BMI), illness symptoms, and alcohol consumption on inflammation, these factors were controlled for (in addition to age and race) in all analyses involving inflammatory outcomes. Additionally, due to the skewed nature of the circulating cytokine data, all analyses on circulating cytokines were performed on natural log-transformed values. Finally, given the number of self-report measures assessed, a family-wise Simes correction for correlated outcomes was applied to reduce the potential for Type I error and is reported for reference.
2.4.2. Gene expression and bioinformatics analyses
Transcript-per-million values for each transcript were log2-transformed for analysis by a standard linear statistical model estimating the magnitude of change in transcript abundance over time (difference score: post-intervention – pre-intervention) as a function of experimental condition (generativity vs. control), with ancillary analyses additionally controlling for individual differences in age, BMI, white vs. non-white race, presence of illness symptoms near the time of blood sampling, and alcohol consumption (history of smoking was also measured but was absent in all subjects), or controlling for mRNA transcripts indicating the relative prevalence of leukocyte subsets within the total PBMC pool (CD3D, CD3E, CD4, CD8A, CD19, NCAM1/CD56, FCGR3A/CD16, and CD14). For exploratory analyses of the 2-month follow-up data, gene expression values were analyzed by standard linear statistical models estimating the magnitude of difference in transcript abundance as a function of experimental condition.
Genes showing > 1.2-fold differential expression across condition served as input into higher-order bioinformatics analyses testing a priori-specified hypotheses regarding transcription control pathways involved in inflammation (NF-κB, measured by the TRANSFAC position-specific weight matrix V$NFKAPPAB_01) using TELiS promoter sequence analysis (Cole, Yan, Galic, Arevalo, & Zack, 2005), and assessing the relative contribution of CD16− classical monocytes versus CD16+ non-classical monocytes to the observed transcriptome differences using Transcript Origin Analysis (Cole, Hawkley, Arevalo, & Cacioppo, 2011) with reference data from a previous study of isolated monocyte subsets (GSE26913; Wong et al., 2011). Statistical testing was based on standard errors derived from bootstrap resampling of linear model residual vectors (controlling for potential correlation across genes).
3. Results
3.1. Characteristics of the sample
As described above, the final sample analyzed consisted of 73 participants (100% female; mean age 70.9 ± 6.5 years; range: 60–86 years; 80.8% white). Of these 73 participants, 35 were randomized into the control condition and 38 were randomized into the generativity condition. The groups were not significantly different on covariates of interest (i.e., age, race, BMI, cold symptoms, alcohol consumption). For a demographic table, please see Supplementary Material.
3.2. Weekly intervention
There was a high completion rate of the intervention, with 72 out of the 73 participants completing 100% of the weekly writing assignments (the remaining participant completed five out of six assignments). There were no between-group differences in the number of words written each week (F(1,71)=.58, p = .45; generativity mean = 364; control mean = 395).
3.3. Effects on weekly post-writing feelings of momentary generativity
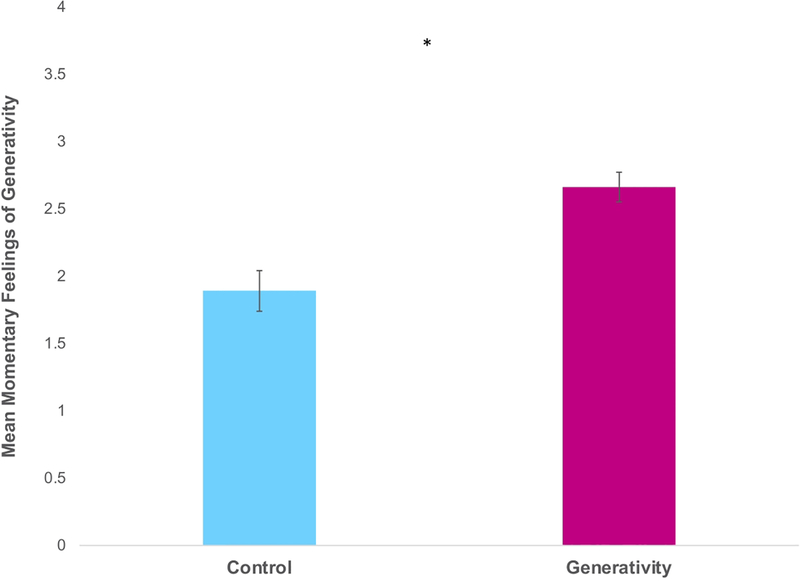
We examined differences in participants’ feelings of momentary generativity immediately post-writing. The generativity group reported feeling more generative (Figure 1; F(1,70)=19.54, p < .001; η2= .21, pSimes=.004) post-writing, averaged across all 6 weeks.
