Abstract
Background
Protective mechanical ventilation is recommended for patients with acute respiratory distress syndrome (ARDS), but it usually requires controlled ventilation and sedation. Using neurally adjusted ventilatory assist (NAVA) or pressure support ventilation (PSV) could have additional benefits, including the use of lower sedative doses, improved patient–ventilator interaction and shortened duration of mechanical ventilation. We designed a pilot study to assess the feasibility of keeping tidal volume (VT) at protective levels with NAVA and PSV in patients with ARDS.
Methods
We conducted a prospective randomized crossover trial in five ICUs from a university hospital in Brazil and included patients with ARDS transitioning from controlled ventilation to partial ventilatory support. NAVA and PSV were applied in random order, for 15 min each, followed by 3 h in NAVA. Flow, peak airway pressure (Paw) and electrical activity of the diaphragm (EAdi) were captured from the ventilator, and a software (Matlab, Mathworks, USA), automatically detected inspiratory efforts and calculated respiratory rate (RR) and VT. Asynchrony events detection was based on waveform analysis.
Results
We randomized 20 patients, but the protocol was interrupted for five (25%) patients for whom we were unable to maintain VT below 6.5 mL/kg in PSV due to strong inspiratory efforts and for one patient for whom we could not detect EAdi signal. For the 14 patients who completed the protocol, VT was 5.8 ± 1.1 mL/kg for NAVA and 5.6 ± 1.0 mL/kg for PSV (p = 0.455) and there were no differences in RR (24 ± 7 for NAVA and 23 ± 7 for PSV, p = 0.661). Paw was greater in NAVA (21 ± 3 cmH2O) than in PSV (19 ± 3 cmH2O, p = 0.001). Most patients were under continuous sedation during the study. NAVA reduced triggering delay compared to PSV (p = 0.020) and the median asynchrony Index was 0.7% (0–2.7) in PSV and 0% (0–2.2) in NAVA (p = 0.6835).
Conclusions
It was feasible to keep VT in protective levels with NAVA and PSV for 75% of the patients. NAVA resulted in similar VT, RR and Paw compared to PSV. Our findings suggest that partial ventilatory assistance with NAVA and PSV is feasible as a protective ventilation strategy in selected ARDS patients under continuous sedation.
Trial registration ClinicalTrials.gov (NCT01519258). Registered 26 January 2012, https://clinicaltrials.gov/ct2/show/NCT01519258
Keywords: Respiration, artificial; Respiratory distress syndrome, adult; Interactive ventilatory support; Positive-pressure respiration; Neurally adjusted ventilatory assist
Background
Acute respiratory distress syndrome (ARDS) has a high mortality burden [1], especially in low and middle-income countries [2, 3]. Protective mechanical ventilation (MV)—consisting of the use of tidal volume (VT) equal or less than 6 mL/kg of predicted body weight (PBW) and plateau pressure (Pplat) limited to 30 cmH2O—reduces mortality and is recommended for ARDS [4–8]. In the initial phase of ARDS, patients are often ventilated with controlled modes for rigorous control of VT and Pplat, requiring sedation and sometimes, neuromuscular blockade [6, 9–12], which are associated with diaphragmatic weakness [13–15]. On the other hand, overload of the respiratory muscles during acute respiratory failure causes muscle fatigue and is also associated with adverse events [16].
Using partial ventilatory support could be one alternative to prevent respiratory muscles weakness and complications associated with controlled mechanical ventilation [13, 14] while also preventing muscle fatigue [16]. Partial ventilatory support in ARDS could have additional benefits, including the use of lower sedative doses, improved patient–ventilator interaction and shortened duration of MV [12, 17, 18].
Neurally adjusted ventilatory assist (NAVA) is a proportional ventilatory mode that uses the electrical activity of the diaphragm (EAdi) to trigger, cycle and provide inspiratory assistance in proportion to patient’s effort [19–21]. Studies have shown that NAVA prevents excessive lung distension, reduces the work of breathing and improves patient–ventilator synchrony when compared with pressure support ventilation (PSV) [22–28].
We designed a pilot study to assess the feasibility of using NAVA and PSV for ARDS patients transitioning from controlled ventilation to partial ventilatory support. We hypothesized that it would be feasible to keep VT at protective levels with NAVA and PSV.
Methods
We conducted a crossover study in five intensive care units of a university hospital in São Paulo, Brazil, from November 2012 to November 2015. The Institution’s Ethics committee (CaPPesq 02874612.6.0000.0068) approved the study and informed consent was obtained from the families of patients and attending physicians. The study was registered on ClinicalTrials.gov (NCT01519258).
We screened intubated and mechanically ventilated patients with ARDS according to the Berlin definition [29] and included a pilot sample of 20 patients. We could not calculate a sample size since data on the performance of NAVA during protective ventilation for ARDS was not available in the literature. Inclusion criteria were MV for more than 24 h; diagnosis of ARDS; indication of protective MV, by the ICU team; presence of inspiratory efforts triggering the ventilator for more than 6 h. Exclusion criteria were age < 18 years, pregnancy, severe hemodynamic instability, contraindications to the placement of the esophageal catheter and participation in other clinical trials.
We randomized patients who fulfilled all inclusion criteria and no exclusion criteria to the order of ventilation in NAVA and PSV using a computer generated randomization list (http://www.R-project.org/, Vienna, Austria), and numbered, opaque and sealed envelopes.
Patients were ventilated with the Servoi Ventilator (Maquet Critical Care, Solna, Sweden), and ventilator settings before initiation of the protocol were adjusted by ICU team (Baseline). PEEP (positive end-expiratory pressure) and FIO2 (fraction of inspired oxygen) were kept constant during the study. We registered baseline ventilatory parameters and demographic data. Sedation was adjusted by the ICU team according to the ICU sedation protocol, targeting a RASS of − 5 for patients receiving neuromuscular blocker and RASS − 2 to 0 for patients transitioning to assisted ventilation.
