Abstract
Paediatric biliary tract and gallbladder diseases include a variety of entities with a wide range of clinical presentations. Cholestasis represents an impaired secretion of bilirubin by hepatocytes, manifesting with high blood levels of conjugated bilirubin and jaundice. Various causes may be involved, which can be recognised analysing blood tests and hepatobiliary imaging, while sometimes liver biopsy or surgery may be necessary. High-resolution real-time ultrasonography is an important tool for differentiation of obstructive and non-obstructive causes of jaundice in infants and children. In this paper, we briefly review the normal anatomy and the ultrasound aspects of main pathologies affecting gallbladder and biliary tree in neonatal and paediatric age.
Keywords: Ultrasonography, Cholestasis, Choledochal cyst, Biliary atresia, Cholelithiasis, Neonatal sclerosant cholangitis
Introduction
Paediatric biliary tract diseases include a variety of entities with a wide range of clinical presentations. They frequently manifest with clinical picture and/or laboratory findings of cholestasis. The term cholestasis is commonly referred to an impairment of the biliary flow causing retention of substances, which should normally be excreted through the bile. From a clinical point of view, symptoms of cholestasis are sub-icterus, jaundice, pale stools, dark urine and itching. The latter does not present within 6 months after birth and may become the main clinical sign in older children and teenagers. Despite the fact that up to 15% of breast-fed infants may have prolonged jaundice over 3 weeks and only 0.04–0.2% of them have cholestatic jaundice, it is important to early exclude bile ducts impairment, which are sometimes related to poor prognosis if not promptly recognised and treated. Therefore, a prolonged jaundice over 2 weeks must always be investigated to rule out cholestatic diseases [1].
Objective examination may find hepatomegaly, sometimes associated with splenomegaly. Laboratory findings often include conjunct hyperbilirubinemia, increased serum levels of biliary acids, gamma-glutamyl transferase (GGT) and alkaline phosphatase. Traditionally, cholestasis can be divided into intrahepatic forms (infections, autoimmune hepatic pathologies, pharmacologically induced hepatic toxicity and parenteral nutrition) and extrahepatic forms (stones, biliary ways malformations and tumours of the biliary ways or the surrounding areas).
The most common extrahepatic disorder in newborns is biliary atresia and it has to be distinguished from other causes of neonatal obstructive cholestasis, choledochal cysts in particular. High-resolution real-time ultrasonography (US) helps to distinguish between obstructive and non-obstructive causes of jaundice in infants and children, detecting the two most common causes of neonatal cholestasis: biliary atresia and choledochal cyst [1, 2]. In this paper, we review the normal anatomy and the US aspects of main pathologies affecting gallbladder and biliary tree in neonatal and paediatric age.
Anatomy, technique and sonographic aspects
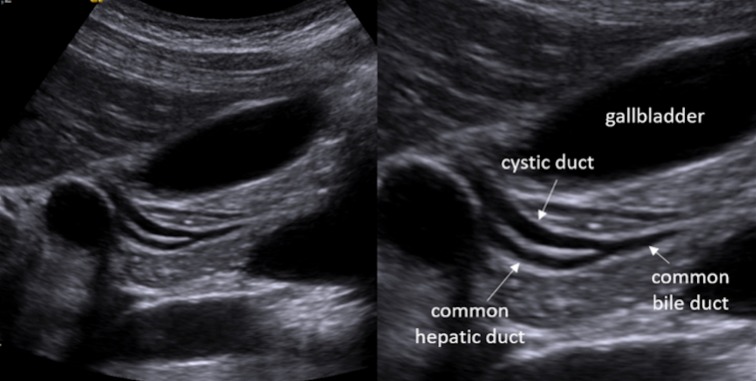
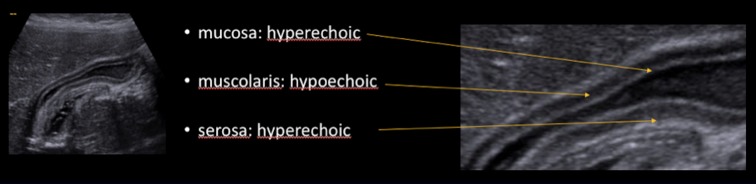
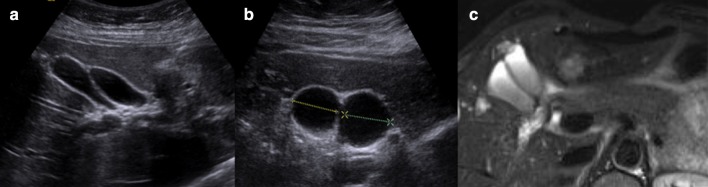
Right and left-hand biliary ducts merge into a single hepatic duct at the level of hepatic hilum (Fig. 1). The common bile duct originates from the junction of the common hepatic duct and the cystic duct (Fig. 2). It runs along the portal vein and then courses posteriorly to the duodenum and to the pancreas, where it joins the Wirsung duct to finally end into the duodenum at the level of the ampulla of Vater. The gallbladder is located on the inferior surface of the liver: it has a piriform shape, which appears lengthened on the sagittal plane and roundish on axial scan. It is divided in a proximal portion (or infundibulum), a body and a lower portion and it joins the common bile duct through the cystic duct. In normal conditions, its wall should not be thicker than 3 mm and it should present anechoic content (Figs. 3, 4). Sometimes, there can be found some congenital gallbladder variants of shape (phrygian cap, multiseptated, diverticula), position (ectopic), size or number (agenesis or duplication) (Fig. 5).
Fig. 1.
Ultrasonography anatomy of intrahepatic biliary tract: the right and left-hand biliary converge into a single hepatic duct
Fig. 2.
Ultrasonography anatomy of extrahepatic biliary tract: the coledocus originates from the junction between the hepatic and the cystic duct
Fig. 3.
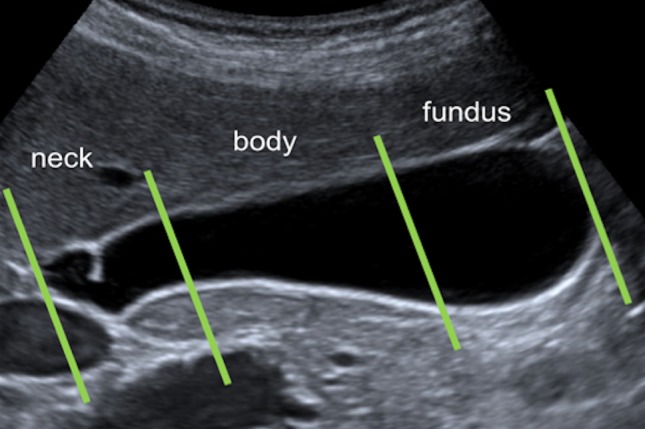
Ultrasonography anatomy of the gallbladder: the gallbladder is divided in a proximal (or neck) portion, a body and lower (or fundus) portion
Fig. 4.
Ultrasonography of the gallbladder wall stratification. From the outside to the inside we recognise: hyperechoic serosa, hypoechoic muscularis and hyperechoic mucosa
Fig. 5.
