Abstract
Paulownia species are important ecological, economic and ornamental species, but their phylogenetic relationship remains unclear, which seriously affects the development and utilization of these important resources. The complete chloroplast genomes of six Paulownia species were assembled by next-generation sequencing data. By adding two known Paulownia chloroplast genomes to these six assembled genomes, we performed the comparative analysis and phylogenetic tree reconstruction of Paulownia. The results indicated that the chloroplast genomes of Paulownia species ranged in size from 154,107 to 154,694 bp. These chloroplast genomes contained 117 unique functional genes, including 80 protein-coding genes, four rRNA genes, and 33 tRNA genes. Twelve hotspot regions, five protein-coding genes and seven noncoding regions, were identified in the chloroplast genomes that showed high levels of sequence variation. Additionally, positive selection was observed in three genes, rps2, rbcL and ndhG. The maximum likelihood (ML) and Bayesian (BI) analysis strongly supported the monophyletic origin of Paulownia species, which clustered into two major clades: One clade included P. coreana, P. tomentosa and P. kawakamii, while the other clade comprised the 5 other species including P. fargesii and P. australis. This study provides useful genetic information for phylogenetic reconstruction, taxonomic discrepancies, and studying species evolution and phylogeography in Paulownia.
Subject terms: Ecology, Evolution, Molecular biology, Plant sciences
Introduction
Paulownia is a general term for plants from the genus Paulownia, which includes a total of eight species, P. coreana, P. tomentosa, P. kawakamii, P. fargesii, P. australis, P. fortunei, P. elongata and P. catalpifolia1,2. Paulownia originated in China and has a long history of cultivation in China. To date, it has been introduced in Japan, Australia, Brazil, Europe and the United States3,4. Paulownia is a fast-growing tree, and its wood has a series of excellent characteristics, such as its light weight and lack of splitting and deformation and its moisture-proof, sound-insulating, fire-resistance, and corrosion-resistance properties, which enable its use in building materials, furniture, agricultural tools, handicrafts, cultural articles and musical instruments5–7. In addition, Paulownia flowers, leaves, fruits, and bark can also be used as medicine, with anti-inflammatory, cough-relieving, diuretic, and antihypertensive effects8. Moreover, Paulownia is also an ornamental plant with lush inflorescence and various flower colors and is often used as a street tree9. In short, Paulownia is an important ecological, economic and ornamental tree with a wide range of uses.
Researchers have studied the genetic relationship among Paulownia species based on morphological characteristics and variations in DNA information, but their inferences on the evolutionary relationship of Paulownia species were affected by unstable morphological characteristics or insufficient genetic information, leading to significant differences between research results10–12. The unclear phylogenetic relationship of Paulownia species, especially the uncertainty of the parental source of the hybrid species, has seriously affected the further development of these important resources and hindered the progress of Paulownia breeding. Therefore, it is necessary to clarify the evolutionary relationship of Paulownia species in the current forest practice of Paulownia.
In most angiosperms, chloroplast DNAs are maternally inherited and do not recombine, making them suitable for the analysis of phylogenetic relationships among species, especially related species13–15. The evolution rate or genetic diversity of different regions of the chloroplast genome varies greatly, and the successful development of common primers in those high-variability regions make these loci widely used in the study of phylogenetic relationships among species; among these loci, matK and rbcL are most commonly used16,17. With the application of high-throughput sequencing technology, the cost of sequencing has been greatly reduced, which makes it possible to reveal the phylogenetic relationships among species by using the genetic information of the whole chloroplast genome in many plant groups15,18–20. The abundant genetic variation information and maternal genetic characteristics of the chloroplast genome are particularly suitable for reconstructing the phylogeny of low-level taxonomic hierarchies with complex relationships. Based on 1564 single-nucleotide variants in the chloroplast genome, Carbbonell-Caballero et al. constructed highly credible phylogenetic trees for wild and cultivated citrus, and Wambugu et al. also used chloroplast genome data to construct a clear pedigree relationship among wild rice species and cultivars21,22.
In the long process of coevolution, most of the genes of the chloroplast genome have been transferred to the nuclear genome23, but approximately 120 genes remain in the chloroplast genome and participate in the physiological processes of chloroplast photosynthesis, transcription and translation, making the chloroplast a semiautonomous organelle15. In the long process of evolution, some chloroplast genes underwent adaptive selection to the environment24,25. This study applied high-throughput sequencing technology to assemble chloroplast genomes of six Paulownia species to explore the following topics: The genetic diversity of the chloroplast genomes of Paulownia; the hypervariable regions of the chloroplast genomes of Paulownia; the loci in the chloroplast genome involved in adaptive selection during the evolution of Paulownia; and the phylogenetic relationships of the eight Paulownia species.
