Abstract
Purpose
Ultrasound is a non-invasive quantitative method to characterize sonographic textures of skeletal muscles. To date, there is no information available on the trapezius muscle. This study assessed the trapezius muscles of patients with myofascial pain compared with normal healthy participants.
Methods
The trapezius muscles of 15 healthy and 17 myofascial pain participants were assessed using B-mode ultrasound to obtain 120 images for healthy and 162 images from myofascial pain participants. Texture features such as blob area, count and local binary patterns (LBP) were calculated. Multi-feature classification and analysis were performed using principal component analysis (PCA) and MANOVA to determine whether there were statistical differences.
Results
We demonstrate the two principal components composed of a combination of LBP and blob parameters which explain 92.55% of the cumulative variance of our data set. In addition, blob characteristics were significantly different between healthy and myofascial pain participants.
Conclusion
Our study provides evidence that texture analysis techniques can differentiate between healthy and myofascial pain affected trapezius muscles. Further research is necessary to evaluate the nature of these differences.
Electronic supplementary material
The online version of this article (10.1007/s40477-018-0330-5) contains supplementary material, which is available to authorized users.
Keywords: Ultrasound, Myofascial pain, Trapezius muscle, Quantitation, Texture analysis
Sommario
Obiettivi
L’ecografia è un metodo quantitativo non invasivo utile per caratterizzare la texture sonografica dei muscoli scheletrici. Allo stato attuale, vi sono pochi dati in letteratura riguardo il muscolo trapezio. Questo studio ha valutato i muscoli trapezi dei pazienti con dolore miofasciale confrontandoli con quelli dei soggetti sani.
Metodi
L’ecografia B-mode è stata utilizzata per valutare i muscoli trapezi di 15 soggetti sani e di 17 pazienti con dolore miofasciale , ottenendo 120 immagini per i soggetti sani e 162 per i pazienti con dolore miofasciale. Sono state calcolate caratteristiche di texture come la blob area, i count ed i local binary pattern (LBP). La classificazione e l'analisi multiparametrica sono state eseguite utilizzando l'analisi delle componenti principali (PCA) e MANOVA per valutare se vi fossero differenze statistiche.
Risultati
Abbiamo dimostrato che due componenti principali, composte da una combinazione di LBP e parametri blob, spiegano il 92.55% della varianza cumulativa dei nostri dati. Inoltre, le caratteristiche blob erano significativamente differenti tra i pazienti con dolore miofasciale ed i soggetti sani.
Conclusioni
Il nostro studio fornisce l’evidenza che le tecniche di texture analysis possono differenziare i muscoli trapezi dei soggetti sani da quelli dei pazienti affetti da dolore miofasciale. Ulteriori studi sono necessari per valutare la natura di tali differenze.
Introduction
One of the most common causes attributable to chronic pain is myofascial pain syndrome (MPS) [1, 2]. It has been reported in approximately 30% of primary care clinic patients [3] and in 85–93% of patients in specialty pain centers [2, 4]. There are approximately 9 million people suffering from myofascial pain in the United States alone [5], and thus many more worldwide who also suffer from this condition.
Myofascial pain syndrome has both direct and indirect implications. Direct costs involve the provision of health care services and the allocation of health resources and indirect costs involve loss in productivity and disability payments. These costs combined results in a significant burden on health care systems and society as a whole. Therefore, efficient and innovative methodologies are required to reduce the burden and to provide better health outcomes for patients.
Ultrasound imaging is an imaging technique widely used in many different medical specialties and provides a non-invasive, safe, reliable and relatively cost-effective imaging modality. At present, ultrasound imaging is used to evaluate muscles, tendons, ligaments, and determine the presence of space occupying lesions (for example, determine whether they are solid or cystic, and if cystic whether they are loculated and fluid filled) [6, 7]. Doppler ultrasound and elastography can also provide quantitative characterization of tested region of interest such as the blood velocity and tissue stiffness. Although useful, these are all qualitative methods of analysis.
Echo intensity has been analyzed by a variety of methods. Semi-quantitative reporting was proposed by Heckmatt et al. [8] using a four-point scale on the presence or absence of a structure on an image. But this has been superseded with the introduction of computer-assisted gray-scale analysis [9]. The benefits include less dependence upon user experience [10], sensitivity to subtle structural changes [11] and allowing more comprehensive statistical analyses [11]. Normative data are available for sternocleidomastoid, biceps brachii, forearm flexors, quadriceps, tibialis anterior [11] supraspinatus and vastus lateralis [12, 13]. However, similar data are not available for the trapezius muscle.
