Abstract
The rates of opioid use disorder during pregnancy have more than quadrupled in the last decade, resulting in numerous infants suffering exposure to opioids during the perinatal period, a critical period of central nervous system (CNS) development. Despite increasing use, the characterization and definition of the molecular and cellular mechanisms of the long-term neurodevelopmental impacts of opioid exposure commencing in utero remains incomplete. Thus, in consideration of the looming public health crisis stemming from the multitude of infants with prenatal opioid exposure entering school age, we undertook an investigation of the effects of perinatal methadone exposure in a novel preclinical model. Specifically, we examined the effects of opioids on the developing brain to elucidate mechanisms of putative neural cell injury, to identify diagnostic biomarkers and to guide clinical studies of outcome and follow-up. We hypothesized that methadone would induce a pronounced inflammatory profile in both dams and their pups, and be associated with immune system dysfunction, sustained CNS injury, and altered cognition and executive function into adulthood. This investigation was conducted using a combination of cellular, molecular, biochemical, and clinically translatable biomarker, imaging and cognitive assessment platforms. Data reveal that perinatal methadone exposure increases inflammatory cytokines in the neonatal peripheral circulation, and reprograms and primes the immune system through sustained peripheral immune hyperactivity. In the brain, perinatal methadone exposure not only increases chemokines and cytokines throughout a crucial developmental period, but also alters microglia morphology consistent with activation, and upregulates TLR4 and MyD88 mRNA. This increase in neuroinflammation coincides with reduced myelin basic protein and altered neurofilament expression, as well as reduced structural coherence and significantly decreased fractional anisotropy on diffusion tensor imaging. In addition to this microstructural brain injury, adult rats exposed to methadone in the perinatal period have significant impairment in associative learning and executive control as assessed using touchscreen technology. Collectively, these data reveal a distinct systemic and neuroinflammatory signature associated with prenatal methadone exposure, suggestive of an altered CNS microenvironment, dysregulated developmental homeostasis, complex concurrent neural injury, and imaging and cognitive findings consistent with clinical literature. Further investigation is required to define appropriate therapies targeted at the neural injury and improve the long-term outcomes for this exceedingly vulnerable patient population.
Keywords: methadone, white matter, microglia, diffusion tensor imaging, cognition, pregnancy
INTRODUCTION
The United States is experiencing unprecedented rates of drug overdose deaths and drug related problems driven by opioids.1 While most responses to the opioid epidemic are focused on preventing harm to adults, there is a rapid rise in the number of children and young adults in the USA with a neonatal and perinatal history of opioid exposure.1 To this end, clinical and research efforts to address the opioid crisis are considered major priorities by the US congress,2,3 the March of Dimes Foundation,2,4 and the World Health Organization,5 with significant financial, social and health expenditures.2 In particular, opioid use disorder (OUD) is on the rise among women of reproductive age, contributing markedly to the opioid epidemic and increasing the incidence of adverse health outcomes in pregnant women and children.6,7 There is a marked increase in the use of prescription opioids among women of childbearing age and pregnant women8–12, with 22–30% of women filling at least one prescription for an opioid analgesic during pregnancy.9,10 Recently, the Substance Abuse and Mental Health Services Administration reported that 1.1% of pregnant women misused opioids (0.9% used prescription opioids and 0.2% used heroin).13,14 Prenatal opioid exposure (POE) includes the use and misuse of prescription opioids, such as oxycodone, morphine, codeine, illicit opioids (e.g., heroin), and exposure to medications used to manage OUD, such as methadone and buprenorphine.11,12,15 Misuse of opioids in pregnancy is associated with gaps in prenatal care, preterm birth, low birth weight, respiratory depression and neonatal withdrawal.1,16
As prenatal opioid use has increased, the proportion of infants who experience opioid withdrawal after birth, known clinically as Neonatal Opioid Withdrawal Syndrome (NOWS, formerly NAS- neonatal abstinence syndrome), has similarly increased. NOWS is used as a proxy for opioid exposure during pregnancy17,18 and approximately 5.8 infants in every 1000-hospital births have NOWS, accounting for an estimated 1.5 billion dollars in hospital charges, in addition to the cumulative individual, familial and societal burdens.12,18–20
Despite the known costs, there remains a dearth of preclinical knowledge on the direct effects and putatively negative cellular and molecular mechanisms of perinatal opioid exposure on developing neural circuitry to inform optimization of neurodevelopment outcomes. Opioids rapidly cross the placenta, and via the fetal circulation have a direct impact on developing organ systems, including the central nervous system (CNS).21–23 Neonates exposed to opioids in utero have significantly smaller brains and basal ganglia, and reduced cerebellar volumes compared to non-exposed infants.21 Similarly, prenatal opioid exposure is associated with microstructural brain injury seen on high-resolution MRI and impaired neurodevelopment.24 Children born to women who have been prescribed opioids, including methadone, are also at risk of neurodevelopmental impairment,24,25 with lower Mental Development Index and Psychomotor Development Index scores than unexposed children, as well as microstructural alterations in major white matter tracts that are present at birth and can be longitudinally visualized throughout childhood.24,25
Undoubtedly, the relationship between opioid exposure, neural circuitry changes and psychosocial factors are complex.26–29 Indeed, pre- and postnatal environmental factors and stressors are important variables in the maturation of brain circuitry.26,27,30–32 Nontheless, the principal mechanisms of neural injury induced by opioids early in development remain a gap in knowledge. Thus, in consideration of the looming public health crisis that will stem from the multitude of infants with prenatal opioid exposure (treated or untreated NOWS) who will soon be school-age children, we undertook an investigation of the effects of perinatal methadone exposure in a novel preclinical model. Specifically, we examined the effects of opioids on the developing brain to elucidate mechanisms of putative neural cell injury, to identify diagnostic biomarkers and to guide clinical studies of outcome and follow-up. We hypothesized that methadone would induce a pronounced inflammatory profile in both dams and their pups, and be associated with immune system dysfunction, sustained CNS injury, and altered cognition and executive function into adulthood.
METHODS
Animals
Two hundred male and female Sprague Dawley rat pups (Charles River) were used, with an equal male to female ratio. In total there were 86 saline-exposed and 114 methadone-exposed offspring used for outcome measures. All experiments were designed in line with ARRIVE guidelines and experimental procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee.
Methadone Exposure
On embryonic day 16 (E16) prior to complete oligodendrocyte, microglial and astrocyte maturation,33 osmotic mini pumps were implanted subcutaneously in the nape of the neck of pregnant rat dams for 28 days of continuous methadone (8–16mg/kg, 0.25μL/h flow rate) or sterile saline infusion. Specifically, a small incision was made in the dorsal neck region, after which the subcutaneous area was opened by blunt dissection. An osmotic mini pump (ALZET, Cupertine, California) previously primed with methadone or saline was placed in the subcutaneous space and the area was closed with suture. Pumps with saline only, 8 mg/kg methadone, 12 mg/kg methadone and 16 mg/kg methadone were utilized. The dosage range of 8–16 mg/kg is physiologically relevant to human exposure.34 Methadone is a synthetic, long-acting μ-opioid receptor agonist, which crosses the placenta and blood-brain barrier.24 On E22, rat pups were born and remained with their dams, receiving methadone or saline through the maternal milk supply until weaning on P21, approximately pre-adolescent equivalent (Fig. 1).35,36Pups were observed for overall health daily, and weighed on P1 and P21. The first two-weeks in rat postnatal life is equivalent to the human third trimester (P10 is roughly human term equivalent).33 Upon establishment of methadone dose response, 12mg/kg was used as the dose for all subsequent experiments.
Figure 1. Animal Model Summary.
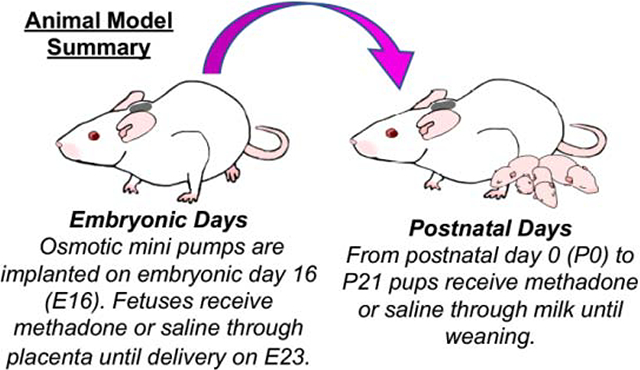
Osmotic mini pumps were implanted into rat dams on embryonic day 16 (E16). Rat fetuses then received methadone or saline through the placenta until delivery at E23. After birth, from postnatal day (P) 0 to P21 pups received methadone or saline through maternal milk until weaning.
Measurement of Urine Methadone Concentration
Urine was collected from pups and dams at multiple time points, and stored at −80°C. Methadone ELISA (MaxSignal Methadone ELISA Kit, Bioo Scientific) was performed per manufacturer’s specifications. Briefly, urine samples were centrifuged at 4000 × g for 5 minutes, and supernatant collected. After which, 100 μL of samples or methadone standards were added into wells on a microtiter plate in duplicate. Subsequently, antibodies were added and after incubation, and washing, HRP-conjugated antibodies were applied. The plate was washed and 3,3’,5,5’-tetramethylbenzidine (TMB) substrate added. Stop buffer was then added and the plate was read with a 450 nm wavelength.
Peripheral Blood Mononuclear Cell (PBMC) Isolation
PBMCs from sham or methadone pups were isolated on postnatal day 7 (P7) using a Ficoll gradient separation consistent with previously published methods.37,38 Specifically, venous blood was collected from the right atrium in pyrogen-free, K2 EDTA vacutainer tubes (BD Vacutainer, Franklin Lakes, NJ, USA). Two milliliters of blood was mixed with 2mL Roswell Park Memorial Institute (RPMI) 1640 media (Gibco, Waltham, MA, USA). The blood mixture was layered on 3mL of Ficoll-Paque Plus 1,084 (GE Healthcare, Chicago, IL, USA) media and centrifuged at 400g for 30 minutes at room temperature (RT). Using sterile technique, the mononuclear cell fraction was then transferred to a new tube, resuspended and centrifuged. Cells were isolated and plated from two saline and two methadone treated pups for each experiment. The experiment was performed twice using a total of 4 brains per condition.
PBMC Treatment with Lipopolysaccharide (LPS)
PBMCs from sham or methadone pups were plated in 3.5 cm culture dishes at a density of 2×106 cells per dish. PBMCs were then stimulated without or with LPS (100 ng/mL) and/or naloxone (10μM) for 3h or 24h, and the supernatants were collected consistent with prior reports.37,38 Each experimental condition and exposure was performed in triplicate.
