Abstract
Background & Aims:
Nearly all studies of gastric adenocarcinoma in the United States have relied on national cancer databases, which do not include data on Helicobacter pylori infection, the most well-known risk factor for gastric cancer. We collected data from a large cohort of patients in the United States to calculate the incidence of and risk factors for non-proximal gastric adenocarcinomas after detection of H pylori. Secondary aims included identifying how treatment and eradication affect cancer risk.
Methods:
We performed a retrospective cohort study, collecting data from the Veterans Health Administration on 371,813 patients (median age, 62 years; 92.3% male) who received a diagnosis of H pylori infection from January 1, 1994 through December 31, 2018. The primary outcome was a diagnosis of distal gastric adenocarcinoma 30 days or more after detection of H pylori infection. We performed time to event with competing risk analysis (death before cancer a competing risk).
Results:
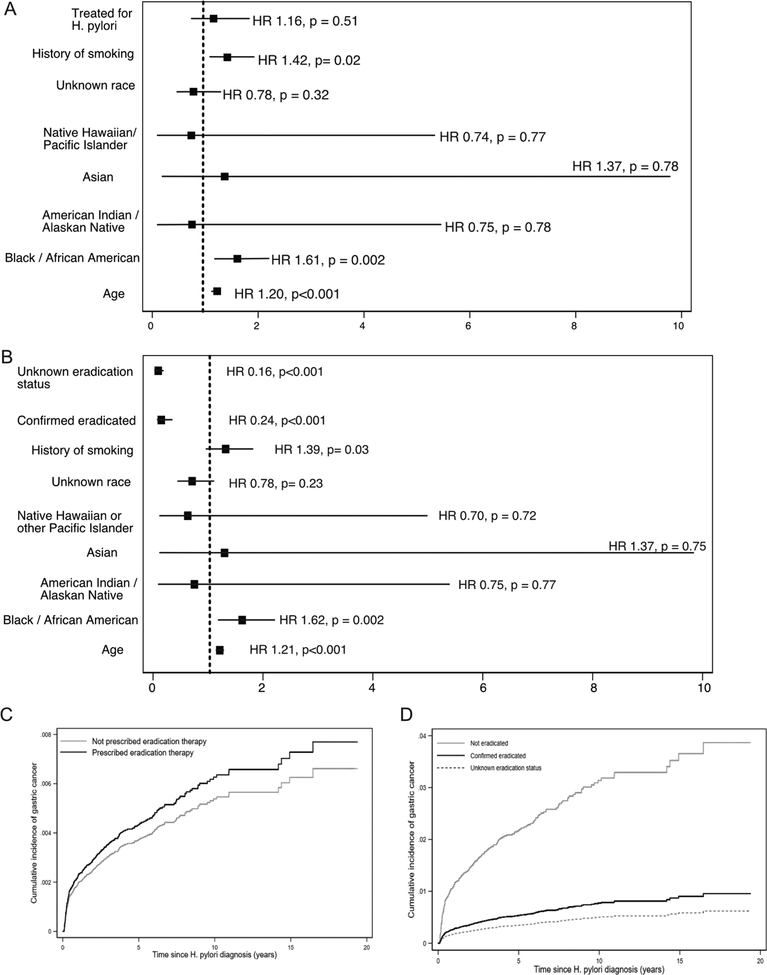
The cumulative incidence of cancer at 5, 10, and 20 years after detection of H pylori infection was 0.37%, 0.5%, and 0.65%, respectively. Factors associated with cancer included older age at time of detection of H pylori infection (sub-hazard ratio [SHR], 1.13; 95% CI, 1.11–1.15; P<.001), black/African American race (SHR, 2.00; 95% CI, 1.80–2.22), Asian race (SHR, 2.52; 95% CI, 1.64–3.89) (P<.001 for race), Hispanic or Latino ethnicity (SHR, 1.59; 95% CI, 1.34–1.87; P<.001), or history of smoking (SHR, 1.38; 95% CI, 1.25–1.52; P<.001). Women had decreased risk of gastric adenocarcinoma compared with men (SHR, 0.52; 95% CI, 0.40–0.68; P<.001); patients whose H pylori infection was detected based on serum antibody positivity also had a reduced risk of cancer (SHR 0.74; 95% CI, 0.54–1.04; P=0.04). Patients who received treatment for their H pylori infection still had an increased risk of gastric cancer (SHR, 1.16; 95% CI, 0.74–1.83; P=.51), but confirmed H pylori eradication after treatment reduced risk of gastric cancer (SHR, 0.24; 95% CI, 0.15–0.41; P<.001).
Conclusions:
In a study of 371,813 veterans with a diagnosis of H pylori infection, we found significantly higher risks of gastric cancer in racial and ethnic minorities and smokers. Treatment of H pylori infection only decreased risk if eradication was successful. Studies are needed on the effects of screening high-risk persons and to identify quality measures for diagnosis, resistance patterns, and treatment efficacy.
Keywords: stomach cancer, antibacterial therapy, microbe, screening
Among US patients with H pylori, racial/ethnic minorities and smokers have a higher risk of future gastric cancer. Eradication of H pylori infection decreases the risk.