Diagnostic imaging of anomalous gallbladder duplication: ultrasonography (a, b) and magnetic resonance imaging (c)
To obtain an accurate ultrasonographic evaluation of gallbladder and biliary ducts, the patient needs to lie in supine position, or, occasionally, to rest on the right side. Oblique and longitudinal scans are obtained below the costal margin along the right hypochondrium, while axial scans are acquired at the epigastric level. Sometimes, intercostal scans through right intercostal gaps may be necessary. 4–6-h fasting is necessary to allow visualization of a distended organ.
Biliary system US study requires a systematic approach thorough the examination of the right upper quadrant viscera including liver, bile ducts, gallbladder, pancreas and portal vein. However, the entire abdomen and pelvis should be scanned.
Normally, the right hepatic lobe should not extend more than 1 cm below the costal margin in a young infant without pulmonary hyperaeration and should not extend below the right costal margin in older infants and children. The normal echotexture of the hepatic parenchyma in paediatric patients does not differ from those seen in normal adult livers. The echogenicity is normally homogeneous, low to medium, and the peripheral portal venous vasculature is clearly seen [2].
Intra- and extrahepatic biliary ducts should be carefully measured to exclude ductal dilatation. However, although there are several studies regarding common bile duct measurement in children, there is no unanimous agreement on its normal size in different paediatric age groups [3–5]. Actually, vagueness of the age criteria and various conditions, such as previous cholecystectomy, drug treatment and the imaging modality itself can affect the diameter measurement [6]. Nonetheless, it is crucial to recognise an abnormal common bile duct dilatation because of its possible association with congenital malformation and pathologic conditions, such as infection, stones, biliary dysfunction and malignancy [3, 7, 8]. Thus, a common bile duct diameter greater than 7 mm is an arbitrary widely accepted cut-off to suggest further investigations in order not to overlook significant biliary tract pathologies [6, 9–13].
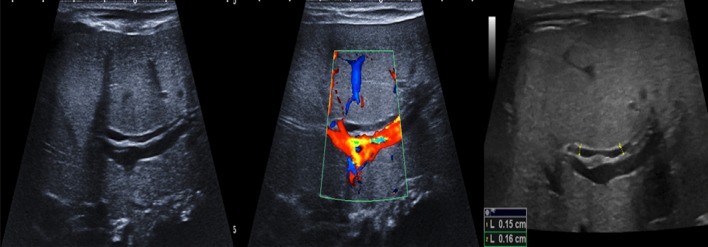
As far as it concerns the gallbladder, a normal length is considered 1.5–3 cm in infants (< 1-year-old) and 3–7 cm in older children. The length of the gallbladder should not exceed the adjacent kidney [2–4]. Furthermore, the gallbladder should also be evaluated for stones, sludge masses and pericholecystic fluid (Fig. 6).
Fig. 6.
Ultrasonography of the gallbladder shows inhomogeneous content: different type of biliary sludge (upper and central line) and micro lithiasis (lower line)
Postnatally US is the initial diagnostic modality of choice, as it allows precise measurements of intra- or extrahepatic duct calibre and identification of stones and sludge.
Choledochal cyst
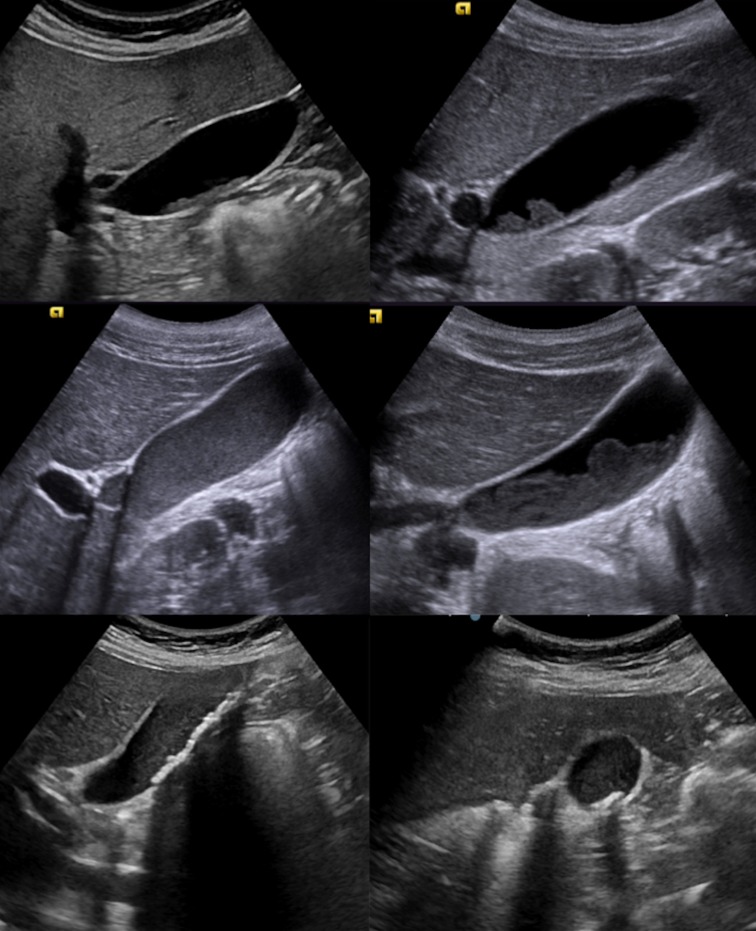
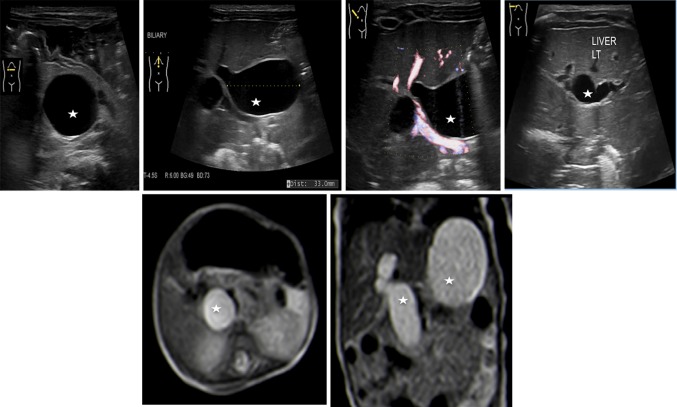
Choledochal cyst is a rare and benign congenital cystic dilation of the biliary tree [14]. There are different degrees of dilatation, ranging from a simple fusiform dilatation of the common bile duct to an anechoic roundish lesion at the hepatic hilum (Figs. 7, 8, 9 and 10).
Fig. 7.