Multiplex Electrochemiluminescent Immunoassay (MECI)
Cytokine and chemokine biomarker profile analysis was performed on serum (1:4 dilution), supernatants from cultured PBMCs (1:4 dilution), or brain tissue (100μg) using a V-plex rat pro-inflammatory panel for TNFα and interleukin 1β (IL-1β), IL-6 and CXCL1 consistent with prior reports (MesoScale Discovery, Gaithersburg, MD, USA).38–47 Plates were read on a Quickplex SQ 120 Imager.
qPCR
Micro-dissected cortical samples were harvested at P10 for transcriptional analyses using quantitative PCR. Gene-of-interest primers were for TLR4 (Fwd: 5’-CCC TGC CAC CAT TTA CAG TTC G-3’; Rev: 5’-GAG TCC CAG CCA GAT GCA AGA G-3’) and MyD88 (Fwd: 5’-CAA CCA GCA GAA ACA GGA GTC T-3’; Rev: 5’-ATT GGG GCA GTA GCA GAT GAA G-3’). Primers and cDNA synthesized from 0.9μg RNA were added to power SYBR green master mix (Life Technologies, Grand Island, NY), and run in triplicate on a LifeTech Step-One Plus (Life Technologies). Gene-of-Interest transcription was normalized to 18s endogenous controls (Fwd: 5’-TCC CTA GTG ATC CCC GAG AAG T-3’; Rev: 5’-CCC TTA ATG GCA GTG ATA GCG A-3’). Only experimental triplicates with a SD less than 0.25 were included in all analyses, consistent with prior reports.48,49
Immunohistochemistry
To evaluate microglia/macrophages, immunofluorescent labeling was performed against ionized calcium binding protein-1 (Iba1) at P21.48,50 Briefly, 20μm floating sections were permeabilized with 0.4% Triton X-100 in PBS, then blocked for 1 hour with PBS plus 0.1% tween20 (PBST), 0.5% bovine serum albumin (BSA), and 10% normal goat serum (NGS). Next, tissue sections were incubated overnight at 4°C with Iba1 primary antibodies(1:500, Wako, Richmond VA) diluted in PBST with 0.5% BSA. After incubation, tissue sections were washed 5 times in PBST, and incubated in biotinylated secondary antibody (1:250, Life Technologies, Grand Island, NY) diluted in 0.5% BSA in PBST for 1 hour. After 5 washes in PBS, tissue sections were mounted on glass slides and coverslipped with 4′,6‐Diamidino‐2‐Phenylindole, Dihydrochloride (DAPI) containing Fluoromount-G (Life Technologies).
The confocal z‐stacks of immunolabeled microglia in the somatosensory cortex at the level of the dorsal hippocampus (bregma −2.50) were obtained using a Leica DMi8 TCS SP8 confocal inverted microscope (Wetzlar, Germany), equipped with an Olympus 63X objective, hybrid spectral detectors, and white light laser. Gain parameters, zoom, pinhole size, step size, scan speed, and resolution were uniform across scans. Within Imaris software (Bitplane, Concord MA), cell process reconstruction was performed within the three-dimensional viewing platform on acquired confocal z-stacks. Filaments protruding from the cell surface were manually traced for individual cells and color coded for branch level by observers (JN) blinded to injury group.51,52
Western Blot
Micro-dissected cortical samples from sham and methadone rats at P21 were homogenized, sonicated, and centrifuged at 4200 × g for 10 min consistent with prior reports53–56. Protein concentration in the whole cell fraction was determined with a Bradford assay (BioRad, Hercules, CA). Thirty micrograms of protein were loaded on 4–20% tris HCl gels or 4–12% bis-tris HCl gels (BioRad), separated by electrophoresis, and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with 5% non-fat dry milk in TBST and incubated with primary antibody overnight at 4 degrees. A species appropriate horseradish-peroxidase-conjugated secondary antibody (ThermoFisher Scientific, Grand Island, NY) was applied, and after washing, detected with chemiluminescence (ThermoFisher Scientific) using a LAS 4000 imager (GE, Healthcare, PA). Primary antibodies against the following targets were used consistent with prior publications: phosphoneurofilament (pNF, Millipore, Temecula, CA, 1:500) or neurofilament (NF, SMI-312, Covance, Princeton, NJ, 1:1000).55,57 Blots were imaged using an ImageQuant LAS 4000 (GE) and bands of interest were quantified using ImageQuant Software (GE) normalized to the loading control, β-actin (Sigma, St. Louis, MO, 1:5000). At least two blots were used to quantify each protein. Data was then normalized to the sham group consistent with previous reports.49,53,55,58
Diffusion Tensor Imaging (DTI)
Ex vivo MRI using diffusion sequences was performed on a Bruker Biospec 7T 70/30 Ultra Shield Refrigerated (USR) nuclear MRI system, consistent with prior published methods.40,41,46,47,57 Echo-planar diffusion tensor imaging (EP-DTI) of twenty contiguous coronal 1 mm slices were obtained with TR:8000ms; TE:40.0ms; slice thickness; 1mm; FOV (field-of-view): 250mm; and matrix: 198×198. Region of interest (ROI) analyses were performed in the corpus callosum and external capsule by a blinded observer (JRM; JCN; TRY) using Bruker’s Paravision 5.1. imaging software. Fractional anisotropy (FA), axial (λ1) and radial ((λ2+λ3)/2) diffusivity eigenvectors were measured in the above mentioned ROI. Directionally encoded diffusion color maps and color-coded FA maps were created.
Touchscreen Assessment of Visual Discrimination and Reversal Learning
Consistent with our prior published methodology, cognition and executive function in adult rats was assessed using an interactive,59–63 touchscreen platform analogous to the Cambridge Neuropsychological Testing Automated Battery (CANTAB) platform and the NIH Tool Box for the Assessment of Neurological and Behavioral Function in humans. Briefly, operant behavior was conducted in a sound and light attenuating chamber (Med Associates, St. Albans, VT), with a pellet dispenser and a touch-sensitive screen (Conclusive Solutions, Sawbridgeworth UK). Stimulus presentation in the response window and touches were controlled and recorded by KLimbic Software (Conclusive Solutions). Following mild food restriction to 10% of ad lib weight, and pretraining to initiate and respond to stimuli, all rats were tested on a pairwise visual discrimination-reversal paradigm. Each rat performed daily sessions for a maximum of 60 minutes or 60 trials. Responses to one stimulus yielded a reward (20 mg dustless pellets; BioServ, Frenchtown, NJ), whereas responses to the other stimulus resulted in 5s time out (signaled by extinguishing the house light). Designation of the initial reward stimulus was counterbalanced across treatment. Rats were trained a priori to a criterion of greater than ≥80% correct responses for two consecutive days. Assessment of reversal learning began after visual discrimination (VD) performance criteria were attained. For reversal learning evaluation, the designation of stimuli as correct versus incorrect was reversed for each rat. Rats were similarly tested on daily 60-trial sessions for reversal to an a priori criterion of ≥80% correct responses for two consecutive sessions. A correction procedure was utilized whereby an initial error was followed by correction trials in which the same stimuli and left/right position was presented until a correct response was made. Failing criteria were set a priori at 28 sessions (days) for both VD and reversal. Specifically, if an individual rat failed to acquire the task and reach criterion ( ≥80% correct responses for two consecutive sessions) within 28 sessions (days), they failed and were removed from the study. In total, 3 rats failed to meet performance criteria for reversal learning and their data was removed from the study.
In total, 10 male rats (4 saline and 6 methadone exposed) and 11 female (6 saline and 5 methadone) rats were used for touchscreen analyses. We recorded the following dependent measures during VD and reversal: total sessions, correct responses made, errors (incorrect responses), correction errors (correction trials, reversal only), reaction time (time from touchscreen stimuli presentation to touchscreen response) and magazine latency (time from touchscreen response to reward retrieval). Discrimination performance was analyzed across all sessions required to reach criterion. To examine distinct phases of reversal (early perseverative and late learning), we analyzed errors and correction errors for sessions where performance was <50% and from 50% to criterion, respectively.
Statistical Analyses
Data are represented as mean ± the standard error of the mean (SEM). Data were tested for normality with the Shapiro-Wilk Test. Parametric statistical differences between two groups were established with a t-test, and non-parametric differences between two groups were established with a Mann-Whitney U-test. Parametic statistical differences between 3 or more groups were established with a one-way ANOVA with boneferroni correction; p<0.05 was considered statistically significant.
RESULTS
Methadone Reduces Neonatal and Perinatal Body Weight
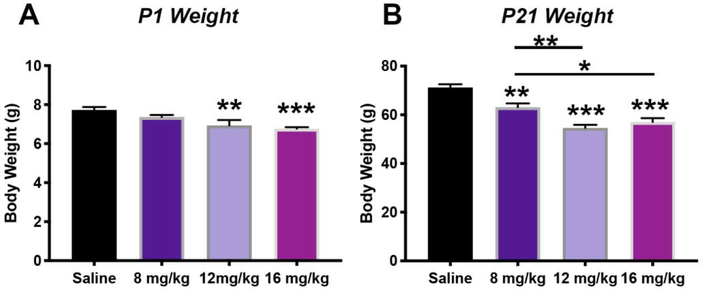
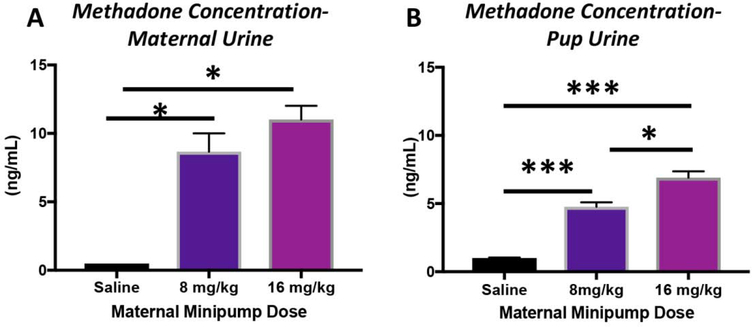
A unique challenge within neurodevelopmental research is to accurately recapitulate the intact maternal-placental-fetal unit, in which the compartment-specific responses to individual insults, such as opioids, can be precisely studied. All rat pups, irrespective of treatment group, survived the duration of the study. Rat pups of both sexes exposed to methadone in utero, and continuing through the perinatal, and neonatal period, had significantly reduced body weight (Fig. 2), similar to what is observed in human neonates following methadone exposure.64–66 Specifically, rats pups born to mothers with methadone containing mini-osmotic pumps were smaller at both P1 and P21 compared to rat pups born to mothers with saline mini-osmotic pumps (P<0.01, and p<0.001 for both ages, n=10–21/group). To confirm levels of methadone were measurable in pups through milk intake, we quantified methadone levels in urine (Fig. 3). Methadone levels were confirmed in both dams and pups, with an average level of 9 ng/mL and 5.5 ng/mL in urine, respectively. As expected, the maternal urine concentration of methadone significantly increased proportionally to the dose of methadone in the mini-osmotic pumps (Fig. 3A, p<0.05, n=4/group). Similarly, in pups, methadone concentration in the urine also increased proportionally to maternal dose (Fig. 3B, p<0.001, n=9–10/group). Given the dose-response observed in both the dams and pups, we selected the intermediate 12mg/kg dose of methadone for the remainder of the study.
