Abstract
Background
The success of platelet rich plasma (PRP) applications in conservative treatment of moderate gonarthrosis has increased over time. Two different PRP formulations that buffy coat (Leukocyte rich PRP: LR-PRP) and plasma-based (Leukocyte poor PRP: LP-PRP) are obtained by different centrifugation methods. This prospective randomized trial was whether LP-PRP will be more effective combination for moderate gonarthrosis when compared to LR-PRP or HA.
Methods
A total 90 patients suffering from moderate knee osteoarthritis were enrolled. Patients were divided equally into three groups and treated with 3 times LR-PRP, LP-PRP and HA injections. A prospective evaluation was done at baseline, and then at 2, 6 and 12 months of follow-up using VAS, WOMAC and Likert scoring systems.
Results
The 2nd, 6th and 12th month VAS and WOMAC scores of LR-PRP demonstrated the most obvious improvement. Recurrence of symptoms was statistically lower (3; 10%) in LR-PRP group (p < 0.001). Male gender had lower recurrence rate than females (1 vs. 18; p = 0.043). Only high BMI had statistically negative effect on recovery and recurrence rates (p = 0.004). Local adverse effects were more common in LR-PRP group (p < 0.05).
Conclusions
PRP injections produced superior results than HA. LR-PRP seems to be the most effective treatment modality for moderate gonarthrosis especially in normal weighted men at the 6th decade of age.
Keywords: Platelet rich plasma, Leukocyte rich PRP, Leukocyte poor PRP, Buffy coat method, Leukocytes, Hyaluronic acid, Moderate gonarthrosis
1. Introduction
Osteoarthritis (OA) is caused by proinflammatory cytokines such as iL-1β and TNFa, which result in the deterioration of chondrocyte metabolism.1 It was shown that growth factors such as TGF-β have important roles in the resolution of the inflammatory process and in the cartilage repair process.2 The effectiveness of growth factors in the repair of damaged cartilage tissue has been investigated in vitro and in vivo and it has been shown that the PRP technique, which contains a large amount of growth factor, causes predictable clinical improvement after one year of follow-up.3
PRP, due to its high platelet concentration contains hyperphysiological levels of clotting and growth factors. These are Insulin-Like Growth Factor (IGF-1), Transforming Growth Factor-β (TGF-β), Platelet-Derived Growth Factor (PDGF), Vascular Endothelial Growth factor (VEGF) and Basic Fibroblast Growth Factor (b-FGF). In this regard, on the contrary to traditional treatments, basic principle of PRP is triggering the inflammation instead of suppression. Increased cell proliferation, collagen synthesis and vascularity have been thought to be activity of PRP.
PRP applications have not yet been reached a consensus; there are many unsolved issues such as activation modalities, storage methods, and injection protocols. One of them is what the efficacy of serial injections is. Addition of local anesthetic into the injection for patient comfort as well as to reduce inflammatory pain may dilute the solution and change the pH, which may reduce the effectiveness of PRP. Adding bicarbonate to provide appropriate pH value or the addition of platelet-activating agents into PRP such as calcium chloride and thrombin to secrete an optimal level of growth factors also have not been standardized. The effectiveness of these differences on PRP applications is not yet known. Moreover, there is no consensus on the optimal formulation of the ratio of platelet and leukocyte to PRP to be used in the repair of damaged cartilage.4
Leukocytes also secrete several molecules involved in wound healing such as TGF-b, interleukins, and TNF-a, as well as cytokines and a number of enzymes that are important in fighting infection. Proteases and products such as Ros are also secreted by leukocytes. In the presence of cartilage damage, Mc Carrel and colleagues allege that leukocyte-poor products are more effective because of the presence of ROS and proteases.5 In an in vitro study targeting the optimization of leukocyte concentration in PRP, the combination of high platelet and low leukocyte combination (LP-PRP) and combination (LR-PRP) containing both at high concentration were compared in cartilage repair and at the end of the seventh day the LP-PRP group promoted cartilage tissue anabolism, whereas LR-PRP has been shown to activate the catabolic pathways.4 In contrast, Marianni and colleagues in a study investigating local and systemic responses in two groups of patients receiving LR-PRP or hyaluronic acid (HA) due to gonarthrosis have shown that LR-PRP does not lead to an increase in proinflammatory mediators including IL-1b, IL-6, IL-8 and IL-17.6 Because of lack of established evidence, it has not been determined whether the above mentioned combinations are more efficient in relieving symptoms related to gonarthrosis.
The research question of this study was whether LP-PRP will be more effective combination for moderate gonarthrosis when compared to LR-PRP or HA. For this purpose, we evaluated midterm effect of these two combinations and compared with HA injections in patients with moderate gonarthrosis.
