Abstract
Objective
To determine independent risk factors for inappropriate antibiotic prescribing for acute respiratory tract infections (ARIs) in internal medicine (IM) residency–based primary care offices.
Patients and Methods
A retrospective study was conducted to measure antibiotic prescribing rates, and multivariable analysis was utilized to identify predictors of inappropriate prescribing among patients presenting to IM residency–based primary care office practices. Patients with an office visit at either of 2 IM residency–based primary care office practices from January 1, 2016, through December 31, 2016, with a primary encounter diagnosis of ARI were included.
Results
During the study period, 911 unique patient encounters were included with 518 for conditions for which antibiotics were considered always inappropriate. Antibiotics were not indicated in 85.8% (782 of 911) of encounters. However, antibiotics were prescribed in 28.4% (222 of 782) of these encounters. Inappropriate antibiotic prescribing occurred in 111 of 518 (21.4%) encounters for conditions for which antibiotics are always inappropriate. Using multivariable logistic regression analysis to assess for independent risk factors when adjusted for other potential risk factors for office visits at which antibiotics were not indicated, IM resident–associated visits (odds ratio, 0.25; 95% CI, 0.18-0.36) was the only variable independently associated with lower risk of inappropriate antibiotic prescribing.
Conclusion
For ARI visits at which antibiotics were not indicated, IM resident comanagement was associated with lower rates of inappropriate prescribing.
Abbreviations and Acronyms: AHN, Allegheny Health Network; ARI, acute respiratory tract infection; ASP, antimicrobial stewardship program; EHR, electronic health record; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; IM, internal medicine; OR, odds ratio; URI, upper respiratory tract infection
The US Centers for Disease Control and Prevention estimates that 2 million infections are caused by antibiotic-resistant pathogens, leading to more than 23,000 deaths per year and $20 billion in additional health care costs in the United States alone, with overuse of antibiotics as the main propagator of antibiotic resistance.1,2 The development of strategies aimed at reducing antibiotic exposure is critical.2, 3, 4, 5, 6 Antimicrobial stewardship programs (ASPs) have largely focused on hospitalized patients, while the bulk of antibiotic prescribing occurs in the ambulatory setting. The ambulatory setting accounts for more than 60% of antibiotic expenditures in the United States, and 40% to 75% of these prescriptions are inappropriate.7, 8, 9 The White House’s National Action Plan for Combating Antibiotic-Resistant Bacteria set a goal of a 50% reduction in inappropriate ovutpatient use by 2020.10 In order to accomplish this goal, ASPs will have to focus on commonly encountered outpatient conditions for which antibiotics are frequently overprescribed. Acute respiratory tract infections (ARIs) account for nearly 60% of all outpatient antibiotic prescriptions and are predominantly viral in origin.11, 12, 13, 14, 15, 16 Thus, ARIs represent an ideal opportunity for ASPs to target.
Previous studies have attempted to determine risk factors for unnecessary antibiotic prescribing in the outpatient setting17, 18, 19, 20, 21, 22; however, a paucity of data exists for antibiotic prescribing practices at residency-based primary care settings, which have unique forces impacting the practice of evidenced-based medicine. Before implementing ASP initiatives to optimize ARI-associated antibiotic use within the Allegheny Health Network (AHN), we aimed to identify characteristics associated with inappropriate antibiotic prescribing for ARIs among physicians in internal medicine (IM) residency–based primary care offices.
Patients and Methods
Study Setting and Population
The study was performed at 2 primary care practices of the IM residency program within AHN in Pittsburgh, Pennsylvania. The IM residency is comprised of 90 categorical residents with 30 in each of 3 postgraduate years. Residents are divided between 2 primary care office practices, each affiliated with a large teaching hospital. The practices account for approximately 14,000 patient encounters annually.
Residents have a regular schedule of 5 clinic sessions every 5 weeks, including urgent care visits. Each resident has a panel of approximately 90 patients. Cohorts of 8 to 10 residents are assigned to 1 to 2 primary attending physician clinic preceptors. During the study period, there were 21 attending physicians between the 2 practices, 17 of whom served as clinic preceptors. All clinic preceptors are key faculty members in the IM program, are involved in providing didactic education, and receive regular faculty development support. In addition to evaluating patients in a supervisory and comanagement role with IM residents, clinic preceptors also spend time in independent practice without residents. There are 4 attending physicians at the 2 practices who evaluate patients in the same clinic space but are not preceptors for IM residents.
