Abstract
Background & Aims:
Adult zymogen-producing (zymogenic) chief cells (ZCs) in the mammalian gastric gland base are believed to arise from descending mucous neck cells, which arise from stem cells. Gastric injury, such as from Helicobacter pylori infection in patients with chronic atrophic gastritis, can cause metaplasia, characterized by gastric cell expression of markers of wound-healing; these cells are called spasmolytic polypeptide-expressing metaplasia (SPEM) cells. We investigated differentiation and proliferation patterns of neck cells, ZCs, and SPEM cells in mice.
Methods:
C57BL/6 mice were given intraperitoneal injections of high-dose tamoxifen to induce SPEM or gavaged with H pylori (PMSS1) to induce chronic gastric injury. Mice were then given pulses of 5-bromo-2'-deoxyuridine (BrdU) in their drinking water, followed by chase periods without BrdU, or combined with intraperitoneal injections of 5-ethynyl-2’-deoxyuridine. We collected gastric tissues and performed immunofluorescence and immunohistochemical analyses to study gastric cell proliferation, differentiation, and turnover.
Results:
After 8 weeks of continuous BrdU administration, less than 10% of homeostatic ZCs incorporated BrdU whereas 88% of neck cells were labeled. In pulse–chase experiments, various chase periods decreased neck cell label but did not increase labeling of ZCs. When mice were given BrdU at the same time as tamoxifen, more than 90% of cells were labeled in all gastric lineages. After 3 months’ recovery (no tamoxifen), ZCs became the predominant BrdU-labeled population whereas other cells—including neck cells—were mostly negative. When we tracked the labeled cells in such mice over time, we observed that the proportion of BrdU-positive ZCs remained greater than 60% up to 11 months. In mice whose ZCs were the principal BrdU-positive population, acute injury by tamoxifen or chronic injury by H pylori infection resulted in SPEM cells becoming the principal BrdU-positive population. After withdrawal of tamoxifen, BrdU-positive ZCs reappeared.
Conclusions:
We studied mice in homeostasis or with tamoxifen- or H pylori-induced SPEM. Our findings indicated that mucous neck cells do not contribute substantially to generation of ZCs during homeostasis and that ZCs maintain their own census, likely through infrequent self-replication. After metaplasia-inducing injury, ZCs can become SPEM cells, and then redifferentiate into ZCs upon injury resolution.
Keywords: lineage tracking, pre-cancer, plasticity
Graphical Abstract

INTRODUCTION
Lineage relationships among stem, progenitor, and differentiated cells in the adult mammalian stomach corpus are poorly defined, largely due to lack of specific markers and genetic tools. All epithelial cells in the healthy stomach body are posited to continually renew from a multipotent stem cell at the narrowing (isthmus) between the superficial pit (foveolar) zone, contiguous with the surface of the gastric lumen, and the middle (neck) of the gastric unit1. In this model, stem cells produce immature progenitors of each cell lineage: acid-secreting parietal cells then mature in the isthmus, mucus-secreting pit cells mature ascending to the stomach surface, and mucous neck cells mature descending into the gland. The model also posits that mature neck cells originating from isthmal stem cells take about two weeks to descend to the bottom of the neck where they eventually transdifferentiate into zymogenic (digestive-enzyme secreting) chief cells (ZCs) 2-4. The conversion of neck cells to ZCs is supported by: a) the existence of ‘transitional’ cells that express markers for both populations5; b) experiments showing that labeled nucleotides like 3H-Thymidine incorporate first into upper neck cells and are found ~2 weeks later throughout the neck and in occasional transitional cells and ZCs1, 2; and c) experiments showing increased transitional cells upon delayed ZC maturation6.
Certain types of gastric injury – almost always involving death of acid-secreting parietal cells in the stomach – cause metaplasia characterized by cells co-expressing ZC and neck genes as well as markers associated with wound-healing (eg TFF2, aka Spasmolytic Polypeptide). This lesion, Spasmolytic Polypeptide-Expressing Metaplasia (SPEM)7, occurs in mice and humans following Helicobacter pylori infection in the setting of chronic atrophic gastritis, a potentially pre-cancerous condition8, 9. Various genetic lineage-tracing experiments indicate that SPEM cells can arise via ZC plasticity. For example, the Mist1(Bhlha15) locus predominantly labels ZCs, and SPEM cells can be traced from Mist1CreERT2-expressing cells10, 11. Similar conclusions have been drawn based on lineage tracing driven from Troy(Tnfrsf19), Lgr5, and Runx1 promoters11-13. Nevertheless, some have questioned a predominantly ZC origin of SPEM, at least based on lineage-tracing data, so there remains controversy in the field14-16. In the absence of chronic injury, SPEM resolves after a few weeks. We and others have shown that high doses of the selective estrogen receptor modulator tamoxifen cause rapid SPEM within 3 days that is reversible by 2-3 weeks17, 18. The fate of individual SPEM cells as tissue damage resolves is unknown.
Here, we use 5-bromo-2'-deoxyuridine (BrdU) to probe gastric cell population dynamics at homeostasis and following injury. Our findings support some aspects of the current model: isthmal progenitors rapidly label with BrdU, and BrdU can be chased into pit, neck, and parietal lineages with turnover dynamics consistent with extant models. The findings also support that ZCs are a key source of SPEM cells following acute and chronic injury. Additionally, we present the first direct evidence that, upon recovery from injury, metaplastic cells can become ZCs again.
Finally, our multiple pulse-chase experiments call into question certain canonical gastric lineage relationships. Namely, our data are difficult to reconcile with the model that during adult homeostasis, the main fate of neck cells is to become ZCs and that ZCs are predominantly supplied by neck cell transdifferentiation. We suggest a model wherein ZCs are predominately a self-maintaining cell population during homeostasis and following injury. Thus, ZCs in this regard resemble related exocrine cells in salivary glands and pancreas that self-replicate rarely during homeostasis but re-enter the cell cycle during large-scale injury.19-21
METHODS AND MATERIALS
Animals and Injections
All animal experiments followed protocols approved by the Washington University School of Medicine Animal Studies Committee. Mice were maintained in a specified pathogen-free barrier facility under a 12-hour light cycle. Wild type C57BL/6 mice were purchased from Jackson Laboratories. Littermate controls were housed together when possible. To induce SPEM, tamoxifen (5 mg/20 g mass; Toronto Research Chemicals, Inc) was injected intraperitoneally daily for 3 days (‘TAM’ hereafter). Tamoxifen was dissolved in 10% ethanol and 90% sunflower oil (Millipore Sigma). 5-bromo-2’-deoxyuridine (BrdU; Millipore Sigma) was administered via drinking water (800 mg/L) for up to 8 weeks, changed every three days. 5-ethynyl-2’-deoxyuridine (EdU; Baseclick) was injected intraperitoneally daily for 4 days (30 mg/kg mass, dissolved in PBS).
Immunofluorescence/Immunohistochemistry
After euthanasia, stomachs were immediately excised, flushed with phosphate buffered saline (PBS), inflated, and fixed overnight in cold formalin (3.7% formaldehyde in PBS; Millipore Sigma). Stomachs were transferred to 70% ethanol, cut into rings, embedded in 3% agar, and subjected to routine paraffin processing and sectioning. Slides were deparaffinized and rehydrated, with antigen retrieval via pressure cooking in Tris-based solution (pH 9; Vector Labs). Slides were blocked with 1% bovine serum albumin and 0.2% Triton X-100 in PBS for 1 hour then primary antibodies were incubated overnight at 4°C. Secondary antibodies were applied for 1 hour then slides were mounted using ProLong Gold antifade reagent with DAPI (Invitrogen). Immunohistochemistry was done as previously described22.