Results and Analysis
Molecular features of the chloroplast genomes
The chloroplast genome lengths of six Paulownia species ranged in size from 154,107 bp for P. kawakamii to 154,694 bp for P. catalpifolia (Fig. 1 and Table 1). As in most land plants, the six Paulownia plastid genomes exhibited a typical quadripartite structure consisting of a large single-copy region (LSC; 84,807–85,420 bp) and a small single-copy region (SSC; 17,731–17,740 bp) separated by two inverted repeats (IRs; 51,540–51,560 bp). These genomes had similar GC contents, with values from 37.96% to 37.99%, consistent with other previously reported Paulownia chloroplast genomes26. In addition, the gene content, order, and orientation were identical in the chloroplast genomes of the six Paulownia species. They contained 117 unique functional genes, including 80 protein-coding genes, four rRNA genes, and 33 tRNA genes (Supplementary Table 1). Among these genes, 17 genes were duplicated, with six protein-coding genes, four rRNAs, and seven tRNAs. In addition, seventeen of the genes contained one or two introns (Supplementary Table 1).
Figure 1.
Map of the chloroplast genome of the six Paulownia species. Genes drawn inside the circle are transcribed clockwise, and those outside the circle are transcribed counterclockwise. The dashed gray area in the inner circle shows the percent GC content of the corresponding genes. LSC, SSC, and IR denote large single copy, small single copy, and inverted repeats, respectively. The circular map of the chloroplast genome was drawn using OGDRAW 1.3.1 (http://ogdraw.mpimp-golm.mpg.de/).
Table 1.
Comparison of the chloroplast genome features of the eight Paulownia species.
| Species | P. elongata | P. australis | P. kawakamii | P. fargesii | P. catalpifolia | P. fortunei | P. coreana | P. tomentosa |
|---|---|---|---|---|---|---|---|---|
| Accession number | MK618176 | MK618177 | MK618178 | MK618179 | MK618180 | MK618181 | KP718622 | KP718624 |
| Total chloroplast genome size (bp) | 154,688 | 154,247 | 154,107 | 154,692 | 154,694 | 154,676 | 154,545 | 154,540 |
| LSC (bp) | 85,415 | 84,972 | 84,807 | 85,418 | 85,420 | 85,400 | 85,241 | 85,236 |
| IR (bp) | 51,540 | 51,544 | 51,560 | 51,540 | 51,540 | 51,540 | 51,568 | 51,568 |
| SSC (bp) | 17,733 | 17,731 | 17,740 | 17,734 | 17,734 | 17,736 | 17,736 | 17,736 |
| Total number of genes | 134 | 134 | 134 | 134 | 134 | 134 | 134 | 134 |
| Protein-coding genes | 86 | 86 | 86 | 86 | 86 | 86 | 86 | 86 |
| rRNAs | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| tRNAs | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| GC content (%) | 37.97% | 37.99% | 37.99% | 37.96% | 37.97% | 37.97% | 38.00% | 38.00% |
Sequence variation
A total of 216 single-nucleotide polymorphisms (SNPs) were detected among the chloroplast genomes of the eight Paulownia species, of which 97 were base transitions, accounting for 44.9% of the total base mutations, and the remaining 119 mutations were base transversions, accounting for 55.1% of the total mutations (Table 2). Of these mutations, T-A was the most common mutation with 61 occurrences, followed by C-T and G-A with 49 and 48 occurrences, respectively; C-G was the least common with only five occurrences.
Table 2.
Nucleotide mutation type.
| Nucleotide mutation | Number of mutations | |
|---|---|---|
| Conversion | A-G | 48 |
| T-C | 49 | |
| Transversion | A-C | 29 |
| A-T | 61 | |
| T-G | 24 | |
| C-G | 5 | |
| Total | 216 |
Sequence divergence analysis indicated that there was a low level of nucleotide diversity (Pi = 0.00066) across the eight Paulownia species. IR regions were the most conserved feature, with the lowest Pi value of 0.00012; the SSC region had relatively high sequence variation, with a Pi value of 0.00106; and the LSC region showed a medium Pi value of 0.00089 (Table 3). In addition, the Pi value (0.00032) in the coding regions was lower than that (Pi = 0.00102) in the noncoding regions (Table 3). In the coding regions, the greatest variability was detected in the genes rps12 and rpl36 with Pi values of 0.0047; other genes with Pi values above 0.00200 were rps11, rpl16 and ycf3 (Fig. 2). In the noncoding regions, seven regions (ccsA-ndhD, trnG-trnfM, psbT-psbN, trnR-atpA, psbM-trnD, rps14-psaB, and trnH-psbA) showed high levels of sequence variation (Pi ≥ 0.00731); among them, the region ccsA-ndhD had the highest Pi (0.02644) (Fig. 3). These hotspot regions could be used as potential markers for species identification and molecular breeding within this genus in the future.
Table 3.
The values of nucleotide diversity (Pi) in different regions among the eight Paulownia species.
| Structural region | Noncoding region | Coding region | |||
|---|---|---|---|---|---|
| LSC | IRa | SSC | |||
| Total number of sites | 83,984 | 25,763 | 17,716 | 75,487 | 79,429 |
| Number of polymorphic sites | 165 | 6 | 38 | 161 | 55 |
| Pi values | 0.00089 | 0.00012 | 0.00106 | 0.00102 | 0.00032 |
| Theta-W | 0.00076 | 0.00009 | 0.00083 | 0.00084 | 0.00027 |
Figure 2.
