Abstract
Background
The role of the airway microbiome in the development of recurrent wheezing and asthma remains uncertain, particularly in the high-risk group of infants hospitalized for bronchiolitis.
Objective
We sought to examine the relation of the nasal microbiota at bronchiolitis-related hospitalization and 3 later points to the risk of recurrent wheezing by age 3 years.
Methods
In 17 US centers researchers collected clinical data and nasal swabs from infants hospitalized for bronchiolitis. Trained parents collected nasal swabs 3 weeks after hospitalization and, when healthy, during the summer and 1 year after hospitalization. We applied 16S rRNA gene sequencing to all nasal swabs. We used joint modeling to examine the relation of longitudinal nasal microbiota abundances to the risk of recurrent wheezing.
Results
Among 842 infants hospitalized for bronchiolitis, there was 88% follow-up at 3 years, and 31% had recurrent wheezing. The median age at enrollment was 3.2 months (interquartile range, 1.7-5.8 months). In joint modeling analyses adjusting for 16 covariates, including viral cause, a 10% increase in relative abundance of Moraxella or Streptococcus species 3 weeks after day 1 of hospitalization was associated with an increased risk of recurrent wheezing (hazard ratio [HR] of 1.38 and 95% high-density interval [HDI] of 1.11-1.85 and HR of 1.76 and 95% HDI of 1.13-3.19, respectively). Increased Streptococcus species abundance the summer after hospitalization was also associated with a greater risk of recurrent wheezing (HR, 1.76; 95% HDI, 1.15-3.27).
Conclusions
Enrichment of Moraxella or Streptococcus species after bronchiolitis hospitalization was associated with recurrent wheezing by age 3 years, possibly providing new avenues to ameliorate the long-term respiratory outcomes of infants with severe bronchiolitis.
Key words: Bronchiolitis, recurrent wheezing, Moraxella species, Streptococcus species, Haemophilus species, respiratory syncytial virus, rhinovirus, longitudinal studies, microbiome
Abbreviations used: HDI, High-density interval; HR, Hazard ratio; MARC-35, 35th Multicenter Airway Research Collaboration; NPA, Nasopharyngeal aspirate; RSV, Respiratory syncytial virus
Bronchiolitis is an acute respiratory tract infection and the leading cause of hospitalization for US infants, accounting for approximately 130,000 hospitalizations annually.1 In addition to this acute severity of illness, these hospitalized infants are 3 to 4 times more likely than healthy control subjects to have recurrent wheezing and childhood asthma.2 Unfortunately, the infants who will have this long-term respiratory morbidity after a bronchiolitis hospitalization remain incompletely defined.
To date, most longitudinal bronchiolitis cohort studies have used 1 of the 2 most common viral causes of bronchiolitis, respiratory syncytial virus (RSV) and rhinovirus, as the primary exposure.2 However, respiratory tract viruses infect infants in airways colonized with highly functional bacteria.3 , 4 Emerging evidence suggests that the bacterial composition of the airway is associated not only with acute severity outcomes5 but also long-term respiratory outcomes.6
More than a decade ago, Bisgaard et al,7 using conventional culture, reported that hypopharyngeal colonization by Moraxella, Haemophilus, or Streptococcus species in asymptomatic 1-month-old infants was associated with recurrent wheezing and childhood asthma. More recently, cross-sectional, culture-independent studies have shown that when compared with healthy control subjects, children and adults with asthma have airway microbiota compositions enriched with Proteobacteria (eg, Moraxella and Haemophilus species).4 , 8, 9, 10, 11 Furthermore, longitudinal microbiota studies in the first 2 years of life have found that Moraxella, Haemophilus, and Streptococcus species dominate nasopharyngeal communities during acute respiratory tract infection6 , 12 and in sensitized children are associated with chronic wheeze at age 5 years.6
To date, however, no study has examined in a multicenter cohort of infants hospitalized for bronchiolitis the association between the longitudinal composition of the nasal microbiota and risk of recurrent wheezing of childhood. Indeed, this is the first longitudinal microbiota analysis of the present cohort.5 , 13 We hypothesized that a posthospitalization increase in Moraxella species relative abundance is associated with a greater risk of recurrent wheezing by age 3 years.
Methods
Study design, setting, and participants
As previously described,5 site teams at 17 hospitals in 14 US states enrolled infants (age <1 year) hospitalized for bronchiolitis into the 35th Multicenter Airway Research Collaboration (MARC-35; http://www.emnet-usa.org/Marc_35/M35.htm). Attending physicians diagnosed an infant’s bronchiolitis based on the American Academy of Pediatrics definition.14 Enrollment occurred over 3 consecutive winter seasons (ie, November-April) from 2011-2014. The institutional review board at the 17 participating hospitals approved this study, and all families signed an informed consent form before participation.
Data collection
Research teams at each of the sites not only extracted clinical data from the emergency department and inpatient charts but also conducted structured interviews with parents/legal guardians during hospitalization for demographic, historical, and environmental information.
Nasopharyngeal aspirate collection
All site researchers collected nasopharyngeal aspirates (NPAs) within 24 hours of hospitalization by using the same equipment (eg, sample traps and suction catheters from Medline Industries [Mundelein, Ill]) and a standardized protocol, as previously described.15
Nasal swab collection
Within 24 hours of hospitalization, site teams collected an index nasal swab from both anterior nares by using a single nylon pediatric FLOQSwab (Copan, Brescia, Italy).15 This swab was collected either before the NPA collection or more than 2 hours after the NPA collection. During hospitalization, site teams taught parents how to collect the nasal swab sample. When possible, during the index sample collection, the site team swabbed 1 nare and had the parent practice their technique by swabbing the other nare. Three weeks after the date of hospitalization, using the same sample collection methods, trained parents collected the “clearance swab.” The summer (June, July, or August) after the hospitalization, when the children were healthy, the parents collected the “summer swab.” The year after the hospitalization, children were randomly assigned to a specific season for their healthy swab collection. Therefore one fourth of the parents were assigned to collect the “seasonal swab” over summer (June, July, and August), fall (September, October, and November), winter (December, January, and February), or spring (March, April, and May). All collected swabs were processed, as previously described.15
16S rRNA gene sequencing
As previously described,15 we sequenced the 16S rRNA gene V4 region of the nasal swab bacteria on the Illumina MiSeq platform (Illumina, San Diego, Calif). We used microbiota data with sufficient sequence depth (ie, ≥1000 reads per sample) for all analyses.
Quantitative RT-PCR assays
By using the NPA samples from hospitalization, quantitative RT-PCR assays were conducted at Baylor College of Medicine, as previously described, for 17 viruses: RSV (types A and B), rhinovirus, influenza (types A and B and 2009 novel H1N1), human metapneumovirus, human bocavirus, parainfluenza viruses (types 1, 2, and 3), adenovirus, enterovirus, and coronaviruses (NL-63, OC-43, HKU1, and 229E).16
Outcome measures
Based on biannual parent telephone interviews, the primary outcome for the present analysis was recurrent wheezing by age 3 years, as defined by the 2007 National Institutes of Health’s Expert Panel Report 3: having at least 2 corticosteroid-requiring exacerbations in 6 months or having at least 4 wheezing episodes in 1 year that last at least 1 day and affect sleep.17 There is no required or specified time frame between episodes of wheezing. Because wheezing in early childhood can be transient,18 we extended this outcome to children who had recurrent wheezing by age 3 years and also had asthma at age 4 years. We defined asthma at age 4 years using an epidemiologic definition: having both a physician’s diagnosis of asthma and either asthma medication use (eg, albuterol inhaler, and inhaled corticosteroids) or asthma-related symptoms (eg, wheezing and nocturnal cough) between the ages of 3 and 4 years.19
Statistical analyses
Analyses only included subjects with an index microbiota sample of sufficient quality. Microbiota samples collected after the onset of recurrent wheezing were excluded to maintain consistency between cross-sectional and longitudinal time-to-event analyses. Covariates for patients’ characteristics and clinical presentation collected at the index visit were compared between children with and without recurrent wheezing by using χ2 or Wilcoxon rank sum tests, as appropriate.
The unrarefied 16S rRNA gene sequence read counts were combined at the genus level. We defined the top 10 genera as those with the greatest mean relative abundance across all samples. Kruskal-Wallis rank sum tests assessed the significance of differences in age, α-diversity (Shannon index), and relative abundances of the top genera across all 4 time points, with P values for the top genera comparisons determined by using the Bonferroni correction for multiple testing.
To examine the relation of genus abundance to recurrent wheezing, we first performed cross-sectional analyses on the data. We calculated the overall mean microbial abundances for each genus of interest using the relative abundance across all time points. Based on microbial abundances at each of the 4 time points, we categorized the nasal samples into 2 groups: those greater than and those less than the mean. χ2 Tests determined the significance of the association between these groups and recurrent wheezing. Multiple comparison correction was not used in these analyses because they served as exploratory analyses for the joint modeling.