This study was approved and granted exempt status from the AHN Institutional Review Board.
Study Design
We conducted a retrospective analysis using electronic health records (EHRs). Inclusion criteria required patients to have an office visit at either IM residency–based primary care office practice from January 1, 2016, through December 31, 2016, with a primary encounter diagnosis of ARI using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) coding data. The search codes included acute nasopharyngitis (460, ICD-9-CM; J00, ICD-10-CM), acute laryngitis and tracheitis (464, ICD-9-CM; J04, J05, ICD-10-CM), acute laryngopharyngitis/upper ARI (465, ICD-9-CM; J06, ICD-10-CM), acute bronchitis (466, ICD-9-CM; J20.9, J21.0, J21.8, ICD-10-CM), bronchitis not specified as acute or chronic (490, ICD-9-CM; J40, ICD-10-CM), acute rhinosinusitis (461, ICD-9-CM; J01, ICD-10-CM), and acute pharyngitis (462, ICD-9-CM; J02.9, ICD-10-CM). For patients with multiple encounters during the study period, each episode was reviewed independently. Using a standardized data collection instrument, study investigators verified the encounter diagnosis and demographic data and obtained information regarding patient comorbidities, microbiological data, radiographic studies, antimicrobial therapy, and subsequent inpatient clinical encounters at the 2 sites during the 30 days following the index office encounter.
Patients were excluded if they were younger than 18 years of age or had a concomitant non-ARI bacterial infection that required systemic antibiotic therapy, neutropenia defined as an absolute neutrophil count of less than 1000/μL (to convert to ×109/L, multiply by 0.001), moderate to severe cell-mediated immunodeficiency, or a history of chronic lung disease (defined as chronic obstructive pulmonary disease, chronic emphysema, chronic bronchiectasis, or cystic fibrosis). Patients with a history of asthma were included. Investigators also excluded any patients whose duration of prescribed antibiotics could not be determined, those who solely had a telephone encounter, and patients with moderate to severe immunodeficiency (defined as use of long-term immunosuppressive therapy at the time of admission [equivalent of >10 mg prednisone daily], human immunodeficiency virus with a CD4 cell count of less than 350 cells/mm3, active malignancy with receipt of systemic chemotherapy within the 30 days before the index office encounter, or previous solid organ transplant or hematopoietic stem cell transplant).
Study Outcomes and Definitions
The primary aims of the study were to measure antibiotic prescription rates and to identify predictors of inappropriate antibiotic prescribing among patients presenting to IM residency–based primary care practices for ARIs. Secondary aims included appropriateness of antibiotic agent selection, antibiotic duration of therapy, and optimal antibiotic prescribing when deemed appropriate.
Acute respiratory tract infections were separated into diagnoses for which antibiotics are always deemed inappropriate and conditions for which antibiotics are potentially appropriate. Diagnoses for which antibiotics are always inappropriate were upper ARIs, acute bronchitis, acute laryngitis, and acute tracheitis. Diagnoses for which antibiotics are potentially appropriate were pharyngitis and rhinosinusitis. For encounters for pharyngitis, antibiotics were considered appropriate if either the result of a group A streptococcal rapid antigen diagnostic test was positive or if a throat culture revealed growth of a β-hemolytic streptococci or other pathogenic bacteria.23 Appropriate pharyngitis antibiotic therapies included either 10 days of oral penicillin V or oral amoxicillin or a single intramuscular dose of benzathine penicillin G. For patients with a documented penicillin allergy, oral antibiotics that were considered appropriate were either 10 days of cephalexin, cefadroxil, clindamycin, or clarithromycin or 5 days of azithromycin.23
In accordance with the Infectious Diseases Society of America practice guidelines, antibiotics were considered appropriate for rhinosinusitis encounters for any of the following clinical presentations24: (1) onset with persistent symptoms or signs compatible with acute rhinosinusitis lasting for 10 or more days without any evidence of clinical improvement, (2) onset with severe symptoms or signs of high fever (≥39°C) and purulent nasal discharge or facial pain/pressure for 3 to 4 days at the beginning of illness, and (3) onset with worsening symptoms or signs with focal findings of rhinosinusitis characterized by new onset of fevers, headache, and increasing nasal discharge after initial improvement of typical upper respiratory tract infection (URI) symptoms (ie, “double-worsening”). Appropriate rhinosinusitis antibiotic therapy included amoxicillin/clavulanate for 5 to 7 days. For patients with a documented penicillin allergy, antibiotics that were considered appropriate were 5 to 7 days of doxycycline, levofloxacin, or moxifloxacin.24
Duration of therapy was defined as the number of days for which an antibiotic was prescribed. Optimal antibiotic prescribing was defined as use of a preferred, first-line antibiotic agent as well as appropriate duration of therapy when antibiotics were deemed appropriate.
