Abstract
Stannylation of calcium carbide followed by Sn–hypervalent iodine(III) exchange reaction cleanly afforded the electrophilic ethynylating agent ethynyl(phenyl)-λ3-iodane in high yield. This two-step method uses very inexpensive materials and is readily operable without any special precautions.
Keywords: hypervalent, iodine, stannane, calcium carbide, ethynyl
Introduction
Hypervalent ethynyl(phenyl)-λ3-iodane 1 is an efficient electrophilic ethynylating agent for a variety of nucleophiles (C, N, O, P, As, S, Se, and halides) in the presence or absence of transition metal catalysts (Figure 1A; Ochiai et al., 1990; Stang et al., 1990; Varvoglis, 1992; Ochiai, 2003; Waser, 2016; Yoshimura and Zhdankin, 2016). However, its synthetic utility is restricted by its high cost and heat/moisture-sensitive character: it gradually decomposes at room temperature in air (Ochiai et al., 2003; Yudasaka et al., 2019). Therefore, an inexpensive, rapid, and facile preparation method of 1 has long been highly desired. The current approach to the synthesis of 1 relies on electrophilic Si/Sn–I(III) exchange reaction on acetylenic carbon atoms, and has remained essentially unchanged since the early days. In 1990, Stang and Ochiai independently reported pioneering approaches for the synthesis of 1. Stang et al. prepared ethynyl(phenyl)(triflato)-λ3-iodane (1b) from ethynyl(tributyl)stannane (3) and Tf2O-activated iodosylbenzene (2) (Figure 1B; Stang et al., 1990). On the other hand, a two-step procedure for the synthesis of 1a via [β-(trimethylsilyl)ethynyl](phenyl)-λ3-iodane 5a was developed by Ochiai and co-workers (Figure 1C; Ochiai et al., 1990). Then, in 2011, Kitamura reported another practical stepwise approach using PhI(OAc)2 (6) and bis(tert-butyldimethylsilyl)acetylene (4b) (Figure 1D; Kitamura et al., 2011). Although these methods provide short-step approaches to 1, they have several disadvantages from the viewpoint of cost/safety of reagents. In particular, 3 is still expensive and concentrated aqueous HF is notoriously toxic (Mckee et al., 2014). We report here a safe, low-cost, two-step method for the synthesis of 1a using calcium carbide CaC2 (7) as an ethynyl group source.
Figure 1.
(A) Electrophilic ethynylation of nucleophile with 1. (B) Stang's approach. (C) Ochiai's approach. (D) Kitamura's approach.
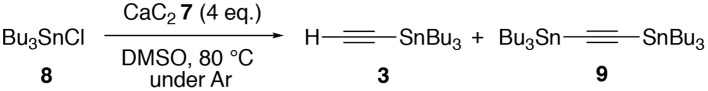
Calcium carbide CaC2 (7) is a widely utilized industrial material that is very inexpensive (0.05 $/g; 3.2 $/mol) (Sigma-Aldrich Co., LLC.). However, its synthetic use has been limited by its poor solubility: it is not soluble in non-reactive common organic solvents (Barber and Sloan, 1961). In recent decades, several approaches using strongly coordinating solvents (DMSO, DMF, etc.) and/or coordinating additives (F−, , HO−, water, etc.) have been reported, for which 7 served as a practical source (Rodygin et al., 2016). It occurred to us that the treatment of 7 with inexpensive chloro(tributyl)stannane (8) (0.3 $/g; 98 $/mol) (Sigma-Aldrich Co., LLC.), followed by Sn–I(III) exchange reaction, might provide more convenient and straightforward access to 1.
Materials and Methods
Calcium carbide (~80%), ethynyl(tributyl)stannane (95%), bis(tributylstannyl)acetylene (95%) were purchased from Sigma Aldrich and used as received. Chloro(tributyl)stannane (>97%), and boron trifluoride etherate (>98%) were purchased from TCI Japan and used as received. (Diacetoxyiodo)benzene (98+%) was purchased from FUJIFILM Wako Pure Chemical or prepared according to the literature (Watanabe et al., 2018). Potassium carbonate (>99.5%) was purchased from FUJIFILM Wako Pure Chemical and used as received. Iodosylbenzene was prepared from (diacetoxyiodo)benzene according to the literature (Sharefkin and Saltzman, 1963). Anhydrous grade of dimethyl sulfoxide, tetrahydrofuran, and N, N-dimethylformamide was purchased from Kanto Chemical and degassed by purging with argon and/or dried with a solvent purification system containing a one-meter column of activated alumina.