Figure 2. Perinatal Methadone Exposure Yields Smaller Offspring.
Osmotic minipumps containing methadone or saline were implanted in pregnant rats on E16. Pups were born and body weights measured on postnatal day 1 (P1, A) and P21 (B). Pups in the 12mg/kg and 16 mg/kg methadone group were already significantly smaller by P1 (A). A dose-response effect was more evident by P21, with pups exposed to 8mg/kg, 12 mg/kg and 16 mg/kg of methadone in utero and through the perinatal period, being smaller compared to pups born to dams who had osmotic mini pumps with saline. *p<0.05, ***p<0.001, n=9–10/group.
Figure 3. Methadone Dose-Response.
Osmotic minipumps were implanted in pregnant rats on E16 and urine methadone concentrations established in the dams at E19 (A) and in offspring pups on P14 (B). The urine concentration of methadone significantly increased as the methadone dose in the minipump was increased. *p<0.05, ***p<0.001, n=9–10/group.
Methadone Increases Inflammatory Cytokines in Peripheral Circulation
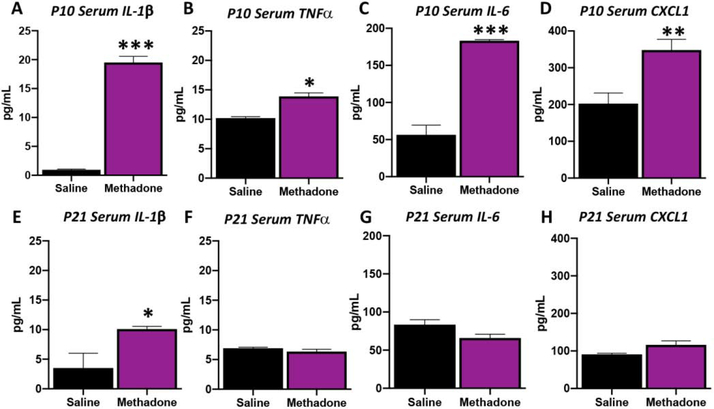
Fetal inflammatory response syndrome (FIRS), the fetal equivalent of systemic inflammatory response syndrome (SIRS), is defined by a robust and diverse systemic inflammatory protein network profile.43,67 Increased concentration of inflammatory cytokines in the fetal circulation are a hallmark of FIRS, including upregulation of IL-6, TNFα and IL-1β.39,68–73 Together with reduced body size and methadone dose-response in urine, we found that methadone induces a SIRS that, like in humans, persists for weeks postnatally (Fig 4).42,45,74–77 Specifically, at P10, representing approximately 17 days of methadone exposure, IL-1β increased by 19-fold (1954%, p<0.001, n=3–4/group), TNFα by 36% (p<0.05, n=3–4/group), IL-6 by 2-fold (225%, p<0.001, n=3–4/group) and CXCL1 by 72% (p<0.01, n=3–4/group). By P21, serum cytokines stabilized such that TNFα, IL-6 and CXCL1 were not different from saline controls. However, IL-1β remained significantly increased and was ~2-fold (188%) of the levels measured in the saline controls (p<0.05) after 28 days of methadone exposure. Together, these data demonstrate perinatal methadone exposure induces an increased inflammatory protein network signature, including elevations in pro-inflammatory cytokines such as IL-1β, TNFα, IL-6 and CXCL1. These elevations persist through the first postnatal week with elevated IL-1β levels over the month of exposure, indicative of a prolonged alteration in peripheral inflammation.
Figure 4. Perinatal Methadone Exposure Causes a Sustained Neonatal Increase in Serum Inflammatory Biomarkers.
Osmotic minipumps containing methadone or saline were implanted in pregnant rats on E16. Pups were born and serum collected on P10(A-D) and P21 (E-H). Using a translational multiplex electrochemiluminescent platform, biomarkers were assayed. On P10, those pups exposed to perinatal methadone had significantly increased IL-1β (A), TNFα (B), IL-6 (C) and CXCL1 (D) compared to saline pups. By P21, serum levels of IL-1β (E) remained significantly increased compared to methadone pups, while other cytokines and chemokines normalized. These data reflect significant and persistent increases in pro-inflammatory proteins in the serum throughout the perinatal period, consistent with a diverse inflammatory response syndrome. *p<0.05, **p<0.01, ***p<0.001, n=3–4/group.
Methadone Reprograms and Primes the Immune System
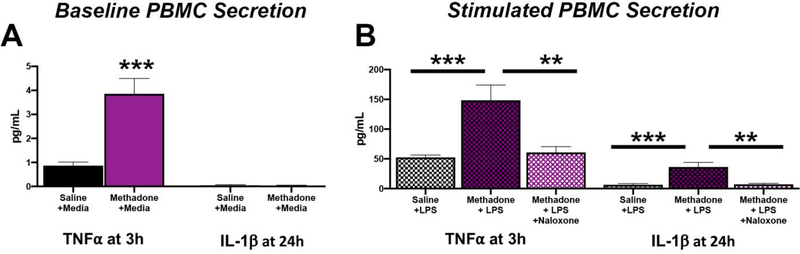
Similar to the CNS, the immune system develops and matures over the course of gestation and the perinatal period.68 Both PBMCs from children born preterm with cerebral palsy,37 and PBMCs from rats with a spastic gait from exposure to chorioamnionitis,38,48 exhibit hyper-responsivity to LPS challenge in vitro, suggesting that perinatal brain injury can co-exist with a sustained alteration in peripheral immunity. Given that we found a unique inflammatory signature in serum over an extended postnatal time course in this new model of perinatal methadone exposure, we examined the PBMC secretome at baseline and responsivity to an in vitro LPS challenge at P7 to assess methadone’s ability to modulate peripheral immune reactivity. Consistent with our hypothesis, we found that PBMCs isolated from methadone exposed pups were primed for inflammation (Fig. 5). PBMCs from methadone exposed pups hyper-sectreted pro-inflammatory cytokines at baseline (TNFα: 0.865±0.148 vs. 3.86±0.641 pg/mL; Fig. 5A, p<0.05, n=4). PBMCs from methadone exposed pups also hyper-secreted pro-inflammatory mediators compared to PBMCs from saline-exposed pups when challenged with LPS (TNFα: 52.4±4.07 vs. 148.5±25.6 pg/mL; IL-1β:6.43±1.99 vs. 36.3±7.59 pg/mL; Fig. 5B, p<0.001 for both, n=4). These data are consistent with enhanced immune activation after methadone exposure and lymphocyte hyper-reactivity following exposure to a secondary inflammatory stimuli. Interestingly, pre-treatment of PBMCs with naloxone, a μ-opioid receptor antagonist, ameliorated the hypersecretion from methadone exposed pups (Fig. 5B). Together, these data indicate that perinatal methadone exposure induces sustained peripheral immune hyper-reactivity (SPIHR),38 a phenomenon that can be blocked by naloxone.
Figure 5. Methadone Peripheral Blood Mononuclear Cells (PBMCs) are Primed and Hypersecrete Pro-Inflammatory Cytokines Following LPS stimulation but are Blocked by Naloxone.
PBMCs were isolated from P7 saline or methadone pups and stimulated with control (+media) (A) or lipopolysaccharide (+LPS) (B) for 3 or 24h. Secreted levels of TNFα from PBMCs were significantly increased at baseline (A). When PBMCs were challenged with LPS (100ng/mL), secreted levels of TNFα and IL-1β were also significantly increased compared to the response observed in saline PBMCs (B). Notably, treatment of PBMCs with naloxone (10μM) blocked the hypersecretion of TNFα and IL-1β (B). Together, these data indicate methadone induces a sustained peripheral inflammatory hyper response (SPIHR), a phenomenon that can be blocked by naloxone (n=4,*p<0.05, **p<0.01, ***p<0.001).
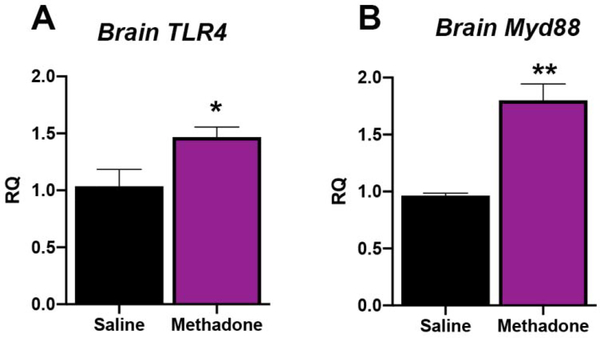
Methadone Increases Key Cerebral Molecular and Cellular Inflammatory Mediators
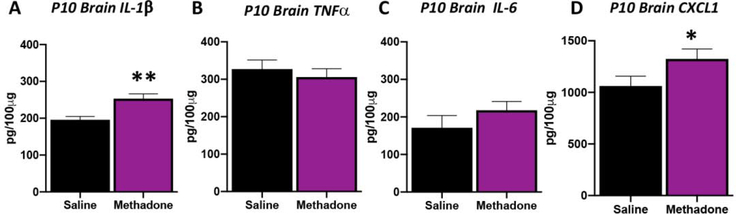
One common pathway for inflammatory signaling in perinatal brain injury involves activation of toll-like receptor 4 (TLR4), and its initial downstream mediator myeloid differentiation primary response protein (Myd88, Innate Immune Signal Transduction Adaptor).78 Initiation of this pathway activates transcription factor NFκβ, and other essential proinflammatory effectors. To begin to assess the cerebral microenvironment with perinatal methadone exposure, we performed qPCR for brain levels of these key inflammatory genes. As expected, perinatal methadone exposure increased cerebral cortical TLR4 and MyD88 mRNA at P10 (Fig. 6, p<0.05 and p<0.01 respectively; n=4–6/group). Specifically, TLR4 mRNA expression increased by 42% and MyD88 mRNA expression increased by 87% in the brains of pups exposed to methadone compared to those exposed to saline (Fig. 6). Complimenting these gene changes, changes in cerebral pro-inflammatory cytokine protein levels were also observed at P10 (Fig. 7). Indeed, brain cytokine and chemokine analyses confirmed a 29% increase in cortical IL-1β (p<0.01, n=6–7/group) and a 25% increase in cortical CXCL1 (p<0.05, n=6–7/group) levels with perinatal methadone exposure compared to saline-exposed controls. Brain levels of TNFα and IL-6 at P10 were unchanged with methadone exposure (Fig. 7). Collectively, these data demonstrate both inflammatory chemokine/cytokine increases and receptor imbalances in crucial inflammatory signaling cascades at a critical period of CNS development.
