Abstract
A growing body of evidence implicates endoplasmic reticulum (ER) stress in the pathogenesis of chronic inflammatory and autoimmune disorders. Here, we demonstrate that the proinflammatory cytokine TNFα stimulates matrix metalloproteinase 9 (MMP9) at the ocular surface through a c-Fos-dependent mechanism of ER stress. We found positive reactivity of the molecular chaperone BiP/GRP78 in conjunctival epithelium of patients with ocular cicatricial pemphigoid and increased levels of BiP/GRP78, sXBP1 and GRP94 in human corneal epithelial cells treated with TNFα. Pharmacological blockade of ER stress in vitro using dexamethasone or the chemical chaperones TUDCA and 4PBA attenuated MMP9 expression and secretion in the presence of TNFα. Moreover, expression analysis of genes associated with inflammation and autoimmunity identified the c-Fos proto-oncogene as a mediator of ER stress responses in epithelial cells. Substantially less TNFα-induced MMP9 expression occurred when c-Fos signaling was suppressed with a function-blocking antibody. Taken together, these results indicate that activation of ER stress contributes to promote inflammation-mediated proteolytic activity and uncovers a target for restoring tissue homeostasis in ocular autoimmune disease.
Subject terms: Endoplasmic reticulum, Corneal diseases
Introduction
About two percent of the human genome is thought to encode proteolytic enzymes responsible for important physiological processes such as development, tissue remodeling and the immune response1. The activity of these enzymes must be tightly regulated within an organism to prevent the abnormal degradation of extracellular and cell surface proteins and, consequently, the development or progression of disease. Dysregulation of protease expression has been documented in autoimmune diseases, a group of chronic inflammatory conditions that occur when the immune system turns its antimicrobial defenses against normal components of the body2. In the eye, these can be tissue-specific (e.g., Mooren’s ulcerative keratitis), systemic (e.g., cicatricial pemphigoid, Sjögren’s syndrome), or secondary to other autoimmune diseases (e.g., rheumatoid arthritis)3.
The loss of self-tolerance in autoimmune disease results in excessive production of cytokines. TNFα is one of the major proinflammatory cytokines contributing to autoimmune pathogenesis due to its direct action on the proliferation and differentiation of immune cells and ability to induce production of additional cytokines4. Binding of TNFα to its receptors also induces upregulation of matrix metalloproteinases (MMPs), a family of extracellular endopeptidases long thought to be involved in the remodeling of basement membranes and interstitial connective tissue. In keratinocytes, TNFα exerts a powerful effect on the synthesis of MMP9, a metalloproteinase capable of degrading type IV collagen and long postulated to contribute to the pathogenesis of autoimmune disease5,6. Elevated levels of TNFα have been found in epithelial cells of conjunctival tissue from patients with ocular cicatricial pemphigoid7,8, but the mechanisms by which this cytokine regulates proteolytic activity during pathological conditions have not been completely elucidated.
The endoplasmic reticulum (ER) is a network of membrane-enclosed tubules and sacs that serve as the first compartment of the secretory pathway in eukaryotic cells9. Under normal conditions, a group of molecular chaperones, such as BiP/GRP78, and folding enzymes assist with the assembly of newly synthesized proteins and prevent the misfolding and aggregation of pre-existing proteins. Another group of proteins called the ER-associated degradation (ERAD) pathway assist with the clearance of misfolded proteins. Disruption of ER homeostasis and accumulation of unfolded proteins can overload the ER and induce activation of specific stress signaling pathways, collectively known as the unfolded protein response (UPR)10. It involves activation of transcription factors such as XBP1 that direct the expression of chaperones, folding enzymes and components of the ERAD pathway to relieve ER stress and restore homeostasis11,12. In pathological conditions, however, prolonged UPR activation initiates cell death. All these pathways have recently gained much attention in autoimmune disease research due to the potential of ER stress proteins to act as autoantigens and their function as quality control factors13. Here, we provide evidence indicating that ER stress plays a role in promoting inflammation-mediated proteolytic activity in ocular autoimmune disease and identify the c-Fos proto-oncogene as a key mediator of these events.
Results
ER stress is elevated in ocular autoimmune disease
Past work has evidenced an association between ER stress and autoimmune diseases14,15, which at the ocular surface include Sjögren’s syndrome16. To expand these findings, we examined tissue from three patients with ocular cicatricial pemphigoid, a type of autoimmune disease that can lead to severe scarring of the conjunctiva and keratopathy17. Immunohistochemical analysis demonstrated increased staining of the molecular chaperone BiP/GRP78 in pathological specimens compared to control tissue (Fig. 1a). In these experiments, the antibody bound to the innermost layer of the epithelium in contact with the basement membrane, as well as suprabasal epithelial cells in two of the three pathological specimens. Quantification of the abundance of BiP/GRP78 in the epithelium revealed a 5-fold increase over control tissue (Fig. 1b).
Figure 1.