Next, we performed longitudinal analyses by constructing joint models for the longitudinal microbial data to quantify their relationship with the onset of recurrent wheezing.20 Specifically, we created a longitudinal mixed-effects generalized linear model to predict relative genus abundances over time using the 8 most abundant taxa and clinical covariates as the fixed effects, time as the random slope, and subject as the random intercept. These relative abundances and their interaction by time point were both scaled such that a 10% change in abundance was equivalent to a unit change and incorporated as time-dependent covariates into a Cox proportional hazards model with the outcome recurrent wheezing by age 3 years. We used Bayesian methods through a modified version of the rstanarm R package, assuming scaled default prior distributions. This Bayesian approach produces conservative model estimates without the multiple comparison problems found in frequentist approaches.21 Significance was determined by using the 95% high-density interval (HDI) of the posterior distribution of the log hazard ratio (HR) within a region of practical equivalence around zero.
To confirm joint model results, we generated Kaplan-Meier curves stratified by groups of participants identified by the joint model as being at greater risk for recurrent wheezing and used log-rank tests to determine statistical significance. We also conducted a joint model sensitivity analysis restricting the outcome to children who had recurrent wheezing by age 3 years and also had asthma at age 4 years.
For each of the high-risk groups identified by using the joint model, we generated locally weighted scatterplot smoothing (ie, loess) curves with 95% CIs for the relative abundances for the time after day 1 of hospitalization. The process of creating smoothed curves for each of the risk groups was repeated to include 8 of the top 10 genera; we removed 2 genera that showed imperceptible changes over time. To further improve clarity, we used a rug plot of sample times (instead of 95% CIs) to indicate the distribution of the observations.
Overlap between participant memberships in the risk groups identified by using the joint model was assessed with a Venn diagram. After removing group overlap, patients’ characteristics and clinical presentation were compared between the risk groups by using Fisher exact and Kruskal-Wallis tests, as appropriate.
The analysis used R version 3.3.3 software. All P values were 2-tailed, with P values of less than .05 were considered statistically significant.
Results
Patients’ characteristics
Of 921 infants in the longitudinal cohort, 842 had sufficient sequence depth (ie, ≥1000 reads per sample) and were included in the present analysis. The analytic and nonanalytic cohorts differed in antibiotic use before hospitalization (P = .02), with a greater percentage of infants in the nonanalytic cohort having used antibiotics (see Table E1 in this article’s Online Repository at www.jacionline.org). The analytic cohort had a median age at hospitalization of 3.2 months (interquartile range, 1.7-5.8 months), 60% were male, and 80% had no history of wheezing. We characterized the bacterial microbiota using 2086 nasal swabs (842 index, 599 clearance, 379 summer, and 266 seasonal swabs) collected during and after hospitalization for bronchiolitis. We had 88% follow-up at age 3 years. Overall, 265 (31%) infants had recurrent wheezing by age 3 years. Table I summarizes characteristics among children who did and did not have recurrent wheezing. In general, infants who had recurrent wheezing were older on study entry and had a mother with asthma, a personal history of eczema, and RSV-negative bronchiolitis.
Table I.
Characteristics and clinical presentations for 842 infants hospitalized for bronchiolitis by recurrent wheezing outcome
| Variables | Recurrent wheezing by 36 mo |
P value | |
|---|---|---|---|
| No, n = 577 (69%) | Yes, n = 265 (31%) | ||
| Characteristics | |||
| Age at index (mo), median (IQR) | 3.0 (1.5-5.8) | 3.6 (2.1-5.9) | .01 |
| Female sex | 232 (40) | 106 (40) | 1.00 |
| Race/ethnicity | .11 | ||
| Non-Hispanic white | 245 (43) | 127 (48) | |
| Non-Hispanic black | 127 (22) | 65 (25) | |
| Hispanic | 180 (31) | 67 (25) | |
| Other | 25 (4) | 6 (2) | |
| Maternal asthma | 100 (17) | 77 (29) | <.001 |
| Maternal smoking during pregnancy | 69 (12) | 45 (17) | .06 |
| Cesarean section delivery | 188 (33) | 90 (34) | .76 |
| Low birth weight (<2.3 kg) | 29 (5) | 22 (8) | .09 |
| Mostly breast-fed during first 3 mo | 254 (44) | 121 (46) | .71 |
| Postnatal smoke exposure | 90 (16) | 38 (14) | .71 |
| Child history of eczema | 72 (13) | 49 (19) | .03 |
| Daycare attendance | 129 (22) | 67 (25) | .40 |
| Presentation at hospitalization for bronchiolitis | |||
| History of antibiotics use before index visit | 172 (30) | 88 (33) | .36 |
| History of hospitalization before index visit | 87 (15) | 44 (17) | .64 |
| RSV∗ | 494 (86) | 196 (74) | <.001 |
| Rhinovirus∗ | 111 (19) | 62 (23) | .20 |
| IgE sensitization† | 118 (21) | 46 (17) | .34 |
| Intensive care use‡ | 82 (14) | 45 (17) | .35 |
All data are presented as numbers (percentages), unless otherwise indicated. P values were computed by using χ2 tests (categorical variables) and Wilcoxon rank sum tests (continuous variables).
IQR, Interquartile range.
Detected through NPA samples collected at the index visit.
Defined as detection of any positive values for serum allergen-specific IgE at the index visit.
Defined as admission to intensive care unit and/or use of mechanical ventilation (continuous positive airway pressure and/or intubation during inpatient stay, regardless of location) at any time during the index hospitalization.
Composition of the nasal microbiota during and after bronchiolitis-related hospitalization
The median age at the time of nasal swab collection, Shannon index scores, and abundances of top 10 nasal microbiota genera by swab collection time point are shown in Table E2 in this article’s Online Repository at www.jacionline.org. Nasal swab collections continued until a median age of 16 months. The α-diversity was lowest at the clearance time point and greatest at the seasonal time point. Among the genera of interest, Moraxella, Haemophilus, and Streptococcus species were all in the top 10 genera by relative abundance.
Relative abundance of Moraxella, Haemophilus, and Streptococcus species by time point and risk of recurrent wheezing by age 3 years
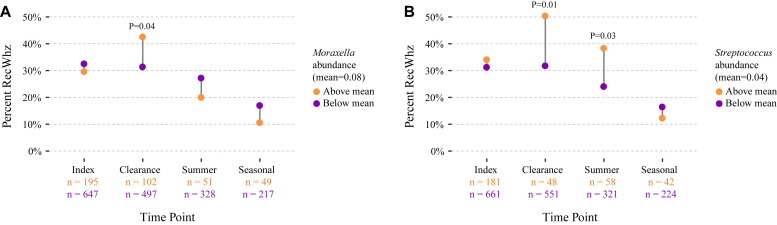
In cross-sectional analyses, on hospitalization, we found that the relative abundances of risk bacteria (ie, Moraxella, Haemophilus, and Streptococcus species) were not associated with recurrent wheezing by age 3 years. By contrast, at the clearance time point (ie, 3 weeks after day 1 of hospitalization), infants with a relative abundance of Moraxella species greater than the mean had significantly greater risk of recurrent wheezing by age 3 years than infants with relative abundances of less than the mean (P = .04; Fig 1 , A). In parallel analyses the association of Haemophilus species abundance with recurrent wheezing did not reach significance at any of the time points (see Fig E1 in this article’s Online Repository at www.jacionline.org). However, infants with Streptococcus species abundance of greater than the mean at either the clearance or summer time points had significantly greater risk of recurrent wheezing by age 3 years when compared with infants who had less than mean Streptococcus species abundance (P = .01 and P = .03, respectively; Fig 1, B).
Fig 1.
Cross-sectional comparison of Moraxella and Streptococcus species relative abundance and percentage of recurrent wheezing (RecWhz) outcomes. Microbiota samples were classified into those at greater than mean abundance (orange circles) and less than mean abundance (purple circles) by comparing the relative abundance of a genus at one of 4 time points (ie, index, clearance, summer, and seasonal) with the mean of this genus across all time points. The percentage of recurrent wheezing by age 3 years was computed at every time point for each group. The association between the abundance group (ie, greater than or less than the mean) and recurrent wheezing outcome at each time point was analyzed by using χ2 tests. Abundance group membership was not consistent across time points; a taxon might be protective at one time point and a risk factor for recurrent wheezing at a different time point. Samples collected after the onset of recurrent wheeze were excluded. A, Increased abundance of Moraxella species (mean = 0.08) at the clearance time point is significantly associated with a greater percentage of recurrent wheezing (P = .04). B, Increased abundance of Streptococcus species (mean = 0.04) at the clearance (P = .01) and summer (P = .03) time points is significantly associated with a greater percentage of recurrent wheezing.
