Abstract
Advances in genomics have transformed our ability to identify the genetic causes of rare diseases (RDs), yet we have a limited understanding of the mechanistic roles of most genes in health and disease. When a novel RD gene is first discovered, there is minimal insight into its biological function, the pathogenic mechanisms of disease-causing variants, and how therapy might be approached. To address this gap, the Canadian Rare Diseases Models and Mechanisms (RDMM) Network was established to connect clinicians discovering new disease genes with Canadian scientists able to study equivalent genes and pathways in model organisms (MOs). The Network is built around a registry of more than 500 Canadian MO scientists, representing expertise for over 7,500 human genes. RDMM uses a committee process to identify and evaluate clinician-MO scientist collaborations and approve 25,000 Canadian dollars in catalyst funding. To date, we have made 85 clinician-MO scientist connections and funded 105 projects. These collaborations help confirm variant pathogenicity and unravel the molecular mechanisms of RD, and also test novel therapies and lead to long-term collaborations. To expand the impact and reach of this model, we made the RDMM Registry open-source, portable, and customizable, and we freely share our committee structures and processes. We are currently working with emerging networks in Europe, Australia, and Japan to link international RDMM networks and registries and enable matches across borders. We will continue to create meaningful collaborations, generate knowledge, and advance RD research locally and globally for the benefit of patients and families living with RD.
Keywords: model organisms, rare genetic diseases, functional insight, gene discovery
Main Text
Introduction
Advances in genome sequencing have transformed our ability to identify the mutations that cause rare diseases (RDs), with 300 new gene-disease associations added to the Online Mendelian Inheritance in Man (OMIM) database each year.1,2 Yet, despite the impressive pace of gene discoveries following the introduction of next-generation sequencing,3 little is known about the mechanistic roles of most genes in health and disease. Recent analyses have shown that biomedical research on human protein-coding genes is focused on a small fraction of the 19,000 genes of the human genome, primarily guided by the generic chemical and biological properties of genes which facilitated experimentation 30 years ago, rather than the physiological importance of the individual genes or relevance to human disease.4 For example, only 16% of all known genes had been reported in the scientific literature by 1991 and yet they accounted for 49% of the literature published during 2015. Therefore, it is not surprising that when an RD gene is discovered, there is often a lack of insight into its biological function, let alone an understanding of how disease-causing variants cause a complex phenotype, or what therapeutic approaches might be considered. As a result, RD gene discovery is inherently a descriptive, hypothesis-generating milestone that requires subsequent studies on the basic function of the gene and the functional consequences of specific pathogenic variants in a biological context.
As genomic and other ‘omic technologies evolve, it is likely that thousands more RDs and genes will be discovered,3 further widening the gap in our understanding of the pathomolecular mechanisms underlying RDs. This incomplete understanding of the biology of many disease genes has prevented the successful development of therapies.4 Model organisms (MOs) have enormous potential for the study of gene function as few, if any, biological processes are unique to humans at the molecular level.5 Indeed, the International Rare Disease Research Consortium (IRDiRC) recognizes that MOs such as yeast, worm, fly, zebrafish, and mouse represent powerful experimental systems to confirm the pathogenicity of candidate RD genes, characterize their biological function, and identify potential therapies.6 Given the rapid pace of RD gene discovery, there is an urgent need for MO research platforms to position disease-causing genes into their biological context.7 Furthermore, most known RD genes are not the dedicated focus of a research laboratory or any specific translational research program. Therefore, for the foreseeable future, we must focus on the next grand challenge: determining the biological functions of genes associated with RDs, how dysregulation or dysfunction of these genes causes disease, and how to use this information to prevent and treat disease.
The Canadian RDMM Network: Addressing the Grand Challenge
To address the RD grand challenge and accelerate the uptake of RD genes into MO laboratories, the Canadian Institutes of Health Research recognized the need to dramatically increase collaboration between clinicians and MO scientists in Canada. In October 2014, the Canadian Rare Diseases Models and Mechanisms (RDMM) Network was established—a world-first—to accelerate and support collaborations between clinicians discovering RD genes and MO scientists conducting functional studies. The RDMM Network has created a rapid and direct pathway from gene discovery to functional characterization studies in MOs to test pathophysiological hypotheses or novel therapies (Figure 1). A formal committee structure (Figure S1; Terms of Reference) was established to identify connections between clinicians and MO scientists and to catalyze research on disease gene function. This structure was supported by a registry of Canadian MO scientists and the genes they study (the Canadian RDMM Registry). Following discovery of human genetic variants likely to cause RD, clinicians submit a two-page Connection Application (Figure S2) for their gene of interest to the Clinical Advisory Committee (CAC). The CAC considers the following criteria when evaluating connection applications: quality of the genetic data as disease-causing, disease severity and medical need, potential therapeutic tractability, impact on a unique Canadian population or community, and novelty of the implicated biological pathway. If approved, the CAC sends the human gene to the Scientific Advisory Committee (SAC), which attempts to match it, with help from the Bioinformatics Core (BIC) (Figure S3; BIC Report), to one or more MO scientists who have registered expertise in that gene in the Canadian RDMM Registry. The SAC then reviews identified matches (including those suggested by the clinician or BIC) and decides whether to invite MO scientists to submit a two-page Model Organism Proposal Application (Figure S4) for review. If the application is successful, and if they have approval from the clinician partner, the MO scientist then receives $25,000 CAD to seed immediate collaborative experiments (Figure S5; Memorandum of Agreement). This is a remarkably efficient yet rigorous process: catalyst funding can be secured within four weeks following three rounds of scientific review (CAC review, SAC invitation review, SAC proposal review). The crux of this process, and critical to its success, is the engagement of the clinical and MO communities in Canada.
