Abstract
Ultrasound scan is a painless and radiation-free imaging modality and, therefore, it is widely considered the first-choice diagnostic tool in the setting of hepatopathies in paediatric patients. This article focuses on the normal ultrasound anatomy of the liver in neonatal and paediatric age and reviews the ultrasound appearance of the most common diffuse and focal liver affections.
Keywords: Ultrasound, Liver, Paediatric, Diffuse hepatopathies, Focal liver lesions, Cholestasis
Introduction
Ultrasound (US) scan is the first-choice diagnostic tool in the setting of hepatopathies in paediatric patients. According to their duration, less or more than 6 months, hepatopathies are, respectively, defined acute or chronic. In addition, there are focal and diffuse liver diseases. Diagnostic imaging plays a crucial role in the evaluation of liver affections as they may both present with non-specific clinical and laboratory picture or represent an incidental finding, especially when signs and symptoms arise late. Nevertheless, also imaging presentation of liver injuries extensively overlaps, therefore, correlation with clinical data is mandatory [1, 2].
Sonography is a quick, painless and radiation-free tool; thus, it is particularly helpful in the diagnostic approach in children. In some cases, further imaging modalities are needed, while in other conditions, it is considered enough for the assessment of liver diseases or for their follow-up.
Anatomy, technique and ultrasonographic aspects
Liver is located in the right upper quadrant of the abdomen and is divided into a right lobe on the right-hand side of the falciform ligament and a left lobe on the opposite side, while the quadrate and the caudate lobes are near to the hilum. According to Couinaud Codification, each lobe is composed of many segments on the basis of portal and venous branches (Fig. 1), so that each one has its own vascular peduncle. The right portal vein separates anterior and posterior segments of the right lobe, the left portal branch, instead, identifies Segment II and Segment III on the left hepatic lobe. Segment I is an autonomously vascularised lobe situated behind the venous ligament [3]. A complete US study of the liver requires systematic study through longitudinal, axial and oblique scans; it also includes the evaluation of intrahepatic vascular structures (intrahepatic and portal veins) and the study of the gallbladder. When necessary, basic B-mode study should be completed by colour-Doppler technique [1, 4].
Fig. 1.
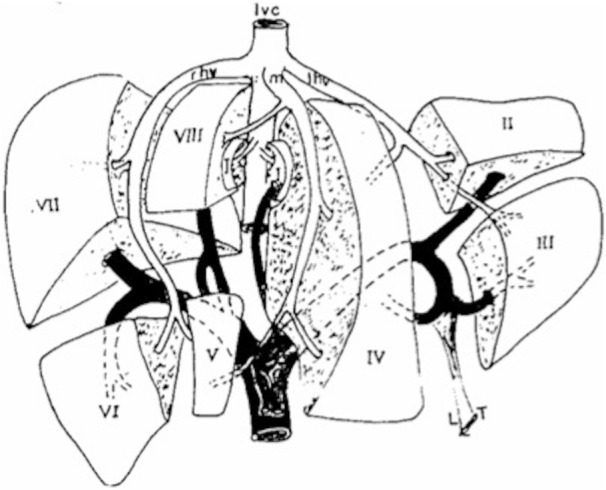
Schematic overview of segmental anatomy of the liver. Commonly, segments are identified following portal and venous branches ramifications
US examination of the liver is performed while the patient lies in the supine position with a convex probe. However, as their abdominal wall is thin, in young children a high-frequency linear probe (5–20 MHz) may be used for obtaining clearer images with improved spatial resolution.
In addition, when possible, it is useful to acquire scans during deep breaths to allow better visualisation of abdominal organs.
A standardised approach is achievable: first, longitudinal and axial scans along the midline are acquired to evaluate the left lobe; secondly, longitudinal and oblique subcostal scans are performed to image the right lobe (Fig. 2).
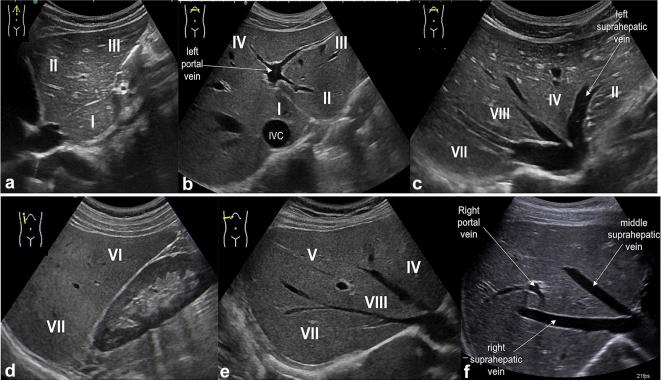
Fig. 2.
US study of the liver: longitudinal and transverse scan along the midline (a, b) show the left hepatic lobe (segments II and III) separated from segment I (close to the inferior vena cava, IVC) and the left portal vein with its feeding branches for segments II, III and IV; in the oblique subcostal scan (c) there are right, middle and left hepatic veins which separate segments VII, VIII, IV and II; longitudinal scan along hemiclavear line (d) shows segments VII and VI; oblique subcostal scans (e, f) show right portal vein separating segments VIII and V
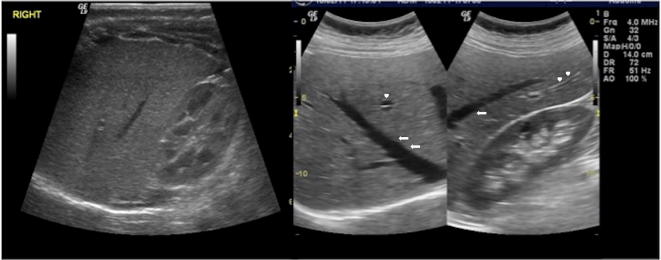
Normally, the liver presents homogeneous echotexture with evenly distributed medium- to low-intensity echoes. Such homogeneous texture is disrupted by tubular anechoic structures representing vessels (portal vein and hepatic veins branches) crossing the hepatic parenchyma (Fig. 3). Hyperechoic rim, due to peri-portal connective tissue, always surrounds the portal vein branches in the hepatic parenchyma [1, 2, 4].
Fig. 3.
US study of the liver, longitudinal and oblique scans: normal hepatic echostructure is characterised by homogenous low-to-intermediate echoes similar to the adjacent renal cortex; there are also tubular anechoic structures representing intrahepatic veins (arrows) and portal vein branches (arrowheads)
Routinely, a longitudinal scan along the hemiclavear line is used to measure the longitudinal diameter of the right lobe, which is reported to evaluate the hepatic size (Fig. 4) [5–7]. Generally, the right lobe is bigger and extends to the lower pole of the ipsilateral kidney while the left lobe reaches the left of the aorta. By contrast, in neonatal period, the liver is relatively broad and may extend much over the midline with the left hepatic lobe being physiologically hypertrophic. Later, the volume of the right lobe increases more than the left one [1].
Fig. 4.
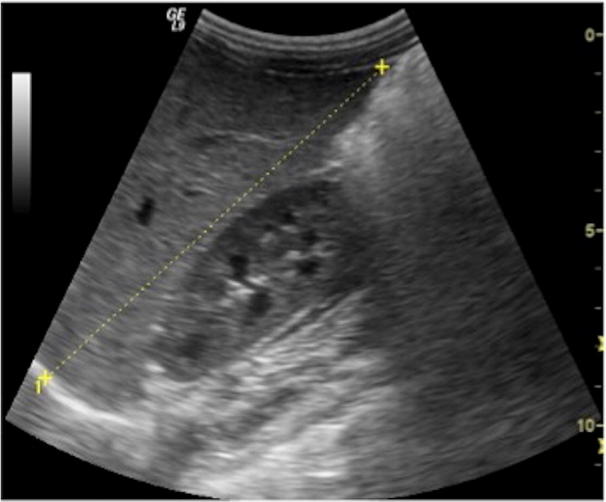
US study of the liver: longitudinal scan along the right hemiclavear line is commonly used to measure the longitudinal diameter of the right hepatic lobe
Furthermore, neonatal liver is characterised by bright echostructure and sharp margins, which progressively become smooth.
Table 1 reports normal range of hepatic diameter related to the patient age [7].
Table 1.
Normal range of hepatic diameter related to the patients age
Modified from Konuş OL et al. [7]
| Age versus longitudinal dimensions of right lobe of liver | |
|---|---|
| Age range (month) | Longitudinal dimensions (mm) |
| 1–3 | 64 |
| 4–6 | 73 |
| 7–9 | 79 |
| 12–30 | 85 |
| 36–59 | 86 |
| 60–83 | 100 |
| 84–107 | 105 |
| 108–131 | 105 |
| 132–155 | 115 |
| 156–179 | 118 |
| 180–200 | 121 |
As well as the liver volume, with the child growth also increases the portal vein calibre, which is measured at the hilum, if possible during a deep breath (Fig. 5). The normal range is considered 3–5 mm in newborns and up to 7–11 mm in older children [8].