2. Materials and methods
2.1. Patients and included study
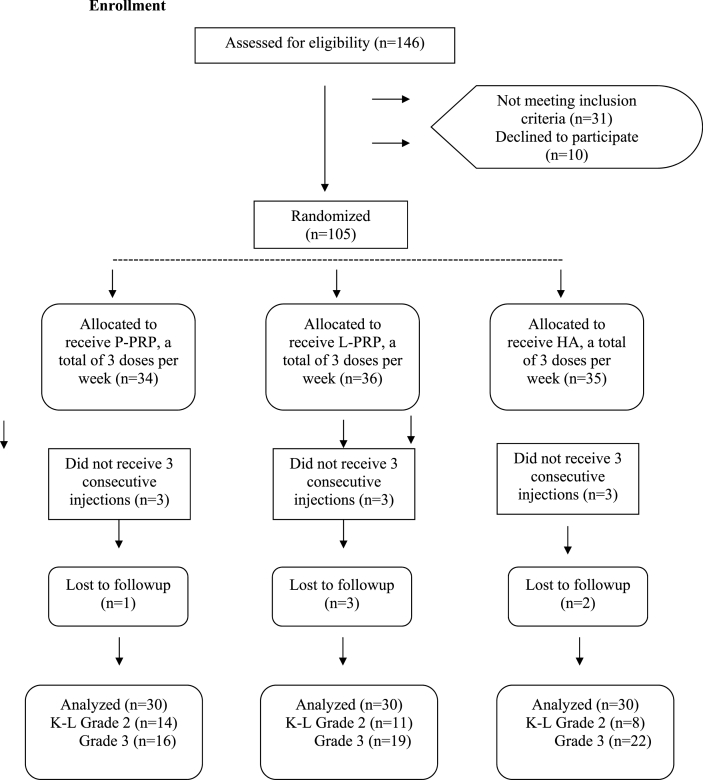
This study included 146 patients who were admitted to outpatient clinic between August 2016 and August 2017 for knee pain, which increased by walking or by using stairs but relieved with rest. Institutional review board approval was obtained and only the participants who signed a written consent form were included. Upon admission, all patients were performed clinical evaluation including physical examination for any meniscal pathologies and stability maneuvers and radiographic evaluation including weight-bearing anteroposterior, lateral and Merchant's view of the knees. Inclusion criteria were as follows: Kellgren-Lawrence Grade 2 or 3 symptomatic knee OA, aged 38–80 years and stable knees. Patients with inflammatory diseases, major malalignment of the knee (>15° of varus or >5° of valgus), hematologic diseases, anemia (hb < 11 mg/dl) and having severe cardiac diseases were excluded.In the patients with bilateral symptoms, only the side with significant symptoms was taken into consideration. When a total of 105 patients who were having fulfilled inclusion criteria reached, participants were randomly assigned into three groups by a computer-based protocol. The patients were given appointments for a fixed day of the week and they were separated into three groups according to the treatment they received: LP-PRP group, (n = 34), LR-PRP group (n = 36) and HA group(n = 35). Of them, a total of 15 patients either did not receive 3 consecutive injections (n = 9) or loss to follow up (n = 6). Finally, 90 participants that were distributed equally in each group were prospectively evaluated (Fig. 1).
Fig. 1.
Flow diagram of the study.
2.2. Randomization and blinding
SPSD version 22.0 (IBM Corporation, Armonk, NY, USA) was used to assign a serial number to the 105 participants and to randomly allocate 35of them into each group. The serial number codes were inserted into opaque envelopes that were sealed and kept in a double-locked cabinet, and opened in the presence of the patient and a guardian. The research coordinator constituted the allocation sequence, enroll participants and assign participants to interventions. For blinding, all syringes, which were used in this study were covered by an opaque obscuring sleeve and patients in Group 3 were harvested blood samples. A senior resident trained in clinical trials before participation in this study did outcome measurements to the patients being unaware of the intervention applied. Also, to standardize the PRP intervention, another senior resident was trained for manual PRP preparation by performing a minimum of 20 PRP solutions before the beginning of the study.