Primary antibodies and lectins used in this project include: rabbit anti-human gastric intrinsic factor (1:10,000; Dr. David Alpers, Washington University), goat anti-5-bromo-2’-deoxyuridine (1:20,000; Dr. Jeffrey Gordon, Washington University), Rabbit anti-Ki67 (1:100; Abcam), Sheep anti-pepsinogen II (1:1000, Abcam), Rabbit anti-αSMA (1:200, Abcam), goat anti-clusterin (1:200, Santa Cruz), rat anti-BrdU (1:200, Abcam), rat anti-CD44 v10-e16 (1:200, Cosmo Bio), rabbit anti-Chromogranin A (1:100, Abcam), AAA Lectin (1:500, EY Labs), and 1 g/mL fluorescently labeled GSII lectin (Molecular Probes). Secondary antibodies included AlexaFluor conjugated donkey anti-goat, anti-rabbit, anti-rat, or anti-mouse antibodies (1:500; Molecular Probes). EdU was imaged using the BCK-EdU488 kit following the manufacturer’s instructions.
Helicobacter pylori infection
The mouse-adapted, wild-type pre-mouse Sydney strain of Helicobacter pylori, PMSS1, was provided by Dr. Rick Peek (Vanderbilt University). PMSS1 strain growth prior to mouse inoculation was previously described23. Mice were fasted 4-6 hours prior to oral gavage with 200 μL of overnight H pylori culture (~1 × 108 cfu/mouse), then fasted 1-2 hours following infection. Mice were then given feed ad libitum then sacrificed 8-12 weeks after infection. Stomachs were excised and opened along the lesser curvature. Food was gently scraped away, and the forestomach removed. Stomachs fixed overnight at 4 °C, then cut in longitudinal sections for routine paraffin processing.
Immunofluorescence Quantification
All quantifications encompassed at least three mice, with both sexes used. Quantifications were done on stomachs stained fluorescently for anti–BrdU, GSII lectin, and anti-GIF. GIF+ cells were scored as ZCs, GSII+ cells as neck cells, and GIF+/GSII+ double-positive cells considered transitional or metaplastic. Slides were imaged on a Zeiss Axiovert 200 microscope with an Axiocam MRM camera. For BrdU-retention assays, only fully imaged units with >3 label-retaining ZCs, SPEM, or neck cells were quantified, with >20 label-retaining units quantified per stomach. Axiovision LE64 software and Adobe Photoshop CS6 were used to selectively overlay color channels to count label-retaining cells co-staining with GIF and/or GSII and to prepare figures, as previously described24. Counts were averaged over the total number of units quantified per mouse, then the average from each mouse per a given condition was then averaged to achieve a mean of means, expressed ±Standard Error of the Mean (SEM). Graphs were completed in GraphPad Prism, using one- or two-tailed student t-tests (based on hypothesis) for two groups or one-tailed ANOVA for >2 groups to determine significance, *P<0.05, **P<0.01, and ***P<0.001.
RESULTS
Even with Extended Continuous BrdU, Labeled Chief Cells Are Rare
We administered BrdU (which incorporates permanently into DNA during S-phase of mitosis) in mouse drinking water for 2-8 weeks continuously until euthanization. Most cell populations accrued label over time1, 25. Consistent with their published short lifespan and high turnover, pit cells labeled quickest, with nearly all BrdU+ by 2 weeks (Supplemental Figure 1A, B). Also behaving consistent with published data were: GSII+ neck cells (2 weeks:70±3% BrdU+; 8 weeks: 88±1%), GSII+/GIF+ transitional cells (2 weeks: 28±2%; 8 weeks: 63±4%) and acid-secreting parietal cells (2 weeks: 24.±2%; 8 weeks: 51±4%; Figure 1A, Supplemental Figure 1C). Surprisingly, GIF+ ZCs never labeled appreciably (2 weeks: 4.3±0.7%; 8 weeks: 9.4±1.6%; Figure 1A, Supplemental Figure 1C).
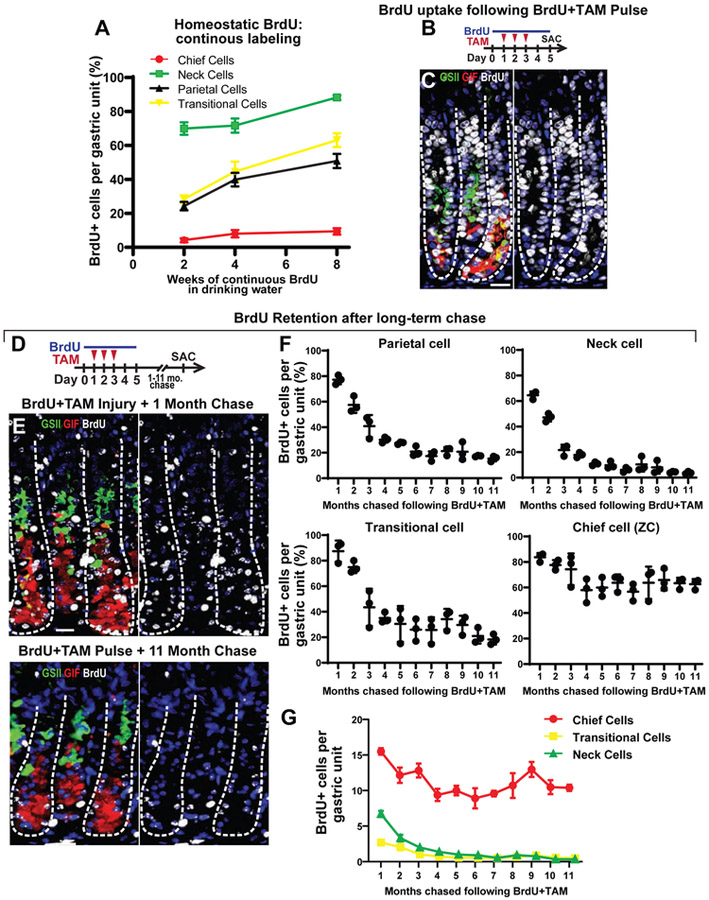
Figure 1: Continuous BrdU labeling marks few chief cells in the healthy stomach.
A) Quantification of BrdU label incorporation in major gastric cell types of mice administered BrdU continuously in the drinking water for 2-8 weeks during homeostasis. B) Experimental paradigm for panel C. Mice were given BrdU via drinking water one day prior to, during, and an additional 2 days following three days of tamoxifen (total of 5 days BrdU infusion, mice sacrificed on the fifth day). C) Representative corpus units stained for BrdU (white nuclei) and main cell types of the damaged gastric epithelium: neck cells (GSII, green), ZCs (GIF, red), SPEM cells (GSII+GIF+, yellow), and nuclei (DAPI, blue). Left- merge; Right- nuclei and BrdU. Scale bar, 20 μm. D) Experimental paradigm for (E, F). Mice were treated as for B,C then chased (no additional BrdU) for 1 to 11 months. E) Representative corpus units for 1-month chase (top) or 11 months chase (bottom) stained for neck cells (GSII, green), ZCs (GIF, red), transitional cells (GSII+GIF+, yellow), and nuclei (DAPI, blue). Scale bar, 20 μm. F) BrdU retention for each cell population quantified from images of 1-11 months chase. G) Per unit cell numbers for Chief, neck, and transitional cells across 11 months of chase.
Acute, Metaplastic Injury Can Be Used to Label Nearly All Epithelial Cells with BrdU and Then Track Label Retention
Because continuous homeostatic BrdU labeling was inefficiently labeling the ZC population, we took a different tack to label these cells and track their behavior thereafter. We have previously shown that high-dose tamoxifen (TAM) causes nearly all corpus epithelial cells to become proliferative within three days as part of the SPEM response17, 18. Accordingly, administering BrdU in drinking water one day prior to TAM through two days following TAM resulted in BrdU marking nearly all the epithelial cells, including 98.3±0.2% of neck cells and 96.3±0.6% of SPEM cells (Figure 1B-C).