Fig E1.
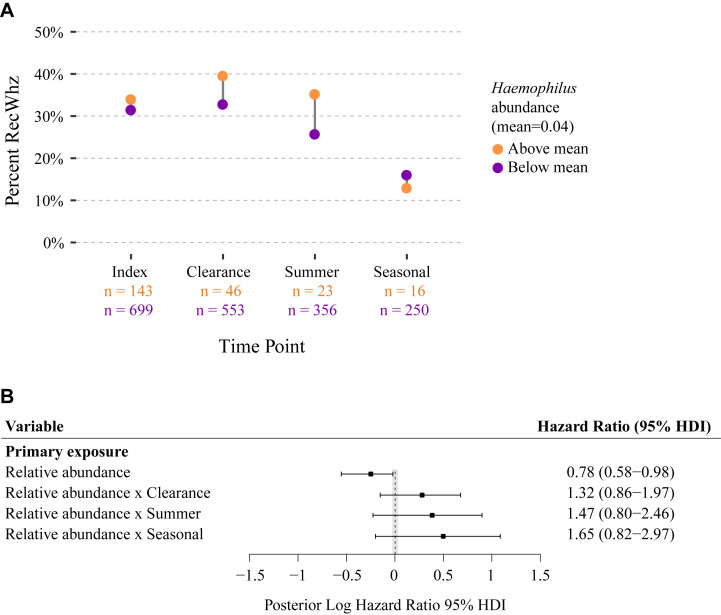
Relation of Haemophilus species relative abundance to recurrent wheezing (RecWhz) by age 3 years. A, Based on the mean abundance of Haemophilus species across all time points (mean = 0.04), microbiota samples were classified into greater than mean (orange circles) and less than mean (purple circles) groups. The percentage of recurrent wheezing was computed at every time point for each abundance group. Abundance group membership was not consistent across time points, and samples collected after onset of recurrent wheeze were excluded. B, HRs for relative abundance and abundance by time point interaction for Haemophilus species were calculated by using a Bayesian implementation of the joint model for longitudinal and time-to-event data. Bars and points indicate 95% HDIs and medians, respectively, for posterior distributions of log HRs. The shaded area represents the region of practical equivalence (ROPE) around zero, which was defined as [−0.05, 0.05]. Variables with HDIs that did not overlap the ROPE were considered statistically significant. The time-to-event model adjusted for clinical variables recorded at the index time point, with maternal asthma and RSV infection being significant in both models (data not shown).
Longitudinal Moraxella, Haemophilus, and Streptococcus species abundance after hospitalization and hazard of having recurrent wheezing by age 3 years
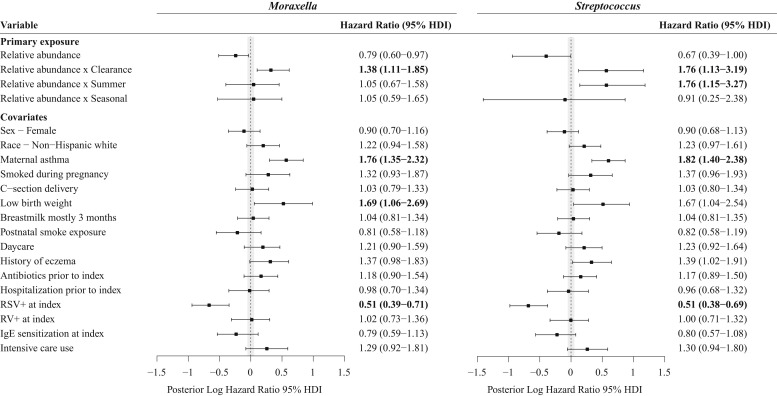
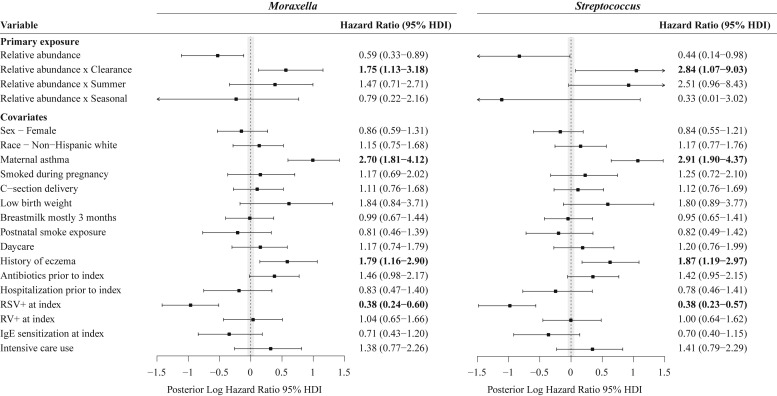
The aforementioned cross-sectional results were also shown in our longitudinal analysis by using joint modeling. In the joint model increased Moraxella species relative abundance at the clearance time point was associated with development of recurrent wheezing by age 3 years after adjusting for 16 confounders, such as maternal asthma, birth mode, history of eczema, sensitization, viral cause, and intensive care use (HR, 1.38; 95% HDI, 1.11-1.85; Fig 2 ). Thus for every 10% increase in the abundance of Moraxella species at the clearance time point, there was a 38% increase in the risk of recurrent wheezing by age 3 years. Similar to our cross-sectional analyses, Haemophilus species abundance did not reach significance in the joint model analyses (see Fig E1). By contrast, we found that increased Streptococcus species abundance at the clearance time point and the summer after hospitalization were both associated with recurrent wheezing by age 3 years (HR of 1.76 and 95% HDI of 1.13-3.19 and HR of 1.76 and 95% HDI of 1.15-3.27, respectively; Fig 2).
Fig 2.
Associations between longitudinal Moraxella and Streptococcus species relative abundances and hazard of recurrent wheezing by age 3 years. The HRs for the relative abundance and abundance by time point interaction for Moraxella (left) and Streptococcus (right) species were calculated by using a Bayesian implementation of the joint model for longitudinal and time-to-event data. Bars and points indicate 95% HDIs and medians, respectively, for posterior distributions of log HRs. The shaded area represents the region of practical equivalence (ROPE) around zero, which was defined as [−0.05, 0.05]. Variables with HDIs that do not overlap the ROPE were considered statistically significant. The time-to-event models adjusted for clinical variables recorded at the index time point, with maternal asthma and RSV infection being significant in both models. RV, Rhinovirus.
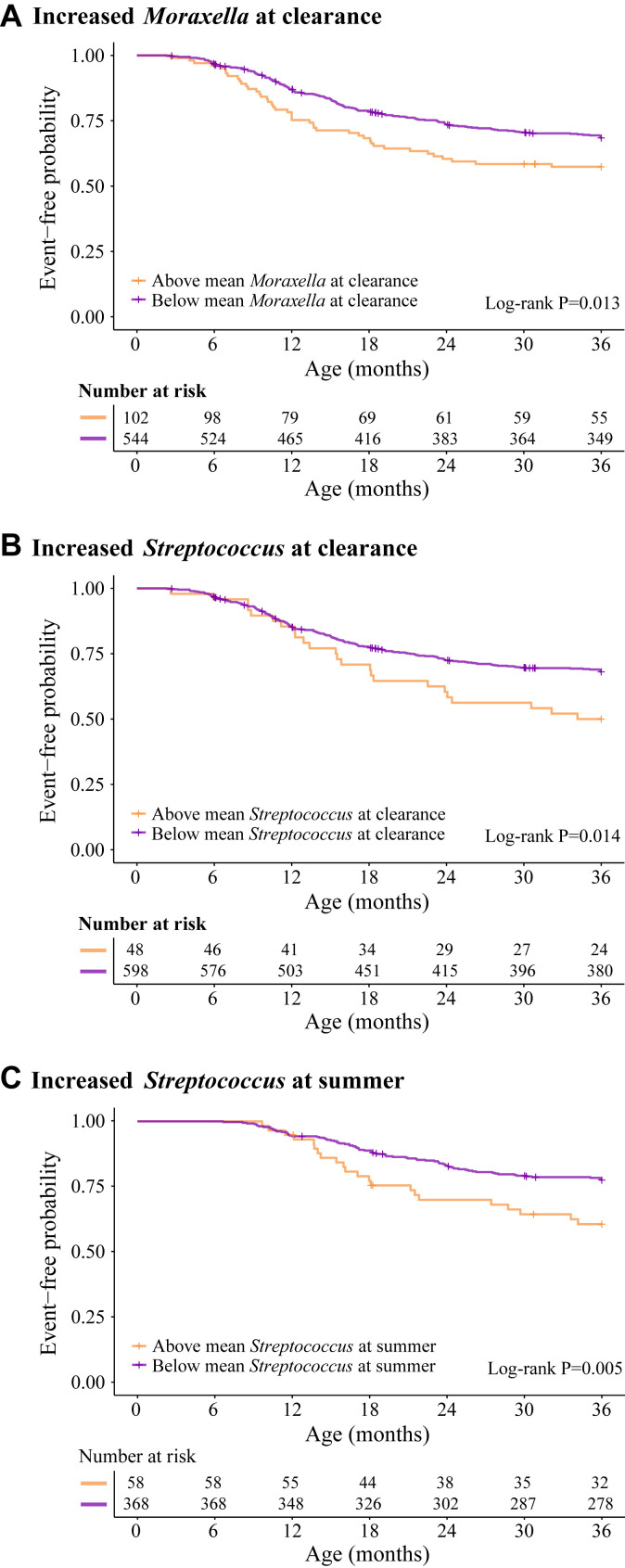
In sensitivity analyses we created Kaplan-Meier curves stratified by the 3 risk groups (ie, increased Moraxella species abundance at the clearance time point, increased Streptococcus species abundance at the clearance time point, and increased Streptococcus species abundance at the summer time point) identified in our cross-sectional and joint modeling analyses (Fig 3 ). In these survival analyses we again demonstrated the increased hazard of recurrent wheezing by age 3 years for infants with high Moraxella or Streptococcus species abundance at the clearance time point (both P = .01) or high Streptococcus species abundance at the summer time point (P < .01).