2.3. PRP preparation
In a sterile manner, a total of 25 ml venous blood sample was harvested for each treatment day from venous antecubital vein and it was separated equally into five 1 ml of 0.106 M sodium citrate containing vacutainer polypropylene blood collection tubes. In order to obtain LP-PRP, samples were centrifuged at 460 g for 5 min. The resultant superior layer made up of platelets from three tubes were gently collected by a sterile pipette into another vacutainer for injection. To obtain LR-PRP, however, samples were centrifuged twice; the first time at 780 g for 5 min, and the second time at 3300 g for 10 min. After that, the supernatant from three tubes (1 ml from top 20% of the centrifuged solution) both rich with platelets and leukocytes were collected to obtain the injection solution. In both methods, after re-suspending for at least 10 min for allowing equal distribution of platelets, one sample was sent to laboratory for cell counting by hematology analyzer system. Examination of the peripheral blood smear to provide platelet count was done manually to the last sample. While the sample was keeping on the rocker to ensure evenly distribution of the platelets a transmission-light microscope at 400× magnification was used and cell counting was performed. The injections were performed from superolateral portal 3 times in 1 week interval. The PAW classification was applied for two samples to organize the results.7 This system covers absolute number of platelets, activation method of platelets and existence of leukocytes. In all three consecutive injections, platelet counts, total WBC's and number of neutrophils were noted. Due to endogenous activation, a designation was not given to samples in PAW classification. Till 24 h post injection, forceful use of the leg and non-steroidal medication were restrained and afterwards normal recreational activities were allowed, as tolerated.
2.4. HA injection
The Ostenil® syringe is a pre-filled 2 ml of syringe containing20.0 mg sodium hyaluronat. The molecular weight of HA is 1–2 million Daltons and claimed to provide effective pain relief up to 12 months’ post injection. The recommended treatment cycle consists of consecutive 3–5 injections. All patients in the HA group (Group 3) was performed injections from the superolateral portal 3 times in 1 week interval. The same post injection protocol was used in these patients for blinding purposes.
2.5. Follow-up and outcome assessment
Radiographic progression of knee OA according to Kellgren-Lawrence classification was made by standard radiographs. Initial and last radiographs were evaluated by two fellowship-trained orthopaedic surgeons. For intraobserver reliability, radiographs were reassessed with 4-week intervals. Evaluation was made prospectively by Visual Analogue Scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores at baseline, at the last intervention and then 2, 6, and 12 months after the last intervention. Range of motion (ROM) of both the index and contralateral knees were noted. Before randomization, all patients completed a 100 mm VAS to state the severity of their knee pain over the past week. Participants also completed the 5-point Likert scale at the second and last follow-up, which was evaluated with the only question that included general satisfaction with the patients’ injection.
Patients whose 12th functional scores returned to initially scores and who wanted to re-injection were evaluated as recurrence. Patients were advised not to use NSAI drugs during the follow-up and paracetamol was recommended in case of complaints.
2.6. Ethics approval and consent to participate
This study was approved by the Institutional review board under the case number12.07.2016/13143278876-929-2422-4586 and informed consent was obtained from all subjects.
2.7. Power analysis
The sample size was determined based on the difference in the primary outcome, which was calculated WOMAC score. We used the previously mentioned clinically relevant difference of 6.4 points on the WOMAC scores [8]. We calculated that with 10% loss to follow up after 12 months, sample size estimation at an alpha of 0.05 and power of 80% indicated 29 patients would be required for each arm.
2.8. Statistical analysis
The Statistical Package for Social Sciences (SPSS) software (Version 13) was used to analyze the data. The Kolmogorov-Smirnov test was used to test the normal distribution of the data. T-test and Chi-square were respectively used for comparison of continuous or parametric variables (Mann-Whitney and Fisher exact test when appropriated). The interobserver and intraobserver reliabilities were quantified using the interclass coefficient. Associations between patient outcomes (ROM, WOMAC scores, Likert scale and VAS) and patient characteristics (including age, sex, stage of osteoarthritis) were examined and comparison between injection methods were made. An alpha level of p < 0.05 was used to determine statistical significance.