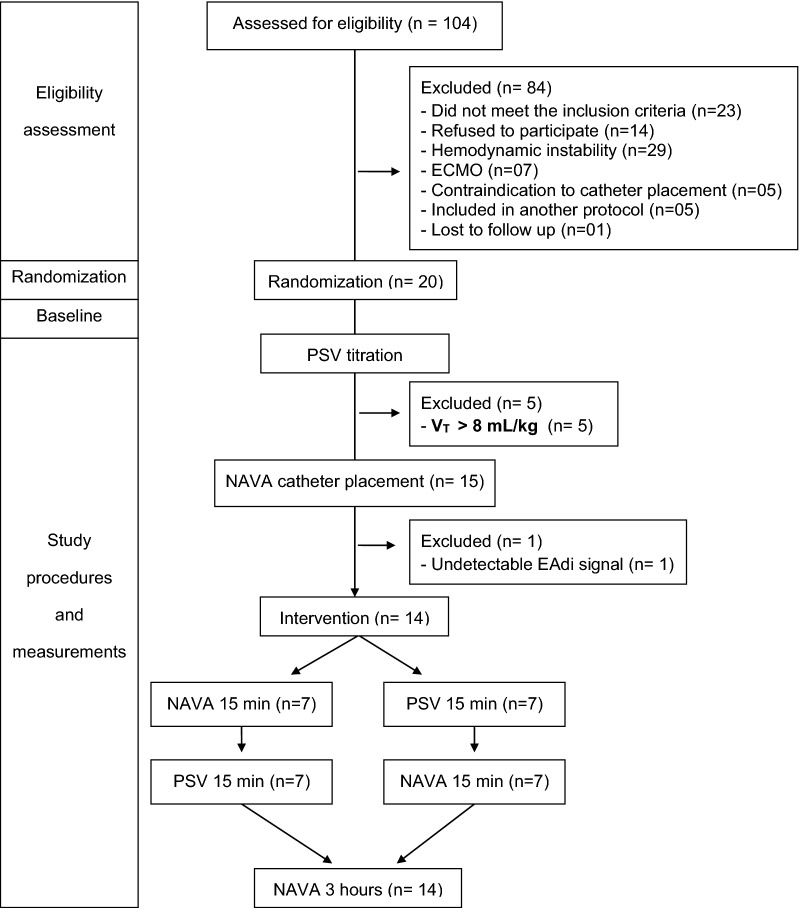
Patients were switched from Baseline to PSV and we titrated PSV to generate a VT ≤ 6 mL/kg PBW. We started with a PS level of 10 cmH2O and increased or decreased it to reach the target VT. If VT was ≥ 6.5 mL/kg despite using PS ≤ 3 cmH2O, the protocol was interrupted, and Baseline was resumed (Fig. 1). If we were able to provide protective MV with PSV, we placed the NAVA catheter and titrated NAVA. Triggering sensitivity and cycling criteria in PSV were adjusted by the ICU team. The ICU team was instructed to use flow triggering adjusted to be as sensitive as possible without generating auto-triggering.
Fig. 1.
Flow diagram of study patients and procedures. NAVA neurally adjusted ventilatory assist, PSV pressure support ventilation, EAdi electrical activity of the diaphragm
As previously described [30], we measured EAdi using a dedicated NAVA catheter (Maquet, Sweden) and titrated NAVA level to generate the same peak pressure generated with PSV. Triggering sensitivity in NAVA was fixed at 0.5 µV, and cycling criteria was fixed at 70% of peak EAdi. Pneumatic triggering sensitivity was adjusted by the ICU team and kept constant.
Then, patients were ventilated in NAVA and PSV for 15 min each, in the order determined by randomization, followed by ventilation with NAVA for 3 h (NAVA3h).
We collected hemodynamic and respiratory parameters at the end of NAVA and PSV periods and every 15 min in the NAVA3h and obtained arterial blood gases at the end of NAVA and PSV periods and NAVA3h.
We recorded peak airway pressure (Paw), flow, and EAdi continuously from the ventilator at a sampling rate of 100 Hz, using dedicated software (ServoTracker V.4.2; Maquet, Solna, Sweden). We analyzed 3-min recordings of the end of the period of Baseline, NAVA and PSV ventilation. For analysis of NAVA3h, data from several consecutive recordings were processed in order to analyze at least 10 min of recording for every 30 min.
We processed and analyzed the data using a custom computer routine (Matlab, Mathworks, MA, USA), which automatically detected the moment of initiation and termination of inspiratory efforts and ventilator cycles, and calculated VT, Paw, respiratory rate (RR), minute ventilation, mean airway pressure (MAP), EAdi peak, and neural inspiratory time (TIn): as the difference of time (in seconds) between the initiation of an inspiratory effort and EAdi Peak. We averaged all cycles in each situation to generate a mean value for the above variables. We calculated EAdi/TIn for each respiratory cycle to have an index of respiratory drive.
Asynchrony event detection was based on a custom computer routine (Matlab, Mathworks, MA, USA) which detected the beginning and termination of each ventilator cycle, the beginning and termination of each respiratory effort and calculated cycle times and mean inspiratory times. This analysis was followed by visual inspection of Paw, flow and EAdi waveforms to confirm the presence of asynchrony. We defined the types of asynchrony in accordance with previous publications [31–34] as: auto-triggering, a ventilator cycle not preceded by an inspiratory effort; triggering delay, delay between start of patient effort and triggering > 25% mean inspiratory time; ineffective effort, an inspiratory effort not accompanied by a ventilator cycle; double triggering, two cycles separated by an expiratory time less than half of mean inspiratory time; prolonged cycle, inspiratory time greater than twice the mean inspiratory time; short cycle, inspiratory time less than half the mean inspiratory time.
The asynchrony index (AI) was calculated as the number of cycles with major asynchronies (auto-triggering, ineffective efforts, double triggering and short cycle) divided by the number of monitored neural cycles, expressed as a percentage.
The primary endpoint was VT in mL/kg of predictive body weight, and secondary endpoints were Paw, EAdi, RR and asynchrony index.
Statistical analysis
Continuous variables are expressed by mean and standard deviation or median and 25–75% interquartile range. We used paired t tests to compare continuous variables. For non-normally distributed variables, we used paired Wilcoxon signed-rank tests. Statistical analysis was done with R (http://www.R-project.org/, Vienna, Austria). A p value less than 0.05 was considered significant.
Results
We assessed 104 patients with P/F ratio less than 300, and excluded 84, mainly because they did not meet the inclusion criteria, refused to participate or had severe hemodynamic instability (Fig. 1). Twenty patients were randomized and included in the trial, and 14 completed the protocol. The protocol was interrupted for five patients (25%) for whom we were unable to maintain VT below 6.5 mL/kg PBW in PSV due to strong inspiratory efforts and for one patient for whom we could not detect the EAdi signal.