Figure 1.
The Canadian Rare Diseases Models and Mechanisms Network Pathway: from Discovery to Functional Insight and Long-Term Collaborations
The Canadian RDMM Network was developed to expedite collaboration between scientists and clinicians in model-based functional studies of rare disease (RD) genes. The RDMM Network has created a rapid and direct pathway from RD gene discovery to functional characterization studies in model organisms. The network supports the investigation of biological functions of RD genes and their roles in disease, testing of pathophysiological hypotheses, identification of therapeutic targets, and the development of long-term collaborations. This figure is a representation of the homepage of the RDMM website, where clicking on the circles provides access to relevant forms and information about the committees and adjudication processes. MO—model organism.
Establishing a National Network: Engaging the Clinical and MO Communities
At the outset, there was a consensus that the Canadian RDMM Network would have the greatest impact if it were a national network. Members of the Governance and Oversight Committee, the CAC, and the SAC reached out directly to each of their respective communities to engage their members. In addition, the RDMM Network was promoted through numerous presentations and publications, as well as the presence of committee members at national and international conferences, and also broadly distributed communiques and regular e-newsletters, including through the Maternal Infant Child and Youth Research Network (MICYRN), whose members include the child health research organizations at academic health centers affiliated with the 17 medical schools in Canada.
Clinical Community
The RDMM Network began with links to clinicians discovering RD genes by directly involving leaders of Canadian RD discovery projects such as FORGE8 and Care4Rare Canada, and TIDE BC.9 These initiatives provided significant reach into their respective clinical networks, and almost the entire Canadian RD clinical community across career stages and geographical regions was thereby engaged.
Model Organism Community
To recruit the MO scientific community, members of the SAC acted as advocates, developing a comprehensive list of Canadian scientists working with their MO (yeast, worm, fly, zebrafish, or mouse and/or rat) and contacting each scientist individually or through their networks. A total of 543 scientists have registered, representing ∼88% of all Canadian MO scientists across geographical regions and MO communities in the Canadian RDMM Registry.
The Canadian RDMM Registry
To meet the goal of registering MO scientists across Canada, a web-based software platform was developed to minimize data entry demands for researchers while allowing comprehensive searches by the BIC for reporting to the SAC (Figure 2). In addition to basic contact information and a brief research description, researchers enter genes for each MO they use. Genes are entered directly into two tiers: Tier 1 represents genes scientists directly study, while Tier 2 represents related genes they could quickly set up to study. The scientist can also select relevant Gene Ontology (GO) terms suggested by the system based on analysis of the scientist’s profile to generate a Tier 3 set of genes. The Canadian RDMM Registry currently contains 12,182 unique genes (across all organisms and all tiers), corresponding to 7,632 human genes available for matching.
Figure 2.
The Canadian Rare Diseases Models and Mechanisms Registry
The RDMM Registry is a model organism (MO) matchmaking platform and the central resource for the Canadian Rare Diseases Models and Mechanisms (RDMM) Network. The RDMM Registry is a secure web-based platform with a user-friendly interface into which MO scientists enter their information and describe their research and relevant expertise with brief textual description, a list of relevant publications and, most importantly, a list of genes they are able to study. Registrants enter genes as Tier 1 (genes they are currently working on in a MO) or Tier 2 (genes they could quickly set up in a MO). The scientists then select appropriate gene ontology (GO) terms suggested by the RDMM Registry (based on the Tier 1 and 2 genes they list in their profile); the genes associated with the selected GO terms are added to their profile as Tier 3 genes.
The system supports searches by researcher name, keywords, or genes. Gene searches leverage orthology information to match human genes across multiple MOs. The Canadian RDMM Registry software is open source (see Web Resources) and includes extensive customization features to allow flexible re-use. During the registration process, users can also select their desired level of privacy for their information (public, shared, or private) which is relevant for activities related to international matching. Private data are only accessible to the BIC; shared data are accessible to the other registered users, and public data can be browsed on the registry’s public search webpage. The platform has an application programming interface (API) that enables remote querying and fetching of data between different software instances, restricted by the appropriate security settings. This enables seamless sharing of data, while the API also enables public data to be accessed by third-party applications and resources. These features were developed to facilitate MO-scientist matching across networks and with international groups, which is now possible.
Making Connections across the Canadian RDMM Network
The goal of the RDMM network is to expedite and foster collaborations among scientists and clinicians on model-based functional studies of RD genes (Figure 3). Several types of gene-to-MO scientist connections are supported by the RDMM Network, but we prioritize those that have the potential to achieve our key objectives: (A) validate discoveries (candidate genes not previously associated with disease or novel mutations in known disease genes causing a distinct disease via a presumed alternative mechanism); (B) provide functional data (novel genes supported by genetic evidence); (C) reveal biological insights (known disease genes that are of interest for a unique Canadian population or community [e.g., Hutterite, First Nation, French Canadian, etc.] and published by a Canadian group); and (D) identify therapeutic targets (via a new model for a known disease gene of interest for a unique Canadian population). The RDMM Network also considers understudied, but well established, disease genes associated with an RD that lack a model organism (MO).
Figure 3.