3. Results
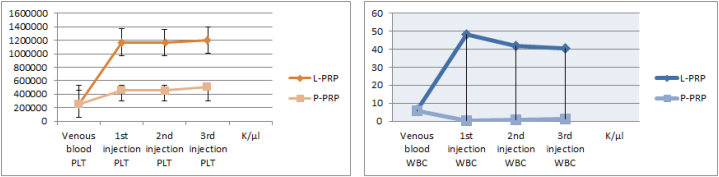
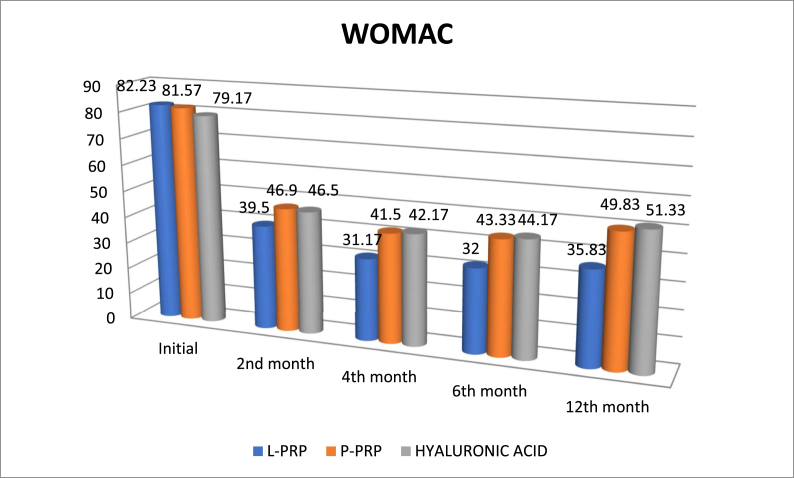
Mean follow-up was 14 ± 3.0 months (range, 12–18 months). And mean age was 60.6 ± 8.65 years. Of them, 11 (12.2%) were male and 79 (87.8%) were female. Forty one patient had left, 49 had right injections. According to body mass index (BMI), 6 were classified as normal, 19 were overweight, 37 were obese, 20 were severe obese and 8 patients were classified as malign obese. Of them, 24 (26.6%) patient had at least one comorbidity. With regard to demographic data including age, gender, BMI, affected side or comorbidity, there were not statistically significant differences among patients (Table 1). The ANOVA test revealed that repetitive measurements of platelet WBC and neutrophil counts had no difference between three injections (F = 219,718; p < 0.001, F = 4.226; p < 0.001 and F = 1.388; p < 0.001, respectively). The mean platelet count for LR-PRP solution was 1,178,177.7 ± 320,69platelet/μlt and 475,733.3 ± 126.74 platelet/μlt for P-PRP solution. While venous blood samples contained a mean of 252,400 platelet/μlt and 247,266 platelet/μlt for LR-PRP and LP-PRP solutions, respectively, the resultant mean platelet increase for LR-PRP was 4.6 fold and 1.9 fold for LP-PRP. Accordingly, the mean WBC and neutrophil counts for LR-PRP and LP-PRP were 43,575.4 ± 13.8WBC/μlt and 23,133 ± 7.3 neutrophil/μlt, 0,866 ± 1.5 WBC/μlt and 0,244 ± 2.5 neutrophil/μlt, respectively (Fig. 2). Finally, for the two PRP groups, the PAW classification demonstrated that LR-PRP had P3-Aα and LP-PRP had P2-Bβ. The measurements of OA grade between two observers had very good to excellent interobserver and intraobserver reliability. Initial evaluation of patients’ radiographs for OA severity yielded 35 patients with grade II and 55 patients with grade III OA. Radiographic progression of OA was noted at 11 patients at the last follow-up and 6 of them progressed (two in each group) from grade 3 to grade 4. Our data did not find any association between rates of osteoarthritis progression and intervention type (p = 0,223). Comparing the clinical outcomes between groups with pre-intervention scores demonstrated significant improvements (Table 2). The mean initial VAS scores of LP-PRP, LR-PRP and HA groups were 8.83 ± 1.2, 8.93 ± 0.94 and 8.77 ± 1.22, respectively. All patients showed significant decrease in VAS scores at the last follow-up. However, the 2nd, 6th and 12th month VAS scores of LP-PRP, LR-PRP and HA groups showed that LR-PRP had the most obvious improvement. Interestingly, the VAS scores diminished from the beginning of second month but begun to increase from the end of 6th month, especially at HA group. According to the analysis results, there was no significant difference in VAS score between the groups regardless of age, gender, BMI, side and comorbidity, regardless of the difference between the groups (p > 0.05). Similar to VAS scores with regard to pre-intervention, WOMAC scores demonstrated significant improvements. At different time intervals, all patients had statistically significant changes at WOMAC scores. However, the mean WOMAC scores of patients at HA group (mean WOMAC = 52.67), LR-PRP group (mean WOMAC = 44.15) and LP-PRP group (mean WOMAC = 52.63) were different; the LR-PRP group had the lowest WOMAC scores (Fig. 3). According to the analysis, relationship between WOMAC score and group interaction was found significant. There was also a significant difference between the different measures of WOMAC scores in these groups (p < 0.05). Evaluation of the data revealed that WOMAC scores begun to decrease from the beginning of the second month, but the scores of LR-PRP group decreased the most with regard to other two groups. In all groups, WOMAC scores increased from the beginning of 6th month, while the greatest increase was found in HA group and LP-PRP group. As a result of the analysis of the temporal changes of WOMAC scores in different groups, the change of WOMAC scores with respect to time was significant in all groups. Also, there was no significant difference between WOMAC scores in term of age, gender, BMI, side and comorbidities in groups, regardless of the difference of WOMAC scores (p > 0.05). At the end of 12th month, despite deterioration of WOMAC and VAS, 24 (80%) patients at LR-PRP group had Likert 5 points whereas LP-PRP group had 9 (30%) and HA group had 4 (13%) patients with Likert 5 points. Finally, all three injection types worked well at patients at the 6th decade of age, male gender and obese patients with regard to severe or malign obese patients (Table 3). Recurrence of symptoms was statistically lower (3; 10%) in LR-PRP group (p < 0.001). Male gender had lower recurrence rate than females (1 vs. 18; p = 0.043). Only high BMI had statistically negative effect on recovery and recurrence rates (p = 0.004). Any relationship between comorbidity and recurrence or recovery was not seen (p = 0.526). Regarding major adverse effects, we did not see any case of deep infection. However, we had 17 patients (12 patients at group LR-PRP,3patients at group LP-PRP, 2 patients at HA group) with local adverse reactions including post-injection pain, burning sense, swelling limitation of daily activities. These side effects were resolved by the first week in all patients after anti-inflammatory medication, activity modification and cold application.