Patient’s demographic characteristics are shown in Table 1.
Table 1.
Patient demographics at admission and at diagnosis of ARDS
| ID | Age | Gender | SAPS 3 | Cause of ARDS | Charlson | P/F |
|---|---|---|---|---|---|---|
| 1 | 51 | M | 43 | Aspiration | 3 | 102 |
| 2 | 54 | F | 67 | Aspiration | 2 | 136 |
| 3 | 79 | M | 71 | Pneumonia | 5 | 114 |
| 4 | 56 | M | 27 | Pneumonia | 2 | 90 |
| 5 | 63 | M | 74 | Pneumonia | 5 | 120 |
| 6 | 42 | M | 75 | Pneumonia | 2 | 160 |
| 7 | 73 | F | 52 | Pneumonia | 4 | 192 |
| 8 | 60 | F | 58 | Pneumonia | 3 | 162 |
| 9 | 41 | M | 39 | Pneumonia | 1 | 70 |
| 10 | 48 | M | 46 | Pneumonia | 1 | 127 |
| 11 | 79 | M | 63 | Pneumonia | 5 | 175 |
| 12 | 45 | F | 52 | Pneumonia | 1 | 190 |
| 13 | 61 | F | 96 | Anaphylactic shock | 4 | 58 |
| 14 | 40 | F | 31 | Pneumonia | 2 | 139 |
| 15 | 33 | F | 38 | Pneumonia | 2 | 289 |
| 16 | 51 | F | 73 | Pneumonia | 1 | 96 |
| 17 | 46 | M | 61 | Pneumonia | 1 | 194 |
| 18 | 56 | M | 51 | Pneumonia | 3 | 140 |
| 19 | 37 | M | 70 | Pneumonia | 6 | 106 |
| 20 | 46 | F | 67 | Pneumonia | 1 | 140 |
ID patient identification, Age age in years, SAPS 3 Simplified Acute Physiology Score 3 calculated at admission, ARDS acute respiratory distress syndrome, Charlson Charlson comorbidity index at admission, P/F ratio of arterial oxygen pressure divided by the fraction of inspired oxygen on the day of the diagnosis of ARDS
The baseline mode was volume-controlled for six patients and pressure-controlled for 14 patients. Ventilatory settings during the study protocol are shown in Table 2. Most patients received neuromuscular blockage on the first 48 h of the diagnosis, but none were still receiving it at the time of the study. Sedation was used for most patients at ICU team discretion, targeting a RASS of − 2 to 0, but since we studied patients a few hours after NMB interruption, some patients were still deeply sedated. The most commonly sedatives used were fentanyl, propofol and midazolam (Table 2).
Table 2.
Ventilator parameters and patient characteristics during the study protocol
| ID | MVdays | Mode | PS | Cycloff | NAVAL | PEEP | FIO2 | P/F | Sedation | RASS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | PCV | 13 | 30% | 2.4 | 11 | 0.45 | 151 | F | − 5 |
| 2 | 7 | PCV | NA | NA | NA | 16 | 0.35 | 183 | None | − 2 |
| 3 | 3 | PCV | NA | NA | NA | 14 | 0.60 | 129 | F, P | − 4 |
| 4 | 3 | PCV | 5 | 30% | 0.2 | 15 | 0.30 | 376 | F, P | − 4 |
| 5 | 1 | PCV | 8 | 25% | 1.2 | 10 | 0.50 | 296 | None | 0 |
| 6 | 3 | PCV | 10 | 30% | 0.6 | 10 | 0.45 | 136 | F | − 4 |
| 7 | 4 | PCV | 6 | 40% | 1.4 | 8 | 0.40 | 265 | F, P | − 4 |
| 8 | 3 | VCV | 6 | 35% | 0.4 | 8 | 0.30 | 281 | F, M | − 5 |
| 9 | 3 | PCV | NA | NA | NA | 8 | 0.50 | 114 | F, M, D | + 1 |
| 10 | 2 | PCV | 5 | 30% | 0.8 | 15 | 0.50 | 187 | F, P | − 4 |
| 11 | 3 | PCV | NA | NA | NA | 8 | 0.30 | 213 | P | − 3 |
| 12 | 3 | VCV | 5 | 30% | 0.6 | 10 | 0.30 | 240 | F | − 2 |
| 13 | 3 | PCV | 7 | 30% | 0.3 | 10 | 0.35 | 193 | F, M | − 4 |
| 14 | 3 | PCV | 6 | 30% | 0.5 | 10 | 0.30 | 280 | D | − 1 |
| 15 | 2 | VCV | 8 | 30% | 2.0 | 10 | 0.50 | 137 | F, P, M | − 3 |
| 16 | 4 | PCV | 12 | 15% | 2.2 | 10 | 0.45 | 170 | None | − 3 |
| 17 | 2 | VCV | 7 | 30% | 0.5 | 8 | 0.40 | 215 | F, M | − 4 |
| 18 | 4 | VCV | 10 | 30% | 0.8 | 12 | 1.00 | 133 | F | 0 |
| 19 | 5 | PCV | NA | NA | NA | 13 | 1.00 | 314 | F, P | − 4 |
| 20 | 4 | VCV | NA | NA | NA | 10 | 0.45 | 149 | F, M | − 3 |
ID patient identification, MVdays days of mechanical ventilation before inclusion on the study, PS pressure support, Cycloff cycling off in pressure support mode, NAVAL level of neurally adjusted ventilatory assist (NAVA) during ventilation with NAVA mode, PEEP positive pressure at the end of expiration, FIO2 fraction of inspired oxygen, P/F ratio of arterial oxygen pressure divided by the fraction of inspired oxygen on the day of the study, F continuous fentanyl, P continuous propofol, M continuous midazolam, D continuous dexmedetomidine, RASS Richmond Agitation–Sedation Scale at the day of study, NA not applicable
The VT stayed within protective levels for the 14 patients who completed the protocol, and there was no difference between NAVA and PSV (Table 3). There was also no statistical difference in RR, minute ventilation, MAP, EAdi and EAdi/TIn comparing the two modes. Paw was greater in NAVA than in PSV, but it remained at protective levels. Median PaO2 and median P/F were greater in NAVA than in PSV. There were no differences for other blood gas variables between NAVA and PSV (Table 4) or in the blood gases comparing baseline and NAVA3h (Additional file 1: Table S1).