INTRODUCTION
Gastric cancer is the third most common cause of cancer death worldwide.1 Although less common in the United States, with an annual incidence of 26,000 cases, it is among the most fatal, with 5-year survival under 30%.2, 3 Helicobacter pylori (HP), a class I carcinogen, is a causative agent in the cascade leading to gastric adenocarcinoma (GAC), particularly non-proximal cancers.4–6 Yet infection alone is not sufficient for carcinogenesis, illustrated by the discordant association of high HP prevalence with low GAC incidence in sub-Saharan Africa (the “African enigma”) or disparate rates of GAC throughout Middle Eastern countries despite high HP burden.7–10
Despite robust data on GAC and the role of HP from Asia, nearly all large studies in the US have used national cancer databases, which contain limited demographic and environmental exposures, medical history, and most importantly, no data on HP.11–16 As a result, there are critical knowledge gaps in our understanding of the incidence of non-proximal GAC in the setting of HP in the US, risk factors associated with future GAC, and the impact of HP treatment. Modeling studies have been performed to assess which populations in the US may benefit from screening, but were limited by a lack of robust data on the incidence of GAC in a US population.17, 18
We aim to determine the incidence of non-proximal GAC after detection of HP infection in a large US cohort and further define how demographics, environmental factors, and treatment of HP infection impact the incidence of GAC. In doing so, we sought to overcome current knowledge gaps in GAC, including potentially modifiable risk factors in a US population, to best inform screening strategies.
METHODS
This retrospective cohort study was conducted within the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW), which includes data from the unified electronic medical record of all VHA facilities (i.e., hospitals and outpatient) since 10/01/1999.
Study cohort
We first identified patients with HP infection based on any of the following: 1) HP infection on endoscopic pathology by natural language processing (Supplemental Methods), 2) positive stool antigen test, 3) positive urea breath test, 4) prescription for one of 11 accepted eradication regimens for HP therapy as recommended by the American College of Gastroenterology, or 5) HP-associated International Classification of Diseases (ICD) 9/10 codes (ICD-9: 041.86; ICD-10: B96.81.19–22
For patients with multiple criteria, the criterion with the earliest date was used. (Unique identifiers assured no duplications.) For those who had a prescription or administrative code as initial HP diagnosis without a diagnostic test that confirmed infection, we queried to identify if serum antibody was tested within 90 days of HP diagnosis.
Study outcome
The outcome for all analyses was GAC, identified using Veterans Affairs Central Cancer Registry and/or ICD 9/10 codes for non-proximal gastric adenocarcinoma (ICD-9: 151.1–151.9; ICD-10: C16.1–C16.9).23 The Central Cancer Registry is a comprehensive, national database of cancers diagnosed and treated in the VHA since 1995. The diagnosis of GAC was minimum 30 days after HP diagnosis to ensure testing was for HP, not a malignancy workup. We filtered to include intestinal type non-cardia GACs, to avoid capturing non-adenocarcinomas and cardiac/gastroesophageal junction tumors, which are less clearly associated with HP.24–26 Additional natural language processing was performed (Supplemental Methods).
Study exposures and statistical analysis
We performed a time to event analysis using competing risk models, with start time the date of HP diagnosis. Follow-up time ended at development of GAC, death prior to GAC, or end of follow-up. Death was considered a competing risk to GAC as patients could die from another cause, precluding the development of GAC, and factors associated with mortality might also be associated with GAC. Among the entire HP cohort, we evaluated covariates shown to be associated with GAC15, 27, 28: age at HP diagnosis, gender, race, ethnicity, history of ever smoking (current or prior diagnostic code)29, and zip code-level poverty at HP diagnosis. Zip code-level poverty was based on 2010 census data, categorized based on percentage of people within a zip code below the federal poverty line. Stata/IC 15.1 (College Station, TX) was used to perform backward selection, with inclusion of all clinically significant sub-hazard ratios (SHRs), where P<0.10.
After evaluating baseline factors associated with GAC, we sought to evaluate the association between treatment and eradication status on the incidence of GAC. Because one of our larger cohort inclusion criteria was receipt of eradication therapy, these analyses were restricted to those for whom the HP diagnosis was made using the gold standard of histology, stool antigen, and/or urea breath testing. Receipt of HP treatment was defined as receiving a recommended antibiotic regimen after HP diagnosis at the VHA using prescription filling data at any inpatient or outpatient VHA facility (Supplemental Methods Table 1). Because guidelines recommend that all patients treated for HP have confirmed eradication (i.e., cure) given the presence of resistant HP strains, we sought to evaluate whether HP treatment only decreased the risk of GAC among those with confirmed eradication (i.e., those who were successfully treated). Eradication was based on having either a negative stool antigen, urea breath test, and/or pathology (gastric biopsy on endoscopy) upon repeat testing. Failed eradication was defined as a positive stool antigen, urea breath test, and/or pathology, or a positive HP test after a prior negative test given that true re-infection is exceedingly rare. Patients without any eradication testing were considered as ‘unknown’ eradication status. HP status on pathology was determined by repeat natural language processing (Supplemental Methods). For this analysis, we excluded patients who had eradication testing via endoscopy within 90 days of eventual cancer diagnosis, as this was possibly performed for alarm symptoms, versus eradication testing alone. The Institutional Review Boards of the Corporal Michael J. Crescenz VA Medical Center and the University of Pennsylvania approved this study.