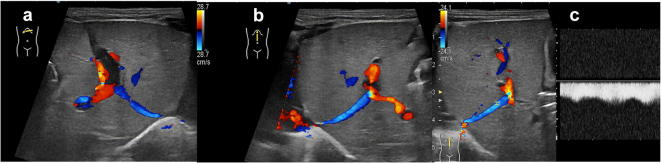
Fig. 5.
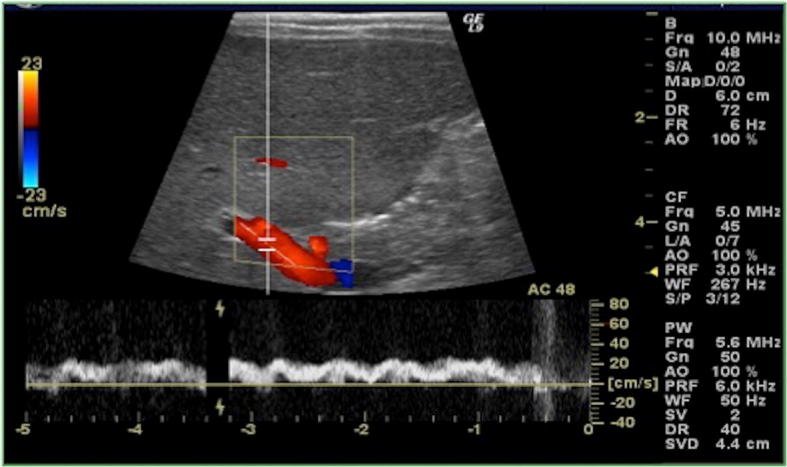
US of the liver, colour-Doppler study of the portal vein at the hilum. Portal flow is usually directed towards the liver, with normal mean velocity of 15–18 cm/s
US study in newborns should also evaluate the presence of still patent ductus venosus, which may be a useful peripheral venous access through the umbilical vein (Fig. 6) [1].
Fig. 6.
US of the liver with colour-Doppler (a, b) and colour-pulse-Doppler (c) study in a newborn depicts patent ductus venosus (with hepatofugal flow in blue)
Commonly, intra-hepatic bile ducts are not clearly identified at US study. Conversely, common hepatic duct and common bile duct are detected near to the hilum. There is not complete agreement about the normal calibre of common bile duct, however, it is generally considered pathological a diameter greater than 7 mm. Gallbladder lies on the inferior surface of the liver and is characterised by a normal length of 1.5–3 cm in infants < 1-year-old and 3–7 cm in older children [9–11].
Diffuse hepatopathies
When considering differential diagnosis among numerous diffuse liver injuries, it is important to correlate clinical picture with laboratory findings and also with the age of the patient. This latter may be crucial as often there is different distribution of hepatopathies among various age groups. For example, newborns mostly suffer from metabolic disorders, cardiac failure, and hepatitis. On the contrary, in older children steatosis, cirrhosis, venous-occlusive disease or cystic fibrosis related hepatopathy are more common [1, 12].
Acute hepatitis
Imaging is only marginally helpful in the evaluation of acute hepatitis. Actually, the diagnosis of this condition is mostly based on clinical and laboratory parameters. Aside from hepatomegaly, there are no specific US signs of hepatitis. The “starred sky” effect, given by a diffuse hypo-echogenicity of the liver with hyperechoic spots along the small portal branches, has been considered specific of acute hepatitis (Fig. 7), but it was demonstrated that it could be found also in healthy subjects. Gallbladder can show thickened walls, due to parietal hypertonus and/or associated oedema, sometimes with a triple-layer effect [1]. Sometimes, there is also ascites.
Fig. 7.
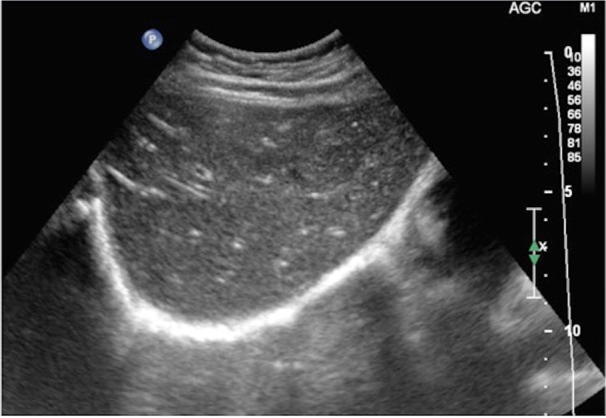
US of the liver, oblique scan in patient affected by acute hepatitis: there is an increase of hepatic volume and slight decrease of echostructure, while portal branches wall appears brighter
Hepatic steatosis
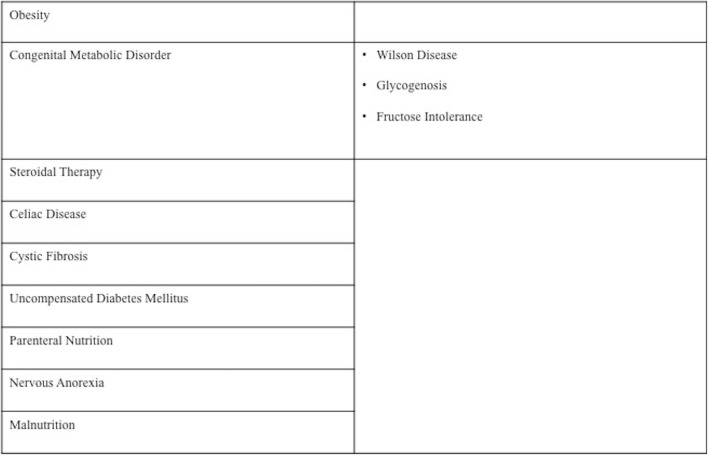
In most of cases, in paediatric patients hyper-echogenicity of the liver is related to steatosis. This quite common changing is usually a reversible injury, characterised by an estimated prevalence of 10% [10]. It may present with a diffuse or focal pattern and is related to hepatocyte fat deposition depending on both abnormal alimentation, such as obesity, malnutrition, parenteral nutrition, nervous anorexia, or steroid therapy, or several metabolic/enzymatic disorders such as uncompensated diabetes mellitus, celiac disease, cystic fibrosis, Wilson disease, glycogenosis, fructose intolerance (Table 2) [13–16].
Table 2.
Main causes of steatosis in paediatric age
At US examination, diffuse fatty liver appears bright, markedly hyperechoic compared to the adjacent renal cortex and with blurred appearance of intra-hepatic vessel walls. The degree of these changing reflects the severity of the fat deposits. In particular, Grade I refers to diffusely bright echo-pattern with still well-defined diaphragmatic profile, Grade II to bright liver with shadowing of periportal echogenicity, Grade III to very bright liver with loss of diaphragmatic profile (Fig. 8) [16].
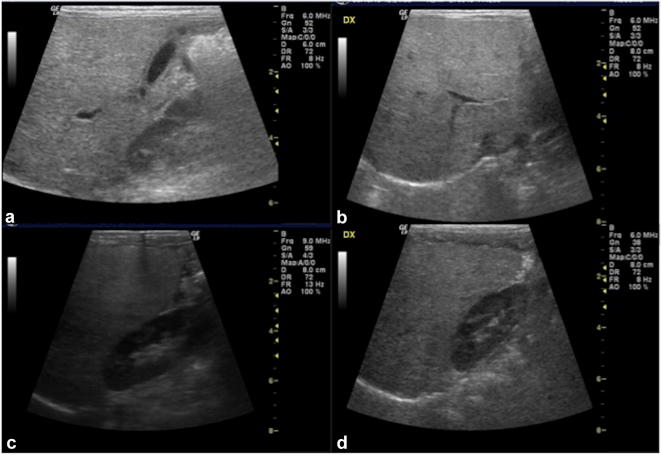
Fig. 8.
US of the liver, longitudinal and oblique scans (a–d): the echogenicity of the liver is particularly bright, especially when compared to the adjacent renal cortex (c, d); moreover, there is blurred appearance of intrahepatic vessels walls and loss of diaphragmatic profile
Sometimes, there are areas not involved in fat deposition, known as “fat sparing”, which could mimic focal lesions and need magnetic resonance imaging (MRI) to be clarified. Conversely, focal pattern of steatosis may result as geometrical or finger-shaped bright areas without any “mass effect” (Fig. 9). These areas are usually found close to the falciform ligament, the “porta hepatis” or the gallbladder fossa [17].