Table 3.
Respiratory variables during the study protocol
| Variable | NAVA | PSV | p value |
|---|---|---|---|
| RR (rpm) | 24 ± 7 | 23 ± 7 | 0.661 |
| VT/kg (mL/kg) | 5.8 ± 1.1 | 5.6 ± 1.0 | 0.455 |
| VE (L/min) | 8.2 ± 2.5 | 8.0 ± 2.5 | 0.431 |
| MAP (cmH2O) | 12.7 ± 2.4 | 12.5 ± 2.3 | 0.364 |
| Paw (cmH2O) | 21 ± 3 | 19 ± 3 | 0.001 |
| EAdi (µV) | 12.9 ± 6.8 | 11.9 ± 6.9 | 0.285 |
| EAdi/TIn (µV/s) | 19.3 ± 9.4 | 17.3 ± 8.8 | 0.156 |
RR respiratory rate, VT/kg tidal volume for predicted body weight, VE minute ventilation, MAP mean airway pressure, Paw peak airway pressure, EAdi peak of electrical activity of the diaphragm, EAdi/TIn peak of electrical activity of the diaphragm divided for neural inspiratory time − index of respiratory drive
Data presented as mean ± standard deviation, p value obtained by paired t test
Table 4.
Blood gases during the study protocol
| Variable | NAVA | PSV | p value |
|---|---|---|---|
| pH | 7.37 (7.36–7.41) | 7.37 (7.36–7.40) | 0.481 |
| PaO2 | 88 (69–96) | 80 (66–96) | 0.045 |
| P/F | 241 (203–265) | 236 (144–260) | 0.050 |
| PaCO2 | 39 (36–44) | 40 (37–45) | 0.290 |
| HCO3 | 23 (21–24) | 23 (21–25) | 0.575 |
| BE | − 1.1 (− 2.7 to 0.1) | − 0.1 (− 3.7 to 0.4) | 0.906 |
| SaO2 | 95 (92–97) | 96 (92–96) | 0.861 |
pH hydrogen potential, PaO2 arterial oxygen pressure, P/F ratio of arterial oxygen pressure divided by the fraction of inspired oxygen, PaCO2 arterial pressure of carbon dioxide, HCO3 bicarbonate, BE base excess, SaO2 oxygen saturation
Data presented as median and 25–75% interquartile range. p value obtained by Wilcoxon signed-rank test
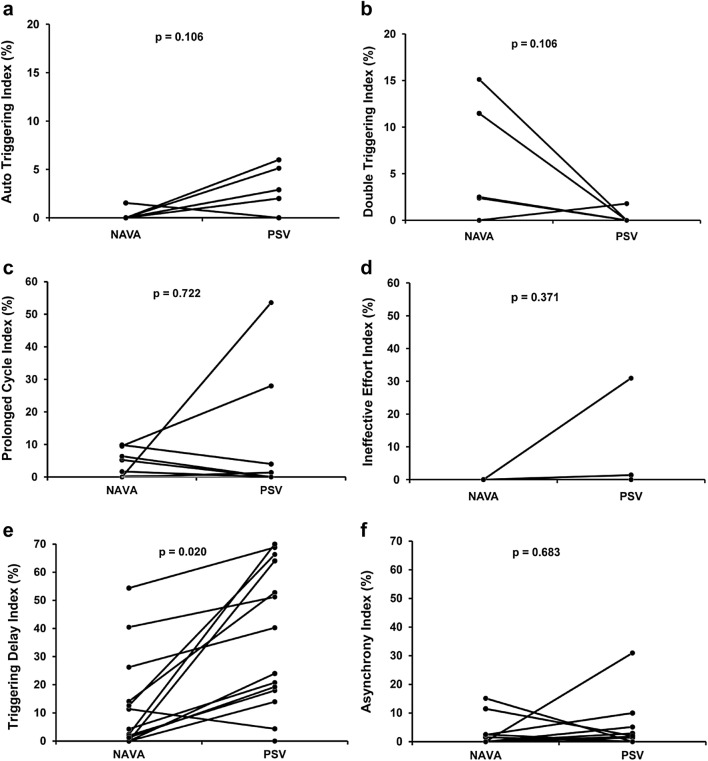
Comparisons of the incidence of asynchronies between NAVA and PSV for each patient are shown in Fig. 2. Auto-triggering (Fig. 2a) was similar for both modes; Double triggering (Fig. 2b) was observed in four patients during NAVA and in one patient during PSV, but the difference was not significant. In NAVA, the second cycle in a double triggering event had very low or zero flow (Fig. 3). Prolonged cycle (Fig. 2c) was similar in NAVA and PSV; ineffective effort was observed in two patients during PSV, and absent during NAVA (Fig. 2d). Triggering delay (Fig. 2e) was observed in 13 patients during PSV and in 10 patients during NAVA. Short cycle was observed in only one patient in PSV. AI was greater than 10% in two patients in both PSV and NAVA and was not significantly different between the two modes of ventilation (Fig. 2f), with a median of 0.7% (0–2.7) in PSV and 0% (0–2.2) in NAVA (p = 0.6835).
Fig. 2.
Asynchrony analysis in NAVA and PSV. Lines represent each patient asynchrony index for each type of asynchrony. NAVA neurally adjusted ventilatory assist, PSV pressure support ventilation. p values obtained with the Wilcoxon signed-rank test. The asynchrony index (AI) includes the following major asynchrony types: auto-triggering, ineffective efforts, double triggering and short cycle
Fig. 3.