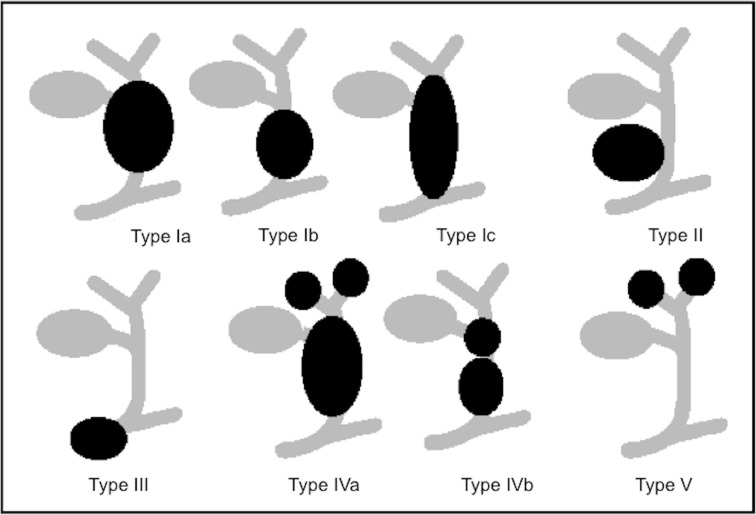
Schematic overview of choledochal cyst (Todani’s classification).
Modified from Soares et al. [14]
Fig. 8.
Ultrasonography scan of the liver, axial image, a, b show simple fusiform dilatation of common biliary duct (yellow arrow) in keeping with the type 1A cyst and coarse liver echotexture thought to be secondary to cholestasis
Fig. 9.
Colour Doppler ultrasonography images a, b show at the level of the main biliary tract a non-vascularized anechoic formation in keeping with the type II cyst or diverticular choledochus (yellow arrow)
Fig. 10.
Multiple dilatation of both intrahepatic and extrahepatic biliary duct (stars) in keeping with the type IVa cysts at ultrasonography (upper line) and at magnetic resonance imaging (lower line)
The estimated incidence of choledochal cyst is 1/100,000–1/150,000 individuals in Western countries with an even higher incidence in some Asian countries (e.g. 1/13,000 in Japan).
Some authors hold that different mechanisms contribute to the formation of the disease [14–16]. The most common aetiology is bile duct obstruction, which may present as acute abdominal pain and/or jaundice and is revealed by abnormal liver function test [17, 18].
The most widely used classification of choledochal cysts (by Todani) is based on the site of cystic changes in the biliary tree [19]. Type 1 (the most common, 80–90% of all choledochal cyst) is characterised by cystic or fusiform dilatation of common bile duct (CBD). Type 2 is associated with a true CBD diverticulum. Type 3 presents as an intraduodenal choledochocele. Type 4 consists of two other subtypes: type 4a is characterised by multiple intra- and extrahepatic cysts, whereas type 4b is quite rare and it is associated with multiple extrahepatic cysts. Type 5, also known as Caroli disease, is associated with single or multiple intrahepatic cysts in combination with simultaneous extrahepatic diseases [20]. At US, Caroli’s disease has focal or diffuse dilated ducts, varies from few millimetres to few centimetres, with or without sludge inside due to biliary stasis, surrounding branches of the portal vein; this arrangement of the cysts is configured as “central dot” sign at colour Doppler where portal radicles are observed within dilated intrahepatic bile duct. The latter is the best diagnostic clue (Fig. 11) [21].
Fig. 11.
Transverse scans of the liver a, b show coarse liver pattern with dilated ducts (calliper) converging to the hilum. Colour Doppler imaging modality b shows colour flow in portal radicles surrounded by dilated intrahepatic ducts as “central dot” sign (arrows). These features are in keeping with type V cysts or Caroli’s disease
Clinical presentations vary among different age groups, the classic triad of jaundice, abdominal pain and abdominal right upper quadrant mass is found only in the minority of patients [4, 22]. Sometimes, delay in diagnosis leads to severe complications such as malignant transformations, cholangitis, pancreatitis and cholelithiasis.
Differential diagnosis of choledochal cysts is with other several cystic lesions, including hepatic cyst, enteric duplication cyst, pancreatic pseudocyst, hepatic artery aneurysm and spontaneous perforation of the CBD. Mostly, these entities can be detected through a careful US scan with colour Doppler imaging. In particular, an enteric duplication cyst is most often characterised by the intestinal wall sign, the “muscular rim sign,” which consists of a bright echoic inner rim (mucosa) and a hypoechoic outer rim (muscular layer) [4, 23–25]. A hepatic artery aneurysm may be instead recognised with colour Doppler US.
Biliary ways atresia
Biliary atresia is a destructive inflammatory obliterative cholangiopathy of neonates that affects both intrahepatic and extrahepatic bile ducts (Fig. 12). It has an incidence of 1/17 000–19000 live born in Western Europe, but is most frequent in East Asia with an incidence of 1/5000 [26]. Female newborns are slightly more affected than males (M:F = 1:1.5). There are two different kinds of biliary atresia: an embryonal form, with very early signs and associated with other, more severe, malformations and a perinatal form.
Fig. 12.
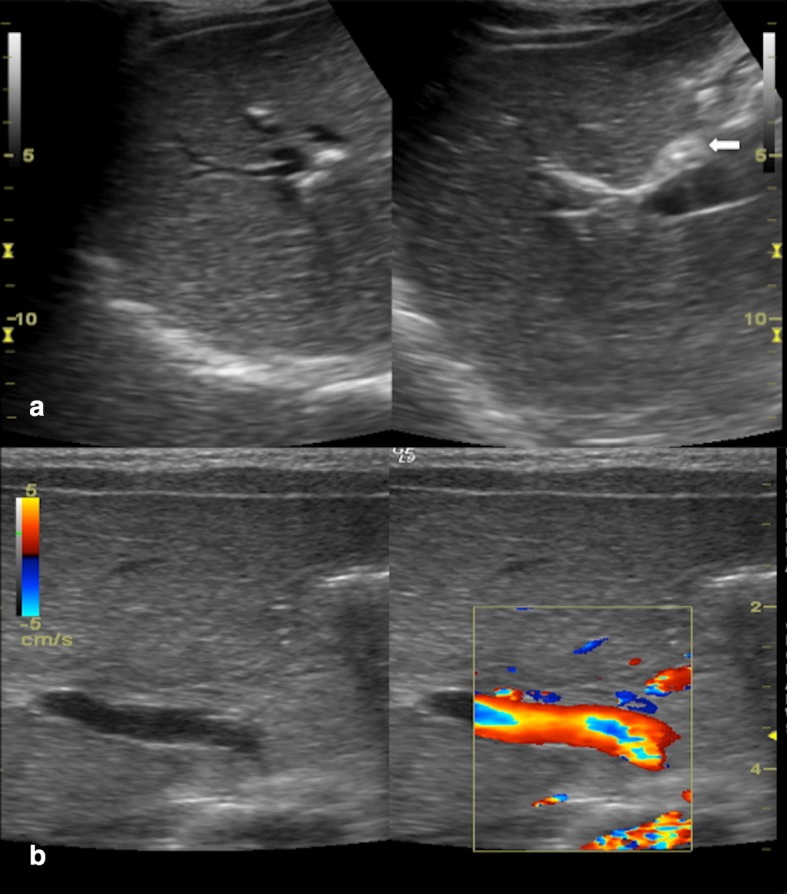
Ultrasonography in biliary atresia (a, b): fibrous non-vascularized triangular-shaped tissue anteriorly to the right portal vein, close to the confluence of right and left hepatic ducts (arrow)
The pathogenesis of the disease is still unknown, but is likely related to multifactorial aetiology due to genetical, infectious or inflammatory factors leading to the development of an obliterative cholangiopathy. Current researches are evaluating the possibility that perinatal infection (e.g. reovirus or rotavirus) may interact with genetic factors that influence biliary morphogenesis, inflammatory response or the presence of associated malformations [26, 27].