Figure 1.
Effects of the intervention on momentary feelings of generativity immediately post-writing in the generativity and control groups. Scores were averaged across all 6 weeks of the intervention. Errors bars depict the standard error of the mean. Asterisk reflects p-value of p < .001 from the reported ANCOVA analysis.
3.4. Effects on global feelings of generativity
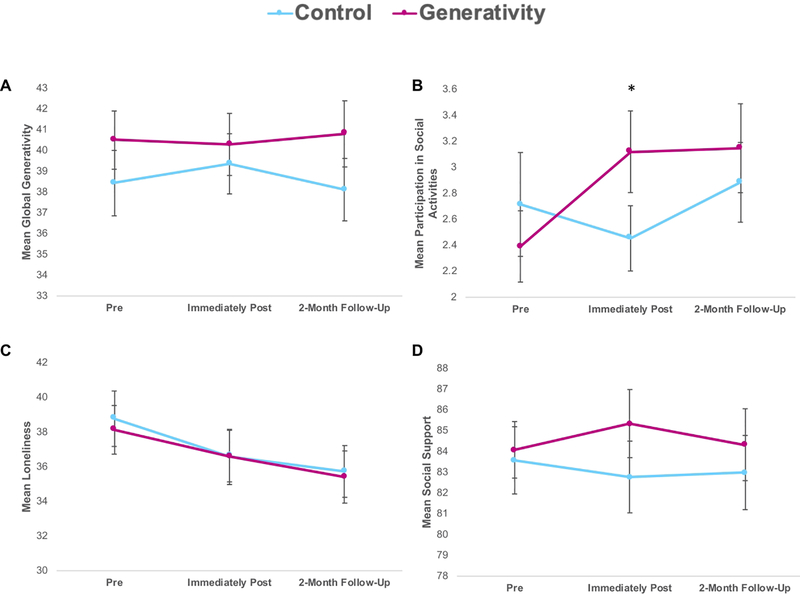
We then tested the effects of the generativity intervention on global feelings of generativity by looking at post-intervention differences between the groups on the Loyola Generativity Scale (LGS). Contrary to our hypotheses, the generativity group (vs. control) did not show increases in global feelings generativity (Figure 2A; F(1,70)=.53, p = .48, pSimes=.53).
Figure 2.
Effects of the intervention on global feelings of generativity and social well-being in the generativity and control groups. Mean pre-intervention, immediately post-intervention, and 2-month follow-up scores are depicted for visual purposes; all analyses were ANCOVAs, examining differences between conditions on post-intervention values (for the primary aim) and differences between conditions on follow-up values (for the exploratory aim), controlling for pre-intervention values (and white/non-white race and age, if p < .1 as covariates). Mean values at pre-intervention, post- intervention, and 2-month follow-up shown for a) Loyola Generativity Scale (global feelings of generativity), b) Participation in social activities on the Lifestyle Activities Questionnaire, c) UCLA Loneliness Scale, and d) Total score on Social Provisions Scale. Error bars depict the standard error of the mean. Asterisk reflects p-value of p < .01 from the reported ANCOVA analysis.
3.5. Effects on social well-being
In order to test the effect of the intervention on social well-being, we examined post-intervention differences between the groups on participation in social activities, feelings of loneliness, and social support. As hypothesized, the generativity group (vs. control group) reported increased participation in social activities post-intervention (Figure 2B; F(1,69)=7.61 p = .007; η2=.06, pSimes=.032).
However, the two groups did not significantly differ in feelings of loneliness (Figure 2C; F(1,70) = .27, p =.61, pSimes=.61) or social support (Figure 2D; F(1,70)=2.52, p = .12, pSimes=.18) post-intervention.