Figure 6. Methadone Increases TLR4 and Myd88 Inflammatory Gene Expression in the Developing Brain.
Osmotic minipumps containing methadone or saline were implanted in pregnant rats on E16. Brain cortex on postnatal day 10 (P10) was assayed for levels of TLR4 (A) and Myd88 (B) gene expression. Methadone significantly increased cerebral TLR4 and Myd88 mRNA expression compared to cortex from pups born to dams with osmotic minipumps containing saline. These data are consistent with a cerebral inflammatory microenvironment during a crucial period of neurodevelopment. *p<0.05, **p<0.01, n=4–6/group.
Figure 7. Perinatal Methadone Exposure Causes Sustained Neuroinflammation.
Osmotic minipumps containing methadone or saline were implanted in pregnant rats on E16. Cerebral cortex collected on postnatal day 10 (P10) was assayed using a translational multiplex electrochemiluminescent platform to quantify cytokine and chemokine levels. Those pups exposed to methadone had significantly increased IL-1β (A), and CXCL1 (D) compared to saline control pups. Levels of TNFα (B) and IL-6 remained unchanged. These data reflect a robust and prolonged increase in pro-inflammatory proteins the brain during a critical period of neurodevelopment. *p<0.05, **p<0.01, ***p<0.001, n=6–7/group.
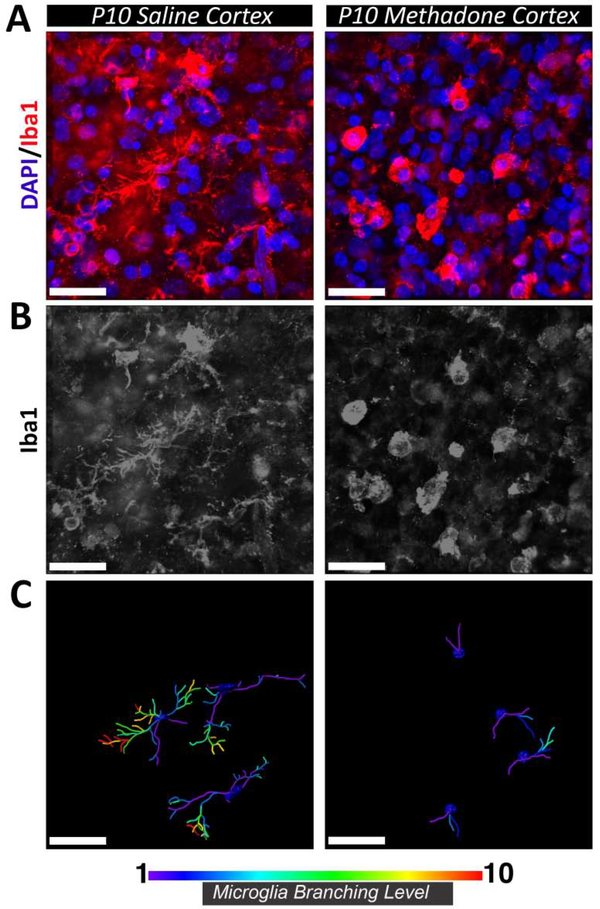
As we observed marked differences in mRNA and proteins associated with neuroinflammation, we then assessed the local cellular response by immunostaining for microglia/macrophage/monocyte marker Iba1. High-resolution confocal microscopy with 3D rendering and filament reconstruction was used to compare and quantify the cellular morphology of Iba1-positive cells residing within the cortex of saline- and methadone-exposed animals at P10 (Fig.8). Visualization of Iba1 immunofluorescence demonstrated clear changes in the morphology of cells following methadone exposure. Specifically, Iba1-positive cells in the cortex of saline-exposed controls were characterized by multiple protruding processes that were further branched as they extended from the central cell body, yielding increased branch levels. In contrast, Iba1-positive cells in methadone-exposed animals were relatively condensed and lacked branch complexity. The sparse branches that were present appeared comparatively short and retracted. These pronounced morphological distinctions were supported by filament reconstruction and quantification within Imaris software, confirming the reduced arborization of cellular processes in methadone exposed animals and clear demonstration of increased branch levels in microglia/macrophage/monocytes from saline exposed pups compared to those from methadone exposed animals (Fig. 8, n=4/group). Together these data indicate altered inflammatory cellular morphology consistent with increased neuroinflammation in methadone exposed pups.
Figure 8. Perinatal methadone exposure alters microglia/macrophage morphology.
Cortex from methadone-exposed and saline-exposed P10 pups was immunolabeled for microglia/macrophage marker Iba1 (red) and cellular nuclei (DAPI; blue) (A). The morphology of Iba1+ microglia/macrophages in methadone-exposed pups was distinct compared to saline-exposed microglia/macrophages (B). Microglia/macrophages in methadone-exposed pups exhibited fewer cellular processes with a diminished degree of branching (C). Scale bars = 30 μm. n=4/group.
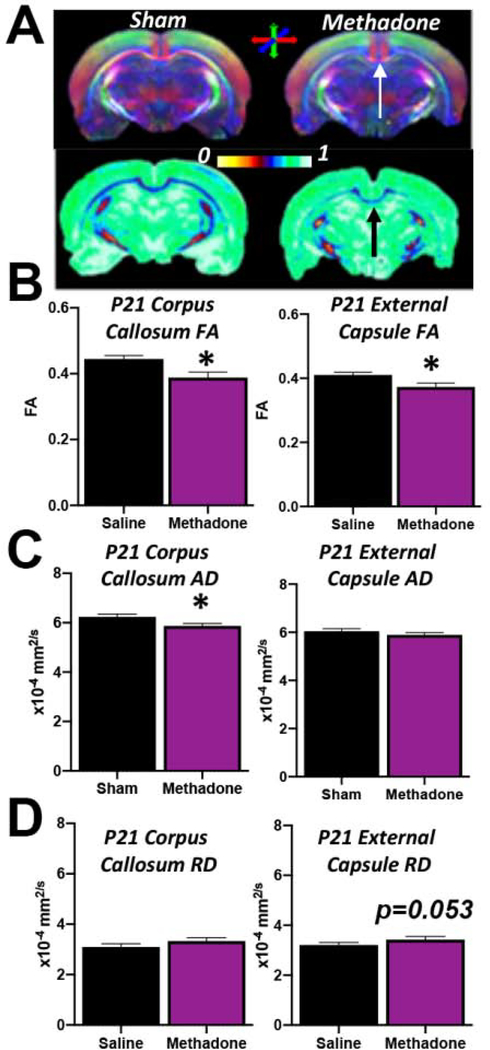
Methadone Induces Structural and Microstructural Brain Injury
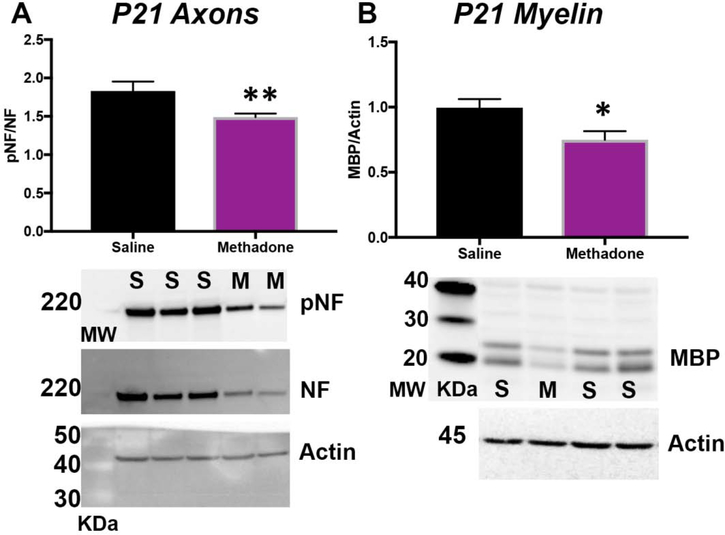
With significant evidence supporting a methadone-induced pro-inflammatory cerebral microenvironment, we next sought to define the structural consequences of methadone exposure in the CNS. We performed a biochemical assessment of axons and myelin, and an ex vivo magnetic resonance imaging evaluation of cerebral diffusion. First, we performed immunoblots for key axonal and myelin proteins. There was a significant reduction in axonal integrity and myelin expression in methadone pups at P21 (pre-adolescent equivalent)35,36 compared to those exposed to saline (Fig. 9). Specifically, the ratio of phosphoneurofilament to total neurofilament (pNF:NF), an indicator of healthy axons, was significantly decreased in methadone pups compared to sham pups (p<0.01, n=6–7/group). Similarly, levels of myelin basic protein (MBP), a marker of mature myelin, was also significantly reduced in methadone treated pups compared to shams (p<0.05, n=6–7/group). To complement this biochemical approach, we then examined the brain using ex vivo diffusion tensor imaging (DTI). At P21, methadone exposed rat offspring had decreased fractional anisotropy (FA) compared to rat pups exposed to saline, consistent with abnormal diffusion in the corpus callosum (methadone:0.388±0.02 vs. saline:0.445±0.01) and external capsule (methadone:0.373±0.01 vs. saline:0.410±0.01) (Fig. 10). Specifically, FA was decreased by 13% in the corpus callosum (p<0.05) and 10% in the external capsule (p<0.05) compared to saline controls (n=4–5/group, Fig. 10A, 10B). Additionally, axial diffusivity (AD) was significantly decreased in the corpus callosum of methadone exposed pups compared to saline (methadone:5.87±0.1 vs. saline:6.24±0.12×10−4 mm2/s, Fig. 10C) and radial diffusivity (RD) was significantly increased in the external capsule of methadone pups compared to saline controls (methadone:3.53±0.09 vs. saline:3.21±0.10×10−4mm2/s, Fig. 10D). Together, these data indicate structural and microstructural changes to axons and myelin with perinatal methadone exposure.
Figure 9. Methadone Reduces the Expression of Essential Cerebral White Matter Proteins.
Osmotic minipumps containing methadone or saline were implanted in pregnant rats on E16. Pups were born and then cerebral white matter collected on P21. Immunoblotting for the axonal proteins phopho-neurofilament and total neurofilament (pNF/NF, A), and myelin basic protein (MBP, B) demonstrate reduced axonal and myelin health in pups exposed to methadone (M) compared to saline (S) controls. *p<0.05, **p<0.01, n=6–7/group.