High variability in echo intensity (EI) impedes robust first-order statistical analyses’ reliability [14]. For these reasons, higher-order statistical analytical techniques such as texture analysis techniques were introduced. Myofacial pain syndrome consists of regional pain with a MTrP, and referred pain [15–17]. Within myofascial pain, Turo et al. [18] provided an association between entropy characteristics of the palpable active myofascial trigger point (MTrP). MTrPs have been defined as hard, palpable, localized nodules located within taut bands of skeletal muscle that are painful on compression [5, 19]. MTrPs may be active or latent, with the active ones showing spontaneous pain, while latent MTrPs becoming painful when firmly palpated [20]. Turo et al.’s [18] study consisted of a convenience sample and was not blinded. They examined 14 patients with chronic neck pain and active trigger points. Receiver operator curves were generated for entropy threshold levels of 3, 4, and 5 and demonstrated that all were able to discriminate effectively between “normal” and “active” locations (p < 0.05). The discriminate analysis performed using data from the whole region of interest (ROI) show sensitivity and specificity equal to 0.69 and 0.81, respectively. Active sites had lower entropy and were small (< 8 mm2). They were also able to show in one patient that entropy was repeatable over six measurements with 2.18% of the mean value for the whole ROI and 6.75% of the mean value for an entropy < 4. Further research using more advanced quantitative techniques has not been described for the trapezius muscle. We believe that the lack of quantitative techniques is a critical gap, since the trapezius is one of the most commonly affected muscles in clinical evaluation of neck pain [21]. Our study addresses this deficit by characterizing the upper fibers of the trapezius muscle using B-mode ultrasound. The images acquired allow higher-order features to be extracted including blob area, blob count and local binary pattern (LBP) textural features. Nielson et al. [22] refer to spatially connected and echo intensity similar regions as “blobs.” Entropy was computed over the entire region of interest. Entropy is a measure of global texture and, in addition, we were interested in local texture patterns and thought that blob and LBP texture features may provide more information. Therefore, our study aim is to provide evidence that these textural features can differentiate between myofascial pain and healthy study participants.
Materials and methods
Subject recruitment and selection
The study protocol was reviewed and approved by the Ethics Review Board of University Health Network (UHN). The research in this study was conducted in accordance with the Declaration of the World Medical Association of Helsinki. Healthy, gender-independent subjects between the ages of 20 and 50 years were recruited via advertisements placed on notice boards in our hospital and surrounding community. A total of 15 healthy subjects, eight men, seven females with a mean age of 22.7 SD 7.1 and 17 participants with myofascial pain, five males, ten females with mean age 31.8 SD 7.3 were recruited. Informed consent was obtained from each study participant prior to participating in the study. Participants were included if they demonstrated clinical evidence of a myofascial trigger point, currently complained of regional neck pain in the distribution of the trapezius muscle and local twitch response. They were excluded if there was a history of recent direct trauma to the neck region, or the clinical presence of signs and symptoms compatible with cervical radiculopathy, severe osteoarthritis of the cervical spine or shoulder, polymyositis, dermatomyositis, other neuromuscular diseases or chronic pain syndrome. None of the participants had performed any physical exercise within 2–3 days prior to entry into the study.
Ultrasound and acquisition procedure
Images were acquired on an Ultrasonix ultrasound system model Sonixtouch Q+ (BK Ultrasound, Richmond, British Colombia, Canada) with a linear array ultrasonic transducer (L14-5). The acquisition used a frequency bandwidth range of 5–15 MHz at a depth of 2.0 cm. The image settings, time gain compensation (TGC), depth, and sector size were kept constant for all subjects. During the acquisition procedure, each subject was sitting upright in a chair with their arms relaxed at their side with their forearms resting on their thighs. The transducer was placed perpendicular to the skin surface and its angle was adjusted to obtain the brightest image. The location on the upper trapezius muscle at the mid-point of the muscle belly was standardized across study participants by the middle point between the C7 spinous process and the acromioclavicular joint. The transducer was carefully placed upon the skin with enough gel to entirely coat its surface. The amount of downwards force was controlled: enough to make complete contact of the transducer with the skin but not enough to cause any deformation of the skin or image. The downward force can create image deformation, changes in thickness of the layer of muscle, and its echo intensity. To mitigate these issues, we controlled the extent of downward force. These issues were carefully explained to each ultrasound user (two users) prior to commencing the study. Sample images were acquired and the procedure was scrutinized by an experienced ultrasonographic technologist to ensure optimal image quality. At this location, B-mode ultrasound images were acquired along the longitudinal orientation of the underlying trapezius muscle. In total, 8 images per subject were acquired, where two observers acquired one image each from each site. Regions of interest (ROI) were manually selected within the trapezius muscle as determined by visual inspection. Higher-order features were then extracted such as blob area, blob count and local binary pattern features.