3.6. Effects on mental health
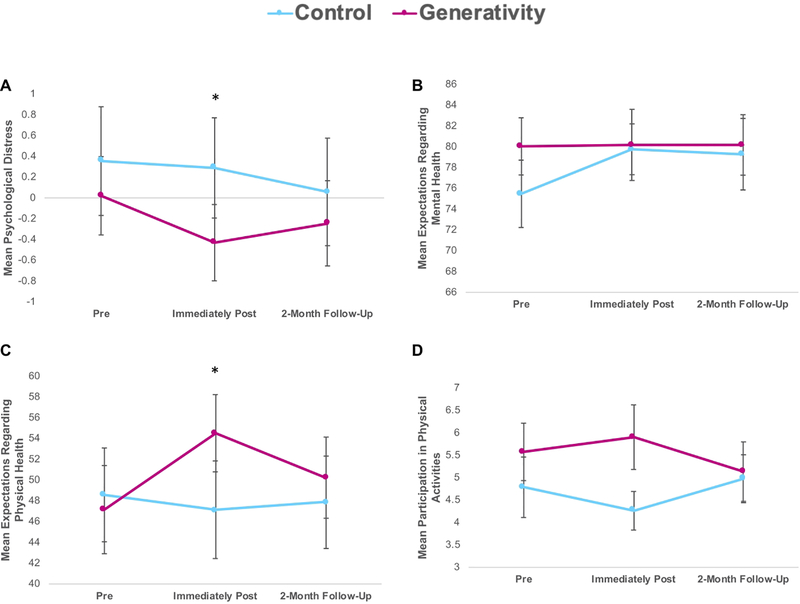
We also examined the impact of the intervention on mental health by testing differences between the groups in psychological distress and in their expectations regarding aging in the mental health domain post-intervention. As hypothesized, the generativity intervention had a positive impact on psychological distress, with the generativity group (vs. control group) reporting lower though perhaps marginal psychological distress post-intervention (Figure 3A; F(1,69)=4.22, p = .044; η2=.01, pSimes=.10).
Figure 3.
Effects of the intervention on mental health and physical health in the generativity and control groups. Mean pre-intervention, immediately post-intervention, and 2-month follow-up scores are depicted for visual purposes; all analyses were ANCOVAs, examining differences between conditions on post-intervention values (for the primary aim) and differences between conditions on follow-up values (for the exploratory aim), controlling for pre-intervention values (and white/non-white race and age, if p < .1 as covariates). Mean values at pre-intervention, post- intervention, and 2-month follow-up shown for: a) Psychological distress (composite of Beck Depression Inventory, Spielberger Trait Anxiety, and Perceived Stress Scale), b) Expectations Regarding Mental Health on Expectations Regarding Aging Survey, c) Expectations Regarding Physical Health on Expectations Regarding Aging Survey, and d) Participation in physical activities on the Lifestyle Activities Questionnaire. Error bars depict the standard error of the mean. Asterisks reflect p-values of p < .05 from the reported ANCOVA analyses.
However, the intervention did not have an impact on expectations regarding aging in the mental health domain at post-intervention (Figure 3B; F(1,69)=.57, p = .46, pSimes=.59).
3.7. Effects on physical health
To probe the effects of the intervention on physical health, we tested whether the intervention led to improvements in participation in physical activity and expectations regarding aging in the physical health domain. As hypothesized, the intervention led to significantly more positive expectations regarding aging in the physical domain (Figure 3C; F(1,69)=6.47, p = .013; η2= .03, pSimes = .039) though unclear improvements in participation in physical activities (Figure 3D; F(1,70)=3.30, p = .074; η2= .02, pSimes=.14) post-intervention.
3.8. Effects on inflammation
3.8.1. Circulating cytokines
In order to test whether the generativity intervention led to decreases in cytokines, we examined differences between the groups on circulating plasma levels of IL-6 and TNF-α post-intervention (see Supplementary Material for mean plasma levels). However, the intervention did not lead to any significant differences in plasma concentrations of IL-6 (F(1,64)=.75, p = .40) or TNF-α (F(1,64)=.74, p = .40) between the two groups at post-intervention.
3.8.2. Gene expression and bioinformatics
To identify the impact of the generativity intervention on transcriptional control pathways, we conducted promoter-based bioinformatics analyses to evaluate genes showing a ≥ 1.2-fold difference in the magnitude of change from pre- to post-intervention in response to the generativity (vs. control) condition. A total of 2300 distinct gene transcripts were up-regulated in the generativity group relative to the control group and 811 were down-regulated. Among the genes down-regulated in response to the generativity condition (vs. control) were transcripts encoding the key pro-inflammatory cytokines, IL1B and IL6.