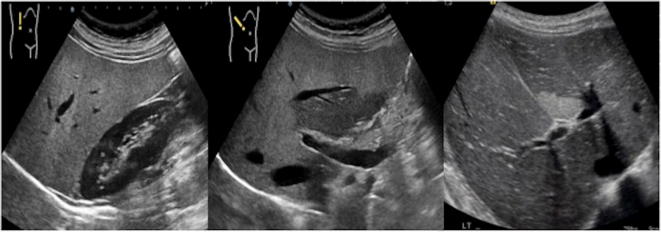
Fig. 9.
US of the liver, longitudinal, axial and oblique scan: there is hyperechogenic liver parenchyma (particularly when compared to the cortex of right kidney) with quite well-defined area of relatively hypoechogenicity (a, b) due to fat sparing-area; in the right figure (c) there is a geometrical shaped bright area which does not cause any mass effect and is therefore in keeping with focal hepatic steatosis
Cirrhosis
Liver cirrhosis is the result of chronic inflammation which leads to cellular regeneration and reparative process finally resulting in fibrosis. It is associated with many different conditions and it is considered rare in newborns. Most frequent forms of cirrhosis in paediatric population are biliary and post-necrotic [18–21]. US scan generally shows volume change of cirrhotic liver, with a small right lobe and hypertrophic caudate and left lobes. Moreover, margins become irregular, typically finely indented in micronodular pattern and roughly lobulated in macronodular one. Fibrosis makes the overall hepatic echostructure appear diffusely and roughly heterogeneous (coarse pattern) (Fig. 10).
Fig. 10.
Abdominal US study shows changes in hepatic cirrhosis, with evidence of volume decrease of the right lobe, irregular margins and roughly heterogeneous echogenicity (coarse pattern) in the right (a) and left lobe (b)
In addition, the portal vein calibre is usually increased due to portal hypertension, which is often found with ascites, splenomegaly, collateral vascular circles and slow or inverted spleno-portal flow [18]. The progressively decreased flow velocity in portal vein may lead to thrombosis. This situation may be followed by lysis of the thrombus and sometimes the cavernous transformation of portal vein (cavernoma), which is constituted by venous channels with hepatopetal flow (Fig. 11) [22]. Furthermore, the hepatic veins are reduced in calibre and may sometimes show parietal irregularities due to nodular regenerative pattern.
Fig. 11.
US evaluation in cirrhotic liver with colour-Doppler study: at the hilum, the portal vein is replaced by portal cavernoma, composed by many small tortuous vessels as consequence of portal hypertension and vein thrombosis
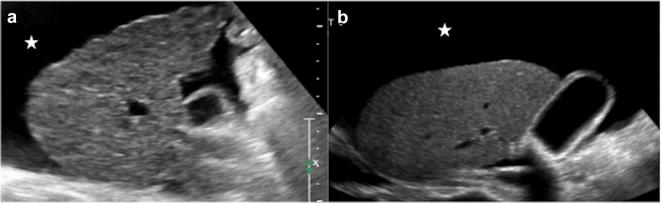
Finally, the complete loss of normal hepatic structure leads to extremely low protein synthesis, which contributes to the development of ascites. This latter is usually found in peri-hepatic, peri-splenic and pelvic spaces (Fig. 12).
Fig. 12.
Abdominal US in advanced cirrhotic condition shows hepatic changes do to regeneration process and fibrosis (a, b), with also ascites, that is identified as anechoic fluid around the perihepatic space (star)
The final step of hepatic changes in cirrhosis is diffused fibrosis. However, before this process becomes irreversible, there are several degrees of injuries that progressively accumulate.
Thus, recently, new diagnostic tools have been introduced to evaluate the mounting fibrotic damages in the liver. Elastography is one of these, which allows evaluation of hepatic fibrosis by measurement of hepatic stiffness. Even though biopsy has been largely considered the gold standard for the diagnosis of liver fibrosis, recently, many authors underlined several disadvantages connected to this procedure. In fact, it is invasive, with risk of haemorrhage, and, because of heterogeneous distribution of fibrosis, it is also associated to high risk of sampling errors. Therefore, elastography is progressively becoming the modality of choice for the assessment of hepatic fibrosis [18, 19]. Transient elastography is a non-invasive technique consisting of US transducer that follows the propagation and the velocity of a shear wave previously transmitted to the tissue. Actually, it has been demonstrated that stiffness is related to the degree of fibrosis and it affects the wave velocity. One more elastographic technique is the point shear-wave elastography, based on the evaluation of the speed of the shear waves propagating through a defined ROI and which result from compression of the tissue by push pulse of standard transducer. A total of 10–12 ROIs are selected placing the probe on the intercostal spaces and mean, median and average velocities are then automatically calculated (Fig. 13). The usefulness of an accurate evaluation of the liver fibrosis is to understand if there is the possibility for the patient to benefit from medical treatment [23–25].
Fig. 13.
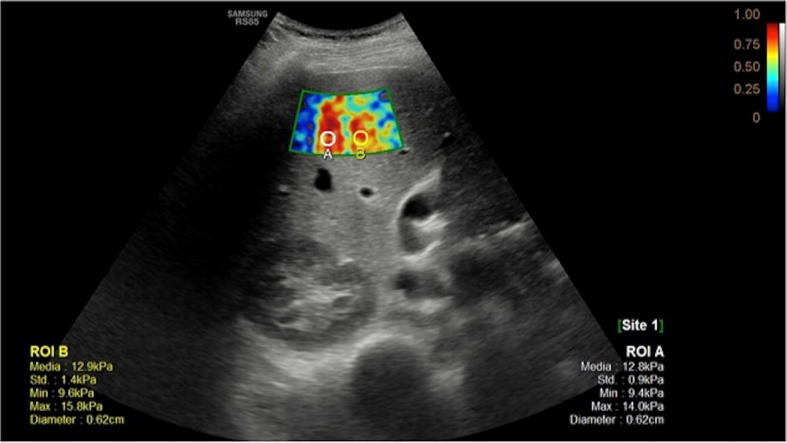
Elastography of cirrhotic liver allows to evaluate stiffness of the parenchyma, which is related to the degree of fibrosis, as it affects the wave velocity measured by the probe
Cystic fibrosis
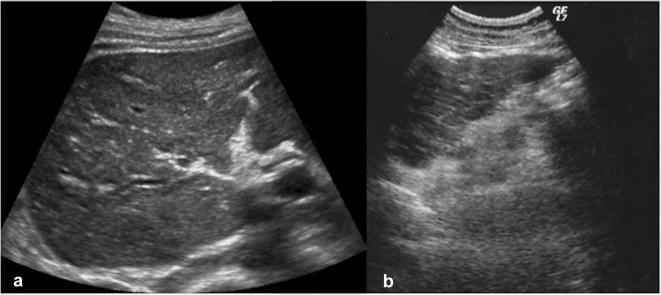
Hepatic manifestations of cystic fibrosis are encountered in older patients surviving to respiratory complications. At US study, liver may be affected by a wide spectrum of alterations, varying from increased volume with hyperechoic structure to a coarse pattern with irregular margins and concurrent hyperechoic areas. In addition, there are injuries to the biliary tree that becomes irregular with tapering of intrahepatic branches and also strictures and beading (Fig. 14) [1, 26].
Fig. 14.
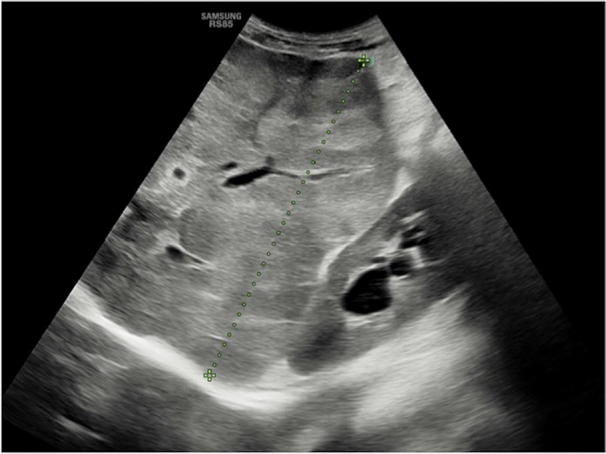
US study of the liver in patient with cystic fibrosis shows diffuse echostructure changes with alternation of hypoechoic and hyperechoic areas
Venous-occlusive disease
Venous-occlusive disease is characterised by damage to the central veins of hepatic lobule resulting in hepatic congestion. It usually follows a primary injury to sinusoidal endothelial cells due to bone marrow transplantation or, very rarely, chemo- or radio-therapy. Ultrasonographic findings are non-specific and the diagnosis may be suspected when there is an increase of liver volume associated to splenomegaly, ascites, gallbladder wall thickening, decreased hepatic vein calibre (< 3 cm), portal vein dilatation. Progressively, liver volume reduces, except for caudate lobe because of its autonomous vascularisation. At colour-Doppler reversed portal flow may be detected [27, 28].