Figure 10. Methadone Reduces White Matter Fractional Anisotropy, Increases Axial Diffusivity and Impairs Cerebral Structural Coherence.
Osmotic minipumps containing methadone or saline were implanted in pregnant rats on E16. Pups were born and underwent ex vivo diffusion tensor imaging on postnatal day 21 (P21). Compared to sham, methadone pups have abnormal directional diffusion (top panel) and reduced fractional anisotropy (FA, A and B) in major white matter tracts. Notably, axial diffusivity (AD) is significantly decreased in the corpus callosum of methadone pups (C), while radial diffusivity (RD) remained unchanged (D). While AD in the external capsule was not statistically different, RD was increased in methadone pups compared to shams. These data are consistent with reduced integrity of white matter microstructure, axon and myelin injury. *p<0.05 n=4–5/group.
Methadone Induces Functional Brain Injury and Cognitive Deficits
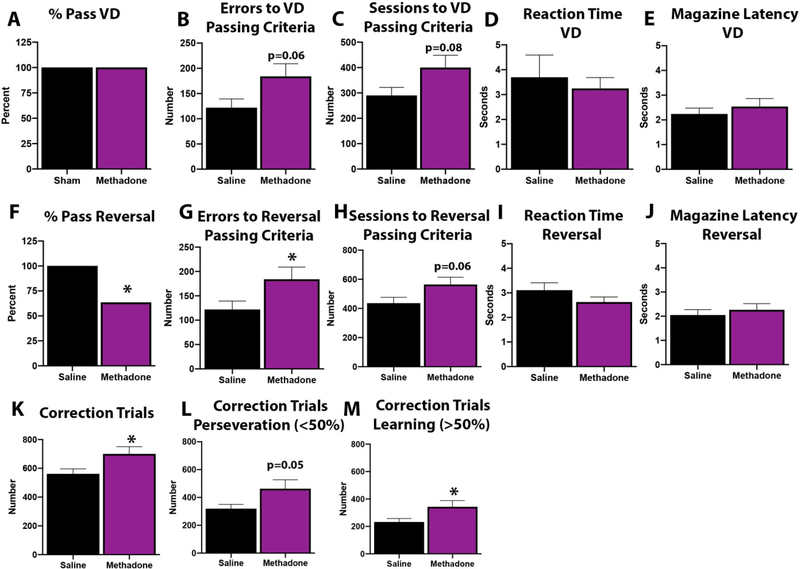
Considering the evidence of structural and microstructural brain injury together, we then investigated whether there was functional impairment in methadone exposed animals through adulthood. Specifically, we chose a translatable touchscreen operant platform to assess executive function and cognition (Fig. 11). We first validated the touchscreen platform in our methadone rats to determine whether adult rats subjected to methadone in the perinatal period could successfully pass touchscreen training and complete a visual discrimination task. Adult rats exposed to methadone (n=11) or saline (n=10) in the perinatal period successfully completed all aspects of touchscreen habituation and training. Additionally, all rats attained the performance criterion for discrimination learning (Fig. 11A). However, rats exposed to perinatal methadone showed a trend to make more incorrect responses (Fig. 11B, p=0.06) and to require more sessions to pass (Fig. 11C, p=0.08), although these differences did not reach statistical significance. There were no significant differences in reaction time (Fig. 11D) or latency to retrieve reward from the magazine (Fig. 11E) between groups, indicating that the observed deficits were not due to impaired sensorimotor-related performance, vision deficits or lack of motivation.
Figure 11. Methadone Significantly Impairs Cognitive Function in Adult Rats Following Perinatal Exposure.
Osmotic minipumps were implanted in pregnant rats on E16. Pups were born and in adulthood were tested on a touchscreen cognitive assessment platform for visual discrimination and reversal learning. All rats exposed to saline and methadone were able to pass the visual discrimination (VD) task (A). However, those exposed to methadone committed more discrimination errors (B) and required more testing sessions (C) in order to achieve passing criteria. There were no significant differences in reaction time (D) or latency to retrieve reward from the magazine (E) between groups. After achieving VD criterion, reversal learning was assessed. Adult rats exposed to methadone during the perinatal period had diminished ability to reverse the previously learned association (F). Methadone animals made significantly more incorrect responses (G) across the reversal paradigm, and also required more sessions to achieve passing criterion (H). As in discrimination, no differences were seen in motor function or motivation as measured by reaction time (I) or magazine latency (J) during reversal. In order to determine whether poor performance during reversal was associated with deficits in cognitive control or learning acquisition, we assessed the number of correction trials (K) required during the initial perseverative phase (accuracy <50%, L) and later learning phase (accuracy ≥50%, M). Consistent with findings in sessions and errors, methadone rats required significantly more correction trials compared to sham. Methadone rats committed significantly more correction errors both during the perseverative phase (L) and during the later learning phase (M) compared to saline control animals. Together, these data indicate that adult rats exposed to perinatal methadone are impaired in both early and late reversal learning, consistent with widespread learning and executive control dysfunction. *p<0.05, n=10–11/group.
After achieving VD criterion, reversal learning was assessed. Notably, adult rats exposed to methadone during the perinatal period had a diminished ability to reverse the previously learned association. Only 63.6% of methadone animals successfully passed criteria for reversal compared to 100% of saline controls (Fig. 11F, p=0.03). Methadone animals made significantly more incorrect responses (Fig. 11G, p=0.02) across the reversal paradigm versus saline controls. Methadone animals also showed a trend to require more sessions to achieve passing criterion (Fig. 11H, p=0.06), although this difference was not significant. As in discrimination, no differences were seen in motor function or motivation as measured by reaction time (Fig. 11I) or magazine latency (Fig. 11J) during reversal.
In order to determine whether poor performance during reversal was associated with deficits in cognitive control or learning acquisition, we assessed the number of correction trials required during the initial perseverative phase (accuracy <50%) and later learning phase (accuracy ≥50%) of reversal.61,63,79,80 Consistent with findings in sessions and errors, methadone rats required significantly more correction trials compared to sham (Fig. 11K, p<0.05). Reversal phase analysis revealed that methadone rats committed significantly more correction errors both during the perseverative phase (Fig. 11L, p=0.05) and during the later learning phase (Fig. 11M, p<0.05) compared to saline control animals. Together, these data indicate that adult rats exposed to perinatal methadone are impaired in both early and late reversal learning, consistent with widespread learning and executive control dysfunction.
DISCUSSION
The mechanisms by which opioids affect children are multifold. They include child overdose, opioid use during pregnancy, disrupted parenting and attachment, maternal deprivation, and parental separation.1 In the current opioid crisis, it is estimated that up to 14.4% of pregnant women have opioids prescriptions dispensed during pregnancy.9,17,24,81 Thus, in order to facilitate translational investigations of the long-term outcomes of prenatal opioid exposure and to define new avenues of diagnosis and treatment for this vulnerable patient population, we developed a preclinical model of perinatal methadone exposure to study the molecular and cellular mechanisms of developmental injury. To our knowledge, this is the first report that rats exposed to methadone in the perinatal period have a signature of exposure defined by neural-immune dysfunction, microstructural injury on MRI and functional, cognitive deficits on a translatable touchscreen platform. This is concomitant with a robust inflammatory response defined by hallmarks of SIRS and ongoing cerebral inflammation. Significantly, our data indicate a distinct systemic and neuroinflammatory signature associated with methadone exposure that commences in utero, suggestive of an altered CNS microenvironment, dysregulated neurodevelopmental homeostasis, and complex concurrent neural injury.38,46,47,82 Importantly, our data corroborate early reports of the detrimental effects of methadone exposure in the perinatal period on neuro-otongeny and neural biochemistry.83–85 Together these data reinforce the importance of the timing and duration of opioid treatment and neurobiological responses.
In addition to reduced body weight in response to perinatal methadone exposure, we observed a robust SIRS and persistent elevations in multiple systemic pro-inflammatory proteins that did not accommodate or normalize with continued methadone exposure. This persistent neuroinflammation is striking in the context of the systemic inflammatory environment and the rapid periods of growth and development during this critical period for multiple organ systems. While a fetal inflammatory response syndrome is believed to commence with direct contact with inflammatory infiltrate into amniotic fluid and/or inflammatory cell transfer from uteroplacental circulation, a SIRS, or systemic inflammatory response syndrome, occurs later in the postnatal course and is associated with complex pathophysiology and multi-organ dysfuction.86–88 Notably, preterm newborns that have elevated levels of biomarkers of systemic inflammation on two occasions one week apart are at a higher risk of brain injury and impaired neurodevelopment.43–45,89,90 Specifically, the increasing breadth of early neonatal inflammation, indexed by the number of protein elevations or the number of functional protein classes elevated, is associated with increased structural and functional brain injury.42–45,91,92 Moreover, adverse outcomes are more strongly associated with a combination of antenatal and postnatal inflammation, than either circumstance alone.89 Together, these data indicate that the sustained inflammatory microenvironment in methadone exposed pups is representative of an environment primed for abnormal neural circuit and cerebral network development, adverse functional outcomes, and long-term changes in immune response.
Similar to neural cells, fetal and neonatal leukocytes are uniquely responsive to their environment68,93. Taken together with the serum cytokine data, the mechanism(s) for elevated pro-inflammatory mediators in the serum of methadone rats may in part be related to increased secretion of TNFα and IL-1β from PBMCs. Indeed, the immune plasticity altered by in utero insults demonstrated here, may have long-term effects on the inflammatory responses of circulating leukocytes and could serve as a fluid biomarker of persistent or prior neuroinflammation and brain injury.94,95 Significanlty, our data reveals a sustained peripheral inflammatory hyper-responsivity (SPIHR) in methadone-exposed pups. Immune activation is enhanced after opioid exposure and continues to be hyperactivated by secondary inflammatory stimuli. Specifically, PBMCs isolated from methadone-exposed pups are hyper-responsive and hyper-secrete proinflammatory cytokines and chemokines upon a second hit of LPS, similar to humans with cerebral palsy37 and animals with complex gait abnormalities secondary to in utero chorioamnionitis.38 These data confirm that immune cells are primed following perinatal methadone exposure and that peripheral immune responses are altered following opioid exposure commencing in utero. This may be an important mechanism of deleterious feed-forward inflammatory pathophysiology and fetal programming of immune system activation. Previously, we have reported altered sensory sensitivity in infants with prenatal opioid exposure compared to unexposed controls.31 Taken together with the preclinical data shown here, this effect observed in infants, suggests that the priming of the pain response may be mediated by the immune system. Thus, the insidious effects of primed peripheral immune cells may compound brain injury secondary to opioid exposure and increase susceptibility to later-life inflammatory, neuroinflammatory, and neurological disease.