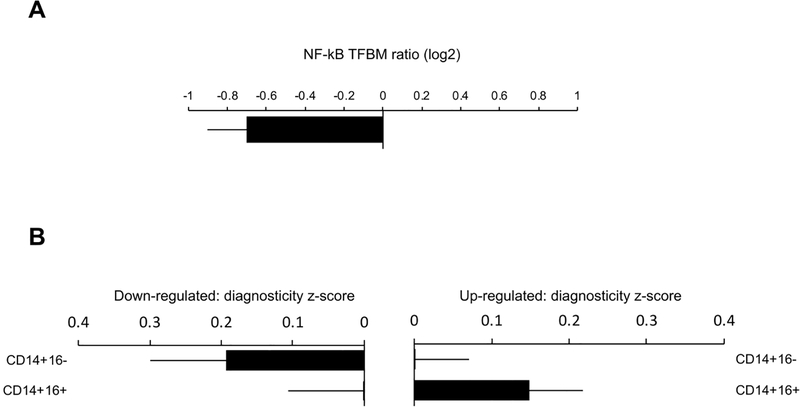
Using TELiS promoter-based bioinformatics analyses, we examined differences in the prevalence of transcription factor-binding motifs for the pro-inflammatory transcription factor, NF-κB, among all 2,300 genes showing ≥ 1.2-fold up-regulation vs. all 811 showing ≥ 1.2-fold down-regulation as a function of intervention condition. These analyses found NF-κB binding sites to be significantly more prevalent within the promoters of genes that were down-regulated in response to the generativity (vs. control) condition (Figure 4A; unadjusted: mean difference = −.699 ± standard error .204 log2 ratio, p = .0007; adjusted for demographic, behavioral, and BMI covariates: −.452 ± .223, p = .0441; adjusted for leukocyte subset distributions: −.441 ± .192, p = .0227). Finally, we tested whether the differentially expressed genes tended to derive from specific cell types known to mediate inflammatory responses – particularly CD16− “classical” monocytes (Powell et al., 2013). Transcript Origin Analyses showed that the genes that were relatively down-regulated as a function of the generativity (vs. control) intervention tended to derive predominately from the immature CD16− pro-inflammatory monocyte subset (Figure 4B (left panel); unadjusted: mean diagnosticity z score = .194 ± .106, p = .0332; adjusted for demographic, behavioral, and BMI covariates: .147 ± .073, p = .0221), whereas genes relatively up-regulated as a function of the generativity (vs. control) intervention derived predominately from the less inflammatory and more reparative CD16+ monocyte subset (Figure 4B (right panel); unadjusted: .148 ± .070, p = .0170; adjusted for demographic, behavioral, and BMI and covariates: .114 ± .067, p = .0438).
Figure 4.
a) Relative change over time in NF-kB activity for the generativity intervention participants relative to the control condition participants, as inferred from NF-kB transcription factor-binding motifs in the promoters of differentially expressed genes (≥ 1.2-fold down or up-regulated as a function of the generativity intervention). b) Transcript origin analyses to determine cellular origins of differentially expressed genes (≥ 1.2-fold down or up-regulated as a function of the generativity intervention). Genes down-regulated as a function of the generativity (vs. control) condition (left panel) tended to derive predominately from the immature CD16− pro-inflammatory monocyte subset, whereas up-regulated genes as a function of the generativity (vs. control) condition (right panel) derived predominately from the less inflammatory and more reparative CD16+ monocyte subset.
3.9. Exploratory aim: effects at 2-month follow-up
Finally, we examined our exploratory aim of whether the groups were different at the 2-month follow-up. No effects were present at the 2-month follow up for behavioral measures (p’s > .2), circulating cytokines (p’s > .2), or inflammatory gene expression (p’s > .5).
4. Discussion
This study assessed the impact of a novel, writing-based intervention aimed at increasing feelings of generativity, or contributing to others, especially younger generations. The generativity intervention led to beneficial changes across various health and well-being domains, including social well-being, mental health, physical health, and pro-inflammatory gene expression immediately post-intervention. Those in the generativity condition reported greater participation in social activities, decreases in psychological distress, more positive expectations of aging regarding physical health, and marginally greater participation in physical activities. Those in the generativity intervention also demonstrated reductions in pro-inflammatory gene expression. Together, these results suggest that this type of brief social psychological intervention can lead to immediate benefits for health and well-being in older adults.
Furthermore, although the intervention did not improve global feelings of generativity (i.e., scores on the Loyola Generativity Scale (LGS)) pre- to post-intervention, participants in the generativity condition did feel more generative immediately post-writing as they completed the intervention, suggesting there was some impact on feelings of momentary generativity. In other words, we observed increases in the psychological state of generativity during the intervention (i.e., increases in momentary feelings of generativity) but no differences in this psychological state (i.e., LGS scores) directly post-intervention. Interestingly, it has been argued that, in the context of interventions, even when the manipulated psychological state is no longer present, that psychological state might have initiated a chain of behaviors that is ultimately responsible for the observed outcomes (Miller, Dannals, & Zlatev, 2017). Thus, the increases we observed in momentary feelings of generativity might have led participants to engage in certain behaviors (e.g., increases in social activities) and led to our observed outcomes.