We reasoned we could use this approach to follow turnover of key cell populations, including ZCs. We loaded gastric epithelial cells by simultaneous TAM and BrdU administration but then allowed mice to recover from injury and chased without additional BrdU administration euthanizing mice every month for nearly one year (Figure 1D-G). The first timepoint (one month) was chosen because at that timepoint, all principal cell types had returned to their pre-injury distributions. At that timepoint, 84±2% of ZCs were BrdU+, along with 77±2% of parietal, and 64±1% of neck cells. As expected, rapidly dividing isthmal and pit cells had almost all become BrdU-negative presumably due to dilution of BrdU signal and/or extrusion of progeny (Figure 1E-F, Supplemental Figure 1D).
Chief and Neck Cell Labeling Dynamics Do Not Directly Correlate
We followed the behavior of BrdU-loaded cells over subsequent months of BrdU-free chase. Parietal cells lost label steadily for the first 5 months, though a stable ~20% remained positive thereafter (Figure 1F; Supplemental Figure 1E). Neck cells lost label rapidly over the first 3 months (though a scant fraction was still detected with 3.8±0.4% BrdU+ even at 11 months; Figure 1F). ZCs lost label more slowly over the first 3 months (decreasing from 84±2% to 75±7% BrdU+); however, from 5 months on, ZCs maintained essentially the same fractional labeling at all chase timepoints. At 11 months, 63±2% were still BrdU+ (Figure 1E-G; Supplemental Figure 2A, B). Cells with transitional neck-ZC features (1-2 cells per unit) largely paralleled neck cell label dynamics, though with more variability due to their sparseness (Figure 1F, G).
The predominant model for the origin of ZCs is that they transdifferentiate – largely without cell division – from neck cells2, 26. The model would predict, therefore, that labeled neck cells would become labeled ZCs. However, Figure 1F shows the predicted drop in labeled neck cells (>80% over months 1-4) but shows unexpectedly that the labeled ZC fraction also decreased during the same timeframe as opposed to gaining the label lost from the neck region. More surprisingly, labeled ZC census remained >60% from 5 months onward, even though during that same timeframe no more than 10% of their putative progenitor neck cells were labeled; hence, some other mechanism, besides migration downward of labeled neck cells, must predominantly account for their retained label.
To further investigate the relationship between neck and ZCs, we did pulse-chase experiments at homeostasis. A canonical previous study of neck and ZC proliferative behavior concluded that maximum labeled nucleotide incorporation into the neck call population occurred after ~2 weeks’ continuous labeling2. We administered BrdU continuously for two weeks and then chased 2-8 weeks. If we euthanized immediately after the 2-week labeling period, 70±3% of neck cells, 28±2% of transitional cells, and only 4.3±0.7% of ZCs had incorporated label (Figure 2A, B). By four weeks’ chase, neck cells showed the first statistically significant change in fractional labeling with only 29±6% now BrdU+ (net loss of 4 cells per unit). BrdU+ ZCs (7.6±0.2%) and transitional cells (41±5%) remained statistically unchanged at ~1 BrdU+ cell per unit. After eight weeks, only 20±2.3% of neck cells retained label (representing a significant loss of 5 cells per unit), while no significant changes occurred in the other populations: 8±1% of ZCs and 36±1% of transitional cells remained BrdU+ (Figure 2A, B).
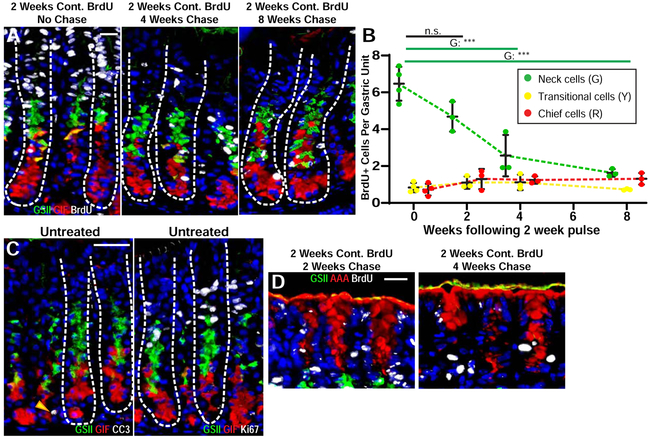
Figure 2: Chief cells are not the final fate for most mucous neck cells.
A) Representative corpus units stained for BrdU (white), neck cells (GSII lectin, green) ZCs (GIF, red), transitional cells (GIF+GSII+, yellow) and nuclei (DAPI, blue). Left panel: two weeks of continuous BrdU, no chase; Middle: two weeks BrdU followed by 4 weeks chase; Right: two weeks BrdU followed by 8 weeks chase. Scale bar, 20 μm. B) Per unit BrdU retention of gastric cell types (neck, green; transitional, yellow; ZCs, red) following pulse-chase experiments. Animals were given BrdU for 2 weeks and chased for 0 (2-week BrdU administration only), 2, 4, and 8 weeks. **p<0.005, ***p<0.001 via one-way ANOVA with Dunnett post-hoc comparison to pulse-only C) Representative corpus units from healthy stomachs stained with GSII (green), GIF (red), DAPI (blue) and either cleaved caspase 3 (white, left) or Ki67 (white, right) to identify cells undergoing apoptosis or in the cell cycle. Scale bar, 40 μm D) Pit and Isthmus regions of corpus units administered BrdU for two weeks then chased for two (left) or four weeks (right) stained for pit cells (AAA lectin, red), neck cells (GSII, green), nuclei (DAPI, blue), and BrdU (white). Scale bar, 20 μm.
If, as the predominant model claims, neck cells become ZCs, the loss of labeled neck cells should correlate with an increase in labeled ZCs, yet there was no such correlation. These experiments cannot rule out that neck cells divide enough times to deplete label and then become ZCs; however, Figure 1F shows that a large population of unlabeled neck cells does not correlate with an increase in unlabeled ZCs. Likewise, there is no correlation between behavior of labeled neck cells and the presumptive neck-ZC transitional cells (Figure 2B). If neck cells do not predominantly transdifferentiate to ZCs, it is unclear what causes their loss of label. Consistent with the literature2, we have never observed an apoptotic neck cell previously or in the current studies, and we also observe that only the neck cells nearest the isthmus are actively proliferative (Figure 2C), as expected. The pit cell population behaved as predicted with nearly all labeled after 2-week pulse and almost none present after 4-week chase (Figure 2A, D), consistent with pit cells being lost steadily from the gastric lumen surface.
Another prediction of the canonical model is that ZCs are born at the transition zone between neck and base as the progenitor neck cells undergo the massive cellular architecture changes needed to become mature ZCs26. In short, dogma describes a downward differentiation model in which new ZCs should arise at the top of the gastric unit base and keep descending thereafter. Thus, in our 2-8-week continuous BrdU labeling experiments, we would predict that BrdU+ ZCs would appear first at the top of the base; however, there is no evidence of such location bias. Of all BrdU+ ZCs after continuous BrdU labeling, only 21% were adjacent to the neck-base transitional zone, and there was likewise no enrichment anywhere within upper, middle, or lower portions of the base (Supplemental Figure 3A, B). One bias we did notice was that wherever BrdU+ ZC nuclei were found, they were frequently adjacent to another BrdU+ ZC. Binuclear ZCs become more common as mice age2, 5, so we used E-cadherin to distinguish cell basolateral borders and found that 34% of the paired nuclei were in adjacent separate cells, consistent with a recent cell division (Supplemental Figure 3C). Unlike neck cells, ZCs have been shown to die during homeostatic conditions2, and our results corroborate this (Figure 2C).