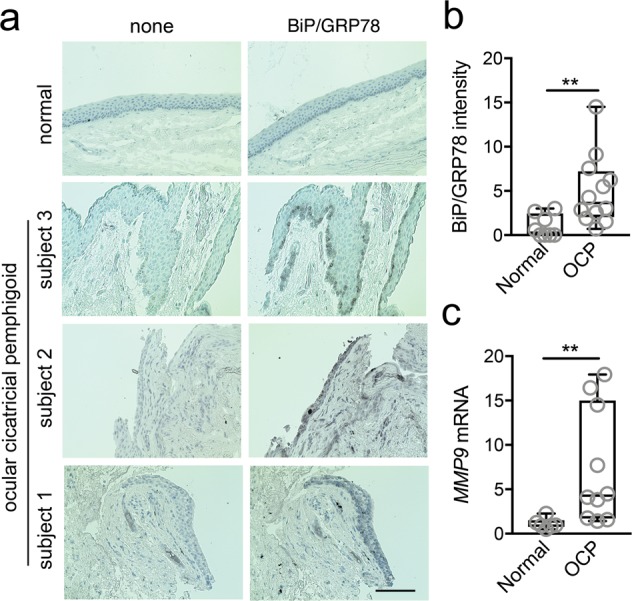
ER stress is elevated in ocular cicatricial pemphigoid. (a) BiP/GRP78 staining was analyzed in conjunctival biopsies of three patients with ocular cicatricial pemphigoid (OCP) and three normal subjects by immunohistochemistry. A representative image is shown for normal tissue. Consecutive sections lacking the primary antibody were used as negative controls. Scale bar, 100 μm. (b) BiP/GRP78 staining intensity in conjunctival epithelium was measured in at least two different histological areas for each subject using ImageJ. (c) Conjunctival epithelium was collected by impression cytology from nine patients with OCP and six normal subjects. The expression of MMP9 was determined by qPCR. The box and whisker plots show the 25 and 75 percentiles (box), the median, and the minimum and maximum data values (whiskers). Significance was determined using Mann-Whitney test. **p < 0.01.
One downstream effect of ER stress following intrinsic or extrinsic challenge is the regulation of processes involved in the remodeling of the extracellular matrix18,19. Therefore, we sought to examine the transcriptional levels of MMP9, a matrix metalloprotease involved in the degradation of denatured collagens, including basement membrane type IV and anchoring fibril type VII collagens. Analysis of gene expression in conjunctival epithelium revealed a significantly higher content of MMP9 transcripts in pathological specimens compared to control (Fig. 1c), suggesting a potential association between ER stress and the regulation of the proteolytic microenvironment in ocular autoimmune disease.
TNFα promotes ER stress at the ocular surface
Increased TNFα expression has been found in ocular autoimmune disease. Therefore, in subsequent experiments, we examined the contribution of TNFα to the activation of the UPR in multilayered cultures of corneal epithelial cells. As shown in Fig. 2a, the expression of spliced XBP1 (sXBP1) encoding an active transcription factor that binds to many UPR target genes peaked at 6 h after treatment with TNFα, and progressively decreased thereafter. This event was followed by the biosynthesis of a subset of downstream ER resident chaperones in the UPR that augment ER folding capacity. Exposure to TNFα for 48 h resulted in a significant increase in both BiP/GRP78 and GRP94 (Fig. 2b). In these experiments we did not observe cell toxicity, as measured by cell viability assay (Fig. 2c), suggesting that TNFα under our assay conditions promotes activation of the UPR without triggering cell death mechanisms.
Figure 2.
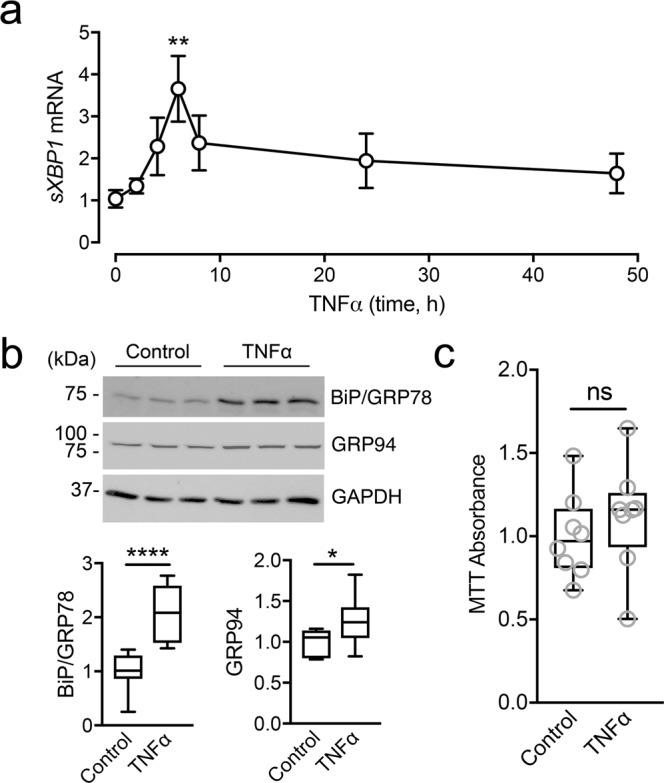
TNFα triggers ER stress in human corneal epithelial cells. (a) Multilayered cultures of human corneal epithelial cells were incubated with 40 ng/ml TNFα at different time points. The expression of sXBP1 was analyzed by qPCR. (b) Cell cultures were treated for 48 h with TNFα. Total protein levels of BiP/GRP78 and GRP94 were assessed by immunoblotting. Full-length blots are presented in Supplemental Figure S1. (c) Cell viability rates after incubation with TNFα for 48 h were determined by using the MTT assay. Results represent at least three independent experiments. Data in (a) represent the mean ± SEM. The box and whisker plots show the 25 and 75 percentiles (box), the median, and the minimum and maximum data values (whiskers). Significance was determined using Kruskal-Wallis with Dunn’s post hoc test (a) and Student’s t test (b,c). *p < 0.05; **p < 0.01; ****p < 0.0001; ns, not significant.