Although this is the first investigation of the health effects of a writing-based generativity intervention, the results of the study nicely complement the existing literature on generativity and its related constructs. Correlational studies have found that generativity, as well as feeling useful to others, is linked to positive health outcomes, such as well-being, lower disability, and longevity in older adults (An & Cooney, 2006; Gruenewald et al., 2007; Gruenewald et al., 2012). Relatedly, engaging in productive activities such as volunteering, which may increase feelings of generativity, has also been associated with lower C-reactive protein, a marker of inflammation (S. Kim & Ferraro, 2013). Positive health correlates of giving support to others have also been established (Konrath & Brown, 2013), which may be relevant to generativity, particularly if the support-giving is to younger generations.
A few experimental studies also support the notion that generativity may positively impact health and well-being. The Experience Corps program, an intergenerational volunteering program which increases feelings of generativity, has led to improvements in health in older adults (Gruenewald et al., 2015; Hong & Morrow-Howell, 2010). Similarly, a volunteering intervention in adolescents led to decreases in circulating levels of IL-6 (Schreier, Schonert-Reichl, & Chen, 2013). Another trial in a community sample of diverse ages also found that prosocial behavior directed towards others led to decreases in pro-inflammatory gene expression (Nelson-Coffey, Fritz, Lyubomirsky, & Cole, 2017). In sum, these correlational and experimental findings point to the potential for generativity, and its related constructs such as volunteering and prosocial behavior, to positively impact well-being and health in older adults, which support the results of the present study.
Why might a generativity intervention lead to such improvements? There are likely several biopsychosocial mechanisms to explain the benefits, but one potential mechanism is through activation of the mammalian caregiving system, as the caregiving system can dampen threat-related responding, which may ultimately lead to health benefits (Eisenberger & Cole, 2012). For example, giving support to others has been found to lead to reduced threat-related neural activation and decreases in sympathetic nervous system activity (Inagaki & Eisenberger, 2012, 2015), which may have downstream effects on inflammation and health (Eisenberger & Cole, 2012; Irwin & Cole, 2011). Given that an important component of generativity involves feeling one has contributed to younger generations, generativity may have co-opted this caregiving system. Thus, generativity may lead to improvements in inflammation and ultimately health through the dampening of threat-related physiology as part of this caregiving system. Although this study was not designed to test this hypothesis directly, future studies should test these mechanisms (e.g., by testing caregiving-related neural correlates and mediators of generativity).
Additional psychological mechanisms may also account for the intervention’s benefits. For example, by increasing feelings of usefulness and feeling needed by others, the generativity intervention may have also boosted participants’ feelings of self-esteem or competence and self-worth. Interestingly, greater self-esteem is associated with reduced inflammatory (O’Donnell, Brydon, Wright, & Steptoe, 2008) and neuroendocrine reactivity (Seeman et al., 1995) to stress. Furthermore, self-esteem is linked to better mental health (Sowislo & Orth, 2013) and some aspects of physical health (Trzesniewski et al., 2006). Thus, a potential increase in self-esteem from the intervention may also help explain the benefits of the intervention and, as measures of self-esteem were not included in the present study, should be directly tested by future studies.
Although this study suggests that generativity can lead to improvements across several health domains, certain limitations should be considered. It is worth noting that while the generativity intervention did improve at least one outcome in each of the health domains measured, it did not improve all outcomes. There are several reasons that could contribute to the lack of improvement on some measures. First, there is the possibility that the generativity intervention truly only has an impact on certain variables, and not others. Second, there could be floor or ceiling effects on certain variables. For example, while participants in the generativity intervention expressed more positive expectations regarding physical health, they did not improve in their expectations regarding mental health. This may have been partly driven by the fact that the pre-intervention level (across both groups) of expectations regarding aging in the physical health domain were much lower than the mental health domain (physical health mean = 47.8; mental health mean = 77.9). The more positive pre-intervention expectations towards mental health than physical health suggest that one potential contributor to the lack of the intervention’s effect on the mental health domain could be that participants already had more positive expectations of mental health and aging compared to physical health. Finally, it may be possible that certain variables are more reflective of “trait”, stable constructs and generally less likely to be affected by a brief intervention. For example, the intervention impacted in-the-moment feelings of generativity, but not LGS scores. The LGS, with items such as “I have made many commitments to many different kinds of people, groups, and activities in my life,” could perhaps be reflective of more trait-like feelings of generativity and life-long commitments to generative activities, which may be difficult to influence with a brief intervention.