Statistical Analyses
Categorical variables were assessed using a χ2 or Fisher exact test. Normality was assessed for continuous variables; either parametric t tests or nonparametric Wilcoxon rank sum tests were conducted. Simple and multivariable logistic regression on inappropriate antibiotic usage (dependent) was used to determine associations with variables such as patient demographic characteristics, clinically relevant risk factors, and resident-specific demographic characteristics. P<.05 was considered statistically significant in all statistical tests. Variables eligible for inclusion in the multivariable model included those with P<.2 in the univariate analysis. A stepwise multivariable logistic regression model selection technique was used in which variables entered the model if their values were P<.2 initially but exited the model if the variable did not remain P<.2 as other variables were added. SAS Enterprise Guide, version 7.11 HF3 (SAS Institute) was used to conduct the statistical analyses.
Results
During the study period, 1118 patients with a principle diagnosis of ARI were initially identified by ICD-9-CM and ICD-10-CM coding. After manual EHR review, 207 patients were excluded because of a history of chronic obstructive pulmonary disease or structural lung disease (98), a concomitant visit diagnosis necessitating antibiotic therapy (44), long-term use of corticosteroids or immunosuppressive mediations (34), immunosuppressed disease state (26), and active malignancy (5).
The final cohort included 911 unique encounters (Table 1), which included 821 unique patients. There were 518 encounters for conditions for which antibiotics were considered always inappropriate. There were 393 encounters for rhinosinusitis (n=238) and pharyngitis (n=155), conditions for which antibiotics are potentially appropriate. There was an indication for antibiotics in 44.1% of encounters for rhinosinusitis (105 of 238), but only15% of encounters for pharyngitis (24 of 155) had an indication for antibiotics.
Table 1.
Characteristics of Antibiotic Use for Outpatient Acute Upper Respiratory Tract Infection Visits
| Variable | Antibiotics prescribed | Antibiotic duration when prescribed (d) | Duration ≤7 d | Duration ≥10 d | Appropriate antibiotic duration | Appropriate antibiotic agent | Optimal prescribing |
|---|---|---|---|---|---|---|---|
| Antibiotics always inappropriate (n=518) | 111/518 | 6.7 | 80 | 31 | |||
| Acute nasopharyngitis | 2/33 | 6.0 | 2 | 0 | |||
| Acute laryngitis or tracheitis | 5/22 | 8.4 | 2 | 3 | |||
| Acute laryngopharyngitis | 0/1 | 0 | 0 | 0 | |||
| Acute upper respiratory tract infection | 35/275 | 7.0 | 25 | 10 | |||
| Acute bronchitis | 18/36 | 6.4 | 13 | 5 | |||
| Bronchitis not specified | 50/124 | 6.5 | 38 | 12 | |||
| Influenza | 1/27 | 5 | 1 | 0 | |||
| Antibiotics potentially appropriate (n=393) | 238/393 | 8.0 | 115 | 123 | |||
| Sinusitis without indication for antibiotics | 79/133 | 8.0 | 35 | 44 | |||
| Sinusitis with indication for antibiotics | 104/105 | 7.8 | 56 | 49 | 55/105 (52.4%) | 77/105 (73.3%) | 30/105 (28.6%) |
| Pharyngitis without indication for antibiotics | 32/131 | 7.8 | 18 | 14 | |||
| Pharyngitis with indication for antibiotics | 23/24 | 8.8 | 7 | 16 | 18/23 (78.3%) | 11/23 (47.8%) | 12/23 (52.2%) |
Overall, antibiotics were not indicated in 85.8% of encounters (782 of 911). However, antibiotics were prescribed in 28.4% of these encounters (222 of 782). Inappropriate antibiotic prescribing occurred in 111 of 518 encounters (21.4%) for conditions for which antibiotics are always inappropriate. Antibiotics were prescribed in 79 of 133 rhinosinusitis encounters without an indication for antibiotics (59.4%) and in 32 of 131 pharyngitis encounters without an indication for antibiotic therapy (24.4%).