Focal liver lesions
Common paediatric benign liver tumours are infantile haemangioma, mesenchymal hamartoma, focal nodular hyperplasia and adenoma; hepatoblastoma and hepatocarcinoma are most frequent malignant focal liver lesions in children [1, 29–31]. US study generally allows the detection of the tumour both in symptomatic patients and in cases of incidental findings. Further diagnostic modalities may, however , be necessary to better characterise the lesion.
Haemangioma
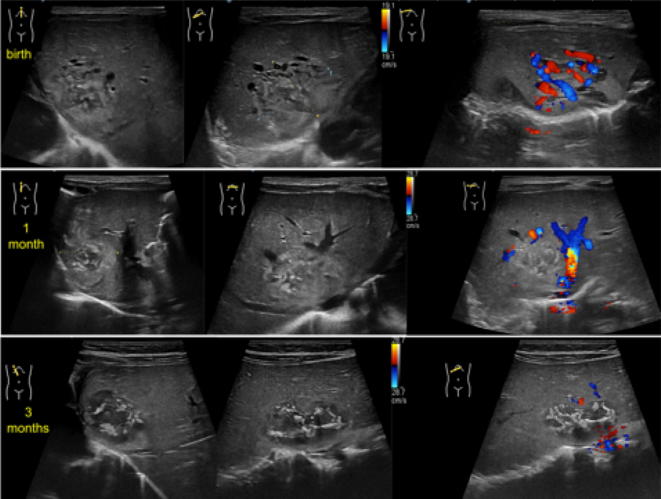
Hepatic haemangiomas are the most frequent hepatic lesions in neonates, detected in patients younger than 6 months. They are also divided into three different sub-types, characterised by different behaviours: focal, multifocal or diffuse. Focal hepatic haemangiomas are congenital variant similar to rapidly involuting congenital haemangioma (RICHs) as they fully disappear usually by 14 months. Multifocal hepatic haemangiomas develops after birth and after a proliferative phase tend to decrease in a longer period (6–10 years). Diffuse lesions replace the entire parenchyma. They all can be asymptomatic or be associated to mass effect or severe complications mostly related to significant arterio-venous shunts and consequent cardiac failure. At US study, focal haemangiomas are detected as heterogeneous mass mostly hypoechoic with central area of necrosis or fibrosis and some anechoic region related to dilated vessels (Fig. 15). All the variants of infantile age haemangioma at colour-Doppler may show high-flow prominent vascular structures, sometimes with well depicted arterio-venous shunts. The most important differential diagnosis is between focal hepatic haemangioma and hepatoblastoma, which is similar in location, main features and epidemiology. However, the lack of persistent elevation of blood alpha-fetoprotein, and, for the diffused form, the presence of very large mass associated with heart failure, will suggest the diagnosis of focal hepatic haemangioma [30, 32–35].
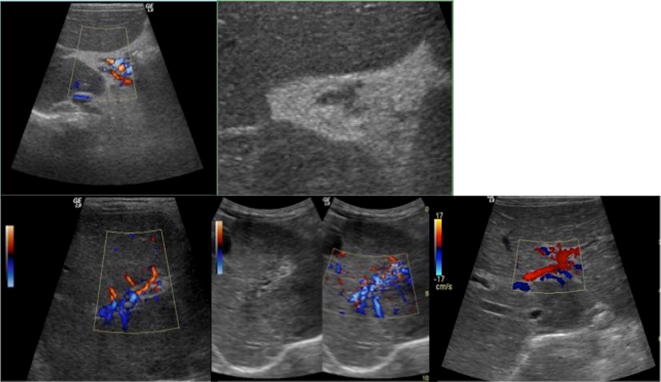
Fig. 15.
US of the liver, oblique B-Mode imaging and colour-Doppler study at birth (upper line), at 1 month (central line) and at 3 months (lower line): there is a large heterogeneous mass, measuring about 20 mm, mostly iso-hypo-echoic, with internal anechoic tubular structures. Colour-Doppler images shows internal vessels characterised by both arterial and venous flow. At follow-up, B-mode evaluation shows slight reduction of diameter with increase of the internal anechoic necrotic component. Moreover, colour-Doppler tool demonstrates significant reduction and finally disappearing of internal vascular flow. These features were in keeping with the diagnosis of focal hepatic haemangioma
Mesenchymal hamartoma
This is the second most common benign liver tumour in paediatric population after infantile haemangioma. Most of cases are diagnosed by 5 years and are associated to asymptomatic abdominal swelling. It is constituted by mixed components including biliary ducts, hepatocytes, vessels and mesenchymal tissue, with a variable amount of fluids. At US, quite often it is described as a multicystic mass, with anechoic components separated by thin or thick echogenic septa. Rarely, solid component may predominate. Differential diagnosis includes hepatoblastoma, which is however typically associated to high alpha-fetoprotein blood levels [30].
Focal nodular hyperplasia
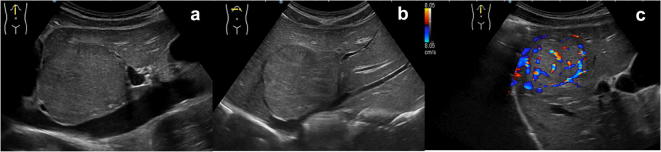
Focal nodular hyperplasia (FNH) is more frequently found in adults rather than in children. Nevertheless, it represents up to 2% of all primitive liver tumours in paediatric population and it is usually recognised incidentally in asymptomatic 2 to 5 years old children [30]. This lesion is characterised by hyperplastic hepatocytes separated by Kupffer cells and sinusoids contained in fibrous septa. These latter radiate from a central vascular axis, which is responsible for the peculiar central stellar scar. At US exam FNH is usually homogenous, with sharp margins, variably iso- hypo- or hyper-echoic, occasionally with hyperechoic central scar (Fig. 16). Colour-Doppler study may detect vascular flow within the scar and its peripheral branches which therefore assume a spoke-wheel like appearance [30, 36, 37].
Fig. 16.
US study of the liver, longitudinal and transverse B-mode images (a, b) and longitudinal colour-Doppler scans (c): there is a well-defined large mass, mostly iso-echoic, showing internal and peripheral vascular flow at colour-Doppler study. This latter takes a spoke-wheel-like appearance which is typical for focal nodular hyperplasia
Adenoma
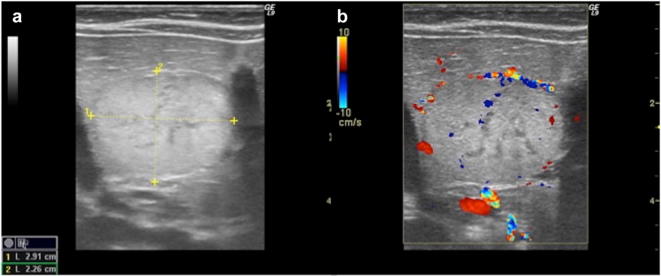
Liver adenoma is a rare finding in children. Most frequently, they are related to systemic conditions such as glycogenosis or chronic steroid therapies. It is usually not associated with specific symptoms, however, in few cases it has been described as abdominal mass. At US it is typically a well-defined lesion relatively hyperechoic, particularly in lipid-rich variants or when there is an amount of blood within (Fig. 17). Less commonly, in steatotic bright liver, the lesion may result relatively hypoechoic. Doppler evaluation may demonstrate peripheral and internal vessels with no central arterial flow (in contrast to FNH) [30, 38].
Fig. 17.
US study of hepatic adenoma with B-mode and colour-Doppler images: there is a well-defined large hyperechoic mass, with some internal anechoic areas, which shows peripheral and internal vessels (a, b)
Hepatoblastoma
Hepatoblastoma is the most frequent primary malignant hepatic tumour in children, being up to 48% of all malignant lesion of the liver. In most of cases, the diagnosis is made by 5 years and the prognosis is unfortunately poor. It typically presents with a large isolated mass in the right lobe and is associated, in up to 90% of cases, with elevated alpha-fetoprotein blood levels [1]. US evaluation can depict a well-defined lesion, mostly hyperechoic but heterogeneous, with internal cystic areas because of necrotic degeneration and with hyperechoic calcific spots (Fig. 18). Doppler study shows high vascularisation. Most important differential diagnosis is with infantile haemangioma as both of them are solid lesions with significant vascularisation, sometimes with calcifications, and they may be found by 1 year. However, hepatoblastoma is characterised by a more aggressive behaviour. Actually, there could be encasement or even invasion of portal branches. Moreover, elevated alpha-fetoprotein blood levels are suggestive for hepatoblastoma [1, 31, 39].