Unless surgically treated within the first 45–60 days since birth, the prognosis is poor because of secondary development of biliary cirrhosis which leads to a two-year life expectancy. Actually, biliary atresia is the most common cause of hepatic transplant in paediatric age (about 75% of hepatic transplant in children aged < 2). 10–20% of biliary atresia cases manifest syndromic forms associated with other malformations, the most common is the biliary atresia splenic malformation (BASM), reported in 10% of all biliary atresia cases. It includes polysplenia, situs inversus and vascular anomalies (such as absence of the inferior vena cava and portal preduodenal vein). In some cases, it can even be associated with cardiac pathologies such as ventricular septal defect (VSD), atrial septal defect (ASD) and left ventricular hypoplasia.
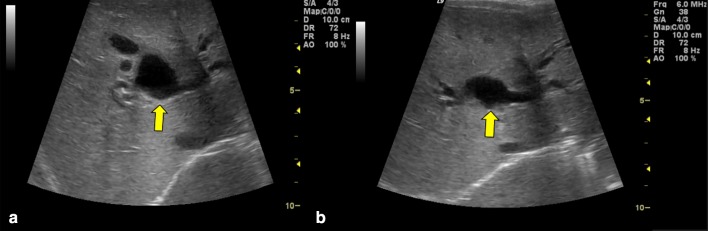
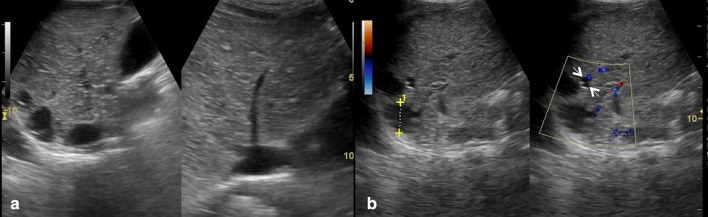
Biliary atresia symptoms usually appear 2–6 weeks after birth. Infants affected by biliary atresia show jaundice, hepatic volume enlargement with increased stiffness, pale stools and dark urine. Due to the fact that all these clinical signs may be overlooked during early clinical checks, an early and accurate preoperative diagnosis of biliary atresia is required. The Kasai procedure (portoenterostomy) has been shown to be more successful when performed within 90 days after birth [28]. Therefore, it is important to quickly differentiate biliary atresia from other medically treatable causes of cholestatic jaundice, even though there are several clinical, histopathologic and radiologic overlaps between biliary atresia and other causes of cholestatic jaundice [29–31]. US plays an important role in the screening of infantile cholestasis: failed visualization of gallbladder (phantom hypoplastic gallbladder) and the presence, anteriorly to the portal vein, of a hyperechoic tubular or triangular tissue (“triangular cord”), which represents fibrotic residuum of atretic biliary ducts (Figs. 13, 14), can be US signs of biliary atresia [4, 32–34].
Fig. 13.
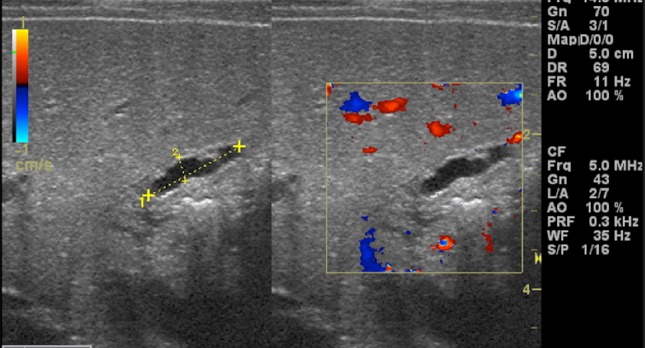
Abnormal gallbladder in biliary atresia. Oblique sonograms show an atrophic small gallbladder with thickened and irregular wall as a phantom hypoplastic gallbladder (calliper)
Fig. 14.
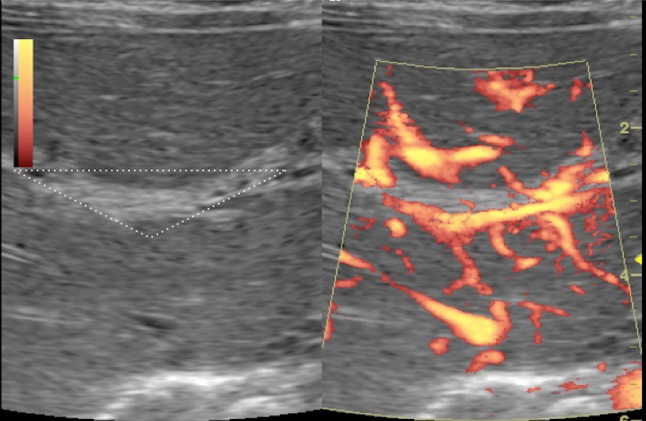
B-mode and power Doppler ultrasonography images show a non-vascularized echogenic fibrous tissue (white triangular border) at hepatic hilum: triangular cord sign
Atretic gallbladder and triangular cord sign have been demonstrated to be useful indicators with variable diagnostic performances. Other US findings which can be related to biliary atresia, such as the visualization of extrahepatic bile duct, the diameter of the hepatic artery and the presence of increased vascular resistance, such as increased portal vein calibre, and hepatic subcapsular flow have been evaluated. Clinical routine diagnosis of biliary atresia considers all these US findings [35–43].
However, there are several limitations, for example, abnormal gallbladder morphology may also be seen in patients without biliary atresia and affected by severe intrahepatic cholestasis [44, 45].
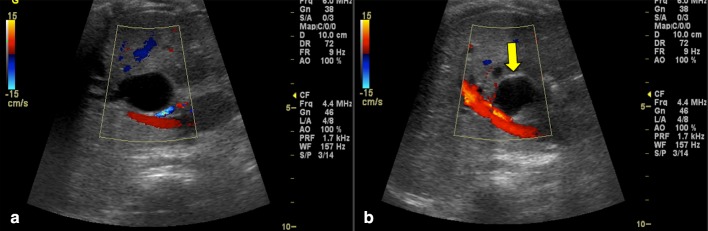
Moreover, signs of increased vascular resistance in the liver, including increased calibre of hepatic arteries, inversion of the subcapsular arterial flow (Fig. 15) and portal hypertension with persistent venous duct, can also be related to cirrhosis. Nonetheless, the presence of hepatic subcapsular flow is useful for differentiating biliary atresia and other causes of neonatal jaundice, as it is characterised by high sensitivity and specificity values (up to, respectively, 100% and 86% [42, 43]).
Fig. 15.