Blob analysis
The ROI within each image was processed using the procedure described by Nielsen et al. [13]. This technique aims at detecting spatially connected and EI similar (and, therefore, homogeneous) regions. The higher-order gray-scale statistical blob analysis consisted of a computation of the number of structures within the ROI. This method is based upon thresholding [13, 22], and therefore, we placed the following limits upon our data. Areas that lay within the 95th and the 99th percentile range of echo intensity (using the data in Table 1) were identified and marked on the filtered ROI images, thereby creating “blobs” within the ROI. As described by Nielsen et al. [13], the following method was used to calculate the mean blob size:
| 1 |
| 2 |
| 3 |
Table 1.
Mean B-Mode Echo Intensity (EI) values for various percentiles of normal upper trapezius musculature to determine threshold values
| Percentiles | 1 | 2.5 | 5 | 10 | 25 | 50 | 75 | 90 | 95 | 97.5 | 99 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EI value | 23.37 | 24.85 | 26.12 | 27.59 | 30.04 | 32.77 | 35.49 | 37.94 | 39.41 | 40.68 | 42.16 |
In the above equations, s is the mean blob size; pj is the size of blob number; j in the blob image; n is the number of blobs; N is the mean number of blobs; ni is the number of blobs in the blob image at threshold i; m is the number of threshold in the range; S is the mean blob size over a range of thresholds; si is the mean blob size at threshold i.
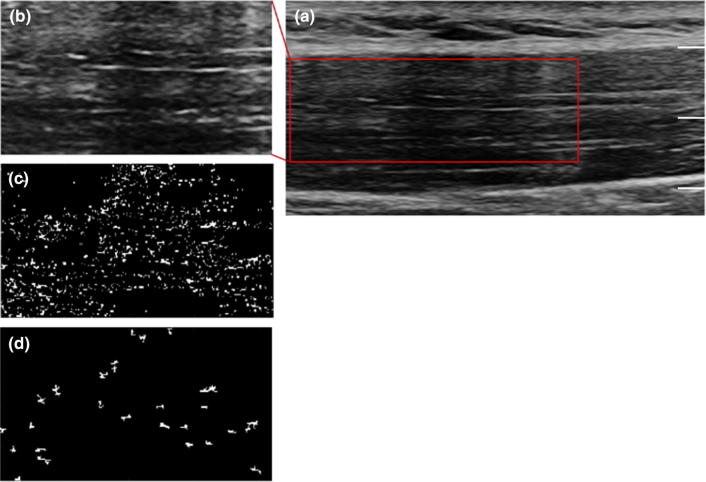
All blobs were enumerated with regard to their number, reported as number per unit area, and size and correlated with the operator responsible for acquiring the image [23]. We had two operators, DK, who had 3 years of experience and SS who did not have any experience with ultrasound. This process is shown in Fig. 1. The statistical distribution of the computed blob areas and the number of the blobs is given in the following Figures 2 and 3.
Fig. 1.
The B-mode Ultrasound gray-scale image of the upper trapezius muscle (a). b shows the selected region of interest for the blob analysis. The first round of identified blobs that have echo intensities outside the specified range is given by c. These blobs are filtered on the basis of their size using the specified area threshold. These residual blobs (shown in d) are counted and measured, generating Figs. 2 and 3. Scale: depth 2.0 cm
Fig. 2.
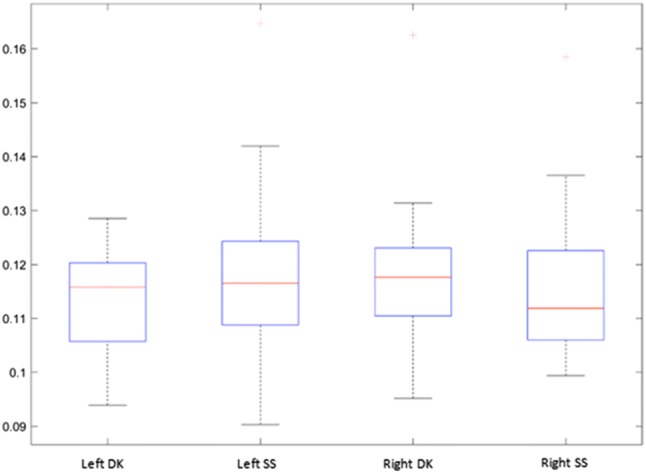
Area of the computed blobs (in mm2), shown for the left and the right upper trapezius musculature, for each of the two operators (DK and SS). Kurtosis can result in a higher SD than the mean
Fig. 3.