RESULTS
We identified 371,813 patients with HP: 26,873 with endoscopic pathology positive for HP, 11,262 with stool antigen, 400 with urea breath test, 266,216 with prescription of an eradication regimen, and 67,062 with administrative codes (Figure 1a). Of 266,216 with a prescription for an eradication regimen for HP, 7,854 (3.0%) had a positive serum antibody within 90 days. Of 67,062 with administrative code for HP, 57,508 (85.8%) had a positive serum antibody within 90 days.
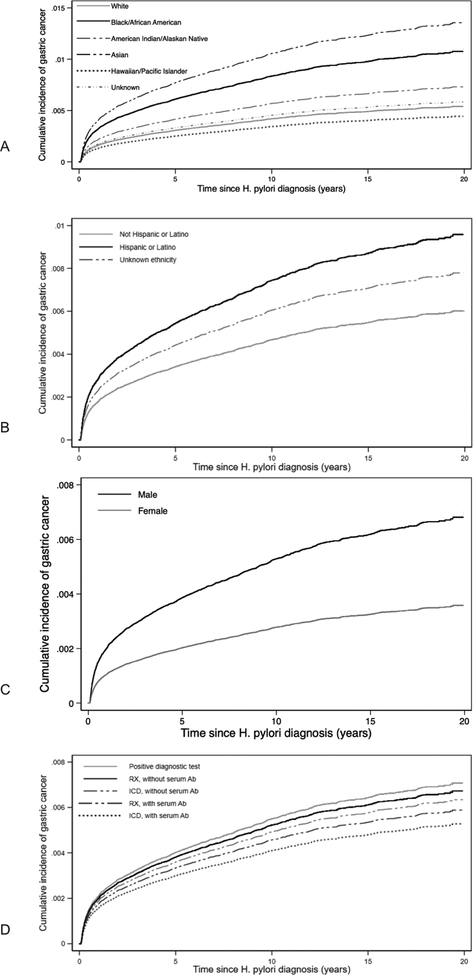
Figure 1a–c:
Cohort of patients: a) with H pylori; b) who developed non-proximal gastric adenocarcinoma; and c) among those a positive diagnostic test (endoscopic pathology, stool antigen, urea breath test) who underwent treatment and eradication testing ICD = International Classification of Diseases (administrative codes)
Among 371,813 patients with HP, 2,024 (0.54%) developed GAC, with median follow-up 7.4 years (Figure 1b). As compared to patients with HP who did not develop cancer, patients with HP who developed cancer were older (median age at HP diagnosis: 65.1 vs 62 years, P<0.001), male (97% vs 92.3%, P<0.001), more likely to be African American (31.7% vs 23.8%, P<0.001), less likely to be Hispanic/Latino (74.5% vs 78.5%, P=0.002), and more likely to have ever smoked (30.7% vs 26.4%, P<0.001). Those who developed cancer were more likely to have died, 67.7% vs 37.2%, P<0.001. Median age at cancer diagnosis was 69 years.
Of the 38,535 with a positive diagnostic test (stool antigen, urea breath test, or pathology), 28,818 (74.8%) were prescribed an eradication regimen and 8,020 (20.8%) then underwent re-testing (Figure 1c). Among those who underwent eradication testing, the infection was successfully eradicated in 7,292 (90.9%).
Multivariable analysis of risk factors associated with GAC
Several factors were associated with incident diagnosis of GAC in multivariable competing-risk models (Table 2). The method of HP diagnosis was associated with GAC, with the lowest risk among those identified as having HP based on only an ICD diagnosis of HP with a positive serum antibody in the adjacent 90 days (SHR, 0.75; 95% CI, 0.62–0.90). For each 5-year increase in age at HP diagnosis, the SHR for future GAC increased 1.13 (95% CI, 1.11–1.15; P<0.001). Black or African American race (SHR, 2.00; 95% CI, 1.80–2.22) and Asian race (SHR, 2.52; 95% CI, 1.64–3.89) were associated with significantly increased risks of GAC, as was Hispanic ethnicity (SHR, 1.59; 95% CI, 1.34–1.87; P<0.001). Smoking history was associated with GAC (SHR, 1.38; 95% CI, 1.25–1.52; P<0.001), while zip code level poverty was not. Figures 2a–2d depict the cumulative incidence curves for each significant covariate, adjusted for other covariates. Sub-analysis of those with a true positive diagnostic test found 5,10, and 20-year incidence of future GAC was 0.40%, 0.55%, and 0.71%, respectively, comparable to the larger cohort.
Table 2.