There were 129 encounters with an indication for antibiotics. Antibiotics were prescribed in 104 of 105 encounters for rhinosinusitis with an indication for antibiotics, and antibiotics were prescribed in 23 of 24 encounters for pharyngitis with an indication for antibiotics. Of the 105 encounters for rhinosinusitis with an indication for antibiotics, 55 (52.4%) received the appropriate duration of 5 to 7 days, 77 (73.3%) received an appropriate agent, and only 30 patients (28.6%) received optimal prescribing with an appropriate drug for the appropriate duration. Of the 24 office visits for pharyngitis with an indication for antibiotics, 18 of the 23 for whom antibiotics were prescribed (78.3%) received the appropriate duration, 11 (47.8%) received an appropriate agent, and 12 (52.2%) patients received optimal prescribing.
Simple logistic regression analysis for physician- and patient-specific predictors of inappropriate antibiotic prescribing for office visits at which antibiotics were not indicated is shown in Table 2. There was an increased risk of inappropriate antibiotic prescription if the attending physician age was older than 40 years (odds ratio [OR], 1.75; 95% CI, 1.3-2.3) and if the attending physician was male (OR, 2.13; 95% CI, 1.5-3.0). Internal medicine resident–associated visits (OR, 0.23; 95% CI, 0.16-0.32) were found to be protective against inappropriate antibiotic prescribing. Among patient-specific factors, there was a higher risk for inappropriate antibiotic prescribing for male patients (OR, 1.62; 95% CI, 1.2-2.2) and for white patients (OR, 2.01; 95% CI, 1.4-2.9).
Table 2.
Simple Logistic Regression of Risk Factors for Antibiotic Prescribing at Visits for Which Antibiotics Were Not Indicated
| Variable | Antibiotics prescribed, No. (%) | Odds ratio (95% CI) | P value |
|---|---|---|---|
| Physician evaluating patient | |||
| Attending physician only | 160/367 (43.6) | Reference | |
| Resident supervised by attending physician clinic preceptor | 62/415 (14.9) | 0.23 (0.16-0.32) | <.001 |
| Attending physician age (y) | |||
| ≤40 | 76/343 (22.2) | Reference | |
| >40 | 146/439 (33.3) | 1.75 (1.3-2.4) | <.001 |
| Attending physician sex | |||
| Female | 61/311 (19.6) | Reference | |
| Male | 161/471 (34.2) | 2.13 (1.5-3.0) | <.001 |
| Patent age (y) | |||
| <65 | 166/593 (28.0) | Reference | |
| ≥65 | 56/189 (29.6) | 1.08 (0.76-1.6) | .66 |
| Patient sex | |||
| Female | 139/548 (25.4) | Reference | |
| Male | 83/234 (35.5) | 1.62 (1.2-2.2) | .004 |
| Patient race | |||
| Non-white | 51/261 (19.5) | Reference | |
| White | 171/521 (32.8) | 2.01 (1.4-2.9) | <.001 |
| Evaluated in the preceding 30 days for same symptoms | |||
| No | 205/717 (28.6) | Reference | |
| Yes | 17/65 (26.2) | 0.89 (0.50-1.6) | .68 |
Table 3 shows the results of a multivariable logistic regression analysis to assess for independent risk factors when adjusted for all other potential risk factors for office visits at which antibiotics were not indicated. In the final model, IM resident–associated visits (OR, 0.25; 95% CI, 0.18-0.36) was the only variable independently associated with lower risk of inappropriate antibiotic prescribing.
Table 3.