Fig 3.
Kaplan-Meier curves stratified by Moraxella and Streptococcus species risk groups for the onset of recurrent wheezing by age 3 years. Using the mean cutoffs for Moraxella (mean = 0.08) and Streptococcus (mean = 0.04) species at the relevant time points, infants were classified into 3 risk groups identified by the joint model: A, increased Moraxella species abundance at the clearance time point (n = 102); B, increased Streptococcus species abundance at the clearance time point (n = 48); and C, increased Streptococcus species abundance at the summer time point (n = 58). Kaplan-Meier curves were generated for each of the 3 risk groups for the outcome of recurrent wheezing by age 3 years. Curves were stratified by membership in the risk group. The table shows the number of subjects still at risk for recurrent wheeze based on event occurrences and study dropout. Infants can be included in more than 1 risk group. Log-rank test results indicated that differences in hazard functions of stratified curves were significant for all risk groups.
Longitudinal Moraxella and Streptococcus species abundance after hospitalization among children with recurrent wheezing by age 3 years who also had asthma at age 4 years
To address the potential heterogeneity of recurrent wheezing, we examined children who not only had recurrent wheezing by age 3 years but also had asthma at age 4 years. Supportive of the recurrent wheezing results, we found in joint models that children with greater Moraxella and Streptococcus species abundance at the clearance time point had an increased risk of recurrent wheezing accompanied by asthma at age 4 years (HR of 1.75 and 95% HDI of 1.13-3.18 and HR of 2.84 and 95% HD of 1.07-9.03, respectively; Fig 4 ). Children with increased Streptococcus species abundance at the summer time point had a borderline significant increased risk of recurrent wheeze with asthma at age 4 years (HR, 2.51; 95% HDI, 0.96-8.43; Fig 4).
Fig 4.
Associations between longitudinal Moraxella and Streptococcus species relative abundances and hazard of recurrent wheezing by age 3 years accompanied by asthma at age 4 years. HRs for the relative abundance and abundance by time point interaction for Moraxella (left) and Streptococcus (right) species were calculated by using a Bayesian implementation of the joint model for longitudinal and time-to-event data. Bars and points indicate 95% HDIs and medians, respectively, for the posterior distributions of log HRs. The shaded area represents the region of practical equivalence (ROPE) around zero, which was defined as [−0.05, 0.05]. Variables with HDIs that do not overlap the ROPE were considered statistically significant. The time-to-event models adjusted for clinical variables were recorded at the index time point, with maternal asthma, RSV infection, and history of eczema being significant in both models. RV, Rhinovirus.
Stability of nasal microbiota risk group composition after hospitalization
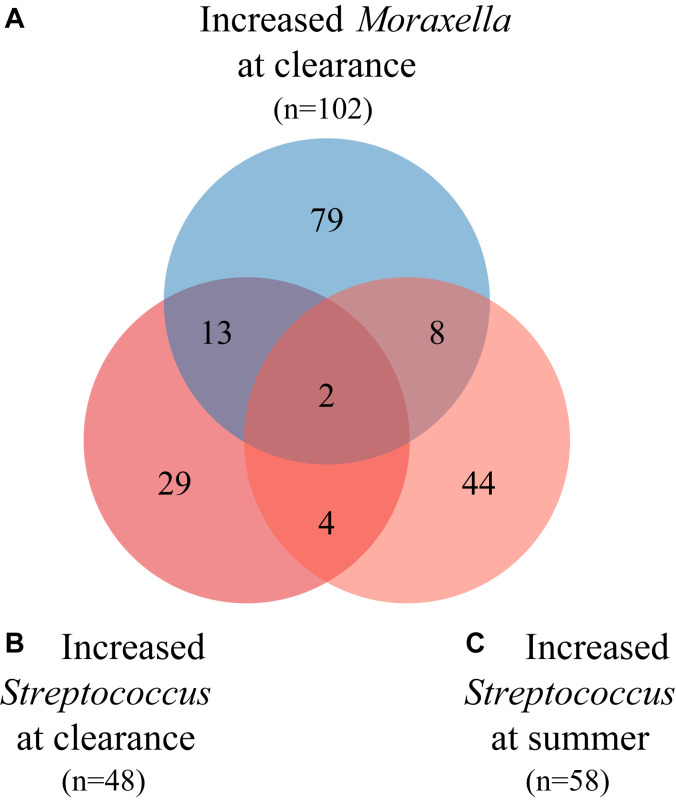
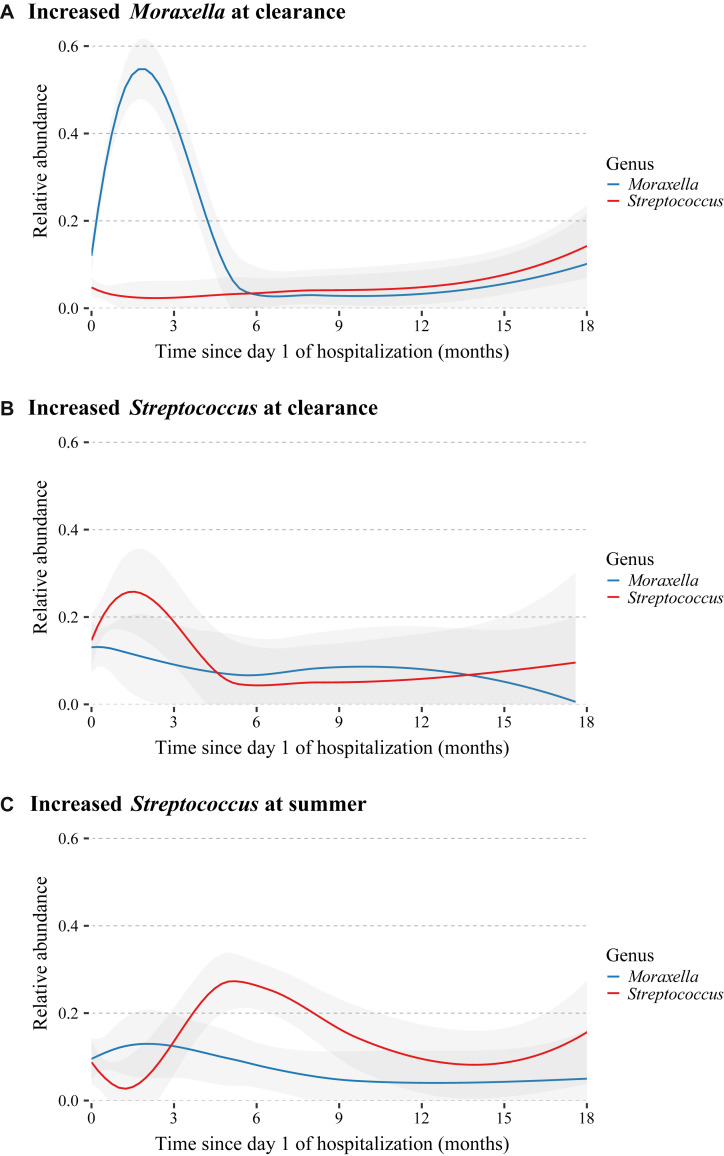
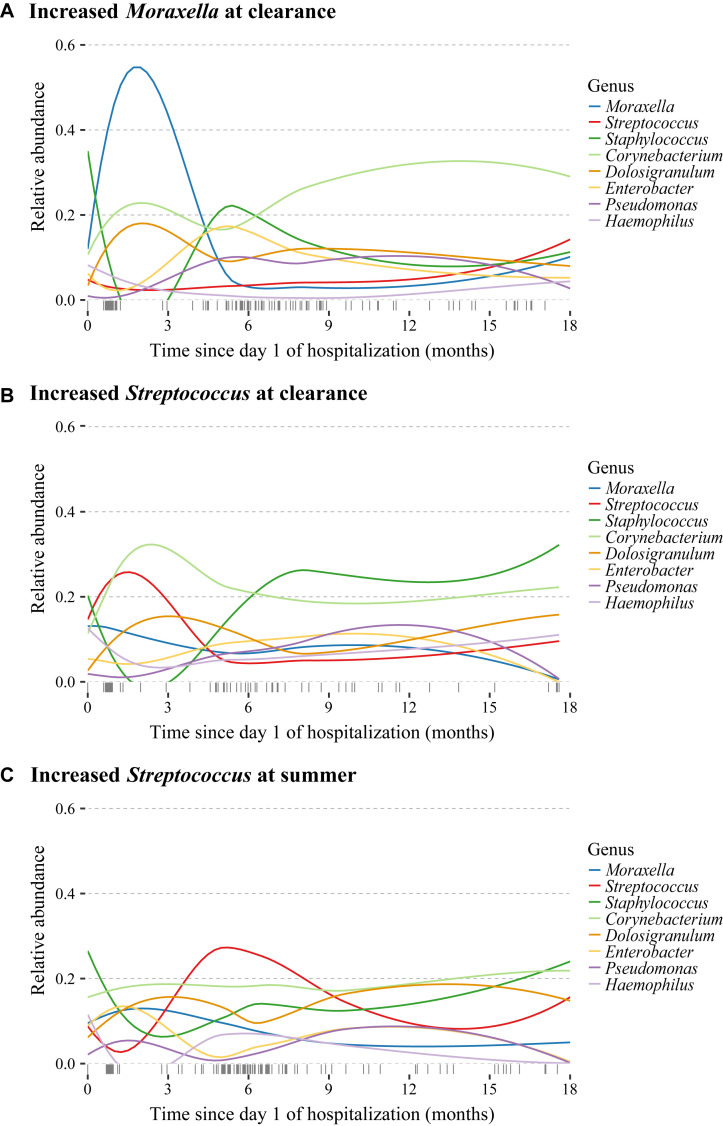
The 3 nasal microbiota risk groups, increased Moraxella species abundance at the clearance time point, increased Streptococcus species abundance at the clearance time point, and increased Streptococcus species abundance at the summer time point, have minimal overlap (see Fig E2 in this article’s Online Repository at www.jacionline.org). Comparing the characteristics and clinical presentation of infants within each of the 3 risk groups after excluding infants in more than 1 risk group, we found that children with increased Moraxella or Streptococcus species abundance at the clearance time point were wheezing on hospitalization more than children with increased Streptococcus species abundance at the summer time point. Otherwise, the 3 risk groups had similar demographics and clinical presentations (see Table E3 in this article’s Online Repository at www.jacionline.org). We also found that the increased abundances found in each of the 3 risk groups were not present at the subsequent sample time point (Fig 5 and see Fig E3 in this article’s Online Repository at www.jacionline.org).
Fig E2.
Overlap of participants in the Moraxella and Streptococcus species risk groups. Using the mean cutoffs for Moraxella (mean = 0.08) and Streptococcus (mean = 0.04) species at the relevant time points, infants were classified into risk groups identified by using the joint model: A (blue), increased Moraxella species abundance at the clearance time point (n = 102); B (red), increased Streptococcus species abundance at the clearance time point (n = 48); and C (light red), increased Streptococcus species abundance at the summer time point (n = 58). The overlap between these 3 risk groups is depicted by using a Venn diagram. There is minimal overlap between the risk groups, with only 2 subjects belonging to all 3 risk groups and 6 subjects having increased Streptococcus species abundance at both the clearance and summer time points.
Fig 5.
Longitudinal variation of relative abundances for Moraxella and Streptococcus species risk groups in the time after hospitalization. Loess curves were fit for the relative abundance of Moraxella (blue lines) and Streptococcus (red lines) species by using the time since day 1 of hospitalization for infants in the 3 risk groups identified by the joint model: A, increased Moraxella species abundance at the clearance time point (n = 102); B, increased Streptococcus species abundance at the clearance time point (n = 48); and C, increased Streptococcus species abundance at the summer time point (n = 58). Infants were classified into risk groups by using the mean cutoffs for Moraxella (mean = 0.08) and Streptococcus (mean = 0.04) species at the relevant time points and might have been included in more than 1 risk group. Shaded regions indicate 95% CIs. Curves show the transience of the increased abundance of the risk genera.