Risk factors for development of gastric cancer using multivariable competing risk time to event model
| SHR (95% Cl) | P- value | |
|---|---|---|
| Agea | 1.13 (1.11–1.15) | P<0.001 |
| Method of H pylori diagnosis | ||
| Positive diagnostic test | REFERENCE | 0.006 |
| RX, no serum Ab | 0.95(0.82–1.10) | |
| ICD, no serum Ab | 0.89(0.65–1.23) | |
| RX, with serum Ab | 0.83(0.59–1.17) | |
| ICD, with serum Ab | 0.75 (0.62–0.90) | |
| Ethnicity | ||
| Not Hispanic or Latino | REFERENCE | |
| Hispanic or Latino | 1.59(1.34–1.87) | P<0.001 |
| Unknown | 1.30 (1.08–1.55) | |
| Race | ||
| White | REFERENCE | |
| Black or African American | 2.00 (1.80–2.22) | P<0.001 |
| American Indian or Alaskan Native | 1.36(0.84–2.19) | |
| Asian | 2.52(1.64–3.89) | |
| Native Hawaiian or other Pacific Islander | 0.82(0.48–1.42) | |
| Unknown | 1.08(0.91–1.29) | |
| History of smoking | 1.38(1.25–1.52) | P<0.001 |
| Female gender | 0.52 (0.40–0.68) | P<0.001 |
| Poverty level of zip code where patient resided at H pylori diagnosis | ||
| < 10% residing below poverty level | REFERENCE | |
| 10 − 24.9% residing below poverty level | 0.86 (0.77–0.97) | 0.09 |
| 25 − 49.9% residing below poverty level | 0.95(0.84–1.09) | |
| ≥50% residing below poverty level | 0.99(0.76–1.29) | |
| Unknown | 0.83(0.65–1.06) |
Age is per 5-year incremental increase in year
RX = prescription therapy; ICD = International Classification of Diseases (administrative codes); Ab = antibody
Figure 2a–d:
Cumulative incidence curves for a) race; b) ethnicity; c) gender; d) method of H pylori diagnosis, amongst entire cohort, adjusted for other covariates RX = prescription therapy; ICD = International Classification of Diseases (administrative codes); Ab = antibody
Secondary analyses of treatment and eradication among those with confirmed HP
Among the 38,535 individuals with a positive diagnostic test within the VHA, receipt of HP treatment was not associated with subsequent development of GAC (SHR, 1.16; 95% CI, 0.74–1.83; P=0.51). Similar to models of the entire cohort, increasing age, tobacco use, and race/ethnicity were associated with GAC in this restricted cohort (Figure 3a–c and Supplemental Table 1).
Figures 3a–d:
plotted sub-hazard ratios for development of gastric cancer after positive diagnostic test (endoscopic pathology, stool antigen, urea breath test), considering a) treatment status and b) eradication status, and cumulative incidence curves for c) treatment of H pylori and d) eradication of H pylori, adjusted for other covariates HR = hazard ratio
Among patients with confirmed HP who had eradication testing, there was a significantly lower risk of GAC among those with confirmed HP eradication (SHR, 0.24; 95% CI, 0.15–0.41; P<0.001). Age, race/ethnicity, and tobacco history were also still associated with development of GAC (Figure 3b–d and Supplemental Table 2).
Sensitivity analyses
Sensitivity analyses were conducted excluding incident cancers within 6 months and 12 months, versus the 3-month exclusion data presented above. The incidence of GAC after HP diagnosis excluding cancers within 6 months at 5, 10, and 20 years was 0.19%, 0.33%, and 0.49%. The incidence of GAC after HP diagnosis excluding cancers within 12 months at 5, 10, and 20 years was 0.24%, 0.38% and 0.53%. The results of subsequent analyses did not vary results significantly, and are presented in Supplemental Tables 3–8.
DISCUSSION
In the largest study of gastric cancer among US patients with diagnosed HP, the incidence of GAC after HP diagnosis at 5, 10, and 20 years was 0.37%, 0.5%, and 0.65%. Non-modifiable risk factors included older age, Black/African American and Asian race, Hispanic ethnicity, and male gender, while modifiable risk factors included tobacco history and eradication of HP. These data provide the first population-level estimates of GAC risk in a US cohort with HP infection which are needed to identify at-risk patients and to inform large-scale gastric cancer screening protocols in the US population.
Most importantly, we demonstrate a stark difference in incidence after HP detection in US patients as compared to patients in countries with high rates of GAC. In Japan, which has among the highest rate of GAC in the world, and where HP eradication is a strong health focus, 2.9% of patients with HP had future GAC, five-fold greater than our findings.30, 31 The reasons for this are not completely clear. The widely touted “hygiene hypothesis” is often used to explain the decreased incidence of GAC in the US, where a decrease in HP due to improved sanitation paralleled the decrease in GAC, but does not explain why we have a significantly lower incidence of GAC among those with known HP.32, 33 There have been findings of HP-negative GAC in the US, with some hypothesizing that this is due to causes of negative testing versus a lack of HP as a requirement for future GAC, but countries with high rates of GAC also have this finding, so it would not account for the discrepancy either.24, 34–36 It is possible that different virulence factors are associated with HP in the US, or that there is some other, not yet elucidated, protective environmental factor in the US, similar to the “African enigma” noted above. These are avenues that should be explored by future studies.