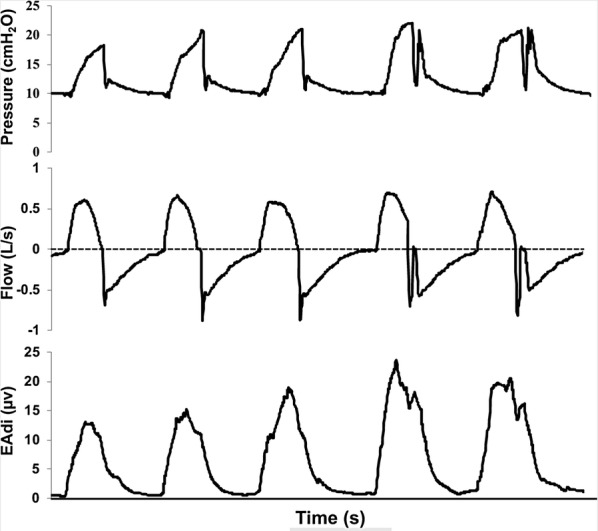
Double triggering in NAVA. Pressure, flow and EAdi vs. time in a representative recording of a patient exhibiting double triggering in NAVA (visible in the last two respiratory cycles). The double triggering was related to EAdi signal showing a biphasic curve. Note that second cycle resulted in an increase in airway pressure, but had zero flow and did not result in breath stacking
To evaluate if protective MV could be sustained with NAVA, we compared the VT, RR and Paw for the 13 patients who completed the period of NAVA3h with controlled ventilation at Baseline. There was no statistical difference in VT (5.8 ± 1.2 mL/kg in NAVA and 5.5±0.8 mL/kg in baseline; p = 0.364), RR (24 ± 7 in NAVA and 25 ± 7 in baseline; p = 0.946), or Paw (20 ± 4 cmH2O in NAVA and 23 ± 4 cmH2O in baseline; p = 0.051). The median P/F ratio was similar for both modes, 216 (172–281) vs. 217 (167–266), p = 0.765. One patient who presented apneas on the first hour of NAVA3h was excluded from this assessment. Mean VT was stable and remained at protective levels over NAVA3h (Fig. 4).
Fig. 4.
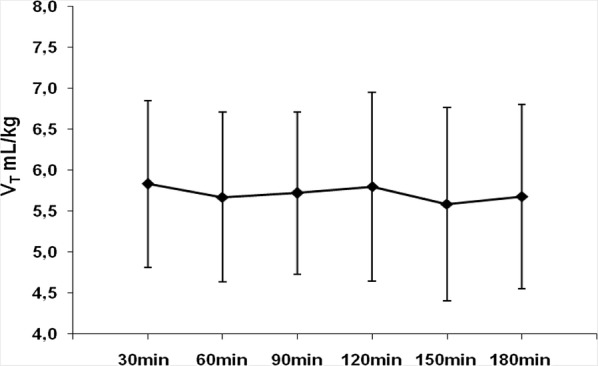
Tidal volume over the 3 h of ventilation with NAVA. Filled diamonds represent the mean tidal volume for predicted body weight of 13 patients who completed the 3 h of NAVA and bars represent standard deviation. VT mL/kg: tidal volume for predicted body weight
Discussion
In this study, we compared VT, RR, Paw, gas exchange and patient–ventilator asynchronies in NAVA and PSV in patients with ARDS on the first day after neuromuscular blockage were interrupted. The main findings were: (1) most patients could be ventilated in PSV and NAVA within protective levels, but one-quarter had strong respiratory efforts that resulted in high tidal volumes that prevented the application of protective ventilation with partial ventilatory support. (2) NAVA and PSV resulted in similar VT and RR; NAVA resulted in greater Paw than PSV, but Paw in both modes remained within protective levels. (3) There was no difference in the asynchrony index between NAVA and PSV. (4) NAVA was well tolerated during a 3-h period and had similar VT, Paw and RR compared to controlled ventilation.
To our knowledge, this is the first study using NAVA while providing protective ventilation in ARDS patients. Our protocol began a few hours after the deep sedation and/or neuromuscular blockage was discontinued and patients had respiratory drive. Other studies using NAVA in patients with ARDS were performed in the weaning phase [25, 34], in patients on ECMO [33] or did not specify timing [24]. The largest trial using NAVA randomized 128 patients to NAVA or PSV in the beginning of the weaning of MV, but the study population was a mix of several causes of respiratory failure [35]. Our intention was to apply partial ventilatory support—NAVA and PSV—while providing protective MV with a potential to reduce the need for sedation and the occurrence of asynchrony, as an improved strategy to avoid ventilator-induced lung injury (VILI) [36].
We found that this approach was feasible for most ARDS patients under continuous sedation, and a short trial on PSV was used to detect patients with strong inspiratory efforts who could not be kept on protective ventilation in partial ventilatory support. Patients who could be ventilated with approximately 6 mL/kg in PSV could also be safely ventilated in NAVA. We included patients on the three categories of ARDS, with a median of 3 days of MV. On the day of the study, the median P/F was still low, 190, and the two patients with P/F above 300 had received recruitment maneuvers and were on high PEEP (13 and 15 cmH2O). Tidal volume was similar in PSV and NAVA and within protective values for the 14 patients who completed the protocol. However, we had to interrupt the protocol and resume deep sedation for five patients (25%) who had strong inspiratory efforts resulting in VT ≥ 8 mL/kg, even with low levels of PS. Our concern was that strong inspiratory efforts and high tidal volumes could contribute to VILI [37]. In such cases, patients may benefit from the use of controlled ventilation and sedation to avoid further injury caused by MV [6].
The breathing pattern was similar in NAVA and PSV, there were no differences in RR, in accordance with previous studies [24, 33]. The respiratory drive was similar in both modes for the 14 patients enrolled, as indicated by the mean values of EAdi/TIn and EAdi. Paw was greater in NAVA than in PSV, despite titration of NAVA level to generate the same Paw in PSV. That happened because Paw in NAVA varies with EAdi, in contrast to PSV, where it is set. However, Paw remained within protective levels in both modes and this difference did not translate into more assistance in NAVA compared to PSV, since RR, VT and EAdi were similar in both modes [21, 38, 39]. The P/F ratio was higher in NAVA when compared with PSV, but the difference was not clinically relevant.
The incidence of the asynchrony was relatively low in our study. AI was greater than 10% in only two patients in both PSV and NAVA, which is considered clinically important and associated with prolonged mechanical ventilation [31, 32, 40]. This finding may be related to the fact that most patients were still sedated during the study and that they were ventilated with low tidal volumes, which may have prevented the occurrence of ineffective triggering [41] the most common type of asynchrony [40, 42].
Triggering delay was the most common type of asynchrony in our study, and it was significantly reduced in NAVA compared to PSV. Since triggering delay greater than 0.15 s may cause considerable discomfort [26, 43] and is easily measured with the NAVA catheter [44–46], we report its value but do not include it in the computation of AI to allow comparison of our results with other studies.