Pharmacological inhibition of ER stress decreases TNFα-induced MMP9 expression
Next, we evaluated whether ER stress was involved in promoting MMP9 expression and secretion under proinflammatory conditions. TNFα is a potent inducer of MMP9 in human corneal epithelial cells20. Consistent with these data, we observed abundant MMP9 transcripts in our multilayered model of corneal epithelium after cytokine treatment (Fig. 3a). To investigate the role of ER stress in this process, we used dexamethasone, a corticosteroid clinically used to control inflammation and with the ability to suppress the activation of the UPR in epithelial cells21. We found that dexamethasone inhibited the expression of sXBP1 following treatment of the epithelial cultures with TNFα (Fig. 3b). Importantly, dexamethasone significantly impaired the transcription and secretion of MMP9 under proinflammatory conditions (Fig. 3c,d), suggesting that this drug could limit MMP9 production by reducing UPR activation. It should be noted, however, that dexamethasone has pleiotropic effects on multiple signaling pathways that limit its utility as a mechanistic probe.
Figure 3.
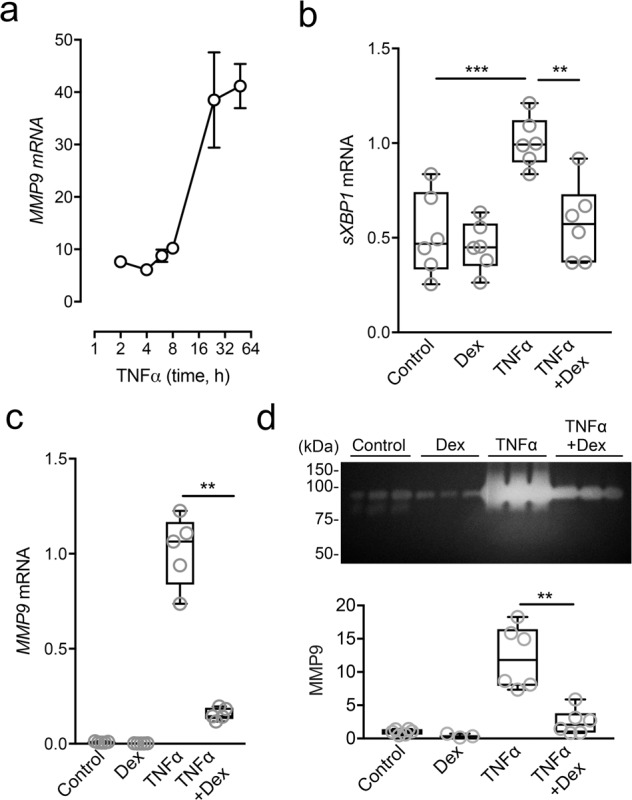
Dexamethasone alleviates ER stress and TNFα-induced MMP9 expression. (a) Multilayered cultures of human corneal epithelial cells were incubated with 40 ng/ml TNFα at different time points. The expression of MMP9 was analyzed by qPCR. (b) The effect of dexamethasone on sXBP1 expression was measured by qPCR following 6 h incubation with TNFα. (c) The effect of dexamethasone on MMP9 expression was measured by qPCR following 48 h incubation with TNFα. (d) Cell culture supernatants in (c) were analyzed by gel zymography. Results in (a) represent at least three independent experiments. Results in (b–d) represent two independent experiments performed in triplicate. Data in (a) represent the mean ± SEM. The box and whisker plots show the 25 and 75 percentiles (box), the median, and the minimum and maximum data values (whiskers). Significance was determined using one-way ANOVA with Tukey’s post hoc test (b) and Mann-Whitney test (c,d). **p < 0.01; ***p < 0.001. Dex, dexamethasone.
Consequently, to further delineate the relationship between ER stress and the production of MMP9 during inflammation, we treated the epithelial cells with two specific inhibitors of ER stress, tauroursodeoxycholic acid (TUDCA) and 4-phenylbutyric acid (4PBA) (Fig. 4a). These are chemical chaperones that reduce ER stress by stabilizing protein conformation and improving ER folding capacity22. Incubation with TUDCA and 4PBA following TNFα stimulation significantly reduced the induction of MMP9 expression as well as its secretion into the cell culture media (Fig. 4b,c). Overall, these results indicate that induction of proteolytic activity in epithelial cells by the proinflammatory cytokine TNFα can be mediated by activation of ER stress.
Figure 4.
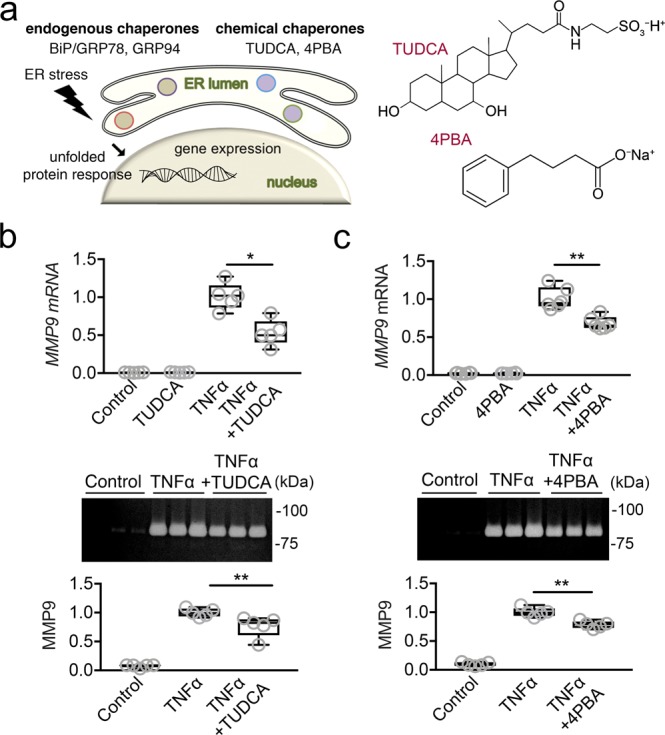
Use of chemical chaperones decreases TNFα-induced MMP9 expression. (a) Diagram showing endogenous and chemical chaperones, which reduce ER stress by stabilizing protein conformation and improving ER folding capacity. (b) The effect of TUDCA on MMP9 expression and secretion into the cell culture media was measured by qPCR and gel zymography, respectively, following 48 h incubation with TNFα. (c) The effect of 4PBA on MMP9 expression and secretion was also measured following 48 h incubation with TNFα. Results represent two independent experiments performed in triplicate. The box and whisker plots show the 25 and 75 percentiles (box), the median, and the minimum and maximum data values (whiskers). Significance was determined using Mann-Whitney test. *p < 0.05; **p < 0.01.