Prior US studies using national level data have demonstrated an increased incidence of GAC among racial/ethnic minorities.17, 37–41 This was hypothesized to be due to a higher prevalence of HP in these populations, but our study offers important insights in that it was restricted to patients with HP, and therefore clearly demonstrates that a higher prevalence of HP in these minority populations alone does not explain their increased incidence of GAC.22, 42 This underscores the need to evaluate other potential genetic, environmental, or HP-specific factors that increase the incidence of HP in these patients. We also found that tobacco smoking was associated with an increased risk of GAC, which speaks to the importance for behavioral modification to mitigate the risks of GAC among smokers, as well as to identify a higher-risk cohort of patients. Male gender was also associated with an increased risk of GAC, consistent with previous trends seen in the US patients with GAC, and posited to be related to a protective effect of estrogen.43–45 Lastly, older age was associated with future GAC, and is likely a proxy for duration of infection and associated inflammation.28
Based on our data, modeling studies could be performed to evaluate the benefit of screening, when targeted towards those patients we can identify as high-risk. Our findings suggest that a larger screening or surveillance program is likely not warranted given the low incidence of GAC after detection of HP, since even those patients we can identify as high-risk have an absolute risk that is small for future GAC. And while some may argue that the absolute risk is so low that no patient should be considered high-risk, our findings have some parallel with esophageal adenocarcinoma after Barrett’s esophagus, where the risk is approximately 2.5% at 18 years among patients with established intestinal metaplasia (an arguably higher risk group than our cohort), but robust screening and surveillance protocols are recommended.46, 47 Moreover, the incidence of gastric cancer is higher than the incidence of esophageal cancer, underscoring the disparity in screening protocols for these two malignancies.48
Importantly, patients who were apparently given a diagnosis of HP based on serum antibody positivity, which has been explicitly recommended against, had a lower risk of GAC, emphasizing the importance of testing with appropriate methods to establish a diagnosis and facilitate appropriate treatment.22 While we are not able to conclusively demonstrate that many patients are treated without a diagnostic test, as they may have undergone testing outside the VHA, there seems to be continued reliance on the serum antibody. Quality measures are important to ensure appropriate testing, and the widespread availability of the serum antibody could be reconsidered, given its lack of a role in diagnosing HP.
Among patients with positive diagnostic test, subsequent treatment was not associated with decreased incidence of future GAC, but failing eradication testing was associated with increased risk. This speaks to the ability of HP eradication to modify future risks of GAC, and the need to not only treat those diagnosed with HP, but to confirm eradication, and re-treat those who fail eradication. Treatment was prescribed in almost three-quarters of patients with a positive diagnostic test, and the majority (90.9%) of patients who were prescribed therapy and tested for eradication had their infections eradicated. Yet only one in five had post-treatment testing, an essential component of treatment. Though confirming eradication is guideline recommended, the rate of eradication testing is known to be subpar.22 Testing for eradication is especially important given the now alarming rates of HP antibiotic resistance, a well-known issue.22, 49–52 Two recent studies from Sweden and Hong Kong suggest that in comparison to the background population or matched controls, treatment decreases risk of future GAC, but other studies have presented limited and conflicting evidence for which populations would most benefit from treatment of HP.16, 21, 33, 53–59 Our study adds to these findings, and further suggests that inadequate eradication may explain disparate findings. Our findings that failed eradication is associated with future GAC also lend further credence to the importance of HP in GAC.
It is important to note, there have been proponents cautioning against the mass eradication of HP, given its role in microecology and antibiotic resistance, suggesting that eradication may fuel unintended diseases, including esophageal adenocarcinoma.60–62 In our study, those confirmed to have their infections eradicated and those who had unknown eradication status both had a lower risk of GAC. Those patients with unknown eradication status may have been re-tested outside of the VHA, treated successfully with a course of antibiotics (though not re-tested), or have some other reason to be lower-risk for GAC than those persons who were tested for eradication, apart from HP. Future studies should continue to assess the global risk-benefit of eradication of HP infection.
There are several limitations to this study. First, its retrospective nature diminishes the ability to determine causality. Second, the cohort has some inherent selection bias (those tested for HP are being tested for some clinical reason), and we are unable to compare to it a confirmed background or control population without HP. The majority were also included based on prescription therapy or administrative code, however, we evaluated those with a true positive diagnostic test, and found incidence of future GAC was comparable to the larger cohort, as noted above. Furthermore, we adjusted for method of diagnosis in the model (Figure 2d). Third, despite its robust nature, the VHA is overwhelmingly male, and our cohort was > 90% male, limiting generalizability, though our findings are consistent with known trends in GAC. Fourth, we were unable to determine indication for HP testing, country of origin, or family history of GAC, potential confounders. Fifth, there are possibilities for false-negative/positive testing. Lastly, there are measurement issues, including patients receiving care outside the VHA (limiting HP diagnosis, testing, treatment ascertainment, lack of endoscopic histopathology, as well as oncologic diagnoses and cancer registry inputs) and limitations in longitudinal follow up. However, misclassification of HP status would only affect inclusion into the cohort, and misclassification of the outcome, inadvertently including a proximal GAC, would only bias towards the null, since risk factors for distal and proximal cancer differ in terms of age, race, and ethnicity.63 Misclassification of treatment/eradication are also possible, with outside treatment and eradication testing under-capturing those who were treated and eradicated. Patients may have not obtained or taken their HP eradication regimen appropriately, which would explain the non-differential finding for GAC after treatment prescription. However, even with fill data, there would be no way to confirm that patients completed their treatment regimen. There may be misclassifications in our available data within the VHA, limiting those patients we are able to identify as diagnosed, treated, or cured, leading us to underestimate the number of patients diagnosed, treated, tested thereafter, and confirmed cured.