The nucleotide diversity of coding regions among the eight Paulownia species.
Figure 3.
The nucleotide diversity of nocoding regions among the eight Paulownia species.
Repeat sequence variation
Among the chloroplast genomes of the eight Paulownia species, seven simple sequence repeats (SSR) types were identified, mononucleotide, dinucleotide, trinucleotide, tetranucleotide, pentanucleotide, hexanucleotide and compound (Fig. 4a). The eight Paulownia species contained similar numbers of SSRs. The maximum number was 70 in P. coreana, and the minimum was 65 in P. elongata, P. australis, P. catalpifolia and P. fortunei. The mononucleotide repeat was the most common type of microsatellite, and tetranucleotide motifs were the second most abundant in the Paulownia plastomes. For example, in P. tomentosa, there were 69 SSRs, of which 52 were mononucleotide repeats with a ratio of 75%, seven were tetranucleotide repeats, five were dinucleotide repeats, three were trinucleotide repeats. A/T repeats were the most common mononucleotides with ratios ranging from 93.9% to 96.2% in the eight Paulownia species, while AT/TA repeats were the most abundant dinucleotide with ratios of 100%; other SSR types had only one to two copies (Supplementary Table 2). Most SSR loci were located in the LSC region. Among the eight Paulownia species, 49 to 55 SSR loci were in the LSC region, and only six and ten (or nine) SSRs were distributed in the IR and SSC regions, respectively (Fig. 4b).
Figure 4.
Analyses of repeated sequences in the chloroplast genomes of the eight Paulownia species. (A) The number of repeats in the eight Paulownia chloroplast genomes. p1: Mono. p2: Di. p3: Tri. p4: Tetra. p5: Penta. p6: Hexa. c: Imperfect repeat. (B) Distribution of SSRs in the eight Paulownia chloroplast genomes.
Positive selection analysis
To determine which genes in the chloroplast genome of Paulownia were involved in adaptive evolution, we conducted a neutral test of protein-coding genes with genetic variation by calculating the ratio (dN/dS) of the nonsynonymous to synonymous substitution. The results indicated that 3 protein-coding genes were subject to positive selection (dN/dS > 1). These genes under positive selection exhibited functional diversity, including one NADPH dehydrogenase subunit gene (ndhG), one ribosomal protein gene (rps2) and one RuBisCO gene (rbcL).
Phylogenetic relationships of the eight Paulownia species
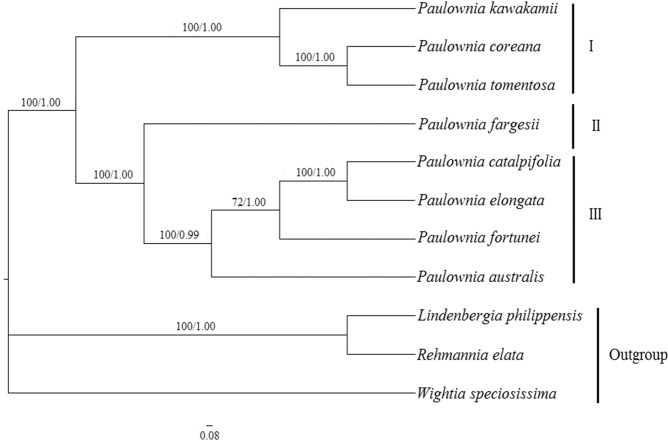
The robust phylogenetic relationships of Paulownia species were reconstructed based on entire chloroplast genome sequences with three positively selected genes being removed using Wightia speciosissima, Rehmannia elata and Lindenbergia philippensis as outgroups (Fig. 5). The maximum likelihood (ML) and Bayesian (BI) analysis strongly suggested that the eight Paulownia species formed a monophyletic group, and these species clustered into two major clades, a small clade and a large clade, with 100% bootstrap values and 1.00 posterior probabilities, respectively. The small clade (I) included P. coreana, P. tomentosa and P. kawakamii, in which the closely related P. coreana and P. tomentosa form a sister branch with P. kawakamii. In another clade, P. fargesii was the earliest species to diverge, forming one subclade (II). The remaining four species clustered into the second subclade (III) with high bootstrap support, in which P. australis was sister to the combined clade of the other three species. In the combined clade, P. elongata was most closely related to P. catalpifolia.
Figure 5.
Phylogenetic tree based on whole plastomes with three positively selected genes being removed. The numbers to the left of the slashes on the braches show the bootstrap values obtained by maximum likelihood analyses, and those to the right show the posterior probabilities according to Bayesian inference.
Discussion
Genetic diversity of the chloroplast genome in different plant groups
Because the divergent time and the rate of chloroplast genome evolution are different in different plant groups, the genetic diversity of chloroplast genome in these plant groups is quite different. According to morphological and ecological data combined with geological records, the following results were obtained: In the early tertiary period, there was only one species of Paulownia, and it was divided into two primitive species of Paulownia, P. kawakamii and P. tomentosa, in the Miocene period. Later, other species of Paulownia were formed through evolution and hybridization27. Our results showed that the nucleotide polymorphism (Pi value) of the Paulownia chloroplast genomes was only 0.00066, which is significantly lower than that of many other groups. The Pi value of the chloroplast genome of 5 Rosa species was 0.00154, with a nucleotide polymorphism 3 times that of the genus Paulownia28. The average Pi value of the chloroplast genome of 6 species of Ipomoea was 0.0045, nearly 10 times the Pi value of the chloroplast genome of Paulownia29; the chloroplast genome of Aristolochia has a higher nucleotide polymorphism than the chloroplast genome of Paulownia, and its Pi was 0.01717, which is 31 times that of Paulownia20.