Following Gastric Injury, SPEM Cells Can Derive from Chief Cells
We next reasoned we could use the TAM-BrdU loading strategy to label the bulk of ZCs and follow what happens to such labeled cells after a second injury. We pulsed gastric units via the TAM+BrdU loading protocol (Figure 1B-C) then chased for 3 months without BrdU (Figure 3A, B). Following the 3-month chase, 75±7% of ZCs were labeled (Figure 1F, 3B), while pit, isthmus, and upper neck cells were nearly completely unlabeled (Supplemental Figure 1D, Figure 1F, 3B). The remaining BrdU+ cells that lacked both neck and ZC markers included, as expected, the relatively long-lived parietal cell population. Also, we observed 0.8±0.03 BrdU+ epithelial cells per unit that likely comprised other rarer populations of label-retaining cells like endocrine and tuft cells (Supplemental Figure 1F). Thus, at the 3-month chase timepoint, almost the only BrdU+ epithelial cells were ZCs and scattered parietal cells (which are non-proliferative).
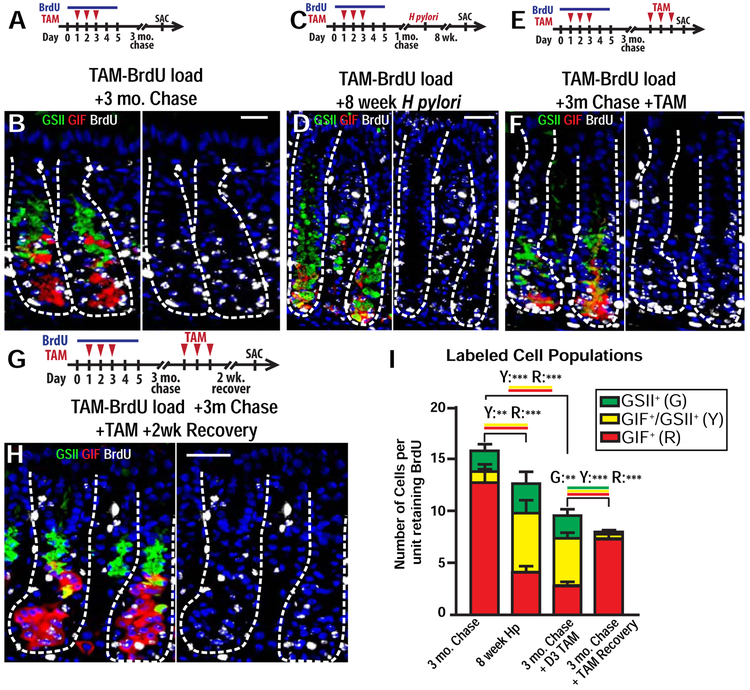
Figure 3: Metaplastic cells can arise from chief cells following chronic injury.
A) Experimental paradigm for (B). Mice were given BrdU during tamoxifen injury (total of 5 days BrdU administration) then chased for 3 months (no additional BrdU). B) Representative corpus units following 3-month chase stained for BrdU (white), neck cells (GSII, green), ZCs (GIF, red), transitional cells (GIF+GSII+, yellow) and nuclei (DAPI, blue). Scale bar, 20 μm. C) Experimental paradigm for (D). Mice were given BrdU during tamoxifen injury (total of 5 days BrdU administration). After BrdU administration, mice were chased for 1 month, infected with H. Pylori, then chased a subsequent 8 weeks with no additional BrdU. D) Representative metaplastic units following 8-week H pylori infection stained for BrdU (white), neck cells (GSII lectin, green), ZCs (GIF, red), SPEM cells (GIF+GSII+, yellow) and nuclei (DAPI, blue). Scale bar, 40 μm. E) Experimental paradigm for (F). Mice were given BrdU during tamoxifen injury (total of 5 days BrdU administration) then chased for 3 months (no additional BrdU). At 3 months, mice were reinjured with tamoxifen for 3 days and sacrificed one day afterwards. F) Representative corpus units for mice described in E stained for BrdU (white), necks cells (GSII, green), ZCs (GIF, red), SPEM (GIF+GSII+, yellow), and nuclei (DAPI, blue). Left: merge; Right: DAPI and BrdU only. Scale bar, 20 μm. G Experimental paradigm for (H). Same setup as E except mice were sacrificed 2 weeks after the second tamoxifen injury (a recovery timepoint). H) Representative corpus units for mice described in G stained for BrdU (white), necks cells (GSII lectin, green), ZCs (GIF, red), transitional cells (GIF+GSII+, yellow), and nuclei (DAPI, blue). Left: merge; Right: DAPI and BrdU only. Scale bar, 20 μm. I) BrdU retention for neck (green), ZCs (red), and SPEM/transitional (yellow) cells quantified from: First column: Mice given BrdU during tamoxifen injury then chased for 3 months (no additional BrdU; described in A,B); second column: Mice were given BrdU during tamoxifen injury (total of 5 days BrdU administration). After BrdU administration, mice were chased for 1 month, infected with H. Pylori, then chased a subsequent 8 weeks with no additional BrdU (described in C,D). third column: Mice were given BrdU during tamoxifen injury (total of 5 days BrdU administration) then chased for 3 months (no additional BrdU). At 3 months, mice were reinjured with tamoxifen for 3 days and sacrificed one day afterwards (described in E,F). Forth column: Mice were given BrdU during tamoxifen injury (total of 5 days BrdU administration) then chased for 3 months (no additional BrdU). At 3 months, mice were reinjured with tamoxifen for 3 days and sacrificed two weeks afterwards (described in G,H). *P<0.05, **P<0.01, and ***P<0.001 in one-tailed Student’s t tests.
To examine the relationship between ZCs and SPEM, we first induced SPEM with H pylori infection. H pylori induces SPEM in the 10-20 corpus gastric units bordering the antrum beginning around 2 months after infection23. After TAM+BrdU loading as above, we allowed mice to rest for 1 month, gavaged H pylori, then waited an additional 2 months to allow H pylori infection to initiate corpus metaplasia (ie euthanasia occurred, in total, 3 months after initial TAM-BrdU loading; Figure 3C). Within metaplastic foci, we observed 67±4% of SPEM cells were BrdU+ (Figure 3D, I). BrdU+ ZC census was statistically significantly lower in H pylori-infected metaplastic units (loss of ~8 ZCs/unit) compared to uninfected TAM-BrdU loaded mice at 3-month chase (Figure 3I). The number of BrdU+ SPEM cells/unit (~6) induced by H pylori almost matched this relative decrease in ZCs (Figure 3I). H pylori infection did not statistically change the number of labeled neck cells/unit (2.0±0.3 vs. 2.8±0.5; Figure 3I). Similar results were seen in 15 mice over 8–12 weeks’ infection (Supplemental Figure 4A, B). Summarizing, in control units, about ¾ of ZCs were BrdU+ (with much of any other proliferative population unlabeled), whereas in H pylori-infected units, ⅔ of SPEM cells were BrdU+ with the number of emerging BrdU+ SPEM cells nearly equaling the decreased BrdU+ ZCs. Only ⅓ of SPEM cells were unlabeled, so most SPEM cells could not have derived from unlabeled precursors.