Characterization
NMR spectra were obtained on a Bruker AVANCE 500 spectrometer. Chemical shifts are expressed in δ (ppm) values. 1H NMR, 13C NMR, and 19F NMR spectra were referenced to tetramethylsilane (0 ppm), CHCl3 (7.26, 77.2 ppm), CHD2CN (1.94 ppm), and CD3CN (118.3 ppm), CFCl3 (0 ppm) as internal standards. IR spectra were obtained on a JASCO FT/IR-4700 spectrometer. Kieselgel 60 (Merck, 230-400 mesh) was used for column chromatography.
Synthesis of Ethynyl(Tributyl)Stannanes
These reactions were carried out in a two-necked round bottom flask. In a typical reaction: To a stirred suspension of well-ground calcium carbide (7) (2.48 g, 38.7 mmol) in DMSO (20 mL) were added chloro(tributyl)stannane (8) (3.26 g, 10.0 mmol) and water (0.40 mL, 22.2 mmol) at room temperature under argon. The resulting grayish suspension was warmed to 80°C for 1 h (the disappearance of 8 was monitored by GCMS analysis), then allowed to cool to room temperature. Hexane was added to it, and the organic phase was filtered under reduced pressure through a K2CO3-silica gel (1:9) mixture and transferred to a separating funnel. The combined organic phase was washed with water several times, then filtered, and the filtrate was concentrated under reduced pressure to give an oil, which was further purified by chromatography (ø5 mm) on a column packed with K2CO3-silica gel (1:9). Elution with hexane gave a pale yellow oil (2.51 g). 1H NMR analysis (mesitylene as an internal standard) showed the formation of a mixture of ethynyl(tributyl)stannane (3) (3.4 mmol, 34%) and bis(tributylstannyl)acetylene (9) (2.3 mmol, 45%). Capillary GC analysis (n-dodecane as an internal standard; Bruker BR-5ms column 0.25 mm × 30 m, 100°C) showed different yields of 3 (3.6 mmol, 36%) and 9 (1.60 mmol, 32%), probably reflecting partial decomposition of 9 during the GC analysis. This product mixture was used directly for the synthesis of 1a. Spectroscopic data of 3 and 9 were compared to the authentic samples synthesized according to the literatures (Supplementary Material).
Ethynyl(tributyl)stannane (3) (Stille and Simpson, 1987): a colorless oil; 1H NMR (CDCl3, 500 MHz) δ 2.20 (s, lH), 1.61–1.54 (m, 6H), 1.40–1.29 (m, 6H), 1.02 (t, J = 8.2 Hz, 6H), 0.91 (t, J = 7.3 Hz, 9H). 13C NMR (CDCl3, 125 MHz) δ 96.9, 89.1, 29.0, 27.1, 13.8, 11.2.
Bis(tributylstannyl)acetylene (9) (Brown and Eichler, 2011): a colorless oil; 1H NMR (CDCl3, 500 MHz) δ 1.69–1.48 (m, 12H), 1.40–1.29 (m, 12H), 1.13–0.94 (m, 12H), 0.90 (t, J = 7.3 Hz, 18H). 13C NMR (CDCl3, 125 MHz) δ 116.6, 29.1, 27.1, 13.8, 11.4.
The same procedure was adopted for other conditions shown in Table 1.
Table 1.
Stannylation of CaC2 7 with Bu3SnCl 8.
 | ||||
|---|---|---|---|---|
| Entry |
H2O (equiv) |
Time (h) |
Yield (%)a | |
| 3 | 9 | |||
| 1 | 2 | 1 | (20) | (43) |
| 2b | 2 | 1 | (34) [36]c | (45) [32]c |
| 3 | 2 | 6 | (10) | (67) |
| 4 | 2 | 28 | 0 | 72 |
| 5 | 0 | 28 | 0 | 65 |
| 6 | 12 | 3 | [1]c | [50]c |
Reactions were carried out on 0.3–1 mmol scale.
Isolated yields based on 8, numbers in parentheses are 1H NMR yields.
10 mmol scale.
GC yields.