Fig E3.
Longitudinal variation of relative abundances for highly abundant genera in the time after hospitalization categorized by the Moraxella and Streptococcus species risk groups. Loess curves were fit for relative abundances of 8 of the top 10 genera by using the time since day 1 of hospitalization for infants in each of the risk groups identified by the joint model: A, increased Moraxella species abundance at the clearance time point (n = 102); B, increased Streptococcus species abundance at the clearance time point (n = 48); and C, increased Streptococcus species abundance at the summer time point (n = 58). Infants were classified into risk groups using the mean cutoffs for Moraxella (mean = 0.08) and Streptococcus (mean = 0.04) species abundance at the relevant time points and might have been included in more than 1 risk group. The rug plot along the x-axis denotes the distribution of observation times for subjects in the specified risk group. Acinetobacter and Bacillus species abundances did not have noticeable variation over time and were excluded to improve readability.
Examining whether the transient nature of the increased abundances could be due to antibiotic administration, we found that most patients did not receive antibiotics either before or during the hospitalization in accordance with the current bronchiolitis practice guidelines.14 Moreover, there were no significant differences in antibiotic administration between patients with taxa abundances greater than or less than the mean (see Table E4 in this article’s Online Repository at www.jacionline.org).
Discussion
In this prospective multicenter cohort of 842 infants hospitalized for bronchiolitis, we identified in cross-sectional, survival, and joint modeling analyses that having increased relative abundance of nasal Moraxella or Streptococcus species 3 weeks after day 1 of hospitalization or increased nasal Streptococcus species abundance the summer after hospitalization was associated with recurrent wheezing by age 3 years. Our data corroborate the relation of infant Moraxella and Streptococcus species abundance to childhood wheezing outcomes6 , 12 and extend this prior research by focusing not only on a high-risk cohort of infants hospitalized for bronchiolitis but also by analyzing hospitalization and posthospitalization samples using robust longitudinal analytic techniques (ie, joint modeling). Although replication is needed and causality has not been established, we might have identified, for infants hospitalized with bronchiolitis, a short window of opportunity (ie, the weeks after hospitalization) to influence the secondary succession of the nasal microbial ecosystem and potentially the risk of recurrent wheezing by age 3 years22 , 23 and recurrent wheezing accompanied by asthma at age 4 years.
Over the past 60 years, multiple cohort studies have demonstrated that up to 60% of infants hospitalized with bronchiolitis will have recurrent wheezing within 3 years of hospitalization.2 Furthermore, compared with healthy control subjects, children hospitalized with bronchiolitis are also at much greater risk of childhood asthma2 and even longer-term respiratory morbidity.24 Many of these bronchiolitis cohorts focused on viral infections (eg, RSV). However, viruses infect infants within a complex microbial ecosystem (ie, microbiome),25 and research results over the last decade have demonstrated that airway bacteria, including nasal microbiota, are also associated with wheezing outcomes in children.6 , 7 , 12 , 26
Several studies of airway microbiota have implicated Moraxella, Haemophilus, and/or Streptococcus species as risk bacteria for wheezing and asthma by using both cross-sectional4 , 8, 9, 10, 11 , 26 and prospective6 , 7 , 12 study designs. Teo et al12 found in the Australian Childhood Asthma Study (n = 234) that asymptomatic nasopharyngeal colonization with a high abundance of Streptococcus species around age 2 months was associated with wheeze at age 5 years.12 In the same cohort greater frequency of detecting nasopharyngeal Moraxella, Haemophilus, or Streptococcus species at times of health between the ages of 6 months and 2 years was associated in sensitized children (n = 73) with greater odds of chronic wheeze at age 5 years and in nonsensitized children (n = 64) with any wheeze in the first 3 years of life (ie, transient wheeze).6
For the first time, we have examined the longitudinal nasal abundances of these 3 risk bacteria (ie, Moraxella, Haemophilus, and Streptococcus species) in a large and diverse multicenter cohort of US infants hospitalized for bronchiolitis. The relative abundances of these risk bacteria on hospitalization were not associated with recurrent wheezing by age 3 years. However, in the weeks after severe viral infection, enrichment of an infant’s nasal ecosystem with either Moraxella or Streptococcus species increased the risk of recurrent wheezing by age 3 years and recurrent wheezing accompanied by asthma at age 4 years. Similar to the Childhood Asthma Study,12 we also found a separate group of children who at a median age of 9 months (ie, around 6 months after hospitalization), although healthy, had increased Streptococcus species abundance and a greater risk of recurrent wheezing by age 3 years. However, it remains unclear why these infants have a later increase in Streptococcus species abundance and, as seen in Fig 3, a later onset of recurrent wheezing. The lack of a significant association with Haemophilus species might have been due to inadequate power, a weaker association, or both. Importantly, we confirmed all of these findings using multiple analytic techniques. Moreover, our joint models demonstrated that the present microbiota findings are independent of multiple other potential confounding factors associated with childhood asthma, including viral cause and IgE sensitization in infancy.