Additionally, it is worth highlighting that although the intervention led to improvements when testing our primary hypotheses (pre- to post-intervention changes), none of the effects that were present at post-intervention were present when examining our exploratory aim (i.e., the outcomes at the 2-month follow-up visit). This suggests that this type of intervention may need to be ongoing, with continued engagement in the activity, in order to confer benefits. Future generativity and related interventions should also test whether improvements in outcomes are only seen while the intervention is occurring or whether other types of generativity interventions lead to sustained changes even after the intervention has ended. Finally, the study sample was comprised of exclusively women, who were relatively healthy and predominantly white. Future studies should build on this intervention by examining the impact of a writing-based generativity intervention in men, clinical samples, and more diverse samples
Despite these limitations, the study also has several important strengths. Importantly, it provides the first evidence that a writing-based intervention to increase generativity can impact health and well-being in older adults. The study also included a neutral control group, whereas some other positive psychological studies have used negative or “listing of hassles” control conditions (e.g., counting of blessings vs. burdens; Emmons & McCullough, 2003). Another strength of the study is the examination of multiple domains of well-being and health, including social well-being, and mental and physical health. Furthermore, not only is this the first study to examine the influence of generativity on inflammation, but it also included multiple levels of analysis of inflammatory biology including both circulating and gene expression measures of inflammation.
Overall, this study introduces an innovative intervention with positive effects on social, mental, and physical well-being, as well as inflammatory biology. Additionally, the study involved minimal time commitment and physical exertion on the part of the participants, providing a potential intervention that may improve health for large segments of the older adult population who may not be able to or wish to participate in more intensive interventions. Indeed, given the limited physical mobility, time, and cost needed to complete this intervention, this could be a potentially impactful, low-cost, low-effort intervention to improve health and well-being in an aging population.
Future work could build on the results of these findings, furthering the scientific study of psychosocial interventions in older adults, particularly interventions intended to increase feelings of being useful to, needed by, and giving back to others. Furthermore, creating opportunities for additional generative activity may not only improve the health and well-being of those doing the generative acts but may also provide numerous benefits for the people and society on the receiving end of these actions. Indeed, generativity interventions could eventually have broad implications not only for the well-being of the fastest-growing segment of the global population but also for the well-being of the world they will ultimately leave behind.
Supplementary Material
Highlights.
Examined effects of a novel, writing-based generativity intervention in older women
Generativity intervention led to improvements in multiple domains of well-being
Generativity intervention led to decreases in inflammation
Suggests a low-cost, low effort intervention to improve well-being in older adults
Acknowledgments
This research was funded by an R03 from NIA to NIE (R03AG049254). This project was also supported by the UCLA Clinical and Translational Science Institute (UL1TR000124; Seed Grant) and the UCLA Older Adults Independence Center (5P30AG028748; Rapid Pilot Grant). Additionally, the first author was supported by a pre-doctoral NRSA fellowship from NIA (F31AG048668) and a post-doctoral fellowship from the UCLA Norman Cousins Center for Psychoneuroimmunology. The aforementioned funders provided financial support for the study, but they were not involved in the conduct of the study in any other capacity (e.g., design, data collection, manuscript preparation, etc.).
We would also like to thank the staff of the UCLA Clinical and Translational Research Center, staff of the UCLA Pathology Research Portal, staff of the UCLA Inflammatory Biology Core (including Stephanie Esquivel, Nancy Herrera-Morales, Nabil Aziz, Robert Bang, and Christian Perez), and staff of the UCLA Social Genomics Core. Additionally, we would like to thank Kanika Shirole, Nina Sadeghi, Carmen Carrillo, Richard Olmstead, Heather McCreath, and Kate E. Byrne Haltom, for their assistance with various aspects of the project. Thank you to members, past and present, of the UCLA SCAN labs for providing valuable feedback on several aspects of this study. Finally, thank you to Spencer Bujarski, Ph.D., for providing statistical consulting.
Footnotes
Competing Interests
The authors declare no conflicts of interest.