Table 1.
Baseline characteristics of the patients who completed interventions.
| HA (n = 30) | LR-PRP (n = 30) | LP-PRP (n = 30) | p | |
|---|---|---|---|---|
| Age, mean ± SD years | 63 ± 9.17 | 60.3 ± 7.65 | 58.93 ± 6.25 | 0.274 |
| <60 | 13 | 15 | 19 | |
| 60-69 | 10 | 11 | 7 | |
| >70 | 7 | 4 | 4 | |
| BMI, mean (SD) kg/m3 | 32.4 ± 4.2 | 31.27 ± 4.08 | 32.53 ± 6.25 | 0.370 |
| Normal | 0 | 2 | 4 | |
| Overweighed | 8 | 7 | 4 | |
| Obese | 11 | 15 | 11 | |
| Severe obese | 7 | 5 | 8 | |
| Morbidly obese | 4 | 1 | 3 | |
| Gender, (Female), no. (%) | %86.7 | %86.7 | %90 | 0.902 |
| Comorbidities | 0.561 | |||
| None | 22 | 20 | 24 | |
| HT + DM | 8 | 10 | 6 | |
| VAS | 8.77 ± 1.22 | 8.93 ± 0.94 | 8.83 ± 1,21 | 0.356 |
| WOMAC | 79.17 ± 13.27 | 82.23 ± 8.37 | 81.57 ± 13.74 | 0.322 |
Abbreviations: LP-PRP, leukocyte poor PRP; LR-PRP, leukocyte rich PRP; BMI, body mass index; DM, diabetes mellitus; HT, hypertension.
Fig. 2.
The platelet and WBC count at three consecutive injections by hematology analyzer system.
Table 2.
VAS and WOMAC scores at follow-up in the treatment groups (mean ± SD).
| Group | 2 Months | 6 Months | 12 Months | P | |
|---|---|---|---|---|---|
| VAS | HA | 3.57 ± 2.49 | 3.70 ± 2.51 | 4.97 ± 2.67 | |
| L-PRP | 2.60 ± 2.25 | 1.83 ± 2.00 | 2.23 ± 2.33 | <0.001 | |
| P-PRP | 2.87 ± 1.94 | 2.97 ± 1.48 | 4.17 ± 2.34 | ||
| WOMAC | |||||
| Total | HA | 46.50 ± 21.90 | 44.17 ± 12.01 | 51.33 ± 21.89 | |
| L-PRP | 39.50 ± 17.54 | 32.00 ± 17.00 | 35.83 ± 19.35 | 0.048 | |
| P-PRP | 46.90 ± 18.36 | 43.33 ± 15.55 | 49.83 ± 19.83 | ||
| Pain | HA | 10.80 ± 5.90 | 9.17 ± 2.01 | 12.23 ± 4.89 | |
| L-PRP | 4.50 ± 2.44 | 3.50 ± 1.70 | 4.13 ± 2.35 | 0.020 | |
| P-PRP | 10.20 ± 6.26 | 9.73 ± 5.35 | 9.83 ± 5.83 | ||
| Stiffness | HA | 3.90 ± 1.90 | 4.17 ± 2.01 | 4.23 ± 1.89 | |
| L-PRP | 3.60 ± 2.14 | 3.70 ± 1.70 | 3.83 ± 1.32 | 0.230 | |
| P-PRP | 3.70 ± 1.86 | 3.90 ± 1.55 | 4.13 ± 1.84 | ||
| Function | HA | 33.50 ± 17.90 | 30.17 ± 2.01 | 35.33 ± 21.89 | |
| L-PRP | 30.50 ± 13.54 | 25.00 ± 17.00 | 29.83 ± 19.35 | 0.090 | |
| P-PRP | 33.90 ± 14.36 | 30.33 ± 15.55 | 34.83 ± 19.83 | ||
| Likert | HA | 2.9 ± 1.1 (1–5) | |||
| L-PRP | 4.5 ± 0.4 (3–5) | 0.001 | |||
| P-PRP | 3.4 ± 0.9 (1–5) | ||||
Fig. 3.