ER stress induces MMP9 expression by promoting FOS transcription
To gain insight into the mechanism underlying the regulation of MMP9 expression by ER stress, we treated corneal epithelial cells with tunicamycin, a potent inhibitor of N-glycosylation that disrupts protein maturation and induces ER stress. Changes in the relative expression of genes involved in the inflammatory response were evaluated with a human inflammatory response and autoimmunity PCR array. Scatterplots using a twofold cutoff showed that induction of ER stress evoked a significant increase in PTGS2, TNFα, CXCL2, CXCL3 and FOS (Fig. 5a, Supplemental Table S1). Of interest was FOS, which encodes a leucine zipper protein that can dimerize with proteins of the JUN family to form a transcription factor complex known as AP-1. AP-1 has been shown to bind the promoter region of human MMP9 to regulate its expression23.
Figure 5.
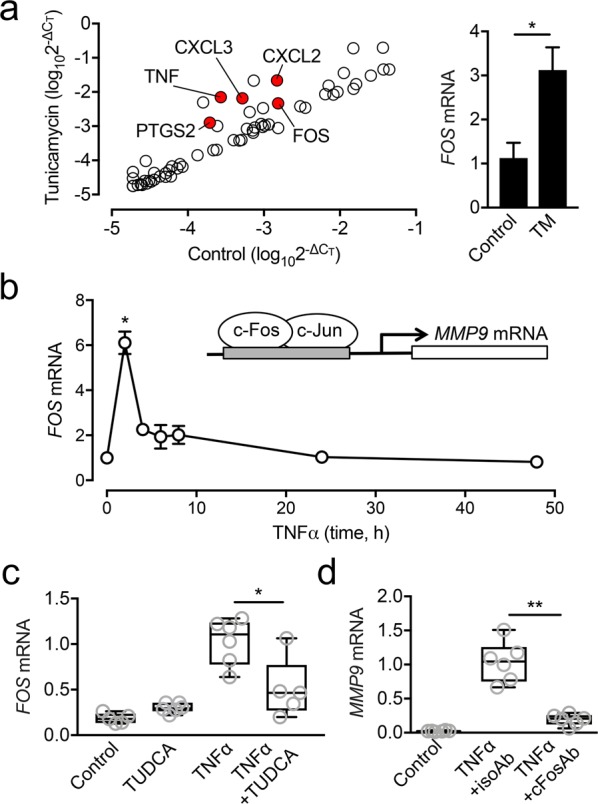
ER stress induces MMP9 expression by promoting FOS transcription. (a) Monolayer cultures of human corneal epithelial cells were incubated with 10 µg/ml tunicamycin for 2 h. The relative expression of genes involved in the inflammatory response was evaluated with a human inflammatory response and autoimmunity PCR array. The red dots in the scatter diagram indicate at least 2-fold significant upregulation compared to control. No gene was significantly downregulated. (b) Multilayered cultures of human corneal epithelial cells were incubated with 40 ng/ml TNFα at different time points. The expression of FOS was analyzed by qPCR. (c) The effect of TUDCA on FOS expression was measured by qPCR following 2 h incubation with TNFα. (d) The effect of a function-blocking antibody to human c-Fos (cFosAb) on MMP9 expression was measured by qPCR following 48 h incubation with TNFα. An isotype-matched antibody (isoAb) served as control. Results in (a,b) represent three independent experiments. Results in (c,d) represent two independent experiments performed in triplicate. Data in (a,b) represent the mean ± SEM. The box and whisker plots show the 25 and 75 percentiles (box), the median, and the minimum and maximum data values (whiskers). Significance was determined using Student’s t test (a), Kruskal-Wallis with Dunn’s post hoc test (b) and Mann-Whitney test (c,d). *p < 0.05; **p < 0.01. TM, tunicamycin.
We continued to examine whether TNFα would promote the transcription of FOS in a similar fashion as tunicamycin. In these experiments, we found that the level of expression of FOS peaked at 2 h following treatment with TNFα (Fig. 5b). Furthermore, we observed that the expression of FOS was reduced by TUDCA (Fig. 5c), implicating ER stress in the expression of components of the AP-1 complex under proinflammatory conditions. Upon further examination, we determined that suppression of c-Fos signaling with a function-blocking antibody significantly impaired MMP9 expression in our model of TNFα-induced inflammation (Fig. 5d). Together, these data indicate that activation of ER stress during inflammation plays a role in regulating FOS expression and the induction of MMP9.