The occurrence of ineffective effort was rare and observed in only two patients in PSV. This is a contrast with previous studies comparing NAVA and PSV [22, 23, 25, 26, 45]. One possible explanation for this difference is that the most important risk factor for ineffective effort is over assistance and air trapping. Since ARDS patients have low respiratory system compliance and we titrated NAVA and PSV to deliver low VT, risk factors for air trapping were reduced.
Double triggering was more common in NAVA than in PSV. This result is consistent with results obtained in other studies [26, 47]. The presence of double triggering in NAVA is related to EAdi signal showing a biphasic curve. In our study, the second cycle in NAVA usually resulted in zero flow and did not result in breath stacking and high VT, which is a concern related to double triggering in assisted-controlled modes [24, 48–50]. Auto-triggering was observed in four patients in PSV and in only one patient during NAVA.
The low incidence of prolonged cycle compared to previous studies [22, 23, 25, 26] is related to patients’ mechanics and influenced by the cycling criterion used. In NAVA, the cycling criterion is fixed at 70% of peak EAdi, and in PSV it was adjusted by the ICU team.
NAVA marginally improved PaO2 and P/F compared with PSV, but the difference was not clinically relevant. Previous studies showed that spontaneous breathing efforts are associated with improved gas exchange compared with controlled ventilation [24, 51, 52], but we did not observe differences in gas exchange comparing NAVA3h and controlled ventilation. We found that NAVA was well tolerated for 3 h, and had similar RR, VT, Paw and hemodynamics compared to controlled ventilation.
Limitations
Our study has several limitations: first, it was a pilot, single-center study, we recruited a small sample size and patients included in the study showed a great variability regarding the number of days of MV, PEEP levels and P/F ratio. In addition, each ventilatory mode was studied for a short period. Therefore, more studies are necessary to test the feasibility and safety of using NAVA continuously in patients with ARDS to deliver protective MV. Second, we opted to interrupt the protocol for 25% of patients due to strong inspiratory efforts that prevented us from maintaining low VT in PSV. This choice was made to address a safety issue, since there is uncertainty about deleterious effects of strong inspiratory efforts at the early phase of ARDS. Since the patients had already been randomized, our power to detect differences between NAVA and PSV was reduced. Therefore, our conclusions about the safety of NAVA and PSV to deliver protective MV cannot be extrapolated to all patients with ARDS. And third, we used the EAdi to identify patients’ inspiratory efforts and asynchrony events; therefore, inspiratory efforts initiated by accessory muscles were not detected, which might impact asynchrony detection. In addition, EAdi is a processed signal, which may have impacted precision.
Conclusions
In this pilot study, we found that it was feasible to keep tidal volume within protective levels with NAVA and PSV for 75% of the patients with ARDS under continuous sedation. Tidal volume was similar in PSV and NAVA and remained within protective levels for 3 h with NAVA. These findings suggest that using partial ventilatory assistance in ARDS can be used as part of a protective ventilation strategy, with potential benefits of less sedation and less muscle paralysis.
Supplementary information
Additional file 1: Table S1. Comparison of blood gases between controlled ventilation and NAVA at the end of 3 h.
Acknowledgements
We acknowledge and thank Dr. Ho Yeh Li and Dr. Leandro Taniguchi for their support in inclusion of patients. We also acknowledge the MECOR program, ATS and ALAT, for their support for research in Latin America.
Abbreviations
- AI
Asynchrony index
- ARDS
Acute respiratory distress syndrome
- EAdi
Electrical activity of the diaphragm
- EAdi peak
Peak of electrical activity of the diaphragm
- ECMO
Extracorporeal membrane oxygenation
- FIO2
Fraction of inspired oxygen
- ICU
Intensive care unit
- MAP
Mean airway pressure
- MV
Mechanical ventilation
- NAVA
Neurally adjusted ventilatory assist
- NAVA3H
Neurally adjusted ventilatory assist delivered for three hours
- Paw
Peak airway pressure
- PBW
Predicted body weight
- PEEP
Positive end-expiratory pressure
- P/F
Ratio of arterial oxygen pressure divided by the fraction of inspired oxygen
- Pplat
Plateau pressure
- PSV
Pressure support ventilation
- RASS
Richmond Agitation–Sedation Scale
- RR
Respiratory rate
- SAPS 3
Simplified acute physiology score
- TIn
Neural inspiratory time
- VE
Minute ventilation
- VT
Tidal volume
Author’s contributions
FDS and JCF designed the study, participated in data collection and interpretation, and drafted the initial manuscript. HTM and AMA participated in data collection and interpretation and critically revised the manuscript. MBPA and CRRC participated in study design, data interpretation, and critically revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
This study was funded by FAPESP (Project Number 2011/20225-1), a government funding agency in Brazil.
Availability of data and materials
All data are available in the manuscript.
Ethics approval and consent to participate
The Institution’s Ethics committee (CaPPesq 02874612.6.0000.0068) approved the study and informed consent was obtained from the families of patients and attending physician. The study was registered on ClinicalTrials.gov (NCT01519258).
Consent for publication
Figure 4 shows a double triggering of one patient of the study, but the image is entirely unidentifiable.
Competing interests
Dr. Juliana Ferreira received fees for lecturing from Medtronic from 2016 to 2019. Dr. Marcelo B.P. Amato, MD, reports that his research laboratory has received grants in the last 5 years from the Covidien/Medtronic (mechanical ventilation), Orange Med/Nihon Kohden (mechanical ventilation) and Timpel S.A (Electrical Impedance Tomography). Dr. Marcelo B.P. Amato is also a minority shareholder in Timpel. The authors, FDS, HTM, AML, and CRRC have no conflicts of interest to disclose related to the contents of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fabia Diniz-Silva, Email: fabiadiniz@yahoo.com.br.
Henrique T. Moriya, Email: htmoriya@gmail.com
Adriano M. Alencar, Email: aalencar@if.usp.br
Marcelo B. P. Amato, Email: amato.marcelo.bp@gmail.com
Carlos R. R. Carvalho, Email: crrcarvalho@uol.com.br
Juliana C. Ferreira, Email: juliana.ferreira@hc.fm.usp.br
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13613-020-0638-0.