Connections funded for genes submitted to the Canadian Rare Diseases Models and Mechanisms Network
From 2014 to 2019, the clinical advisory committee (CAC) considered 135 rare disease (RD) genes, of which 95 were approved by the CAC for model organism (MO)-scientist matching. In addition, 116 known yet understudied genes were sent directly to the Bioinformatics Core (BIC) for MO-scientist matching. Of the 211 RD genes reviewed by the BIC, 140 genes had one (or more) MO-scientist match in the registry, so we removed the 71 genes with no MO-scientist match. After review of all potential clinician-MO scientist connections for these 140 genes, the scientific advisory committee (SAC) invited one (or more) MO proposal(s) for 101 genes, removing an additional 39 genes with no MO-scientist match. After review of the submitted MO proposals, for 77 genes, at least one MO proposal was funded, removing an additional 24 genes with no MO-scientist match. For eight of these 77 genes, two separate MO awards were made, for a total of 85 catalyst projects funded. In addition, the Network funded 10/17 follow-up studies of previously funded genes, as well as 10/29 projects from targeted calls with Canadian RD foundations. RD—rare disease; MO—model organism; CAC—clinical advisory committee; BIC—bioinformatics core; SAC—scientific advisory committee.
From January 2015 to September 2019, the CAC approved 95 genes submitted by the clinical network (70% of the 135 submissions) for MO matching (Figure 3). Submissions to CAC addressed all four objectives (some addressed more than one): validated discoveries (n = 73), functional data (n = 35), biological insights (n = 13), and therapeutic targets (n = 18). The most common reason for failure to move forward was lack of substantive supporting genetic data for unpublished novel candidate genes. An additional 99 previously understudied RD genes from the FORGE (Finding of Rare Disease Genes) and Care4Rare Canada projects and 17 understudied RD genes from the United States Undiagnosed Diseases Network’s centralized Model Organisms Screening Center (MOSC) were also included, bringing the total number of RD genes submitted for MO matching to the RDMM registry to 211 during this period. The BIC identified candidate MO matches in the registry for 140 (66%) of the genes. The candidate MO-scientist matches for these 140 RD genes were assessed by the SAC using information from the standardized reports generated by the BIC (Figure S3), and for 101 genes (72%), at least one MO researcher was invited to submit a MO proposal. After review by the SAC of all submitted MO proposals, 77 RD genes had at least one MO proposal approved for support (for eight of these genes, two separate awards were made to scientists working in different MOs, for a total of 85 catalyst awards approved). SAC also approved a follow study for 10 of 17 genes previously awarded catalyst funding. Funded projects addressed all objectives (some addressed more than one): validated discoveries (n = 38), functional data (n = 25), biological insights (n = 14), and therapeutic targets (n = 11). Beyond submitted genes, the RDMM Network partnered with several Canadian RD Foundations to co-fund MO applications specific to their diseases (10/29 applications approved), providing the scientific and administrative oversight for the research interests of these patient and family-led organizations. In total, the RDMM network has facilitated 85 clinician-MO scientist connections and supported 105 functional characterization studies to date. The 105 functional studies use modeling in mouse (n = 38), fly (n = 10), zebrafish (n = 41), worm (n = 7), yeast (n = 8), and protozoa (n = 1).
Meaningful Collaborations Generate New Knowledge
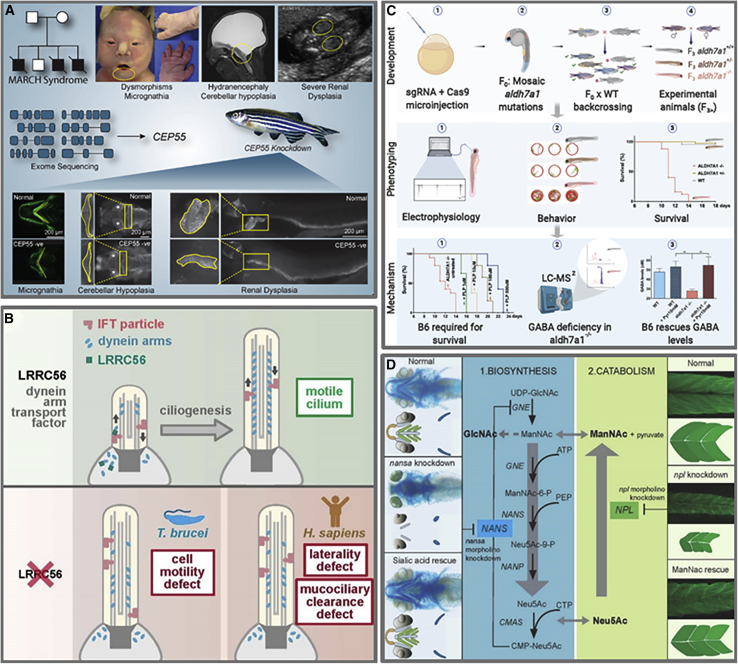
By making connections and providing catalyst funding, the Canadian RDMM network has led to scientific publications of original work and abstracts at scientific meetings. Based on our PubMed review of publications from RDMM-funded MO scientists and clinicians, there are a minimum of 20 published manuscripts resulting from catalyst awards. Further, our review of final reports from funded projects reveals at least 42 abstracts. Published manuscripts delineate the molecular pathogenesis of RDs using a variety of models (e.g., zebrafish,10, 11, 12, 13, 14, 15, 16, 17, 18, 19 mouse,18,20, 21, 22, 23, 24 fly,25 worm,26 and trypanosome27). These publications also demonstrate how the Canadian RDMM network is achieving its key objectives: five validate discoveries,11,20,21,26,28 eleven provide functional data,12, 13, 14, 15,17, 18, 19,23, 24, 25,27 two reveal biological insights,10,16 and six explore therapeutic targets.13,15,17,18,20,22 In many instances, the work supported by RDMM on a particular gene has had an impact on multiple objectives. Figure 4 highlights, for each objective, the range of new knowledge attained from projects supported through the Canadian RDMM network.