Discussion
Biochemical and functional studies have implicated abnormal proteolytic activity in the pathogenesis of autoimmune disease. These analyses indicate that active MMPs cleave a diverse range of substrates such as cell surface molecules, adhesion proteins and components of the extracellular matrix to influence signaling and tissue remodeling processes24. The expression and activity of these enzymes is regulated by a number of factors, and accumulating evidence points to the participation of ER stress proteins in some of these pathways. Here, we report the presence of ER stress at the ocular surface epithelia of patients with ocular cicatricial pemphigoid and demonstrate a role for the UPR in mediating the expression of MMP9 under proinflammatory conditions. Moreover, we identify the c-Fos proto-oncogene as a transcription factor that governs these events.
Induction of ER stress and subsequent triggering of the adaptive UPR mechanism are processes that have recently gained much attention for their contributions to autoimmune disease. Examples include pemphigus and inflammatory bowel disease, where ER stress and the UPR have been shown to be associated with disease progression and susceptibility, respectively25,26. The mechanism by which ER stress contributes to the pathobiology of autoimmune disease appears to reside in the immunogenic nature of misfolded proteins and the function of ER stress proteins as autoantigens13. More recent evidence indicates that ER stress proteins also have pathogenic relevance by participating in additional biological processes. An example is the ER chaperone BiP/GRP78, which promotes synovial cell proliferation and angiogenesis through a VEGF-dependent mechanism in rheumatoid arthritis27. In our hands, ER stress contributes to the pathobiology of ocular autoimmune disease by regulating the proteolytic microenvironment at the ocular surface. Central to these processes are the proinflammatory cytokines. Addition of TNFα to synoviocytes, hepatocytes or fibrosarcoma cells has been shown to increase the expression of BiP/GRP78 and to activate the UPR27–29. Consistent with these results, we also find that TNFα upregulates BiP/GRP78 expression in corneal epithelial cells, supporting the role of proinflammatory cytokines as ER stressors in autoimmune diseases and revealing an important overlap between inflammation and ER stress pathways.
The presence of elevated levels of MMP9 is a common feature in autoimmune conditions such as lupus, Sjögren’s syndrome, multiple sclerosis, and rheumatoid arthritis. In multiple sclerosis, the finding of increased expression of MMP9 in areas of damaged tissue has suggested that this enzyme plays a role in the disruption of vascular basement membranes30 whereas in patients with Sjögren’s syndrome it has been linked to the progressive atrophy of the salivary glands and the extensive infiltration by fibrous tissue31. MMP9 is also elevated in the tear fluid of patients with ocular cicatricial pemphigoid32,33, but its regulation has not been thoroughly investigated so far. Glucocorticoids are a group of steroid compounds with anti-inflammatory properties that can reduce ER stress on epithelial surfaces. In the intestine, dexamethasone alleviates ER stress by enhancing protein folding and the degradation of misfolded proteins21. Consistent with these data, we found that dexamethasone reduced the levels of sXBP1 of corneal epithelial cells under inflammatory stress. Importantly, we observed a concomitant reduction in the expression and secretion of MMP9, suggesting an association between ER stress and proteolytic activity at the ocular surface. The use of the chemical chaperones TUDCA and 4PBA conclusively demonstrated that ER stress supported the actions of TNFα as a regulator of MMP9 transcription in epithelial cells. Based on these results, it is likely that ER stress contributes to the excessive induction of MMP9 under continued exposure to proinflammatory mediators at the ocular surface.
One question that remains is how ER stress modulates MMP9 expression under proinflammatory conditions. Using a PCR array we identified FOS as a downstream gene targeted for induction by ER stress in corneal epithelial cells. These results were not completely unexpected, since the MMP9 promoter has multiple functional cis-regulatory regions for binding transcriptional factors, including three for AP-1, a heterodimer of c-Jun and c-Fos23. Indeed, previous research using the immortalized HaCaT keratinocyte cell line has shown that activation of c-Jun by TNFα is a critical step in the regulation of MMP9 expression5,34. Crucially, these reports point out to mitogen-activated protein kinases (MAPK) as the enzymes responsible for the activation of the AP-1 complex and the expression of MMP9. It is well established that there are points of crosstalk between the MAPK signaling cascade and the UPR. MAPK signaling networks are activated in response to extracellular signals that include not only proinflammatory cytokines but also multiple cellular assaults including ER stress35. Therefore, it is possible to envision that expression of MMP9 in epithelial cells is a consequence of the direct action of proinflammatory cytokines on signaling pathways but also the activation of the UPR itself. Future work should focus on determining the relative contribution of each pathway to the expression of proteolytic enzymes during inflammation.
Collectively, our data advance the understanding of the contribution of ER stress to the pathogenesis of ocular autoimmune disease and highlight the complexity of signal transduction pathways activated by proinflammatory cytokines in the regulation of the proteolytic microenvironment. The results also suggest that use of chemical chaperones to alleviate ER stress has potential as a therapeutic strategy for patients with ocular autoimmune disorders.
Methods
Human specimens
Conjunctival epithelium was collected by impression cytology from ten eyes of nine patients with ocular cicatricial pemphigoid stage II at the Ocular Surface Center at Campus Bio-Medico University of Rome, Italy. Cells were collected by placing a sterile disc of nitrocellulose membrane on the temporal bulbar conjunctiva using sterile forceps as described36. The mean age of the patients was 66.1 ± 13.1 years (range, 44–81 years). Six specimens from six age-matched normal subjects were used as a control group. The mean age of the control group was 62 ± 6.1 years (range, 53–71 years). Exclusion criteria for the control group included history of ocular disease or eye surgery and contact lens wear37. Informed consent was obtained from each recruited patient. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the Institutional Review Board of Campus Bio-Medico University of Rome. Human conjunctival biopsies stored in paraffin from three normal subjects were obtained as archived material from a previously published study38. Conjunctival tissue sections from three patients with ocular cicatricial pemphigoid were obtained from Campus Bio-Medico University of Rome.