Multivariable Logistic Regression Analysis to Assess for Independent Risk Factors for Inappropriate Antibiotic Prescription When Adjusted for All Other Potential Risk Factors for Office Visits at Which Antibiotics Were Not Indicateda
| Variable | OR (95% CI) | P value |
|---|---|---|
| Patient sex, male | 1.35 (0.95-1.9) | .09 |
| Patient race, white | 1.45 (0.99-2.1) | .06 |
| Resident supervised by attending physician clinic preceptor | 0.25 (0.18-0.36) | <.001 |
For stepwise selection of the multivariable model, the final model included variables that maintained a P value <.2. Model’s C statistic was 0.699.
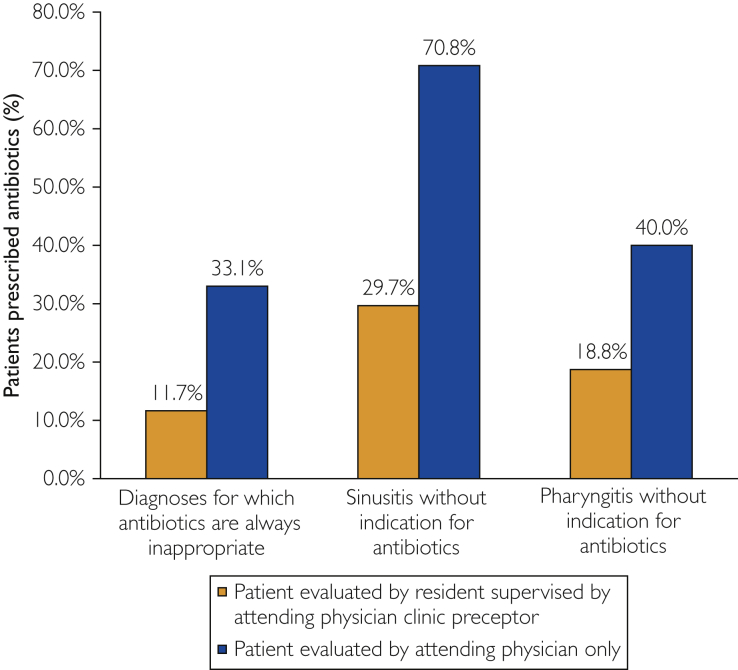
For all 3 diagnosis categories in which antibiotics were not indicated—conditions for which antibiotics are always inappropriate, rhinosinusitis without an indication for antibiotics, and pharyngitis without an indication for antibiotics—antibiotic prescribing rates were lower for IM resident–associated visits than for attending physician–only visits (Figure).
Figure.
Utilization of antibiotics for primary care office visits at which antibiotic therapy was deemed inappropriate.
Additionally, for encounters in which antibiotic prescribing was deemed inappropriate, IM resident–associated visits were associated with significantly lower antibiotic prescribing rates when compared with visits at which an attending physician residency clinic preceptor evaluated the patient without a resident (14.9% [62 of 415] vs 24.4% [30 of 123]; P=.02).
Discussion
We observed that antibiotics were inappropriately prescribed in 28.4% of encounters for ARI. To identify independent risk factors for inappropriate prescribing, we performed a multivariable logistical regression analysis and found that attending physician–only visit was the only independent risk factor for inappropriate antibiotic use, while comanagement with an IM resident was protective.
Although the literature continues to demonstrate ongoing issues with unnecessary antibiotic exposure for ARIs and risk factors for inappropriate prescribing in the outpatient setting,17, 18, 19, 20, 21, 22 a paucity of data exists on the impact of residents on antibiotic use in primary care. Gaur et al25 reported that during outpatient visits for a diagnosis suggestive of a viral URI in children, antibiotic prescribing occurred more commonly among attending staff physicians than trainees (36.5% vs 19.5%; OR, 0.44; 95% CI, 0.33-0.59). This difference between house staff and attending physicians persisted even within teaching hospitals (32.5% vs 19.5%; OR, 0.5; 95% CI, 0.4-0.7). Gonzales et al26 evaluated antibiotic prescribing patterns for ARIs in an emergency department setting. Although antibiotic prescription rates were similar for antibiotic-responsive diagnoses and acute bronchitis for visits comanaged with house staff compared with visits managed by attending physicians only, the antibiotic prescription rate for URIs was greater for attending-only visits compared with house staff–associated visits (48% vs 15%; P=.01). The authors noted that it was possible that the attending physicians who comanaged visits with house staff were different from attending physicians who managed patients without house staff because they were unable to explore this possibility.26 In our analysis, however, we were able to demonstrate that the attending physicians who managed patients behaved differently when they comanaged with IM residents compared with when they managed patients alone. This finding would suggest that behavioral-based, rather than just knowledge-based, interventions aimed at these attending physicians should be deployed.