Figure 4.
New Knowledge Generated by the Canadian Rare Diseases Models and Mechanisms (RDMM) Network
Graphical summaries illustrating examples of the new knowledge attained through the Canadian RDMM Network.
(A) Validate novel gene discoveries. Morpholino or CRISPR/Cas9-mediated disruption of the CEP55 ortholog in zebrafish embryos recapitulated the phenotypic features of MARCH syndrome (MIM: 236500).11 This provided functional evidence of the pathogenicity of homozygous truncating mutations in CEP55, a novel gene discovery in a single family with MARCH syndrome.
(B) Functional data. Biallelic variants in LRRC56 were identified in three unrelated families with laterality defects and chronic pulmonary infections (MIM: 618254) where cultured epithelial cells showed severely dyskinetic cilia.27 Investigations in Trypanosoma brucei revealed LRRC56’s interactions with intracellular transport protein IFT88 in dynein transport during cilia assembly.
(C) Biological insight. This represents the first animal model for pyridoxine-dependent epilepsy (MIM: 266100), a rare epilepsy syndrome first described more than 60 years ago. CRISPR/Cas9-mediated disruption of the ALDH7A1 ortholog in zebrafish recapitulated the human phenotype, including an almost immediate response to pyridoxine and pyridoxal 5′-phosphate.10 This work suggested a role for gamma aminobutyric acid (GABA) homeostasis in disease pathogenesis.
(D) Identify therapeutic targets. Knockdown of nansa and npl in zebrafish embryos provided functional evidence for the role of NANS and NPL in sialic acid metabolism.13,15 Addition of sialic acid to embryo water partially rescued the skeletal phenotype associated with NANS, and the addition of N-acetyl mannosamine (ManNAc) rescued the myopathy phenotype associated with NPL. These data suggest potential treatment methods for human NANS and NPL deficiencies.
Validate Novel Gene Discoveries
Gene discovery projects are increasingly studying ultra-rare RDs, where a promising novel candidate gene identified in a particular family presents the N-of-1 challenge. Identifying another family with an overlapping phenotype with variants in the same candidate gene can provide the evidence needed to confirm the disease-gene association. Finding such ultra-rare families requires global data sharing, and tools such as Matchmaker Exchange have been developed for this purpose, now connecting over 70,000 cases.29 Even with these tools, most ultra-rare cases remain unmatched so MOs play a critical role in providing the functional evidence needed to support causality: this has been an area of focus for the Canadian RDMM. For example, a novel autosomal-recessive syndrome characterized by multinucleated neurons, anhydramnios, renal dysplasia, cerebellar hypoplasia and hydranencephaly (MARCH syndrome; MIM: 236500) was described in a single Canadian Mennonite family associated with a homozygous nonsense variant in CEP55, encoding centrosomal protein 55.11 As part of this primary discovery, the authors demonstrated that morpholino or CRISPR/Cas9-mediated disruption of the CEP55 ortholog in zebrafish embryos resulted in craniofacial abnormalities, micrognathia, cerebellar hypoplasia, and renal tubular atrophy (Figure 4A). Similarly, in a single family with periventricular nodular heterotopia, intellectual disability, and nocturnal seizures, compound heterozygous variants in TMTC3, encoding transmembrane and tetratricopeptide repeat containing 3, were identified.25 This gene had previously been associated with cobblestone lissencephaly, a distinct brain malformation (MIM: 617255). Further support for disease causation and the neurobiological role of TMTC3 was obtained by generating flies with post-mitotic neuron-specific knockdown of the highly conserved Drosophila melanogaster TMTC3 ortholog, CG4050/tmtc3. These flies demonstrated increased susceptibility to induced seizures, and this phenotype was rescued by neuron-specific expression of human TMTC3. These examples highlight the contribution that MOs can make to advancing diagnostic possibilities for patients with RDs.
Functional Data
Novel gene discoveries where the N-of-1 challenge has been addressed still benefit from functional studies to assess, for the first time, how alteration of the gene impacts protein function in the biological context of a MO. For example, heterozygous variants in PMEL encoding the premelanosome protein were identified in two families with Pigmentary glaucoma, a subtype of glaucoma that results from release of pigment from the iris and its deposition throughout the anterior chamber of the eye.14 Further screening for PMEL variants in relevant cohorts identified seven additional affected individuals. CRISPR/Cas9-mediated disruption of the PMEL ortholog in zebrafish caused profound developmental defects and enlarged anterior segments in the eye, further supporting its role in ocular pigmentation and function. Similarly, biallelic variants in LRRC56 were identified in three unrelated families with laterality defects and chronic pulmonary infections.27 Using the protist Trypanosoma brucei, it was shown that LRRC56 is recruited to the cilium during axoneme construction, where it co-localizes with intraflagellar transport trains and is required for the addition of dynein arms to the distal end of the flagellum during cilia assembly (Figure 4B). In summary, these examples highlight the significant mechanistic insight that can accompany the reporting of a novel disease gene.