Fig. 18.
Ultrasonographic images of hepatic hepatoblastoma: there is an ill-defined heterogeneous mass, involving almost completely the right lobe, which shows hyper- and isoechoic areas (a–c); many internal vessels are identified at colour-Doppler image (d)
Hepatocarcinoma
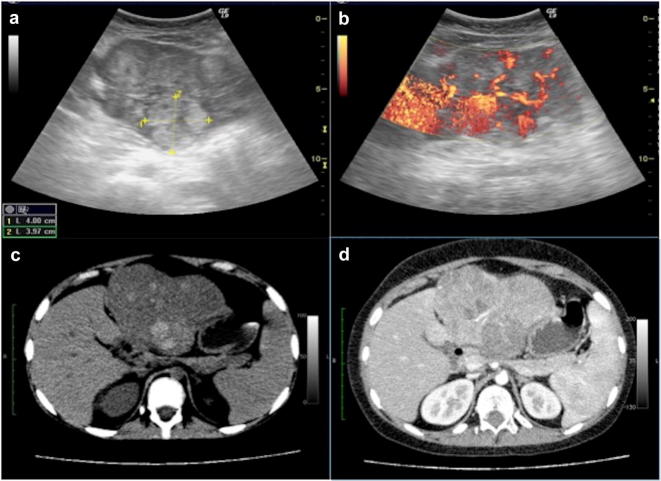
Hepatocarcinoma is the second most common primary malignant hepatic tumour in children and therefore it represents up to 20% of lesions in this group [1]. It has been described in association with several predisposing conditions such as primary biliary atresia, glycogenosis type I, hepatitis by virus B or C. As well as for adults, even for children hepatocarcinoma may present as isolated mass, multifocal or diffuse. At US study, small lesions appear hypoechoic to the liver, bigger ones are more heterogenous, with hyper- or hypo-echoic internal areas, respectively, due to fat infiltration and/or haemorrhages or necrosis (Fig. 19). Doppler usually shows arterial internal flows. Main differential diagnosis is with other vascularised solid lesions, particularly with adenoma and FNH [31, 40].
Fig. 19.
Hepatocarcinoma at US B-Mode and Doppler images (a, b) and axial computed tomography and contrast-enhanced computed tomography images (c, d). At US the tumour appears as large heterogeneous lesion that bulges the liver surface, with significant internal vascular flow (a, b). Computed tomography images show large lesion with hyperdense components at un-enhanced scan (c), which demonstrates heterogeneous enhancement (d)
Liver abscess
Liver abscess is a quite rare diagnosis in developed countries, nonetheless, it is to consider among differential diagnoses of focal liver lesions. In particular, umbilical region infections or complicated appendicitis should be considered in children with depressed immunity, who are more vulnerable to sepsis. Other known predisposing conditions are congenital abnormalities of biliary tree, which can be associated to obstruction and therefore determine bacterial proliferation. In most of cases pyogenic bacteria (80%) are involved, generally associated to single abscess that frequently is localised in the right hepatic lobe. Histologically, it is constituted by central necrotic and purulent portion with well-defined fibrotic wall. Clinical picture is usually characterised by abdominal pain and fever, less commonly there is vague pain, fatigue and weight loss. At US evaluation, pyogenic abscess appears as roundish lesion with hypo to anechoic centre, sometimes with mixed echoes, delimited by thick wall. Less frequently are found fungal abscesses that usually are multiple small lesions scattered throughout the liver parenchyma. Their US appearance may vary from target lesions to small anechoic ones. Moreover, there is the amoebic abscess, the result of a rare parasitic infection. At US evaluation it is difficult to differentiate from other liver abscesses as it is characterised by central anechoic component, which can show some low-level internal echoes, and variably thick wall (Fig. 20) [41].
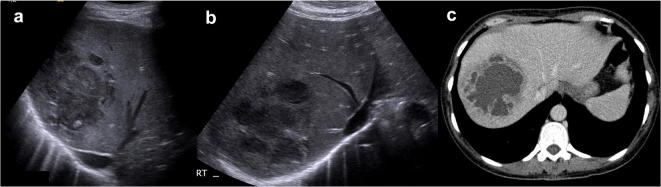
Fig. 20.
US study (a, b) and computed tomography axial scans venous phase (c) of amoebic abscess: there is a large well-defined lesion involving VII/VIII segments, which shows internal hypo and anechoic areas at US. Computed tomography scans demonstrates internal fluid component with only enhancement of the irregularly thick peripheral wall
Hepatic complications associated with umbilical venous catheter
As previously mentioned, US study in newborns should also evaluate the presence of still patent ductus venosus, which may be a useful peripheral venous access through the umbilical vein.
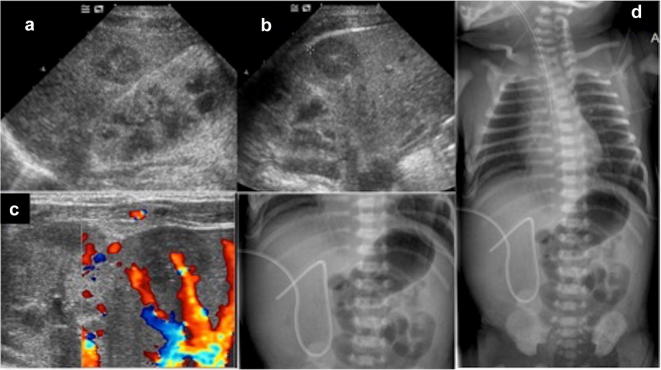
Usually, the right progression of umbilical venous catheter (UVC) from single umbilical vein to the inferior vena cava, passing through the ductus venosus, is found by abdominal radiography [42]. Some authors proposed US as radiation-free imaging modality to assess the correct position of the catheter tip at the level of ductus venosus or inferior vena cava distal to the right atrium [40]. In addition, US may become necessary when the catheter erroneously perforates the wall of intrahepatic vessels, secondary causing intrahepatic hematoma. This is an uncommon complication of malpositioned catheter which could be suspected when there is poor blood return from the catheter or there is difficult administration of fluids through it. US study may detect the presence of well-defined hepatic mass, initially anechoic and later heterogeneous, which normally resolves spontaneously and may progressively become calcific (Fig. 21). Anyway, US monitoring is advisable [42, 43].
Fig. 21.
US study of the liver demonstrates the presence of roundish hypo- anechoic area, probably due to parenchymal hematoma caused by displaced UVC (a–c). Frontal abdominal radiography of a newborn showing the UVC ending at the level of right portal vein (d)
Cholestasis
There are several conditions associated to cholestasis. Here we will focus on biliary atresia and choledochal cyst. Biliary atresia is a condition mostly found in newborns by 3 months, presenting with persistent jaundice. Conversely, choledochal cysts may not be associated with jaundice and present only in more advanced age. Diagnostic imaging plays an important role in the diagnosis and management of these conditions. Actually, if not recognised, they may lead to hepatic transplant by 1 year of life.
Choledochal cysts
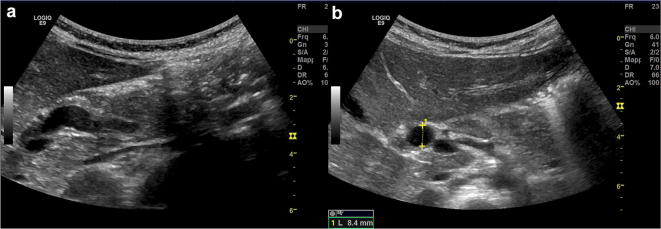
Choledochal cysts represent congenital dilatations of the biliary tree. There are many variants of choledochal cysts, all related to congenital conditions. The clinical picture is variable, related to the age of the patient: obstructive jaundice is the main sign in children, while abdominal pain is more common in adults. Only the 8% of children present symptoms by 10 years. In older children and teenagers, it can be recognised the typical triad including fever, jaundice and abdominal pain. Rarely, choledochal cysts may present as palpable abdominal mass [44–46]. The current classification (Todani’s) includes (Fig. 22): type I, the most common variant that is characterised by cystic or fusiform dilatation of extrahepatic bile duct (Fig. 23); type II, that is quite rare, represents a diverticulum of the extrahepatic bile duct; type III is a cystic dilatation of extrahepatic bile duct within the duodenal wall; type IV is a cystic dilatation of both main biliary duct and intrahepatic biliary tree; in type V there is a diffused cystic dilatation of intrahepatic biliary ducts (also known as Caroli’s disease) [47].