The change of WOMAC scores with respect to time.
Table 3.
Final analysis of the patients for recurrence of symptoms with regard to type of injection and baseline characteristics [no. (%)].
| No recurrence | Recurrence | P | |
|---|---|---|---|
| Group | |||
| HA | 20 (%66.7) | 10 (%33.3) | |
| LR-PRP | 27 (%90) | 3 (%10) | 0.001 |
| LP-PRP | 24 (%80) | 6 (%20) | |
| Age | |||
| <60 | 30 (%81) | 7 (%19) | |
| 61-69 | 32 (%80) | 8 (%20) | 0.152 |
| >70 | 9 (%69) | 4 (%31) | |
| Gender | |||
| Male | 10 (%90.9) | 1 (%9.1) | 0.043 |
| Female | 61 (%77.2) | 18 (%22.8) | |
| BMI | |||
| Normal | 6 (%100) | 0 | |
| Overweighed | 17 (%89.5) | 2 (%10.5) | |
| Obese | 34 (%91.9) | 3 (%8.1) | 0.004 |
| Severe obese | 9 (%45) | 11 (%55) | |
| Morbidly obese | 5 (%62.5) | 3 (%37.5) | |
| Comorbidity | |||
| None | 53 (%81.5) | 12 (%18.5) | |
| DM | 4 (%66.7) | 2 (%33.3) | 0.526 |
| HT | 8 (%66.7) | 4 (%33.3) | |
| DM + HT | 5 (%83.3) | 1 (%16.7) | |
4. Discussion
Osteoarthritis of the knee is a chronic degenerative disease, which has several treatment methods and its prevalence increases because of the aging population.8 Total Knee Arthroplasty (TKA) is the mainly suggested treatment method for advanced stage knee osteoarthritis, but conservative methods are more popular for the earlier stages. For Kellgren-Lawrence grade 2 and 3 osteoarthritis, oral glycosaminoglycan preparations and non-steroidal anti-inflammatory drugs (NSAID), lifestyle modifications and physiotherapy are utilized, and if these methods fail then hyaluronic acid (HA), ozone, steroids and platelet-rich plasma (PRP) are tried for regenerating the avascular joint cartilage and relieving the symptoms.9 HA is commonly thought to increase lubrication on joint surface and diminish the surface tension with its visco-induction feature and with these properties have an active role in treatment of knee osteoarthritis.10 In the last years, with increasing popularity, PRP consisted of active platelets having high concentrations biologically active proteins is produced by several methods and used to induce morphogenesis of chondral tissue and diminish inflammation. In this study, midterm effects of two kinds of PRP solutions and HA on moderate staged knee osteoarthritis were compared. All of three methods were significantly effective in diminishing the symptoms on the admission in the midterm. These methods were effective especially on the sixth-decade old male patients who are not obese. On the other hand, these methods were less effective on over 70 years old, female and obese patients, and these patients had tendency for recurrence of symptoms in the midterm. The most significant result was, with more transient local side effects, L-PRP was more effective than P-PRP and HA.
This study compare two kinds of PRP solutions which weren't compared directly each other for gonartrosis in literature. However, this study has some drawbacks. First, amount of the growth factors and cytokines in the PRP was not evaluated. According to the literature, amount of growth factors is correlated well with platelet count and amounts of IL-1 and TNFα are correlated with leukocyte count. Only platelet and leukocyte counts in PRP solutions were documented in this study. Second is the difficulty in RPM and g correlation in obtaining leukocyte-deficient PRP with manual methods. We made standardization easily with the help of our pilot study conducted with 20 patients. Third, long-term results were missing because of the relatively short follow-up (mean 14 months). Lastly, according to the prior power analysis, 30 patients in each group was enough for 80% power, but more patients are needed for a more powerful result.