Biological Insights
To begin to address the grand challenge of understanding the biological functions of genes associated with RDs, the Canadian RDMM network also considers understudied but well established RD genes that lack a MO. For example, pyridoxine-dependent epilepsy (PDE; MIM: 266100) is a rare epilepsy that was first described over 60 years ago, yet it lacks an animal model to further study the biological context of the disease process. Autosomal recessive mutations in the lysine degradation gene, ALDH7A1, lead to recurrent seizures with onset in utero, which are uniquely responsive to high doses of pyridoxine or pyridoxal 5′-phosphate (vitamin B6 vitamers). However, despite treatment, most affected individuals still suffer from neurodevelopmental issues, and a subset requires anticonvulsant medications to help control the seizures, highlighting the need for further study to optimize treatment approaches. CRISPR/Cas9-mediated disruption of the ALDH7A1 ortholog in zebrafish resulted in deficient lysine metabolism and spontaneous and recurrent seizures in the larval stage; remarkably, as is the case in human PDE, the seizures demonstrated an almost immediate sensitivity to pyridoxine and pyridoxal 5′-phosphate, with an increase in lifespan, while lysine supplementation induced earlier seizure onset and death.10 Further insights into PDE pathogenesis were identified by studying the untreated fish by mass spectrometry approaches, which showed several changes in amino acid levels, most remarkably in the lysine metabolism pathway. In addition, low B6 vitamers and gamma aminobutyric acid (GABA) levels were observed, suggesting that PDE is, at least in part, a disorder of GABA homeostasis (Figure 4C). In another example, pathogenic variants in the LRPPRC gene are causative for a distinct monogenic form of Leigh syndrome in the French-Canadian population (LSFC; MIM: 220111). Although patients with LSFC exhibit many hallmarks of inherited mitochondrial diseases (including Leigh syndrome), observed perturbations in lipid metabolism are poorly understood. An H-Lrpprc–/– mouse corroborated most of the plasma lipid perturbations observed in LSFC patients, including changes supporting a contribution from dysregulated peroxisomal lipid metabolism.23 This work revealed unexpected mechanisms underlying lipid dyshomeostasis resulting from mitochondrial disease; the recognized involvement of the peroxisome in the pathophysiology of LSFC may be generalizable to other mitochondrial diseases. Insufficient understanding of biological processes of many disease genes has prevented the successful development of effective therapies, and supporting the study of such diseases is an important contribution for the Network.
Identify Therapeutic Targets
Most connection applications addressed mechanistic and functional objectives, but a subset proposed to explore therapeutic opportunities and, on occasion, the MO supported novel gene discovery in addition to facilitating investigation of a possible approach to treatment. For example, biallelic pathogenic variants in NANS, the gene encoding N-acetylneuraminic acid synthase, were identified in nine individuals with infantile-onset severe developmental delay and skeletal dysplasia.15 Studies in patient cells showed reduced NANS activity and inability to incorporate sialic acid precursors into sialylated glycoproteins. This confirmed the functional impairment of NANS activity in the metabolic pathway of sialic acid biosynthesis and protein sialylation and suggested that exogeneously administered sialic acid might be used to bypass the enzymatic block. Studies in zebrafish embryos showed that knockdown of nansa resulted in abnormal skeletal development (Figure 4D). The addition of sialic acid to the water in which the zebrafish embryos were maintained resulted in partial rescue of the skeletal phenotype dependent on the timing of its addition to the water. Further work to examine sialic acid biosynthesis in different tissues and developmental phases is planned to explore other therapeutic strategies for NANS deficiency. In a similar application of a MO to investigate biology and potential therapeutic approaches, the zebrafish was again used to model a novel disorder of sialic acid catabolism.13 In a single family, biallelic pathogenic variants in NPL, encoding N-acetylneuraminate pyruvate lyase, were shown to be associated with sialuria, exercise intolerance, muscle wasting, and cardiac symptoms. A knockdown of npl in zebrafish resulted in severe skeletal myopathy and cardiac edema; the myopathy phenotype was rescued by treatment with the catabolic products of NPL: N-acetyl glucosamine (GlcNAc) and N-acetyl mannosamine (ManNAc); the cardiac phenotype was rescued with ManNAc (Figure 4D), suggesting monosaccharide replacement as a therapeutic strategy for patients. As only 6% of RDs have an available treatment, of which fewer than 1% are curative,30 the focus of translational research laboratories on developing therapies for understudied RDs is of paramount importance and MOs provide an excellent platform to do so.
Long-Term Collaborations
A key objective of the Canadian RDMM network is to facilitate longer term collaborations between basic scientists and clinicians that will lead to subsequent funding in support of outstanding basic and/or applied research. This objective is of central importance to the overall success of the RDMM Network as it reflects the longer-term impact of catalyzing connections focused on understudied RDs. To date, preliminary data generated from 11 of the understudied RD genes funded by RDMM catalyst grants have gone on to facilitate peer-reviewed funding totaling $10M CAD, primarily from CIHR but also from the private sector. For families affected by these RDs, this effectively means that their diseases are now the focus of a research laboratory, a significant milestone in the translational journey of a RD.
The Way Forward
The Canadian RDMM Network has comprehensively engaged the clinician and MO scientist communities in Canada to begin unraveling the molecular pathogenesis of understudied RDs. However, much broader collaboration will be needed to comprehensively understand the human RD genome, as it has been previously noted that most research in this area focuses on only approximately 2,000 of the 19,000 genes of the human genome.4 Important partners will include academic networks, patient organizations, and industry.