These findings suggest that interventions that alter the nasal microbiome after hospitalization for bronchiolitis might be beneficial. Indeed, Beigelman et al23 found in a small (n = 39) randomized placebo-controlled trial that azithromycin for 14 days in infants hospitalized with RSV-related bronchiolitis reduced nasal lavage Moraxella species abundance at 2 weeks.22 Furthermore, in concordance with our findings, decreased Moraxella species abundance at 14 days, no matter the group assignment, was associated with reduced odds of recurrent wheezing (ie, ≥3 wheezing episodes) over the subsequent 12 months.22 This potential approach to bronchiolitis treatment needs further study and thoughtful consideration about possible unintended consequences of widespread antibiotic use. Indeed, bronchiolitis and its long-term respiratory outcomes might be best understood as a complex adaptive system.27
Taken together, the potential health effects of the airway microbiome are tantalizing. However, we note that early Moraxella species colonization in healthy infants was associated with lower rates of respiratory tract infection.28 Thus understanding the potential protective role of Moraxella species in healthy populations, compared with its consistent identification as a risk bacterium (not only before and during acute respiratory tract infections6 , 11 but also in asthmatic patients8), will be critical to advancing our understanding of the role of this genus (and ultimately the strain of Moraxella species) in respiratory conditions. It is also possible that the posthospitalization increases in Moraxella or Streptococcus species abundance might simply be markers of children who are already predisposed to recurrent wheezing. Alternatively, the association between posthospitalization increases in Moraxella or Streptococcus species abundance and recurrent wheezing might be causal (ie, microbiota might influence host immune response to viral infection).23 , 29 , 30 If true, influencing microbiome reassembly after hospitalization for bronchiolitis either with antibiotics22 , 23 or, preferably, with intranasal administration of protective species31 might be an approach to help prevent asthma in this high-risk group.
This study has potential limitations. First, although bronchiolitis is the leading cause of hospitalization in infants, we suggest caution when generalizing our results beyond the approximately 130,000 US infants hospitalized with bronchiolitis each year.32
Second, bronchiolitis is a condition that affects the entire airway from the nose to the lungs.32 Although our samples from the nose might not represent pathobiology in the lung, nasal and bronchial cells have similar responses to stimulation and significant mediator release correlations.33
Third, there was no healthy control group in MARC-35, but our study objective was not to examine the association between the microbiome and the development of bronchiolitis within the general population but rather to determine the relation of the nasal microbiota to recurrent wheezing within the high-risk population of infants hospitalized with bronchiolitis.
Fourth, although we have 88% follow-up at age 3 years, the number of nasal swabs included in the analyses decreased over time because of parents forgetting to collect swabs and the exclusion of samples collected after the onset of recurrent wheezing, as required by the joint model.
Fifth, the present results are unable to untangle how host environmental conditions, including bacterial-bacterial interactions at the time of infection, might enhance the survival of certain species.34 However, our group is currently examining the role of airway microbe-host relations in the development of childhood asthma.
Sixth, these analyses will need to be repeated when the children in the cohort turn 6 years of age and are formally evaluated for asthma.
Finally, the present analysis accounted for neither potential genetic differences within our sample32 nor different strains of Moraxella and Streptococcus species or microbiome function. We are currently pursuing these important research questions.
In summary, we have identified in a large, prospective, multicenter cohort of diverse US infants hospitalized with bronchiolitis that increased abundance of nasal Moraxella or Streptococcus species in the weeks and months after hospitalization was related to recurrent wheezing by age 3 years. These results not only confirm that Moraxella and Streptococcus species are risk bacteria but also identify a potential window of opportunity for clinicians to ameliorate the chronic respiratory outcomes for this high-risk group of children. Indeed, these findings might serve as a starting point to begin investigations that would change management for a common pediatric condition that has no proven effective intervention beyond supportive care.
Clinical implications.
In a prospective multicenter cohort of infants hospitalized with bronchiolitis, we found the posthospitalization reassembly of the nasal microbial ecosystem was associated with recurrent wheezing by age 3 years.
Acknowledgments
We thank the MARC-35 hospitals and research personnel for their ongoing dedication to bronchiolitis and asthma research (see Table E5 in this article’s Online Repository at www.jacionline.org). We also thank Alkis Togias, MD, for his ongoing support.
Footnotes
This work was supported by grants U01 AI-087881, R01 AI-114552, R01 AI-108588, R01 AI-137091, and R01 AI-134940 from the National Institute of Allergy and Infectious Diseases and UG3/UH3 OD-023253 from the Office of the Director at the National Institutes of Health (Bethesda, Md). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure of potential conflict of interest: J. F. Petrosino owns shares at Diversigen, a microbiome research company. P. A. Piedra provided bronchiolitis-related consultation for Gilead, Novavax, Ablynx, and Regeneron and received grant support from Novavax, Gilead, Regeneron, Janssen, and Ablynx. The rest of the authors declare that they have no relevant conflicts of interest.
Supplementary data
Table E1.
Comparison of characteristics and clinical presentations for analytic and nonanalytic cohorts
| Variables | Analytic cohort, n = 842∗ | Nonanalytic cohort, n = 78 | P value |
|---|---|---|---|
| Genus abundances, mean (SD) | |||
| Moraxella species at the clearance time point | 0.07 (0.19) | 0.10 (0.24) | .56 |
| Streptococcus species at the clearance time point | 0.02 (0.09) | 0.06 (0.18) | .46 |
| Streptococcus species at the summer time point | 0.04 (0.14) | 0.04 (0.13) | .97 |
| Outcomes | |||
| Recurrent wheezing by age 3 y | 265 (31) | 31 (40) | .17 |
| Recurrent wheezing by age 3 y accompanied by asthma at age 4 y | 119 (14) | 19 (24) | .03 |
| Characteristics | |||
| Age at index (mo), median (IQR) | 3.19 (1.68-5.78) | 3.73 (1.61-7.32) | .17 |
| Female sex | 338 (40) | 29 (37) | .70 |
| Race/ethnicity | .47 | ||
| Non-Hispanic white | 372 (44) | 28 (36) | |
| Non-Hispanic black | 192 (23) | 18 (23) | |
| Hispanic | 247 (29) | 28 (36) | |
| Other | 31 (4) | 4 (5) | |
| Maternal asthma | 177 (21) | 18 (23) | .82 |
| Maternal smoking during pregnancy | 114 (14) | 12 (15) | .78 |
| Cesarean section delivery | 278 (33) | 33 (42) | .12 |
| Low birth weight (<2.3 kg) | 51 (6) | 8 (10) | .23 |
| Mostly breast-fed during first 3 mo | 375 (45) | 36 (46) | 1.00 |
| Postnatal smoke exposure | 128 (15) | 10 (13) | .69 |
| Child history of eczema | 121 (14) | 16 (21) | .20 |
| Daycare attendance | 196 (23) | 15 (19) | .50 |
| Presentation at hospitalization for bronchiolitis | |||
| History of antibiotic use before index visit | 260 (31) | 35 (45) | .02 |
| History of hospitalization before index visit | 131 (16) | 16 (21) | .33 |
| RSV† | 690 (82) | 61 (78) | .51 |
| Rhinovirus† | 173 (21) | 13 (17) | .50 |
| IgE sensitization‡ | 164 (19) | 18 (23) | .54 |
| Intensive care use§ | 127 (15) | 14 (18) | .61 |
All data are presented as numbers (percentages), unless otherwise indicated. P values were computed by using χ2 tests (categorical variables), Wilcoxon rank sum tests (continuous variables), and Kruskal-Wallis rank sum tests (relative abundances).