SPEM Cells Can Redifferentiate into Chief Cells Following Recovery from Injury
We next asked what happens to SPEM cells if metaplasia resolves. First, we performed an experiment similar to the one just described, except we induced metaplasia in TAM-BrdU loaded mice with a second round of TAM rather than H pylori (Figure 3E). As with H pylori, reinjury with TAM induced BrdU+ SPEM cells and coordinately decreased BrdU+ ZCs, with BrdU+ neck cells again unchanged (Figure 3F, I). We confirmed that the new BrdU+ cells were SPEM using additional SPEM markers Clusterin and CD44v27, 28 (Supplemental Figure 5A, B). Overall labeling decreased in the TAM SPEM units: total BrdU+ cells were ~9 in 2nd round TAM SPEM vs. ~15 at 3-month chase and ~13 in H pylori SPEM (Figure 3I). This could be due to label dilution, with TAM-induced SPEM cells more proliferative than H pylori-induced SPEM cells during the timing of the experiment (Supplemental Figure 5C, D).
To track SPEM cell fate upon recovery, we performed the same experiment but then allowed mice to recover for another two weeks to restore homeostatic cell lineage census. (Figure 3G). Analyzing units that had retained most of their label (≥4 BrdU+ GIF and/or GSII+ cells), we found that nearly half the ZCs (46.8±5.4%) were BrdU+ (Figure 3G, H). Specifically, in mice euthanized immediately after the second SPEM, 2.8±0.1 ZCs and 4.6±0.2 SPEM cells per unit were BrdU+; in mice allowed to recover, 7.3±0.2 ZCs were BrdU+. The number of BrdU+ ZCs following recovery was nearly identical to the sum of BrdU+ ZCs and SPEM cells at the height of injury (Figure 3I).
Thus, after BrdU-loading and 3 months’ chase, the vast majority of BrdU is concentrated in cells (ZCs and/or SPEM cells) in the base of the gastric unit and remains in those basal cells during and after metaplastic injury. In other words, during metaplasia ZCs can give rise to SPEM and, if mice are allowed to recover, SPEM cells can again become ZCs.
Lack of Evidence that Migrating Progenitor Cells Substantially Contribute to SPEM
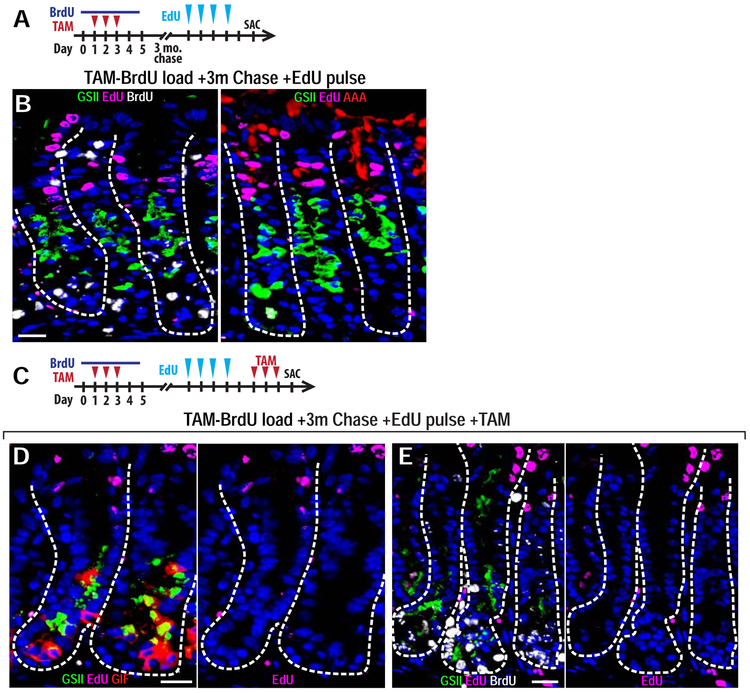
To further address the origin of SPEM cells14, 15, we injected TAM-BrdU-loaded mice at the 3-month chase timepoint (described above) with EdU on 4 consecutive days (Figure 4A). After this 4-day continuous labeling period, EdU incorporated nearly exclusively into the expected populations of rapidly proliferating cells, including those of the isthmus, pit, and upper neck regions (Figure 4B). We observed no BrdU+ ZCs that were also EdU+. We then injured such mice that had been TAM-BrdU-loaded 3 months prior and short-term homeostatically EdU-loaded with a second round of TAM (Figure 4C). If isthmal/neck progenitors contributed substantially to SPEM, we would see the EdU in those cells trace into SPEM, or those cells would divide several times and become EdU−, and we would see that many SPEM cells would be EdU−/BrdU−. However, neither happened: we observed no EdU+ SPEM cells, and most SPEM cells were BrdU+, indicating no large influx of EdU−/BrdU− cells (Figure 4D-E).
Figure 4: Migrating Progenitor Cells Do Not Substantially Contribute to SPEM.
A) Experimental paradigm for (B). Mice were given BrdU during tamoxifen injury then chased for 3 months. At 3 months, mice were given 4 daily injections of EdU and sacrificed one day afterwards. B) Representative corpus units for mice described in (A). Left: BrdU (white), EdU (fuchsia), neck cells (GSII, green), and nuclei (DAPI, blue), Right: EdU (fuchsia), neck cells (GSII, green), pit cells (AAA, red), and nuclei (DAPI, blue). Scale bar, 20 μm. C) Experimental paradigm for (D and E). Mice were given BrdU during tamoxifen injury then chased for 3 months. At 3 months, mice were given 4 daily injections of EdU, 1-day break, a second 3-day tamoxifen injury, and sacrificed one day afterwards. D,E) Representative corpus units for mice described in (C). D) EdU (fuchsia), necks cells (GSII, green), ZCs (GIF, red), SPEM (GIF+GSII+, yellow), and nuclei (DAPI, blue). Left: merge; Right: DAPI and EdU only. Scale bar, 20 μm. J) EdU (fuchsia), BrdU (white), necks cells (GSII lectin, green), and nuclei (DAPI, blue). Left: merge; Right: DAPI and EdU only. Scale bar, 20 μm.
DISCUSSION
Here, we use multiple-timepoint, pulse-chase BrdU and EdU labeling experiments with and without acute and chronic injury to trace the behavior of gastric corpus epithelial cells in homeostasis, during metaplasia, and during recovery from metaplasia.
Using this approach, we can confirm several aspects of the predominant model for homeostatic epithelial dynamics. For one, we observe that the isthmus of the gastric unit harbors the most actively proliferating cells. Progeny emerging from those cells migrate bidirectionally up towards the gastric lumen as pit cells and downward into the gland as neck cells. In pulse-chase experiments, labeled pit cells were lost rapidly, consistent with their predicted rapid extrusion into the gastric lumen. Parietal cells also behaved as expected with our TAM-BrdU loading experiments indicating a parietal cell half-life of about 2 months (Figure 1F), consistent with published estimates29.
Over the last 30 years, the consensus model for neck and ZC dynamics has been that isthmus-derived neck cells mature as they migrate down the neck until they transdifferentiate into ZCs via a transitional cell intermediate2, 26. The first ZCs (pre-ZCs) emerging from the transition zone continue to age as they descend towards the base and eventually die after 4-6 months. Some genetic lineage tracing experiments have also been interpreted as supporting this model3, 14. Additionally, mice with mutant genes that block chief cell maturation can also have altered neck and transitional cell dynamics, which could indicate a direct relationship between the lineages30-32.
However, a model in which ZCs arise solely from neck cells has not always been uniformly accepted. For one, investigators have questioned the teleology of why neck cells would elaborate one essentially mature-cell phenotype only to dismantle that and adopt another one33. And others have used multiple experimental modalities – including labeled nucleotide pulse-chases – to argue that ZCs maintain themselves independent of other lineages34-36. Genetic lineage tracing experiments, either using Cre-Lox11, 13 or mutagenesis37 approaches have also argued for a non-canonical relationship between ZCs and other lineages.