Connecting the World: RDMM International
To maximize our ability to study RD genes in MOs, the Canadian RDMM Network is establishing international linkages with emerging networks around the world. The 134 RD genes (out of the 211 RD genes considered by RDMM) that did not result in a funded clinician-MO scientist connection are just the tip of the iceberg when it comes to the potential benefits of broader, international collaboration. The opportunity currently underway is that RD genes discovered in other countries can be studied by experts in Canada, while genes discovered here that lack a Canadian MO scientist can be studied by an expert in another country using their regional funding. To enable international linkages, the Registry software is portable, with streamlined security, improved performance and maintenance, easy customization of the user interface and documentation, and allows for different types of administrative access to the database. The software has been distributed to RD network initiatives in Europe (RDMM Europe, as part of the European Commission funded Solve-RD research project), Australia (Australian Functional Genomics Network, as part of the Australian Genomics Health Alliance), and Japan (J-RDMM, as part of the Initiative on Rare and Undiagnosed Diseases). These networks are now operational, emulating our organizational structure, informatics pipeline, and committee processes, and most have also adopted our Registry software. The next step will be to establish the policy and approaches for global interactions on a broad scale, irrespective of software, such that experts modeling a particular gene anywhere in the world can be connected to the clinical experts on the RD, with resultant collaborations catalyzed with seed funding. Facilitated by the Canadian RDMM registry’s API, connections between international RDMMs have already been established. As such, a small number of RD genes submitted for a connection from Canada are now being studied in Europe (via Solve-RD – RDMM Europe) and at Monash University, Australia (via Monash University Network of Excellence in Functional Genomics), and RD genes submitted from these countries are being studied by scientists in Canada. We look forward to facilitating meaningful collaborations on a much broader scale as similar networks are established around the world.
Partnerships with Patient Organizations and Rare Disease Funders
Partnerships with stakeholders will continue to be of importance as we address the grand challenge of determining the biological functions of genes associated with RD, how dysregulation of these genes causes disease, and how to use this information to prevent and treat disease. RDMM has partnered with Canadian patient advocacy organizations on several occasions, providing the scientific and administrative oversight for the research interests of these patient and family-led organizations. For example, we have partnered with Dravet Canada and La Fondation du Grand défi Pierre Lavoie to co-fund projects related to epilepsy and metabolic disease, respectively. These organizations found our streamlined processes and scientific expertise invaluable for efficient peer-review of submitted proposals. The partnership resulted in significant co-funding that was used toward ten RD catalyst projects. Partnerships with Canadian patient organizations will continue to be fostered to co-fund future RDMM Network projects and increase capacity to provide catalyst funding and generate new knowledge for the benefit of the broader RD community.
Conclusion
The Canadian RDMM Network has recently been awarded renewed funding for an additional five years and will continue to accelerate translational research and foster international connections. As early as possible following the discovery of a candidate gene variant, RDMM enables efficient mechanisms for catalyzing connections, collaboration, and cross-talk between clinicians and MO scientists to investigate pathogenic mechanisms. In a similar fashion, RDMM will continue to increase awareness of the value of fundamental research in understanding biological mechanisms underlying human disease. For patients, families, and government decision makers, the realization that fundamental aspects of most human disorders can often be best informed through analysis of orthologous genes and pathways in experimentally tractable organisms is profound. RDMM has had the good fortune to launch and operate the Network in Canada for nearly five years, and in that time have caught the attention of the international RD community. Successful collaborations with Japanese, European, and Australian networks strongly suggest that the establishment of a global network of RDMM networks is within reach. A coordinated and large-scale international effort will accelerate elucidation of RD gene and genome function and facilitate the development of rational approaches to disease prevention and therapy for decades to come. Most importantly, a strong RDMM network of networks will increase research opportunities for the millions of families living with RD, yielding benefits such as accurate diagnosis, informed care, and the possibility for treatment.
Acknowledgments
The authors would like to thank the Canadian clinical and model organism RD communities for their enthusiastic participation and strong collaborative spirit. We are grateful to the patients with RD and their families for their participation in our studies. We thank Paul Lasko for his support and guidance as we established this Network. We would also like to thank the leadership teams of RDMM Europe, Australian Functional Genomics Network, and J-RDMM in Japan for their commitment to international collaborations for the benefit of scientific progress for families living with RDs. The authors thank Koroboshka Brand-Arzamendi for the artwork drawing of panel D of Figure 4. The Canadian Rare Diseases Models and Mechanisms (RDMM) Network is funded by the Canadian Institutes of Health Research (CIHR) (grant numbers RCN-137793 and RCN-160422) with additional support from Genome Canada and Genome British Columbia. The database for the Australian Functional Genomics Network was supported by a Monash University International Networks of Excellence Grant.
Declarations of Interest
The authors declare no competing interests.
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2020.01.009.
Contributor Information
Kym M. Boycott, Email: kboycott@cheo.on.ca.
Philip Hieter, Email: hieter@msl.ubc.ca.