Given the retrospective, observational design of our study, we were unable to determine the reasons for lower rates of inappropriate antibiotic prescribing for ARIs when patients were comanaged by IM residents. Clinicians have reported prescribing antibiotics because of a perceived lack of time necessary to explain why antibiotics were not needed27 or due to the belief that writing a prescription was faster than communicating nonantibiotic treatment strategies.18 Thus, it is plausible that attending physicians with high caseloads may be more likely to prescribe antibiotics than those with fewer patients, such as house staff.20,22 Furthermore, it is possible that the Hawthorne effect, in which an individual modifies or improves an aspect of their behavior in response to their awareness of being observed, may have played a role in attending physicians’ improved prescribing of antibiotics when a resident was present.28
Interestingly, the overall rate of inappropriate antibiotic prescribing of 28.4% in our cohort for ARI visits is lower than rates in the literature, in which inappropriate prescribing rates vary from 40% to 50%.29, 30, 31, 32 One possible explanation is the presence of a long-standing and robust hospital-based ASP, which regularly interacts with trainees and medical staff via didactics on antimicrobial stewardship principles and philosophies, prior authorization for restricted antimicrobials, prospective audit with real-time intervention and feedback for nonrestricted antimicrobials and targeted disease states such as lower ARIs and skin and soft tissue infections, ASP newsletters, and a yearly antimicrobial guide. Although the efforts of the ASP are focused on the inpatient setting, it is likely that the promoted concepts of evidence-based, high-value, cost-conscious, and judicious antibiotic prescribing indirectly led to alterations in outpatient prescribing patterns as well.
Although our practitioners have frequent exposure to our local ASP, there remain substantial opportunities to improve prescribing when antibiotics are indicated. For patients with rhinosinusitis who met criteria for antibiotics, 47.6% (50 of 105) received inappropriately prolonged duration of therapy, and nearly three-quarters did not receive an appropriate first-line agent. Thus, even when antibiotics are indicated for certain common outpatient conditions, less than 30% of patients received optimal prescribing for rhinosinusitis and 50% for pharyngitis.
In addition to patient and clinical factors, antibiotic prescribing in the outpatient setting for ARIs is driven by psychosocial factors.18,22,27,29,33 Many outpatient antimicrobial stewardship interventions have proven successful at reducing unnecessary antibiotic prescribing, including communications training, accountable justification, feedback with peer comparison, and public commitment posters.22,29,33 Given our findings that attending physicians were more likely to inappropriately prescribe antibiotics when evaluating patients independently compared with when they comanaged patients with a resident, this suggested that there was less of a knowledge deficit among the attending physicians and that sociobehavioral factors may be contributing to unnecessary use when attending physicians cared for patients on their own. Thus, our health network’s ASP collaborated with the IM residency–based practices to create a bundled intervention aimed at optimizing antibiotic use in the outpatient setting for ARIs by incorporating sociobehavioral interventions. Similar to the public commitment letters posted by Meeker et al29 as a behavioral “nudge,” we created 20 × 30-inch antibiotic pledge posters and placed them in all the examination rooms of the 2 primary care practices. These posters display the attending physicians’ pictures and signatures with a message written at a sixth-grade reading level that indicates their commitment to reducing inappropriate antibiotic use for ARIs. We also created antibiotic pledge handouts, with the same verbiage as the posters, that could be distributed to patients presenting for ARIs by the front office staff or clinicians. An ASP newsletter was created and disseminated to all medical staff and trainees with antibiotic prescribing recommendations for common outpatient conditions, including when antibiotics are indicated, first-line and second-line antibiotic agent selection, dosage and duration recommendations, and adjuvant nonantibiotic options. We plan to measure the impact of these interventions in the near future.