Our results best support a model in which neck cells and ZCs largely operate independently. Continuous BrdU labeling during homeostasis for 2 weeks caused nearly all neck cells to label with BrdU, yet as those labelled neck cells were lost in subsequent chase, there was no commensurate increase in labeled ZCs. Transdifferentiation of labeled neck into labeled ZCs should have been easy to detect, because at the start of the chase, ZCs were ~90% BrdU− and neck cells were over 70% BrdU+. Similarly, when both neck and ZCs were TAM-BrdU-loaded, both populations lost label during chase over the first 3 months, not supporting a model wherein labeled neck cells sustained the labeled ZC population. Moreover, in subsequent months, when there were almost no labeled neck cells remaining, the labeled ZC population remained stable, indicating that: a) unlabeled neck cells were not becoming unlabeled ZCs and diluting the BrdU+ ZC fraction and b) some other mechanism must be at work to maintain the same fraction of labeled ZCs (assuming ZCs do have a set lifespan of a few months). In short, we find neither transfer of unlabeled or labeled neck cells into ZCs at any timepoint using different approaches. Finally, the distribution of labeled ZCs appeared random within the base and was not biased -- as would be predicted by the predominant model -- upwards towards neck/transitional zone. The only pattern we observed was that labeled ZCs often appeared as pairs, consistent with their having arisen from a ZC that self-replicated.
Overall, we favor a model in which ZCs during adult homeostasis largely maintain their own census through infrequent division, independent of contribution from the neck or putative stem cells in the isthmus (Figure 5). Our proposed model is consistent with that of others who also performed longer pulse-chase experiments with timings different from those used by Karam and Leblond36. It is also consistent with dynamics in other organs with zymogenic cells: pancreatic and salivary acinar cells largely self-replicate19-21. We stress that our model describes only the bulk behavior in adults at homeostasis. We cannot rule out that neck cells transdifferentiate to ZCs occasionally, and this transdifferentiation process may increase in mice with genetic mutations that affect ZC homeostasis or in other types of injury.
Figure 5: Suggested model of neck and chief cell behavior in homeostasis and metaplasia.
A) The canonical model of cell lineage dynamics in a healthy unit. Isthmal stem cells (grey) give rise to pit cells (purple), parietal cells (blue), and neck cells (green), which subsequently transdifferentiate into ZCs (red). Colors on arrows denote cell conversions. B) Left: Our proposed model for cell lineage dynamics at homeostasis. Stem cells give rise to pit cells, neck cells, and parietal cells. ZCs form a self-maintaining population at the base with minimal input from upper cell populations. Middle: Upon metaplasia-inducing injury such as infection with Helicobacter species or acute drugs, units lose parietal cells and see proliferative expansion of the isthmus and neck cells. ZCs undergo paligenosis to proliferative, metaplastic cells (yellow). Right: As injury resolves, SPEM cells re-become ZCs and the unit reverts to its normal homeostatic maintenance.
Here we also provide additional evidence that the bulk of SPEM arises from existing ZCs. We cannot rule out contribution from progenitors in the isthmus or neck, as has been proposed by others14; however, stem/progenitor cells do not directly transdifferentiate into SPEM, because when such cells were loaded with EdU by 4 days’ continuous injection, they did not contribute to SPEM. On the other hand, it is possible that rapidly proliferating progenitor cells might contribute to SPEM after multiple rounds of cell division depleted the EdU they originally harbored. However, such progenitor-derived SPEM cells would have to be the minority, because any such SPEM cells would also be BrdU−, and BrdU− SPEM cells were the minor population. Thus, most SPEM is evidently derived from cells that divide infrequently, because inducing metaplasia 3 months after the last exposure to BrdU still results in SPEM cells being BrdU+.
Finally, our results examine for the first time the behavior of SPEM cells in mice allowed to recover from metaplastic injury. In TAM-BrdU-loaded mice, chased for 3 months, almost all the BrdU concentrates in chief cells (excluding amitotic parietal cells which are also depleted in SPEM). During subsequent metaplasia, almost all the BrdU+ cells are SPEM cells. Upon recovery, BrdU is again concentrated in ZCs. The results indicate that SPEM cells can redifferentiate into ZCs and are consistent with experiments showing metaplastic cell cycle reentry (paligenosis) of pancreatic acinar cells with return to the mature state after injury resolution as well as with some models of tumorigenesis38. By delving more deeply into the dynamics of cellular differentiation and turnover, we make a better blueprint to understand how organs either successfully repair or embark on aberrant pathways that lead to cancer.
Supplementary Material
Supplemental Figure 1: BrdU retention in other cell types in the stomach. A) Pit and isthmus region of a gastric corpus unit from mice given two weeks’ continuous BrdU via drinking water. Staining for neck cells (GSII lectin, green), BrdU (white), nuclei (DAPI, blue) and pit cells (AAA lectin, red). Scale bar, 20 μm. B) Wide field immunohistochemistry for BrdU with hematoxylin and eosin counterstain; same experiment as (A). Scale bar, 50 μm. C) Mice administered BrdU continuously for two (left) or eight weeks (right) stained for BrdU (white nuclei) incorporation into main gastric epithelial cell types. Left panel pair: neck cells (GSII, green), ZCs (GIF, red), transitional cells (GSII+GIF+, yellow), and nuclei (DAPI, blue). Right panel pair: parietal cells (Ezrin, red). Scale bars, 40 μm. D) Mice were given BrdU in drinking water during tamoxifen injury (total of 5 days of BrdU treatment). After 5 days for BrdU treatment, animals were chased for 1 (left panel), 2 (middle panel), or 3 (right panel) months. Staining for BrdU (white), neck cells (GSII lectin, green), pit cells (AAA lectin, red), and nuclei (DAPI, blue). Scale bar, 20 μm. E) Same BrdU labeling experiment as (D). Mice were chased for 1 (left panel) or 9 (right panel) months. Representative corpus units stained for BrdU (white), neck cells (GSII lectin, green), PCs (Ezrin, red), and nuclei (DAPI, blue). Scale bar, 40 μm. F) Same BrdU labeling experiment as in (D). Mice were chased for 3 months and stained for BrdU (white), nuclei (DAPI, blue), and endocrine cells (left panel; Chromogranin A, red), tuft cells (middle panel; DCLK1, red), or stromal cells (right panel; α-SMA, red). Scale bar, 10 μm.
Supplemental Figure 2: IHC of Long-term BrdU Chase Mice were given BrdU via drinking water one day prior to, during, and an additional 2 days following three days of tamoxifen (total of 5 days BrdU infusion) and chased with no additional BrdU for 1 (A) or 11 (B) months. A) Representative BrdU immunohistochemistry after 1-month chase. B) Representative BrdU immunohistochemistry after 11-month chase. Dashed yellow boxes correspond to adjacent higher power image. Scale bar, 50 μm (left images), 25 μm (right images).
Supplemental Figure 3: BrdU is incorporated into chief cells nonadjacent to the transitional zone A) Bases of units from mice administered BrdU continuously for eight weeks and stained for BrdU (white), neck cells (GSII, green), ZCs (GIF, red), transitional cells (GSII+GIF+, yellow), and nuclei (DAPI, blue). Scale bar, 20 μm. B) Histogram of the location within the base of each BrdU+ ZC compiled from all continuous BrdU-infusion experiments. ZCs adjacent to the lowest GSII+ (or GIF+GSII+) cell are labeled ‘uppermost,’ with ‘lowermost’ denoting the bottom ZC in a unit. All other BrdU+ ZCs were scored as upper or lower half of the base. C) Bases of gastric units from mice administered BrdU continuously for 8 weeks and stained for E-Cadherin (red), BrdU (white), GSII lectin (green), and DAPI (blue). Adjacent BrdU+ nuclei were counted as individual cells if E-Cadherin separated the nuclei (eg top panels; arrowheads), otherwise they were considered binuclear ZCs (bottom). Scale bar, 20 μm.