Genetic diversity in different chloroplast regions
The genetic polymorphisms in different regions of the chloroplast genome vary substantially. In general, the single copy (SC) regions (containing LSC and SSC regions) of the chloroplast genome have higher genetic diversity than the IR regions in most plant groups30. In our study, the Pi values of SSC and LSC in the Paulownia chloroplast genomes were 0.00104 and 0.00089, respectively, both of which were significantly higher than the Pi value (0.00012) in the IR regions. Similar results were also found in other plant groups. The Pi values of LSC and SSC in the chloroplast genomes of Aristolochia were 0.02182 and 0.03114, respectively, which were also much higher than the Pi value (0.00411) in the IR regions20. The difference in genetic diversity among regions of the chloroplast genome also appeared at the family level. The IR regions of Apiaceae species were far more conserved than the SC regions, with an average Pi value of 0.002 for the former and 0.009 for the latter31. The percentage of nucleotide variation in the SC sequences (12.7%) was also higher than that in IRs (4.14%) in the chloroplast genomes of 6 Adoxaceae species32. However, the opposite was found in some groups. For example, in Caprifoliaceae chloroplast genomes, the percentage of nucleotide variation in the SC regions (17.61%) was slightly lower than that in the IR regions (21.25%)32.
The coding region of the chloroplast genome is more conserved due to its functional limitations; therefore, the genetic diversity of the coding region is lower than that of the noncoding region. The genetic diversity (Pi = 0.00102) of the noncoding region was significantly higher than that of the coding region (Pi = 0.00033) in Paulownia chloroplast genomes, which was consistent with the results of other groups with ratio differences. The genetic polymorphism of the noncoding region was 3.1 times that of the coding region in Paulownia, 3.9 times that in six Adoxaceae species, 3.5 times that in eight Caprifoliaceae species and 2.4 times that in six Ipomoea species29,32. Because of their abundant nucleotide variations, which can provide rich genetic information, noncoding regions are often employed to analyze the phylogenetic relationship of species and probe into plant evolution and colonization33–35. Many studies have shown that genetic diversities also differ greatly among noncoding regions of the chloroplast genome, and the regions with the greatest variation are usually called hotspot regions20. In different plant groups, hotspot regions vary. Dong et al. compared the chloroplast genomes of 29 plant species from 12 genera and identified 19 noncoding regions with high variability, of which pl32-rnL and trnH-psbA had the highest genetic variation14. The most variable noncoding regions included trnH-GUG-psbA, trnR-UCU-atpA, trnC-GCA-petN, ycf3-trnS-GGA, and trnL-UAA-trnF-GAA in six Adoxaceae chloroplast genomes32; and TrnN-GUU-ndhF is the hotspot region in Capsicum36. The regions with the highest percentage of sequence variation were ccs-trnL-UAG, psbI-trnS-GCU, rpl32-ndhF, trnT-UGU-TrnL-UAA and petN-psbM in Echinacea19. In three closely related East Asian wild roses, matK-trnK, psbI-trnS-trnG, rps16-trnG, rpoB-trnC and rps4-trnT were the most divergent intergenic regions, with Pi values exceeding 0.00628.
There were also significant differences in the degree of variation among chloroplast protein-coding regions. Some coding regions show high variability in most plant groups, such as ycf1, nahF, rbcL, and matK, which are often used for barcoding14. Other coding regions show high polymorphism only in some groups, such as trnK, rpl22, ndhI, clpP, and rps1614,32. In the chloroplast genomes of Paulownia, the high-polymorphism coding regions included rpl36, rps12, rps11, rpl16, and ycf3, most of which are genes that encode ribosomal proteins.
In short, although many universal primers for chloroplast DNA have been used, the overall variation in the chloroplast genome of target groups should be detected before selecting certain DNA fragments for further research because of the difference in hotspot regions in different plant groups. The hypervariable loci found in Paulownia in this study, including coding regions and noncoding regions, can provide abundant variation information, which can be used to identify Paulownia species and study species differentiation, population genetics and phylogeography.