Web Resources
Australian Functional Genomics Network, https://www.functionalgenomics.org.au/
Canadian RDMM Network, http://rare-diseases-catalyst-network.ca/
Canadian RDMM Registry software, https://github.com/PavlidisLab/rgr
Care4Rare Canada, http://www.care4rare.ca
Japanese Rare Disease Models and Mechanisms Network, https://j-rdmm.org/indexEn.html
Matchmaker Exchange, https://www.matchmakerexchange.org/
MICYRN, https://www.micyrn.ca
Online Mendelian Inheritance in Man (OMIM), https://omim.org/
RDMM Europe, http://solve-rd.eu/rdmm-europe/
TIDE BC, http://www.tidebc.org/
Supplemental Information
References
- 1.Hartley T., Balcı T.B., Rojas S.K., Eaton A., Canada C.R., Dyment D.A., Boycott K.M. The unsolved rare genetic disease atlas? An analysis of the unexplained phenotypic descriptions in OMIM®. Am. J. Med. Genet. C. Semin. Med. Genet. 2018;178:458–463. doi: 10.1002/ajmg.c.31662. [DOI] [PubMed] [Google Scholar]
- 2.Amberger J.S., Bocchini C.A., Scott A.F., Hamosh A. OMIM.org: leveraging knowledge across phenotype-gene relationships. Nucleic Acids Res. 2019;47(D1):D1038–D1043. doi: 10.1093/nar/gky1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boycott K.M., Rath A., Chong J.X., Hartley T., Alkuraya F.S., Baynam G., Brookes A.J., Brudno M., Carracedo A., den Dunnen J.T. International cooperation to enable the diagnosis of all rare genetic diseases. Am. J. Hum. Genet. 2017;100:695–705. doi: 10.1016/j.ajhg.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoeger T., Gerlach M., Morimoto R.I., Nunes Amaral L.A. Large-scale investigation of the reasons why potentially important genes are ignored. PLoS Biol. 2018;16:e2006643. doi: 10.1371/journal.pbio.2006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolinski K., Botstein D. Orthology and functional conservation in eukaryotes. Annu. Rev. Genet. 2007;41:465–507. doi: 10.1146/annurev.genet.40.110405.090439. [DOI] [PubMed] [Google Scholar]
- 6.Lochmüller H., Torrent I Farnell J., Le Cam Y., Jonker A.H., Lau L.P., Baynam G., Kaufmann P., Dawkins H.J., Lasko P., Austin C.P., Boycott K.M., IRDiRC Consortium Assembly The International Rare Diseases Research Consortium: Policies and Guidelines to maximize impact. Eur. J. Hum. Genet. 2017;25:1293–1302. doi: 10.1038/s41431-017-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wangler M.F., Yamamoto S., Chao H.-T., Posey J.E., Westerfield M., Postlethwait J., Hieter P., Boycott K.M., Campeau P.M., Bellen H.J., Members of the Undiagnosed Diseases Network (UDN) Model organisms facilitate rare disease diagnosis and therapeutic research. Genetics. 2017;207:9–27. doi: 10.1534/genetics.117.203067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaulieu C.L., Majewski J., Schwartzentruber J., Samuels M.E., Fernandez B.A., Bernier F.P., Brudno M., Knoppers B., Marcadier J., Dyment D., FORGE Canada Consortium FORGE Canada Consortium: outcomes of a 2-year national rare-disease gene-discovery project. Am. J. Hum. Genet. 2014;94:809–817. doi: 10.1016/j.ajhg.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Karnebeek C.D., Stockler-Ipsiroglu S. Early identification of treatable inborn errors of metabolism in children with intellectual disability: The Treatable Intellectual Disability Endeavor protocol in British Columbia. Paediatr. Child Health. 2014;19:469–471. doi: 10.1093/pch/19.9.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pena I.A., Roussel Y., Daniel K., Mongeon K., Johnstone D., Weinschutz Mendes H., Bosma M., Saxena V., Lepage N., Chakraborty P. Pyridoxine-dependent epilepsy in zebrafish caused by Aldh7a1 deficiency. Genetics. 2017;207:1501–1518. doi: 10.1534/genetics.117.300137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frosk P., Arts H.H., Philippe J., Gunn C.S., Brown E.L., Chodirker B., Simard L., Majewski J., Fahiminiya S., Russell C., FORGE Canada Consortium. Canadian Rare Diseases: Models & Mechanisms Network A truncating mutation in CEP55 is the likely cause of MARCH, a novel syndrome affecting neuronal mitosis. J. Med. Genet. 2017;54:490–501. doi: 10.1136/jmedgenet-2016-104296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodehl A., Rezazadeh S., Williams T., Munsie N.M., Liedtke D., Oh T., Ferrier R., Shen Y., Jones S.J.M., Stiegler A.L., FORGE Canada Consortium Mutations in ILK, encoding integrin-linked kinase, are associated with arrhythmogenic cardiomyopathy. Transl. Res. 2019;208:15–29. doi: 10.1016/j.trsl.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen X.Y., Tarailo-Graovac M., Brand-Arzamendi K., Willems A., Rakic B., Huijben K., Da Silva A., Pan X., El-Rass S., Ng R. Sialic acid catabolism by N-acetylneuraminate pyruvate lyase is essential for muscle function. JCI Insight. 2018;3:e122373. doi: 10.1172/jci.insight.122373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahola-Chomiak A.A., Footz T., Nguyen-Phuoc K., Neil G.J., Fan B., Allen K.F., Greenfield D.S., Parrish R.K., Linkroum K., Pasquale L.R. Non-Synonymous variants in premelanosome protein (PMEL) cause ocular pigment dispersion and pigmentary glaucoma. Hum. Mol. Genet. 2019;28:1298–1311. doi: 10.1093/hmg/ddy429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Karnebeek C.D.M., Bonafé L., Wen X.-Y., Tarailo-Graovac M., Balzano S., Royer-Bertrand B., Ashikov A., Garavelli L., Mammi I., Turolla L. NANS-mediated synthesis of sialic acid is required for brain and skeletal development. Nat. Genet. 2016;48:777–784. doi: 10.1038/ng.3578. [DOI] [PubMed] [Google Scholar]