The strengths of our study are primarily related to the unique nature of the cohort, being the largest study of HP-related GACs in the US. National level data lacks the ability to identify preceding HP status, which we were able to do. We were able to define that HP status even further in granularity by identifying positive testing and those who were deemed positive by serology. With our diverse, nationwide population, and up to 20 years of follow up, this is the first study to identify risk factors and the importance of HP infection among US individuals. We demonstrate that in a diverse group of US patients with HP infection, future GAC incidence is low. Those at highest risk are males, Black or African Americans, Asians, and Hispanic and Latino patients. Smoking is an important modifiable risk factor for future GAC development. Treatment and eradication of infection are not uniformly performed at the VHA, and testing for HP continues to be performed by serum antibody, despite recommendations against this modality. Eradication of HP infection is associated with decreased future GAC. Future studies should focus on consideration of screening of high-risk persons, identification of what host and HP factors predispose to increased risk, quality measures to ensure appropriate diagnosis of HP infection, and resistance patterns and efficacy of treatment of HP infection.
Supplementary Material
Table 1.
Comparison of patients with H pylori who did and did not develop gastric cancer
| H pylori (n=369,789) | H pylori with future non-proximal gastric adenocarcinoma (n = 2,024) | P-value | |
|---|---|---|---|
| Age in years at H pylori, median (IQR) | 62.0 (52.7, 70.9) | 65.1 (57.4, 73.3) | P<0.001 |
| Male (%) | 341,367(92.3%) | 1,963 (97.0%) | P<0.001 |
| Race (%) | P<0.001 | ||
| White | 214,090 (57.9%) | 984 (48.6%) | |
| Black or African American | 87,989 (23.8%) | 641 (31.7%) | |
| American Indian or Alaskan Native | 3,322 (0.9%) | 17 (0.8%) | |
| Asian | 2,483 (0.7%) | 21 (1.0%) | |
| Native Hawaiian / Pacific Islander | 3,837 (1.0%) | 13 (0.6%) | |
| Unknown / Declined to Answer | 58,068 (15.7%) | 348 (17.2%) | |
| Ethnicity | P<0.001 | ||
| Hispanic / Latino | 290,286 (78.5%) | 1,507 (74.5%) | |
| Not Hispanic or Latino | 33,306 (9.0%) | 213 (10.5%) | |
| Unknown / Declined to Answer | 46,197 (12.5%) | 304 (15.0%) | |
| Ever smoker (%) | 97,571 (26.4%) | 621 (30.7%) | P<0.001 |
| Method of H pylori diagnosis | 0.009 | ||
| Pathology | 26,725 (7.2%) | 148 (7.3%) | |
| Stool Antigen | 11,205 (3.0%) | 57 (2.8%) | |
| Urea Breath Test | 399 (0.1%) | 1 (0.05%) | |
| RX, no serum Ab | 256,883 (69.5%) | 1,479 (73.1%) | |
| ICD, no serum Ab | 9,507 (2.6%) | 47 (2.3%) | |
| RX, with serum Ab | 7,815 (2.1%) | 39 (1.9%) | |
| ICD, with serum Ab | 57,255 (15.5%) | 253 (12.5%) | |
| Poverty level of zip code where patient resided at H pylori diagnosis | P<0.001 | ||
| < 10% residing below poverty level | 69,754 (18.9%) | 386 (19.1%) | |
| 10 − 24.9% residing below poverty level | 184,848 (50.0%) | 912 (45.1%) | |
| 25 − 49.9% residing below poverty level | 88,986 (24.1%) | 572 (28.3%) | |
| ≥50% residing below poverty level Unknown | 9,880 (2.7%) 16,321 (4.4%) | 76 (3.8%) 78 (3.9%) | |
| Follow up time in years, median (IQR) | 7.4 (3.3, 12.3) | 1.8 (0.4,5.8) | P<0.001 |
| Deceased | 137,575 (37.2%) | 1,371 (67.7%) | P<0.001 |
IQR = inter-quartile range; RX = prescription therapy; ICD = International Classification of Diseases (administrative codes); Ab = antibody
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT:
We collected data from a large cohort of patients in the United States to calculate the incidence of and risk factors for non-proximal gastric adenocarcinomas after detection of Helicobacter pylori.
NEW FINDINGS:
In a study of 371,813 veterans with a diagnosis of H pylori infection, we found significantly higher risks of gastric cancer in racial and ethnic minorities and smokers. Treatment of H pylori infection only decreased risk if eradication was successful.
LIMITATIONS:
This was a retrospective study of US veterans.
IMPACT:
Eradication of H pylori infection reduces the risk of gastric cancer. Studies are needed on the effects of screening high-risk persons and to identify quality measures for diagnosis, resistance patterns, and treatment efficacy.