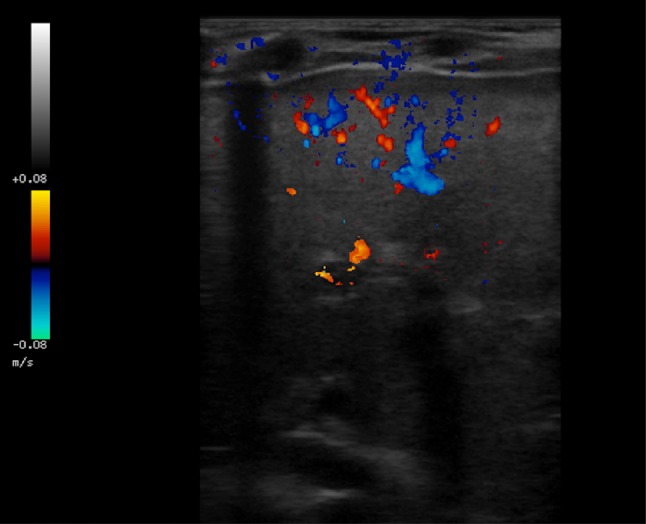
Colour Doppler ultrasonography image shows hepatic subcapsular flow
The triangular cord sign has been reported to be a highly specific finding for the diagnosis of biliary atresia in many studies [4, 35, 36, 38, 41, 42, 46–50]. Several authors used a triangular cord thickness of 4 mm as the objective criterion for triangular cord sign in biliary atresia, many other used 3 mm [37, 41, 42, 49–52]. Lee et al. reported 3.4 mm as a cut-off value for the thickness of triangular cord sign, which alone was associated to a sensitivity of 78.2%, a specificity of 100% and a diagnostic accuracy of 90% [53]. Moreover, since other studies demonstrated that the triangular cord sign may be not evaluable in the early stage of some biliary atresia cases and evolve later it was underlined that the absence of the triangular cord sign cannot exclude the diagnosis of biliary atresia at all and, therefore, the presence of other US findings, such as abnormal gallbladder morphology, should be more carefully evaluated [36, 38, 53].
Despite the usefulness of US in the diagnosis of biliary atresia, there are several limitations, only partially related to the operator’s ultrasound experience. Sometimes the common hepatic duct is not visualized in healthy infants and, in some cases of intrahepatic cholestasis, is not associated with a remarkable reduction of the biliary flow. A few cases of biliary atresia (about 20%) show normal gallbladder; despite the fact that it is a specific sign, the detection of the triangular cord depends on the operator’s ability and the quality of the US machine [54].
Other available diagnostic techniques are magnetic resonance cholangiopancreatography (MRCP) and HIDA (hepatoiminodiacetic acid) scan. However, the diagnostic value of MR cholangiography for biliary atresia is still under debate (sensitivity: 90–100%; specificity: 36–96%; diagnostic accuracy: 71–98%) as well as the usefulness of HIDA [48, 49]. Frequently, a definitive diagnosis is achieved only in the surgical theatre through direct exploration of the biliary way, preferably combined with intraoperative cholangiography [55, 56].
Cholelithiasis
Cholelithiasis in children is a rare disease with prevalence between 0.13 and 0.22% [57]. Nonetheless, it is detected more frequently than in the past because of the more numerous abdominal US examinations [58].
Gallstones may be idiopathic or secondary. There are several clinical conditions associated to their development such as obesity, diabetes, prematurity, characterised by different incidence among the various age groups. In infants and children under 5 years of age, the conditions most frequently associated with lithiasis are prematurity, total parenteral nutrition, systemic infections and genetic diseases; in school-age children, and even more in adolescents, the most frequently reported causes are familiarity, obesity, haemolytic diseases, ileal diseases and cystic fibrosis [4, 59–61].
Although approximately 80% of adults with gallstones are asymptomatic, this situation is much less frequent in children (17–35%) [59, 60]. Moreover, also the composition of gallstones is different: adult gallstones are mostly composed of cholesterol; paediatric stones can be composed of black pigments (associated with haemolytic disease, total parenteral nutrition), cholesterol, or calcium carbonate (associated with systemic illness) [62].
Generally, the most common presenting sign in infants < 1 year is jaundice, while in children > 1 year is vomiting.
However, clinical presentation and diagnosis of biliary colic in children may be challenging, especially when patients are very young. Different clinical pictures have been described varying from abdominal pain (often located in the upper quadrants and sometimes associated with dyspeptic symptoms) to a real biliary colic (characterised by acute abdominal pain in the right hypochondrium and/or epigastrium, which can radiate to the shoulder homolaterally, generally accompanied by jaundice, nausea and vomiting). Moreover, there could be signs and symptoms of pancreatitis/acute cholecystitis. Sometimes, gallstones are an incidental finding, not associated to specific symptoms [59, 60, 63].
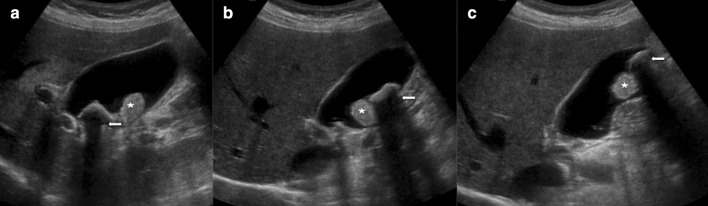
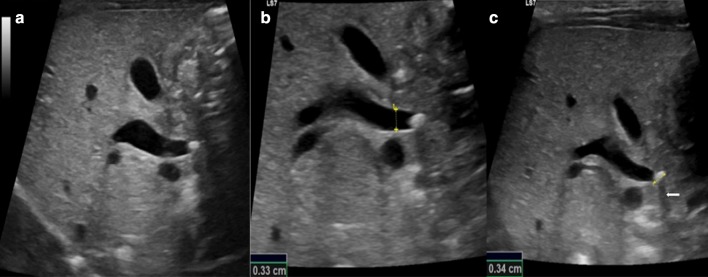
When not complicated, laboratory findings are not helpful in the diagnosis of cholelithiasis as only half of the patients present abnormal results. Therefore, the diagnosis of gallbladder stones needs imaging modalities [64]. The abdominal US is often the first choice as it is characterised by a high level of accuracy with a sensitivity and a specificity of 95%. Gallstones appear as hyperechogenic formations in the gallbladder lumen, with a rear shadow cone, sometimes movable with the patient’s decubitus changes (Figs. 6, 16 and 17) [4, 64].
Fig. 16.
Ultrasonography of the gallbladder shows sludge (star) and hyperechoic gallstones with acoustic shadow cone (arrow) moving with the decubitus of the patients from the neck (a) and body (b) to the fundus (c)
Fig. 17.
Standard longitudinal ultrasonography scans of the extrahepatic biliary tract (a–c) show a dilated common bile duct (b, calliper) obstructed by a hyperechoic gallstone (c, calliper) with acoustic shadow cone (c, arrow)
Although it is not regularly used in diagnostic imaging of uncomplicated cholelithiasis, MRC is of great use in selected cases, particularly in case of common duct stones. MRC is also useful to better delineate the anatomy of the bilio-pancreatic tree when anatomical malformations are suspected [64].
Endoscopic retrograde cholangiopancreatography (ERCP) is considered the gold standard for choledocholithiasis not only in adult age but also in paediatric age, as it is both diagnostic and therapeutic.