Immunohistochemistry
Six-micron tissue sections of conjunctival epithelium were subjected to routine deparaffinization with xylene and rehydration with graded ethanol. Epitope exposure was performed using citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) at 95 °C for 15 min with subsequent cooling at room temperature for 20 min. Sections were blocked for 1 h in 1% bovine serum albumin and 10% goat serum in phosphate buffered saline and incubated with rabbit anti-human BiP/GRP78 antibody (C50B12; 1:200; Cell Signaling Technology) in 1% bovine serum albumin and 5% goat serum in phosphate buffered saline at 4 °C overnight. Tissues were exposed to 0.3% hydrogen peroxide in phosphate buffered saline for 10 min to quench endogenous peroxidase activity, followed by sequential application of a secondary anti-rabbit antibody (VWR) conjugated to biotin for 45 min, and the HRP-streptavidin complex (Vector Laboratories) for 30 min at room temperature. Immunoreactivity was detected using SigmaFast™ DAB with metal enhancer (Sigma-Aldrich) and nuclei counterstained with hematoxylin QS (Vector Laboratories). Coverslips were mounted using aqueous mounting medium, and staining was visualized by light microscopy. Incubation with primary antibody was routinely omitted in control experiments to demonstrate lack of endogenous peroxidase activity. Positive staining was analyzed by hand-outlining areas of conjunctival epithelium in ImageJ (National Institutes of Health) and measuring the density of brown staining per area using the H DAB vector. Mean staining per area in sections treated without the primary antibody was subtracted as background.
Cell culture
Cultures of telomerase-immortalized human corneal epithelial cells were grown as previously reported36. Briefly, cells were plated at a seeding density of 1 × 105 cells/cm2 and maintained in keratinocyte serum-free medium (Thermo Fisher Scientific) supplemented with 0.3 mM CaCl2, 25 µg/ml bovine pituitary extract, 0.2 ng/ml epidermal growth factor, and 1% penicillin/streptomycin until confluence. Thereafter, cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 supplemented with 10% calf serum, 10 ng/ml epidermal growth factor, and 1% penicillin/streptomycin for 7 days to promote stratification and differentiation. Where indicated, multilayered cultures were serum-starved for 1 h and incubated with TNFα (40 ng/ml; PeproTech) in serum-free DMEM/F12 in the presence and absence of dexamethasone (10 µg/ml; Sigma-Aldrich), 4PBA (2.5 mM; Sigma-Aldrich), TUDCA (1 mM; Merck Millipore), a blocking antibody to human c-Fos (6–2H-2F; 2 µg/ml, Santa Cruz Biotechnology) or mouse IgG control (sc-2025; 2 µg/ml, Santa Cruz Biotechnology). Monolayer cultures were treated with tunicamycin (10 µg/ml; Sigma-Aldrich) or DMSO control.
Immunoblotting
Cells were lysed in RIPA buffer supplemented with Complete™ EDTA-free Protease Inhibitor Cocktail (Roche Diagnostics) as described39. After homogenization with a pellet pestle, the cell extracts were centrifuged at 17,115 × g for 45 min at 4 °C, and the protein concentration of the supernatant determined using the Pierce BCA™ Protein Assay Kit (Thermo Fisher Scientific). Proteins were separated by SDS-PAGE (10% resolving gel) and electroblotted onto nitrocellulose membranes. Nonspecific binding to the nitrocellulose was blocked by incubation with 5% nonfat milk in 0.1% Tween 20 in Tris-buffered saline at room temperature for 1 h. Membranes were incubated with primary antibodies to BiP/GRP78 (1:1,000; C50B12, Cell Signaling Technology) and GRP94 (1:1,000; #2104; Cell Signaling Technology) in blocking buffer overnight at 4 °C. GAPDH (1:3,000; FL-335; Santa Cruz Biotechnology) served as a sample loading control. Membranes were then incubated with the appropriate secondary antibodies coupled to horseradish peroxidase (1:5,000; Santa Cruz Biotechnology) for 1 h at room temperature. Peroxidase activity was visualized using chemiluminescence. Densitometry was performed using ImageJ software.
Gel zymography
Cell culture medium from 12-well plates was collected and centrifuged at 1,150 × g for 5 min to remove cells and cellular debris as described40. The supernatant (15 μl) was mixed with non-reducing loading buffer (50 mM Tris-HCl pH 6.8, 10% glycerol, 1% SDS, and 0.01% Bromophenol Blue) and resolved on 7.5% SDS-PAGE gels containing 1 mg/ml gelatin (bovine skin type B). Gels were then incubated in 50 mM Tris containing 5 mM CaCl2 and 2.5% Triton X-100 for two h at room temperature, refreshing the buffer every 30 min. After washing with distilled water, gels were incubated in collagenase buffer (50 mM Tris-HCl pH 7.6, 5 mM CaCl2) for 24 h at 37 °C followed by staining in Coomassie Brilliant Blue solution (40% methanol, 10% acetic acid, 0.025% Coomassie Brilliant Blue R-250). Gels were then washed in distilled water for 2 h and photographed. Gelatinase activity was quantified using ImageJ software.