Supplemental Figure 4: Additional H. Pylori infection data A) Mice were given BrdU in drinking water during tamoxifen injury (total of 5 days of BrdU treatment). After 5 days of BrdU treatment, mice were chased for 1 month, infected with H. Pylori, and chased for an additional 8 weeks. Representative non-metaplastic units stained for BrdU (white), neck cells (GSII lectin, green), ZCs (GIF, red), transitional cells (GIF+GSII+, yellow) and nuclei (DAPI, blue). Scale bar, 20 μm. B) Same experiment as in (A) except HP infection was for 12 weeks. Representative metaplastic units following 12-week HP infection stained for BrdU (white), neck cells (GSII lectin, green), ZCs (GIF, red), transitional cells (GIF+GSII+, yellow) and nuclei (DAPI, blue). Scale bar, 20 μm.
Supplemental Figure 5: Additional metaplasia data following tamoxifen reinjury and HP infection A) Mice were given BrdU in drinking water during tamoxifen injury (total of 5 days of BrdU treatment). After 5 days of BrdU treatment, animals were chased for 3 months (no additional BrdU). At 3 months, mice were reinjured with tamoxifen for 3 days and sacrificed one day afterwards. Representative metaplastic units stained for BrdU (white), neck cells (GSII lectin, green), clusterin (red), and nuclei (DAPI, blue). Scale bar, 20 μm. B) Same experiment as in (A). Staining for BrdU (white), neck cells (GSII lectin, green), CD44v (red), and nuclei (DAPI, blue). Scale bar, 20 μm. C) Mice were given BrdU in drinking water during tamoxifen injury then chased for 1 month, infected with H. Pylori, and chased for an additional 8 weeks. Representative metaplastic units stained for BrdU (white), neck cells (GSII lectin, green), Ki67 (red), and nuclei (DAPI, blue). Scale bar, 20 μm. D) Same experiment as in (A). Representative metaplastic stained for BrdU (white), neck cells (GSII lectin, green), Ki67 (red), and nuclei (DAPI, blue). Scale bar, 20 μm.
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT: Digestive enzyme-secreting chief cells in the stomach are believed to arise from mucous neck cell progenitors. Gastric injury, such as from Helicobacter pylori infection, can cause formation of spasmolytic polypeptide-expressing metaplastic cells. We studied this process.
NEW FINDINGS: In healthy mice, most neck cells do not differentiate into chief cells. After injury, chief cells can become metaplastic cells, and then return to being chief cells upon injury resolution.
LIMITATIONS: We cannot rule out minor contributions to the gastric chief cell lineage or to metaplasia from progenitors or other gastric lineages. This study was performed in mice
IMPACT: In our model, chief cells are predominately a separate, self-maintaining cell population during homeostasis. Injury can induce chief cells to change into proliferating metaplastic cells, which can return to chief cells upon recovery.
Lay Summary: We found that chief cells, which produce enzymes in they stomach, change into a different cell type, with different functions, in response to injury. Once the injury resolves, chief cells return to their normal activities.
Acknowledgements:
We would like to thank the Vanderbilt University Digestive Disease Research Center (H Pylori experiments) and Washington University Digestive Disease Research Core Center (imaging and histology) for technical support.
Grant Support: This work was supported by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (DK094989, DK105129, DK110406), by the Alvin J. Siteman Cancer Center/Barnes Jewish Hospital Foundation Cancer Frontier Fund, NIH National Cancer Institute grant P30CA091842 to Jason C. Mills; by the National Institute of General Medical Sciences Cell and Molecular Biology training grant GM007067 and the Philip and Sima Needleman Student Fellowship in Regenerative Medicine to Joseph Burclaff; and by the National Cancer Institute training grant CA00954731 and AACR-Debbie’s Dream Foundation Fellowship to Spencer G. Willet; Postdoctoral Enrichment Program Award from the Burroughs Wellcome Fund and American Gastroenterology Association Gastric Cancer Foundation Research Scholar Award to José B. Sáenz. Imaging and Histology was performed by the Digestive Disease Research Core Centers and Advanced Imaging and Tissue Analysis Core (P30DK052574). Training and support for H Pylori experiments was provided by the Vanderbilt Digestive Disease Research Center (P30DK058404)
Abbreviations:
- BrdU
5-bromo-2'-deoxyuridine
- EdU
5-ethynyl-2’-deoxyuridine
- SPEM
Spasmolytic Polypeptide-Expressing Metaplasia
- TAM
Tamoxifen
- ZC
zymogenic cell
Footnotes
The authors disclose no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
Author names in bold designate shared co-first authorship
- 1.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 1993;236:259–79. [DOI] [PubMed] [Google Scholar]
- 2.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec 1993;236:297–313. [DOI] [PubMed] [Google Scholar]
- 3.Quante M, Marrache F, Goldenring JR, et al. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology 2010;139:2018–2027 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldenring JR, Nam KT, Mills JC. The origin of pre-neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp Cell Res 2011;317:2759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki S, Tsuyama S, Murata F. Cells intermediate between mucous neck cells and chief cells in rat stomach. Cell Tissue Res 1983;233:475–84. [DOI] [PubMed] [Google Scholar]
- 6.Huh WJ, Mysorekar IU, Mills JC. Inducible activation of Cre recombinase in adult mice causes gastric epithelial atrophy, metaplasia, and regenerative changes in the absence of "floxed" alleles. Am J Physiol Gastrointest Liver Physiol 2010;299:G368–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest 1999;79:639–46. [PubMed] [Google Scholar]
- 8.Petersen CP, Mills JC, Goldenring JR. Murine Models of Gastric Corpus Preneoplasia. Cell Mol Gastroenterol Hepatol 2017;3:11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willet SG, Mills JC. Stomach Organ and Cell Lineage Differentiation: from Embryogenesis to Adult Homeostasis. Cell Mol Gastroenterol Hepatol 2016;2:546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam KT, Lee HJ, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 2010;139:2028–2037 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stange DE, Koo BK, Huch M, et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 2013;155:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leushacke M, Tan SH, Wong A, et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol 2017;19:774–786. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo J, Kimura S, Yamamura A, et al. Identification of Stem Cells in the Epithelium of the Stomach Corpus and Antrum of Mice. Gastroenterology 2017;152:218–231 e14. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa Y, Ariyama H, Stancikova J, et al. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell 2015;28:800–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinoshita H, Hayakawa Y, Niu Z, et al. Mature gastric chief cells are not required for the development of metaplasia. Am J Physiol Gastrointest Liver Physiol 2018;314:G583–G596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phesse TJ, Sansom OJ. Lgr5 joins the club of gastric stem cell markers in the corpus. Nat Cell Biol 2017;19:752–754. [DOI] [PubMed] [Google Scholar]