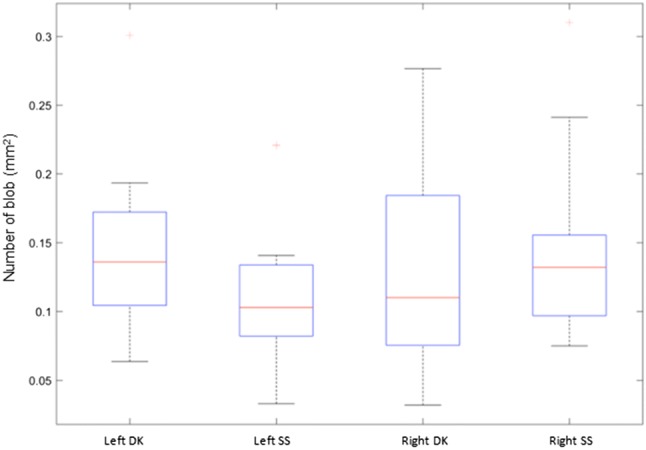
Number of the computed blobs (per mm2) shown for the left and the right upper trapezius musculature, for each of the two operators (DK and SS). Kurtosis can result in a higher SD than the mean
Local binary pattern analyses
Local binary pattern features were originally introduced by Ojala et al. for use with facial recognition and texture analysis [24, 25]. The local binary difference is obtained between the gray level of a pixel and the gray levels of “p” pixels in a local neighborhood which has a radius around the start-point (central) pixel. This technique compares the pixel intensity of the central pixel with its neighbors in a circular local neighborhood. The central pixel intensity is used to threshold and a binary image is created using the following technique. The pixel in the neighborhood is assigned 1 if its intensity is higher than the threshold and zero if it is lower. Then, this is multiplied by a matrix containing the powers of 2 (Fig. 4). The number of non-uniform patterns can occur at “p + 1”. The histogram of dimension “p + 2” is extracted. This contains the number of occurrences of the non-uniform patterns and “p + 1” types of uniform patterns. Using this technique, nine LBP parameters were calculated for each ROI and gathered into LBP feature vectors; these are typically labeled LBP1–LBP10.
Fig. 4.
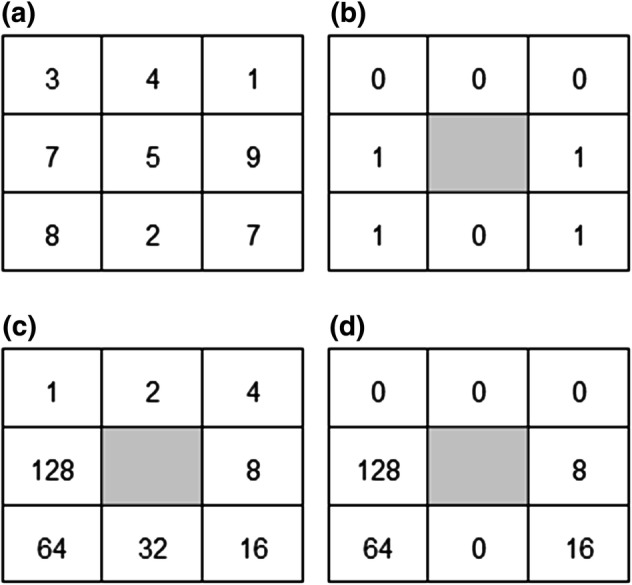
Local binary pattern example showing the various matrices that are created during the calculation of LBP. a The central pixel echo intensity is used to calculated the values of the surrounding pixels (in a circular pattern) to generate the matrix in b. Then, the values are multiplied by the matrix in c to obtain the final matrix in d
A linear support vector machine (SVM) was trained on the set of 13 features comprised of mean blob area, mean blob count, entropy, and ten LBP features followed by a tenfold cross validation run. In total, twelve features were identified to characterize ultrasound images of the upper trapezius muscle images of participants with myofascial pain and healthy individuals. A variable reduction using principal components analysis (PCA) on all twelve features in healthy individuals and in patients with myofascial trigger points (MTrP) was conducted to determine which features account for a majority of the observed variability in the data. The ability for these features to be predicted by group membership was confirmed using a multivariate analysis of variance (MANOVA), with clinical status (healthy or myofascial pain) as the independent variable and the identified features as dependent variables. Univariate analyses of variance (ANOVA), with Bonferroni corrections, were then conducted on each of the identified features in the MANOVA to determine which features are different between participants with myofascial pain and healthy.