Grant support:
Shria Kumar, MD is supported by an NIH training grant (5 T32 DK 7740–22)
Disclosures:
Shria Kumar, MD: Travel (Boston Scientific Corporation, Olympus Corporation)
David C. Metz, MBBCh: Consulting (Takeda, Lexicon, AAA. Novartis), Grant Support (Lexicon, Wren Laboratories, Ipsen, AAA)
Susan Ellenberg, PhD: Data monitoring committee (BMS, Marinus, Merck), Consulting (Janssen, Shionogi, Insmed, Alkermes, Novartis)
David E. Kaplan, MD, MSc: Research grant support (Gilead, Bayer)
David S. Goldberg, MD, MSCE: Research grant support (Gilead, Merck, AbbVie, Zydus)
Abbreviations:
- CDW
Corporate Data Warehouse
- GAC
gastric adenocarcinoma
- HP
Helicobacter pylori
- ICD
International Classification of Diseases
- VHA
Veterans Health Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Jim MA, Pinheiro PS, Carreira H, et al. Stomach cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer 2017;123 Suppl 24:4994–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noone AM HN, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2015, National Cancer Institute; Bethesda, MD. [Google Scholar]
- 4.Wang F, Meng W, Wang B, et al. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett 2014;345:196–202. [DOI] [PubMed] [Google Scholar]
- 5.Correa P Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol 1995;19 Suppl 1:S37–43. [PubMed] [Google Scholar]
- 6.Crowe SE. Helicobacter pylori Infection. N Engl J Med 2019;380:1158–1165. [DOI] [PubMed] [Google Scholar]
- 7.Mbulaiteye SM, Hisada M, El-Omar EM. Helicobacter Pylori associated global gastric cancer burden. Front Biosci (Landmark Ed) 2009;14:1490–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussein NR. Helicobacter pylori and gastric cancer in the Middle East: a new enigma? World J Gastroenterol 2010;16:3226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correa P, Piazuelo MB. Helicobacter pylori Infection and Gastric Adenocarcinoma. US Gastroenterol Hepatol Rev 2011;7:59–64. [PMC free article] [PubMed] [Google Scholar]
- 10.Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017;153:420–429. [DOI] [PubMed] [Google Scholar]
- 11.Corral JE, Delgado Hurtado JJ, Dominguez RL, et al. The descriptive epidemiology of gastric cancer in Central America and comparison with United States Hispanic populations. J Gastrointest Cancer 2015;46:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunz PL, Gubens M, Fisher GA, et al. Long-term survivors of gastric cancer: a California population-based study. J Clin Oncol 2012;30:3507–15. [DOI] [PubMed] [Google Scholar]
- 13.Lui FH, Tuan B, Swenson SL, et al. Ethnic disparities in gastric cancer incidence and survival in the USA: an updated analysis of 1992–2009 SEER data. Dig Dis Sci 2014;59:3027–34. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Sun Y, Bertagnolli MM. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: results from the Surveillance Epidemiology and End Results (SEER) database. Ann Surg Oncol 2015;22:2965–71. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Tao L, Murphy JD, et al. Race/Ethnicity-, Socioeconomic Status-, and Anatomic Subsite-Specific Risks for Gastric Cancer. Gastroenterology 2019;156:59–62 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YC, Chiang TH, Chou CK, et al. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016;150:1113–1124 e5. [DOI] [PubMed] [Google Scholar]
- 17.Kim GH, Liang PS, Bang SJ, et al. Screening and surveillance for gastric cancer in the United States: Is it needed? Gastrointest Endosc 2016;84:18–28. [DOI] [PubMed] [Google Scholar]
- 18.Saumoy M, Schneider Y, Shen N, et al. Cost Effectiveness of Gastric Cancer Screening According to Race and Ethnicity. Gastroenterology 2018;155:648–660. [DOI] [PubMed] [Google Scholar]
- 19.Thirumurthi S, Desilva R, Castillo DL, et al. Identification of Helicobacter pylori infected patients, using administrative data. Aliment Pharmacol Ther 2008;28:1309–16. [DOI] [PubMed] [Google Scholar]
- 20.Takenaka R, Okada H, Kato J, et al. Helicobacter pylori eradication reduced the incidence of gastric cancer, especially of the intestinal type. Aliment Pharmacol Ther 2007;25:805–12. [DOI] [PubMed] [Google Scholar]
- 21.Leung WK, Wong IOL, Cheung KS, et al. Effects of Helicobacter pylori Treatment on Incidence of Gastric Cancer in Older Individuals. Gastroenterology 2018;155:67–75. [DOI] [PubMed] [Google Scholar]
- 22.El-Serag HB, Kao JY, Kanwal F, et al. Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States. Clin Gastroenterol Hepatol 2018;16:992–1002 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zullig LL, Sims KJ, McNeil R, et al. Cancer Incidence Among Patients of the U.S. Veterans Affairs Health Care System: 2010 Update. Mil Med 2017;182:e1883–e1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helicobacter, Cancer Collaborative G. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001;49:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JH, Cheung DY. Must-Have Knowledge about the Helicobacter pylori-Negative Gastric Cancer. Gut Liver 2016;10:157–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Long JM, Ginsberg GG, et al. The role of endoscopy in the management of hereditary diffuse gastric cancer syndrome. World J Gastroenterol 2019;25:2878–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zali H, Rezaei-Tavirani M, Azodi M. Gastric cancer: prevention, risk factors and treatment. Gastroenterol Hepatol Bed Bench 2011;4:175–85. [PMC free article] [PubMed] [Google Scholar]