Gene selective analysis
Chloroplasts are organelles that carry out photosynthesis in green plants and are the most abundant energy converters on earth. Some enzymes and structural proteins within chloroplasts are encoded by genes of chloroplast genomes24. During chloroplast genome evolution, most genes were subjected to purifying selection due to functional limitations; some of these genes were involved in adaptation to the environment and underwent positive selection, while others were under neutral evolution. By calculating the ratio of dN to dS (dN/dN) for the coding genes with genetic variation, we identified 3 genes (rps2, rbcL and ndhG) under positive selection in the chloroplast genomes of Paulownia, and each of three selected genes performed different physiological functions. A few genes undergoing positive selection also occurred in some other plant groups. Five plastid genes (rbcL, clpP, atpF, ycf1 and ycf2) were subject to positive selection in 7 Panax species30, and only three chloroplast genes (clpP, ycf1 and ycf2) underwent positive selection in the chloroplast genomes of seven Sileneae species37. In many other groups, multiple chloroplast genes show a positive selection effect. One-third of the chloroplast genes in PACMAD grasses, 27 genes in the genus Iodes, 19 genes in Dipsacales species, and 10 genes in Gossypium evolved under positive selection25,32,38,39. Those identified selected genes may be underwent certain functional diversification during their evolutionary history.
Among the selected chloroplast genes in Paulownia, the rbcL gene encodes the large subunit of RuBisCO, which plays an important role in plant photosynthesis. Previous studies showed that rbcL is often under positive selection because of being the target of selection in relation to the changes in temperature, drought and carbon dioxide concentration24,30,32,39. So, the rbcL gene could be a positively selected site during the evolutionary process of Paulownia. The ndhG gene is another selected gene in Paulownia. In higher plants, chloroplast NAD(P)H dehydrogenase can protect plants from photoinhibition or photooxidation stress caused by strong light and alleviate the decrease in the photosynthetic rate and growth delay caused by drought40,41. This enzyme has important functions and is composed of many subunits. Due to adaptations to the environment, some of the genes encoding these subunits (ndh) are involved in adaptive evolution and exhibit positive selection25,38,42. For example, in Australian Citrus, ndhF exhibited a positive selection effect for its involvement in the adaptation to hot and dry climates21,43, and ndhG were also subjected to positive selection in Iodes38. The positive selection signal of ndhG in the Paulownia genus might be the result of adaptation to different environments because the climate of the growth areas of different Paulownia species is different.
Phylogenetic relationships of Paulownia species
Due to the frequent hybridization among Paulownia species, there is a general genetic introgression among these species, which leads to a complex phylogenetic relationship of Paulownia species11. Although the phylogenetic relationships of Paulownia species have been investigated based on morphological, structural, physiological, biochemical and genetic information, a reliable phylogenetic tree for Paulownia species has not been established. Using the complete chloroplast genome information, we constructed a highly reliable pedigree tree of Paulownia. In our study, the Paulownia genus was of monophyletic origin, and its eight species clustered into two clades. P. coreana, P. tomentosa and P. kawakamii formed one clade, while the five other species of the genus formed another clade. Our results were generally consistent with those obtained based on the morphological traits of Paulownia. Fan selected 22 independent traits to conduct comparative analysis of Paulownia species9. According to the Q cluster of these morphological traits, he concluded that P. elongata, P. catalpifolia and P. fortunei were clustered together, forming a white flower Paulownia group with other species, while P. tomentosa and P. kawakamii were included in another Paulownia group. In addition, some of our results are also supported by studies based on molecular data. For example, by analyzing random amplified polymorphic DNA (RAPD) data, Lu et al. categorized P. fargesii, P. australis, P. catalpifolia and P. fortunei into one group44.
The systematic positions of P. fargesii and P. australis have always been the most controversial issue. Fan’s study indicated that P. fargesii, P. tomentosa and P. kawakamii clustered into one clade, while P. australis formed a separate clade9. The phylogenetic relationship established by Mo based on inter simple sequence repeat (ISSR) data suggested that P. fargesii and P. australis were also divided into two different groups10. However, according to morphological traits, Xiong et al. proposed that P. fargesii and P. australis were closely related to P. tomentosa and P. kawakamii, and all four species were classified into one group45. In our study, P. fargesii and P. australis form a large clade together with P. elongata, P. catalpifolia and P. fortunei with high bootstrap support.
Based on the above analysis, it is very clear that, in the Paulownia genus, P. coreana, P. tomentosa and P. kawakamii form an evolutionary branch, while P. fortunei, P. elongata and P. catalpifolia are involved in forming another branch. In addition, in our study, the most controversial systematic positions of P. fargesii and P. australis have been well resolved.
Materials and Methods
Extraction, genome sequencing and assembly of the plant materials
Fresh leaves of six Paulownia species, P. kawakamii, P. fargesii, P. australis, P. fortunei, P. elongata and P. catalpifolia, were collected from different provinces in China (Supplementary Table 3). The genomic DNA, which was extracted by using the modified CTAB method, was used to construct a library with an inserted fragment ~270 bp in size and was sequenced according to the strategy of 150 bp paired-end reads on the Illumina HiSeq. 2500 platform. The sequencing depth was 20 × . After that, six genomic libraries were established. Clean data, which were obtained after filtering raw data, were assembled by SOAPdenovo_v2.04 (http://soap.genomics.org.cn/soapdenovo.html)46 according to the chloroplast genome sequence of P. tomentosa (KP718624). The optimal assembly results were obtained after adjusting the multiple parameters repeatedly, and then GapCloser_v1.12: (http://soap.genomics.org.cn/soapdenovo.html) was used to fill gaps. The boundaries of LSC-IR and SSC-IR were validated using PCR-based sequencing. Primers were designed by Primer Premier 5.0. The complete chloroplast genome sequences of the six species were deposited in GenBank, accession numbers are MK618176- MK618181 (Table 1).