Results
We obtained mean blob Area = 1122.3 mm2, SD = 1186.3 mm2; Mean blob Count = 21 #/mm2, SD = 10 #/mm2 for participants with myofascial pain and Mean blob Area = 844 mm2, SD = 1723.2 mm2; Mean blob Count = 47 #/mm2, SD = 21 #/mm2.
The overall distribution of ultrasound B-mode gray-scale pixel values was normally distributed using the Kolomogorov–Smirinov test (p < 0.0001). The overall B-mode gray-scale Echo Intensity (EI) distribution obtained had a mean of 32.77, a standard deviation of 4.04, was slightly leptokurtic (kurtosis of 3.58), and was marginally skewed to the right side (skewness 0.37). Based on this, percentile values for normal upper trapezius B-mode gray-scale ultrasound values are presented in Table 1.We constructed a histogram that displayed the distribution of EI across healthy subjects. Once that was created, we then fit this to a normal distribution which provided percentiles and are reported in Table 1. The blobs consisted of pixels that had an EI value between the 95th and the 99th percentile range and together formed a contiguous structure of at least 4.5 mm × 4.5 mm. Overall, the mean number of blobs was found to be 0.132 blobs/mm2 and their area was 0.12 mm2 ± 0.01.
Principal components analysis
Principal component analysis was utilized to reduce our data without any apriori bias. Eleven of the twelve features assessed were correlated with at least another feature at 0.3 or above, indicating adequate conditions for factorability (refer to Appendix for inter-feature correlations). The Kaiser–Meyer–Olkin measure of sampling adequacy was 0.89, which is above the recommended value of 0.6. Bartlett’s test of sphericity was significant X2 (78) = 13,987.6, p < 0.001. The communalities values for all features were above 0.6. Rotated component solutions indicated that the two components explained a cumulative variance of 92.55%. The three features with the highest loading factors in component one were features LBP2, LBP6 and LBP10. The features with the highest loading factors in component two were Blob area and Blob count (Table 2).
Table 2.
Rotated Component Solutions
| Component | Highest loading variables | % of variance |
|---|---|---|
| Component 1 | LBP10, LBP6, LBP2 | 80.25 |
| Component 2 | Blob area, Blob count | 12.30 |
| Cumulative variance | 92.55% | |
Group differences using MANOVA
MANOVA was used to examine group differences of texture features provided by the PCA (independent variables) between healthy and myofascial pain clinical designations (dependent variables). MANOVA results were significant for an association between the identified features and the clinical designations (myofascial pain or healthy) [F (5, 276) = 812.2, p < 0.001 Wilk’s Lambda = 0.064]. Univariate ANOVAs were used to examine individual features within each clinical designation for their significance to take into account multiple comparisons. The ANOVA results indicated that Blob area was not significantly different between healthy individuals and patients with myofascial pain (p = 0.11). All of the remaining variables were statistically different between healthy individuals and patients with myofascial pain (p < 0.001) (Table 3) taking into account multiple comparisons.
Table 3.
MANOVA results for identified features between healthy and myofascial pain groups
| Feature | Healthy mean (SD) | Myofascial pain mean (SD) |
|---|---|---|
| Blob area | 844.50 (1723.21) | 1121.92 (1181.64) |
| Blob count | 47.07 (21.31) | 20.96 (10.0) |
| LBP2 | 0.02 (0.00536) | 0.0001 (0.00001) |
| LBP6 | 0.45 (0.09038) | 0.0048 (0.00081) |
| LBP10 | 0.05 (0.01) | 0.000005 (0.000015) |
The tenfold cross-validated accuracy was found to be 98.58%, along with a sensitivity of 95.6% and specificity of 97.3%. These indicate the high predictive power of the features used for the classification.