Fig. 22.
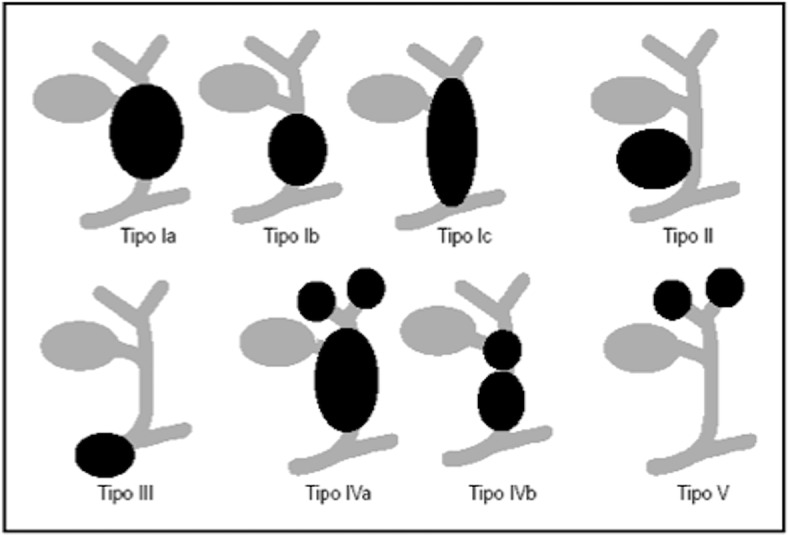
Schematic overview of choledochal cyst (Todani’s classification)
Modified from Soares et al. [44]
Fig. 23.
US study of choledochal cyst type I shows fusiform dilatation (a) of extrahepatic bile duct measuring 8.4 mm (b)
Caroli’s disease is characterised by biliary ducts dilatation, which appears at US as intrahepatic single or multiple cysts. Portal branches may be partially or totally rounded by dilated ducts; frequently, there is also dilatation of the main bile duct.
US is the imaging modality of choice for the first evaluation of intra and extra-hepatic biliary ducts. Main differential diagnosis of choledochal cysts is the ileal duplication cyst, which is depicted as a round anechoic formation close to the hepatic hilum and the gastric antrum. However, ileal duplication cyst shows the peculiar stratified wall sign that cannot be recognised in the choledochal cyst [48]. Other cystic lesions to include in the differential diagnosis are simple cysts of the liver, cystic teratoma and pancreatic pseudocysts, which may require further diagnostic techniques to be excluded. In addition, magnetic resonance cholangiopancreatography (MRCP) and endoscopic retrograde cholangiopancreatography (ERCP) are also useful to obtain a more precise biliary tree anatomy, which is crucial for surgical plan preparation. [49].
Biliary atresia
Biliary atresia is characterised by a progressive obstruction of extrahepatic biliary ducts with subsequent possible involvement of intrahepatic ducts too. It has been postulated a multifactorial aetiology including genetic, infective and toxic factors leading to an obstructive progressive process. If not surgically treated by 45–60 days of life, it is associated to a poor prognosis because of the development of hepatic cirrhosis. Clinical picture is characterised by jaundice, hepatomegaly, acholic stools and brown urine [50–52].
Differential diagnosis between biliary atresia and other causes of obstructive jaundice is crucial to undertake the best therapeutic management and avoid severe complications.
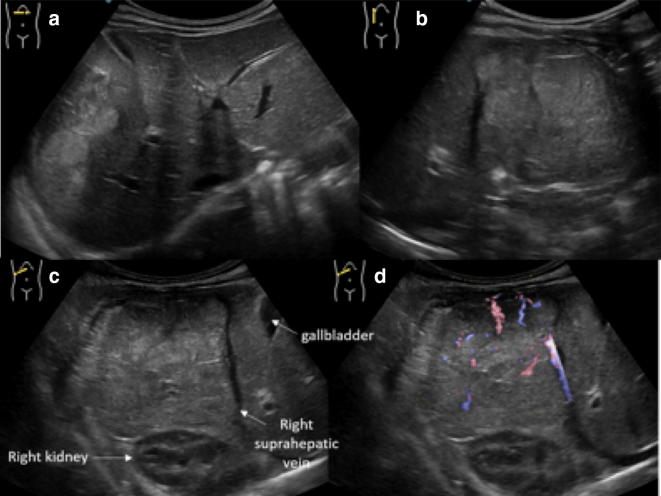
Abdominal US plays a pivotal role in the screening of infantile cholestasis: biliary atresia can be suspected when gallbladder is not visualised (ghost gallbladder) and if there is a hyperechoic tissue anterior to the main portal vein, probably representing fibrotic residuum of atretic biliary ducts (triangular cord sign) (Fig. 24). This latter sign is associated to a sensibility of 78.2% and specificity of 100% in the diagnosis of biliary atresia [53–58].
Fig. 24.
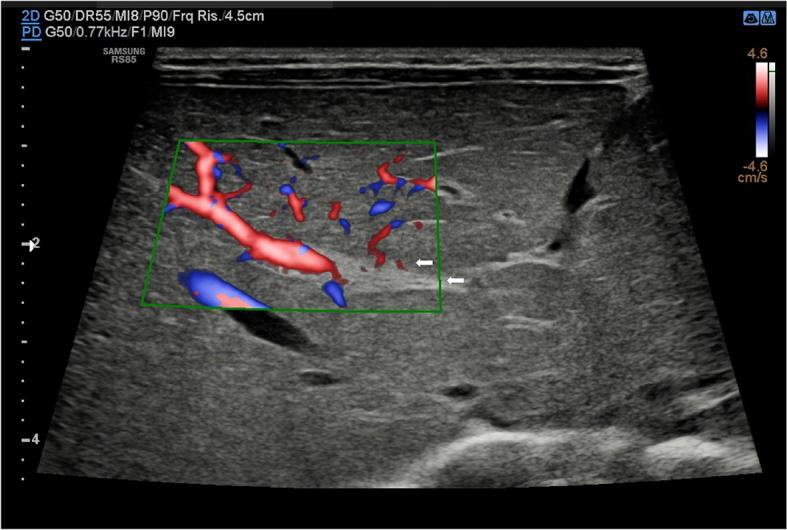
US study of the liver with B-mode (a) and colour-Doppler (b) in a newborn with biliary atresia shows hyperechoic tissue (arrows) just near to the hepatic hilum
In addition, there could be US signs of increased hepatic vascular resistances, such as increased portal vein calibre and increased flow in the subcapsular arteries (Fig. 25) [58, 59].
Fig. 25.
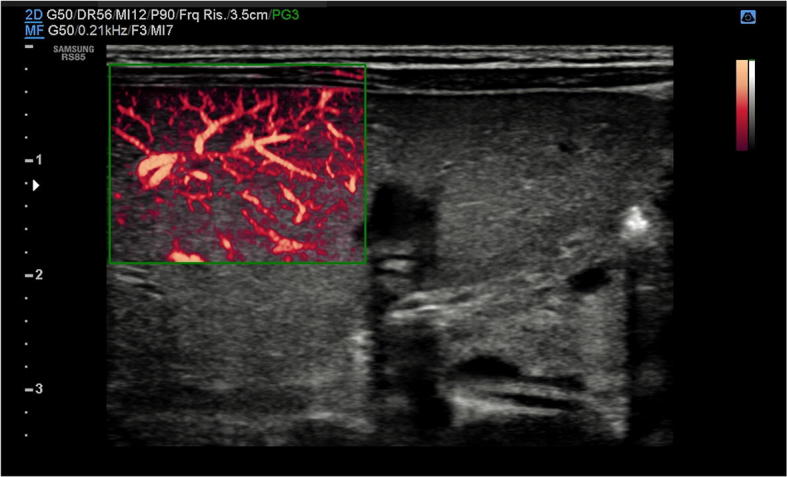
US study of the liver with B-Mode and power Doppler modalities in a newborn with biliary atresia: there is hepatic arterial flow extending to the hepatic surface (subcapsular flow)
However, US study has some limitations: extrahepatic bile duct may sometimes not be visualised even in normal newborns; a not clearly evaluable gallbladder may be also related to other causes of cholestasis; there are, on the contrary, cases of biliary atresia with normal gallbladder (about 20%); depending on operator’s expertise and US scanner quality, triangular cord sign could be sometimes missed [60].