In the mature CNS, a significant relationship has been demonstrated between opioids and inflammation mediated by TLR4.96,97 Peripheral and CNS immune cells express a wide array of receptors, including opioid receptors and TLR4. Classical TLR4 activation occurs upon the recognition of molecular patterns and danger signals (lipolpolysaccharide/LPS, danger-associated molecular patterns/DAMPs, Alarmins) to trigger an innate immune response.98 Upon recognition of these patterns, TLR4 dimerizes and signals through adaptor proteins like Myd88 to phosphorylate NFκB, resulting in the production of pro-inflammatory mediators, such as cytokines IL-1β, TNFα and IL-6 and chemokines MCP-1 and CXCL1.78 In addition to this classical signaling pathway through Myd88, TLR4 signaling also occurs in response to all natural, semisynthetic and fully synthetic opioids, including methadone, morphine, and oxycodone.99 While LPS is the classical TLR4 agonist, major differences exist between LPS and opioids as TLR4-agonists, and the nature of their TLR4-mediated immune responses. Specifically, LPS-inflammation relies upon peripheral immune signals to activate glia due to poor brain- and placental-barrier penetrance.99 Opioids, however, rapidly cross the placenta and blood-brain barrier and directly activate glia and other immune cells.99 Opioids increase the expression of GFAP and Iba1, key astrocyte and microglial markers, and alter cellular morphology from a ramified to an amoeboid state.100 In adults, opioid-induced glial activation opposes opioid analgesia and enhances opioid tolerance, dependence and reward.100,101 Opioids also induce a central immune response by increasing major pro-inflammatory cytokines including IL-1β, TNFα and IL-6 and chemokines MCP-1 and CXCL1 following acute and chronic exposure.102 Here, we confirm that perinatal methadone exposure augments brain levels of TLR4 and Myd88, concomitant with upregulation of brain chemokines and changes in microglia/macrophage morphology, thereby inducing a molecular and cellular neuroinflammatory response. An increase in cerebral inflammatory mediators such IL-1β and CXCL1 could contribute to perinatal brain injury through multiple mechanisms, including direct initiation of programed cell death pathways, microglial activation, and neutrophil and peripheral immune cell recruitment. When the trajectory of brain development over gestation and the perinatal period is considered, these data support a signature of neural injury linked to a toxic inflammatory profile induced by methadone exposure. Interestingly, IL-1β failed to normalize over the time course examined in this study, but it is difficult to speculate whether IL-1β is instrumental to the cognitive and microstructural injury observed following exposure to methadone or is simply a bystander effect. Unquestionably, future work on molecular mechanism is required and will be needed to define spatiotemporal analyses of IL-1β, IL-1R expression and downstream signal transduction.
Opioids impair adult brain function and cognitive skills acutely.2,103 However, their effects on the developing brain may be subtle and long-lasting.2 Our data confirm that opioid exposure during crucial in utero and perinatal periods of neurodevelopment induce lasting and permanent changes in brain structure and function in rats. Notably, methadone pups have decreased expression of MBP and altered pNF:NF expression, synonymous with impaired myelination and axonal injury, similar to that which has been reported in animal models of placental insufficiency and chorioamnionitis.40,55 These data also corroborate prior data demonstrating that buprenorphine and methadone disrupt complex sequences of molecular events essential to connectivity in the developing brain, including oligodendrocyte maturation and timing of myelination.104,105 To this end, using high-resolution, translational neuroimaging with diffusion sequences, we found that FA was decreased in major white matter tracts, including the corpus callosum, in methadone pups at P21 compared to controls. FA is a marker of tract microstructure that reflects fiber density, axonal diameter, wrapping by pre-myelinating oligodendrocytes and myelination.24 Therefore, these data suggests that rats exposed to perinatal methadone have more immature, less coherently organized fiber tracts compared to controls, consistent with microstructural brain injury. Interestingly, white matter injury can be driven by immune dysregulation.82,106,107 Our data are similar to those in human neonates exposed to methadone in utero,24 and are consistent with the observation that FA is reduced through the white matter of neonates born to mothers who were prescribed methadone.17,24
Reductions in neonatal FA are associated with neurodevelopmental impairment.17,24,108 Thus, because many infants with prenatal opioid exposure from the current US epidemic will soon be school-age children, we assessed learning and cognition in adult rats following perinatal methadone exposure using a touchscreen platform. Adult rats exposed to methadone in the perinatal period performed markedly slower in the acquisition of pairwise visual discrimination in adulthood compared to controls. Moreover, when the reinforced contingencies of the learned association were reversed, methadone rats were significantly impaired compared to controls. Analysis of reversal performance by stage revealed that methadone rats were impaired both on the early, perseverative phase of reversal, as well as the later learning stage. Numerous previous studies have shown that discrimination, as well as the learning stage of reversal, is mediated primarily by the dorsal striatum, while efficient early reversal requires intact cortical functioning.61,63,109,110 Our results suggest that methadone significantly impairs both the efficient learning of a new association during discrimination, as well as cortically-mediated early phases of reversal. Not surprisingly, the deficits in associative learning in the methadone rats also impaired the learning of the new association during late reversal. Importantly, these impairments were not due to sensorimotor-related performance or nonspecific lack of motivation, as evidenced by normal scores on response reaction times and reward retrieval latencies. Collectively, these data show that methadone exposure in utero leads to long term impairments in both associative learning and executive control. Perhaps more compelling, however, is that these data support clinical literature, and are in direct alignment with a recent meta analysis demonstrating prenatal opioid exposure is negatively associated with neurocognitive and physical development111, along with numerous other smaller studies.112–115 Specifically, prenatal opioid exposure is associated with lower cognitive scores, with the largest differences observed between ages 6 months and 6 years. Indeed, data show that neurodevelopment does not improve after preschool and worsens by school age.111 This persistence of deficits through school age is significant, as these children are also often vulnerable to multiple tenuous social and environmental factors. They are at increased risk of neglect and abuse, and have a greater likelihood of behavioral and attention deficits, all of which contribute to poorer academic, social and lifestyle factors116,117 and are contingent on cognitive function, associative learning and executive control.111
We acknowledge limitations to the design and implementation of our study. These limitations will be the subject of additional, future investigations. First, our study was not powered to exclude sex differences in outcome measures despite both sexes being used in each outcome measure. Now that this study is complete, further investigations beyond the scope of the present investigation will examine sex differences in executive function and cognitive control as has been done in similar studies with prenatal exposures including alcohol63 This is essential as sex-specific modifiers of opioid induced inflammation may yield important data about molecular and biobehavioural outcomes and be central to the development of novel therapeutic approaches. Similarly, differentiation of the postnatal versus the in utero onset of opioid induced neuroinflammation and definition of the inflammatory signature in utero will be essential for defining therapeutic window and putative timing for neurotherapeutic intervention. Second, we used Iba1 as a cellular marker of microglia. However, Iba1 can also be expressed by monocytes and macrophages. Given the degree of inflammation shown here, and because opioid use has been shown to disrupt the blood-brain barrier, further studies on the contribution of resident and infiltrating immune cells are needed. Indeed, it is very likely that resident and infiltrating immune cell populations are also contributing to opioid induced pathophysiology and future studies beyond the present scope will more fully elucidate the nature of the inflammatory response with complete flow cytometry panels, individual immune cell gene analyses, RNA seq and differential immunolabeling. Third, we used a laboratory grade ELISA kit to perform urinalysis for methadone levels in both rat dams and pups. Sensitive, clinical assays are needed to distinguish between methadone, endogenous opioids and their metabolites. Future studies will also address regional differences in diffusion metrics and expanded MRI analyses, as well as assessment of the nociceptive phenotype in animals exposed to methadone in the perinatal period.
In conclusion, we provide evidence that methadone exposure in the perinatal period leads to a unique immune, neural and behavioral phenotype, associated with a systemic pro-inflammatory signature indicative of widespread brain and immune system injury. This signature reflects a significantly altered cerebral and immune microenvironment concomitant with dysregulated developmental homeostasis in the perinatal period. This disruption not only alters early CNS development, but also induces lifelong changes in brain structure and function. The structural and functional brain injury observed in this investigation as pups exposed to methadone aged into adulthood is consistent with other forms of perinatal brain injury hallmarked by neuroinflammation.40,47,59,60 However, it is the differences between various forms of perinatal brain injury that may be truly informative to outcomes. For example, we previously have shown a much more robust and persistent CXCL1 signature in preclinical models of chorioamnionitis and different biobehavioral and cognitive control phenotypes in models of prenatal alcohol exposure, infant trauma and cerebral palsy.38,46,47,60 Interestingly, naloxone or interventions that are anti-inflammatory, neurorestorative,118 and support myelination and cognition, such as erythropoietin and melatonin,40,41,49,53,55,59,60 may have utility during the perinatal period and potential clinical applications in this patient population with methadone exposure. Undoubtedly, these data support the need for further study of the mecahnisms of neural and immune cell dysfunction in the context of opioid exposure and demand concern considering the rapid rise of prescription opioid use and misuse around the world.
Highlights.
Perinatal methadone increases inflammatory cytokines in the peripheral circulation
Methadone reprograms and primes lymphocytes and induces a sustained peripheral immune hyper-reactivity
Perinatal methadone increases cerebral TLR4 and Myd88 gene expression concomitant with increased microglial activation
Perinatal methadone decreases essential myelin and axon protein expression and induces abnormalities on diffusion tensor imaging
Perinatal methadone induces functional brain injury and cognitive deficits in adult animals
Acknowledgements
This study was supported by generous funding from the Dedicated Health Research Funds from the University of New Mexico, the Department of Pediatrics at the University of New Mexico Health Sciences Center, and the National Institutes of Health 1R01HL139492 to LJ. We are grateful to1S10OD021598 for 7T MRI resources at the University of New Mexico, and the exceptional MRI expertise of Yirong Yang, PhD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Feder KA, Letourneau EJ, Brook J. Children in the Opioid Epidemic: Addressing the Next Generation’s Public Health Crisis. Pediatrics 2019;143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oei JL, Melhuish E, Uebel H, et al. Neonatal Abstinence Syndrome and High School Performance. Pediatrics 2017;139. [DOI] [PubMed] [Google Scholar]
- 3.Congress U. Protecting Our Infants Act of 2015. S799 2015;114th Congress (2015–2016). [Google Scholar]
- 4.Howse J March of Dimes Foundation letter of support. March 18, 2015 2015;Available at: www.marchofdimes.org/materials/HR-1462-March-of-Dimes-LetterofSupport-March-18-2015.pdf.