Discussion
Quantitative analysis adds important information and extends the clinician’s knowledge beyond the current qualitative assessment. Based on the PCA, our study results demonstrate that a combination of texture features such as local binary patterns and blob area and count can be used to describe differences between study participants with myofascial pain and healthy individuals. We show that the size and number of blobs differ between these two clinical groups. It is possible that this difference could be related to myofascial pain and could also be related to the muscle attributes consistent with the integrated hypothesis [16]. Within this hypothesis, it is believed that there is a change in the muscle to create a taut band (a painful, firm region). The taut band is believed to contain muscle fiber bundles that have a different alignment than usual muscle fiber positioning. The combination of blobs and LBP features may be an ultrasound representation of this. Other possibilities include intramuscular adipose tissue, non-contractile elements or the residua of previous muscle injuries unrelated to the current episode. Further research is necessary to elucidate the actual cause of blob area, count and LBP differences.
Regions of interest
Our study used regions of interest within the ultrasound image that were manually drawn, not acquired using an automated process, in an effort to exclude as much non-contractile tissue as possible within the normal portions of the muscle. In addition, the visually apparent characteristics of the MTrP described in the literature were used, including hypoechoic regions, elliptical or spherical shapes with distinct borders. The ROIs of our myofascial pain participants’ were demarcated based upon these characteristics. The differences in texture analyses suggest that congruence with the processes was also responsible for the hypoechoic-shaped regions. Further research is suggested to elucidate the impact of the integrated hypothesis on quantitative muscle ultrasound characteristics.
Clinical implications
Currently, the criteria for diagnosis of MPS are clinical, dependent on the detection of the trigger point and subjective in nature [17, 26]. The clinical detection of the trigger point has poor interrater reliability [27]. Therefore, the results of our study would augment the current diagnostic criteria with the addition of reliable and objective information about the underlying muscle tissue characteristics obtained through quantitative assessment as described above. This may lead to the development of new diagnostic criteria as well as clinical decision rule for MPS. This may allow physicians to provide more specific management recommendations for their patients who suffer from MPS. The results may also benefit future research through the creation of homogenous experimental groups.
Operator variability
One important methodological consideration addressed by our study is operator experience. It has been well established that operator error contributes a significant degree to the variance of the end-product image [28]. We show that, given appropriate training, an inexperienced user can obtain results that are similar to an experienced one. The inter- and intra-rater reliability results are very similar for both operators, see Table 4. The detailed scanning protocol as well as the quantitative analyses contributed to the reliability results obtained. These results are similar to those reported by Pillen et al. [28] who concluded that quantification of EI was more objective and accurate method. Their inter-observer agreements when using computer-assisted gray-scale analyses of muscle EI were Cohen’s κ = 0.86 compared with visual evaluation, Cohen’s κ = 0.53. In fact, they suggested that quantitative analyses could be used as a method of screening for neuromuscular disorders in children [28]. For these reasons, we suggest the adoption of a standardized method of image acquisition from the upper fibers of trapezius muscle. One such methodology is presented in our methods section.
Table 4.
Intra-rater reliabilities (ρ) for both users on axial and longitudinal orientations of image acquisition
| Experienced user | Inexperienced user | |
|---|---|---|
| Axial (A) | 0.73 | 0.83 |
| Longitudinal (L) | 0.72 | 0.88* |
| Overall (A + L) | 0.72 | 0.86* |
The values that are significantly different (p < 0.05) from the set threshold (0.6) are shown with an asterisk (*)
Echo intensity
Since echo intensity values have high variability, the first-order statistical results are unreliable and therefore, we performed higher-order analyses based upon relative pixel intensities of regions of the image. We used the “blob analysis” technique first outlined by Nielsen et al. [22] within the supraspinatus muscle. This is a preliminary study investigating the speckling patterns within normal muscle to understand the underlying anatomical structures. For example, a tendinous region is associated with higher intensity EI and few, large blobs. This type of analysis is important since each muscle has unique anatomical structural characteristics: extent of fascia, number of fascicles, pennation angles, aponeuroses, tendons, etc. Future research could compile normative data for each clinically relevant muscle and then be used to make clinical decisions.