Genome annotation
The protein-coding sequences and noncoding RNAs of the complete chloroplast genome were predicted using the software DOGMA (http://dogma.ccbb.utexas.edu/)47 and revised based on the referential chloroplast genome of P. tomentosa and the start and stop codons.
The coding genes were homologously aligned using BLAST48 in different databases, including NR (http://www.ncbi.nlm.nih.gov/), KEGG (http://www.genome.jp/kegg/), COG (http://www.ncbi.nlm.nih.gov/COG/), GO (http://geneontology.org/) and Swiss-Prot (http://www.ebi.ac.uk/uniprot/) databases for functional annotation. Finally, the circular maps of the six chloroplast genomes were drawn using OGDRAW 1.3.1(http://ogdraw.mpimp-golm.mpg.de/)49.
Comparative analysis
We compared the whole chloroplast genome sequences of Paulownia species using Geneious, and then DnaSP version 5.150 was used to calculate the Pi value, the SNP sites in the eight Paulownia chloroplast genomes and the nucleotide substitutions in the coding regions of the eight Paulownia genomes. The values of dN and dS for each protein-coding exon with genetic variation were calculated using the codeml package (seqtype = 1, model = 0) in PAMLX51. The SSRs in the eight Paulownia chloroplast genomes were identified using MISA with the parameters set to ten repeat units for mononucleotide SSRs, five repeat units for dinucleotide, four repeat units for trinucleotide, and three repeat units for tetranucleotide, pentanucleotide, and hexanucleotide SSRs. The imperfect repeat sequences were limited to interruptions between 2 SSRs that did not exceed 10 bp.
Phylogenetic analysis
The complete chloroplast genomes of the eight Paulownia species, W. speciosissima, R. elata (NC_034312) and L. philippensis (NC_022859) were aligned by MAFFT in Geneious V.9.152. The complete chloroplast genome sequences of W. speciosissima, R. elata and L. philippensis were included as the outgroups downloaded from NCBI. Phylogenetic trees were constructed by maximum like lihood (ML) and Bayesian analysis (BI) methods using chloroplast genome sequences with positively selected genes being removed. ML analyses were performed using RAxML-HPC BlackBox v.8.2.10 with the GTR + G model and 1,000 bootstrap replicates with the CIPRES Science Gateway website53,54. BI was performed with MrBayes 3.2.655 with the following settings: Markov chain Monte Carlo simulations for 1,000,000 generations with four incrementally heated chains, starting from random trees and sampling one out of every 1,000 generations. The first 25% of the trees were regarded as burn-ins. The ML tree and BI tree were visualized using FigTree version 1.4.256.
Supplementary information
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (Grant U1404302) and the Natural Science Foundation of Henan (Grant 162300410146). We thank H.-Z.S., Y.-Y.L. and Y.-N.C. for their assistance in data analysis.
Author contributions
H.-W.W. and Y.-Q.C. designed the study. P.-P.L. and G.-L.L. performed the experiments. P.-P.L., B.Z., X.-R.C., and G.-L.L. conducted data analyses. H.-W.W., P.-P.L., and Y.-Q.C. wrote and revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yueqin Cheng, Email: Chengyq126@126.com.
Hongwei Wang, Email: whwcas@163.com.
Supplementary information
is available for this paper at 10.1038/s41598-020-59204-y.
References
- 1.Olmstead R, et al. Disintegration of the Scrophulariaceae. Am. J. Bot. 2001;88:348–361. doi: 10.2307/2657024. [DOI] [PubMed] [Google Scholar]
- 2.The Angiosperm Phylogeny Group (APG). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016;181:1–20. doi: 10.1111/boj.12385. [DOI] [Google Scholar]
- 3.Peng HF, Fan GQ, Ye YZ. Study on the relationship of Paulownia genus plants. Henan Sci. 1999;17:23–27. doi: 10.13537/j.issn.1004-3918.1999.s1.008. [DOI] [Google Scholar]
- 4.Yaycili O, Alikamanoglu S. The effect of magnetic field on Paulownia tissue cultures. Plant Cell Tiss. Org. 2005;83:109–114. doi: 10.1007/s11240-005-4852-0. [DOI] [Google Scholar]
- 5.Gong T. Studies on Chinese Paulownia Sieb. et Zucc. J. Syst. Evol. 1976;14:38–50. [Google Scholar]
- 6.López F, Pérez A, Zamudio MAM, Alva HED, García JC. Paulownia as raw material for solid biofuel and cellulose pulp. Biomass Bioenerg. 2012;45:77–86. doi: 10.1016/j.biombioe.2012.05.010. [DOI] [Google Scholar]
- 7.Pozoga M, Olewnicki D, Jablonska L. In Vitro propagation protocols and variable cost comparison in commercial production for Paulownia tomentosa × Paulownia fortunei hybrid as a renewable energy source. Appl. Sci. 2019;9:2272. doi: 10.3390/app911272. [DOI] [Google Scholar]
- 8.Li K, Zhang DG, Yao FC. Molecular identification of Paulownia plants from XiangxiHongcheng pharmaceutical companies. J. Jishou Univ. 2012;4:83–87. doi: 10.3969/j.issn.1007-2985.2011.04.020. [DOI] [Google Scholar]
- 9.Fan, Y. M. Morphological variation and new classification system of Paulowniaceae plants, Henan Agricultural University (2018).