- 16.Samarut É., Swaminathan A., Riché R., Liao M., Hassan-Abdi R., Renault S., Allard M., Dufour L., Cossette P., Soussi-Yanicostas N., Drapeau P. γ-Aminobutyric acid receptor alpha 1 subunit loss of function causes genetic generalized epilepsy by impairing inhibitory network neurodevelopment. Epilepsia. 2018;59:2061–2074. doi: 10.1111/epi.14576. [DOI] [PubMed] [Google Scholar]
- 17.Johnstone D.L., Al-Shekaili H.H., Tarailo-Graovac M., Wolf N.I., Ivy A.S., Demarest S., Roussel Y., Ciapaite J., van Roermund C.W.T., Kernohan K.D., Care4Rare Canada Consortium PLPHP deficiency: clinical, genetic, biochemical, and mechanistic insights. Brain. 2019;142:542–559. doi: 10.1093/brain/awy346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Karnebeek C.D.M., Ramos R.J., Wen X.-Y., Tarailo-Graovac M., Gleeson J.G., Skrypnyk C., Brand-Arzamendi K., Karbassi F., Issa M.Y., van der Lee R. Bi-allelic GOT2 mutations cause a treatable malate-aspartate shuttle-related encephalopathy. Am. J. Hum. Genet. 2019;105:534–548. doi: 10.1016/j.ajhg.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Kuilenburg A.B.P., Tarailo-Graovac M., Richmond P.A., Drögemöller B.I., Pouladi M.A., Leen R., Brand-Arzamendi K., Dobritzsch D., Dolzhenko E., Eberle M.A. Glutaminase deficiency caused by short tandem repeat expansion in GLS. N. Engl. J. Med. 2019;380:1433–1441. doi: 10.1056/NEJMoa1806627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Smith C., Parboosingh J.S., Khan A., Innes M., Hekimi S. Pathogenicity of two COQ7 mutations and responses to 2,4-dihydroxybenzoate bypass treatment. J. Cell. Mol. Med. 2017;21:2329–2343. doi: 10.1111/jcmm.13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrell S.A., Sodhi S., Marshall C.R., Guerin A., Slavotinek A., Paton T., Chong K., Sirkin W.L., Scherer S.W., Bérubé-Simard F.-A., Pilon N. HLX is a candidate gene for a pattern of anomalies associated with congenital diaphragmatic hernia, short bowel, and asplenia. Am. J. Med. Genet. A. 2017;173:3070–3074. doi: 10.1002/ajmg.a.38354. [DOI] [PubMed] [Google Scholar]
- 22.Xue Y., Meehan B., Macdonald E., Venneti S., Wang X.Q.D., Witkowski L., Jelinic P., Kong T., Martinez D., Morin G. CDK4/6 inhibitors target SMARCA4-determined cyclin D1 deficiency in hypercalcemic small cell carcinoma of the ovary. Nat. Commun. 2019;10:558. doi: 10.1038/s41467-018-06958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz M., Cuillerier A., Daneault C., Deschênes S., Frayne I.R., Bouchard B., Forest A., Legault J.T., Vaz F.M., Rioux J.D., LSFC Consortium Lipidomics unveils lipid dyshomeostasis and low circulating plasmalogens as biomarkers in a monogenic mitochondrial disorder. JCI Insight. 2019;4:e123231. doi: 10.1172/jci.insight.123231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray M.J., Kannu P., Sharma S., Neyt C., Zhang D., Paria N., Daniel P.B., Whetstone H., Sprenger H.-G., Hammerschmidt P. Mutations preventing regulated exon skipping in MET cause osteofibrous dysplasia. Am. J. Hum. Genet. 2015;97:837–847. doi: 10.1016/j.ajhg.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farhan S.M.K., Nixon K.C.J., Everest M., Edwards T.N., Long S., Segal D., Knip M.J., Arts H.H., Chakrabarti R., Wang J., FORGE Canada Consortium Identification of a novel synaptic protein, TMTC3, involved in periventricular nodular heterotopia with intellectual disability and epilepsy. Hum. Mol. Genet. 2017;26:4278–4289. doi: 10.1093/hmg/ddx316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoyagi K., Rossignol E., Hamdan F.F., Mulcahy B., Xie L., Nagamatsu S., Rouleau G.A., Zhen M., Michaud J.L. A gain-of-function mutation in NALCN in a child with intellectual disability, ataxia, and arthrogryposis. Hum. Mutat. 2015;36:753–757. doi: 10.1002/humu.22797. [DOI] [PubMed] [Google Scholar]
- 27.Bonnefoy S., Watson C.M., Kernohan K.D., Lemos M., Hutchinson S., Poulter J.A., Crinnion L.A., Berry I., Simmonds J., Vasudevan P., Care4Rare Canada Consortium Biallelic mutations in LRRC56, encoding a protein associated with intraflagellar transport, cause mucociliary clearance and laterality defects. Am. J. Hum. Genet. 2018;103:727–739. doi: 10.1016/j.ajhg.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roston T.M., Guo W., Krahn A.D., Wang R., Van Petegem F., Sanatani S., Chen S.R.W., Lehman A. A novel RYR2 loss-of-function mutation (I4855M) is associated with left ventricular non-compaction and atypical catecholaminergic polymorphic ventricular tachycardia. J. Electrocardiol. 2017;50:227–233. doi: 10.1016/j.jelectrocard.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Philippakis A.A., Azzariti D.R., Beltran S., Brookes A.J., Brownstein C.A., Brudno M., Brunner H.G., Buske O.J., Carey K., Doll C. The Matchmaker Exchange: a platform for rare disease gene discovery. Hum. Mutat. 2015;36:915–921. doi: 10.1002/humu.22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin C.P., Cutillo C.M., Lau L.P.L., Jonker A.H., Rath A., Julkowska D., Thomson D., Terry S.F., de Montleau B., Ardigò D., International Rare Diseases Research Consortium (IRDiRC) Future of rare diseases research 2017-2027: An IRDiRC perspective. Clin. Transl. Sci. 2018;11:21–27. doi: 10.1111/cts.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.