References
- 1.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.Azevedo LCP, Park M, Salluh JIF, Rea-Neto A, Souza-Dantas VC, Varaschin P, et al. Clinical outcomes of patients requiring ventilatory support in Brazilian intensive care units: a multicenter, prospective, cohort study. Crit Care. 2013;17(2):R63. doi: 10.1186/cc12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz JV, Riviello ED, Papali A, Adhikari NKJ, Ferreira JC. Global critical care: moving forward in resource-limited settings. Ann Glob Health. 2019;85(1):3, 1–11. [DOI] [PMC free article] [PubMed]
- 4.Barbas CSV, Ísola AM, Farias AMC, Cavalcanti AB, Gama AMC, Duarte ACM, et al. Brazilian recommendations of mechanical ventilation 2013. Parte I. Rev Bras Ter Intensiva. 2014;26(2):89–121. doi: 10.5935/0103-507X.20140017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 6.Chiumello D, Brochard L, Marini JJ, Slutsky AS, Mancebo J, Ranieri VM, et al. Respiratory support in patients with acute respiratory distress syndrome: an expert opinion. Crit Care. 2017;21:240. doi: 10.1186/s13054-017-1820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walkey AJ, Goligher EC, Del Sorbo L, Hodgson CL, Adhikari NKJ, Wunsch H, et al. Low tidal volume versus non-volume-limited strategies for patients with acute respiratory distress syndrome—a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14:S271–S279. doi: 10.1513/AnnalsATS.201704-337OT. [DOI] [PubMed] [Google Scholar]
- 8.Amato MBP, Barbas CSV, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 9.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 10.Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 12.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 13.Sassoon CSH. Ventilator-associated diaphragmatic dysfunction. Am J Respir Crit Care Med. 2002;166:1017–1018. doi: 10.1164/rccm.2207008. [DOI] [PubMed] [Google Scholar]
- 14.Vassilakopoulos T, Petrof BJ. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;169:336–341. doi: 10.1164/rccm.200304-489CP. [DOI] [PubMed] [Google Scholar]
- 15.Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183:364–371. doi: 10.1164/rccm.201004-0670OC. [DOI] [PubMed] [Google Scholar]
- 16.Kallet RH. Patient–ventilator interaction during acute lung injury, and the role of spontaneous breathing: part 1: respiratory muscle function during critical illness. Respir Care. 2011;56:181–189. doi: 10.4187/respcare.00964. [DOI] [PubMed] [Google Scholar]
- 17.Girard TD, Kress JP, Fuchs BD, Thomason JWW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 18.Chanques G, Kress JP, Pohlman A, Patel S, Poston J, Jaber S, Hall JB. Impact of ventilator adjustment and sedation—analgesia practices on severe asynchrony in patients ventilated in assist-control mode. Crit Care Med. 2013;41:2177–2187. doi: 10.1097/CCM.0b013e31828c2d7a. [DOI] [PubMed] [Google Scholar]
- 19.Sinderby C, Beck J. Proportional assist ventilation and neurally adjusted ventilatory assist—better approaches to patient ventilator synchrony? Clin Chest Med. 2008;29:329–342. doi: 10.1016/j.ccm.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, et al. Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999;5(12):1433–1436. doi: 10.1038/71012. [DOI] [PubMed] [Google Scholar]
- 21.Sinderby C, Beck J, Spahija J, de Marchie M, Lacroix J, Navalesi P, Slutsky AS. Inspiratory muscle unloading by neurally adjusted ventilatory assist during maximal inspiratory efforts in healthy subjects. Chest. 2007;131:711–717. doi: 10.1378/chest.06-1909. [DOI] [PubMed] [Google Scholar]
- 22.Colombo D, Cammarota G, Bergamaschi V, De Lucia M, Della Corte F, Navalesi P. Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med. 2008;34:2010–2018. doi: 10.1007/s00134-008-1208-3. [DOI] [PubMed] [Google Scholar]
- 23.Spahija J, de Marchie M, Albert M, Bellemare P, Delisle S, Beck J, et al. Patient–ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. 2010;38:518–526. doi: 10.1097/CCM.0b013e3181cb0d7b. [DOI] [PubMed] [Google Scholar]
- 24.Doorduin J, Sinderby CA, Beck J, van der Hoeven JG, Heunks LMA. Assisted ventilation in patients with acute respiratory distress syndrome—lung-distending pressure and patient–ventilator interaction. Anesthesiology. 2015;123:181–190. doi: 10.1097/ALN.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 25.Terzi N, Pelieu I, Guittet L, Ramakers M, Seguin A, Daubin C, et al. Neurally adjusted ventilatory assist in patients recovering spontaneous breathing after acute respiratory distress syndrome: physiological evaluation. Crit Care Med. 2010;38:1830–1837. doi: 10.1097/CCM.0b013e3181eb3c51. [DOI] [PubMed] [Google Scholar]
- 26.Piquilloud L, Vignaux L, Bialais E, Roeseler J, Sottiaux T, Laterre P-F, et al. Neurally adjusted ventilatory assist improves patient–ventilator interaction. Intensive Care Med. 2011;37:263–271. doi: 10.1007/s00134-010-2052-9. [DOI] [PubMed] [Google Scholar]
- 27.Haro C, Lopez-Aguilar J, Magrans R, Montanya J, Fernández-Gonzalo S, Turon M, et al. Double cycling during mechanical ventilation frequency, mechanisms and physiologic implications. Crit Care Med. 2018;46(9):1385–1392. doi: 10.1097/CCM.0000000000003256. [DOI] [PubMed] [Google Scholar]
- 28.Carteaux G, Córdoba-Izquierdo A, Lyazidi A, Heunks L, Thille AW, Brochard L. Comparison between neurally adjusted ventilatory assist and pressure support ventilation levels in terms of respiratory effort. Crit Care Med. 2016;44:503–511. doi: 10.1097/CCM.0000000000001418. [DOI] [PubMed] [Google Scholar]
- 29.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 30.Barwing J, Ambold M, Linden N, Quintel M, Moerer O. Evaluation of the catheter positioning for neurally adjusted ventilatory assist. Intensive Care Med. 2009;35:1809–1814. doi: 10.1007/s00134-009-1587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao DC, Scheinhorn DJ, Stearn-Hassenpflug M. Patient–ventilator trigger asynchrony in prolonged mechanical ventilation. Chest. 1997;112:1592–1599. doi: 10.1378/chest.112.6.1592. [DOI] [PubMed] [Google Scholar]