Limitations
Although we used standard technique to acquire and import B-mode ultrasound images, there is potential for “noise” to be present. Noise is unwanted reflections caused by neighboring matter which are captured by the ultrasound. The process of median filtering as described above minimized this but, theoretically, it may not have been sufficient. However, our statistically significant results illustrate that noise contamination was kept to a minimum and did not affect the features being investigated. Moreover, since the features computed are second-order features, they are not directly affected by EI variables, which are first-order statistics. The users were not blinded to the clinical status of the participants. Although this could theoretically impose bias, our study outcome measures would not theoretically be affected since they are second-order statistical measures. Another potential limitation is in establishing the size of the smallest blob. We addressed this by connected regions of at least three pixels corresponding to an area of 0.05 mm2. This is similar to Nielsen et al. [13] who initially proposed the “blob” technique. Finally, the most important issue is that while this study provides information that extends beyond first-order statistics, its results are not directly anatomically correlated. Further research correlating ultrasound characteristics with anatomical structures is crucial to improving our understanding of and interpretation of quantitative analytical techniques using B-mode ultrasound images.
Conclusions
This study provides information to begin to develop quantitative B-mode ultrasound assessment for the trapezius muscle. It assists in defining the blob and LBP features associated with statistically significant discriminative ability in the quantitative analysis of B-mode ultrasound images. This may provide the clinician with a reliable and objective method of discerning normal muscle from those affected with myofascial pain. Further research is recommended to improve our understanding of the impact of the integrated hypothesis on the target muscle as well as the anatomical attributes of a muscle, myofascial pain and correlation with the B-mode ultrasound characteristics.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflict of interest
The authors and author institutions have no conflict of interest to declare. This includes financial or personal relationships, dual commitments, competing interests or competing loyalties.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Harden RN, Bruehl SP, Gass S, Niemiec C, Barbick B. Signs and symptoms of the myofascial pain syndrome: a national survey of pain management providers. Clin J Pain. 2000;16(1):64–72. doi: 10.1097/00002508-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Gerwin RD. Classification, epidemiology, and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412–420. doi: 10.1007/s11916-001-0052-8. [DOI] [PubMed] [Google Scholar]
- 3.Skootsky SA, Jaeger B, Oye RK. Prevalence of myofascical pain in general internal medicine practice. Retrieved From West J Med. 1989;151(20):157–160. [PMC free article] [PubMed] [Google Scholar]
- 4.Fishbain DA, Goldberg M, Meagher BR, Steele R, Rosomoff H. Male and female chronic pain patients categorized by DSM-III psychiatric diagnostic criteria. Pain. 1986;26(2):181–197. doi: 10.1016/0304-3959(86)90074-6. [DOI] [PubMed] [Google Scholar]
- 5.Fleckenstein J, Zaps D, Ruger LJ, Lehmeyer L, Freiberg F, Lang PM, Irnich D. Discrepancy between prevalence and perceived effectiveness of treatment methods in myofascial pain syndrome: results of a cross-sectional, nationwide survey. BMC Musculoskelet Disord. 2010;11:32. doi: 10.1186/1471-2474-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mourtzakis M, Wischmeyer P. Bedside ultrasound measurement of skeletal muscle. Curr Opin Clin Nutr Metab Care. 2014;17:389–395. doi: 10.1097/MCO.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 7.Shirley IM, Blackwell R, Cusick G, Farman DJ, Vicary FR. Ultrasound. In: Shirley IM, Blackwell R, Cusick G, Farman DJ, Vicary FR, editors. A user’s guide to diagnostic ultrasound. London: Pitman Medical Publishing Co LtD; 1988. pp. 32–39. [Google Scholar]
- 8.Heckmatt JZ, Leeman S, Dubowitz V. Ultrasound imaging in the diagnosis of muscle disease. J Pediatr. 1982;101:656–660. doi: 10.1016/S0022-3476(82)80286-2. [DOI] [PubMed] [Google Scholar]
- 9.Scholten RR, Pillen S, Verrips A, Zwarts MJ. Quantitative ultrasonography of skeletal muscles in children: normal values. Muscle Nerve. 2003;27:693–698. doi: 10.1002/mus.10384. [DOI] [PubMed] [Google Scholar]