Neonatal sclerosant cholangitis
Neonatal sclerosant cholangitis (NSC) is an idiopathic disease characterised by inflammation and fibrosis of intra- and extra-hepatic bile ducts. It is described in a limited number of patients presenting in early infancy with jaundice, hepatosplenomegaly, pale stools and high serum γ-glutamyl transferase activity (GGT) [4, 65]. A genetic origin of consanguinity of the parents was hypothesized in 40% of cases, suggesting an autosomal recessive transmission. In 50% of cases, NSC comes in association with extrahepatic manifestations, including aortic stenosis with or without obstructive hypertrophic cardiomyopathy. The diagnosis is suspected in the presence of low birth weight and onset with cholestatic jaundice and clay-coloured stools within the first 2 weeks of life, often indistinguishable from biliary atresia. Liver US may show irregularity of bile ducts calibre. However, definitive diagnosis is made by cholangiography which demonstrates the bile duct patency and the typical intra- and extrahepatic ducts alterations. Hepatic histology is not very useful because it shows signs that are indistinguishable from those of the biliary atresia such as portal fibrosis, focal parenchymal necrosis, ductular proliferation and mixed inflammatory infiltrate [4, 66]. MRCP has a low diagnostic sensitivity and specificity. For the definitive diagnosis, exploratory laparotomy is used in combination with intraoperative cholangiography to identify the irregular and rarefied intrahepatic bile ducts, to define the opacification of the common bile duct and to exclude biliary atresia (Fig. 18).
Fig. 18.
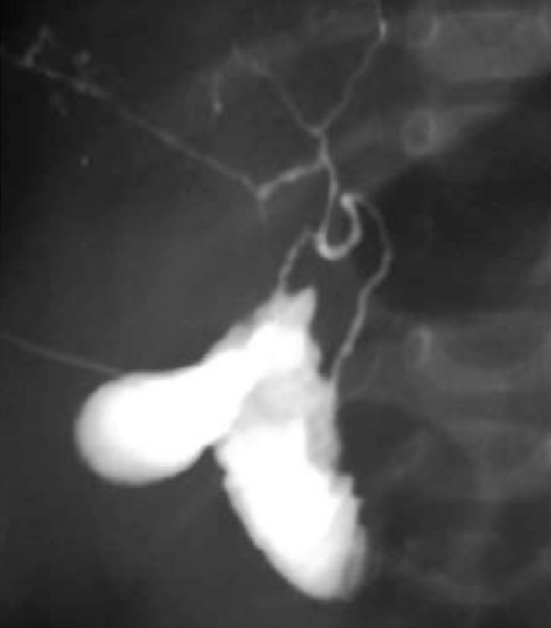
Magnetic resonance cholangiopancreatography image with maximum intensity projection (MIP) reconstruction shows irregular, thin and poorly branched biliary tree
Conclusion
Paediatric biliary tract diseases include a variety of entities with a wide range of clinical presentations. Thus, correlation of sonographic, clinical, laboratory and epidemiological findings is crucial. High-resolution real-time ultrasonography is an easy and non-invasive first diagnostic tool to differentiate between obstructive and non-obstructive causes of jaundice in infants and children.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and its late amendments. Additional informed consented was obtained from all patients for which identifying information is not included in this article.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Behrman RE, editor. Nelson textbook of pediatrics. 14. Philadelphia: Saunders; 1992. p. 478. [Google Scholar]
- 2.Frank SJ, Kurian J. Three-dimensional sonography of biliary tract disorders. J US Med. 2016;35(4):791–804. doi: 10.7863/ultra.15.04044. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Wang XL, Li SX, Bai YZ, Ren WD, Xie LM, et al. Ultrasonographic dimensions of the common bile duct in Chinese children: results of 343 cases. J Pediatr Surg. 2013;48:1892–1896. doi: 10.1016/j.jpedsurg.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 4.Di Serafino M, Severino R, Gioioso M, et al. Pediatric liver ultrasound: a pictorial essay. J Ultrasound. 2019 doi: 10.1007/s40477-018-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernanz-Schulman M, Ambrosino MM, Freeman PC, Quinn CB. Common bile duct in children: sonographic dimensions. Radiology. 1995;195:193–195. doi: 10.1148/radiology.195.1.7892467. [DOI] [PubMed] [Google Scholar]
- 6.Di Serafino M, Vitale V, Severino R, Barbuto L, Vezzali N, Ferro F, Rossi E, Caprio MG, Raia V, Vallone G. Pediatric ultrasonography of the pancreas: normal and abnormal findings. J Ultrasound. 2018;25:8. doi: 10.1007/s40477-018-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon J, Song SY, Lee KT, Lee KH, Bae MH, Lee JK. Clinical significance and long-term outcome of incidentally found bile duct dilatation. Dig Dis Sci. 2013;58:3293–3299. doi: 10.1007/s10620-013-2792-9. [DOI] [PubMed] [Google Scholar]
- 8.Tas A, Koklu S. Unusual cause of common bile duct dilatation in asymptomatic elderly patient: right hepatic artery syndrome. Ann Hepatol. 2012;11:150–151. [PubMed] [Google Scholar]
- 9.Gore RM, Levine MS. Textbook of gastrointestinal radiology. 3. Philadelphia: Elsevier; 2007. [Google Scholar]
- 10.Oppong KW, Mitra V, Scott J, Anderson K, Charnley RM, Bonnington S, et al. Endoscopic US in patients with normal liver blood tests and unexplained dilatation of common bile duct and or pancreatic duct. Scand J Gastroenterol. 2014;49:473–480. doi: 10.3109/00365521.2014.881547. [DOI] [PubMed] [Google Scholar]
- 11.Kim JE, Lee JK, Lee KT, Park DI, Hyun JG, Paik SW, et al. The clinical significance of common bile-duct dilatation in patients without biliary symptoms or causative lesions on ultrasonography. Endoscopy. 2001;33:495–500. doi: 10.1055/s-2001-15088. [DOI] [PubMed] [Google Scholar]
- 12.Carroll BA, Oppenheimer DA, Muller HH. High-frequency real-time US of the neonatal biliary system. Radiology. 1982;145:437–440. doi: 10.1148/radiology.145.2.7134449. [DOI] [PubMed] [Google Scholar]
- 13.Teele RL, Share JC. The liver. In: Teele RL, Share JC, editors. Ultrasonography of infants and children. Philadelphia: Saunders; 1991. pp. 416–451. [Google Scholar]
- 14.Soares KC, Arnaoutakis DJ, Kamel I, et al. Choledochal cysts: presentation, clinical differentiation, and management. J Am Coll Surg. 2014;219:1167–1180. doi: 10.1016/j.jamcollsurg.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin SF, Lee HC, Yeung CY, Jiang CB, Chan WT. Common bile duct dilatations in asymptomatic neonates: incidence and prognosis. Gastroenterol Res Pract. 2014 doi: 10.1155/2014/392562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato M, Ishida H, Konno K, et al. Choledochal cyst due to anomalous pancreatobiliary junction in the adult: sonographic findings. Abdom Imaging. 2001;26:395–400. doi: 10.1007/s002610000184. [DOI] [PubMed] [Google Scholar]