General Procedure for Synthesis of Ethynyl-λ3-Iodane 1a From PhI(OAc)2 6
To a stirred solution of (diacetoxyiodo)benzene (6) (159 mg, 0.49 mmol) in dichloromethane (1 mL) was added BF3-Et2O (130 μL, 1.04 mmol) at −78°C, and then a 60:40 mixture of stannanes 3 and 9 (301 mg, 0.70 mmol) was slowly added. The reaction mixture was stirred at the same temperature for 1 h, then allowed to warm to room temperature, and the solvent was removed under reduced pressure. The resulting pale yellow solid was washed several times with hexane and Et2O at 0°C to give 1a (114 mg, 73%).
Ethynyl(phenyl)(tetrafluoroborato)-λ3-iodane (1a) (Ochiai et al., 1990): a white solid; IR (ATR-FTIR) ν 3,241, 3,080, 2,056, 1,480, 1,442, 1,170–840, 735, 672 cm−1; 1H NMR (CD3CN, 500 MHz) δ 8.18 (d, J = 8.5 Hz, 2H), 7.82 (t, J = 7.6 Hz, 1H), 7.64 (dd, J = 8.5, 7.6 Hz, 2H), 3.89 (s, 1H). 19F NMR (CD3CN, 470 MHz) δ −151.8 (s, 4F). 13C NMR (CDCl3, 125 MHz) δ 136.1, 134.6, 133.8, 116.5, 99.0, 26.7 (see also Supplementary Material).
General Procedure for Synthesis of Ethynyl-λ3-Iodane 1a From PhIO 2
To a stirred solution of iodosylbenzene (2) (77.6 mg, 0.35 mmol) in dichloromethane (0.7 mL) was added BF3-Et2O (100 μL, 0.77 mmol) at −78°C, and then a 38:62 mixture of stannanes 3 and 9 (218 mg, 0.46 mmol) was slowly added. The reaction mixture was stirred at the same temperature for 1 h, then allowed to warm to room temperature, and the solvent was removed under reduced pressure. The resulting pale brown solid was washed several times with hexane and Et2O at 0°C to give 1a (90.4 mg, 81%); 1H NMR analysis shows this product contained a small amount of impurities. 1H NMR yield: 67% (mesitylene as an internal standard).
Results and Discussion
We commenced our study by trapping CaC2 7 with 8. Exposure of well-ground 7 (4 equiv) to 8 in DMSO at room temperature did not give any alkynylstannanes. Addition of small amount of water (2 equiv), which has been reported to be effective for the electrophilic trapping of 7, was fruitless (Rodygin et al., 2016). On the other hand, heating at 80°C resulted in smooth consumption of 8 and after 1 h, a 6:4 mixture of 3 and bis(tributylstannyl)acetylene (9) was obtained in 79% yield (Table 1, entry 2). The ratio of 3 and 9 has changed in a range of ca. 3:7–6:4 through several runs, partly due to the reaction scale and the surface area of 7 (entries 1 and 2). Use of longer reaction time increased the ratio of 9 (entries 3 and 4). Under the conditions, the addition of water did not significantly change the yield of 9, but it accelerated the bis-stannylation (entries 4–6). Interestingly, this stannylation did not occur in other aprotic solvents such as THF and DMF, even at elevated temperatures (≤110°C) (Cochran et al., 1990). It should be noted that these alkynylstannanes 3 and 9 could be separated from other organostannane impurities on a short column packed with K2CO3-silica gel (1:9) mixture (Harrowven et al., 2010). Other crystallogen analog, trimethylsilyl chloride did not afford corresponding ethynyl(trimethyl)silanes under optimized conditions, partly because of the more moisture sensitive character of silyl chloride.
Next, we focused on the synthesis of ethynyl-λ3-iodane 1a using a mixture of alkynylstannanes 3 and 9. After screening various reaction conditions, we found an efficient method. Exposure of a 6:4 mixture of 3 and 9 (obtained from the reaction shown in entry 1 in Table 1) to a combination of PhI(OAc)2 6 and BF3-Et2O in dichloromethane at −78°C resulted in smooth Sn–I(III) exchange, and after 1 h, 1a was selectively obtained in 73% yield (Figure 2A). The standard PhIO 2–BF3-Et2O system also afforded 1a in high yield. It should be emphasized that these methods do not require time-consuming work-up. Simple washing of the reaction mixture with hexane and Et2O by decantation gave pure 1a and a mixture of Bu3SnX-type organostannanes thus formed by I(III)–Sn exchange was recovered quantitatively in the supernatant. As we expected, these optimized conditions could also be applied to authentic 3 and 9 individually to provide 1a in moderate to high yields (Figure 2B). In these cases, the combination of I(III)–organostannane pairs (6–3 and 2–9) gave better yields of 1a than opposite pairs (6–9 and 2–3), although the reason remains unclear. From a mechanistic point of view, our results using 9 is somewhat surprising since the Sn–I(III) exchange of 9 with cyano(trifluoromethylsulfonyloxy)iodobenzene (10) selectively affords bis[phenyl(triflato)-λ3-iodanyl]acetylene (11) (Figure 2C; Stang and Zhdankin, 1990, 1991). The moderately electrophilic nature of iodine center of the intermediates such as PhI(OAc)2-BF3 (Izquierdo et al., 2016) or PhIO-BF3 (Ochiai, 2007) might be partly responsible for the selective formation of 1a.