- 10.Mo, W. J. Studies on genetic diversiy of germplasm resources in genus Paulownia by ISSR marker, Central South University Of Forestry And Technology (2010).
- 11.Hou, T. Study on the development of the system of Paulownia, Henan Agricultural University, (2016).
- 12.Xia Z, Wen J, Gao ZM. Does the enigmatic wightia belong to Paulowniaceae (Lamiales)? Front. Plant sci. 2019;10:528. doi: 10.3389/fpls.2019.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goremykin VV, Holland B, Hirsch-Ernst KI, Hellwig FH. Analysis of Acorus calamus chloroplast genome and its phylogenetic implications. Mol. Biol. Evol. 2005;22:1813–1822. doi: 10.1093/molbev/msi173. [DOI] [PubMed] [Google Scholar]
- 14.Dong WP, Liu J, Yu J, Wang L, Zhou S. Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PLoS ONE. 2012;7:e35071. doi: 10.1371/journal.pone.0035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniell H, Lin CS, Yu M, Chang WJ. Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17:134. doi: 10.1186/s13059-016-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagnon E, Ringelberg JJ, Bruneau A, Lewis GP, Hughes CE. Global Succulent Biome phylogenetic conservatism across the pantropical Caesalpinia Group (Leguminosae) New Phytol. 2019;222:1994–2008. doi: 10.1111/nph.15633. [DOI] [PubMed] [Google Scholar]
- 17.Costa Joana, Torices Rubén, Barrett Spencer C. H. Evolutionary history of the buildup and breakdown of the heterostylous syndrome in Plumbaginaceae. New Phytologist. 2019;224(3):1278–1289. doi: 10.1111/nph.15768. [DOI] [PubMed] [Google Scholar]
- 18.Jansen RK, et al. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc. Natl. Acad. Sci. USA. 2007;104:19369–19374. doi: 10.1073/pnas.0709121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, et al. An analysis of Echinacea chloroplast genomes: Implications for future botanical identification. Sci. Rep. 2017;7:216. doi: 10.1038/s41598-017-00321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li XQ, Zuo YJ, Zhu XX, Liao S, Ma JS. Complete chloroplast genomes and comparative analysis of sequences evolution among seven Aristolochia (Aristolochiaceae) medicinal species. Int. J. Mol. Sci. 2019;20:1045. doi: 10.3390/ijms20051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carbonell-Caballero J, et al. phylogenetic analysis of 34 chloroplast genomes elucidates the relationships between wild and domestic species within the genus. Citrus. Mol. Biol. Evol. 2015;32:2015–2035. doi: 10.1093/molbev/msv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wambugu PW, Brozynska M, Furtado A, Waters DL, Henry RJ. Relationships of wild and domesticated rices (Oryza AA genome species) based upon whole chloroplast genome sequences. Sci. Rep. 2015;5:13957. doi: 10.1038/srep13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timmis JN, Ayliffe MA, Huang CY, Martin W. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet. 2004;5:123–135. doi: 10.1038/nrg1271. [DOI] [PubMed] [Google Scholar]
- 24.Bock DG, Andrew RL, Rieseberg LH. On the adaptive value of cytoplasmic genomes in plants. Mol. Ecol. 2014;23:4899–4911. doi: 10.1111/mec.12920. [DOI] [PubMed] [Google Scholar]
- 25.Piot A, Hackel J, Christin PA, Besnard G. One-third of the plastid genes evolved under positive selection in PACMAD grasses. Planta. 2018;247:255–266. doi: 10.1007/s00425-017-2781-x. [DOI] [PubMed] [Google Scholar]
- 26.Yi DK, Kim KJ. Two complete chloroplast genome sequences of genus. Paulownia (Paulowniaceae): Paulownia coreana and P. tomentosa. Mitochondr. DNA Part B. 2016;1:627–629. doi: 10.1080/23802359.2016.1214546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen ZY, Yao CH, Hu HR, L ZY. The origin phylogeny and distribution of Paulownia. J. Wuhan Bot. Res. 2000;18:325–328. doi: 10.3969/j.issn.2095-0837.2000.04.012. [DOI] [Google Scholar]
- 28.Jeon JH, Kim SC. Comparative analysis of the complete chloroplast genome sequences of three closely related East-Asian wild roses (Rosa sect. Synstylae; Rosaceae) Genes. 2019;10:23. doi: 10.3390/genes10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park I, et al. The complete chloroplast genomes of six Ipomoea species and indel marker development for the discrimination of authentic pharbitidissemen (Seeds of I. nil or I. purpurea) Front. Plant Sci. 2018;9:965. doi: 10.3389/fpls.2018.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang P, et al. Positive selection driving cytoplasmic genome evolution of the medicinally important ginseng plant genus. Panax. Front. Plant Sci. 2018;9:359. doi: 10.3389/fpls.2018.