- 17.Naji O, Hussain A, Baker D, Habib N, El-Hasani S. Obstructive jaundice due to autoimmune cholangiopathy. BMJ Case Rep. 2009 doi: 10.1136/bcr.11.2008.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riederer J. Obstructive jaundice du to sludge in the common bile duct. Dtsch Med Wochenschr. 2000;125:11–14. doi: 10.1055/s-2007-1023877. [DOI] [PubMed] [Google Scholar]
- 19.Todani Takuji, et al. Congenital bile duct cysts. Am J Surg. 1977;134(2):263–269. doi: 10.1016/0002-9610(77)90359-2. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi T, Wakai T, Kubota M, et al. Risk of subsequent biliary malignancy in patients undergoing cyst excision for congenital choledochal cysts. J Gastroenterol Hepatol. 2013;28:243–247. doi: 10.1111/j.1440-1746.2012.07260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahuja AT, Griffith JF et al (2007) Caroli disease. In: Amirsys (ed) Diagnostic imaging: ultrasound, vol 1, pp 36–37
- 22.Shah OJ, Shera AH, Zargar SA, et al. Choledochal cysts in children and adults with contrasting profiles: 11-year experience at a tertiary care center in Kashmir. World J Surg. 2009;33:2403–2411. doi: 10.1007/s00268-009-0184-2. [DOI] [PubMed] [Google Scholar]
- 23.Di Serafino M, Mercogliano C, Vallone G. US evaluation of the enteric duplication cyst: the gut signature. J Ultrasound. 2015;19(2):131–133. doi: 10.1007/s40477-015-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Serafino M, Severino R, Mercogliano C, Lisanti F, Martino C, Rocca R, Abate R, Salata M, Vallone G, Maroscia D. A complicated ileal duplication cyst in a young adult: the value of the “Gut Signature”. Open J Radiol. 2016;6:100–104. [Google Scholar]
- 25.Segal SR, Sherman NH, Rosenberg HK, et al. Ultrasonographic features of gastrointestinal duplications. J US Med. 1994;13:863–870. doi: 10.7863/jum.1994.13.11.863. [DOI] [PubMed] [Google Scholar]
- 26.Mack CL, Sokol RJ. Unraveling the pathogenesis and etiology of biliary atresia. Pediatr Res. 2005;57:87R–94R. doi: 10.1203/01.PDR.0000159569.57354.47. [DOI] [PubMed] [Google Scholar]
- 27.Bezerra JA. Potential etiologies of biliary atresia. Pediatr Transplant. 2005;9:646–651. doi: 10.1111/j.1399-3046.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 28.Valayer J. Conventional treatment of biliary atresia: long-term results. J Pediatr Surg. 1996;31:1546–1551. doi: 10.1016/s0022-3468(96)90174-8. [DOI] [PubMed] [Google Scholar]
- 29.Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, et al. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology. 1996;23:1682–1692. doi: 10.1002/hep.510230652. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda S, Sera Y, Ohshiro H, Uchino S, Akizuki M, Kondo Y. Gallbladder contraction in biliary atresia: a pitfall of US diagnosis. Pediatr Radiol. 1998;28:451–453. doi: 10.1007/s002470050380. [DOI] [PubMed] [Google Scholar]
- 31.Nicotra JJ, Kramer SS, Bellah RD, Redd DC. Congenital and acquired biliary disorders in children. Semin Roentgenol. 1997;32:215–227. doi: 10.1016/s0037-198x(97)80008-9. [DOI] [PubMed] [Google Scholar]
- 32.Choi SO, Park WH, Lee HJ, Woo SK. ‘Triangular cord’: a sonographic finding applicable in the diagnosis of biliary atresia. J Pediatr Surg. 1996;31:363–366. doi: 10.1016/s0022-3468(96)90739-3. [DOI] [PubMed] [Google Scholar]
- 33.Iorio R, Liccardo D, Di Dato F, Puoti MG, Spagnuolo MI, Alberti D, Vallone G. US scanning in infants with biliary atresia: the different implications of biliary tract features and liver echostructure. Ultraschall Med. 2013;34(5):463–467. doi: 10.1055/s-0033-1335455. [DOI] [PubMed] [Google Scholar]
- 34.Giannattasio A, Cirillo F, Liccardo D, Russo M, Vallone G, Iorio R. Diagnostic role of US for biliary atresia. Radiology. 2008;247(3):912. doi: 10.1148/radiol.2473071715. [DOI] [PubMed] [Google Scholar]
- 35.Visrutaratna P. Biliary atresia: making the diagnosis by the gallbladder ghost triad. Pediatr Radiol. 2003;33:902. doi: 10.1007/s00247-003-1022-6. [DOI] [PubMed] [Google Scholar]
- 36.Park WH, Choi SO, Lee HJ, Kim SP, Zeon SK, Lee SL. A new diagnostic approach to biliary atresia with emphasis on the ultrasonographic triangular cord sign: comparison of ultrasonography, hepatobiliary scintigraphy, and liver needle biopsy in the evaluation of infantile cholestasis. J Pediatr Surg. 1997;32:1555–1559. doi: 10.1016/s0022-3468(97)90451-6. [DOI] [PubMed] [Google Scholar]
- 37.Lee HJ, Lee SM, Park WH, Choi SO. Objective criteria of triangular cord sign in biliary atresia on US scans. Radiology. 2003;229:395–400. doi: 10.1148/radiol.292020472. [DOI] [PubMed] [Google Scholar]
- 38.Choi SO, Park WH, Lee HJ, Woo SK. ‘Triangular cord’: a sonographic finding applicable in the diagnosis of biliary atresia. J Pediatr Surg. 1996;31:363–366. doi: 10.1016/s0022-3468(96)90739-3. [DOI] [PubMed] [Google Scholar]
- 39.Di Serafino M, Esposito F, Mercogliano C, Vallone G. The triangular cord sign. Abdom Radiol (NY). 2016;41(9):1867–1868. doi: 10.1007/s00261-016-0734-7. [DOI] [PubMed] [Google Scholar]
- 40.Azuma T, Nakamura T, Nakahira M, Harumoto K, Nakaoka T, Moriuchi T. Pre-operative ultrasonographic diagnosis of biliary atresia–with reference to the presence or absence of the extrahepatic bile duct. Pediatr Surg Int. 2003;19:475–477. doi: 10.1007/s00383-003-0962-0. [DOI] [PubMed] [Google Scholar]
- 41.Kim WS, Cheon JE, Youn BJ, Yoo SY, Kim WY, Kim IO, et al. Hepatic arterial diameter measured with US: adjunct for US diagnosis of biliary atresia. Radiology. 2007;245:549–555. doi: 10.1148/radiol.2452061093. [DOI] [PubMed] [Google Scholar]
- 42.Lee MS, Kim MJ, Lee MJ, Yoon CS, Han SJ, Oh JT, et al. Biliary atresia: color doppler US findings in neonates and infants. Radiology. 2009;252:282–289. doi: 10.1148/radiol.2522080923. [DOI] [PubMed] [Google Scholar]