Figure 2.
Synthesis of ethynyl(phenyl)-λ3-iodane 1a. (A) Reaction of a mixture of alkynylstannanes 3 and 9 obtained from CaC2 7. (B) Individual reactions of authentic 3 and 9. (C) Stang and Zhdankin's approach.
Conclusion
In summary, we have developed a safe and inexpensive two-step method for the synthesis of 1a using readily available CaC2 7 as an ethynyl group source. This method not only provides time-/cost-/labor-saving methodology to prepare unstable ethynyl-λ3-iodane 1, but also serves as an effective approach for synthetically useful but costly bis(stannyl)acetylene 9 (Brend'amour et al., 2018).
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
KM and MU conceived and designed the experiments and wrote the manuscript. TH and TO conducted the experiments. All authors participated in data analyses and discussions.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by grants from JSPS KAKENHI (S) (17H06173), KAKENHI (B) (17H03017), NAGASE Science & Technology Development Foundation, and Sumitomo Foundation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2020.00012/full#supplementary-material
References
- Barber W. A., Sloan C. L. (1961). Solubility of calcium carbide in fused salt system. J. Phys. Chem. 65, 2026–2028. 10.1021/j100828a025 [DOI] [Google Scholar]
- Brend'amour S., Gilmer J., Bolte M., Lerner H. W., Wagner M. (2018). C-halogenated 9,10-diboraanthracenes: how the halogen load and distribution influences key optoelectronic properties. Chem. Eur. J. 24, 16910–16915. 10.1002/chem.201804288 [DOI] [PubMed] [Google Scholar]
- Brown A. E., Eichler B. E. (2011). Symmetric diarylacetylenes: one-pot syntheses and solution photoluminescence. Tetrahedron Lett. 52, 1960–1963. 10.1016/j.tetlet.2011.02.062 [DOI] [Google Scholar]
- Cochran J. C., Lemieux R. P., Giacobbe R. C., Roitstein A. (1990). Synthesis of bis-stannylacetylenes from calcium carbide; assisted by ultrasound. Synth. React. Inorg. Met. Org. Chem. 20, 251–261. 10.1080/00945719008048132 [DOI] [Google Scholar]
- Harrowven D. C., Curran D. P., Kostiuk S. L., Wallis-Guy I. L., Whiting S., Stenning K. J., et al. (2010). Potassium carbonate–silica: a highly effective stationary phase for the chromatographic removal of organotin impurities. Chem. Commun. 46, 6335–6337. 10.1039/C0CC01328E [DOI] [PubMed] [Google Scholar]
- Izquierdo S., Essafi S., del Rosal I., Vidossich P., Pleixats R., Vallribera A., et al. (2016). Acid activation in phenyliodine dicarboxylates: direct observation, structures, and implications. J. Am. Chem. Soc. 138, 12747–12750. 10.1021/jacs.6b07999 [DOI] [PubMed] [Google Scholar]
- Kitamura T., Morshed M. H., Tsukada S., Miyazaki Y., Iguchi N., Inoue D. (2011). Alkynylation of benzotriazole with silylethynyliodonium triflates. Regioselective synthesis of 2-ethynyl-2H-benzotriazole derivatives. J. Org. Chem. 76, 8117–8120. 10.1021/jo2015467 [DOI] [PubMed] [Google Scholar]
- Mckee D., Thoma A., Bailey K., Fish J. (2014). A review of hydrofluoric acid burn management. Plast. Surg. 22, 95–98. [PMC free article] [PubMed] [Google Scholar]
- Ochiai M. (2003). Reactivities, properties and structures, Topic in Current Chemistry, Vol. 224, ed Wirth T. (Cham: Springer; ), 5–68. [Google Scholar]