Despite the fact that MRCP and ERCP are potentially useful in the diagnosis of biliary atresia, their routine use in infants is still not accepted. Therefore, definitive diagnosis is often made with cholangiography which may also provide accurate evaluation of biliary tree [61, 62].
Conclusions
Ultrasonography is an essential imaging modality for the first detection and the management of hepatic injuries in children. Therefore, it is important to be aware of basic normal ultrasonographic anatomy and of most common liver focal or diffuse pathologies in order to recognise them and eventually ask for laboratory tests and/or further diagnostic modalities.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and its late amendments. Additional informed consented was obtained from all patients for which identifying information is not included in this article.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Bruyn R (2005) The liver, spleen and pancreas. In: Pediatric US How, Why and When. Elsevier, 131–154
- 2.Riccabona M (2013) Liver and Bile System. In: Pediatric US, ed. Springer 213–245
- 3.Lafortune M, Madore F, Patriquin H, Breton G. Segmental anatomy of the liver: a sonographic approach to the Couinaud nomenclature. Radiology. 1991;181:443–448. doi: 10.1148/radiology.181.2.1924786. [DOI] [PubMed] [Google Scholar]
- 4.Draghi F, Rapaccini GL, Fachinetti C, et al. US examination of the liver: normal vascular anatomy. J US. 2007;10:5–11. doi: 10.1016/j.jus.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niederau C, Sonnenberg A, Müller JE, Erckenbrecht JF, Scholten T, Fritsch WP. Sonographic measurements of the normal liver, spleen, pancreas, and portal vein. Radiology. 1983;149:537–540. doi: 10.1148/radiology.149.2.6622701. [DOI] [PubMed] [Google Scholar]
- 6.Holder LE, Strife J, Padikal TN, Perkins PJ, Kereiakes JG. Liver size determination in pediatrics using sonographic and scintigraphic techniques. Radiology. 1975;117:349–353. doi: 10.1148/117.2.349. [DOI] [PubMed] [Google Scholar]
- 7.Konuş OL, Ozdemir A, Akkaya A, Erbaş G, Celik H, Işik S. Normal liver, spleen, and kidney dimensions in neonates, infants, and children: evaluation with sonography. Am J Roentgenol. 1998;171:1693–1698. doi: 10.2214/ajr.171.6.9843315. [DOI] [PubMed] [Google Scholar]
- 8.Soyupak S, Gunesli A, Seydaoğlu G, Binokay F, Celiktas M, Inal M. Portal venous diameter in children: normal limits according to age, weight and height. Eur J Radiol. 2010;75:245–247. doi: 10.1016/j.ejrad.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 9.Hernanz-Schulman M, Ambrosino MM, Freeman PC, Quinn CB. Common bile duct in children: sonographic dimensions. Radiology. 1995;195(1):193–195. doi: 10.1148/radiology.195.1.7892467. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Wang XL, Li SX, Bai YZ, Ren WD, Xie LM, Zhang SC. Ultrasonographic dimensions of the common bile duct in Chinese children: results of 343 cases. J Pediatr Surg. 2013;48(9):1892–1896. doi: 10.1016/j.jpedsurg.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 11.McGahan JP, Phillips HE, Cox KL. Sonography of the normal pediatric gallbladder and biliary tract. Radiology. 1982;144(4):873–875. doi: 10.1148/radiology.144.4.7111740. [DOI] [PubMed] [Google Scholar]
- 12.Zwiebel WJ. Sonographic diagnosis of diffuse liver disease. Semin US CT MRI. 1995;16:8–15. doi: 10.1016/0887-2171(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 13.Özcan HN, Oğuz B, Haliloğlu M, Orhan D, Karçaaltıncaba M. Imaging patterns of fatty liver in pediatric patients. Diagn Int Radiol. 2015;21:355–360. doi: 10.5152/dir.2015.14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marion AW, Baker AJ, Dhawan A. Fatty liver disease in children. Arch Dis Child. 2004;89:648–652. doi: 10.1136/adc.2003.029942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vajro P, Mandato C, Licenziati MR, Franzese A, Vitale DF, Lenta S, Caropreso M, Vallone G, Meli R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52(6):740–743. doi: 10.1097/MPG.0b013e31821f9b85. [DOI] [PubMed] [Google Scholar]
- 16.Qayyum A, Chen DM, Breiman RS, et al. Evaluation of diffuse liver steatosis by US, computed tomography, and magnetic resonance imaging: which modality is best? Clin Imaging. 2009;33:110–115. doi: 10.1016/j.clinimag.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Décarie P-O, Lepanto L, Billiard J-S, et al. Fatty liver deposition and sparing: a pictorial review. Insights Imaging. 2011;2:533–538. doi: 10.1007/s13244-011-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeom SK, Lee CH, Cha SH, Park CM. Prediction of liver cirrhosis, using diagnostic imaging tools. World J Hepatol. 2015;7:2069–2079. doi: 10.4254/wjh.v7.i17.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Gaetano AM, Lafortune M, Patriquin H, De Franco A, Aubin B, Paradis K. Cavernous transformation of the portal vein: patterns of intrahepatic and splanchnic collateral circulation detected with Doppler sonography. Am J Roentgenol. 1995;165(5):1151–1155. doi: 10.2214/ajr.165.5.7572494. [DOI] [PubMed] [Google Scholar]
- 20.Pinto RB, Schneider ACR, da Silveira TR. Cirrhosis in children and adolescents: an overview. World J Hepatol. 2015;7:392–405. doi: 10.4254/wjh.v7.i3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dehghani SM, Imanieh MH, Haghighat M, Malekpour A, Falizkar Z. Etiology and complications of liver cirrhosis in children: report of a single center from southern Iran. Middle East J Dig Dis. 2013;5:41–46. [PMC free article] [PubMed] [Google Scholar]
- 22.Ranucci G, Cirillo F, Della Corte C, Vecchione R, Vallone G, Iorio R. Successful use of ursodeoxycholic acid in nodular regenerative hyperplasia of the liver. Ann Pharmacother. 2011;45(4):e20. doi: 10.1345/aph.1P742. [DOI] [PubMed] [Google Scholar]
- 23.Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, et al. Elastography assessment of liver fibrosis: society of radiologists in US consensus conference statement. Radiology. 2015;276(3):845–861. doi: 10.1148/radiol.2015150619. [DOI] [PubMed] [Google Scholar]
- 24.Babu AS, Wells ML, Teytelboym OM, Mackey JE, Miller FH, Yeh BM, et al. Elastography in chronic liver disease: modalities, techniques, limitations, and future directions. Radiographics. 2016;36:1987–2006. doi: 10.1148/rg.2016160042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frulio N, Trillaud H. US elastography in liver. Diagn Interv Imaging. 2013;94:515–534. doi: 10.1016/j.diii.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Williams SM, Goodman R, Thomson A, McHugh K, Lindsell DRM. US evaluation of liver disease in cystic fibrosis as part of an annual assessment clinic: a 9-year review. Clin Radiol. 2002;57:365–370. doi: 10.1053/crad.2001.0861. [DOI] [PubMed] [Google Scholar]
- 27.McCarville MB, Hoffer FA, Howard SC, Goloubeva O, Kauffman WM. Hepatic veno-occlusive disease in children undergoing bone-marrow transplantation: usefulness of sonographic findings. Pediatr Radiol. 2001;31(2):102–105. doi: 10.1007/s002470000373. [DOI] [PubMed] [Google Scholar]
- 28.Bayraktar UD, Seren S, Bayraktar Y. Hepatic venous outflow obstruction: three similar syndromes. World J Gastroenterol. 2007;13(13):1912–1927. doi: 10.3748/wjg.v13.i13.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunelle F, Chaumont P. Hepatic tumors in children: ultrasonic differentiation of malignant from benign lesions. Radiology. 1984;150:695–699. doi: 10.1148/radiology.150.3.6695069. [DOI] [PubMed] [Google Scholar]