- 5.Organization WH. Guidelines for the identification and management of substance use and substance use disorders in pregnancy. Available at: http://appswhoint/iris/bitstream/10665/107130/1/9789241548731_engpdf 2016. [PubMed]
- 6.Azuine RE, Ji Y, Chang HY, et al. Prenatal Risk Factors and Perinatal and Postnatal Outcomes Associated With Maternal Opioid Exposure in an Urban, Low-Income, Multiethnic US Population. JAMA Netw Open 2019;2:e196405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salihu HM, Salinas A, Medina I, Krishnaswami J, Aliyu MH. Biopsychosocial determinants of opioid use disorder (OUD) and implications for maternal and child health research: A scoping review. J Opioid Manag 2019;15:77–91. [DOI] [PubMed] [Google Scholar]
- 8.Ailes EC, Dawson AL, Lind JN, et al. Opioid prescription claims among women of reproductive age--United States, 2008–2012. MMWR Morb Mortal Wkly Rep 2015;64:37–41. [PMC free article] [PubMed] [Google Scholar]
- 9.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol 2014;123:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein RA, Bobo WV, Martin PR, et al. Increasing pregnancy-related use of prescribed opioid analgesics. Ann Epidemiol 2013;23:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patrick SW, Dudley J, Martin PR, et al. Prescription opioid epidemic and infant outcomes. Pediatrics 2015;135:842–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patrick SW, Schiff DM, Committee On Substance USE, Prevention. A Public Health Response to Opioid Use in Pregnancy. Pediatrics 2017;139. [DOI] [PubMed] [Google Scholar]
- 13.Kocherlakota P Neonatal abstinence syndrome. Pediatrics 2014;134:e547–61. [DOI] [PubMed] [Google Scholar]
- 14.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA : the journal of the American Medical Association 2012;307:1934–40. [DOI] [PubMed] [Google Scholar]
- 15.McQueen K, Murphy-Oikonen J. Neonatal Abstinence Syndrome. N Engl J Med 2016;375:2468–79. [DOI] [PubMed] [Google Scholar]
- 16.Hudak ML, Tan RC, Committee On D, Committee On F, Newborn, American Academy of P. Neonatal drug withdrawal. Pediatrics 2012;129:e540–60. [DOI] [PubMed] [Google Scholar]
- 17.Monnelly VJ, Hamilton R, Chappell FM, Mactier H, Boardman JP. Childhood neurodevelopment after prescription of maintenance methadone for opioid dependency in pregnancy: a systematic review and meta-analysis. Developmental medicine and child neurology 2019;61:750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol 2015;35:650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiff DM, Patrick SW. Treatment of Opioid Use Disorder During Pregnancy and Cases of Neonatal Abstinence Syndrome. JAMA pediatrics 2017. [DOI] [PubMed] [Google Scholar]
- 20.Brandt L, Finnegan LP. Neonatal abstinence syndrome: where are we, and where do we go from here? Curr Opin Psychiatry 2017. [DOI] [PubMed] [Google Scholar]
- 21.Sirnes E, Oltedal L, Bartsch H, Eide GE, Elgen IB, Aukland SM. Brain morphology in school-aged children with prenatal opioid exposure: A structural MRI study. Early Hum Dev 2017;106–107:33–9. [DOI] [PubMed] [Google Scholar]
- 22.Nekhayeva IA, Nanovskaya TN, Deshmukh SV, Zharikova OL, Hankins GD, Ahmed MS. Bidirectional transfer of methadone across human placenta. Biochem Pharmacol 2005;69:187–97. [DOI] [PubMed] [Google Scholar]
- 23.Gerdin E, Rane A, Lindberg B. Transplacental transfer of morphine in man. J Perinat Med 1990;18:305–12. [DOI] [PubMed] [Google Scholar]
- 24.Monnelly VJ, Anblagan D, Quigley A, et al. Prenatal methadone exposure is associated with altered neonatal brain development. NeuroImage Clinical 2018;18:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monnelly VJ, Hamilton R, Chappell FM, Mactier H, Boardman JP. Childhood neurodevelopment after prescription of maintenance methadone for opioid dependency in pregnancy: a systematic review and meta-analysis. Developmental medicine and child neurology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansson LM, Velez M, McConnell K, et al. Maternal buprenorphine treatment and fetal neurobehavioral development. American journal of obstetrics and gynecology 2017;216:529 e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansson LM, Velez ML, McConnell K, et al. Maternal buprenorphine treatment and infant outcome. Drug Alcohol Depend 2017;180:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velez ML, McConnell K, Spencer N, Montoya L, Tuten M, Jansson LM. Prenatal buprenorphine exposure and neonatal neurobehavioral functioning. Early human development 2018;117:7–14. [DOI] [PubMed] [Google Scholar]
- 29.Jansson LM, Dipietro JA, Velez M, et al. Fetal neurobehavioral effects of exposure to methadone or buprenorphine. Neurotoxicol Teratol 2011;33:240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaltenbach K, O’Grady KE, Heil SH, et al. Prenatal exposure to methadone or buprenorphine: Early childhood developmental outcomes. Drug Alcohol Depend 2018;185:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakhireva LN, Holbrook BD, Shrestha S, et al. Association between prenatal opioid exposure, neonatal opioid withdrawal syndrome, and neurodevelopmental and behavioral outcomes at 5–8 months of age. Early human development 2019;128:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansson LM, Di Pietro JA, Elko A, Williams EL, Milio L, Velez M. Pregnancies exposed to methadone, methadone and other illicit substances, and poly-drugs without methadone: a comparison of fetal neurobehaviors and infant outcomes. Drug Alcohol Depend 2012;122:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Progress in neurobiology 2013;106–107:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiu JR, Ensom MH. Dosing and monitoring of methadone in pregnancy: literature review. Can J Hosp Pharm 2012;65:380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabriel SM, Roncancio JR, Ruiz NS. Growth hormone pulsatility and the endocrine milieu during sexual maturation in male and female rats. Neuroendocrinology 1992;56:619–25. [DOI] [PubMed] [Google Scholar]
- 36.Agoston DV. How to Translate Time? The Temporal Aspect of Human and Rodent Biology. Frontiers in neurology 2017;8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin CY, Chang YC, Wang ST, Lee TY, Lin CF, Huang CC. Altered inflammatory responses in preterm children with cerebral palsy. Annals of neurology 2010;68:204–12. [DOI] [PubMed] [Google Scholar]
- 38.Yellowhair TR, Noor S, Mares B, et al. Chorioamnionitis in Rats Precipitates Extended Postnatal Inflammatory Lymphocyte Hyperreactivity. Developmental neuroscience 2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maxwell JR, Denson JL, Joste NE, Robinson S, Jantzie LL. Combined in utero hypoxia-ischemia and lipopolysaccharide administration in rats induces chorioamnionitis and a fetal inflammatory response syndrome. Placenta 2015;36:1378–84. [DOI] [PubMed] [Google Scholar]
- 40.Robinson S, Corbett CJ, Winer JL, et al. Neonatal erythropoietin mitigates impaired gait, social interaction and diffusion tensor imaging abnormalities in a rat model of prenatal brain injury. Experimental neurology 2017;302:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson S, Winer JL, Berkner J, et al. Imaging and serum biomarkers reflecting the functional efficacy of extended erythropoietin treatment in rats following infantile traumatic brain injury. Journal of neurosurgery Pediatrics 2016;17:739–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dammann O, Allred EN, Fichorova RN, et al. Duration of Systemic Inflammation in the First Postnatal Month Among Infants Born Before the 28th Week of Gestation. Inflammation 2016;39:672–7. [DOI] [PubMed] [Google Scholar]
- 43.Kuban KC, O’Shea TM, Allred EN, et al. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatric neurology 2015;52:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leviton A, Kuban KC, Allred EN, et al. Early postnatal blood concentrations of inflammation-related proteins and microcephaly two years later in infants born before the 28th post-menstrual week. Early human development 2011;87:325–30. [DOI] [PubMed] [Google Scholar]
- 45.O’Shea TM, Allred EN, Kuban KC, et al. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. J Pediatr 2012;160:395–401 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yellowhair TR, Newville JC, Noor S, et al. CXCR2 Blockade Mitigates Neural Cell Injury Following Preclinical Chorioamnionitis. Front Physiol 2019;10:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yellowhair TR, Noor S, Maxwell JR, et al. Preclinical chorioamnionitis dysregulates CXCL1/CXCR2 signaling throughout the placental-fetal-brain axis. Experimental neurology 2018;301:110–9. [DOI] [PubMed] [Google Scholar]
- 48.Jantzie LL, Corbett CJ, Berglass J, et al. Complex pattern of interaction between in utero hypoxia-ischemia and intra-amniotic inflammation disrupts brain development and motor function. J Neuroinflammation 2014;11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jantzie LL, Getsy PM, Firl DJ, Wilson CG, Miller RH, Robinson S. Erythropoietin attenuates loss of potassium chloride co-transporters following prenatal brain injury. Molecular and cellular neurosciences 2014;61:152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson S, Berglass JB, Denson JL, et al. Microstructural and microglial changes after repetitive mild traumatic brain injury in mice. Journal of neuroscience research 2017;95:1025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cengiz P, Zafer D, Chandrashekhar JH, et al. Developmental differences in microglia morphology and gene expression during normal brain development and in response to hypoxia-ischemia. Neurochem Int 2019;127:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Combes AN, Short KM, Lefevre J, Hamilton NA, Little MH, Smyth IM. An integrated pipeline for the multidimensional analysis of branching morphogenesis. Nature protocols 2014;9:2859–79. [DOI] [PubMed] [Google Scholar]
- 53.Jantzie LL, Corbett CJ, Firl DJ, Robinson S. Postnatal Erythropoietin Mitigates Impaired Cerebral Cortical Development Following Subplate Loss from Prenatal Hypoxia-Ischemia. Cereb Cortex 2015;25:2683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jantzie LL, Getsy P, Denson JL, Firl DJ, Wilson CG, Robinson S. Prenatal hypoxia-ischemia induces potassium chloride cotransporter 2 loss and abnormalities in inhibitory tone. Front Cell Neurosci 2015;3:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jantzie LL, Winer JL, Corbett CJ, Robinson S. Erythropoietin Modulates Cerebral and Serum Degradation Products from Excess Calpain Activation following Prenatal Hypoxia-Ischemia. Developmental neuroscience 2016;38:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jantzie LL, Talos DM, Jackson MC, et al. Developmental Expression of N-Methyl-d-Aspartate (NMDA) Receptor Subunits in Human White and Gray Matter: Potential Mechanism of Increased Vulnerability in the Immature Brain. Cereb Cortex 2015;25:482–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yellowhair TR, Noor S, Maxwell JR, et al. Preclinical chorioamnionitis dysregulates CXCL1/CXCR2 signaling throughout the placental-fetal-brain axis. Experimental neurology 2017;301(B):110–9. [DOI] [PubMed] [Google Scholar]