Due to technical issues, one of the items in the mental health domain of the Expectations Regarding Aging Survey was from the 38-item version of the scale (Sarkisian, Hays, Berry, & Mangione, 2002) rather than the intended 12-item version. The item in the 12-item scale that reads “as people get older they worry more” (item #7) instead read “quality of life declines as people age.” Removing this item from the scale does not change the results of the analyses.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An JS, & Cooney TM (2006). Psychological well-being in mid to late life: The role of generativity development and parent–child relationships across the lifespan. International Journal of Behavioral Development, 30(5), 410–421. [Google Scholar]
- Beck AT, Steer RA, & Carbin MG (1988). Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical psychology review, 8(1), 77–100. [Google Scholar]
- Carlson MC, Parisi JM, Xia J, Xue Q-L, Rebok GW, Bandeen-Roche K, & Fried LP (2011). Lifestyle activities and memory: variety may be the spice of life. The women’s health and aging study II. Journal of the International Neuropsychological Society, 18(2), 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MC, Seeman T, & Fried LP (2000). Importance of generativity for healthy aging in older women. Aging clinical and experimental research, 12(2), 132–140. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of health and social behavior, 385–396. [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, & Cacioppo JT (2011). Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proceedings of the National Academy of Sciences, 108(7), 3080–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Yan W, Galic Z, Arevalo J, & Zack JA (2005). Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics, 21(6), 803–810. [DOI] [PubMed] [Google Scholar]
- Cutrona CE (1984). Social support and stress in the transition to parenthood. Journal of abnormal psychology, 93(4), 378. [DOI] [PubMed] [Google Scholar]
- Cutrona CE, & Russell DW (1987). The provisions of social relationships and adaptation to stress. Advances in personal relationships, 1(1), 37–67. [Google Scholar]
- Eisenberger NI, & Cole SW (2012). Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nature neuroscience, 15(5), 669–674. [DOI] [PubMed] [Google Scholar]
- Emmons RA, & McCullough ME (2003). Counting blessings versus burdens: an experimental investigation of gratitude and subjective well-being in daily life. Journal of personality and social psychology, 84(2), 377. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti M-C, Cohen HJ,… Havlik RJ (1999). Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc, 47(6), 639–646. [DOI] [PubMed] [Google Scholar]
- Fisher BJ (1995). Successful aging, life satisfaction, and generativity in later life. The International Journal of Aging and Human Development, 41(3), 239–250. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Karlamangla AS, Greendale GA, Singer BH, & Seeman TE (2007). Feelings of usefulness to others, disability, and mortality in older adults: The MacArthur study of successful aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 62(1), P28–P37. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Karlamangla AS, Greendale GA, Singer BH, & Seeman TE (2009). Increased mortality risk in older adults with persistently low or declining feelings of usefulness to others. Journal of aging and health, 21(2), 398–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Liao DH, & Seeman TE (2012). Contributing to others, contributing to oneself: Perceptions of generativity and health in later life. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 67(6), 660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Tanner EK, Fried LP, Carlson MC, Xue Q-L, Parisi JM,… Seeman TE (2015). The Baltimore Experience Corps Trial: Enhancing Generativity via Intergenerational Activity Engagement in Later Life. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, gbv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, & Morrow-Howell N (2010). Health outcomes of Experience Corps®: A high-commitment volunteer program. Social science & medicine, 71(2), 414–420. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, & Eisenberger NI (2012). Neural correlates of giving support to a loved one. Psychosom Med, 74(1), 3–7. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, & Eisenberger NI (2015). Giving support to others reduces sympathetic nervous system-related responses to stress. Psychophysiology. [DOI] [PubMed] [Google Scholar]
- Irwin MR, & Cole SW (2011). Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology, 11(9), 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes CLM, & Ryff CD (1998). Generativity in adult lives: Social structural contours and quality of life consequences. [Google Scholar]
- Kim D, Langmead B, & Salzberg SL (2015). HISAT: a fast spliced aligner with low memory requirements. Nature methods, 12(4), 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, & Ferraro KF (2013). Do Productive Activities Reduce Inflammation in Later Life? Multiple Roles, Frequency of Activities, and C-Reactive Protein. The Gerontologist, gnt090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrath S, & Brown S (2013). The effects of giving on givers In Roberts N & Newman M (Eds.), Handbook of Health and Social Relationships: APA Books. [Google Scholar]