- 30.Chung EM, Cube R, Lewis RB, Conran RM. Pediatric liver masses: radiologic-pathologic correlation part 1. Benign tumors. Radiographics. 2010;30:801–826. doi: 10.1148/rg.303095173. [DOI] [PubMed] [Google Scholar]
- 31.Chung EM, Lattin GE, Jr, Cube R, Lewis RB, Marichal-Hernández C, Shawhan R, et al. From the archives of the AFIP: pediatric liver masses: radiologic-pathologic correlation part 2. Malignant tumors. Radiographics. 2011;31:483–507. doi: 10.1148/rg.312105201. [DOI] [PubMed] [Google Scholar]
- 32.Roebuck D. Focal liver lesion in children . Pediatr Radiol. 2008;38(Suppl 3):518. doi: 10.1007/s00247-008-0850-9. [DOI] [PubMed] [Google Scholar]
- 33.Gnarra M, Behr G, Kitajewski A, et al. History of the infantile hepatic hemangioma: from imaging to generating a differential diagnosis. World J Clin Pediatr. 2016;5(3):273–280. doi: 10.5409/wjcp.v5.i3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roos JE, Pfiffner R, Stallmach T, Stuckmann G, Marincek B, Willi U. Infantile hemangioendothelioma. Radiographics. 2003;23:1649–1655. doi: 10.1148/rg.236035024. [DOI] [PubMed] [Google Scholar]
- 35.Kim EH, Koh KN, Park M, Kim BE, Im HJ, Seo JJ. Clinical features of infantile hepatic hemangioendothelioma. Korean J Pediatr. 2011;54:260–266. doi: 10.3345/kjp.2011.54.6.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cha DI, Yoo S-Y, Kim JH, Jeon TY, Eo H. Clinical and imaging features of focal nodular hyperplasia in children. Am J Roentgenol. 2014;202:960–965. doi: 10.2214/AJR.13.11856. [DOI] [PubMed] [Google Scholar]
- 37.Zhuang L, Ni C, Din W, et al. Huge focal nodular hyperplasia presenting in a 6-year-old child: a case presentation. Int J Surg Case Rep. 2016;29:76–79. doi: 10.1016/j.ijscr.2016.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grazioli L, Federle MP, Brancatelli G, Ichikawa T, Olivetti L, Blachar A. Hepatic adenomas: imaging and pathologic findings. Radiographics. 2001;21:877–892. doi: 10.1148/radiographics.21.4.g01jl04877. [DOI] [PubMed] [Google Scholar]
- 39.Pan F, Xu M, Wang W, Zhou L, Xie X. Infantile hepatic hemangioendothelioma in comparison with hepatoblastoma in children: clinical and US features. Hepat Mon. 2013;13:e11103. doi: 10.5812/hepatmon.11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walther A, Tiao G. Approach to pediatric hepatocellular carcinoma. Clin Liver Dis. 2013;2:219–222. doi: 10.1002/cld.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishra K, Basu S, Roychoudhury S, Kumar P. Liver abscess in children: an overview. World J Pediatr. 2010;6(3):210–216. doi: 10.1007/s12519-010-0220-1. [DOI] [PubMed] [Google Scholar]
- 42.Schlesinger AE, Braverman RM, Di Pietro MA. Neonates and umbilical venous catheters: normal appearance, anomalous positions, complications, and potential aid to diagnosis. Am J Roentgenol. 2003;180(4):1147–1153. doi: 10.2214/ajr.180.4.1801147. [DOI] [PubMed] [Google Scholar]
- 43.Simanovsky N, Ofek-Shlomai N, Rozovsky K, Ergaz-Shaltiel Z, Hiller N, Bar-Oz B. Umbilical venous catheter position: evaluation by US. Eur Radiol. 2011;21(9):1882–1886. doi: 10.1007/s00330-011-2129-z. [DOI] [PubMed] [Google Scholar]
- 44.Soares KC, Arnaoutakis DJ, Kamel I, Rastegar N, Anders R, Maithel S, Pawlik TM. Choledochal cysts: presentation, clinical differentiation, and management. J Am Coll Surg. 2014;219(6):1167–1180. doi: 10.1016/j.jamcollsurg.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin SF, Lee HC, Yeung CY, Jiang CB, Chan WT. Common bile duct dilatations in asymptomatic neonates: incidence and prognosis. Gastroenterol Res Pract. 2014;2014:392562. doi: 10.1155/2014/392562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah OJ, Shera AH, Zargar SA, Shah P, Robbani I, Dhar S, Khan AB. Choledochal cysts in children and adults with contrasting profiles: 11-year experience at a tertiary care center in Kashmir. World J Surg. 2009;33(11):2403–2411. doi: 10.1007/s00268-009-0184-2. [DOI] [PubMed] [Google Scholar]
- 47.Lee HK, Park SJ, Yi BH, Lee AL, Moon JH, Chang YW. Imaging features of adult choledochal cysts: a pictorial review. Korean J Radiol. 2009;10(1):71–80. doi: 10.3348/kjr.2009.10.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Serafino M, Mercogliano C, Vallone G. US evaluation of the enteric duplication cyst: the gut signature. J US. 2015;19(2):131–133. doi: 10.1007/s40477-015-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis VA, Adam SZ, Nikolaidis P, Wood C, Wu JG, Yaghmai V, Miller FH. Imaging of choledochal cysts. Abdom Imaging. 2015;40(6):1567–1580. doi: 10.1007/s00261-015-0381-4. [DOI] [PubMed] [Google Scholar]
- 50.Mack CL, Sokol RJ. Unraveling the pathogenesis and etiology of biliary atresia. Pediatr Res. 2005;57(5 Pt 2):87R–94R. doi: 10.1203/01.PDR.0000159569.57354.47. [DOI] [PubMed] [Google Scholar]
- 51.Bezerra JA. Potential etiologies of biliary atresia. Pediatr Transplant. 2005;9(5):646–651. doi: 10.1111/j.1399-3046.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 52.Davenport M. Biliary atresia: clinical aspects. Semin Pediatr Surg. 2012;21(3):175–184. doi: 10.1053/j.sempedsurg.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Iorio R, Liccardo D, Di Dato F, Puoti MG, Spagnuolo MI, Alberti D, Vallone G. US scanning in infants with biliary atresia: the different implications of biliary tract features and liver echostructure. Ultraschall Med. 2013;34(5):463–467. doi: 10.1055/s-0033-1335455. [DOI] [PubMed] [Google Scholar]
- 54.Giannattasio A, Cirillo F, Liccardo D, Russo M, Vallone G, Iorio R. Diagnostic role of US for biliary atresia. Radiology. 2008;247(3):912. doi: 10.1148/radiol.2473071715. [DOI] [PubMed] [Google Scholar]
- 55.Choi SO, Park WH, Lee HJ, Woo SK. ʻTriangular cord’: a sonographic finding applicable in the diagnosis of biliary atresia. J Pediatr Surg. 1996;31(3):363–366. doi: 10.1016/s0022-3468(96)90739-3. [DOI] [PubMed] [Google Scholar]
- 56.Di Serafino M, Esposito F, Mercogliano C, Vallone G. The triangular cord sign. Abdom Radiol (NY). 2016;41(9):1867–1868. doi: 10.1007/s00261-016-0734-7. [DOI] [PubMed] [Google Scholar]
- 57.Tan Kendrick AP, Phua KB, Ooi BC, Tan CE. Biliary atresia: making the diagnosis by the gallbladder ghost triad. Pediatr Radiol. 2003;33(5):311–315. doi: 10.1007/s00247-003-0867-z. [DOI] [PubMed] [Google Scholar]
- 58.Lee MS, Kim MJ, Lee MJ, Yoon CS, Han SJ, Oh JT, Park YN. Biliary atresia: color doppler US neonates and infants. Radiology. 2009;252(1):282–289. doi: 10.1148/radiol.2522080923. [DOI] [PubMed] [Google Scholar]
- 59.El-Guindi MA, Sira MM, Konsowa HA, El-Abd OL, Salem TA. Value of hepatic subcapsular flow by color Doppler ultrasonography in the diagnosis of biliary atresia. J Gastroenterol Hepatol. 2013;28(5):867–872. doi: 10.1111/jgh.12151. [DOI] [PubMed] [Google Scholar]
- 60.Ikeda S, Sera Y, Ohshiro H, Uchino S, Akizuki M, Kondo Y. Gallbladder contraction in biliary atresia: a pitfall of US diagnosis. Pediatr Radiol. 1998;28(6):451–453. doi: 10.1007/s002470050380. [DOI] [PubMed] [Google Scholar]
- 61.Han SJ, Kim MJ, Han A, Chung KS, Yoon CS, Kim D, Hwang EH. J Magnetic resonance cholangiography for the diagnosis of biliary atresia. Pediatr Surg. 2002;37(4):599–604. doi: 10.1053/jpsu.2002.31617. [DOI] [PubMed] [Google Scholar]
- 62.Tang ST, Li SW, Ying Y, Mao YZ, Yong W, Tong QS. The evaluation of laparoscopy-assisted cholangiography in the diagnosis of prolonged jaundice in infants. J Laparoendosc Adv Surg Tech A. 2009;19(6):827–830. doi: 10.1089/lap.2008.0432. [DOI] [PubMed] [Google Scholar]