- 58.Veenstra M, Ransohoff RM. Chemokine receptor CXCR2: physiology regulator and neuroinflammation controller? J Neuroimmunol 2012;246:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jantzie LL, Oppong AY, Conteh FS, et al. Repetitive Neonatal Erythropoietin and Melatonin Combinatorial Treatment Provides Sustained Repair of Functional Deficits in a Rat Model of Cerebral Palsy. Frontiers in neurology 2018;9:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson S, Winer JL, Chan LAS, et al. Extended Erythropoietin Treatment Prevents Chronic Executive Functional and Microstructural Deficits Following Early Severe Traumatic Brain Injury in Rats. Frontiers in neurology 2018;9:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brigman JL, Daut RA, Wright T, et al. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nat Neurosci 2013;16:1101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graybeal C, Feyder M, Schulman E, et al. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat Neurosci 2011;14:1507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marquardt K, Sigdel R, Caldwell K, Brigman JL. Prenatal ethanol exposure impairs executive function in mice into adulthood. Alcoholism, clinical and experimental research 2014;38:2962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zedler BK, Mann AL, Kim MM, et al. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta-analysis of safety in the mother, fetus and child. Addiction 2016;111:2115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mactier H, Shipton D, Dryden C, Tappin DM. Reduced fetal growth in methadone-maintained pregnancies is not fully explained by smoking or socio-economic deprivation. Addiction 2014;109:482–8. [DOI] [PubMed] [Google Scholar]
- 66.Wouldes TA, Woodward LJ. Maternal methadone dose during pregnancy and infant clinical outcome. Neurotoxicol Teratol 2010;32:406–13. [DOI] [PubMed] [Google Scholar]
- 67.Francis F, Bhat V, Mondal N, et al. Fetal inflammatory response syndrome (FIRS) and outcome of preterm neonates - a prospective analytical study. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2019;32:488–92. [DOI] [PubMed] [Google Scholar]
- 68.Madsen-Bouterse SA, Romero R, Tarca AL, et al. The transcriptome of the fetal inflammatory response syndrome. American journal of reproductive immunology 2010;63:73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. American journal of obstetrics and gynecology 1998;179:194–202. [DOI] [PubMed] [Google Scholar]
- 70.Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. American journal of obstetrics and gynecology 1998;179:186–93. [DOI] [PubMed] [Google Scholar]
- 71.Galinsky R, Polglase GR, Hooper SB, Black MJ, Moss TJ. The consequences of chorioamnionitis: preterm birth and effects on development. Journal of pregnancy 2013;2013:412831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. American journal of obstetrics and gynecology 2006;195:803–8. [DOI] [PubMed] [Google Scholar]
- 73.Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clinical obstetrics and gynecology 2007;50:652–83. [DOI] [PubMed] [Google Scholar]
- 74.Dowling O, Chatterjee PK, Gupta M, et al. Magnesium sulfate reduces bacterial LPS-induced inflammation at the maternal-fetal interface. Placenta 2012;33:392–8. [DOI] [PubMed] [Google Scholar]
- 75.O’Shea TM, Joseph RM, Kuban KC, et al. Elevated blood levels of inflammation-related proteins are associated with an attention problem at age 24 mo in extremely preterm infants. Pediatric research 2014;75:781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97:210–5. [PubMed] [Google Scholar]
- 77.Watterberg KL, Scott SM, Naeye RL. Chorioamnionitis, cortisol, and acute lung disease in very low birth weight infants. Pediatrics 1997;99:E6. [DOI] [PubMed] [Google Scholar]
- 78.Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal 2007;7:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem 2008;15:50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brigman JL, Rothblat LA. Stimulus specific deficit on visual reversal learning after lesions of medial prefrontal cortex in the mouse. Behavioural brain research 2008;187:405–10. [DOI] [PubMed] [Google Scholar]
- 81.Bateman BT, Hernandez-Diaz S, Rathmell JP, et al. Patterns of opioid utilization in pregnancy in a large cohort of commercial insurance beneficiaries in the United States. Anesthesiology 2014;120:1216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boardman JP, Ireland G, Sullivan G, et al. The Cerebrospinal Fluid Inflammatory Response to Preterm Birth. Front Physiol 2018;9:1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McLaughlin PJ, Zagon IS, White WJ. Perinatal methadone exposure in rats. Effects on body and organ development. Biol Neonate 1978;34:48–54. [DOI] [PubMed] [Google Scholar]
- 84.Zagon IS, McLaughlin PJ. Perinatal methadone exposure and its influence on the behavioral ontogeny of rats. Pharmacol Biochem Behav 1978;9:665–72. [DOI] [PubMed] [Google Scholar]
- 85.Zagon IS, McLaughlin PJ. Perinatal methadone exposure and brain development: a biochemical study. J Neurochem 1978;31:49–54. [DOI] [PubMed] [Google Scholar]
- 86.Anblagan D, Pataky R, Evans MJ, et al. Association between preterm brain injury and exposure to chorioamnionitis during fetal life. Sci Rep 2016;6:37932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ackerman WEt, Buhimschi IA, Eidem HR, et al. Comprehensive RNA profiling of villous trophoblast and decidua basalis in pregnancies complicated by preterm birth following intra-amniotic infection. Placenta 2016;44:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sampson JE, Theve RP, Blatman RN, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. American journal of obstetrics and gynecology 1997;176:77–81. [DOI] [PubMed] [Google Scholar]
- 89.Yanni D, Korzeniewski SJ, Allred EN, et al. Both antenatal and postnatal inflammation contribute information about the risk of brain damage in extremely preterm newborns. Pediatric research 2017;82:691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leviton A, Kuban K, O’Shea TM, et al. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J Pediatr 2011;158:897–903 e1–5. [DOI] [PubMed] [Google Scholar]
- 91.Leviton A, Fichorova R, Yamamoto Y, et al. Inflammation-related proteins in the blood of extremely low gestational age newborns. The contribution of inflammation to the appearance of developmental regulation. Cytokine 2011;53:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leviton A, Allred EN, Fichorova RN, et al. Systemic inflammation on postnatal days 21 and 28 and indicators of brain dysfunction 2years later among children born before the 28th week of gestation. Early human development 2016;93:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Claus CP, Tsuru-Aoyagi K, Adwanikar H, et al. Age is a determinant of leukocyte infiltration and loss of cortical volume after traumatic brain injury. Developmental neuroscience 2010;32:454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci 2005;25:8000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci 2005;119:293–301. [DOI] [PubMed] [Google Scholar]
- 96.Hutchinson MR, Northcutt AL, Hiranita T, et al. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 2012;32:11187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lewis SS, Loram LC, Hutchinson MR, et al. (+)-naloxone, an opioid-inactive toll-like receptor 4 signaling inhibitor, reverses multiple models of chronic neuropathic pain in rats. J Pain 2012;13:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grace PM, Maier SF, Watkins LR. Opioid-induced central immune signaling: implications for opioid analgesia. Headache 2015;55:475–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jacobsen JH, Watkins LR, Hutchinson MR. Discovery of a novel site of opioid action at the innate immune pattern-recognition receptor TLR4 and its role in addiction. Int Rev Neurobiol 2014;118:129–63. [DOI] [PubMed] [Google Scholar]
- 100.Hutchinson MR, Coats BD, Lewis SS, et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain, behavior, and immunity 2008;22:1178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci 2009;30:581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther 2012;134:219–45. [DOI] [PubMed] [Google Scholar]
- 103.Younger JW, Chu LF, D’Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain 2011;152:1803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vestal-Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE, Sato-Bigbee C. The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Developmental neuroscience 2014;36:409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sanchez ES, Bigbee JW, Fobbs W, Robinson SE, Sato-Bigbee C. Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia 2008;56:1017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Back SA, Miller SP. Brain injury in premature neonates: A primary cerebral dysmaturation disorder? Annals of neurology 2014;75:469–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hagberg H, Mallard C, Ferriero DM, et al. The role of inflammation in perinatal brain injury. Nat Rev Neurol 2015;11:192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Kooij BJ, de Vries LS, Ball G, et al. Neonatal tract-based spatial statistics findings and outcome in preterm infants. AJNR American journal of neuroradiology 2012;33:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marquardt K, Josey M, Kenton JA, Cavanagh JF, Holmes A, Brigman JL. Impaired cognitive flexibility following NMDAR-GluN2B deletion is associated with altered orbitofrontal-striatal function. Neuroscience 2019;404:338–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bergstrom HC, Lipkin AM, Lieberman AG, et al. Dorsolateral Striatum Engagement Interferes with Early Discrimination Learning. Cell Rep 2018;23:2264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yeoh SL, Eastwood J, Wright IM, et al. Cognitive and Motor Outcomes of Children With Prenatal Opioid Exposure: A Systematic Review and Meta-analysis. JAMA Netw Open 2019;2:e197025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nygaard E, Slinning K, Moe V, Walhovd KB. Cognitive function of youths born to mothers with opioid and poly-substance abuse problems during pregnancy. Child Neuropsychol 2017;23:159–87. [DOI] [PubMed] [Google Scholar]
- 113.Konijnenberg C, Melinder A. Executive function in preschool children prenatally exposed to methadone or buprenorphine. Child Neuropsychol 2015;21:570–85. [DOI] [PubMed] [Google Scholar]
- 114.Nygaard E, Moe V, Slinning K, Walhovd KB. Longitudinal cognitive development of children born to mothers with opioid and polysubstance use. Pediatric research 2015;78:330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nygaard E, Slinning K, Moe V, Due-Tonnessen P, Fjell A, Walhovd KB. Neuroanatomical characteristics of youths with prenatal opioid and poly-drug exposure. Neurotoxicol Teratol 2018;68:13–26. [DOI] [PubMed] [Google Scholar]
- 116.Maguire DJ, Taylor S, Armstrong K, et al. Long-Term Outcomes of Infants with Neonatal Abstinence Syndrome. Neonatal Netw 2016;35:277–86. [DOI] [PubMed] [Google Scholar]
- 117.Messinger DS, Bauer CR, Das A, et al. The maternal lifestyle study: cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics 2004;113:1677–85. [DOI] [PubMed] [Google Scholar]
- 118.Volpe JJ. Dysmaturation of Premature Brain: Importance, Cellular Mechanisms, and Potential Interventions. Pediatric neurology 2019;95:42–66. [DOI] [PubMed] [Google Scholar]