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park I, et al. Sequencing and comparative analysis of the chloroplast genome of Angelica polymorpha and the development of a novel Indel marker for species identification. Molecules. 2019;24:1038. doi: 10.3390/molecules24061038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan WB, Wu Y, Yang J, Shahzad K, Li ZH. Comparative chloroplast genomics of Dipsacales species: Insights into sequence variation, adaptive evolution, and phylogenetic relationships. Front. Plant Sci. 2018;9:689. doi: 10.3389/fpls.2018.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang HW, Ge S. Phylogeography of the endangered Cathaya argyrophylla (Pinaceae) inferred from sequence variation of mitochondrial and nuclear DNA. Mol. Ecol. 2006;5:4109–4123. doi: 10.1111/j.1365-294x.2006.03086.x. [DOI] [PubMed] [Google Scholar]
- 34.Morris AB, Shaw J. Markers in time and space: A review of the last decade of plant phylogeographic approaches. Mol.Ecol. 2018;27:2317–2333. doi: 10.1111/mec.14695. [DOI] [PubMed] [Google Scholar]
- 35.Ye JW, Li DZ, Hampe A. Differential Quaternary dynamics of evergreen broadleaved forests in subtropical China revealed by phylogeography of Lindera aggregata (Lauraceae) J. Biogeogr. 2019;46:1112–1123. doi: 10.1111/jbi.13547. [DOI] [Google Scholar]
- 36.D’Agostino N, et al. The complete plastome sequences of eleven Capsicum genotypes: insights into DNA variation and molecular evolution. Genes. 2018;9:503. doi: 10.3390/genes9100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sloan, D. B. et al. A recurring syndrome of accelerated plastid genome evolution in the angiosperm tribe Sileneae (Caryophyllaceae). Mol. Phylogenet. Evol. 72, 82–89, https://doi.org/10.10.1016/j.ym pev.2013.12.004 (2014). [DOI] [PubMed]
- 38.Wang LQ, et al. Complete plastome sequence of Iodes cirrhosaTurcz., the first in the Icacinaceae, comparative genomic analyses and possible split of Idoes species in response to climate changes. PeerJ. 2019;7:e6663. doi: 10.7717/peerj.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y, et al. Comparative chloroplast genomics of Gossypium species: Insights into repeat sequence variations and phylogeny. Front. Plant Sci. 2018;9:376. doi: 10.3389/fpls.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casano LM, Zapata JM, Martin M, Sabater B. Chlororespiration and poising of cyclic electron transport. J. Biol. Chem. 2000;275:942–948. doi: 10.1074/jbc.275.2.942. [DOI] [PubMed] [Google Scholar]
- 41.Horvath EM, et al. Targeted inactivation of the plastid ndhB gene in tobacco results in an enhanced sensitivity of photosynthesis to moderate stomatal closure. Plant Physiol. 2000;123:1337–1350. doi: 10.1104/pp.123.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng H, et al. The complete chloroplast genome sequence of strawberry (Fragaria×ananassa Duch.) and comparison with related species of Rosaceae. PeerJ. 2017;5:e3919. doi: 10.7717/peerj.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caspermeyer J. Most comprehensive study to date reveals evolutionary history of Citrus. Mol. Biol. Evol. 2015;32:2217–2218. doi: 10.1093/molbev/msv101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu LD, Xie LX, Du QY, Chang CJ. RAPD analysis of seven species in Paulownia. Guihaia. 2001;4:83–87. doi: 10.3969/j.issn.1000-3142.2001.04.009. [DOI] [Google Scholar]
- 45.Xiong JQ, Chen ZY. A study on numerical taxonomy of the genus Paulownia. Bull. Bot. Res. 1992;2:185–188. [Google Scholar]
- 46.Luo R, et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1:18. doi: 10.1186/2047-217x-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20:3252–3255. doi: 10.1093/bioinformatics/bth352. [DOI] [PubMed] [Google Scholar]
- 48.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1006/jmbi.1990.9999. [DOI] [PubMed] [Google Scholar]
- 49.Lohse M, Drechsel O, Kahlau S, Bock R. Organellar genome DRAW- a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013;41:575–581. doi: 10.1093/nar/gkt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 51.Xu B, Yang ZH. PAMLX: A Graphical User Interface for PAML. Mol. Biol. Evol. 2013;30:2723–2724. doi: 10.1093/molbev/mst179. [DOI] [PubMed] [Google Scholar]
- 52.Kearse M, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 54.Miller M, Pfeiffer W, Schwartz T. Creating the CIPRES science gateway for inference of large phylogenetic trees. Proc. Gatew. Comput. Environ. 2010;14:1–8. doi: 10.1109/gce.2010.5676129. [DOI] [Google Scholar]
- 55.Ronquist F, M.P.D LAS, et al. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rambaut, A. FigTree v 1.4. 2: Molecular Evolution, Phylogenetics and Epidemiology. Edinburgh: University of Edinburgh (2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.