- 32.Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L. Patient–ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006;32:1515–1522. doi: 10.1007/s00134-006-0301-8. [DOI] [PubMed] [Google Scholar]
- 33.Mauri T, Bellani G, Grasselli G, Confalonieri A, Rona R, Patroniti N, Pesenti A. Patient–ventilator interaction in ARDS patients with extremely low compliance undergoing ECMO: a novel approach based on diaphragm electrical activity. Intensive Care Med. 2013;39:282–291. doi: 10.1007/s00134-012-2755-1. [DOI] [PubMed] [Google Scholar]
- 34.Ferreira JC, Diniz-Silva F, Moriya HT, Alencar AM, Amato MBP, Carvalho CRR. Neurally adjusted ventilatory assist (NAVA) or pressure support ventilation (PSV) during spontaneous breathing trials in critically ill patients: a crossover trial. BMC Pulm Med. 2017;17:139–147. doi: 10.1186/s12890-017-0484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demoule A, Clavel M, Rolland-Debord C, Perbet S, Terzi N, Kouatchet A, et al. Neurally adjusted ventilatory assist as an alternative to pressure support ventilation in adults: a French multicenter randomized trial. Intensive Care Med. 2016;42:1723–1732. doi: 10.1007/s00134-016-4447-8. [DOI] [PubMed] [Google Scholar]
- 36.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida T, Torsani V, Gomes S, De Santis RR, Beraldo MA, Costa EL, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med. 2013;188:1420–1427. doi: 10.1164/rccm.201303-0539OC. [DOI] [PubMed] [Google Scholar]
- 38.Lecomte F, Brander L, Jalde F, Beck J, Qui H, Elie C, et al. Physiological response to increasing levels of neurally adjusted ventilatory assist (NAVA) Respir Physiol Neurobiol. 2009;166(2):117–124. doi: 10.1016/j.resp.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Brander L, Leong-Poi H, Beck J, Brunet F, Hutchison SJ, Slutsky AS, Sinderby C, et al. Titration and implementation of neurally adjusted ventilatory assist in critically ill patients. Chest. 2009;135(3):695–703. doi: 10.1378/chest.08-1747. [DOI] [PubMed] [Google Scholar]
- 40.Blanch L, Villagra A, Sales B, Montanya J, Lucangelo U, Luján M, et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med. 2015;41:633–641. doi: 10.1007/s00134-015-3692-6. [DOI] [PubMed] [Google Scholar]
- 41.Thille AW, Cabello B, Galia F, Lyazidi A, Brochard L. Reduction of patient–ventilator asynchrony by reducing tidal volume during pressure-support ventilation. Intensive Care Med. 2008;34:1477–1486. doi: 10.1007/s00134-008-1121-9. [DOI] [PubMed] [Google Scholar]
- 42.Sousa MLA, Magrans R, Hayashi FK, Blanch L, Kacmarek R, Ferreira JC. Predictors of asynchronies during assisted ventilation and its impact on clinical outcomes: the EPISYNC cohort study. J Crit Care. 2020 doi: 10.1016/j.jcrc.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 43.De la Oliva P, Schüffelmann C, Gómez-Zamora A, Villar J, Kacmarek RM. Asynchrony, neural drive, ventilatory variability and COMFORT: NAVA versus pressure support in pediatric patients. A non-randomized cross-over trial. Intensive Care Med. 2012;38(5):838–846. doi: 10.1007/s00134-012-2535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mortamet G, Larouche A, Ducharme-Crevier L, Fléchelles O, Constantin G, Essouri S, et al. Patient–ventilator asynchrony during conventional mechanical ventilation in children. Ann Intensive Care. 2017;7:122–133. doi: 10.1186/s13613-017-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colombo D, Cammarota G, Alemani M, Carenzo L, Barra FL, Vaschetto R, et al. Efficacy of ventilator waveforms observation in detecting patient–ventilator asynchrony. Crit Care Med. 2011;39:2452–2457. doi: 10.1097/CCM.0b013e318225753c. [DOI] [PubMed] [Google Scholar]
- 46.Bellani G, Coppadoro A, Patroniti N, Turella M, Arrigoni Marocco S, Grasselli G, et al. Clinical assessment of auto-positive end-expiratory pressure by diaphragmatic electrical activity during pressure support and neurally adjusted ventilatory assist. Anesthesiology. 2014;121:563–571. doi: 10.1097/ALN.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 47.Yonis H, Crognier L, Conil J-M, Serres I, Rouget A, Virtos M, et al. Patient–ventilator synchrony in Neurally Adjusted Ventilatory Assist (NAVA) and Pressure Support Ventilation (PSV): a prospective observational study. BMC Anesthesiol. 2015;15:117–126. doi: 10.1186/s12871-015-0091-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beitler JR, Sands SA, Loring SH, Owens RL, Malhotra A, Spragg RG, et al. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: the BREATHE criteria. Intensive Care Med. 2016;42(9):1427–1436. doi: 10.1007/s00134-016-4423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sottile PD, Albers D, Higgins C, Mckeehan J, Moss MM. The association between ventilator dyssynchrony, delivered tidal volume, and sedation using a novel automated ventilator dyssynchrony detection algorithm. Crit Care Med. 2018;46(2):e151–e157. doi: 10.1097/CCM.0000000000002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holanda MA, Vasconcelos RDS, Ferreira JC, Pinheiro BV. Patient–ventilator asynchrony. J Bras Pneumol. 2018;44(4):321–333. doi: 10.1590/s1806-37562017000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Putensen C, Zech S, Wrigge H, Zinserling J, Stüber F, Von Spiegel T, Mutz N. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164:43–49. doi: 10.1164/ajrccm.164.1.2001078. [DOI] [PubMed] [Google Scholar]
- 52.Gama de Abreu M, Güldner A, Pelosi P. Spontaneous breathing activity in acute lung injury and acute respiratory distress syndrome. Curr Opin Anaesthesiol. 2012;25:148–155. doi: 10.1097/ACO.0b013e3283504bde. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Comparison of blood gases between controlled ventilation and NAVA at the end of 3 h.
Data Availability Statement
All data are available in the manuscript.