- 17.Huh WJ, Khurana SS, Geahlen JH, et al. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 2012;142:21–24 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saenz JB, Burclaff J, Mills JC. Modeling Murine Gastric Metaplasia Through Tamoxifen-Induced Acute Parietal Cell Loss. Methods Mol Biol 2016;1422:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aure MH, Konieczny SF, Ovitt CE. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev Cell 2015;33:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sangiorgi E, Capecchi MR. Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proc Natl Acad Sci U S A 2009;106:7101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai BM, Oliver-Krasinski J, De Leon DD, et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest 2007;117:971–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willet SG, Lewis MA, Miao ZF, et al. Regenerative proliferation of differentiated cells by mTORC1-dependent paligenosis. EMBO J 2018;37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saenz JB, Vargas N, Mills JC. Tropism for Spasmolytic Polypeptide-Expressing Metaplasia Allows Helicobacter pylori to Expand Its Intragastric Niche. Gastroenterology 2019;156:160–174 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radyk MD, Burclaff J, Willet SG, et al. Metaplastic Cells in the Stomach Arise, Independently of Stem Cells, via Dedifferentiation or Transdifferentiation of Chief Cells. Gastroenterology 2018;154:839–843 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec 1993;236:280–96. [DOI] [PubMed] [Google Scholar]
- 26.Ramsey VG, Doherty JM, Chen CC, et al. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 2007;134:211–22. [DOI] [PubMed] [Google Scholar]
- 27.Weis VG, Sousa JF, LaFleur BJ, et al. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut 2013;62:1270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wada T, Ishimoto T, Seishima R, et al. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci 2013;104:1323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec 1993;236:314–32. [DOI] [PubMed] [Google Scholar]
- 30.Huh WJ, Esen E, Geahlen JH, et al. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology 2010;139:2038–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore BD, Jin RU, Lo H, et al. Transcriptional Regulation of X-Box-binding Protein One (XBP1) by Hepatocyte Nuclear Factor 4alpha (HNF4Alpha) Is Vital to Beta-cell Function. J Biol Chem 2016;291:6146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lennerz JK, Kim SH, Oates EL, et al. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol 2010;177:1514–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanby AM, Poulsom R, Playford RJ, et al. The mucous neck cell in the human gastric corpus: a distinctive, functional cell lineage. J Pathol 1999;187:331–7. [DOI] [PubMed] [Google Scholar]
- 34.Stoffels GL, Preumont AM, De Reuck M. Cell differentiation in human gastric gland as revealed by nuclear binding of tritiated actinomycin. Gut 1979;20:693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willems G, Galand P, Vansteenkiste Y, et al. Cell population kinetics of zymogen and parietal cells in the stomach of mice. Z Zellforsch Mikrosk Anat 1972;134:505–18. [DOI] [PubMed] [Google Scholar]
- 36.Willems G, Lehy T. Radioautographic and quantitative studies on parietal and peptic cell kinetics in the mouse. A selective effect of gastrin on parietal cell proliferation. Gastroenterology 1975;69:416–26. [PubMed] [Google Scholar]
- 37.Bjerknes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. Am J Physiol Gastrointest Liver Physiol 2002;283:G767–77. [DOI] [PubMed] [Google Scholar]
- 38.Burclaff J, Mills JC. Plasticity of differentiated cells in wound repair and tumorigenesis, part I: stomach and pancreas. Dis Model Mech 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: BrdU retention in other cell types in the stomach. A) Pit and isthmus region of a gastric corpus unit from mice given two weeks’ continuous BrdU via drinking water. Staining for neck cells (GSII lectin, green), BrdU (white), nuclei (DAPI, blue) and pit cells (AAA lectin, red). Scale bar, 20 μm. B) Wide field immunohistochemistry for BrdU with hematoxylin and eosin counterstain; same experiment as (A). Scale bar, 50 μm. C) Mice administered BrdU continuously for two (left) or eight weeks (right) stained for BrdU (white nuclei) incorporation into main gastric epithelial cell types. Left panel pair: neck cells (GSII, green), ZCs (GIF, red), transitional cells (GSII+GIF+, yellow), and nuclei (DAPI, blue). Right panel pair: parietal cells (Ezrin, red). Scale bars, 40 μm. D) Mice were given BrdU in drinking water during tamoxifen injury (total of 5 days of BrdU treatment). After 5 days for BrdU treatment, animals were chased for 1 (left panel), 2 (middle panel), or 3 (right panel) months. Staining for BrdU (white), neck cells (GSII lectin, green), pit cells (AAA lectin, red), and nuclei (DAPI, blue). Scale bar, 20 μm. E) Same BrdU labeling experiment as (D). Mice were chased for 1 (left panel) or 9 (right panel) months. Representative corpus units stained for BrdU (white), neck cells (GSII lectin, green), PCs (Ezrin, red), and nuclei (DAPI, blue). Scale bar, 40 μm. F) Same BrdU labeling experiment as in (D). Mice were chased for 3 months and stained for BrdU (white), nuclei (DAPI, blue), and endocrine cells (left panel; Chromogranin A, red), tuft cells (middle panel; DCLK1, red), or stromal cells (right panel; α-SMA, red). Scale bar, 10 μm.
Supplemental Figure 2: IHC of Long-term BrdU Chase Mice were given BrdU via drinking water one day prior to, during, and an additional 2 days following three days of tamoxifen (total of 5 days BrdU infusion) and chased with no additional BrdU for 1 (A) or 11 (B) months. A) Representative BrdU immunohistochemistry after 1-month chase. B) Representative BrdU immunohistochemistry after 11-month chase. Dashed yellow boxes correspond to adjacent higher power image. Scale bar, 50 μm (left images), 25 μm (right images).
Supplemental Figure 3: BrdU is incorporated into chief cells nonadjacent to the transitional zone A) Bases of units from mice administered BrdU continuously for eight weeks and stained for BrdU (white), neck cells (GSII, green), ZCs (GIF, red), transitional cells (GSII+GIF+, yellow), and nuclei (DAPI, blue). Scale bar, 20 μm. B) Histogram of the location within the base of each BrdU+ ZC compiled from all continuous BrdU-infusion experiments. ZCs adjacent to the lowest GSII+ (or GIF+GSII+) cell are labeled ‘uppermost,’ with ‘lowermost’ denoting the bottom ZC in a unit. All other BrdU+ ZCs were scored as upper or lower half of the base. C) Bases of gastric units from mice administered BrdU continuously for 8 weeks and stained for E-Cadherin (red), BrdU (white), GSII lectin (green), and DAPI (blue). Adjacent BrdU+ nuclei were counted as individual cells if E-Cadherin separated the nuclei (eg top panels; arrowheads), otherwise they were considered binuclear ZCs (bottom). Scale bar, 20 μm.
Supplemental Figure 4: Additional H. Pylori infection data A) Mice were given BrdU in drinking water during tamoxifen injury (total of 5 days of BrdU treatment). After 5 days of BrdU treatment, mice were chased for 1 month, infected with H. Pylori, and chased for an additional 8 weeks. Representative non-metaplastic units stained for BrdU (white), neck cells (GSII lectin, green), ZCs (GIF, red), transitional cells (GIF+GSII+, yellow) and nuclei (DAPI, blue). Scale bar, 20 μm. B) Same experiment as in (A) except HP infection was for 12 weeks. Representative metaplastic units following 12-week HP infection stained for BrdU (white), neck cells (GSII lectin, green), ZCs (GIF, red), transitional cells (GIF+GSII+, yellow) and nuclei (DAPI, blue). Scale bar, 20 μm.
Supplemental Figure 5: Additional metaplasia data following tamoxifen reinjury and HP infection A) Mice were given BrdU in drinking water during tamoxifen injury (total of 5 days of BrdU treatment). After 5 days of BrdU treatment, animals were chased for 3 months (no additional BrdU). At 3 months, mice were reinjured with tamoxifen for 3 days and sacrificed one day afterwards. Representative metaplastic units stained for BrdU (white), neck cells (GSII lectin, green), clusterin (red), and nuclei (DAPI, blue). Scale bar, 20 μm. B) Same experiment as in (A). Staining for BrdU (white), neck cells (GSII lectin, green), CD44v (red), and nuclei (DAPI, blue). Scale bar, 20 μm. C) Mice were given BrdU in drinking water during tamoxifen injury then chased for 1 month, infected with H. Pylori, and chased for an additional 8 weeks. Representative metaplastic units stained for BrdU (white), neck cells (GSII lectin, green), Ki67 (red), and nuclei (DAPI, blue). Scale bar, 20 μm. D) Same experiment as in (A). Representative metaplastic stained for BrdU (white), neck cells (GSII lectin, green), Ki67 (red), and nuclei (DAPI, blue). Scale bar, 20 μm.