IQR, Interquartile range.
One index swab was lost during shipment.
Detected through NPA samples collected at the index visit.
Defined as detection of any positive values for serum allergen-specific IgE at the index visit for both food allergens and aeroallergens.
Defined as admission to the intensive care unit and/or use of mechanical ventilation (continuous positive airway pressure and/or intubation during inpatient stay, regardless of location) at any time during the index hospitalization.
Table E2.
Nasal swab microbiota summaries by collection time point
| Variable | Collection time point |
P value∗ | |||
|---|---|---|---|---|---|
| Index, n = 842 | Clearance, n = 599 | Summer, n = 379 | Seasonal, n = 266 | ||
| Age (mo), median (IQR) | 3.2 (1.7-5.8) | 3.8 (2.3-6.5) | 8.9 (7.1-10.9) | 16.4 (12.5-19.8) | <.001 |
| α-Diversity, median (IQR) | |||||
| Shannon index | 0.61 (0.09-1.12) | 0.57 (0.19-0.94) | 0.68 (0.32-1.05) | 0.77 (0.42-1.12) | <.001 |
| Relative abundances of top 10 genera, mean (SD) | |||||
| Staphylococcus species | 0.40 (0.42) | 0.32 (0.40) | 0.26 (0.37) | 0.19 (0.33) | <.001 |
| Corynebacterium species | 0.11 (0.22) | 0.13 (0.24) | 0.19 (0.28) | 0.16 (0.26) | <.001 |
| Dolosigranulum species | 0.05 (0.14) | 0.08 (0.18) | 0.08 (0.17) | 0.12 (0.22) | <.001 |
| Moraxella species | 0.09 (0.20) | 0.07 (0.19) | 0.05 (0.14) | 0.07 (0.16) | <.001 |
| Streptococcus species | 0.05 (0.13) | 0.02 (0.09) | 0.04 (0.14) | 0.04 (0.13) | <.001 |
| Enterobacter species | 0.06 (0.21) | 0.13 (0.29) | 0.13 (0.30) | 0.13 (0.29) | .004 |
| Acinetobacter species | 0.02 (0.12) | 0.06 (0.21) | 0.03 (0.14) | 0.05 (0.16) | .41 |
| Bacillus species | 0.02 (0.14) | 0.03 (0.15) | 0.04 (0.15) | 0.05 (0.17) | <.001 |
| Pseudomonas species | 0.01 (0.08) | 0.05 (0.18) | 0.04 (0.17) | 0.07 (0.20) | <.001 |
| Haemophilus species | 0.07 (0.20) | 0.02 (0.11) | 0.02 (0.07) | 0.02 (0.08) | <.001 |
Comparisons across time points use Kruskal-Wallis rank sum tests. Relative abundances were calculated by using unrarefied sequence read counts.
IQR, Interquartile range.
P values for relative abundance were adjusted for multiple testing by using the Bonferroni correction.
Table E3.
Characteristics and clinical presentations for infants hospitalized for bronchiolitis by identified risk groups
| Variable | Risk group |
P value | ||
|---|---|---|---|---|
| Moraxella species clearance, n = 79 | Streptococcus species clearance, n = 29 | Streptococcus species summer, n = 44 | ||
| Characteristics | ||||
| Age at time of collection (mo), median (IQR) | 4.3 (2.8-7.1) | 6.1 (3.4-10.1) | 9.6 (7.7-11.1) | <.001 |
| Female sex | 37 (47) | 10 (35) | 20 (46) | .52 |
| Race/ethnicity | .21 | |||
| Non-Hispanic white | 38 (48) | 14 (48) | 27 (61) | |
| Non-Hispanic black | 13 (17) | 7 (24) | 8 (18) | |
| Hispanic | 26 (33) | 6 (21) | 6 (14) | |
| Other | 2 (3) | 2 (7) | 3 (7) | |
| Maternal asthma | 17 (22) | 4 (14) | 8 (18) | .70 |
| Maternal smoking during pregnancy | 10 (13) | 2 (7) | 7 (16) | .52 |
| Birth season | .15 | |||
| Spring | 8 (10) | 5 (17) | 4 (9) | |
| Summer | 11 (14) | 8 (28) | 10 (23) | |
| Fall | 43 (54) | 7 (24) | 19 (43) | |
| Winter | 17 (22) | 9 (31) | 11 (25) | |
| Cesarean section delivery | 32 (41) | 12 (41) | 13 (30) | .40 |
| Low birth weight (<2.3 kg) | 4 (5) | 0 (0) | 3 (7) | .45 |
| Mostly breast-fed during first 3 mo | 41 (52) | 16 (55) | 24 (55) | .93 |
| Postnatal smoke exposure | 13 (17) | 2 (7) | 7 (16) | .48 |
| Child history of eczema | 14 (18) | 7 (24) | 6 (14) | .50 |
| Daycare attendance | 21 (27) | 9 (31) | 11 (25) | .83 |
| Presentation at hospitalization for bronchiolitis | ||||
| Age at index (mo), median (IQR) | 3.6 (2.0-6.3) | 5.5 (2.2-8.7) | 3.9 (2.1-5.2) | .25 |
| History of antibiotic use before index visit | 28 (35) | 10 (35) | 19 (43) | .66 |
| History of hospitalization before index visit | 11 (14) | 6 (21) | 5 (11) | .56 |
| RSV positive at index visit∗ | 62 (79) | 22 (76) | 36 (82) | .80 |
| RV positive at index visit∗ | 15 (19) | 6 (21) | 12 (27) | .59 |
| IgE sensitization at index visit† | 16 (20) | 8 (28) | 9 (21) | .70 |
| Wheezing at index visit | .01 | |||
| No | 31 (39) | 2 (7) | 17 (39) | |
| Yes | 45 (57) | 24 (83) | 25 (57) | |
| Unknown | 3 (4) | 3 (10) | 2 (5) | |
| Antibiotics during preadmission and/or hospitalization | 21 (27) | 13 (45) | 18 (41) | .11 |
| Intensive care use‡ | 6 (8) | 4 (14) | 7 (16) | .31 |
All data are presented as numbers (percentages), unless otherwise indicated. Subjects in more than 1 risk group are excluded. P values were computed by using Fisher exact tests (categorical variables) and Wilcoxon rank sum tests (continuous variables).
IQR, Interquartile range; RV, rhinovirus.
Detected through NPA samples collected at the index visit.
Defined as detection of any positive values for serum allergen-specific IgE at the index visit for both food allergens and aeroallergens.
Defined as admission to the intensive care unit and/or use of mechanical ventilation (continuous positive airway pressure and/or intubation during inpatient stay, regardless of location) at any time during the index hospitalization.
Table E4.
Comparison of greater than and less than mean taxa abundances by antibiotic administration
| Genus abundance | History of antibiotic use before index visit |
P value | |
|---|---|---|---|
| No, n = 562 (67%) | Yes, n = 280 (33%) | ||
| Moraxella species abundance at the clearance time point | .10 | ||
| Less than mean | 486 (87) | 254 (91) | |
| Greater than mean | 76 (14) | 26 (9) | |
| Streptococcus species abundance at the clearance time point | .26 | ||
| Less than mean | 534 (95) | 260 (93) | |
| Greater than mean | 28 (5) | 20 (7) | |
| Streptococcus species abundance at the summer time point | .95 | ||
| Less than mean | 524 (93) | 260 (93) | |
| Greater than mean | 38 (7) | 20 (7) | |
All data are presented as numbers (percentages). P values were computed by using χ2 tests.
Table E5.