- 28.Blaser MJ, Chyou PH, Nomura A. Age at establishment of Helicobacter pylori infection and gastric carcinoma, gastric ulcer, and duodenal ulcer risk. Cancer Res 1995;55:562–5. [PubMed] [Google Scholar]
- 29.Wiley LK, Shah A, Xu H, et al. ICD-9 tobacco use codes are effective identifiers of smoking status. J Am Med Inform Assoc 2013;20:652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 31.Tsuda M, Asaka M, Kato M, et al. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter 2017;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol 2019;14:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 2013;62:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S, Metz DC, Kaplan DE, et al. Seroprevalence of H. pylori infection in a national cohort of veterans with non-cardia gastric adenocarcinoma. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomura A, Stemmermann GN, Chyou PH, et al. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med 1991;325:1132–6. [DOI] [PubMed] [Google Scholar]
- 36.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 1991;325:1127–31. [DOI] [PubMed] [Google Scholar]
- 37.Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin 2012;62:283–98. [DOI] [PubMed] [Google Scholar]
- 39.Martinson HA, Shelby NJ, Alberts SR, et al. Gastric cancer in Alaska Native people: A cancer health disparity. World J Gastroenterol 2018;24:2722–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Islami F, DeSantis CE, Jemal A. Incidence Trends of Esophageal and Gastric Cancer Subtypes by Race, Ethnicity, and Age in the United States, 1997–2014. Clin Gastroenterol Hepatol 2019;17:429–439. [DOI] [PubMed] [Google Scholar]
- 41.Pabla BS, Shah SC, Corral JE, et al. Increasing Incidence and Mortality of Gastric Cancer in Immigrant Populations from High to Low Regions of Incidence - a Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epplein M, Signorello LB, Zheng W, et al. Race, African ancestry, and Helicobacter pylori infection in a low-income United States population. Cancer Epidemiol Biomarkers Prev 2011;20:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014;120:1290–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- 45.Sheh A, Ge Z, Parry NM, et al. 17beta-estradiol and tamoxifen prevent gastric cancer by modulating leukocyte recruitment and oncogenic pathways in Helicobacter pylori-infected INS-GAS male mice. Cancer Prev Res (Phila) 2011;4:1426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med 2011;365:1375–83. [DOI] [PubMed] [Google Scholar]
- 47.Committee ASoP, Evans JA, Early DS, et al. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc 2012;76:1087–94. [DOI] [PubMed] [Google Scholar]
- 48.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 49.Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018;18:318–327. [DOI] [PubMed] [Google Scholar]
- 50.Zullo A, De Francesco V, Manes G, et al. Second-line and rescue therapies for Helicobacter pylori eradication in clinical practice. J Gastrointestin Liver Dis 2010;19:131–4. [PubMed] [Google Scholar]
- 51.Savoldi A, Carrara E, Graham DY, et al. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2018;155:1372–1382 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fallone CA, Moss SF, Malfertheiner P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology 2019;157:44–53. [DOI] [PubMed] [Google Scholar]
- 53.Ford AC, Forman D, Hunt RH, et al. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ 2014;348:g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu CY, Kuo KN, Wu MS, et al. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology 2009;137:1641–8 e1–2. [DOI] [PubMed] [Google Scholar]
- 55.Dong ECQ, Wu BU. Residual risk of gastric cancer following Helicobacter pylori treatment Digestive Disease Week San Diego, CA, 2019:Abstract 439. [Google Scholar]
- 56.Annibale B, Aprile MR, D’Ambra G, et al. Cure of Helicobacter pylori infection in atrophic body gastritis patients does not improve mucosal atrophy but reduces hypergastrinemia and its related effects on body ECL-cell hyperplasia. Aliment Pharmacol Ther 2000;14:625–34. [DOI] [PubMed] [Google Scholar]
- 57.Shiotani A, Haruma K, Graham DY. Metachronous gastric cancer after successful Helicobacter pylori eradication. World J Gastroenterol 2014;20:11552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Take S, Ishiki K, Mizuno M. [Helicobacter pylori eradication therapy does not prevent gastric cancer development in all patients]. Gan To Kagaku Ryoho 2011;38:353–7. [PubMed] [Google Scholar]
- 59.Doorakkers E, Lagergren J, Engstrand L, et al. Helicobacter pylori eradication treatment and the risk of gastric adenocarcinoma in a Western population. Gut 2018;67:2092–2096. [DOI] [PubMed] [Google Scholar]
- 60.Hadley C The infection connection. Helicobacter pylori is more than just the cause of gastric ulcers--it offers an unprecedented opportunity to study changes in human microecology and the nature of chronic disease. EMBO Rep 2006;7:470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blaser MJ. Our missing microbes: Short-term antibiotic courses have long-term consequences. Cleve Clin J Med 2018;85:928–930. [DOI] [PubMed] [Google Scholar]
- 62.MJ B Missing microbes: how the overuse of antibiotics is fueling our modern plagues New York: Henry Holt and Company, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilkinson NW, Howe J, Gay G, et al. Differences in the pattern of presentation and treatment of proximal and distal gastric cancer: results of the 2001 gastric patient care evaluation. Ann Surg Oncol 2008;15:1644–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.