- 43.El-Guindi MA, Sira MM, Konsowa HA, El-Abd OL, Salem TA. Value of hepatic subcapsular flow by color Doppler ultrasonography in the diagnosis of biliary atresia. J Gastroenterol Hepatol. 2013;28:867–872. doi: 10.1111/jgh.12151. [DOI] [PubMed] [Google Scholar]
- 44.Burton EM, Babcock DS, Heubi JE, Gelfand MJ. Neonatal jaundice: clinical and ultrasonographic findings. South Med J. 1990;83:294–302. doi: 10.1097/00007611-199003000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Kirks DR, Coleman RE, Filston HC, Rosenberg ER, Merten DF. An imaging approach to persistent neonatal jaundice. AJR Am J Roentgenol. 1984;142:461–465. doi: 10.2214/ajr.142.3.461. [DOI] [PubMed] [Google Scholar]
- 46.Takamizawa S, Zaima A, Muraji T, Kanegawa K, Akasaka Y, Satoh S, et al. Can biliary atresia be diagnosed by ultrasonography alone? J Pediatr Surg. 2007;42:2093–2096. doi: 10.1016/j.jpedsurg.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 47.Park WH, Choi SO, Lee HJ. The ultrasonographic ‘triangular cord’ coupled with gallbladder images in the diagnostic prediction of biliary atresia from infantile intrahepatic cholestasis. J Pediatr Surg. 1999;34:1706–1710. doi: 10.1016/s0022-3468(99)90650-4. [DOI] [PubMed] [Google Scholar]
- 48.Lee SY, Kim GC, Choe BH, Ryeom HK, Jang YJ, Kim HJ, et al. Efficacy of US-guided percutaneous cholecystocholangiography for the early exclusion and type determination of biliary atresia. Radiology. 2011;261:916–922. doi: 10.1148/radiol.11110665. [DOI] [PubMed] [Google Scholar]
- 49.Kanegawa K, Akasaka Y, Kitamura E, Nishiyama S, Muraji T, Nishijima E, et al. Sonographic diagnosis of biliary atresia in pediatric patients using the “triangular cord” sign versus gallbladder length and contraction. AJR Am J Roentgenol. 2003;181:1387–1390. doi: 10.2214/ajr.181.5.1811387. [DOI] [PubMed] [Google Scholar]
- 50.Mittal V, Saxena AK, Sodhi KS, Thapa BR, Rao KL, Das A, et al. Role of abdominal sonography in the preoperative diagnosis of extrahepatic biliary atresia in infants younger than 90 days. AJR Am J Roentgenol. 2011;196:W438–W445. doi: 10.2214/AJR.10.5180. [DOI] [PubMed] [Google Scholar]
- 51.Han SJ, Kim MJ, Han A, Chung KS, Yoon CS, Kim D, et al. Magnetic resonance cholangiography for the diagnosis of biliary atresia. J Pediatr Surg. 2002;37:599–604. doi: 10.1053/jpsu.2002.31617. [DOI] [PubMed] [Google Scholar]
- 52.Liu B, Cai J, Xu Y, Peng X, Zheng H, Huang K, et al. Three-dimensional magnetic resonance cholangiopancreatography for the diagnosis of biliary atresia in infants and neonates. PLoS One. 2014;9:e88268. doi: 10.1371/journal.pone.0088268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee SM, Cheon JE, Choi YH, Kim WS, Cho HH, Kim IO, You SK. Ultrasonographic diagnosis of biliary atresia based on a decision-making tree model Korean. J Radiol. 2015;16(6):1364–1372. doi: 10.3348/kjr.2015.16.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ikeda S, Sera Y, Ohshiro H, Uchino S, Akizuki M, Kondo Y. Gallbladder contraction in biliary atresia: a pitfall of US diagnosis. Pediatr Radiol. 1998;28(6):451–453. doi: 10.1007/s002470050380. [DOI] [PubMed] [Google Scholar]
- 55.Han SJ, Kim MJ, Han A, Chung KS, Yoon CS, Kim D, Hwang EH. J magnetic resonance cholangiography for the diagnosis of biliary atresia. Pediatr Surg. 2002;37(4):599–604. doi: 10.1053/jpsu.2002.31617. [DOI] [PubMed] [Google Scholar]
- 56.Tang ST, Li SW, Ying Y, Mao YZ, Yong W, Tong QS. The evaluation of laparoscopy-assisted cholangiography in the diagnosis of prolonged jaundice in infants. J Laparoendosc Adv Surg Tech A. 2009;19(6):827–830. doi: 10.1089/lap.2008.0432. [DOI] [PubMed] [Google Scholar]
- 57.Bălănescu RN, Bălănescu L, Drăgan G, Moga A, Caragaåã R. Biliary lithiasis with choledocolithiasis in children. Chirurgia (Bucur). 2015;110(6):559–561. [PubMed] [Google Scholar]
- 58.Jeanty C, Derderian SC, Courtier J, Hirose S. Clinical management of infantile cholelithiasis. J Pediatr Surg. 2015;50(8):1289–1292. doi: 10.1016/j.jpedsurg.2014.10.051. [DOI] [PubMed] [Google Scholar]
- 59.Wesdorp I, Bosman D, de Graaff A, et al. Clinical presentations and predisposing factors of cholelithiasis and sludge in children. JPGN. 2000;31:411–417. doi: 10.1097/00005176-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 60.Della Corte C, Falchetti D, Nebbia G, et al. Management of cholelithiasis in Italian children: a national multicenter study. World J Gastroenterol. 2008;14:1383–1388. doi: 10.3748/wjg.14.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matos C, Avni EF, Van Gansbeke D, Pardou A, Struyven J. Total parenteral nutrition (TPN) and gallbladder diseases in neonates. Sonographic assessment. J Ultrasound Med. 1987;6(5):243–248. doi: 10.7863/jum.1987.6.5.243. [DOI] [PubMed] [Google Scholar]
- 62.Poffenberger C, Gausche-Hill M, Ngai S, et al. Cholelithiasis and its complications in children and adolescents. Pediatr Emerg Care. 2012;28:68–79. doi: 10.1097/PEC.0b013e31823f5b1e. [DOI] [PubMed] [Google Scholar]
- 63.Svensson J, Makin E. Gallstone disease in children. Semin Pediatr Surg. 2012;21:255–265. doi: 10.1053/j.sempedsurg.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 64.Rothstein D, Harmon CM. Gallbladder disease in children. Sem Ped Surg. 2016;25:225–231. doi: 10.1053/j.sempedsurg.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 65.Amedee-Manesme O, Bernard O, Brunelle F, Hadchouel M, Polonovski C, Baudon JJ, Beguet P, et al. Sclerosing cholangitis with neonatal onset. J Pediatr. 1987;111:225–229. doi: 10.1016/s0022-3476(87)80072-0. [DOI] [PubMed] [Google Scholar]
- 66.Baker AJ, Portmann B, Westaby D, Wilkinson M, Karani J, Mowat AP. Neonatal sclerosing cholangitis in two siblings: a category of progressive intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 1993;17:317–322. doi: 10.1097/00005176-199310000-00016. [DOI] [PubMed] [Google Scholar]