There are some randomized controlled trials in the literature examining the effect of PRP on the knee osteoarthritis, in which sodium hyaluronate was used as control but manually prepared PRP solutions were also studied.11, 12, 13, 14 Protocols for PRP preparation are open for development. Marcaci, landesberg and Filardo described different methods for harvesting PRP, with spin rates between 1 and 3 and different spin times, with different tubes and activators.3,15,16 As understood from this fact, there is no consensus on the PRP harvesting procedures. The main difference between these procedures is the leukocyte count in these solutions. Supporters of the LR-PRP method argued that high leukocyte count is necessary for regulation of inflammatory processes and also for the effectivity of growth factors. These leucocytes are also thought to have an antimicrobial effect.17 Supporters of P-PRP discussed that LR-PRP inhibits cartilage healing via increased free oxygen radicals and metalloproteinases by the high leukocyte concentration.18,19 Browning et al. showed the cytokine and matrix metalloproteinase ingredient of PRP and claimed that these mediators increase cartilage metabolism and induce synoviocyte damage in in-vivo cell culture, but also according to this study, which compared the clinical results of both LR-PRP and LP-PRP with HA as a control group, LR-PRP had better results than LP-PRP, a similar result with the study of Paterson et al.14,18
The second important difference of these methods is platelet concentrations. Mishra et al. described a basal value of four-folds more platelet counts than peripheral blood for the effectivity of PRP, and developed a classification, called Mishra Classification according to this.20 Marx et al. described this amount as five-folds.21 This threshold is commonly overrun in LR-PRP method, but not in LP-PRP method and this is another point for the supporters of LR-PRP method. Cavallo et al. compared the effect of LR-PRP on cartilage regeneration with LP-PRP in an in-vitro study and found that catabolic activity is prominent in LR-PRP group and anabolic activity is prominent in LP-PRP group on 7th day.4 LP-PRP is found to be more effective to stimulate the tendon stem cells than LR-PRP in an animal study.22 Animal studies and in vitro studies discussed above trigger us in favor of LP-PRP but in our clinical study it was shown that LR-PRP is more effective in the treatment of moderate-stage osteoarthritis in midterm. Mariani et al. made periodical joint aspirations after LR-PRP and HA injections for a clinical study and showed similar increments of the proinflammatory cytokines, especially IL-1.6 This may explain the discordance between clinical results and in-vitro and in-vivo studies.
The radiological progress of osteoarthritis after intraartricular injections is usually poorly studied. In our study, the radiograms of the patients in the last control examinations were evaluated for radiological staging. On follow-up, 11 patients (12.2%) showed progress, 6 patients (6.7%) became last stage. No statistically significant difference between the groups were found, in terms of Kellgren-Lawrence stages (p = 0.223). After one-year follow-up, three patients, whose radiological stage became 4 and symptoms aggravated, were referred for surgical treatment. If TKA is accepted as the last step of the treatment of knee osteoarthritis, then there were failures in two patients of HA group and one patient (3.3%) in LP-PRP groups. We can conclude that knee injections do not decelerate the progress of knee osteoarthritis.
It is hard to obtain long-term results of intraartricular injections, because the disease is progressively degenerative, and recurrence of symptoms is common, so treatment method is usually altered in the follow-up course. The patients, whose functional scores diminished to the start point were re-evaluated. A profile of patients who will likely to have a recurrence of symptoms was obtained from demographical data and groups. It is known that therapeutical effect of PRP, especially LP-PRP decreases after 6th month of treatment.13 Filardo et al. showed that LR-PRP and HA injections have similar clinical results in terms of functional scores and post-injection pain and swelling is more common in PRP group and claimed that leukocyte depletion can yield more favorable results after 12 months.11 Besides the similar effectivity of LR-PRP with HA in terms of clinical results shown in this study, PRP obtained with a completely biological and manual method was shown to be superior.
Riboh et al. compared side effects with functional scores and found a transient aggravation of symptoms with LR-PRP.23 No aggravation was found after LP-PRP injection and this suggests that high concentrations of leucocytes are associated with transient inflammation and worsening of symptoms. Despite the different results reported, PRP is more commonly associated with a transient aggravation of symptoms and local adverse reactions.
In conclusion, PRP is more effective on knee osteoarthritis than HA in terms of functional results. Patients were more satisfied with PRP injections. Among the PRP obtaining methods, two-spin method for LR-PRP is better in short-term and its effect lasts longer. Female gender, comorbidities and obesity are risk factors for recurrence of symptoms after treatment. Local side effects are more common with LR-PRP.
Funding
This work was supported by Hospital's education planning committee; award no. 43278876-929-2424/4701.
Declaration of competing interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgement
We express our appreciation to XY, MD, whose contribution to statistical analysis was of great significance.