- 10.Pillen S, van Keimpema M, Nievelstein RA, Verrips A, Kruijsbergen-Raijmann W, Zwarts MJ. Skeletal muscle ultrasonography: visual versus quantitative evaluation. Ultrasound Med Biol. 2006;32:1315–1321. doi: 10.1016/j.ultrasmedbio.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Arts IMP, Pillen S, Schelhaas HJ, Overeem S, Zwarts MJ. Normal values for quantitative muscle ultrasonography in adults. Muscle Nerve. 2010;41:32–41. doi: 10.1002/mus.21458. [DOI] [PubMed] [Google Scholar]
- 12.Jensen BR, Bakke M. Prolonged work with shoulder muscles and other small muscle groups: use, function, and pain. In: Capodaglio P, Narici MV, editors. Muscle atrophy: disuse and disease. Pavia: Le Collane della Fondazione Salvatore Maugeri; 1998. pp. 149–161. [Google Scholar]
- 13.Nielsen PK, Jensen BR, Darvann T, et al. Quantitative ultrasound tissue characterization in supraspinatus and thigh muscles—a new approach. BMC Musculoskel Disord. 2006;7:2–13. doi: 10.1186/1471-2474-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillen S. Skeletal muscle ultrasound. Eur J Transl Myol. 2010;20(4):145–156. doi: 10.4081/bam.2010.4.145. [DOI] [Google Scholar]
- 15.Rivers WE, Garrigues D, Graciosa J, Harden RN. Signs and symptoms of myofascial pain: an international survey of pain management providers and proposed preliminary set of diagnostic criteria. Pain Med. 2015;16(9):1794–1805. doi: 10.1111/pme.12780. [DOI] [PubMed] [Google Scholar]
- 16.Gerwin RD. Diagnosis of myofascial pain syndrome. Phys Med Rehabil Clin N Am. 2014;25(2):341–355. doi: 10.1016/j.pmr.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Tough EA, White AR, Richards S, Campbell J. Variability of criteria used to diagnose myofascial trigger point pain syndrome—evidence from a review of the literature. Clin J Pain. 2007;23:278–286. doi: 10.1097/AJP.0b013e31802fda7c. [DOI] [PubMed] [Google Scholar]
- 18.Turo D, Otto P, Shah JP, Heimur J, Gebreab T, Zaazhoa M, Armstrong K, Gerber LH, Sikdar S. Ultrasonic characterization of the upper trapezius muscle in patients with chronic neck pain. Ultrason Imaging. 2013;35:173–187. doi: 10.1177/0161734612472408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah JP, Thaker N, Heimur J, Aredo JV, Sikdar S, Gerber L. Myofascial trigger points then and now: a historical and scientific perspective. PM&R. 2015;7(7):746–761. doi: 10.1016/j.pmrj.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons DG, Travell JG, Simons PT. Travell and Simon’s myofascial pain and dysfunction: the trigger point manual. 2. Baltimore: Williams and Wilkins; 1999. [Google Scholar]
- 21.McDermid J, Gross AR, Galea V, McLaughlin LM, Parkinson W, Woodhouse L. Developing biologically based assessment tools for physical therapy management of neck pain. J Orthop Sports Phys Ther. 2009;39(5):388–399. doi: 10.2519/jospt.2009.3126. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen PK, Jensen BR, Darvann T, Jørgensen K, Bakke M. Quantitative ultrasound image analyses of the supraspinatus muscle. Clin Biomech. 2000;15(Suppl 1):S13–S16. doi: 10.1016/S0268-0033(00)00053-X. [DOI] [PubMed] [Google Scholar]
- 23.Lindeberg T (1991) Discrete scale-space theory and the scale-space primal sketch (Doctoral dissertation, KTH Royal Institute of Technology)
- 24.Ojala T, Pietikäinen M, Harwood D. A comparative study of texture measures with classification based on featured distributions. Pattern Recogn. 1996;29(1):51–59. doi: 10.1016/0031-3203(95)00067-4. [DOI] [Google Scholar]
- 25.Ojala T, Pietikainen M, Maenpaa T. Multiresolution gray-scale and rotation invariant texture classification with local binary patterns. IEEE Trans Pattern Anal Mach Intell. 2002;24(7):971–987. doi: 10.1109/TPAMI.2002.1017623. [DOI] [Google Scholar]
- 26.Myburgh C, Lauridsen HH, Larsen AH, Hartvigsen J. Standardized manual palpation of myofascial trigger points in relation to neck/shoulder pain; the influence of clinical experience on inter-examiner reproducibility. Man Ther. 2011;16(2):136–140. doi: 10.1016/j.math.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Rathbone AT, Grosman-Rimon L, Kumbhare DA. Interrater agreement of manual palpation for identification of myofascial trigger points. Clin J Pain. 2017;33(8):715–729. doi: 10.1097/AJP.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 28.Pillen S, Arts IM, Zwarts MJ. Muscle ultrasound in neuromuscular disorders. Muscle Nerve. 2008;37(6):679–693. doi: 10.1002/mus.21015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.