RNA isolation and cDNA synthesis
Total RNA was extracted from impression cytology samples and cell cultures using a Qiagen RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s protocol. Up to 1 μg total RNA was used for cDNA synthesis (iScript™ cDNA Synthesis; Bio-Rad).
qPCR
Gene expression levels were detected by quantitative real-time PCR using the KAPA SYBR® FAST qPCR kit (Kapa Biosystems) in a Mastercycler ep realplex thermal cycler (Eppendorf). Primer sequences for sXBP1 (forward 5′-CTGAGTCCGAATCAGGTGCAG-3′; reverse 5′-ATCCATGGGGAGATGTTCTGG-3′) mRNA have been previously published41. Primer sequences for MMP9 (Unique Assay ID qHsaCID0011597), FOS (Unique Assay ID qHsaCED0046695) and GAPDH (Unique Assay ID qHsaCED0038674) mRNA were obtained from Bio-Rad. The following parameters were used: 2 min at 95 °C, followed by 40 cycles of 5 seconds at 95 °C and 30 seconds at 60 °C. All samples were normalized using GAPDH housekeeping gene expression. The comparative ΔΔCT method was used for relative quantitation of the number of transcripts37. No template controls were run in each assay to confirm lack of DNA contamination in the reagents used for amplification.
Human inflammatory response and autoimmunity PCR array
The analysis of 84 genes encoding for inflammatory cytokines and chemokines and their receptors was carried out using a human inflammatory response and autoimmunity PCR array (PAHS-077Z; RT2 Profiler PCR array, SABiosciences) according to the manufacturer’s instructions. Expression values were corrected for the housekeeping gene GAPDH. The ΔCT method was used for relative quantitation of the number of transcripts.
MTT assay
Cell viability was assessed by means of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay following the manufacturer’s instructions (Molecular Probes). Briefly, cultures were incubated with a 1.2 mM MTT solution at 37 °C for 4 h as described37. The absorbance values of blue formazan were determined at 540 nm. Cell viability was expressed as MTT uptake in treated cells normalized to untreated cells.
Statistical analyses
All statistical analyses were performed using Prism 7 (GraphPad Software) for Mac OS X.
Supplementary information
Acknowledgements
This work was supported by the National Institutes of Health, NEI Grant R01EY026147 (PA) and the National Institutes of Health, NEI Core Grant P30EY003790 (PA). The authors thank Dr. Ilene Gipson for providing the HCLE telomerase-immortalized human corneal epithelial cell line. PCR array experiments were performed with the hTCEpi telomerase-immortalized human corneal epithelial cell line kindly provided by Dr. James Jester. We also appreciate the aid of Ms. Bianai Fan in performing paraffin sectioning.
Author contributions
A.M.W. designed, performed and interpreted experiments, and co-wrote the manuscript. A.D.Z. and S.B. commented on the study, provided technical and material support, and reviewed the manuscript. P.A. conceived the project, designed and interpreted experiments, and co-wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-59237-3.
References
- 1.Turk B. Targeting proteases: successes, failures and future prospects. Nat. Rev. Drug. Discov. 2006;5:785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- 2.Goodnow CC. Multistep pathogenesis of autoimmune disease. Cell. 2007;130:25–35. doi: 10.1016/j.cell.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 3.Stern ME, et al. Autoimmunity at the ocular surface: pathogenesis and regulation. Mucosal Immunol. 2010;3:425–442. doi: 10.1038/mi.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mateen S, Zafar A, Moin S, Khan AQ, Zubair S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta. 2016;455:161–171. doi: 10.1016/j.cca.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Bahar-Shany K, Ravid A, Koren R. Upregulation of MMP-9 production by TNFalpha in keratinocytes and its attenuation by vitamin D. J. Cell. Physiol. 2010;222:729–737. doi: 10.1002/jcp.22004. [DOI] [PubMed] [Google Scholar]
- 6.Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. J. Clin. immunology. 2006;26:299–307. doi: 10.1007/s10875-006-9022-6. [DOI] [PubMed] [Google Scholar]
- 7.Cordero Coma M, Yilmaz T, Foster CS. Tumour necrosis factor-alpha in conjunctivae affected by ocular cicatricial pemphigoid. Acta ophthalmologica Scandinavica. 2007;85:753–755. doi: 10.1111/j.1600-0420.2007.00941.x. [DOI] [PubMed] [Google Scholar]
- 8.Saw VP, et al. Tumor necrosis factor-alpha in ocular mucous membrane pemphigoid and its effect on conjunctival fibroblasts. Investig. Ophthalmol. Vis. Sci. 2009;50:5310–5317. doi: 10.1167/iovs.08-3345. [DOI] [PubMed] [Google Scholar]
- 9.Almanza A, et al. Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J. 2019;286:241–278. doi: 10.1111/febs.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nat. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida H, et al. A time-dependent phase shift in the mammalian unfolded protein response. Developmental Cell. 2003;4:265–271. doi: 10.1016/S1534-5807(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 12.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morito D, Nagata KER. Stress Proteins in Autoimmune and Inflammatory Diseases. Front. Immunol. 2012;3:48. doi: 10.3389/fimmu.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrera MJ, et al. Endoplasmic reticulum stress in autoimmune diseases: Can altered protein quality control and/or unfolded protein response contribute to autoimmunity? A critical review on Sjogren’s syndrome. Autoimmun. Rev. 2018;17:796–808. doi: 10.1016/j.autrev.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Cipolla GA, Park JK, Lavker RM, Petzl-Erler ML. Crosstalk between Signaling Pathways in Pemphigus: A Role for Endoplasmic Reticulum Stress in p38 Mitogen-Activated Protein Kinase Activation? Front. Immunol. 2017;8:1022. doi: 10.3389/fimmu.2017.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coursey TG, Tukler Henriksson J, Barbosa FL, de Paiva CS, Pflugfelder SC. Interferon-gamma-Induced Unfolded Protein Response in Conjunctival Goblet Cells as a Cause of Mucin Deficiency in Sjogren Syndrome. Am. J. Pathol. 2016;186:1547–1558. doi: 10.1016/j.ajpath.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razzaque MS, Foster CS, Ahmed AR. Tissue and molecular events in human conjunctival scarring in ocular cicatricial pemphigoid. Histol. Histopathol. 2001;16:1203–1212. doi: 10.14670/HH-16.1203. [DOI] [PubMed] [Google Scholar]