References
- 1.Henrotin Y.E., Bruckner P., Pujol J.P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2013;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 2.Wang-Saegusa A., Cugat R., Ares O., Seijas R., Cusco X., Garcia-Balletbo M. Infiltration of plasma rich in growth factors for osteoarthritis of the knee short-term effects on function and quality of life. Arch Orthop Trauma Surg. 2011;131:311–317. doi: 10.1007/s00402-010-1167-3. [DOI] [PubMed] [Google Scholar]
- 3.Filardo G., Kon E., Di Martino A. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Muscoskel Disord. 2012;23(13):229. doi: 10.1186/1471-2474-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavallo C., Filardo G., Mariani E. Comparison of platelet-rich plasma formulations for cartilage healing: an in vitro study. J Bone Joint Surg Am. 2014;5(5):423–429. doi: 10.2106/JBJS.M.00726. 96. [DOI] [PubMed] [Google Scholar]
- 5.McCarrel T.M., Minas T., Fortier Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;3(1-8):e143. doi: 10.2106/JBJS.L.00019. 94(19) [DOI] [PubMed] [Google Scholar]
- 6.Mariani E., Canella V., Cattini L. Leukocyte-rich platelet-rich plasma injections do not up-modulate intra-articular pro-inflammatory cytokines in the osteoarthritic knee. PloS One. 2016;3(6) doi: 10.1371/journal.pone.0156137. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLong J.M., Rusdell R.P., Mazzocca A.D. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28:998–1009. doi: 10.1016/j.arthro.2012.04.148. [DOI] [PubMed] [Google Scholar]
- 8.Dennison E, Cooper C. Osteoarthritis: epidemiology and classification. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH (eds). Rheumatol. 2003; third ed. Mosby, Edinburgh, pp 1781-1791.
- 9.Mathieu Pierre, Conrozier Thierry, Vignon Eric, Rozand Yves, Rinaudo Marguerite. Rheologic behavior of osteoarthritic synovial fluid after addition of hyaluronic acid: a pilot study. Clin Orthop Relat Res. 2003;467:3002–3009. doi: 10.1007/s11999-009-0867-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duymus T.M., Mutlu S., Dernek B., Komur B., Aydogmus S., Kesiktas F.N. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc. 2017;25(2):485–492. doi: 10.1007/s00167-016-4110-5. [DOI] [PubMed] [Google Scholar]
- 11.Filardo G., Di Matteo B., Di Martino A. Platelet-rich plasma intra-articular knee injections show No superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43(7):1575–1582. doi: 10.1177/0363546515582027. [DOI] [PubMed] [Google Scholar]
- 12.Görmeli G., Görmeli C.A., Ataoğlu B., Çolak C., Aslantürk O., Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958–965. doi: 10.1007/s00167-015-3705-6. [DOI] [PubMed] [Google Scholar]
- 13.Patel S., Dhillon M.S., Aggarwal S., Marwaha N., Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 14.Paterson K.L., Nicholls M., Bennell K.L., Bates D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: a double-blind, randomized controlled pilot study. BMC Muscoskel Disord. 2016;9:67. doi: 10.1186/s12891-016-0920-3. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kon E., Buda R., Filardo G. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):472–479. doi: 10.1007/s00167-009-0940-8. [DOI] [PubMed] [Google Scholar]
- 16.Landesberg R., Roy M., Glickman R.S. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297–300. doi: 10.1016/s0278-2391(00)90058-2. [DOI] [PubMed] [Google Scholar]
- 17.Dohan Ehrenfest D.M., Andia I., Zumstein M.A., Zhang C.Q., Pinto N.R., Bielecki T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;8(4):3–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Browning S.R., Weiser A.M., Woolf N. Platelet-rich plasma increases matrix metalloproteinases in cultures of human synovial fibroblasts. J Bone Joint Surg Am. 2012;5(23):e1721–e1727. doi: 10.2106/JBJS.K.01501. 94. [DOI] [PubMed] [Google Scholar]
- 19.DeLong J.M., Beitzel K., Mazzocca A.D., Shepard D., Roller B.L., Hanypsiak B.T. Update on platelet-rich plasma. Curr Orthop Pract. 2011;22:514–522. [Google Scholar]
- 20.Mishra A., Harmon K., Woodall J., Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharmaceut Biotechnol. 2012;13:1185–1195. doi: 10.2174/138920112800624283. [DOI] [PubMed] [Google Scholar]
- 21.Marx R.E. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Yin W., Qi X., Zhang Y. Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in promoting repair of bone defects. J Transl Med. 2016;14:73. doi: 10.1186/s12967-016-0825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampson S., Gerhardt M., Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3-4):165–174. doi: 10.1007/s12178-008-9032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]