- Ochiai M. (2007). Stoichiometric and catalytic oxidations with hypervalent organo-λ3-iodanes. Chem. Rec. 7, 12–23. 10.1002/tcr.20104 [DOI] [PubMed] [Google Scholar]
- Ochiai M., Ito T., Takaoka Y., Masaki Y., Kunishima M., Tani S., et al. (1990). Synthesis of ethynyl(phenyl)iodonium tetrafluoroborate. A new reagent for ethynylation of 1,3-dicarbonyl compounds. J. Chem. Soc. Chem. Commun. 1990, 118–119. 10.1039/C39900000118 [DOI] [Google Scholar]
- Ochiai M., Miyamoto K., Suefuji T., Sakamoto S., Yamaguchi K., Shiro M. (2003). Synthesis, characterization, and reaction of ethynyl(phenyl)-λ3-iodane complex with [18]crown-6. Angew. Chem. Int. Ed. 42, 2191–2194. 10.1002/anie.200250866 [DOI] [PubMed] [Google Scholar]
- Rodygin K. S., Werner G., Kucherov F. A., Ananikov V. P. (2016). Calcium carbide: a unique reagent for organic synthesis and nanotechnology. Chem. Asian J. 11, 965–976. 10.1002/asia.201501323 [DOI] [PubMed] [Google Scholar]
- Sharefkin J. G., Saltzman H. (1963). Iodosobenzene. Org. Synth. 43:60 10.15227/orgsyn.043.0060 [DOI] [Google Scholar]
- Stang P. J., Arif A. M., Crittell C. M. (1990). Ethynyl(phenyl)iodonium triflate, [HCCIPh][OSO2CF3]: preparation, spectral properties, mechanism of formation and X-ray molecular structure. Angew. Chem. Int. Ed. Engl. 29, 287–288. 10.1002/anie.199002871 [DOI] [Google Scholar]
- Stang P. J., Zhdankin V. V. (1990). Bis[phenyl[(perfluoroalkanesulfonyl)oxy]iodo]acetylene, PhI+CCI+Ph•2RF, and 1,4-bis[phenyl[(perfluoroalkanesulfonyl)oxy]iodo]-1,3-butadiyne, PhI+CC-CCI+Ph•2 RF. J. Am. Chem. Soc. 112, 6437–6438. 10.1021/ja00173a064 [DOI] [Google Scholar]
- Stang P. J., Zhdankin V. V. (1991). Preparation and chemistry of PhI+CCI+Ph•2−OTf, bis[phenyl[[(trifluoromethyl)sulfonyl]oxy]iodo]acetylene, a novel difunctional acetylene, bis(iodonium) species and a stable C2-transfer agent. J. Am. Chem. Soc. 113, 4571–4576. 10.1021/ja00012a028 [DOI] [Google Scholar]
- Stille J. K., Simpson J. H. (1987). Stereospecific palladium-catalyzed coupling reactions of vinyl iodides with acetylenic tin reagents. J. Am. Chem. Soc. 109, 2138–2152. 10.1021/ja00241a035 [DOI] [Google Scholar]
- Varvoglis A. (1992). The Organic Chemistry of Polycoordinated Iodine. New York, NY: VCH. [Google Scholar]
- Waser J. (2016). Alkynylation with hypervalent iodine reagents, Topic in Current Chemistry, Vol. 373, ed Wirth T. (Cham: Springer; ), 187–222. [DOI] [PubMed] [Google Scholar]
- Watanabe A., Miyamoto K., Okada T., Asawa T., Uchiyama M. (2018). Safer synthesis of (diacetoxyiodo)arenes using sodium hypochlorite pentahydrate. J. Org. Chem. 83, 14262–14268. 10.1021/acs.joc.8b02541 [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Zhdankin V. V. (2016). Advances in synthetic applications of hypervalent iodine compounds. Chem. Rev. 116, 3328–3435. [DOI] [PubMed] [Google Scholar]
- Yudasaka M., Shimbo D., Maruyama T., Tada N., Itoh A. (2019). Synthesis, characterization, and reactivity of an ethynyl benziodoxolone (EBX)–acetonitrile complex. Org. Lett. 21, 1098–1102. 10.1021/acs.orglett.9b00005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.