Our analysis has several important limitations. The retrospective and observational nature prohibited complete analysis of some potentially significant risk factors because the accuracy of the data was dependent on documentation entered into the EHR. One of these potential risk factors was time of day the office visit took place. Office visits later in the day are known to be associated with higher rates of antibiotic prescribing, which has been attributed to decision fatigue.20,22 Also, we did not evaluate duration of visits for ARIs or number of visits per clinic session. Additionally, this analysis only evaluated residents from one IM residency, so the results may not be generalizable to other practices or residencies, especially those that do not have exposure to a robust ASP.
A major strength of our study is that we evaluated disease states for which antibiotics are always inappropriate as well as those for which antibiotics are potentially appropriate. Rather than simply describing rates of prescribing, we were able to review records and apply criteria for antibiotic administration to determine if management was guideline concordant and consistent with best practice.
Conclusion
The findings of this study strongly suggest that for ARI visits at which antibiotics are not indicated, IM resident comanagement is associated with higher-quality, guideline-concordant care. Further exploration into the impact of both knowledge-based and behavioral-based interventions aimed at optimizing use of antibiotics for ARIs in this setting is urgently needed, given the evolving epidemic of antimicrobial resistance.
Acknowledgments
We thank Drs Patrick Sleckman, Ryan Cooper, Alison O’Donnell, Peter Leehan, Lauren Mathos, James Bruce, Steven Goodnow, Anita Chandra, Leslie DiRuzza, and Brian Lamb for assistance with data extraction and entry.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Centers for Disease Control and Prevention Antibiotic/antimicrobial resistance. Centers for Disease Control and Prevention website. http://www.cdc.gov/drugresistance/ Updated September 10, 2018. Accessed October 26, 2018.
- 2.Spellberg B., Guidos R., Gilbert D., et al. Infectious Diseases Society of America The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 3.Deshpande A., Pasupuleti V., Thota P., et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother. 2013;68(9):1951–1961. doi: 10.1093/jac/dkt129. [DOI] [PubMed] [Google Scholar]
- 4.Brown K.A., Khanafer N., Daneman N., Fisman D.N. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. 2013;57(5):2326–2332. doi: 10.1128/AAC.02176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher H.W., Talbot G.H., Bradley J.S., et al. Bad bugs, no drugs: no ESKAPE! an update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Overview and evidence to support appropriate antibiotic use. Centers for Disease Control and Prevention website. https://www.cdc.gov/antibiotic-use/healthcare/evidence.html Updated October 1, 2019. Accessed October 26, 2018.
- 7.Suda K.J., Hicks L.A., Roberts R.M., Hunkler R.J., Danziger L.H. A national evaluation of antibiotic expenditures by healthcare setting in the United States, 2009. J Antimicrob Chemother. 2013;68(3):715–718. doi: 10.1093/jac/dks445. [DOI] [PubMed] [Google Scholar]
- 8.Shively N.R., Buehrle D.J., Clancy C.J., Decker B.K. Prevalence of inappropriate antibiotic prescribing in primary care clinics within a Veterans Affairs health care system. Antimicrob Agents Chemother. 2018;62(8):e00337–e00418. doi: 10.1128/AAC.00337-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming-Dutra K.E., Hersh A.L., Shapiro D.J., et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864–1873. doi: 10.1001/jama.2016.4151. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Office-related antibiotic prescribing for persons aged ≤ 14 years—United States, 1993-1994 to 2007-2008. MMWR Morb Mortal Wkly Rep. 2011;60(34):1153–1156. [PubMed] [Google Scholar]
- 11.Grijalva C.G., Nuorti J.P., Griffin M.R. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302(7):758–766. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinman M.A., Gonzales R., Linder J.A., Landefeld C.S. Changing use of antibiotics in community-based outpatient practice, 1991-1999. Ann Intern Med. 2003;138(7):525–533. doi: 10.7326/0003-4819-138-7-200304010-00008. [DOI] [PubMed] [Google Scholar]
- 13.Fairlie T., Shapiro D.J., Hersh A.L., Hicks L.A. National trends in visit rates and antibiotic prescribing for adults with acute sinusitis. Arch Intern Med. 2012;172(19):1513–1514. doi: 10.1001/archinternmed.2012.4089. [DOI] [PubMed] [Google Scholar]
- 14.Barnett M.L., Linder J.A. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020–2022. doi: 10.1001/jama.2013.286141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzales R., Malone D.C., Maselli J.H., Sande M.A. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757–762. doi: 10.1086/322627. [DOI] [PubMed] [Google Scholar]
- 16.National Action Plan for Combating Antibiotic-Resistant Bacteria. The White House website. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf Published March 2015. Accessed October 26, 2018.