- 18.Limia Celia, Sauzay Chloé, Urra Hery, Hetz Claudio, Chevet Eric, Avril Tony. Emerging Roles of the Endoplasmic Reticulum Associated Unfolded Protein Response in Cancer Cell Migration and Invasion. Cancers. 2019;11(5):631. doi: 10.3390/cancers11050631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owusu BY, Zimmerman KA, Murphy-Ullrich JE. The role of the endoplasmic reticulum protein calreticulin in mediating TGF-beta-stimulated extracellular matrix production in fibrotic disease. J. Cell Commun. Signal. 2018;12:289–299. doi: 10.1007/s12079-017-0426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li DQ, et al. Regulation of MMP-9 production by human corneal epithelial cells. Exp. eye Res. 2001;73:449–459. doi: 10.1006/exer.2001.1054. [DOI] [PubMed] [Google Scholar]
- 21.Das I, et al. Glucocorticoids alleviate intestinal ER stress by enhancing protein folding and degradation of misfolded proteins. J. Exp. Med. 2013;210:1201–1216. doi: 10.1084/jem.20121268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Sci. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanjul-Fernandez M, Folgueras AR, Cabrera S, Lopez-Otin C. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochimica et. biophysica acta. 2010;1803:3–19. doi: 10.1016/j.bbamcr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Hiroyasu S, Turner CT, Richardson KC, Granville DJ. Proteases in Pemphigoid Diseases. Front. Immunol. 2019;10:1454. doi: 10.3389/fimmu.2019.01454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaser A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanza A, et al. Deregulation of PERK in the autoimmune disease pemphigus vulgaris occurs via IgG-independent mechanisms. Br. J. dermatology. 2011;164:336–343. doi: 10.1111/j.1365-2133.2010.10084.x. [DOI] [PubMed] [Google Scholar]
- 27.Yoo SA, et al. A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J. Exp. Med. 2012;209:871–886. doi: 10.1084/jem.20111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue X, et al. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J. Biol. Chem. 2005;280:33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 30.Kieseier BC, et al. Matrix metalloproteinase-9 and -7 are regulated in experimental autoimmune encephalomyelitis. Brain: a J. Neurol. 1998;121(Pt 1):159–166. doi: 10.1093/brain/121.1.159. [DOI] [PubMed] [Google Scholar]
- 31.Perez, P. et al. Differential expression of matrix metalloproteinases in labial salivary glands of patients with primary Sjogren’s syndrome. Arthritis and rheumatism43, 2807–2817, https://doi.org/10.1002/1529-0131(200012)43:12<2807::AIDANR22>3.0.CO;2-M (2000). [DOI] [PubMed]
- 32.Arafat SN, et al. Neutrophil collagenase, gelatinase, and meloperoxidase in tears of patients with stevens-johnson syndrome and ocular cicatricial pemphigoid. Ophthalmol. 2014;121:79–87. doi: 10.1016/j.ophtha.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan MF, et al. Membrane array analysis of tear proteins in ocular cicatricial pemphigoid. Optometry Vis. science: Off. Publ. Am. Acad. Optometry. 2011;88:1005–1009. doi: 10.1097/OPX.0b013e31821ddc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holvoet S, Vincent C, Schmitt D, Serres M. The inhibition of MAPK pathway is correlated with down-regulation of MMP-9 secretion induced by TNF-alpha in human keratinocytes. Exp. Cell Res. 2003;290:108–119. doi: 10.1016/s0014-4827(03)00293-3. [DOI] [PubMed] [Google Scholar]
- 35.Darling NJ, Cook SJ. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochimica et. biophysica acta. 2014;1843:2150–2163. doi: 10.1016/j.bbamcr.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Argueso P, Gipson IK. Assessing mucin expression and function in human ocular surface epithelia in vivo and in vitro. Methods Mol. Biol. 2012;842:313–325. doi: 10.1007/978-1-61779-513-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodward AM, et al. Inflammatory Stress Causes N-Glycan Processing Deficiency in Ocular Autoimmune Disease. Am. J. Pathol. 2019;189:283–294. doi: 10.1016/j.ajpath.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Argueso P, et al. The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Investig. Ophthalmol. Vis. Sci. 2003;44:86–92. doi: 10.1167/iovs.02-0181. [DOI] [PubMed] [Google Scholar]
- 39.Taniguchi T, et al. N-Glycosylation affects the stability and barrier function of the MUC16 mucin. J. Biol. Chem. 2017;292:11079–11090. doi: 10.1074/jbc.M116.770123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mauris J, Woodward AM, Cao Z, Panjwani N, Argueso P. Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. J. Cell Sci. 2014;127:3141–3148. doi: 10.1242/jcs.148510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hrincius ER, et al. Acute Lung Injury Results from Innate Sensing of Viruses by an ER Stress Pathway. Cell Rep. 2015;11:1591–1603. doi: 10.1016/j.celrep.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