- Layous K, Nelson SK, & Lyubomirsky S (2012). What is the optimal way to deliver a positive activity intervention? The case of writing about one’s best possible selves. Journal of Happiness Studies, 1–20. [Google Scholar]
- Lyubomirsky S, & Layous K (2013). How Do Simple Positive Activities Increase Well-Being? Current Directions in Psychological Science, 22(1), 57–62. [Google Scholar]
- McAdams DP, & De St Aubin E (1992). A theory of generativity and its assessment through self-report, behavioral acts, and narrative themes in autobiography. Journal of personality and social psychology, 62(6), 1003. [Google Scholar]
- McAdams DP, & de St Aubin E (1998). Generativity and adult development. Washington, DC: American Psychological Association. [Google Scholar]
- Mendiondo MS, Ashford JW, Kryscio RJ, & Schmitt FA (2003). Designing a brief alzheimer screen (BAS). Journal of Alzheimer’s Disease, 5(5), 391–398. [DOI] [PubMed] [Google Scholar]
- Miller DT, Dannals JE, & Zlatev JJ (2017). Behavioral processes in long-lag intervention studies. Perspectives on psychological science, 12(3), 454–467. [DOI] [PubMed] [Google Scholar]
- Nelson-Coffey SK, Fritz MM, Lyubomirsky S, & Cole SW (2017). Kindness in the Blood: A Randomized Controlled Trial of the Gene Regulatory Impact of Prosocial Behavior. Psychoneuroendocrinology. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Brydon L, Wright CE, & Steptoe A (2008). Self-esteem levels and cardiovascular and inflammatory responses to acute stress. Brain, behavior, and immunity, 22(8), 1241–1247. [DOI] [PubMed] [Google Scholar]
- Parisi JM, Kuo J, Rebok GW, Xue Q-L, Fried LP, Gruenewald TL,… Tanner EK (2015). Increases in lifestyle activities as a result of Experience Corps® participation. Journal of Urban Health, 92(1), 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi JM, Rebok GW, Seeman TE, Tanner EK, Tan EJ, Fried LP,… Carlson MC (2012). Lifestyle Activities in Sociodemographically At-Risk Urban, Older Adults Prior to Participation in the Baltimore Experience Corps® Trial. Activities, adaptation & aging, 36(3), 242–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M, Kim D, Pertea GM, Leek JT, & Salzberg SL (2016). Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nature Protocols, 11(9), 1650–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piber D, Olmstead R, Cho JH-J, Witarama T, Perez C, Dietz N,… Irwin MR (2019). Inflammaging: Age and Systemic, Cellular, and Nuclear Inflammatory Biology in Older Adults. The Journals of Gerontology: Series A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Population Reference Bureau. (2011). World Population Aging: Clocks Illustrate Growth in Population Under Age 5 and Over Age 65. Retrieved from http://www.prb.org/Publications/Articles/2011/agingpopulationclocks.aspx
- Russell DW (1996). UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. Journal of personality assessment, 66(1), 20–40. [DOI] [PubMed] [Google Scholar]
- Sarkisian CA, Hays RD, Berry S, & Mangione CM (2002). Development, reliability, and validity of the expectations regarding aging (ERA-38) survey. The Gerontologist, 42(4), 534–542. [DOI] [PubMed] [Google Scholar]
- Sarkisian CA, Steers WN, Hays RD, & Mangione CM (2005). Development of the 12-item expectations regarding aging survey. The Gerontologist, 45(2), 240–248. [DOI] [PubMed] [Google Scholar]
- Schoklitsch A, & Baumann U (2012). Generativity and aging: A promising future research topic? Journal of Aging Studies, 26(3), 262–272. [Google Scholar]
- Schreier HM, Schonert-Reichl KA, & Chen E (2013). Effect of volunteering on risk factors for cardiovascular disease in adolescents: A randomized controlled trial. JAMA pediatrics, 167(4), 327–332. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Berkman LF, Gulanski BI, Robbins RJ, Greenspan SL, Charpentier PA, & Rowe JW (1995). Self-esteem and neuroendocrine response to challenge: MacArthur studies of successful aging. Journal of psychosomatic research, 39(1), 69–84. [DOI] [PubMed] [Google Scholar]
- Sowislo JF, & Orth U (2013). Does low self-esteem predict depression and anxiety? A meta-analysis of longitudinal studies. Psychological Bulletin, 139(1), 213–240. [DOI] [PubMed] [Google Scholar]
- Spielberger CD (2010). State-Trait Anxiety Inventory: Wiley Online Library. [Google Scholar]
- Trzesniewski KH, Donnellan MB, Moffitt TE, Robins RW, Poulton R, & Caspi A (2006). Low self-esteem during adolescence predicts poor health, criminal behavior, and limited economic prospects during adulthood. Developmental psychology, 42(2), 381. [DOI] [PubMed] [Google Scholar]
- Van Breukelen GJ (2006). ANCOVA versus change from baseline had more power in randomized studies and more bias in nonrandomized studies. Journal of clinical epidemiology, 59(9), 920–925. [DOI] [PubMed] [Google Scholar]
- Wong KL, Tai JJ-Y, Wong W-C, Han H, Sem X, Yeap W-H,… Wong S-C (2011). Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood, 118(5), e16–e31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.