Principal investigators at the 17 participating sites in MARC-35
| Amy D. Thompson, MD | Alfred I. duPont Hospital for Children, Wilmington, Delaware |
| Federico R. Laham, MD, MS | Arnold Palmer Hospital for Children, Orlando, Florida |
| Jonathan M. Mansbach, MD, MPH | Boston Children's Hospital, Boston, Massachusetts |
| Vincent J. Wang, MD, MHA, and Susan Wu, MD | Children's Hospital of Los Angeles, Los Angeles, California |
| Michelle B. Dunn, MD, and Jonathan M. Spergel, MD, PhD | Children's Hospital of Philadelphia, Philadelphia, Pennsylvania |
| Juan C. Celedon, MD, DrPH | Children's Hospital of Pittsburgh, Pittsburgh, Pennsylvania |
| Michael R. Gomez, MD, MS-HCA, and Nancy Inhofe, MD | Children's Hospital at St Francis, Tulsa, Oklahoma |
| Brian M. Pate, MD, and Henry T. Puls, MD | Children's Mercy Hospital & Clinics, Kansas City, Missouri |
| Stephen J. Teach, MD, MPH | Children's National Medical Center, Washington, DC |
| Richard T. Strait, MD, and Stephen C. Porter, MD, MSc, MPH | Cincinnati Children's Hospital and Medical Center, Cincinnati, Ohio |
| Ilana Y. Waynik, MD | Connecticut Children's Medical Center, Hartford, Connecticut |
| Sujit S. Iyer, MD | Dell Children's Medical Center of Central Texas, Austin, Texas |
| Michelle D. Stevenson, MD, MS | Norton Children's Hospital, Louisville, Kentucky |
| Wayne G. Schreffler, MD, PhD, and Ari R. Cohen, MD | Massachusetts General Hospital, Boston, Massachusetts |
| Anne K Beasley, MD, and Cindy S. Bauer, MD | Phoenix Children's Hospital, Phoenix, Arizona |
| Thida Ong, MD, and Markus Boos, MD, PhD | Seattle Children's Hospital, Seattle, Washington |
| Charles G. Macias, MD, MPH | Texas Children's Hospital, Houston, Texas |
References
- 1.Hasegawa K., Tsugawa Y., Brown D.F., Mansbach J.M., Camargo C.A., Jr. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132:28–36. doi: 10.1542/peds.2012-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasegawa K., Mansbach J.M., Camargo C.A., Jr. Infectious pathogens and bronchiolitis outcomes. Expert Rev Anti Infect Ther. 2014;12:817–828. doi: 10.1586/14787210.2014.906901. [DOI] [PubMed] [Google Scholar]
- 3.Lemon K.P., Klepac-Ceraj V., Schiffer H.K., Brodie E.L., Lynch S.V., Kolter R. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. MBio. 2010;1 doi: 10.1128/mBio.00129-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilty M., Burke C., Pedro H., Cardenas P., Bush A., Bossley C. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa K., Mansbach J.M., Ajami N.J., Espinola J.A., Henke D.M., Petrosino J.F. Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalised for bronchiolitis. Eur Respir J. 2016;48:1329–1339. doi: 10.1183/13993003.00152-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teo S.M., Tang H.H.F., Mok D., Judd L.M., Watts S.C., Pham K. Airway microbiota dynamics uncover a critical window for interplay of pathogenic bacteria and allergy in childhood respiratory disease. Cell Host Microbe. 2018;24:341–352.e5. doi: 10.1016/j.chom.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bisgaard H., Hermansen M.N., Buchvald F., Loland L., Halkjaer L.B., Bonnelykke K. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 8.Depner M., Ege M.J., Cox M.J., Dwyer S., Walker A.W., Birzele L.T. Bacterial microbiota of the upper respiratory tract and childhood asthma. J Allergy Clin Immunol. 2017;139:826–834. doi: 10.1016/j.jaci.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y.J., Nelson C.E., Brodie E.L., Desantis T.Z., Baek M.S., Liu J. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2010;127:372–381. doi: 10.1016/j.jaci.2010.10.048. e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marri P.R., Stern D.A., Wright A.L., Billheimer D., Martinez F.D. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2012;131:346–352. doi: 10.1016/j.jaci.2012.11.013. e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosas-Salazar C., Shilts M.H., Tovchigrechko A., Chappell J.D., Larkin E.K., Nelson K.E. Nasopharyngeal microbiome in respiratory syncytial virus resembles profile associated with increased childhood asthma risk. Am J Respir Crit Care Med. 2016;193:1180–1183. doi: 10.1164/rccm.201512-2350LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teo S.M., Mok D., Pham K., Kusel M., Serralha M., Troy N. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansbach J.M., Hasegawa K., Piedra P.A., Avadhanula V., Petrosino J.F., Sullivan A.F. Haemophilus-dominant nasopharyngeal microbiota is associated with delayed clearance of respiratory syncytial virus in infants hospitalized for bronchiolitis. J Infect Dis. 2019;219:1804–1808. doi: 10.1093/infdis/jiy741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ralston S.L., Lieberthal A.S., Meissner H.C., Alverson B.K., Baley J.E., Gadomski A.M. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 15.Luna P.N., Hasegawa K., Ajami N.J., Espinola J.A., Henke D.M., Petrosino J.F. The association between anterior nares and nasopharyngeal microbiota in infants hospitalized for bronchiolitis. Microbiome. 2018;6:2. doi: 10.1186/s40168-017-0385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansbach J.M., Piedra P.A., Teach S.J., Sullivan A.F., Forgey T., Clark S. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166:700–706. doi: 10.1001/archpediatrics.2011.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 18.Martinez F.D., Wright A.L., Taussig L.M., Holberg C.J., Halonen M., Morgan W.J. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 19.Camargo C.A., Jr., Ingham T., Wickens K., Thadhani R., Silvers K.M., Epton M.J. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–e187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 20.Hickey G.L., Philipson P., Jorgensen A., Kolamunnage-Dona R. Joint modelling of time-to-event and multivariate longitudinal outcomes: recent developments and issues. BMC Med Res Methodol. 2016;16:117. doi: 10.1186/s12874-016-0212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelmon A., Hill J., Yajima M. Why we (usually) don't have to worry about multiple comparisons. J Res Educ Effect. 2012;5:189–211. [Google Scholar]
- 22.Zhou Y., Bacharier L.B., Isaacson-Schmid M., Baty J., Schechtman K.B., Sajol G. Azithromycin therapy during respiratory syncytial virus bronchiolitis: upper airway microbiome alterations and subsequent recurrent wheeze. J Allergy Clin Immunol. 2016;138:1215–1219.e5. doi: 10.1016/j.jaci.2016.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beigelman A., Isaacson-Schmid M., Sajol G., Baty J., Rodriguez O.M., Leege E. Randomized trial to evaluate azithromycin's effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2015;135:1171–1178.e1. doi: 10.1016/j.jaci.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dharmage S.C., Erbas B., Jarvis D., Wjst M., Raherison C., Norback D. Do childhood respiratory infections continue to influence adult respiratory morbidity? Eur Respir J. 2009;33:237–244. doi: 10.1183/09031936.00062907. [DOI] [PubMed] [Google Scholar]
- 25.Mansbach J.M., Hasegawa K., Henke D.M., Ajami N.J., Petrosino J.F., Shaw C.A. Respiratory syncytial virus and rhinovirus severe bronchiolitis are associated with distinct nasopharyngeal microbiota. J Allergy Clin Immunol. 2016;137:1909–1913. doi: 10.1016/j.jaci.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fazlollahi M., Lee T.D., Andrade J., Oguntuyo K., Chun Y., Grishina G. The nasal microbiome in asthma. J Allergy Clin Immunol. 2018;142:834–843.e2. doi: 10.1016/j.jaci.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickson R.P., Erb-Downward J.R., Huffnagle G.B. Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir Med. 2014;2:238–246. doi: 10.1016/S2213-2600(14)70028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biesbroek G., Tsivtsivadze E., Sanders E.A., Montijn R., Veenhoven R.H., Keijser B.J. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med. 2014;190:1283–1292. doi: 10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- 29.de Steenhuijsen Piters W.A., Heinonen S., Hasrat R., Bunsow E., Smith B., Suarez-Arrabal M.C. Nasopharyngeal microbiota, host transcriptome, and disease severity in children with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2016;194:1104–1115. doi: 10.1164/rccm.201602-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folsgaard N.V., Schjorring S., Chawes B.L., Rasmussen M.A., Krogfelt K.A., Brix S. Pathogenic bacteria colonizing the airways in asymptomatic neonates stimulates topical inflammatory mediator release. Am J Respir Crit Care Med. 2013;187:589–595. doi: 10.1164/rccm.201207-1297OC. [DOI] [PubMed] [Google Scholar]
- 31.Spacova I., Petrova M.I., Fremau A., Pollaris L., Vanoirbeek J., Ceuppens J.L. Intranasal administration of probiotic Lactobacillus rhamnosus GG prevents birch pollen-induced allergic asthma in a murine model. Allergy. 2019;74:100–110. doi: 10.1111/all.13502. [DOI] [PubMed] [Google Scholar]
- 32.Meissner H.C. Viral bronchiolitis in children. N Engl J Med. 2016;374:62–72. doi: 10.1056/NEJMra1413456. [DOI] [PubMed] [Google Scholar]
- 33.McDougall C.M., Blaylock M.G., Douglas J.G., Brooker R.J., Helms P.J., Walsh G.M. Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol. 2008;39:560–568. doi: 10.1165/rcmb.2007-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sande C.J., Njunge J.M., Mwongeli Ngoi J., Mutunga M.N., Chege T., Gicheru E.T. Airway response to respiratory syncytial virus has incidental antibacterial effects. Nat Commun. 2019;10:2218. doi: 10.1038/s41467-019-10222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]