- 17.Fleming-Dutra K.E., Bartoces M., Roberts R.M., Hicks L.A. Characteristics of primary care physicians associated with high outpatient antibiotic prescribing volume. Open Forum Infect Dis. 2018;15(1):ofx279. doi: 10.1093/ofid/ofx279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dempsy P.P., Businger A.C., Whaley L.E., Gagne J.J., Linder J.A. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract. 2014;15:194. doi: 10.1186/s12875-014-0194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez G.V., Roberts R.M., Albert A.P., Johnson D.D., Hicks L.A. Effects of knowledge, attitudes, and practices of primary care providers on antibiotic selection, United States. Emerg Infect Dis. 2014;20(12):2041–2047. doi: 10.3201/eid2012.140331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linder J.A., Doctor J.N., Friedberg M.W., et al. Time of day and the decision to prescribe antibiotics. JAMA Intern Med. 2014;174(12):2029–2031. doi: 10.1001/jamainternmed.2014.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerber J.S., Prasad P.A., Russell Localio A., et al. Variation in antibiotic prescribing across a pediatric primary care network. J Pediatric Infect Dis Soc. 2015;4(4):297–304. doi: 10.1093/jpids/piu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King L.M., Fleming-Dutra K.E., Hicks L.A. Advances in optimizing the prescription of antibiotics in outpatient settings. BMJ. 2018;363:k3047. doi: 10.1136/bmj.k3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shulman S.T., Bisno A.L., Clegg H.W., et al. Infectious Diseases Society of America Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America [published correction appears in Clin Infect Dis. 2014;58(10):1496] Clin Infect Dis. 2012;55(10):e86–e102. doi: 10.1093/cid/cis629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow A.W., Benninger M.S., Brook I., et al. Infectious Diseases Society of America IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):e72–e112. doi: 10.1093/cid/cir1043. [DOI] [PubMed] [Google Scholar]
- 25.Gaur A.H., Hare M.E., Shorr R.I. Provider and practice characteristics associated with antibiotic use in children with presumed viral respiratory tract infections. Pediatrics. 2005;115(3):635–641. doi: 10.1542/peds.2004-0670. [DOI] [PubMed] [Google Scholar]
- 26.Gonzales R., Camargo C.A., Jr., MacKenzie T., et al. IMPAACT Trial Investigators Antibiotic treatment of acute respiratory infections in acute care settings. Acad Emerg Med. 2006;13(3):288–294. doi: 10.1197/j.aem.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Szymczak J.E., Feemster K.A., Zaoutis T.E., Gerber J.S. Pediatrician perceptions of an outpatient antimicrobial stewardship intervention. Infect Control Hosp Epidemiol. 2014;35(3):S69–S78. doi: 10.1086/677826. [DOI] [PubMed] [Google Scholar]
- 28.McCarney R., Warner J., Iliffe S., van Haselen R., Griffin M., Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meeker D., Knight T.K., Friedberg M.W., et al. Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med. 2014;174(3):425–431. doi: 10.1001/jamainternmed.2013.14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebell M.H., Radke T. Antibiotic use for viral acute respiratory tract infections remains common. Am J Manag Care. 2015;21(10):e567–e575. [PubMed] [Google Scholar]
- 31.Alweis R., Greco M., Wasser T., Wenderoth S. An initiative to improve adherence to evidence-based guidelines in the treatment of URIs, sinusitis, and pharyngitis. J Community Hosp Intern Med Perspect. 2014;4:22958. doi: 10.3402/jchimp.v4.22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hicks L.A., Bartoces M.G., Roberts R.M., et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308–1316. doi: 10.1093/cid/civ076. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez G.V., Fleming-Dutra K.E., Roberts R.M., Hicks L.A. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1–12. doi: 10.15585/mmwr.rr6506a1. [DOI] [PubMed] [Google Scholar]